A Simplified Methodology for Solvent Screening in Selective Extraction of Lipids from Microalgae Based on Hansen Solubility Parameters
Abstract
1. Introduction
2. Results and Discussion
2.1. Application of the Proposed Methodology to the Selective Extraction of Fatty Acid Esters from Microalgae
2.2. Gibbs Free Energy of Solvation and Partition Coefficients
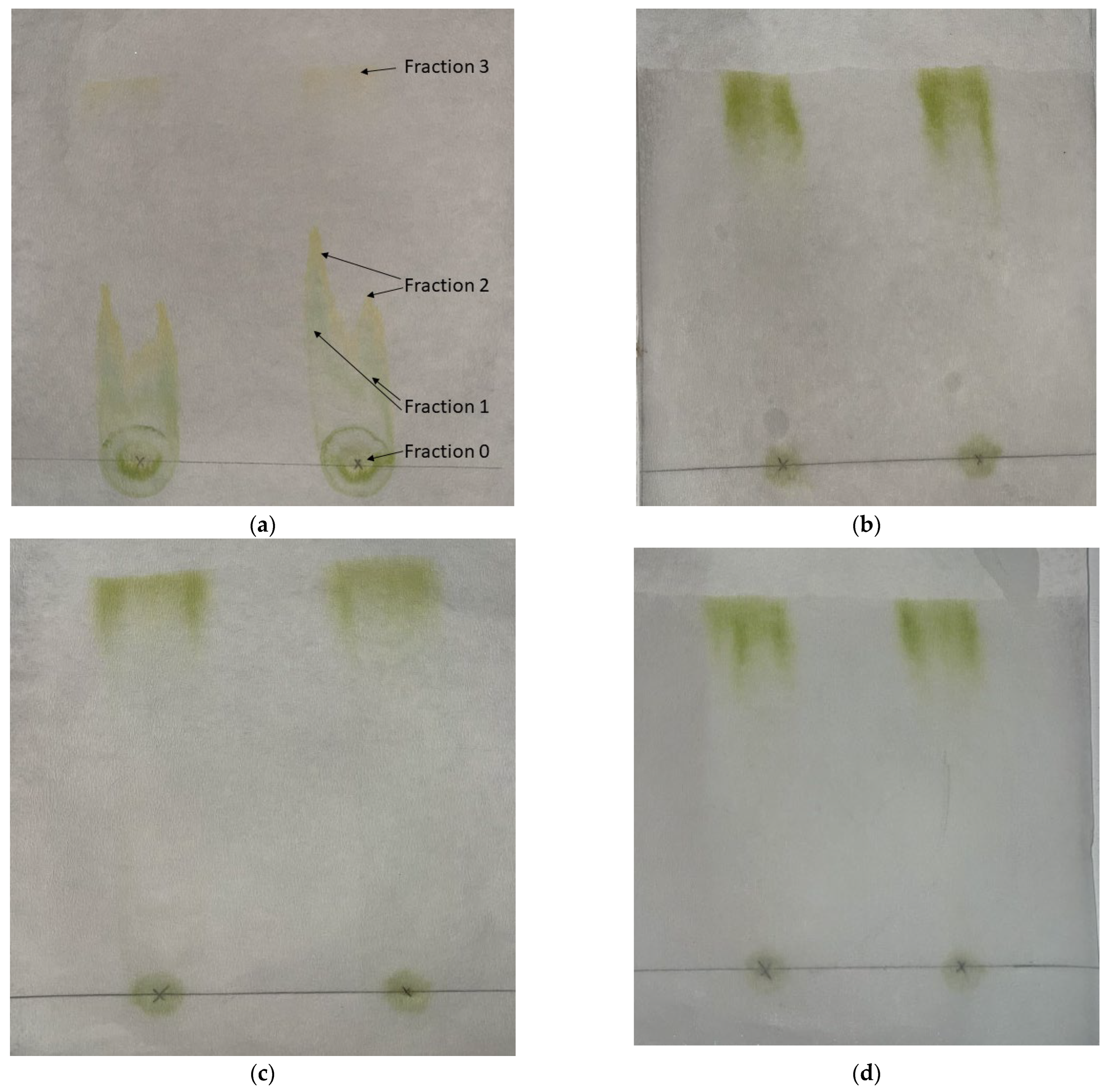
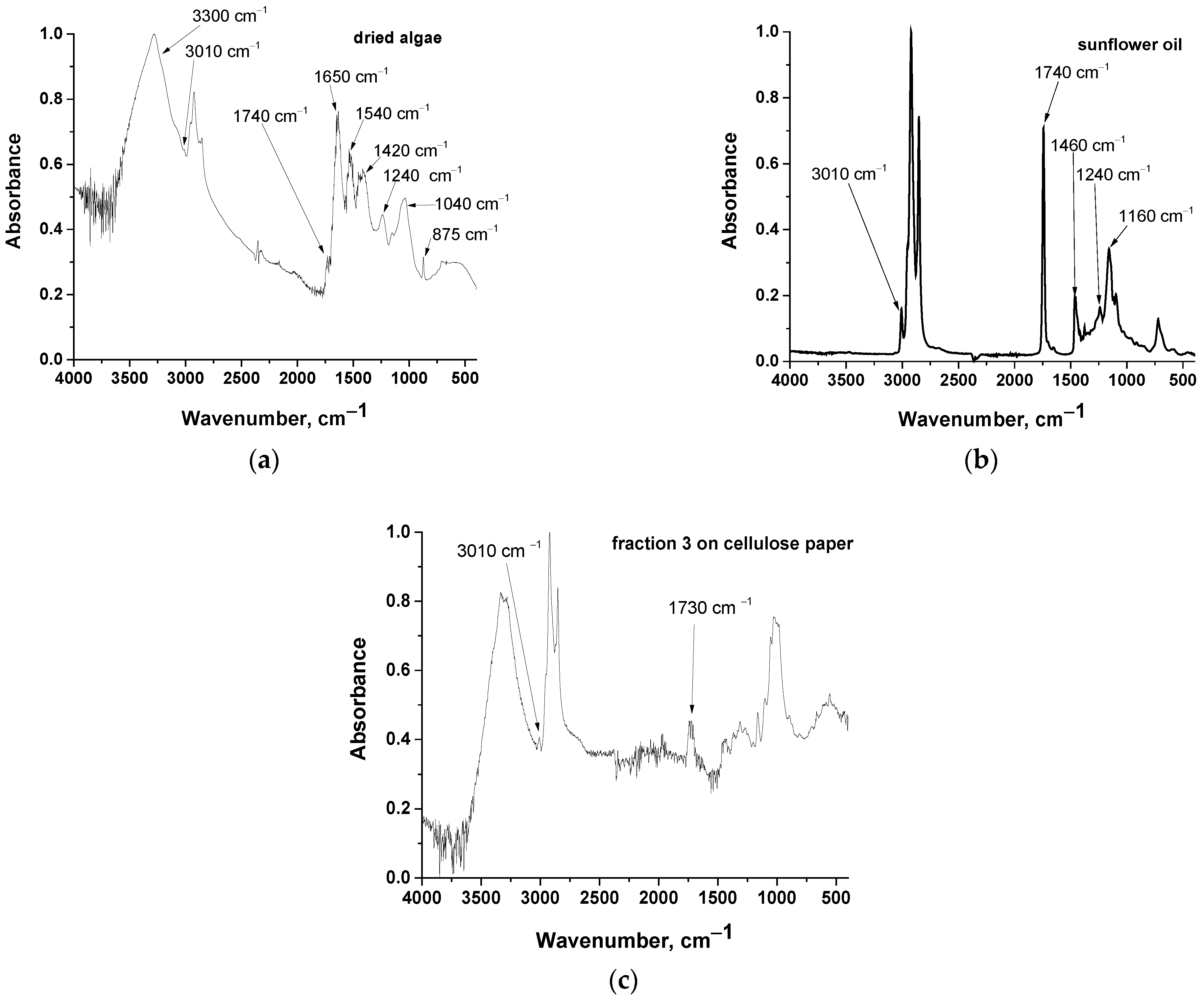
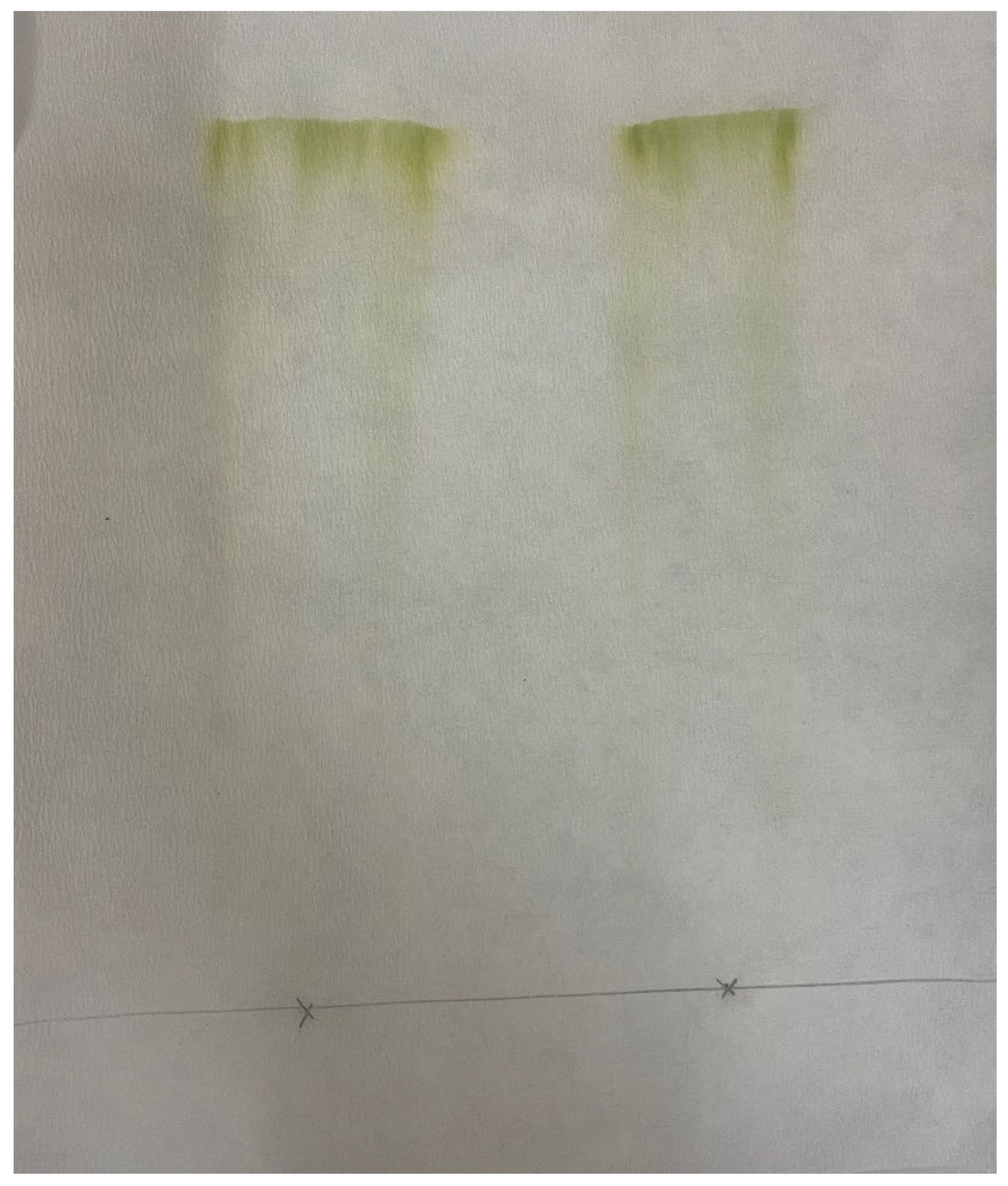
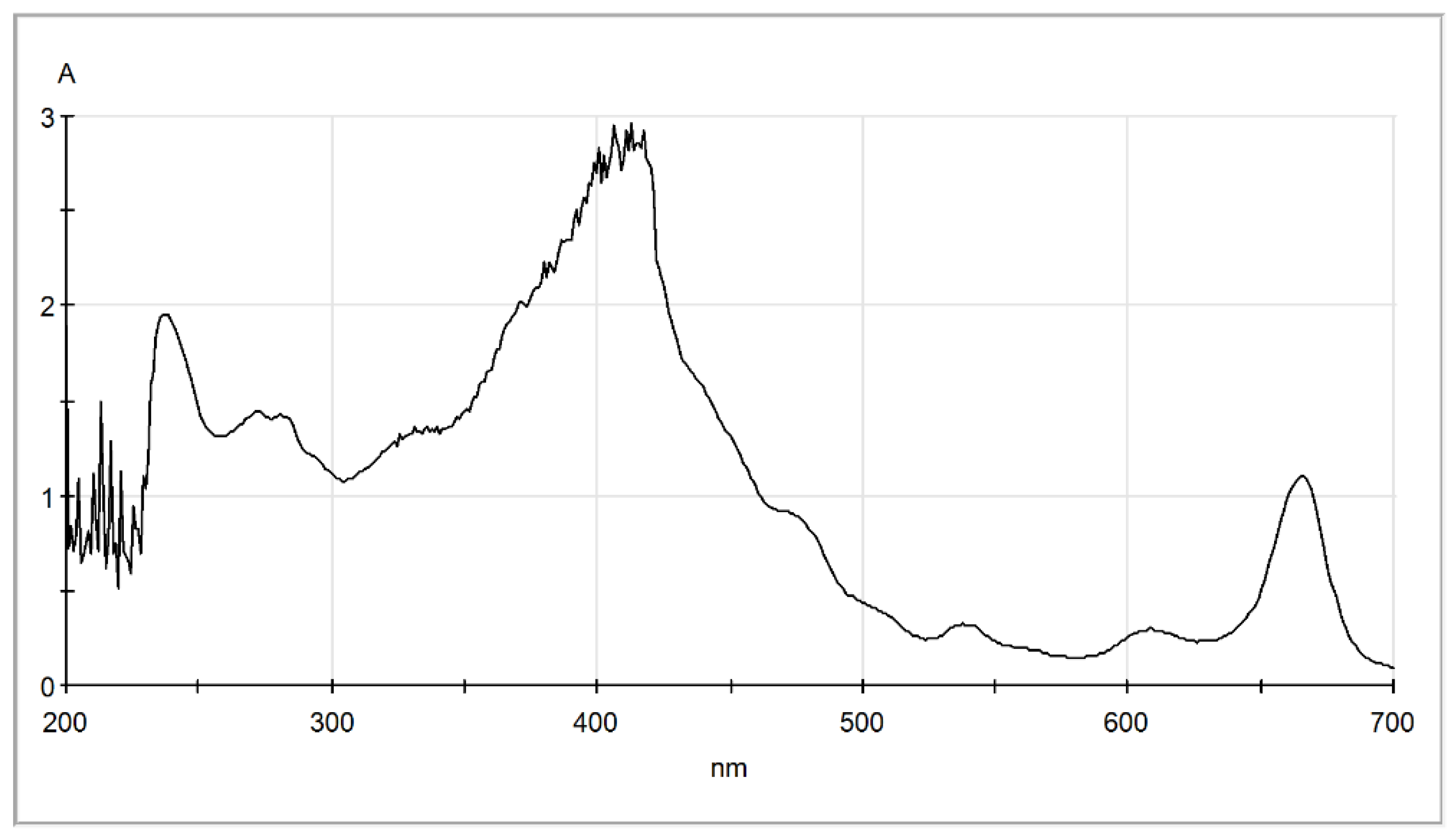
2.3. Paper Chromatography of Solvent Extracted Microalgae Lipids
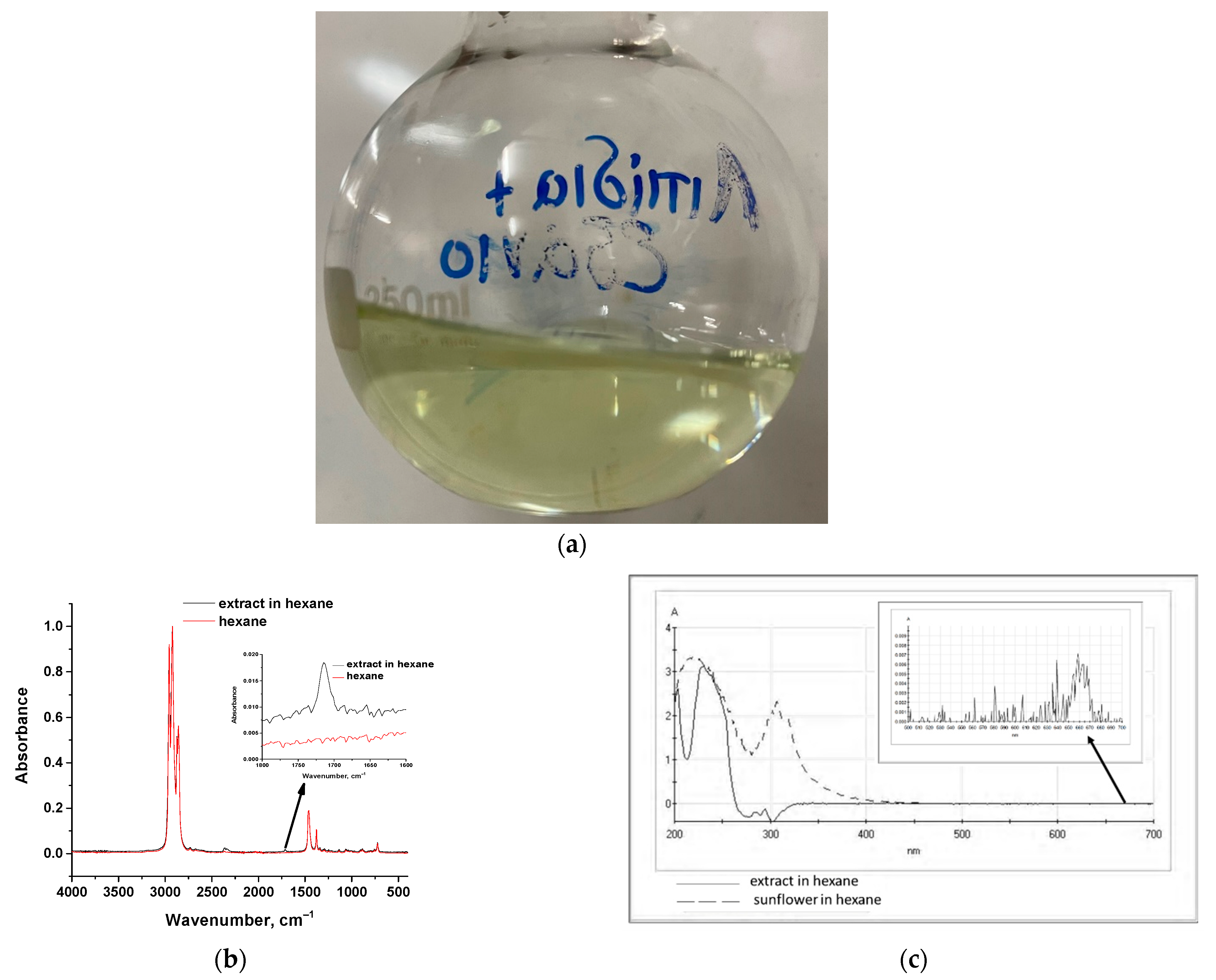
2.4. Extraction from Algae with Hexane and Chloroform/Methanol Mixture
3. Theoretical Calculations
3.1. Methodology for Solvent Screening for Selective Extraction Based on HSP
- : the dispersion HSP of the solute or solvent in MPa1/2;
- : the polar HSP of the solute or solvent in MPa1/2;
- : the hydrogen bonding HSP of the solute or solvent in MPa1/2.
- : the value of of the ith non-desired solute;
- : the average value of of all desired solutes.
3.2. Ab Initio DFT Calculations of Gibbs Free Energy of Solvation and Partition Coefficients
4. Experimental
4.1. Materials and Instruments
4.2. Paper Chromatography
4.3. Extraction of Fatty Acid Esters from Algae Liquor
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thibert, V.; Legeay, P.; Chapuis-Hugon, F.; Pichon, V. Molecularly imprinted polymer for the selective extraction of cocaine and its metabolites, benzoylecgonine and ecgonine methyl ester, from biological fluids before LC–MS analysis. J. Chromatogr. B 2014, 949–950, 16–23. [Google Scholar] [CrossRef]
- Zhou, D.-D.; Zhang, H.; Zhang, Q.; Qian, Z.-M.; Li, W.-J.; Li, C.-H.; Yang, F.-Q.; Chen, H. Preparation of titanium ion functionalized polydopamine coated ferroferric oxide core-shell magnetic particles for selective extraction of nucleotides from Cordyceps and Lentinus edodes. J. Chromatogr. A 2019, 1591, 24–32. [Google Scholar] [CrossRef]
- Zhang, P.; Yi, W.H.; Lei, B.; Zhou, J.F.; Tian, Y.L.; Ren, W.Y. Effects of the Molecular Weight of PCz on Selective Extraction of Large-Diameter Semiconducting Single-Walled Carbon Nanotubes. J. Nano Res. 2021, 69, 11–21. [Google Scholar] [CrossRef]
- Saad, E.M.; El Gohary, N.A.; Abdel-Halim, M.; Handoussa, H.; Mohamed El Nashar, R.; Mizaikoff, B. Molecularly imprinted polymers for selective extraction of rosmarinic acid from Rosmarinus officinalis L. Food Chem. 2021, 335, 127644. [Google Scholar] [CrossRef] [PubMed]
- Missopolinou, D.; Tsioptsias, C.; Lambrou, C.; Panayiotou, C. Selective extraction of oxygenated compounds from oregano with sub-critical water. J. Sci. Food Agric. 2012, 92, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Olea, F.; Valenzuela, M.; Zurob, E.; Parraguez, B.; Abejón, R.; Cabezas, R.; Merlet, G.; Tapia, R.; Romero, J.; Quijada-Maldonado, E. Hydrophobic eutectic solvents for the selective solvent extraction of molybdenum (VI) and rhenium (VII) from a synthetic pregnant leach solution. J. Mol. Liq. 2023, 385, 122415. [Google Scholar] [CrossRef]
- Huang, Y.; Shao, P.; Yang, L.; Zheng, Y.; Sun, Z.; Fang, L.; Lv, W.; Yao, Z.; Wang, L.; Luo, X. Thermochemically driven crystal phase transfer via chlorination roasting toward the selective extraction of lithium from spent LiNi1/3Co1/3Mn1/3O2. Resour. Conserv. Recycl. 2021, 174, 105757. [Google Scholar] [CrossRef]
- Martins, M.A.R.; Sharma, G.; Pinho, S.P.; Gardas, R.L.; Coutinho, J.A.P.; Carvalho, P.J. Selection and characterization of non-ideal ionic liquids mixtures to be used in CO2 capture. Fluid Phase Equilibria 2020, 518, 112621. [Google Scholar] [CrossRef]
- Gonzalez-Miquel, M.; Talreja, M.; Ethier, A.L.; Flack, K.; Switzer, J.R.; Biddinger, E.J.; Pollet, P.; Palomar, J.; Rodriguez, F.; Eckert, C.A.; et al. COSMO-RS Studies: Structure–Property Relationships for CO2 Capture by Reversible Ionic Liquids. Ind. Eng. Chem. Res. 2012, 51, 16066–16073. [Google Scholar] [CrossRef]
- Scheffczyk, J.; Redepenning, C.; Jens, C.M.; Winter, B.; Leonhard, K.; Marquardt, W.; Bardow, A. Massive, automated solvent screening for minimum energy demand in hybrid extraction–distillation using COSMO-RS. Chem. Eng. Res. Des. 2016, 115, 433–442. [Google Scholar] [CrossRef]
- Lubben, M.J.; Canales, R.I.; Lyu, Y.; Held, C.; Gonzalez-Miquel, M.; Stadtherr, M.A.; Brennecke, J.F. Promising Thiolanium Ionic Liquid for Extraction of Aromatics from Aliphatics: Experiments and Modeling. Ind. Eng. Chem. Res. 2020, 59, 15707–15717. [Google Scholar] [CrossRef]
- Wojeicchowski, J.P.; Ferreira, A.M.; Abranches, D.O.; Mafra, M.R.; Coutinho, J.A.P. Using COSMO-RS in the Design of Deep Eutectic Solvents for the Extraction of Antioxidants from Rosemary. ACS Sustain. Chem. Eng. 2020, 8, 12132–12141. [Google Scholar] [CrossRef]
- Rezaei Motlagh, S.; Harun, R.; Awang Biak, D.R.; Hussain, S.A.; Wan Ab Karim Ghani, W.A.; Khezri, R.; Wilfred, C.D.; Elgharbawy, A.A.M. Screening of Suitable Ionic Liquids as Green Solvents for Extraction of Eicosapentaenoic Acid (EPA) from Microalgae Biomass Using COSMO-RS Model. Molecules 2019, 24, 713. [Google Scholar] [CrossRef]
- Peng, D.; Picchioni, F. Prediction of toxicity of Ionic Liquids based on GC-COSMO method. J. Hazard. Mater. 2020, 398, 122964. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters, A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- van Krevelen, D.W.; Te Nijenhuis, K. Properties of Polymers, 4th ed.; Elsevier Science: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Lawley, M.D.; Boon, D.; Stein, L.Y.; Sauvageau, D. Switchable Solvents for the Reversible Dissolution of Poly(3-hydroxybutyrate). ACS Sustain. Chem. Eng. 2022, 10, 2602–2608. [Google Scholar] [CrossRef]
- Stefanis, E.; Panayiotou, C. A new expanded solubility parameter approach. Int. J. Pharm. 2012, 426, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, D.; Hirano, M.; Ohta, R. Nontoxic organic solvents identified using an a priori approach with Hansen solubility parameters. Chem. Commun. 2017, 53, 4096–4099. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kwak, M.; Chang, Y.K.; Kim, D. Green solvent-based extraction of chlorophyll a from Nannochloropsis sp. Using 2,3-butanediol. Sep. Purif. Technol. 2021, 276, 119248. [Google Scholar] [CrossRef]
- Vásquez-Villanueva, R.; Plaza, M.; García, M.C.; Marina, M.L. Recovery and determination of cholesterol-lowering compounds from Olea europaea seeds employing pressurized liquid extraction and gas chromatography-mass spectrometry. Microchem. J. 2020, 156, 104812. [Google Scholar] [CrossRef]
- Vásquez-Villanueva, R.; Plaza, M.; García, M.C.; Turner, C.; Marina, M.L. A sustainable approach for the extraction of cholesterol-lowering compounds from an olive by-product based on CO2-expanded ethyl acetate. Anal. Bioanal. Chem. 2019, 411, 5885–5896. [Google Scholar] [CrossRef]
- Sánchez-Camargo, A.d.P.; Bueno, M.; Parada-Alfonso, F.; Cifuentes, A.; Ibáñez, E. Hansen solubility parameters for selection of green extraction solvents. TrAC Trends Anal. Chem. 2019, 118, 227–237. [Google Scholar] [CrossRef]
- Zhang, Q.; Tan, X.; Wang, W.; Yu, Q.; Wang, Q.; Miao, C.; Guo, Y.; Zhuang, X.; Yuan, Z. Screening Solvents Based on Hansen Solubility Parameter Theory to Depolymerize Lignocellulosic Biomass Efficiently under Low Temperature. ACS Sustain. Chem. Eng. 2019, 7, 8678–8686. [Google Scholar] [CrossRef]
- Deneme, I.; Yıldız, T.A.; Kayaci, N.; Usta, H. The Hansen solubility approach towards green solvent processing: N-channel organic field-effect transistors under ambient conditions. J. Mater. Chem. C 2024, 12, 3854–3864. [Google Scholar] [CrossRef]
- Stefanis, E.; Panayiotou, C. Prediction of Hansen Solubility Parameters with a New Group-Contribution Method. Int. J. Thermophys. 2008, 29, 568–585. [Google Scholar] [CrossRef]
- Vilas-Boas, S.M.; Coutinho, J.A.; Pinho, S.P.; Ferreira, O. Solvent screening for the purification of monoterpenoids by countercurrent and centrifugal partition chromatography. J. Chem. Technol. Biotechnol. 2025, 100, 166–175. [Google Scholar] [CrossRef]
- Jalalinejad, A.; Seyf, J.Y.; Funke, A.; Dahmen, N. Solvent Screening for Separation of Lignin-Derived Molecules Using the NIST-UNIFAC Model. ACS Sustain. Chem. Eng. 2023, 11, 7863–7873. [Google Scholar] [CrossRef]
- Vilas-Boas, S.M.; Cordova, I.W.; Kurnia, K.A.; Almeida, H.H.S.; Gaschi, P.S.; Coutinho, J.A.P.; Pinho, S.P.; Ferreira, O. Comparison of two computational methods for solvent screening in countercurrent and centrifugal partition chromatography. J. Chromatogr. A 2022, 1666, 462859. [Google Scholar] [CrossRef] [PubMed]
- Zapata-Boada, S.; Gonzalez-Miquel, M.; Jobson, M.; Cuéllar-Franca, R.M. Integrating Technoeconomic, Environmental, and Safety Criteria in Solvent Screening for Extraction Processes: The Case of Algae Lipid Extraction. ACS Sustain. Chem. Eng. 2022, 10, 472–485. [Google Scholar] [CrossRef]
- Zapata-Boada, S.; Gonzalez-Miquel, M.; Jobson, M.; Cuéllar-Franca, R.M. Techno-economic and Environmental Analysis of Algae Biodiesel Production via Lipid Extraction Using Alternative Solvents. Ind. Eng. Chem. Res. 2022, 61, 18030–18044. [Google Scholar] [CrossRef]
- Valcareggi Morcelli, A.; da Silva Andrade, W.; Frankenberg, C.L.C.; Rech, R.; Marcílio, N.R. Extraction of Chlorophylls and Carotenoids from Microalgae: COSMO-SAC-Assisted Solvent Screening. Chem. Eng. Technol. 2021, 44, 1227–1232. [Google Scholar] [CrossRef]
- Xu, D.; Chow, J.; Weber, C.C.; Packer, M.A.; Baroutian, S.; Shahbaz, K. Evaluation of deep eutectic solvents for the extraction of fucoxanthin from the alga Tisochrysis lutea—COSMO-RS screening and experimental validation. J. Environ. Chem. Eng. 2022, 10, 108370. [Google Scholar] [CrossRef]
- König-Mattern, L.; Linke, S.; Rihko-Struckmann, L.; Sundmacher, K. Computer-aided solvent screening for the fractionation of wet microalgae biomass. Green Chem. 2021, 23, 10014–10029. [Google Scholar] [CrossRef]
- König-Mattern, L.; Rihko-Struckmann, L.; Sundmacher, K. Systematic solvent selection enables the fractionation of wet microalgal biomass. Sep. Purif. Technol. 2025, 354, 129462. [Google Scholar] [CrossRef]
- Anto, S.; Premalatha, M.; Mathimani, T. Tertiary amine as an efficient CO2 switchable solvent for extracting lipids from hypersaline microalgae. Chemosphere 2022, 288, 132442. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.; Rodriguez, C. Lipid extraction from Chlorella vulgaris & Haematococcus pluvialis using the switchable solvent DMCHA for biofuel production. Energy 2023, 278, 127983. [Google Scholar]
- Banskota, A.H.; Stefanova, R.; Hui, J.P.M.; Bermarija, T.; Stemmler, K.; McGinn, P.J.; O’Leary, S.J.B. Comprehensive Analysis of Biomass from Chlorella sorokiniana Cultivated with Industrial Flue Gas as the Carbon Source. Molecules 2024, 29, 3368. [Google Scholar] [CrossRef]
- Voshall, A.; Christie, N.T.M.; Rose, S.L.; Khasin, M.; Van Etten, J.L.; Markham, J.E.; Riekhof, W.R.; Nickerson, K.W. Sterol Biosynthesis in Four Green Algae: A Bioinformatic Analysis of the Ergosterol Versus Phytosterol Decision Point. J. Phycol. 2021, 57, 1199–1211. [Google Scholar] [CrossRef]
- Lu, S.; Wang, J.; Ma, Q.; Yang, J.; Li, X.; Yuan, Y.J. Phospholipid metabolism in an industry microalga Chlorella sorokiniana: The impact of inoculum sizes. PLoS ONE 2013, 8, e70827. [Google Scholar] [CrossRef]
- Hansen Solubility. 2025. Available online: https://hansen-solubility.com/ (accessed on 4 September 2025).
- Snyder, L.R. Classification of the solvent properties of common liquids. J. Chromatogr. A 1974, 92, 223–230. [Google Scholar] [CrossRef]
- Haynes, W.M. (Ed.) Handbook of Chemistry and Physics, 97th ed.; CRC Press LLC: Boca Raton, FL, USA, 2016–2017. [Google Scholar]
- Available online: https://www.atamanchemicals.com/dimethylcyclohexylamine-dmcha_u33716/ (accessed on 7 November 2025).
- Du, Y.; Schuur, B.; Samorì, C.; Tagliavini, E.; Brilman, D.W.F. Secondary amines as switchable solvents for lipid extraction from non-broken microalgae. Bioresour. Technol. 2013, 149, 253–260. [Google Scholar] [CrossRef]
- Lo, C.; Wijffels, R.H.; Eppink, M.H.M. Lipid recovery from deep eutectic solvents by polar antisolvents. Food Bioprod. Process. 2024, 143, 21–27. [Google Scholar] [CrossRef]
- Stuart, B. Infrared Spectroscopy: Fundamentals and applications; John Wiley and Sons Ltd.: Chichester, UK, 2004. [Google Scholar]
- Poletto, M.; Pistor, V.; Zeni, M.; Zattera, A.J. Crystalline properties and decomposition kinetics of cellulose fibers in wood pulp obtained by two pulping processes. Polym. Degrad. Stab. 2011, 96, 679–685. [Google Scholar] [CrossRef]
- Rosa, M.F.; Medeiros, E.S.; Malmonge, J.A.; Gregorski, K.S.; Wood, D.F.; Mattoso, L.H.C.; Glenn, G.; Orts, W.J.; Imam, S.H. Cellulose nanowhiskers from coconut husk fibers: Effect of preparation conditions on their thermal and morphological behavior. Carbohydr. Polym. 2010, 81, 83–92. [Google Scholar] [CrossRef]
- Inouye, L.S.; Lotufo, G.R. Comparison of macro-gravimetric and micro-colorimetric lipid determination methods. Talanta 2006, 70, 584–587. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Didham, M.; Truong, V.K.; Chapman, J.; Cozzolino, D. Sensing the Addition of Vegetable Oils to Olive Oil: The Ability of UV–VIS and MIR Spectroscopy Coupled with Chemometric Analysis. Food Anal. Methods 2020, 13, 601–607. [Google Scholar] [CrossRef]
- Wellburn, A.R. The Spectral Determination of Chlorophylls a and b, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminformatics 2012, 4, 17. [Google Scholar] [CrossRef]
- Avogadro, S. An Open-Source Molecular Builder and Visualization Tool, Version 1.1.0; Avogadro: Fontenilles, France, 2009.
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system—Version 5.0. WIREs Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Valeev, E.F. Libint: A Library for the Evaluation of Molecular Integrals of Many-Body Operators over Gaussian Functions, Version 2.9.0, 2024. Available online: http://libint.valeyev.net/ (accessed on 3 May 2025).
- Lehtola, S.; Steigemann, C.; Oliveira, M.J.T.; Marques, M.A.L. Recent developments in libxc—A comprehensive library of functionals for density functional theory. SoftwareX 2018, 7, 1–5. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Bursch, M.; Mewes, J.-M.; Hansen, A.; Grimme, S. Best-Practice DFT Protocols for Basic Molecular Computational Chemistry. Angew. Chem. Int. Ed. 2022, 61, e202205735. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Neese, F. An improvement of the resolution of the identity approximation for the formation of the Coulomb matrix. J. Comput. Chem. 2003, 24, 1740–1747. [Google Scholar] [CrossRef]
- Bykov, D.; Petrenko, T.; Izsák, R.; Kossmann, S.; Becker, U.; Valeev, E.; Neese, F. Efficient implementation of the analytic second derivatives of Hartree–Fock and hybrid DFT energies: A detailed analysis of different approximations. Mol. Phys. 2015, 113, 1961–1977. [Google Scholar] [CrossRef]
- Garcia-Ratés, M.; Neese, F. Effect of the Solute Cavity on the Solvation Energy and its Derivatives within the Framework of the Gaussian Charge Scheme. J. Comput. Chem. 2020, 41, 922–939. [Google Scholar] [CrossRef]
- Neese, F. The SHARK integral generation and digestion system. J. Comput. Chem. 2023, 44, 381–396. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. WIREs Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Neese, F. Approximate second-order SCF convergence for spin unrestricted wavefunctions. Chem. Phys. Lett. 2000, 325, 93–98. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum Calculation of Molecular Energies and Energy Gradients in Solution by a Conductor Solvent Model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Garrido, N.M.; Queimada, A.J.; Jorge, M.; Macedo, E.A.; Economou, I.G. 1-Octanol/Water Partition Coefficients of n-Alkanes from Molecular Simulations of Absolute Solvation Free Energies. J. Chem. Theory Comput. 2009, 5, 2436–2446. [Google Scholar] [CrossRef]
- Psachoulia, P.; Chatzidoukas, C.; Samaras, P. Study of Chlorella sorokiniana Cultivation in an Airlift Tubular Photobioreactor Using Anaerobic Digestate Substrate. Water 2024, 16, 485. [Google Scholar] [CrossRef]
- Gerde, J.A.; Montalbo-Lomboy, M.; Yao, L.; Grewell, D.; Wang, T. Evaluation of microalgae cell disruption by ultrasonic treatment. Bioresour. Technol. 2012, 125, 175–181. [Google Scholar] [CrossRef]
| Solutes | δd, MPa1/2 | δp, MPa1/2 | δhb, MPa1/2 |
|---|---|---|---|
| desired solutes | |||
| glycerides (fatty acid esters) | |||
| glyceryl Monostearate | 16.1 | 4.5 | 9.8 |
| glyceryl Monooleate | 16.2 | 4.6 | 9.4 |
| glyceryl Tributyrate | 16.3 | 2.5 | 7.0 |
| glyceryl Trioleate | 16.0 | 3.8 | 3.2 |
| non-desired solutes | |||
| sterols | |||
| β-sitosterol | 17.2 | 1.8 | 3.4 |
| pigments | |||
| chlorophyll | 20.2 | 15.6 | 18.2 |
| lutein | 17.8 | 1.5 | 5.1 |
| phospholipids | |||
| phosphatidylethanolamine | 16.2 | 7.1 | 9.8 |
| phosphatidylserine | 17.6 | 12.2 | 18.7 |
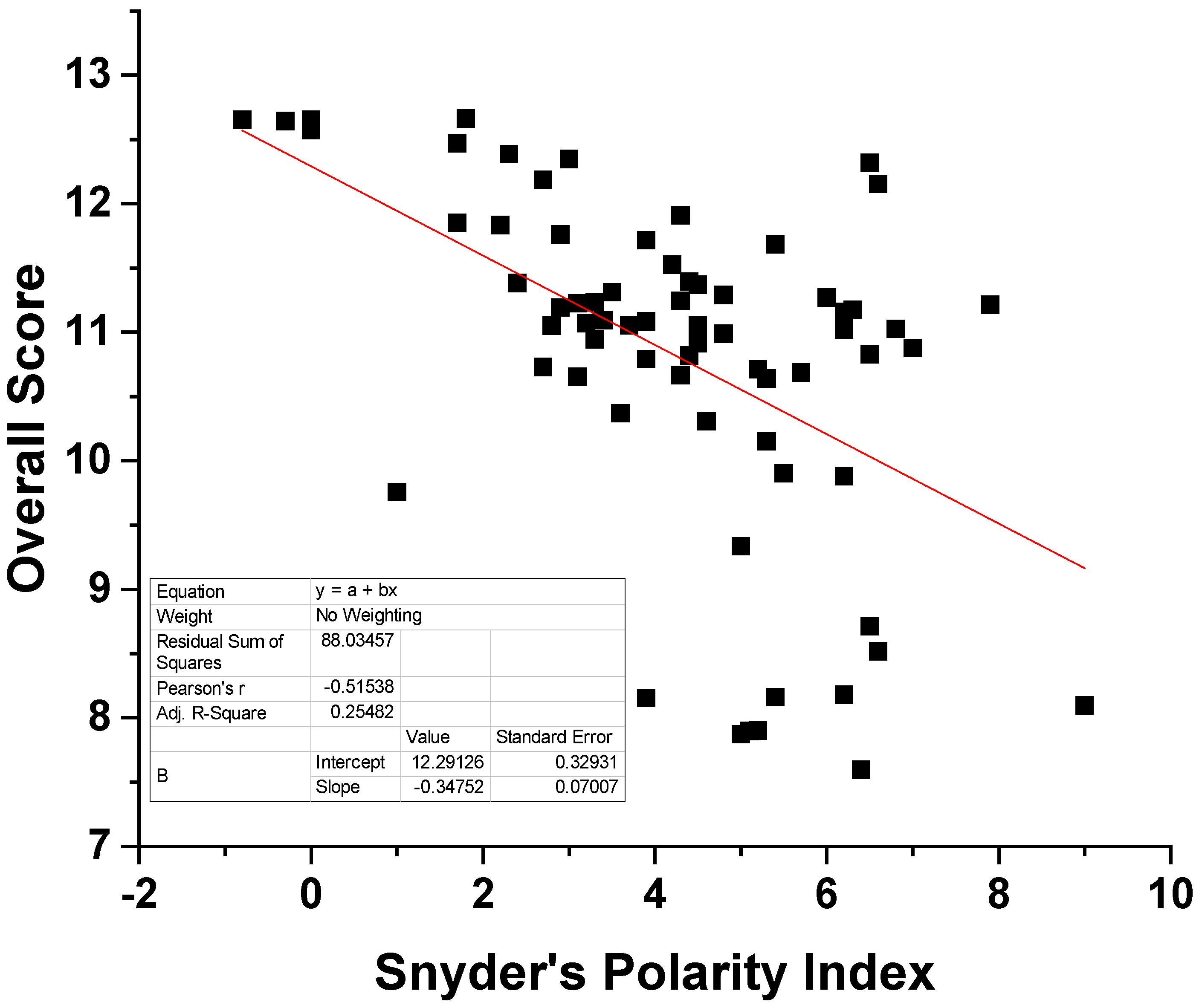
| Solvent | Snyder’s Polarity Index [42] | Dielectric Constant [43] | Overall Score | Classification (Out of 5632 Solvents) |
|---|---|---|---|---|
| hexane | 0 | 1.8865 | 12.653 | 188 |
| DMCHA | triethyl amine 1.8 | 2.86 * | 12.211 | 775 |
| ethyl acetate | 4.3 | 6.0814 | 11.910 | 1531 |
| 1-pentanol | isopentanol 3.6 | 15.13 | 11.143 | 4545 |
| methanol/chloroform/water mixture in volume ratios of 2/1/0.8 | chloroform 4.4 | methanol 33 | 8.567 | |
| methanol 6.6 | chloroform 4.8069 | |||
| water 9 | water 80.1 | rejected |
| Solute | ΔG of Solvation Hexane, kJ/Mol | ΔG of Solvation 1-Pentanol, kJ/Mol | ΔG of Solvation Water, kJ/Mol |
|---|---|---|---|
| glyceryl triolate | −169.8 | −166.2 | −23.2 |
| β-sitosterol | −58.7 | −63.9 | −8.4 |
| chlorophyll a | −158.0 | −176.5 | −88.9 |
| phosphatidylethanolamine | −48.4 | −91.9 | −82.3 |
| lutein | −102.9 | −118.6 | −46.9 |
| Solute | logphexane/water | logp1-pentanol/water |
|---|---|---|
| glyceryl triolate | 25.70 | 25.06 |
| β-sitosterol | 8.80 | 9.72 |
| chlorophyll a | 12.10 | 15.35 |
| phosphatidylethanolamine | −5.94 | 1.69 |
| lutein | 9.82 | 12.57 |
| Non-Desired Solute | ΔΔGsolvation, Hexane, kJ/Mol | ΔΔGsolvation, 1-Pentanol, kJ/Mol | ΔΔGsolvation, Water, kJ/Mol |
|---|---|---|---|
| β-sitosterol | −111.2 | −102.3 | −14.8 |
| chlorophyll a | −11.8 | 10.3 | 65.7 |
| phosphatidylethanolamine | −121.4 | −74.3 | 59.1 |
| lutein | −66.9 | −47.6 | 23.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsioptsias, C.; Mitis, S.; Rentzela, A.; Alvanou, K.; Kelesi, D.; Manolis, C.; Stergiou, A.; Kalamaras, S.D.; Samaras, P. A Simplified Methodology for Solvent Screening in Selective Extraction of Lipids from Microalgae Based on Hansen Solubility Parameters. Molecules 2025, 30, 4428. https://doi.org/10.3390/molecules30224428
Tsioptsias C, Mitis S, Rentzela A, Alvanou K, Kelesi D, Manolis C, Stergiou A, Kalamaras SD, Samaras P. A Simplified Methodology for Solvent Screening in Selective Extraction of Lipids from Microalgae Based on Hansen Solubility Parameters. Molecules. 2025; 30(22):4428. https://doi.org/10.3390/molecules30224428
Chicago/Turabian StyleTsioptsias, Costas, Stefania Mitis, Alexandra Rentzela, Kalitsa Alvanou, Dimitra Kelesi, Christos Manolis, Anastasia Stergiou, Sotirios D. Kalamaras, and Petros Samaras. 2025. "A Simplified Methodology for Solvent Screening in Selective Extraction of Lipids from Microalgae Based on Hansen Solubility Parameters" Molecules 30, no. 22: 4428. https://doi.org/10.3390/molecules30224428
APA StyleTsioptsias, C., Mitis, S., Rentzela, A., Alvanou, K., Kelesi, D., Manolis, C., Stergiou, A., Kalamaras, S. D., & Samaras, P. (2025). A Simplified Methodology for Solvent Screening in Selective Extraction of Lipids from Microalgae Based on Hansen Solubility Parameters. Molecules, 30(22), 4428. https://doi.org/10.3390/molecules30224428








