On the Question of the Application Potential and the Molecular Mechanism of the Formation of 1,3-Diaryl-5-Nitropyrazoles from Trichloromethylated Diarylnitropyrazolines
Abstract
1. Introduction
- (i)
- The molecular mechanism of chloroform elimination
- (ii)
- The generality of the elimination mechanism and the role of substituents
- (iii)
- The influence of solvent polarity
- (iv)
- The electronic nature of the transition states
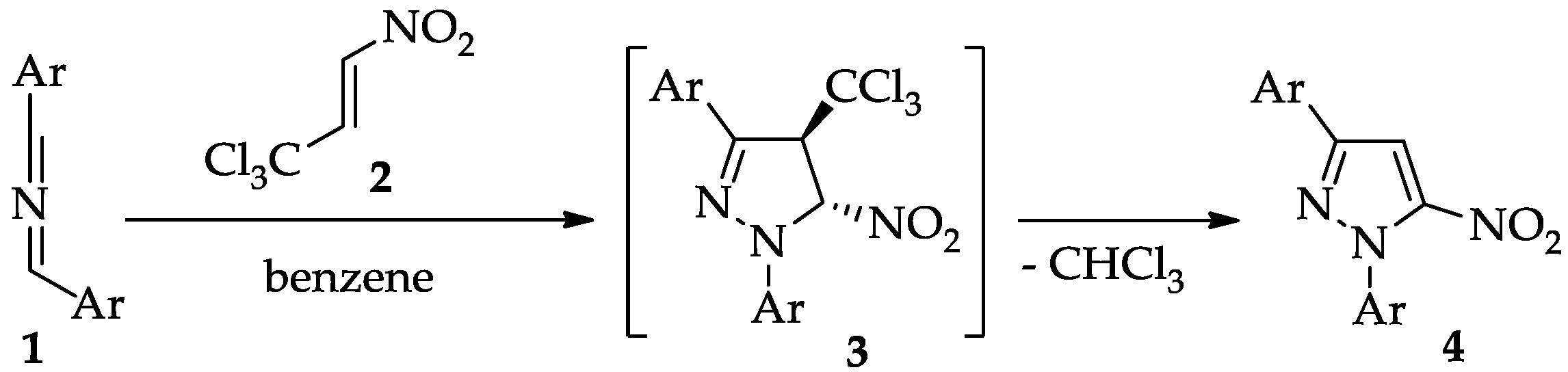
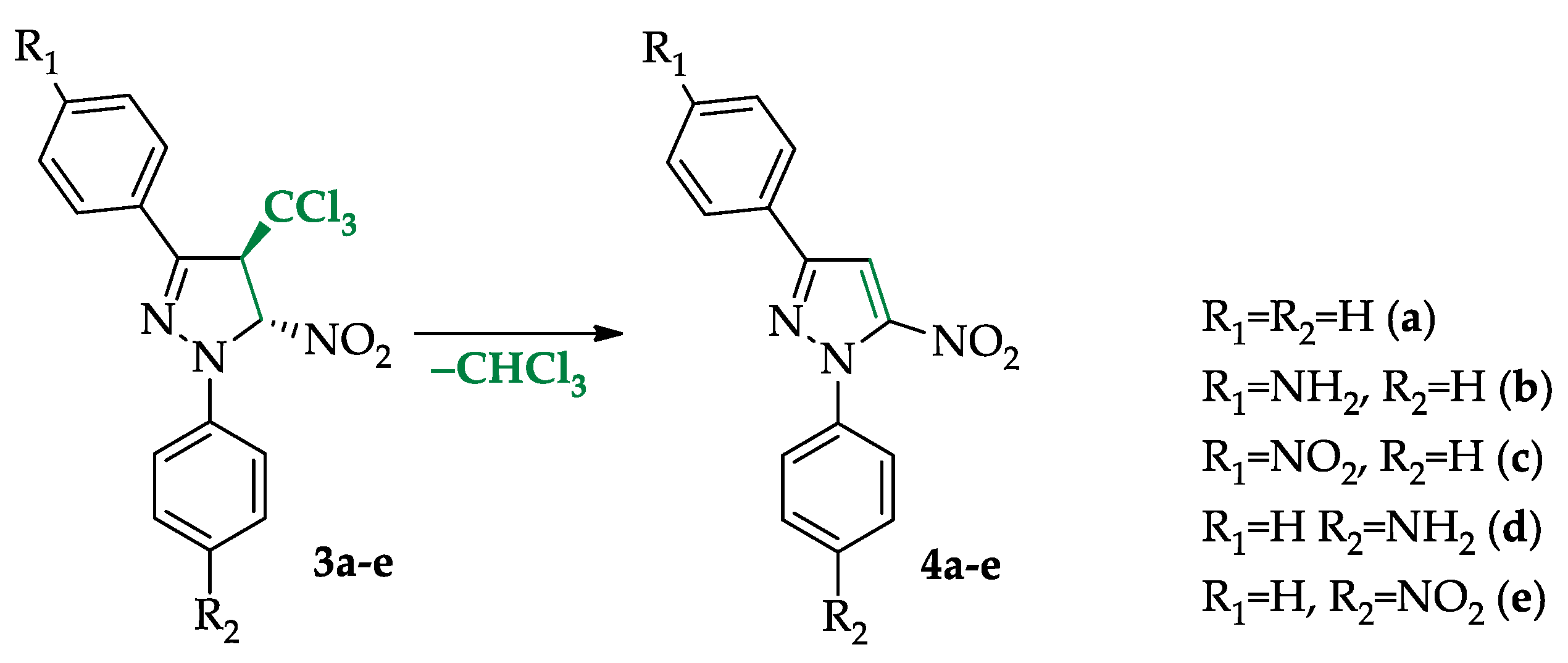
2. Results and Discussion
2.1. Mechanistic Consideration
2.2. In Silico Evaluation of Biological Potential
2.2.1. ADME In Silico Evaluation of Pharmacokinetic Properties
2.2.2. PASS in Silico Assessment of Biological Activity
3. Computational Details
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations and Symbols
| MW | Molecular Weight |
| #heavy atoms | Number of Heavy Atoms |
| #arom. heavy atoms | Number of Aromatic Heavy Atoms |
| #rotatable bonds | Number of Rotatable Bonds |
| #H-bond acceptors | Number of Hydrogen Bond Acceptors |
| #H-bond donors | Number of Hydrogen Bond Donors |
| MR | Molecular Refractivity |
| TPSA | Topological Polar Surface Area |
| Log Po/w (iLOGP) | Octanol/Water Partition Coefficient (iLOGP method [115]) |
| Log Po/w (XLOGP3) | Octanol/Water Partition Coefficient (XLOGP3 method [116]) |
| Log Po/w (WLOGP) | Octanol/Water Partition Coefficient (WLOGP method [117]) |
| Log Po/w (MLOGP) | Octanol/Water Partition Coefficient (MLOGP method [118]) |
| Log Po/w (SILICOS-IT) | Octanol/Water Partition Coefficient (SILICOS-IT method [119]) |
| Consensus Log Po/w | Consensus Octanol/Water Partition Coefficient |
| Log S (ESOL) | Predicted Aqueous Solubility (ESOL method [77]) |
| Log S (Ali) | Predicted Aqueous Solubility (Ali method [120]) |
| Log S (SILICOS-IT) | Predicted Aqueous Solubility (SILICOS-IT method [121]) |
| IG absorption | Gastrointestinal Absorption |
| BBB permeant | Blood–Brain Barrier Permeability |
References
- Faria, J.V.; Vegi, P.F.; Miguita, A.G.C.; Silva dos Santos, M.; Boechat, N.; Bernardino, A.M.R. Recently reported biological activities of pyrazole compounds. Bioorg. Med. Chem. 2017, 25, 5891–5903. [Google Scholar] [CrossRef] [PubMed]
- Odeh, D.M.; Odeh, M.M.; Hafez, T.S.; Hassan, A.S. Bioactive Fused Pyrazoles Inspired by the Adaptability of 5-Aminopyrazole Derivatives: Recent Review. Molecules 2025, 30, 366. [Google Scholar] [CrossRef] [PubMed]
- Lelyukh, M.I.; Komarenska, Z.M.; Chaban, T.I.; Chaban, I.H. An Overview of the Synthetic Routes toward [1,2,4]Triazolo [3,4-b][1,3,4]Thiadiazoles (Microreview). Chem. Heterocycl. Compd. 2024, 60, 342–344. [Google Scholar] [CrossRef]
- Lyapustin, D.N.; Fayzullina, D.F.; Marusich, I.V.; Kotovskaya, S.K.; Melekhin, V.V.; Tokhtueva, M.D.; Ulomsky, E.N.; Rusinov, V.L. A synthesis of novel 5-methylsulfanylazolo[1,5-a]pyrimidin-7(4H)-ones and investigation of their chemical and cytotoxic properties. Chem. Heterocycl. Compd. 2024, 60, 52–57. [Google Scholar] [CrossRef]
- Kosylo, N.; Hotynchan, A.; Skrypska, O.; Horak, Y.; Obushak, M. Synthesis and prediction of toxicological and pharmacological properties of Schiff bases containing arylfuran and pyrazole moiety. Sci. Radices 2024, 3, 62–73. [Google Scholar] [CrossRef]
- Ustinov, I.I.; V Khlytin, N.V.R. Selective reduction of 5,7-dinitro-8-hydroxyquinoline and synthesis of 2-substituted 5-nitrooxazolo[4,5-h]quinolines. Chem. Heterocycl. Compd. 2024, 60, 524–528. [Google Scholar] [CrossRef]
- Boguszewska-Czubara, A.; Łapczuk-Krygier, A.; Rykała, K.; Biernasiuk, A.; Wnorowski, A.; Popiolek, Ł.; Maziarka, A.; Hordyjewska, A.; Jasiński, R. Novel Synthesis Scheme and In Vitro Antimicrobial Evaluation of a Panel of (E)-2-aryl-1-cyano-1-nitroethenes. J. Enzym. Inhib. Med. Chem. 2016, 31, 900–907. [Google Scholar] [CrossRef]
- Zawadzińska, K.; Zavecz, I.; Hirka, S. The recent progress in the field of the applications of isoxazoles and their hydrogenated analogs: Mini review. Sci. Radices 2024, 3, 228–247. [Google Scholar] [CrossRef]
- Noriega, S.; Cardoso-Ortiz, J.; López-Luna, A.; Cuevas-Flores, M.D.R.; Flores De La Torre, J.A. The Diverse Biological Activity of Recently Synthesized Nitro Compounds. Pharmaceuticals 2022, 15, 717. [Google Scholar] [CrossRef]
- Jaber, A.A.; Bitar, L.; Jaber, A. The role of nitro groups in pharmaceuticals: Effects and interactions with biological systems. Artic. Afr. J. Pharm. Sci. 2025, 5, 1–7. [Google Scholar] [CrossRef]
- Latif, N.; Girgis, N.S.; Assad, F.M.; Grant, N. (Nitroethenyl) Salicylic Acid Anilides and Related Substances. A New Group of Molluscicidal and Microbicidal Compounds. Liebigs Ann. 1985, 6, 1202–1209. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, C.; Cong, X.; Geng, G.; Liu, L.L.; Luo, M.; Zeng, X. Cyclic (Alkyl)(amino)carbene Ligand-Promoted Nitro Deoxygenative Hydroboration with Chromium Catalysis: Scope, Mechanism, and Applications. J. Am. Chem. Soc. 2021, 143, 1618–1629. [Google Scholar] [CrossRef]
- Ballini, R.; Petrini, M.; Rosini, G. Nitroalkanes as Central Reagents in the Synthesis of Spiroketals. Molecules 2008, 13, 319–330. [Google Scholar] [CrossRef]
- Al-Najjar, H.J.; Barakat, A.; Al-Majid, A.M.; Mabkhot, Y.N.; Weber, M.; Ghabbour, H.A.; Fun, H.-K. A Greener, Efficient Approach to Michael Addition of Barbituric Acid to Nitroalkene in Aqueous Diethylamine Medium. Molecules 2014, 19, 1150–1162. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Lim, T.; Park, B.Y.; Han, M.S. Metal-Free, Rapid, and Highly Chemoselective Reduction of Aromatic Nitro Compounds at Room Temperature. J. Org. Chem. 2022, 87, 910–919. [Google Scholar] [CrossRef]
- Kula, K.; Kuś, E. In Silico Study About Substituent Effects, Electronic Properties, and the Biological Potential of 1,3-Butadiene Analogues. Int. J. Mol. Sci. 2025, 26, 8983. [Google Scholar] [CrossRef] [PubMed]
- Deka, D.C.; Kakati, H.S. Selective reduction of aromatic nitro groups in the presence of amide functionality. J. Chem. Res. 2006, 4, 223–224. [Google Scholar] [CrossRef]
- Barton, D.H.; Motherwell, W.B.; Zard, S.Z. A mild reduction of aliphatic nitro compounds to imines for further in situ reactions: A simple synthesis of pyrroles. Tetrahedron Lett. 1984, 25, 3707–3710. [Google Scholar] [CrossRef]
- Maugein, N.; Wagner, A.; Mioskowski, C. New conditions for the generation of nitrile oxides from primary nitroalkanes. Tetrahedron Lett. 1997, 38, 1547–1550. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, Z.; Lan, D.; Jia, Q.; Liu, N.; Zhang, J.; Kou, K. Recent Advances in Synthesis and Properties of Nitrated-Pyrazoles Based Energetic Compounds. Molecules 2020, 25, 3475. [Google Scholar] [CrossRef]
- Zapol’skii, V.A.; Munoz Castillo, D.C.; Pawletta, B.; Bilitewski, U.; Gjikaj, M.; Brüdigam, C.; Kaufmann, D.E. Synthesis and Microbiological Activities of 3-Nitropyrazolo-[1,5-d][1,2,4]triazin-7(6H)-ones and Derivatives. Molecules 2025, 30, 3792. [Google Scholar] [CrossRef]
- De Lucchi, O.; Modena, G. Acetylene equivalents in cycloaddition reactions. Tetrahedron 1984, 40, 2585–2632. [Google Scholar] [CrossRef]
- Voronin, V.V.; Ledovskaya, M.S.; Bogachenkov, A.S.; Rodygin, K.S.; Ananikov, V.P. Acetylene in Organic Synthesis: Recent Progress and New Uses. Molecules 2018, 23, 2442. [Google Scholar] [CrossRef]
- Neochoritis, C.G.; Zarganes–Tzitzikas, T.; Stephanidou-Stephanatou, J. Dimethyl Acetylenedicarboxylate: A Versatile Tool in Organic Synthesis. Synthesis 2014, 46, 0537–0585. [Google Scholar] [CrossRef]
- Jasiński, R.; Dresler, E. On the Question of Zwitterionic Intermediates in the [3+2] Cycloaddition Reactions: A Critical Review. Organics 2020, 1, 49–69. [Google Scholar] [CrossRef]
- Correia, C.M.; Silva, A.M.S.; Silva, V.L.M. The Role of Flow Chemistry on the Synthesis of Pyrazoles, Pyrazolines and Pyrazole-Fused Scaffolds. Molecules 2025, 30, 1582. [Google Scholar] [CrossRef]
- Jasiński, R. Nitroacetylene as dipolarophile in [2 + 3] cycloaddition reactions with allenyl-type three-atom components: DFT computational study. Monatsh. Chem. 2015, 146, 591–599. [Google Scholar] [CrossRef]
- Rall, K.B.; Vil’Davskaya, A.I.; Petrov, A.A. Nitroacetylenes. Russ. Chem. Rev. 1975, 44, 373–379. [Google Scholar] [CrossRef]
- Sharko, A.V.; Senchyk, G.A.; Rusanov, E.B.; Domasevitch, K.V. Preparative synthesis of 3(5),3′(5′)-dimethyl-4,4′-bipyrazole. Tetrahedron Lett. 2015, 56, 6089–6092. [Google Scholar] [CrossRef]
- Kula, K.; Jasiński, R. Synthesis of bis(het)aryl systems via domino reaction involving (2E,4E)-2,5-dinitrohexa-2,4-diene: DFT mechanistic considerations. Chem. Heterocycl. Compd. 2024, 60, 600–610. [Google Scholar] [CrossRef]
- Kula, K.; Łapczuk, A.; Sadowski, M.; Kras, J.; Zawadzińska, K.; Demchuk, O.M.; Gaurav, G.K.; Wróblewska, A.; Jasiński, R. On the Question of the Formation of Nitro-Functionalized 2,4-Pyrazole Analogs on the Basis of Nitrylimine Molecular Systems and 3,3,3-Trichloro-1-Nitroprop-1-Ene. Molecules 2022, 27, 8409. [Google Scholar] [CrossRef]
- Kula, K.; Dobosz, J.; Jasiński, R.; Kącka-Zych, A.; Łapczuk-Krygier, A.; Mirosław, B.; Demchuk, O.M. [3 + 2] Cycloaddition of diaryldiazomethanes with (E)-3,3,3-trichloro-1-nitroprop-1-ene: An experimental, theoretical and structural study. J. Mol. Struct. 2020, 1203, 127473. [Google Scholar] [CrossRef]
- Kula, K.; Kącka-Zych, A.; Łapczuk-Krygier, A.; Wzorek, Z.; Nowak, A.K.; Jasiński, R. Experimental and Theoretical Mechanistic Study on the Thermal Decomposition of 3,3-diphenyl-4-(trichloromethyl)-5-nitropyrazoline. Molecules 2021, 26, 1364. [Google Scholar] [CrossRef]
- Kącka, A.; Domingo, L.R.; Jasiński, R. Does a fluorinated Lewis acid catalyst change the molecular mechanism of the decomposition process of nitroethyl carboxylates? Res. Chem. Intermed. 2018, 44, 325. [Google Scholar] [CrossRef]
- Dresler, E. The participation of oleic acid and its esters in [3 + 2] cycloaddition reactions: A mini-review. Sci. Radices 2024, 3, 53–61. [Google Scholar] [CrossRef]
- Karpov, I.D.; Kolobov, A.V.; Filippov, I.P.; Rostovskii, N.V.; Ovchinnikov, K.L. A One-Pot Synthesis of 3-Nitro-2H-Thiopyrans and Their Selective Reduction to 3-Nitro-3,4-Dihydro-2H-Thiopyrans. Chem. Heterocycl. Compd. 2024, 60, 251–256. [Google Scholar] [CrossRef]
- Pannequin, A.; Laurini, E.; Giordano, L.; Muselli, A.; Pricl, S.; Tintaru, A. Caution: Chemical Instability of Natural Biomolecules During Routine Analysis. Molecules 2020, 25, 3292. [Google Scholar] [CrossRef] [PubMed]
- Kącka, A.; Jasiński, R. A dramatic change of kinetic conditions and molecular mechanism of decomposition processes of nitroalkyl carboxylates catalyzed by ethylammonium cations. Comput. Theor. Chem. 2017, 1104, 37–42. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, S.; Gao, H.; Wen, D. Metal-Modified Zeolites for Catalytic Dehydration of Bioethanol to Ethylene: Mechanisms, Preparation, and Performance. Catalysts 2025, 15, 791. [Google Scholar] [CrossRef]
- Kącka-Zych, A.; Jasiński, R. Unexpected molecular mechanism of trimethylsilyl bromide elimination from 2-(trimethylsilyloxy)-3-bromo-3-methyl-isoxazolidines. Theor. Chem. Acc. 2019, 138, 81–86. [Google Scholar] [CrossRef]
- Domingo, L.R. Molecular Electron Density Theory: A Modern View of Reactivity in Organic Chemistry. Molecules 2016, 21, 1319. [Google Scholar] [CrossRef]
- Chiacchio, M.A.; Legnani, L. Density Functional Theory Calculations: A Useful Tool to Investigate Mechanisms of 1,3-Dipolar Cycloaddition Reactions. Int. J. Mol. Sci. 2024, 25, 1298. [Google Scholar] [CrossRef] [PubMed]
- Fałowska, A.; Grzybowski, S.; Kapuściński, D.; Sambora, K.; Łapczuk, A. Modeling of the General Trends of Reactivity and Regioselectivity in Cyclopentadiene–Nitroalkene Diels–Alder Reactions. Molecules 2025, 30, 2467. [Google Scholar] [CrossRef] [PubMed]
- Karaś, A.; Łapczuk, A. Computational Model of the Formation of Novel Nitronorbornene Analogs via Diels–Alder Process. React. Kinet. Mech. Catal. 2025, 138, 2671–2689. [Google Scholar] [CrossRef]
- Ameur, S.; Barhoumi, A.; El Abdallaoui, H.A.; Syed, A.; Belghiti, M.E.; Elgorban, A.M.; Wong, L.S.; Wang, S.; El Idrissi, M.; Zeroual, A.; et al. Molecular docking, exploring diverse selectivities and mechanistic insights in the cycloaddition reaction between 3-benzoylpyrrolo [1,2-a]quinoxaline-1,2,4(5H)-triones and butyl vinyl ether. Chem. Heterocycl. Compd. 2024, 60, 584–591. [Google Scholar] [CrossRef]
- Baammi, S.; Aitouna, A.O.; Zeroual, A.; Chekroun, A.; Mohammad-Salim, H.; Al-Sadoon, M.K.; Benharref, A. Quantum evaluation of novel epoxides: Molecular docking, dynamics simulation, pharmacokinetics, stereoselectivity, and mechanistic insights into cis-himachalone and cis-himachalol epoxidation. Chem. Heterocycl. Compd. 2024, 60, 575–583. [Google Scholar] [CrossRef]
- Di, L.; Kerns, E. Drug-Like Properties: Concepts, Structure Design and Methods from ADME to Toxicity Optimization; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Filimonov, D.A.; Lagunin, A.A.; Gloriozova, T.A.; Rudik, A.V.; Druzhilovskii, D.S.; Pogodin, P.V.; Poroikov, V.V. Prediction of the biological activity spectra of organic compounds using the PASS online web resource. Chem. Heterocycl. Compd. 2014, 50, 444–457. [Google Scholar] [CrossRef]
- Ryachi, K.; Mohammad-Salim, H.; Al-Sadoon, M.K.; Zeroual, A.; de Julián-Ortiz, J.V.; Idrissi, M.E.; Tounsi, A. Quantum study of the [3 + 2] cycloaddition of nitrile oxide and carvone oxime: Insights into toxicity, pharmacokinetics, and mechanism. Chem. Heterocycl. Comp. 2024, 60, 646–654. [Google Scholar] [CrossRef]
- Demidov, M.R.; Osyanin, V.A. [3 + 3] Annulation of diethyl 2, 3-dicyanofumarate and cyclic 1, 3-dicarbonyl compounds: Synthesis of fused diethyl 2-amino-4-cyano-4 H-pyran-3, 4-dicarboxylates. Chem. Heterocycl. Comp. 2024, 60, 415–418. [Google Scholar] [CrossRef]
- Woliński, P.; Kącka-Zych, A.; Dziuk, B.; Ejsmont, K.; Łapczuk-Krygier, A.; Dresler, E. The structural aspects of the transformation of 3-nitroisoxazoline-2-oxide to 1-aza-2,8-dioxabicyclo[3.3.0]octane derivatives: Experimental and MEDT theoretical study. J. Mol. Struct. 2019, 1192, 27–34. [Google Scholar] [CrossRef]
- Becke, A.D.; Edgecombe, K.E. A Simple Measure of Electron Localization in Atomic and Molecular Systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Ameur, S.; Kącka-Zych, A.; Moussa, Z.; Alsantali, R.I.; Zeroual, A.; Alluhaibi, M.S.; Alsimaree, A.A.; Ahmed, S.A. Study of 1,3-Dipolar Cycloaddition Between 4-Acyl-1H-pyrrole-2,3-diones Fused at the [e]-Side with a Heterocyclic Moiety and Diphenylnitrone: A Comprehensive MEDT, Docking Approach and MD Simulation. Molecules 2025, 30, 3718. [Google Scholar] [CrossRef]
- Kula, K.; Sadowski, M. Regio- and stereoselectivity of [3 + 2] cycloaddition reactions Between (Z)-C-(9-anthryl)-N-methylnitrone and analogues of trans-β-nitrostyrene in the light of MEDT computational study. Chem. Heterocycl. Comp. 2023, 59, 138–144. [Google Scholar] [CrossRef]
- Sadowski, M.; Dresler, E.; Jasiński, R. On the Question of the Regio-Orientation, Stereo-Orientation and Molecular Mechanism in the Cascade Cycloaddition/Rearrangement/Elimination Processes Leading to Nitro-Substituted Thiopyran Analogs: DFT Computational Study. Int. J. Mol. Sci. 2025, 26, 8948. [Google Scholar] [CrossRef]
- Kula, K.; Kącka-Zych, A.; Łapczuk-Krygier, A.; Jasiński, R. Analysis of the Possibility and Molecular Mechanism of Carbon Dioxide Consumption in the Diels-Alder Processes. Pure Appl. Chem. 2021, 93, 427–446. [Google Scholar] [CrossRef]
- Messaadia, S.; Nacereddine, A.K.; Djerourou, A. Exploring the factors controlling the mechanism and the high stereoselectivity of the polar [3 + 2] cycloaddition reaction of the N,N′-cyclic azomethine imine with 3-nitro-2-phenyl-2H-chromene. A Molecular Electron Density Theory study. Chem. Heterocycl. Compd. 2023, 59, 128–137. [Google Scholar] [CrossRef]
- Abdoul-Hakim, M.; Idrissi, K.E.; Zeroual, A.; Garmes, H. Investigation of the solvent effect, regioselectivity, and the mechanism of the cycloaddition reaction Between 2-chlorobenzimidazole and benzonitrile oxide. Chem. Heterocycl. Compd. 2023, 59, 155–164. [Google Scholar] [CrossRef]
- Karrouchi, K.; Radi, S.; Ramli, Y.; Taoufik, J.; Mabkhot, Y.N.; Al-aizari, F.A.; Ansar, M. Synthesis and Pharmacological Activities of Pyrazole Derivatives: A Review. Molecules 2018, 23, 134. [Google Scholar] [CrossRef] [PubMed]
- Boguszewska-Czubara, A.; Kula, K.; Wnorowski, A.; Biernasiuk, A.; Popiolek, Ł.; Miodowski, D.; Demchuk, O.M.; Jasiński, R. Novel Functionalized β-nitrostyrenes: Promising Candidates for New Antibacterial Drugs. Saudi Pharm. J. 2019, 27, 593–601. [Google Scholar] [CrossRef]
- Wei, J.; Chen, L.; Zhu, K.; Liu, Y.; Li, H.; Zhao, M.; Wu, Y. Design, Synthesis, and Fungicidal Activity Evaluation of 2-Methyl-5-Phenylthiazole-4-Carboxamides Bearing Morpholine, Thiomorpholine, or Thiomorpholine 1,1-Dioxide Moiety. Chem. Heterocycl. Compd. 2024, 60, 536–543. [Google Scholar] [CrossRef]
- Kruczyński, T.; Łapczuk, A. Prediction of biological activity and pharmacokinetic properties of novel nitronorbornene analogs. Sci. Radices 2025, 4, 223–233. [Google Scholar] [CrossRef]
- Swiss ADME. Swiss Institute of Bioinformatics. Available online: http://www.swissadme.ch/ (accessed on 19 September 2025).
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanadhan, V.N.; Wendoloski, J.J. A knowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. A qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Prediction of drug absorption using multivariate statistics. J. Med. Chem. 2000, 43, 3867–3877. [Google Scholar] [CrossRef]
- Muegge, I.; Heald, S.L.; Brittelli, D. Simple selection criteria for drug-like chemical matter. J. Med. Chem. 2001, 44, 1841–1846. [Google Scholar] [CrossRef] [PubMed]
- Way2Drug. PASS Online. Available online: http://www.way2drug.com/passonline/ (accessed on 21 September 2025).
- Mugnaini, C.; Kostrzewa, M.; Casini, M.; Kumar, P.; Catallo, V.; Allarà, M.; Guastaferro, L.; Brizzi, A.; Paolino, M.; Tafi, A.; et al. Systematic Modification of the Substitution Pattern of the 7-Hydroxy-5-oxopyrazolo[4,3-b]pyridine-6-carboxamide Scaffold Enabled the Discovery of New Ligands with High Affinity and Selectivity for the Cannabinoid Type 2 Receptor. Molecules 2023, 28, 4958. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Dulsat, J.; López-Nieto, B.; Estrada-Tejedor, R.; Borrell, J.I. Evaluation of Free Online ADMET Tools for Academic or Small Biotech Environments. Molecules 2023, 28, 776. [Google Scholar] [CrossRef]
- Sumontri, S.; Eiamart, W.; Tadtong, S.; Samee, W. Utilizing ADMET Analysis and Molecular Docking to Elucidate the Neuroprotective Mechanisms of a Cannabis-Containing Herbal Remedy (Suk-Saiyasna) in Inhibiting Acetylcholinesterase. Int. J. Mol. Sci. 2025, 26, 3189. [Google Scholar] [CrossRef] [PubMed]
- Ertl, P.; Rohde, B.; Selzer, P. Fast Calculation of Molecular Polar Surface Area as a Sum of Fragment-Based Contributions and Its Application to the Prediction of Drug Transport Properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef]
- Mauri, A.; Bertola, M. Alvascience: A New Software Suite for the QSAR Workflow Applied to the Blood–Brain Barrier Permeability. Int. J. Mol. Sci. 2022, 23, 12882. [Google Scholar] [CrossRef]
- Lopez-Mercado, S.; Enríquez, C.; Valderrama, J.A.; Pino-Rios, R.; Ruiz-Vásquez, L.; Ruiz Mesia, L.; Vargas-Arana, G.; Buc Calderon, P.; Benites, J. Exploring the Antibacterial and Antiparasitic Activity of Phenylaminonaphthoquinones—Green Synthesis, Biological Evaluation and Computational Study. Int. J. Mol. Sci. 2024, 25, 10670. [Google Scholar] [CrossRef]
- Delaney, J.S. ESOL: Estimating Aqueous Solubility Directly from Molecular Structure. J. Chem. Inf. Comput. Sci. 2004, 44, 1000–1005. [Google Scholar] [CrossRef]
- Ali, J.; Camilleri, P.; Brown, M.B.; Hutt, A.J.; Kirton, S.B. In Silico Prediction of Aqueous Solubility Using Simple QSPR Models: The Importance of Phenol and Phenol-like Moieties. J. Chem. Inf. Model. 2012, 52, 2950–2957. [Google Scholar] [CrossRef] [PubMed]
- Arnott, J.A.; Planey, S.L. The influence of lipophilicity in drug discovery and design. Expert Opin. Drug Discov. 2012, 7, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug solubility: Importance and enhancement techniques. ISRN Pharm. 2012, 2012, 195727. [Google Scholar] [CrossRef] [PubMed]
- Mannhold, R.; Poda, G.I.; Ostermann, C.; Tetko, I.V. Calculation of molecular lipophilicity: State-of-the-art and comparison of log P methods on more than 96,000 compounds. J. Pharm. Sci. 2009, 98, 861–893. [Google Scholar] [CrossRef]
- He, Z.; Yang, D.; Fan, X.; Zhang, M.; Li, Y.; Gu, X.; Yang, M. The Roles and Mechanisms of lncRNAs in Liver Fibrosis. Int. J. Mol. Sci. 2020, 21, 1482. [Google Scholar] [CrossRef]
- Nicze, M.; Borówka, M.; Dec, A.; Niemiec, A.; Bułdak, Ł.; Okopień, B. The Current and Promising Oral Delivery Methods for Protein- and Peptide-Based Drugs. Int. J. Mol. Sci. 2024, 25, 815. [Google Scholar] [CrossRef]
- Wu, K.; Kwon, S.H.; Zhou, X.; Fuller, C.; Wang, X.; Vadgama, J.; Wu, Y. Overcoming Challenges in Small-Molecule Drug Bioavailability: A Review of Key Factors and Approaches. Int. J. Mol. Sci. 2024, 25, 13121. [Google Scholar] [CrossRef] [PubMed]
- Muller, T.; Demizieux, L.; Troy-Fioramonti, S.; Buch, C.; Leemput, J.; Belloir, C.; Pais de Barros, J.-P.; Jourdan, T.; Passilly-Degrace, P.; Fioramonti, X.; et al. Chemical Synthesis, Pharmacokinetic Properties and Biological Effects of JM-00266, a Putative Non-Brain Penetrant Cannabinoid Receptor 1 Inverse Agonist. Int. J. Mol. Sci. 2022, 23, 2923. [Google Scholar] [CrossRef]
- Apan, A.; Casoni, D.; Leonte, D.; Pop, C.; Iaru, I.; Mogoșan, C.; Zaharia, V. Heterocycles 52: The Drug-Likeness Analysis of Anti-Inflammatory Thiazolo[3,2-b][1,2,4]triazole and Imidazo[2,1-b][1,3,4]thiadiazole Derivatives. Pharmaceuticals 2024, 17, 295. [Google Scholar] [CrossRef]
- Filimonov, D.A.; Rudik, A.V.; Dmitriev, A.V.; Poroikov, V.V. Computer-Aided Estimation of Biological Activity Profiles of Drug-Like Compounds Taking into Account Their Metabolism in Human Body. Int. J. Mol. Sci. 2020, 21, 7492. [Google Scholar] [CrossRef]
- Janczy-Cempa, E.; Mazuryk, O.; Kania, A.; Brindell, M. Significance of Specific Oxidoreductases in the Design of Hypoxia-Activated Prodrugs and Fluorescent Turn off–on Probes for Hypoxia Imaging. Cancers 2022, 14, 2686. [Google Scholar] [CrossRef]
- Previti, S.; Ettari, R. Inhibitors of Proteases: A Well-Grounded Strategy in Drug Development. Molecules 2025, 30, 2909. [Google Scholar] [CrossRef]
- Sadowski, M.; Synkiewicz-Musialska, B.; Kula, K. (1E,3E)-1,4-Dinitro-1,3-butadiene—Synthesis, Spectral Characteristics and Computational Study Based on MEDT, ADME and PASS Simulation. Molecules 2024, 29, 542. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, M.; Kula, K. Unexpected Course of Reaction Between (1E,3E)-1,4-Dinitro-1,3-butadiene and N-Methyl Azomethine Ylide—A Comprehensive Experimental and Quantum-Chemical Study. Molecules 2024, 29, 5066. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Chai, J.-D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom-atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef]
- Hariharan, P.C.; Pople, J.A. The influence of polarization functions on molecular orbital hydrogenation energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Abbasi, M.; Farnia, S.M.F.; Tahghighi, A. The possibility of applying some heteroatom-decorated g-C3N4 heterocyclic nanosheets for delivering 5-aminosalicylic acid anti-inflammatory agent. Chem. Heterocycl. Comp. 2024, 60, 655–662. [Google Scholar] [CrossRef]
- Łapczuk-Krygier, A.; Kazimierczuk, K.; Pikies, J.; Ríos-Gutiérrez, M. A Comprehensive Experimental and Theoretical Study on the [{(η5-C5H5)2Zr[P(µ-PNEt2)2P(NEt2)2P]}]2O Crystalline System. Molecules 2021, 26, 7282. [Google Scholar] [CrossRef]
- Demchuk, O.M.; Jasiński, R.; Strzelecka, D.; Dziuba, K.; Kula, K.; Chrzanowski, J.; Krasowska, D. A clean and simple method for deprotection of phosphines from borane complexes. Pure Appl. Chem. 2018, 90, 49–62. [Google Scholar] [CrossRef]
- Fryźlewicz, A.; Olszewska, A.; Zawadzińska, K.; Woliński, P.; Kula, K.; Kącka-Zych, A.; Łapczuk-Krygier, A.; Jasiński, R. On the Mechanism of the Synthesis of Nitrofunctionalised Δ2-Pyrazolines via [3 + 2] Cycloaddition Reactions between α-EWG-Activated Nitroethenes and Nitrylimine TAC Systems. Organics 2022, 3, 59–76. [Google Scholar] [CrossRef]
- Abdoul-Hakim, M.; Kenzy, C.; Subramaniam, M.; Zeroual, A.; Syed, A.; Bahkali, A.H.; Garmes, H.; Verma, M.; Wang, S. Elucidating chemoselectivity and unraveling the mechanism of 1,3-dipolar cycloaddition between diphenyl nitrilimine and (isoxazol-3-yl)methylbenzimidazole through molecular electron density theory. Chem. Heterocycl. Comp. 2024, 60, 617–626. [Google Scholar] [CrossRef]
- Łapczuk-Krygier, A.; Korotaev, V.Y.; Barkov, A.Y.; Sosnovskikh, V.Y.; Jasińska, E.; Jasiński, R. A DFT computational study on the molecular mechanism of the nitro group migration in the product derived from 3-nitro-2-(trifluoromethyl)-2H-chromene and 2-(1-phenylpropylidene) malononitrile. J. Fluor. Chem. 2014, 168, 236–239. [Google Scholar] [CrossRef]
- Cramer, C.J. Essentials of Computational Chemistry: Theories and Models; Wiley: Louisville, KY, USA, 2013; ISBN 978-0-470-09182-1. [Google Scholar]
- Tomasi, J.; Persico, M. Molecular Interactions in Solution: An Overview of Methods Based on Continuous Distributions of the Solvent. Chem. Rev. 1994, 94, 2027–2094. [Google Scholar] [CrossRef]
- Cossi, M.; Barone, V.; Cammi, R.; Tomasi, J. Ab initio study of solvated molecules: A new implementation of the polarizable continuum model. Chem. Phys. Chem. 1996, 225, 327–335. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M.; Tomasi, J. Geometry optimization of molecular structures in solution by the polarizable continuum model. J. Comput. Chem. 1998, 19, 404–417. [Google Scholar] [CrossRef]
- Schlegel, H.B. Optimization of equilibrium geometries and transition structures. J. Comput. Chem. 1982, 3, 214–218. [Google Scholar] [CrossRef]
- Schlegel, H.B. Geometry Optimization on Potential Energy Surfaces. In Modern Electronic Structure Theory; Yarkony, D.R., Ed.; World Scientific Publishing: Singapore, 1994; pp. 459–501. [Google Scholar] [CrossRef]
- Fukui, K. Formulation of the reaction coordinate. J. Phys. Chem. 1970, 74, 4161–4163. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. Reaction path following in mass-weighted internal coordinates. J. Phys. Chem. 1990, 94, 5523–5527. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. Improved algorithms for reaction path following: Higher order implicit algorithms. J. Chem. Phys. 1991, 95, 5853–5860. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural population analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Noury, S.; Krokidis, X.; Fuster, F.; Silvi, B. Computational tools for the electron localization function topological analysis. Comput. Chem. 1999, 23, 597–604. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 6.0; Semichem Inc.: Shawnee, KS, USA, 2016. [Google Scholar]
- Ayachit, U. The ParaView Guide: A Parallel Visualization Application; Kitware Inc.: New York, NY, USA, 2015. [Google Scholar]
- Daina, A.; Michielin, O.; Zoete, V. ILOGP: A Simple, Robust, and Efficient Description of n -Octanol/Water Partition Coefficient for Drug Design Using the GB/SA Approach. J. Chem. Inf. Model. 2014, 54, 3284–3301. [Google Scholar] [CrossRef]
- Cheng, T.; Zhao, Y.; Li, X.; Lin, F.; Xu, Y.; Zhang, X.; Li, Y.; Wang, R.; Lai, L. Computation of Octanol−Water Partition Coefficients by Guiding an Additive Model with Knowledge. J. Chem. Inf. Model. 2007, 47, 2140–2148. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A BOILED-Egg to Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef]
- Moriguchi, I.; Hirono, S.; Liu, Q.; Nakagome, I.; Matsushita, Y. Simple Method of Calculating Octanol/Water Partition Coefficient. Chem. Pharm. Bull. 1992, 40, 127–130. [Google Scholar] [CrossRef]
- Moriguchi, I.; Hirono, S.; Nakagome, I.; Hirano, H. Comparison of Reliability of Log P Values for Drugs Calculated by Several Methods. Chem. Pharm. Bull. 1994, 42, 976–978. [Google Scholar] [CrossRef]
- Ali, J.; Camilleri, P.; Brown, M.B.; Hutt, A.J.; Kirton, S.B. Revisiting the general solubility equation: In silico prediction of aqueous solubility incorporating the effect of topographical polar surface area. J. Chem. Inf. Model. 2012, 52, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Duffy, E.M. Prediction of drug solubility from structure. Adv. Drug Deliv. Rev. 2002, 54, 355–366. [Google Scholar] [CrossRef] [PubMed]
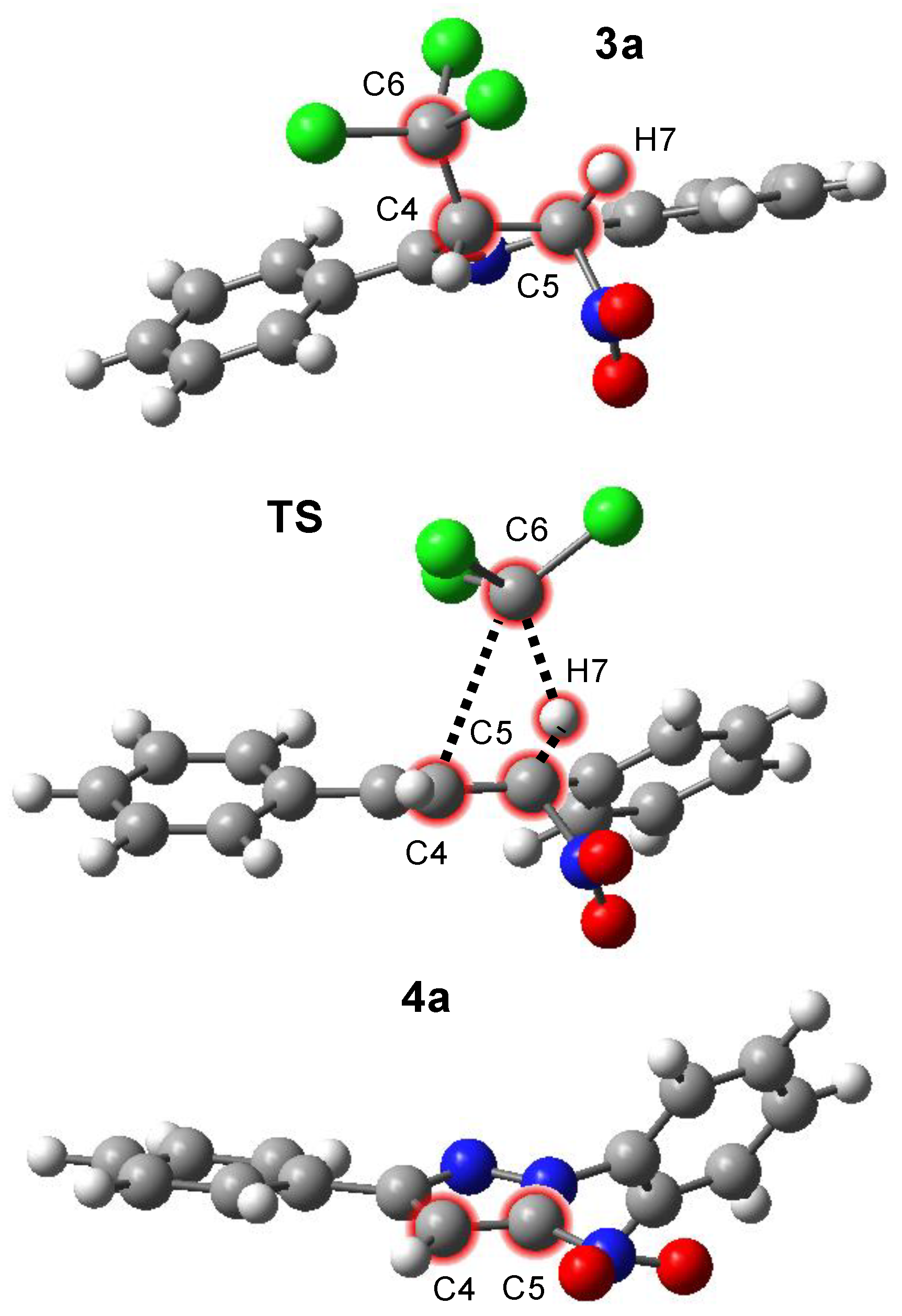
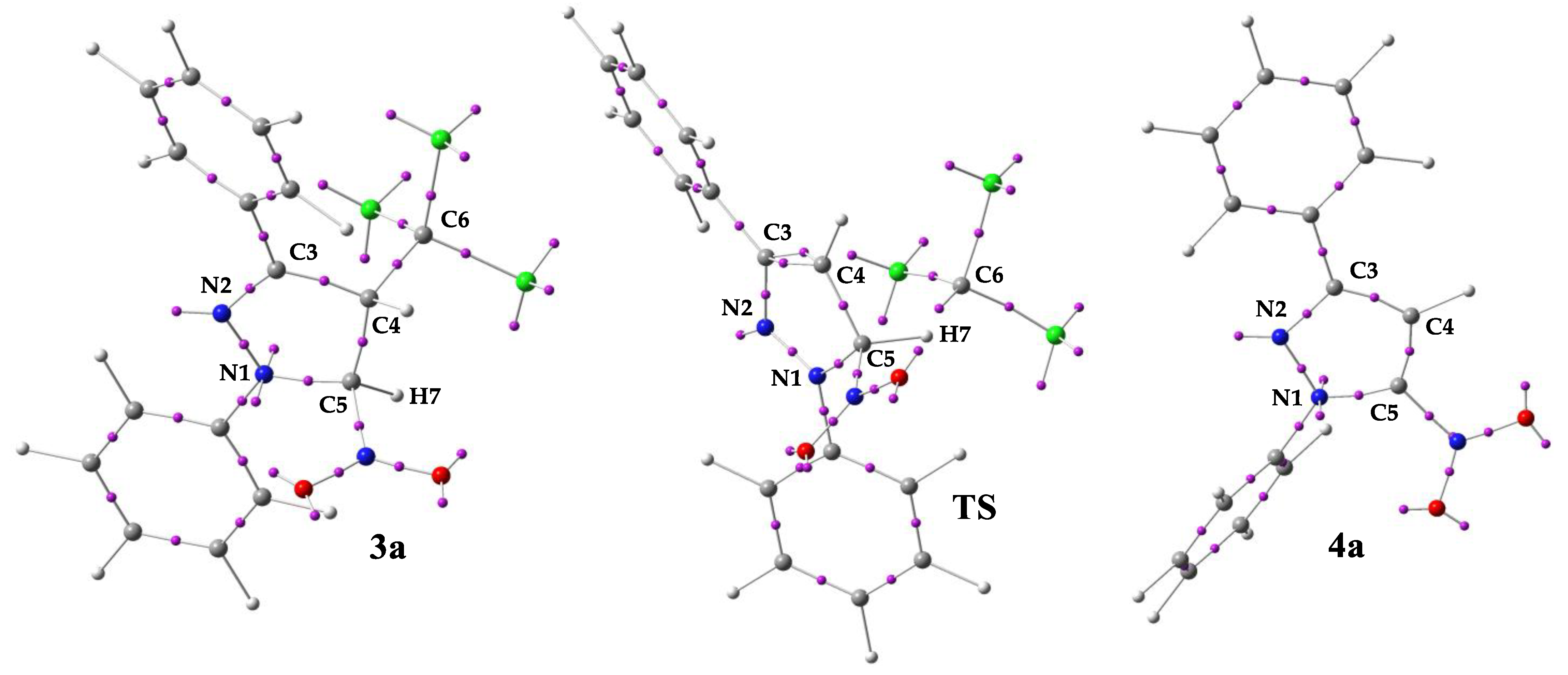
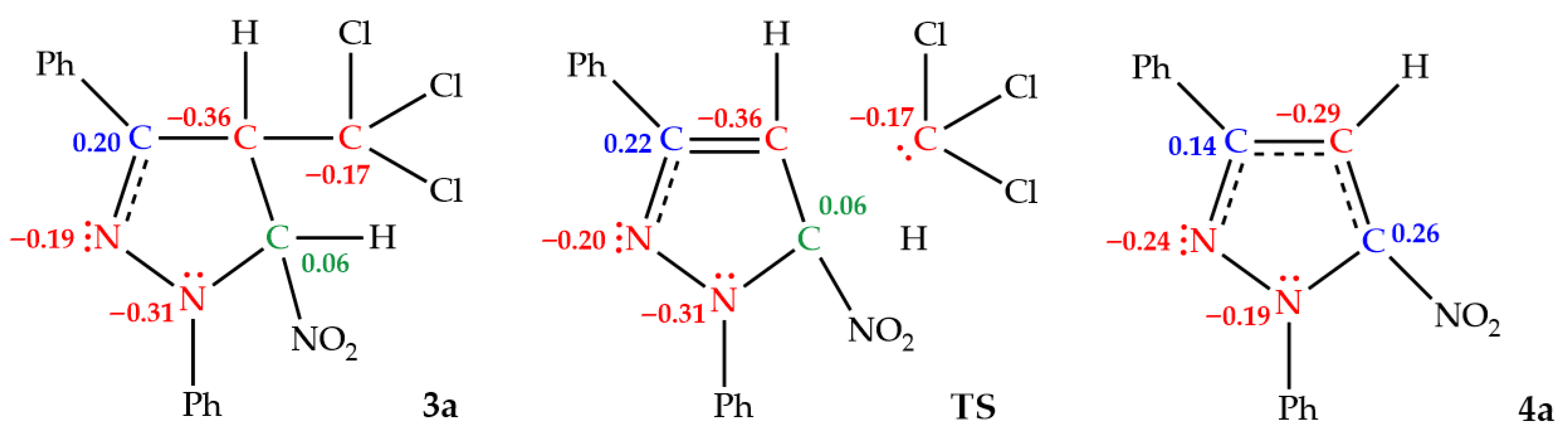
| Solvent | Substrate | Transition | ΔH | ΔS | ΔG |
|---|---|---|---|---|---|
| Benzene | 3a | 3a → TS | 34.7 | 8.2 | 32.2 |
| (ε = 2.2706) | 3a → 4a | −40.4 | 45.2 | −53.9 | |
| Acetone | 3a | 3a → TS | 30.6 | 9.0 | 27.9 |
| (ε = 20.493) | 3a → 4a | −41.1 | 45.8 | −54.7 | |
| Nitromethane | 3a | 3a → TS | 30.3 | −0.9 | 30.6 |
| (ε = 36.562) | 3a → 4a | −41.1 | 35.7 | −51.7 | |
| Benzene | 3b | 3b → TS | 33.5 | 9.4 | 30.7 |
| 3b → 4b | −39.8 | 47.1 | −53.9 | ||
| Benzene | 3c | 3c → TS | 36.1 | 8.7 | 33.5 |
| 3c → 4c | −41.0 | 45.5 | −54.6 | ||
| Benzene | 3d | 3d → TS | 28.2 | 8.2 | 25.7 |
| 3d → 4d | −42.6 | 47.4 | −56.8 | ||
| Benzene | 3e | 3e → TS | 40.5 | 11.4 | 37.1 |
| 3e → 4e | −37.4 | 48.6 | −51.9 |
| Solvent | Reaction | Structure | Interatomic Distances [Å] | |||
|---|---|---|---|---|---|---|
| C4–C6 | C6–H7 | H7–C5 | C4–C5 | |||
| Benzene | 3a → 4a | 3a | 1.552 | 2.666 | 1.090 | 1.539 |
| (ε = 2.2706) | TS | 3.339 | 2.155 | 1.106 | 1.482 | |
| 4a | 1.375 | |||||
| Acetone | 3a → 4a | 3a | 1.552 | 2.674 | 1.089 | 1.538 |
| (ε = 20.493) | TS | 3.398 | 2.261 | 1.101 | 1.481 | |
| 4a | 1.375 | |||||
| Nitromethane | 3a → 4a | 3a | 1.552 | 2.675 | 1.089 | 1.538 |
| (ε = 36.562) | TS | 3.402 | 2.270 | 1.101 | 1.481 | |
| 4a | 1.375 | |||||
| Benzene | 3b → 4b | 3b | 1.552 | 2.664 | 1.089 | 1.539 |
| TS | 3.355 | 2.173 | 1.104 | 1.480 | ||
| 4b | 1.374 | |||||
| Benzene | 3c → 4c | 3c | 1.553 | 2.661 | 1.089 | 1.540 |
| TS | 3.295 | 2.136 | 1.108 | 1.481 | ||
| 4c | 1.374 | |||||
| Benzene | 3d → 4d | 3d | 1.552 | 2.670 | 1.090 | 1.539 |
| TS | 3.452 | 2.195 | 1.101 | 1.483 | ||
| 4d | 1.375 | |||||
| Benzene | 3e → 4e | 3e | 1.553 | 2.664 | 1.090 | 1.536 |
| TS | 3.327 | 2.129 | 1.109 | 1.480 | ||
| 4e | 1.372 | |||||
| Valence Basin Populations, N [e] | |||
|---|---|---|---|
| ELF Basins | 3a | TS | 4a |
| V(N1) | 1.01 | 0.87 | |
| V′(N1) | 1.33 | 0.88 | |
| V(N1,N2) | 1.44 | 2.99 | 1.62 |
| V(N2) | 2.81 | 2.67 | 3.06 |
| V(N2,C3) | 3.12 | 2.07 | 2.55 |
| V(C3,C4) | 2.02 | 1.80 | 2.91 |
| V′(C3,C4) | 1.80 | ||
| V(C4,C5) | 1.96 | 2.07 | 3.39 |
| V(N1,C5) | 1.78 | 2.00 | 2.20 |
| V(C4,C6) | 2.02 | ||
| V(C6) | 2.28 | ||
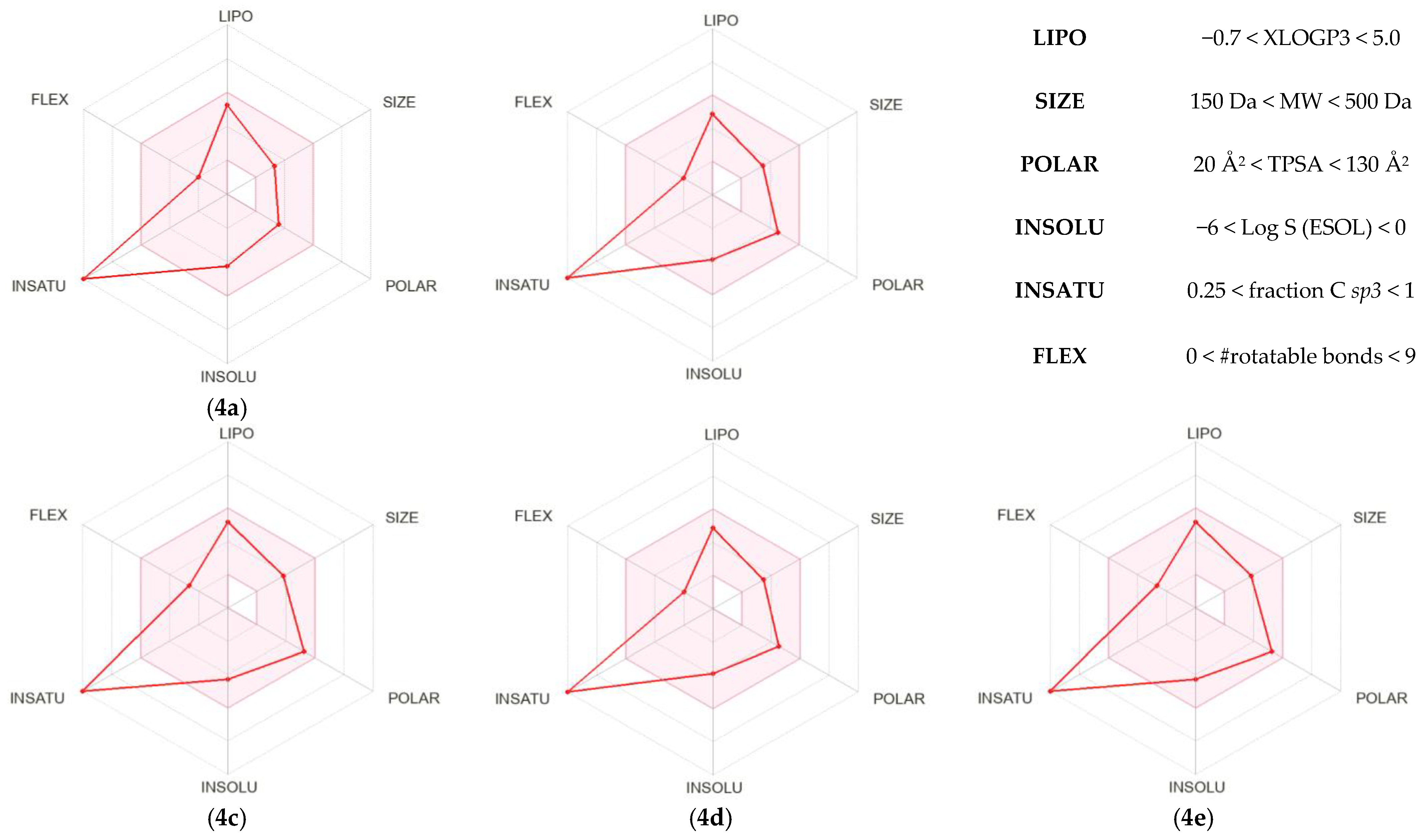
| 4a | 4b | 4c | 4d | 4e | ||
|---|---|---|---|---|---|---|
| Physchem. properties | MW [g/mol] | 265.27 | 280.28 | 310.26 | 280.28 | 310.26 |
| #heavy atoms | 20 | 21 | 23 | 21 | 23 | |
| #arom. heavy atoms | 17 | 17 | 17 | 17 | 17 | |
| #rotatable bonds | 3 | 3 | 4 | 3 | 4 | |
| #H-bond acceptors | 3 | 3 | 5 | 3 | 5 | |
| #H-bond donors | 0 | 1 | 0 | 1 | 0 | |
| MR | 77.82 | 82.23 | 86.64 | 82.23 | 86.64 | |
| TPSA [Å2] | 63.64 | 89.66 | 109.46 | 89.66 | 109.46 | |
| Lipophilicity | Log Po/w (iLOGP) | 2.32 | 1.93 | 2.03 | 1.89 | 2.02 |
| Log Po/w (XLOGP3) | 3.72 | 3.03 | 3.54 | 3.03 | 3.54 | |
| Log Po/w (WLOGP) | 3.97 | 3.56 | 4.40 | 3.56 | 4.40 | |
| Log Po/w (MLOGP) | 3.63 | 2.27 | 2.64 | 2.27 | 2.64 | |
| Log Po/w (SILICOS-IT) | 1.21 | 0.48 | −0.58 | 0.48 | −0.58 | |
| Consensus Log Po/w | 2.97 | 2.26 | 2.41 | 2.25 | 2.41 | |
| Water solubility | Log S (ESOL) | −4.26 | −3.89 | −4.28 | −3.89 | −4.28 |
| solubility [mg/mL] | 0.0146 | 0.0363 | 0.0164 | 0.0363 | 0.0164 | |
| Log S (Ali) | −4.75 | −4.58 | −5.52 | −4.58 | −5.52 | |
| solubility [mg/mL] | 0.0047 | 0.0074 | 0.0093 | 0.0074 | 0.0093 | |
| Log S (SILICOS-IT) | −5.30 | −4.94 | −5.15 | −4.94 | −5.15 | |
| solubility [mg/mL] | 0.0013 | 0.0032 | 0.0021 | 0.0032 | 0.0021 | |
| Pharmacokinetics * | CYP1A2 inhibitor | Yes | Yes | Yes | Yes | Yes |
| CYP2C19 inhibitor | Yes | Yes | Yes | Yes | Yes | |
| CYP2C9 inhibitor | Yes | Yes | Yes | Yes | Yes | |
| CYP2D6 inhibitor | No | No | No | No | No | |
| CYP3A4 inhibitor | No | No | No | No | No | |
| IG absorption | Yes | Yes | Yes | Yes | Yes | |
| BBB permeant | Yes | No | No | No | No | |
| Druglikeness | Lipinski et al. [64] (Pfizer) | ✔ | ✔ | ✔ | ✔ | ✔ |
| Ghose et al. [65] (Amgen) | ✔ | ✔ | ✔ | ✔ | ✔ | |
| Veber et al. [66] (GSK) | ✔ | ✔ | ✔ | ✔ | ✔ | |
| Egan et al. [67] (Pharmacia) | ✔ | ✔ | ✔ | ✔ | ✔ | |
| Muegge et al. [68] (Bayer) | ✔ | ✔ | ✔ | ✔ | ✔ |
| 4a | 4c | 4e | |
|---|---|---|---|
| 5-O-(4-coumaroyl)-D-quinate 3′-monooxygenase inhibitor | 0.751 | - | - |
| Hyponitrite reductase inhibitor | 0.741 | 0.758 | 0.758 |
| (R)-6-hydroxynicotine oxidase inhibitor | 0.737 | 0.747 | 0.747 |
| Acrocylindropepsin inhibitor | 0.748 | 0.808 | 0.808 |
| Saccharopepsin inhibitor | 0.748 | 0.808 | 0.808 |
| Chymosin inhibitor | 0.748 | 0.808 | 0.808 |
| Glycosylphosphatidylinositol phospholipase D inhibitor | 0.728 | - | - |
| Phospholipid-translocating ATPase inhibitor | 0.708 | - | - |
| Arylacetonitrilase inhibitor | 0.704 | 0.712 | 0.712 |
| (R)-6-hydroxynicotine oxidase inhibitor | - | 0.747 | 0.747 |
| Fusarinine-C ornithinesterase inhibitor | - | 0.741 | 0.741 |
| Ubiquinol-cytochrome-c reductase inhibitor | - | 0.765 | 0.765 |
| Bisphosphoglycerate phosphatase inhibitor | - | 0.728 | 0.728 |
| Phospholipid-translocating ATPase inhibitor | - | 0.716 | 0.716 |
| Interatomic Distances [Å] | ||||
|---|---|---|---|---|
| Level of Theory | C4–C6 | C6–H7 | H7–C5 | C4–C5 |
| ωB97X-D/6-31+G(d,p) | 3.339 | 2.155 | 1.106 | 1.482 |
| ωB97X-D/6-311G(d) | 3.333 | 2.168 | 1.103 | 1.479 |
| ωB97X-D/6-311G(d,p) | 3.320 | 2.168 | 1.104 | 1.478 |
| ωB97X-D/6-311+G(d) | 3.344 | 2.182 | 1.102 | 1.479 |
| ωB97X-D/6-311+G(d,p) | 3.337 | 2.180 | 1.104 | 1.479 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kula, K.; Jasiński, R. On the Question of the Application Potential and the Molecular Mechanism of the Formation of 1,3-Diaryl-5-Nitropyrazoles from Trichloromethylated Diarylnitropyrazolines. Molecules 2025, 30, 4306. https://doi.org/10.3390/molecules30214306
Kula K, Jasiński R. On the Question of the Application Potential and the Molecular Mechanism of the Formation of 1,3-Diaryl-5-Nitropyrazoles from Trichloromethylated Diarylnitropyrazolines. Molecules. 2025; 30(21):4306. https://doi.org/10.3390/molecules30214306
Chicago/Turabian StyleKula, Karolina, and Radomir Jasiński. 2025. "On the Question of the Application Potential and the Molecular Mechanism of the Formation of 1,3-Diaryl-5-Nitropyrazoles from Trichloromethylated Diarylnitropyrazolines" Molecules 30, no. 21: 4306. https://doi.org/10.3390/molecules30214306
APA StyleKula, K., & Jasiński, R. (2025). On the Question of the Application Potential and the Molecular Mechanism of the Formation of 1,3-Diaryl-5-Nitropyrazoles from Trichloromethylated Diarylnitropyrazolines. Molecules, 30(21), 4306. https://doi.org/10.3390/molecules30214306







