Synergistic Enhancement of Photocatalytic H2O2 Production over Carbon Nitride Oxide/Biochar Composites
Abstract
1. Introduction
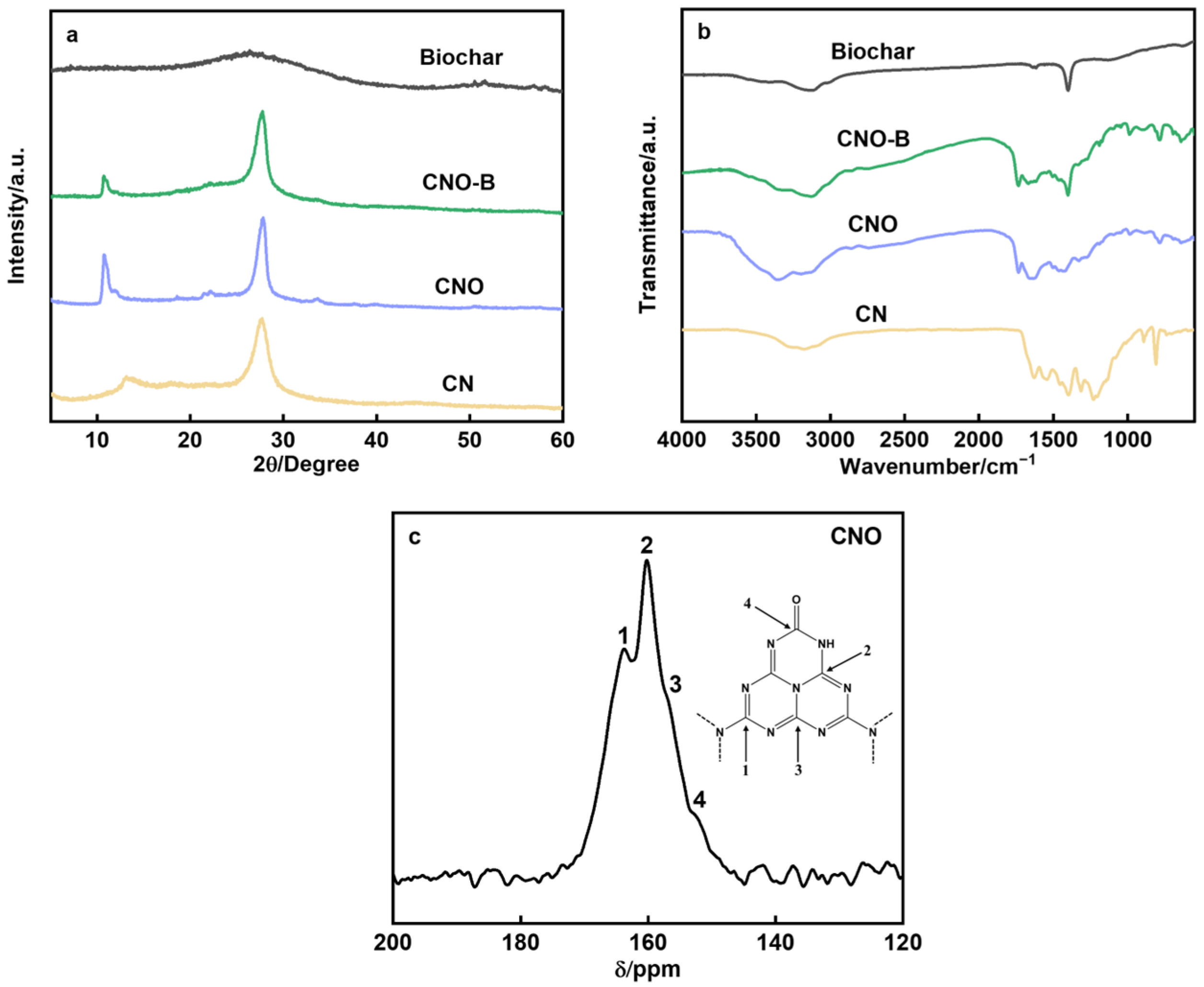
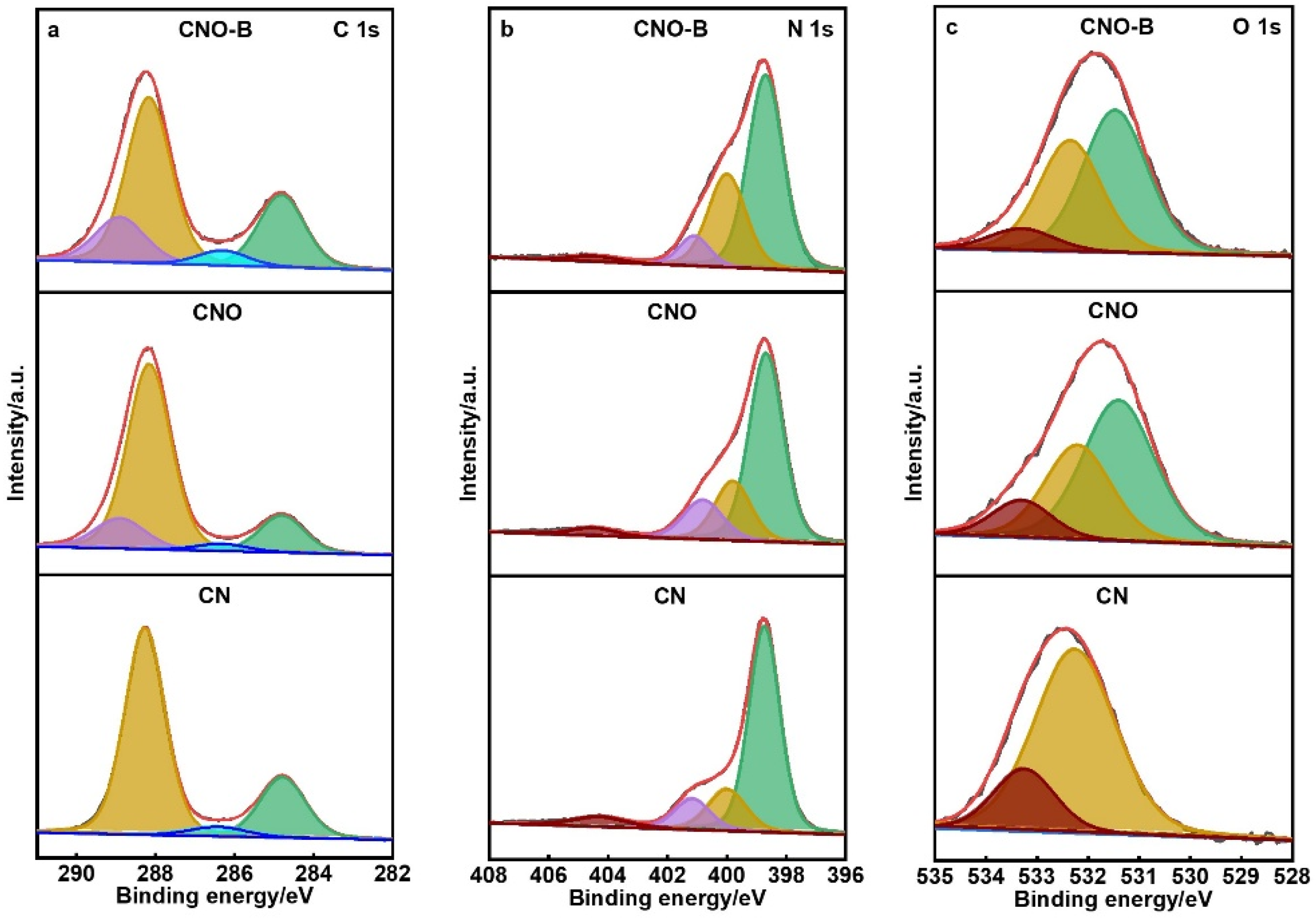
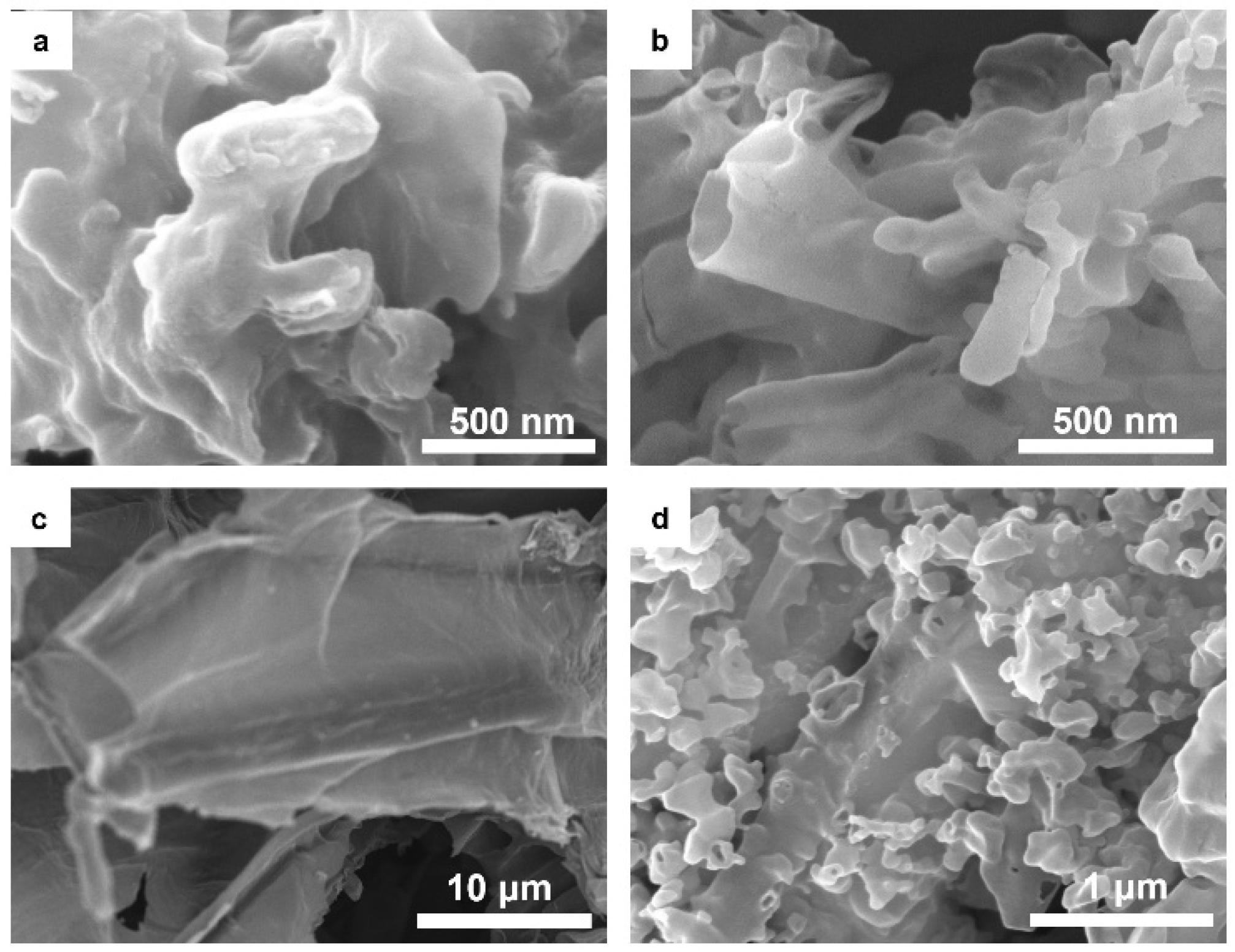
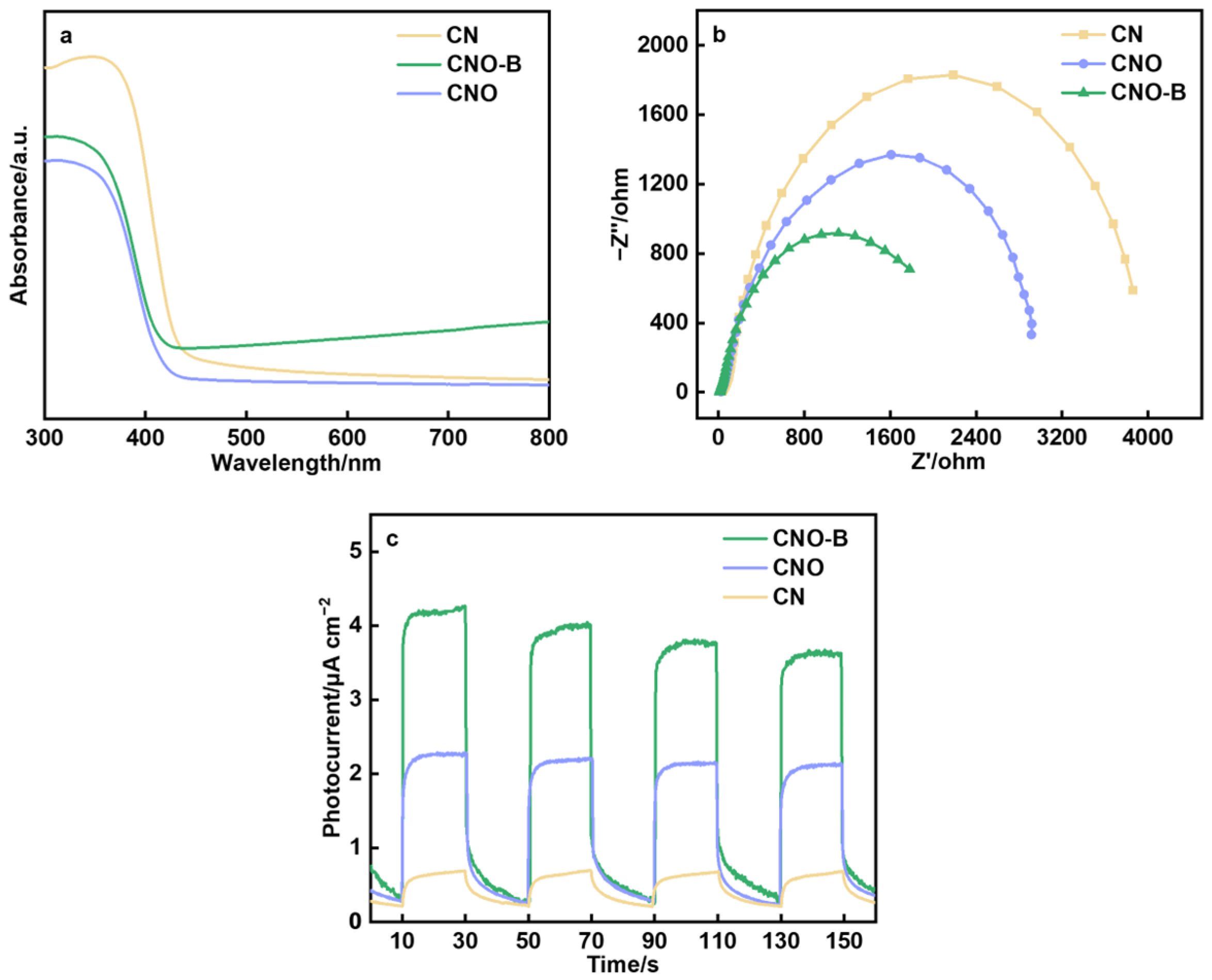
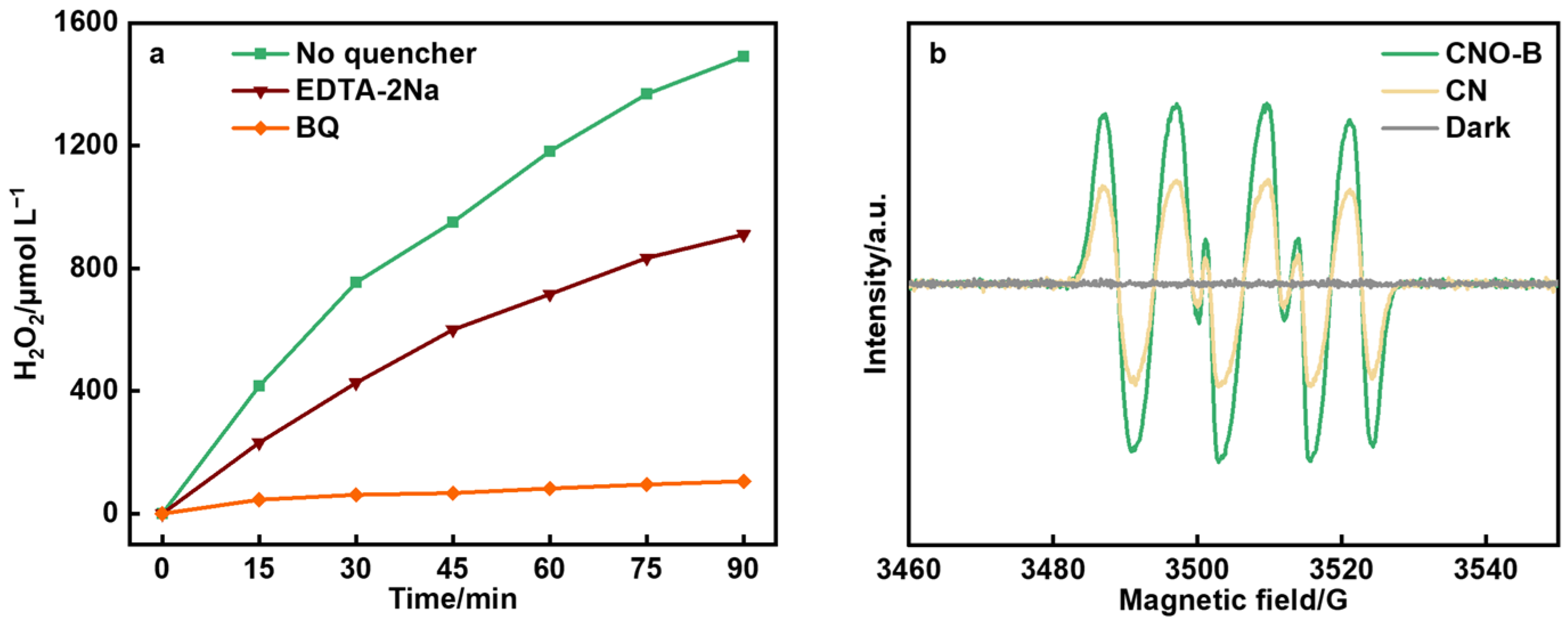
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Synthesis of g-C3N4 (CN)
3.3. Synthesis of Carbon Nitride Oxide/Biochar Composites (CNO-B)
3.4. Characterization
3.5. Photocatalytic H2O2 Production
3.6. Photoelectrochemical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Tu, H.; Zhao, Z.C.; Chen, S.S.; Wang, Y.; Chen, S.H.; Zhang, J.; Wu, J. Unveiling the Impact of Microstructure Alterations on Photocatalytic Hydrogen Peroxide Preparation via DFT Prediction and Analysis. Energ. Environ. Mater. 2025, 8, e70016. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, C.C.; Zhang, Y.; Sun, C.H.; Yu, J.G.; Zhang, L.Y. Harnessing S-scheme BiOCl/COF heterojunctions for sustainable and efficient photocatalytic hydrogen peroxide synthesis under visible light. J. Colloid Interf. Sci. 2025, 692, 137544. [Google Scholar] [CrossRef]
- Qi, W.J.; He, X.X.; Gong, Z.H.; Wang, Q.; Peng, D.Y.; Chen, X.J.; Wang, J.; Li, J.S.; Ma, J.; Zhu, Y.F. Enhanced photocatalytic performance for hydrogen peroxide photosynthesis over bayberry tannin-formaldehyde resin with a dynamically modulated donor-acceptor structure. Appl. Catal. B 2025, 371, 125264. [Google Scholar] [CrossRef]
- Wang, C.; Qiu, T.Y.; Zhao, Y.N.; Lang, Z.L.; Li, Y.G.; Su, Z.M.; Tan, H.Q. Phosphorus-Alkynyl Functionalized Covalent Triazine/Heptazine-Based Frameworks for High-Performance Photocatalytic Hydrogen Peroxide Production. Adv. Energy Mater. 2023, 13, 23. [Google Scholar] [CrossRef]
- Jiang, B.S.; Chen, D.Y.; Li, N.J.; Xu, Q.F.; Li, H.; Lu, J.M. A quaternary ammonium cyclodextrin polymer for efficient photocatalytic production of hydrogen peroxide from pure water. Chem. Eng. J. 2024, 487, 150609. [Google Scholar] [CrossRef]
- Fu, J.; Chen, S.; Liu, X.; Yang, H.; Xie, Y.; Zhang, Q.; Chen, J.; Cao, M. Soluble polymer microenvironments promote photocatalytic hydrogen peroxide production and self-Fenton reactions. Chem. Catal. 2025, 5, 101260. [Google Scholar] [CrossRef]
- Abebe, A.; Kaushik, R.; Kumar, S.; Mondal, I.C.; Ghosh, S.; Halder, A. Resorcinol-Formaldehyde/TiO2 Polymer Composites: Efficient Photocatalysts for Oxidation of Water to Hydrogen Peroxide. ACS Appl. Nano Mater. 2025, 8, 1882–1892. [Google Scholar] [CrossRef]
- Jang, S.B.; Yoon, S.Y.; Wong, K.T.; Choong, C.E.; Yoon, Y.; Ha Choi, E.; Jang, M. Enhanced in-situ oxygen evolution and hydrogen peroxide production by a floatable ZnO-incorporated polyurethane photocatalyst for sulfamethoxazole degradation. Chem. Eng. J. 2023, 467, 143470. [Google Scholar] [CrossRef]
- Zhang, K.L.; Chen, H.C.; Wang, L.; Tang, H.; Liu, Z.Q. Compressive interatomic distance stimulates photocatalytic oxygen-oxygen coupling to hydrogen peroxide. Sci. Bull. 2024, 70, 536–545. [Google Scholar] [CrossRef]
- Zhang, Y.G.; Huang, H.W.; Wang, L.C.; Zhang, X.L.; Zhu, Z.J.; Wang, J.J.; Yu, W.Y.; Zhang, Y.H. Cooperation of congenital and acquisitus sulfur vacancy for excellent photocatalytic hydrogen peroxide evolution of CdS nanorods from air. Chem. Eng. J. 2023, 454, 140420. [Google Scholar] [CrossRef]
- Choi, J.Y.; Check, B.; Fang, X.Y.; Blum, S.; Pham, H.T.B.; Tayman, K.; Park, J. Photocatalytic Hydrogen Peroxide Production through Functionalized Semiconductive Metal-Organic Frameworks. J. Am. Chem. Soc. 2024, 146, 11319–11327. [Google Scholar] [CrossRef]
- Shu, C.; Xie, P.X.; Yang, X.J.; Yang, X.; Gao, H.; Tan, B.; Wang, X.Y. Integrating β-ketoenamine linkages into covalent organic frameworks toward efficient overall photocatalytic hydrogen peroxide production. J. Mater. Chem. A 2024, 12, 25927–25933. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Zeng, D.; Wang, W.J.; Wang, J.X.; Wang, W.M.; Wang, W.Z. An efficient strategy for photocatalytic hydrogen peroxide production over oxygen-enriched graphitic carbon nitride with sodium phosphate. Chin. J. Catal. 2022, 43, 2690–2698. [Google Scholar] [CrossRef]
- Liu, T.Y.; Chen, F.; An, Y.; Huang, H.W.; Liu, J.A.; Yu, W.Y.; Li, M.T.; Bai, L.Q.; Zhang, Y.H.; Tian, N. Oxygen vacancy induced robust interfacial electric field for efficient photocatalytic hydrogen peroxide production. Chem. Eng. J. 2024, 479, 147724. [Google Scholar] [CrossRef]
- Wen, D.; Su, Y.R.; Fang, J.Y.; Zheng, D.; Xu, Y.S.; Zhou, S.; Meng, A.Y.; Han, P.G.; Wong, C.P. Synergistically boosted photocatalytic production of hydrogen peroxide via protonation and oxygen doping on graphitic carbon nitride. Nano Energ. 2023, 117, 108917. [Google Scholar] [CrossRef]
- Li, Q.; Jiao, Y.Q.; Tang, Y.Q.; Zhou, J.; Wu, B.G.; Jiang, B.J.; Fu, H.G. Shear Stress Triggers Ultrathin-Nanosheet Carbon Nitride Assembly for Photocatalytic H2O2 Production Coupled with Selective Alcohol Oxidation. J. Am. Chem. Soc. 2023, 145, 20837–20848. [Google Scholar] [CrossRef]
- Liu, W.; Che, H.N.; Liu, B.; Ao, Y.H. Unveiling the mechanism on photocatalytic singlet oxygen generation over rationally designed carbonylated carbon nitride. J Mater Chem. A. 2024, 12, 13427–13434. [Google Scholar] [CrossRef]
- Kharlamov, A.; Bondarenko, M.; Kharlamova, G.; Gubareni, N. Features of the synthesis of carbon nitride oxide (g-C3N4)O at urea pyrolysis. Diam. Relat. Mat. 2016, 66, 16–22. [Google Scholar] [CrossRef]
- Kharlamov, A.; Bondarenko, M.; Kharlamova, G. Method for the synthesis of water-soluble oxide of graphite-like carbon nitride. Diam. Relat. Mat. 2016, 61, 46–55. [Google Scholar] [CrossRef]
- Yu, X.N.; Liu, Z.X.; Wang, Y.M.; Luo, H.Y.; Tang, X. Fabrication of corncob-derived biomass charcoal decorated g-C3N4 photocatalysts for removing 2-mercaptobenzothiazole. New J. Chem. 2020, 44, 15908–15918. [Google Scholar] [CrossRef]
- Kong, L.Q.; Yan, J.Q.; Liu, S.Z. Carbonyl Linked Carbon Nitride Loading Few Layered MoS2 for Boosting Photocatalytic Hydrogen Generation. ACS Sustain. Chem. Eng. 2019, 7, 1389–1398. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, N.; Liu, X.M.; He, F.T.; Lu, Y.M.; Wang, S.L.; Zhao, C.C.; Wang, Y.Q.; Zhang, J.Q.; Wang, S.B. Crystalline Oxygen-Modified Carbon Nitride Photocatalyst with Enhanced Internal Electric Field and Strong Resistance to Ionic Interference for Robust Seawater Splitting. ACS Catal. 2024, 14, 13768–13780. [Google Scholar] [CrossRef]
- Meng, L.R.; Yin, W.H.; Wang, S.S.; Wu, X.G.; Hou, J.H.; Yin, W.Q.; Feng, K.; Ok, Y.S.; Wang, X.Z. Photocatalytic behavior of biochar-modified carbon nitride with enriched visible-light reactivity. Chemosphere 2020, 239, 124713. [Google Scholar] [CrossRef]
- Lin, M.X.; Li, F.Y.; Cheng, W.Y.; Rong, X.M.; Wang, W. Facile preparation of a novel modified biochar-based supramolecular self-assembled g-C3N4 for enhanced visible light photocatalytic degradation of phenanthrene. Chemosphere 2022, 288, 132610. [Google Scholar] [CrossRef]
- Yang, C.W.; Chen, Y.B.; Chen, T.Z.; Low, J.; Rajendran, S.; Zeng, Z.Y.; Zhang, X.Y.; Qin, J.Q. Active site modulation of porous g-C3N4 nanofragment via defect assemblage for enhancing visible-light driven hydrogen evolution. Fuel 2023, 337, 126894. [Google Scholar] [CrossRef]
- Motlagh, P.Y.; Vahid, B.; Babazadeh, N.; Karimpour, D.; Kayan, B.; Baran, T.; Yoon, Y.; Khataee, A. Palladium nanoparticles supported on biochar/graphitic carbon nitride as a heterogeneous catalyst for pharmaceutical degradation. J. Environ. Chem. Eng. 2024, 12, 113150. [Google Scholar] [CrossRef]
- Rajesh, Y.; Pilli, S.R.; Ali, W.; Motana, S.; Khan, M.E.; Bashiri, A.H.; Zakri, W. Synthesis of activated biochar from sustainable bamboo resources: An environment-friendly and low-cost solution for palladium (II) removal from wastewater. Chemosphere 2023, 341, 139944. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, D.; Deng, J.; Zhou, F.; Duan, Z.; Su, Q.; Pang, S. Mosquito’s Compound Eyes as Inspiration for Fabrication of Conductive Superhydrophobic Nanocarbon Materials from Waste Wheat Straw. ACS Sustain. Chem. Eng. 2019, 7, 3883–3894. [Google Scholar] [CrossRef]
- Chen, C.; Du, S.; Wang, Y.; Han, Z.; Zhang, S.; Ma, W.; Xu, H.; Fang, P. Hollow MoSe2/N-doped carbon composited with ZnIn2S4 constructing dual Z-scheme heterojunction for enhanced visible-light photocatalytic performances. J. Colloid Interf. Sci. 2026, 701, 138758. [Google Scholar] [CrossRef]
- Liang, H.; Xu, Q.; Cheng, R.; Jing, S.; Chen, F.; Tsiakaras, P. Triggering n→π∗ electronic transitions by thiazole modified graphitic carbon nitride for enhanced photocatalytic hydrogen peroxide production and Rhodamine B degradation. Carbon 2025, 244, 120603. [Google Scholar] [CrossRef]
- Li, K.X.; Huang, Z.; Zhu, S.Y.; Luo, S.L.; Yan, L.S.; Dai, Y.H.; Guo, Y.H.; Yang, Y.X. Removal of Cr(VI) from water by a biochar-coupled g-C3N4 nanosheets composite and performance of a recycled photocatalyst in single and combined pollution systems. Appl. Catal. B 2019, 243, 386–396. [Google Scholar] [CrossRef]
- Liang, H.G.; Wang, H.R.; Wang, A.H.; Cheng, R.L.; Jing, S.Y.; Chen, F.; Kannan, P.; Balkourani, G.; Tsiakaras, P. Efficient photocatalytic hydrogen peroxide production over S-scheme InS/molten salt modified CN heterojunction. J. Colloid Interf. Sci. 2024, 669, 506–517. [Google Scholar] [CrossRef]
- Li, J.C.; Shi, H.; Li, Z.A.; Wang, J.X.; Si, H.L.; Liao, F.; Huang, H.; Liu, Y.; Kang, Z.H. Interaction of metal ions in high efficiency seawater hydrogen peroxide production by a carbon-based photocatalyst. Appl. Catal. B 2024, 343, 123541. [Google Scholar] [CrossRef]
- Sun, S.; Gao, F.; Yang, H. Construction of Multiple Asymmetric Catalytic Sites on Carbon Nitrides Toward Efficient Solar Hydrogen Peroxide Production. Adv. Sci. 2025, e13453. [Google Scholar] [CrossRef]
- Liu, B.; Du, J.; Ke, G.; Jia, B.; Huang, Y.; He, H.; Zhou, Y.; Zou, Z. Boosting O2 Reduction and H2O Dehydrogenation Kinetics: Surface N-Hydroxymethylation of g-C3N4 Photocatalysts for the Efficient Production of H2O2. Adv. Funct. Mater. 2022, 32, 2111125. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, J.; Wang, D.; Zhang, F.; Zhao, Y.T.; Yan, M.; Zheng, C.; Wang, Q.; Long, M.; Chen, C. Modification of g-C3N4 with hydroxyethyl cellulose as solid proton donor via hydrogen bond to enhance H2O2 production. Appl. Catal. B 2022, 318, 121749. [Google Scholar] [CrossRef]
- Shi, H.; Li, Y.; Wang, X.; Yu, H.; Yu, J. Selective modification of ultra-thin g-C3N4 nanosheets on the (110) facet of Au/BiVO4 for boosting photocatalytic H2O2 production. Appl. Catal. B 2021, 297, 120414. [Google Scholar] [CrossRef]
- Shen, Y.; Shi, J.; Wang, Y.; Shi, Y.; Shan, P.; Zhang, S.; Hou, J.; Guo, F.; Li, C.; Shi, W. Incorporation of hydroxyl groups and π-rich electron domains into g-C3N4 framework for boosted sacrificial agent-free photocatalytic H2O2 production. Chem. Eng. J. 2024, 498, 155744. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, G.; Li, W.; Yuan, Z.; Si, C. Broad-spectrum responsive lignin-KOH co-modified graphitic carbon nitride for synergetic photocatalytic H2O2 production via carbon-ring embedding and defect engineering. Chem. Eng. J. 2025, 503, 158655. [Google Scholar] [CrossRef]
- Zhou, L.; Feng, J.; Qiu, B.; Zhou, Y.; Lei, J.; Xing, M.; Wang, L.; Zhou, Y.; Liu, Y.; Zhang, J. Ultrathin g-C3N4 nanosheet with hierarchical pores and desirable energy band for highly efficient H2O2 production. Appl. Catal. B 2020, 267, 118396. [Google Scholar] [CrossRef]
- Zhou, J.; Shan, T.; Zhang, F.; Boury, B.; Huang, L.; Yang, Y.; Liao, G.; Xiao, H.; Chen, L. A Novel Dual-Channel Carbon Nitride Homojunction with Nanofibrous Carbon for Significantly Boosting Photocatalytic Hydrogen Peroxide Production. Adv. Fiber Mater. 2024, 6, 387–400. [Google Scholar] [CrossRef]
- Liu, W.; Song, C.; Kou, M.; Wang, Y.; Deng, Y.; Shimada, T.; Ye, L. Fabrication of ultra-thin g-C3N4 nanoplates for efficient visible-light photocatalytic H2O2 production via two-electron oxygen reduction. Chem. Eng. J. 2021, 425, 130615. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, C.; Tayyab, M.; Wei, Z.; Zheng, X.; Shangguan, W.; Zhang, S.; Chen, S.; Meng, S. Regulating electron-hole pairs of g-C3N4 efficiently separated and fully utilized for photosynthesis of H2O2 under visible light. Chem. Eng. J. 2025, 509, 161409. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, R.; Wang, Y.; Lu, S. Synergistic Enhancement of Photocatalytic H2O2 Production over Carbon Nitride Oxide/Biochar Composites. Molecules 2025, 30, 4323. https://doi.org/10.3390/molecules30224323
Cheng R, Wang Y, Lu S. Synergistic Enhancement of Photocatalytic H2O2 Production over Carbon Nitride Oxide/Biochar Composites. Molecules. 2025; 30(22):4323. https://doi.org/10.3390/molecules30224323
Chicago/Turabian StyleCheng, Ruolin, Yue Wang, and Shijian Lu. 2025. "Synergistic Enhancement of Photocatalytic H2O2 Production over Carbon Nitride Oxide/Biochar Composites" Molecules 30, no. 22: 4323. https://doi.org/10.3390/molecules30224323
APA StyleCheng, R., Wang, Y., & Lu, S. (2025). Synergistic Enhancement of Photocatalytic H2O2 Production over Carbon Nitride Oxide/Biochar Composites. Molecules, 30(22), 4323. https://doi.org/10.3390/molecules30224323