From Chains to Chromophores: Tailored Thermal and Linear/Nonlinear Optical Features of Asymmetric Pyrimidine—Coumarin Systems
Abstract
1. Introduction
2. Results and Discussion
2.1. Syntheses
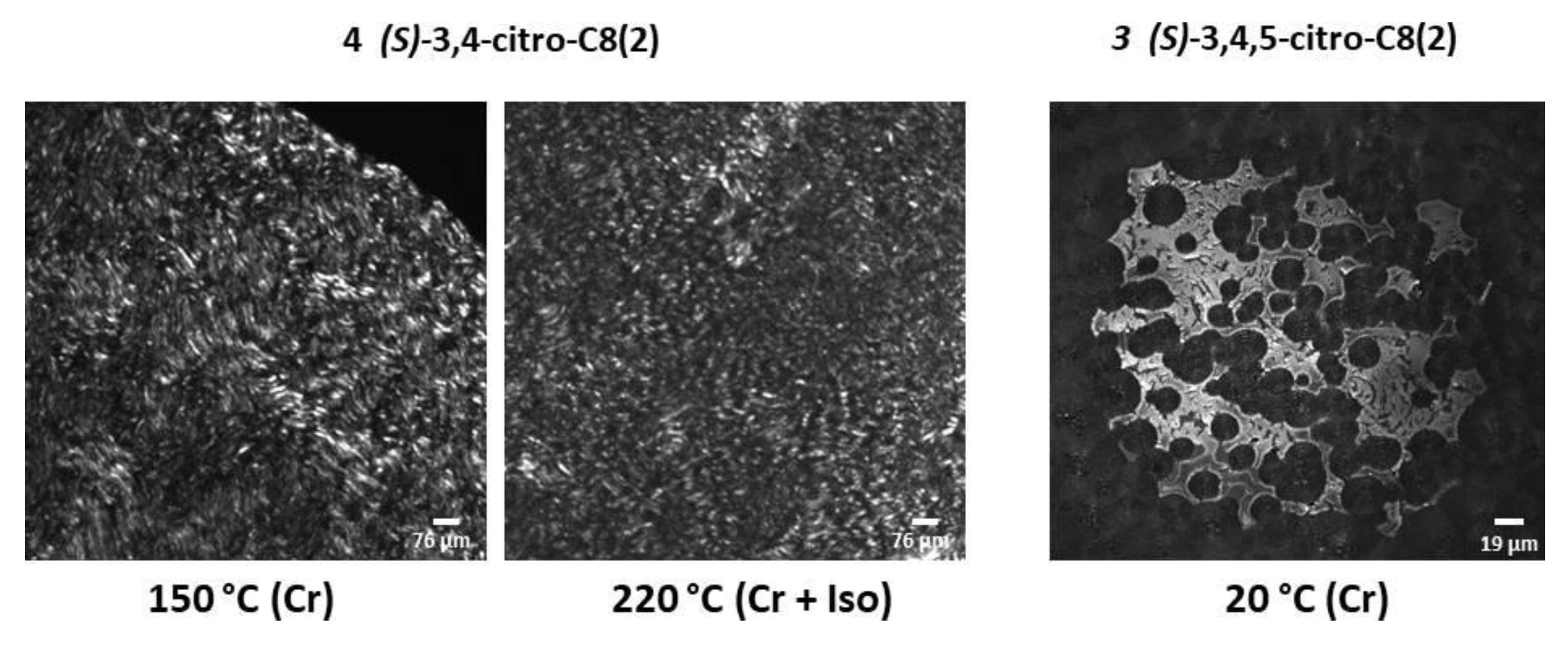
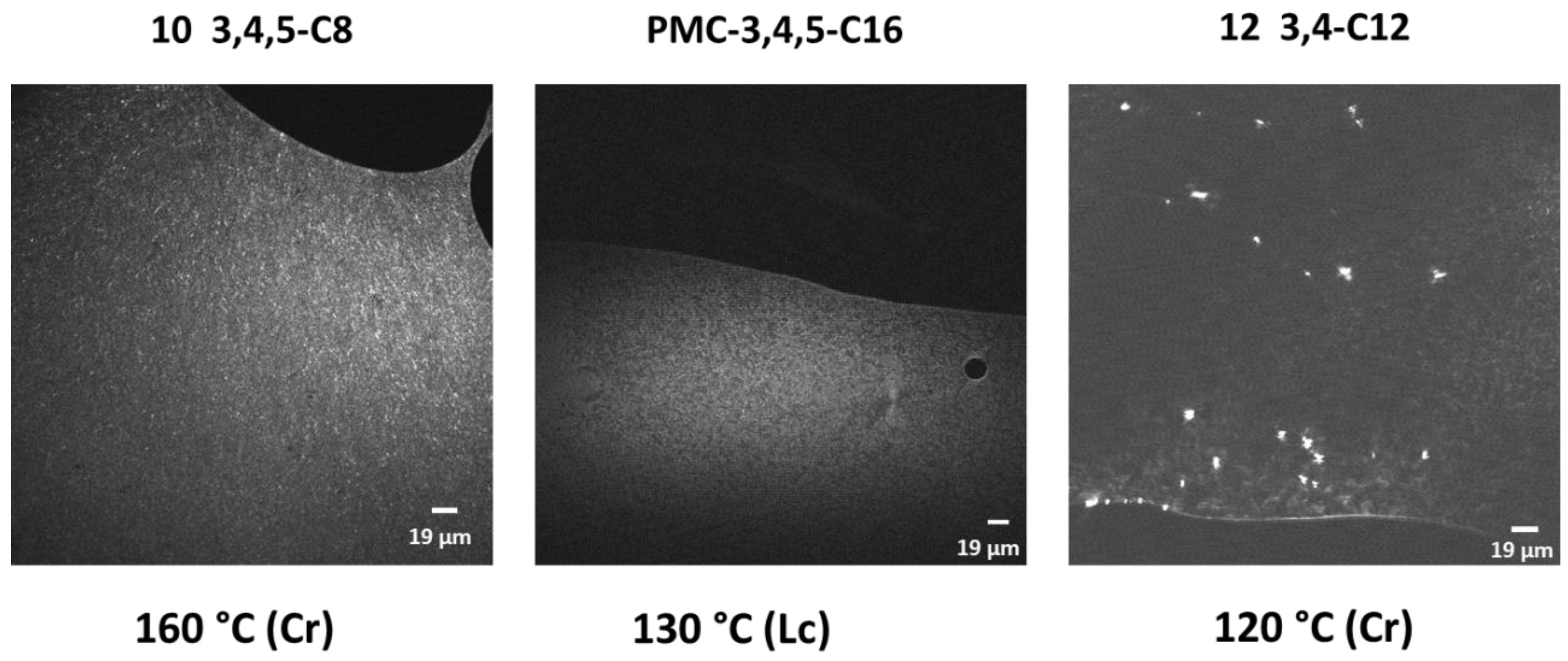
2.2. Thermal Properties
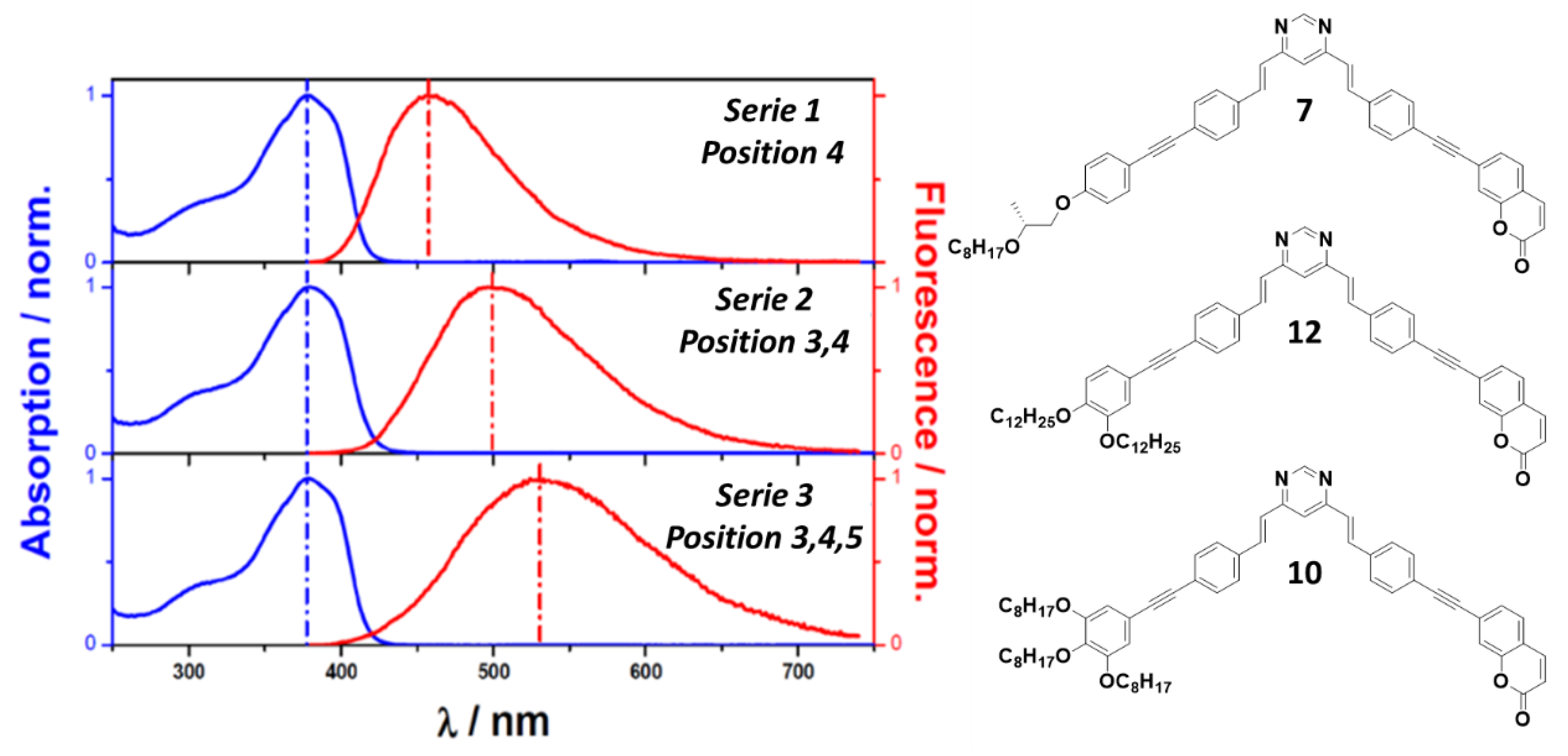
2.3. Linear One-Photon Absorption (1PA)
- Series 1 is the most luminescent, with higher fluorescence quantum yields and lower non-radiative losses.
- Series 2 maintains good optical properties with fluorescence yields slightly lower than series 1, although the non-radiative constant is higher than series 1.
- Series 3 presents significant losses through non-radiative conversion, strongly reducing its luminescent efficiency.
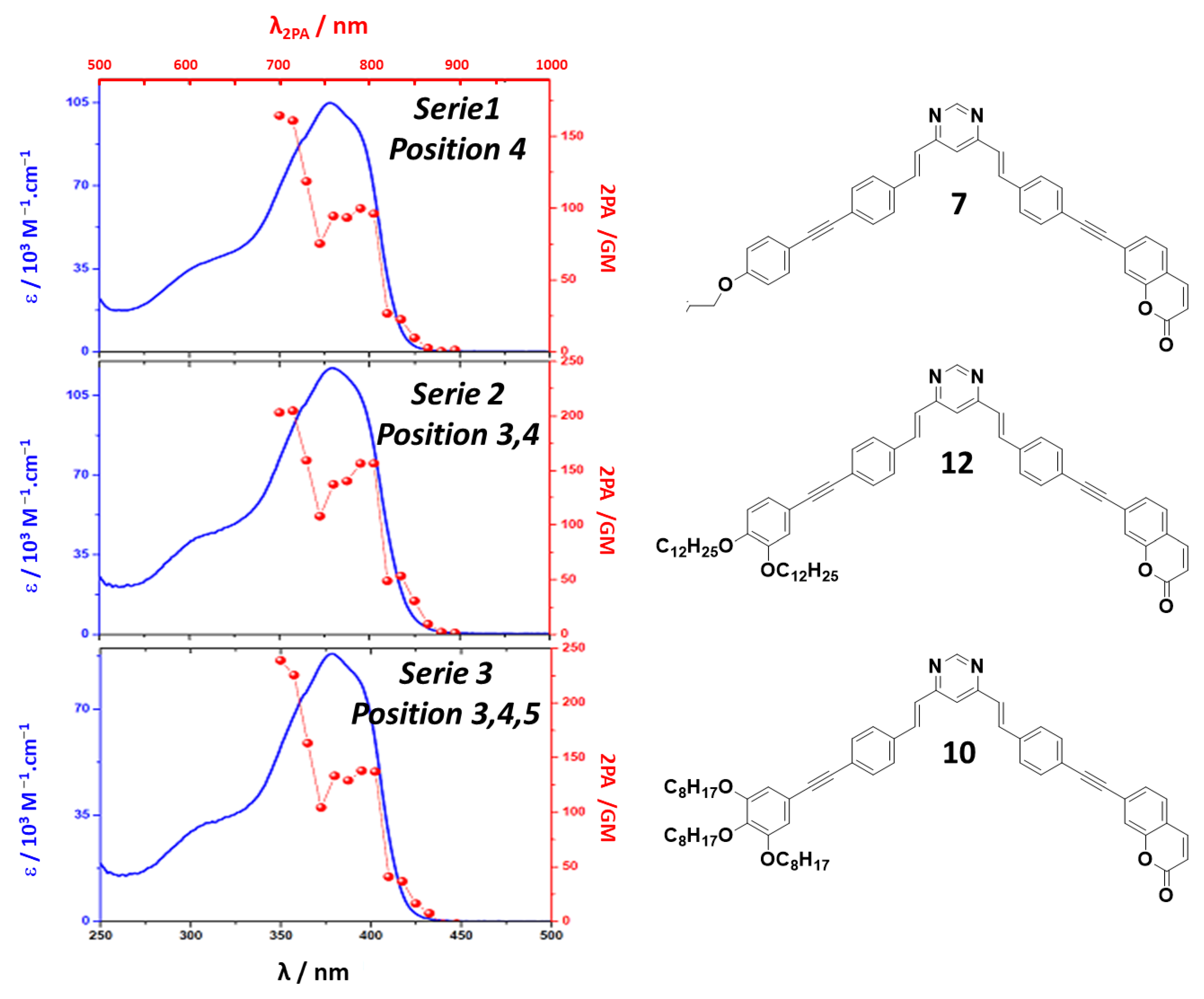
2.4. Nonlinear Two-Photon Absorption
3. Second-Harmonic Generation
3.1. Citronellol-Derived Series
3.2. Lactate-Derived Series
3.3. Diol-Derived Series
3.4. Linear Chains Series
4. Conclusions
5. Experimental Section/Methods
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Achelle, S.; Baudequin, C. Recent advances in pyrimidine derivatives as luminescent, photovoltaic and non-linear optical materials. Targets Heterocycl. Syst. 2013, 17, 1–34. Available online: http://www.chim.it/sites/default/files/ths/old/vol_17_2013.pdf (accessed on 24 September 2025).
- Achelle, S.; Ple, N. Pyrimidine Ring as Building Block for the Synthesis of Functionalized π-Conjugated Materials. Curr. Org. Synth. 2012, 9, 163–187. [Google Scholar] [CrossRef]
- Achelle, S.; Barsella, A.; Caro, B.; Robin-Le Guen, F. Donor–linker–acceptor (D–π–A) diazine chromophores with extended π-conjugated cores: Synthesis, photophysical and second order nonlinear optical properties. RSC Adv. 2015, 5, 39218–39227. [Google Scholar] [CrossRef]
- Petrov, V.F. Pyrimidine as a Structural Fragment in Calamitic Liquid Crystals. Mol. Cryst. Liq. Cryst. 2006, 457, 121–149. [Google Scholar] [CrossRef]
- Fecková, M.; le Poul, P.; Bureš, F.; Robin-Le Guen, F.; Achelle, S. Nonlinear optical properties of pyrimidine chromophores. Dye. Pigment. 2020, 182, 108659. [Google Scholar] [CrossRef]
- Achelle, S.; Verbitskiy, E.V.; Fecková, M.; Bureš, F.; Barsella, A.; Robin-le Guen, F. V-Shaped Methylpyrimidinium Chromophores for Nonlinear Optics. ChemPlusChem 2021, 86, 758–762. [Google Scholar] [CrossRef]
- Murthy Potla, K.; Asiri, Y.I.; Usha Rani, N.; Osorio, F.A.P.; Valverde, C.; Raja, M.; Armakovi, S.J.; Armakovi, S. Investigation of the linear and nonlinear optical properties in the crystalline phase of a pyrimidine derivative—A potential nonlinear optical material: Analysis of its structure, reactivity, and docking studies. RSC Adv. 2024, 14, 37709–37724. [Google Scholar] [CrossRef]
- Achelle, S.; Malval, J.-P.; Aloïse, S.; Barsella, A.; Spangenberg, A.; Mager, L.; Akdas-Kilig, H.; Fillaut, J.-L.; Caro, B.; Robin-le Guen, F. Synthesis, Photophysics and Nonlinear Optical Properties of Stilbenoid Pyrimidine-Based Dyes Bearing Methylenepyran Donor Groups. ChemPhysChem 2013, 14, 2725–2736. [Google Scholar] [CrossRef]
- Pascal, S.; David, S.; Andraud, C.; Maury, O. Near-infrared dyes for two-photon absorption in the short-wavelength infrared: Strategies towards optical power limiting. Chem. Soc. Rev. 2021, 50, 6613–6658. [Google Scholar] [CrossRef]
- Chien, C.W.; Liu, K.T.; Lai, C.K. Heterocyclic columnar pyrimidines: Synthesis, characterization and mesomorphic properties. Liq. Cryst. 2004, 31, 1007–1017. [Google Scholar] [CrossRef]
- Rahman, M.L.; Hegde, G.; Yusoff, M.M.; Malek, M.N.F.A.; Srinivasa, H.T.; Kumar, S. New pyrimidine-based photo-switchable bent-core liquid crystals. New J. Chem. 2013, 37, 2460–2467. [Google Scholar] [CrossRef]
- Nicolas, P.; Minon, C.; Abdallah, S.; Chen, D.; Rizzi, G.; de Coene, Y.; Liu, W.; Jeannin, O.; Verbiest, T.; Clays, K.; et al. Multiphoton-And SHG-Active Pyrimidine-Based Liquid Crystalline Thin Films Toward 3D Optical Data Storage. Adv. Opt. Mater. 2025, 13, 2402083. [Google Scholar] [CrossRef]
- Iliopoulos, K.; Krupka, O.; Gindre, D.; Sallé, M. Reversible Two-Photon Optical Data Storage in Coumarin-Based Copolymers. J. Am. Chem. Soc. 2010, 132, 14343–14345. [Google Scholar] [CrossRef] [PubMed]
- Gindre, D.; Champigny, E.; Iliopoulos, K.; Krupka, O.; Morille, Y.; Sallé, M. Image storage in coumarin-based copolymer thin films by photoinduced dimerization. Opt. Lett. 2013, 38, 4636–4639. [Google Scholar] [CrossRef]
- Gu, B.; Zhao, C.; Baev, A.; Yong, K.-T.; Wen, S.; Prasad, P.N. Molecular nonlinear optics: Recent advances and applications. Adv. Opt. Photonics 2016, 8, 328–369. [Google Scholar] [CrossRef]
- Verbiest, T.; Clays, K.; Rodriguez, V. Second-Order Nonlinear Optical Characterization Techniques: An Introduction, 1st ed.; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar] [CrossRef]
- Yasuda, T.; Shimizu, T.; Liu, F.; Ungar, G.; Kato, T. Electro-Functional Octupolar π-Conjugated Columnar Liquid Crystals. J. Am. Chem. Soc. 2011, 133, 13437–13444. [Google Scholar] [CrossRef]
- Chen, Y.; He, J.; Lin, H.; Wang, H.F.; Hu, P.; Wang, B.Q.; Zhao, K.Q.; Donnio, B. Efficient synthesis of fluorinated triphenylenes with enhanced arene–perfluoroarene interactions in columnar mesophases. Beilstein J. Org. Chem. 2024, 20, 3263–3273. [Google Scholar] [CrossRef]
- Camerel, F.; Ulrich, G.; Retailleau, P.; Ziessel, R. Ethynyl–Boron Subphthalocyanines Displaying Efficient Cascade Energy Transfer and Large Stokes Shifts. Angew. Chem. Int. Ed. 2008, 47, 8876–8880. [Google Scholar] [CrossRef]
- Jankowiak, A.; Pociecha, D.; Szczytko, J.; Kaszyński, P. Chiral discotic derivatives of 1,3,5-triphenyl-6-oxoverdazyl radical. Liq. Cryst. 2014, 41, 1653–1660. [Google Scholar] [CrossRef]
- Sankaranarayanan, S.; Sharma, A.; Chattopadhyay, S. Synthesis of the 1,5-dimethylic chiron enantiomers, 3,7,11-trimethyldodec-10-en-1-ol: Application to enantiomeric syntheses of tribolure and a marine fatty acid. Tetrahedron Asymmetry 2002, 13, 1373–1378. [Google Scholar] [CrossRef]
- Kaller, M.; Tussetschläger, S.; Fischer, P.; Deck, C.; Baro, A.; Giesselmann, F.; Laschat, S. Columnar Mesophases Controlled by Counterions in Potassium Complexes of Dibenzo[18]crown-6 Derivatives. Chem. A Eur. J. 2009, 15, 9530–9542. [Google Scholar] [CrossRef]
- Fayzullin, R.R.; Antonovich, O.A.; Zakharychev, D.V.; Bredikhina, Z.A.; Kurenkov, A.V.; Bredikhin, A.A. Synthesis and some features of phase behavior of chiral p-alkoxyphenyl glycerol ethers. Russ. J. Org. Chem. 2015, 51, 202–209. [Google Scholar] [CrossRef]
- Glang, S.; Rieth, T.; Borchmann, D.; Fortunati, I.; Signorini, R.; Detert, H. Arylethynyl-Substituted Tristriazolotriazines: Synthesis, Optical Properties, and Thermotropic Behavior. Eur. J. Org. Chem. 2014, 2014, 3116–3126. [Google Scholar] [CrossRef]
- Cardolaccia, T.; Li, Y.; Schanze, K.S. Phosphorescent Platinum Acetylide Organogelators. J. Am. Chem. Soc. 2008, 130, 2535–2545. [Google Scholar] [CrossRef] [PubMed]
- Parker, R.R.; Liu, D.; Yu, X.; Whitwood, A.C.; Zhu, W.; Williams, J.A.G.; Wang, Y.; Lynam, J.M.; Bruce, D.W. Synthesis, mesomorphism, photophysics and device performance of liquid-crystalline pincer complexes of gold(III). J. Mater. Chem. C 2021, 9, 1287–1302. [Google Scholar] [CrossRef]
- Collings, P.J.; Hird, M. Introduction to Liquid Crystals, 1st ed.; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar] [CrossRef]
- Goossens, K.; Wellens, S.; Vana Hecke, K.; Van Meervelt, L.; Cardinaels, T.; Binnemans, K. T-Shaped Ionic Liquid Crystals Based on the Imidazolium Motif: Exploring Substitution of the C-2 Imidazolium Carbon Atom. Chem. A Eur. J. 2011, 17, 4291–4306. [Google Scholar] [CrossRef]
- Etxebarria, J.; Blanca Ros, M. Bent-core liquid crystals in the route to functional materials. J. Mater. Chem. 2008, 18, 2919–2926. [Google Scholar] [CrossRef]
- Akdas-Kilig, H.; Godfroy, M.; Fillaut, J.-L.; Donnio, B.; Heinrich, B.; Kedziora, P.; Malval, J.-P.; Spangenberg, A.; Van Cleuvenbergen, S.; Clays, K.; et al. Mesogenic, Luminescence, and Nonlinear Optical Properties of New Bipyrimidine-Based Multifunctional Octupoles. J. Phys. Chem. C 2015, 119, 3697–3710. [Google Scholar] [CrossRef]
- Malval, J.P.; Achelle, S.; Bodiou, L.; Spangenberg, A.; Gomez, L.C.; Soppera, O.; Robin-Le Guen, F. Two-photon absorption in a conformationally twisted D–π–A oligomer: A synergic photosensitizing approach for multiphoton lithography. J. Mater. Chem. C 2014, 2, 7869–7880. [Google Scholar] [CrossRef]
- Meech, R.; Phillips, D. Photophysics of some common fluorescence standards. J. Photochem. 1983, 23, 193–217. [Google Scholar] [CrossRef]
- Connor, D.V.; Phillips, D. Time Correlated Single Photon Counting; Academic Press: London, UK, 1984. [Google Scholar]
- Lewis, D.; Kalgutkar, R.S.; Yang, J.-S. The Photochemistry of trans-ortho-, -meta-, and -para-Aminostilbenes. J. Am. Chem. Soc. 1999, 121, 12045–12053. [Google Scholar] [CrossRef]
- Nicolas, P.; Abdallah, S.; Dok, A.; de Coene, Y.; Jeannin, O.; Bellec, N.; Malval, J.-P.; Verbiest, T.; Clays, K.; Van Cleuvenbergen, S.; et al. Non-Linear Optical Activity of Chiral Bipyrimidine-Based Thin Films. Chem. Asian J. 2024, 19, e202400112. [Google Scholar] [CrossRef]
- Schreivogel, A.; Dawin, U.; Baro, A.; Giesselmann, F.; Laschat, S. Chiral tetraphenylethenes as novel dopants for calamitic and discotic liquid crystals. Phys. Org. Chem. 2009, 22, 484–494. [Google Scholar] [CrossRef]


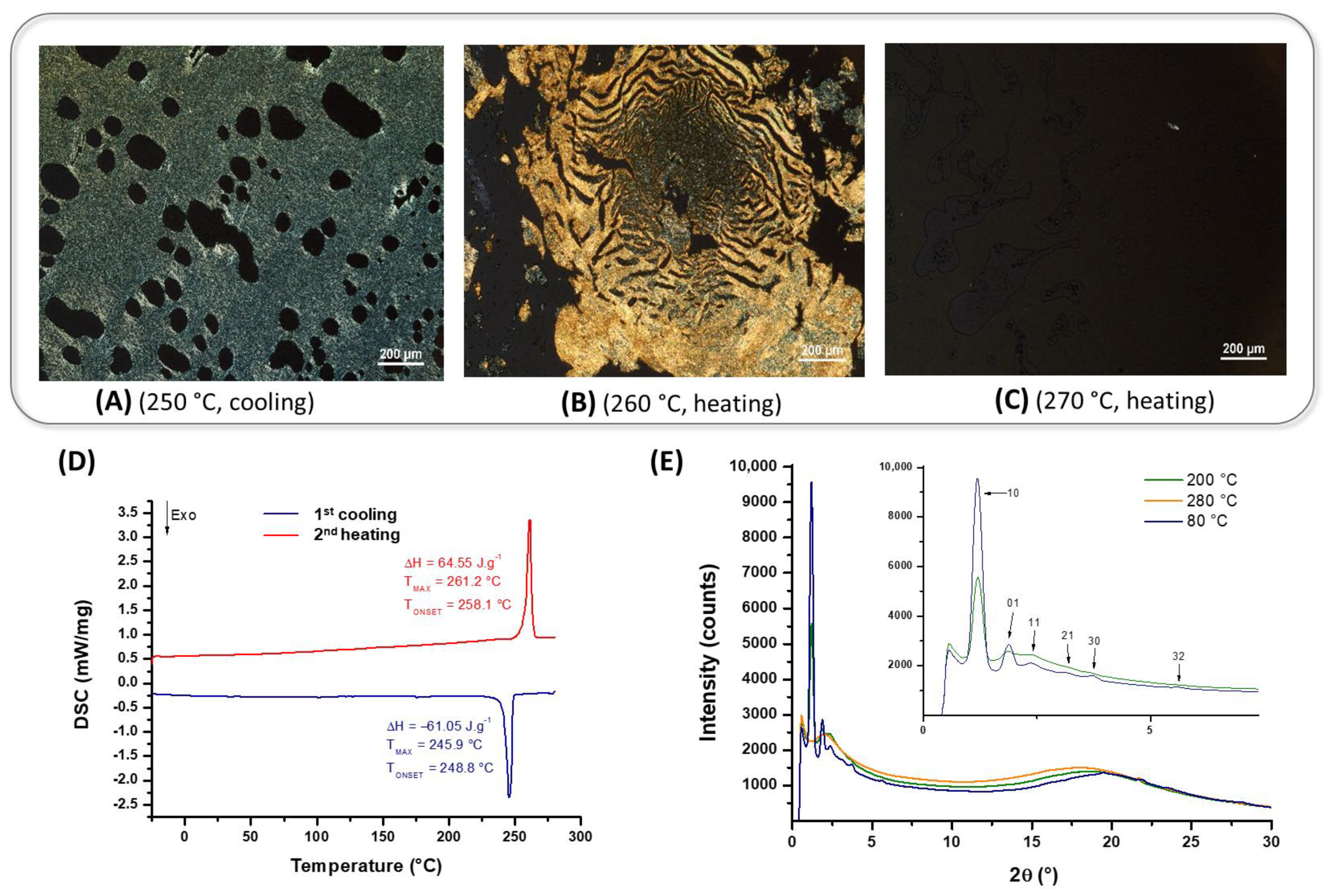
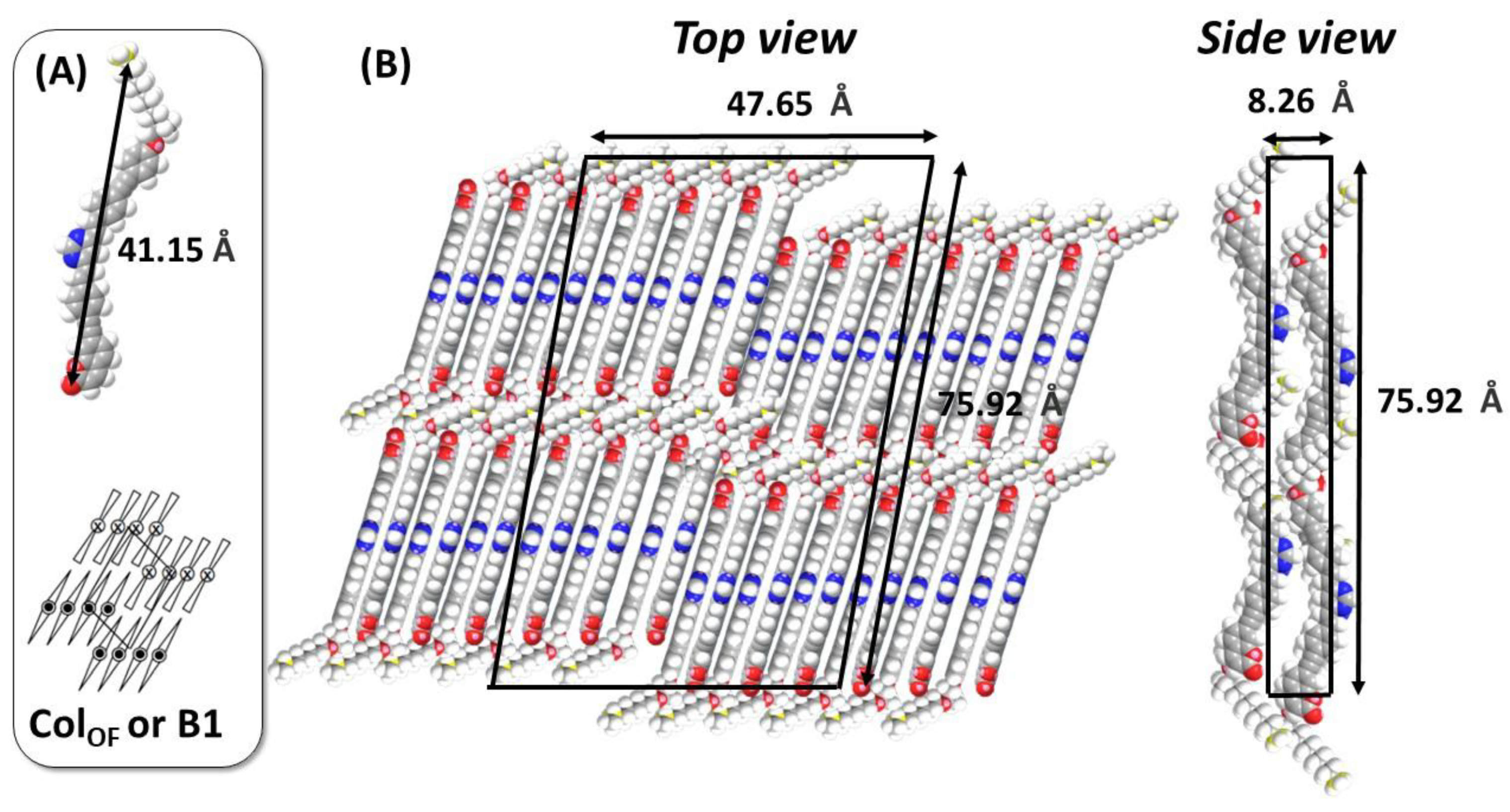
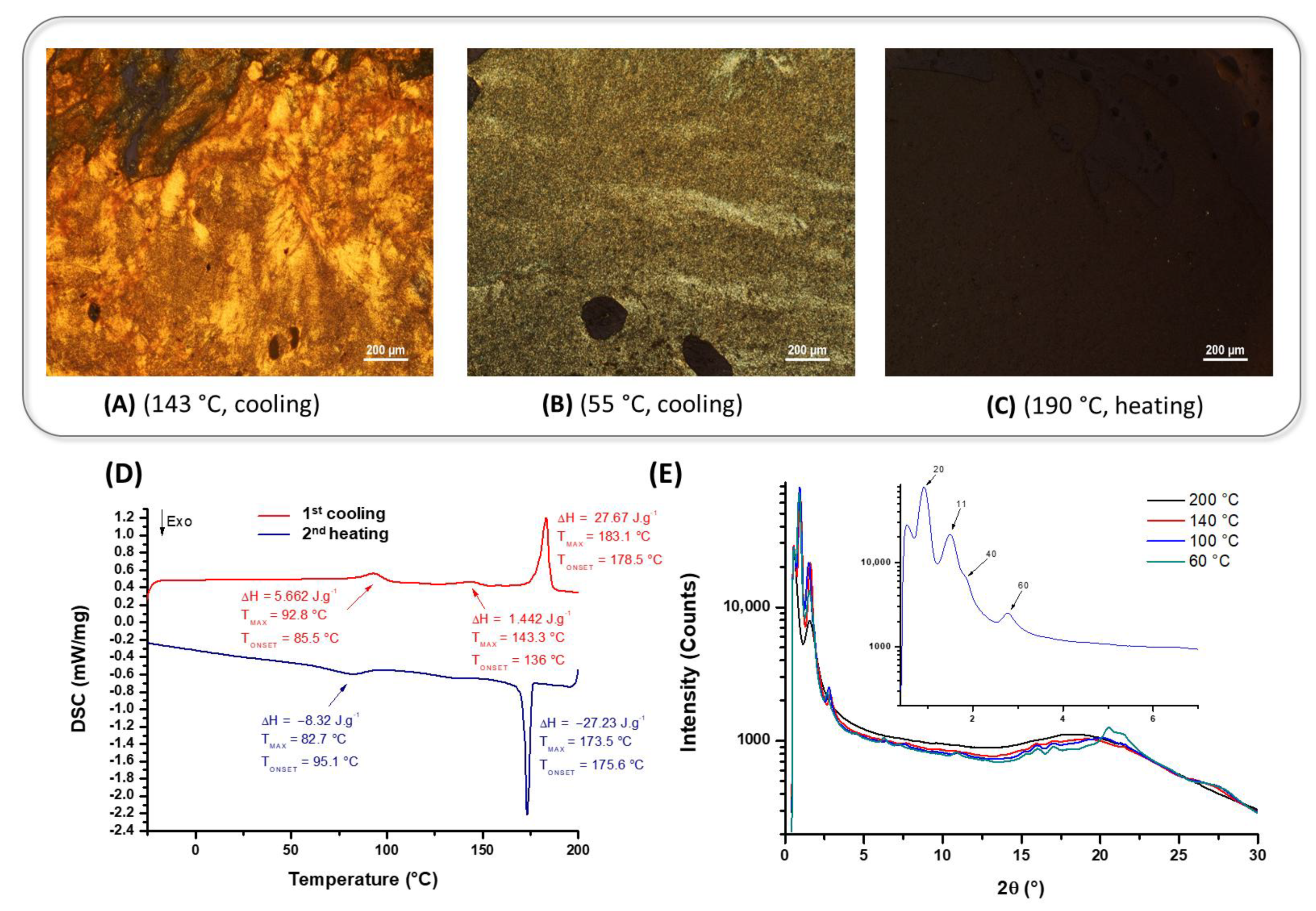
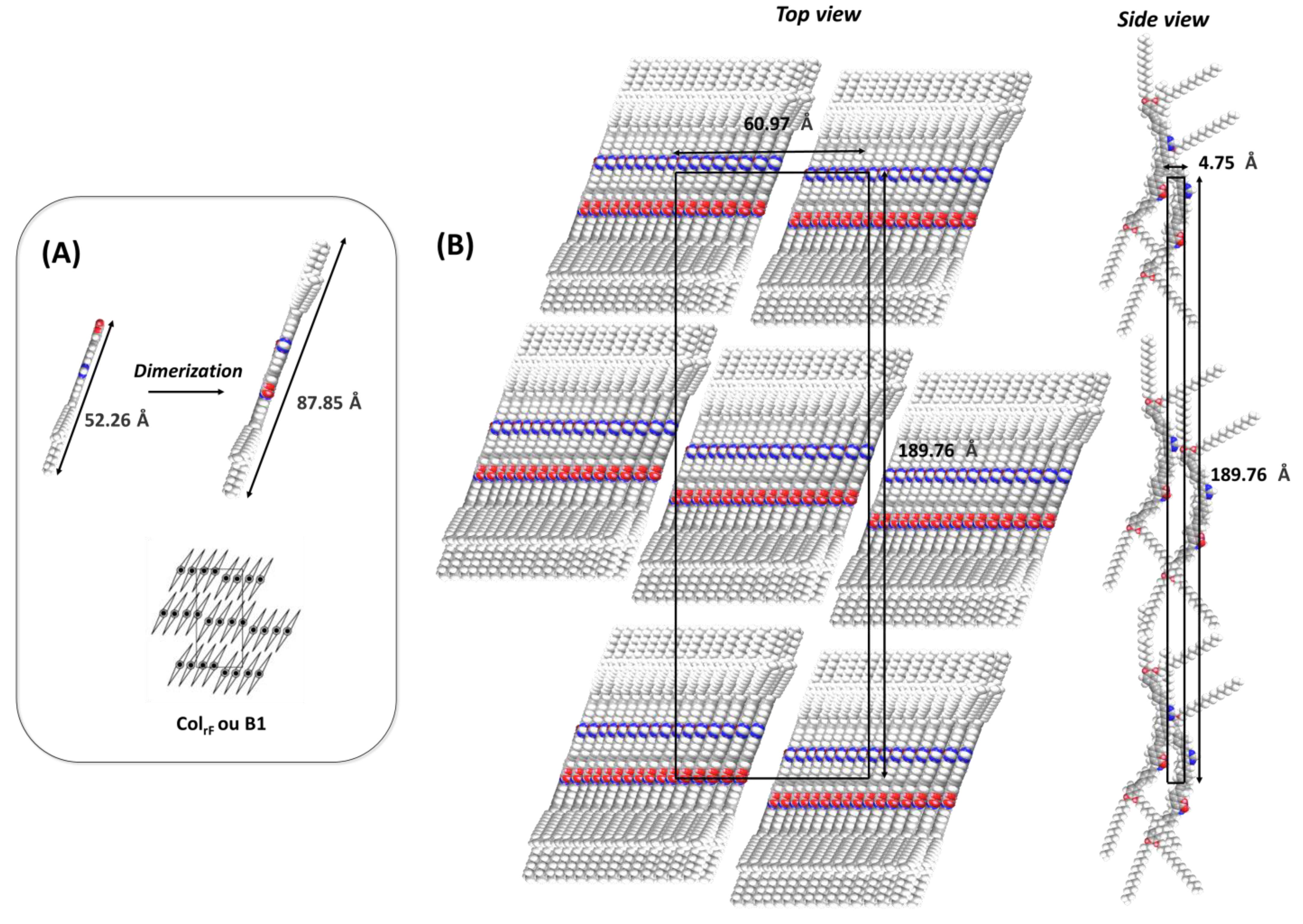
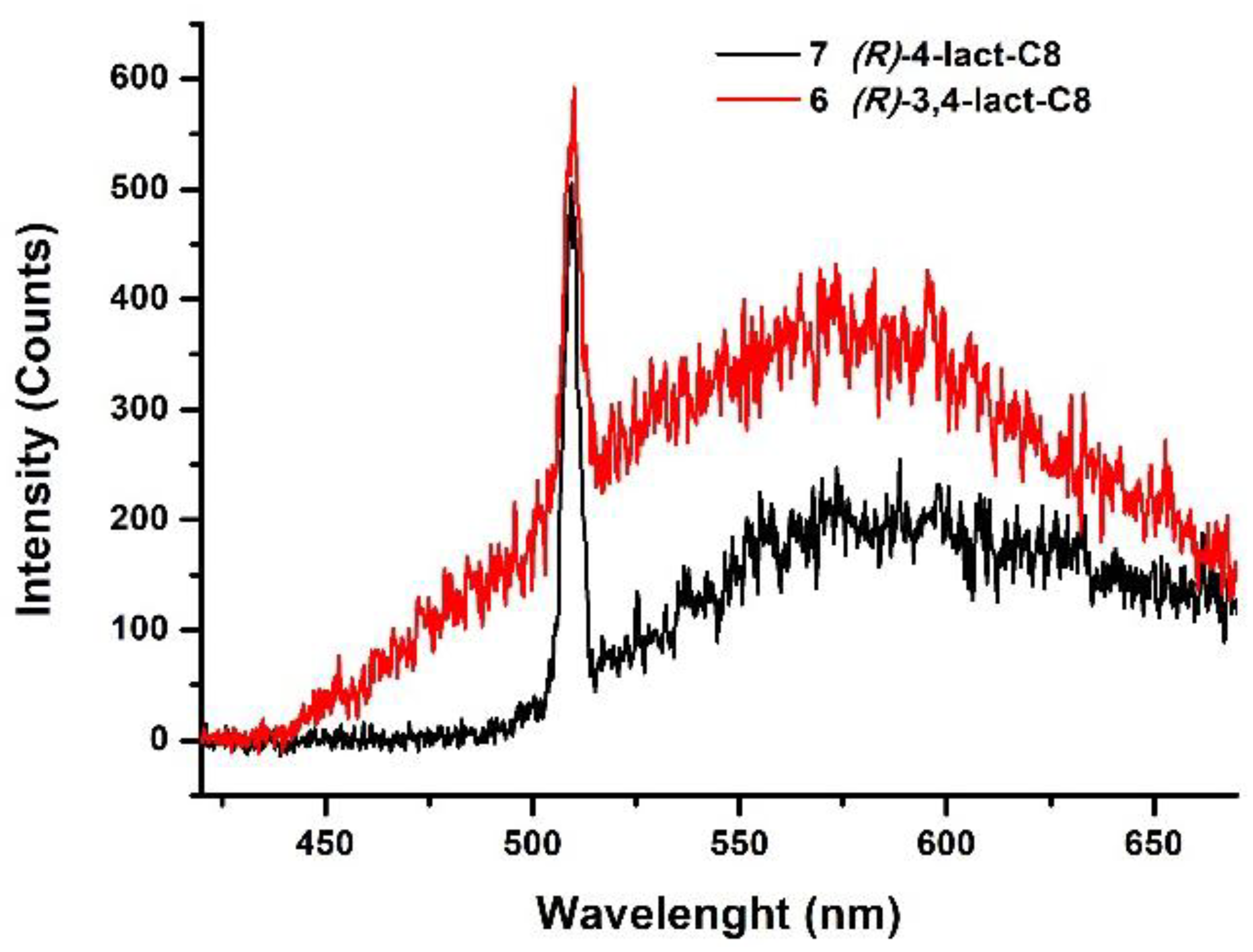
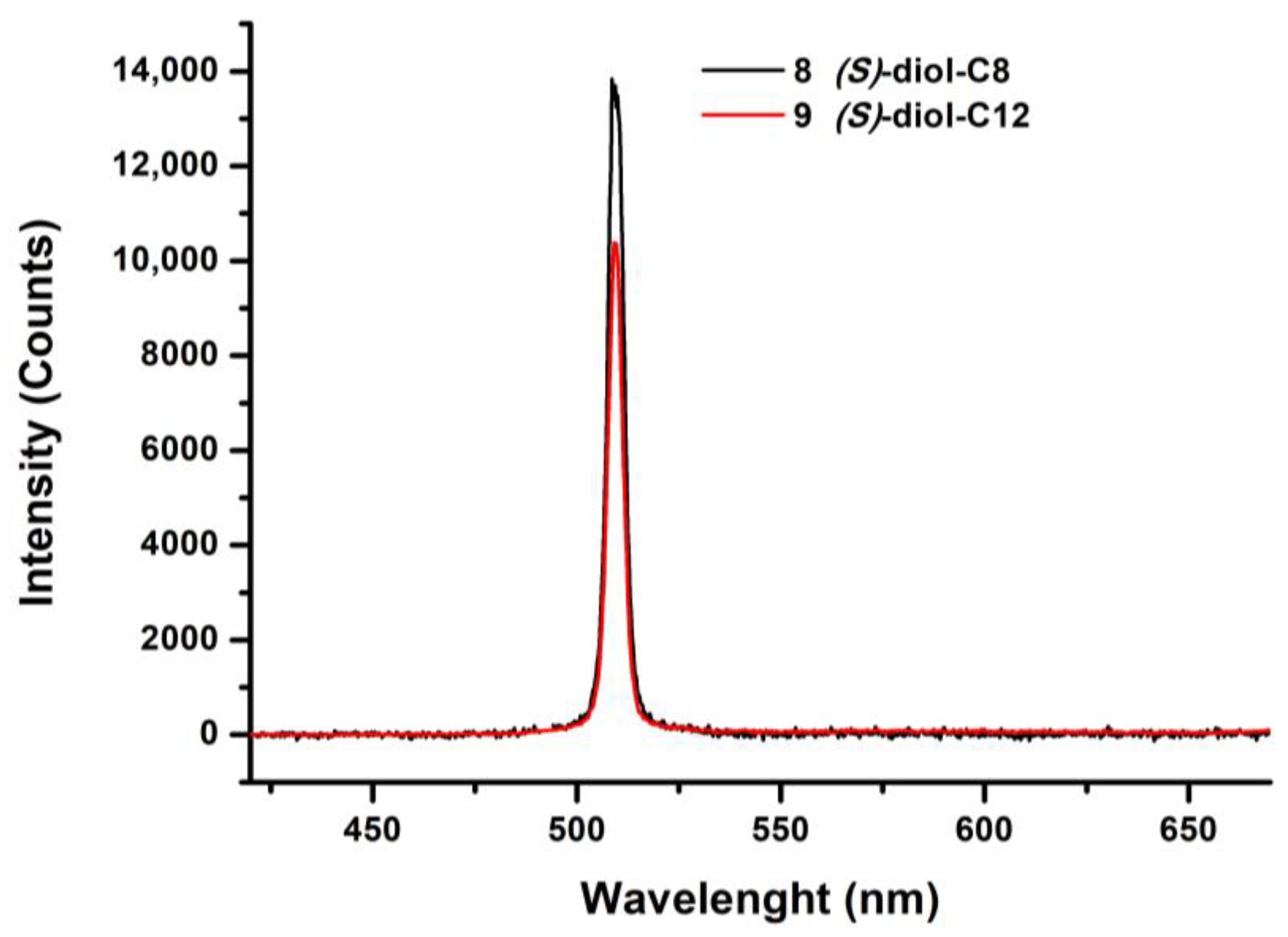
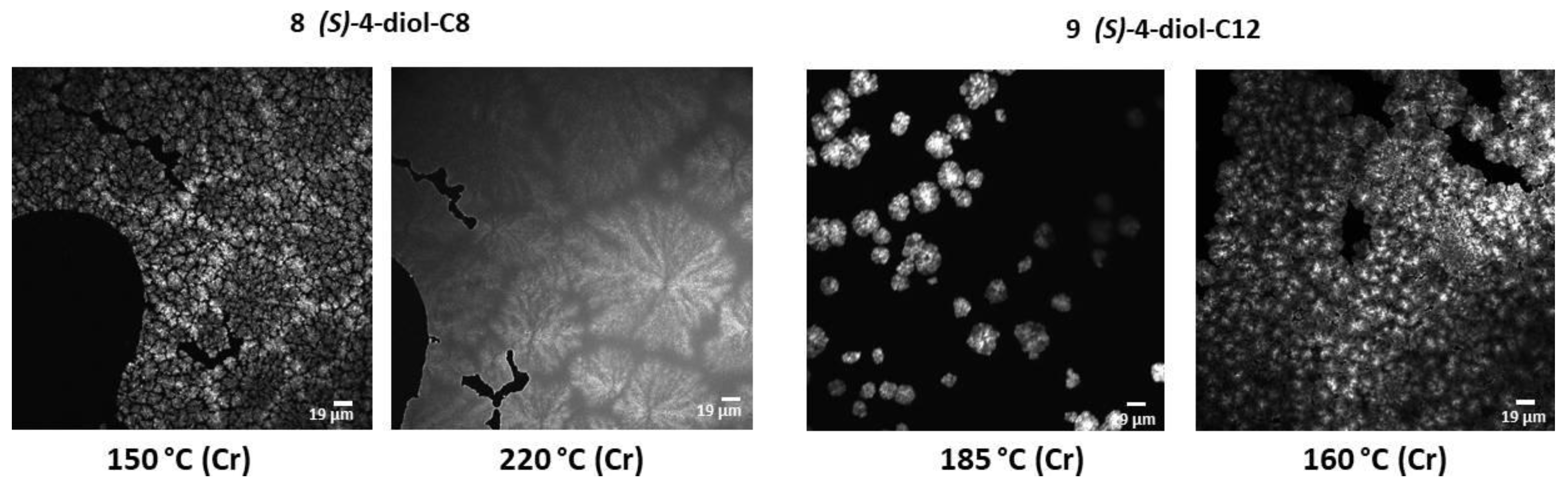
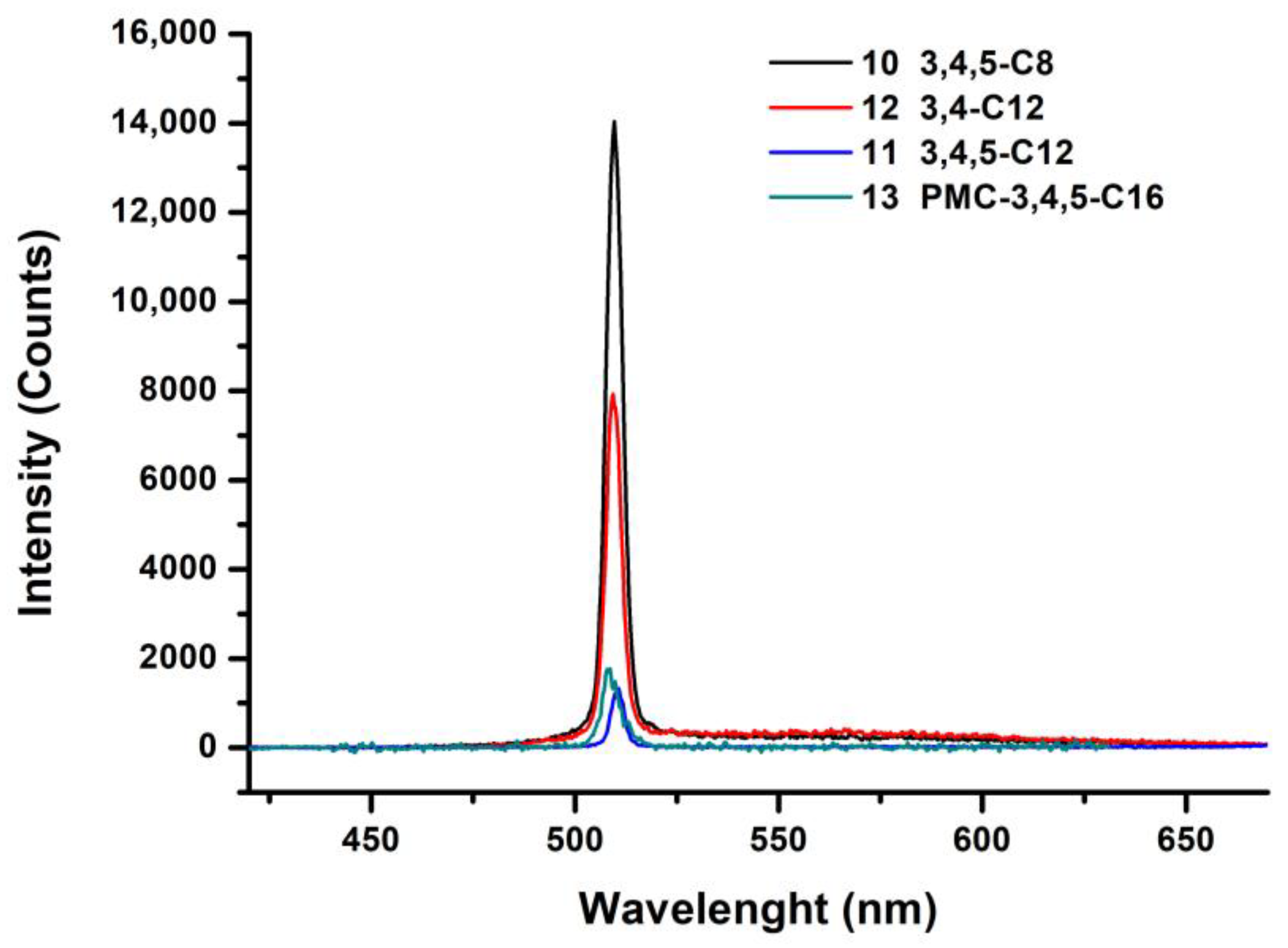
| Compounds | Grafting | Transition Temperatures T(°C) [ΔH, kJ/mol] |
|---|---|---|
| 3 | (S)-3,4,5-citro-C8(2) | Heating → Cr 156.7 [37.01] Iso Cooling → Iso 126.5 [−37.68] Cr |
| 4 | (S)-3,4-citro-C8(2) | Heating → Cr1 61.8 [4.75] Cr2 190.8 [32.23] Iso Cooling → Iso 180.2 [−0.31] Cr3 172.6 [−32.49] Cr2 14.8 [−4.17] Cr1 |
| 5 | (S)-4-citro-C8(2) | Heating → Cr1 150.9 [0.25] Cr2 190.6 [0.57] Cr3 250 Decomp cooling → Cr3 185 [−1.12] Cr1 |
| 6 | (R)-3,4-lact-C8 | Heating → Cr 168.3 [26.6] Iso Cooling → Iso 158.2 [−26.22] Cr |
| 7 | (R)-4-lact-C8 | Heating → LC 261.2 [47.7] Iso Cooling → Iso 245.9 [−45.11] LC |
| 8 | (S)-4-diol-C8 | Heating → Cr1 161.1 [−15.6] Cr2 201.4 [36.24] Cr3 + Iso 250.1 [4.91] Iso Cooling → Iso 235.4 [−6.79] Cr3 191.2 [−16.62] Cr2 114.2 [−0.97] Cr1 |
| 9 | (S)-4-diol-C12 | Heating → Cr1 42.9 [4.4] Cr2 192 [47.08] Iso Cooling → Iso 192.9 [−1.56] Cr3 179.7 [−43.71] Cr2 39.3 [−6.79] Cr1 |
| 10 | 3,4,5-C8 | Heating → Cr1 73.1 [5.3] Cr2 184 [50.69] Iso Cooling → Iso 157.3 [−47.39] Cr2 36.8 [−3.61] Cr1 |
| 11 | 3,4,5-C12 | Heating → Cr 139.9 [42.02] Iso Cooling → Iso 122.5 [−45.62] Cr |
| 12 | 3,4-C12 | Heating → Cr1 93.9 [2.05] Cr2 123 [1.04] Cr3 196.6 [32.6] Iso Cooling → Iso 183.5 [−31.58] Cr2 81.7 [−2.86] Cr1 |
| 13 | 3,4-C16 | Heating → Cr1 92.8 [5.85] LC143.3 [1.49] LC’ 183.1 [28.6] Iso Cooling → Iso 173.5 [−28.14] LC 82.7 [−8.6] Cr1 |
| PMC-3,4,5-C16 [12] | 3,4,5-C16 | Heating → Cr1 128.6 [53.0] Sm 136.0 [-] Iso Cooling → Iso 135.4 [−0.32] Sm 82.7 [−57.81] Cr1 |
| 2θ (°) | d(Å) Measured | d(Å) Calculated (Colo Cell) | Δ (d) | Indexation | Cell Parameters |
|---|---|---|---|---|---|
| 1.18 | 74.77 | 75.92 | 1.15 | 10 | a = 75.92 Å |
| 1.86 | 47.44 | 47.65 | 0.21 | 01 | b = 47.65 Å |
| 2.39 | 36.92 | 36.96 | 0.04 | 11 | β = 100 ° |
| 3.15 | 28.01 | 27.04 | −0.97 | 21 | Cell area = 3563 Å2 |
| 3.67 | 24.05 | 24.92 | 0.87 | 30 | Vmolecule = 1227 Å3 |
| 5.59 | 15.79 | 15.77 | −0.02 | 32 | Vcell = 29,448 Å3 |
| 21.68 (halo) | 4.09 | / | / | / | M = 738.93 g·mol−1 |
| 2θ (°) | d(Å) Measured | d(Å) Calculated (Colr cell) | Δ (d) | Indexation | Cell Parameters |
|---|---|---|---|---|---|
| 0.93 1.52 1.85 2.83 21.52 (halo) | 94.78 58.05 47.7 31.18 4.12 | 94.88 58.05 47.44 31.62 / | 0.1 0.0 −0.26 0.44 / | 20 11 40 60 / | a = 189.76 Å b = 60.97 Å β = 90 ° Cell area = 11.570 Å2 Vmolecule = 1717 Å3 Vcell = 54,944 Å3 M = 1033.49 g·mol−1 |
| Compound | λabs (ε)/nm (103 M−1·cm−1) | λfluo (nm) | Φfluo | E00 (a)/eV | τfluo (b)/ns | kr (c) /109 s−1 | knr (c) /109 s−1 | knr/kr | |
|---|---|---|---|---|---|---|---|---|---|
| SERIE 1 | 5 (S)-4-citro-C8(2) | / | / | / | / | / | / | / | / |
| 7 (R)-4-lact-C8 | 378 (104.8) | 457 | 0.82 | 3.00 | 1.43 | 0.57 | 0.13 | 0.23 | |
| 8 (S)-4-diol-C8 | 378 (100.9) | 456 | 0.78 | 3.00 | 1.38 | 0.57 | 0.16 | 0.28 | |
| 9 (S)-4-diol-C12 | 378 (105.6) | 458 | 0.78 | 3.00 | 1.41 | 0.55 | 0.16 | 0.28 | |
| SERIE 2 | 6 (R)-3,4-lact-C8 | 379 (109.1) | 497 | 0.80 | 2.88 | 2.09 | 0.38 | 0.10 | 0.25 |
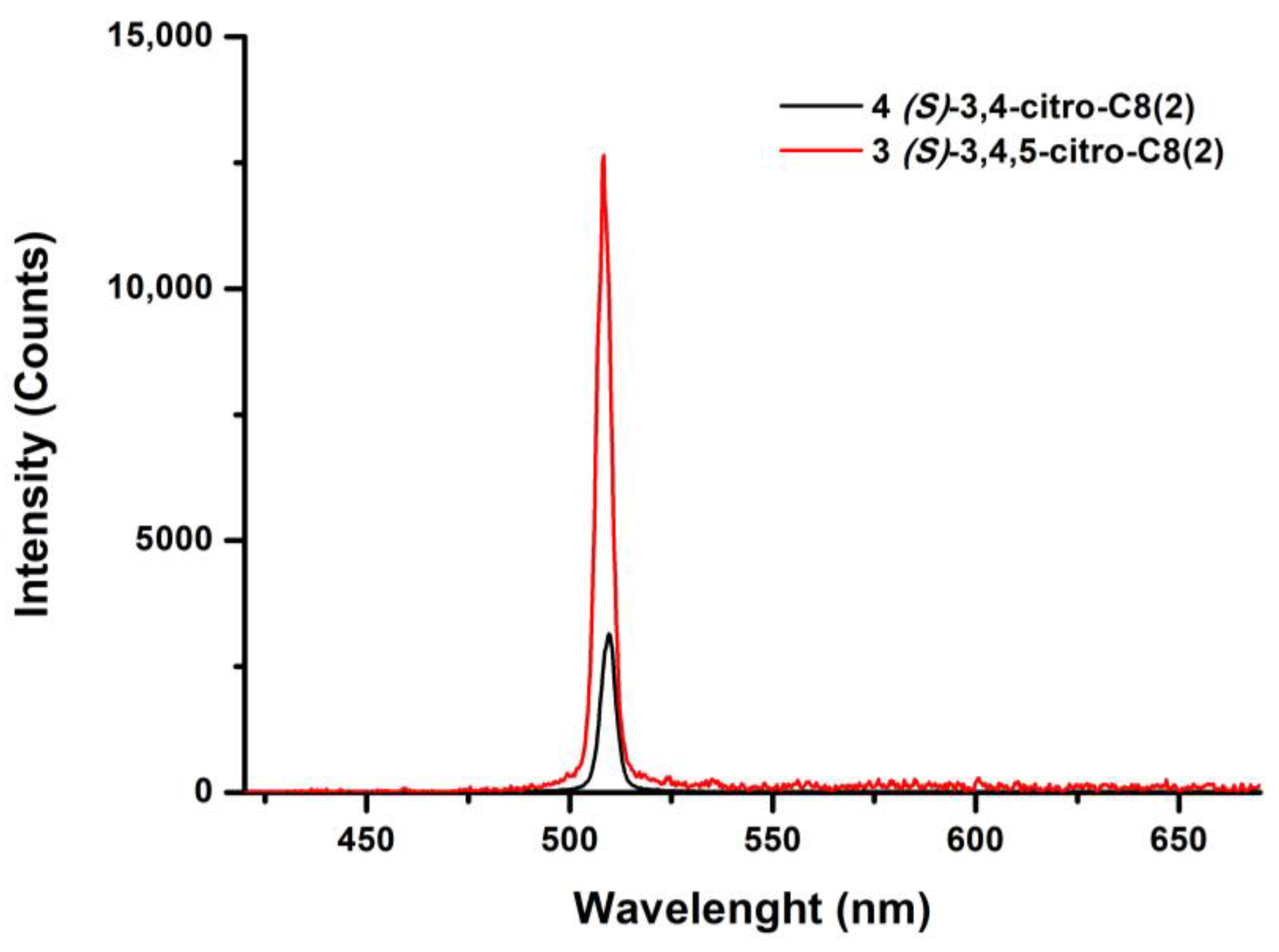
| 4 (S)-3,4-citro-C8(2) | 379 (101.4) | 498 | 0.82 | 2.88 | 2.13 | 0.38 | 0.08 | 0.22 | |
| 12 3,4-C12 | 379 (117.1) | 499 | 0.76 | 2.88 | 2.14 | 0.36 | 0.11 | 0.31 | |
| 13 3,4-C16 | / | / | / | / | / | / | / | / | |
| SERIE 3 | 3 (S)-3,4,5-citro-C8(2) | 377 (79.4) | 524 | 0.46 | 2.83 | 1.56 | 0.29 | 0.35 | 1.17 |
| 10 3,4,5-C8 | 378 (88.2) | 532 | 0.35 | 2.81 | 1.53 | 0.23 | 0.42 | 1.82 | |
| 11 3,4,5-C12 | 378 (91.5) | 533 | 0.35 | 2.81 | 1.49 | 0.23 | 0.44 | 1.86 | |
| PMC-3,4,5-C16 3,4,5-C16 [12] | 378 (93.3) | 532 | 0.39 | 2.81 | 1.48 | 0.26 | 0.41 | 1.58 |
| High-Energy Band | Low-Energy Band | |||||
|---|---|---|---|---|---|---|
| Compound | δ2PA/GM | λ2PA/nm | δ2PA/GM | λ2PA/nm | Ratio δ2PA | |
| SERIE 1 | 5 (S)-4-citro-C8(2) | / | / | / | / | / |
| 7 (R)-4-lact-C8 | 164 | 700 | 99 | 790 | 1.66 | |
| 8 (S)-4-diol-C8 | 123 | 700 | 77 | 790 | 1.60 | |
| 9 (S)-4-diol-C12 | 157 | 700 | 102 | 790 | 1.54 | |
| SERIE 2 | 6 (R)-3,4-lact-C8 | 143 | 715 | 110 | 805 | 1.30 |
| 4 (S)-3,4-citro-C8(2) | 175 | 715 | 136 | 805 | 1.29 | |
| 12 3,4-C12 | 205 | 700 | 156 | 805 | 1.31 | |
| 13 3,4-C16 | / | / | / | / | / | |
| SERIE 3 | 3 (S)-3,4,5-citro-C8(2) | 238 | 715 | 150 | 775 | 1.59 |
| 10 3,4,5-C8 | 238 | 700 | 136 | 805 | 1.75 | |
| 11 3,4,5-C12 | 118 | 700 | 73 | 790 | 1.62 | |
| PMC-3,4,5-C16 [12] | 121 | 715 | 76 | 790 | 1.59 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolas, P.; Abdallah, S.; Chen, D.; Rizzi, G.; Jeannin, O.; Clays, K.; Bellec, N.; Bilgin-Eran, B.; Akdas-Kiliç, H.; Malval, J.-P.; et al. From Chains to Chromophores: Tailored Thermal and Linear/Nonlinear Optical Features of Asymmetric Pyrimidine—Coumarin Systems. Molecules 2025, 30, 4322. https://doi.org/10.3390/molecules30214322
Nicolas P, Abdallah S, Chen D, Rizzi G, Jeannin O, Clays K, Bellec N, Bilgin-Eran B, Akdas-Kiliç H, Malval J-P, et al. From Chains to Chromophores: Tailored Thermal and Linear/Nonlinear Optical Features of Asymmetric Pyrimidine—Coumarin Systems. Molecules. 2025; 30(21):4322. https://doi.org/10.3390/molecules30214322
Chicago/Turabian StyleNicolas, Prescillia, Stephania Abdallah, Dong Chen, Giorgia Rizzi, Olivier Jeannin, Koen Clays, Nathalie Bellec, Belkis Bilgin-Eran, Huriye Akdas-Kiliç, Jean-Pierre Malval, and et al. 2025. "From Chains to Chromophores: Tailored Thermal and Linear/Nonlinear Optical Features of Asymmetric Pyrimidine—Coumarin Systems" Molecules 30, no. 21: 4322. https://doi.org/10.3390/molecules30214322
APA StyleNicolas, P., Abdallah, S., Chen, D., Rizzi, G., Jeannin, O., Clays, K., Bellec, N., Bilgin-Eran, B., Akdas-Kiliç, H., Malval, J.-P., Van Cleuvenbergen, S., & Camerel, F. (2025). From Chains to Chromophores: Tailored Thermal and Linear/Nonlinear Optical Features of Asymmetric Pyrimidine—Coumarin Systems. Molecules, 30(21), 4322. https://doi.org/10.3390/molecules30214322







