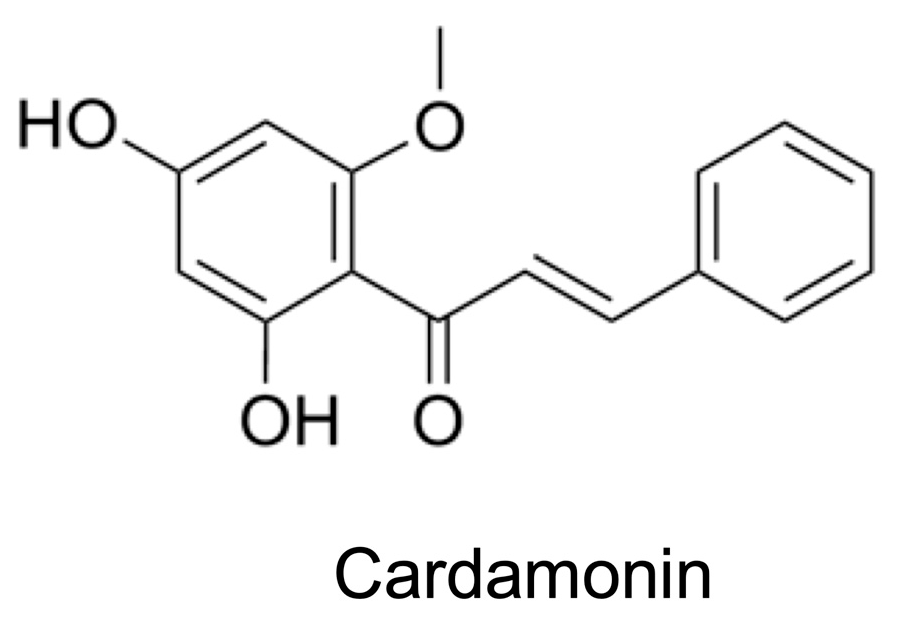
Cardamonin Inhibits the Nuclear Translocation and DNA Binding of RelA in the Tumor Necrosis Factor-α-Induced NF-κB Signaling Pathway in Human Lung Adenocarcinoma A549 Cells
Abstract
1. Introduction
2. Results
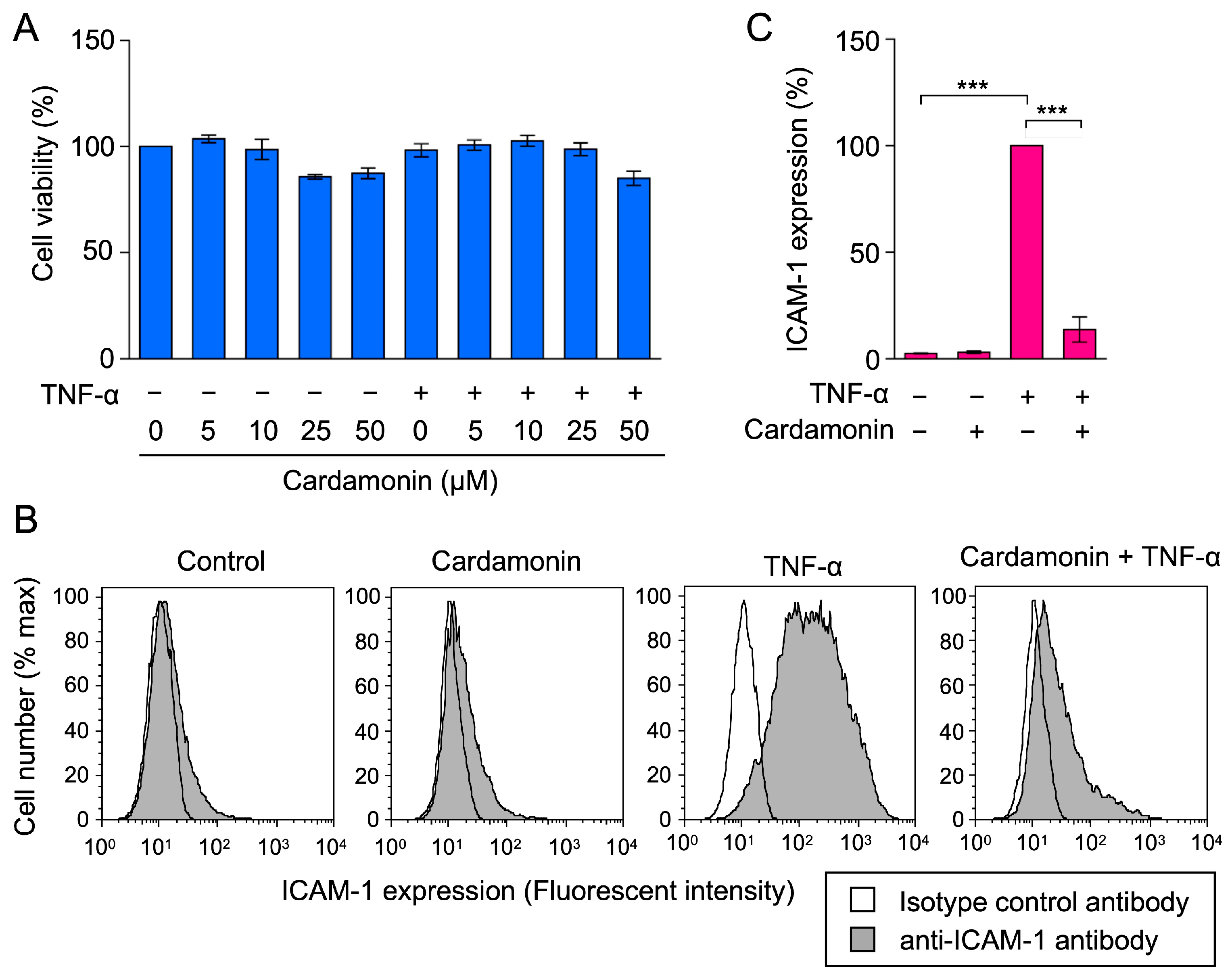
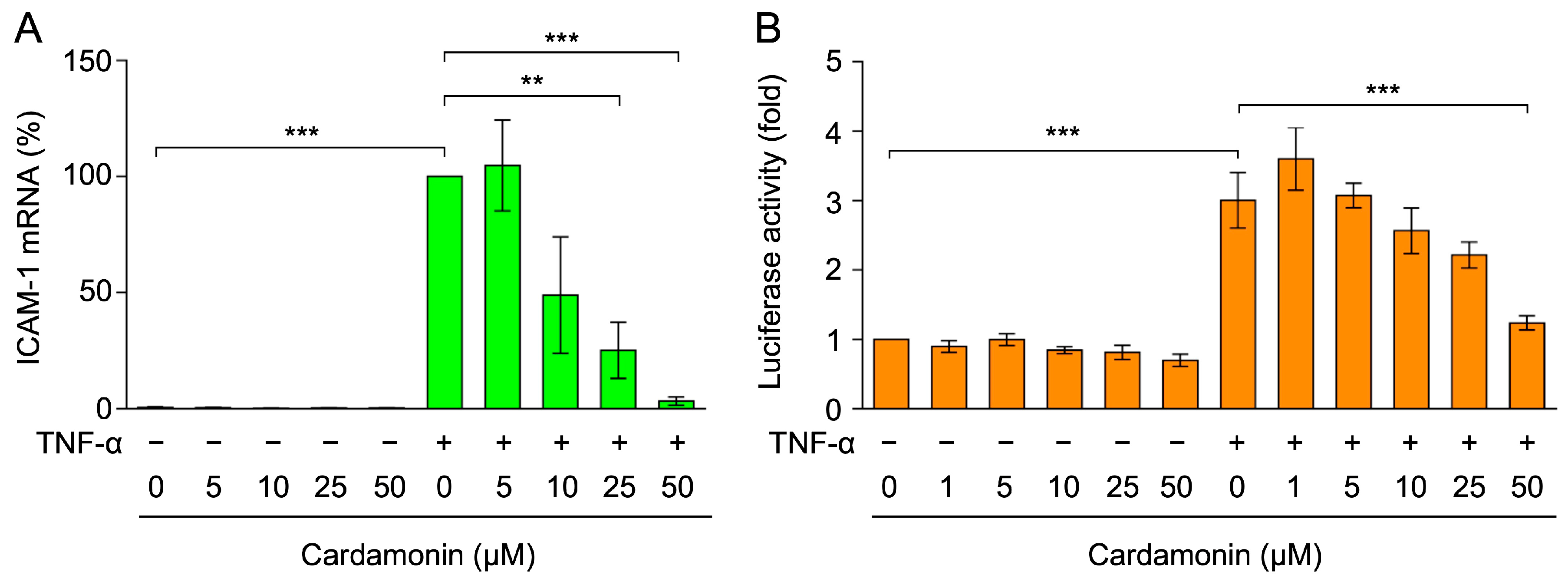
2.1. Cardamonin Inhibited TNF-α-Induced ICAM-1 mRNA Expression in A549 Cells
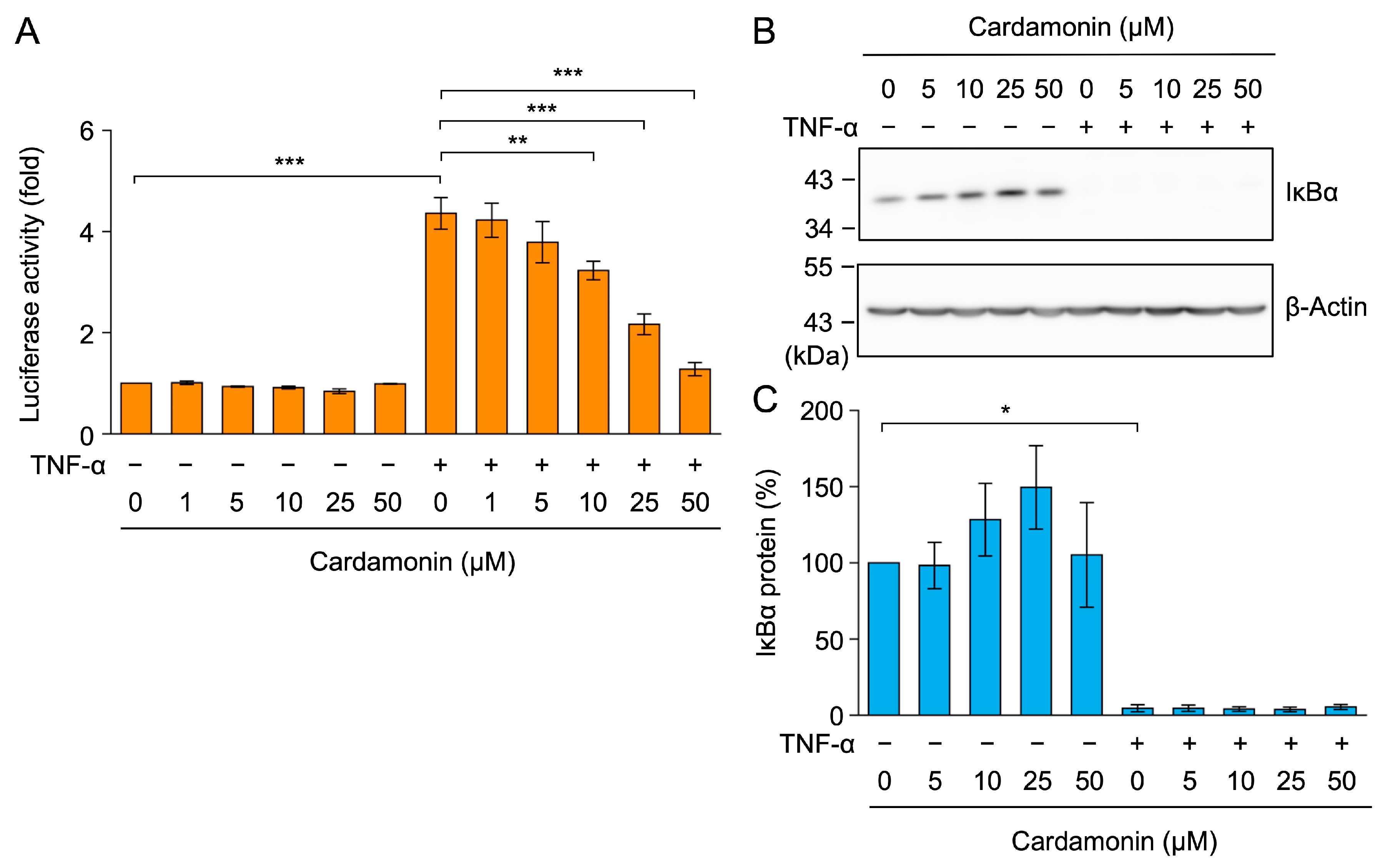
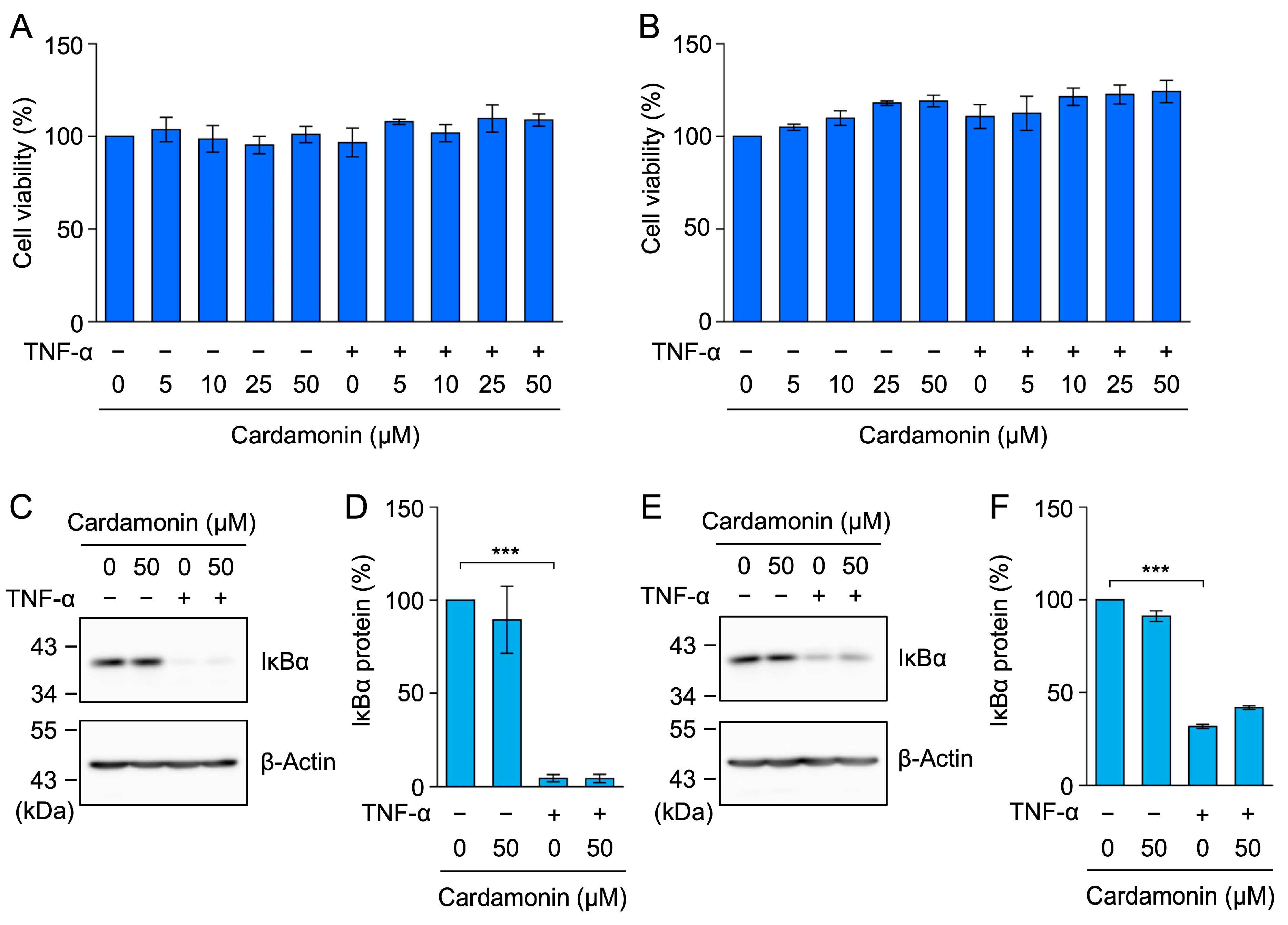
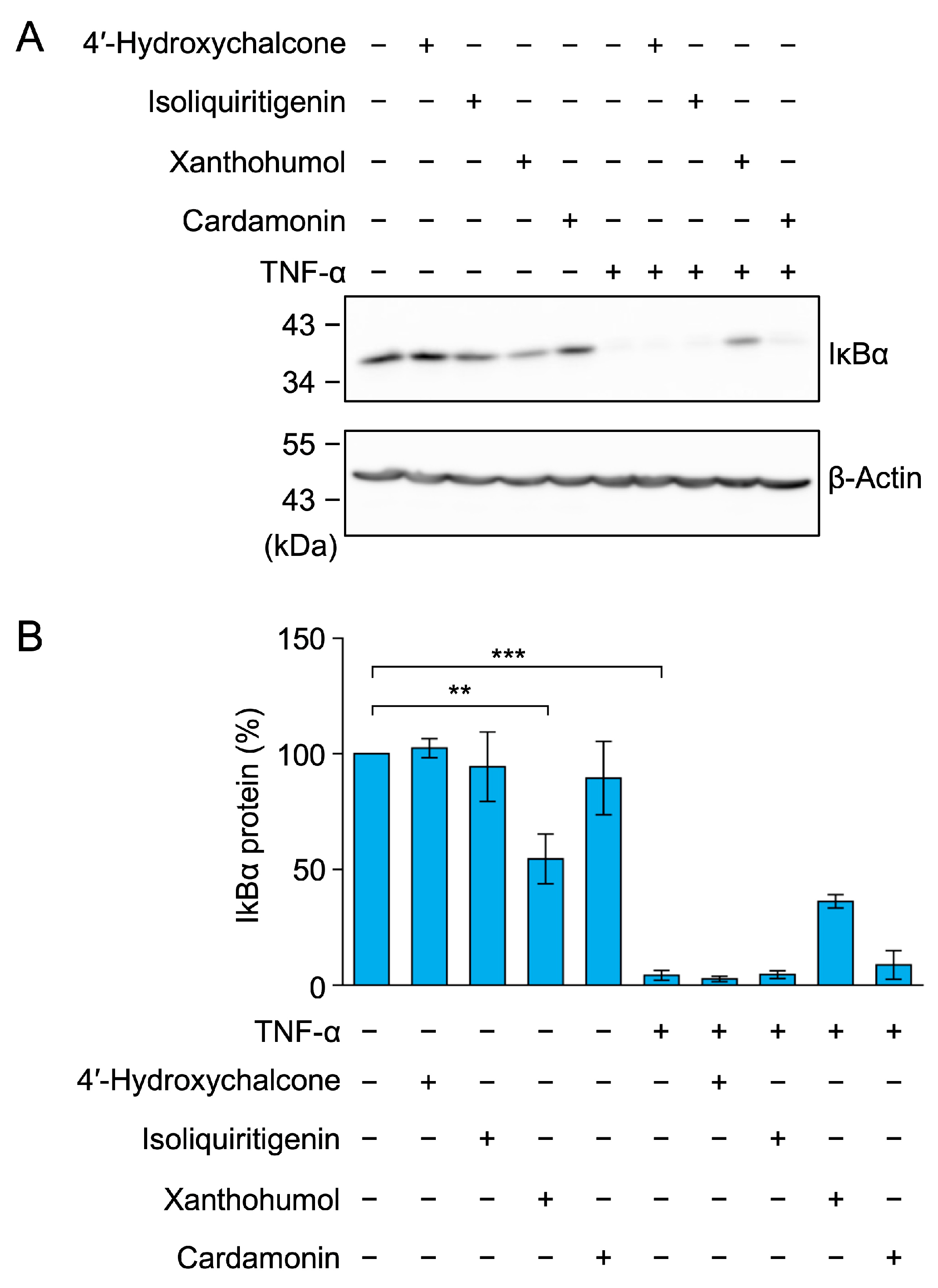
2.2. Cardamonin Did Not Affect TNF-α-Induced IκBα Degradation in A549 Cells
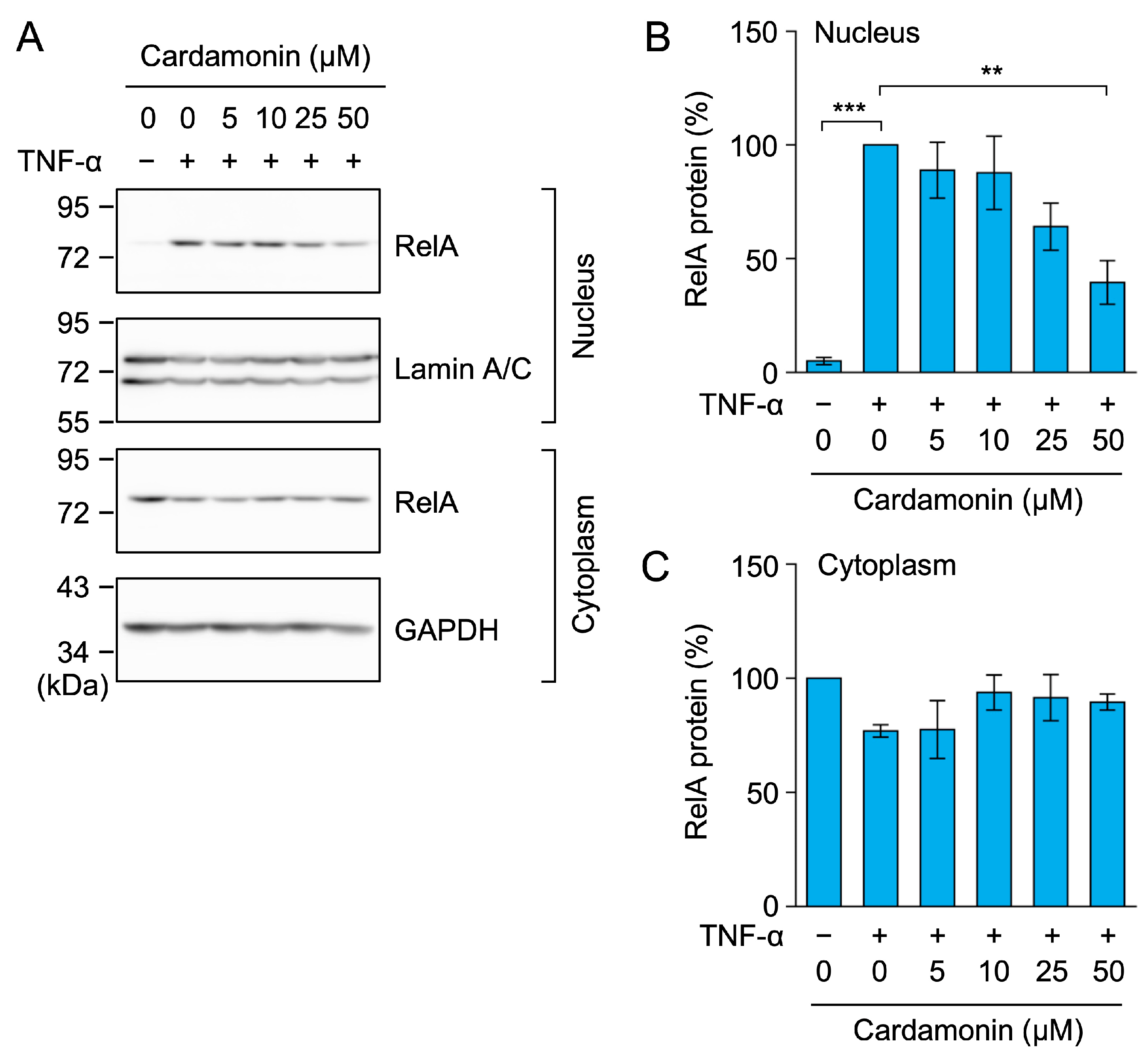
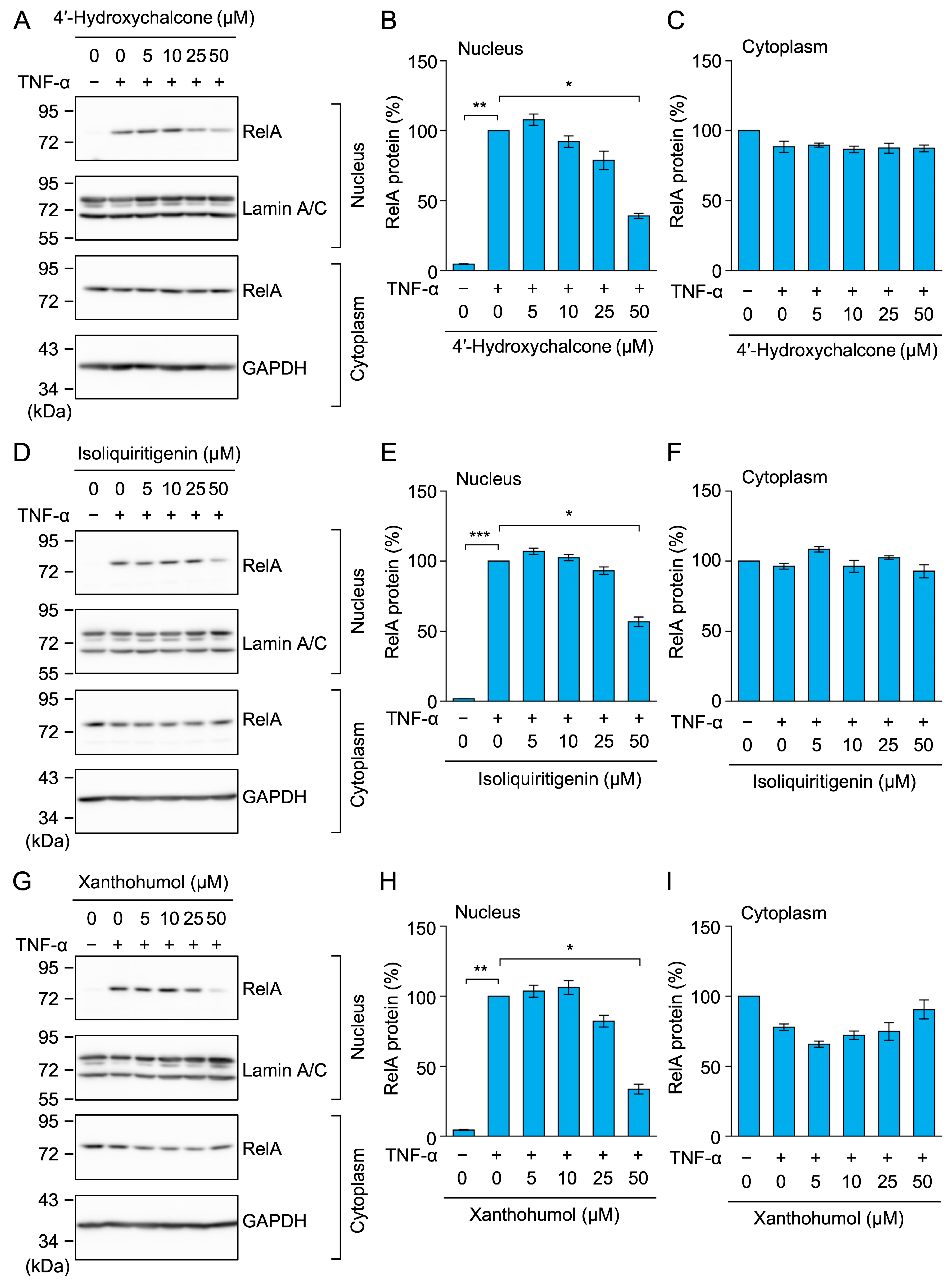
2.3. Cardamonin Inhibited the TNF-α-Induced Nuclear Translocation of RelA in A549 Cells
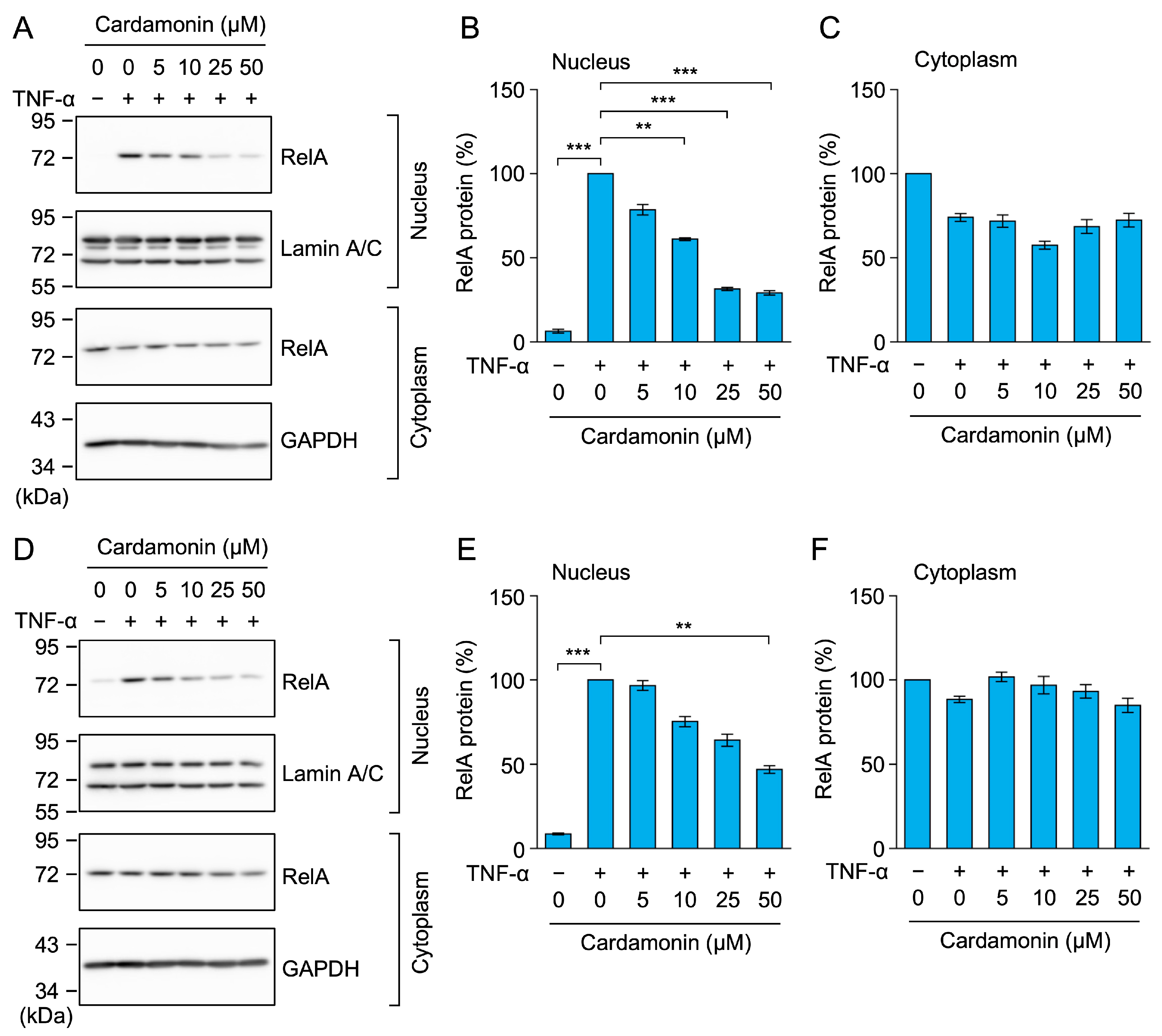
2.4. Cardamonin Did Not Affect TNF-α-Induced IκBα Degradation, but Inhibited the Nuclear Translocation of RelA in MCF-7 and HT-1080 Cells
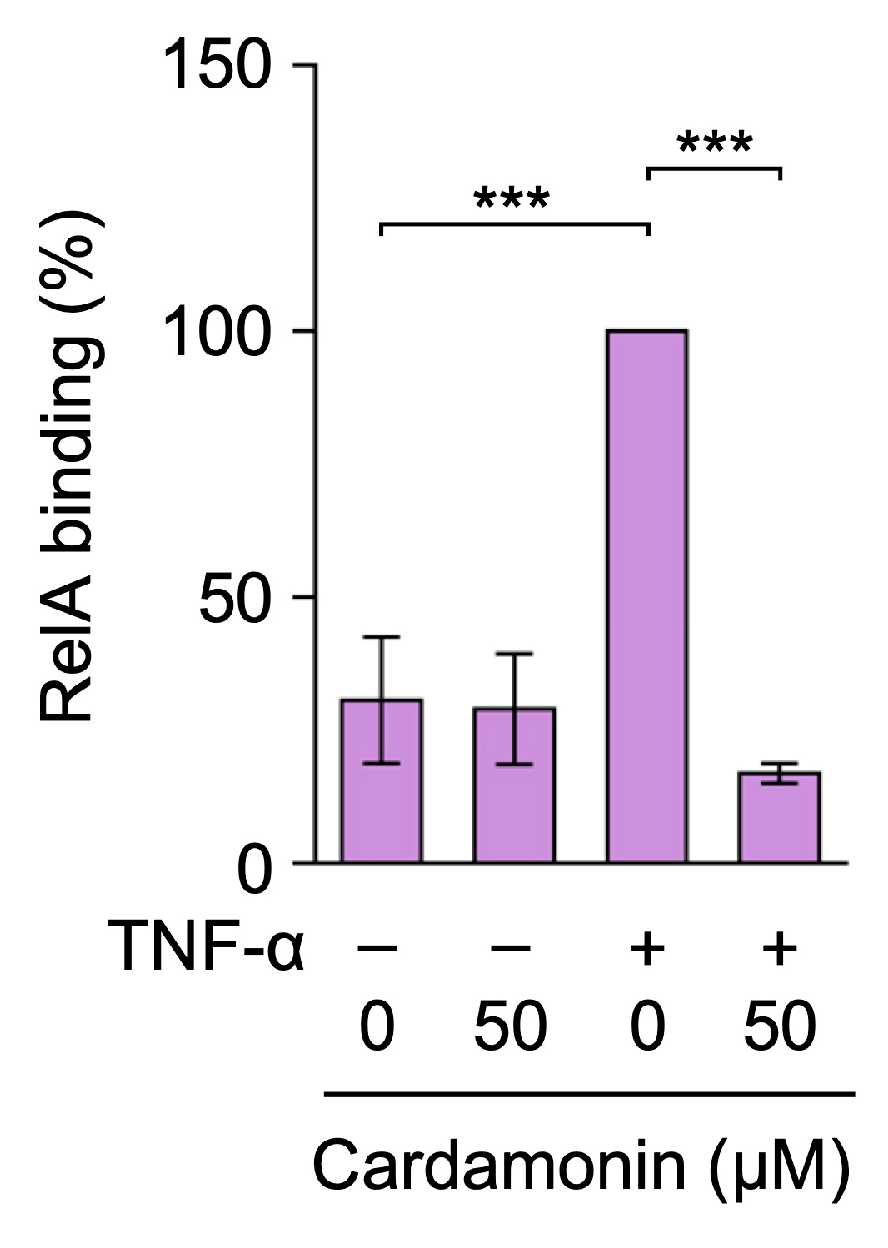
2.5. Cardamonin Inhibited TNF-α-Induced RelA Binding to the ICAM-1 Promoter in A549 Cells
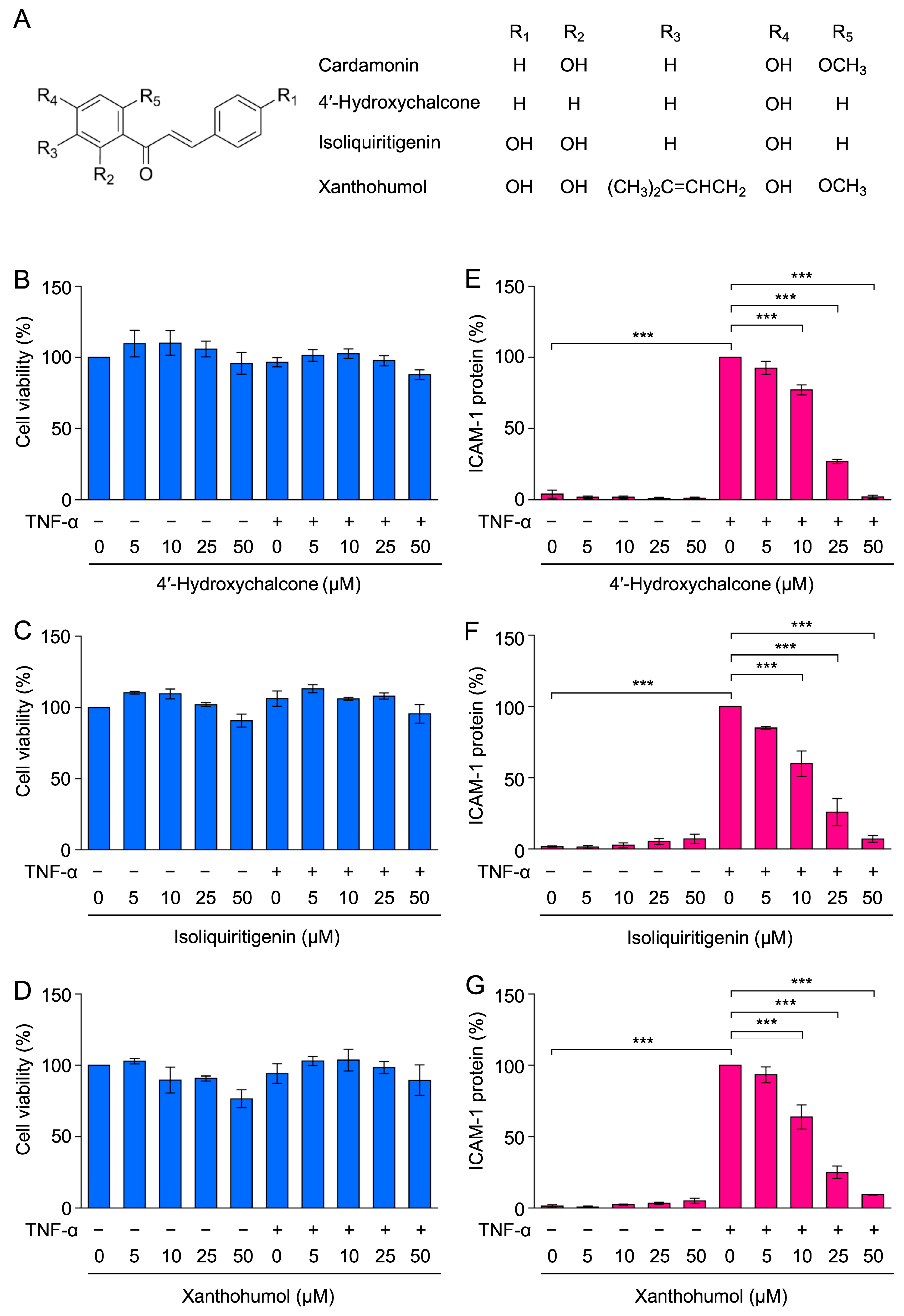
2.6. 4′-Hydroxychalcone, Isoliquiritigenin, and Xanthohumol Inhibited TNF-α-Induced ICAM-1 Expression in A549 Cells
2.7. 4′-Hydroxychalcone, Isoliquiritigenin, and Xanthohumol Did Not Markedly Inhibit TNF-α-Induced IκBα Degradation in A549 Cells
2.8. 4′-Hydroxychalcone, Isoliquiritigenin, and Xanthohumol Inhibited TNF-α-Induced RelA Nuclear Translocation in A549 Cells
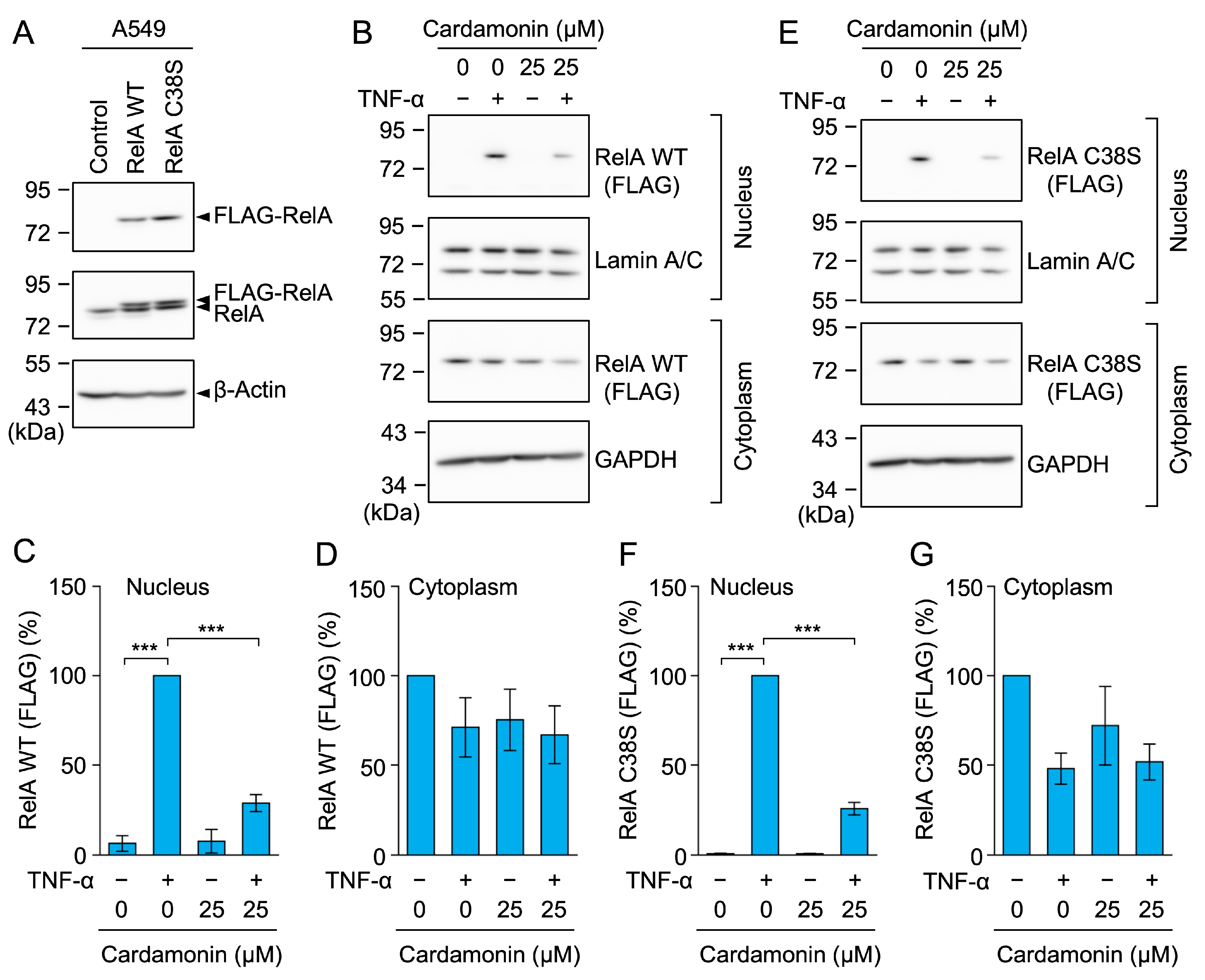
2.9. Cardamonin Inhibited the Nuclear Translocation of the RelA C38S Mutant
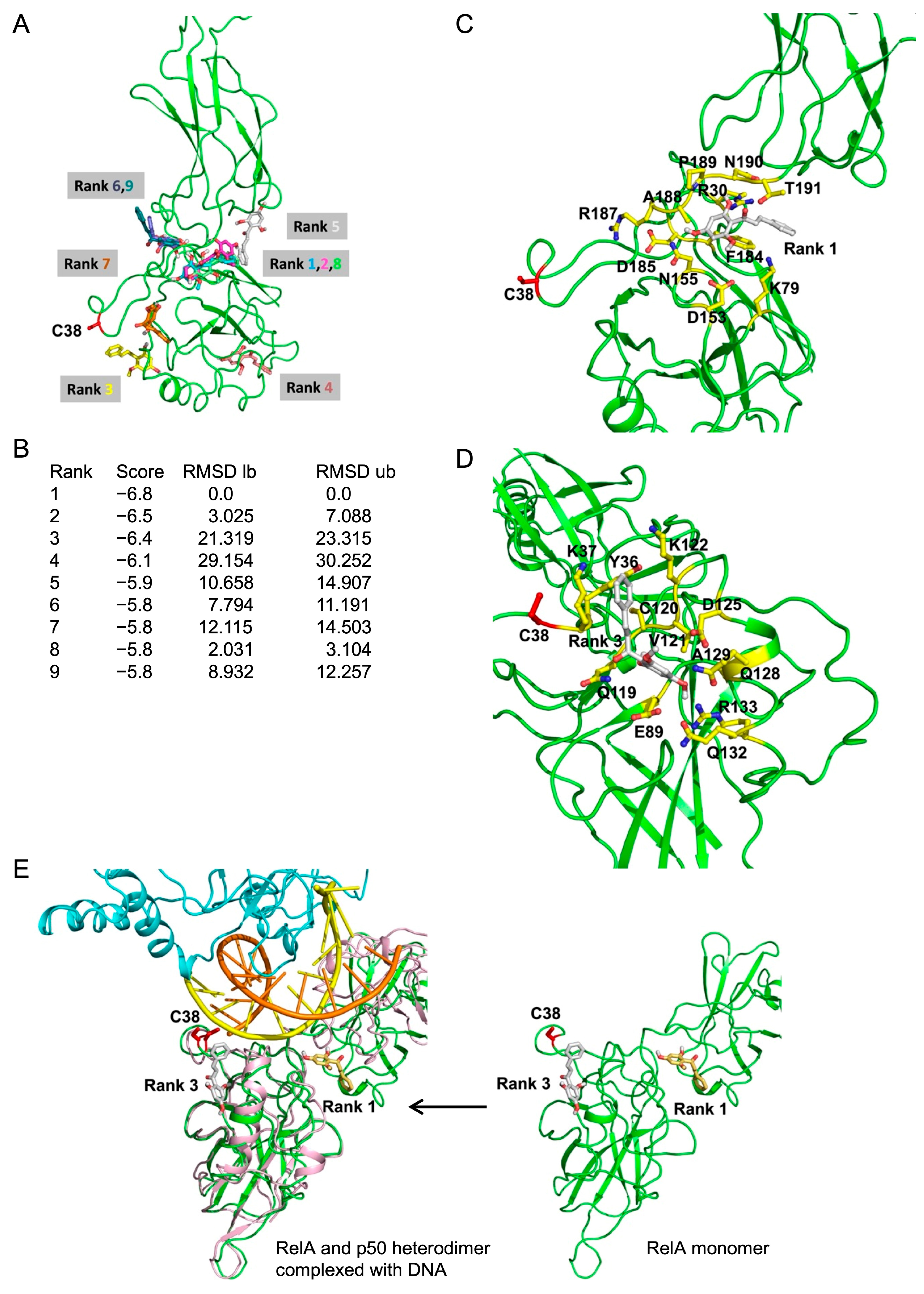
2.10. In Silico Molecular Docking Showed a Potential Interaction Between Cardamonin and RelA
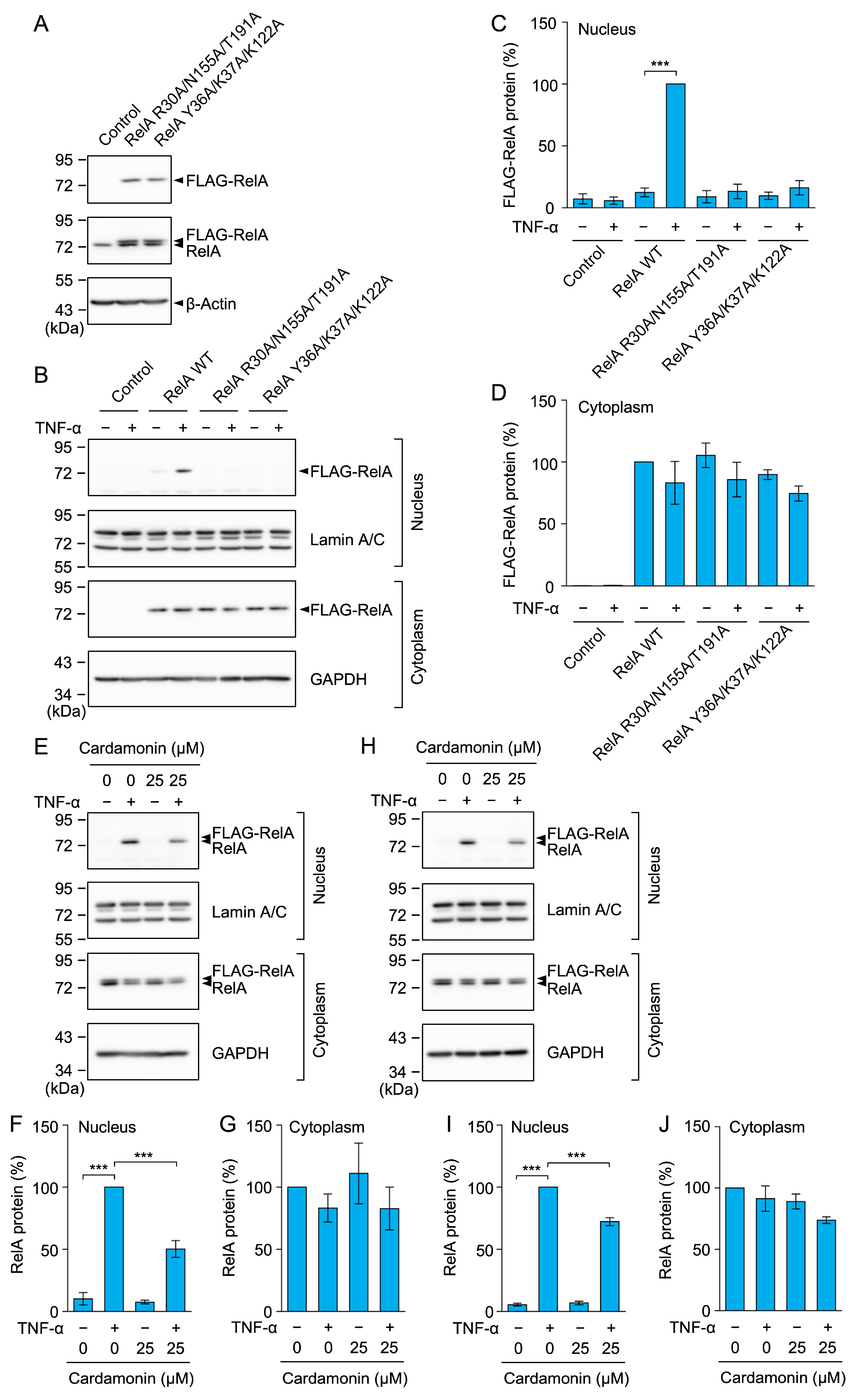
2.11. RelA R30A/N155A/T191A and Y36A/K37A/K122A Mutants Did Not Undergo Nuclear Translocation in Response to the TNF-α Stimulation
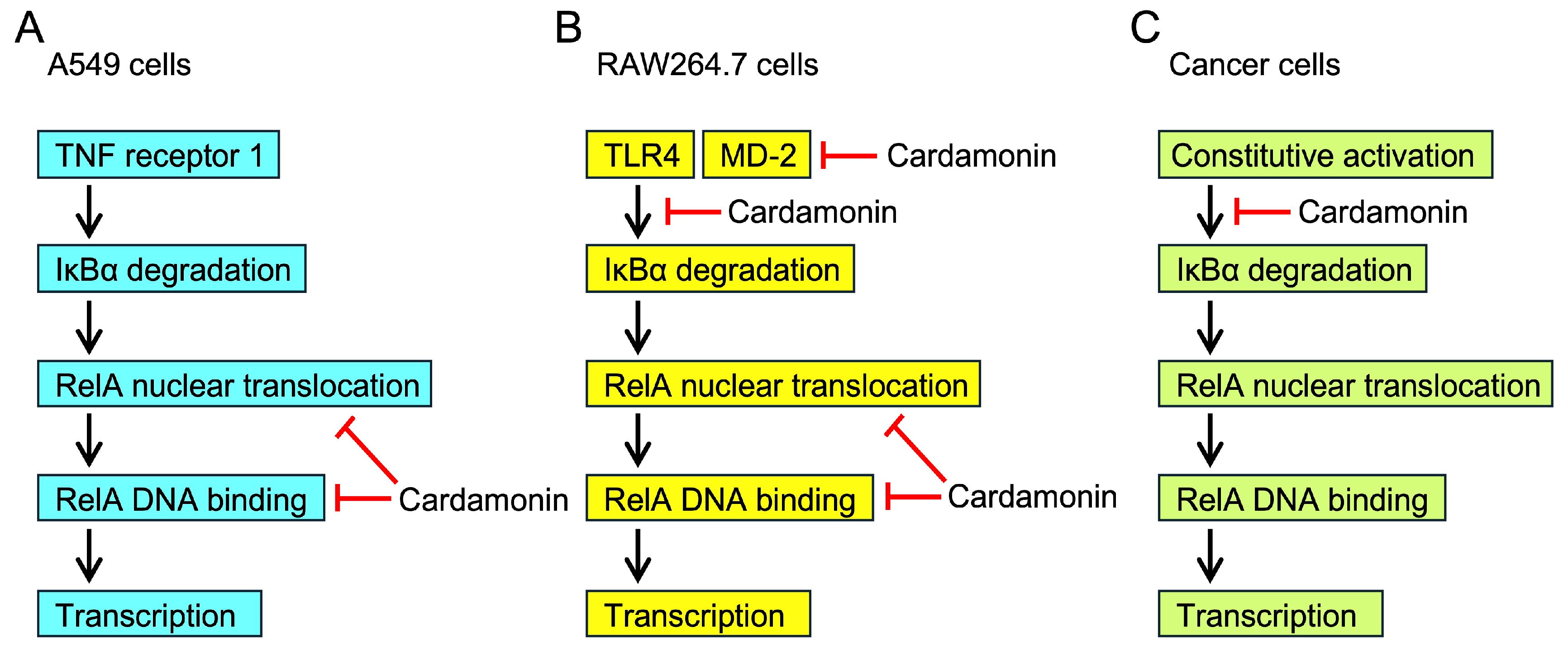
3. Discussion
4. Materials and Methods
4.1. Cells
4.2. Reagents
4.3. Antibodies
4.4. Plasmids
4.5. Cell Culture
4.6. Cell Viability Assay
4.7. Flow Cytometry
4.8. Quantitative PCR
4.9. Luciferase Reporter Assay
4.10. Western Blotting
4.11. ChIP Assay
4.12. Cell ELISA
4.13. Statistical Analysis
4.14. In Silico Molecular Docking Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ICAM-1 | Intercellular adhesion molecule 1 |
| TNF | Tumor necrosis factor |
| NF-κB | Nuclear factor κB |
| IκB | Inhibitor of nuclear factor κB |
| RIPK1 | Receptor-interacting protein kinase 1 |
| TRADD | TNF receptor-associated death domain protein |
| TRAF | TNF receptor-associated factor |
| LPS | Lipopolysaccharide |
| ELISA | Enzyme-linked immunosorbent assay |
| PCR | Polymerase chain reaction |
| ChIP | Chromatin immunoprecipitation |
| SRC2 | Santonin-related compound 2 |
| WT | Wild type |
| TLR | Toll-like receptor |
| MD-2 | Myeloid differentiation factor 2 |
| IFN-γ | Interferon-γ |
| mTOR | Mammalian target of rapamycin |
References
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Wang, T.; He, C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018, 44, 38–50. [Google Scholar] [CrossRef]
- Gerhardt, T.; Ley, K. Monocyte trafficking across the vessel wall. Cardiovasc. Res. 2015, 107, 321–330. [Google Scholar] [CrossRef]
- Vestweber, D. How leukocytes cross the vascular endothelium. Nat. Rev. Immunol. 2015, 15, 692–704. [Google Scholar] [CrossRef]
- Qu, X.; Tang, Y.; Hua, S. Immunological approaches towards cancer and inflammation: A cross talk. Front. Immunol. 2018, 9, 563. [Google Scholar] [CrossRef]
- Bhat, A.A.; Nisar, S.; Singh, M.; Ashraf, B.; Masoodi, T.; Prasad, C.P.; Sharma, A.; Maacha, S.; Karedath, T.; Hashem, S.; et al. Cytokine- and chemokine-induced inflammatory colorectal tumor microenvironment: Emerging avenue for targeted therapy. Cancer Commun. 2022, 42, 689–715. [Google Scholar] [CrossRef] [PubMed]
- Habanjar, O.; Bingula, R.; Decombat, C.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. Crosstalk of inflammatory cytokines within the breast tumor microenvironment. Int. J. Mol. Sci. 2023, 24, 4002. [Google Scholar] [CrossRef] [PubMed]
- Harjunpää, H.; Llort Asens, M.; Guenther, C.; Fagerholm, S.C. Cell adhesion molecules and their roles and regulation in the immune and tumor microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef]
- Bui, T.M.; Wiesolek, H.L.; Sumagin, R. ICAM-1: A master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J. Leukoc. Biol. 2020, 108, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Wang, Y.; Zhang, Z.; Qin, R.; Peng, Y.; Tang, W.; Xi, Y.; Tian, G.; Zhang, Y. Roles of intercellular cell adhesion molecule-1 (ICAM-1) in colorectal cancer: Expression, functions, prognosis, tumorigenesis, polymorphisms and therapeutic implications. Front. Oncol. 2022, 12, 1052672. [Google Scholar] [CrossRef]
- Webster, J.D.; Vucic, D. The balance of TNF mediated pathways regulates inflammatory cell death signaling in healthy and diseased tissues. Front. Cell Dev. Biol. 2020, 8, 365. [Google Scholar] [CrossRef]
- Siegmund, D.; Wajant, H. TNF and TNF receptors as therapeutic targets for rheumatic diseases and beyond. Nat. Rev. Rheumatol. 2023, 19, 576–591. [Google Scholar] [CrossRef] [PubMed]
- van Loo, G.; Bertrand, M.J.M. Death by TNF: A road to inflammation. Nat. Rev. Immunol. 2023, 23, 289–303. [Google Scholar] [CrossRef]
- Fischer, R.; Kontermann, R.E.; Pfizenmaier, K. Selective targeting of TNF receptors as a novel therapeutic approach. Front. Cell Dev. Biol. 2020, 8, 401. [Google Scholar] [CrossRef]
- Chen, A.Y.; Wolchok, J.D.; Bass, A.R. TNF in the era of immune checkpoint inhibitors: Friend or foe? Nat. Rev. Rheumatol. 2021, 17, 213–223. [Google Scholar] [CrossRef]
- Wajant, H.; Siegmund, D. TNFR1 and TNFR2 in the control of the life and death balance of macrophages. Front. Cell Dev. Biol. 2019, 7, 91. [Google Scholar] [CrossRef]
- Atretkhany, K.N.; Gogoleva, V.S.; Drutskaya, M.S.; Nedospasov, S.A. Distinct modes of TNF signaling through its two receptors in health and disease. J. Leukoc. Biol. 2020, 107, 893–905. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, C.; Zhang, Z.; Zhang, H.; Hu, H. NF-κB signaling in inflammation and cancer. MedComm 2021, 2, 618–653. [Google Scholar] [CrossRef] [PubMed]
- Iacobazzi, D.; Convertini, P.; Todisco, S.; Santarsiero, A.; Iacobazzi, V.; Infantino, V. New insights into NF-κB signaling in innate immunity: Focus on immunometabolic crosstalks. Biology 2023, 12, 776. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target. Ther. 2020, 5, 209. [Google Scholar] [CrossRef]
- Pavitra, E.; Kancharla, J.; Gupta, V.K.; Prasad, K.; Sung, J.Y.; Kim, J.; Tej, M.B.; Choi, R.; Lee, J.H.; Han, Y.K.; et al. The role of NF-κB in breast cancer initiation, growth, metastasis, and resistance to chemotherapy. Biomed. Pharmacother. 2023, 163, 114822. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Zhao, X.; Sun, S.C. NF-κB in inflammation and cancer. Cell Mol. Immunol. 2025, 22, 811–839. [Google Scholar] [CrossRef] [PubMed]
- Courtois, G.; Fauvarque, M.O. The many roles of ubiquitin in NF-κB signaling. Biomedicines 2018, 6, 43. [Google Scholar] [CrossRef] [PubMed]
- El Yaagoubi, O.M.; Oularbi, L.; Bouyahya, A.; Samaki, H.; El Antri, S.; Aboudkhil, S. The role of the ubiquitin-proteasome pathway in skin cancer development: 26S proteasome-activated NF-κB signal transduction. Cancer Biol. Ther. 2021, 22, 479–492. [Google Scholar] [CrossRef]
- Puar, Y.R.; Shanmugam, M.K.; Fan, L.; Arfuso, F.; Sethi, G.; Tergaonkar, V. Evidence for the involvement of the master transcription factor NF-κB in cancer initiation and progression. Biomedicines 2018, 6, 82. [Google Scholar] [CrossRef]
- Millar, M.W.; Fazal, F.; Rahman, A. Therapeutic targeting of NF-κB in acute lung injury: A double-edged sword. Cells 2022, 11, 3317. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khan, J.; Dukhyil, A.A.B.; Alarousy, R.M.I.I.; Attah, E.I.; Sharma, T.; Khairnar, S.J.; Bendale, A.R. Chalcone scaffolds, bioprecursors of flavonoids: Chemistry, bioactivities, and pharmacokinetics. Molecules 2021, 26, 7177. [Google Scholar] [CrossRef]
- Dhaliwal, J.S.; Moshawih, S.; Goh, K.W.; Loy, M.J.; Hossain, M.S.; Hermansyah, A.; Kotra, V.; Kifli, N.; Goh, H.P.; Dhaliwal, S.K.S.; et al. Pharmacotherapeutics applications and chemistry of chalcone derivatives. Molecules 2022, 27, 7062. [Google Scholar] [CrossRef]
- Gonçalves, L.M.; Valente, I.M.; Rodrigues, J.A. An overview on cardamonin. J. Med. Food 2014, 17, 633–640. [Google Scholar] [CrossRef]
- Ramchandani, S.; Naz, I.; Dhudha, N.; Garg, M. An overview of the potential anticancer properties of cardamonin. Explor. Target. Antitumor Ther. 2020, 1, 413–426. [Google Scholar] [CrossRef]
- Nawaz, J.; Rasul, A.; Shah, M.A.; Hussain, G.; Riaz, A.; Sarfraz, I.; Zafar, S.; Adnan, M.; Khan, A.H.; Selamoglu, Z. Cardamonin: A new player to fight cancer via multiple cancer signaling pathways. Life Sci. 2020, 250, 117591. [Google Scholar] [CrossRef]
- Daimary, U.D.; Parama, D.; Rana, V.; Banik, K.; Kumar, A.; Harsha, C.; Kunnumakkara, A.B. Emerging roles of cardamonin, a multitargeted nutraceutical in the prevention and treatment of chronic diseases. Curr. Res. Pharmacol. Drug Discov. 2020, 2, 100008. [Google Scholar] [CrossRef]
- Lee, J.H.; Jung, H.S.; Giang, P.M.; Jin, X.; Lee, S.; Son, P.T.; Lee, D.; Hong, Y.S.; Lee, K.; Lee, J.J. Blockade of nuclear factor-κB signaling pathway and anti-inflammatory activity of cardamomin, a chalcone analog from Alpinia conchigera. J. Pharmacol. Exp. Ther. 2006, 316, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Hatziieremia, S.; Gray, A.I.; Ferro, V.A.; Paul, A.; Plevin, R. The effects of cardamonin on lipopolysaccharide-induced inflammatory protein production and MAP kinase and NFκB signalling pathways in monocytes/macrophages. Br. J. Pharmacol. 2006, 149, 188–198. [Google Scholar] [CrossRef]
- Israf, D.A.; Khaizurin, T.A.; Syahida, A.; Lajis, N.H.; Khozirah, S. Cardamonin inhibits COX and iNOS expression via inhibition of p65NF-κB nuclear translocation and Iκ-B phosphorylation in RAW 264.7 macrophage cells. Mol. Immunol. 2007, 44, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Sun, A.; Deng, C.; Zhang, J.; Wu, X.; Wei, X.; Mani, S.; Dou, W.; Wang, Z. The anti-inflammatory effect and potential mechanism of cardamonin in DSS-induced colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G517–G527. [Google Scholar] [CrossRef]
- Kim, A.Y.; Shim, H.J.; Kim, S.Y.; Heo, S.; Youn, H.S. Differential regulation of MyD88- and TRIF-dependent signaling pathways of Toll-like receptors by cardamonin. Int. Immunopharmacol. 2018, 64, 1–9. [Google Scholar] [CrossRef]
- Yang, L.; Luo, W.; Zhang, Q.; Hong, S.; Wang, Y.; Samorodov, A.V.; Chattipakorn, N.; Pavlov, V.N.; Liang, G. Cardamonin inhibits LPS-induced inflammatory responses and prevents acute lung injury by targeting myeloid differentiation factor 2. Phytomedicine 2021, 93, 153785. [Google Scholar] [CrossRef]
- Badroon, N.A.; Abdul Majid, N.; Alshawsh, M.A. Antiproliferative and apoptotic effects of cardamonin against hepatocellular carcinoma HepG2 cells. Nutrients 2020, 12, 1757. [Google Scholar] [CrossRef] [PubMed]
- Harrold, A.P.; Cleary, M.M.; Bharathy, N.; Lathara, M.; Berlow, N.E.; Foreman, N.K.; Donson, A.M.; Amani, V.; Zuercher, W.J.; Keller, C. In vitro benchmarking of NF-κB inhibitors. Eur. J. Pharmacol. 2020, 873, 172981. [Google Scholar] [CrossRef]
- Ding, Q.; Niu, P.; Zhu, Y.; Chen, H.; Shi, D. Cardamonin inhibits the expression of P-glycoprotein and enhances the anti-proliferation of paclitaxel on SKOV3-Taxol cells. J. Nat. Med. 2022, 76, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Tanigaki, R.; Takahashi, R.; Nguyen, M.T.T.; Nguyen, N.T.; Do, T.V.N.; Nguyen, H.X.; Kataoka, T. 4-Hydroxypanduratin A and isopanduratin A inhibit tumor necrosis factor α-stimulated gene expression and the nuclear factor κB-dependent signaling pathway in human lung adenocarcinoma A549 cells. Biol. Pharm. Bull. 2019, 42, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, C.; Tanigaki, R.; Miyake, Y.; Vo, N.T.; Nguyen, M.T.T.; Nguyen, N.T.; Do, T.N.V.; Nguyen, H.X.; Kataoka, T. Isopanduratin A inhibits tumor necrosis factor (TNF)-α-induced nuclear factor κB signaling pathway by promoting extracellular signal-regulated kinase-dependent ectodomain shedding of TNF receptor 1 in human lung adenocarcinoma A549 cells. BioChem 2021, 1, 174–189. [Google Scholar] [CrossRef]
- Vu, Q.V.; Baba, K.; Sasaki, S.; Kawaguchi, K.; Hirano, H.; Osada, H.; Kataoka, T. Alantolactone derivatives inhibit the tumor necrosis factor α-induced nuclear factor κB pathway by a different mechanism from alantolactone. Eur. J. Pharmacol. 2024, 969, 176458. [Google Scholar] [CrossRef]
- Vu, Q.V.; Vu, N.T.; Baba, K.; Sasaki, S.; Tamura, R.; Morimoto, K.; Hirano, H.; Osada, H.; Kataoka, T. Porphyrin derivatives inhibit tumor necrosis factor α-induced gene expression and reduce the expression and increase the cross-linked forms of cellular components of the nuclear factor κB signaling pathway. Eur. J. Pharmacol. 2024, 977, 176747. [Google Scholar] [CrossRef]
- Roebuck, K.A.; Finnegan, A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J. Leukoc. Biol. 1999, 66, 876–888. [Google Scholar] [CrossRef]
- Singh, M.; Thakur, M.; Mishra, M.; Yadav, M.; Vibhuti, R.; Menon, A.M.; Nagda, G.; Dwivedi, V.P.; Dakal, T.C.; Yadav, V. Gene regulation of intracellular adhesion molecule-1 (ICAM-1): A molecule with multiple functions. Immunol. Lett. 2021, 240, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Morimoto, K.; Kondo, T.; Hiramatsu, R.; Okina, Y.; Muko, R.; Matsuda, I.; Kataoka, T. Quinacrine inhibits ICAM-1 transcription by blocking DNA binding of the NF-κB subunit p65 and sensitizes human lung adenocarcinoma A549 cells to TNF-α and the Fas ligand. Int. J. Mol. Sci. 2017, 18, 2603. [Google Scholar] [CrossRef]
- Vo, N.T.; Sasaki, S.; Miyake, Y.; Nguyen, N.T.; Dang, P.H.; Nguyen, M.T.T.; Kataoka, T. α-Conidendrin inhibits the expression of intercellular adhesion molecule-1 induced by tumor necrosis factor-α in human lung adenocarcinoma A549 cells. Eur. J. Pharmacol. 2021, 890, 173651. [Google Scholar] [CrossRef]
- Gilmore, T.D.; Herscovitch, M. Inhibitors of NF-κB signaling: 785 and counting. Oncogene 2006, 25, 6887–6899. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, T. Chemical biology of inflammatory cytokine signaling. J. Antibiot. 2009, 62, 655–667. [Google Scholar] [CrossRef]
- Pande, V.; Sousa, S.F.; Ramos, M.J. Direct covalent modification as a strategy to inhibit nuclear factor-κB. Curr. Med. Chem. 2009, 16, 4261–4273. [Google Scholar] [CrossRef]
- Tamura, R.; Morimoto, K.; Hirano, S.; Wang, L.; Zhao, M.; Ando, M.; Kataoka, T. Santonin-related compound 2 inhibits the nuclear translocation of NF-κB subunit p65 by targeting cysteine 38 in TNF-α-induced NF-κB signaling pathway. Biosci. Biotechnol. Biochem. 2012, 76, 2360–2363. [Google Scholar] [CrossRef]
- Liang, S.T.; Chen, C.; Chen, R.X.; Li, R.; Chen, W.L.; Jiang, G.H.; Du, L.L. Michael acceptor molecules in natural products and their mechanism of action. Front. Pharmacol. 2022, 13, 1033003. [Google Scholar] [CrossRef]
- Chen, F.E.; Huang, D.B.; Chen, Y.Q.; Ghosh, G. Crystal structure of p50/p65 heterodimer of transcription factor NF-κB bound to DNA. Nature 1998, 391, 410–413. [Google Scholar] [CrossRef]
- Jaiswal, S.; Sharma, A.; Shukla, M.; Lal, J. Gender-related pharmacokinetics and bioavailability of a novel anticancer chalcone, cardamonin, in rats determined by liquid chromatography tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2015, 986–987, 23–30. [Google Scholar] [CrossRef]
- Jaiswal, S.; Shukla, M.; Sharma, A.; Rangaraj, N.; Vaghasiya, K.; Malik, M.Y.; Lal, J. Preclinical pharmacokinetics and ADME characterization of a novel anticancer chalcone, cardamonin. Drug Test. Anal. 2017, 9, 1124–1136. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Cochet, F.; Peri, F. The role of carbohydrates in the lipopolysaccharide (LPS)/Toll-like Receptor 4 (TLR4) signalling. Int. J. Mol. Sci. 2017, 18, 2318. [Google Scholar] [CrossRef]
- Marongiu, L.; Gornati, L.; Artuso, I.; Zanoni, I.; Granucci, F. Below the surface: The inner lives of TLR4 and TLR9. J. Leukoc. Biol. 2019, 106, 147–160. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like receptors and the control of immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Ko, H.; Park, J.S.; Han, I.H.; Amor, E.C.; Lee, J.W.; Yang, H.O. Dimethyl cardamonin inhibits lipopolysaccharide-induced inflammatory factors through blocking NF-κB p65 activation. Int. Immunopharmacol. 2010, 10, 1127–1134. [Google Scholar] [CrossRef]
- Chaturvedi, M.M.; Sung, B.; Yadav, V.R.; Kannappan, R.; Aggarwal, B.B. NF-κB addiction and its role in cancer: ‘one size does not fit all’. Oncogene 2011, 30, 1615–1630. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Shen, S.; Verma, I.M. NF-κB, an active player in human cancers. Cancer Immunol. Res. 2014, 2, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Pires, B.R.B.; Silva, R.C.M.C.; Ferreira, G.M.; Abdelhay, E. NF-κB: Two sides of the same coin. Genes 2018, 9, 24. [Google Scholar] [CrossRef]
- Qin, Y.; Sun, C.Y.; Lu, F.R.; Shu, X.R.; Yang, D.; Chen, L.; She, X.M.; Gregg, N.M.; Guo, T.; Hu, Y. Cardamonin exerts potent activity against multiple myeloma through blockade of NF-κB pathway in vitro. Leuk. Res. 2012, 36, 514–520. [Google Scholar] [CrossRef]
- Jia, D.; Tan, Y.; Liu, H.; Ooi, S.; Li, L.; Wright, K.; Bennett, S.; Addison, C.L.; Wang, L. Cardamonin reduces chemotherapy-enriched breast cancer stem-like cells in vitro and in vivo. Oncotarget 2016, 7, 771–785. [Google Scholar] [CrossRef]
- Ruibin, J.; Bo, J.; Danying, W.; Jianguo, F.; Linhui, G. Cardamonin induces G2/M phase arrest and apoptosis through inhibition of NF-κB and mTOR pathways in ovarian cancer. Aging 2020, 12, 25730–25743. [Google Scholar] [CrossRef]
- Kusagawa, E.; Okuda, C.; Yamaguchi, R.; Nakano, K.; Miyake, Y.; Kataoka, T. Cucurbitacin B down-regulates TNF receptor 1 expression and inhibits the TNF-α-dependent nuclear factor κB signaling pathway in human lung adenocarcinoma A549 cells. Int. J. Mol. Sci. 2022, 23, 7130. [Google Scholar] [CrossRef]
- Tang, Y.; Fang, Q.; Shi, D.; Niu, P.; Chen, Y.; Deng, J. mTOR inhibition of cardamonin on antiproliferation of A549 cells is involved in a FKBP12 independent fashion. Life Sci. 2014, 99, 44–51. [Google Scholar] [CrossRef]
- Break, M.K.B.; Hossan, M.S.; Khoo, Y.; Qazzaz, M.E.; Al-Hayali, M.Z.K.; Chow, S.C.; Wiart, C.; Bradshaw, T.D.; Collins, H.; Khoo, T.J. Discovery of a highly active anticancer analogue of cardamonin that acts as an inducer of caspase-dependent apoptosis and modulator of the mTOR pathway. Fitoterapia 2018, 125, 161–173. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, R.; Li, Q.; Jie, X.; Hong, J.; Zong, Y.; Dong, X.; Zhang, S.; Li, Z.; Wu, G. Cardamonin inhibits the proliferation and metastasis of non-small-cell lung cancer cells by suppressing the PI3K/Akt/mTOR pathway. Anti-Cancer Drugs 2019, 30, 241–250. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, R.; Li, W.; Ma, X.C.; Qiu, F.; Sun, C.P. IκB kinase β (IKKβ): Structure, transduction mechanism, biological function, and discovery of its inhibitors. Int. J. Biol. Sci. 2023, 19, 4181–4203. [Google Scholar] [CrossRef]
- Amin, H.; Althagafy, H.S.; El-Maksoud, M.S.A.; Ibrahim, I.M.; Hassanein, E.H.M. Flavonoids in combating renal fibrosis: Targeting NF-κB signal and in silico support. Chem. Biodivers. 2025, 22, e202403022. [Google Scholar] [CrossRef] [PubMed]
- Badroon, N.; Abdul Majid, N.; Al-Suede, F.S.R.; Nazari, V.M.; Giribabu, N.; Abdul Majid, A.M.S.; Eid, E.E.M.; Alshawsh, M.A. Cardamonin exerts antitumor effect on human hepatocellular carcinoma xenografts in athymic nude mice through inhibiting NF-κβ pathway. Biomedicines 2020, 8, 586. [Google Scholar] [CrossRef] [PubMed]
- Vo, N.T.; Kusagawa, E.; Nakano, K.; Moriwaki, C.; Miyake, Y.; Haruyama, S.; Fukuhara, S.; Nguyen, N.T.; Dang, P.H.; Nguyen, M.T.T.; et al. Biological evaluation of alkyl triphenylphosphonium ostruthin derivatives as potential anti-inflammatory agents targeting the nuclear factor κB signaling pathway in human lung adenocarcinoma A549 cells. BioChem 2021, 1, 107–121. [Google Scholar] [CrossRef]
- Dohrman, A.; Kataoka, T.; Cuenin, S.; Russell, J.Q.; Tschopp, J.; Budd, R.C. Cellular FLIP (long form) regulates CD8+ T cell activation through caspase-8-dependent NF-κB activation. J. Immunol. 2005, 174, 5270–5278. [Google Scholar] [CrossRef]
- Fukuoka, N.; Harada, M.; Nishida, A.; Ito, Y.; Shiota, H.; Kataoka, T. Eomesodermin promotes interferon-γ expression and binds to multiple conserved noncoding sequences across the Ifng locus in mouse thymoma cell lines. Genes Cells 2016, 21, 146–162. [Google Scholar] [CrossRef]
- Wan, M.; Liu, J.; Ouyang, X. Nucleotide-binding oligomerization domain 1 regulates Porphyromonas gingivalis-induced vascular cell adhesion molecule 1 and intercellular adhesion molecule 1 expression in endothelial cells through NF-κB pathway. J. Periodontal. Res. 2015, 50, 189–196. [Google Scholar] [CrossRef]
- Zhang, Y.; Lian, F.; Zhu, Y.; Xia, M.; Wang, Q.; Ling, W.; Wang, X.D. Cyanidin-3-O-β-glucoside inhibits LPS-induced expression of inflammatory mediators through decreasing IκBα phosphorylation in THP-1 cells. Inflamm. Res. 2010, 59, 723–730. [Google Scholar] [CrossRef]
- Xue, J.; Thippegowda, P.B.; Hu, G.; Bachmaier, K.; Christman, J.W.; Malik, A.B.; Tiruppathi, C. NF-κB regulates thrombin-induced ICAM-1 gene expression in cooperation with NFAT by binding to the intronic NF-κB site in the ICAM-1 gene. Physiol. Genom. 2009, 38, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, J.; Santos-Martins, D.; Tillack, A.F.; Forli, S. AutoDock Vina 1.2.0: New docking methods, expanded force field, and python bindings. J. Chem. Inf. Model. 2021, 61, 3891–3898. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vu, N.T.; Vu, Q.V.; Vo, N.T.; Tanigaki, R.; Quach, H.T.; Miyake, Y.; Shiba, T.; Kataoka, T. Cardamonin Inhibits the Nuclear Translocation and DNA Binding of RelA in the Tumor Necrosis Factor-α-Induced NF-κB Signaling Pathway in Human Lung Adenocarcinoma A549 Cells. Molecules 2025, 30, 4324. https://doi.org/10.3390/molecules30224324
Vu NT, Vu QV, Vo NT, Tanigaki R, Quach HT, Miyake Y, Shiba T, Kataoka T. Cardamonin Inhibits the Nuclear Translocation and DNA Binding of RelA in the Tumor Necrosis Factor-α-Induced NF-κB Signaling Pathway in Human Lung Adenocarcinoma A549 Cells. Molecules. 2025; 30(22):4324. https://doi.org/10.3390/molecules30224324
Chicago/Turabian StyleVu, Nhat Thi, Quy Van Vu, Nghia Trong Vo, Riho Tanigaki, Hue Tu Quach, Yasunobu Miyake, Tomoo Shiba, and Takao Kataoka. 2025. "Cardamonin Inhibits the Nuclear Translocation and DNA Binding of RelA in the Tumor Necrosis Factor-α-Induced NF-κB Signaling Pathway in Human Lung Adenocarcinoma A549 Cells" Molecules 30, no. 22: 4324. https://doi.org/10.3390/molecules30224324
APA StyleVu, N. T., Vu, Q. V., Vo, N. T., Tanigaki, R., Quach, H. T., Miyake, Y., Shiba, T., & Kataoka, T. (2025). Cardamonin Inhibits the Nuclear Translocation and DNA Binding of RelA in the Tumor Necrosis Factor-α-Induced NF-κB Signaling Pathway in Human Lung Adenocarcinoma A549 Cells. Molecules, 30(22), 4324. https://doi.org/10.3390/molecules30224324






