Synthesis of 2-Oxazolines from N-Allyl and N-Propargyl Amides
Abstract
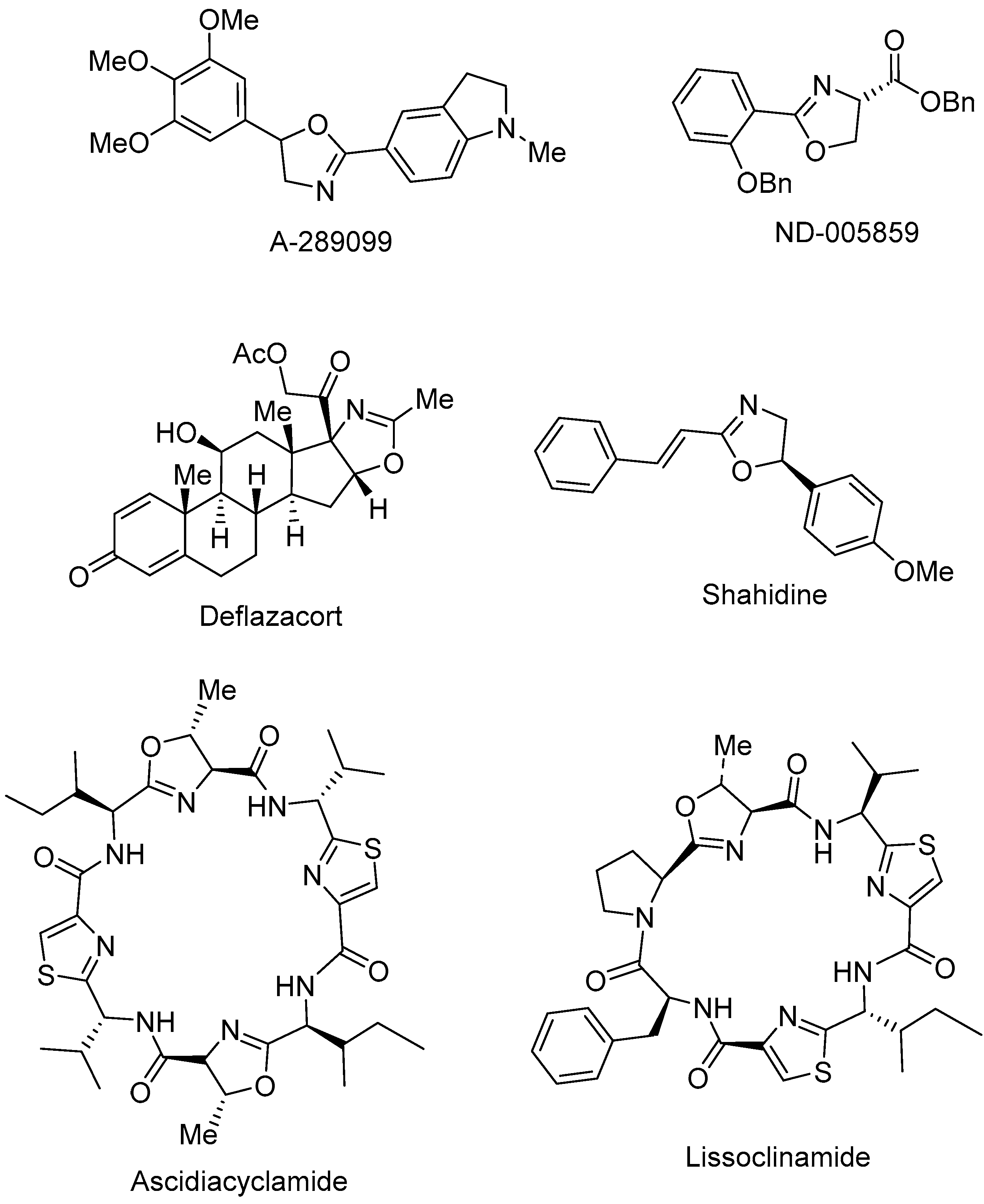
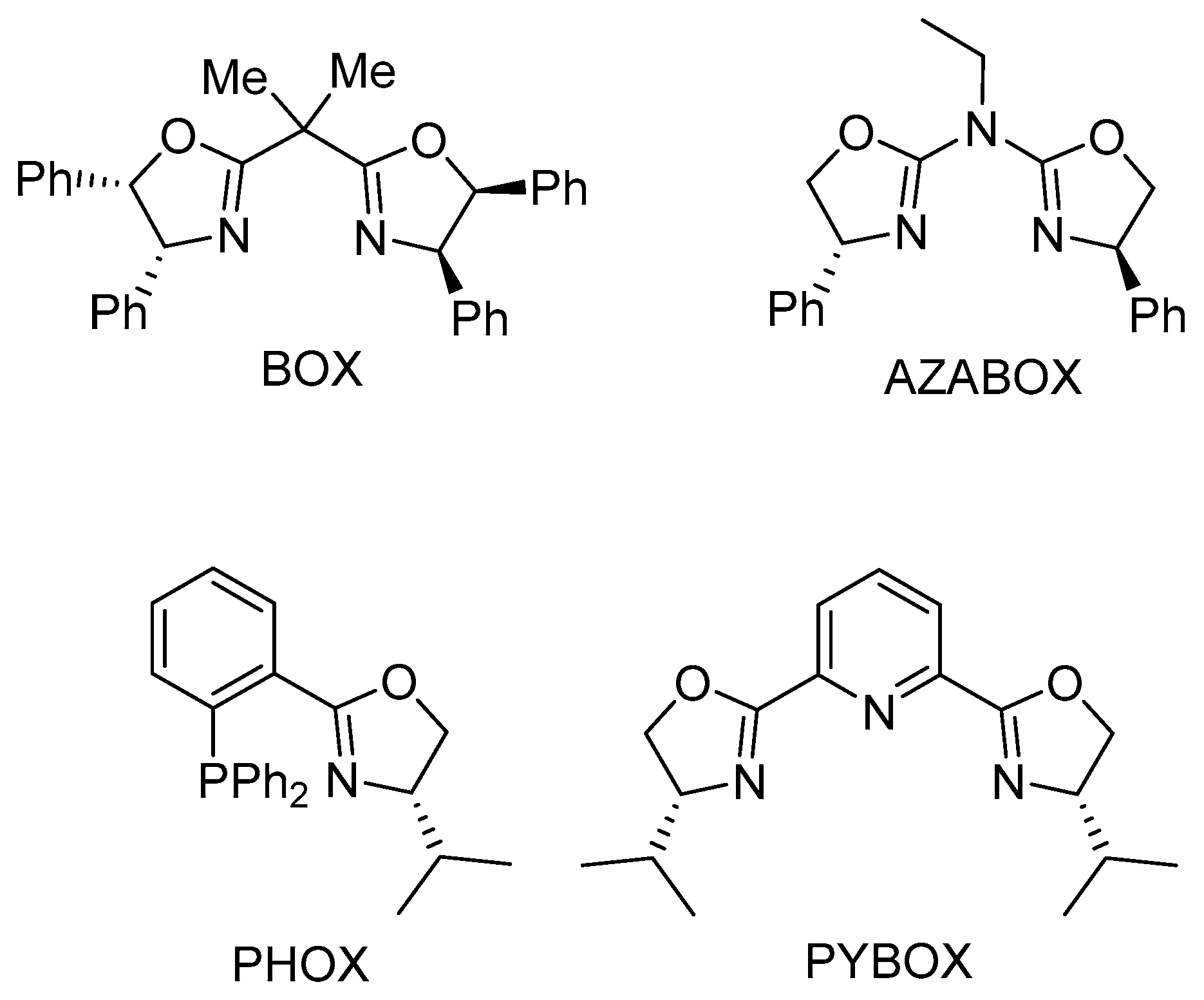
1. Introduction
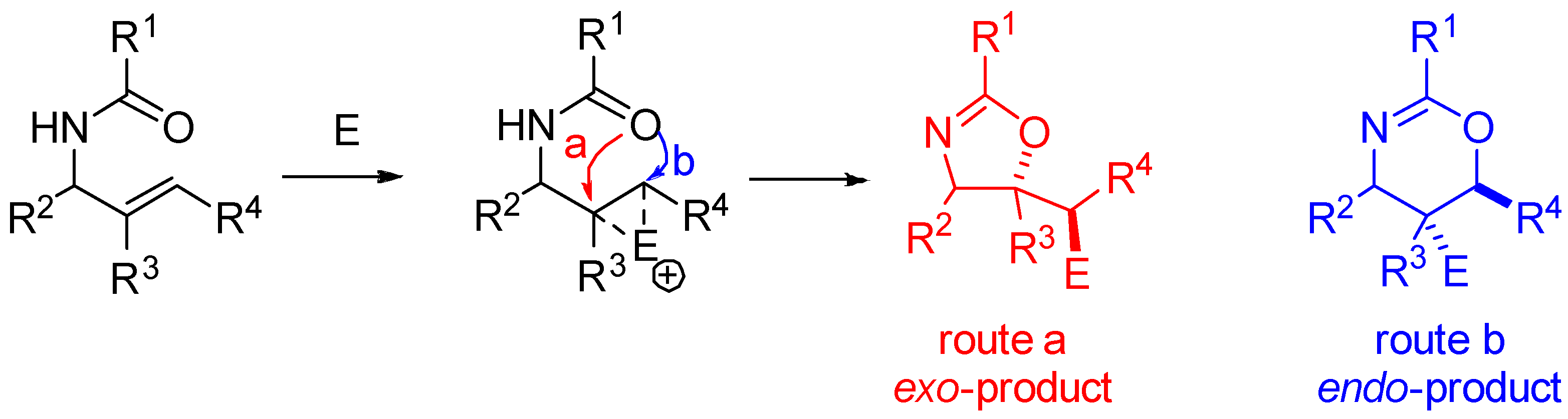
2. Synthesis of Oxazolines from N-Allyl Amides
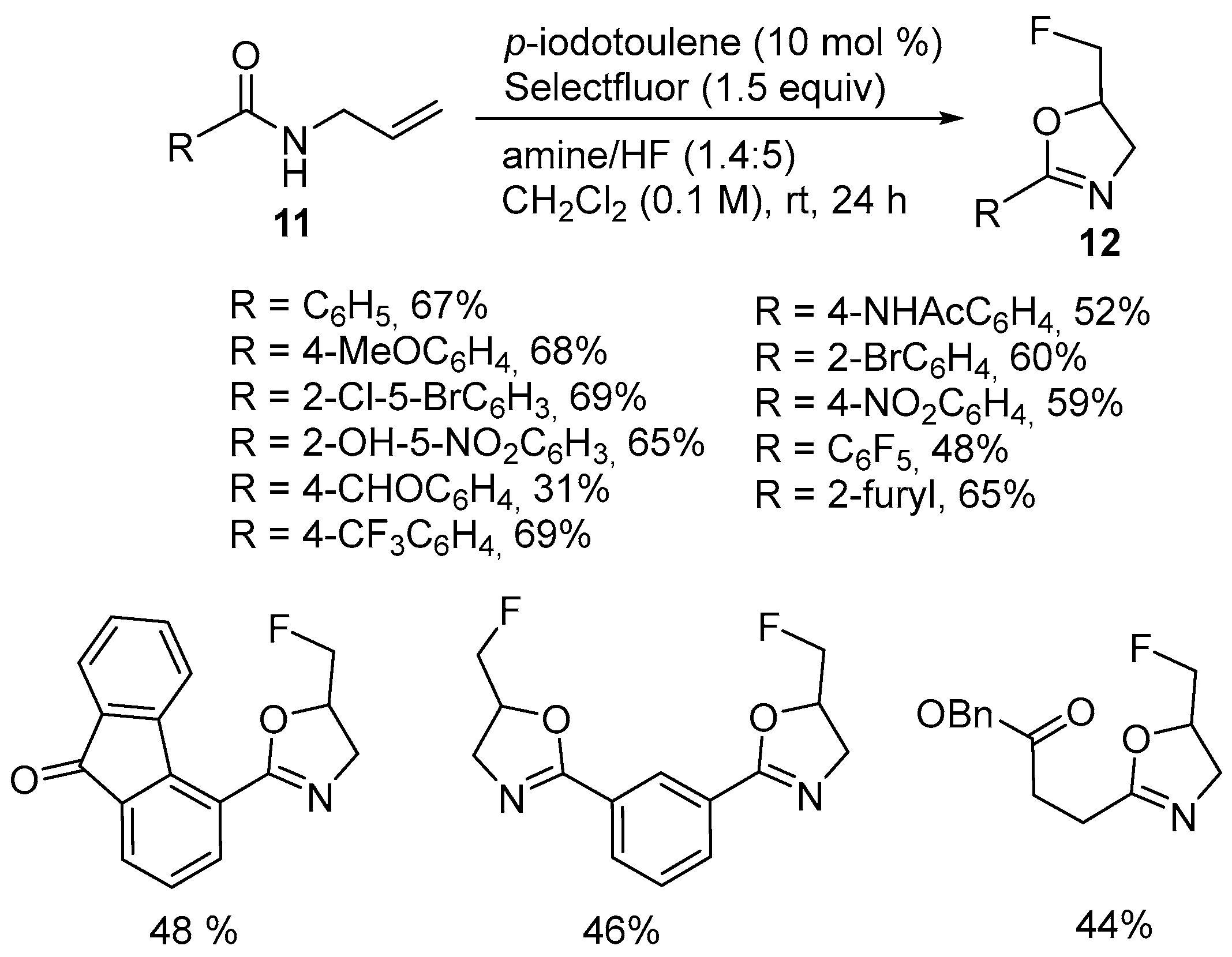
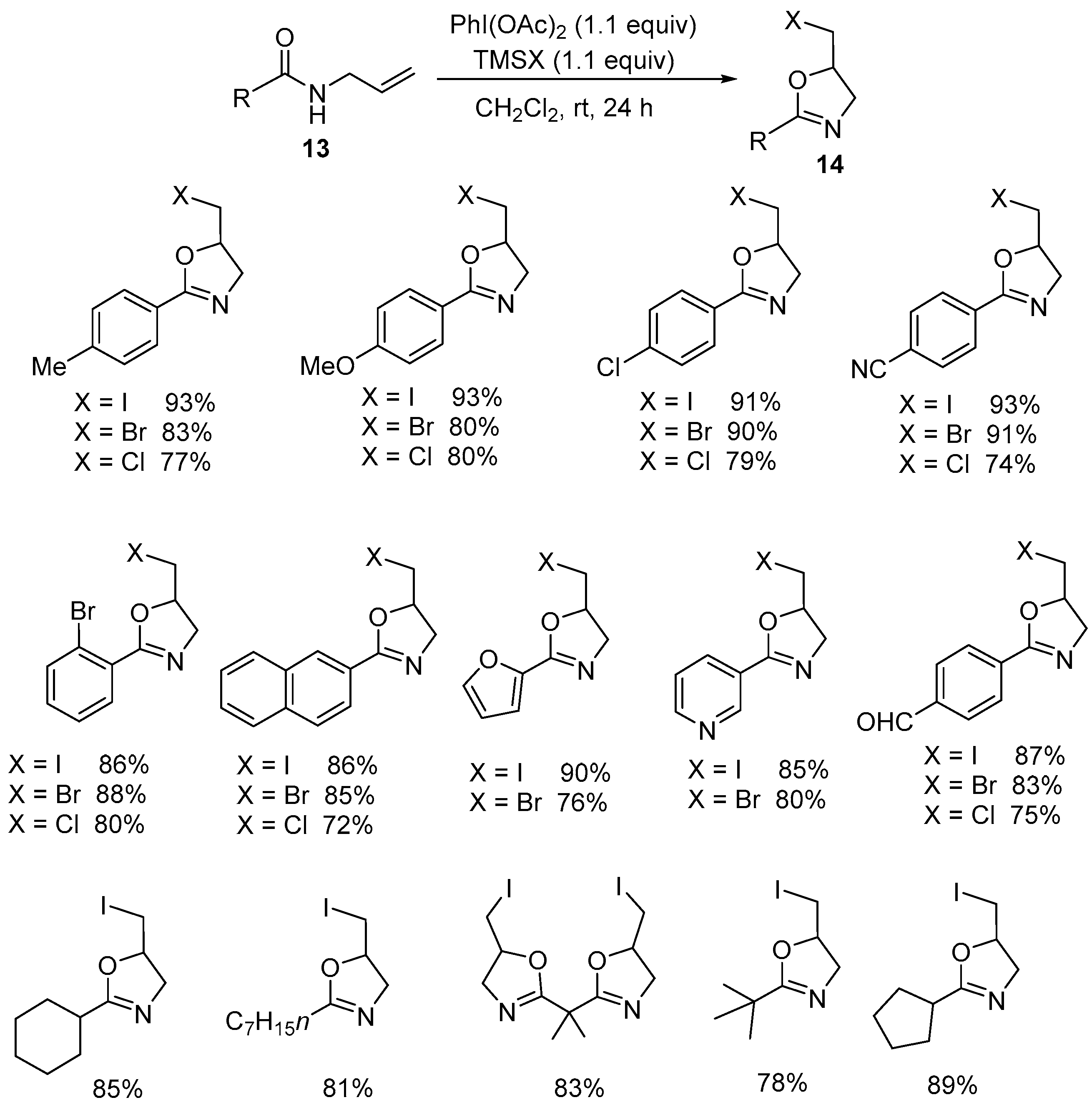
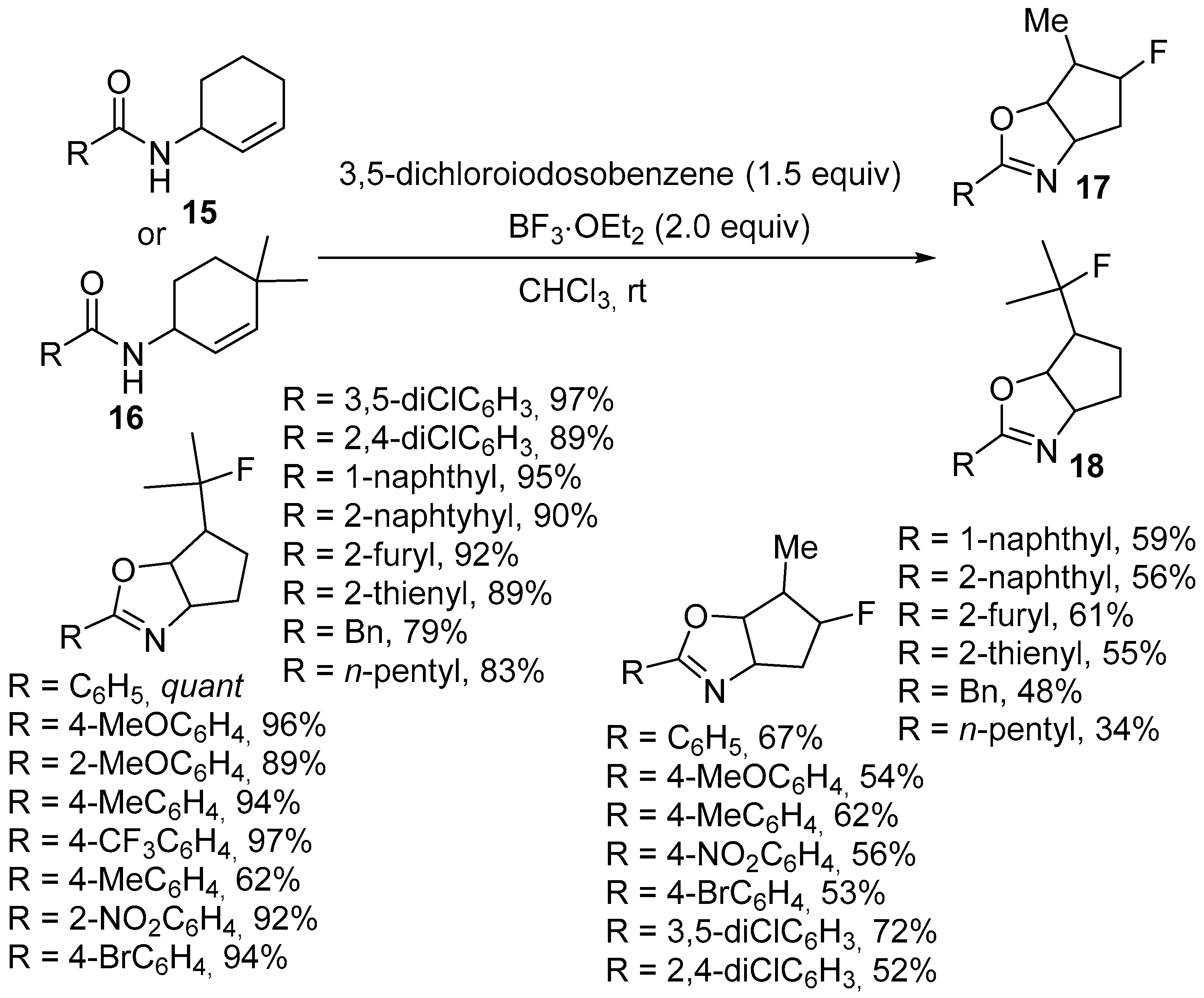
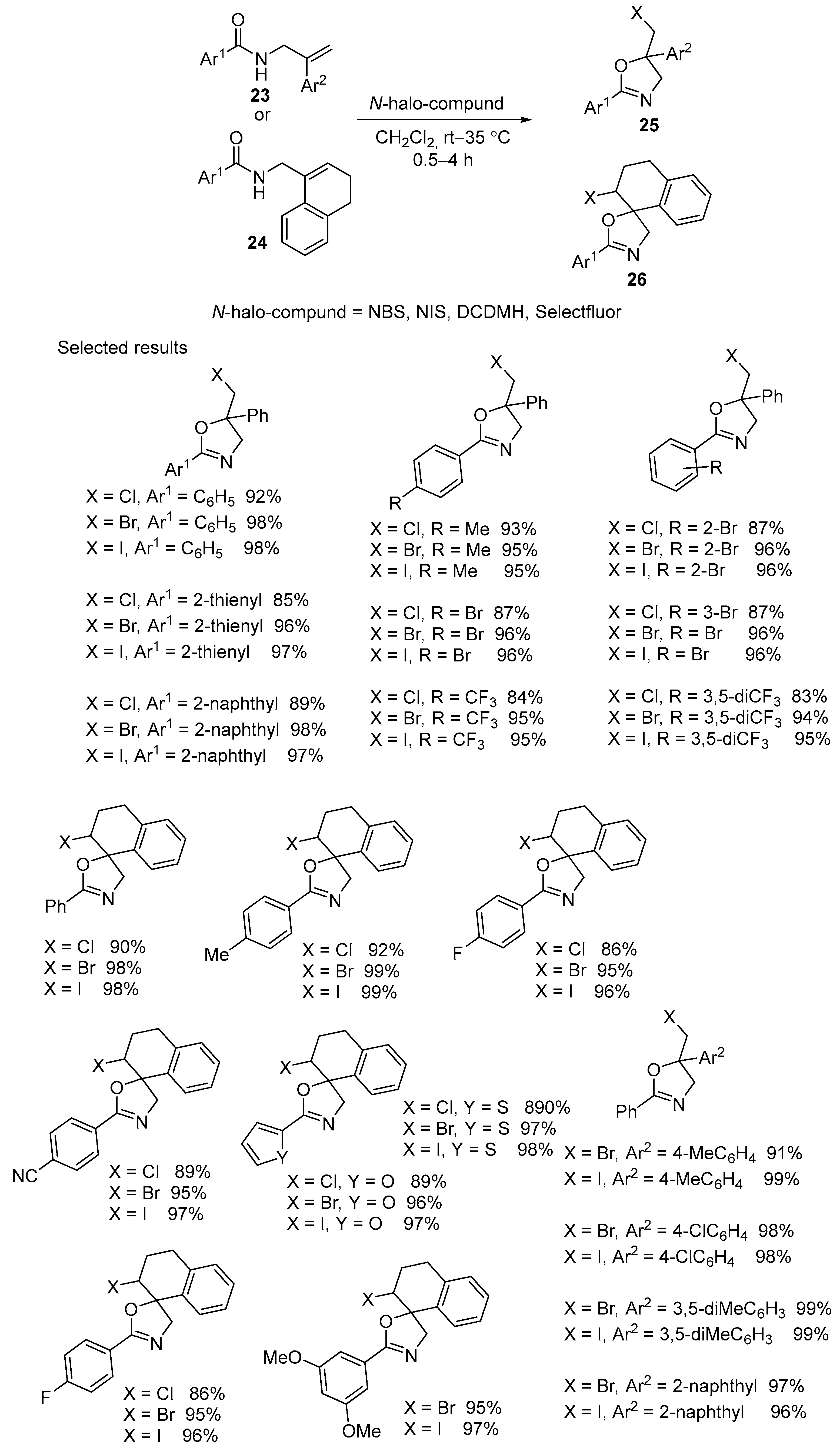
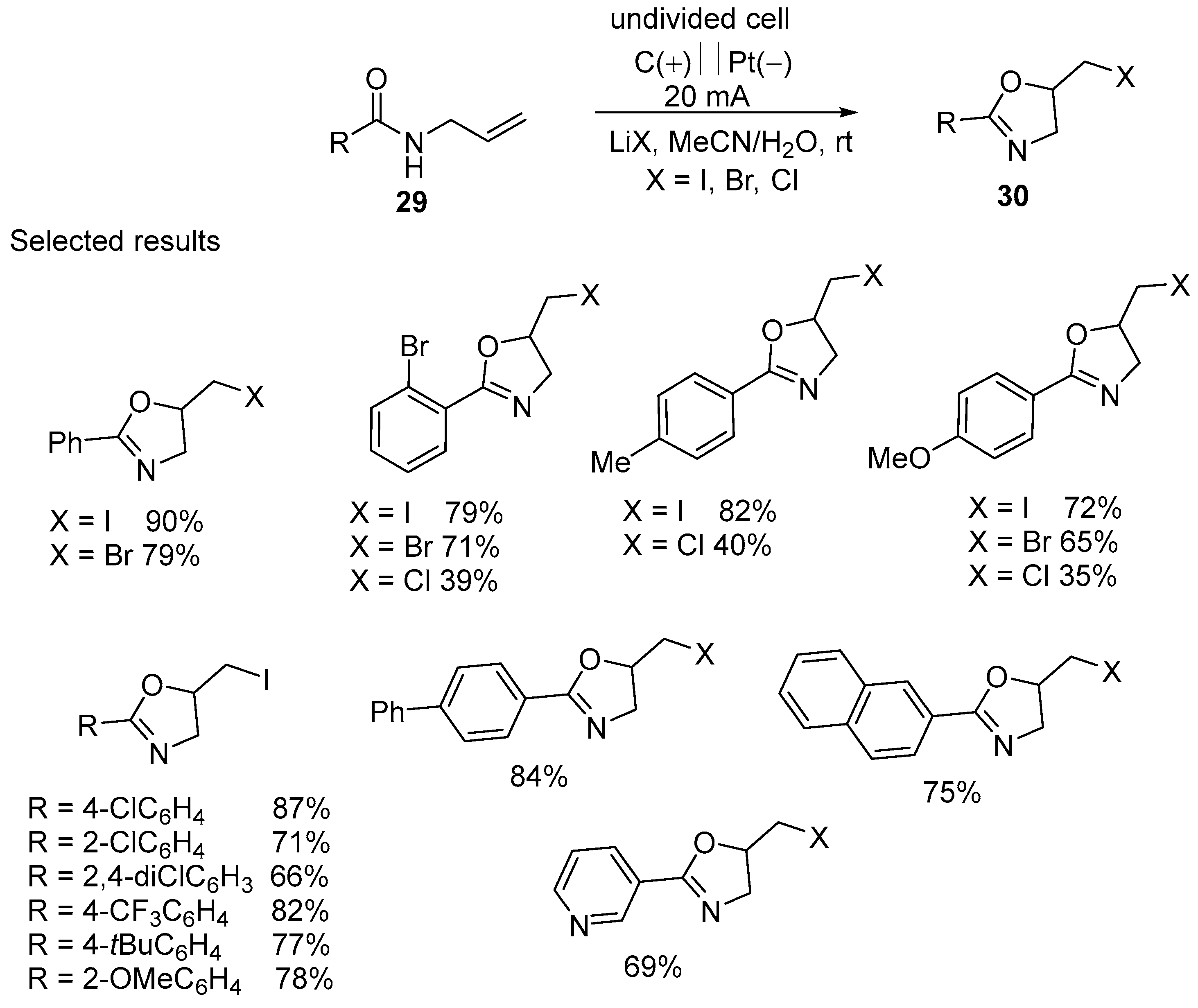
2.1. Halofunctionalized Oxazolines
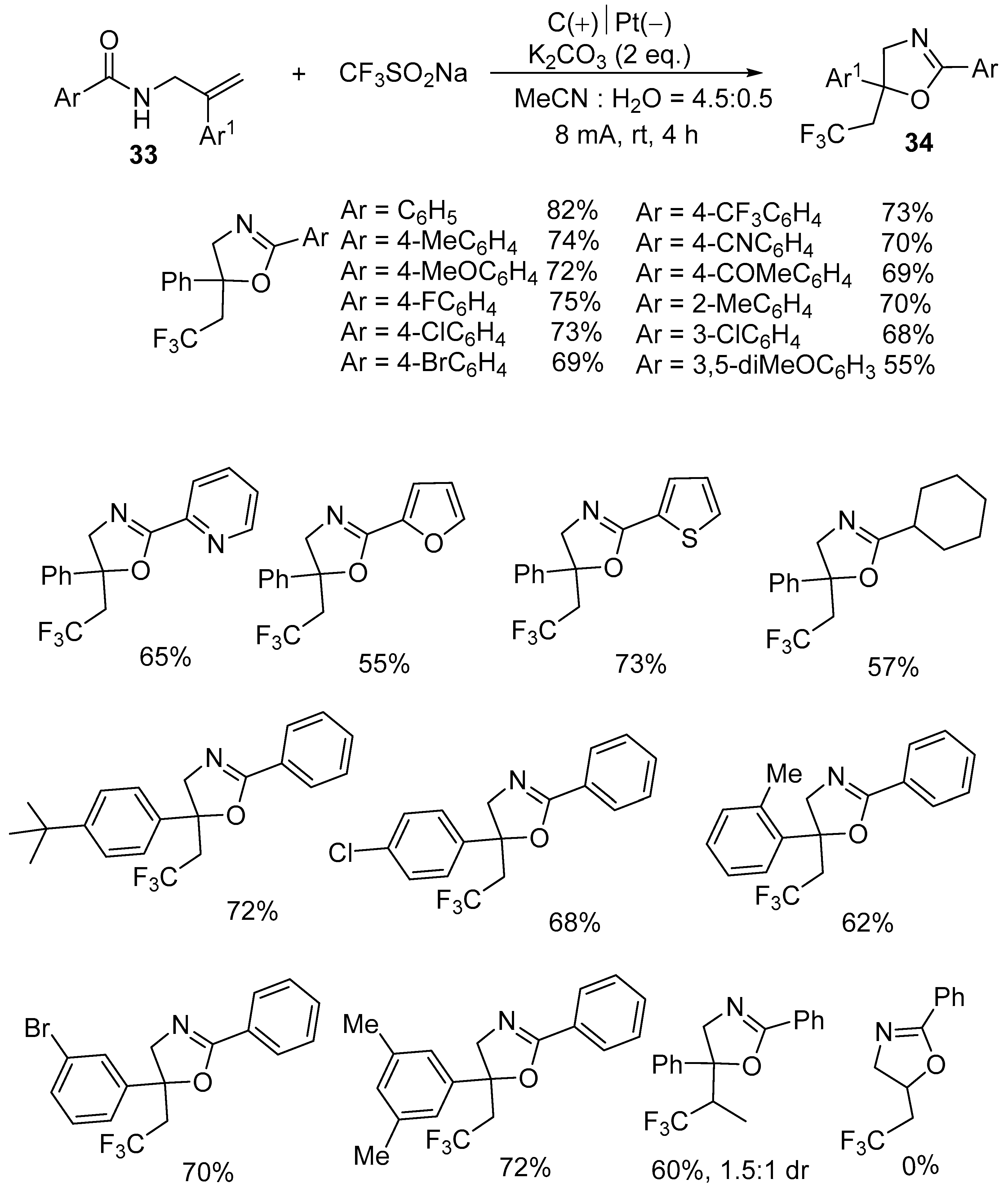
2.2. Trifluoromethyl Functionalized Oxazolines
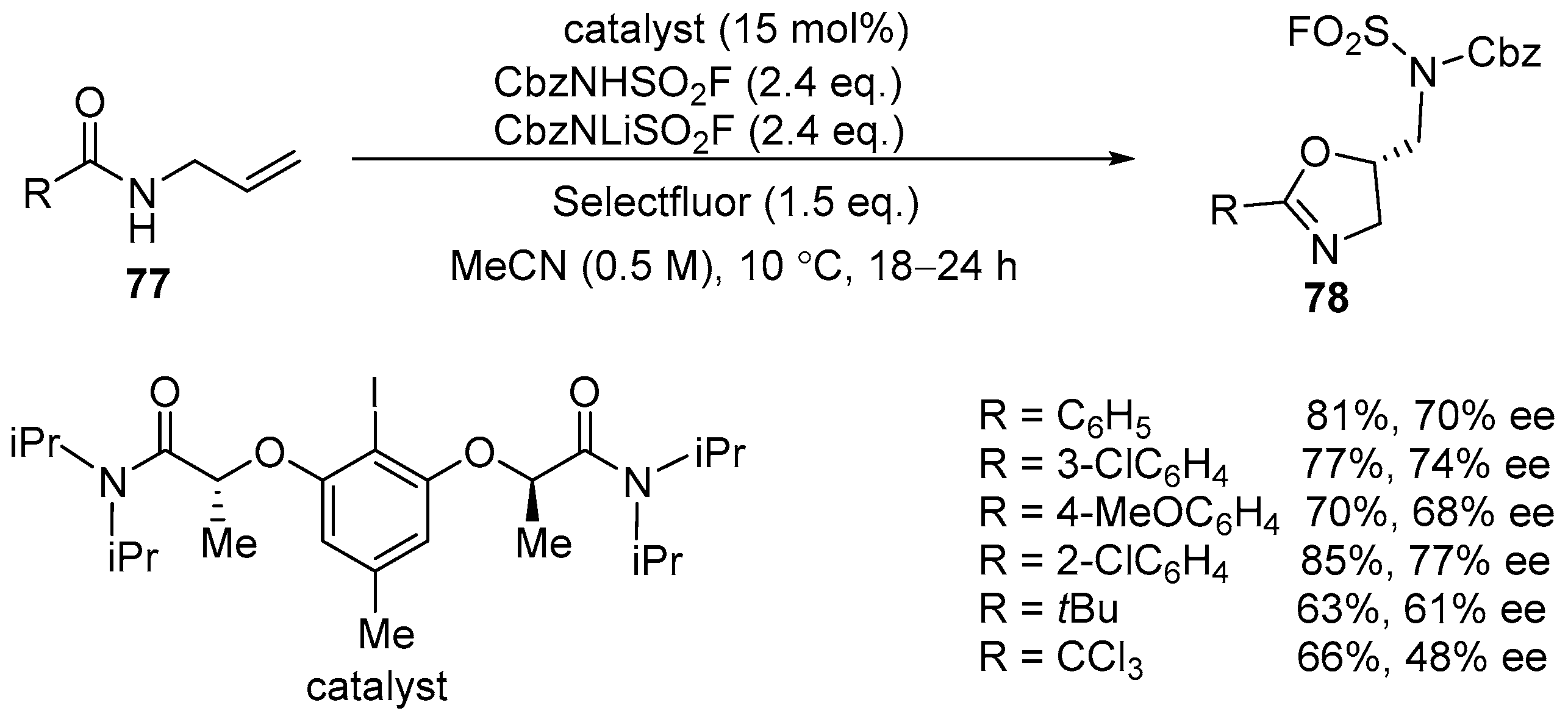
2.3. Sulfonyl Funtionalized Oxazolines
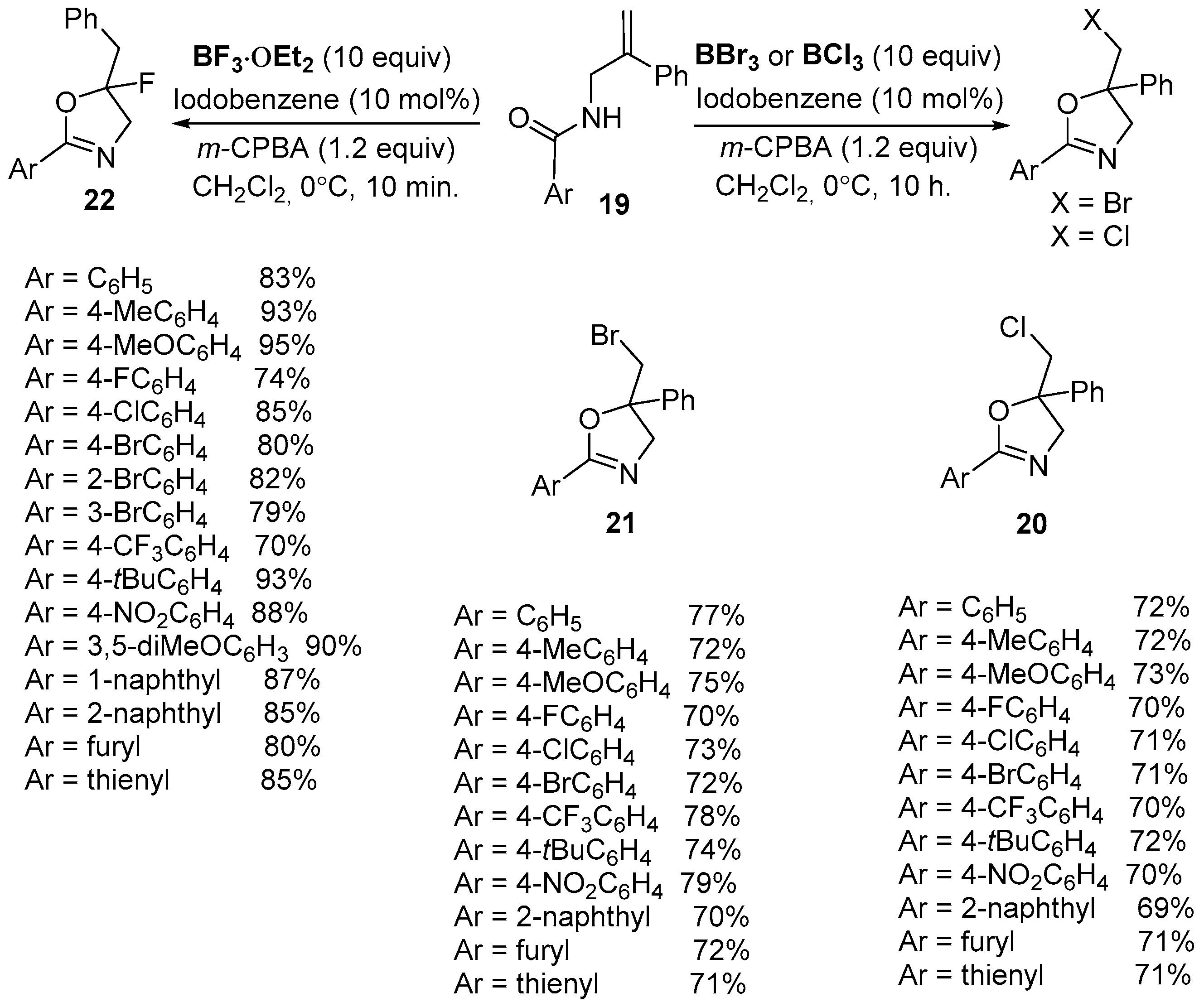
2.4. Sulfenyl-Functionalized Oxazolines
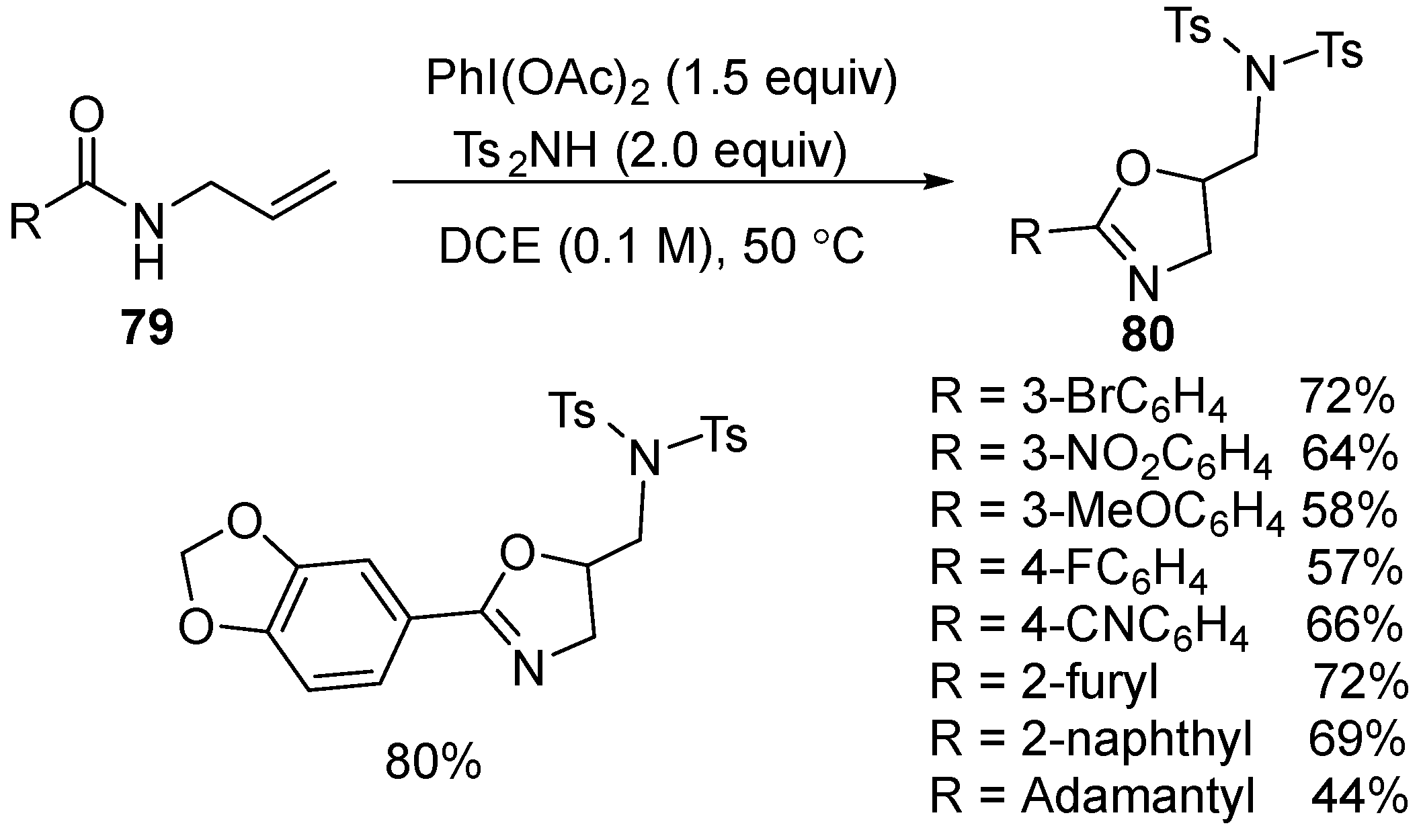
2.5. Selenyl-Functionalized Oxazolines
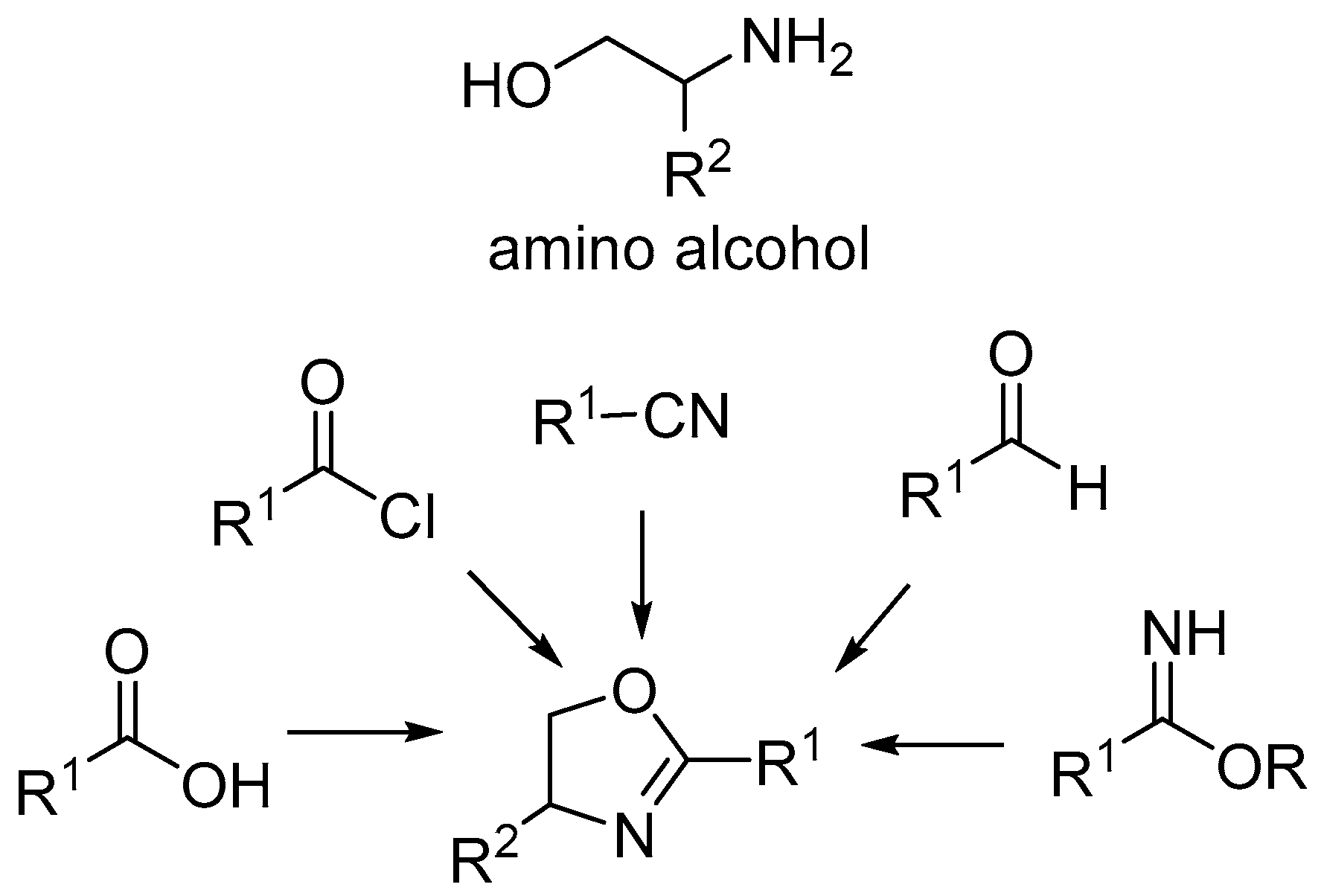
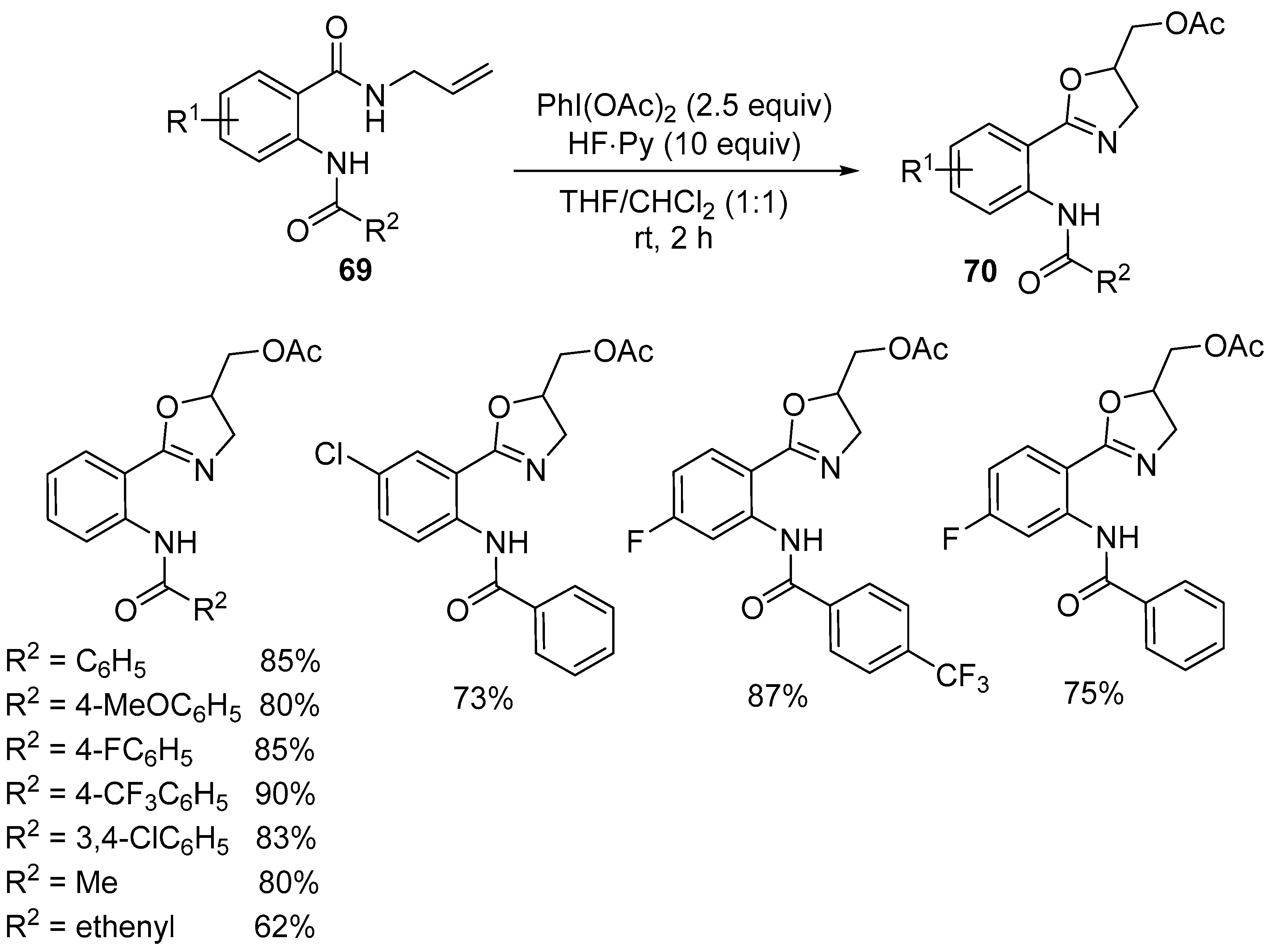
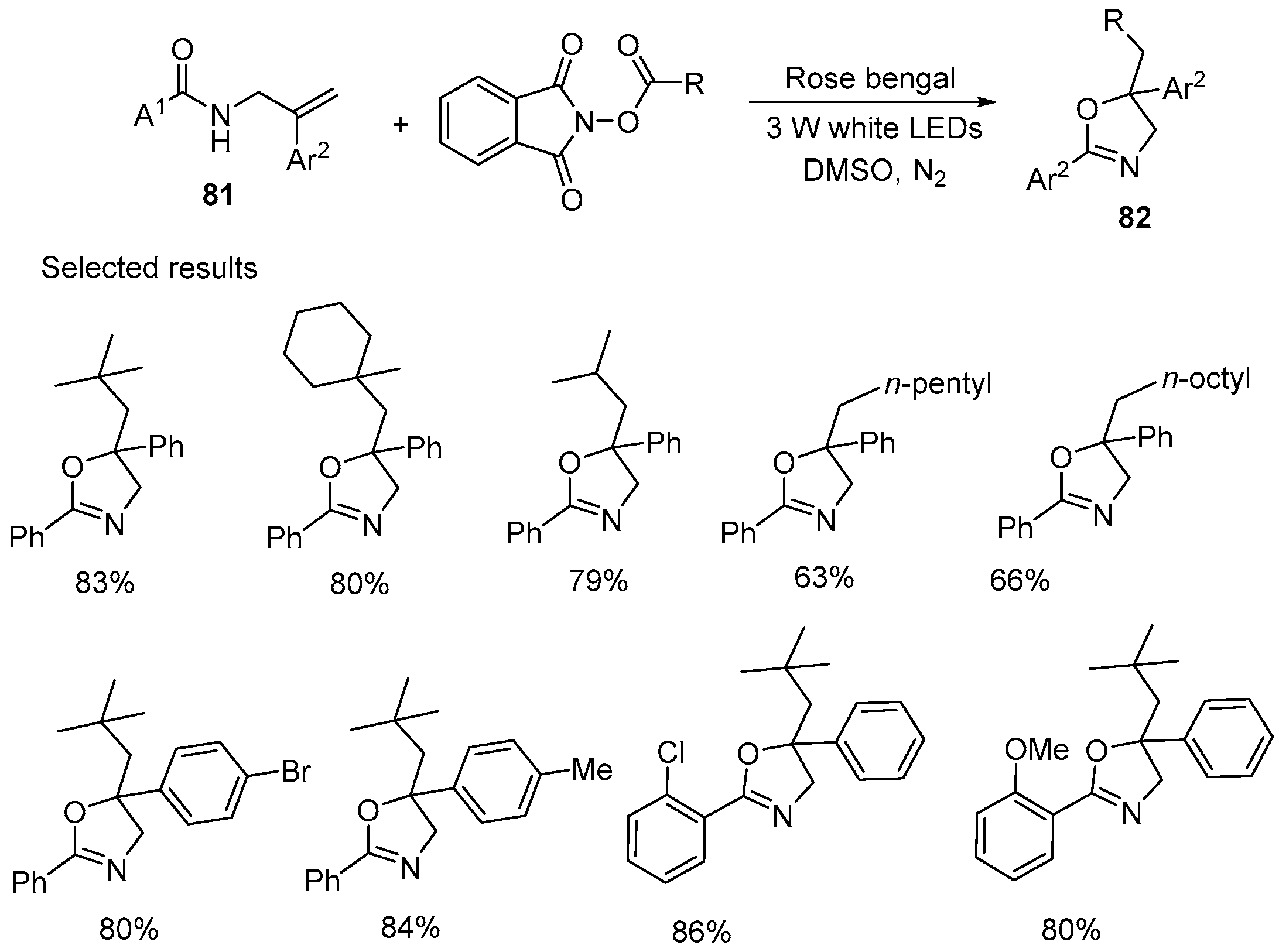
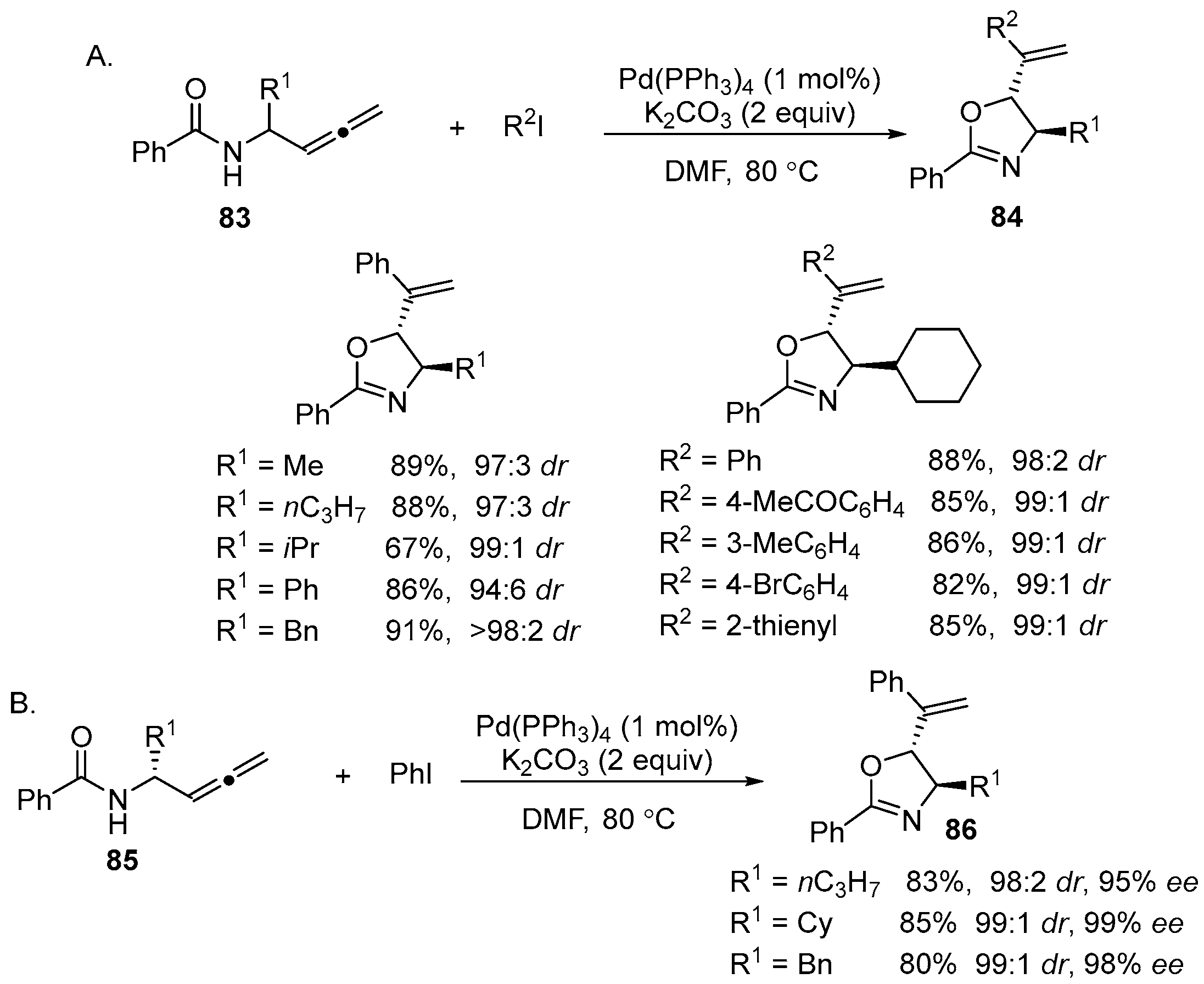
2.6. Acetoxy-, Hydroxyl-, Amino-, and Alkyl-Functionalized Oxazolines
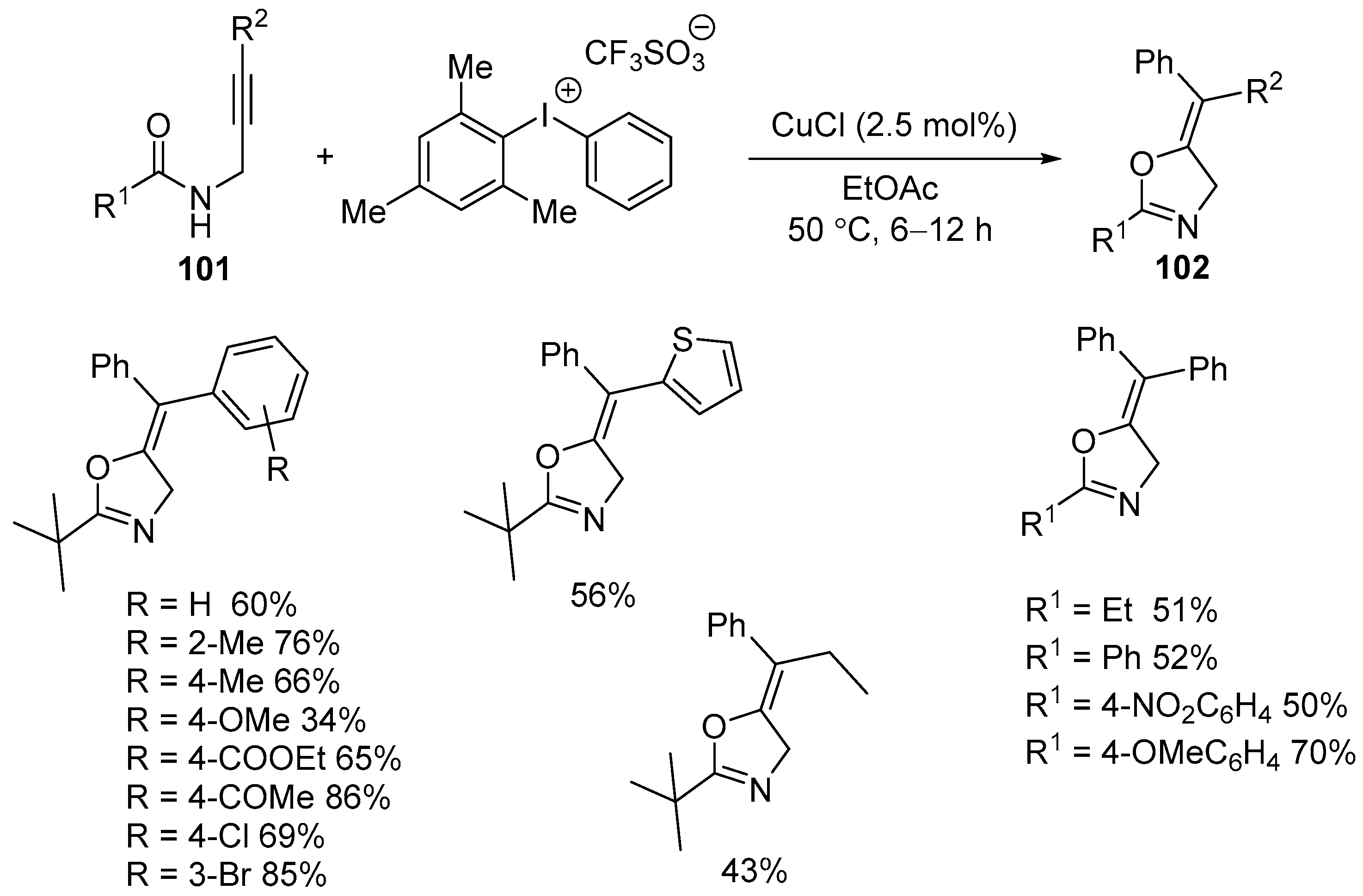
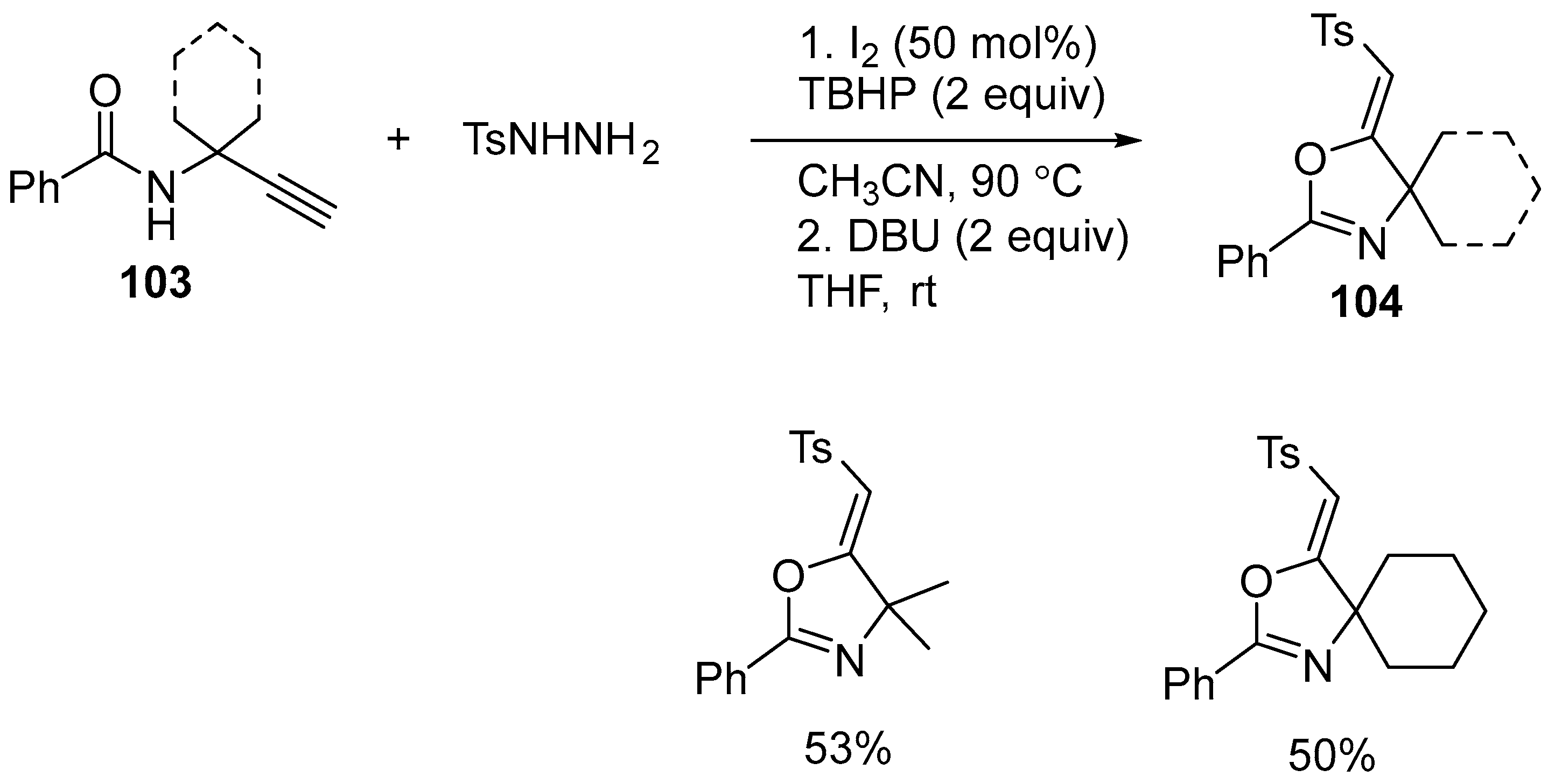
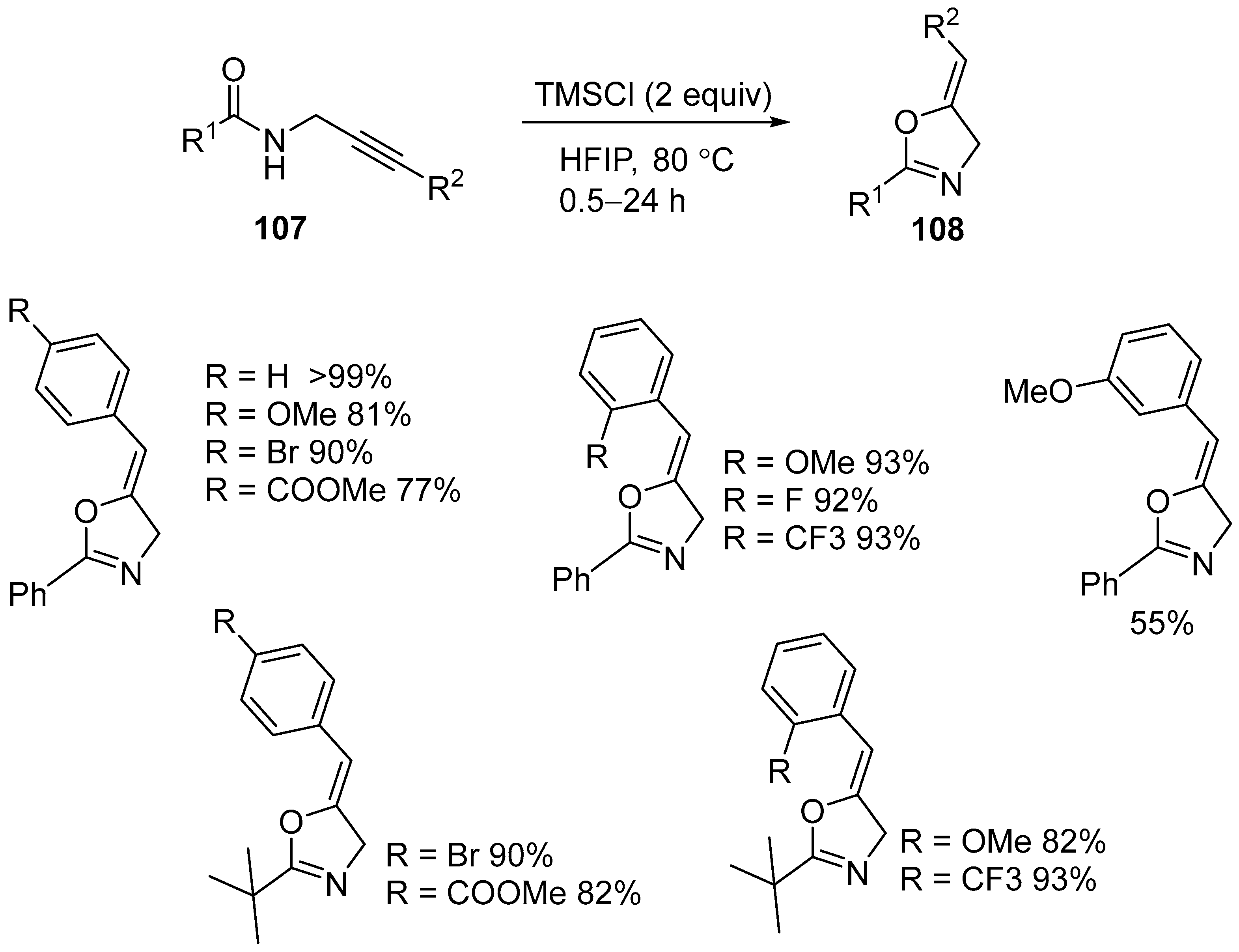
3. Synthesis of Oxazolines from N-Propargyl Amides
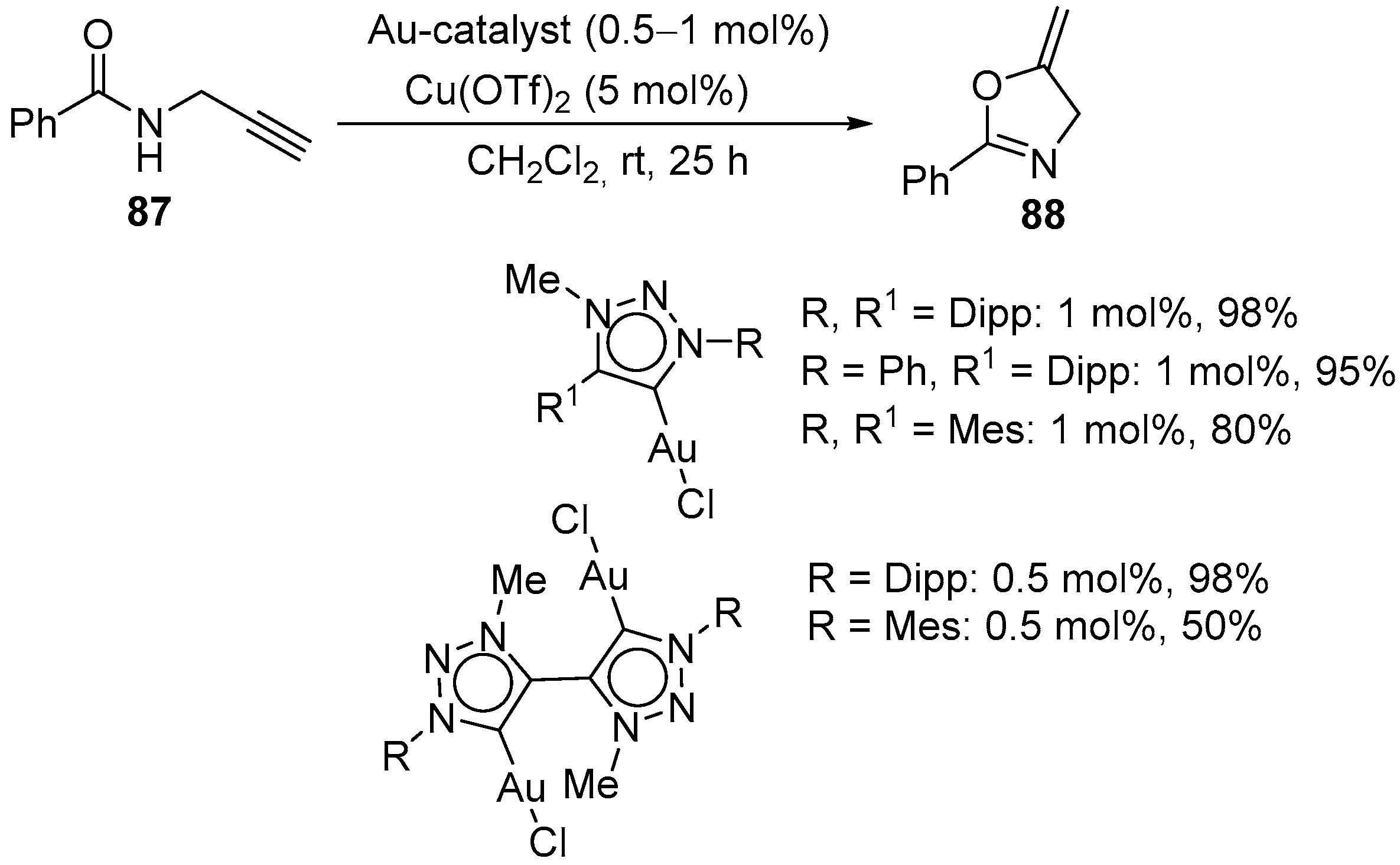
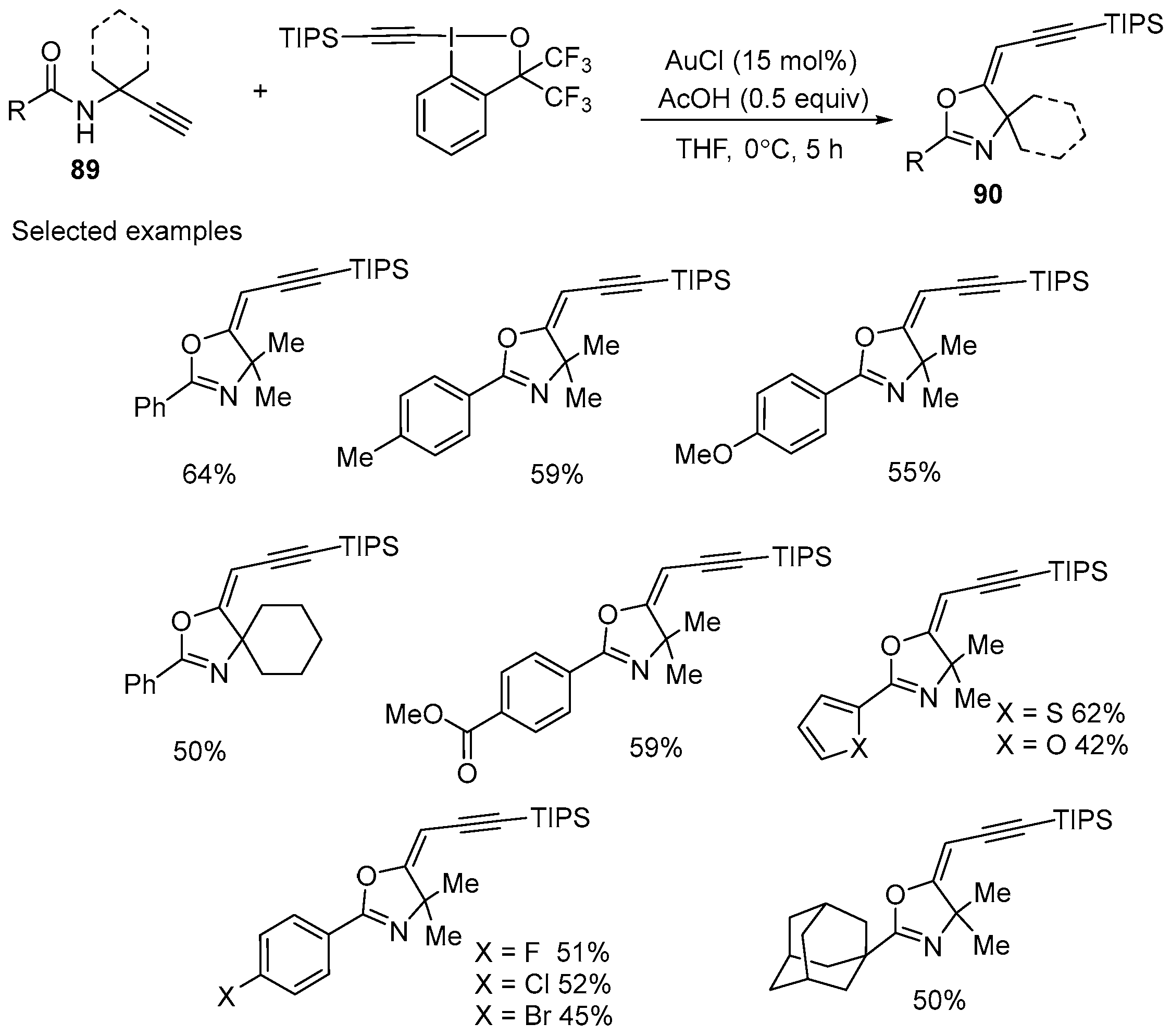
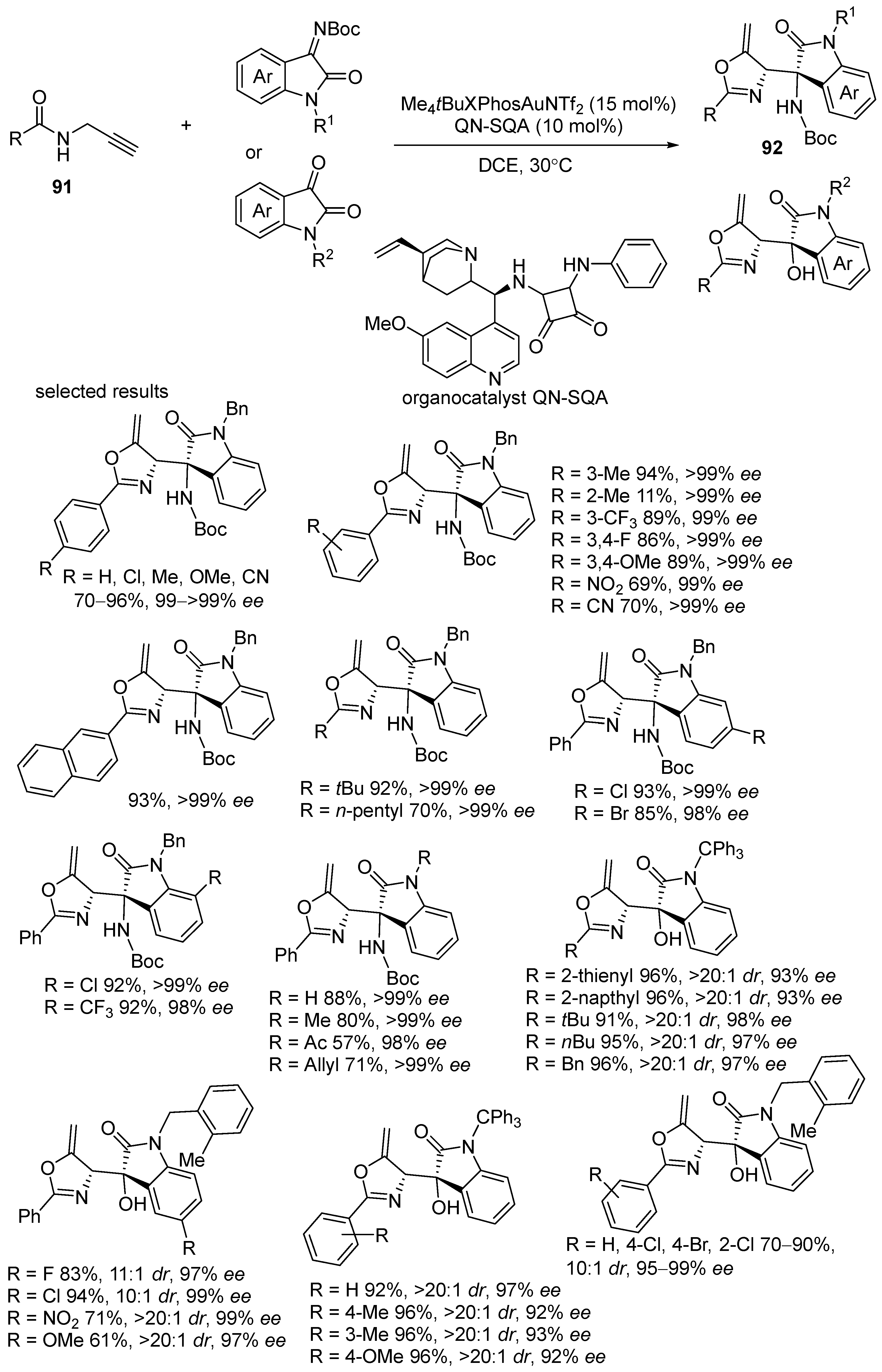
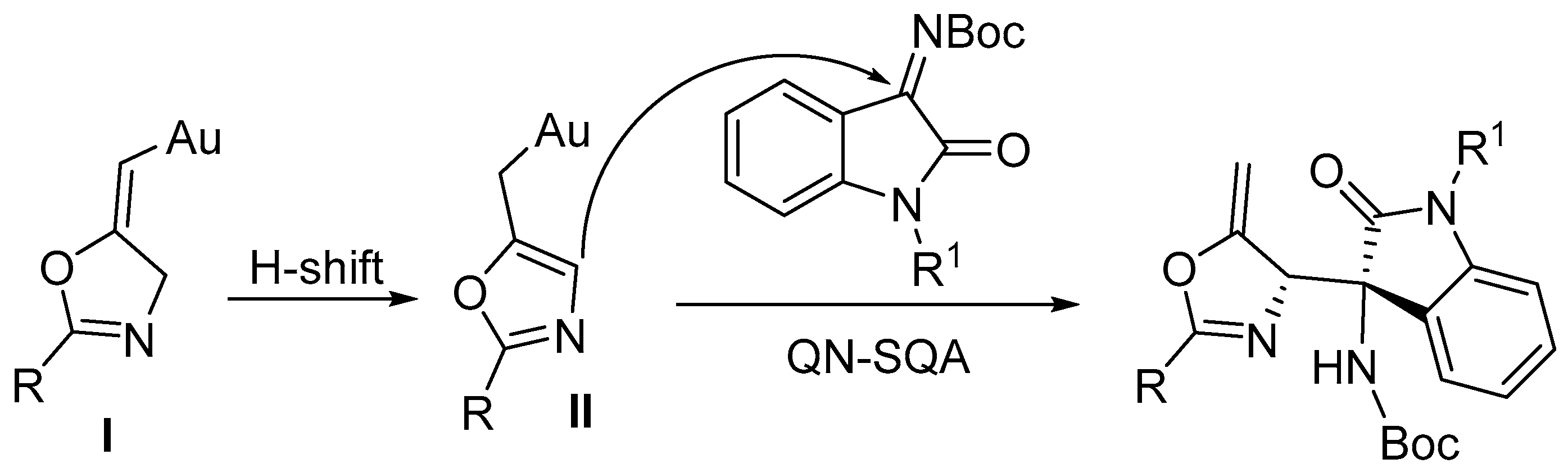
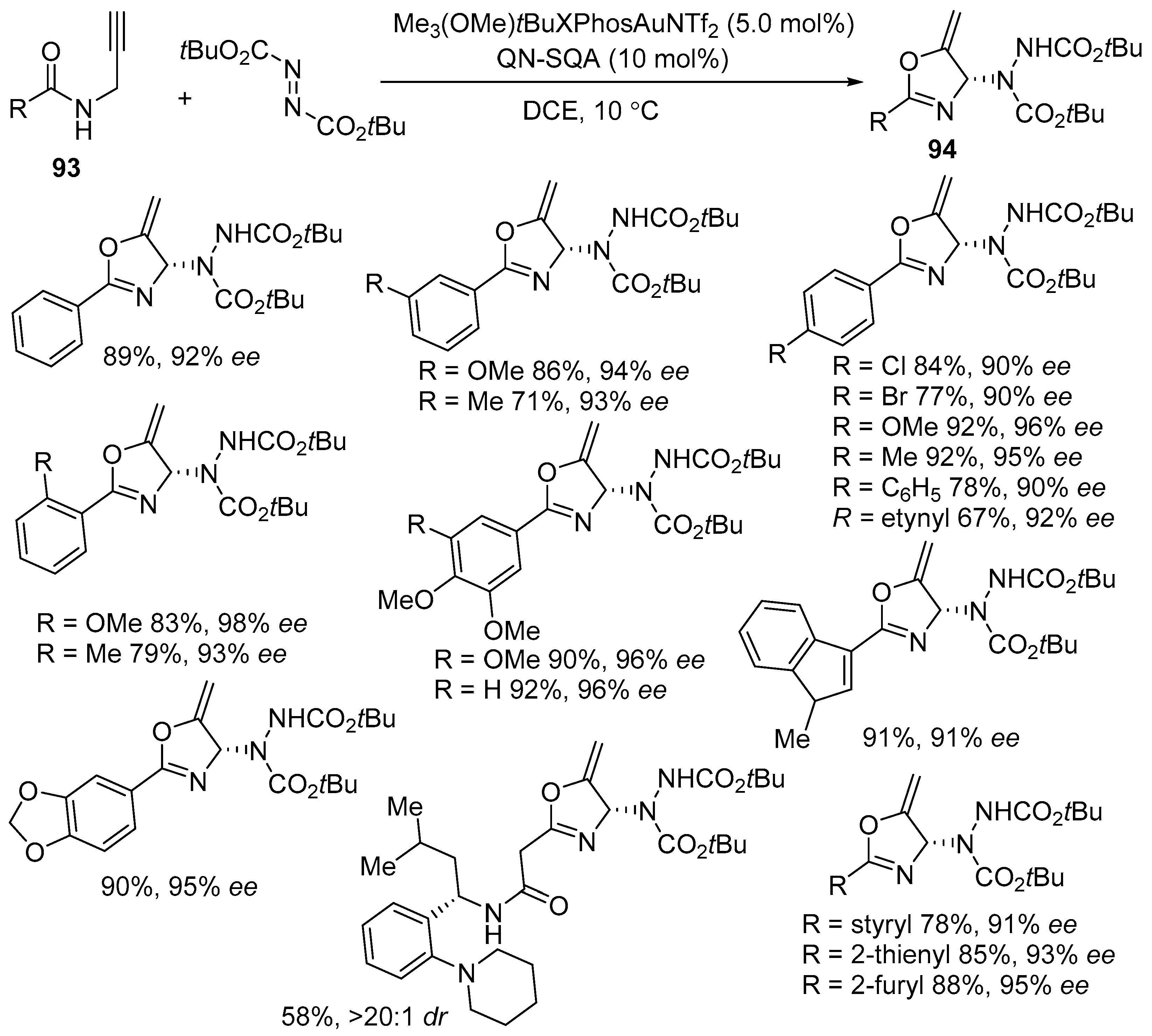
3.1. Gold-Catalyzed Electrophilic Intramolecular Cyclization of N-Propargyl Amides
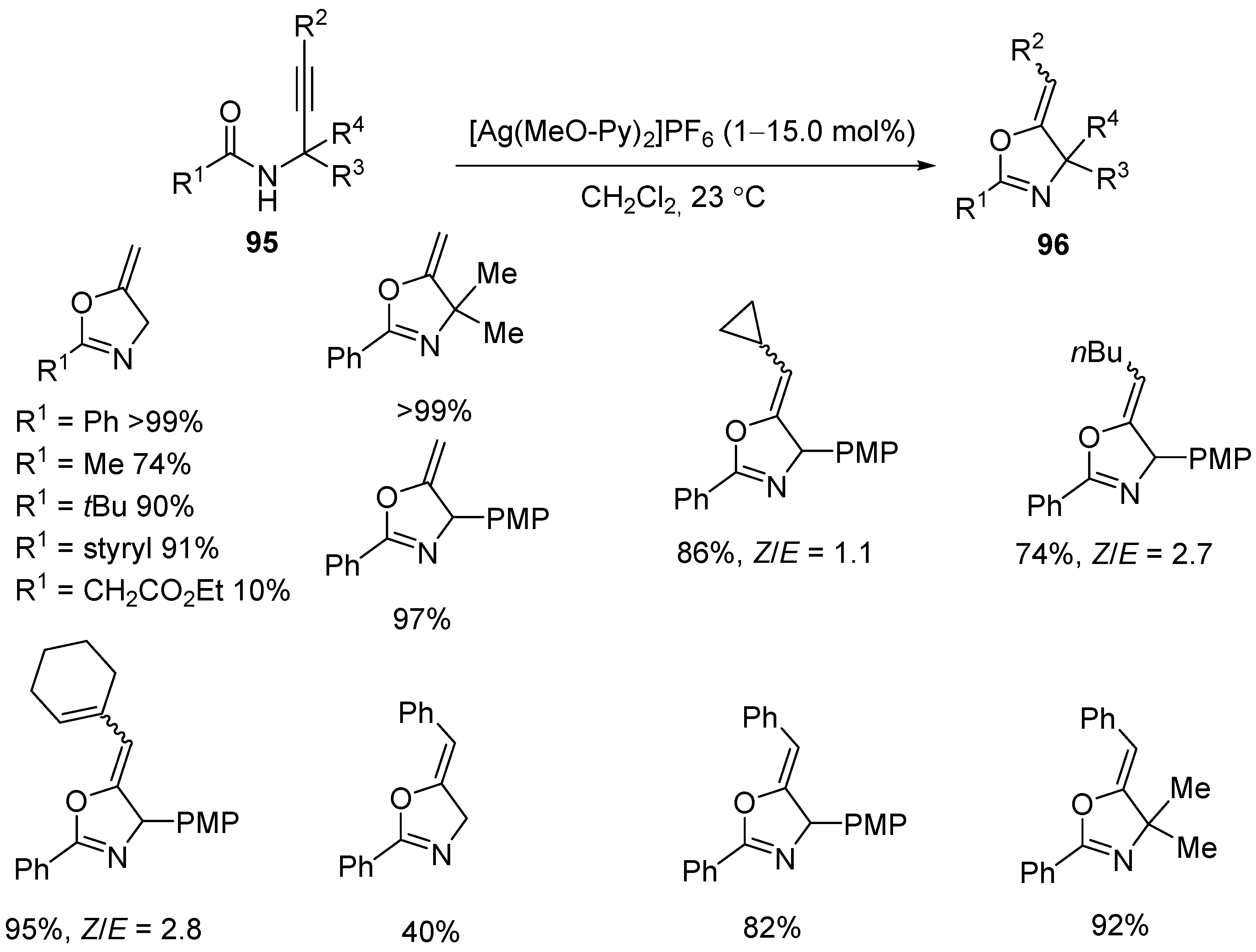
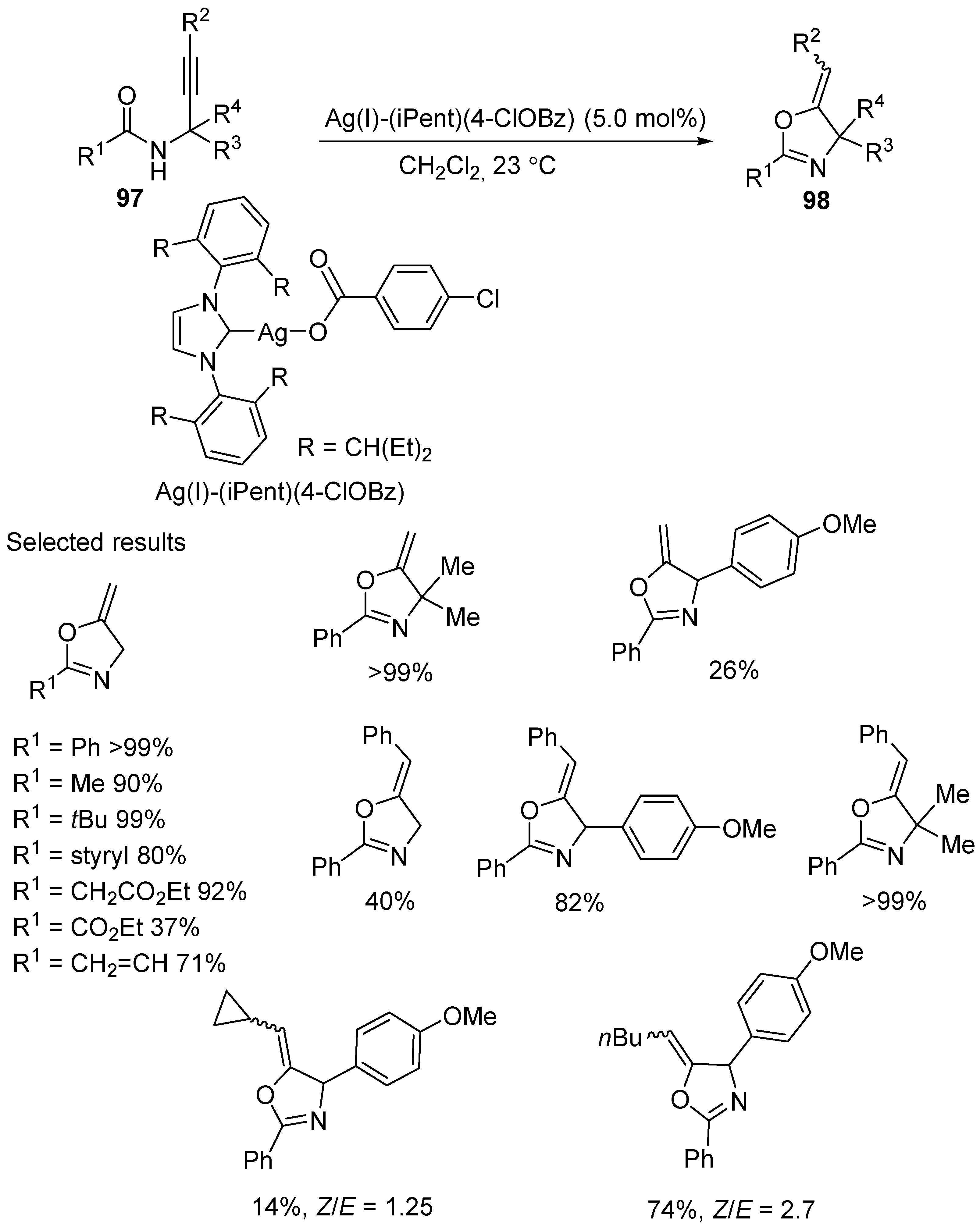
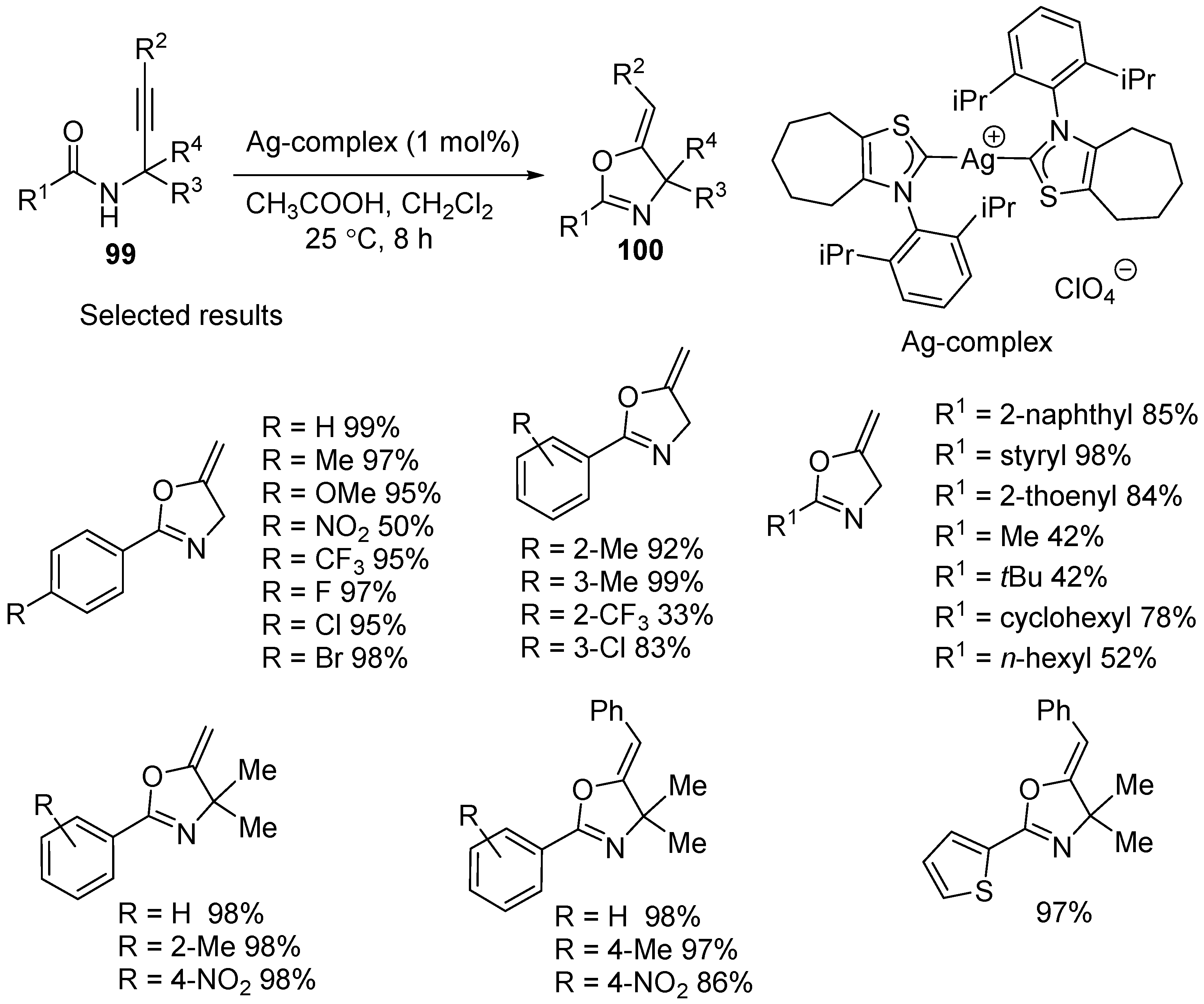
3.2. Silver- and Copper-Catalyzed Electrophilic Intramolecular Cyclization of N-Propargyl Amides
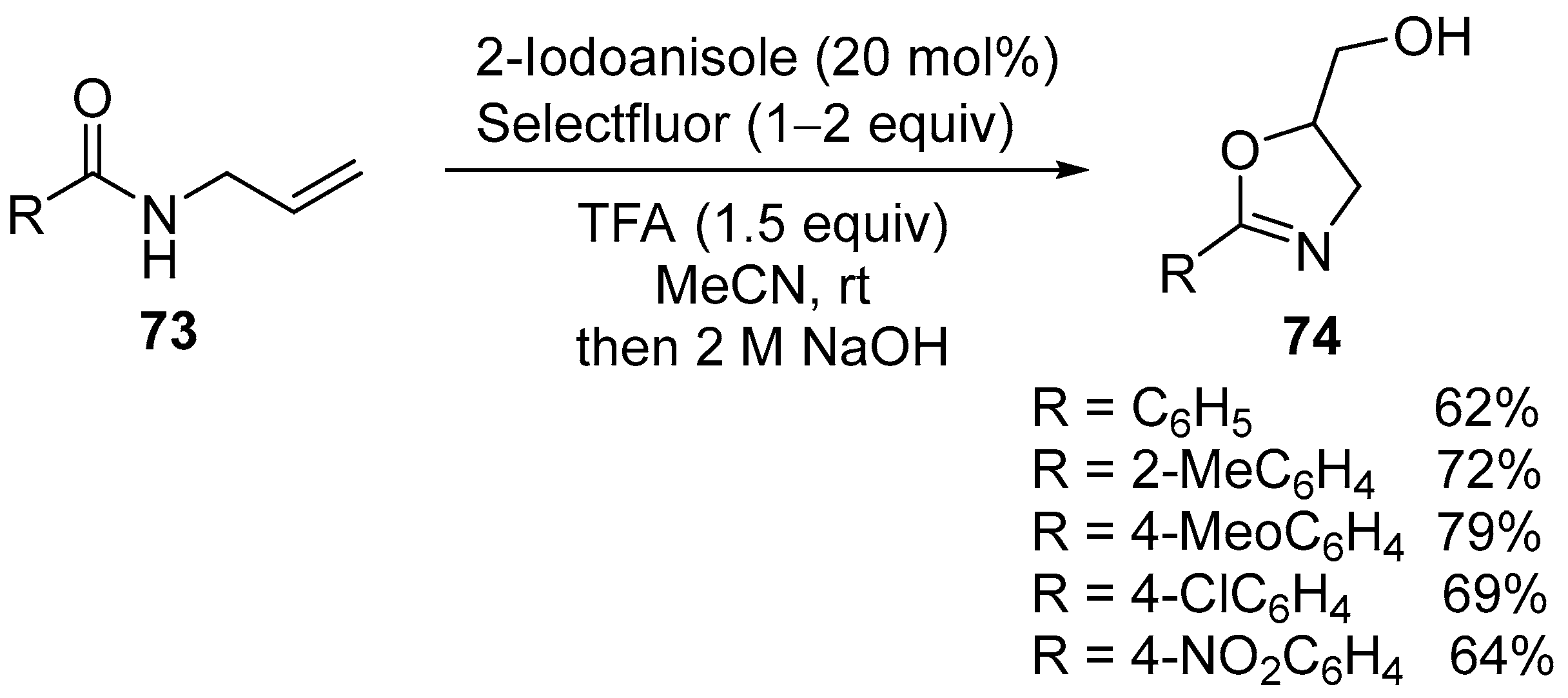
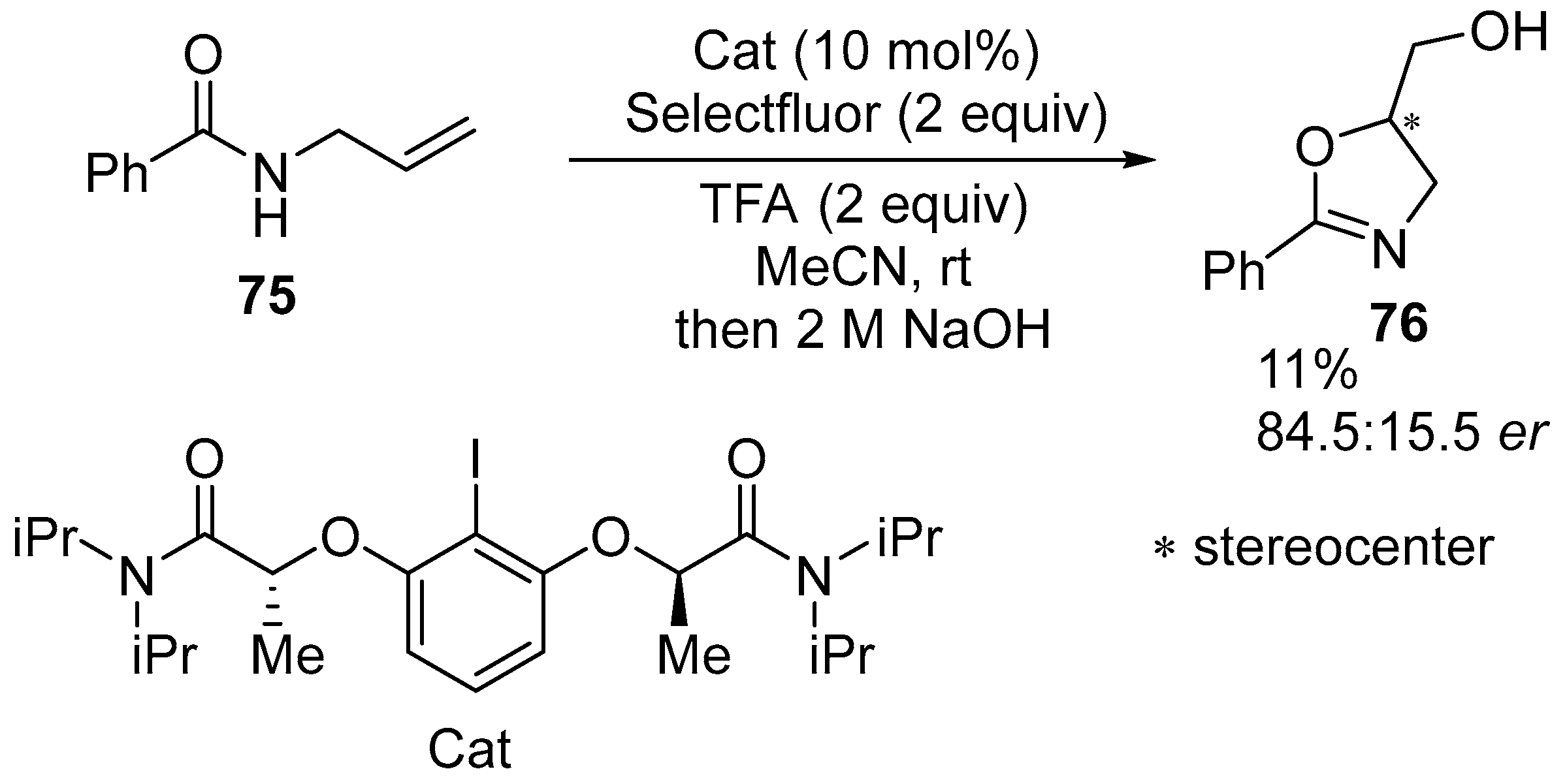
3.3. Transition Metal-Free Electrophilic Intramolecular Cyclization of N-Propargyl Amides
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bansal, S.; Halve, A.K. Oxazolines: Their synthesis and biological activity. Int. J. Pharm. Sci. Res. 2014, 5, 4601–4616. [Google Scholar]
- Moraski, G.C.; Chang, M.; Villegas-Estrada, A.; Franzblau, S.G.; Möllmann, U.; Miller, M.J. Structure–Activity Relationship of New Anti-Tuberculosis Agents Derived from Oxazoline and Oxazole Benzyl Esters. Eur. J. Med. Chem. 2010, 45, 1703–1716. [Google Scholar] [CrossRef]
- Li, Q.; Woods, K.W.; Claiborne, A.; Gwaltney, I.S.L.; Barr, K.J.; Liu, G.; Gehrke, L.; Credo, R.B.; Hui, Y.H.; Lee, J.; et al. Synthesis and Biological Evaluation of 2-Indolyloxazolines as a New Class of Tubulin Polymerization Inhibitors. Discovery of A-289099 as an Orally Active Antitumor Agent. Bioorg. Med. Chem. Lett. 2002, 12, 465–469. [Google Scholar] [CrossRef]
- Faizi, S.; Farooqi, F.; Zikr-Ur-Rehman, S.; Naz, A.; Noor, F.; Ansari, F.; Ahmad, A.; Khan, S.A. Shahidine, a Novel and Highly Labile Oxazoline from Aegle Marmelos: The Parent Compound of Aegeline and Related Amides. Tetrahedron 2009, 65, 998–1004. [Google Scholar] [CrossRef]
- Lunagariya, N.A.; Shah, P. Aegeline: A Biologically Important Alkaloid from Nature. Phytomed. Plus 2025, 5, 100765. [Google Scholar] [CrossRef]
- Ishida, T.; Tanaka, M.; Nabae, M.; Inoue, M.; Kato, S.; Hamada, Y.; Shioiri, T. Solution and Solid-State Conformations of Ascidiacyclamide, a Cytotoxic Cyclic Peptide from Ascidian. J. Org. Chem. 1988, 53, 107–112. [Google Scholar] [CrossRef]
- Degnan, B.M.; Hawkins, C.J.; Lavin, M.F.; McCaffrey, E.J.; Parry, D.L.; Van Den Brenk, A.L.; Watters, D.J. New Cyclic Peptides with Cytotoxic Activity from the Ascidian Lissoclinum patella. J. Med. Chem. 1989, 32, 1349–1354. [Google Scholar] [CrossRef] [PubMed]
- Franchino, A.; Jakubec, P.; Dixon, D.J. Enantioselective Synthesis of (−)-Chloramphenicol via Silver-Catalysed Asymmetric Isocyanoacetate Aldol Reaction. Org. Biomol. Chem. 2016, 14, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Arsenie, L.V.; Lapinte, V. A Recent State of Art on Polyoxazoline-Containing Antifouling Coatings for Biological and Environmental Applications. Prog. Org. Coat. 2024, 186, 107993. [Google Scholar] [CrossRef]
- Sedlacek, O.; Hoogenboom, R. Drug Delivery Systems Based on Poly(2-Oxazoline)s and Poly(2-Oxazine)s. Adv. Ther. 2020, 3, 1900168. [Google Scholar] [CrossRef]
- Brunner, H.; Obermann, U.; Wimmer, P. Asymmetrische katalysen: XXXII. Enantioselektive phenylierung von cis-cyclohexan-1, 2-diol und meso-butan-2, 3-diol. J. Organomet. Chem. 1986, 316, C1–C3. [Google Scholar] [CrossRef]
- Fritschi, H.; Leutenegger, U.; Pfaltz, A. Chiral Copper-Semicorrin Complexes as Enantioselective Catalysts for the Cyclopropanation of Olefins by Diazo Compounds. Angew. Chem. Int. Ed. Engl. 1986, 25, 1005–1006. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, W. Renaissance of Pyridine-Oxazolines as Chiral Ligands for Asymmetric Catalysis. Chem. Soc. Rev. 2018, 47, 1783–1810. [Google Scholar] [CrossRef]
- Wolińska, E.; Wysocki, W.; Branowska, D.; Karczmarzyk, Z. Synthesis and Structures of Three New Pyridine-Containing Oxazoline Ligands of Complexes for Asymmetric Catalysis. Acta Crystallogr. Sect. C Struct. Chem. 2021, 77, 529–536. [Google Scholar] [CrossRef]
- Wolińska, E. Chiral Oxazoline Ligands Containing a 1,2,4-Triazine Ring and Their Application in the Cu-Catalyzed Asymmetric Henry Reaction. Tetrahedron 2013, 69, 7269–7278. [Google Scholar] [CrossRef]
- Wolińska, E. Asymmetric Henry Reactions Catalyzed by Copper (II) Complexes of Chiral 1,2,4-Triazine-Oxazoline Ligands: The Impact of Substitution in the Oxazoline Ring on Ligand Activity. Tetrahedron Asymmetry 2014, 25, 1122–1128. [Google Scholar] [CrossRef]
- Wolińska, E. A Study of Chiral Oxazoline Ligands with a 1,2,4-Triazine and Other Six-Membered Aza-Heteroaromatic Rings and Their Application in Cu-Catalysed Asymmetric Nitroaldol Reactions. Tetrahedron Asymmetry 2014, 25, 1478–1487. [Google Scholar] [CrossRef]
- Wolińska, E. Chiral Oxazoline Ligands with Two Different Six-Membered Azaheteroaromatic Rings—Synthesis and Application in the Cu-Catalyzed Nitroaldol Reaction. Heterocycl. Commun. 2016, 22, 85–94. [Google Scholar] [CrossRef]
- Wolińska, E.; Karczmarzyk, Z.; Wysocki, W. Structural Characterization of Copper Complexes with Chiral 1,2,4-Triazine-Oxazoline Ligands. Heterocycl. Commun. 2016, 22, 265–274. [Google Scholar] [CrossRef]
- Wolińska, E.; Rozbicki, P.; Branowska, D. Chiral Pyridine Oxazoline and 1,2,4-Triazine Oxazoline Ligands Incorporating Electron-Withdrawing Substituents and Their Application in the Cu-Catalyzed Enantioselective Nitroaldol Reaction. Monatshefte Chem. Chem. Mon. 2022, 153, 245–256. [Google Scholar] [CrossRef]
- Karczmarzyk, Z.; Wolińska, E.; Fruziński, A. N-{2-[(4S)-4-Tert-Butyl-4,5-Dihydro-1,3-Oxazol-2-Yl]Phenyl}-5,6-Diphenyl-1,2,4-Triazin-3-Amine. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67, o651. [Google Scholar] [CrossRef]
- Das, D.; Mal, R.; Mittal, N.; Zhu, Z.; Emge, T.J.; Seidel, D. Chiral Bisoxazoline Ligands Designed to Stabilize Bimetallic Complexes. Beilstein J. Org. Chem. 2018, 14, 2002–2011. [Google Scholar] [CrossRef]
- Fekner, T.; Müller-Bunz, H.; Guiry, P.J. Synthesis and Application of Quinazoline−Oxazoline-Containing (Quinazox) Ligands. Org. Lett. 2006, 8, 5109–5112. [Google Scholar] [CrossRef]
- Hargaden, G.C.; Müller-Bunz, H.; Guiry, P.J. New Proline–Oxazoline Ligands and Their Application in the Asymmetric Nozaki–Hiyama–Kishi Reaction. Eur. J. Org. Chem. 2007, 2007, 4235–4243. [Google Scholar] [CrossRef]
- McKeon, S.C.; Müller-Bunz, H.; Guiry, P.J. Synthesis of Thiazoline–Oxazoline Ligands and Their Application in Asymmetric Catalysis. Eur. J. Org. Chem. 2011, 2011, 7107–7115. [Google Scholar] [CrossRef]
- Hargaden, G.C.; O’Sullivan, T.P.; Guiry, P.J. Synthesis of Non-Symmetric Bis (Oxazoline)-Containing Ligands and Their Application in the Catalytic Enantioselective Nozaki–Hiyama–Kishi Allylation of Benzaldehyde. Org. Biomol. Chem. 2008, 6, 562–566. [Google Scholar] [CrossRef]
- Connon, R.; Roche, B.; Rokade, B.V.; Guiry, P.J. Further Developments and Applications of Oxazoline-Containing Ligands in Asymmetric Catalysis. Chem. Rev. 2021, 121, 6373–6521. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, S.; Guiry, P. Recent Applications of C 1-Symmetric Bis (Oxazoline)-Containing Ligands in Asymmetric Catalysis. Synthesis 2014, 46, 722–739. [Google Scholar] [CrossRef]
- Hargaden, G.C.; Guiry, P.J. Recent Applications of Oxazoline-Containing Ligands in Asymmetric Catalysis. Chem. Rev. 2009, 109, 2505–2550. [Google Scholar] [CrossRef] [PubMed]
- Wenker, H. The Synthesis of ▵2-Oxazolines and ▵2-Thiazolines from N-Acyl-2-Aminoethanols. J. Am. Chem. Soc. 1935, 57, 1079–1080. [Google Scholar] [CrossRef]
- Phillips, A.J.; Uto, Y.; Wipf, P.; Reno, M.J.; Williams, D.R. Synthesis of Functionalized Oxazolines and Oxazoles with DAST and Deoxo-Fluor. Org. Lett. 2000, 2, 1165–1168. [Google Scholar] [CrossRef]
- Lafargue, P.; Guenot, P.; Lellouche, J.P. (Diethylamino) Sulfur Trifluoride (DAST) as a Useful Reagent for the Preparation of 2-Oxazolines from 1,2-Amido Alcohols. Heterocycles 1995, 41, 947. [Google Scholar] [CrossRef]
- Pouliot, M.-F.; Angers, L.; Hamel, J.-D.; Paquin, J.-F. Synthesis of 2-Oxazolines and Related N-Containing Heterocycles Using [Et2NSF2]BF4 as a Cyclodehydration Agent. Tetrahedron Lett. 2012, 53, 4121–4123. [Google Scholar] [CrossRef]
- Mollo, M.C.; Orelli, L.R. Microwave-Assisted Synthesis of 2-Aryl-2-Oxazolines, 5,6-Dihydro-4H-1,3-Oxazines, and 4,5,6,7-Tetrahydro-1,3-Oxazepines. Org. Lett. 2016, 18, 6116–6119. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Li, Z. A Facile Synthesis of 2-Oxazolines Using a PPh3–DDQ System. Tetrahedron Lett. 2009, 50, 6838–6840. [Google Scholar] [CrossRef]
- Roush, D.M.; Patel, M.M. A Mild Procedure for the Preparation of 2-Oxazolines. Synth. Commun. 1985, 15, 675–679. [Google Scholar] [CrossRef]
- Liyanage, W.; Weerasinghe, L.; Strong, R.K.; Del Valle, J.R. Synthesis of Carbapyochelins via Diastereoselective Azidation of 5-(Ethoxycarbonyl) Methylproline Derivatives. J. Org. Chem. 2008, 73, 7420–7423. [Google Scholar] [CrossRef]
- Jadhav, K.A.; Bhosle, S.D.; Itage, S.V.; Bhosale, R.S.; Eppa, G.; Yadav, J.S. A Novel Method for the Synthesis of 2-Oxazolines. Tetrahedron Lett. 2022, 106, 154048. [Google Scholar] [CrossRef]
- Pérez-Piña, V.M.; Argüello-Velasco, R.O.; Ordoñez, M.; Romero-Estudillo, I. Synthesis of Novel 2-Alkyl and 2-Aryl 4-Phosphorylated Oxazolines from Dimethyl (±)-Phosphoserinate. Tetrahedron 2025, 176, 134471. [Google Scholar] [CrossRef]
- Schwekendiek, K.; Glorius, F. Efficient Oxidative Synthesis of 2-Oxazolines. Synthesis 2006, 2006, 2996–3002. [Google Scholar] [CrossRef]
- Karade, N.; Tiwari, G.; Gampawar, S. Efficient Oxidative Conversion of Aldehydes to 2-Substituted Oxazolines and Oxazines Using (Diacetoxyiodo) Benzene. Synlett 2007, 2007, 1921–1924. [Google Scholar] [CrossRef]
- Ishihara, M.; Togo, H. Direct Oxidative Conversion of Aldehydes and Alcohols to 2-Imidazolines and 2-Oxazolines Using Molecular Iodine. Tetrahedron 2007, 63, 1474–1480. [Google Scholar] [CrossRef]
- Deng, H.; Wang, J.; He, W.; Ye, Y.; Bai, R.; Zhang, X.; Ye, X.-Y.; Xie, T.; Hui, Z. Microwave-Assisted Rapid Synthesis of Chiral Oxazolines. Org. Biomol. Chem. 2023, 21, 2312–2319. [Google Scholar] [CrossRef]
- Hassani, R.; Requet, A.; Marque, S.; Gaucher, A.; Prim, D.; Kacem, Y.; Hassine, B.B. Efficacious and Rapid Metal- and Solvent-Free Synthesis of Enantiopure Oxazolines. Tetrahedron Asymmetry 2014, 25, 1275–1279. [Google Scholar] [CrossRef]
- Theodorou, A.; Triandafillidi, I.; Kokotos, C.G. Organocatalytic Synthesis of Oxazolines and Dihydrooxazines from Allyl-Amides: Bypassing the Inherent Regioselectivity of the Cyclization. Adv. Synth. Catal. 2018, 360, 951–957. [Google Scholar] [CrossRef]
- Miller, E.; Kim, S.; Gibson, K.; Derrick, J.S.; Toste, F.D. Regio- and Enantioselective Bromocyclization of Difluoroalkenes as a Strategy to Access Tetrasubstituted Difluoromethylene-Containing Stereocenters. J. Am. Chem. Soc. 2020, 142, 8946–8952. [Google Scholar] [CrossRef]
- Hirokawa, R.; Nakahara, Y.; Uchida, S.; Yamashita, K.; Hamashima, Y. Enantioselective Synthesis of Bromodifluoromethyl-containing Oxazolines by Concerted Lewis/Brønsted Base Catalysis with Chiral Bisphosphine Oxide. Chem. Asian J. 2023, 18, e202300141. [Google Scholar] [CrossRef]
- Moschona, F.; Misirlaki, C.; Karadimas, N.; Koutiva, M.; Savvopoulou, I.; Rassias, G. Organocatalytic Asymmetric Halocyclization of Allylic Amides to Chiral Oxazolines Using DTBM-SEGPHOS—Mechanistic Implications from Hammett Plots. Symmetry 2022, 14, 989. [Google Scholar] [CrossRef]
- Kawato, Y.; Kubota, A.; Ono, H.; Egami, H.; Hamashima, Y. Enantioselective Bromocyclization of Allylic Amides Catalyzed by BINAP Derivatives. Org. Lett. 2015, 17, 1244–1247. [Google Scholar] [CrossRef]
- Yang, C.-H.; Xu, Z.-Q.; Duan, L.; Li, Y.-M. CuBr2-Promoted Intramolecular Bromocyclization of N-Allylamides and Aryl Allyl Ketone Oximes. Tetrahedron 2017, 73, 6747–6753. [Google Scholar] [CrossRef]
- Yoshimura, A.; Saito, A.; Yusubov, M.S.; Zhdankin, V.V. Synthesis of Oxazoline and Oxazole Derivatives by Hypervalent-Iodine-Mediated Oxidative Cycloaddition Reactions. Synthesis 2020, 52, 2299–2310. [Google Scholar] [CrossRef]
- Scheidt, F.; Thiehoff, C.; Yilmaz, G.; Meyer, S.; Daniliuc, C.G.; Kehr, G.; Gilmour, R. Fluorocyclisation via I(I)/I(III) Catalysis: A Concise Route to Fluorinated Oxazolines. Beilstein J. Org. Chem. 2018, 14, 1021–1027. [Google Scholar] [CrossRef]
- Liu, G.-Q.; Yang, C.-H.; Li, Y.-M. Modular Preparation of 5-Halomethyl-2-Oxazolines via PhI(OAc)2-Promoted Intramolecular Halooxygenation of N-Allylcarboxamides. J. Org. Chem. 2015, 80, 11339–11350. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-C.; Zhang, Y.-D.; Jia, Q.; Cui, J.; Zhang, C. Hypervalent-Iodine-Mediated Ring-Contraction Monofluorination Affording Monofluorinated Five-Membered Ring-Fused Oxazolines. Org. Lett. 2017, 19, 5300–5303. [Google Scholar] [CrossRef]
- Qin, Y.; Qi, L.; Zhen, X.; Wang, X.; Chai, H.; Ma, X.; Jiang, X.; Cai, X.; Zhu, W. Different Performances of BF3, BCl3, and BBr3 in Hypervalent Iodine-Catalyzed Halogenations. J. Org. Chem. 2023, 88, 4359–4371. [Google Scholar] [CrossRef]
- Chai, H.; Zhen, X.; Wang, X.; Qi, L.; Qin, Y.; Xue, J.; Xu, Z.; Zhang, H.; Zhu, W. Catalytic Synthesis of 5-Fluoro-2-Oxazolines: Using BF3 Et2O as the Fluorine Source and Activating Reagent. ACS Omega 2022, 7, 19988–19996. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Qi, L.; Ma, X.; Zhou, Y.; Jiang, X.; Zhu, W. Halogenation of Unsaturated Amides: Synthesis of Halogenated (Spiro) Oxazolines. ChemistrySelect 2022, 7, e202203419. [Google Scholar] [CrossRef]
- Haupt, J.D.; Berger, M.; Waldvogel, S.R. Electrochemical Fluorocyclization of N-Allylcarboxamides to 2-Oxazolines by Hypervalent Iodine Mediator. Org. Lett. 2019, 21, 242–245. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yin, Y.; Liu, C.; Wu, X.-F.; Yin, Z. Electrochemical Oxidative Cyclization of N-Allylcarboxamides: Efficient Synthesis of Halogenated Oxazolines. New J. Chem. 2022, 46, 663–667. [Google Scholar] [CrossRef]
- Yu, J.; Yang, H.; Fu, H. Transition Metal-Free Trifluoromethylation of N-Allylamides with Sodium Trifluoromethanesulfinate: Synthesis of Trifluoromethyl-Containing Oxazolines. Adv. Synth. Catal. 2014, 356, 3669–3675. [Google Scholar] [CrossRef]
- Chen, S.-J.; Zhong, W.-Q.; Huang, J.-M. Electrochemical Trifluoromethylation and Sulfonylation of N-Allylamides: Synthesis of Oxazoline Derivatives. J. Org. Chem. 2023, 88, 12630–12640. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Zhou, S.; He, G.; Zhou, Y. Electrochemical Oxidative Cyclization of N-Allylamides for the Synthesis of CF3-Containing Benzoxazines and Oxazolines. RSC Adv. 2024, 14, 154–159. [Google Scholar] [CrossRef]
- Noto, N.; Miyazawa, K.; Koike, T.; Akita, M. Anti-Diastereoselective Synthesis of CF3-Containing Spirooxazolines and Spirooxazines via Regiospecific Trifluoromethylative Spirocyclization by Photoredox Catalysis. Org. Lett. 2015, 17, 3710–3713. [Google Scholar] [CrossRef]
- Kawamura, S.; Sekine, D.; Sodeoka, M. Synthesis of CF3-Containing Oxazolines via Trifluoromethylation of Allylamides with Togni Reagent in the Presence of Alkali Metal Iodides. J. Fluor. Chem. 2017, 203, 115–121. [Google Scholar] [CrossRef]
- Han, J.; Gao, F.; Chen, L.; He, Z.; Lin, Y.; Ye, K. Cobalt-Catalyzed Oxytrifluoromethylation of N-Allylamides toward Trifluoromethyl-Containing Oxazolines. Asian J. Org. Chem. 2024, 13, e202300579. [Google Scholar] [CrossRef]
- Chen, Z.; He, M.; Zheng, H.; Weng, W.; Sun, L.; Xie, X. Silver-Mediated Radical Cascade Cyclization of N-Allylamides with Sodium Sulfinates to Access Sulfonated Oxazolines. New J. Chem. 2023, 47, 12487–12491. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, H.; Zhou, S.-F.; Cui, X. Photoredox-Catalyzed Synthesis of Sulfonated Oxazolines from N-Allylamides through the Insertion of Sulfur Dioxide. Org. Chem. Front. 2022, 9, 364–369. [Google Scholar] [CrossRef]
- Yu, J.; Tian, H.; Gao, C.; Yang, H.; Jiang, Y.; Fu, H. Boron-Catalyzed Arylthiooxygenation of N-Allylamides: Synthesis of (Arylsulfanyl) Oxazolines. Synlett 2015, 26, 676–680. [Google Scholar] [CrossRef]
- Nagao, Y.; Hiroya, K. Improved Synthetic Method for 5-[(Phenylthio)Methyl]Oxazoline Derivatives: Electrophilic Cyclization of Allylic Amide Using a Brønsted Acid and Tetrabutylammonium Chloride under Mild Conditions. Synlett 2020, 31, 813–817. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Mitani, N.; Yanagi, R.; Kashimura, S.; Suga, S.; Yoshida, J.; Matsumoto, K. Synthesis of Oxazolines Form N-Allylamides Using an Electrochemically Generated Ras(ArSS)Ar+ Pool. Heterocycles 2018, 96, 1373–1382. [Google Scholar] [CrossRef]
- Lu, F.; Xu, J.; Li, H.; Wang, K.; Ouyang, D.; Sun, L.; Huang, M.; Jiang, J.; Hu, J.; Alhumade, H.; et al. Electrochemical Oxidative Radical Cascade Cyclization of Olefinic Amides and Thiophenols towards the Synthesis of Sulfurated Benzoxazines, Oxazolines and Iminoisobenzofurans. Green Chem. 2021, 23, 7982–7986. [Google Scholar] [CrossRef]
- Senadi, G.C.; Guo, B.-C.; Hu, W.-P.; Wang, J.-J. Iodine-Promoted Cyclization of N-Propynyl Amides and N-Allyl Amides via Sulfonylation and Sulfenylation. Chem. Commun. 2016, 52, 11410–11413. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Li, R.; You, Q.; Fu, W.; Guo, W. Synthesis of SCF3-Containing Benzoxazines and Oxazolines via a Photoredox-Catalyzed Radical Trifluoromethylthiolation-Cyclization of Olefinic Amides. Asian J. Org. Chem. 2019, 8, 2002–2005. [Google Scholar] [CrossRef]
- Mallick, S.; Baidya, M.; Mahanty, K.; Maiti, D.; De Sarkar, S. Electrochemical Chalcogenation of β,γ-Unsaturated Amides and Oximes to Corresponding Oxazolines and Isoxazolines. Adv. Synth. Catal. 2020, 362, 1046–1052. [Google Scholar] [CrossRef]
- Alzaidi, O.; Wirth, T. Continuous Flow Electroselenocyclization of Allylamides and Unsaturated Oximes to Selenofunctionalized Oxazolines and Isoxazolines. ACS Org. Inorg. Au 2024, 4, 350–355. [Google Scholar] [CrossRef]
- Wang, P.-F.; Yi, W.; Ling, Y.; Ming, L.; Liu, G.-Q.; Zhao, Y. Preparation of Selenofunctionalized Heterocycles via Iodosobenzene-Mediated Intramolecular Selenocyclizations of Olefins with Diselenides. Chin. Chem. Lett. 2021, 32, 2587–2591. [Google Scholar] [CrossRef]
- Moon, N.G.; Harned, A.M. Iodine(III)-Promoted Synthesis of Oxazolines from N-Allylamides. Tetrahedron Lett. 2013, 54, 2960–2963. [Google Scholar] [CrossRef]
- Ranjith, J.; Rajesh, N.; Sridhar, B.; Radha Krishna, P. Intramolecular Oxyacetoxylation of N-Allylamides: An Expeditious Synthesis of Oxazolines and Oxazines by Using a PhI(OAc)2/Hydrogen Fluoride–Pyridine System. Org. Biomol. Chem. 2016, 14, 10074–10079. [Google Scholar] [CrossRef]
- Abazid, A.H.; Hollwedel, T.-N.; Nachtsheim, B.J. Stereoselective Oxidative Cyclization of N-Allyl Benzamides to Oxaz(Ol)Ines. Org. Lett. 2021, 23, 5076–5080. [Google Scholar] [CrossRef] [PubMed]
- Alhalib, A.; Kamouka, S.; Moran, W.J. Iodoarene-Catalyzed Cyclizations of Unsaturated Amides. Org. Lett. 2015, 17, 1453–1456. [Google Scholar] [CrossRef]
- Butt, S.E.; Das, M.; Sotiropoulos, J.-M.; Moran, W.J. Computationally Assisted Mechanistic Investigation into Hypervalent Iodine Catalysis: Cyclization of N-Allylbenzamide. J. Org. Chem. 2019, 84, 15605–15613. [Google Scholar] [CrossRef]
- Wata, C.; Hashimoto, T. Organoiodine-Catalyzed Enantioselective Intramolecular Oxy aminations of Alkenes with N-(Fluorosulfonyl) Carbamate. Synthesis 2021, 53, 2594–2601. [Google Scholar] [CrossRef]
- Jeon, H.; Kim, D.; Lee, J.H.; Song, J.; Lee, W.S.; Kang, D.W.; Kang, S.; Lee, S.B.; Choi, S.; Hong, K.B. Hypervalent Iodine-Mediated Alkene Functionalization: Oxazoline and Thiazoline Synthesis via Inter-/Intramolecular Aminohydroxylation and Thioamination. Adv. Synth. Catal. 2018, 360, 779–783. [Google Scholar] [CrossRef]
- Yan, Z.; Sun, B.; Huang, P.; Zhao, H.; Ding, H.; Su, W.; Jin, C. Visible-Light-Promoted Radical Alkylation/Cyclization of Allylic Amide with N-Hydroxyphthalimide Ester: Synthesis of Oxazolines. Chin. Chem. Lett. 2022, 33, 1997–2000. [Google Scholar] [CrossRef]
- Wang, N.; Chen, B.; Ma, S. A Practical Synthesis of Chiral Oxazolines through a Highly Diastereoselective Coupling–Cyclization Reaction of N-(Buta-2,3-dienyl)Amides and Aryl Iodides. Asian J. Org. Chem. 2014, 3, 723–730. [Google Scholar] [CrossRef]
- Dorel, R.; Echavarren, A.M. Gold(I)-Catalyzed Activation of Alkynes for the Construction of Molecular Complexity. Chem. Rev. 2015, 115, 9028–9072. [Google Scholar] [CrossRef]
- Zuccarello, G.; Escofet, I.; Caniparoli, U.; Echavarren, A.M. New-Generation Ligand Design for the Gold-Catalyzed Asymmetric Activation of Alkynes. ChemPlusChem 2021, 86, 1283–1296. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Weyrauch, J.P.; Frey, W.; Bats, J.W. Gold Catalysis: Mild Conditions for the Synthesis of Oxazoles from N-Propargylcarboxamides and Mechanistic Aspects. Org. Lett. 2004, 6, 4391–4394. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Schuster, A.M.; Rominger, F. Gold Catalysis: Isolation of Vinylgold Complexes Derived from Alkynes. Angew. Chem. Int. Ed. 2009, 48, 8247–8249. [Google Scholar] [CrossRef] [PubMed]
- Sargentoni, N.; Galassi, R.; Luciani, L.; Rominger, F.; Rudolph, M.; Hashmi, A.S.K. Oxidation State and Halogen Influence on the NHC-Gold-Halide-Catalyzed Cyclization of Propargylic Amides. Adv. Synth. Catal. 2024, 366, 5108–5122. [Google Scholar] [CrossRef]
- Cao, Z.; Bassani, D.M.; Bibal, B. Photoreduction of Thioether Gold (III) Complexes: Mechanistic Insight and Homogeneous Catalysis. Chem. Eur. J. 2018, 24, 18779–18787. [Google Scholar] [CrossRef]
- Pérez-Sánchez, J.C.; Herrera, R.P.; Concepción Gimeno, M. Ferrocenyl Dinuclear Gold (I) Complexes. Study of Their Structural Features and the Influence of Bridging and Phosphane Ligands in a Catalytic Cyclization Reaction. Chem. Eur. J. 2024, 30, e202303585. [Google Scholar] [CrossRef]
- Ali, H.S.; Hussein, A.A.; Obies, M. Impact of Counteranions on N-Heterocyclic Carbene Gold (I)-Catalyzed Cyclization of Propargylic Amide. RSC Adv. 2023, 13, 2896–2902. [Google Scholar] [CrossRef]
- Ma, Y.; Ali, H.S.; Hussein, A.A. A Mechanistic Study on the Gold (I)-Catalyzed Cyclization of Propargylic Amide: Revealing the Impact of Expanded-Ring N-Heterocyclic Carbenes. Catal. Sci. Technol. 2022, 12, 674–685. [Google Scholar] [CrossRef]
- Hettmanczyk, L.; Schulze, D.; Suntrup, L.; Sarkar, B. Mono- and Digold (I) Complexes with Mesoionic Carbenes: Structural Characterization and Use in Catalytic Silver-Free Oxazoline Formation. Organometallics 2016, 35, 3828–3836. [Google Scholar] [CrossRef]
- Zhao, X.; Tian, B.; Yang, Y.; Si, X.; Mulks, F.F.; Rudolph, M.; Rominger, F.; Hashmi, A.S.K. Gold-Catalyzed Stereoselective Domino Cyclization/Alkynylation of N-Propargylcarboxamides with Benziodoxole Reagents for the Synthesis of Alkynyloxazolines. Adv. Synth. Catal. 2019, 361, 3155–3162. [Google Scholar] [CrossRef]
- Dong, G.; Bao, M.; Xie, X.; Jia, S.; Hu, W.; Xu, X. Asymmetric Allylation by Chiral Organocatalyst-Promoted Formal Hetero-Ene Reactions of Alkylgold Intermediates. Angew. Chem. Int. Ed. 2021, 60, 1992–1999. [Google Scholar] [CrossRef]
- Yuan, H.; Bao, M.; Chen, K.; Huang, J.; Pan, Y.; Gryko, D.; Xu, X. Cocatalyst-Dependent Divergent Amination of Alkylgold Intermediates with Azodicarboxylates. ACS Catal. 2025, 15, 5211–5218. [Google Scholar] [CrossRef]
- Wong, V.H.L.; White, A.J.P.; Hor, T.S.; Hii, K.K. Silver-Catalyzed Cyclization of Propargylic Amides to Oxazolines. Adv. Synth. Catal. 2015, 357, 3943–3948. [Google Scholar] [CrossRef]
- Wong, V.H.L.; Vummaleti, S.V.C.; Cavallo, L.; White, A.J.P.; Nolan, S.P.; Hii, K.K. Synthesis, Structure and Catalytic Activity of NHC–AgI Carboxylate Complexes. Chem. Eur. J. 2016, 22, 13320–13327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, T.; Li, X.; Lv, A.; Li, X.; Wang, Z.; Wang, R.; Ma, Y.; Fang, R.; Szostak, R.; et al. Thiazol-2-Ylidenes as N-Heterocyclic Carbene Ligands with Enhanced Electrophilicity for Transition Metal Catalysis. Commun. Chem. 2022, 5, 60. [Google Scholar] [CrossRef]
- Sinai, Á.; Vangel, D.; Gáti, T.; Bombicz, P.; Novák, Z. Utilization of Copper-Catalyzed Carboarylation–Ring Closure for the Synthesis of New Oxazoline Derivatives. Org. Lett. 2015, 17, 4136–4139. [Google Scholar] [CrossRef]
- Wang, J.; Niu, J.-Q.; Li, J.-C.; Liu, L.; Shang, X.; Zhou, P.-X.; Chao, S. Synthesis of SeCF3-Substituted Oxazines and Oxazolines through Electrophilic Trifluoromethylselenolation Cyclization of Propargylic Amides. J. Org. Chem. 2025, 90, 8353–8365. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, N.; Krause, N. Efficient and Stereoselective Synthesis of (Z)-2-Oxazolines by Transition Metal-Free Cycloisomerization of Internal Propargylic Amides in Hexafluoroisopropanol (HFIP). Asian J. Org. Chem. 2025, 14, e202500262. [Google Scholar] [CrossRef]
- Sigmund, L.M.; Assante, M.; Johansson, M.J.; Norrby, P.-O.; Jorner, K.; Kabeshov, M. Computational Tools for the Prediction of Site- and Regioselectivity of Organic Reactions. Chem. Sci. 2025, 16, 5383–5412. [Google Scholar] [CrossRef] [PubMed]

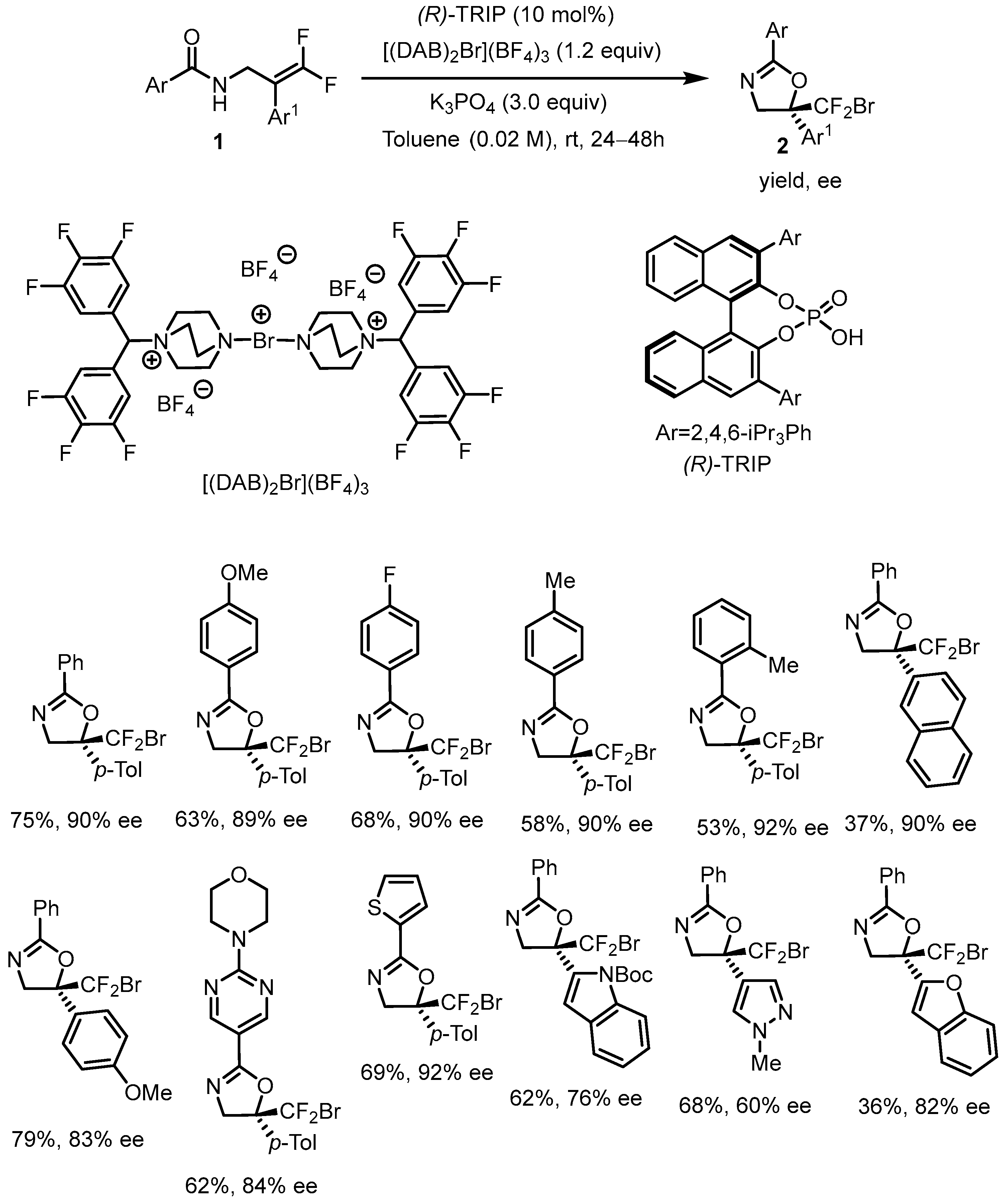
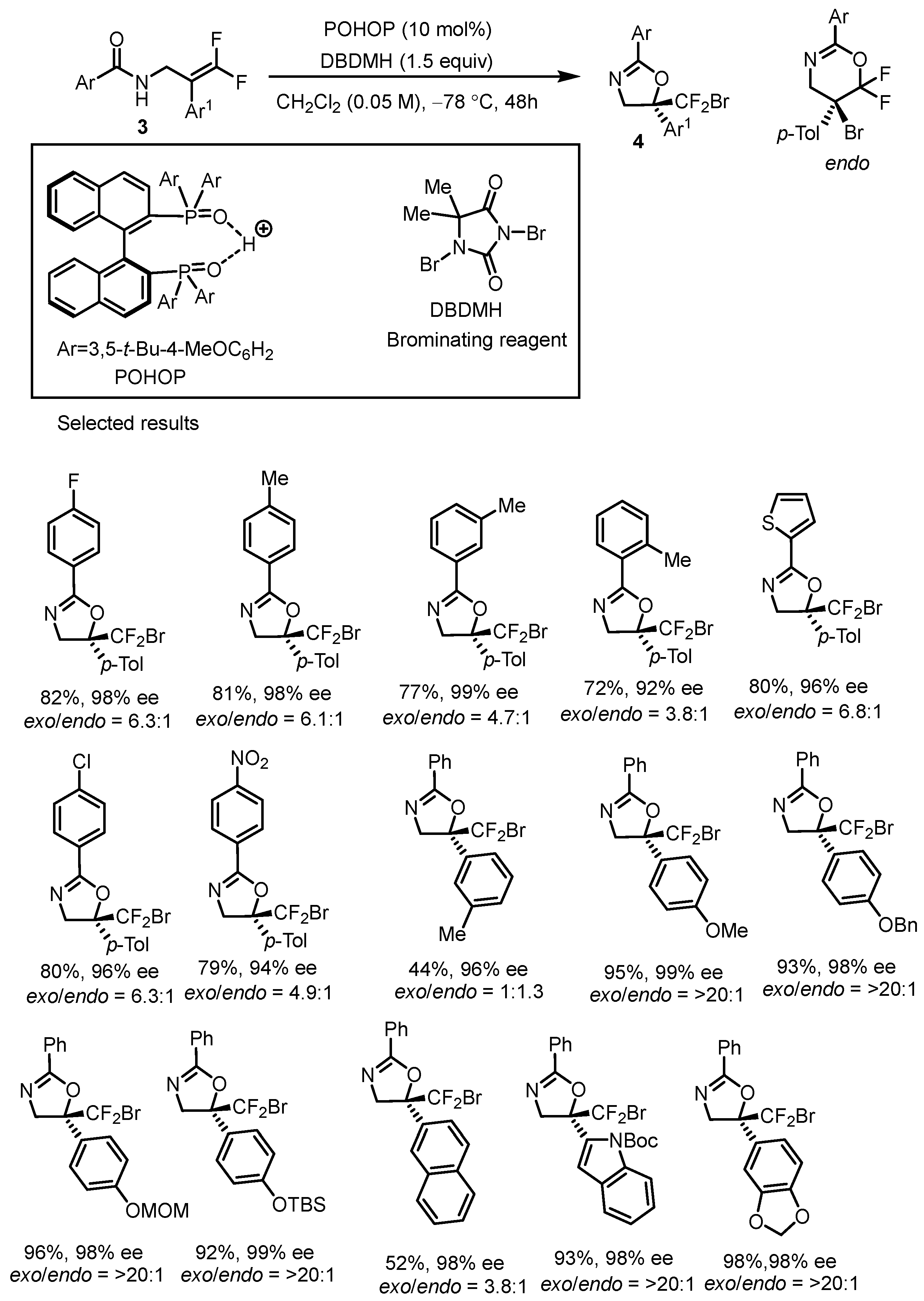

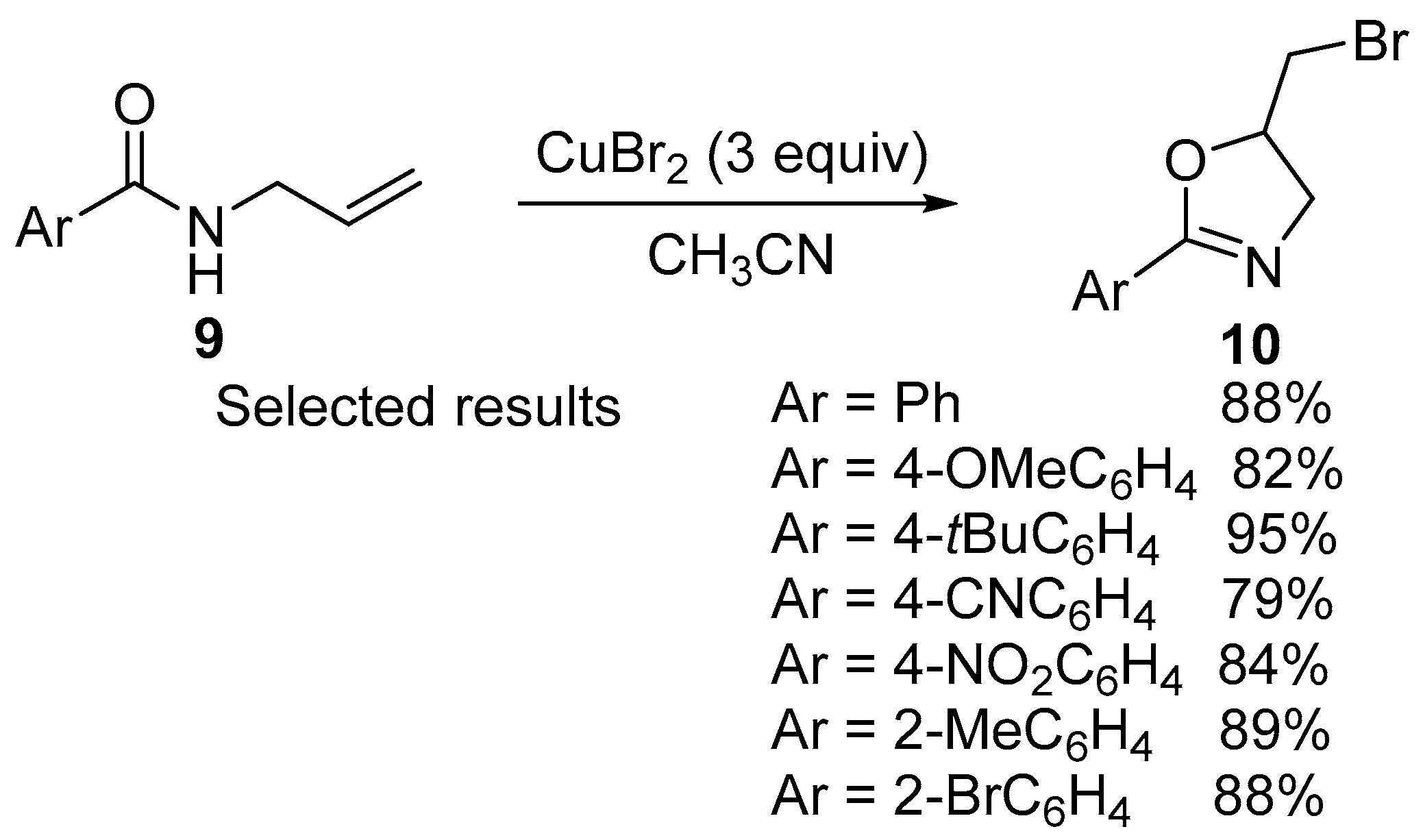

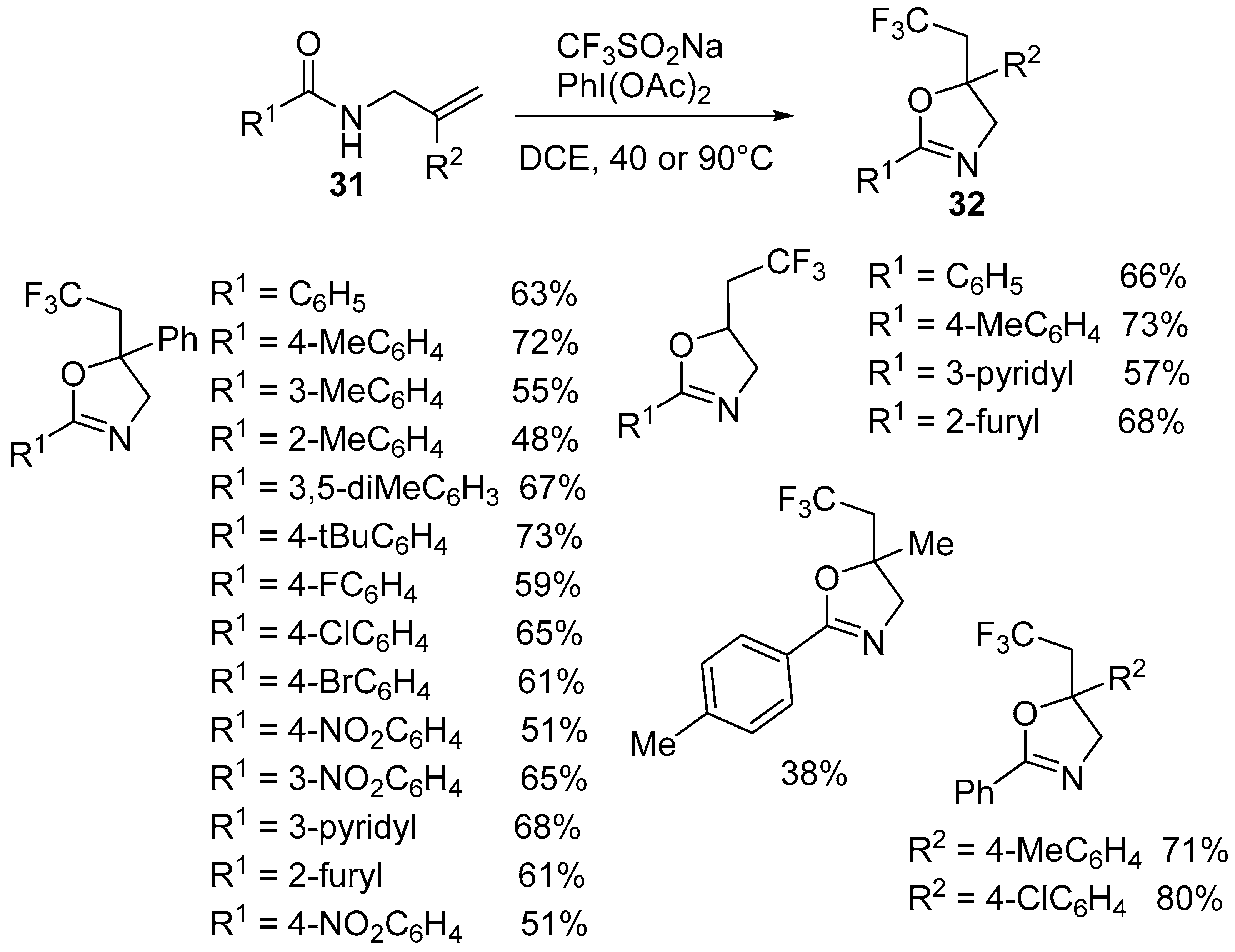

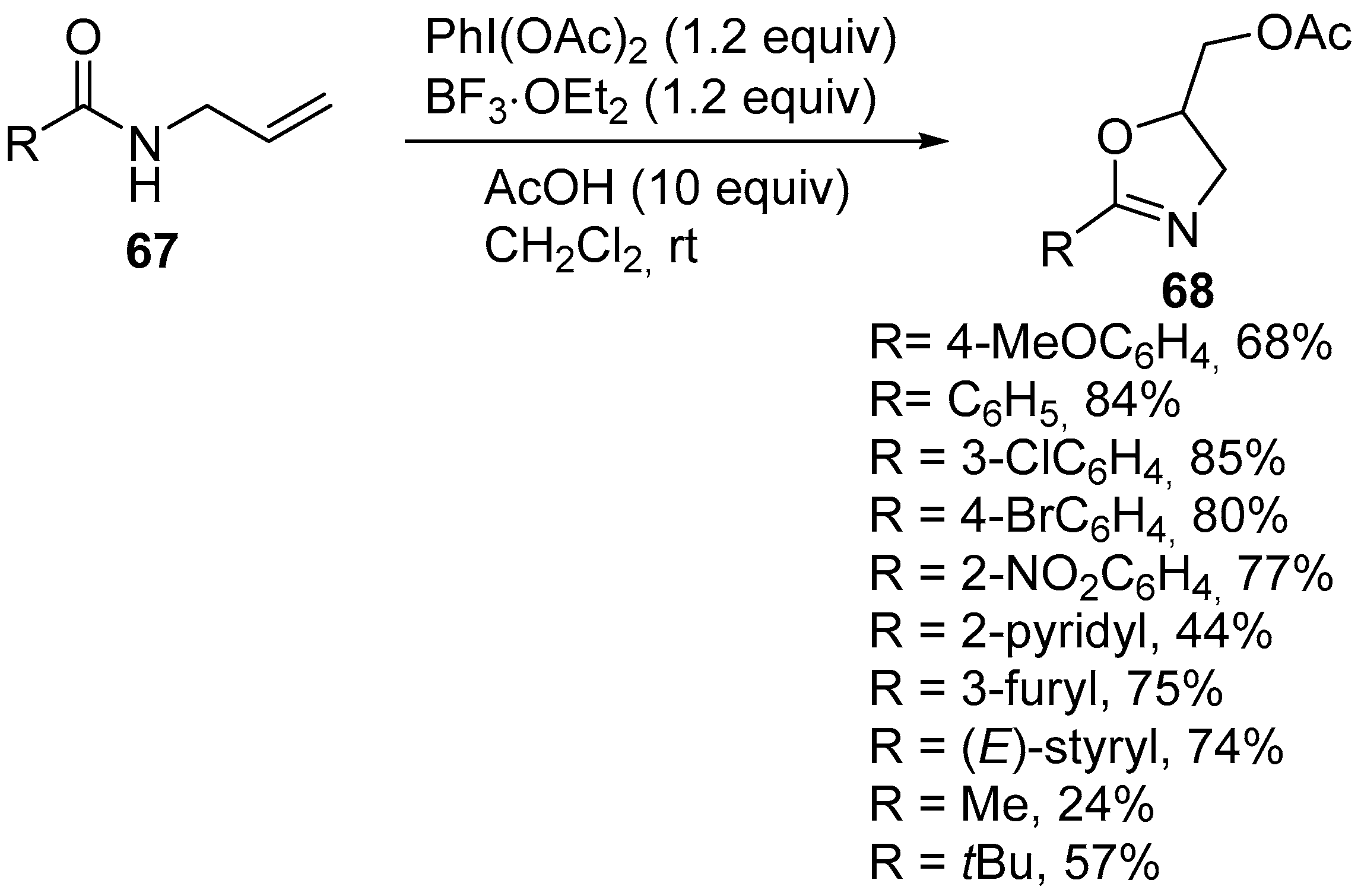


| Toste Method | Hirokawa Method | |
|---|---|---|
| Brominating reagent | [(DAB)2Br](BF4)3 | DBDMH |
| Brominating reagent loading | 1.2 equiv. | 1.5 equiv. |
| Phase-transfer catalyst | (R)-TRIP | POHOP |
| Phase-transfer cat. loading | 10 mol% | 10 mol% |
| Solvent | Toluene | Dichloromethane |
| Temperature | rt | −78 °C |
| Base | K3PO4 | - |
| Yield | 33–79% | 44–98% |
| ee | 60–92% | 92–99% |
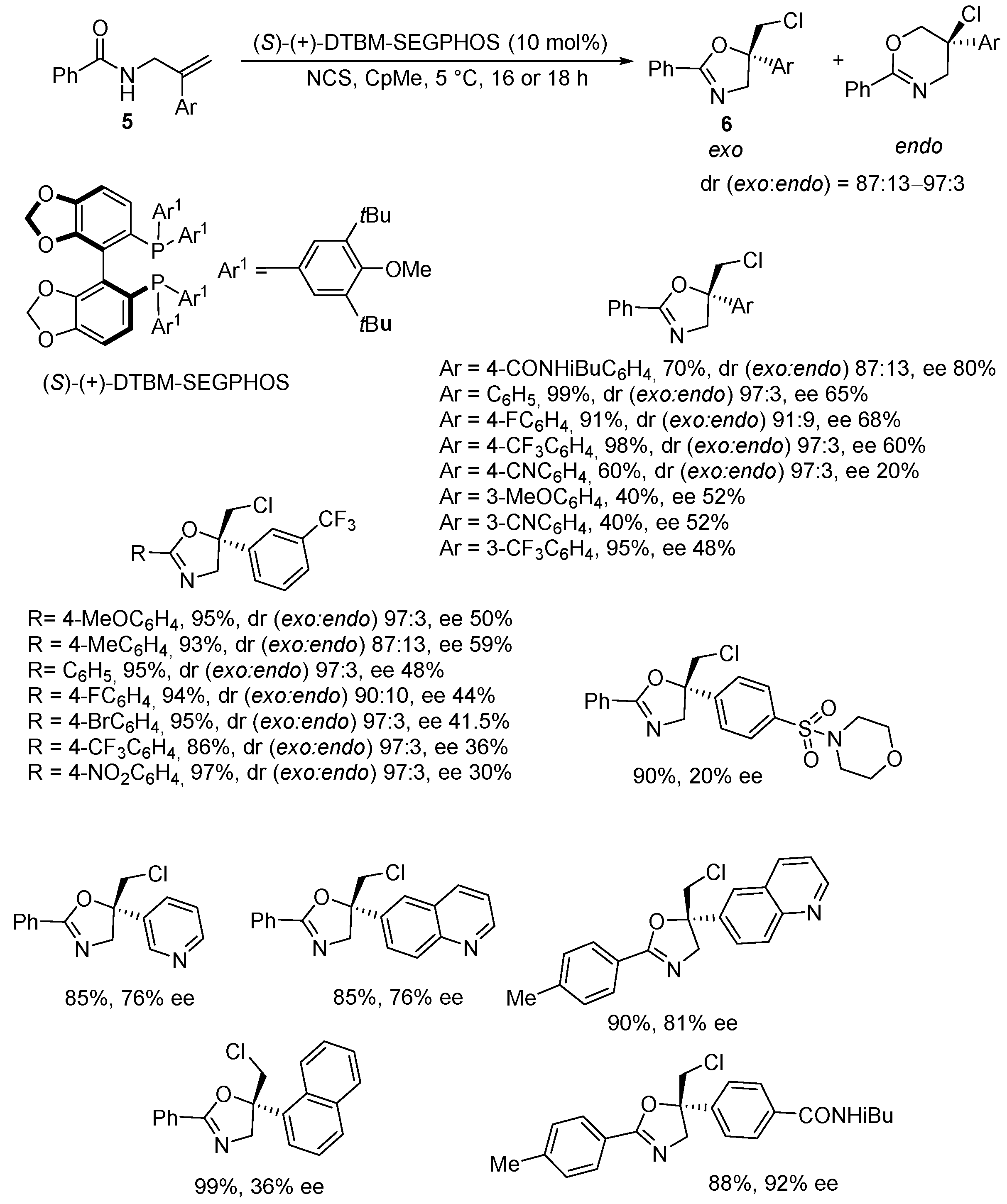
| Rassias Chlorocyclization | Hamashima Bromocyclization | |
|---|---|---|
| Halogenating reagent | NCS | NBS |
| Halogenating reagent loading | 1.3 equiv. | 1.2 equiv. |
| Organocatalyst | (S)-(+)-DTBM-SEGPHOS | (S)-DTBM-BINAP |
| Organocatalyst loading | 10 mol% | 10 mol% |
| Solvent | CpMe | Dichloromethane |
| Temperature | 5 °C | −78 °C |
| Time | 16 or 18 h | 12–24 h |
| Yield | 40–79% | 40–99% |
| ee | 20–97% | 39–99% |
| Entry | Substrate | CF3 Source | Conditions | Product | Yield |
|---|---|---|---|---|---|
| 1 | 31 | CF3SO2Na | PhI(OAc)2, DCE 40 or 90 °C | 32 | 48–80% |
| 2 | 33 | CF3SO2Na | C(+)‖Pt(−) K2CO3 (2 equiv)) MeCN:H2O = 4.5:1 8 mA, rt, 4 h | 34 | 55–82% |
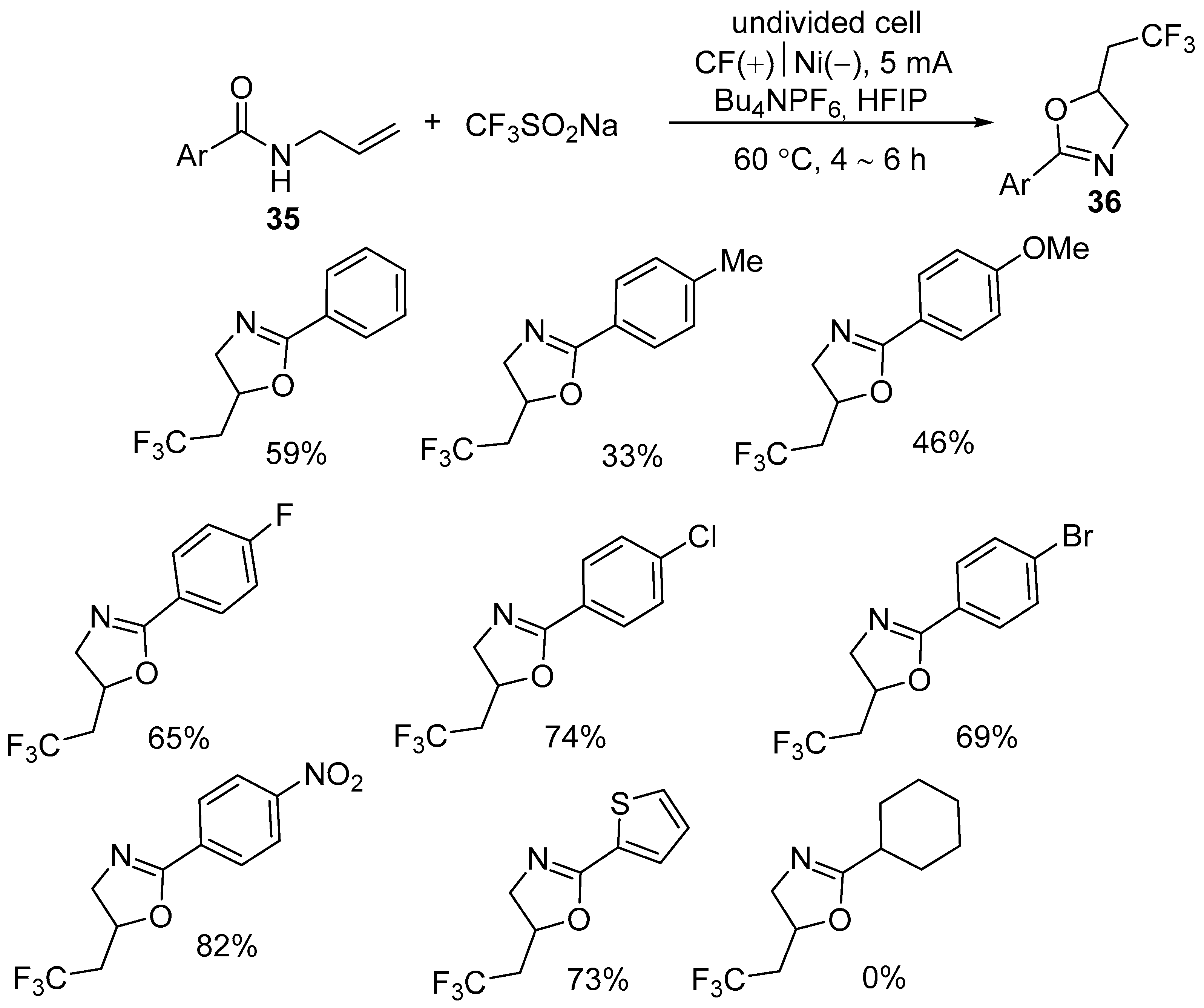
| 3 | 35 | CF3SO2Na/ CF2HSO2Na | CF(+)‖Ni(−) Bu4NPF6, HFIP 5 mA, 60 °C, 4–6 h | 36 | 33–82% |
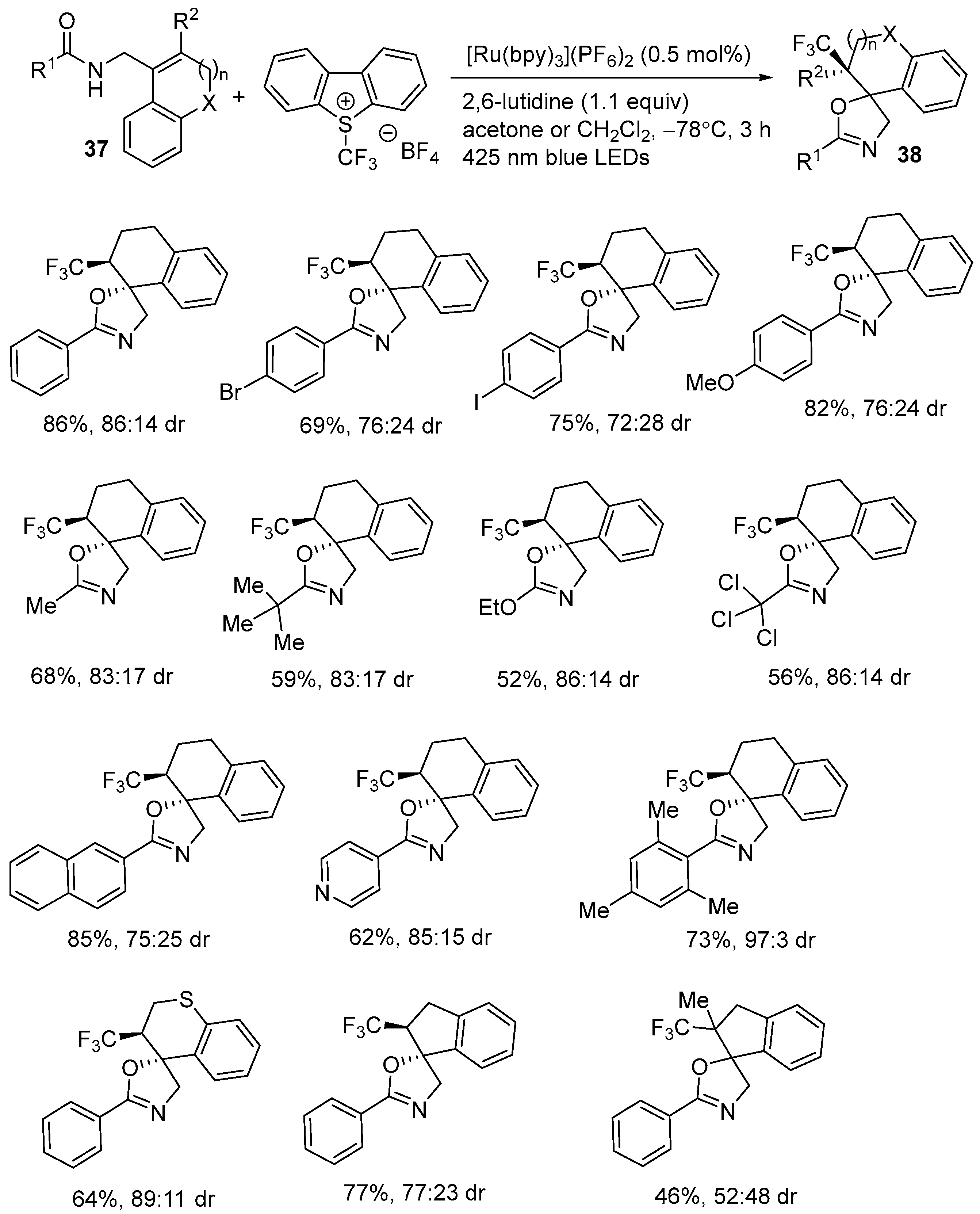
| 4 | 37 | Umemoto reagent | [Ru(bpy)3](PF6)2 (0.5 mol%) 2,6-lutidine (1.1 equiv) Acetone or DCM 425 nm blue LEDs −78 °C, 3 h | 38 | 46–86% |
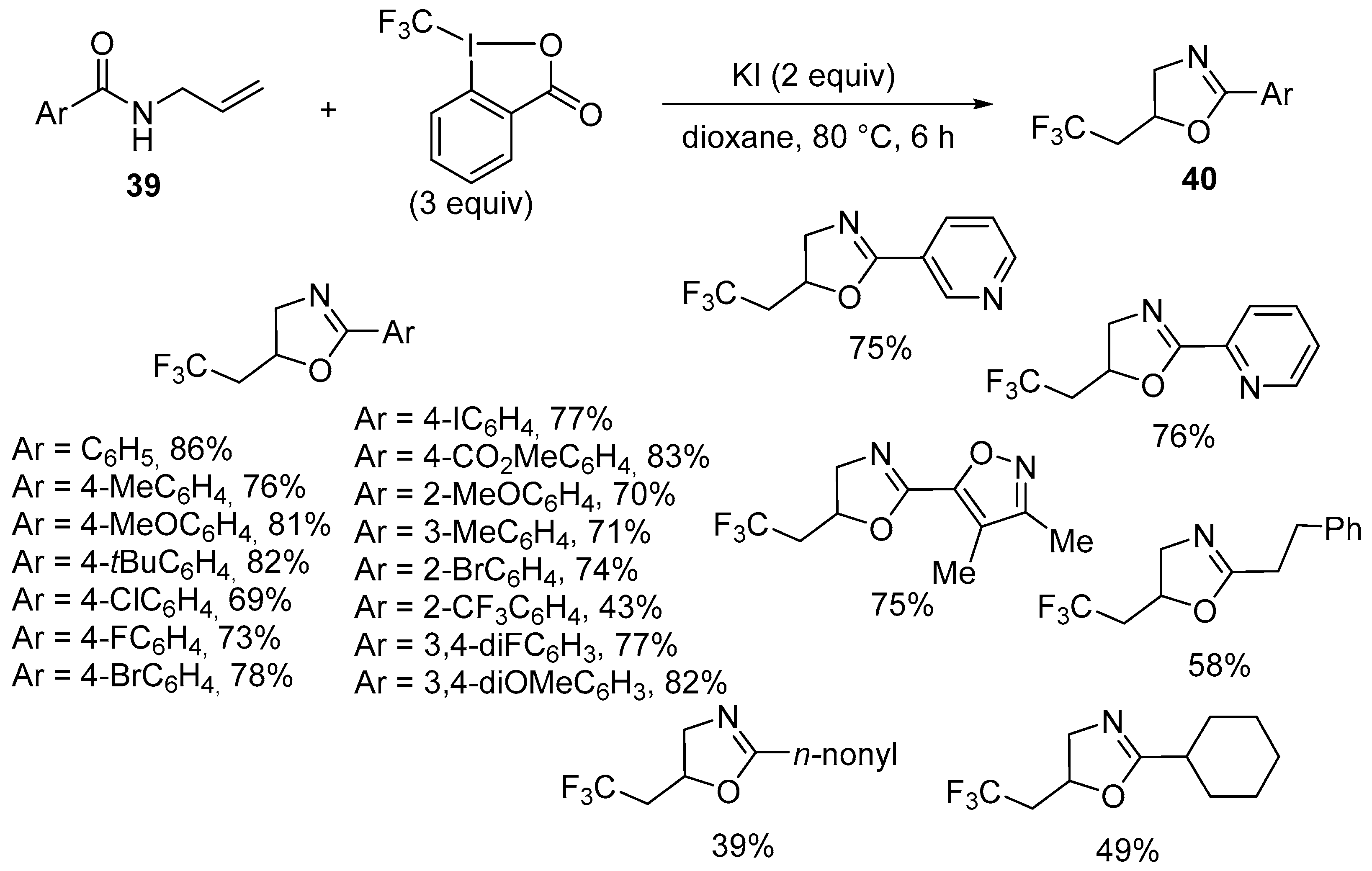
| 5 | 39 | Togni reagent | KI (2 equiv) Dioxane, 80 °C, 6 h | 40 | 39–86% |
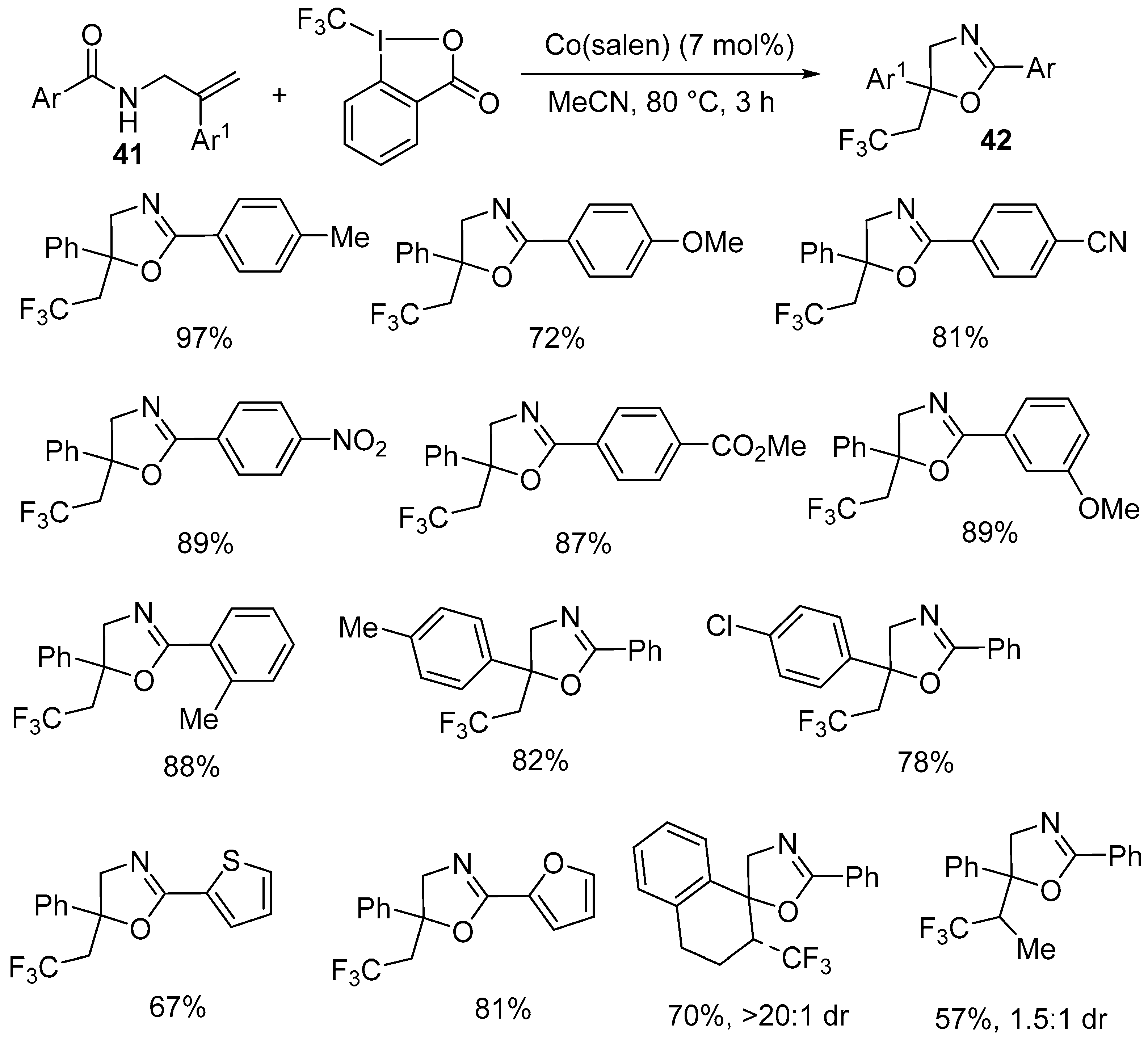
| 6 | 41 | Togni reagent | Co(salen) (7 mol%) MeCN, 80 °C, 3 h | 42 | 57–97% |
| Entry | Substrate | Sulfonyl Source | Conditions | Product | Yield |
|---|---|---|---|---|---|
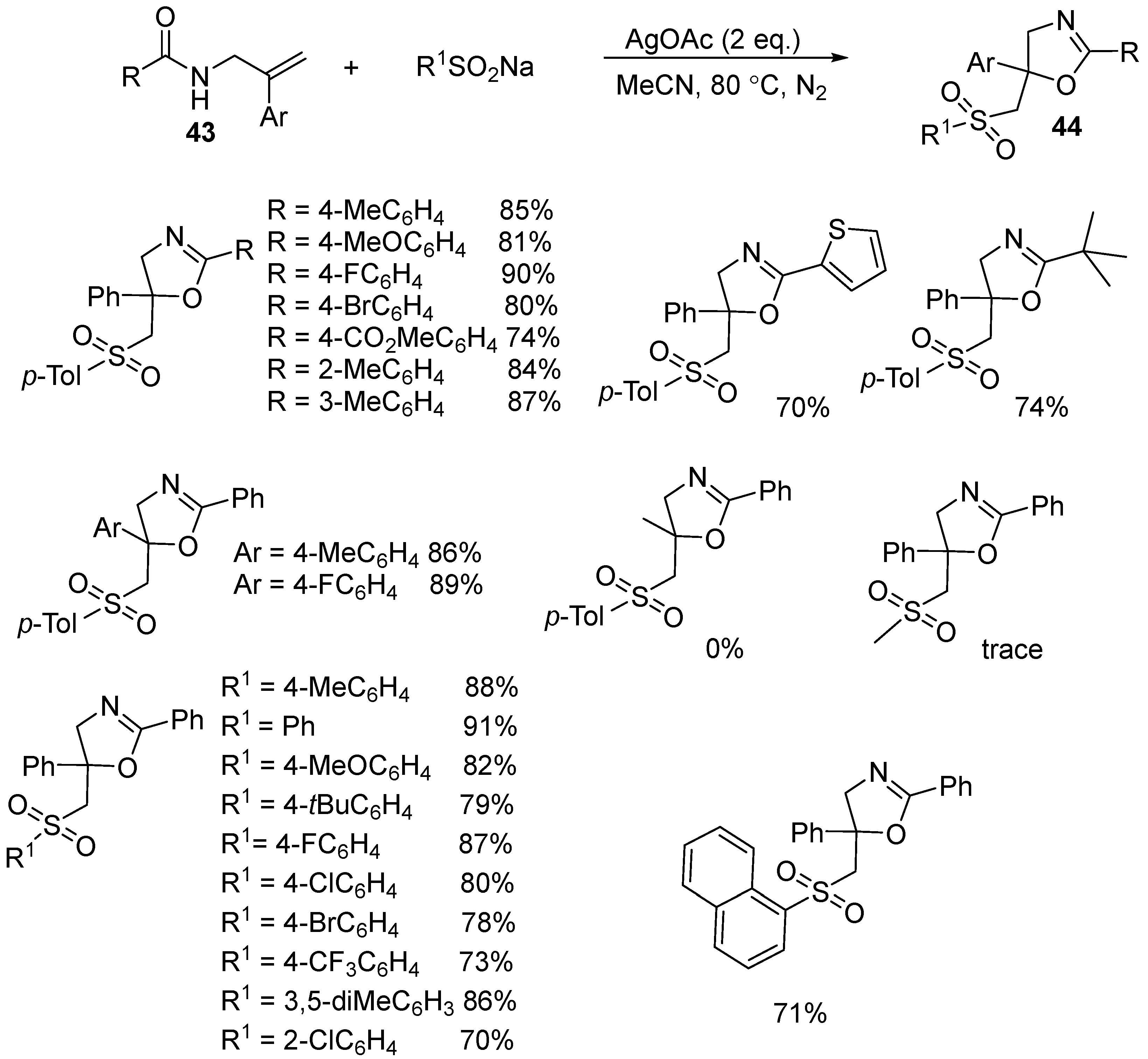
| 1 | 43 | R1SO2Na | Ag(OAc)2 (2 equiv) MeCN, 80 °C | 44 | 70–91% |
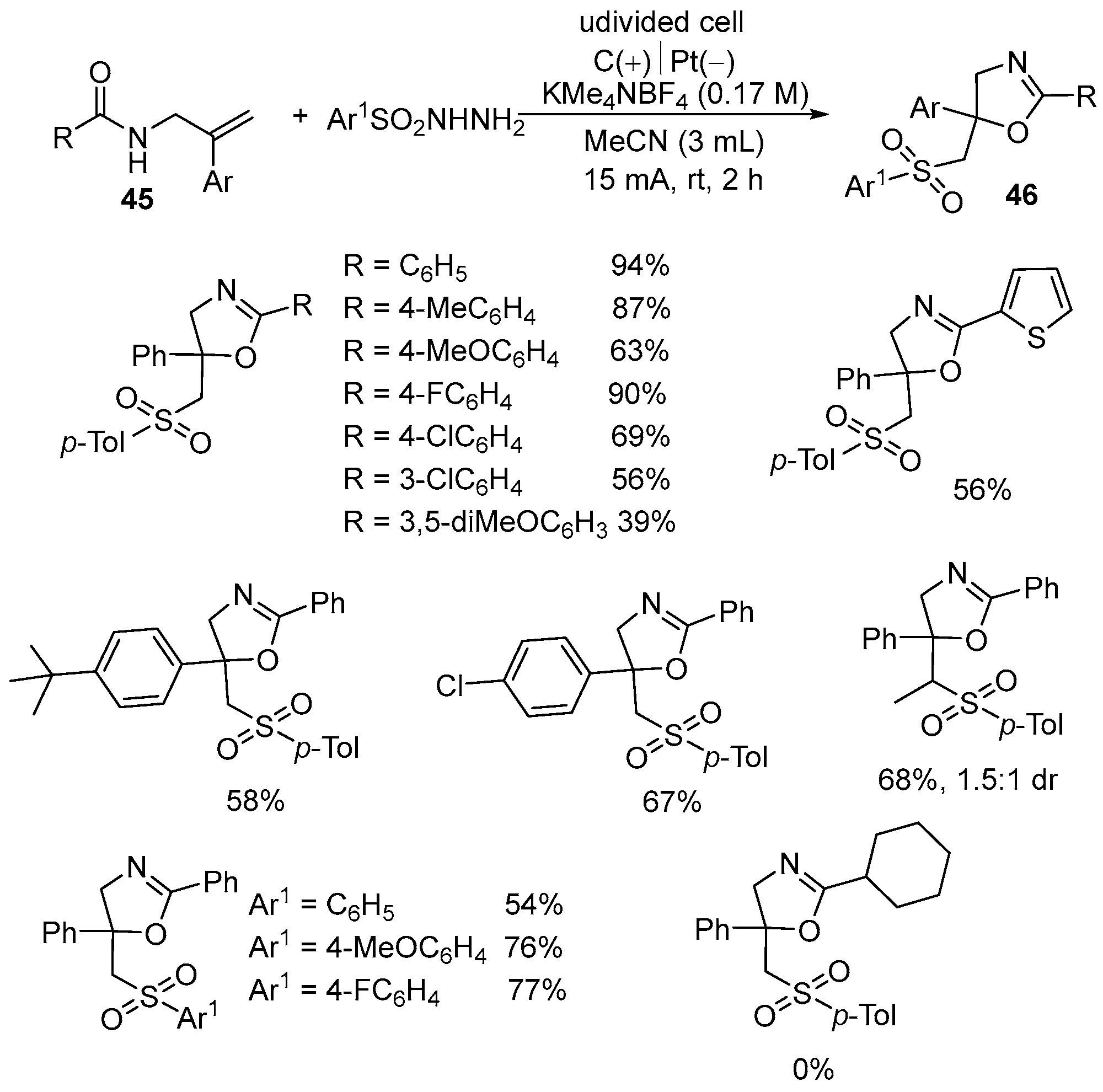
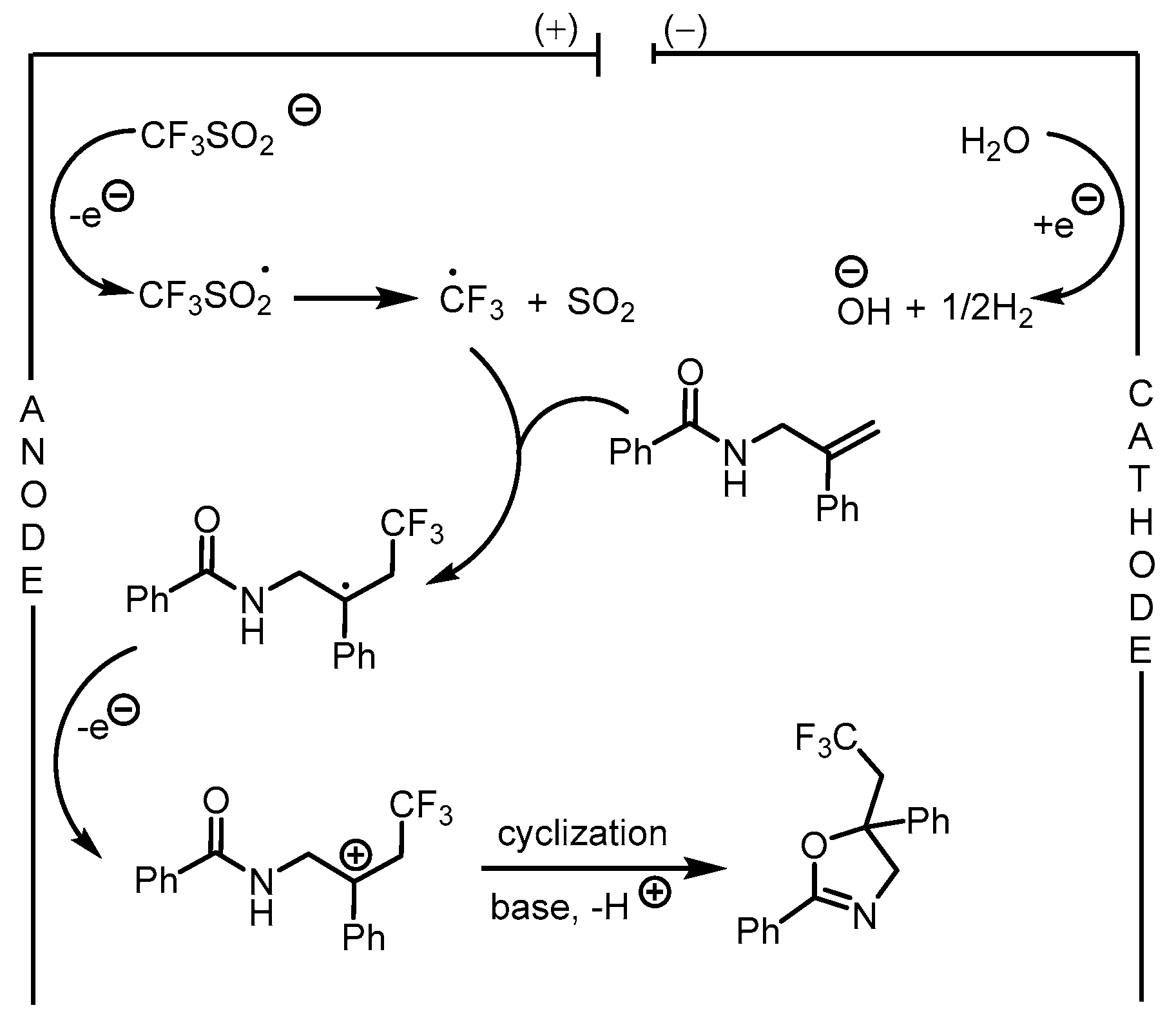
| 2 | 45 | Ar1SO2NHNH2 | C(+)‖Pt(−) KMe4NBF4 (0.17 M)) MeCN (3 mL) 15 mA, rt, 2 h | 46 | 39–94% |
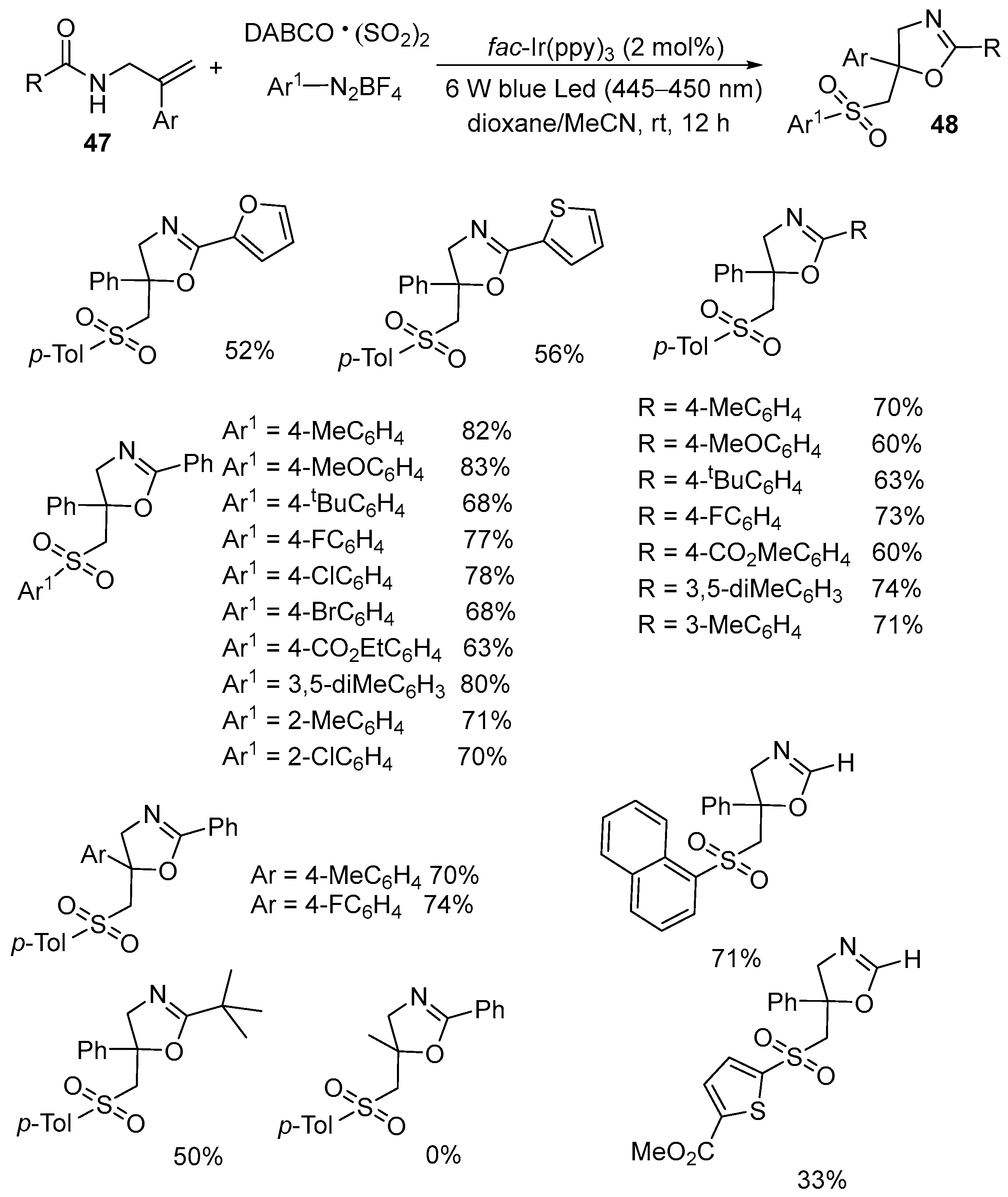
| 3 | 47 | DABCO·(SO2)2 | Ar1N2BF4 fac-Ir(ppy)3 (2 mol%) 6 W blue LEDs (445–450 nm) Dioxane/MeCN rt, 12 h | 48 | 33–82% |
| Entry | Substrate | Sulfenyl Source | Conditions | Product | Yield |
|---|---|---|---|---|---|
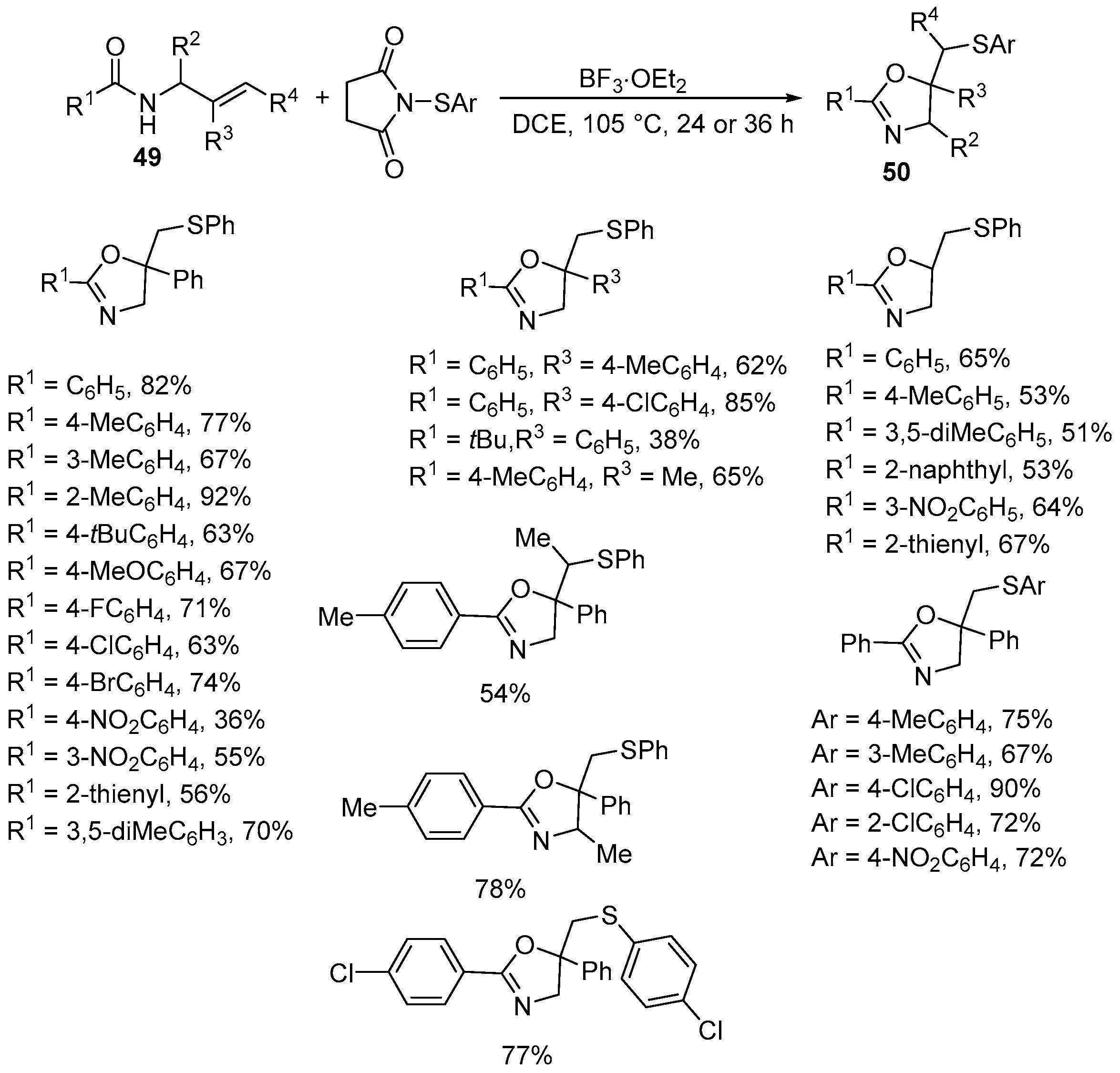
| 1 | 49 | ArS-succinimide | BF3·OEt2 DCE, 105 °C 24 or 36 h | 50 | 36–92% |
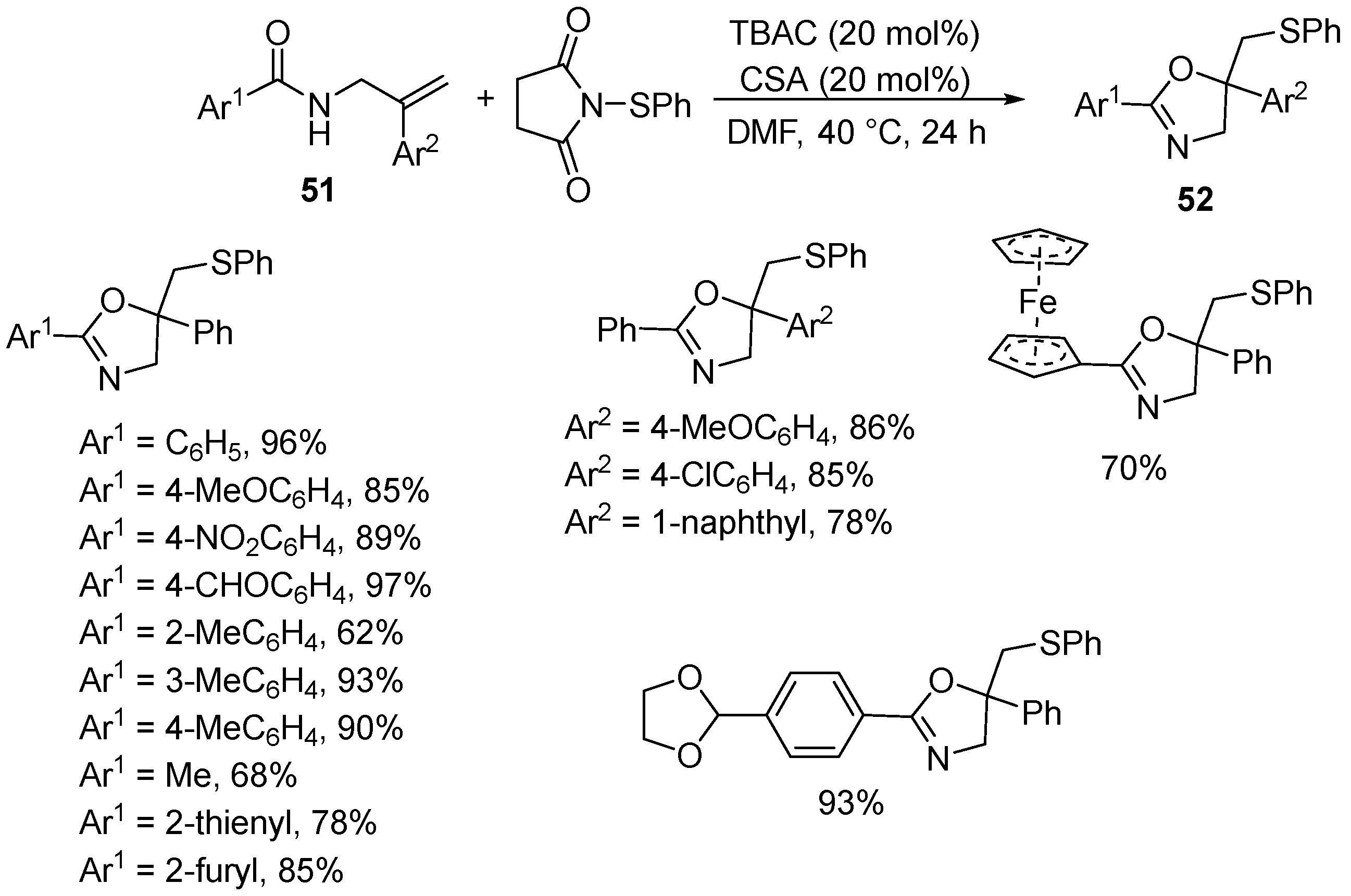
| 2 | 51 | ArS-succinimide | TBAC (20 mol%M) CSA (20 mol%) DMF, 40 °C, 24 h | 52 | 62–97% |
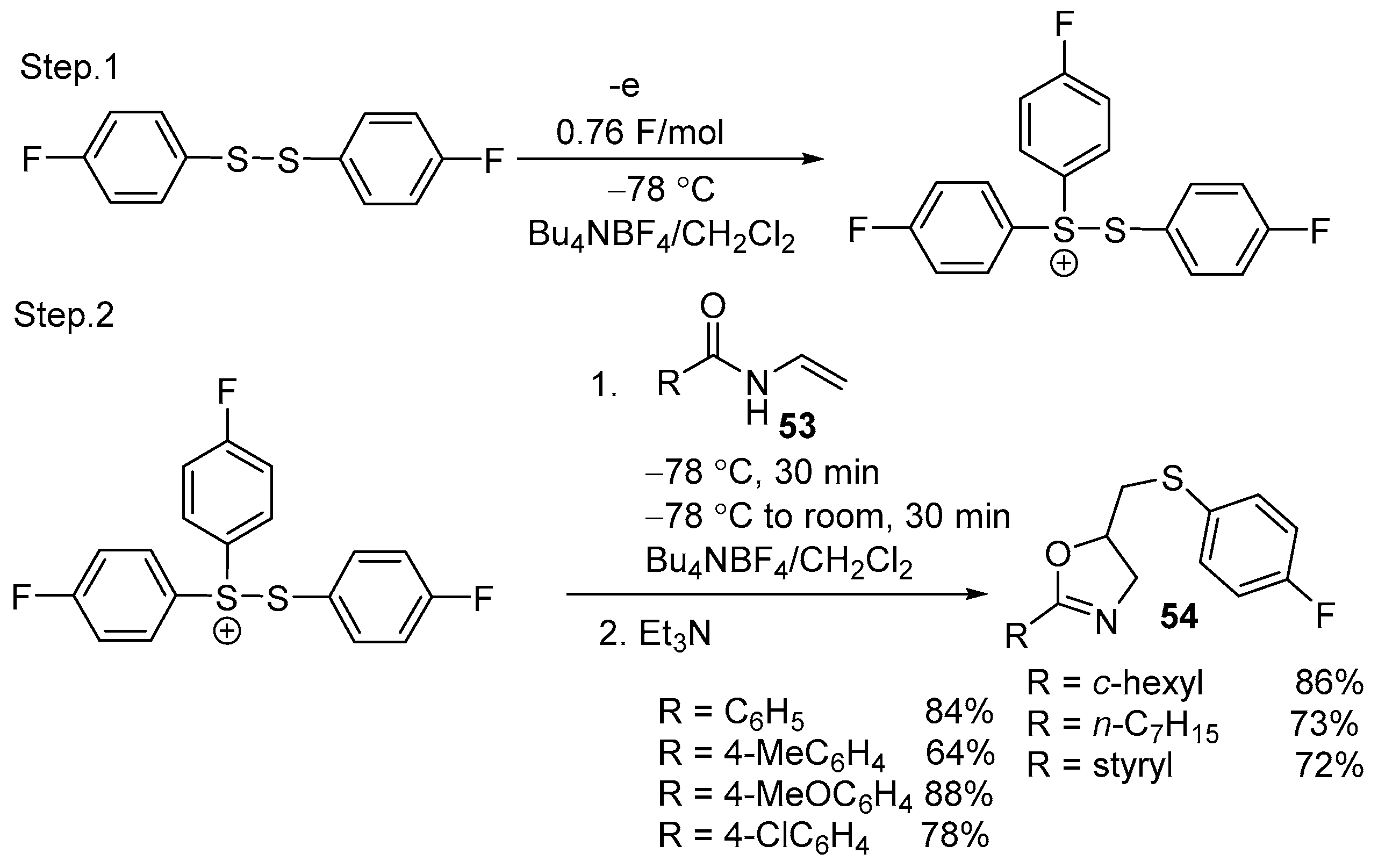
| 3 | 53 | ArSSAr | 0.76 F/mol Bu4NBF4/DCM −78 °C, 30 min. | 54 | 72–88% |
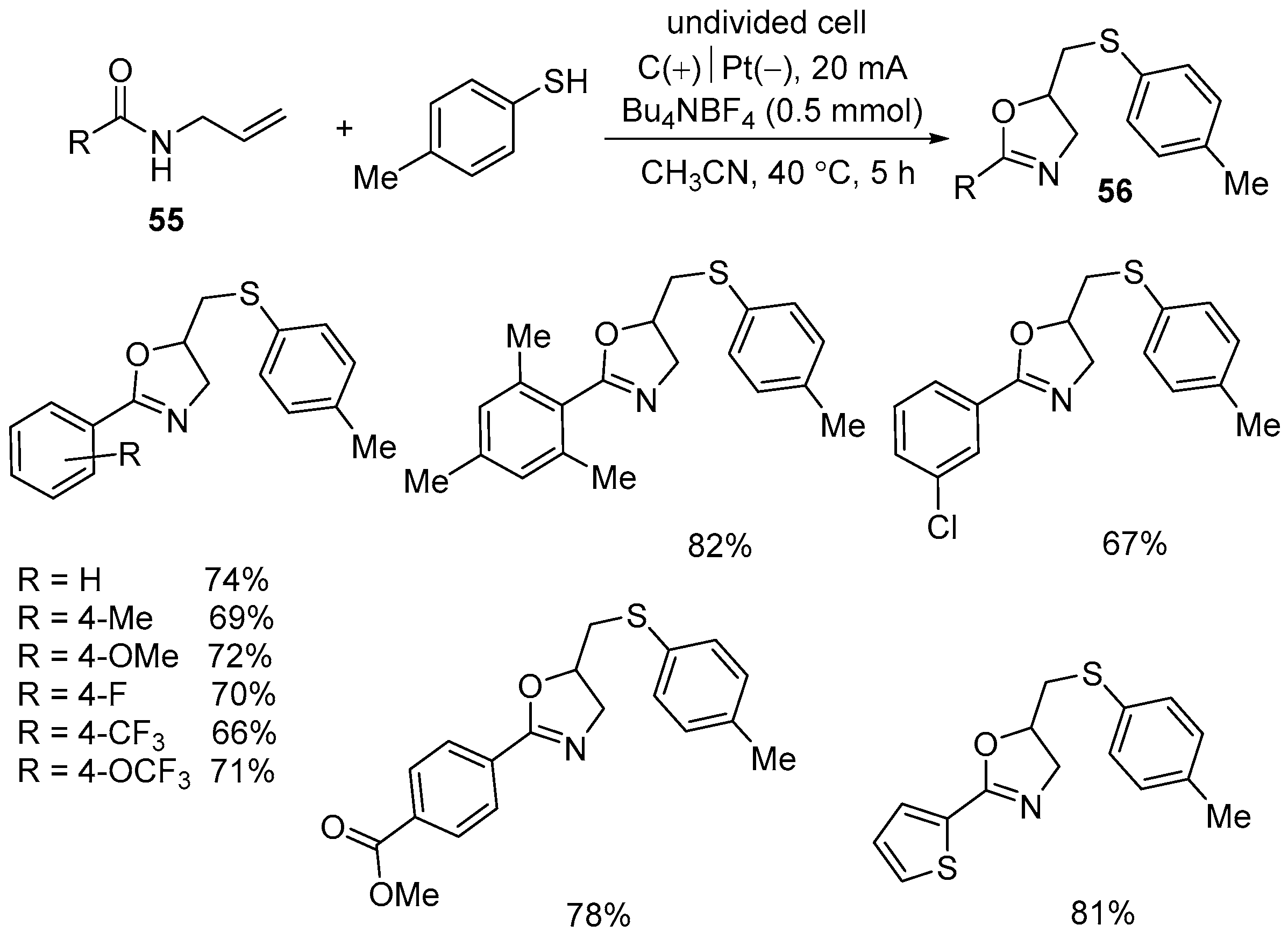
| 4 | 55 | 4-methylbenzenethiol | C(+)‖Pt(−) Bu4NBF4/MeCN 20 mA, 40 °C, 5 h | 56 | 66–82% |
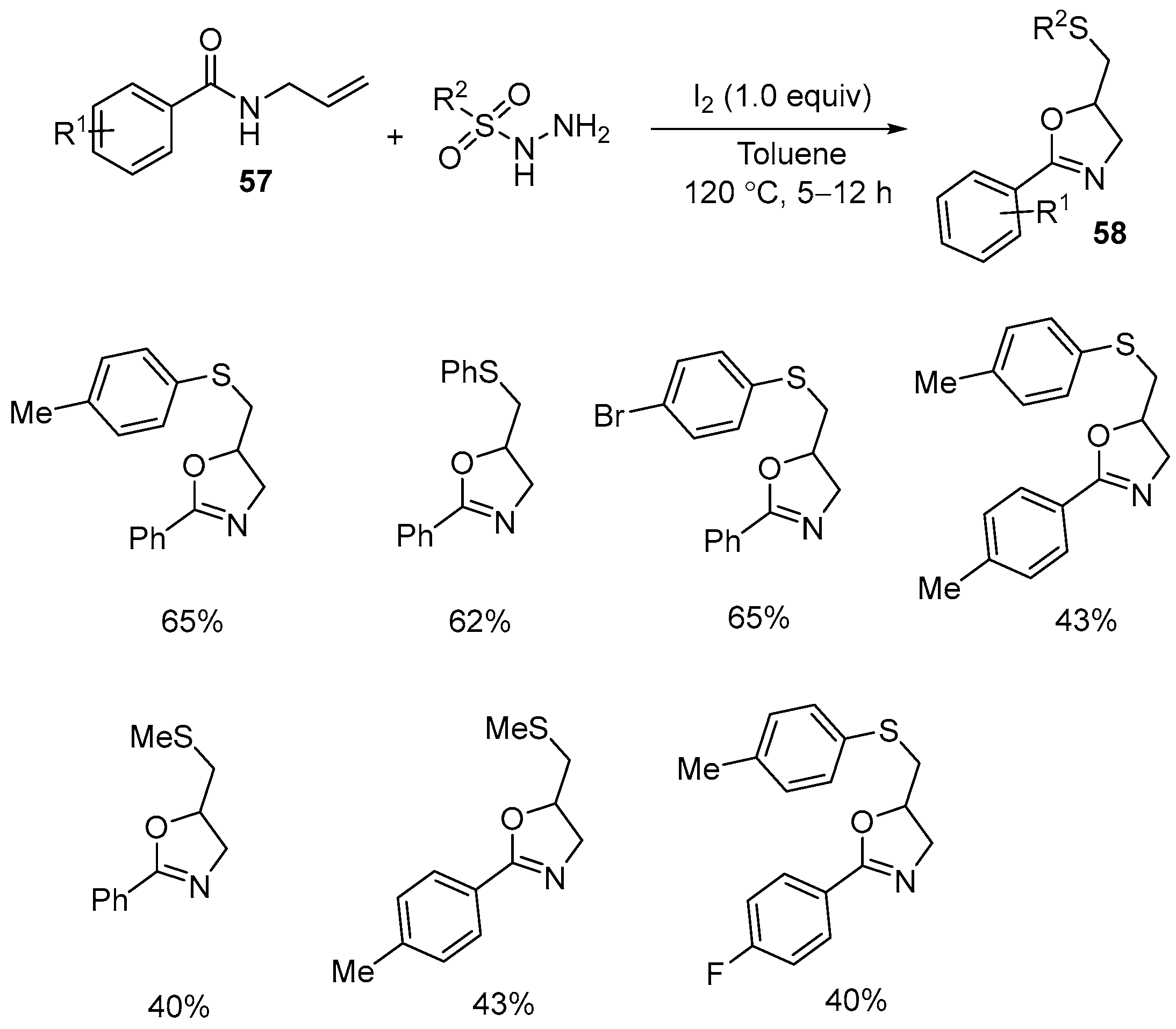
| 5 | 57 | RSO2NHNH2 | I2 (1 equiv) Toluene 120 °C, 5–12 h | 58 | 40–65% |
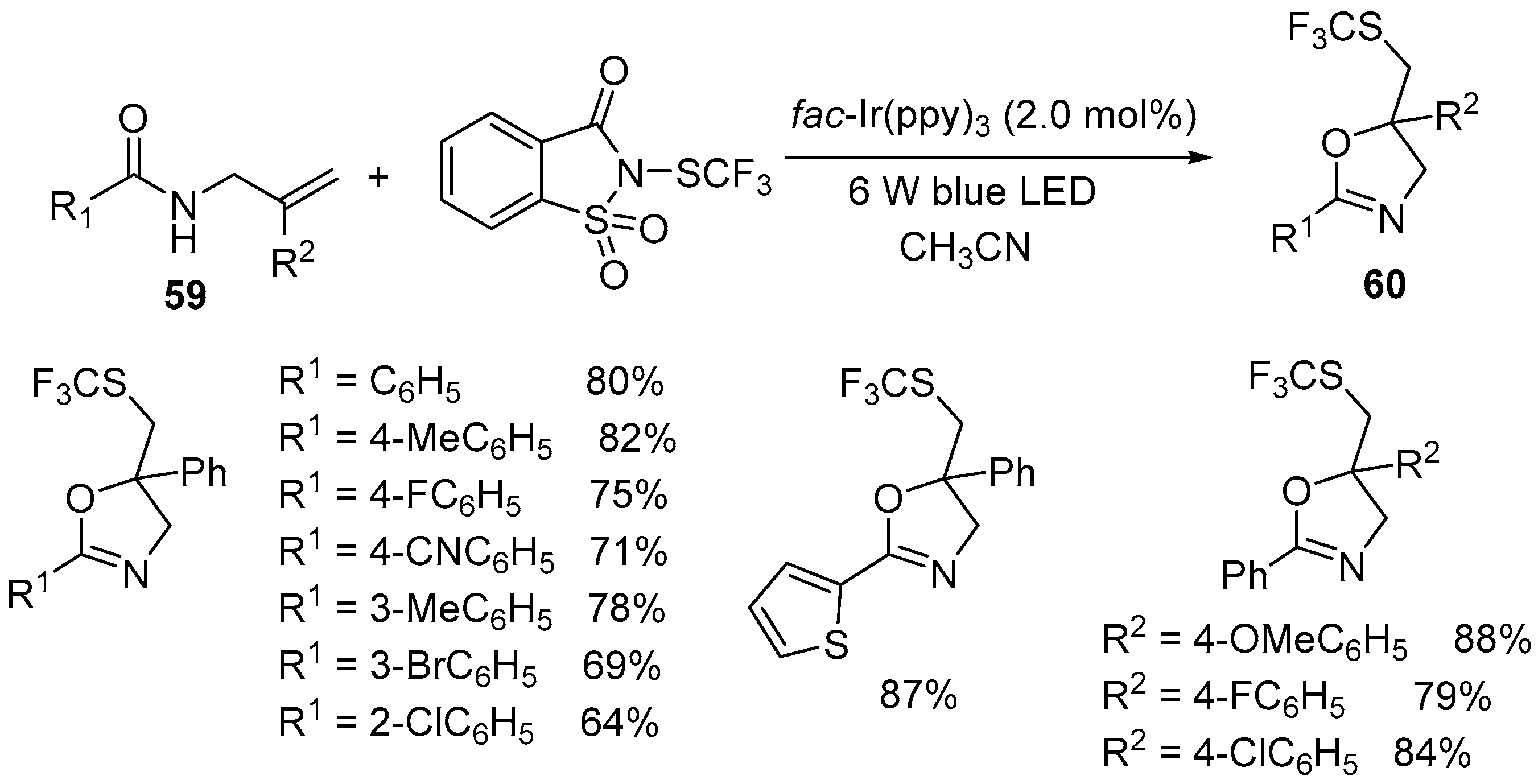
| 6 | 59 | N-trifluoromethylthiosaccharin | fac-Ir(ppy)3 (2 mol %) 6 W blue LED MeCN | 60 | 64–88% |
| Entry | Substrate | Selenyl Source | Conditions | Product | Yield |
|---|---|---|---|---|---|
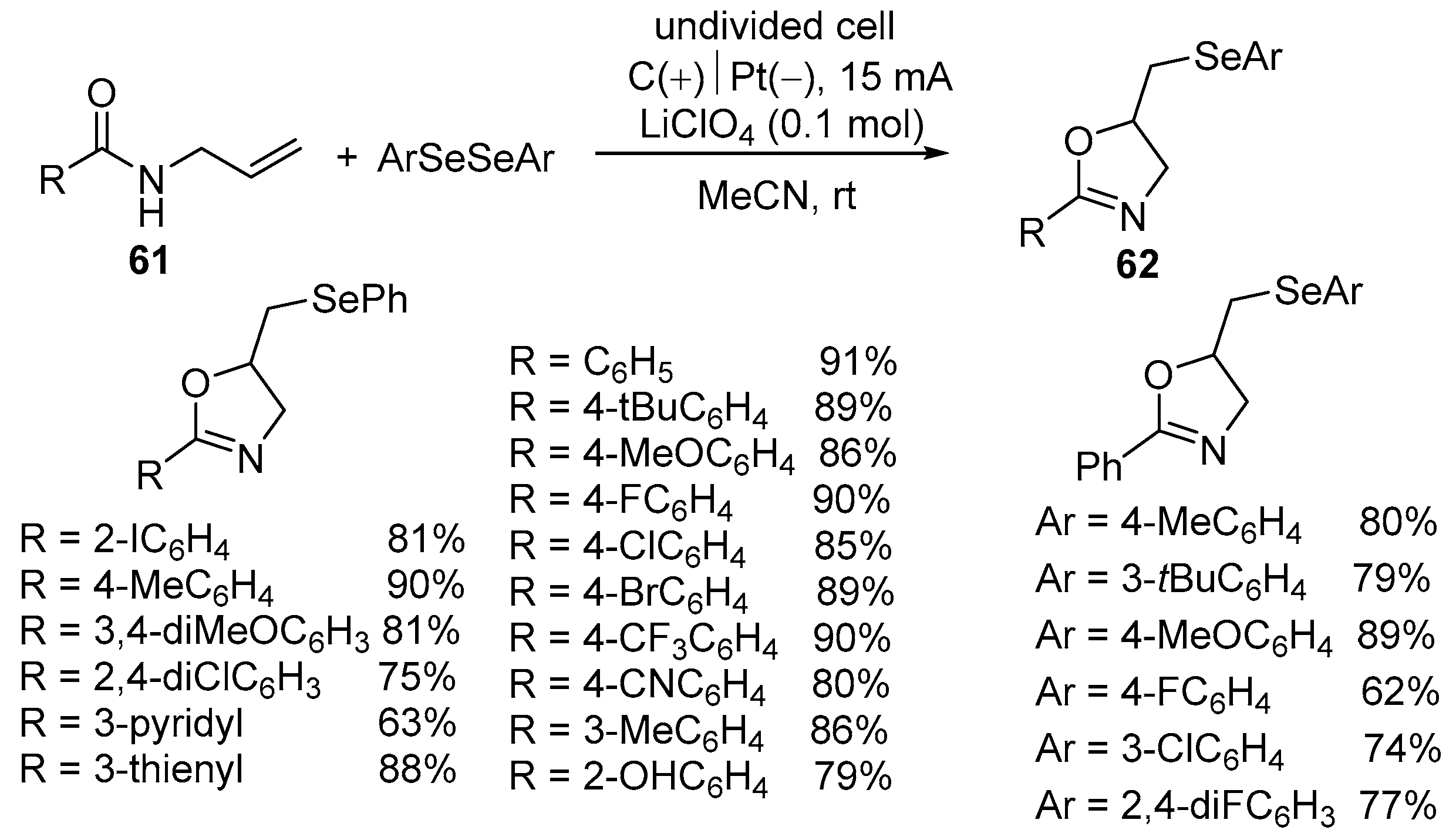
| 1 | 61 | ArSeSeAr | Undivided cell C(+)‖Pt(−) LiClO4,(0.1 mol) 15 mA, MeCN, rt | 62 | 63–91% |
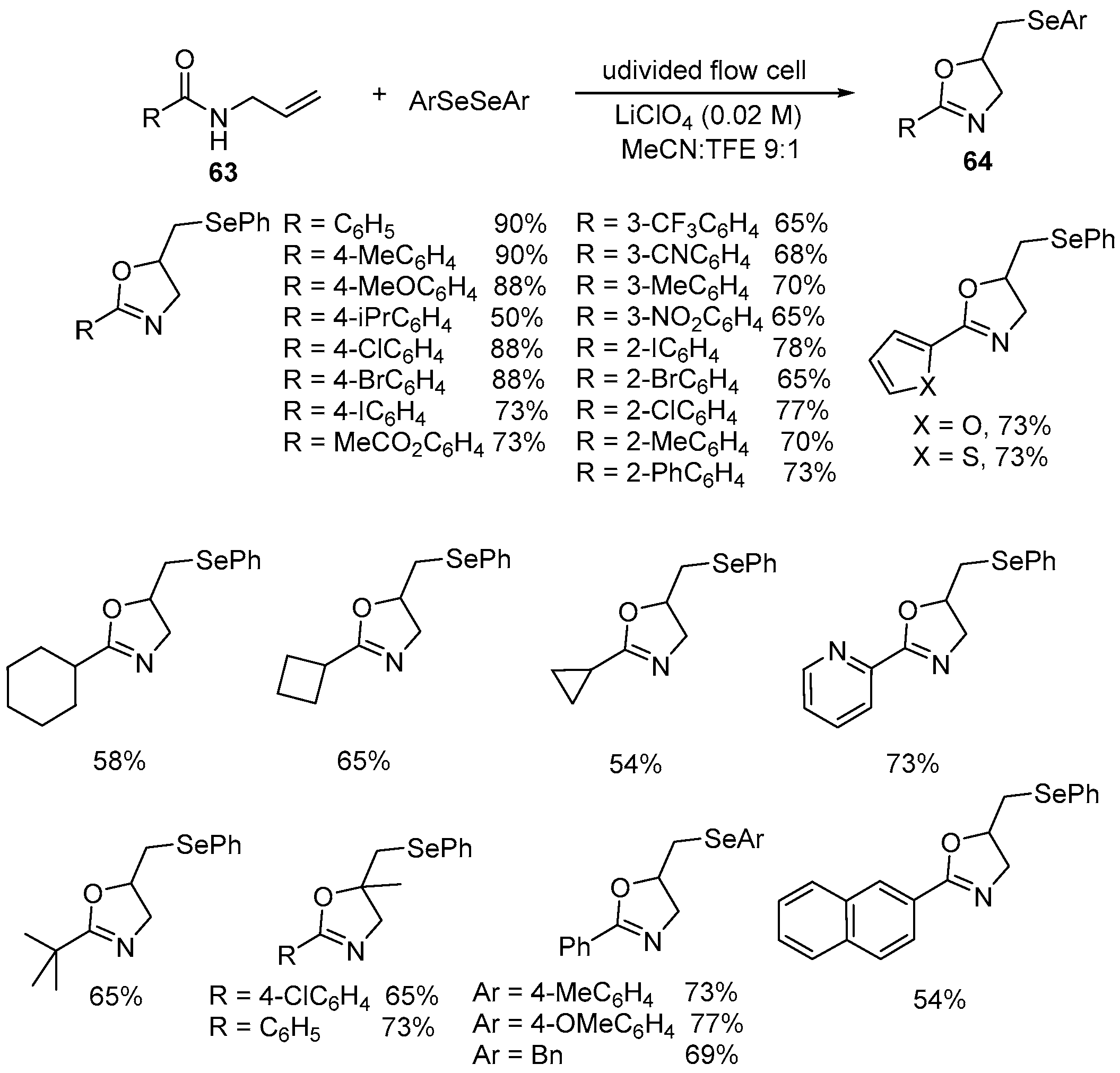
| 2 | 63 | ArSeSeAr | Undivided flaw cell Graphite electrodes LiClO4,(0.02 M) MeCN:TFE 9:1 | 64 | 50–90% |
| 3 | 65 | PhSeSePh | PhIO (1 equiv) DCM, rt | 65 | 71–90% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bojar, K.; Branowska, D.; Wolińska, E. Synthesis of 2-Oxazolines from N-Allyl and N-Propargyl Amides. Molecules 2025, 30, 4369. https://doi.org/10.3390/molecules30224369
Bojar K, Branowska D, Wolińska E. Synthesis of 2-Oxazolines from N-Allyl and N-Propargyl Amides. Molecules. 2025; 30(22):4369. https://doi.org/10.3390/molecules30224369
Chicago/Turabian StyleBojar, Karolina, Danuta Branowska, and Ewa Wolińska. 2025. "Synthesis of 2-Oxazolines from N-Allyl and N-Propargyl Amides" Molecules 30, no. 22: 4369. https://doi.org/10.3390/molecules30224369
APA StyleBojar, K., Branowska, D., & Wolińska, E. (2025). Synthesis of 2-Oxazolines from N-Allyl and N-Propargyl Amides. Molecules, 30(22), 4369. https://doi.org/10.3390/molecules30224369







