Coupling Photothermal Effect in N-Doped Hollow Carbon Spheres with ZnIn2S4 Boosts Solar Hydrogen Evolution
Abstract
1. Introduction
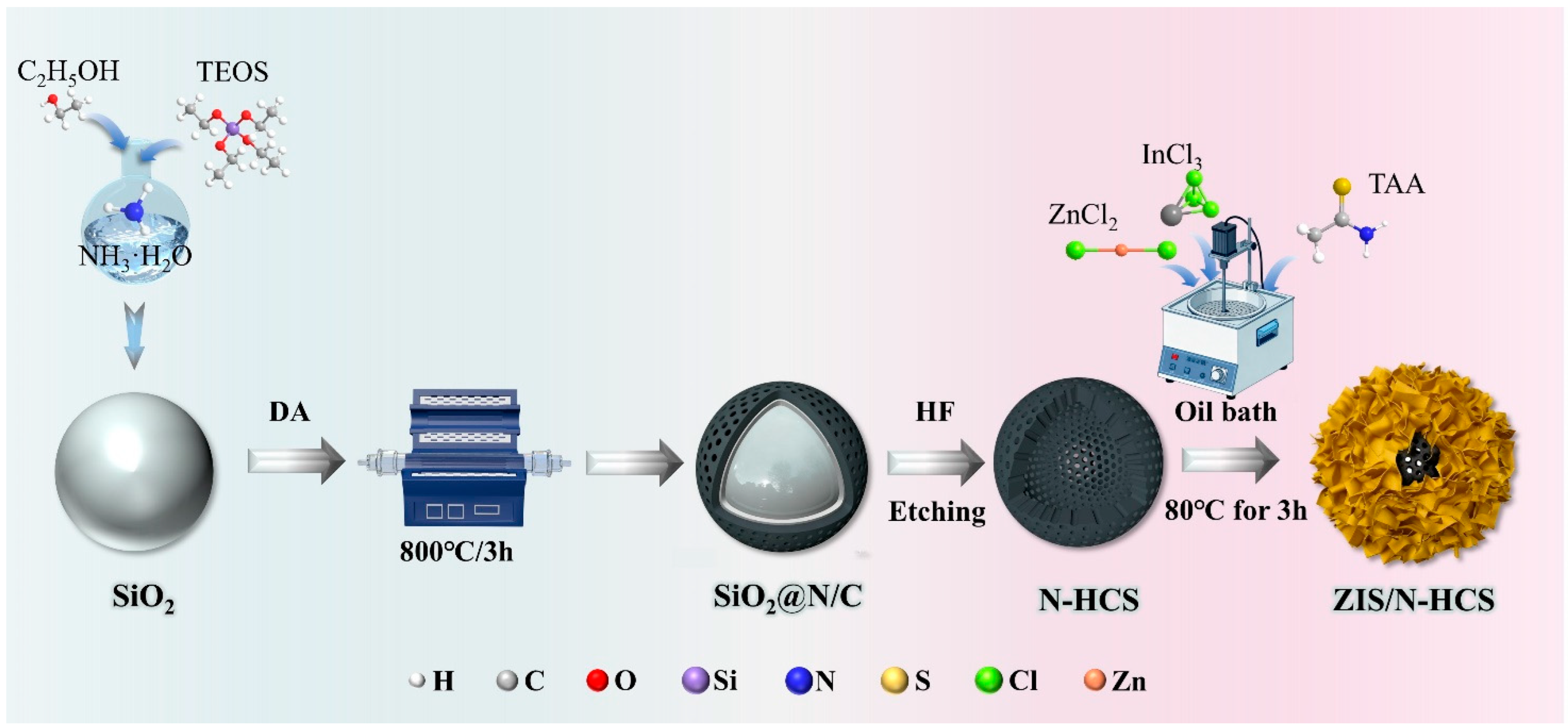
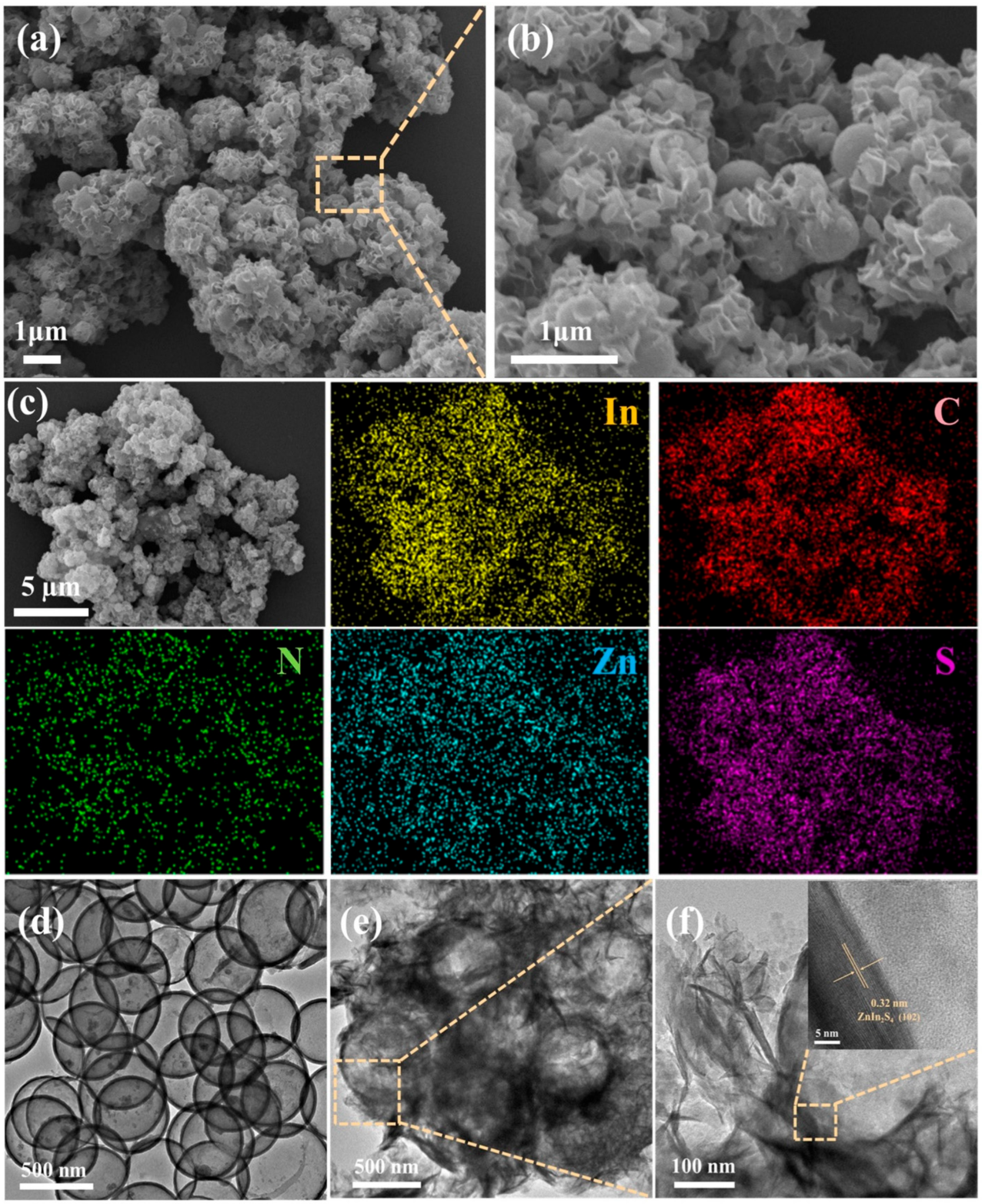
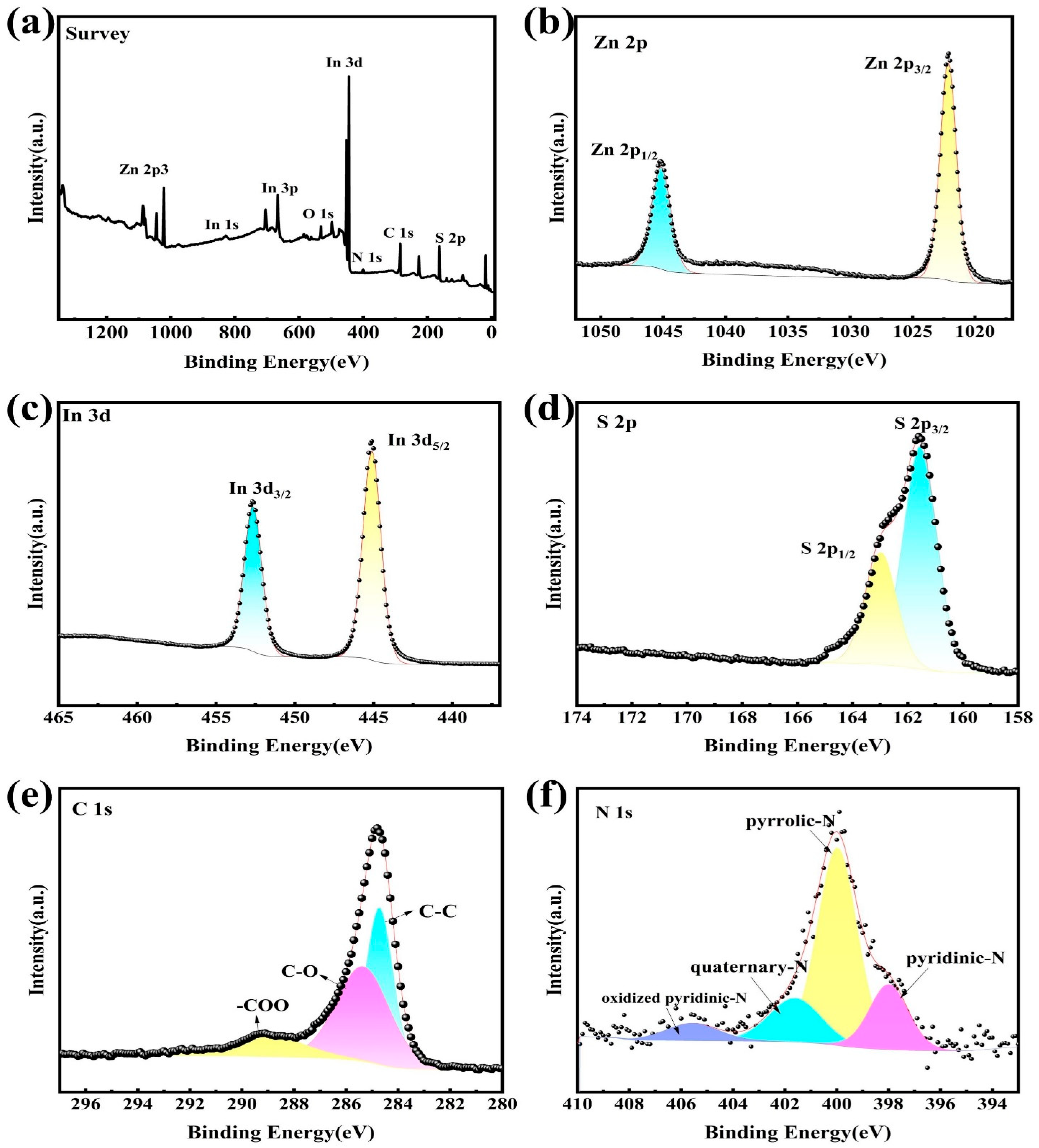
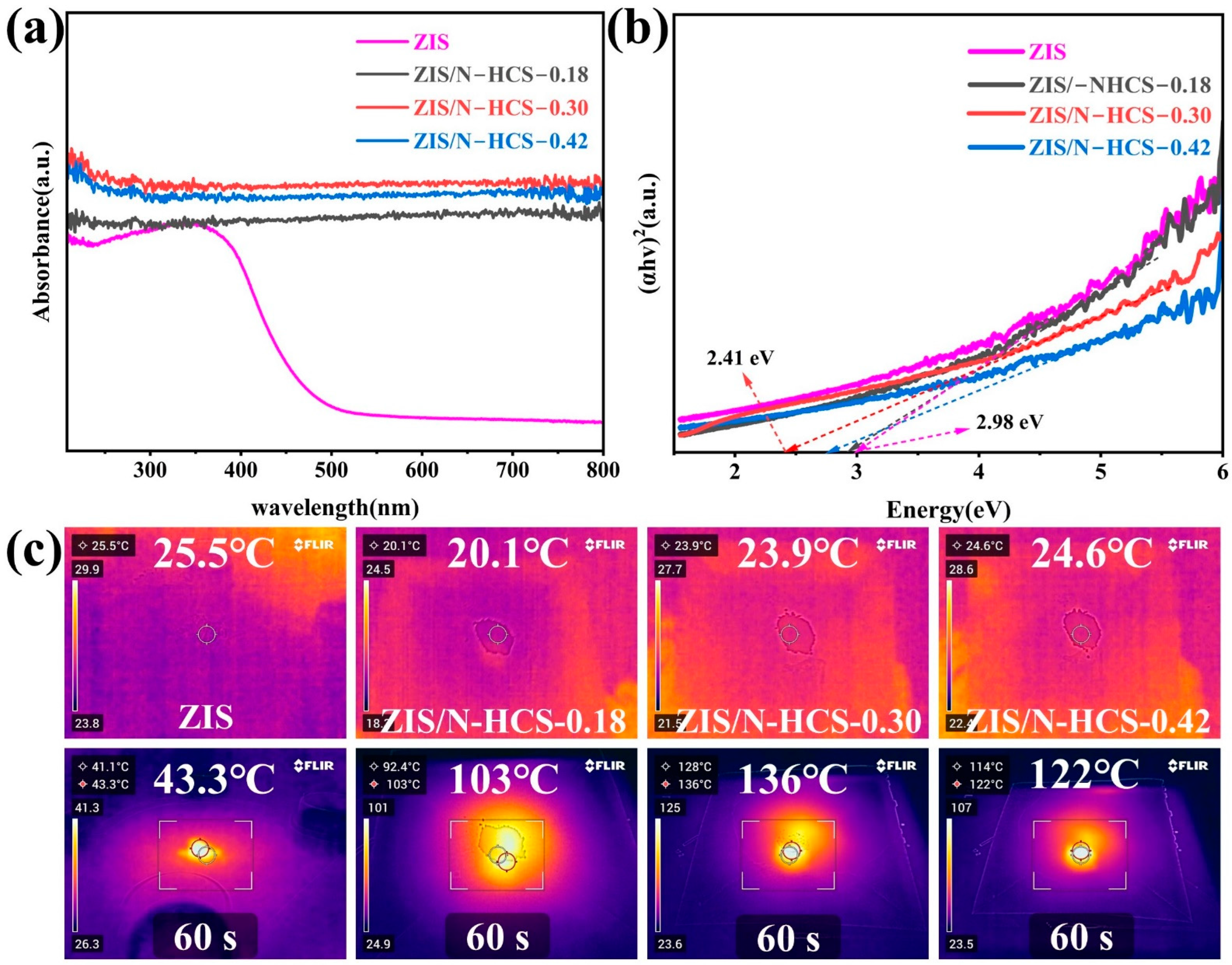
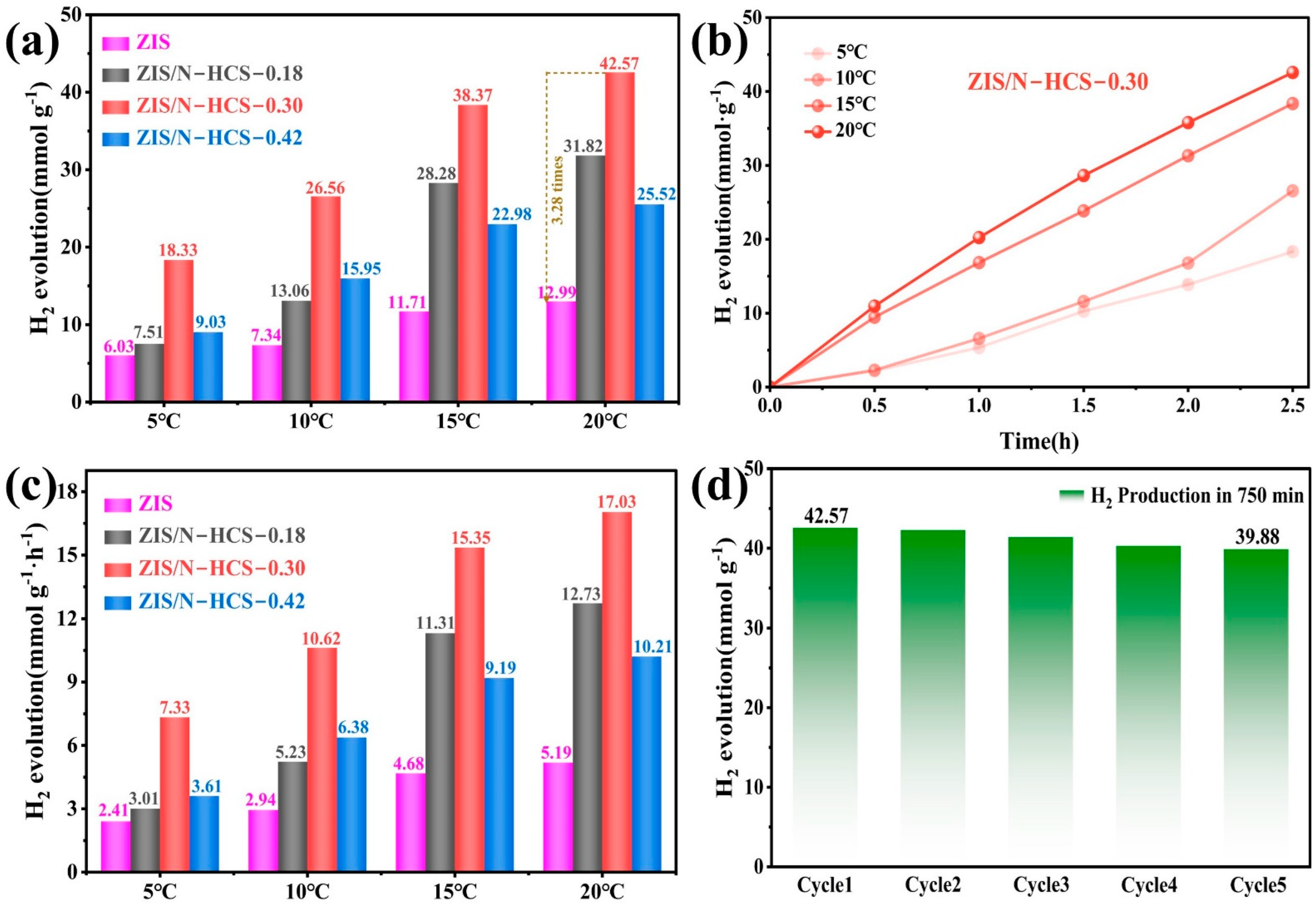
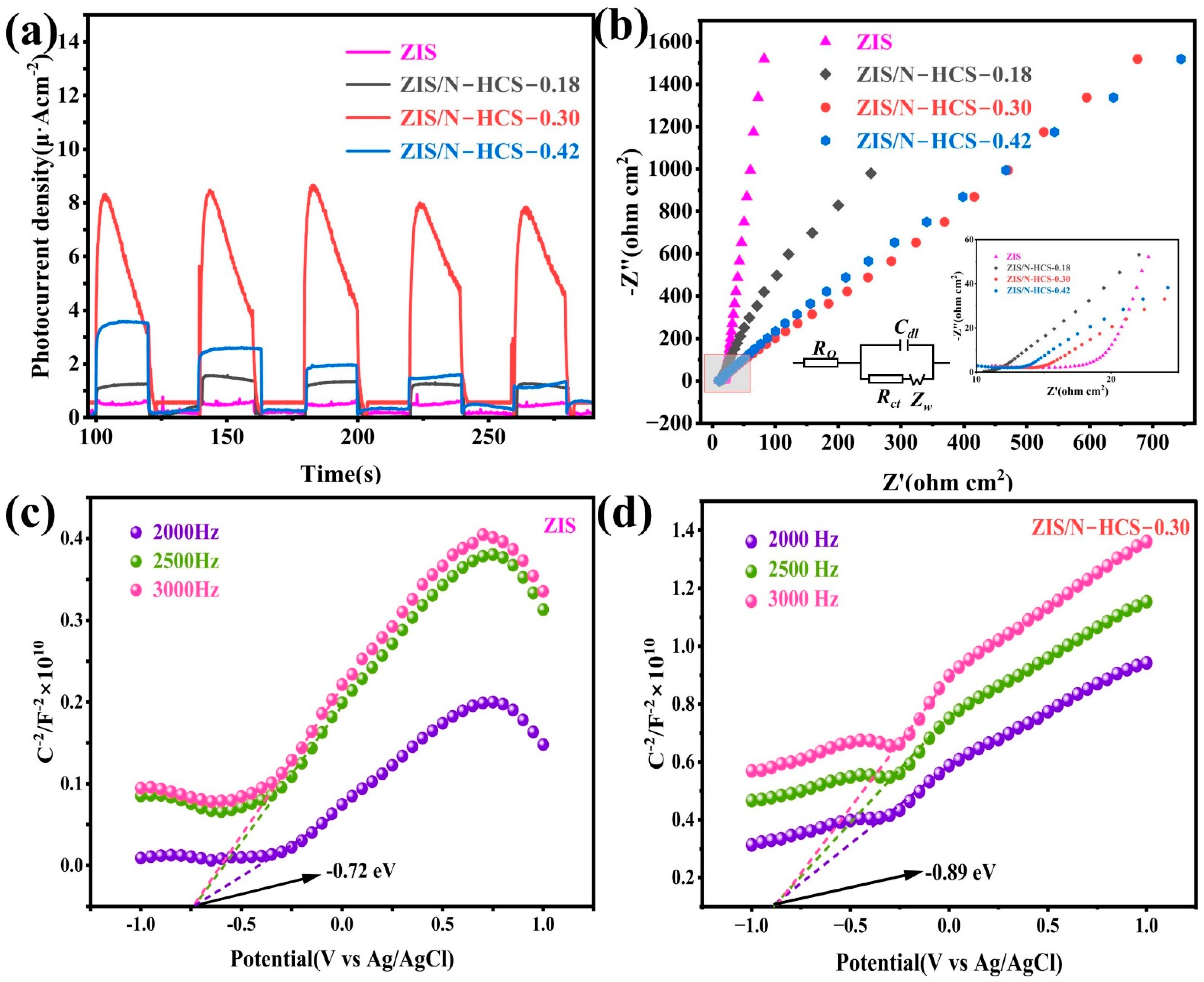
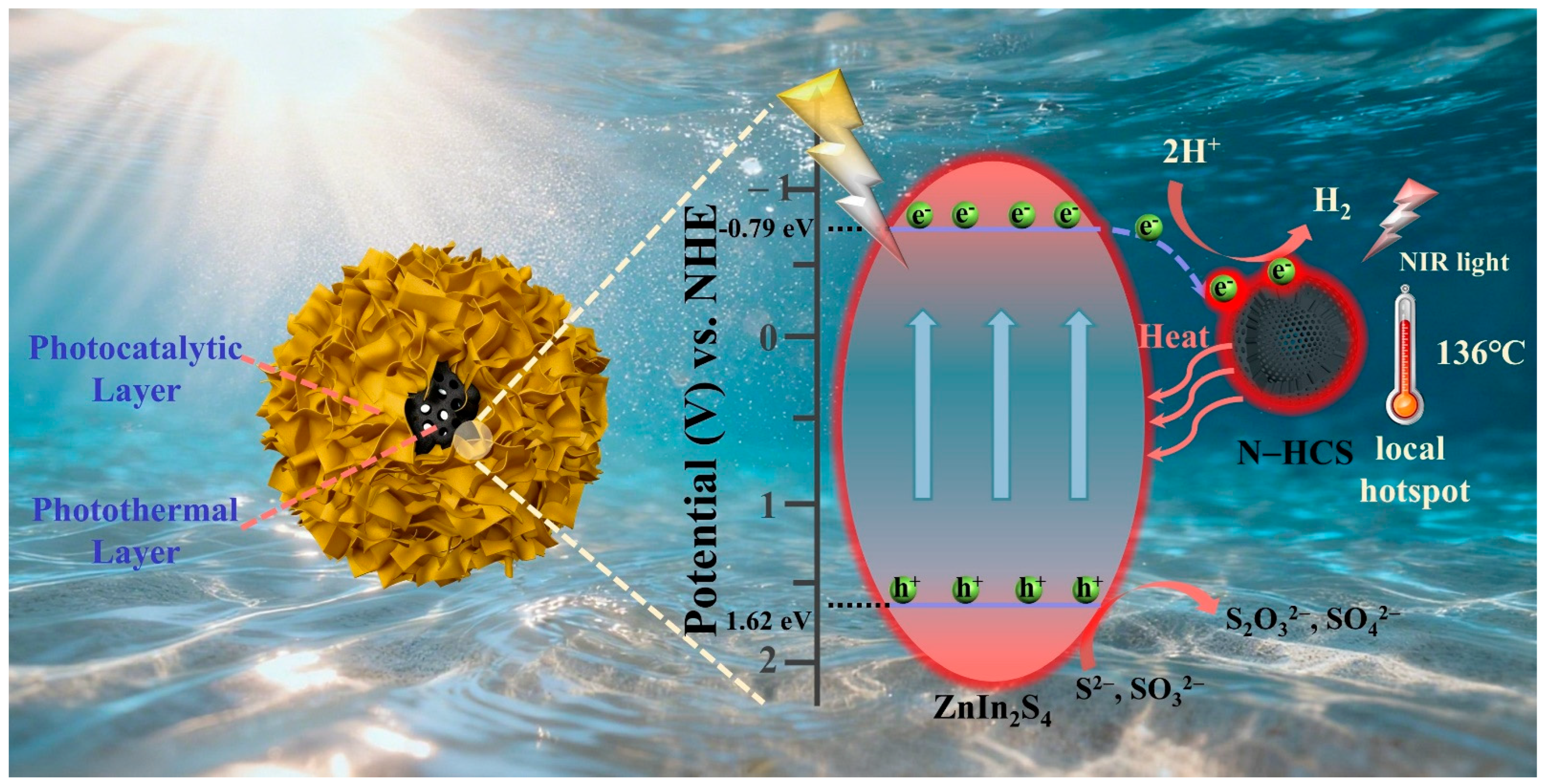
2. Results and Discussion
3. Experimental
3.1. Reagents and Instruments
3.2. Synthesis of SiO2@PDA
3.3. Synthesis of ZnIn2S4 Grown on N-Doped Hollow Carbon Spheres (ZIS/N-HCS)
3.4. Experiments on Photothermal-Assisted Photocatalytic Hydrogen Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ashu Abey, S.; Reis, N.M.; Emanuelsson, E.A.C.; Expósito, A.J. Harnessing visible light: Advanced photocatalytic strategies for sustainable environmental reactions. Chem. Eng. J. 2025, 519, 164951. [Google Scholar] [CrossRef]
- Leggerini, C.; Bannò, M.; Molin, M.D. Hydrogen innovation: An exploration of its determinants across. Eur. Energy Policy 2025, 204, 114675. [Google Scholar] [CrossRef]
- Najafov, B.A.; Nasirov, S.N.; Neymetov, S.R. HYDROGEN technologies for the manufacture of solar-hydrogen Energy objects. Int. J. Hydrogen Energy 2025, 99, 328–339. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor. Electrode Nat. 1972, 238, 37–38. [Google Scholar]
- Zheng, X.; Song, Y.; Wang, C.; Gao, Q.; Shao, Z.; Lin, J.; Zhai, J.; Li, J.; Shi, X.; Wu, D.; et al. Properties, applications, and challenges of copper- and zinc-based multinary metal sulfide photocatalysts for photocatalytic hydrogen evolution. Chin. J. Catal. 2025, 74, 22–70. [Google Scholar] [CrossRef]
- Lv, H.; Zhao, Y.; Wan, B.; Shen, Q.; Xi, W.; Ji, W.; Wang, G.; Wang, G.; Liu, Y. Sulfur vacancies tuning Schottky barrier of Mo2C/Mn0.5Cd0.5S heterojunction for improved photocatalytic hydrogen evolution. Chem. Eng. J. 2025, 522, 167448. [Google Scholar] [CrossRef]
- Li, S.; Xiao, X.; Zuo, L.; Li, R.; Liu, L.; Fan, H.; Li, B.; Wang, L. Synthesis of Mn-doped hollow octahedron ZnIn2S4 to enhance photocatalytic hydrogen production performance utilizing metal–organic framework as template. J. Colloid Interface Sci. 2025, 684, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Luo, Y.; Xiong, Y.; Wang, F.; Dai, Y.; Ma, Y.; Xie, Y.; Zhang, J. V-doped ZnIn2S4 induces S vacancy modulation to enhance photocatalytic hydrogen production under visible light. Sep. Purif. Technol. 2025, 364, 132537. [Google Scholar] [CrossRef]
- Zheng, Z.; Du, T.; Chen, P.; Yue, Q.; Wang, H.; Zhou, L.; Wang, Y. 2D/1D nested hollow porous ZnIn2S4/g-C3N4 heterojunction based on morphology modulation for photocatalytic CO2 reduction. J. Environ. Chem. Eng. 2024, 12, 112971. [Google Scholar] [CrossRef]
- Ding, S.; Medic, I.; Steinfeldt, N.; Dong, T.; Voelzer, T.; Haida, S.; Rabeah, J.; Hu, J.; Strunk, J. Ultrathin Defective Nanosheet Subunit ZnIn2S4 Hollow Nanoflowers for Efficient Photocatalytic Hydrogen Evolution. Small Struct. 2023, 4, 2300091. [Google Scholar] [CrossRef]
- Su, H.; Gong, Y.; Lou, H.; Pang, Y.; Yang, D.; Gao, D.; Qiu, X. The open core-shell TiO2@ZnIn2S4 step-scheme heterojunction to enhance mass transfer and light utilization for efficient photocatalytic performance. J. Clean. Prod. 2023, 419, 138034. [Google Scholar] [CrossRef]
- Xiong, R.; Ke, X.; Jia, W.; Xiao, Y.; Cheng, B.; Lei, S. Photothermal-coupled solar photocatalytic CO2 reduction with high efficiency and selectivity on a MoO3−x@ZnIn2S4 core–shell S-scheme heterojunction. J. Mater. Chem. A 2023, 11, 2178–2190. [Google Scholar] [CrossRef]
- Song, L.; Lin, K.; Feng, D.; Ma, B.; Zhang, F. Homogeneous core-shell cocatalyst of amorphous CoZnSx with capacitance enhancement for synergistic photocatalytic hydrogen evolution and selective oxidation. Appl. Catal. B 2025, 379, 125657. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, S.; Liu, W.; Liu, J.; Liu, T.X. In situ synthesis of three-dimensional core–shell structure Bi2WO6/BiOCl and photocatalytic degradation of trinitrotoluene wastewater. Adv. Compos. Hybrid Mater. 2025, 8, 102. [Google Scholar] [CrossRef]
- Zou, Z.; Wang, Y.; Zhang, T.; Lu, J.; Turkevich, V.; Yu, Y.; Xu, J.; Lin, H.; Li, Y.; Wang, L. Hollow p-n heterostructures with vacancy engineering to enhance Z-scheme photocatalytic CO2 reduction. Chem. Eng. J. 2025, 520, 165595. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, S.; Yang, K.; Yang, X.; Liu, X. Controlled gold–palladium cores in ceria hollow spheres as nanoreactor for plasmon-enhanced catalysis under visible light irradiation. J. Colloid Interface Sci. 2023, 633, 11–23. [Google Scholar] [CrossRef]
- Shan, P.; Geng, K.; Guo, L.; Kuang, L.; Shen, Y.; Xiong, B.; Hou, J.; Guo, F.; Wang, G.; Shi, W. Synergistic effects of photothermal response and Schottky junction for enhanced photothermal-assisted photocatalytic hydrogen production. Chem. Eng. J. 2025, 513, 162801. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, Y.; Sun, K.; Tan, L.; Guo, F.; Du, X.; Shi, W. Plasmonic coupling-boosted photothermal composite photocatalyst for achieving near-infrared photocatalytic hydrogen production. J. Colloid Interface Sci. 2024, 661, 12–22. [Google Scholar] [CrossRef]
- Sun, Z.; Yi, L.; Zhang, G. Plasmon Resonance Coupling and Amplification Promoting Photothermal Catalytic Hydrogen Production from Water and Seawater under High Temperature. ACS Sustain. Chem. Eng. 2025, 13, 13518–13532. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, M.; Li, H.; Wang, Z.; Wang, Y. Multi-channel charge transfer in self-supporting B-g-C3Nx/Bi2S3/CdS dual S-scheme heterojunction toward enhanced photothermal-photocatalytic performance. Nano Energy 2024, 120, 109164. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, H.; Zhu, X.; Ye, D.; Yang, Y.; Wang, H.; Chen, R.; Liao, Q. Photo-thermo catalytic hydrogen production along with pollutant degradation by cost-effective carbon materials. J. Energy Storage 2023, 72, 108624. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Wu, C.; Gao, J.; Li, M.; Xing, Z.; Li, Z.; Zhou, W. Hollow Nanoboxes Cu2-xS@ZnIn2S4 Core-Shell S-Scheme Heterojunction with Broad-Spectrum Response and Enhanced Photothermal-Photocatalytic Performance. Small 2022, 18, 2202544. [Google Scholar] [CrossRef]
- Li, W.; Zuo, G.; Ma, S.; Xi, L.; Ouyang, C.; He, M.; Wang, Y.; Ji, Q.; Yang, S.; Zhu, W.; et al. Localized photothermal effect mediated hollow S-scheme NiCo2O4@ZnIn2S4 for enhanced photocatalytic hydrogen evolution. Appl. Catal. B 2025, 365, 124971. [Google Scholar] [CrossRef]
- Qi, Y.; Jiang, J.; Liang, X.; Ouyang, S.; Mi, W.; Ning, S.; Zhao, L.; Ye, J. Fabrication of Black In2O3 with Dense Oxygen Vacancy through Dual Functional Carbon Doping for Enhancing Photothermal CO2 Hydrogenation. Adv. Funct. Mater. 2021, 31, 2100908. [Google Scholar] [CrossRef]
- Cheng, C.; Wang, J.; Guo, X.; Xing, F.; Huang, C.; Song, M. Thermal-assisted photocatalytic H2 production over sulfur vacancy-rich Co0.85Se/Mn0.3Cd0.7S nanorods under visible light. Appl. Surf. Sci. 2021, 557, 149812. [Google Scholar] [CrossRef]
- Xia, Y.; Cheng, B.; Fan, J.; Yu, J.; Liu, G. Unraveling Photoexcited Charge Transfer Pathway and Process of CdS/Graphene Nanoribbon Composites toward Visible-Light Photocatalytic Hydrogen Evolution. Small 2019, 15, 1902459. [Google Scholar] [CrossRef] [PubMed]
- Martindale, B.C.M.; Hutton, G.A.M.; Caputo, C.A.; Prantl, S.; Godin, R.; Durrant, J.R.; Reisner, E. Enhancing Light Absorption and Charge Transfer Efficiency in Carbon Dots through Graphitization and Core Nitrogen Doping. Angew. Chem. Int. Ed. 2017, 56, 6459–6463. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Liu, Y.; Sun, M.; Guan, J.; Chen, A.; Han, B. Highly Selective Oxygen Electroreduction to Hydrogen Peroxide on Sulfur-Doped Mesoporous Carbon. Angew. Chem. Int. Ed. 2025, 64, e202503385. [Google Scholar] [CrossRef]
- Wang, D.; Liu, C.; Yang, C.; Li, J.; Cheng, X. Enhanced photocatalytic activity of nitrogen-carbon co-doped TiO2 for air pollution mitigation on cement-based surfaces. J. Environ. Chem. Eng. 2025, 13, 116768. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, J.; Huang, H.; Sun, C.; Li, C.; Li, G.; Ji, D.; Fan, Z.; Pan, L. New insights into the role of nitrogen doping in microporous carbon on the capacitive charge storage mechanism: From ab initio to machine learning accelerated molecular dynamics. Carbon 2024, 229, 119498. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, G.; Ren, Z.; Pan, K.; Shi, Y.; Wang, J.; Fu, H. Hierarchical Core–Shell Carbon Nanofiber@ZnIn2S4 Composites for Enhanced Hydrogen Evolution Performance. ACS Appl. Mater. Interfaces 2014, 6, 13841–13849. [Google Scholar] [CrossRef]
- Tan, M.; Yu, C.; Luan, Q.; Liu, C.; Dong, W.; Su, Y.; Qiao, L.; Gao, L.; Lu, Q.; Bai, Y. The Mott–Schottky heterojunction MoC@NG@ZIS with enhanced kinetic response for promoting photocatalytic hydrogen production. J. Mater. Chem. A 2022, 10, 21465–21473. [Google Scholar] [CrossRef]
- Wang, F.; Xia, Y.; He, Z.; He, G.; Zhang, J.; Chen, L.; Chen, G.; Fu, X.; Tang, B.; Xie, Y.; et al. Ni12P5/MgIn2S4 Schottky junctions with sulfur vacancies and oxygen doping synergistic promotes ultraefficient photocatalytic hydrogen evolution. Chem. Eng. J. 2025, 522, 167598. [Google Scholar] [CrossRef]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Li, S.; Ye, J.; Fan, Z.; Dai, Y.; Xie, Y.; Ling, Y.; Xu, M.; Wang, Y. CoP co-catalyst modification ZnIn2S4 driving efficient H2 evolution under visible light. Sep. Purif. Technol. 2025, 358, 130294. [Google Scholar] [CrossRef]
- Gao, Y.; Ren, F.; Lu, Y.; Zhang, S.; Guo, P.; Zhang, J.; Dai, F.; Chen, L.; Zhao, Y. Mo2C-supported ultralow-Pt-loading bifunctional cocatalyst for enhancing photocatalytic H2 evolution on ZnIn2S4. Sep. Purif. Technol. 2025, 377, 133869. [Google Scholar] [CrossRef]
- Liu, X.; Gong, W.; Yan, Z.; Wang, Y.; Guo, H.; Luo, Y.; Li, Y.; Lin, J. Noble-metal-free Ni3C cocatalysts decorated ZnIn2S4 nanosheet microspheres for improved photocatalytic H2 evolution. Int. J. Hydrogen Energy 2025, 150, 150136. [Google Scholar] [CrossRef]
- Geng, K.; Shan, P.; Shi, J.; Shen, Y.; Yan, S.; Zhao, M.; Guo, F.; Wang, G.; Shi, W. Broad spectral absorption cooperates with local plasma resonance for promoted photothermal-assisted photocatalytic hydrogen production. Chem. Eng. J. 2025, 507, 160561. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, W.; Zhang, Y.; Zhu, J. Construction of core-shell W18O49@ZnIn2S4 hollow hierarchical structure for boosted photothermal-assisted photocatalytic H2 production. Int. J. Hydrogen Energy 2024, 85, 165–174. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, J.; Luo, M.; Ma, J.; Xian, T.; Liu, G.; Yang, H. Elevating photocatalytic H2 evolution over ZnIn2S4@Au@Cd0.7Zn0.3S multilayer nanotubes via Au-mediating H–S antibonding-orbital occupancy. Chem. Eng. J. 2024, 499, 156455. [Google Scholar] [CrossRef]
- Shan, P.; Geng, K.; Shen, Y.; Hao, P.; Zhang, S.; Hou, J.; Lu, J.; Guo, F.; Li, C.; Shi, W. Facile synthesis of hierarchical core-shell carbon@ZnIn2S4 composite for boosted photothermal-assisted photocatalytic H2 production. J. Colloid Interface Sci. 2025, 677, 1098–1107. [Google Scholar] [CrossRef]
- Li, Q.; Lu, Q.; Guo, E.; Wei, M.; Pang, Y. Hierarchical Co9S8/ZnIn2S4 Nanoflower Enables Enhanced Hydrogen Evolution Photocatalysis. Energy Fuels 2022, 36, 4541–4548. [Google Scholar] [CrossRef]
- Liu, R.; Cui, Y.; Wang, T.; Liu, J.; Liu, B.; Zuo, S.; Yu, H. A localized surface plasmon resonance effect boosts photocatalytic hydrogen evolution of ZnIn2S4/amorphous MoO3−x nanodot Z-scheme heterojunctions. J. Mater. Chem. A 2025, 13, 8144–8156. [Google Scholar] [CrossRef]
- Yang, W.-N.; Yang, J.; Yang, H.; Sun, L.; Li, H.-X.; Li, D.-C.; Dou, J.-M.; Li, X.-G.; Cao, G.-D. In-situ construction of tubular core–shell noble-metal-free CMT@TiO2/ZnIn2S4 S-scheme heterojunction for superior photothermal-photocatalytic hydrogen evolution. Rare Met. 2025, 44, 2474–2488. [Google Scholar] [CrossRef]
- Jia, J.; Guo, X.; Tang, Y.; Zeng, W.; Zeng, H.; Rui, Z. ZnIn2S4 supported carbon dots/Ni3P hybrid for efficient photothermal catalytic hydrogen production from water. Int. J. Hydrogen Energy 2025, 104, 122–130. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Cao, J.; Yu, J.; Dong, J.; Zhao, Y.; Zhao, F.; Zhang, D.; Pu, X. Anchoring ZnIn2S4 nanosheets on cross-like FeSe2 to construct photothermal-enhanced S-scheme heterojunction for photocatalytic H2 evolution. J. Colloid Interface Sci. 2024, 673, 463–474. [Google Scholar] [CrossRef]

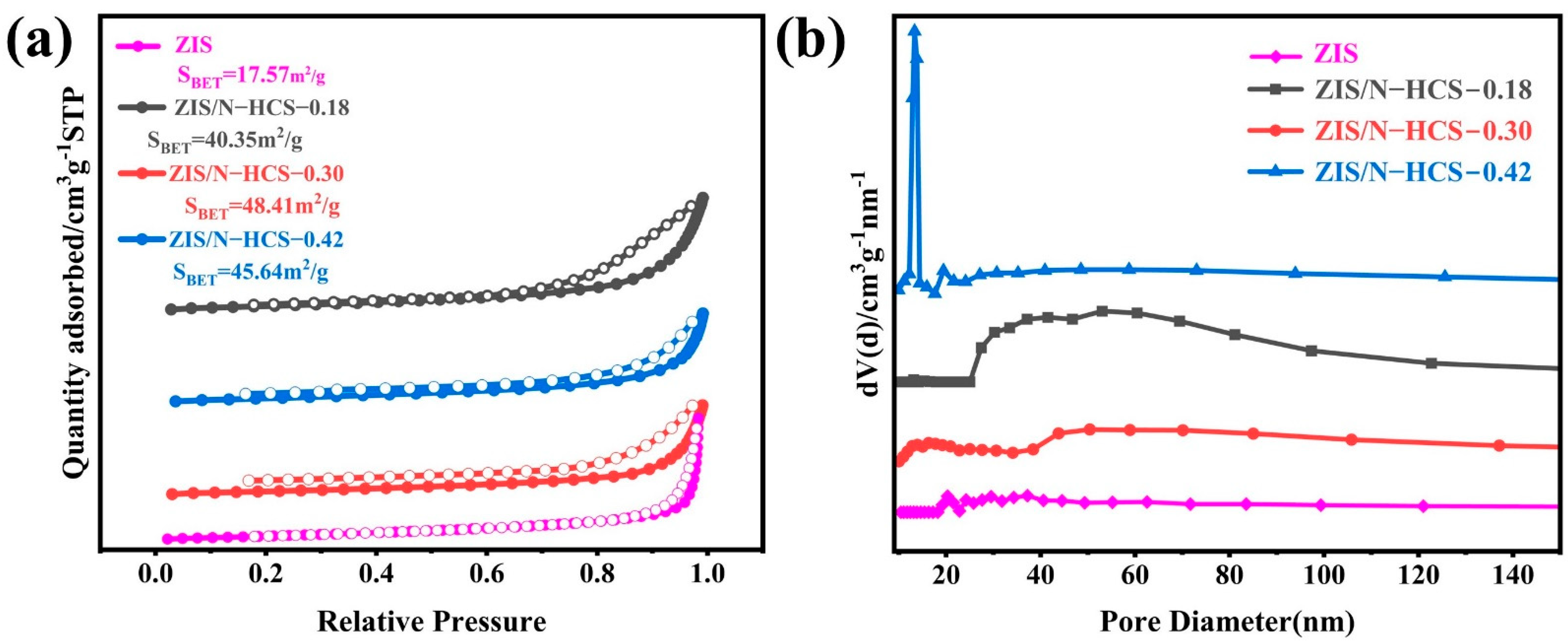
| Sample | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| ZIS | 17.57 | 0.13 | 14.2 |
| ZIS/N-HCS-0.18 | 40.35 | 0.19 | 94.4 |
| ZIS/N-HCS-0.30 | 48.41 | 0.24 | 98.4 |
| ZIS/N-HCS-0.42 | 45.64 | 0.19 | 83.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, S.; Liu, L.; Liu, M.; Tian, J.; Xue, Y.; Wu, K. Coupling Photothermal Effect in N-Doped Hollow Carbon Spheres with ZnIn2S4 Boosts Solar Hydrogen Evolution. Molecules 2025, 30, 4368. https://doi.org/10.3390/molecules30224368
He S, Liu L, Liu M, Tian J, Xue Y, Wu K. Coupling Photothermal Effect in N-Doped Hollow Carbon Spheres with ZnIn2S4 Boosts Solar Hydrogen Evolution. Molecules. 2025; 30(22):4368. https://doi.org/10.3390/molecules30224368
Chicago/Turabian StyleHe, Shanhao, Li Liu, Min Liu, Jinjun Tian, Yan Xue, and Keliang Wu. 2025. "Coupling Photothermal Effect in N-Doped Hollow Carbon Spheres with ZnIn2S4 Boosts Solar Hydrogen Evolution" Molecules 30, no. 22: 4368. https://doi.org/10.3390/molecules30224368
APA StyleHe, S., Liu, L., Liu, M., Tian, J., Xue, Y., & Wu, K. (2025). Coupling Photothermal Effect in N-Doped Hollow Carbon Spheres with ZnIn2S4 Boosts Solar Hydrogen Evolution. Molecules, 30(22), 4368. https://doi.org/10.3390/molecules30224368






