Cation Induced Structural Variation in Topochemically Modified Dion-Jacobson Perovskite Solid Solutions
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| DJ | Dion-Jacobson |
| S | Staggered |
| E | Eclipsed |
| PS | Partially staggered |
| XRD | X-ray powder diffraction |
| DSC | Differential scanning calorimetry |
References
- Sato, M.; Watanabe, J.; Uematsu, K. Crystal-Structure and Ionic Conductivity of a Layered Perovskite, AgLaNb2O7. J. Solid State Chem. 1993, 107, 460–470. [Google Scholar] [CrossRef]
- Byeon, S.H.; Nam, H.J. Neutron diffraction and FT-Raman study of ion-exchangeable layered titanates and niobates. Chem. Mater. 2000, 12, 1771–1778. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Kitada, A.; Uemura, Y.J.; Goko, T.; Aczel, A.A.; Williams, T.J.; Luke, G.M.; Narumi, Y.; Kindo, K.; Nishi, M.; et al. Two-Dimensional S=1 Quantum Antiferromagnet (NiCl)Sr2Ta3O10. Chem. Mater. 2010, 22, 4625–4631. [Google Scholar] [CrossRef]
- Kim, H.J.; Byeon, S.H.; Yun, H.S. Raman Spectra of the Solid Solution between Rb2La2Ti3O10 and RbCa2Nb3O10. Bull. Korean Chem. Soc. 2001, 22, 298–302. [Google Scholar]
- Viciu, L.; Caruntu, G.; Royant, N.; Koenig, J.; Zhou, W.L.L.; Kodenkandath, T.A.; Wiley, J.B. Formation of metal-anion arrays within layered perovskite hosts. Preparation of a series of new metastable transition-metal oxyhalides, (MCl)LaNb2O7 (M = Cr, Mn, Fe, Co). Inorg. Chem. 2002, 41, 3385–3388. [Google Scholar] [CrossRef]
- Gopalakrishnan, J.; Bhat, V.; Raveau, B. A(I)LaNb2O7: A New Series of Layered Perovskites Exhibiting Ion Exchange and Intercalation Behavior. Mater. Res. Bull. 1987, 22, 413–417. [Google Scholar] [CrossRef]
- Josepha, E.A.; Farooq, S.; Mitchell, C.M.; Wiley, J.B. Synthesis and thermal stability studies of a series of metastable Dion-Jacobson double-layered neodymium-niobate perovskites. J. Solid State Chem. 2014, 216, 85–90. [Google Scholar] [CrossRef]
- Viciu, L.; Liziard, N.; Golub, V.; Kodenkandath, T.A.; Wiley, J.B. Transition-metal Dion-Jacobson layered perovskites, M0.5LaNb2O7. Mater. Res. Bull. 2004, 39, 2147–2154. [Google Scholar] [CrossRef]
- Kodenkandath, T.A.; Kumbhar, A.S.; Zhou, W.L.; Wiley, J.B. Construction of copper halide networks within layered perovskites. Syntheses and characterization of new low-temperature copper oxyhalides. Inorg. Chem. 2001, 40, 710–714. [Google Scholar] [CrossRef]
- Hermann, A.T.; Wiley, J.B. Thermal stability of Dion-Jacobson mixed-metal-niobate double-layered perovskites. Mater. Res. Bull. 2009, 44, 1046–1050. [Google Scholar] [CrossRef]
- Armstrong, A.R.; Anderson, P.A. Synthesis and Structure of a New Layered Niobium Blue Bronze—Rb2LaNb2O7. Inorg. Chem. 1994, 33, 4366–4369. [Google Scholar] [CrossRef]
- Kumada, N.; Kinomura, N.; Sleight, A.W. CsLaNb2O7. Acta Crystallogr. Sect. C-Cryst. Struct. Commun. 1996, 52, 1063–1065. [Google Scholar] [CrossRef]
- Sato, M.; Jin, T.; Ueda, H. Structural Change in Lithium intercalation of Layered Perovskite LiLaNb2O7. Chem. Lett. 1994, 23, 161–164. [Google Scholar] [CrossRef]
- Toda, K.; Uematsu, K.; Sato, M. Structure determination of new layered perovskite compound, NaLaTa2O7, synthesized by ion-exchange reaction. J. Ceram. Soc. Jpn. 1997, 105, 482–485. [Google Scholar] [CrossRef][Green Version]
- Sato, M.; Abo, J.; Jin, T.; Ohta, M. Structure Determination of KLaNb2O7 Exhibiting Ion-Exchange Ability by X-ray Powder Diffraction. Solid State Ion. 1992, 51, 85–89. [Google Scholar] [CrossRef]
- Sato, M.; Jin, T.; Uematsu, K. Proton Conduction of MLaNb2O7 (M = K, Na, H) with a Layered Perovskite Structure. J. Solid State Chem. 1993, 102, 557–561. [Google Scholar] [CrossRef]
- Sato, M.; Abo, J.; Jin, T. Structure Examination of NaLaNb2O7 Synthesized by Soft Chemistry. Solid State Ion. 1992, 57, 285–293. [Google Scholar] [CrossRef]
- Cushing, B.L.; Wiley, J.B. A two-step ion exchange route to the new metastable double-layered perovskite, (Rb,Na)1-xCax/2LaNb2O7 (x~0.9). Mater. Res. Bull. 1999, 34, 271–278. [Google Scholar] [CrossRef]
- Takahashi, S.; Nakato, T.; Hayashi, S.; Sugahara, Y.; Kuroda, K. Formation of a Methoxy-Modified Interlayer Surface via the Reaction Between Methanol and Layered Perovskite HLaNb2O7-xH2O. Inorg. Chem. 1995, 34, 5065–5069. [Google Scholar] [CrossRef]
- Shimada, A.; Yoneyama, Y.; Tahara, S.; Mutin, R.H.; Sugahara, Y. Interlayer surface modification of the protonated ion-exchangeable layered perovskite HLaNb2O7-xH2O with organophosphonic acids. Chem. Mater. 2009, 21, 4155–4162. [Google Scholar] [CrossRef]
- Takeda, Y.; Momma, T.; Osaka, T.; Kuroda, K.; Sugahara, Y. Organic derivatives of the layered perovskite HLaNb2O7-xH2O with polyether chains on the interlayer surface: Characterization, intercalation of LiClO4, and ionic conductivity. J. Mater. Chem. 2008, 18, 3581–3587. [Google Scholar] [CrossRef]
- Poduval, A.; Jones, K.D.; LeBan, L.A.; Wiley, J.B. The Grafting of Hydroxyaromatic Organics within Layered Perovskites via a Microwave-Assisted Method. Molecules 2024, 29, 2888. [Google Scholar] [CrossRef] [PubMed]
- Kodenkandath, T.A.; Lalena, J.N.; Zhou, W.L.; Carpenter, E.E.; Sangregorio, C.; Falster, A.U.; Simmons, W.B.; O’Connor, C.J.; Wiley, J.B. Assembly of metal-anion arrays within a perovskite host. Low-temperature synthesis of new layered copper-oxyhalides, (CuX)LaNb2O7, X = Cl, Br. J. Amer. Chem. Soc. 1999, 121, 10743–10746. [Google Scholar] [CrossRef]
- Viciu, L.; Kodenkandath, T.A.; Wiley, J.B. Construction of a double-layered tetrahedral network within a perovskite host: Two-step route to the alkali-metal-halide layered perovskite, (LixCl)LaNb2O7. J. Solid State Chem. 2007, 180, 583–588. [Google Scholar] [CrossRef]
- Choi, J.; Zhang, X.; Wiley, J.B. Building Alkali-Metal-Halide Layers within a Perovskite Host by Sequential Intercalation: (A2Cl)LaNb2O7 (A = Rb, Cs). Inorg. Chem. 2009, 48, 4811–4816. [Google Scholar] [CrossRef]
- Montasserasadi, D.; Mohanty, D.; Huq, A.; Heroux, L.; Payzant, E.A.; Wiley, J.B. Topochemical Synthesis of Alkali-Metal Hydroxide Layers within Double- and Triple-Layered Perovskites. Inorg. Chem. 2014, 53, 1773–1778. [Google Scholar] [CrossRef] [PubMed]
- Ranmohotti, K.G.S.; Montasserasadi, M.D.; Choi, J.; Yao, Y.; Mohanty, D.; Josepha, E.A.; Adireddy, S.; Caruntu, G.; Wiley, J.B. Room temperature oxidative intercalation with chalcogen hydrides: Two-step method for the formation of alkali-metal chalcogenide arrays within layered perovskites. Mater. Res. Bull. 2012, 47, 1289–1294. [Google Scholar] [CrossRef]
- Kusada, K.; Wu, D.S.; Kitagawa, H. New Aspects of Platinum Group Metal-Based Solid-Solution Alloy Nanoparticles: Binary to High-Entropy Alloys. Chem.-Eur. J. 2020, 26, 5105–5130. [Google Scholar] [CrossRef]
- Liu, B.D.; Li, J.; Yang, W.J.; Zhang, X.L.; Jiang, X.; Bando, Y. Semiconductor Solid-Solution Nanostructures: Synthesis, Property Tailoring, and Applications. Small 2017, 13, 1701998. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Wang, M.; Zhong, Y.J.; Huang, M.R.; Long, Y.; Zhu, H.W. Bandgap-tunable double-perovskite thin films by solution processing. Mater. Today 2019, 28, 25–30. [Google Scholar] [CrossRef]
- Straus, D.B.; Cava, R.J. Tuning the Band Gap in the Halide Perovskite CsPbBr3 through Sr Substitution. ACS Appl. Mater. Interfaces 2022, 14, 34884–34890. [Google Scholar] [CrossRef]
- Taskaev, S.; Khovaylo, V.; Ulyanov, M.; Bataev, D.; Basharova, A.; Kononova, M.; Plakhotskiy, D.; Bogush, M.; Zherebtsov, D.; Hu, Z. Magnetic properties and magnetocaloric effect in Dy100-xYx solid solutions. AIP Adv. 2021, 11, 015014. [Google Scholar] [CrossRef]
- Ren, K.X.; Liu, J.; Liang, J.; Zhang, K.; Zheng, X.; Luo, H.D.; Huang, Y.B.; Liu, P.J.; Yu, X.B. Synthesis of the bismuth oxyhalide solid solutions with tunable band gap and photocatalytic activities. Dalton Trans. 2013, 42, 9706–9712. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, A.F.; Assis, M.; Ribeiro, L.K.; Gomes, E.D.; Teodoro, M.D.; Longo, E.; Andrés, J. Disentangling the structure, optical properties, and photoluminescence emissions of NiW1-xMoxO4 (x = 25, 50, and 75%) solid solutions: Experimental and DFT studies. J. Mater. Chem. C 2025, 13, 6788–6798. [Google Scholar] [CrossRef]
- Tekade, A.R.; Yadav, J.N. A Review on Solid Dispersion and Carriers Used Therein for Solubility Enhancement of Poorly Water Soluble Drugs. Adv. Pharm. Bull. 2020, 10, 359–369. [Google Scholar] [CrossRef]
- Lv, S.R.; Qiu, Z.L.; Yu, D.H.; Wu, X.A.; Yan, X.; Ren, Y.P.; Huang, Y.Q.; Jiang, G.; Gao, F.L. Custom-Made Piezoelectric Solid Solution Material for Cancer Therapy. Small 2023, 19, 2300976. [Google Scholar] [CrossRef]
- Yamamoto, H.; Tahara, T.; Sugahara, Y.; Kuroda, K.; Kato, C. Synthesis of La1-xMxTiO3 (M = Na, K, 0 ≤ x ≤ 0.4) and the Electrical-Properties. Phase Transit. 1993, 41, 137–141. [Google Scholar] [CrossRef]
- Lichtenberg, F.; Herrnberger, A.; Wiedenmann, K. Synthesis, structural, magnetic and transport properties of layered perovskite-related titanates, niobates and tantalates of the type AnBnO3n+2, A’Ak-1BkO3k+1 and AmBm-1O3m. Prog. Solid State Chem. 2008, 36, 253–387. [Google Scholar] [CrossRef]
- Wei, Y.L.; Li, J.; Huang, Y.F.; Huang, M.L.; Lin, J.M.; Wu, J.H. Photocatalytic water splitting with In-doped H2LaNb2O7 composite oxide semiconductors. Sol. Energy Mater. Sol. Cells 2009, 93, 1176–1181. [Google Scholar] [CrossRef]
- Denton, A.R.; Ashcroft, N.W. Vegard’s law. Phys. Rev. A 1991, 43, 3161–3164. [Google Scholar] [CrossRef]
- Jehng, J.M.; Wachs, I.E. Structural Chemistry and Raman-Spectra of Niobium Oxides. Chem. Mater. 1991, 3, 100–107. [Google Scholar] [CrossRef]
- Sato, M.; Abo, J.; Jin, T.; Ohta, M. Sturcture and Ionic Conductivity of MLaNb2O7 (M = K, Na, Li, H). J. Alloys Compd. 1993, 192, 81–83. [Google Scholar] [CrossRef]
- Sato, M.; Kono, Y.; Jin, T. Structural Characterization and Ion Conductivity of MCa2NaNb4O13 (M = Rb, Na) with 4 Units of Perovskite Layer. J. Ceram. Soc. Jpn. 1993, 101, 980–984. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic-Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Poduval, A.; Granier, M.; Harpin, M.C.; Rick, S.; Wiley, J.B. Structural Analysis of the Double-Layered Dion-Jacobson Solid Acid, HLaNb2O7. 2025; in preparation. [Google Scholar]
- Poduval, A. Synthesis of Inorganic-Organic Perovskite Hybrid Materials via a Microwave Assisted Method (Chapter 6: Structure Analysis of HLaNb2O7 and DLaNb2O7 Using Neutron Diffraction, pp. 133–140). Doctoral Dissertation, University of New Orleans, New Orleans, LA, USA, 2022. [Google Scholar]
- Peel, M.D.; Ashbrook, S.E.; Lightfoot, P. Unusual Phase Behavior in the Piezoelectric Perovskite System, LixNa1–xNbO3. Inorg. Chem. 2013, 52, 8872–8880. [Google Scholar] [CrossRef]
- CrystalDiffract®: A Powder Diffraction Program for Mac and Windows. CrystalMaker Software Ltd.: Oxford, UK. Available online: www.crystalmaker.com (accessed on 22 September 2025).



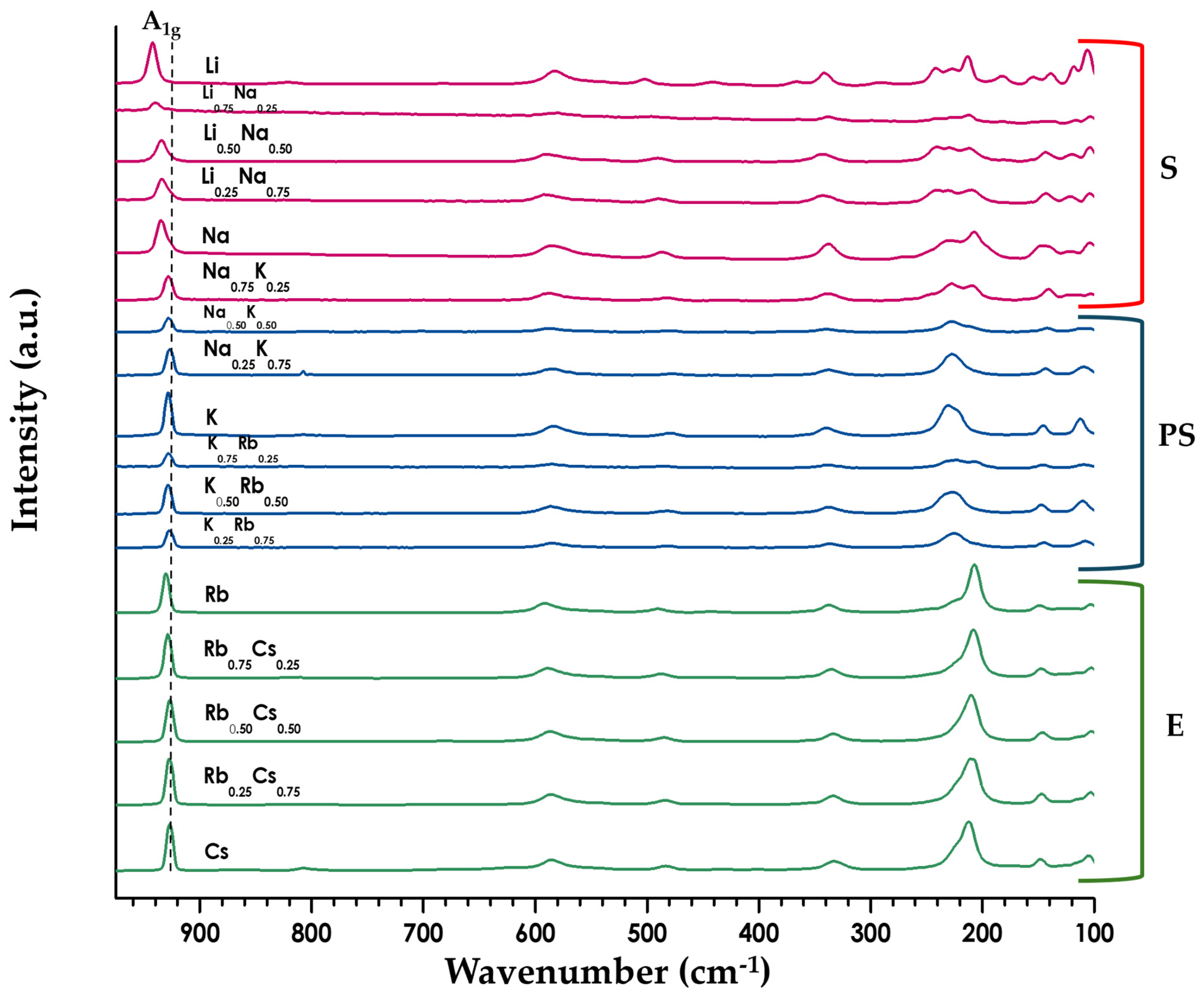
 ), Na1−xKxLaNb2O7 by orange circles (
), Na1−xKxLaNb2O7 by orange circles ( ), K1−xRbxLaNb2O7 by pink squares (
), K1−xRbxLaNb2O7 by pink squares ( ), K1−xCsxLaNb2O7 by purple triangles (
), K1−xCsxLaNb2O7 by purple triangles ( ), and Rb1−xCsxLaNb2O7 by blue crosses (
), and Rb1−xCsxLaNb2O7 by blue crosses ( ).
).
 ), Na1−xKxLaNb2O7 by orange circles (
), Na1−xKxLaNb2O7 by orange circles ( ), K1−xRbxLaNb2O7 by pink squares (
), K1−xRbxLaNb2O7 by pink squares ( ), K1−xCsxLaNb2O7 by purple triangles (
), K1−xCsxLaNb2O7 by purple triangles ( ), and Rb1−xCsxLaNb2O7 by blue crosses (
), and Rb1−xCsxLaNb2O7 by blue crosses ( ).
).
| Unit Cell Parameters for A1−xA′xLaNb2O7 (A/A′ = Li, Na, K, Rb, Cs; 0 ≤ x ≤ 1) | ||||||||
|---|---|---|---|---|---|---|---|---|
| A | x | a (Å) | b (Å) | c (Å) | Unit Cell * | Layer Spacing (Å) | V (Å3) | Normalized V (Å3) ** |
| Li | 3.893(2) | 3.893(2) | 20.435(8) | t | 10.218 | 309.8(2) | 154.9 | |
| Li1−xNax | 0.25 | 3.919(2) | 3.919(2) | 20.649(8) | t | 10.325 | 317.2(3) | 158.6 |
| 0.50 | 3.92(1) | 3.92(1) | 20.65(5) | t | 10.33 | 317(2) | 159 | |
| 0.75 *** | 3.90(1) | 3.90(1) | 21.18(6) | t | 10.59 | 322(1) | 161 | |
| Na ** | 3.900(4) | 3.900(4) | 21.183(2) | t | 10.592 | 322.2(6) | 161.1 | |
| Na1−xKx | 0.25 *** | 3.95(6) | 3.95(6) | 21.1(3) | t | 10.55 | 329(8) | 164.6 |
| 0.50 | 3.901(7) | 21.312(4) | 3.875(7) | o | 10.656 | 322.10(1) | 161.05 | |
| 0.75 | 3.906(7) | 21.455(5) | 3.881(7) | o | 10.728 | 325.28(1) | 162.64 | |
| K | 3.899(1) | 21.658(8) | 3.888(1) | o | 10.829 | 328.4(2) | 164.2 | |
| K1−xRbx | 0.25 | 3.895(2) | 21.706(1) | 3.888(2) | o | 10.853 | 328.73(2) | 164.37 |
| 0.50 | 3.898(4) | 21.698(3) | 3.887(4) | o | 10.849 | 328.68(7) | 164.34 | |
| 0.75 | 3.897(1) | 21.890(6) | 3.882(1) | o | 10.945 | 331.2(2) | 165.6 | |
| Rb | 3.886(3) | 3.886(3) | 10.991(1) | t | 10.991 | 165.95(3) | 165.95 | |
| Rb1−xCsx | 0.25 | 3.889(5) | 3.889(5) | 11.016(2) | t | 11.016 | 166.57(4) | 166.57 |
| 0.50 | 3.895(7) | 3.895(7) | 11.086(2) | t | 11.086 | 168.16(6) | 168.16 | |
| 0.75 | 3.897(6) | 3.897(6) | 11.119(2) | t | 11.119 | 168.83(5) | 168.83 | |
| Cs | 3.906(1) | 3.906(1) | 11.160(3) | t | 11.160 | 170.26(8) | 170.26 | |
| Unit Cell Parameters for K1−xCsxLaNb2O7 (0 ≤ x ≤ 1) | ||||||||
|---|---|---|---|---|---|---|---|---|
| A | x | a (Å) | b (Å) | c (Å) | Unit Cell * | Layer Spacing (Å) | V (Å3) | Normalized V (Å3) ** |
| K | 3.899(1) | 21.658(8) | 3.888(1) | o | 10.829 | 328.4(2) | 164.2 | |
| K1−xCsx | 0.25 | 3.905(2) | 21.93(1) | 3.892(2) | o | 10.97 | 333.2(3) | 166.6 |
| 0.50 | 3.8909(4) | 3.8909(4) | 11.011(1) | t | 11.011 | 166.69(3) | 166.69 | |
| 0.75 | 3.8984(3) | 3.8984(3) | 11.091(1) | t | 11.091 | 168.55(2) | 168.55 | |
| Cs | 3.906(1) | 3.906(1) | 11.160(3) | t | 11.160 | 170.26(8) | 170.26 | |
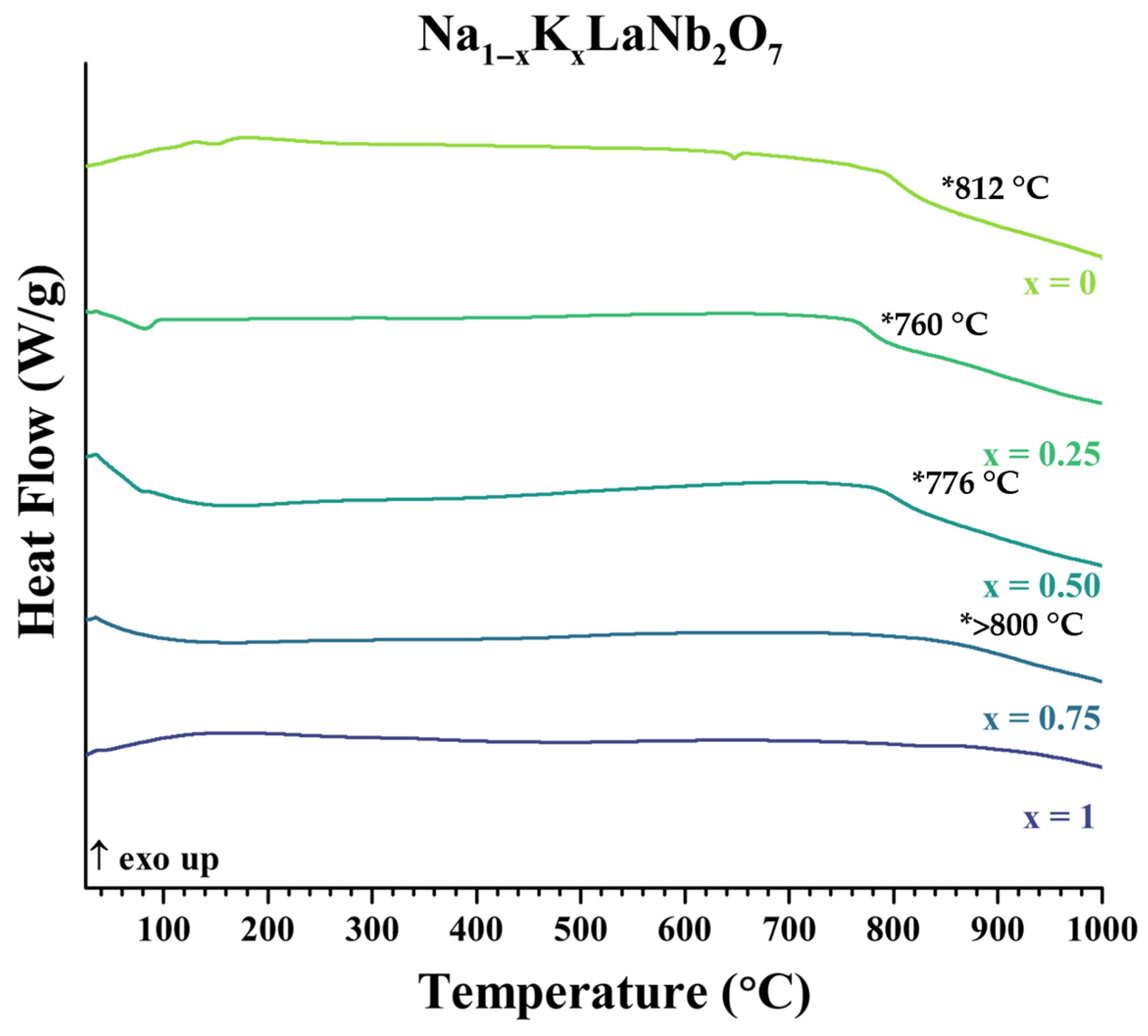
| Compound | Decomposition Temperature (°C) | Decomposition Products | |
|---|---|---|---|
| LiLaNb2O7 | 719 | LiNbO3, LaNbO4 | |
| Li1−xNax | 0.25 | 725 | LiNbO3, NaNbO3, LaNbO4 |
| 0.50 | 734 | LiNbO3, NaNbO3, LaNbO4 | |
| 0.75 | 752 | LiNbO3, NaNbO3, LaNbO4 | |
| NaLaNb2O7 | 812 | NaNbO3, LaNbO4 | |
| Na1−xKx | 0.25 | 760 | KLaNb2O7, NaNbO3, LaNbO4 |
| 0.50 | 773 | KLaNb2O7, NaNbO3, (LaNbO4) | |
| 0.75 | >800 | KLaNb2O7, NaNbO3, (LaNbO4) | |
| KLaNb2O7 | No evidence of decomposition up to 1000 °C | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhuvan, R.; Sandrock, G.J.; Akbarian-Tefaghi, S.; Kelch, M.; Granier, M.; Wiley, J.B. Cation Induced Structural Variation in Topochemically Modified Dion-Jacobson Perovskite Solid Solutions. Molecules 2025, 30, 4430. https://doi.org/10.3390/molecules30224430
Bhuvan R, Sandrock GJ, Akbarian-Tefaghi S, Kelch M, Granier M, Wiley JB. Cation Induced Structural Variation in Topochemically Modified Dion-Jacobson Perovskite Solid Solutions. Molecules. 2025; 30(22):4430. https://doi.org/10.3390/molecules30224430
Chicago/Turabian StyleBhuvan, Roshni, Gary J. Sandrock, Sara Akbarian-Tefaghi, Mya Kelch, Mark Granier, and John B. Wiley. 2025. "Cation Induced Structural Variation in Topochemically Modified Dion-Jacobson Perovskite Solid Solutions" Molecules 30, no. 22: 4430. https://doi.org/10.3390/molecules30224430
APA StyleBhuvan, R., Sandrock, G. J., Akbarian-Tefaghi, S., Kelch, M., Granier, M., & Wiley, J. B. (2025). Cation Induced Structural Variation in Topochemically Modified Dion-Jacobson Perovskite Solid Solutions. Molecules, 30(22), 4430. https://doi.org/10.3390/molecules30224430







