Investigation of Antithrombotic Activity and In Vivo Effective Forms of Kaempferitrin Using FeCl3-Induced Rat Arterial Thrombosis and UHPLC-Q-Exactive Orbitrap MS
Abstract
1. Introduction
2. Results
2.1. Antithrombotic Activities of KAE
2.1.1. Plasma Recalcification Time (PRT) Measurement
2.1.2. In Vitro Inhibitory Effect of KAE on Platelet Aggregation
2.1.3. Evaluation of the In Vivo Antithrombotic Activity of KAE
2.1.4. The Rat Tail Vein Bleeding Time Measurement
2.2. Analysis of the Metabolites of KAE
2.2.1. Phase I Metabolites Characterization
2.2.2. Phase II Metabolites Characterization
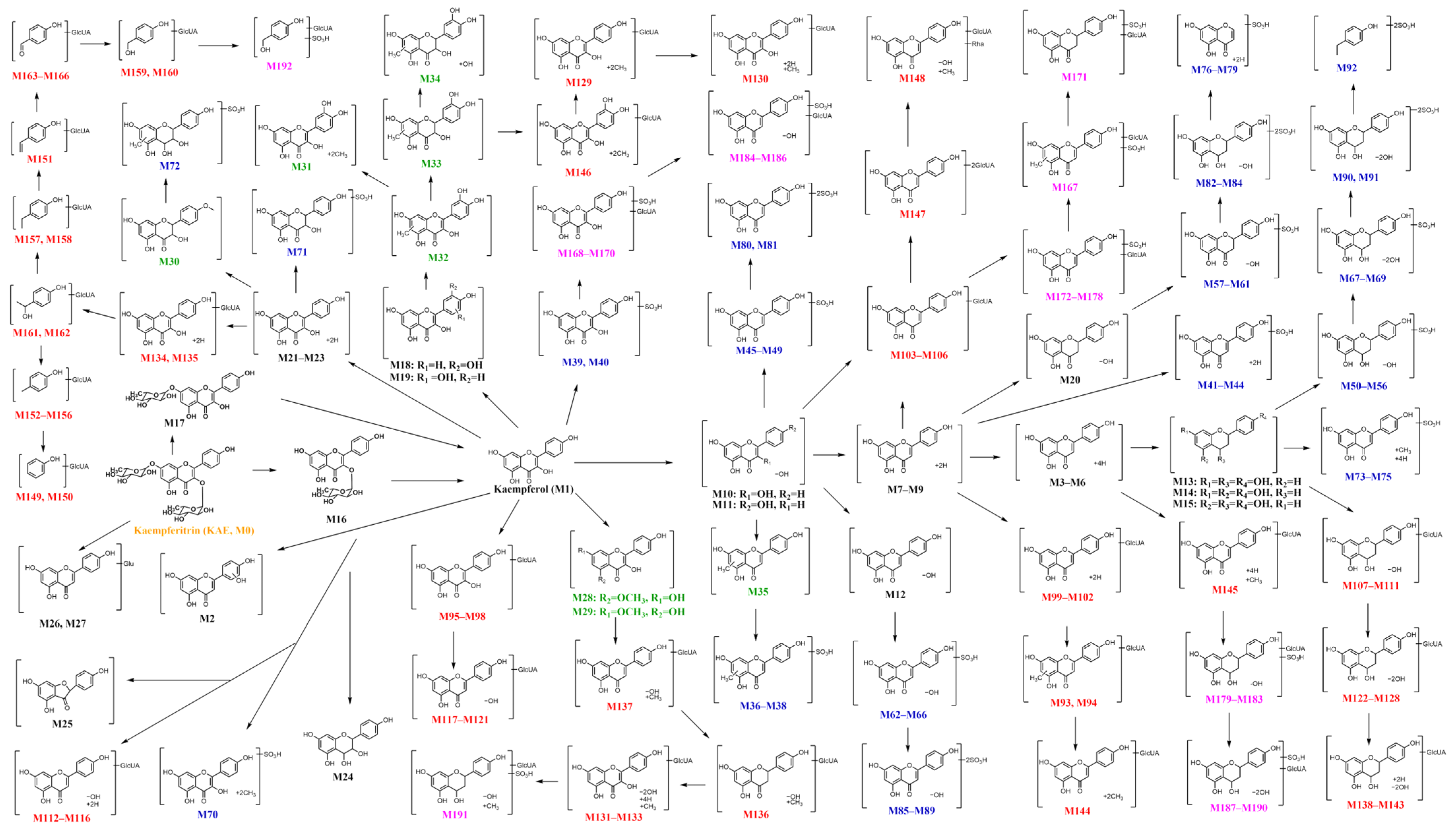
2.2.3. Potential Metabolic Pathway
2.3. Network Pharmacology of KAE and Its Metabolites
2.3.1. Screening “Effective Forms” and Their Antithrombotic Targets and Construction of the “Compound-Target” Network
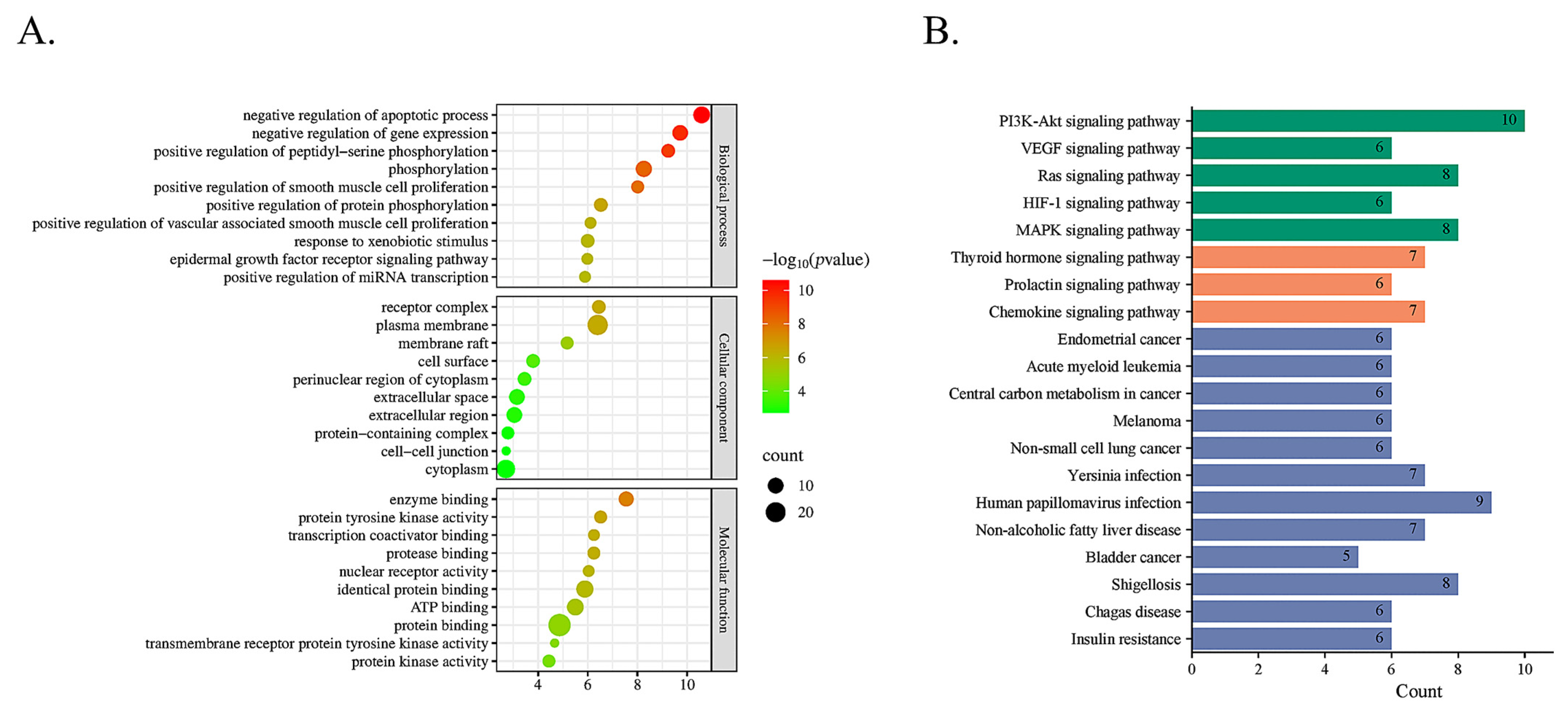
2.3.2. Signaling Pathway Enrichment Analysis with GO and KEGG
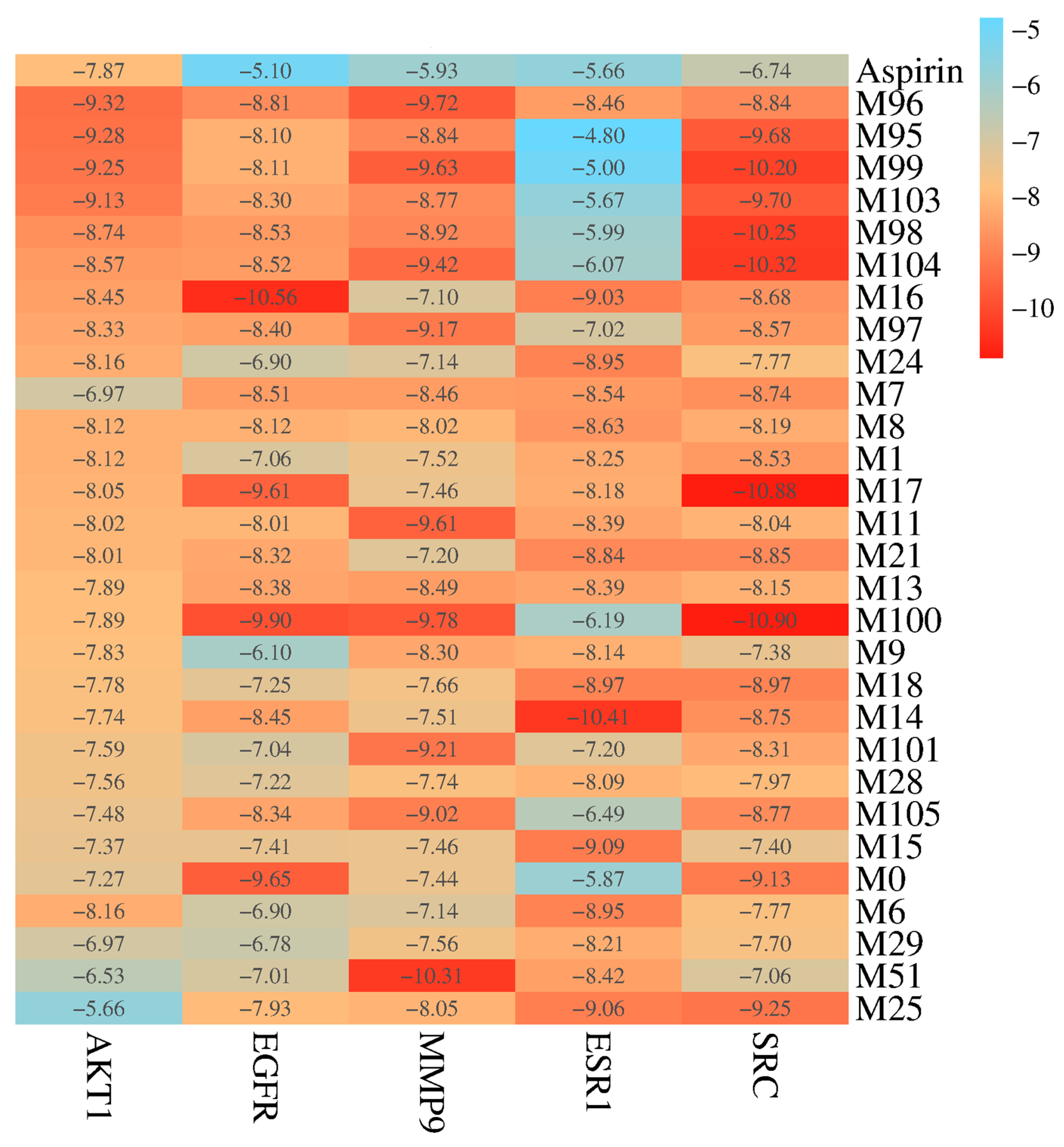
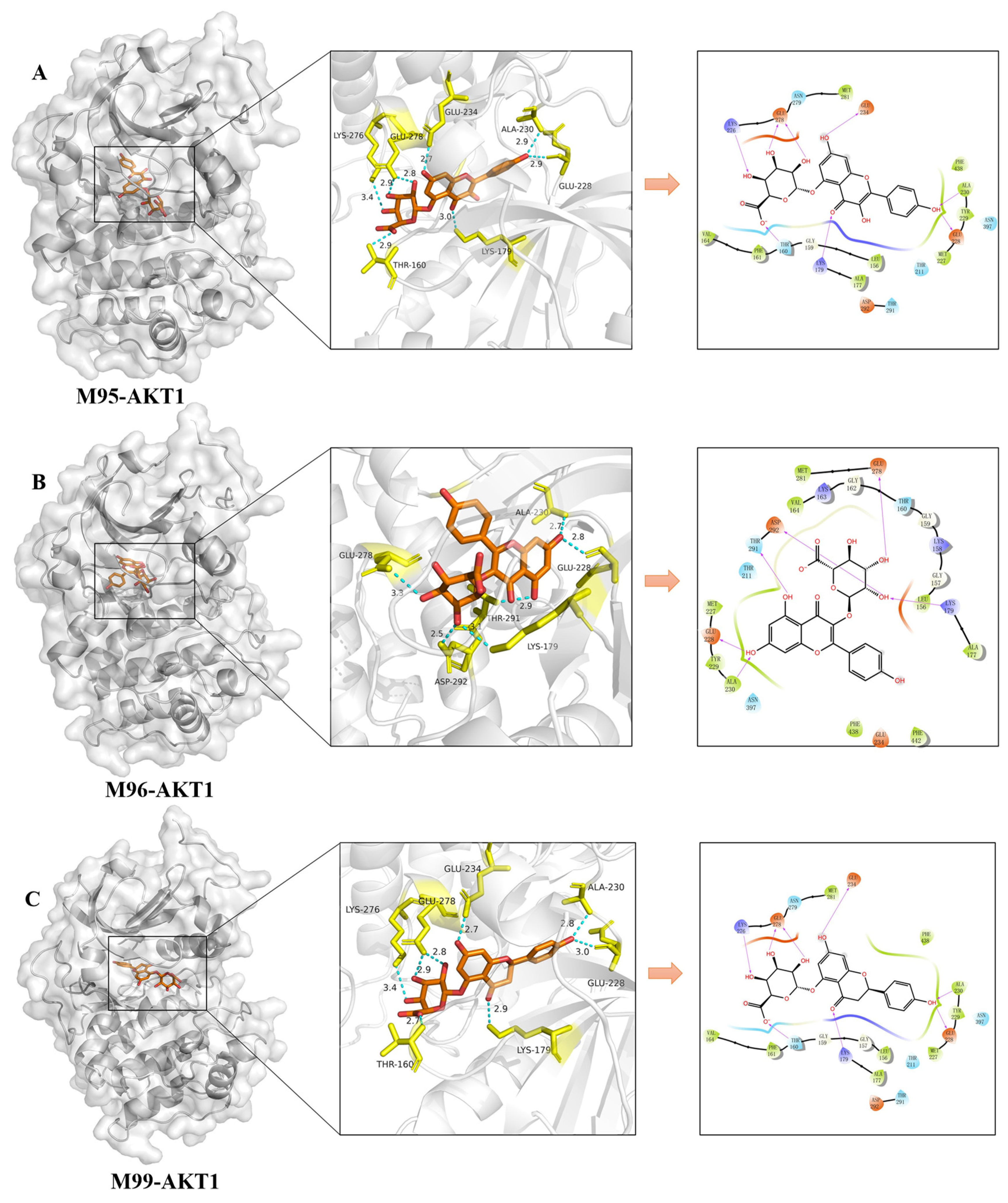
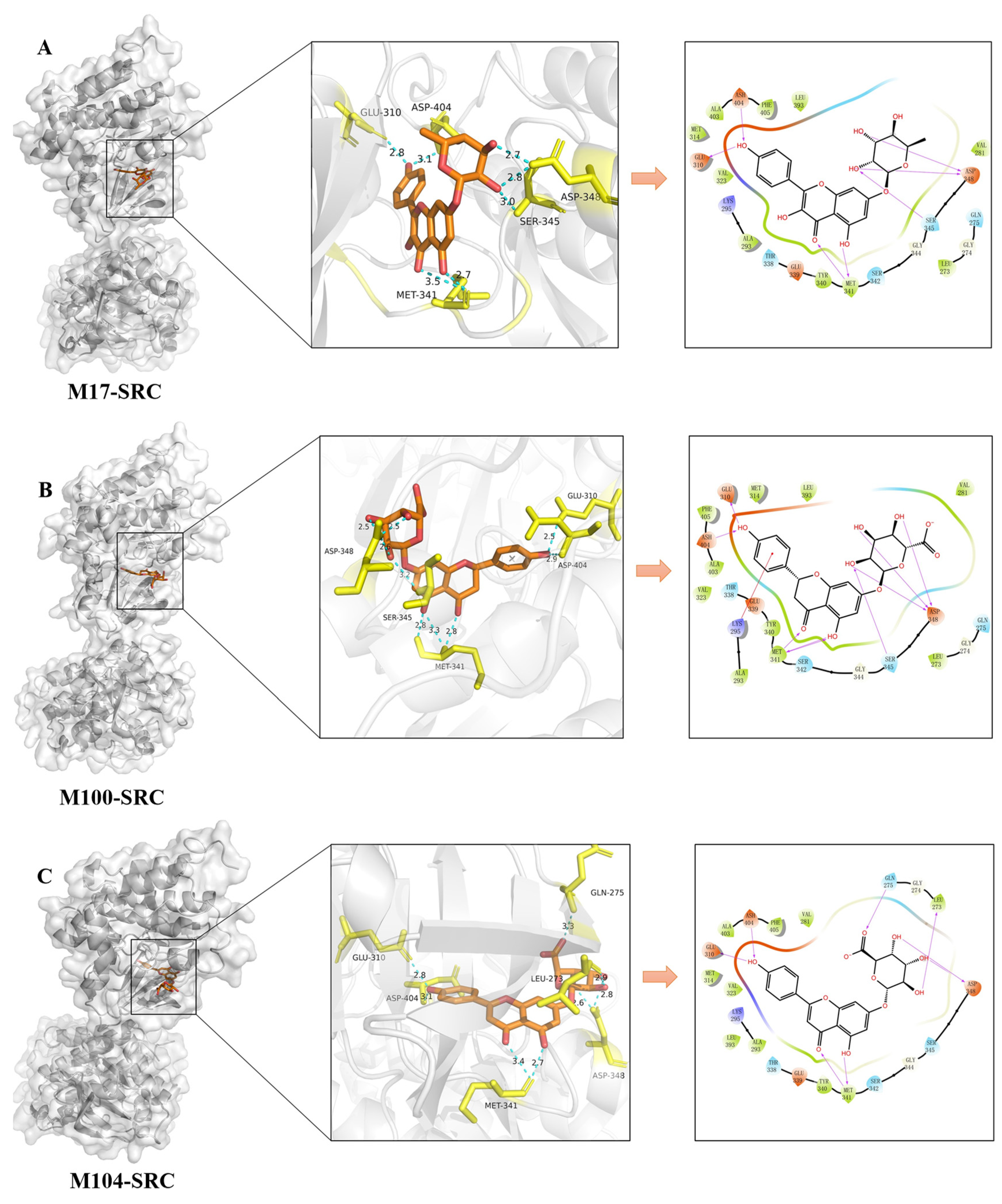
2.4. Molecular Docking Validation Analysis
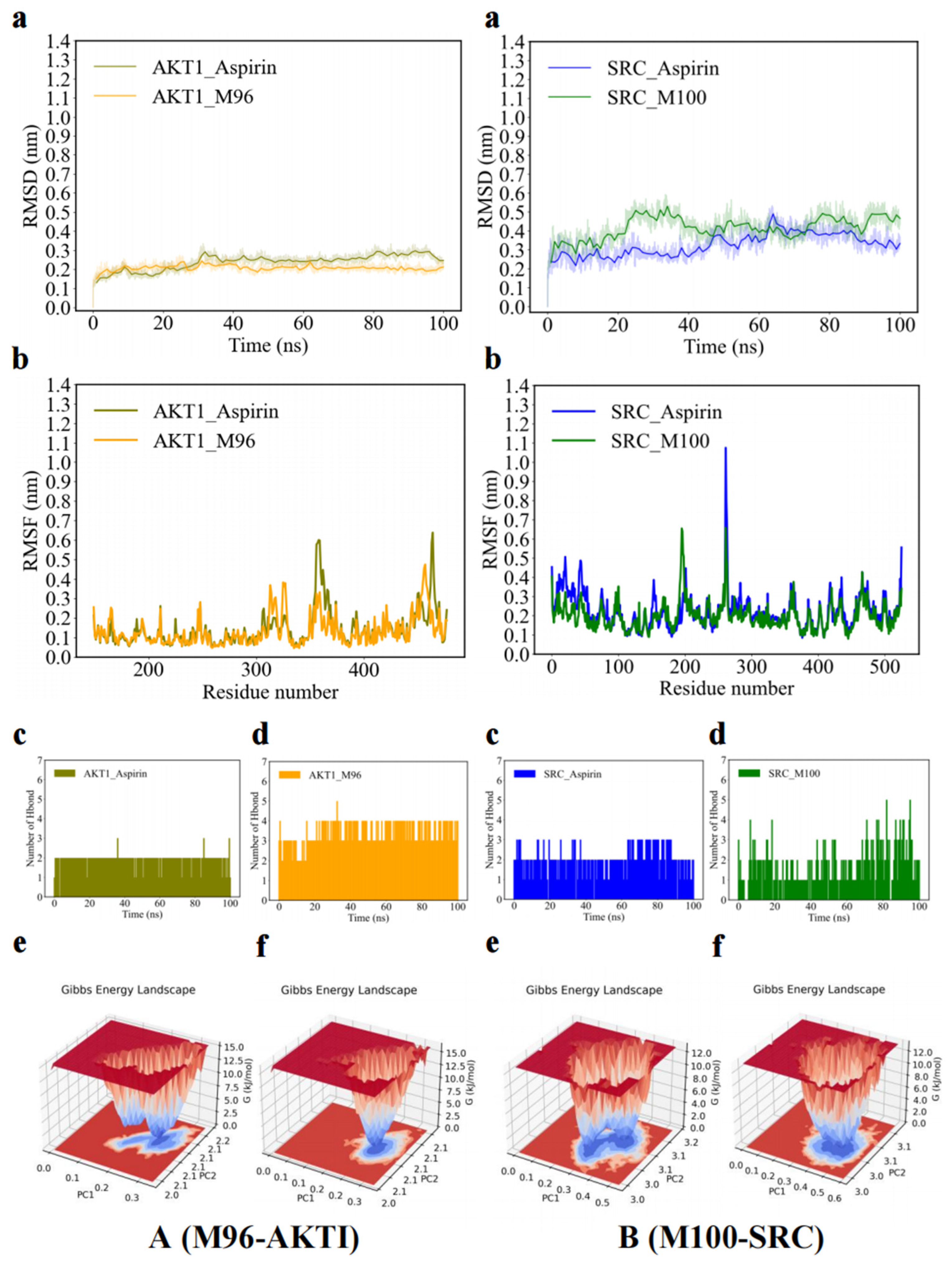
2.5. Molecular Dynamic Simulations
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Experimental Animals
4.3. Antithrombotic Activity of KAE In Vitro
4.4. Platelet Aggregation Assay In Vitro
4.5. Antithrombotic Activity of KAE In Vivo
4.5.1. Rats Administration, Thrombus Formation, and Plasma Collection
4.5.2. Evaluation of Antithrombotic Activity
4.6. Study on the “Effective Forms” of KAE for Antithrombotic Activity
4.6.1. Metabolism Experiment of KAE
4.6.2. Biosamples Collection and Preparation
4.6.3. Instruments and Conditions
4.6.4. Metabolites Characterization
4.7. Network Pharmacology
4.7.1. Targets Collection for KAE Metabolites
4.7.2. Antithrombotic Targets Collection for KAE Metabolites
4.7.3. Key Antithrombotic Targets Analysis for KAE Metabolites
4.7.4. GO and KEGG Pathway Enrichment Analysis
4.8. Molecular Docking
4.8.1. Pretreatment of Receptor Proteins
4.8.2. Pretreatment of Ligand Small Molecules
4.8.3. Generation of Docking Grid Interface
4.8.4. Molecular Docking Parameter Setting
4.9. Molecular Dynamics Simulation
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABCB1 | Adenosine triphosphate binding cassette subfamily B member 1 gene |
| ABCG2 | Breast cancer resistance protein |
| AKT1 | Serine/threonine-protein kinase 1 gene |
| ANOVA | One-way analysis of variance |
| APP | Amyloid precursor protein |
| APTT | activated partial thromboplastin time |
| AR | Androgen receptor |
| BCL2 | B-cell lymphoma-2 |
| BP | Biological processes |
| BRAF | B-raf proto oncogene serine/threonine protein kinase |
| CaCl2 | Calcium chloride |
| C. orbiculatus | Celastrus orbiculatus thunb. |
| CC | Cell composition |
| CLog P | Calculated n-octanol/water partition coefficient |
| CMC-Na | Sodium carboxymethyl cellulose |
| C-T-P | Compound-target-pathway |
| CVDs | Cardiovascular diseases |
| DAVID | Database for annotation, visualization and integrated discovery |
| DBE | Double bond equivalents |
| DMSO | Dimethyl sulfoxide |
| EGFR | Epidermal growth factor receptor |
| EICs | Extracted ion chromatograms |
| ELISA | Enzyme-linked immunosorbent assay |
| ESR1 | Estrogen receptor 1 |
| FeCl3 | Ferric chloride |
| FIB | Fibrinogen |
| GAFF | General amber force field |
| GO | Gene ontology |
| GSK3B | Glycogen synthase kinase 3 beta gene |
| HIF-1 | Hypoxia-inducible factor |
| HPLC | High-performance liquid chromatography |
| 6-keto-PGF1α | 6-keto-prostaglandin F1α |
| IL2 | Interleukin-2 |
| KAE | Kaempferitrin |
| KAE-H | Kaempferitrin high dose group |
| KAE-L | Kaempferitrin low dose group |
| KDR | Kinase domain-containing receptor |
| KEGG | Kyoto encyclopedia of genes and genomes |
| KIT | Kinase inserts domain receptor |
| LC-MS | Liquid chromatograph mass spectrometer |
| LSD | Least significant difference |
| MARK | Mitogen-activated protein kinase |
| MD | Molecular dynamics simulation |
| MeSH | Medical subject headings |
| MET | Mesenchymal to epithelial transition factor |
| MF | Molecular function |
| MMP2 | Matrix metallopeptidase 2 |
| MMP9 | Matrix metallopeptidase 9 |
| MPO | Myeloperoxidase |
| MF | Molecule formula |
| MS2 | Secondary mass spectrometry |
| MW | Molecular weight |
| NADPH-oxidase | Nicotinamide adenine dinucleotide phosphate-oxidase |
| NF-κB | Nuclear factor kappa-B |
| NPT | Isothermal isobaric ensemble |
| NVT | Isothermal isovolumic ensemble |
| OMIM | Online mendelian inheritance in man |
| PAI-1 | Plasminogen activator inhibitor |
| PDB | Protein data bank |
| PGI2 | Prostacyclin |
| PIK3CA | Phosphatidylinositol-4, 5-bisphosphate-3-kinase catalytic subunit α |
| PIK3R1 | Phosphoinositide-3-kinase regulatory subunit 1 gene |
| PIP2 | Phosphatidylinositol-4, 5-bisphosphate |
| PKB | Protein kinase B |
| PLG | Plasminogen gene |
| PPARA | Peroxisome proliferator-activated receptor α |
| PPARG | Peroxisome proliferator activated receptor gamma gene |
| PPI | Protein–protein interaction |
| PRKACA | Cyclic adenosine monophosphate-dependent protein kinase catalytic subunit α |
| PRP | Platelet rich plasma |
| PRT | Plasma recalcification time |
| PT | Prothrombin time |
| PTGS2 | Prostaglandin-endoperoxide synthase 2 |
| RDA | Retro-Diels–Alder |
| RMSD | Root-mean-square deviation |
| RMSF | Root-mean-square fluctuation |
| SD | Standard deviation |
| SERPINE1 | Plasminogen activator inhibitor 1 |
| SMILES | Simplified molecular input line entry system |
| SO3 | Sulfuric acid |
| SPSS | Statistical product and service solutions |
| SRC | Non-receptor tyrosine kinase gene |
| STRING | Search Tool for the Retrieval of Interacting Genes/Proteins |
| TCMs | Traditional Chinese Medicines |
| TERT | Telomerase reverse transcriptase |
| TNF | Tumor necrosis factor |
| t-PA | Tissue-type plasminogen activator |
| tR | Retention time |
| TT | Thrombin time |
| TXA2 | Thromboxane A2 |
| TXB2 | Thromboxane B2 |
| UHPLC | Ultra-high-performance liquid chromatography |
| VEGF | Vascular endothelial growth factor |
References
- Alkarithi, G.; Duval, C.; Shi, Y.; Macrae, F.L.; Ariens, R.A.S. Thrombus Structural Composition in Cardiovascular Disease. Arterioscl. Throm. Vas. 2021, 41, 2370–2383. [Google Scholar] [CrossRef]
- Schmaier, A.A.; Anderson, P.F.; Chen, S.M.; EI-Darzi, E.; Aivasovsky, I.; Kaushik, M.P.; Sack, K.D.; Hartzell, H.C.; Parikh, S.M.; Flaumenhaft, R.; et al. TMEM16E regulates endothelial cell procoagulant activity and thrombosis. J. Clin. Investig. 2023, 133, e163808. [Google Scholar] [CrossRef]
- Hosokawa, K.; Ohnishi-Wada, T.; Nagasato, T.; Sameshima-Kaneko, H.; Oyamada, C.; Dahlen, J. New methodological approaches for assessing thrombus formation in cardiovascular disease. Kardiol. Pol. 2020, 78, 667–673. [Google Scholar] [CrossRef]
- Ząbczyk, M.; Ariëns, R.A.S.; Undas, A. Fibrin clot properties in cardiovascular disease: From basic mechanisms to clinical practice. Cardiovasc. Res. 2023, 119, 94–111. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.W.; Huang, Z.T.; Zhang, X.J. Efficacy and safety of clopidogrel and/or aspirin for ischemic stroke/transient ischemic attack. Medicine 2021, 100, e27804. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Abe, K.; Kodashima, S. Gastrointestinal bleeding and mucosal injury induced by antithrombotic drugs. J. Gastroenterol. 2024, 121, 807–812. [Google Scholar]
- Bhatia, K.; Ladd, L.M.; Carr, K.H.; Di Napoli, M.; Saver, J.L.; McCullough, L.D.; Farahabadi, M.H.; Alsbrook, D.L.; Hinduja, A.; Ortiz Garcia, J.G.; et al. Contemporary Antiplatelet and Anticoagulant Therapies for Secondary Stroke Prevention: A Narrative Review of Current Literature and Guidelines. Curr. Neurol. Neurosci. Rep. 2023, 23, 235–262. [Google Scholar] [CrossRef]
- Kim, T.-H.; Lee, K.M.; Hong, N.D.; Jung, Y.-S. Anti-platelet and anti-thrombotic effect of a traditional herbal medicine Kyung-Ok-Ko. J. Ethnopharmacol. 2016, 178, 172–179. [Google Scholar] [CrossRef]
- Ye, W.L.; Wang, J.J.; Little, P.J.; Zou, J.M.; Zheng, Z.H.; Lu, J.; Yin, Y.J.; Liu, H.; Zhang, D.M.; Liu, P.Q.; et al. Anti-atherosclerotic effects and molecular targets of ginkgolide B from Ginkgo biloba. Acta Pharm. Sin. B. 2024, 14, 1–19. [Google Scholar] [CrossRef]
- Orgah, J.O.; He, S.; Wang, Y.; Jiang, M.M.; Wang, Y.F.; Orgah, E.A.; Duan, Y.J.; Zhao, B.C.; Han, J.H.; Zhu, Y. Pharmacological potential of the combination of Salvia miltiorrhiza (Danshen) and Carthamus tinctorius (Honghua) for diabetes mellitus and its cardiovascular complications. Pharmacol. Res. 2020, 153, 104654. [Google Scholar] [CrossRef]
- Wang, Y.H.; Zhang, X.T.; Zhou, C.X.; Khan, H.; Fu, M.Q.; Cheang, W.S. Citri Reticulatae Pericarpium (Chenpi) Protects against Endothelial Dysfunction and Vascular Inflammation in Diabetic Rats. Nutrients 2022, 14, 5221. [Google Scholar] [CrossRef]
- Sánchez, M.; Romero, M.; Gómez-Guzmán, M.; Tamargo, J.; Pérez-Vizcaino, F.; Duarte, J. Cardiovascular Effects of Flavonoids. Curr. Med. Chem. 2019, 26, 6991–7034. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, H.Y.; Yang, F.; Dong, W.B. The Protective Effects of Myricetin against Cardiovascular Disease. J. Nutr. Sci. Vitaminol. 2019, 65, 470–476. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, S.-L. The role of flavonoids in the prevention and management of cardiovascular complications: A narrative review. Ann. Palliat. Med. 2021, 10, 8254–8263. [Google Scholar] [CrossRef]
- Rolnik, A.; Żuchowski, J.; Stochmal, A.; Olas, B. Quercetin and kaempferol derivatives isolated from aerial parts of Lens culinaris Medik as modulators of blood platelet functions. Ind. Crops Prod. 2020, 152, 112536. [Google Scholar] [CrossRef]
- Wang, S.B.; Chae, Y.H.; Jang, J.Y.; Min, J.H.; Baek, J.Y.; Kim, M.; Park, Y.; Hwang, G.S.; Ryu, J.S.; Chang, T.S. Kaempferol suppresses collagen-induced platelet activation by inhibiting NADPH oxidase and protecting SHP-2 from oxidative inactivation. Free Radic Biol Med. 2015, 83, 41–53. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, Y.; Xin, H. Analysis of flavonoids in rosae laevigatae fructus by UPLC-Q-TOF-MS. Chin. J. Exp. Formulaics. 2017, 23, 71–76. [Google Scholar]
- Guo, F.Y.; Wang, Y.; Hao, Y.J.; Lu, X.Y.; Sang, Y.L. Determination of four flavonoids in Vicia amoena Fisch. and Vicia amoena Fisch. var. angusta Freyn. by HPLC. Chin. J. Pharm. Anal. 2017, 37, 432–437. [Google Scholar]
- Zhou, J.J.; Zhai, J.X.; Zheng, W.L.; Han, N.; Liu, Z.H.; Lv, G.H.; Zheng, X.J.; Chang, S.; Yin, J. The antithrombotic activity of the active fractions from the fruits of Celastrus orbiculatus Thunb through the anti-coagulation, anti-platelet activation and anti-fibrinolysis pathways. J. Ethnopharmacol. 2019, 241, 111974. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.P.; Luo, Q.; Hou, Y.; Zhao, F.L.; Li, F.; Meng, Q.G. Houttuynia Cordata Thunb.: A comprehensive review of traditional applications, phytochemistry, pharmacology and safety. Phytomedicine 2024, 123, 155195. [Google Scholar] [CrossRef]
- Tang, Y.T.; Hou, X.T.; Du, Z.C.; Hao, E.-W. Research progress on chemical constituents and pharmacological effects of Siraitiae Fructus and predictive analysis on quality markers. Chin. Tradit. Herbal. Drugs. 2021, 52, 2843–2850. [Google Scholar]
- Patel, D.K. Pharmacological Activities and Therapeutic Potential of Kaempferitrin in Medicine for the Treatment of Human Disorders: A Review of Medicinal Importance and Health Benefits. Cardiovasc. Hematol. Disord. Drug Targets. 2021, 21, 104–114. [Google Scholar] [CrossRef]
- Sousa, E.D.; Zanatta, L.; Seifriz, I.; Creczynski-Pasa, T.B.; Pizzolatti, M.G.; Szpoganicz, B.; Mena Barreto Silva, F.R. Hypoglycemic effect and antioxidant potential of kaempferol-3,7-O-(alpha)-dirhamnoside from Bauhinia forficata leaves. J. Nat. Prod. 2004, 67, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zheng, L.; Lin, Z.F.; Hou, C.Q.; Liu, W.Q.; Yan, T.M.; Zhu, L.J.; Wang, Y.; Lu, L.L.; Liu, Z.Q. Study of pharmacokinetic profiles and characteristics of active components and their metabolites in rat plasma following oral administration of the water extract of Astragali radix using UPLC–MS/MS. J. Ethnopharmacol. 2015, 169, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Li, H.F.; Xu, F.; Yang, P.; Liu, G.X.; Shang, M.Y.; Wang, X.; Yin, J.; Cai, S.Q. Systematic screening and characterization of prototype constituents and metabolites of total astragalosides using HPLC-ESI-IT-TOF-MSn after oral administration to rats. J. Pharm. Biomed. Anal. 2017, 142, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.Q.; Wang, X.; Shang, M.Y.; Xu, F.; Liu, G.X. “Efficacy theory” may help to explain characteristic advantages of traditional Chinese medicines. Chin. J. Chin. Mater. Med. 2015, 40, 3435–3443. [Google Scholar]
- Chen, M.J.; Xiao, J.B.; El-Seedi, H.R.; Woźniak, K.S.; Daglia, M.; Little, P.J.; Weng, J.P.; Xu, S.W. Kaempferol and atherosclerosis: From mechanism to medicine. Crit. Rev. Food Sci. Nutr. 2024, 64, 2157–2175. [Google Scholar] [CrossRef]
- Bangar, S.P.; Chaudhary, V.; Sharma, N.; Bansal, V.; Ozogul, F.; Lorenzo, J.M. Kaempferol: A flavonoid with wider biological activities and its applications. Crit. Rev. Food Sci. Nutr. 2023, 63, 9580–9604. [Google Scholar] [CrossRef]
- Gogoi, D.; Chattopadhyay, P.; Dolui, S.K.; Khan, M.R.; Mukherjee, A.K. Studies on in vivo antithrombotic activity of quercetin, a natural flavonoid isolated from a traditional medicinal plant, African eggplant (Solanum indicum). J. Ethnopharmacol. 2024, 335, 118686. [Google Scholar] [CrossRef]
- Senis, Y.A.; Mazharian, A.; Mori, J. Src family kinases: At the forefront of platelet activation. Blood 2014, 124, 2013–2024. [Google Scholar] [CrossRef]
- Zhai, Y.H.; Yang, J.; Zhang, J.; Yang, J.; Li, Q.; Zheng, T. Src-family Protein Tyrosine Kinases: A promising target for treating Cardiovascular Diseases. Int. J. Med. Sci. 2021, 18, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Nigam, A.; Manjuprasanna, V.N.; Naik, M.U.; Naik, U.P. Platelet Spreading and Clot Retraction are Regulated by two Distinct αIIbβ3 Outside-in Signaling Pathways. J. Pharmacol. Exp. Ther. 2024, 392, 100012. [Google Scholar] [CrossRef] [PubMed]
- Song, H.D.; Yang, Y.J.; Li, B. Tripeptide Hyp–Asp–Gly from collagen peptides inhibited platelet activation via regulation of PI3K/Akt–MAPK/ERK1/2 signaling pathway. J. Food Sci. 2022, 87, 3279–3293. [Google Scholar] [CrossRef]
- Kwon, H.W. Inhibitory Effects of Ginsenoside Ro on Clot Retraction through Suppressing PI3K/Akt Signaling Pathway in Human Platelets. Prev. Nutr. Food Sci. 2019, 24, 56–63. [Google Scholar] [CrossRef]
- Xie, Z.T.; Liu, B.; Xiong, Y.Y.; Yang, Y.F. Study of Components and Mechanism of Juechuang Against Platelet Aggregation Based on Network Pharmacology. Nat. Prod. Commun. 2020, 15, 227–229. [Google Scholar] [CrossRef]
- Zakaria, N.H.; Tap, F.M.; Aljohani, G.F.; Abdul Majid, F.A. Molecular docking and dynamics simulations revealed the potential inhibitory activity of honey-iQfood ingredients against GSK-3β and CDK5 protein targets for brain health. J. Biomol. Struct. Dyn. 2024, 43, 3429–3448. [Google Scholar] [CrossRef]
- Liu, Y.; Xiong, B.B.; Qiu, X.; Hao, H.Y.; Sha, A. Study on the antithrombotic effect and physiological mechanism of okanin. Biomed. Pharmacother. 2022, 153, 113358. [Google Scholar] [CrossRef]
- Chu, L.X.; Qin, Y.Q.; Zhou, S.X.; Yang, F.; He, L.P.; Liang, Z.S.; Mo, C.G.; Wang, X.D. The effect of pravastatin on carotid artery thrombosis in rats under the stimulus of C-reactive protein. Thromb. Res. 2016, 144, 213–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yao, W.; Zhu, X.D.; Wang, J.J.; Lu, L.H.; Zhu, N.; Lan, T.; Kuang, Y.X.; Zhu, W.F.; Liu, R.H.; et al. Exploring the mechanism of the antithrombotic effects of Pueraria lobata and Pueraria lobata var. thomsonii based on network pharmacology. J. Ethnopharmacol. 2023, 300, 115701. [Google Scholar] [CrossRef]
- Chen, G.L.; Zeng, R.; Wang, X.; Cai, H.Y.; Chen, J.J.; Zhong, Y.X.; Zhong, S.Y.; Jia, X.J. Antithrombotic Activity of Heparinoid G2 and Its Derivatives from the Clam Coelomactra antiquata. Mar. Drugs 2022, 20, 50. [Google Scholar] [CrossRef]
- Mustapha, M.; Nassir, C.M.N.C.M.; Aminuddin, N.; Safri, A.A.; Ghazali, M.M. Cerebral Small Vessel Disease (CSVD)—Lessons From the Animal Models. Front. Physiol. 2019, 10, 1317. [Google Scholar] [CrossRef]
- Van den Kerkhof, D.L.; Nagy, M.; Wichapong, K.; Brouns, S.L.N.; Heemskerk, J.W.M.; Hackeng, T.M.; Dijkgraaf, I. Inhibition of platelet adhesion, thrombus formation, and fibrin formation by a potent αIIbβ3 integrin inhibitor from ticks. Res. Pract. Thromb Hae. 2021, 5, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Ruan, D.T.; Lu, R.; Ruan, K.-H. Redirecting thromboxane A2 and prostacyclin biosyntheses from thrombotic to antithrombotic property by an Enzymelink. Future Med. Chem. 2021, 13, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Jiang, M.; Wang, J.; Gao, J.Y.; Guo, M.; Liu, J.X.; Chen, X.Y.; Lang, H.Y. The hemostatic mechanism of “Treated the Spleen” therapy on immune thrombocytopenia based on the characteristics of vasoactive factors. Ann. Palliat. Med. 2021, 10, 4612–4622. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.T.; Abdurahman, A.; Umar, A.; Iskander, G.; Abdusalam, E.; Berke, B.; Begaud, B.; Moore, N. Effects of Cydonia oblonga Miller extracts on blood hemostasis, coagulation and fibrinolysis in mice, and experimental thrombosis in rats. J. Ethnopharmacol. 2014, 154, 163–169. [Google Scholar] [CrossRef]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Choi, J.H.; Park, S.E.; Kim, S.J.; Kim, S. Kaempferol inhibits thrombosis and platelet activation. Biochimie 2015, 115, 177–186. [Google Scholar] [CrossRef]
- Ding, Y.H.; Xiang, Q.; Zhu, P.Y.; Fan, M.L.; Tong, H.Q.; Wang, M.X.; Cheng, S.Y.; Yu, P.; Shi, H.B.; Zhang, H.W.; et al. Qihuang Zhuyu formula alleviates coronary microthrombosis by inhibiting PI3K/Akt/αIIbβ3-mediated platelet activation. Phytomedicine 2024, 125. [Google Scholar] [CrossRef]
- Lee, D.H. Inhibitory effects of scoparone through regulation of PI3K/Akt and MAPK on collagen-induced human platelets. J. Appl. Biol. Chem. 2020, 63, 131–136. [Google Scholar] [CrossRef]
- Su, W.; Chen, Y.; Wang, C.H.; Ding, X.; Rwibasira, G.; Kong, Y. Human cathelicidin LL-37 inhibits platelet aggregation and thrombosis via Src/PI3K/Akt signaling. Biochem. Biophys. Res. Commun. 2016, 473, 283–289. [Google Scholar] [CrossRef]
- Dhahri, M.; Rodriguez-Ruiz, V.; Aid-Launais, R.; Ollivier, V.; Pavon-Djavid, G.; Journe, C.; Louedec, L.; Chaubet, F.; Letourneur, D.; Maaroufi, R.M.; et al. In vitro and in vivo hemocompatibility evaluation of a new dermatan sulfate-modified PET patch for vascular repair surgery. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 2001–2009. [Google Scholar] [CrossRef]
- Lee, K.O.; Kwon, I.; Nam, H.S.; Park, Y.; Kim, J.; Shim, Y.; Erdenebileg, Z.; Cha, M.J.; Choi, H.J.; Choi, H.Y.; et al. Effect of leukopenia induced by cyclophosphamide on the initial stage of arterial thrombosis in mice. Thromb. Res. 2021, 206, 111–119. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.J.; Gindulyte, A.; He, J.; He, S.Q.; Li, Q.L.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Wu, J.S.; Zhang, F.Q.; Ruan, H.N.; Chang, X.Y.; Wang, J.X.; Li, Z.Z.; Jin, W.Y.; Shi, Y. Integrating Network Pharmacology and RT-qPCR Analysis to Investigate the Mechanisms Underlying ZeXie Decoction-Mediated Treatment of Non-alcoholic Fatty Liver Disease. Front. Pharmacol. 2021, 12, 722016. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Zhang, H.H.; Yao, S.; Zhang, Z.G.; Zhou, C.C.; Fu, F.D.; Bian, Y.S.; Luo, H.; Li, Y.; Yan, S.X.; Ge, Y.Y.; et al. Network Pharmacology and Experimental Validation to Reveal the Pharmacological Mechanisms of Liuwei Dihuang Decoction Against Intervertebral Disc Degeneration. Drug Des. Dev. Ther. 2021, 15, 4911–4924. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Zhao, L.F.; Wang, H.-F.; Wen, Y.T.; Jiang, K.K.; Mao, X.M.; Zhou, Z.Y.; Yao, K.T.; Geng, Q.S.; Guo, D.; et al. GenCLiP 3: Mining human genes’ functions and regulatory networks from PubMed based on co-occurrences and natural language processing. Bioinformatics 2019, 36, 1973–1975. [Google Scholar] [CrossRef] [PubMed]
- Nogales, C.; Mamdouh, Z.M.; List, M.; Kiel, C.; Casas, A.; Schmidt, H.H. Network pharmacology: Curing causal mechanisms instead of treating symptoms. Trends Pharmacol. Sci. 2022, 43, 136–150. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, T.F.; Chen, X.Y.; Ye, Y.; Liu, L.Q.; Wang, Y.F.; Zhang, P. Network pharmacology and molecular docking approach to investigate the mechanism of a Chinese herbal formulation Yougui pills against steroid-related osteonecrosis of the femoral head. Arab. J. Chem. 2024, 17, 105609. [Google Scholar] [CrossRef]
- Fu, F.Y.; Huang, Z.Q.; Ye, H.L.; Tan, B.; Wang, R.T.; Chen, W.H. Mechanisms and Molecular Targets of the Tao-Hong-Si-Wu-Tang Formula for Treatment of Osteonecrosis of Femoral Head: A Network Pharmacology Study. Evid. Based Complement. Alternat. Med. 2020, 2020, 7130105. [Google Scholar] [CrossRef]
- Jiang, C.N.; Meng, A.G.; Shi, X.Y.; Fu, Z.P.; Wang, Y.L.; Zhou, J.J.; Zhang, X.W.; Liu, C.Y. Preparation of antioxidant peptides from yak skin gelatin and their protective effect on myocardial ischemia reperfusion injury. Food Funct. 2024, 15, 7961–7973. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Pall, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef] [PubMed]
- Collier, T.A.; Piggot, T.J.; Allison, J.R. Molecular Dynamics Simulation of Proteins. Methods Mol. Biol. 2020, 2073, 311–327. [Google Scholar] [PubMed]

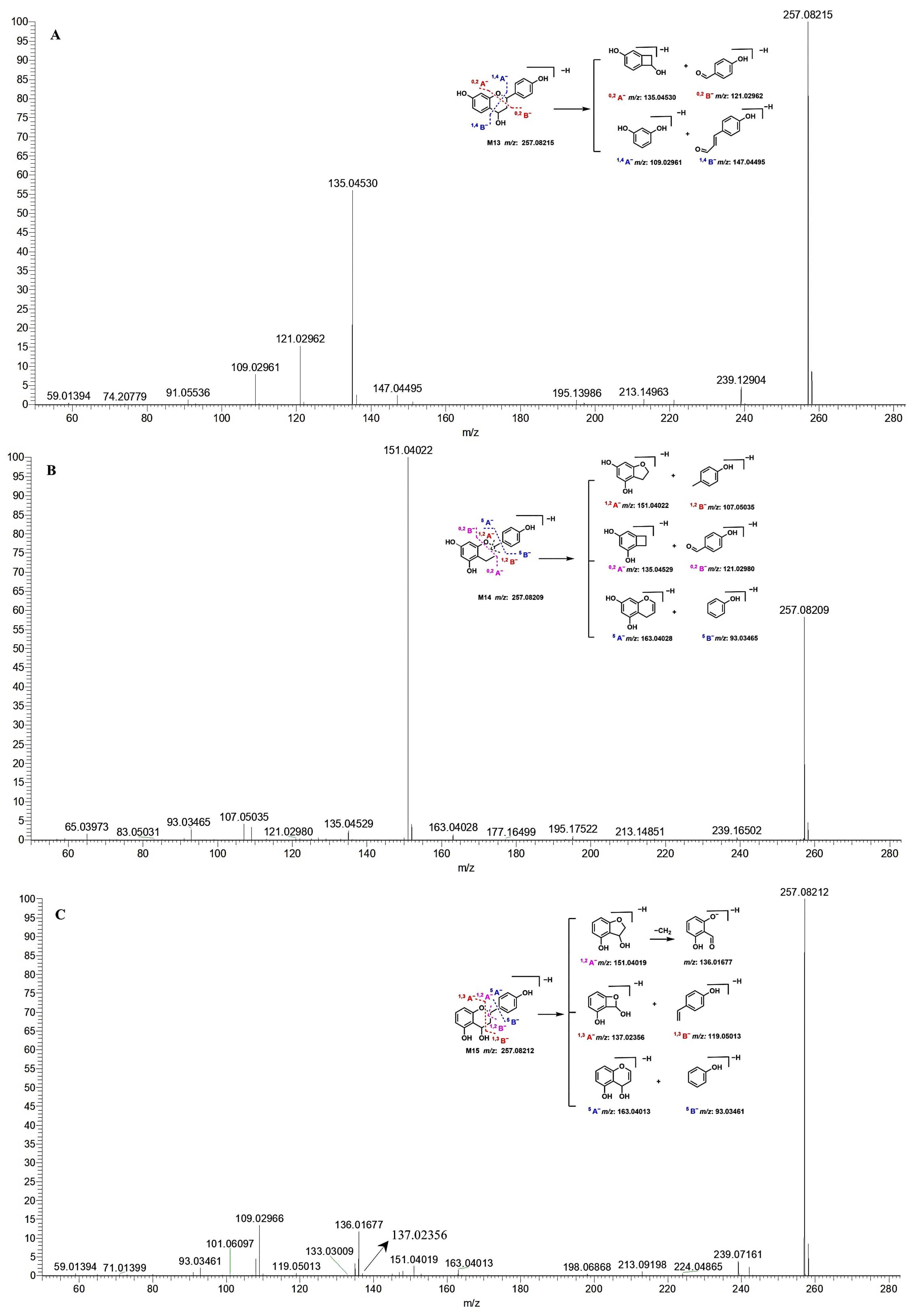
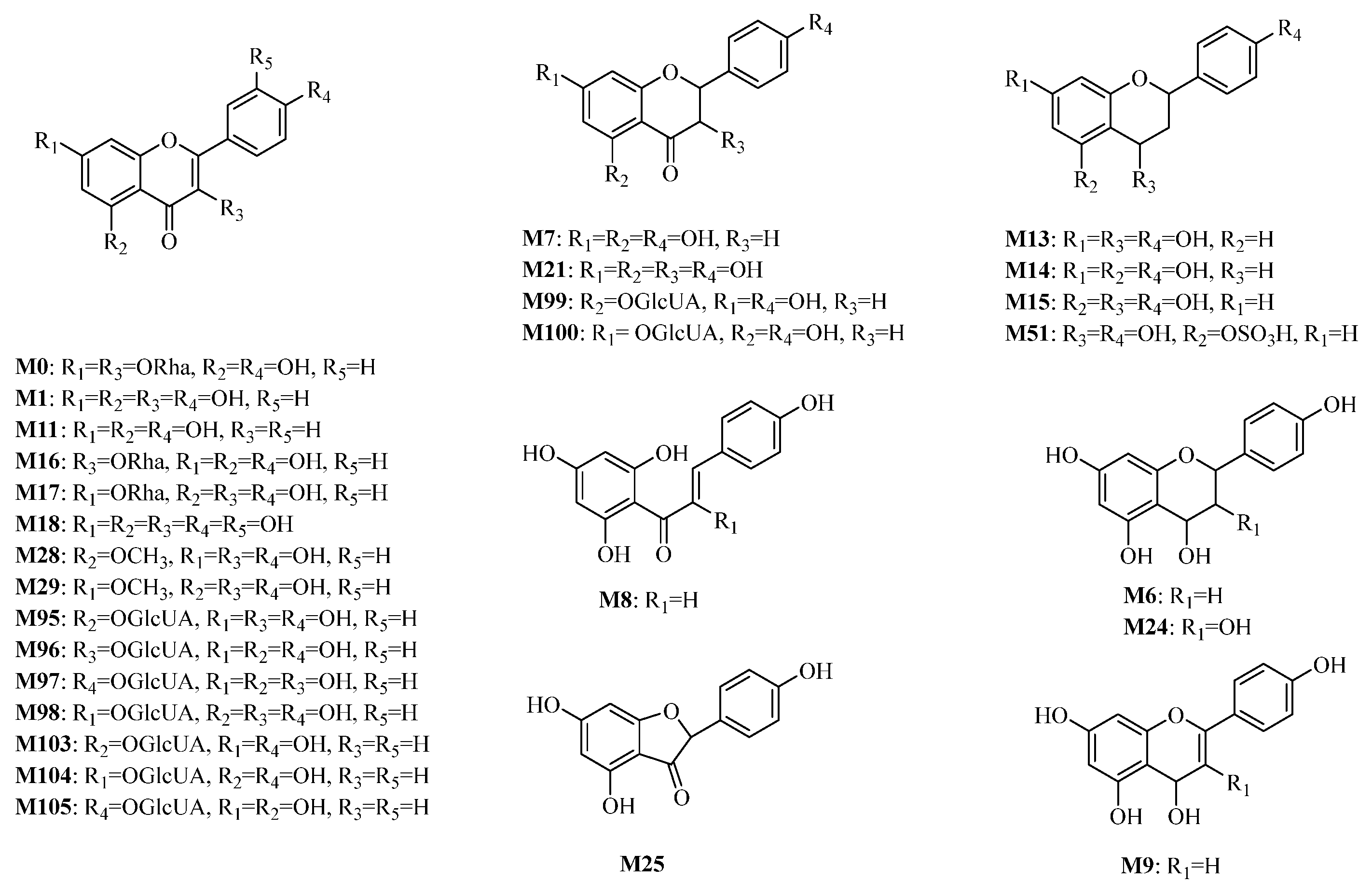
 : unchanged hydroxyl group. (A) M10, galangin; M11, apigenin. (B) M12, A-ring dehydroxylated apigenin. (C) M13–M15, dehydroxylated and hydrogenated apigenin.
: unchanged hydroxyl group. (A) M10, galangin; M11, apigenin. (B) M12, A-ring dehydroxylated apigenin. (C) M13–M15, dehydroxylated and hydrogenated apigenin.
 : unchanged hydroxyl group. (A) M10, galangin; M11, apigenin. (B) M12, A-ring dehydroxylated apigenin. (C) M13–M15, dehydroxylated and hydrogenated apigenin.
: unchanged hydroxyl group. (A) M10, galangin; M11, apigenin. (B) M12, A-ring dehydroxylated apigenin. (C) M13–M15, dehydroxylated and hydrogenated apigenin. 

| Group | Dose (µg/mL) | Platelet Aggregation Rate (%) | Platelet Aggregation Inhibition Rate (%) |
|---|---|---|---|
| Control | — | 56.53 ± 1.30 | — |
| Aspirin | 54.05 | 14.33 ± 0.53 ** | 74.66 ± 1.30 |
| KAE-L | 54.05 | 21.01 ± 0.75 **## | 62.82 ± 1.44 ## |
| KAE-H | 108.10 | 15.34 ± 0.42 ** | 72.87 ± 0.25 |
| No. | Name | MF | Meas. (Da) | Pred. (Da) | Diff (ppm) | DBE | tR (min) | P | U | F | CL |
|---|---|---|---|---|---|---|---|---|---|---|---|
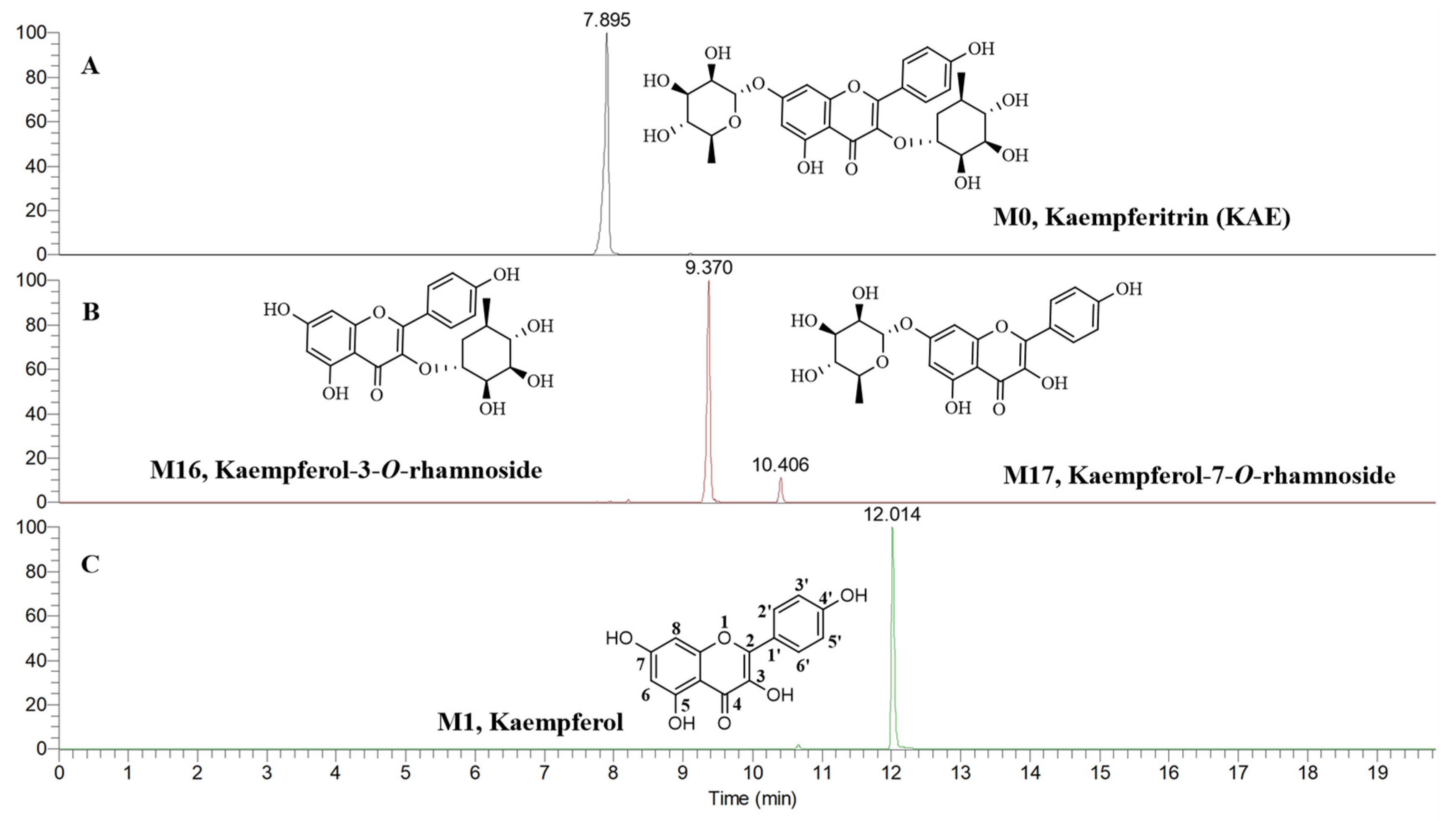
| M0 | Kaempferitrin (KAE) | C27H30O14 | 577.15509 | 577.15628 | −2.06 | 13 | 7.946 | ▲ | ▲ | ▲ | 1 |
| M1 | Kaempferol | C15H10O6 | 285.04010 | 285.04046 | −1.26 | 11 | 12.078 | ▲ | ▲ | 1 | |
| M11 | Apigenin | C15H10O5 | 269.04568 | 269.04555 | 0.48 | 11 | 11.927 | ▲ | ▲ | ▲ | 1 |
| M16 | Kaempferol-3-O-rhamnoside | C21H20O10 | 431.09613 | 431.09837 | −5.20 | 12 | 9.370 | ▲ | 1 | ||
| M17 | Kaempferol-7-O-rhamnoside | C21H20O10 | 431.09766 | 431.09837 | −1.65 | 12 | 10.406 | ▲ | 1 | ||
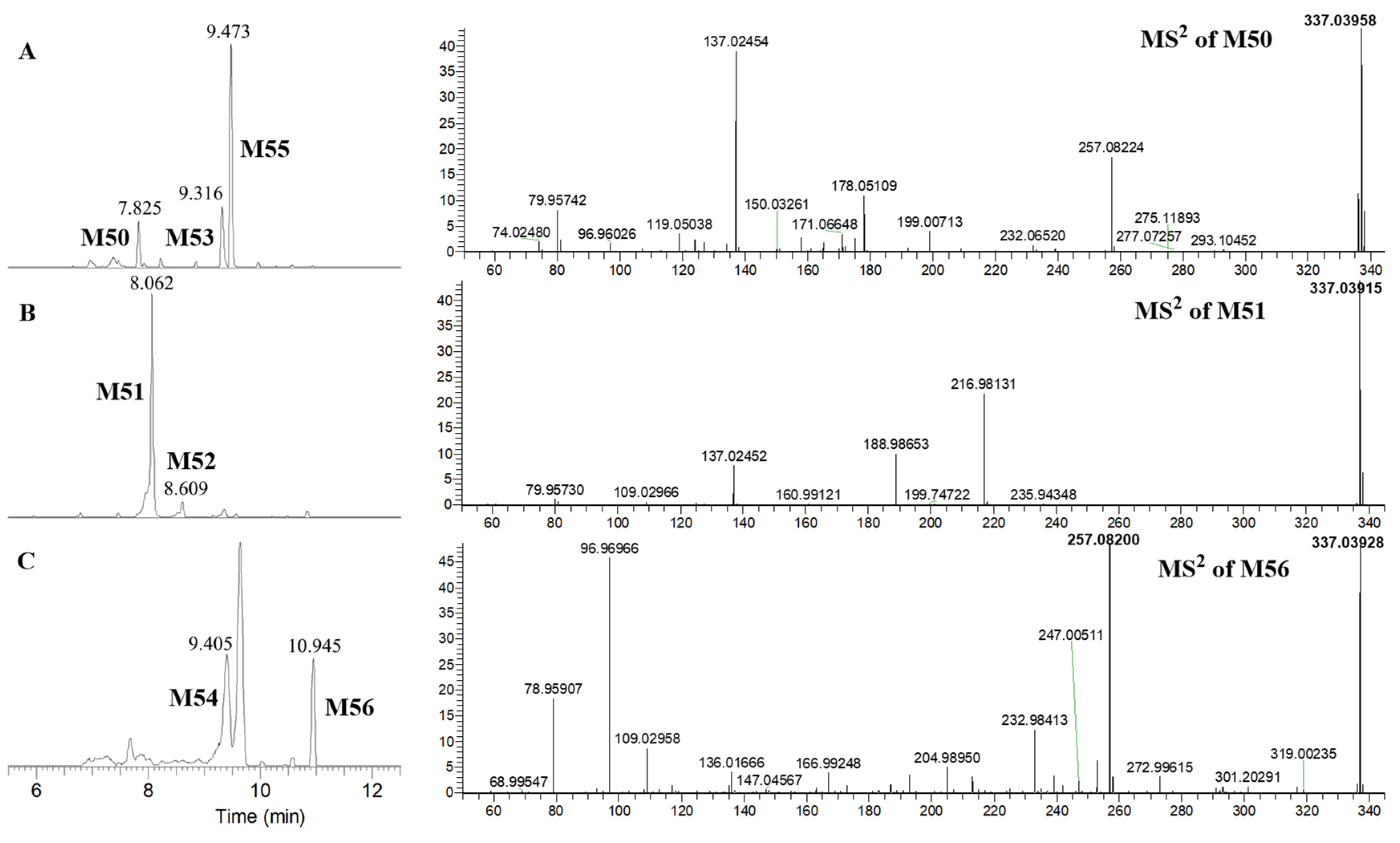
| * M50 | 4, 5, 4′-Trihydroxylated dihydrogenated flavone-4′-O-sulfate | C15H14O7S | 337.03958 | 337.03875 | 2.46 | 9 | 7.829 | ▲ | 2 | ||
| * M51 | 4, 5, 4′-Trihydroxylated dihydrogenated flavone-5-O-sulfate | C15H14O7S | 337.03915 | 337.03875 | 1.19 | 9 | 8.062 | ▲ | 2 | ||
| * M53 | 4, 5, 4′-Trihydroxylated dihydrogenated flavone-4-O-sulfate isomer 1 | C15H14O7S | 337.03903 | 337.03875 | 0.83 | 9 | 9.312 | ▲ | 2 | ||
| * M54 | 4, 5, 4′-Trihydroxylated dihydrogenated flavone-4-O-sulfate isomer 2 | C15H14O7S | 337.03928 | 337.03875 | 1.57 | 9 | 9.405 | ▲ | 2 | ||
| * M56 | 4, 5, 4′-Trihydroxylated dihydrogenated flavone sulfate | C15H14O7S | 337.03928 | 337.03875 | 1.57 | 9 | 10.945 | ▲ | 2 | ||
| * M67 | Dihydroxylated flavane sulfate 1 | C15H14O6S | 321.04330 | 321.04383 | −1.65 | 9 | 9.125 | ▲ | 3 | ||
| * M68 | Dihydroxylated flavane sulfate 2 | C15H14O6S | 321.04337 | 321.04383 | −1.43 | 9 | 9.408 | ▲ | 3 | ||
| * M69 | Dihydroxylated flavane sulfate 3 | C15H14O6S | 321.04345 | 321.04383 | −1.18 | 9 | 9.577 | ▲ | ▲ | 3 | |
| * M72 | A-Ring methylated dihydrogenated kaempferol sulfate | C16H16O9S | 383.04306 | 383.04423 | −3.05 | 9 | 9.430 | ▲ | 2 | ||
| * M73 | B-Ring methylated dihydrogenated apigenin B-ring sulfate | C16H16O8S | 367.04846 | 367.04931 | −2.32 | 9 | 7.782 | ▲ | 2 | ||
| * M74 | A-Ring methylated dihydrogenated apigenin B-ring sulfate 1 | C16H16O8S | 367.04810 | 367.04931 | −3.30 | 9 | 9.518 | ▲ | 2 | ||
| * M75 | A-Ring methylated dihydrogenated apigenin B-ring sulfate 2 | C16H16O8S | 367.04867 | 367.04931 | −1.74 | 9 | 9.616 | ▲ | 2 | ||
| * M76 | A, C-Rings of apigenin cracking sulfate 1 | C9H8O7S | 258.99152 | 258.99180 | −1.08 | 6 | 4.063 | ▲ | 2 | ||
| * M77 | A, C-Rings of apigenin cracking sulfate 2 | C9H8O7S | 258.99158 | 258.99180 | −0.85 | 6 | 4.533 | ▲ | 2 | ||
| * M78 | A, C-Rings of apigenin cracking sulfate 3 | C9H8O7S | 258.99158 | 258.99180 | −0.85 | 6 | 4.570 | ▲ | 2 | ||
| * M79 | A, C-Rings of apigenin cracking sulfate 4 | C9H8O7S | 258.99155 | 258.99180 | −0.97 | 6 | 4.760 | ▲ | 2 | ||
| * M82 | 4, 5, 4′-Trihydroxylated dihydrogenated flavone disulfate | C15H14O10S2 | 416.99478 | 416.99556 | −1.87 | 9 | 6.963 | ▲ | 2 | ||
| * M83 | 4, 7, 4′-Trihydroxylated dihydrogenated flavone disulfate 1 | C15H14O10S2 | 416.99460 | 416.99556 | −2.30 | 9 | 7.378 | ▲ | 2 | ||
| * M84 | 4, 7, 4′-Trihydroxylated dihydrogenated flavone disulfate 2 | C15H14O10S2 | 416.99460 | 416.99556 | −2.30 | 9 | 7.477 | ▲ | 2 | ||
| * M90 | Dihydroxylated flavane disulfate 1 | C15H14O9S2 | 400.99979 | 401.00065 | −2.14 | 9 | 7.203 | ▲ | 3 | ||
| * M91 | Dihydroxylated flavane disulfate 2 | C15H14O9S2 | 401.00009 | 401.00065 | −1.40 | 9 | 7.724 | ▲ | 3 | ||
| * M121 | Dihydroxylated flavane glucuronide 1 | C21H22O9 | 417.11819 | 417.11911 | −2.21 | 11 | 8.534 | ▲ | 3 | ||
| * M122 | Dihydroxylated flavane glucuronide 2 | C21H22O9 | 417.11807 | 417.11911 | −2.49 | 11 | 8.595 | ▲ | 3 | ||
| * M123 | Dihydroxylated flavane glucuronide 3 | C21H22O9 | 417.11801 | 417.11911 | −2.64 | 11 | 8.877 | ▲ | 3 | ||
| * M124 | Dihydroxylated flavane glucuronide 4 | C21H22O9 | 417.11816 | 417.11911 | −2.28 | 11 | 8.904 | ▲ | ▲ | 3 | |
| * M125 | Dihydroxylated flavane glucuronide 5 | C21H22O9 | 417.11810 | 417.11911 | −2.42 | 11 | 9.035 | ▲ | ▲ | 3 | |
| * M126 | Dihydroxylated flavane glucuronide 6 | C21H22O9 | 417.11819 | 417.11911 | −2.21 | 11 | 9.499 | ▲ | 3 | ||
| * M127 | Dihydroxylated flavane glucuronide 7 | C21H22O9 | 417.11821 | 417.11911 | −2.16 | 11 | 9.642 | ▲ | 3 | ||
| * M129 | B-Ring methylated hydrogenated kaempferol glucuronide | C22H22O12 | 477.10306 | 477.10385 | −1.66 | 12 | 9.201 | ▲ | 2 | ||
| * M130 | Methylated dedihydroxylated dihydrogenated kaempferol glucuronide 1 | C22H24O10 | 447.12900 | 447.12967 | −1.50 | 11 | 9.285 | ▲ | ▲ | 3 | |
| * M131 | Methylated dedihydroxylated dihydrogenated kaempferol glucuronide 2 | C22H24O10 | 447.12930 | 447.12967 | −0.83 | 11 | 9.430 | ▲ | ▲ | 3 | |
| * M132 | Methylated dedihydroxylated dihydrogenated kaempferol glucuronide 3 | C22H24O10 | 447.12894 | 447.12967 | −1.63 | 11 | 9.665 | ▲ | ▲ | 3 | |
| * M135 | Methylated dehydroxylated naringenin glucuronide | C22H22O10 | 445.11316 | 445.11402 | −1.93 | 12 | 11.017 | ▲ | 3 | ||
| * M137 | Dihydroxylated dihydrogenated flavane glucuronide 1 | C21H24O9 | 419.13397 | 419.13476 | −1.88 | 10 | 7.651 | ▲ | 3 | ||
| * M138 | Dihydroxylated dihydrogenated flavane glucuronide 2 | C21H24O9 | 419.13403 | 419.13476 | −1.74 | 10 | 7.731 | ▲ | 3 | ||
| * M139 | Dihydroxylated dihydrogenated flavane glucuronide 3 | C21H24O9 | 419.13397 | 419.13476 | −1.88 | 10 | 9.201 | ▲ | ▲ | 3 | |
| * M140 | Dihydroxylated dihydrogenated flavane glucuronide 4 | C21H24O9 | 419.13336 | 419.13476 | −3.34 | 10 | 9.459 | ▲ | ▲ | 3 | |
| * M141 | Dihydroxylated dihydrogenated flavane glucuronide 5 | C21H24O9 | 419.13370 | 419.13476 | −2.53 | 10 | 9.748 | ▲ | ▲ | 3 | |
| * M142 | Dihydroxylated dihydrogenated flavane glucuronide 6 | C21H24O9 | 419.13310 | 419.13476 | −3.96 | 10 | 10.145 | ▲ | 3 | ||
| * M143 | Dimethylated apigenin glucuronide | C23H22O11 | 473.10779 | 473.10893 | −2.41 | 13 | 10.299 | ▲ | 3 | ||
| * M144 | A-Ring methylated dihydrogenated apigenin glucuronide | C22H24O11 | 463.12418 | 463.12458 | −0.86 | 11 | 9.962 | ▲ | 2 | ||
| * M166 | A-Ring methylated apigenin A-ring glucuronyl B-ring sulfate | C22H20O14S | 539.04901 | 539.05010 | −2.02 | 13 | 6.055 | ▲ | 2 | ||
| * M178 | Trihydroxylated dihydrogenated flavone glucuronyl sulfate 1 | C21H22O13S | 513.07007 | 513.07083 | −1.48 | 11 | 7.048 | ▲ | 3 | ||
| * M179 | Trihydroxylated dihydrogenated flavone glucuronyl sulfate 2 | C21H22O13S | 513.07000 | 513.07083 | −1.62 | 11 | 7.183 | ▲ | 3 | ||
| * M180 | Trihydroxylated dihydrogenated flavone glucuronyl sulfate 3 | C21H22O13S | 513.07007 | 513.07083 | −1.48 | 11 | 7.408 | ▲ | 3 | ||
| * M181 | Trihydroxylated dihydrogenated flavone glucuronyl sulfate 4 | C21H22O13S | 513.06995 | 513.07083 | −1.72 | 11 | 7.681 | ▲ | 3 | ||
| * M182 | Trihydroxylated dihydrogenated flavone glucuronyl sulfate 5 | C21H22O13S | 513.06909 | 513.07083 | −3.39 | 11 | 8.522 | ▲ | 3 | ||
| * M183 | Trihydroxylated dihydrogenated flavone glucuronyl sulfate 6 | C21H22O13S | 513.07007 | 513.07083 | −1.48 | 11 | 8.857 | ▲ | 3 | ||
| * M184 | A-Ring dehydroxylated apigenin glucuronyl sulfate 1 | C21H18O13S | 509.03869 | 509.03953 | −1.65 | 13 | 5.417 | ▲ | 2 | ||
| * M185 | A-Ring dehydroxylated apigenin glucuronyl sulfate 2 | C21H18O13S | 509.03855 | 509.03953 | −1.93 | 13 | 5.669 | ▲ | 2 | ||
| * M186 | A-Ring dehydroxylated apigenin glucuronyl sulfate 3 | C21H18O13S | 509.03870 | 509.03953 | −1.63 | 13 | 5.800 | ▲ | 2 | ||
| * M187 | Dihydroxylated flavane glucuronyl sulfate 1 | C21H22O12S | 497.07629 | 497.07592 | 0.74 | 11 | 6.761 | ▲ | 3 | ||
| * M188 | Dihydroxylated flavane glucuronyl sulfate 2 | C21H22O12S | 497.07455 | 497.07592 | −2.76 | 11 | 7.401 | ▲ | 3 | ||
| * M189 | Dihydroxylated flavane glucuronyl sulfate 3 | C21H22O12S | 497.07489 | 497.07592 | −2.07 | 11 | 7.216 | ▲ | 3 | ||
| * M190 | Dihydroxylated flavane glucuronyl sulfate 4 | C21H22O12S | 497.07413 | 497.07592 | −3.60 | 11 | 7.317 | ▲ | 3 | ||
| * M191 | A-Ring methylated dehydroxylated apiferol glucuronyl-4′-O-sulfate | C22H24O13S | 527.08582 | 527.08648 | −1.25 | 11 | 7.862 | ▲ | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Wang, R.; Hou, J.; Qi, Y.; Liu, Y.; Niu, L.; Xia, X.; Shao, J.; Liu, Y.; Liu, C.; et al. Investigation of Antithrombotic Activity and In Vivo Effective Forms of Kaempferitrin Using FeCl3-Induced Rat Arterial Thrombosis and UHPLC-Q-Exactive Orbitrap MS. Molecules 2025, 30, 4434. https://doi.org/10.3390/molecules30224434
Zhou J, Wang R, Hou J, Qi Y, Liu Y, Niu L, Xia X, Shao J, Liu Y, Liu C, et al. Investigation of Antithrombotic Activity and In Vivo Effective Forms of Kaempferitrin Using FeCl3-Induced Rat Arterial Thrombosis and UHPLC-Q-Exactive Orbitrap MS. Molecules. 2025; 30(22):4434. https://doi.org/10.3390/molecules30224434
Chicago/Turabian StyleZhou, Jingjing, Ruixin Wang, Jingchen Hou, Yitong Qi, Yanglu Liu, Linying Niu, Xinyu Xia, Jinchen Shao, Yizhou Liu, Chunyan Liu, and et al. 2025. "Investigation of Antithrombotic Activity and In Vivo Effective Forms of Kaempferitrin Using FeCl3-Induced Rat Arterial Thrombosis and UHPLC-Q-Exactive Orbitrap MS" Molecules 30, no. 22: 4434. https://doi.org/10.3390/molecules30224434
APA StyleZhou, J., Wang, R., Hou, J., Qi, Y., Liu, Y., Niu, L., Xia, X., Shao, J., Liu, Y., Liu, C., & Li, H. (2025). Investigation of Antithrombotic Activity and In Vivo Effective Forms of Kaempferitrin Using FeCl3-Induced Rat Arterial Thrombosis and UHPLC-Q-Exactive Orbitrap MS. Molecules, 30(22), 4434. https://doi.org/10.3390/molecules30224434







