Abstract
Risdiplam is the first approved small-molecule therapy for spinal muscular atrophy (SMA), a severe, progressive neuromuscular disorder. In addition to its clinical significance, risdiplam is of a great interest for organic and medicinal chemistry due to its complex molecular architecture. Its structure incorporates three highly substituted heterocyclic fragments—imidazo[1,2-b]pyridazine, pyrido[1,2-a]pyrimidin-4-one, and 4,7-diazaspiro[2.5]octane—that serve as both versatile synthetic building blocks and critical pharmacophoric elements for drug design and discovery. The increasing scientific interest in risdiplam has led to numerous publications and patent applications that describe alternative synthetic methodologies. Recently, our group has also developed and introduced efficient, scalable manufacturing routes for the preparation of the target substance and the key intermediates of its synthesis. This mini-review systematically analyzes a plethora of risdiplam assembly strategies and synthetic approaches, covering developments from 2013 to the present.
1. Introduction
Spinal muscular atrophy (SMA), an autosomal recessive disorder that affects approximately 1 in 6000–10,000 newborns worldwide due to homozygous deletion of SMN1 gene; it represents one of pediatric medicine’s most urgent therapeutic challenges. The condition is asymptomatic at birth but rapidly progresses to a neuromuscular disease that, in its most severe Type 1 form, typically manifests by three months of age and leads to death or permanent ventilator dependence within the first two years of life in the absence of disease-modifying treatment [1,2]. Risdiplam (Evrysdi) is an orally bioavailable SMN2 splicing modifier for the treatment of 5q spinal muscular atrophy across the full clinical spectrum, from presymptomatic infants to adults [3]. The drug’s safety and efficacy were confirmed in a comprehensive clinical development program (Figure 1). In the pivotal FIREFISH study of risdiplam in infantile-onset SMA, 41% of treated infants achieved sitting without support for ≥5 s at 12 months, and 90% survived without permanent ventilation, establishing risdiplam’s efficacy in the most severe phenotype, where respiratory and nutritional complications typically limit therapeutic options [3,4]. The randomized, placebo-controlled SUNFISH trial has demonstrated in turn, statistically significant motor function improvements in patients with later-onset SMA (Types 2 and 3), with benefits most pronounced in younger participants [3,5].
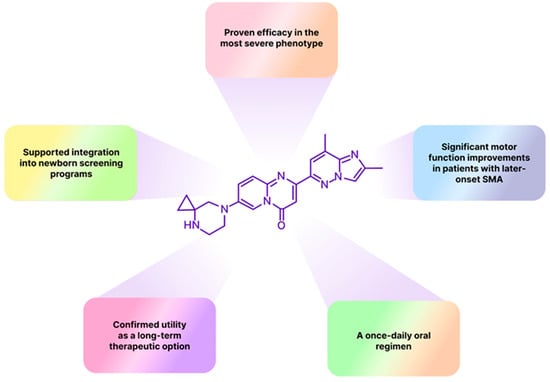
Figure 1.
Outstanding therapeutic and pharmaceutical characteristics of risdiplam.
The JEWELFISH study has provided safety and tolerability data for patients previously treated with other SMA therapies, demonstrating maintained tolerability over 24 months, with adverse event rates decreasing in the second year, supporting risdiplam’s utility as a long-term therapeutic option [6,7]. The RAINBOWFISH study showed that presymptomatic infants receiving risdiplam achieved age-appropriate motor milestones, which underpinned the FDA label expansion in May 2022 and catalyzed broader adoption of newborn screening programs for spinal muscular atrophy [8,9,10]. Finally, in September 2025, a nationwide real-world observational analysis provided results on the risdiplam use in adults. Patients with spinal muscular atrophy, predominantly Type 2 (40.4%) and Type 3 (47.4%), with a median age of 35.7 years and a median disease duration of 29.6 years, exhibited sustained, clinically meaningful functional gains that became most evident beyond 18 months of therapy. This data supports durable long-term treatment with risdiplam in adults and meaningfully closes the previously recognized evidence gap in the adult population treatment [11]. Overall, a once-daily oral dosing combined with efficacy in all SMA phenotypes positions risdiplam as a practical outpatient therapy across the disease spectrum.
However, beyond its exceptional clinical significance, risdiplam also showcases how structurally complex, highly substituted heterocyclic motifs can function as privileged scaffolds in contemporary drug discovery (Figure 2). The imidazo[1,2-b]pyridazine core exemplifies its versatility, most notably in the FDA-approved multi-kinase inhibitor ponatinib (Iclusig®) for chronic myeloid leukemia [12], and across diverse therapeutic programs including brain-penetrant GSK-3β inhibitors [13], orally active TYK2 JH2 inhibitors for autoimmune diseases [14] and various molecules with antimicrobial properties [15,16]. Recent structure–activity relationship studies highlight its capacity for systematic optimization through strategic substitution patterns, enabling precise modulation of potency, selectivity, and pharmacokinetic properties of the target substances [16].
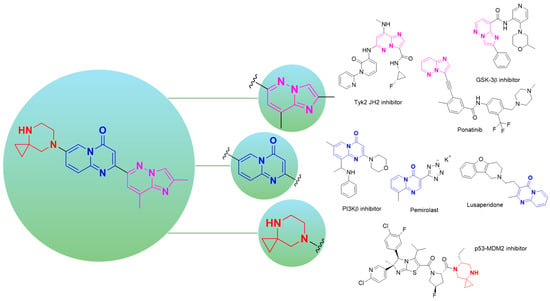
Figure 2.
Highlighting three structural blocks within risdiplam— purple, imidazo[1,2-b]pyridazine; blue, pyrido[1,2-a]pyrimidin-4-one; red, 4,7-diazaspiro[2.5]octane —and a representative set of therapeutics and bioactive small molecules containing these building blocks.
The pyrido[1,2-a]pyrimidin-4-one motif in turn, represents one of the most pharmaceutically successful heterocyclic scaffolds, forming the core of numerous approved drugs including the anti-allergic agent pemirolast [17] and the antidepressant lusaperidone [18]. This framework continues to attract substantial interest from medicinal chemists, with applications spanning aldose reductase inhibitors [19], PI3K inhibitors for oncology [20], and CXCR3 inhibitors for inflammatory diseases [21]. Its synthetic accessibility via diverse methodologies and favorable drug-like properties position it as a valuable building block for pharmaceutical development [22].
Finally, the 4,7-diazaspiro[2.5]octane fragment reflects the growing recognition of spirocyclic systems as privileged building blocks in drug design [23]. While less explored than other spirocyclic frameworks, related diazaspiro systems have demonstrated utility in medicinal chemistry [24], including a promising p53–MDM2 interaction inhibitor [25]. Spirocyclic scaffolds, in general, offer distinct advantages including enhanced three-dimensionality, increased sp3 fraction, and conformational rigidity that enable exploration of previously inaccessible chemical space. This flexibility has been further enhanced by recent advances in novel strain-release spirocyclization methodologies, which have improved access to diverse diazaspiro frameworks, enabling their integration into screening libraries and lead optimization campaigns [26]. The widespread and successful utilization of these heterocyclic frameworks in targeted design of pharmacologically active compounds with a given type of biological activity unequivocally confirms that risdiplam serves not only as a breakthrough therapeutic agent but also as a valuable repository of privileged structural motifs for future drug discovery endeavors.
The synthetic evolution of risdiplam itself, which also represents a compelling case study in modern pharmaceutical process development, encompassing over a decade of innovation from initial discovery through industrial optimization to contemporary academic refinements is also worth paying attention to. The timeline of synthetic innovation (Figure 3) reflects the collaborative efforts of diverse research communities worldwide—from industrial process chemists optimizing manufacturing efficiency to academic groups developing novel metal-free methodologies—each contributing unique perspectives and technical solutions to the synthetic challenges posed by risdiplam’s complex architecture.
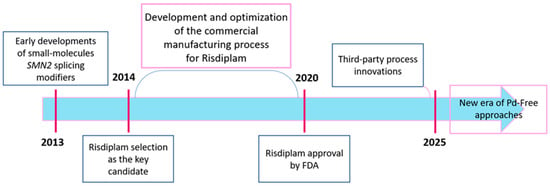
Figure 3.
The timeline of risdiplam synthetic development and innovation.
The original industrial synthetic scheme, documented in Roche’s foundational patents and process disclosures, established a multi-step palladium-catalyzed approach featuring borylation and Suzuki-type cross-coupling reactions to assemble the key heterocyclic fragments (Figure 4) [27,28]. This baseline methodology, while effective for establishing proof-of-concept and early clinical supplies, utilized multiple purification steps that presented scalability challenges for commercial manufacturing.
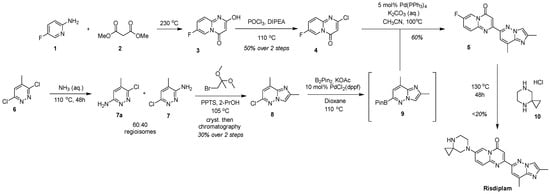
Figure 4.
The originally developed scheme for the synthesis of risdiplam.
The industrial process evolution took around a decade, through late 2014 until early 2024, as evidenced by a growing patent landscape encompassing not only the original Roche intellectual property but also third-party process innovations claiming improved telescoped sequences, alternative protecting group strategies, and enhanced impurity control methodologies [29,30,31,32,33]. This period also witnessed the expansion of the patent landscape to include polymorph patents, formulation improvements, and combination therapy claims, reflecting the comprehensive intellectual property strategy surrounding a commercially successful pharmaceutical [34,35,36,37]. The most recent phase of synthetic development, exemplified by our group’s 2025 academic publications, has introduced paradigm-shifting methodologies that fundamentally challenge the metal-dependence of traditional approaches [38,39]. These innovations not only address cost and supply chain considerations inherent in palladium-based processes, but they also open up new avenues for synthetic exploration and optimization strategies.
This rich tapestry of synthetic evolution—spanning originator industrial chemistry, competitive process development, and cutting-edge academic innovation—has generated a substantial body of literature encompassing diverse strategic approaches, novel intermediates, and innovative methodologies. The accumulated knowledge base now warrants systematic analysis to distill key insights, evaluate synthetic efficiency trends, and identify emerging opportunities for further advancement. This mini-review aims to provide such a comprehensive assessment, leveraging our group’s expertise to critically evaluate the benefits and limitations of each approach while highlighting the most promising directions for future synthetic methodology development.
2. Advances, Limitations, and Major Challenges in Risdiplam Synthesis
Since the first experimental preparation of risdiplam [28], numerous synthetic pathways have been reported. These can be organized into three unequal classes, that differ in fragment preparation and in the sequence used to assemble the target molecule. Risdiplam scaffold can be virtually sliced into three previously defined building blocks, labeled from A to C, to facilitate navigation, structuring, visualization and examination of alternative assembly sequences (Figure 5).
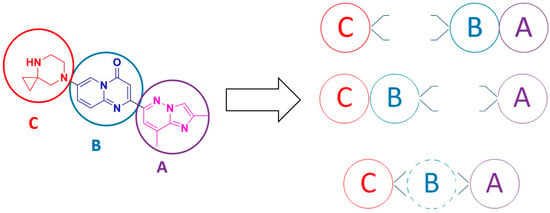
Figure 5.
Structuring and visualization of three main synthetic approaches towards the risdiplam molecule.
2.1. C + BA-Strategies Towards the Target Molecule
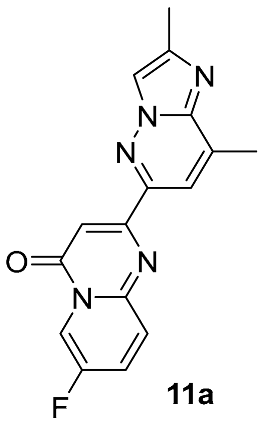
From a retrosynthetic standpoint, most routes of this kind, on the final stages rely on direct substitution of a leaving group at C-7 position in 2-(2,8-dimethylimidazo[1,2-b]pyridazin-6-yl)-7-halo-4H-pyrido[1,2-a]pyrimidin-4-one 11 with the N-4-protected or unprotected 4,7-diazaspiro[2.5]octan-7-amine moiety 12 (Figure 6). Depending on the halogen atom, the C–Hal displacement may proceed catalytically (typically for Cl- or Br-atoms) or in a catalyst-free manner (typically for F-atom). Pd-catalyzed substitutions of Cl and Br atoms are well established in related substrates and span a wide range of conditions and yields [40,41,42,43]. Nevertheless, they have seen limited adoption in risdiplam synthesis, likely because most known and well-described routes to risdiplam are already burdened with additional Pd-catalyzed cross-coupling steps, so it appears mainly as isolated examples in a few patents [44,45].
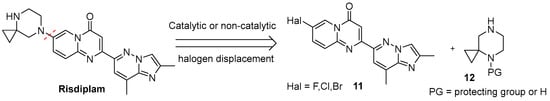
Figure 6.
The first retrosynthetic disconnection (red dotted line) in risdiplam C + BA—synthetic strategies.
In contrast, the non-catalyzed substitution at C–F has been described repeatedly for risdiplam and its bioisosteric analogs. In the early phases of risdiplam development, substitutions of the fluorine atom for cyclic and spirocyclic amine moiety were initially conducted in high-boiling polar aprotic solvents (e.g., DMA, NMP) [46,47], then in DMSO with K2CO3 or triethylamine [48,49], and—once risdiplam had been successfully secured as the lead compound—in DMSO with DIPEA [27]. Notably, during the post-discovery optimization of the final step of such synthetic approaches, the focus has shifted from solvent/base selection to the deliberate choice of a protecting group on the incoming 4,7-diazaspiro[2.5]octane 12 fragment. It is also important to note that amine 12, and its protected forms, are widely available with no restrictions from multiple commercial suppliers. It makes the development of routes from simpler precursors commercially unattractive and highly resource consuming. Accordingly, throughout this review, intermediate 12 and any of its derivatives are considered as readily available synthetic building blocks, or as intermediates, whose preparation from the unprotected/Boc-protected analogs is not expected to be challenging.
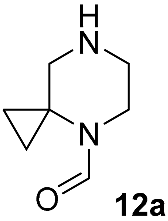
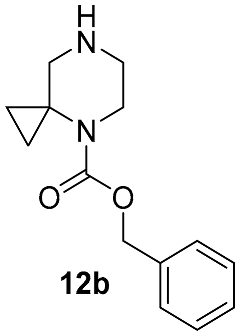
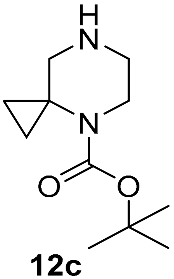
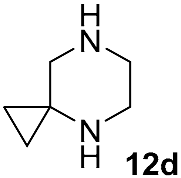
Different kinds of N-acyl protection of the spiroamine using formyl 12a [50], (benzyloxy)carbonyl 12b [33] or tert-butoxycarbonyl 12c [39] provide superior chemo- and regioselectivity in comparison with utilization of the free amine 12d [27], typically increasing the isolated yield of the substitution product (Table 1). Given broadly similar yields, Boc-protection is typically preferred owing to its mild cleavage conditions and the downstream ability to form an isolable risdiplam salt that can be readily converted to the free base.

Table 1.
Comparison of reaction conditions and yields using different protecting groups on the 4,7-diazaspiro[2.5]octane fragment, compared with the unprotected analog.
However, it is quite obvious, that the pivotal step on the path to the target molecule is the rapid, scalable route to the assembly of the key intermediate 11. As a rule, it is prepared by Pd-complex catalyzed cross-coupling of 7-halo-4H-pyrido[1,2-a]pyrimidin-4-one fragment 13, containing a good nucleofuge at C-2 position, and 2,8-dimethylimidazo[1,2-b]pyridazin-6-boronic acid (or its pinacol ester) 14 (Figure 7).
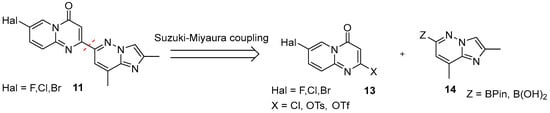
Figure 7.
The second retrosynthetic disconnection (red dotted line) in risdiplam C + BA—synthetic strategies.
In contrast to the diversity seen in the terminal substitution step, converging the two fragments 13 and 14 to afford target 11 is comparatively uniform across reports and offers limited methodological variety.
The pivotal operation is activation at C-2 position of the 7-halo-4H-pyrido[1,2-a]pyrimidin-4-one core 15 by deoxychlorination or O-acylation. For 7-fluoro derivatives, POCl3 with Hünig’s base commonly used [27,28,33,44,47,48,49,51]; neat POCl3 or the PPA/POCl3 system are also effective, particularly for 7-bromo and 7-chloro analogs [52,53,54,55]. When a better nucleofuge is required, O-tosylation (e.g., TsCl/Et3N in CH2Cl2) or triflation (e.g., PhNTf2/NaH) is employed [30,44] (Figure 8). The resulting activated intermediate 13a or 13b/13c then undergoes a standard Suzuki–Miyaura reaction with coupling-ready partner 14 in the presence of Pd(PPh3)4 and aqueous K2CO3 in MeCN at 100 °C, typically furnishing the desired product in ca. 60% yield [27,30,33]. Alternatively, the key intermediate 11 can be assembled without palladium catalysis, but via a Cu(I)-catalyzed heterocyclization between a 3-aminoacrylate 16 and 5-fluoro-2-iodopyridine 17 with 1,10-phenanthroline as the ligand of choice in the DMF/K2CO3 system at 120 °C [39] (Figure 8). It is worth noting that an easier pathway, based upon the cyclocondensation of 3-oxoester 18 and the proper 2-aminopyridine derivative 19 under catalyst-free conditions, although previously effective for closely related analogs [48], proved to be totally unsuccessful for risdiplam synthesis [39].
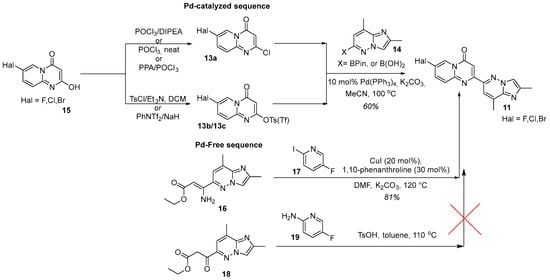
Figure 8.
Pd-catalyzed sequence: standard C-2 activation strategies on the 7-halo-4H-pyrido[1,2-a]pyrimidin-4-one core 15 and conditions for construction of the key intermediate 11; Pd-Free sequence: Cu(I)-catalyzed heterocyclization route and failed alternative approach to the same target intermediate 11.
The Pd-free heterocyclization described above, which within the chosen strategy furnishes the required block 11, is both unique and nontrivial in the context of risdiplam synthesis. In contrast to standard, well-studied palladium-catalyzed cross-coupling reactions, the present transformation is empirically validated yet lacks a definitive description of mechanism. To clarify the underlying chemistry and to enable broader application in assembling related heterocyclic frameworks, we next propose a plausible mechanism for this heterocyclization (Figure 9).
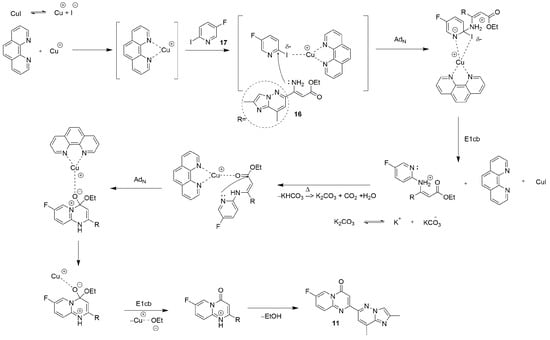
Figure 9.
The plausible mechanism for the Cu(I)-catalyzed heterocyclization of intermediates 16 and 17 to obtain the key building block 11.
Notably, 1,10-phenantroline appears to be better for this reaction, than the corresponding sterically hindered phosphine ligands. This fact is in good agreement with the data of Beletskaya and co-workers [56] and is opposite to the other published data [57].
While the final steps of risdiplam molecule construction include innovative and demanding operations, the true locus of the chemical challenge lies in accessing the requisite building blocks 14 and 15, which enable the foregoing transformations. The synthesis of 7-halo-2-hydroxypyrido[1,2-a]pyrimidin-4-one precursors 15 can be readily accomplished through established Conrad-Limpach methodology variants—employing either thermal condensation of 2-amino-5-halopyridines with dialkyl malonates at elevated temperatures [28,44,47,48,58] or their reaction with malonyl dichloride [54,59] or bis-(2,4,6-trichlorophenyl)malonate under mild conditions [60,61,62]. Among the synthetic steps towards the target compound, this condensation demands particular attention.
While malonyl dichloride, with its potent reactivity, promises the gentlest conditions for this transformation [54,59], its hydrolytic instability and corrosive nature render it a capricious and challenging reagent to handle. Bis-(2,4,6-trichlorophenyl)malonate emerges as a compelling alternative to the troublesome acyl chloride, mirroring its mildness and delivering comparable yields [60,61,62] while boasting superior stability and diminished corrosivity. Here, the 2,4,6-trichlorophenoxy group proves a good nucleofuge—a linchpin of the “addition-elimination” mechanism that drives the reaction to fruition. Conversely, the allure of less expensive dialkyl malonates (dimethyl [47,48], diethyl [58], and di-tert-butyl [63]) fades under scrutiny, their application demanding harsh conditions due to the recalcitrance of methoxy, ethoxy, and especially tert-butoxy groups to anionoid separation. Indeed, the feasibility of employing di-tert-butyl malonate hangs in considerable doubt, its bulky tert-butyl groups exerting both a significant positive inductive effect and a formidable steric hindrance around the ester carbonyl, rendering nucleophilic attack a Herculean task. Tellingly, the lone documented instance of di-tert-butyl malonate’s use resides within a Roche patent detailing the synthesis of risdiplam, standing in stark contrast to the numerous reports showcasing the utility of its more amenable congeners.
The corresponding imidazo[1,2-b]pyridazine coupling partner 14, in turn, poses significantly greater synthetic challenges due to its limited commercial availability and nonconventional substitution pattern. Even the first two retrosynthetic disconnections (Figure 10) toward intermediates of type 14 being quite simple, allow multiple practical implementations.
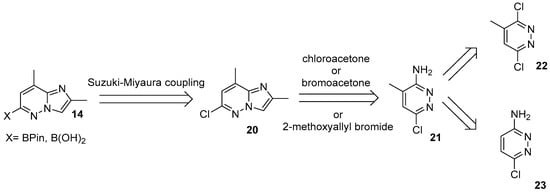
Figure 10.
Retrosynthetic pathway of the key 2,8-dimethylimidazo[1,2-b]pyridazin-6-substituted building-block 14.
Typically, boronic acid (or its pinacol ester) 14 is obtained in an almost quantitative yield, without additional purification via Miyaura borylation of the corresponding 6-chloro-2,8-dimethylimidazo[1,2-b]pyridazine 20 with B2Pin2. Common catalyst/ligand systems include different options: PdCl2(dppf) [27,28,33,51,63,64,65], XPhos–Pd variants [66], and Pd(OAc)2 with PCy3 [30]. Direct routes to the free boronic acid in turn, employ both abovementioned PdCl2(dppf) or Pd2(dba)3/XPhos system [67,68,69,70,71,72].
However, despite the nature of the required boronic acid derivative, the irreplaceable starting material for each synthesis remains imidazo[1,2-b]pyridazine 20. This compound is known for decades [73] and is recognized as an important building block for the synthesis of herbicides [74] and various pharmaceuticals. The most practical syntheses of it start from 3-amino-4-methyl-6-chloropyridazine 21, followed by annulation with bromo- or chloroacetone, or with in situ–generated 2-methoxyallyl bromide [27,67,72,73,75,76].
It is worth noting that in virtually all reported routes to risdiplam, pyridazine 21 constitutes the principal bottleneck and the most problematic intermediate. The cornerstone of the syntheses of 21 is a poor regioselectivity of the chlorine substitution in 3,6-dichloro-4-methylpyridazine 22, leading to a mixture of regioisomers together with the traces of a product of double substitution. These two regioisomers are markedly different in their melting point and can be, more or less, effectively separated either by chromatography on aluminum oxide [77] or by fractional recrystallization from ethanol [78,79]. In both cases the product is obtained by the reaction of 22 with ethanolic ammonia. Up to date, a number of modern protocols describe the use of aqueous ammonia instead for this purpose [27,51,80], but these approaches lead to the formation of the products of hydrolysis, as well, making the purification of the target compound accessible only via chromatographical techniques. Given the relatively harsh conditions, extended reaction times, and frequent reliance on chromatography, the quality of the final risdiplam—and its ability to satisfy pharmacopeial specifications—depends critically on the quality of the starting pyridazine 21. These considerations argue for a fundamentally different strategy to access this building block.
In order to secure reproducible, chromatography-free access to pyridazine 21 synthesis without isomer separation, Roche devised an alternative strategy in the course of the route optimization campaign, which was ultimately adopted in the final commercial synthesis of risdiplam. The fundamental idea was to invert the order of the introduction of the substituents, beginning with commercially available 3-amino-5-chloro-pyridazine 23 as the starting material [30]. This approach enabled precise regiocontrol through sequential functionalization steps (Figure 11).
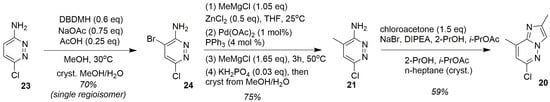
Figure 11.
Optimized manufacturing process for key intermediate 20.
The initial bromination of 23 using elemental bromine provided only 40% yield, accompanied by significant handling and storage challenges. Systematic evaluation of alternative brominating agents led to the identification of 1,3-dibromo-5,5-dimethylhydantoin (DBDMH) as the optimal reagent for this purpose. Using DBDMH in a buffered NaOAc/AcOH (3:1) solution in methanol, followed by Na2SO3 quenching and crystallization, provided compound 24 as a single regioisomer in 70% yield with >99% purity on 200 kg scale [30]. The subsequent methylation via Negishi coupling appeared to be very tedious. Initial attempts using pyrophoric dimethylzinc were deemed unsuitable for large-scale operations. The resolution involved generating the organozinc reagent in situ from readily available ZnCl2 and methylmagnesium chloride (which was added in two portions). The first portion (1.05 eq) was to control methane formation and the second (1.65 eq) was to trigger the methylation. Careful temperature control (50 °C) and Pd/L ratio optimization (1:4) to minimize bis-methylation (<1%), rendered the desired compound 21 in an excellent yield. The addition of K2HPO4 was needed to precipitate any residual zinc and palladium salts, which were filtered off just before the crystallization of the pure product from the MeOH/H2O system. The final cyclization employed 2-chloroacetone, as was mentioned before, in the presence of Hünig’s base at 80 °C, with sodium bromide, serving as a reaction facilitator. Following comprehensive workup and dual crystallization from i-PrOAc/2-PrOH/n-heptane, intermediate 20 was obtained in >99.8% purity and 59% yield from compound 21 [30]. This systematic process development transformed a low-yielding, chromatography-dependent discovery route into a scalable, high-purity manufacturing process, demonstrating the critical importance of regioselectivity control and process optimization in pharmaceutical manufacturing.
Nevertheless, despite the outstanding achievements in preparing intermediate 20, even the developed commercial process suffers from numerous drawbacks that complicate its technical implementation. The challenging palladium-catalyzed modified Negishi coupling, which is a key step in the synthetic scheme, presents several critical issues including stringent requirements for methane evolution control, rigorous removal of palladium residues, and management of bis-methylation side products. Combined with the modest overall yield of 31% for this sequence, these factors render the preparation of these intermediates as still a technically demanding challenge.
While the independent preparation of key building blocks introduces convergent elements into the synthetic strategy, the final risdiplam assembly proceeds fundamentally through a linear C + BA (Figure 5) sequence, creating exceptional opportunities for structural diversification in the design of related therapeutic compounds. This modular framework enables systematic modification of individual heterocyclic components, establishing a versatile platform for structure-activity relationship exploration and analog development.
2.2. CB + A and Convergent C + A (With the Formation of B During the Reaction) Approaches Toward Risdiplam
Contemporary risdiplam synthesis increasingly employs convergent schemes, exemplified by both Roche’s optimized commercial process and recent academic innovations that have redefined synthetic accessibility. These converging methodologies not only amplify manufacturing efficiency but also safeguard the vital flexibility needed for pioneering medicinal chemistry. This reflects a strategic metamorphosis from the nascent pathways of discovery to impeccably refined production processes, poised to meet both the rigors of commercial demand and the ever-evolving frontier of therapeutic innovation.
Besides the C+BA strategy, several convergent syntheses of risdiplam have been described and patented. The 5-(4,7-diazaspiro[2.5]octan-7-yl)pyridin-2-amine 25 turned out to be an important intermediate here. However, in most cases, it contained a protected imino-group [32,63,81]; there is also a sole exception of utilization of this amine in a free form as a key intermediate in this process [82]. Compound 25 can be prepared by a well-described two-step protocol. The first step is the amino-dehalogenation of 5-bromo-2-nitropyridine 26 with the proper spirocyclic amine, yielding the corresponding nitropyridine 27 in a free or acylated form. In most cases, a Boc-protected form of the spirocyclic amine has been used for this purpose [32,38,83,84]. The utilization of a free 4,7-diazaspiro[2.5]octane [85], however, seems doubtful due to regioselectivity issues, connected with the possible hetarylation of the two unequal nitrogen atoms in the structure of the spirocyclic amine. This reaction can be conducted either under metal-complex catalysis: for example, with Pd2(dba)3 in the presence of BINAP and Cs2CO3 in 1,4-dioxane [86], as well, as without it. In the latter cases the solvents of choice were DMSO [63,85,86] or MeCN [32], combined with triethylamine [83], potassium carbonate [32,85] or the “lithium chloride—tetramethylguanidine” system [30,63,84], as a base, giving the desired intermediate 27 (Figure 12).
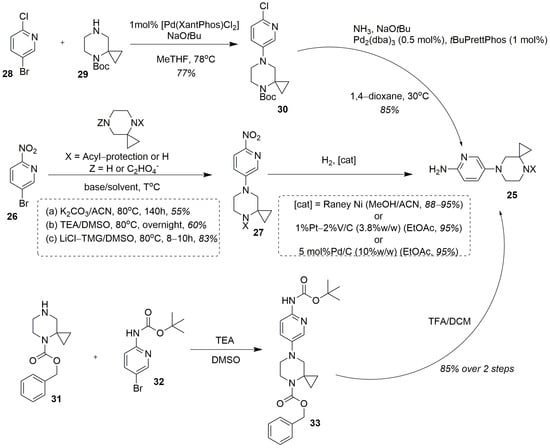
Figure 12.
Three main synthetic approaches to the key intermediate 25.
The thus obtained nitro-compound 27 is then subjected to catalytic hydrogenation (in the presence of Raney nickel [85], platinum [32] or a palladium catalyst [38,81,86]) resulting in the key intermediate 25. In the literature we can also find two synthetic protocols for the preparation of an intermediate of a kind, avoiding the use of 5-bromo-2-nitropyridine. One of them relies on a palladium-complex catalyzed amino-dehalogenation of 5-bromo-2-chloropyridine 28 with Boc-protected spiroamine 29, followed by catalytic chlorine substitution for amino-group [30,63] (Figure 12). Alternatively, the similar Cbz-protected intermediate may be prepared by direct interaction of tert-butyl N-(5-bromopyridin-2-yl)carbamate 32 with 1-Cbz-protected spiroamine 31, followed by acidic cleavage of the Boc-protective group [87]. However, this protocol also seems doubtful due to the absence of the electron-withdrawing group in para-position to the bromine atom, making it much more resistant to the uncatalyzed nucleophilic displacement.
Next, regardless of the chosen synthetic way—the key intermediate 25 may be either introduced into a modified Conrad-Limpach-type synthesis with either bis-(2,4,6-trichlorophenyl) malonate [84,85] or di-tert-butyl malonate [63], or alternatively, it can undergo a modified Gould-Jacobs heterocyclization. In the first case, a N-protected 7-(4,7-diazaspiro[2.5]octan-7-yl)-2-hydroxy-4H-pyrido[1,2-a]pyrimidin-4-one 34, which is a valuable building block for the further preparation of risdiplam, is formed. Then, the already mentioned O-tosylation [63,84] or deoxychlorination [85], followed by a palladium-complex catalyzed cross-coupling reaction, results in an N-protected form of risdiplam, which in turn, can be conveniently converted into the target compound (Figure 13).
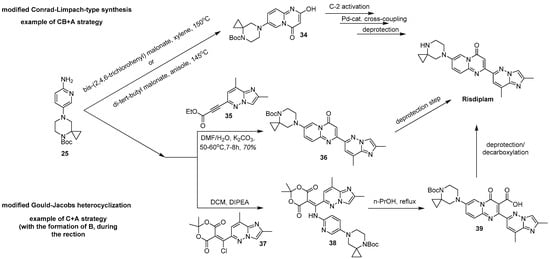
Figure 13.
Two main strategies toward risdiplam in a convergent manner from the key intermediate 25.
A modified Gould-Jacobs reaction, in turn, is based upon the interaction of ethyl ester of 3-(2,8-dimethylimidazo[1,2-b]pyridazin-6-yl)propiolic acid 35 [31,82,88] or its synthetic surrogate: 5-[chloro(2,8-dimethylimidazo[1,2-b]pyridazin-6-yl)methylidene]-2,2-dimethyl-1,3-dioxane-4,6-dione 37 (prepared in situ from the corresponding acylated Meldrum’s acid and Vilsmeyer-Haack regent) [32,38]. This then leads to either risdiplam itself (after deprotection work-up) [82] or its N-protected carboxylated form 39 [32,38] (Figure 13). The latter may be converted into the target compound upon an acidic reflux condition which is needed to afford the deprotection/decarboxylation step.
The main disadvantage of these two pathways is the low stability of both the propiolic acid derivative 35 and the intermediate 37. The first one has a high tendency for oligo- and polymerization due to the presence of a basic nitrogen together with an activated acetylenic bond in the same molecule. The second one is endowed with a highly reactive chlorine atom in its structure, capable of different substitution reactions. Moreover, it is known from the literature that the heterocyclization reaction between these kinds of propiolic acid derivatives and free 2-aminopyrdines does not proceed, due to the relatively low basicity and nucleophilicity of the latter [89]. For heterocyclization to proceed, it is necessary to use an anion-form of the corresponding N-(pyridine-2-yl)formamide, followed by in situ deprotection [89]. Alternatively, silver triflate, as a strong aprotic Lewis acid, can be postulated to catalyze such reaction with a free form of the corresponding 2-aminopyridine [90]. Finally, the use of ethylene glycol as a solvent may also promote the reaction [91]. Therefore, such significant contradictions raise doubts on the synthetic sequences, presented in the above-mentioned patent.
After all, the starting material for the synthesis of intermediates 35 and 37 is still the same: 6-chloro-2,8-dimethylimidazo[1,2-b]pyridazine 20. For the preparation of the first compound, it is introduced into the cross-coupling reaction with ethyl propiolate [31]. The preparation of the second compound is a multi-step process, finishing with the palladium-complex catalyzed carbonylation of 20, leading to the isolation of the corresponding 2,8-dimethylimidazo[1,2-b]pyridazine-6-carboxylic acid [32]. The impurities, remaining from the previous synthetic transformations appear to be critical to the success of both of the methodologies.
To overcome all these difficulties, our group has proposed a simple and convenient way that completely avoids the palladium-complex catalyzed reactions in the synthesis of risdiplam [38]. The key feature of it was the synthesis of 3-hydroxy-4-methylpyridazin-6-carboxylic acid 43 via the Knoevenagel condensation of methyl pyruvate, followed by heterocyclization of the crude reaction product with hydrazine. The thus obtained heterocyclic acid was subjected to esterification, deoxychlorination, and indirect amino-dechlorination yielding an ethyl ester of 3-amino-4-methylpyridazin-6-carboxylic acid 47. Treatment of the latter with chloroacetone led to the formation of ethyl 2,8-dimethylimidazo[1,2-b]pyridazine-6-carboxylate 48 (Figure 14) [38].
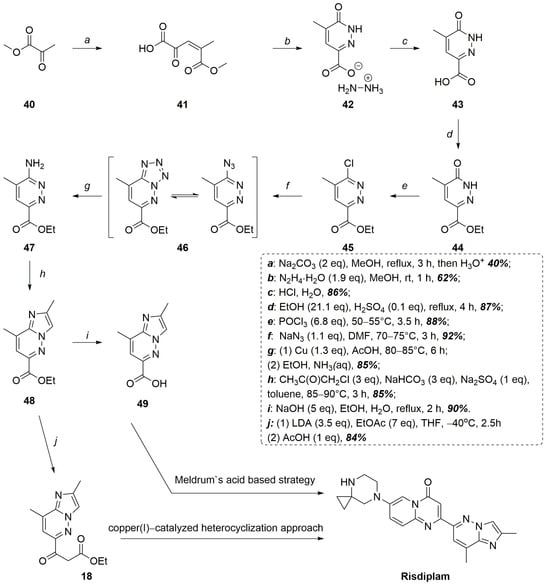
Figure 14.
Novel, total Pd-catalyzed free approaches to the synthesis of risdiplam.
Then, upon Claisen condensation with ethyl acetate it formed a 3-oxoester 18, which was successively converted into the corresponding 3-aminoacrylate 16, which is a key intermediate in the risdiplam preparation that was described earlier [39]. Alternatively, 48 may be hydrolyzed to the corresponding acid 49 and introduced into the Meldrum-acid based synthesis of risdiplam, also described previously [38]. A comprehensive head-to-head comparison of the developed routes with the originators’ optimized manufacturing process is not feasible because of substantial differences in design, logic, and numerous other important non-chemical factors. Nevertheless, a simple metric-based comparison can be performed: the optimal commercial process furnishes risdiplam in 12 steps with an overall yield of approximately 9.6%. The route featuring copper-catalyzed heterocyclization delivers the target molecule in a comparable 11 steps with an overall yield of about 10%, whereas the pathway that employs Meldrum’s acid-derived intermediates proceeds in 12 steps, (four of which are telescoped one-pot sequences), to deliver an overall yield of 17%. It is important to note that these estimates were calculated from intermediates that already incorporate the pyridazine fragment. In practice, each of the developed methodologies adds three initial steps that convert very inexpensive and simple reagents into the starting compound 43 with a modest overall yield of around 20%, thereby eliminating dependence on the commercially supplied intermediates, such as compound 23, while enabling large-scale preparation of a previously scarce and valuable building block 43 of considerable synthetic interest (priced at US $1086 per gram).
Thus, this comprehensive review of risdiplam synthetic methodologies reveals a clear hierarchy of strategies, spanning from questionable patent claims to genuinely innovative breakthroughs. Certain reported routes—particularly those employing unprotected spirocyclic amines or unstable propiolic acid derivatives—raise serious concerns regarding feasibility and scalability. Regardless, the landscape has been dramatically reshaped by the embrace of rigorously validated methodologies. Roche’s systematically optimized commercial process stands as a benchmark in industrial process development, effectively resolving the regioselectivity and scalability challenges that hindered early discovery routes. Equally notable are the recent academic contributions that have achieved complete palladium independence while preserving synthetic efficiency, thereby demonstrating that complex heterocyclic targets can be realized via environmentally sustainable and cost-effective pathways.
3. Conclusions
The synthetic evolution of risdiplam represents a paradigmatic example of modern pharmaceutical process development, where academic innovation converges with industrial necessity to address complex synthetic challenges. This comprehensive analysis reveals a remarkable transformation from the initial palladium-dependent discovery routes, characterized by regioselectivity issues and scalability limitations, to sophisticated contemporary methodologies that have fundamentally redefined the synthetic landscape for complex heterocyclic targets. The successful transition from precious metal catalysis to metal-free methodologies, addresses critical industry concerns including cost containment, supply chain resilience, and environmental sustainability. The modular synthetic frameworks developed for risdiplam itself and its key intermediates provide versatile platforms for structure-activity relationship exploration and next-generation active pharmaceutical substances development. This newfound capacity to selectively sculpt each heterocyclic building block, without compromising synthetic agility, unlocks the systematic fine-tuning of pharmacological profiles for tomorrow’s drug contenders, poised to tackle not only the scourge of spinal muscular atrophy but a plethora of different ailments. The relentless march of synthetic innovation promises a future brimming with therapeutic breakthroughs. These advancements will not only address the urgent needs of today but will also fuel the creation of tomorrow’s life-saving medications, making them more readily available through streamlined production and dramatically reduced costs.
Author Contributions
Conceptualization, G.K.; Data Curation, G.K. and M.B.N.; Funding Acquisition, R.A.I.; Investigation, G.K. and M.B.N.; Methodology, G.K.; Project Administration, R.A.I.; Writing—Original Draft Preparation, G.K. and M.B.N.; Writing—Review and Editing, G.K., M.B.N. and R.A.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation, grant number 23-90-04000.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nishio, H.; Niba, E.T.E.; Saito, T.; Okamoto, K.; Takeshima, Y.; Awano, H. Spinal Muscular Atrophy: The Past, Present, and Future of Diagnosis and Treatment. Int. J. Mol. Sci. 2023, 24, 11939. [Google Scholar] [CrossRef]
- Mendonça, R.H.; de Godoi, J.S.A.; Zanoteli, E. Um Cadastro Brasileiro Autorrelatado de Atrofia Muscular Espinhal 5q: Dados de História Natural, Características Genéticas e Cuidados Multidisciplinares. Arq. Neuropsiquiatr. 2024, 82, s00441792096. [Google Scholar] [CrossRef]
- Paik, J. Risdiplam: A Review in Spinal Muscular Atrophy. CNS Drugs 2022, 36, 401–410. [Google Scholar] [CrossRef]
- Baranello, G.; Darras, B.T.; Day, J.W.; Deconinck, N.; Klein, A.; Masson, R.; Mercuri, E.; Rose, K.; El-Khairi, M.; Gerber, M.; et al. Risdiplam in Type 1 Spinal Muscular Atrophy. N. Engl. J. Med. 2021, 384, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Mercuri, E.; Deconinck, N.; Mazzone, E.S.; Nascimento, A.; Oskoui, M.; Saito, K.; Vuillerot, C.; Baranello, G.; Boespflug-Tanguy, O.; Goemans, N.; et al. Safety and Efficacy of Once-Daily Risdiplam in Type 2 and Non-Ambulant Type 3 Spinal Muscular Atrophy (SUNFISH Part 2): A Phase 3, Double-Blind, Randomised, Placebo-Controlled Trial. Lancet Neurol. 2022, 21, 42–52. [Google Scholar] [CrossRef]
- Chiriboga, C.A.; Bruno, C.; Duong, T.; Fischer, D.; Mercuri, E.; Kirschner, J.; Kostera-Pruszczyk, A.; Jaber, B.; Gorni, K.; Kletzl, H.; et al. Risdiplam in Patients Previously Treated with Other Therapies for Spinal Muscular Atrophy: An Interim Analysis from the JEWELFISH Study. Neurol. Ther. 2023, 12, 543–557. [Google Scholar] [CrossRef]
- Chiriboga, C.A.; Bruno, C.; Duong, T.; Fischer, D.; Mercuri, E.; Kirschner, J.; Kostera-Pruszczyk, A.; Jaber, B.; Gorni, K.; Kletzl, H.; et al. JEWELFISH: 24-Month Results from an Open-Label Study in Non-Treatment-Naïve Patients with SMA Receiving Treatment with Risdiplam. J. Neurol. 2024, 271, 4871–4884. [Google Scholar] [CrossRef] [PubMed]
- Servais, L.; Farrar, M.; Finkel, R.; Vlodavets, D.; Zanoteli, E.; Al-Muhaizea, M.; de Queiroz Campos Araújo, A.P.; Nelson, L.; Jaber, B.; Gorni, K.; et al. RAINBOWFISH: Primary Efficacy and Safety Data in Risdiplam-Treated Infants with Presymptomatic Spinal Muscular Atrophy (SMA) (S37.006). Neurology 2024, 102, 5269. [Google Scholar] [CrossRef]
- Finkel, R.S.; Farrar, M.A.; Vlodavets, D.; Servais, L.; Zanoteli, E.; Al-Muhaizea, M.; Nelson, L.; Prufer, A.; Wang, Y.; Fisher, C.; et al. RAINBOWFISH: Preliminary Efficacy and Safety Data in Risdiplam-Treated Infants with Presymptomatic SMA (P17-5.003). Neurology 2022, 98, 1636. [Google Scholar] [CrossRef]
- Finkel, R.S.; Al-Muhaizea, M.; Farrar, M.A.; Nelson, L.; Prufer, A.; Servais, L.; Wang, Y.; Zanoteli, E.; Palfreeman, L.; El-Khairi, M.; et al. RAINBOWFISH: A Study of Risdiplam in Newborns with Presymptomatic Spinal Muscular Atrophy (SMA) (4281). Neurology 2021, 96, 4281. [Google Scholar] [CrossRef]
- Keritam, O.; Erdler, M.; Fasching, B.; Zulehner, G.; Rath, J.; Krenn, M.; Waldhör, T.; Gruber, V.A.; Langweil, N.; Kiss, C.; et al. Efficacy and Safety of Risdiplam in Adults with 5q-Associated Spinal Muscular Atrophy: A Nationwide Observational Cohort Study in Austria. EClinicalMedicine 2025, 88, 103536. [Google Scholar] [CrossRef]
- Cortes, J.E.; Kantarjian, H.; Shah, N.P.; Bixby, D.; Mauro, M.J.; Flinn, I.; O’Hare, T.; Hu, S.; Narasimhan, N.I.; Rivera, V.M.; et al. Ponatinib in Refractory Philadelphia Chromosome–Positive Leukemias. N. Engl. J. Med. 2012, 367, 2075–2088. [Google Scholar] [CrossRef]
- Hartz, R.A.; Ahuja, V.T.; Sivaprakasam, P.; Xiao, H.; Krause, C.M.; Clarke, W.J.; Kish, K.; Lewis, H.; Szapiel, N.; Ravirala, R.; et al. Design, Structure–Activity Relationships, and In Vivo Evaluation of Potent and Brain-Penetrant Imidazo[1,2- b]Pyridazines as Glycogen Synthase Kinase-3β (GSK-3β) Inhibitors. J. Med. Chem. 2023, 66, 4231–4252. [Google Scholar] [CrossRef]
- Liu, C.; Lin, J.; Moslin, R.; Tokarski, J.S.; Muckelbauer, J.; Chang, C.Y.; Tredup, J.; Xie, D.; Park, H.; Li, P.; et al. Identification of Imidazo[1,2- b] Pyridazine Derivatives as Potent, Selective, and Orally Active Tyk2 JH2 Inhibitors. ACS Med. Chem. Lett. 2019, 10, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.C.; Goldstein, D.M.; Hermann, J.C.; Kuglstatter, A.; Liu, W.; Luk, K.C.; Padilla, F.; Slade, M.; Villaseñor, A.G.; Wanner, J.; et al. Rational Design of Highly Selective Spleen Tyrosine Kinase Inhibitors. J. Med. Chem. 2012, 55, 10414–10423. [Google Scholar] [CrossRef] [PubMed]
- El Akkaoui, A.; Koubachi, J.; Guillaumet, G.; El Kazzouli, S. Synthesis and Functionalization of Imidazo[1,2-b] Pyridazine by Means of Metal-Catalyzed Cross-Coupling Reactions. ChemistrySelect 2021, 6, 8985–9011. [Google Scholar] [CrossRef]
- Kawashima, T.; Iwamoto, I.; Nakagawa, N.; Tomioka, H.; Yoshida, S. Inhibitory Effect of Pemirolast, a Novel Antiallergic Drug, on Leukotriene C4 and Granule Protein Release from Human Eosinophils. Int. Arch. Allergy Immunol. 1994, 103, 405–409. [Google Scholar] [CrossRef] [PubMed]
- Kennis, L.E.J.; Bischoff, F.P.; Mertens, C.J.; Love, C.J.; Van den Keybus, F.A.F.; Pieters, S.; Braeken, M.; Megens, A.A.H.P.; Leysen, J.E. New 2-Substituted 1,2,3,4-Tetrahydrobenzofuro[3,2-c] Pyridine Having Highly Active and Potent Central α 2 -Antagonistic Activity as Potential Antidepressants. Bioorg Med. Chem. Lett. 2000, 10, 71–74. [Google Scholar] [CrossRef]
- La Motta, C.; Sartini, S.; Mugnaini, L.; Simorini, F.; Taliani, S.; Salerno, S.; Marini, A.M.; Da Settimo, F.; Lavecchia, A.; Novellino, E.; et al. Pyrido[1,2-a] Pyrimidin-4-One Derivatives as a Novel Class of Selective Aldose Reductase Inhibitors Exhibiting Antioxidant Activity. J. Med. Chem. 2007, 50, 4917–4927. [Google Scholar] [CrossRef]
- Mohammed, E.U.R.; Porter, Z.J.; Jennings, I.G.; Al-Rawi, J.M.A.; Thompson, P.E.; Angove, M.J. Synthesis and Biological Evaluation of 4H-Benzo[e] [1,3] Oxazin-4-Ones Analogues of TGX-221 as Inhibitors of PI3Kβ. Bioorg. Med. Chem. 2022, 69, 116832. [Google Scholar] [CrossRef]
- Li, A.R.; Johnson, M.G.; Liu, J.; Chen, X.; Du, X.; Mihalic, J.T.; Deignan, J.; Gustin, D.J.; Duquette, J.; Fu, Z.; et al. Optimization of the Heterocyclic Core of the Quinazolinone-Derived CXCR3 Antagonists. Bioorg. Med. Chem. Lett. 2008, 18, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Bhawale, R.T.; Chillal, A.S.; Kshirsagar, U.A. 4H-Pyrido[1,2-a] Pyrimidin-4-One, Biologically Important Fused Heterocyclic Scaffold: Synthesis and Functionalization. J. Heterocycl. Chem. 2023, 60, 1356–1373. [Google Scholar] [CrossRef]
- Moshnenko, N.; Kazantsev, A.; Chupakhin, E.; Bakulina, O.; Dar’in, D. Synthetic Routes to Approved Drugs Containing a Spirocycle. Molecules 2023, 28, 4209. [Google Scholar] [CrossRef]
- Hiesinger, K.; Dar’In, D.; Proschak, E.; Krasavin, M. Spirocyclic Scaffolds in Medicinal Chemistry. J. Med. Chem. 2021, 64, 150–183. [Google Scholar] [CrossRef]
- Miyazaki, M.; Uoto, K.; Sugimoto, Y.; Naito, H.; Yoshida, K.; Okayama, T.; Kawato, H.; Miyazaki, M.; Kitagawa, M.; Seki, T.; et al. Discovery of DS-5272 as a Promising Candidate: A Potent and Orally Active P53-MDM2 Interaction Inhibitor. Bioorg. Med. Chem. 2015, 23, 2360–2367. [Google Scholar] [CrossRef]
- Jiang, Q.; Dong, J.; Lei, F.; Yu, D.; Li, T.; Sun, H.; Xue, D. Strain-Release Driven Spirocyclization of Bicyclo [1.1.0] Butanes: Access to 6,7-Diazaspiro [3.4] Octanes. Chem. Sci. 2025, 16, 12189–12195. [Google Scholar] [CrossRef] [PubMed]
- Ratni, H.; Ebeling, M.; Baird, J.; Bendels, S.; Bylund, J.; Chen, K.S.; Denk, N.; Feng, Z.; Green, L.; Guerard, M.; et al. Discovery of Risdiplam, a Selective Survival of Motor Neuron-2 (SMN2) Gene Splicing Modifier for the Treatment of Spinal Muscular Atrophy (SMA). J. Med. Chem. 2018, 61, 6501–6517. [Google Scholar] [CrossRef] [PubMed]
- Ratni, H.; Green, L.; Naryshkin, N.A.; Weetall, M.L. Compounds for Treating Spinal Muscular Atrophy. WO2015173181, 19 November 2015. [Google Scholar]
- Thirumalai Rajan, S.; Sajja, E.; Mathad, V.T.; Ismail Subuddhi, P. Process for the Preparation of Risdiplam and Its Intermediates. Technical Disclosure Commons. Available online: https://www.tdcommons.org/dpubs_series/5296/ (accessed on 31 July 2022).
- Moessner, C.; Hoffmann-Emery, F.; Adam, J.M.; Fantasia, S.; Fishlock, D.V.; Meier, R.; Wuitschik, G.; Ratni, H. Development and Optimization of the Manufacturing Process for RNA-Splicing Modifier Risdiplam RG7916. In Complete Accounts of Integrated Drug Discovery and Development: Recent Examples from the Pharmaceutical Industry; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2022; Volume 1423, pp. 301–332. [Google Scholar] [CrossRef]
- Kothakonda, K.K.; Pitta, B.R.; Namana, S.B.; Rangisetty, J.B.; Pullagurla, M.R. A Novel Process for the Preparation of 7-(4,7-Diazaspiro[2.5]octan-7-yl)-2-(2,8-Dimethylimidazo[1,2-b]Pyridazin-6-yl)Pyrido-4H-[1,2-a]Pyrimidin-4-one with Novel Intermediates. WO2024003798, 4 January 2024. [Google Scholar]
- Adam, J.-M.; Pfleger, C.; Wuitschik, G. Process for Preparing Risdiplam. WO2022194909, 22 September 2022. [Google Scholar]
- Kandula, S.; Yadlapalli, R.K.; Vipparla, B.B.; Thumati, S.; Nagapuri, S.; Parakala, S.; Suthrapu, S.; Dandala, R.; Muddasani, P.R.; Nannapaneni, V.C. Improved Process for the Preparation of Risdiplam and its Intermediates. WO2024069646, 4 April 2024. [Google Scholar]
- Meier, R.; Schwitter, U.; De Paepe, A.; Kuehl, P.; Thun, J.; Stowasser, F. New Forms of Pyrido[1,2-a]Pyrimidin-4-one Derivatives, its Formulation and its Process of Making. WO2020079203, 23 April 2020. [Google Scholar]
- Pradhan, N.S.; Telange, V.R.; Sonar, Y.S.; Shinde, V.C.; Minhas, H.S.; Minhas, G.S. Novel Crystalline Form of Risdiplam and Process for Preparation Thereof. WO2024236590, 21 November 2024. [Google Scholar]
- Lengauer, H. Crystalline Form of Risdiplam. WO2022162107, 4 August 2022. [Google Scholar]
- Matečić Mušanić, S.; Klarić, D.; Kolenić, M. Solid State Forms of Risdiplam and Process for Preparation Thereof. WO2021021775, 4 February 2021. [Google Scholar]
- Korenev, G.; Gutenev, A.A.; Antipin, F.V.; Chernyshov, V.V.; Korobkina, M.P.; Nawrozkij, M.B.; Ivanov, R.A. A Brand-New Metal Complex Catalyst-Free Approach to the Synthesis of 2,8-Dimethylimidazo[1,2-b] Pyridazine-6-Carboxylic Acid—A Key Intermediate in Risdiplam Manufacturing Process. Molecules 2025, 30, 3011. [Google Scholar] [CrossRef] [PubMed]
- Korenev, G.; Gutenev, A.A.; Antipin, F.V.; Chernyshov, V.V.; Shulgina, J.A.; Korobkina, M.P.; Nawrozkij, M.B.; Ivanov, R.A. A Convenient, Pd-Free Approach to the Synthesis of Risdiplam. Molecules 2025, 30, 3375. [Google Scholar] [CrossRef]
- Hoang, G.L.; Zoll, A.J.; Ellman, J.A. Three-Component Coupling of Aldehydes, 2-Aminopyridines, and Diazo Esters via Rhodium (III)-Catalyzed Imidoyl C-H Activation: Synthesis of Pyrido[1,2-a] Pyrimidin-4-Ones. Org. Lett. 2019, 21, 3886–3890. [Google Scholar] [CrossRef]
- Jones, E.D.; Vandegraaff, N.; Le, G.; Choi, N.; Issa, W.; Macfarlane, K.; Thienthong, N.; Winfield, L.J.; Coates, J.A.V.; Lu, L.; et al. Design of a Series of Bicyclic HIV-1 Integrase Inhibitors. Part 1: Selection of the Scaffold. Bioorg Med. Chem. Lett. 2010, 20, 5913–5917. [Google Scholar] [CrossRef]
- Yu, L. Pyridino [1, 2-a] Pyrimidone Analogue and Application Thereof in Preparation of FGFR (Fibroblast Growth Factor Receptor) inhibitor. CN114853753, 20 April 2022. [Google Scholar]
- Le, G.; Vandegraaff, N.; Rhodes, D.I.; Jones, E.D.; Coates, J.A.V.; Lu, L.; Li, X.; Yu, C.; Feng, X.; Deadman, J.J. Discovery of Potent HIV Integrase Inhibitors Active against Raltegravir Resistant Viruses. Bioorg Med. Chem. Lett. 2010, 20, 5013–5018. [Google Scholar] [CrossRef]
- Qi, H.; Choi, S.; Dakka, A.; Karp, G.M.; Narasimhan, J.; Naryshkin, N.; Turpoff, A.A.; Weetall, M.L.; Welch, E.; Woll, M.G.; et al. Compounds for Treating Spinal Muscular Atrophy. WO2013119916, 15 August 2013. [Google Scholar]
- Jie, F.; Wancheng, G.; Jinfu, D.; Dingjun, C.; Xiaoqiang, X. Synthesis Method of Lisethopram and Intermediate of Lisethopram. CN119504737, 25 February 2025. [Google Scholar]
- Ebeling, M.; Metzger, F.; Sivaramakrishnan, M. Screening Method. WO2015024876, 26 February 2015. [Google Scholar]
- Woll, M.G.; Qi, H.; Turpoff, A.; Zhang, N.; Zhang, X.; Chen, G.; Li, C.; Huang, S.; Yang, T.; Moon, Y.C.; et al. Discovery and Optimization of Small Molecule Splicing Modifiers of Survival Motor Neuron 2 as a Treatment for Spinal Muscular Atrophy. J. Med. Chem. 2016, 59, 6070–6085. [Google Scholar] [CrossRef] [PubMed]
- Ratni, H.; Karp, G.M.; Weetall, M.; Naryshkin, N.A.; Paushkin, S.V.; Chen, K.S.; McCarthy, K.D.; Qi, H.; Turpoff, A.; Woll, M.G.; et al. Specific Correction of Alternative Survival Motor Neuron 2 Splicing by Small Molecules: Discovery of a Potential Novel Medicine to Treat Spinal Muscular Atrophy. J. Med. Chem. 2016, 59, 6086–6100. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, R.J.; Ratni, H.; Sarie, J.C. Compounds for Treating Spinal Muscular Atrophy. WO2016184832, 24 November 2016. [Google Scholar]
- Minhas, H.S.; Minhas, G.S.; Pradhan, N.S.; Telange, V.R.; Arabune, A.S.; Mardhekar, V.V.; Sonar, Y.S. Process for Preparation of Risdiplam, Novel Intermediates, and Process for Preparation Thereof. WO2024154148, 25 July 2024. [Google Scholar]
- McCarthy, K.D.; Metzger, F.; Ratni, H. Compounds for Treating Amyotrophic Lateral Sclerosis. WO2017081111, 18 May 2017. [Google Scholar]
- Roma, G.; Cinone, N.; Di Braccio, M.; Grossi, G.; Leoncini, G.; Signorello, M.G.; Carotti, A. Synthesis, Antiplatelet Activity and Comparative Molecular Field Analysis of Substituted 2-Amino-4 H -Pyrido[1,2-a] Pyrimidin-4-Ones, Their Congeners and Isosteric Analogues. Bioorg Med. Chem. 2000, 8, 751–768. [Google Scholar] [CrossRef]
- Richards, S.; Derudas, M.; Ahlsten, N.; Papachristos, K. MPRO Targeting Antiviral Compounds. WO2023180189, 28 September 2023. [Google Scholar]
- Guan, H.; Wu, C.; Yu, T.; Huang, L.; Hao, D.; Gao, B.; Sun, J.; Shi, N.; Chen, S. Pyridino[1,2-a]Pyrimidone Analogue Used as mTOR/PI3K Inhibitor. WO2015192761, 23 December 2015. [Google Scholar]
- Ahuja, V.; Bi, Y.; Cole, A.G.; Dorsey, B.D.; Fan, Y.; Kakarla, R.; Kirk, S.M.; Nguyen, D.; Ozturk, S.; Quintero, J.; et al. Substituted 1,1′-Biphenyl Compounds and Methods Using Same. WO2021158481, 12 August 2021. [Google Scholar]
- Averin, A.D.; Abel, A.S.; Grigorova, O.K.; Latyshev, G.V.; Kotovshchikov, Y.N.; Mitrofanov, A.Y.; Bessmertnykh-Lemeune, A.; Beletskaya, I.P. Recent Achievements in Copper Catalysis for C–N Bond Formation. Pure Appl. Chem. 2020, 92, 1181–1199. [Google Scholar] [CrossRef]
- Mo, B.; Chen, C.; Peng, J. CuI-Catalyzed Synthesis of Multisubstituted Pyrido [1,2- a] Pyrimidin-4-Ones through Tandem Ullmann-Type C–N Cross-Coupling and Intramolecular Amidation Reaction. RSC Adv. 2023, 13, 24264–24271. [Google Scholar] [CrossRef] [PubMed]
- Gaube, G.; Mutter, J.; Leitch, D.C. A “Neat” Synthesis of Substituted 2-Hydroxy-Pyrido[1,2-a] Pyrimidin-4-Ones. Can. J. Chem. 2024, 102, 206–213. [Google Scholar] [CrossRef]
- Wu, C.; Yu, T.; Chen, S. Pyridino[1,2-a] Pyrimidone Analogue Used as PI3K Inhibitor. WO2015192760, 23 December 2015. [Google Scholar]
- Park, D.S.; Jo, E.; Choi, J.; Lee, M.E.; Kim, S.; Kim, H.Y.; Nam, J.; Ahn, S.; Hwang, J.Y.; Windisch, M.P. Characterization and Structure-Activity Relationship Study of Iminodipyridinopyrimidines as Novel Hepatitis C Virus Inhibitor. Eur. J. Med. Chem. 2017, 140, 65–73. [Google Scholar] [CrossRef]
- Yalduz, S.; Yilmaz, M. Microwave Assisted Synthesis of 2,3-Dihydro-4H- Furo[2,3-d] Pyrido[1,2-a] Pyrimidin-4-Ones and Furo[2,3-d] Pyrido[1,2-a] Pyrimidin-4-One. ChemistrySelect 2023, 8, e202204260. [Google Scholar] [CrossRef]
- Yalduz, S.; Sarı, S.; Yılmaz, M. Microwave Assisted Synthesis, Acetylcholinesterase Inhibition and Molecular Docking Studies of Furo[2,3-d] Pyrido [1,2-a] Pyrimidin-4-One Derivatives. J. Heterocycl. Chem. 2024, 61, 1517–1530. [Google Scholar] [CrossRef]
- Adam, J.M.; Fantasia, S.M.; Fishlock, D.V.; Hoffmann-Emery, F.; Moine, G.; Pfleger, C.; Moessner, C. Process for the Preparation of 7-(4,7-Diazaspiro [2.5] Octan-7-yl)-2-(2,8-Dimethylimidazo[1,2-b] Pyridazin-6-yl) Pyrido[1,2-a] Pyrimidin-4-one Derivatives. WO2019057740, 28 March 2019. [Google Scholar]
- Chen, G.; Bhattacharyya, A.; Jiang, Y.; Karp, G.M.; Narasimhan, J.; Turpoff, A.; Zhang, N. Heteroaryl Compounds for Treating Huntington’s Disease. WO2020005882, 2 January 2020. [Google Scholar]
- Dolente, C.; Grether, N.; O’Hara, F.S.; Piras, M.; Ratni, H.; Reutlinger, M.; Vifian, W.; Zambaldo, C. New Thiadiazolopyrimidone Derivatives. WO2022194802, 22 September 2022. [Google Scholar]
- Reynolds, D.; Seiler, M.W.; Agrawal, A.A.; Vaillancourt, F.; Smith, P.; Prajapati, S.; Hopper, A.T.; Vyskocil, S.; Moreau, B. Compounds and Methods for Modulating Splicing. WO2023097007, 1 June 2023. [Google Scholar]
- Woll, M.G.; Amedzo, L.; Babu, S.; Barraza, S.J.; Bhattacharyya, A.; Karp, G.M.; Mazzotti, A.R.; Narasimhan, J.; Patel, J.; Turpoff, A.; et al. Compounds for Treating Huntington’s Disease. WO2018226622, 13 December 2018. [Google Scholar]
- Reynolds, D.; Seiler, M.W.; Agrawal, A.A.; Vaillancourt, F.; Smith, P.; Prajapati, S.; Hopper, A.T.; Vyskocil, S.; Sirin, G.S. Compounds and Methods for Modulating Splicing. WO2023034811, 9 March 2023. [Google Scholar]
- Wager, T.T.; Weng, Z.; Xi, H.S. Heterocyclic Substituted 1,3,4-Thiadiazole and Pyridazine Compounds and Methods of Using the Same. WO2023092149, 25 May 2023. [Google Scholar]
- Reynolds, D.; Leger, S.; Seiler, M.W.; Agrawal, A.A.; Vaillancourt, F.; Smith, P.; Hopper, A.T.; Prajapati, S.; Soueidan, O. Compounds and Methods for Modulating Splicing. WO2021207554, 14 October 2021. [Google Scholar]
- Reynolds, D.; Seiler, M.W.; Agrawal, A.A.; Vaillancourt, F.; Smith, P.; Prajapati, S.; Hopper, A.T.; Vyskocil, S.; Moreau, B. Compounds and Methods for Modulating Nucleic Acid Splicing. WO2023064880, 20 April 2023. [Google Scholar]
- Xing, Q.; Chandrachud, P.; Tillett, K.; Lopchuk, J. Regioselective Hydroamination of Unactivated Olefins with Diazirines as a Diversifiable Nitrogen Source. Nat. Commun. 2024, 15, 6049. [Google Scholar] [CrossRef]
- Pollak, A.; Stanovnik, B.; Tišler, M. Synthesis of Pyridazine Derivatives—XVI. Tetrahedron 1968, 24, 2623–2629. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kajiwara, Y.; Noguchi, M.; Kajiwara, T.; Tabuchi, T. Fused Heterocyclic Sulfonylurea Compound, Herbicide Containing the Same, and Method of Controlling Weed with the Same. WO2003061388, 31 July 2003. [Google Scholar]
- Almario Garcia, A.; Burnier, P.; Cote-Des Combes, S.; Gilbert, J.-F.; Pacaud, C.; Puech, F.; Chiang, Y.; Davis, L.; Gao, Z.; Zhao, Q. 2-alkyl-6-Cycloamino-3-(Pyridin-4-yl)Imidazo[1,2-b]Pyridazine Derivatives, Preparation Thereof, and Therapeutic Application Thereof. WO2010018327, 18 February 2010. [Google Scholar]
- Jacobson, R.M.; Raths, R.A.; McDonald, J.H. 2-Methoxyallyl Bromide. A Superior Acetonyl Alkylating Agent. J. Org. Chem. 1977, 42, 2545–2549. [Google Scholar] [CrossRef]
- Takahayashi, N. Synthesis of Pyridazine Derivatives. VIII. Nucleophilic Substitution of 3, 6-Dichloro-4-Methylpyridazine. Pharm. Bull. 1957, 5, 229–234. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barlin, G. Imidazo[1,2-B] Pyridazines. I. Some 3-Alkoxy-6-Halogeno-2-Phenyl-(and 4′-Substituted Phenyl) Imidazo[1,2-B] Pyridazines and 3-Methoxy-2,6-Diphenylimidazo[1,2-B] Pyridazine. Aust. J. Chem. 1986, 39, 1803. [Google Scholar] [CrossRef]
- Mori, K. Synthesis of 1, 2-Diazine Derivatives. VII. Yakugaku Zasshi J. Pharm. Soc. Jpn. 1962, 82, 304–308. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Farmer, L.J.; Fournier, P.A.; Lessard, S.; Liu, B.; St-Onge, M.; Sturino, C.; Szychowski, J.; Yannopoulos, C.; Vallee, F.; Lacoste, J.E.; et al. Imidazopyridazines Useful as Inhibitors of the PAR-2 Signaling Pathway. WO2015048245, 2 April 2015. [Google Scholar][Green Version]
- Hu, Z.; He, H.; Zhang, F.; Zhu, X.; Zhong, W. Cdk Inhibitors. WO2020224568, 12 November 2020. [Google Scholar][Green Version]
- Dong, J.; Fang, W.; Guo, W.; Fang, J.; Chu, D.; Xie, X. Synthesis Method of Lisethopram intermediate and Lisethopram Intermediate. CN116947865, 27 October 2023. [Google Scholar][Green Version]
- Jiang, Y. 5-Methyl-2-(Pyridinyl-2-Amino)-8H-Pyrido[2,3-d]Pyrimidin-7-Ketone Compounds. CN105111201, 11 January 2017. [Google Scholar][Green Version]
- Wang, Y.; Zhao, J. Substituted Pyridopyrimidinone Compound, Composition Comprising Same, and Use Thereof. WO2023202501, 26 October 2023. [Google Scholar][Green Version]
- Xu, J.; Han, Z.; Li, K.; Lin, H.; Chen, G. Preparation Method of Lisethopram. CN117050096, 14 November 2023. [Google Scholar][Green Version]
- Wu, L.; You, X.; Zhao, L.; Chen, D.; Chen, S. Nitrogen-Containing Tricyclic Bifunctional Compound, Preparation Method Therefor, and Application Thereof. WO2022206924, 6 October 2022. [Google Scholar][Green Version]
- Kim, M.; Kim, J.S.; Ryu, H.C.; Lim, J.W.; Yoo, D. Compounds for Inhibiting CDK and Medical Use Thereof. KR20210156400A, 27 December 2021. [Google Scholar][Green Version]
- Dong, J.; Fang, W.; Guo, W.; Fang, J.; Chu, D.; Xie, X. Method for Synthesizing Risdiplam Intermediate and Risdiplam INTERMEDIATE. WO2025036129, 20 February 2025. [Google Scholar][Green Version]
- Zhu, Y.; Back, T.G. Preparation of 1,7- and 3,9-Dideazapurines from 2-Amino-3-Iodo- and 3-Amino-4-Iodopyridines and Activated Acetylenes by Conjugate Addition and Copper-Catalyzed Intramolecular Arylation. J. Org. Chem. 2014, 79, 11270–11276. [Google Scholar] [CrossRef]
- Chen, Z.; Wen, Y.; Ding, H.; Luo, G.; Ye, M.; Liu, L.; Xue, J. Silver-Catalyzed Highly Efficient Synthesis of Pyrido[1,2-a] Pyrimidin-4-Ones from 2-Aminopyridines and Alkynoates. Tetrahedron Lett. 2017, 58, 13–16. [Google Scholar] [CrossRef]
- Hussain, M.; Liu, J. Practical Synthesis of 4H-Pyrido [1, 2-a] Pyrimidin-4-Ones Using Ethylene Glycol as a Promoting Solvent. Tetrahedron Lett. 2020, 61, 152269. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).