Comprehensive Risdiplam Synthesis Overview: From Cross-Coupling Reliance to Complete Palladium Independence
Abstract
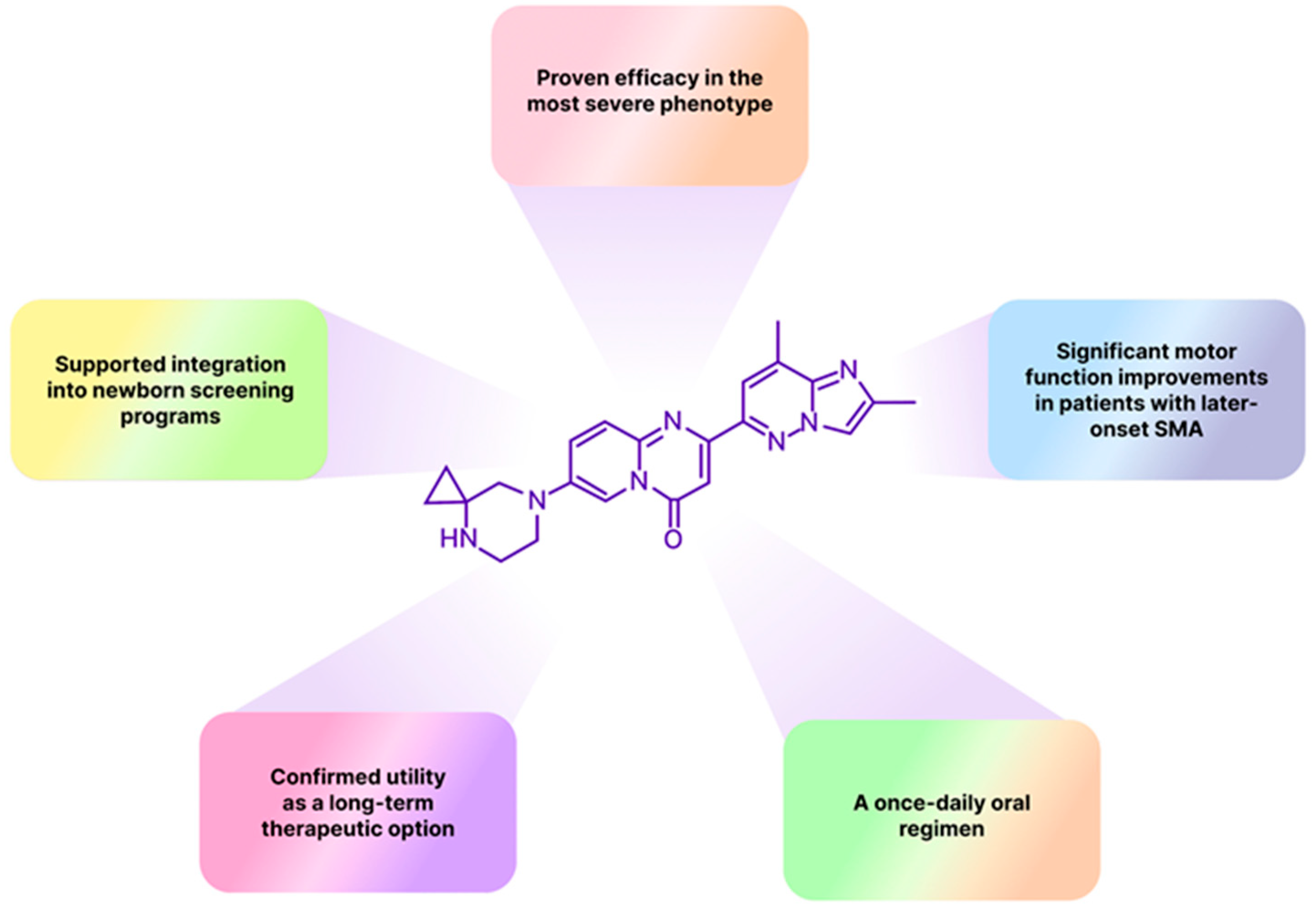
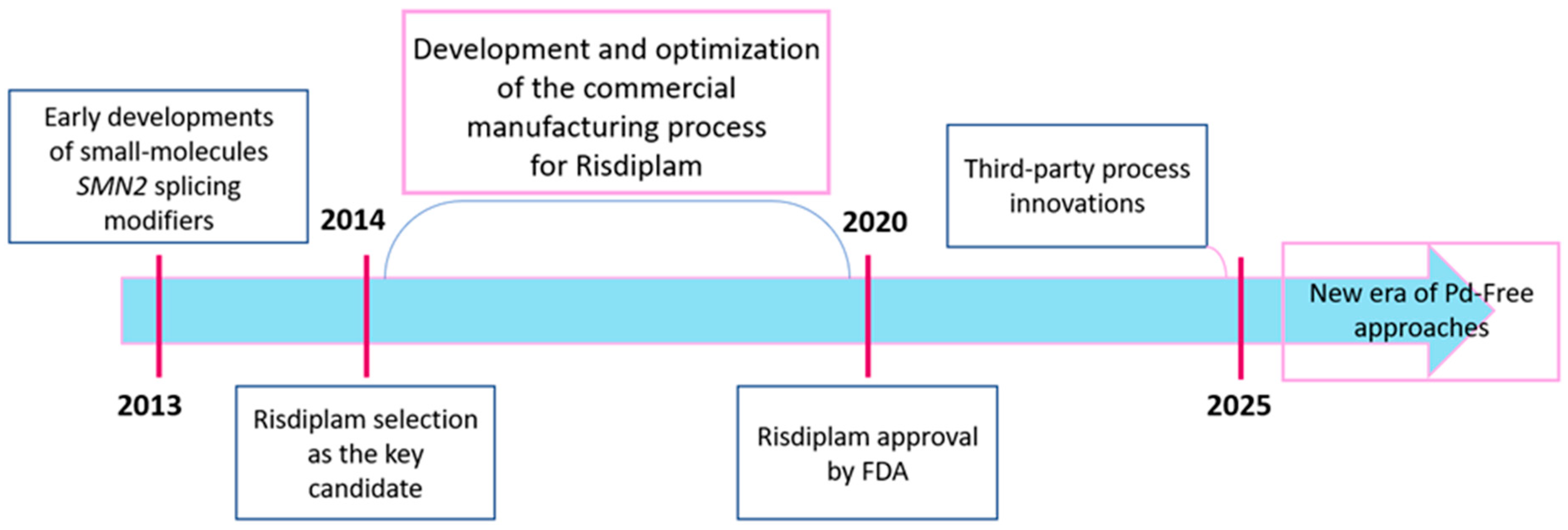
1. Introduction
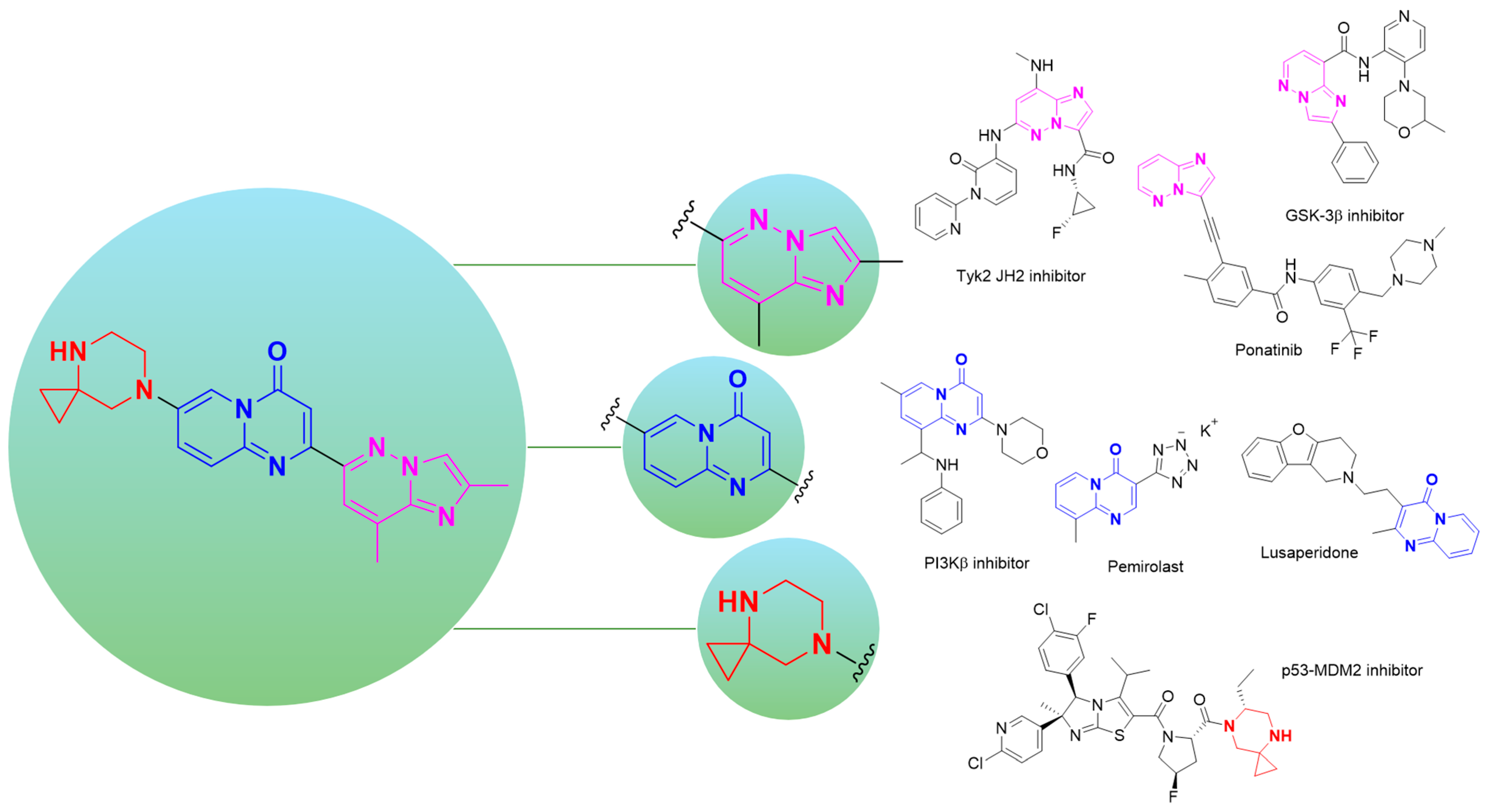
2. Advances, Limitations, and Major Challenges in Risdiplam Synthesis
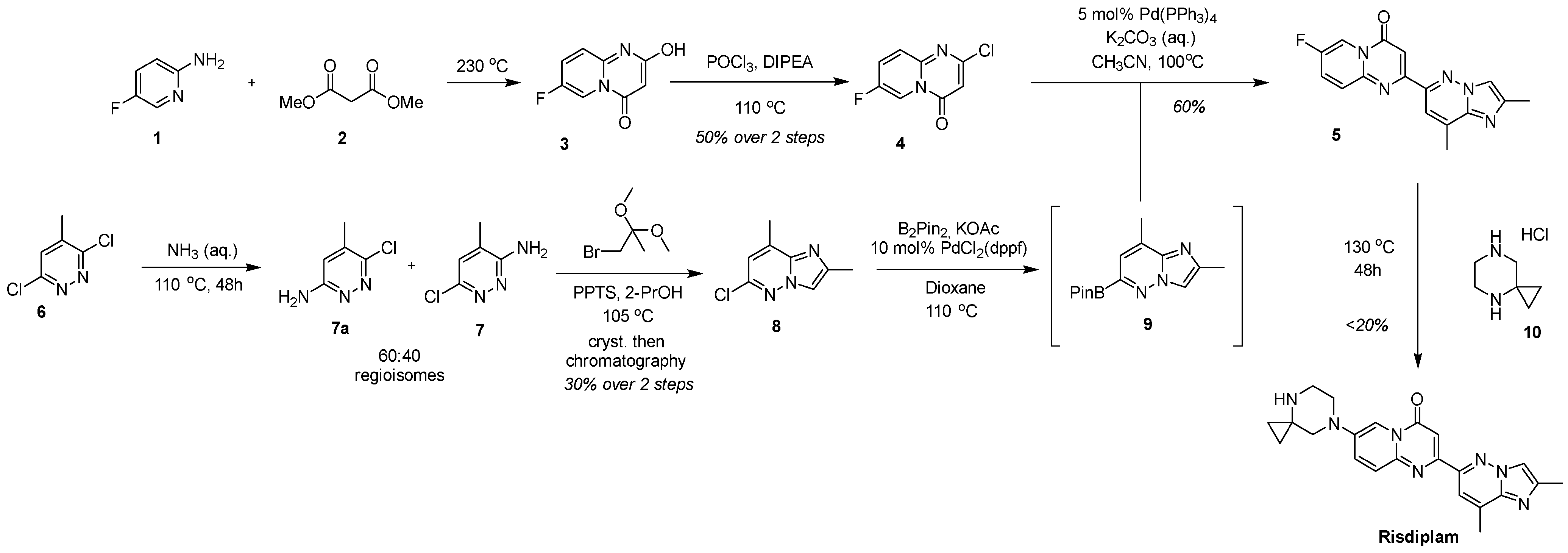
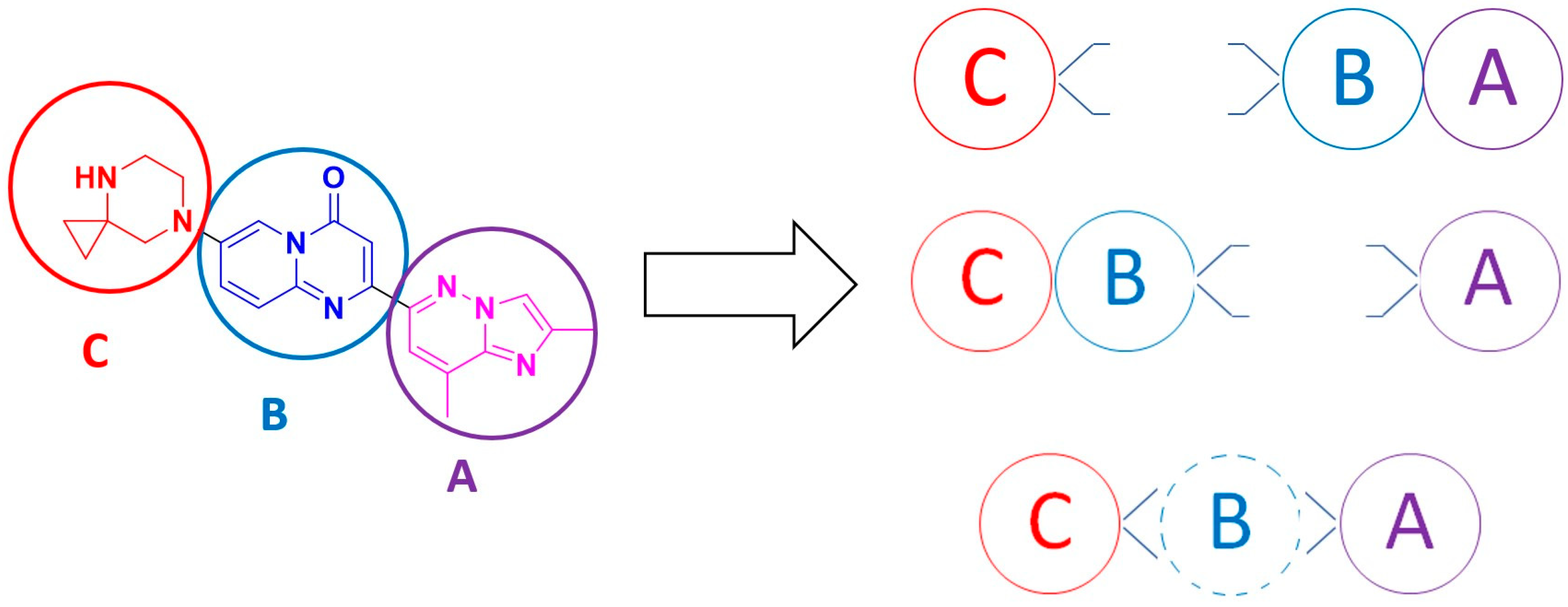
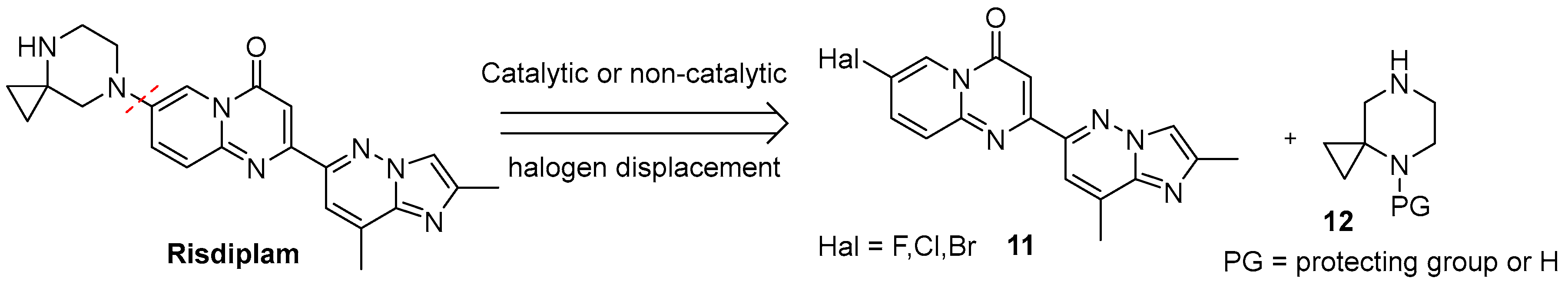
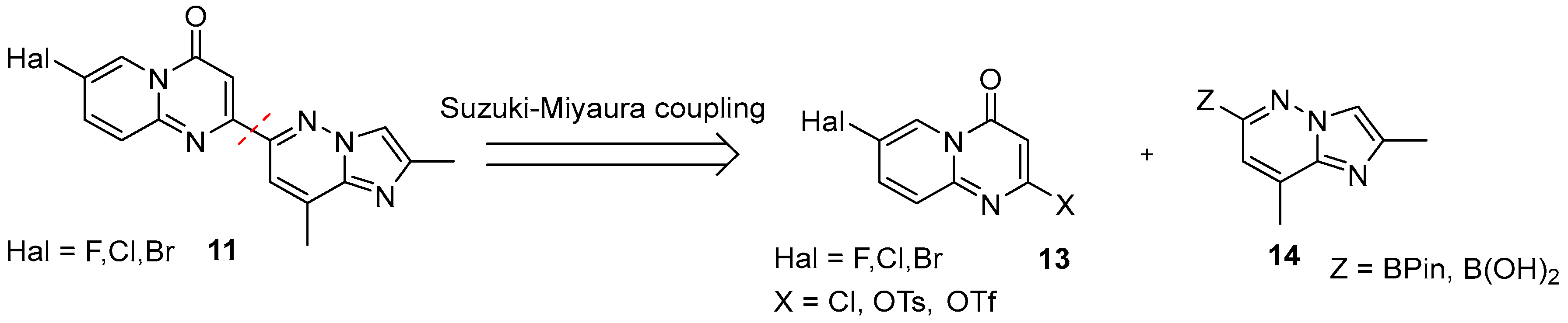
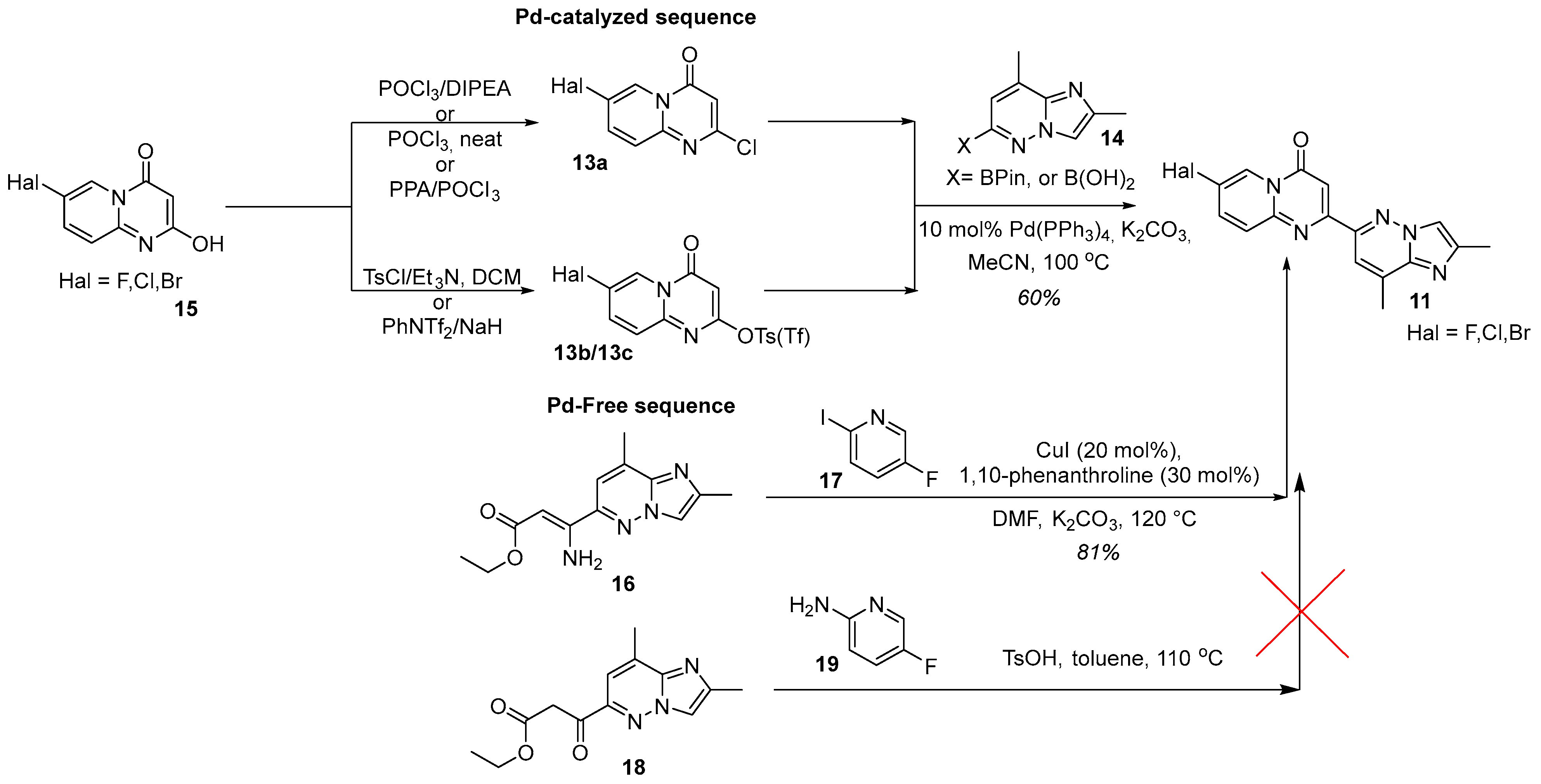
2.1. C + BA-Strategies Towards the Target Molecule
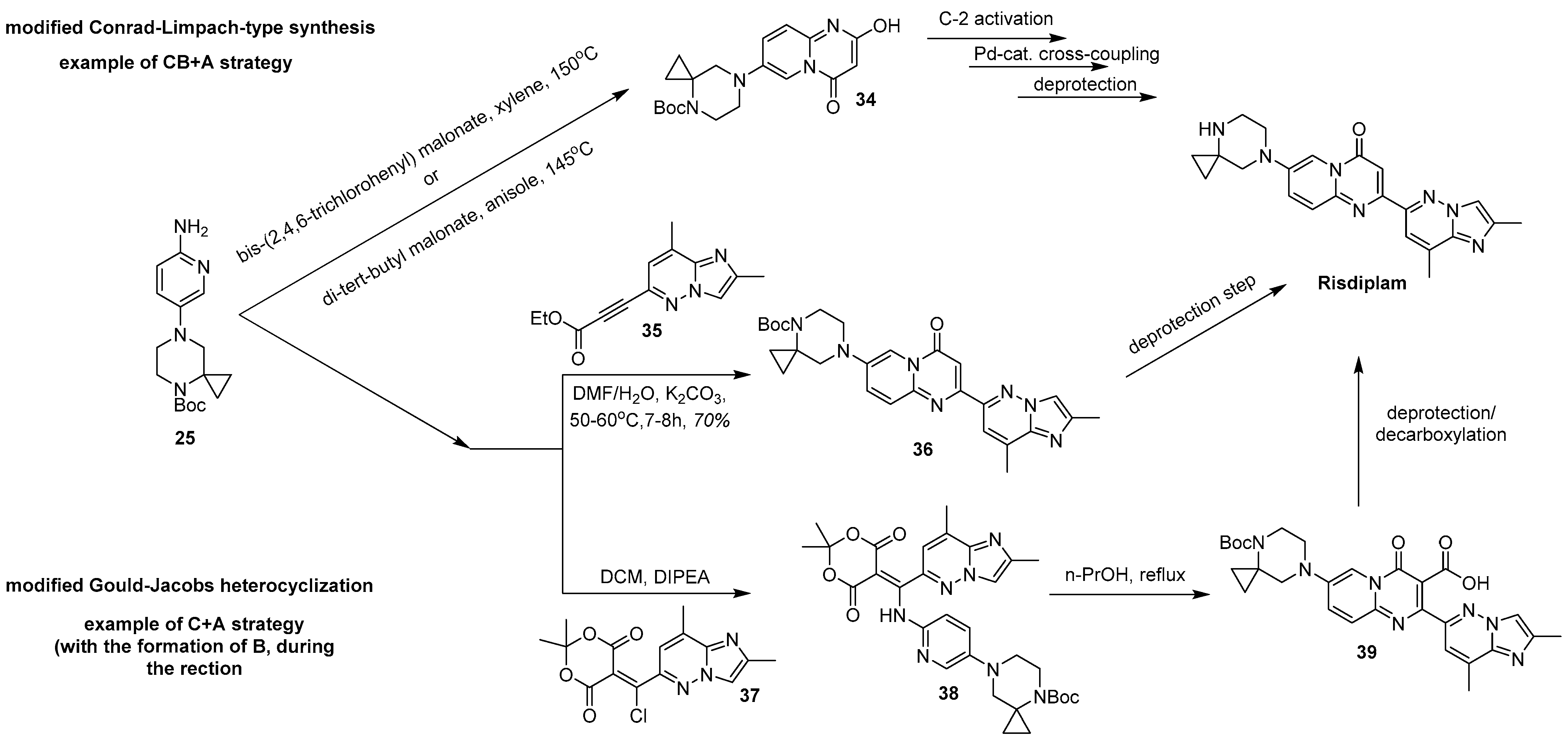
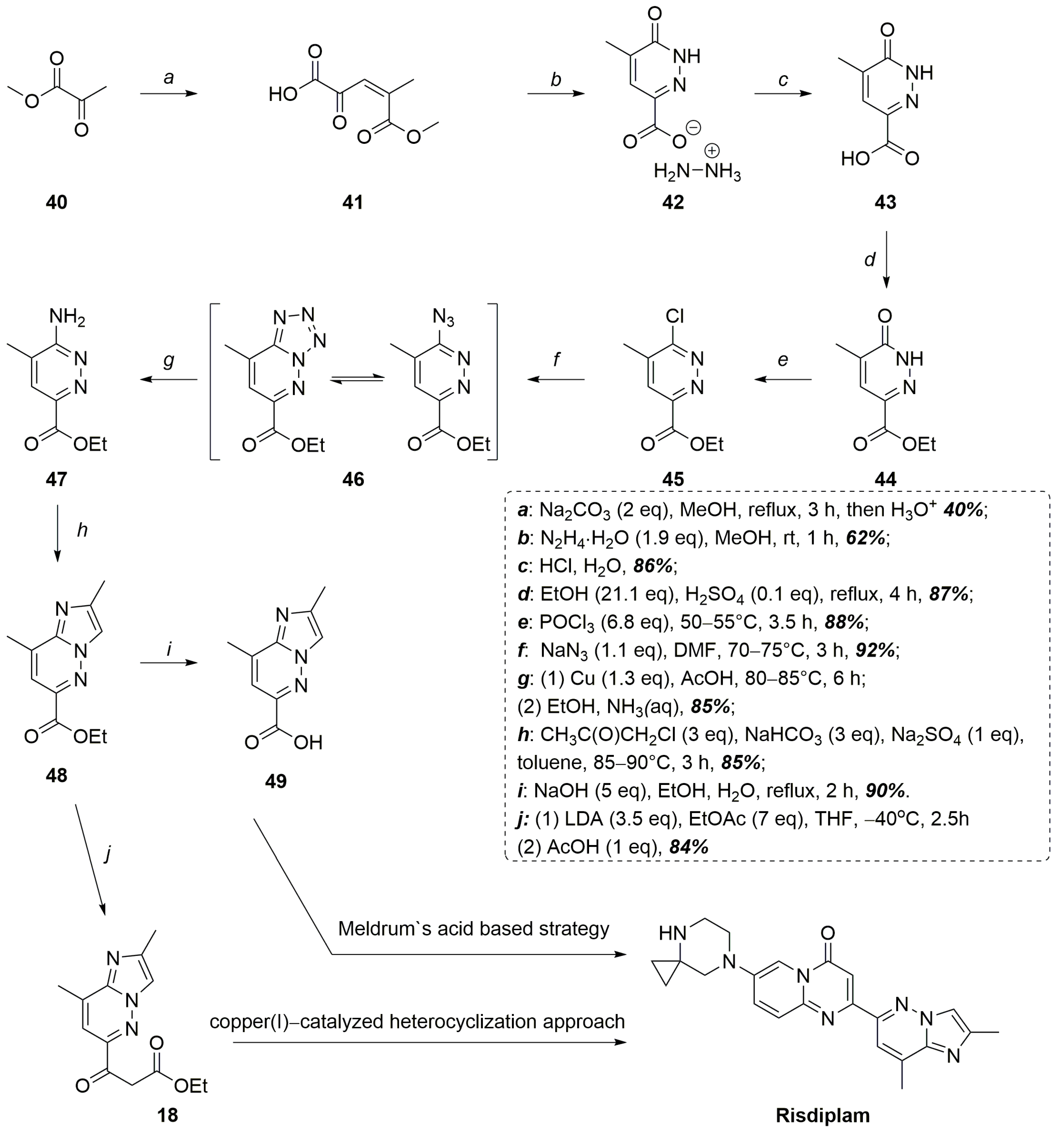
2.2. CB + A and Convergent C + A (With the Formation of B During the Reaction) Approaches Toward Risdiplam
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nishio, H.; Niba, E.T.E.; Saito, T.; Okamoto, K.; Takeshima, Y.; Awano, H. Spinal Muscular Atrophy: The Past, Present, and Future of Diagnosis and Treatment. Int. J. Mol. Sci. 2023, 24, 11939. [Google Scholar] [CrossRef]
- Mendonça, R.H.; de Godoi, J.S.A.; Zanoteli, E. Um Cadastro Brasileiro Autorrelatado de Atrofia Muscular Espinhal 5q: Dados de História Natural, Características Genéticas e Cuidados Multidisciplinares. Arq. Neuropsiquiatr. 2024, 82, s00441792096. [Google Scholar] [CrossRef]
- Paik, J. Risdiplam: A Review in Spinal Muscular Atrophy. CNS Drugs 2022, 36, 401–410. [Google Scholar] [CrossRef]
- Baranello, G.; Darras, B.T.; Day, J.W.; Deconinck, N.; Klein, A.; Masson, R.; Mercuri, E.; Rose, K.; El-Khairi, M.; Gerber, M.; et al. Risdiplam in Type 1 Spinal Muscular Atrophy. N. Engl. J. Med. 2021, 384, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Deconinck, N.; Mazzone, E.S.; Nascimento, A.; Oskoui, M.; Saito, K.; Vuillerot, C.; Baranello, G.; Boespflug-Tanguy, O.; Goemans, N.; et al. Safety and Efficacy of Once-Daily Risdiplam in Type 2 and Non-Ambulant Type 3 Spinal Muscular Atrophy (SUNFISH Part 2): A Phase 3, Double-Blind, Randomised, Placebo-Controlled Trial. Lancet Neurol. 2022, 21, 42–52. [Google Scholar] [CrossRef]
- Chiriboga, C.A.; Bruno, C.; Duong, T.; Fischer, D.; Mercuri, E.; Kirschner, J.; Kostera-Pruszczyk, A.; Jaber, B.; Gorni, K.; Kletzl, H.; et al. Risdiplam in Patients Previously Treated with Other Therapies for Spinal Muscular Atrophy: An Interim Analysis from the JEWELFISH Study. Neurol. Ther. 2023, 12, 543–557. [Google Scholar] [CrossRef]
- Chiriboga, C.A.; Bruno, C.; Duong, T.; Fischer, D.; Mercuri, E.; Kirschner, J.; Kostera-Pruszczyk, A.; Jaber, B.; Gorni, K.; Kletzl, H.; et al. JEWELFISH: 24-Month Results from an Open-Label Study in Non-Treatment-Naïve Patients with SMA Receiving Treatment with Risdiplam. J. Neurol. 2024, 271, 4871–4884. [Google Scholar] [CrossRef] [PubMed]
- Servais, L.; Farrar, M.; Finkel, R.; Vlodavets, D.; Zanoteli, E.; Al-Muhaizea, M.; de Queiroz Campos Araújo, A.P.; Nelson, L.; Jaber, B.; Gorni, K.; et al. RAINBOWFISH: Primary Efficacy and Safety Data in Risdiplam-Treated Infants with Presymptomatic Spinal Muscular Atrophy (SMA) (S37.006). Neurology 2024, 102, 5269. [Google Scholar] [CrossRef]
- Finkel, R.S.; Farrar, M.A.; Vlodavets, D.; Servais, L.; Zanoteli, E.; Al-Muhaizea, M.; Nelson, L.; Prufer, A.; Wang, Y.; Fisher, C.; et al. RAINBOWFISH: Preliminary Efficacy and Safety Data in Risdiplam-Treated Infants with Presymptomatic SMA (P17-5.003). Neurology 2022, 98, 1636. [Google Scholar] [CrossRef]
- Finkel, R.S.; Al-Muhaizea, M.; Farrar, M.A.; Nelson, L.; Prufer, A.; Servais, L.; Wang, Y.; Zanoteli, E.; Palfreeman, L.; El-Khairi, M.; et al. RAINBOWFISH: A Study of Risdiplam in Newborns with Presymptomatic Spinal Muscular Atrophy (SMA) (4281). Neurology 2021, 96, 4281. [Google Scholar] [CrossRef]
- Keritam, O.; Erdler, M.; Fasching, B.; Zulehner, G.; Rath, J.; Krenn, M.; Waldhör, T.; Gruber, V.A.; Langweil, N.; Kiss, C.; et al. Efficacy and Safety of Risdiplam in Adults with 5q-Associated Spinal Muscular Atrophy: A Nationwide Observational Cohort Study in Austria. EClinicalMedicine 2025, 88, 103536. [Google Scholar] [CrossRef]
- Cortes, J.E.; Kantarjian, H.; Shah, N.P.; Bixby, D.; Mauro, M.J.; Flinn, I.; O’Hare, T.; Hu, S.; Narasimhan, N.I.; Rivera, V.M.; et al. Ponatinib in Refractory Philadelphia Chromosome–Positive Leukemias. N. Engl. J. Med. 2012, 367, 2075–2088. [Google Scholar] [CrossRef]
- Hartz, R.A.; Ahuja, V.T.; Sivaprakasam, P.; Xiao, H.; Krause, C.M.; Clarke, W.J.; Kish, K.; Lewis, H.; Szapiel, N.; Ravirala, R.; et al. Design, Structure–Activity Relationships, and In Vivo Evaluation of Potent and Brain-Penetrant Imidazo[1,2- b]Pyridazines as Glycogen Synthase Kinase-3β (GSK-3β) Inhibitors. J. Med. Chem. 2023, 66, 4231–4252. [Google Scholar] [CrossRef]
- Liu, C.; Lin, J.; Moslin, R.; Tokarski, J.S.; Muckelbauer, J.; Chang, C.Y.; Tredup, J.; Xie, D.; Park, H.; Li, P.; et al. Identification of Imidazo[1,2- b] Pyridazine Derivatives as Potent, Selective, and Orally Active Tyk2 JH2 Inhibitors. ACS Med. Chem. Lett. 2019, 10, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.C.; Goldstein, D.M.; Hermann, J.C.; Kuglstatter, A.; Liu, W.; Luk, K.C.; Padilla, F.; Slade, M.; Villaseñor, A.G.; Wanner, J.; et al. Rational Design of Highly Selective Spleen Tyrosine Kinase Inhibitors. J. Med. Chem. 2012, 55, 10414–10423. [Google Scholar] [CrossRef] [PubMed]
- El Akkaoui, A.; Koubachi, J.; Guillaumet, G.; El Kazzouli, S. Synthesis and Functionalization of Imidazo[1,2-b] Pyridazine by Means of Metal-Catalyzed Cross-Coupling Reactions. ChemistrySelect 2021, 6, 8985–9011. [Google Scholar] [CrossRef]
- Kawashima, T.; Iwamoto, I.; Nakagawa, N.; Tomioka, H.; Yoshida, S. Inhibitory Effect of Pemirolast, a Novel Antiallergic Drug, on Leukotriene C4 and Granule Protein Release from Human Eosinophils. Int. Arch. Allergy Immunol. 1994, 103, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Kennis, L.E.J.; Bischoff, F.P.; Mertens, C.J.; Love, C.J.; Van den Keybus, F.A.F.; Pieters, S.; Braeken, M.; Megens, A.A.H.P.; Leysen, J.E. New 2-Substituted 1,2,3,4-Tetrahydrobenzofuro[3,2-c] Pyridine Having Highly Active and Potent Central α 2 -Antagonistic Activity as Potential Antidepressants. Bioorg Med. Chem. Lett. 2000, 10, 71–74. [Google Scholar] [CrossRef]
- La Motta, C.; Sartini, S.; Mugnaini, L.; Simorini, F.; Taliani, S.; Salerno, S.; Marini, A.M.; Da Settimo, F.; Lavecchia, A.; Novellino, E.; et al. Pyrido[1,2-a] Pyrimidin-4-One Derivatives as a Novel Class of Selective Aldose Reductase Inhibitors Exhibiting Antioxidant Activity. J. Med. Chem. 2007, 50, 4917–4927. [Google Scholar] [CrossRef]
- Mohammed, E.U.R.; Porter, Z.J.; Jennings, I.G.; Al-Rawi, J.M.A.; Thompson, P.E.; Angove, M.J. Synthesis and Biological Evaluation of 4H-Benzo[e] [1,3] Oxazin-4-Ones Analogues of TGX-221 as Inhibitors of PI3Kβ. Bioorg. Med. Chem. 2022, 69, 116832. [Google Scholar] [CrossRef]
- Li, A.R.; Johnson, M.G.; Liu, J.; Chen, X.; Du, X.; Mihalic, J.T.; Deignan, J.; Gustin, D.J.; Duquette, J.; Fu, Z.; et al. Optimization of the Heterocyclic Core of the Quinazolinone-Derived CXCR3 Antagonists. Bioorg. Med. Chem. Lett. 2008, 18, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Bhawale, R.T.; Chillal, A.S.; Kshirsagar, U.A. 4H-Pyrido[1,2-a] Pyrimidin-4-One, Biologically Important Fused Heterocyclic Scaffold: Synthesis and Functionalization. J. Heterocycl. Chem. 2023, 60, 1356–1373. [Google Scholar] [CrossRef]
- Moshnenko, N.; Kazantsev, A.; Chupakhin, E.; Bakulina, O.; Dar’in, D. Synthetic Routes to Approved Drugs Containing a Spirocycle. Molecules 2023, 28, 4209. [Google Scholar] [CrossRef]
- Hiesinger, K.; Dar’In, D.; Proschak, E.; Krasavin, M. Spirocyclic Scaffolds in Medicinal Chemistry. J. Med. Chem. 2021, 64, 150–183. [Google Scholar] [CrossRef]
- Miyazaki, M.; Uoto, K.; Sugimoto, Y.; Naito, H.; Yoshida, K.; Okayama, T.; Kawato, H.; Miyazaki, M.; Kitagawa, M.; Seki, T.; et al. Discovery of DS-5272 as a Promising Candidate: A Potent and Orally Active P53-MDM2 Interaction Inhibitor. Bioorg. Med. Chem. 2015, 23, 2360–2367. [Google Scholar] [CrossRef]
- Jiang, Q.; Dong, J.; Lei, F.; Yu, D.; Li, T.; Sun, H.; Xue, D. Strain-Release Driven Spirocyclization of Bicyclo [1.1.0] Butanes: Access to 6,7-Diazaspiro [3.4] Octanes. Chem. Sci. 2025, 16, 12189–12195. [Google Scholar] [CrossRef] [PubMed]
- Ratni, H.; Ebeling, M.; Baird, J.; Bendels, S.; Bylund, J.; Chen, K.S.; Denk, N.; Feng, Z.; Green, L.; Guerard, M.; et al. Discovery of Risdiplam, a Selective Survival of Motor Neuron-2 (SMN2) Gene Splicing Modifier for the Treatment of Spinal Muscular Atrophy (SMA). J. Med. Chem. 2018, 61, 6501–6517. [Google Scholar] [CrossRef] [PubMed]
- Ratni, H.; Green, L.; Naryshkin, N.A.; Weetall, M.L. Compounds for Treating Spinal Muscular Atrophy. WO2015173181, 19 November 2015. [Google Scholar]
- Thirumalai Rajan, S.; Sajja, E.; Mathad, V.T.; Ismail Subuddhi, P. Process for the Preparation of Risdiplam and Its Intermediates. Technical Disclosure Commons. Available online: https://www.tdcommons.org/dpubs_series/5296/ (accessed on 31 July 2022).
- Moessner, C.; Hoffmann-Emery, F.; Adam, J.M.; Fantasia, S.; Fishlock, D.V.; Meier, R.; Wuitschik, G.; Ratni, H. Development and Optimization of the Manufacturing Process for RNA-Splicing Modifier Risdiplam RG7916. In Complete Accounts of Integrated Drug Discovery and Development: Recent Examples from the Pharmaceutical Industry; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2022; Volume 1423, pp. 301–332. [Google Scholar] [CrossRef]
- Kothakonda, K.K.; Pitta, B.R.; Namana, S.B.; Rangisetty, J.B.; Pullagurla, M.R. A Novel Process for the Preparation of 7-(4,7-Diazaspiro[2.5]octan-7-yl)-2-(2,8-Dimethylimidazo[1,2-b]Pyridazin-6-yl)Pyrido-4H-[1,2-a]Pyrimidin-4-one with Novel Intermediates. WO2024003798, 4 January 2024. [Google Scholar]
- Adam, J.-M.; Pfleger, C.; Wuitschik, G. Process for Preparing Risdiplam. WO2022194909, 22 September 2022. [Google Scholar]
- Kandula, S.; Yadlapalli, R.K.; Vipparla, B.B.; Thumati, S.; Nagapuri, S.; Parakala, S.; Suthrapu, S.; Dandala, R.; Muddasani, P.R.; Nannapaneni, V.C. Improved Process for the Preparation of Risdiplam and its Intermediates. WO2024069646, 4 April 2024. [Google Scholar]
- Meier, R.; Schwitter, U.; De Paepe, A.; Kuehl, P.; Thun, J.; Stowasser, F. New Forms of Pyrido[1,2-a]Pyrimidin-4-one Derivatives, its Formulation and its Process of Making. WO2020079203, 23 April 2020. [Google Scholar]
- Pradhan, N.S.; Telange, V.R.; Sonar, Y.S.; Shinde, V.C.; Minhas, H.S.; Minhas, G.S. Novel Crystalline Form of Risdiplam and Process for Preparation Thereof. WO2024236590, 21 November 2024. [Google Scholar]
- Lengauer, H. Crystalline Form of Risdiplam. WO2022162107, 4 August 2022. [Google Scholar]
- Matečić Mušanić, S.; Klarić, D.; Kolenić, M. Solid State Forms of Risdiplam and Process for Preparation Thereof. WO2021021775, 4 February 2021. [Google Scholar]
- Korenev, G.; Gutenev, A.A.; Antipin, F.V.; Chernyshov, V.V.; Korobkina, M.P.; Nawrozkij, M.B.; Ivanov, R.A. A Brand-New Metal Complex Catalyst-Free Approach to the Synthesis of 2,8-Dimethylimidazo[1,2-b] Pyridazine-6-Carboxylic Acid—A Key Intermediate in Risdiplam Manufacturing Process. Molecules 2025, 30, 3011. [Google Scholar] [CrossRef] [PubMed]
- Korenev, G.; Gutenev, A.A.; Antipin, F.V.; Chernyshov, V.V.; Shulgina, J.A.; Korobkina, M.P.; Nawrozkij, M.B.; Ivanov, R.A. A Convenient, Pd-Free Approach to the Synthesis of Risdiplam. Molecules 2025, 30, 3375. [Google Scholar] [CrossRef]
- Hoang, G.L.; Zoll, A.J.; Ellman, J.A. Three-Component Coupling of Aldehydes, 2-Aminopyridines, and Diazo Esters via Rhodium (III)-Catalyzed Imidoyl C-H Activation: Synthesis of Pyrido[1,2-a] Pyrimidin-4-Ones. Org. Lett. 2019, 21, 3886–3890. [Google Scholar] [CrossRef]
- Jones, E.D.; Vandegraaff, N.; Le, G.; Choi, N.; Issa, W.; Macfarlane, K.; Thienthong, N.; Winfield, L.J.; Coates, J.A.V.; Lu, L.; et al. Design of a Series of Bicyclic HIV-1 Integrase Inhibitors. Part 1: Selection of the Scaffold. Bioorg Med. Chem. Lett. 2010, 20, 5913–5917. [Google Scholar] [CrossRef]
- Yu, L. Pyridino [1, 2-a] Pyrimidone Analogue and Application Thereof in Preparation of FGFR (Fibroblast Growth Factor Receptor) inhibitor. CN114853753, 20 April 2022. [Google Scholar]
- Le, G.; Vandegraaff, N.; Rhodes, D.I.; Jones, E.D.; Coates, J.A.V.; Lu, L.; Li, X.; Yu, C.; Feng, X.; Deadman, J.J. Discovery of Potent HIV Integrase Inhibitors Active against Raltegravir Resistant Viruses. Bioorg Med. Chem. Lett. 2010, 20, 5013–5018. [Google Scholar] [CrossRef]
- Qi, H.; Choi, S.; Dakka, A.; Karp, G.M.; Narasimhan, J.; Naryshkin, N.; Turpoff, A.A.; Weetall, M.L.; Welch, E.; Woll, M.G.; et al. Compounds for Treating Spinal Muscular Atrophy. WO2013119916, 15 August 2013. [Google Scholar]
- Jie, F.; Wancheng, G.; Jinfu, D.; Dingjun, C.; Xiaoqiang, X. Synthesis Method of Lisethopram and Intermediate of Lisethopram. CN119504737, 25 February 2025. [Google Scholar]
- Ebeling, M.; Metzger, F.; Sivaramakrishnan, M. Screening Method. WO2015024876, 26 February 2015. [Google Scholar]
- Woll, M.G.; Qi, H.; Turpoff, A.; Zhang, N.; Zhang, X.; Chen, G.; Li, C.; Huang, S.; Yang, T.; Moon, Y.C.; et al. Discovery and Optimization of Small Molecule Splicing Modifiers of Survival Motor Neuron 2 as a Treatment for Spinal Muscular Atrophy. J. Med. Chem. 2016, 59, 6070–6085. [Google Scholar] [CrossRef] [PubMed]
- Ratni, H.; Karp, G.M.; Weetall, M.; Naryshkin, N.A.; Paushkin, S.V.; Chen, K.S.; McCarthy, K.D.; Qi, H.; Turpoff, A.; Woll, M.G.; et al. Specific Correction of Alternative Survival Motor Neuron 2 Splicing by Small Molecules: Discovery of a Potential Novel Medicine to Treat Spinal Muscular Atrophy. J. Med. Chem. 2016, 59, 6086–6100. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, R.J.; Ratni, H.; Sarie, J.C. Compounds for Treating Spinal Muscular Atrophy. WO2016184832, 24 November 2016. [Google Scholar]
- Minhas, H.S.; Minhas, G.S.; Pradhan, N.S.; Telange, V.R.; Arabune, A.S.; Mardhekar, V.V.; Sonar, Y.S. Process for Preparation of Risdiplam, Novel Intermediates, and Process for Preparation Thereof. WO2024154148, 25 July 2024. [Google Scholar]
- McCarthy, K.D.; Metzger, F.; Ratni, H. Compounds for Treating Amyotrophic Lateral Sclerosis. WO2017081111, 18 May 2017. [Google Scholar]
- Roma, G.; Cinone, N.; Di Braccio, M.; Grossi, G.; Leoncini, G.; Signorello, M.G.; Carotti, A. Synthesis, Antiplatelet Activity and Comparative Molecular Field Analysis of Substituted 2-Amino-4 H -Pyrido[1,2-a] Pyrimidin-4-Ones, Their Congeners and Isosteric Analogues. Bioorg Med. Chem. 2000, 8, 751–768. [Google Scholar] [CrossRef]
- Richards, S.; Derudas, M.; Ahlsten, N.; Papachristos, K. MPRO Targeting Antiviral Compounds. WO2023180189, 28 September 2023. [Google Scholar]
- Guan, H.; Wu, C.; Yu, T.; Huang, L.; Hao, D.; Gao, B.; Sun, J.; Shi, N.; Chen, S. Pyridino[1,2-a]Pyrimidone Analogue Used as mTOR/PI3K Inhibitor. WO2015192761, 23 December 2015. [Google Scholar]
- Ahuja, V.; Bi, Y.; Cole, A.G.; Dorsey, B.D.; Fan, Y.; Kakarla, R.; Kirk, S.M.; Nguyen, D.; Ozturk, S.; Quintero, J.; et al. Substituted 1,1′-Biphenyl Compounds and Methods Using Same. WO2021158481, 12 August 2021. [Google Scholar]
- Averin, A.D.; Abel, A.S.; Grigorova, O.K.; Latyshev, G.V.; Kotovshchikov, Y.N.; Mitrofanov, A.Y.; Bessmertnykh-Lemeune, A.; Beletskaya, I.P. Recent Achievements in Copper Catalysis for C–N Bond Formation. Pure Appl. Chem. 2020, 92, 1181–1199. [Google Scholar] [CrossRef]
- Mo, B.; Chen, C.; Peng, J. CuI-Catalyzed Synthesis of Multisubstituted Pyrido [1,2- a] Pyrimidin-4-Ones through Tandem Ullmann-Type C–N Cross-Coupling and Intramolecular Amidation Reaction. RSC Adv. 2023, 13, 24264–24271. [Google Scholar] [CrossRef] [PubMed]
- Gaube, G.; Mutter, J.; Leitch, D.C. A “Neat” Synthesis of Substituted 2-Hydroxy-Pyrido[1,2-a] Pyrimidin-4-Ones. Can. J. Chem. 2024, 102, 206–213. [Google Scholar] [CrossRef]
- Wu, C.; Yu, T.; Chen, S. Pyridino[1,2-a] Pyrimidone Analogue Used as PI3K Inhibitor. WO2015192760, 23 December 2015. [Google Scholar]
- Park, D.S.; Jo, E.; Choi, J.; Lee, M.E.; Kim, S.; Kim, H.Y.; Nam, J.; Ahn, S.; Hwang, J.Y.; Windisch, M.P. Characterization and Structure-Activity Relationship Study of Iminodipyridinopyrimidines as Novel Hepatitis C Virus Inhibitor. Eur. J. Med. Chem. 2017, 140, 65–73. [Google Scholar] [CrossRef]
- Yalduz, S.; Yilmaz, M. Microwave Assisted Synthesis of 2,3-Dihydro-4H- Furo[2,3-d] Pyrido[1,2-a] Pyrimidin-4-Ones and Furo[2,3-d] Pyrido[1,2-a] Pyrimidin-4-One. ChemistrySelect 2023, 8, e202204260. [Google Scholar] [CrossRef]
- Yalduz, S.; Sarı, S.; Yılmaz, M. Microwave Assisted Synthesis, Acetylcholinesterase Inhibition and Molecular Docking Studies of Furo[2,3-d] Pyrido [1,2-a] Pyrimidin-4-One Derivatives. J. Heterocycl. Chem. 2024, 61, 1517–1530. [Google Scholar] [CrossRef]
- Adam, J.M.; Fantasia, S.M.; Fishlock, D.V.; Hoffmann-Emery, F.; Moine, G.; Pfleger, C.; Moessner, C. Process for the Preparation of 7-(4,7-Diazaspiro [2.5] Octan-7-yl)-2-(2,8-Dimethylimidazo[1,2-b] Pyridazin-6-yl) Pyrido[1,2-a] Pyrimidin-4-one Derivatives. WO2019057740, 28 March 2019. [Google Scholar]
- Chen, G.; Bhattacharyya, A.; Jiang, Y.; Karp, G.M.; Narasimhan, J.; Turpoff, A.; Zhang, N. Heteroaryl Compounds for Treating Huntington’s Disease. WO2020005882, 2 January 2020. [Google Scholar]
- Dolente, C.; Grether, N.; O’Hara, F.S.; Piras, M.; Ratni, H.; Reutlinger, M.; Vifian, W.; Zambaldo, C. New Thiadiazolopyrimidone Derivatives. WO2022194802, 22 September 2022. [Google Scholar]
- Reynolds, D.; Seiler, M.W.; Agrawal, A.A.; Vaillancourt, F.; Smith, P.; Prajapati, S.; Hopper, A.T.; Vyskocil, S.; Moreau, B. Compounds and Methods for Modulating Splicing. WO2023097007, 1 June 2023. [Google Scholar]
- Woll, M.G.; Amedzo, L.; Babu, S.; Barraza, S.J.; Bhattacharyya, A.; Karp, G.M.; Mazzotti, A.R.; Narasimhan, J.; Patel, J.; Turpoff, A.; et al. Compounds for Treating Huntington’s Disease. WO2018226622, 13 December 2018. [Google Scholar]
- Reynolds, D.; Seiler, M.W.; Agrawal, A.A.; Vaillancourt, F.; Smith, P.; Prajapati, S.; Hopper, A.T.; Vyskocil, S.; Sirin, G.S. Compounds and Methods for Modulating Splicing. WO2023034811, 9 March 2023. [Google Scholar]
- Wager, T.T.; Weng, Z.; Xi, H.S. Heterocyclic Substituted 1,3,4-Thiadiazole and Pyridazine Compounds and Methods of Using the Same. WO2023092149, 25 May 2023. [Google Scholar]
- Reynolds, D.; Leger, S.; Seiler, M.W.; Agrawal, A.A.; Vaillancourt, F.; Smith, P.; Hopper, A.T.; Prajapati, S.; Soueidan, O. Compounds and Methods for Modulating Splicing. WO2021207554, 14 October 2021. [Google Scholar]
- Reynolds, D.; Seiler, M.W.; Agrawal, A.A.; Vaillancourt, F.; Smith, P.; Prajapati, S.; Hopper, A.T.; Vyskocil, S.; Moreau, B. Compounds and Methods for Modulating Nucleic Acid Splicing. WO2023064880, 20 April 2023. [Google Scholar]
- Xing, Q.; Chandrachud, P.; Tillett, K.; Lopchuk, J. Regioselective Hydroamination of Unactivated Olefins with Diazirines as a Diversifiable Nitrogen Source. Nat. Commun. 2024, 15, 6049. [Google Scholar] [CrossRef]
- Pollak, A.; Stanovnik, B.; Tišler, M. Synthesis of Pyridazine Derivatives—XVI. Tetrahedron 1968, 24, 2623–2629. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kajiwara, Y.; Noguchi, M.; Kajiwara, T.; Tabuchi, T. Fused Heterocyclic Sulfonylurea Compound, Herbicide Containing the Same, and Method of Controlling Weed with the Same. WO2003061388, 31 July 2003. [Google Scholar]
- Almario Garcia, A.; Burnier, P.; Cote-Des Combes, S.; Gilbert, J.-F.; Pacaud, C.; Puech, F.; Chiang, Y.; Davis, L.; Gao, Z.; Zhao, Q. 2-alkyl-6-Cycloamino-3-(Pyridin-4-yl)Imidazo[1,2-b]Pyridazine Derivatives, Preparation Thereof, and Therapeutic Application Thereof. WO2010018327, 18 February 2010. [Google Scholar]
- Jacobson, R.M.; Raths, R.A.; McDonald, J.H. 2-Methoxyallyl Bromide. A Superior Acetonyl Alkylating Agent. J. Org. Chem. 1977, 42, 2545–2549. [Google Scholar] [CrossRef]
- Takahayashi, N. Synthesis of Pyridazine Derivatives. VIII. Nucleophilic Substitution of 3, 6-Dichloro-4-Methylpyridazine. Pharm. Bull. 1957, 5, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Barlin, G. Imidazo[1,2-B] Pyridazines. I. Some 3-Alkoxy-6-Halogeno-2-Phenyl-(and 4′-Substituted Phenyl) Imidazo[1,2-B] Pyridazines and 3-Methoxy-2,6-Diphenylimidazo[1,2-B] Pyridazine. Aust. J. Chem. 1986, 39, 1803. [Google Scholar] [CrossRef]
- Mori, K. Synthesis of 1, 2-Diazine Derivatives. VII. Yakugaku Zasshi J. Pharm. Soc. Jpn. 1962, 82, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Farmer, L.J.; Fournier, P.A.; Lessard, S.; Liu, B.; St-Onge, M.; Sturino, C.; Szychowski, J.; Yannopoulos, C.; Vallee, F.; Lacoste, J.E.; et al. Imidazopyridazines Useful as Inhibitors of the PAR-2 Signaling Pathway. WO2015048245, 2 April 2015. [Google Scholar]
- Hu, Z.; He, H.; Zhang, F.; Zhu, X.; Zhong, W. Cdk Inhibitors. WO2020224568, 12 November 2020. [Google Scholar]
- Dong, J.; Fang, W.; Guo, W.; Fang, J.; Chu, D.; Xie, X. Synthesis Method of Lisethopram intermediate and Lisethopram Intermediate. CN116947865, 27 October 2023. [Google Scholar]
- Jiang, Y. 5-Methyl-2-(Pyridinyl-2-Amino)-8H-Pyrido[2,3-d]Pyrimidin-7-Ketone Compounds. CN105111201, 11 January 2017. [Google Scholar]
- Wang, Y.; Zhao, J. Substituted Pyridopyrimidinone Compound, Composition Comprising Same, and Use Thereof. WO2023202501, 26 October 2023. [Google Scholar]
- Xu, J.; Han, Z.; Li, K.; Lin, H.; Chen, G. Preparation Method of Lisethopram. CN117050096, 14 November 2023. [Google Scholar]
- Wu, L.; You, X.; Zhao, L.; Chen, D.; Chen, S. Nitrogen-Containing Tricyclic Bifunctional Compound, Preparation Method Therefor, and Application Thereof. WO2022206924, 6 October 2022. [Google Scholar]
- Kim, M.; Kim, J.S.; Ryu, H.C.; Lim, J.W.; Yoo, D. Compounds for Inhibiting CDK and Medical Use Thereof. KR20210156400A, 27 December 2021. [Google Scholar]
- Dong, J.; Fang, W.; Guo, W.; Fang, J.; Chu, D.; Xie, X. Method for Synthesizing Risdiplam Intermediate and Risdiplam INTERMEDIATE. WO2025036129, 20 February 2025. [Google Scholar]
- Zhu, Y.; Back, T.G. Preparation of 1,7- and 3,9-Dideazapurines from 2-Amino-3-Iodo- and 3-Amino-4-Iodopyridines and Activated Acetylenes by Conjugate Addition and Copper-Catalyzed Intramolecular Arylation. J. Org. Chem. 2014, 79, 11270–11276. [Google Scholar] [CrossRef]
- Chen, Z.; Wen, Y.; Ding, H.; Luo, G.; Ye, M.; Liu, L.; Xue, J. Silver-Catalyzed Highly Efficient Synthesis of Pyrido[1,2-a] Pyrimidin-4-Ones from 2-Aminopyridines and Alkynoates. Tetrahedron Lett. 2017, 58, 13–16. [Google Scholar] [CrossRef]
- Hussain, M.; Liu, J. Practical Synthesis of 4H-Pyrido [1, 2-a] Pyrimidin-4-Ones Using Ethylene Glycol as a Promoting Solvent. Tetrahedron Lett. 2020, 61, 152269. [Google Scholar] [CrossRef]
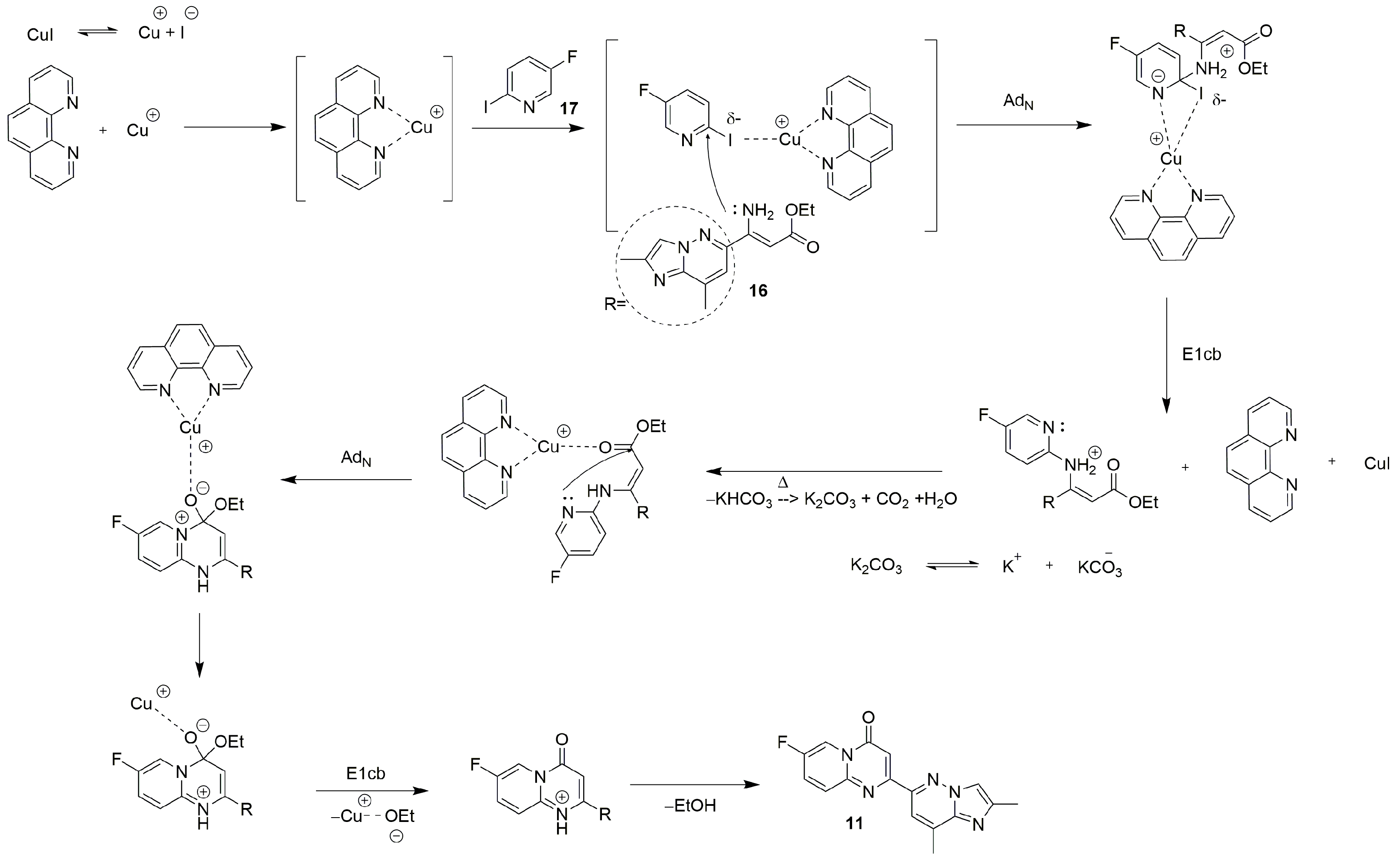
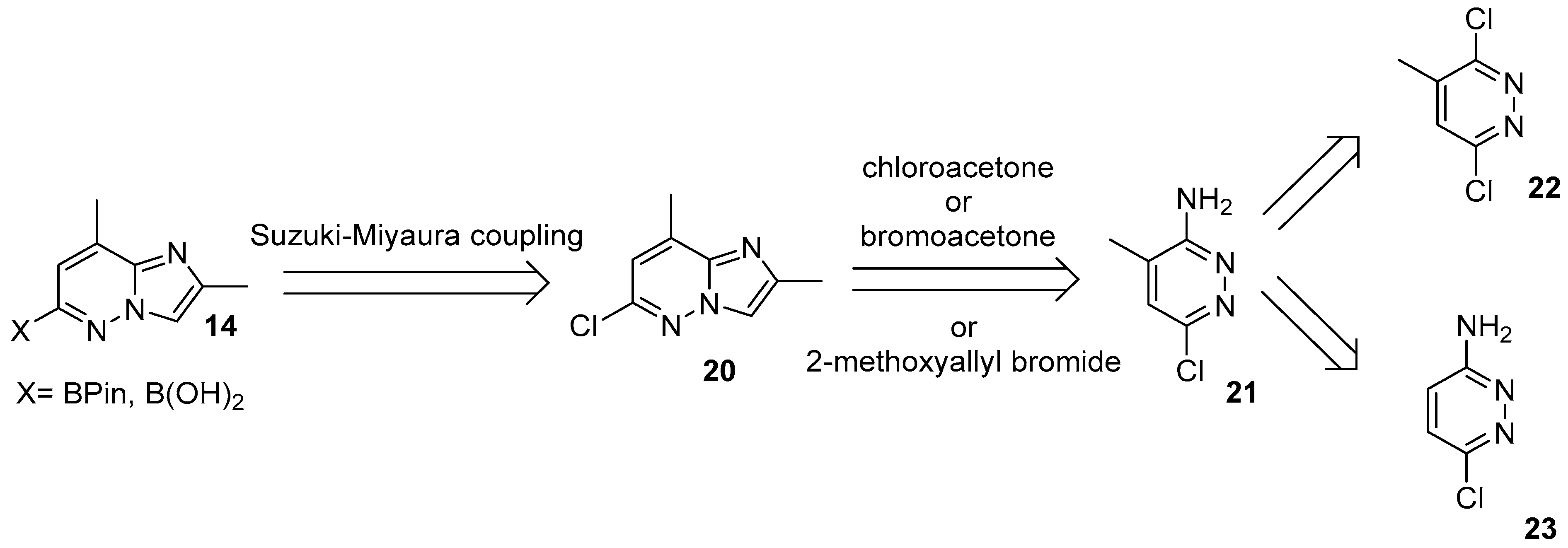
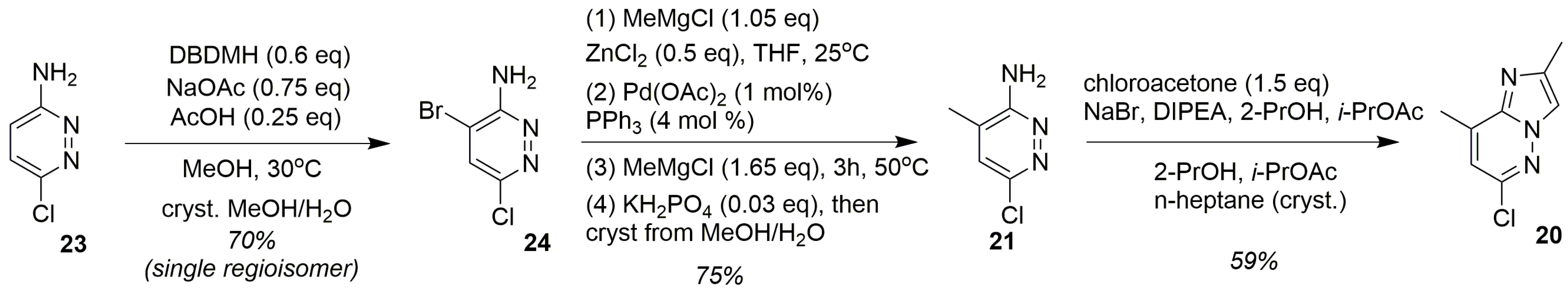
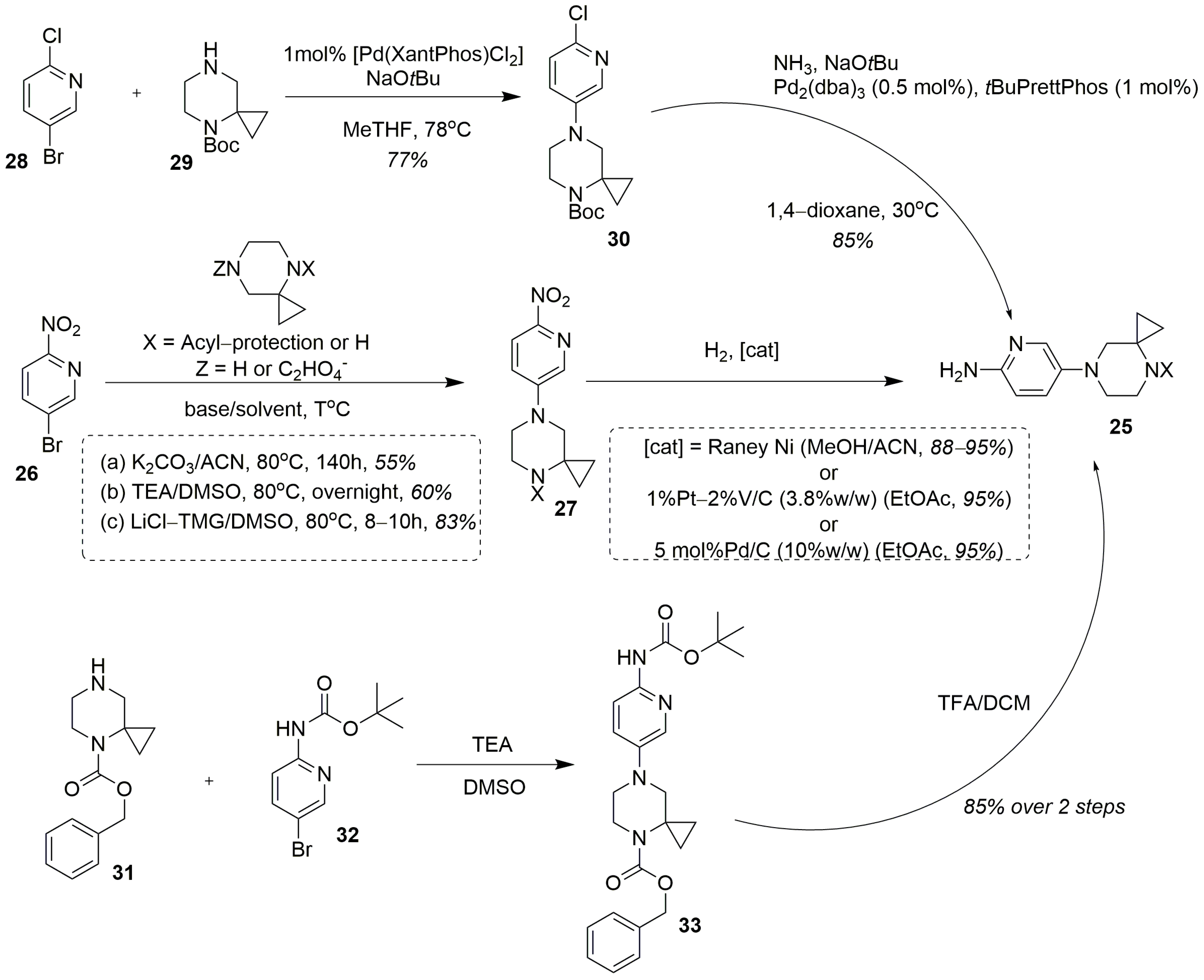
| Substrate | Substitute | Reaction Conditions | Yield (%) | Reference |
|---|---|---|---|---|
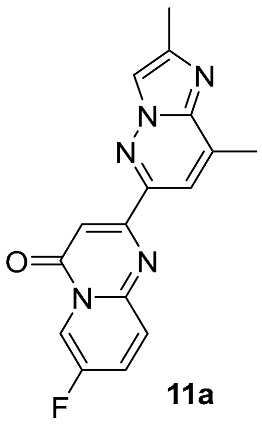 | 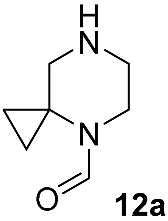 | K2CO3 DMSO, 120–160 °C overnight | 50 | [50] |
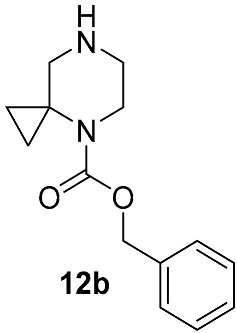 | DIPEA DMSO,130 °C 48 h | 47 | [33] | |
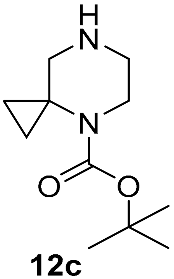 | DBU DMAc,120 °C 6 h | 42 | [39] | |
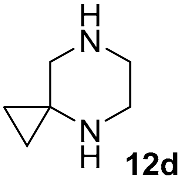 | DIPEA DMSO 130 °C,48 h | 18 | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korenev, G.; Nawrozkij, M.B.; Ivanov, R.A. Comprehensive Risdiplam Synthesis Overview: From Cross-Coupling Reliance to Complete Palladium Independence. Molecules 2025, 30, 4365. https://doi.org/10.3390/molecules30224365
Korenev G, Nawrozkij MB, Ivanov RA. Comprehensive Risdiplam Synthesis Overview: From Cross-Coupling Reliance to Complete Palladium Independence. Molecules. 2025; 30(22):4365. https://doi.org/10.3390/molecules30224365
Chicago/Turabian StyleKorenev, Georgiy, Maxim B. Nawrozkij, and Roman A. Ivanov. 2025. "Comprehensive Risdiplam Synthesis Overview: From Cross-Coupling Reliance to Complete Palladium Independence" Molecules 30, no. 22: 4365. https://doi.org/10.3390/molecules30224365
APA StyleKorenev, G., Nawrozkij, M. B., & Ivanov, R. A. (2025). Comprehensive Risdiplam Synthesis Overview: From Cross-Coupling Reliance to Complete Palladium Independence. Molecules, 30(22), 4365. https://doi.org/10.3390/molecules30224365






