Chemical Profiling and Geographic Differentiation of Ugandan Propolis by GC-MS Through Chemometric Modelling
Abstract
1. Introduction
2. Results and Discussion
2.1. Propolis Colour Variation Across Sample Sites Representing Agroecological Zones
2.2. Chromatographic Profiles of Ugandan Propolis from Different Agroecological Zones
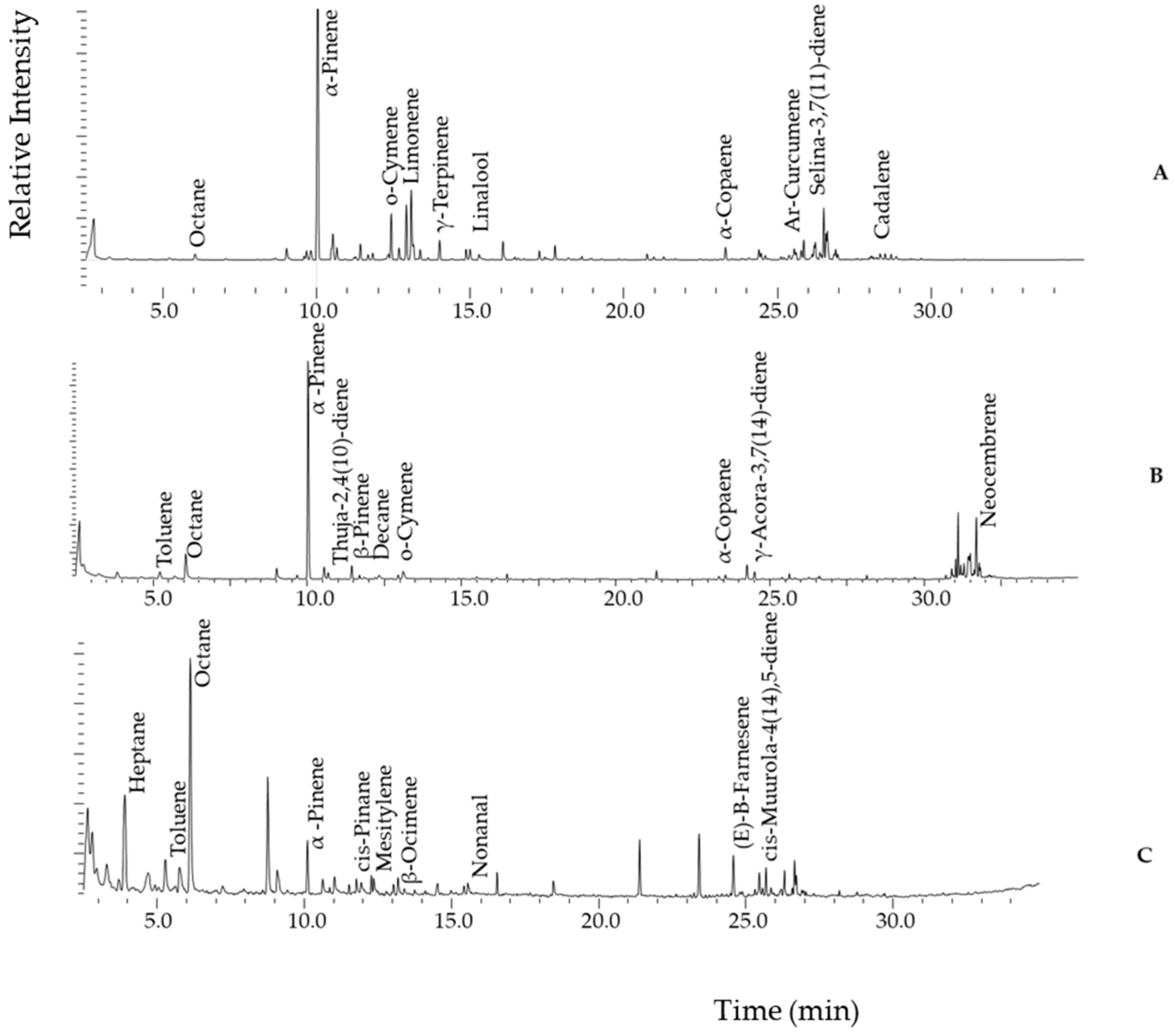
2.2.1. Representative Chromatograms from Propolis Samples Analysed by HS-GC-MS
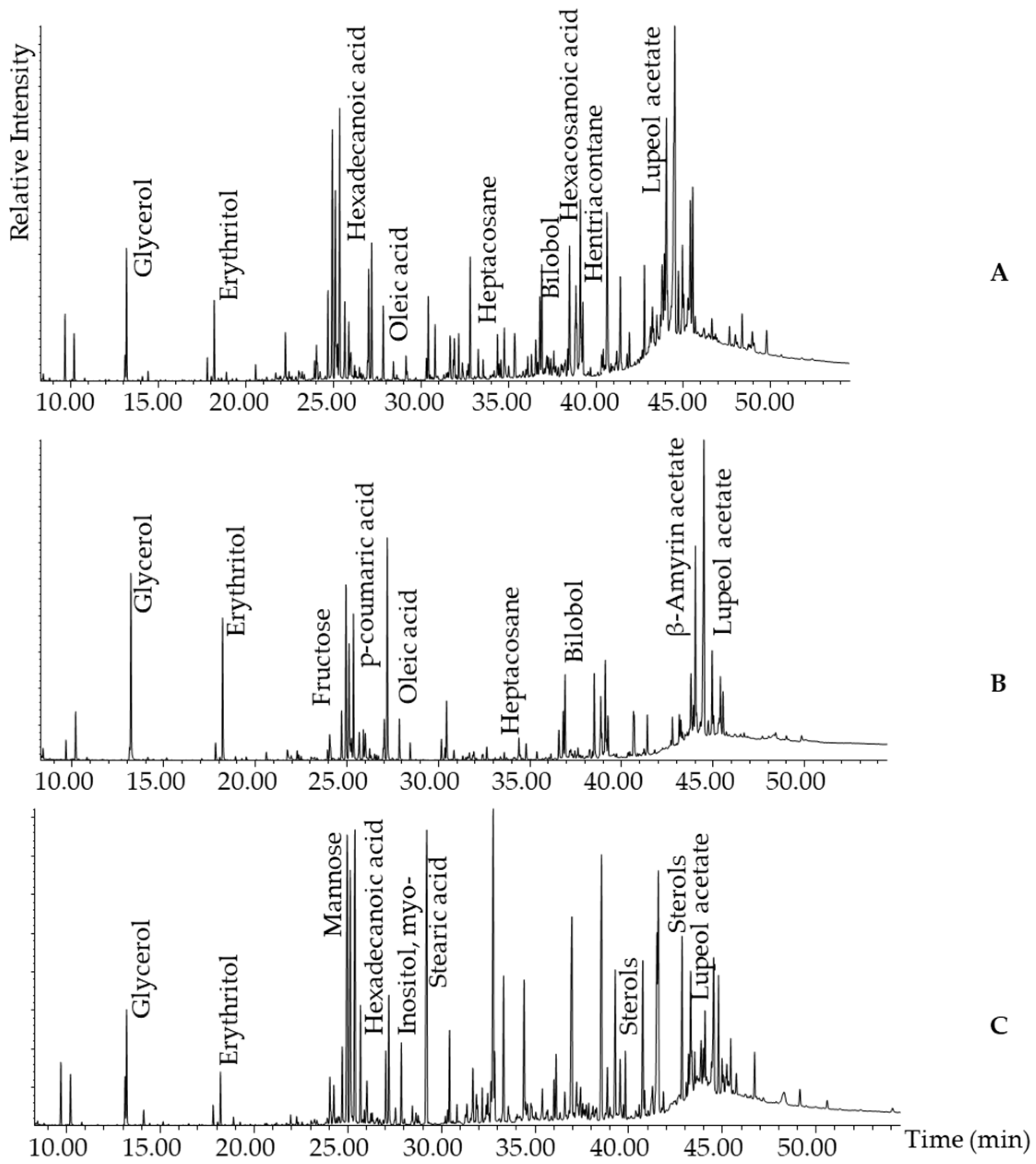
2.2.2. Representative GC-MS Chromatograms from Derivatised Propolis Samples
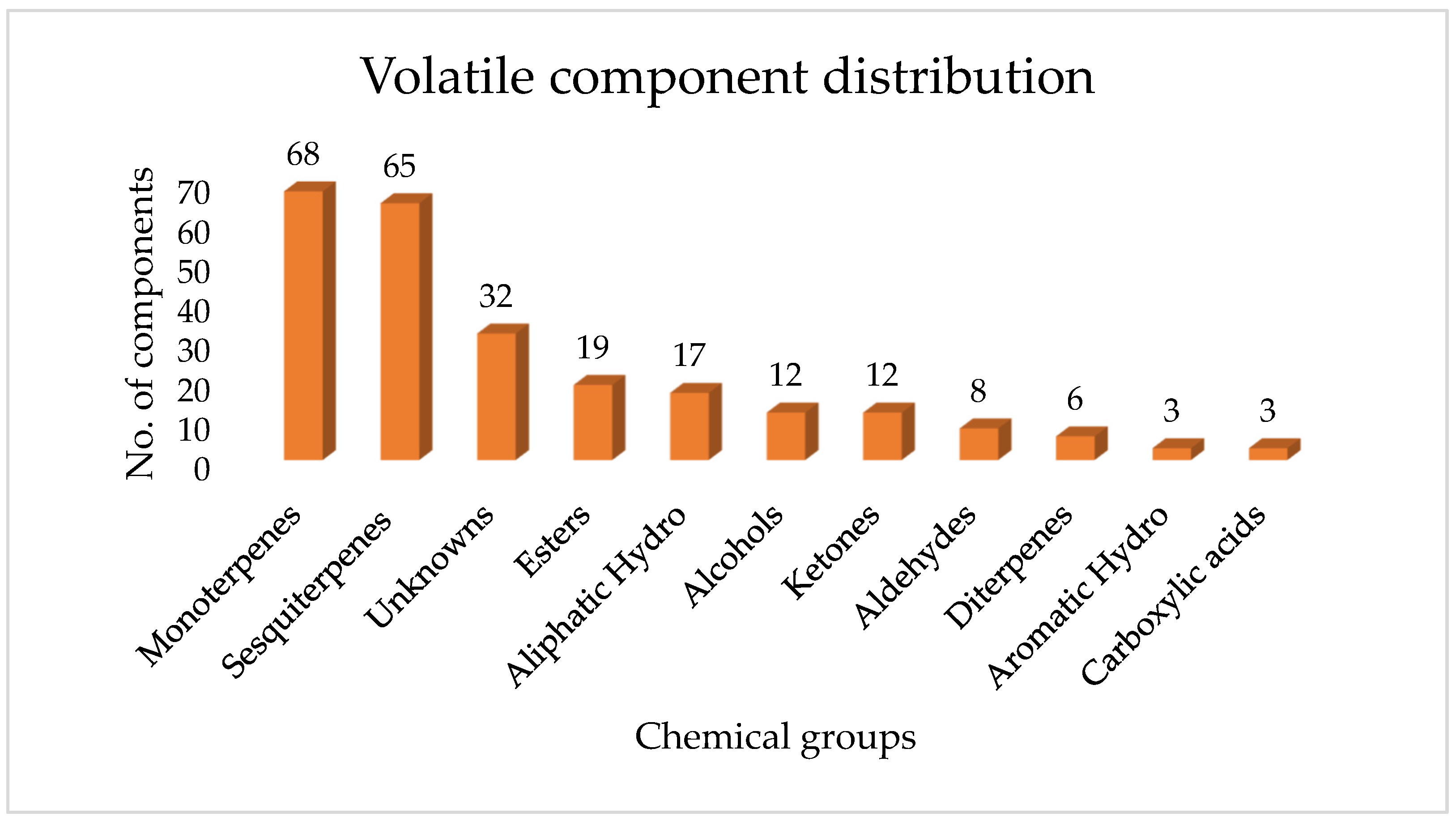
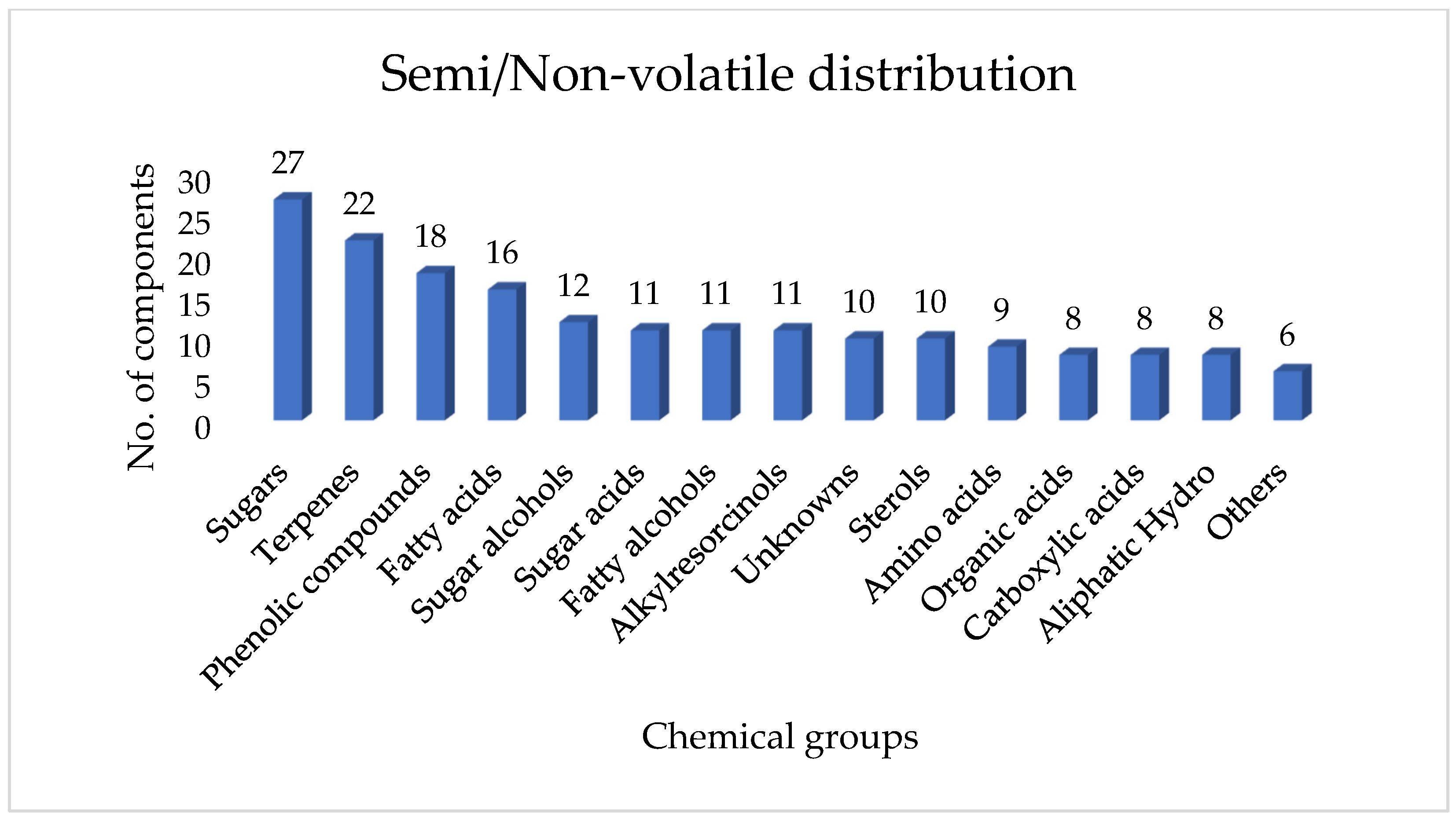
2.3. Detailed Volatile and Semi-Volatile Chemical Composition of Ugandan Propolis
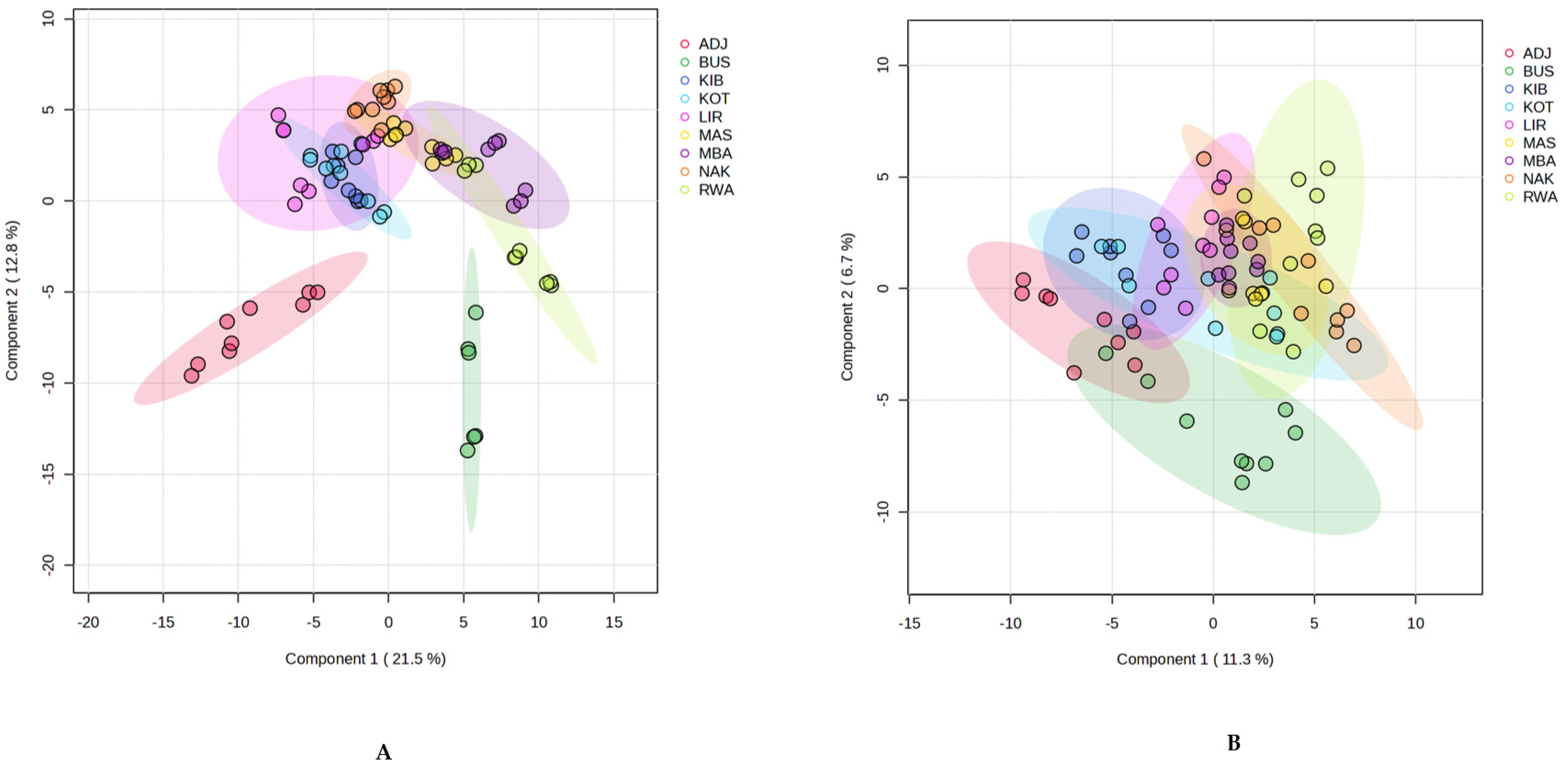
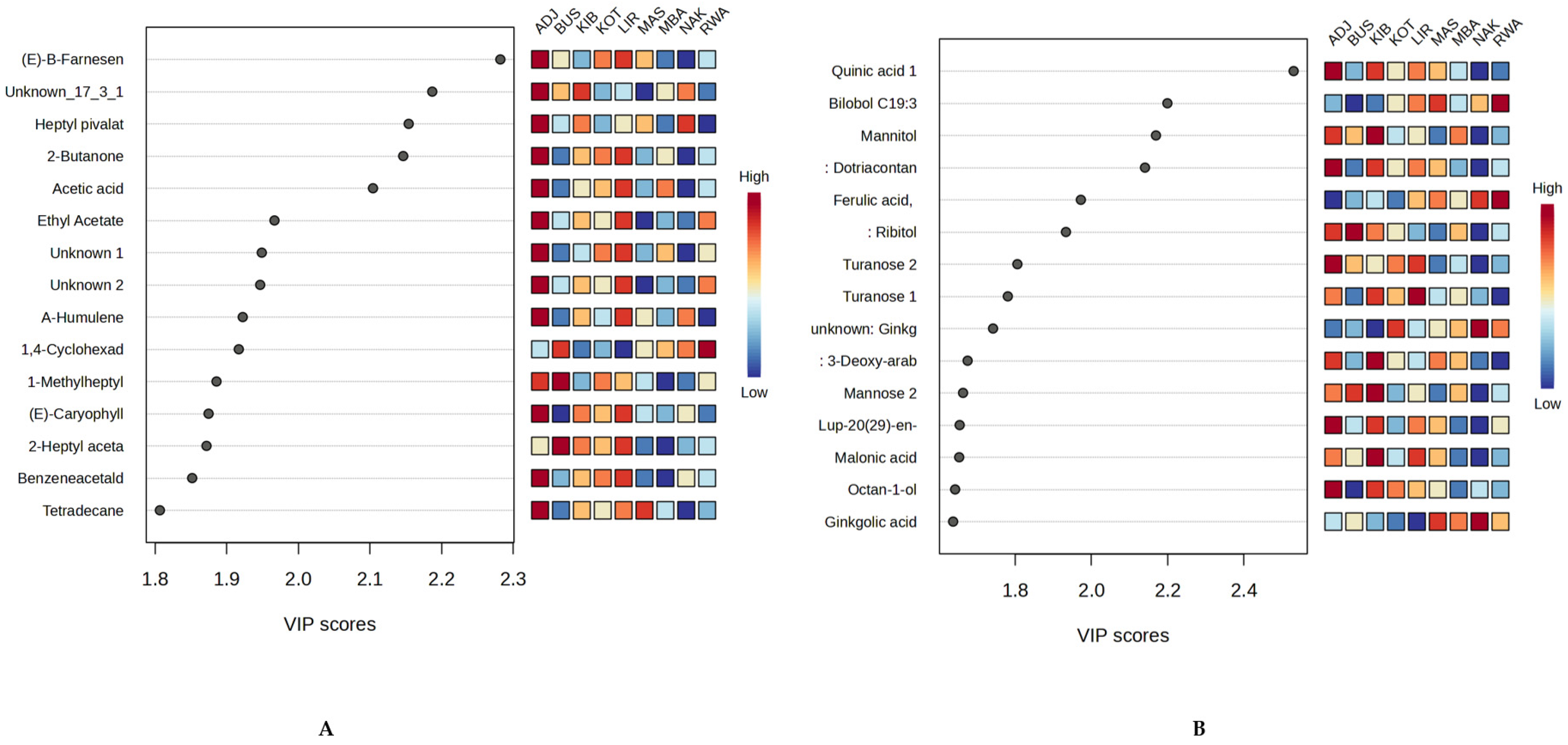
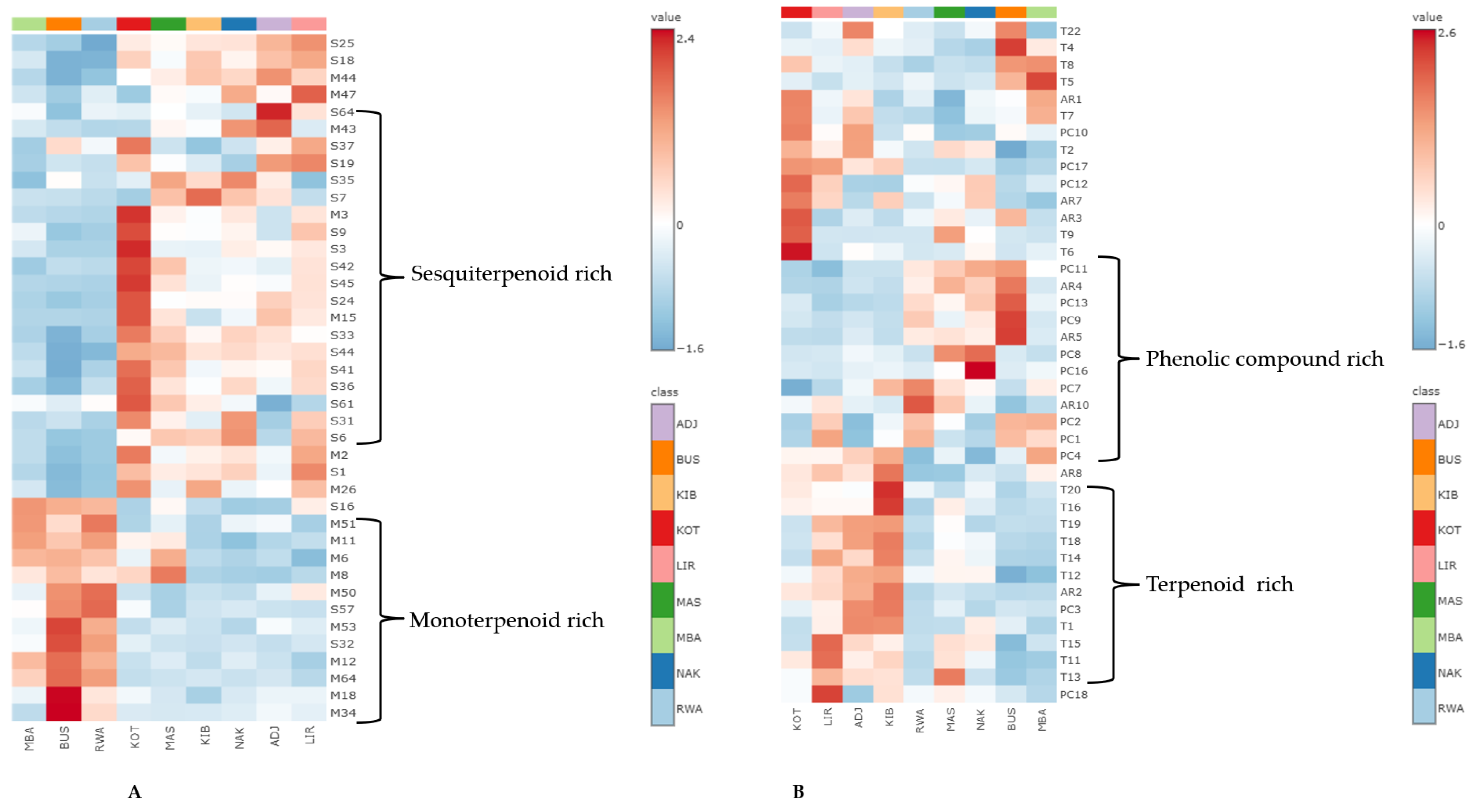
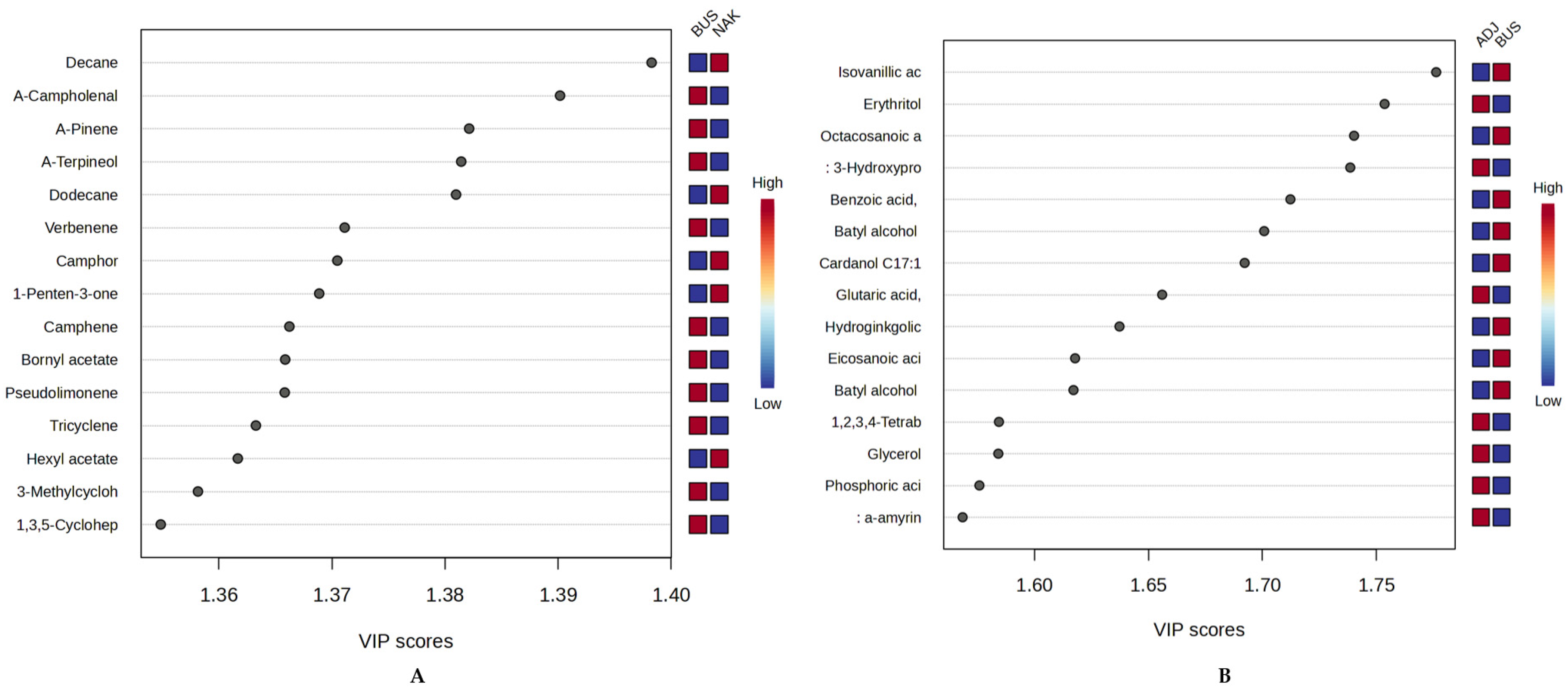
2.4. Multivariate Chemometric Analysis of Propolis Samples from Uganda
2.4.1. PLS-DA for Geographic Discrimination of Propolis Samples
2.4.2. Heatmap Visualisation of Selected Bioactive Secondary Metabolites
2.4.3. Geographic Chemometric Differentiation of Ugandan Propolis Using oPLS-DA
3. Materials and Methods
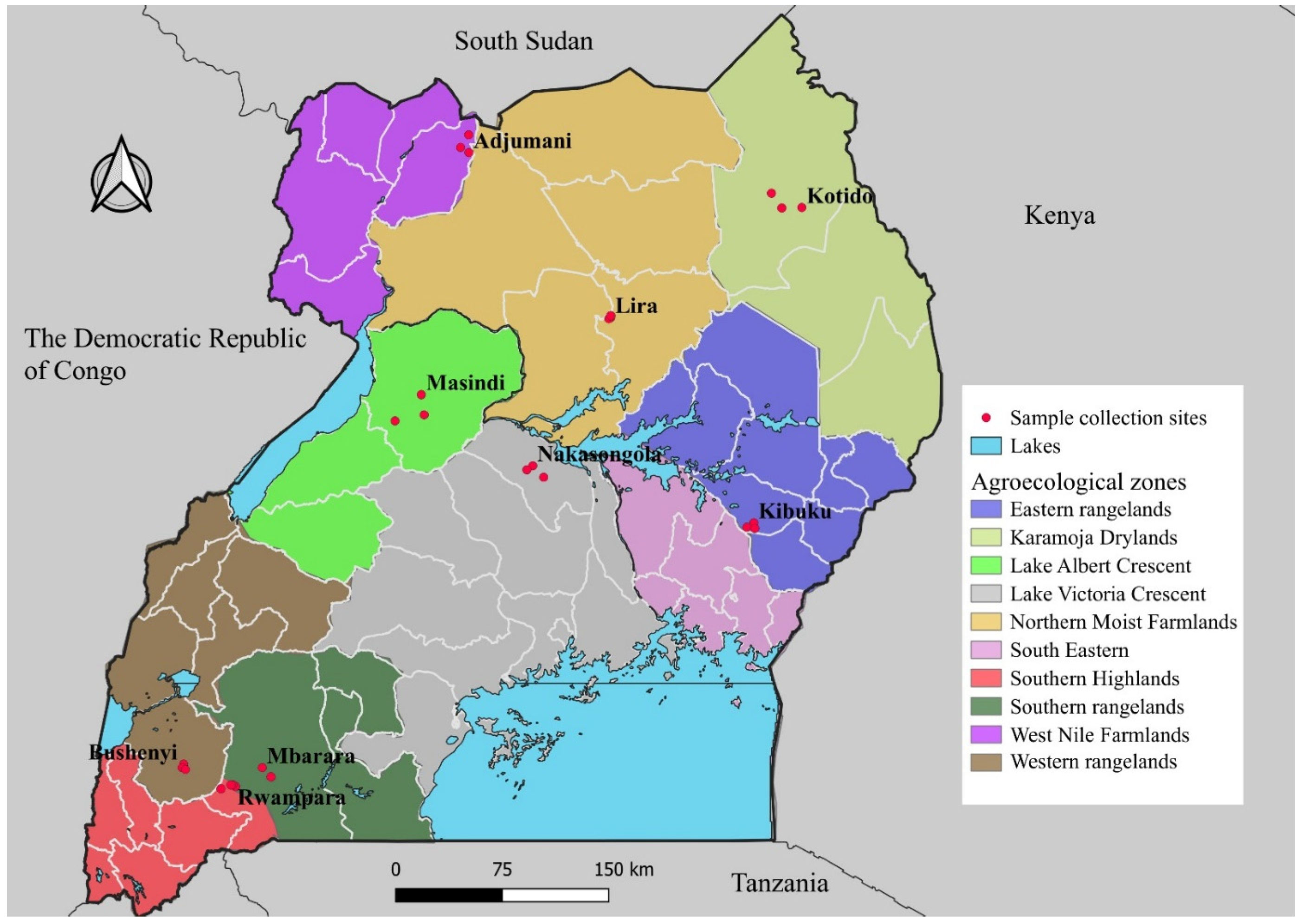
3.1. Sample Collection and Storage
3.2. Homogenization of Propolis Samples
3.3. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
3.3.1. Headspace GC-MS Analysis
3.3.2. GC-MS Analysis of Derivatised Propolis Samples
3.4. Multivariate Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martinotti, S.; Bonsignore, G.; Ranzato, E. Propolis: A Natural Substance with Multifaceted Properties and Activities. Int. J. Mol. Sci. 2025, 26, 1519. [Google Scholar] [CrossRef]
- Kuropatnicki, A.K.; Szliszka, E.; Krol, W. Historical Aspects of Propolis Research in Modern Times. Evid.-Based Complement. Altern. Med. 2013, 2013, 964149. [Google Scholar] [CrossRef]
- Brahimi, H.; Oulebsir-Mohandkaci, H.; Hendel, N.; Sarri, M. Ethnobotanical Knowledge and Traditional Uses of Propolis among the Algerian Population: A Comparative and Multivariate Analysis. Ethnobot. Res. Appl. 2025, 31, 1–18. [Google Scholar] [CrossRef]
- Hellner, M.; Winter, D.; von Georgi, R.; Münstedt, K. Apitherapy: Usage and Experience in German Beekeepers. Evid.-Based Complement. Altern. Med. 2008, 5, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, N.; Hajam, Y.A.; Mushtaq, S.; Kaur, L.; Kumar, R.; Rai, S. A Review on Dynamic Pharmacological Potency and Multifaceted Biological Activities of Propolis. Discov. Sustain. 2024, 5, 185. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Bahador, A. Virtual Screening and Computational Simulation Analysis of Antimicrobial Photodynamic Therapy Using Propolis-Benzofuran A to Control of Monkeypox. Photodiagn. Photodyn. Ther. 2023, 41, 103208. [Google Scholar] [CrossRef] [PubMed]
- Ayad, A.S.; Benchaabane, S.; Daas, T.; Smagghe, G.; Loucif-Ayad, W. Propolis Stands out as a Multifaceted Natural Product: Meta-Analysis on Its Sources, Bioactivities, Applications, and Future Perspectives. Life 2025, 15, 764. [Google Scholar] [CrossRef]
- Nichitoi, M.M.; Josceanu, A.M.; Isopescu, R.D.; Isopencu, G.O.; Geana, E.-I.; Ciucure, C.T.; Lavric, V. Polyphenolics Profile Effects upon the Antioxidant and Antimicrobial Activity of Propolis Extracts. Sci. Rep. 2021, 11, 20113. [Google Scholar] [CrossRef]
- Dezmirean, D.S.; Mărghitaş, L.A.; Chirilă, F.; Copaciu, F.; Simonca, V.; Bobiş, O.; Erler, S. Influence of Geographic Origin, Plant Source and Polyphenolic Substances on Antimicrobial Properties of Propolis against Human and Honey Bee Pathogens. J. Apic. Res. 2017, 56, 588–597. [Google Scholar] [CrossRef]
- Rivero-Cruz, J.F.; Granados-Pineda, J.; Pedraza-Chaverri, J.; Pérez-Rojas, J.M.; Kumar-Passari, A.; Diaz-Ruiz, G.; Rivero-Cruz, B.E. Phytochemical Constituents, Antioxidant, Cytotoxic, and Antimicrobial Activities of the Ethanolic Extract of Mexican Brown Propolis. Antioxidants 2020, 9, 70. [Google Scholar] [CrossRef]
- Pobiega, K.; Gniewosz, M.; Krasniewska, K. Antimicrobial and Antiviral Properties of Different Types of Propolis. Zesz. Probl. Postępów Nauk. Rol. 2017, 589, 69–79. [Google Scholar] [CrossRef]
- Kustiawan, P.M.; Syaifie, P.H.; Al Khairy Siregar, K.A.; Ibadillah, D.; Mardliyati, E. New Insights of Propolis Nanoformulation and Its Therapeutic Potential in Human Diseases. ADMET DMPK 2024, 12, 1–26. [Google Scholar] [CrossRef]
- Tunç, M.; Solmaz, R. A Preliminary Hazard Analysis in Bee Pollen and Propolis Decontamination Process: From the Beehive to the Laboratory. Food Control 2024, 163, 110487. [Google Scholar] [CrossRef]
- Santos, L.M.; Fonseca, M.S.; Sokolonski, A.R.; Deegan, K.R.; Araújo, R.P.C.; Umsza--Guez, M.A.; Barbosa, J.D.V.; Portela, R.D.; Machado, B.A.S. Propolis: Types, Composition, Biological Activities, and Veterinary Product Patent Prospecting. J. Sci. Food Agric. 2020, 100, 1369–1382. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, C.P.; Wang, K.; Li, G.Q.; Hu, F.L. Recent Advances in the Chemical Composition of Propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef]
- Kumazawa, S.; Nakamura, J.; Murase, M.; Miyagawa, M.; Ahn, M.-R.; Fukumoto, S. Plant Origin of Okinawan Propolis: Honeybee Behavior Observation and Phytochemical Analysis. Naturwissenschaften 2008, 95, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Kumazawa, S.; Goto, H.; Hamasaka, T.; Fukumoto, S.; Fujimoto, T.; Nakayama, T. A New Prenylated Flavonoid from Propolis Collected in Okinawa, Japan. Biosci. Biotechnol. Biochem. 2004, 68, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Neunaber, E. Phytochemische Und Mikrobiologische Untersuchungen von Propolis Verschiedener Provenienzen Als Beitrag Zur Kenntnis Der Wirkprinzipien in Propolis. Ph.D. Thesis, Freie Universität Berlin, Berlin, Germany, 1995. [Google Scholar]
- Marquez Hernandez, I.; Cuesta-Rubio, O.; Campo Fernandez, M.; Rosado Perez, A.; Montes de Oca Porto, R.; Piccinelli, A.L.; Rastrelli, L. Studies on the Constituents of Yellow Cuban Propolis: GC-MS Determination of Triterpenoids and Flavonoids. J. Agric. Food Chem. 2010, 58, 4725–4730. [Google Scholar] [CrossRef]
- Feitosa, L.G.P.; Feres, J.M.; Godinho, C.C.; Albernaz, L.C.; Espindola, L.S.; Vessecchi, R.; Guaratini, T.; Lopes, N.P. Characterization of Larvicidal Diterpene Resin Acids in Melipona Quadrifasciata Geopropolis via LC-ESI-MS/MS, GC–MS and Computational Analysis. Rapid Commun. Mass. Spectrom. 2025, 39, e10025. [Google Scholar] [CrossRef]
- Bruschi, M.L. Recent Advances and Future Directions of Propolis Delivery. Expert. Opin. Drug Deliv. 2025, 22, 1689–1708. [Google Scholar] [CrossRef] [PubMed]
- Balwierz, R.; Kasperkiewicz, K.; Straszak, M.; Siodłak, D.; Pokajewicz, K.; Ben Hammouda, I.; Wieczorek, P.P.; Kurek-Górecka, A.; Czuba, Z.P.; Baj, T. From Chemistry to Bioactivity: HS-SPME-GC-MS Profiling and Bacterial Growth Inhibition of Three Different Propolis Samples from Romania, Australia, and Uruguay. Molecules 2025, 30, 4014. [Google Scholar] [CrossRef]
- Sarapa, A.; Peter, A.; Buettner, A.; Loos, H.M. Organoleptic and Chemical Properties of Propolis: A Review. Eur. Food Res. Technol. 2025, 251, 1331–1352. [Google Scholar] [CrossRef]
- Ayad, A.S.; Hébert, M.P.A.; Doiron, J.A.; Loucif-Ayad, W.; Daas, T.; Smagghe, G.; Alburaki, M.; Barnett, D.A.; Touaibia, M.; Surette, M.E. Algerian Propolis from Distinct Geographical Locations: Chemical Profiles, Antioxidant Capacity, Cytotoxicity and Inhibition of 5-Lipoxygenase Product Biosynthesis. Chem. Biodivers. 2024, 21, e202301758. [Google Scholar] [CrossRef]
- Turco, J.F.; Mokochinski, J.B.; Torres, Y.R. Chemical Characterization of the Hydrophilic Fraction of (Geo) Propolis from Brazilian Stingless Bees. Food Biosci. 2024, 61, 104686. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Bakier, S.; Pirożnikow, E.; Zambrzycka, M.; Swiecicka, I. Selective Behaviour of Honeybees in Acquiring European Propolis Plant Precursors. J. Chem. Ecol. 2016, 42, 475–485. [Google Scholar] [CrossRef]
- Omar, R.; Igoli, J.O.; Zhang, T.; Gray, A.I.; Ebiloma, G.U.; Clements, C.J.; Fearnley, J.; Edrada Ebel, R.; Paget, T.; De Koning, H.P. The Chemical Characterization of Nigerian Propolis Samples and Their Activity against Trypanosoma Brucei. Sci. Rep. 2017, 7, 923. [Google Scholar] [CrossRef]
- Kasote, D.; Bankova, V.; Viljoen, A.M. Propolis: Chemical Diversity and Challenges in Quality Control. Phytochem. Rev. 2022, 21, 1887–1911. [Google Scholar] [CrossRef] [PubMed]
- Salatino, A.; Salatino, M.L.F.; Negri, G. How Diverse Is the Chemistry and Plant Origin of Brazilian Propolis? Apidologie 2021, 52, 1075–1097. [Google Scholar] [CrossRef] [PubMed]
- Bertolucci, V.; Ninomiya, A.F.; Longato, G.B.; Kaneko, L.O.; Nonose, N.; Scariot, P.P.M.; Messias, L.H.D. Bioactive Compounds from Propolis on Bone Homeostasis: A Narrative Review. Antioxidants 2025, 14, 81. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Q.; Wang, M.; Zhang, L. Metabolomics Reveals Discrimination of Chinese Propolis from Different Climatic Regions. Foods 2020, 9, 491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cao, X.; Ping, S.; Wang, K.; Shi, J.; Zhang, C.; Zheng, H.; Hu, F. Comparisons of Ethanol Extracts of Chinese Propolis (Poplar Type) and Poplar Gums Based on the Antioxidant Activities and Molecular Mechanism. Evid.-Based Complement. Altern. Med. 2015, 2015, 307594. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, J.; Ping, S.; Ma, Q.; Chen, X.; Xuan, H.; Shi, J.; Zhang, C.; Hu, F. Anti-Inflammatory Effects of Ethanol Extracts of Chinese Propolis and Buds from Poplar (Populus × Canadensis). J. Ethnopharmacol. 2014, 155, 300–311. [Google Scholar] [CrossRef]
- Wang, X.; Hu, H.; Luo, Z.; Liu, Y.; Zhang, H. A Plant Origin of Chinese Propolis: Populus Canadensis Moench. J. Apic. Res. 2018, 57, 228–245. [Google Scholar] [CrossRef]
- Nyandwi, R.; Kılıç, A.S.; Çelik, M.; Oruç, H.H. Determination and Quantification of Gallic Acid in Raw Propolis by High-Performance Liquid Chromatography–Diode Array Detector in Burundi. East. Afr. Sci. 2019, 1, 43–48. [Google Scholar] [CrossRef]
- Didas, R. Beekeeping Project in SW Uganda. Bee World 2005, 86, 69–70. [Google Scholar] [CrossRef]
- Kalanzi, F.; Nansereko, S.; Buyinza, J.; Kiwuso, P.; Turinayo, Y.; Mwanja, C.; Niyibizi, G.; Ongerep, S.; Sekatuba, J.; Mujuni, D. Socio-Economic Analysis of Beekeeping Enterprise in Communities Adjacent to Kalinzu Forest, Western Uganda. Int. J. Res. Land-Use Sustain. 2015, 1, 81–90. [Google Scholar]
- Kajobe, R. Important Bee Plants for African and Other Stingless Bees. In Pot-Honey: A Legacy of Stingless Bees; Springer: Berlin/Heidelberg, Germany, 2012; pp. 315–335. [Google Scholar]
- Baptista Pereira, D.; de Souza Alves, N.; Oliveira Silva, E.; de Menezes Epifanio, N.M.; Siqueira de Almeida Chaves, D. Extraction Kinetics of Brazilian Green Propolis and Chemical Characterization of Its Volatiles. Chem. Biodivers. 2024, 21, e202400610. [Google Scholar] [CrossRef]
- El-Guendouz, S.; Lyoussi, B.; Miguel, M.G.; Figueiredo, A.C. Characterization of Volatiles from Moroccan Propolis Samples. J. Essent. Oil Res. 2019, 31, 27–33. [Google Scholar] [CrossRef]
- Kaškonienė, V.; Kaškonas, P.; Maruška, A.; Kubilienė, L. Chemometric Analysis of Volatiles of Propolis from Different Regions Using Static Headspace GC-MS. Open Chem. 2014, 12, 736–746. [Google Scholar] [CrossRef]
- Bankova, V.; Bertelli, D.; Borba, R.; Conti, B.J.; da Silva Cunha, I.B.; Danert, C.; Eberlin, M.N.; IFalcão, S.; Isla, M.I.; Moreno, M.I.N. Standard Methods for Apis Mellifera Propolis Research. J. Apic. Res. 2019, 58, 1–49. [Google Scholar] [CrossRef]
- Nie, W.; Alimujiang, S.; Zhang, Y.; Zhang, S.; Li, W. A Multi-Omics Approach Combining GC-MS, LC-MS, and FT-NIR with Chemometrics and Machine Learning for Metabolites Systematic Profiling and Geographical Origin Tracing of Artemisia Argyi Folium. J. Chromatogr. A 2025, 1757, 466138. [Google Scholar] [CrossRef] [PubMed]
- Munyuli, M.B.T. Pollinator Biodiversity in Uganda and in Sub-Sahara Africa: Landscape and Habitat Management Strategies for Its Conservation. Int. J. Biodivers. Conserv. 2011, 3, 551–609. [Google Scholar]
- Otim, A.S.; Kajobe, R.; Abila, P.P.; Kasangaki, P.; Echodu, R. Important Plants for Honey Production in Four Agro Ecological Zones of Uganda. Bee World 2019, 96, 81–86. [Google Scholar] [CrossRef]
- Hames-Kocabas, E.E.; Demirci, B.; Uzel, A.; Demirci, F. Volatile Composition of Anatolian Propolis by Headspace-Solid-Phase Microextraction (HS-SPME), Antimicrobial Activity against Food Contaminants and Antioxidant Activity. J. Med. Plants Res. 2013, 7, 2140–2149. [Google Scholar]
- Sakava, P.; Nyemb, J.N.; Matchawe, C.; Kumcho, M.P.; Tagatsing, M.F.; Nsawir, B.J.; Talla, E.; Atchadé, A.D.T.; Laurent, S.; Henoumont, C. Chemical Constituents and Antibacterial Activities of Cameroonian Dark Brown Propolis against Potential Biofilm-Forming Bacteria. Nat. Prod. Res. 2024, 1–14. [Google Scholar] [CrossRef]
- Duru, I.A. Comparative Phytochemical Analysis of Brown, Green and Red Propolis from Umudike, Abia State Nigeria. Adv. J. Chem. B 2020, 3, 86–97. [Google Scholar]
- Boke Sarikahya, N.; Varol, E.; Sumer Okkali, G.; Yucel, B.; Margaoan, R.; Nalbantsoy, A. Comparative Study of Antiviral, Cytotoxic, Antioxidant Activities, Total Phenolic Profile and Chemical Content of Propolis Samples in Different Colors from Turkiye. Antioxidants 2022, 11, 2075. [Google Scholar] [CrossRef]
- Cuesta-Rubio, O.; Piccinelli, A.L.; Campo Fernandez, M.; Márquez Hernández, I.; Rosado, A.; Rastrelli, L. Chemical Characterization of Cuban Propolis by HPLC− PDA, HPLC− MS, and NMR: The Brown, Red, and Yellow Cuban Varieties of Propolis. J. Agric. Food Chem. 2007, 55, 7502–7509. [Google Scholar] [CrossRef]
- Machado, C.S.; Mokochinski, J.B.; Lira, T.O.; de Oliveira, F.d.C.E.; Cardoso, M.V.; Ferreira, R.G.; Sawaya, A.C.H.F.; Ferreira, A.G.; Pessoa, C.; Cuesta-Rubio, O. Comparative Study of Chemical Composition and Biological Activity of Yellow, Green, Brown, and Red Brazilian Propolis. Evid.-Based Complement. Altern. Med. 2016, 2016, 6057650. [Google Scholar] [CrossRef] [PubMed]
- Kurek-Górecka, A.; Keskin, Ş.; Bobis, O.; Felitti, R.; Górecki, M.; Otręba, M.; Stojko, J.; Olczyk, P.; Kolayli, S.; Rzepecka-Stojko, A. Comparison of the Antioxidant Activity of Propolis Samples from Different Geographical Regions. Plants 2022, 11, 1203. [Google Scholar] [CrossRef]
- Bankova, V.; Popova, M.; Trusheva, B. Propolis Volatile Compounds: Chemical Diversity and Biological Activity: A Review. Chem. Cent. J. 2014, 8, 28. [Google Scholar] [CrossRef]
- Kirby, J.; Nishimoto, M.; Park, J.G.; Withers, S.T.; Nowroozi, F.; Behrendt, D.; Rutledge, E.J.G.; Fortman, J.L.; Johnson, H.E.; Anderson, J. V Cloning of Casbene and Neocembrene Synthases from Euphorbiaceae Plants and Expression in Saccharomyces Cerevisiae. Phytochemistry 2010, 71, 1466–1473. [Google Scholar] [CrossRef]
- Ahangari, Z.; Naseri, M.; Vatandoost, F. Propolis: Chemical Composition and Its Applications in Endodontics. Iran. Endod. J. 2018, 13, 285. [Google Scholar]
- Reddy, N.B.P.; Indumathi, C.; Deotale, S.; Nath, P.C.; Ashoksuraj, B.S.R.; Rajam, R.; Thivya, P. Recent Developments and Innovative Application of Propolis in the Food Industry: A Natural Preservative from Honeybee Waste. Food Sci. Biotechnol. 2025, 34, 3153–3173. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Li, J.; Liu, H.; Wang, Y. Effect of Drying Temperature on Composition of Edible Mushrooms: Characterization and Assessment via HS-GC-MS and IR Spectral Based Volatile Profiling and Chemometrics. Curr. Res. Food Sci. 2024, 9, 100819. [Google Scholar] [CrossRef]
- Kamatou, G.; Sandasi, M.; Tankeu, S.; Vuuren, S.V.; Viljoen, A. Headspace Analysis and Characterisation of South African Propolis Volatile Compounds Using GCxGC–ToF–MS. Rev. Bras. Farm. 2019, 29, 351–357. [Google Scholar] [CrossRef]
- Haile, K.; Kebede, T.; Dekebo, A. A Comparative Study of Volatile Components of Propolis (Bee Glue) Collected from Haramaya University and Assela Beekeeping Centers, Ethiopia. Bull. Chem. Soc. Ethiop. 2012, 26, 353–360. [Google Scholar] [CrossRef]
- Toutou, Z.; Fatmi, S.; Chibani, N.; Pokajewicz, K.; Skiba, M.; Wieczorek, P.P.; Iguerouada, M. Exploring the Therapeutic Potential of Algerian Propolis: GC/MS Profiling, Protective Inclusion Complex, and In Silico Evaluation Against SARS-CoV-2 Main Proteases. Pept. Sci. 2025, 117, e24381. [Google Scholar] [CrossRef]
- Wieczorek, P.P.; Hudz, N.; Yezerska, O.; Horčinová-Sedláčková, V.; Shanaida, M.; Korytniuk, O.; Jasicka-Misiak, I. Chemical Variability and Pharmacological Potential of Propolis as a Source for the Development of New Pharmaceutical Products. Molecules 2022, 27, 1600. [Google Scholar] [CrossRef]
- Kong, X.; Luo, H.; Chen, Y.; Shen, H.; Shi, P.; Yang, F.; Li, H.; Yu, L. Elucidating Softening Mechanism of Honey Peach (Prunus Persica L.) Stored at Ambient Temperature Using Untargeted Metabolomics Based on Liquid Chromatography-Mass Spectrometry. Horticulturae 2023, 9, 1210. [Google Scholar] [CrossRef]
- Alcázar Magaña, A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic Acids: Chemistry, Biosynthesis, Occurrence, Analytical Challenges, and Bioactivity. Plant J. 2021, 107, 1299–1319. [Google Scholar] [CrossRef]
- Bonaduce, I.; Colombini, M.P. Characterisation of Beeswax in Works of Art by Gas Chromatography–Mass Spectrometry and Pyrolysis–Gas Chromatography–Mass Spectrometry Procedures. J. Chromatogr. A 2004, 1028, 297–306. [Google Scholar] [CrossRef]
- Horváth, K.; Molnár-Perl, I. Simultaneous GC-MS Quantitation of o-Phosphoric, Aliphatic and Aromatic Carboxylic Acids, Proline, Hydroxymethylfurfurol and Sugars as Their TMS Derivatives: In Honeys. Chromatographia 1998, 48, 120–126. [Google Scholar] [CrossRef]
- Popova, M.; Trusheva, B.; Chimshirova, R.; Antonova, D.; Gechovska, K.; Thanh, L.N.; Lien, N.T.P.; Phuong, D.T.L.; Bankova, V. Chemical Profile and Antioxidant Capacity of Propolis from Tetragonula, Lepidotrigona, Lisotrigona and Homotrigona Stingless Bee Species in Vietnam. Molecules 2022, 27, 7834. [Google Scholar] [CrossRef] [PubMed]
- Gerginova, D.; Simova, S.; Popova, M.; Stefova, M.; Stanoeva, J.P.; Bankova, V. NMR Profiling of North Macedonian and Bulgarian Honeys for Detection of Botanical and Geographical Origin. Molecules 2020, 25, 4687. [Google Scholar] [CrossRef] [PubMed]
- Wanyama, J.; Nakawuka, P.; Bwambale, E.; Kiraga, S.; Kiggundu, N.; Barasa, B.; Katimbo, A. Evaluation of Land Suitability for Surface Irrigation under Changing Climate in a Tropical Setting of Uganda, East Africa. Agric. Syst. 2024, 217, 103937. [Google Scholar] [CrossRef]
- Nuwaha, F.; Okware, J.; Hanningtone, T.; Mwebaze, C. False Teeth” Ebiino” and Millet Disease” Oburo” in Bushenyi District of Uganda. Afr. Health Sci. 2007, 7, 25–32. [Google Scholar] [PubMed]
- Munyuli, M.B.T. Micro, Local, Landscape and Regional Drivers of Bee Biodiversity and Pollination Services Delivery to Coffee (Coffea Canephora) in Uganda. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2012, 8, 190–203. [Google Scholar] [CrossRef]
- Kajobe, R. Pollen Foraging by Apis Mellifera and Stingless Bees Meliponula Bocandei and Meliponula Nebulata in Bwindi Impenetrable National Park, Uganda. Afr. J. Ecol. 2007, 45, 265. [Google Scholar] [CrossRef]
- Maling, S.; Kabakyenga, J.; Muchunguzi, C.; Olet, E.A.; Namaganda, M.; Kahwa, I.; Alele, P.E. Medicinal Plants Used by Traditional Medicine Practitioners in Treatment of Alcohol-Related Disorders in Bushenyi District, Southwestern Uganda. Front. Pharmacol. 2024, 15, 1407104. [Google Scholar] [CrossRef]
- Tumwebaze, S.B.; Byakagaba, P. Soil Organic Carbon Stocks under Coffee Agroforestry Systems and Coffee Monoculture in Uganda. Agric. Ecosyst. Environ. 2016, 216, 188–193. [Google Scholar] [CrossRef]
- Ssebulime, G.; Nyombi, K.; Kagezi, G.; Mpiira, S.; Byabagambi, S.; Tushemereirwe, W.; Karamura, E.; Kiyingi, I.; Balimunsi, H.K.; Staver, C. Assessing the Potential of Trees to Sustain Soil Fertility in the Banana Agroforestry System in Uganda. Agroecol. Sustain. Food Syst. 2019, 43, 662–677. [Google Scholar] [CrossRef]
- De Leeuw, J.; Njenga, M.; Wagner, B.; Iiyama, M. Treesilience: An Assessment of the Resilience Provided by Trees in the Drylands of Eastern Africa; World Agroforestry Centre (ICRAF): Nairobi, Kenya, 2014. [Google Scholar]
- Afata, T.N.; Dekebo, A. Chemical Composition and Antimicrobial Effect of Western Ethiopian Propolis. Chem. Biodivers. 2023, 20, e202200922. [Google Scholar] [CrossRef]
- Mwanjalolo, M.G.J.; Bernard, B.; Paul, M.I.; Joshua, W.; Sophie, K.; Cotilda, N.; Bob, N.; John, D.; Edward, S.; Barbara, N. Assessing the Extent of Historical, Current, and Future Land Use Systems in Uganda. Land 2018, 7, 132. [Google Scholar] [CrossRef]
- Acema, D.; Byakagaba, P.; Banana, A.Y.; Turyahabwe, N. Local Institutions and the Governance of Tree Resources: The Case of the Shea Tree (Vitellaria paradoxa C.F Gaertn.) in West Nile Region of Uganda. Conserv. Soc. 2021, 19, 44–56. [Google Scholar] [CrossRef]
- Zhang, T.; Omar, R.; Siheri, W.; Al Mutairi, S.; Clements, C.; Fearnley, J.; Edrada-Ebel, R.; Watson, D. Chromatographic Analysis with Different Detectors in the Chemical Characterisation and Dereplication of African Propolis. Talanta 2014, 120, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Sha, M.; Li, X.; Liu, Y.; Tian, H.; Liang, X.; Li, X.; Gao, W. Comparative Chemical Characters of Pseudostellaria Heterophylla from Geographical Origins of China. Chin. Herb. Med. 2023, 15, 439–446. [Google Scholar] [CrossRef]
- Hutschenreuther, A.; Kiontke, A.; Birkenmeier, G.; Birkemeyer, C. Comparison of Extraction Conditions and Normalization Approaches for Cellular Metabolomics of Adherent Growing Cells with GC-MS. Anal. Methods 2012, 4, 1953–1963. [Google Scholar] [CrossRef]
- Hackstadt, A.J.; Hess, A.M. Filtering for Increased Power for Microarray Data Analysis. BMC Bioinform. 2009, 10, 11. [Google Scholar] [CrossRef]
- Szymańska, E.; Saccenti, E.; Smilde, A.K.; Westerhuis, J.A. Double-Check: Validation of Diagnostic Statistics for PLS-DA Models in Metabolomics Studies. Metabolomics 2012, 8, 3–16. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kahwa, I.; Kaysser, L.; Wangalwa, R.; Billig, S.; Tusiimire, J.; Wiesner, C. Chemical Profiling and Geographic Differentiation of Ugandan Propolis by GC-MS Through Chemometric Modelling. Molecules 2025, 30, 4435. https://doi.org/10.3390/molecules30224435
Kahwa I, Kaysser L, Wangalwa R, Billig S, Tusiimire J, Wiesner C. Chemical Profiling and Geographic Differentiation of Ugandan Propolis by GC-MS Through Chemometric Modelling. Molecules. 2025; 30(22):4435. https://doi.org/10.3390/molecules30224435
Chicago/Turabian StyleKahwa, Ivan, Leonard Kaysser, Rapheal Wangalwa, Susan Billig, Jonans Tusiimire, and Claudia Wiesner. 2025. "Chemical Profiling and Geographic Differentiation of Ugandan Propolis by GC-MS Through Chemometric Modelling" Molecules 30, no. 22: 4435. https://doi.org/10.3390/molecules30224435
APA StyleKahwa, I., Kaysser, L., Wangalwa, R., Billig, S., Tusiimire, J., & Wiesner, C. (2025). Chemical Profiling and Geographic Differentiation of Ugandan Propolis by GC-MS Through Chemometric Modelling. Molecules, 30(22), 4435. https://doi.org/10.3390/molecules30224435







