1. Introduction
Acetaminophen (APAP), also known as paracetamol or N-(4-hydroxyphenyl)acetamide, is a widely used over-the-counter and prescription non-steroidal analgesic and antipyretic. Its primary pharmacological action involves the inhibition of prostaglandin synthesis through cyclooxygenase (COX-1 and COX-2) pathways, thereby alleviating pain and reducing fever. Global consumption of APAP is substantial, estimated at nearly 1.5 tons annually [
1,
2,
3]. China and India currently lead global production, accounting for over 70% of the total supply [
4,
5].
Despite its therapeutic benefits, APAP has been increasingly recognized as a persistent and ubiquitous contaminant in aquatic environments, posing a pressing environmental and health concern. Its widespread usage, combined with incomplete removal in conventional wastewater treatment plants (WWTPs), contributes to its frequent detection in wastewater effluents, surface waters, groundwater, and even seawater. Reported concentrations range from nanograms to milligrams per liter [
1,
6,
7]. With a solubility of 305 mg/L at 25 °C and a Log K
ow of 0.46, APAP is relatively hydrophilic and poorly adsorbed onto organic matter, contributing to its mobility in aquatic systems. Following administration, approximately 90% of APAP is excreted via urine in both unchanged and conjugated forms [
8]. Additional pathways of environmental contamination include discharge from pharmaceutical manufacturing and improper disposal of expired or unused medications [
1]. The direct release of untreated or partially treated wastewater, a significant source of APAP, can have severe ecotoxic effects on aquatic species and carcinogenic and mutagenic effects on humans [
9].
The environmental occurrence of APAP has consequences. It has been documented in surface water, groundwater, and drinking water sources across 29 countries, with reported concentrations averaging 0.161 μg/L and reaching a maximum of up to 230 μg/L. These concentrations, even at the lower end of the range, are significant and indicate the widespread presence of APAP in our water sources, posing potential risks to both the environment and human health [
10].
Human health risks associated with APAP include severe hepatotoxicity, oxidative stress, DNA damage, and liver failure in overdose scenarios. Environmentally, it is classified as a high-risk pollutant, particularly to aquatic organisms [
11,
12,
13]. Aquatic invertebrates such as
Daphnia magna, as well as fish species like
Danio rerio and trout, exhibit sensitivity to APAP even at trace levels, manifesting in oxidative stress, enzyme inhibition, and developmental impairments [
14,
15]. Notably, paracetamol was identified as the NSAID (nonsteroidal anti-inflammatory drugs) that disrupted the most significant number of oxidative stress biomarkers, underscoring its ecotoxicological relevance [
16]. Plants also experience phytotoxic effects, including reduced concentrations of photosynthetic pigments and inhibited root growth [
13].
While the challenges are significant, the potential of advanced treatment solutions beyond conventional methods is promising. A recent survey of 20 WWTPs in China revealed APAP in 19 of the samples, with concentrations ranging from 0.06 to 29.2 nM. However, despite high removal rates reported for APAP during secondary treatment [
3,
17,
18], more toxic metabolites such as
p-aminophenol have been detected post treatment at elevated concentrations (23.93–108.68 nM), indicating the existence of metabolic bottlenecks in existing treatment processes [
19]. This highlights the need for advanced treatment solutions beyond conventional methods, along with continuous monitoring strategies to mitigate potential environmental and health risks [
8,
20,
21].
Several advanced remediation technologies have been explored for the removal of APAP. Among these, Advanced Oxidation Processes (AOPs) have garnered considerable interest due to their ability to generate highly reactive radicals, particularly hydroxyl (•OH), capable of mineralizing organic pollutants into benign by-products [
3,
22]. In AOPs, hydroxyl radicals play a key role in breaking down organic pollutants, including APAP, into simpler, less harmful compounds, thereby reducing their environmental impact. However, AOPs face limitations, including incomplete mineralization and the formation of toxic intermediates—especially during photocatalytic oxidation. Additionally, UV-based systems are energy-intensive, and their efficiency can be diminished by background organic matter or radical-scavenging ions present in complex water matrices. Some of these problems are being solved using techniques such as cold plasma treatment [
23], which allows organic contaminants to be eliminated with lower energy consumption and at ambient temperature and pressure. Membrane technologies, including microfiltration, ultrafiltration, nanofiltration, and reverse osmosis, provide an advanced treatment pathway. While these systems offer high separation efficiencies, they are often hindered by membrane fouling, increased energy consumption, and high operational costs, particularly in pressure-driven applications such as reverse osmosis [
24,
25]. Additionally, high energy consumption is a notable drawback, particularly in pressure-driven systems, such as reverse osmosis, which require substantial energy inputs to maintain optimal performance. Another problem with membrane filtration is that its results depend on the size of the molecules [
26]. Although it can be a suitable method for separating contaminants when they have some value, its results may not be optimal for complex matrices.
Electrochemical methods, particularly Electrochemical Advanced Oxidation Processes (EAOPs), provide a flexible alternative for degrading pharmaceutical pollutants through redox reactions [
27]. Recent innovations in nanostructured electrodes and catalytic coatings have significantly improved performance [
28]. Integration with biological and membrane-based technologies has also demonstrated synergistic potential in complex treatment scenarios.
Biological remediation approaches, including phytoremediation and microbial biodegradation, have shown promise due to their sustainability and cost-effectiveness. Phytoremediation relies on specific plant species—often hardy or invasive—to absorb and transform pharmaceuticals through enzymatic pathways [
29,
30]. Similarly, microbial biodegradation utilizes bacteria, fungi, and microalgae capable of enzymatically degrading APAP via pathways involving amidases, oxygenases, and deaminases [
13]. The low operational cost and environmental compatibility of biological methods make them an attractive option for sustainable water treatment. However, these methods are sensitive to environmental factors such as temperature, pH, and the presence of co-contaminants, which can impair microbial activity and overall efficiency.
Among the various remediation technologies, adsorption has emerged as one of the most promising strategies for removing emerging contaminants, including pharmaceutical contaminants from water systems [
31,
32,
33,
34,
35]. Adsorbents, such as activated carbon and biochar, capitalize on their high surface area, porosity, and tunable surface chemistries to effectively capture organic contaminants [
36]. Activated carbon, available in both granular (GAC) and powdered (PAC) forms, is particularly valued for its cost-effectiveness, chemical stability, and high adsorption capacity. Surface modifications, including oxidation and amination, further enhance its performance by introducing reactive functional groups that improve affinity toward ionizable or polar compounds such as APAP [
20,
37,
38,
39]. Advanced materials such as metal–organic frameworks (MOFs), carbon nanotubes (CNTs), and graphene derivatives exhibit even higher adsorption capacities. However, their high production costs and limited scalability remain significant barriers to widespread application [
40,
41]. In contrast, activated carbon offers a practical and scalable solution, especially for large-scale water treatment applications. Many of the methods mentioned above (e.g., AOPs) have a clear disadvantage compared to adsorption: although they can be very effective against organic pollutants, they do not usually act on inorganic compounds. Given that in most situations, wastewater contains a complex mixture of both types of products, it is logical to conclude that adsorption should be a widely used option. Despite its advantages, adsorption-based remediation is not without challenges. Regeneration of spent adsorbents via thermal or chemical means is often energy-intensive and can degrade material integrity over time [
42]. Moreover, safe disposal or recycling of exhausted adsorbents is critical to prevent secondary contamination. Nonetheless, continued advancements in adsorbent materials and system design underscore the viability of adsorption, particularly using activated carbon, as a cornerstone strategy for APAP remediation.
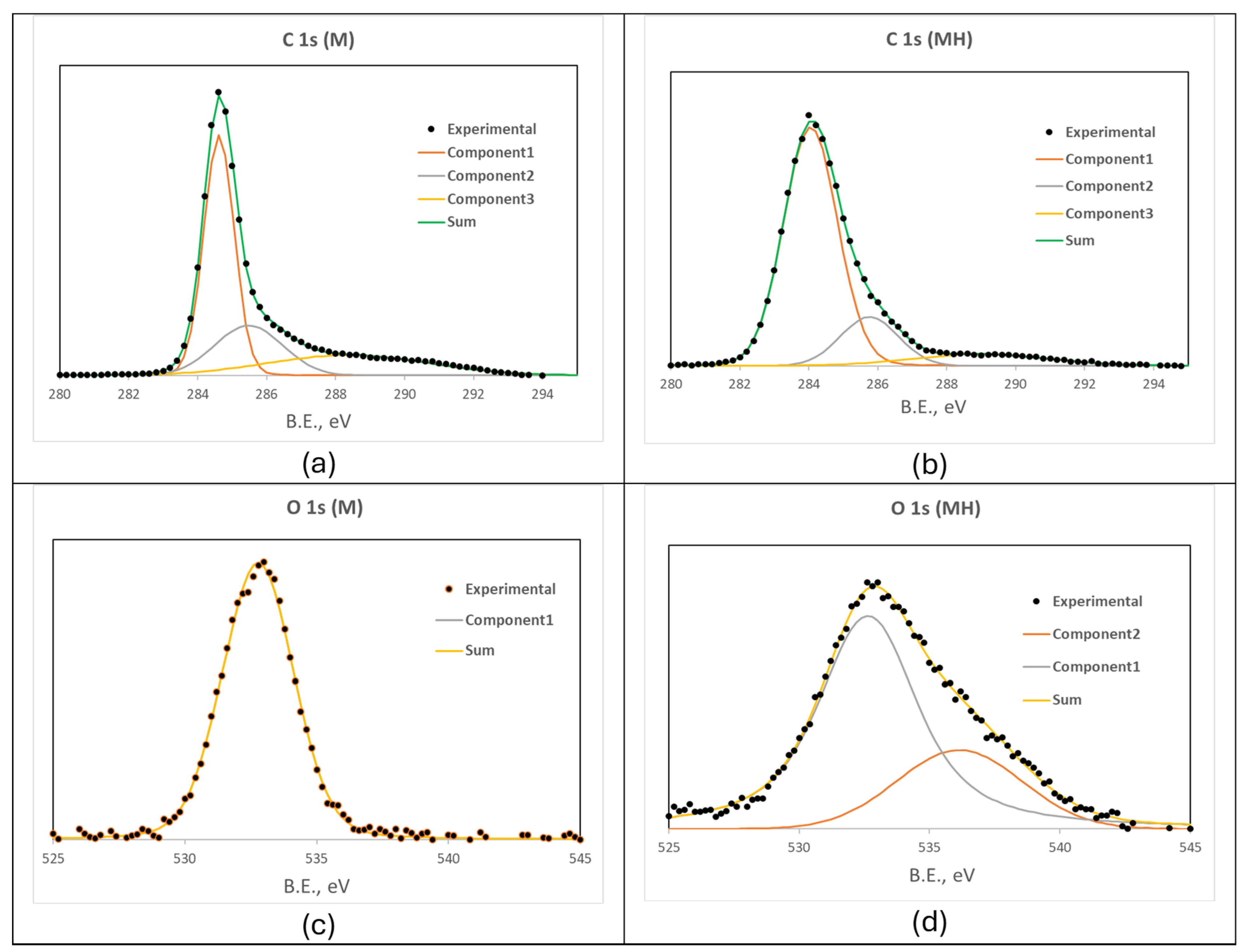
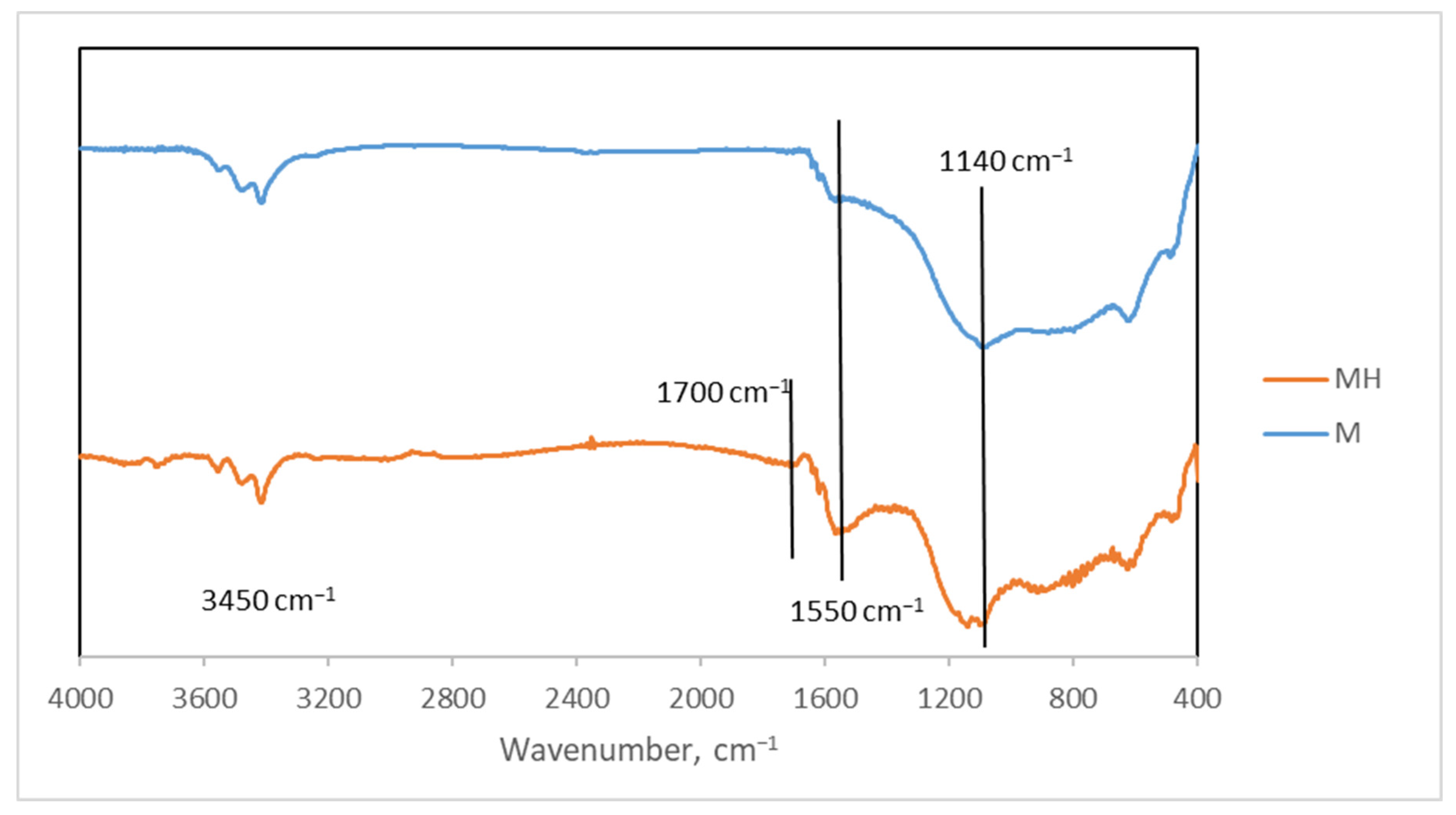
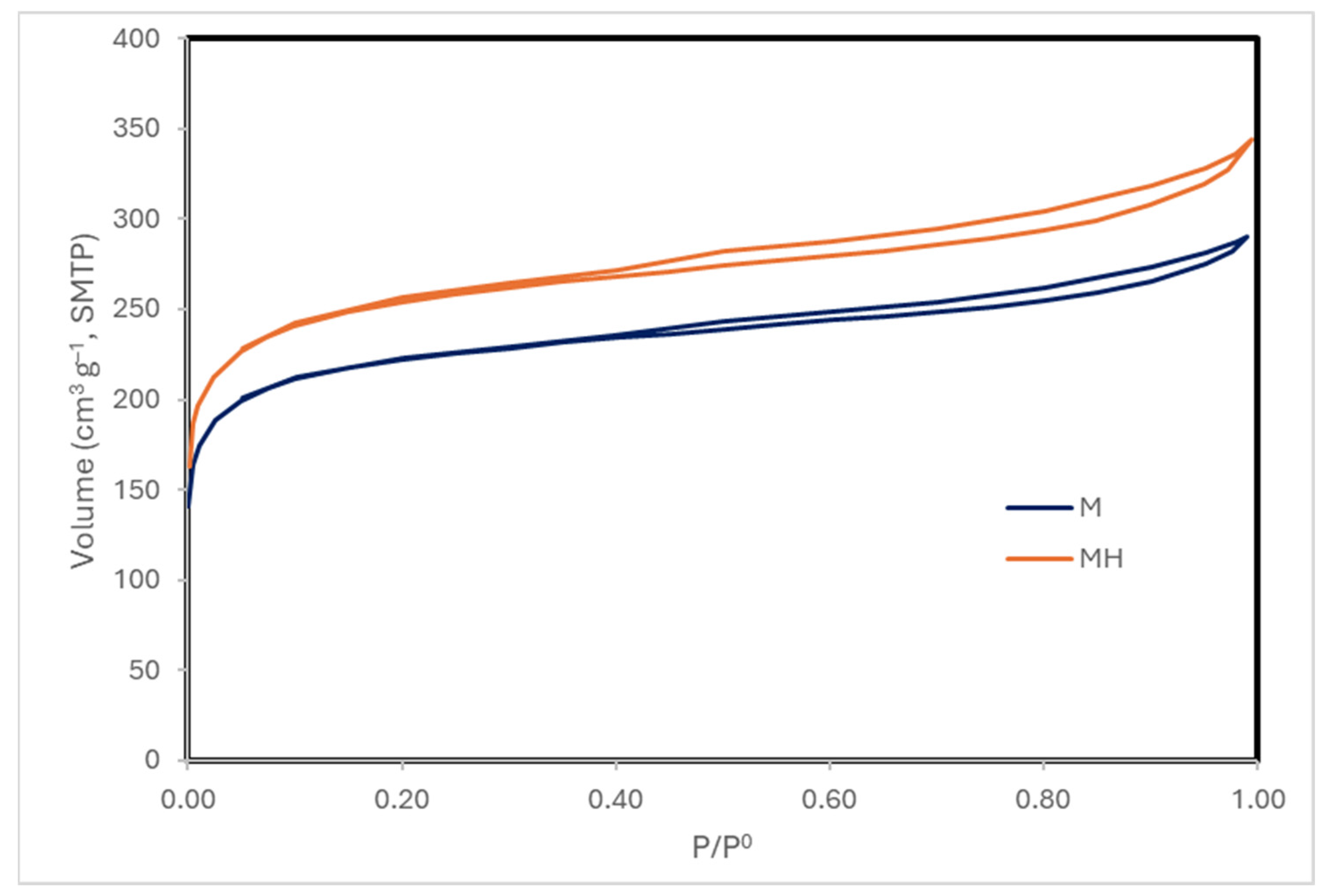
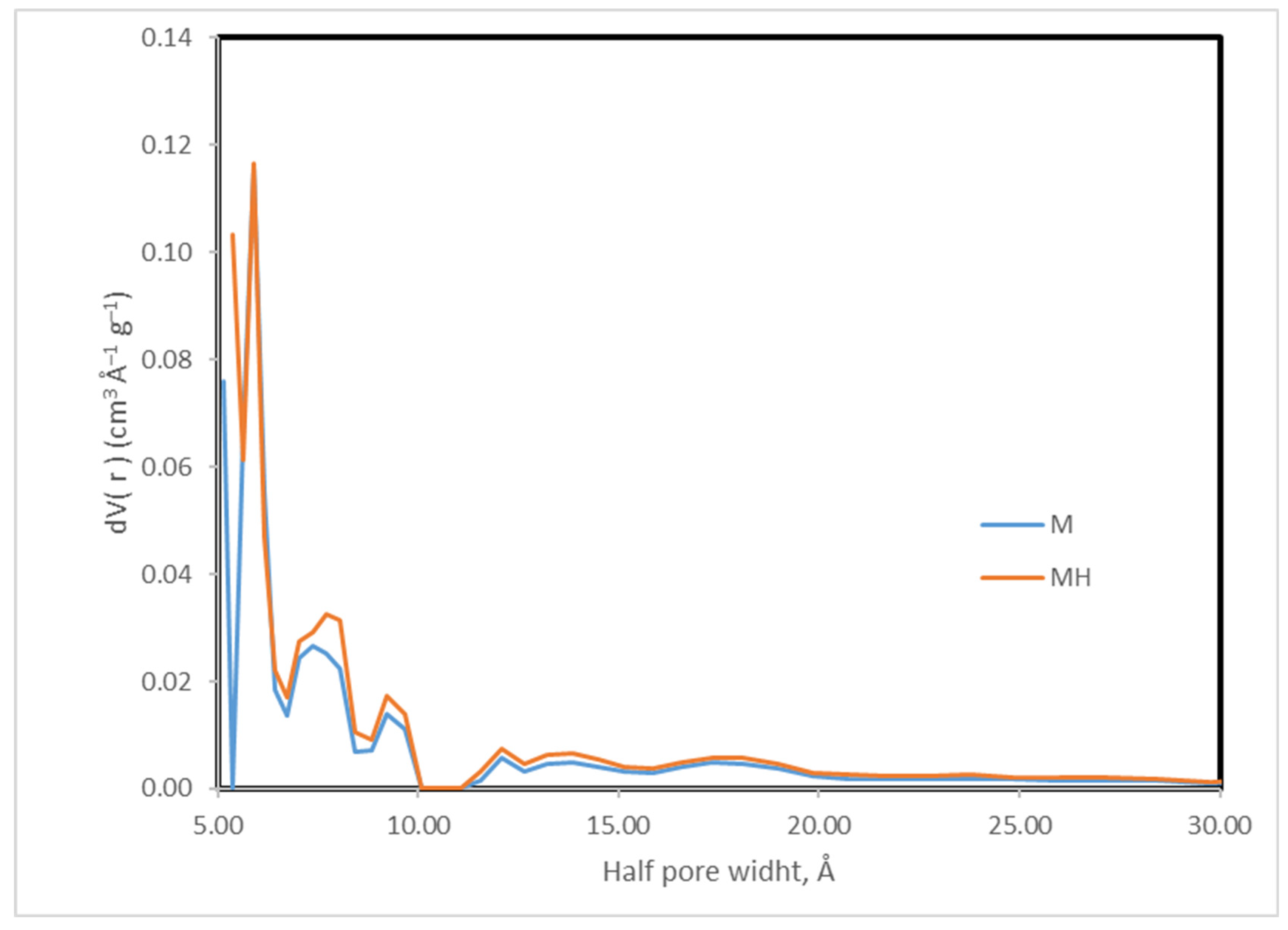
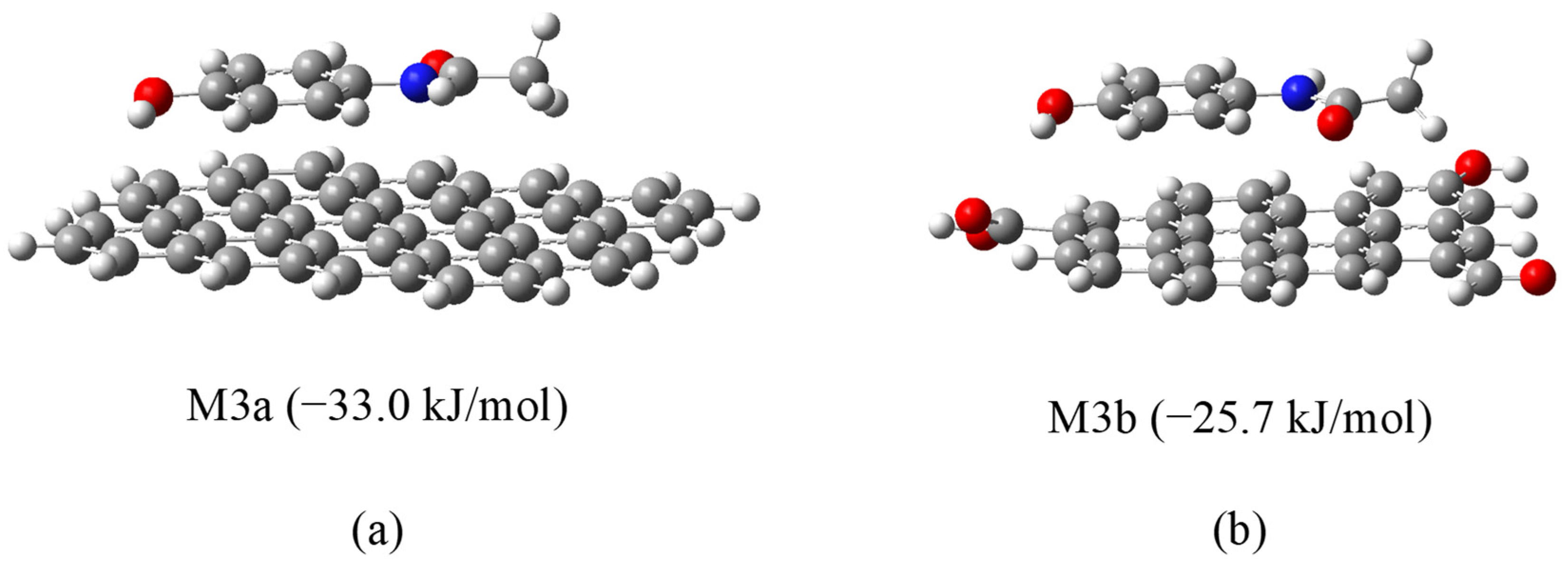
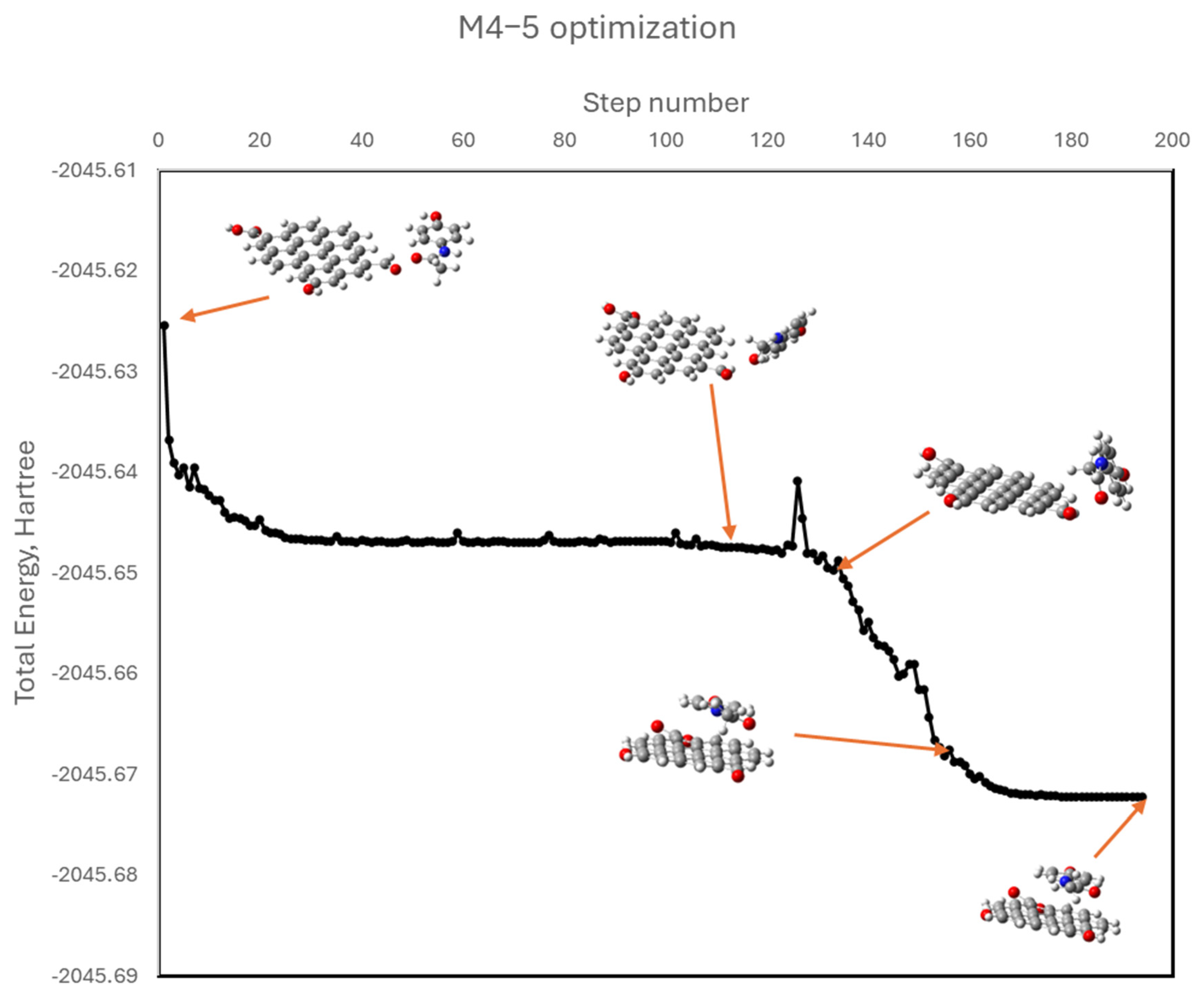
Enhancing the yield and performance of activated carbons can be effectively achieved through modifications to their surface chemistry and porous structure. In this context, we have explored hydrothermal modification as a viable strategy to improve the physicochemical properties of activated carbon. This approach has yielded promising results in previous studies, where the modified materials demonstrated high adsorption capacities for contaminants such as thorium [
43], uranium [
44], and acetamiprid [
45]. Additionally, these materials have shown catalytic activity in reactions relevant to the synthesis of fine chemicals [
46], further highlighting their versatility and potential for multifunctional applications.
In this context, the present study explores the use of activated carbon for the adsorption and removal of acetaminophen from contaminated water. Particular emphasis is placed on elucidating the adsorption mechanisms, evaluating key performance parameters, and assessing the practical feasibility of applying this approach in real-world water treatment systems.
3. Future Perspectives
The modification of adsorbents using hydrothermal methods is a new methodology that, to our knowledge, has only been tested by our research group [
41,
42,
43,
44], so there is little experience in its use in research and none in industrial processes, unlike what happens with the preparation of adsorbents using the hydrothermal method. According to the results we have obtained, its mechanism of operation is similar to that of other adsorbents such as commercial activated carbon. In most of the cases we studied, a more significant improvement over the initial activated carbon is obtained when metal cations are adsorbed than when organic contaminants are removed. However, as shown in this article, there are also cases in which the adsorption of organic compounds is improved. This is an issue that will be resolved with further research to determine when it is appropriate to apply this technology.
In real-world situations, the presence of other contaminants, such as natural organic matter (NOM), must be considered. Similar adsorbents, such as activated carbons, have been used for the removal of NOM [
64]. This suggests that in real wastewater, there is competition between NOM and APAP for adsorption sites. This aspect is particularly important given that most NOM comprises low-polarity organic compounds. However, it should be noted that some pre-treatments, such as coagulation, can remove part of the NOM and avoid some of this competition, leaving adsorption to remove more recalcitrant compounds.
As for its industrial implementation, there should be no major difficulties as it is a simple process. To prepare these materials industrially, a third process (hydrothermal treatment) would have to be added to the two that are common in the manufacture of activated carbon (carbonization and activation). Considering that no reagents are used, as can be the case in activation, and that the working temperature is much lower tan in the other steps, it is expected that this third stage will not be costly and that the improvement obtained will offset this expense. The environmental aspect must also be taken into account: better performance means less production is needed, and since the raw material is usually biomass, there will be less demand for it, which could help combat deforestation in some parts of the world. As for adsorbent regeneration, it is a matter of doubtful use because it is not always cost-effective to regenerate an adsorbent, as it depends on numerous factors. In this regard, hydrothermal treatment could be an option worth considering, as it does not require expensive reagents (only water) and the temperatures required are not as high as in thermal regeneration.
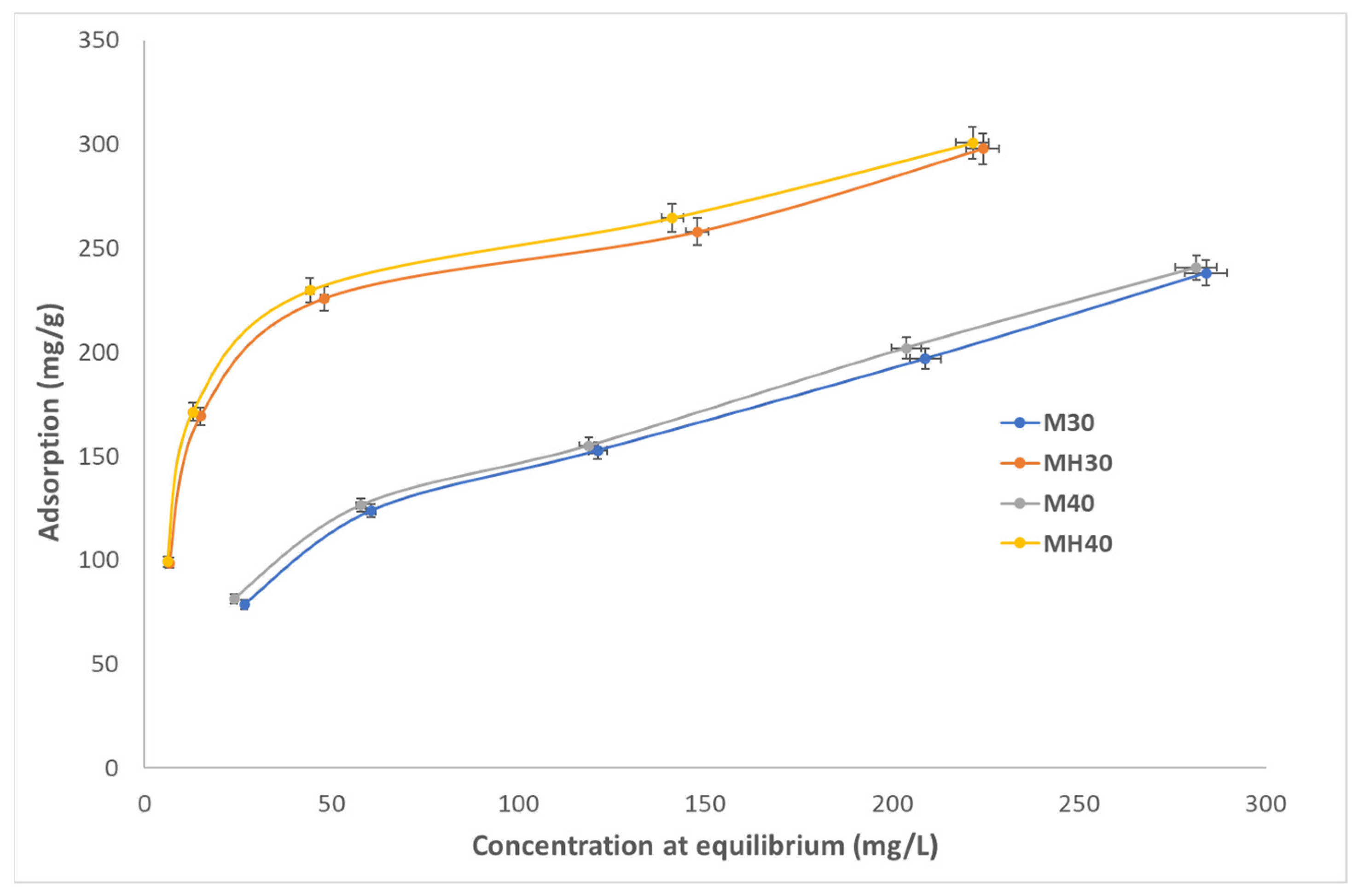
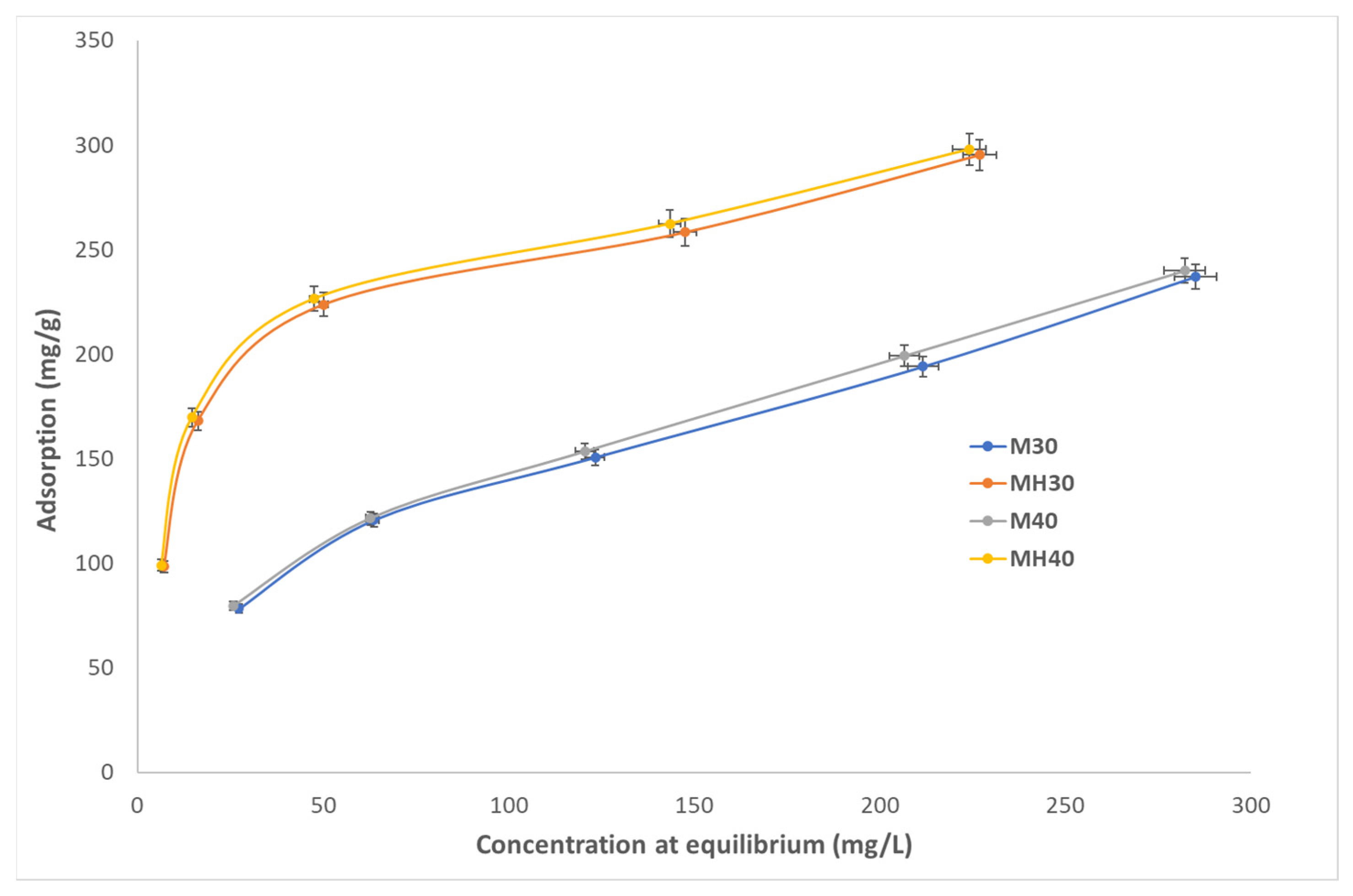
As shown in
Table 10 and further detailed in reference [
63], MH has a comparatively high adsorption capacity and is also a low-cost material, especially when compared to others such as MOFs or CNTs, which do not improve performance enough to justify their higher cost.