Enhancing the Catalytic Performance of Zeolites via Metal Doping and Porosity Control
Abstract
1. Introduction
- (i)
- (ii)
- (iii)
- Incipient wetness impregnation [6] leading to the formation of nanoparticles.
2. Catalytic Application of Hierarchical Zeolites
2.1. Catalytic Pyrolysis
2.1.1. Pyrolysis in the Presence of Metal-Free Zeolites
- (i)
- Bottom-up approach where the well-known template for the ZSM-5 synthesis, tetrapropylammonium hydroxide (TPAOH) [93], was used in combination with NaOH addition and hydrothermal treatment (the sample designated MFI-Meso);
- (ii)
- Top-down approach where desilication was carried out by the treatment with NaOH solutions of different concentrations (MFI-SA-Mild and MFI-SA-Strong) with the addition of a surfactant (CTAB) (the samples designated MFI-DS-Mild and MFI-DS-Strong).
2.1.2. Metal-Doped Zeolites as the Pyrolysis Catalysts
2.2. Transformation of Alcohols to Fuel Hydrocarbons, Aromatics and Olefins
2.2.1. Alcohols to Fuel Hydrocarbons and Aromatics
2.2.2. Methanol to Olefins
2.3. Cracking, Hydrocracking, and Hydroisomerization
2.3.1. Catalytic Cracking and Hydrocracking
2.3.2. Catalytic Hydroisomerization
2.4. Hydrodeoxygenation
3. Conclusions
4. Challenges and Outlook
- (i)
- Development of hierarchical structures from 2D layers, which possess better hydrothermal stability;
- (ii)
- Combination of zeolites with different silica-based materials in composite hierarchical structures with controllable acidity;
- (iii)
- Modification of zeolite surface with different silica or phosphorous species and control over the defect concentration during the synthesis of the hierarchical catalytic material.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APTES | 3-aminopropyltrimethoxy-silane |
| BAS | Brønsted acid sites |
| BCR | biomass-to-catalyst ratio |
| BTX | benzene, toluene, xylene |
| CTAB | cetyltrimethylammonium bromide |
| CFP | catalytic fast pyrolysis |
| EDTA | ethylenediaminetetraacetic acid |
| ETH | ethanol-to-hydrocarbons |
| EV | ethyl valerate |
| GVL | gamma-valerolactone |
| HCP | hydrocarbon pool |
| HDPE | high-density polyethylene |
| LA | levulinic acid |
| LAS | Lewis acid sites |
| LHSV | liquid hourly space velocity |
| LDPE | low-density polyethylene |
| MACFP | microwave-assisted catalytic fast pyrolysis |
| MAHs | mono-aromatic hydrocarbons |
| MeOH | methanol |
| MFBR | microfluidized bed reactor |
| MR | membered ring |
| MTA | methanol-to-aromatics |
| MTH | methanol-to-hydrocarbons |
| MTO | methanol-to-olefins |
| MW | microwave |
| OMMC | ordered macro-mesoporous carbon |
| OSA | organosilane |
| PAHs | poly-cyclic aromatic hydrocarbons |
| PHAPTMS | phenylaminopropyltrimethoxysilane |
| PMMA | poly(methyl methacrylate) |
| PP | polypropylene |
| PTH | propanol-to-hydrocarbons |
| SAR | Si-to-Al ratio |
| SPP | self-pillared pentasil |
| TBAOH | tetrabutylammonium hydroxide |
| TEAOH | tetraethylammonium hydroxide |
| TEOS | tetraethoxysilane |
| TPAOH | tetrapropylammonium hydroxide |
| TPOAC | [3-(trimethoxysilyl)propyl]dimethyloctadecylammonium chloride |
| TPW | torrefied poplar wood sawdust |
| TS | transition state |
| VGO | vacuum gas oil |
| WHSV | weight hourly space velocity |
References
- Ding, Y.; Ye, Y.; Miao, D.; Wang, H.; Feng, J.; Qu, J.; Zhao, Y.; Chen, Z.; Zhang, P.; Yu, R.; et al. Heteroatom-Induced Fractal Growth for Hierarchical Zeolites. Chem. Mater. 2025, 37, 87–96. [Google Scholar] [CrossRef]
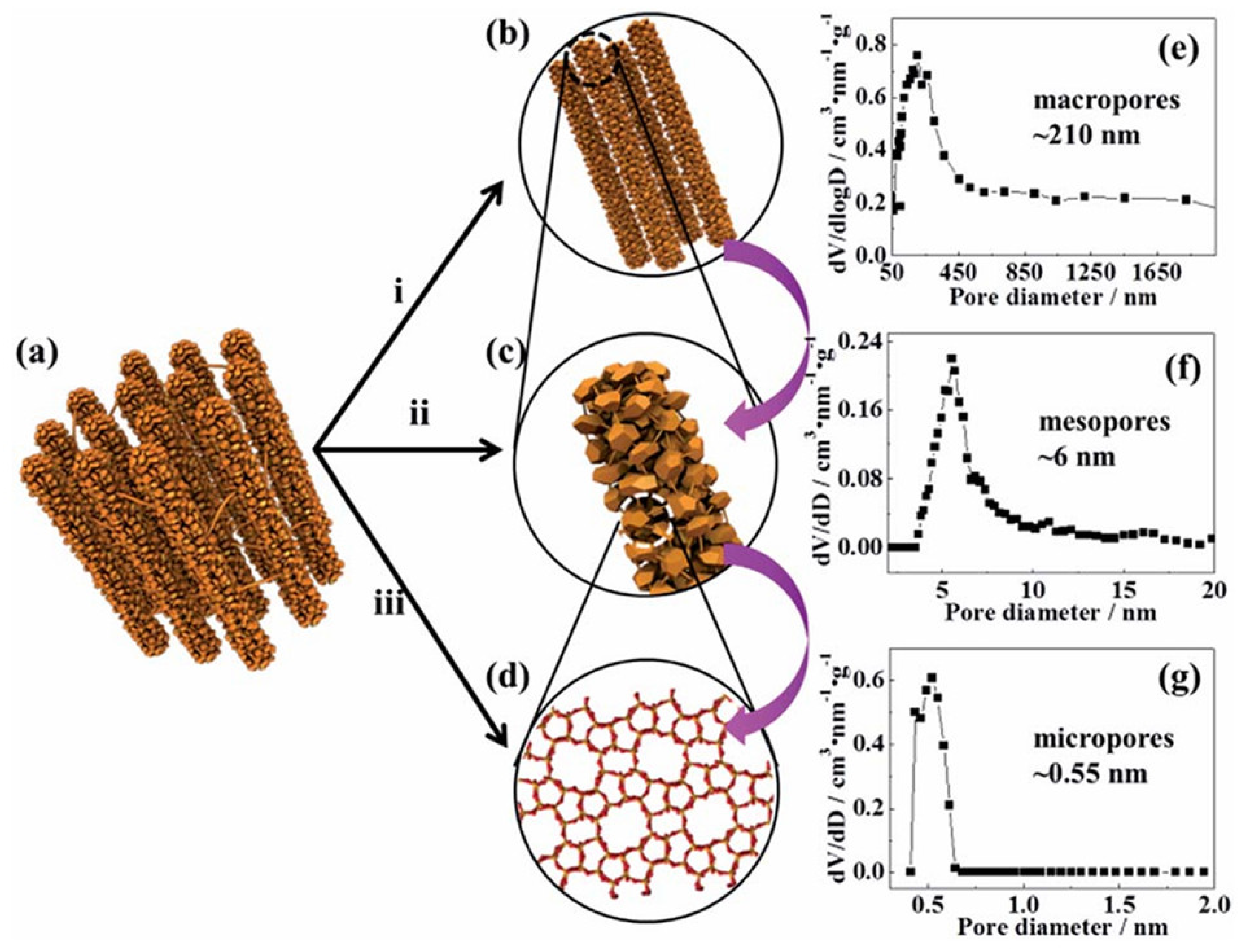
- Feliczak-Guzik, A. Hierarchical zeolites: Synthesis and catalytic properties. Microporous Mesoporous Mater. 2018, 259, 33–45. [Google Scholar] [CrossRef]
- Zhang, X.; Zhuang, Y.; Liang, S.; Gong, H.; Liu, J. A strategy for synthesizing an ordered porous beta zeolite support for Ni2P catalysts with enhanced hydrodenitrogenation performance. Microporous Mesoporous Mater. 2025, 393, 113651. [Google Scholar] [CrossRef]
- Potter, M.E.; Oakley, A.E.; Le Brocq, J.J.M.; Riley, L.N.; Carravetta, M.; King, S.M.; Doherty, C.M.; Vandegehuchted, B.D.; Raja, R. Using small angle neutron scattering to explore porosity, connectivity and accessibility, towards optimised hierarchical solid acid catalysts. J. Mater. Chem. A 2023, 11, 22822–22834. [Google Scholar] [CrossRef]
- Hartmann, M.; Thommes, M.; Schwieger, W. Hierarchically-Ordered Zeolites: A Critical Assessment. Adv. Mater. Interfaces 2021, 8, 2001841. [Google Scholar] [CrossRef]
- You, X.; Zhang, X.; Jiang, S.; Ye, Y.; Gu, L.; Zhou, H.; Ma, P.; Ftouni, J.; Chowdhury, A.D. Efficacy of Ca/ZSM-5 zeolites derived from precipitated calcium carbonate in the methanol-to-olefin process. Chin. J. Struct. Chem. 2024, 43, 100265. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Chu, W.; Gao, Y.; Xie, S.; Li, X.; Xu, L.; Zhu, X. Advances in the green and controllable synthesis of MWW zeolite. Chem. Commun. 2024, 60, 9907–9917. [Google Scholar] [CrossRef]
- Ren, L.; Guo, Q.; Kumar, P.; Orazov, M.; Xu, D.; Alhassan, S.M.; Mkhoyan, K.A.; Davis, M.E.; Tsapatsis, M. Self-Pillared, Single-Unit-Cell Sn-MFI Zeolite Nanosheets and Their Use for Glucose and Lactose Isomerization. Angew. Chem. Int. Ed. 2015, 54, 10848–10851. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Xiao, F.-S. Metal@Zeolite Hybrid Materials for Catalysis. ACS Cent. Sci. 2020, 6, 1685–1697. [Google Scholar] [CrossRef]
- Kosinov, N.; Liu, C.; Hensen, E.J.M.; Pidko, E.A. Engineering of Transition Metal Catalysts Confined in Zeolites. Chem. Mater. 2018, 30, 3177–3198. [Google Scholar] [CrossRef]
- Guo, Y.; Tai, W.; Zhao, M.; Chen, X.; Chai, Y.; Wu, G.; Li, L. Synthesis of self-pillared pentasil zeolites without organic templates and seeds. Nanoscale 2024, 16, 21594–21603. [Google Scholar] [CrossRef]
- Wang, L.-Q.; Li, H.-J.; Diao, J.-Q.; Hou, D.-D.; Qiang, M.-M.; Chen, L.-J. Photocatalytic performance of hierarchical metal-doped framework zeolite. Inorg. Chem. Commun. 2022, 140, 109425. [Google Scholar] [CrossRef]
- Kwok, K.M.; Ong, S.W.D.; Chen, L.; Zeng, H.C. Transformation of Stöber Silica Spheres to Hollow Hierarchical Single-Crystal ZSM-5 Zeolites with Encapsulated Metal Nanocatalysts for Selective Catalysis. ACS Appl. Mater. Interfaces 2019, 11, 14774–14785. [Google Scholar] [CrossRef] [PubMed]
- Kerstens, D.; Smeyers, B.; Van Waeyenberg, J.; Zhang, Q.; Yu, J.; Sels, B.F. State of the Art and Perspectives of Hierarchical Zeolites: Practical Overview of Synthesis Methods and Use in Catalysis. Adv. Mater. 2020, 32, 2004690. [Google Scholar] [CrossRef] [PubMed]
- Da Rocha, J.D.G.; Macuvele, D.L.P.; de Andrade, C.J.; Riella, H.G.; Padoin, N.; Soares, C. Advances and Environmental Aspects on the Synthesis of Hierarchical Zeolites Revisited: A state-of-the-art description. J. Environ. Chem. Eng. 2023, 11, 109397. [Google Scholar] [CrossRef]
- Roth, W.J.; Gil, B.; Tarach, K.A.; Góra-Marek, K. Top-down engineering of zeolite porosity. Chem. Soc. Rev. 2025, 54, 7484–7560. [Google Scholar] [CrossRef]
- Peron, D.V.; Zholobenko, V.L.; de Melo, J.H.S.; Capron, M.; Nuns, N.; de Souza, M.O.; Feris, L.A.; Marcilio, N.R.; Ordomsky, V.V.; Khodakov, A.Y. External surface phenomena in dealumination and desilication of large single crystals of ZSM-5 zeolite synthesized from a sustainable source. Microporous Mesoporous Mater. 2019, 286, 57–64. [Google Scholar] [CrossRef]
- Kononov, P.; Kononova, I.; Moshnikov, V.; Maraeva, E.; Trubetskaya, O. Step-By-Step Modeling and Demetallation Experimental Study on the Porous Structure in Zeolites. Molecules 2022, 27, 8156. [Google Scholar] [CrossRef]
- Hong, L.; Zang, J.; Li, B.; Liu, G.; Wang, Y.; Wu, L. Research Progress on the Synthesis of Nanosized and Hierarchical Beta Zeolites. Inorganics 2023, 11, 214. [Google Scholar] [CrossRef]
- Qin, Z.; Hafiz, L.; Shen, Y.; Van Daele, S.; Boullay, P.; Ruaux, V.; Mintova, S.; Gilson, J.; Valtchev, V. Defect-engineered zeolite porosity and accessibility. J. Mater. Chem. A 2020, 8, 3621–3631. [Google Scholar] [CrossRef]
- Zhu, X.; Gao, Y.; Chen, H.; Jiang, M.; Wang, X.; Miao, C.; Shen, Y.; Ji, Y.; Qin, Z.; Wu, Z.; et al. Constructing Hierarchical Zeolites with Highly Complete Framework via Controlled Desilication. Angew. Chem. Int. Ed. 2024, 63, e202411446. [Google Scholar] [CrossRef]
- Suárez, N.; Pérez-Pariente, J.; Mondragón, F.; Moreno, A. Generation of hierarchical porosity in beta zeolite by post-synthesis treatment with the cetyltrimethylammonium cationic surfactant under alkaline conditions. Microporous Mesoporous Mater. 2019, 280, 144–150. [Google Scholar] [CrossRef]
- Gangwar, M.K.; Pandey, S.; Parsapur, R.K.; Harb, M.; Murugesan, S.; Emwas, A.-H.; Koseoglu, O.R.; Hodgkins, R.P.; Bendjeriou-Sedjerari, A.; Rueping, M.; et al. Surface Organometallic Chemistry on Zeolites: Synthesis of Group IV Metal Alkyls and Metal Hydrides on Hierarchical Mesoporous H-ZSM-5. Chem. Mater. 2022, 34, 8777–8789. [Google Scholar] [CrossRef]
- Meng, X.; Xiao, F.-S. Mesoporous Zeolite Templated from Polymers. In Mesoporous Zeolites; García-Martínez, J., Li, K., Eds.; Wiley: Hoboken, NJ, USA, 2015; pp. 199–226. [Google Scholar] [CrossRef]
- Flores, C.G.; Schneider, H.; Louis, B. Lignin as a Bio-Sourced Secondary Template for ZSM-5 Zeolite Synthesis. Catalysts 2022, 12, 368. [Google Scholar] [CrossRef]
- Ma, Y.; Hu, J.; Fan, K.; Chen, W.; Han, S.; Wu, Q.; Ma, Y.; Zheng, A.; Kunkes, E.; De Baerdemaeker, T.; et al. Design of an Organic Template for Synthesizing ITR Zeolites under Ge-Free Conditions. J. Am. Chem. Soc. 2023, 145, 17284–17291. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Zhuang, Y.; Liu, J.; Zhang, X. Three-dimensionally ordered macroporous ZSM-5 zeolite supported high-dispersed Ni2P: Highly active catalyst for quinoline hydrodenitrogenation. Chem. Eng. J. 2024, 480, 148236. [Google Scholar] [CrossRef]
- Zheng, M.; Chen, Y.; Liu, Z.; Lyu, J.; Ye, B.; Sun, M.-H.; Chen, L.-H.; Su, B.-L. Hierarchically Macroporous Zeolite ZSM-5 Microspheres for Efficient Catalysis. Chem. Res. Chin. Univ. 2024, 40, 704–711. [Google Scholar] [CrossRef]
- Czarna-Juszkiewicz, D.; Cader, J.; Wdowin, M. From coal ashes to solid sorbents for hydrogen storage. J. Clean. Prod. 2020, 270, 122355. [Google Scholar] [CrossRef]
- Schneider, H.; Schmitz, T.; Flores, C.G.; Louis, B.; Tessaro, I.C.; Marcilio, N.R. Template-Free ZSM-5 Synthesis Using Bio-Sourced Waste Materials. Eur. J. Inorg. Chem. 2024, 27, e202400244. [Google Scholar] [CrossRef]
- Liu, Z.; Lyu, J.-M.; Guo, C.-M.; Hu, Z.-Y.; Sun, M.-H.; Chen, L.-H.; Su, B.-L. Tunable acidity and porosity for optimizing liquid-phase catalytic efficiency utilizing hierarchical zeolite ZSM-5 single crystal reactor. Microporous Mesoporous Mater. 2025, 387, 113509. [Google Scholar] [CrossRef]
- Pan, T.; Wu, Z.; Yip, A.C.K. Advances in the Green Synthesis of Microporous and Hierarchical Zeolites: A Short Review. Catalysts 2019, 9, 274. [Google Scholar] [CrossRef]
- Van Santen, R.A.; Ooms, G.; den Ouden, C.J.J.; van Beest, B.W.; Post, M.F.M. Computational Studies of Zeolite Framework Stability. Zeolite Synth. 1989, 398, 617–633. [Google Scholar] [CrossRef]
- Gao, Z.R.; Yu, H.; Chen, F.J.; Mayoral, A.; Niu, Z.; Niu, Z.; Li, X.; Deng, H.; Márquez-Álvarez, C.; He, H.; et al. Interchain-expanded extra-large-pore zeolites. Nature 2024, 628, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.-J.; Wu, S.-M.; Antonietti, M.; Janiak, C.; Terasaki, O.; Yang, X.-Y. Hierarchy design of zeolite mesocrystals. Chem. Eng. J. 2025, 505, 159282. [Google Scholar] [CrossRef]
- Swindlehurst, G.R.; Kumar, P.; Xu, D.; Alhassan, S.M.; Mkhoyan, K.A.; Tsapatsis, M. Nucleation, Growth, and Robust Synthesis of SPP Zeolite: Effect of Ethanol, Sodium, and Potassium. Top. Catal. 2015, 58, 545–558. [Google Scholar] [CrossRef]
- Roth, W.J.; Nachtigall, P.; Morris, R.E.; Čejka, J. Two-Dimensional Zeolites: Current Status and Perspectives. Chem. Rev. 2014, 114, 4807–4837. [Google Scholar] [CrossRef]
- Schulman, E.; Wu, W.; Liu, D. Two-Dimensional Zeolite Materials: Structural and Acidity Properties. Materials 2020, 13, 1822. [Google Scholar] [CrossRef]
- Schwanke, A.; Pergher, S. Lamellar MWW-Type Zeolites: Toward Elegant Nanoporous Materials. Appl. Sci. 2018, 8, 1636. [Google Scholar] [CrossRef]
- Zi, W.; Hu, Z.; Jiang, X.; Zhang, J.; Guo, C.; Qu, K.; Tao, S.; Tan, D.; Liu, F. Morphology Regulation of Zeolite MWW via Classical/Nonclassical Crystallization Pathways. Molecules 2024, 29, 170. [Google Scholar] [CrossRef]
- Zhou, Y.; Mu, Y.; Hsieh, M.-F.; Kabius, B.; Pacheco, C.; Bator, C.; Rioux, R.M.; Rimer, J.D. Enhanced Surface Activity of MWW Zeolite Nanosheets Prepared via a One-Step Synthesis. J. Am. Chem. Soc. 2020, 142, 8211–8222. [Google Scholar] [CrossRef]
- Wang, C.; Jin, F.; Ji, C.; Wu, G. Synthesis of silicate and alumina pillared MWW zeolite as ethane dehydroaromatization catalyst. Microporous Mesoporous Mater. 2021, 327, 111440. [Google Scholar] [CrossRef]
- Bao, R.; Shen, Z.; Ma, C.; Li, L.; Wang, Y.; Sun, H.; Yang, W. Delaminated MWW-type zeolite and its catalytic performance in alkylation of benzene with cyclohexene. Appl. Catal. A Gen. 2022, 643, 118741. [Google Scholar] [CrossRef]
- Shamzhy, M.; Gil, B.; Opanasenko, M.; Roth, W.J.; Čejka, J. MWW and MFI Frameworks as Model Layered Zeolites: Structures, Transformations, Properties, and Activity. ACS Catal. 2021, 11, 2366–2396. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, D.; Xu, D.; Asahina, S.; Cychosz, K.A.; Agrawal, K.V.; Al Wahedi, Y.; Bhan, A.; Al Hashimi, S.; Terasaki, O.; et al. Synthesis of Self-Pillared Zeolite Nanosheets by Repetitive Branching. Science 2012, 336, 1684–1687. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Chawla, A.; Linares, N.; Martínez, J.G.; Rimer, J.D. Spontaneous Pillaring of Pentasil Zeolites. Adv. Mater. 2021, 33, 2100897. [Google Scholar] [CrossRef]
- Ma, Y.; Tang, X.; Hu, J.; Ma, Y.; Chen, W.; Liu, Z.; Han, S.; Xu, C.; Wu, Q.; Zheng, A.; et al. Design of a Small Organic Template for the Synthesis of Self-Pillared Pentasil Zeolite Nanosheets. J. Am. Chem. Soc. 2022, 144, 6270–6277. [Google Scholar] [CrossRef]
- Tai, W.; Dai, W.; Wu, G.; Li, L. A simple strategy for synthesis of b-axis-oriented MFI zeolite macro-nanosheets. Chem. Synth. 2023, 3, 38. [Google Scholar] [CrossRef]
- Shi, J.; Zhang, M.; Zhu, L.; Wu, Q.; Meng, X.; Xiao, F.-S. Recent advances in sustainable synthesis of zeolites. Mater. Today Sustain. 2025, 29, 101065. [Google Scholar] [CrossRef]
- Ruiz-Zamora, E.; De la Rosa, J.R.; Maldonado, C.S.; Lucio-Ortiz, C.J.; De Haro Del Río, D.A.; Garza-Navarro, M.A.; Sandoval-Rangel, L.; Morales-Leal, F.J.; Wi, S. Siliceous self-pillared pentasil (SPP) zeolite with incorporated phosphorus groups in catalytic formation of butadiene by dehydra-decyclization of tetrahydrofuran: Study of catalyst stability by NMR and REDOR analysis. Appl. Catal. A Gen. 2022, 640, 118648. [Google Scholar] [CrossRef]
- Josephson, T.R.; DeJaco, R.F.; Pahari, S.; Ren, L.; Guo, Q.; Tsapatsis, M.; Siepmann, J.I.; Vlachos, D.G.; Caratzoulas, S. Cooperative Catalysis by Surface Lewis Acid/Silanol for Selective Fructose Etherification on Sn-SPP Zeolite. ACS Catal. 2018, 8, 9056–9065. [Google Scholar] [CrossRef]
- Wang, X.; Li, N.; Yang, F.; Yang, X.; Chun, W.; He, G.; Chen, H. Efficient Epoxidation of Cyclohexene Catalyzed by W-Substituted Self-Pillared Pentasil Zeolite Nanosheets. ACS Appl. Nano Mater. 2025, 8, 10526–10536. [Google Scholar] [CrossRef]
- Wang, M.; Li, X.; Huang, Y.; Zeng, X.; Liu, B. Fabrication of pillared MFI zeolite with enhanced stability in the alkylation of phenol and tert-butyl alcohol. Microporous Mesoporous Mater. 2025, 393, 113666. [Google Scholar] [CrossRef]
- Borcuch, A.; Rutkowska, M.; Marzec, A.; Kowalczyk, A.; Michalik, M.; Moreno, J.M.; Díaz, U.; Chmielarz, L. Selective ammonia oxidation over ZSM-5 zeolite: Impact of catalyst’s support porosity and type of deposited iron species. Catal. Today 2020, 348, 223–229. [Google Scholar] [CrossRef]
- Shao, Y.; Wang, H.; Liu, X.; Haydel, P.R.; Li, T.; Chen, J.; Huang, P.; Xiao, Q.; Tatsumi, T.; Wang, J. Single-crystalline hierarchically-porous TS-1 zeolite catalysts via a solid-phase transformation mechanism. Microporous Mesoporous Mater. 2021, 313, 110828. [Google Scholar] [CrossRef]
- Soltan, W.B.; Peng, J.; Cao, Z.; Fu, Z.; Liu, H. Bimetallic Fe-Mn loaded H-ZSM-5 zeolites for excellent VOCs catalytic oxidation at low-temperatures: Synergistic effects and catalytic mechanisms. Chem. Eng. J. 2023, 475, 146251. [Google Scholar] [CrossRef]
- Treu, P.; Iltsiou, D.; Elbuga-Ilica, R.; Maliakkal, C.; Kegnæs, S.; Saraçi, E. Enhancing the oxidative cleavage of vicinal diols on Fe-ZSM-5 catalysts with hierarchical porosity. Sustain. Chem. Environ. 2025, 10, 100232. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, G. Yb-Doped CuY modulates Cu electronic structures for efficient oxidation of anisole to guaiacol. RSC Adv. 2025, 15, 9899–9909. [Google Scholar] [CrossRef]
- Zormpa, F.; Treu, P.; Saraçi, E. Advancing biomass valorization with zeolite catalysts: Focus on oxidative transformations. Sustain. Chem. Environ. 2025, 10, 100249. [Google Scholar] [CrossRef]
- Lv, G.; Ruan, H.; Zou, X.; Chen, Y.; Zhang, X.; Wang, F. Solvent-free synthesis of hierarchical tungsten-doped MFI zeolite for cyclohexene epoxidation reaction. Microporous Mesoporous Mater. 2025, 381, 113345. [Google Scholar] [CrossRef]
- Wei, Y.; Chen, M.; Ren, X.; Wang, Q.; Han, J.; Wu, W.; Yang, X.; Wang, S.; Yu, J. One-Pot Three-Dimensional Printing Robust Self-Supporting MnOx/Cu-SSZ-13 Zeolite Monolithic Catalysts for NH3-SCR. CCS Chem. 2022, 4, 1708–1719. [Google Scholar] [CrossRef]
- Chen, M.; Ren, S.-B.; Han, D.-M. Recent advances in bimetallic small-pore zeolite catalysts for ammonia-assisted selective catalytic reduction of NOx. Chem. Synth. 2025, 5, 33. [Google Scholar] [CrossRef]
- Yao, Y.; Cheng, H.; Zhong, G.; Tang, X.; Yi, H.; Zhao, S.; Gao, F.; Yu, Q. Progress in the construction strategy of noble metal active sites for zeolite-based PNA and VOCs catalysts. Green Energy Environ. 2025, 10, 709–732. [Google Scholar] [CrossRef]
- Yao, X.; Zhuang, H.; Guo, H.; Lu, X.; Xia, Q.; Zhou, D. Catalytic hydration/hydrolysis of bulky and hydrophobic organic compounds in aqueous phase over zeolite-based catalyst efficiently. Appl. Surf. Sci. 2025, 705, 163514. [Google Scholar] [CrossRef]
- Zhang, J.; Zakeri, T.; Yue, Q.; Kubů, M.; Barakov, R.; Přech, J.; Opanasenko, M.; Shamzhy, M. Lewis acid zeolite catalysts via chemical modification of extra-large pore germanosilicates. Catal. Today 2024, 440, 114825. [Google Scholar] [CrossRef]
- Dang, T.; Gong, M.; Li, B.; Zhang, G.; Yang, X.; Wang, X.; Han, D.; Chen, H. One-pot template-free synthesis of metal-doped ETS-10 zeolites for Knoevenagel condensation of benzaldehyde with ethyl cyanoacetate. Results Eng. 2024, 21, 101683. [Google Scholar] [CrossRef]
- Cui, Y.; Chen, B.; Xu, L.; Chen, M.; Wu, C.-E.; Qiu, J.; Cheng, G.; Wang, N.; Xu, J.; Hu, X. CO2 methanation over the Ni-based catalysts supported on the hollow ZSM-5 zeolites: Effects of the hollow structure and alkaline treatment. Fuel 2023, 334, 126783. [Google Scholar] [CrossRef]
- Spataru, D.; Canastreiro, D.; Da Costa, K.Ś.; Quindimil, A.; Lopes, J.M.; Da Costa, P.; Henriques, C.; Bacariza, C. Doping Ni/USY zeolite catalysts with transition metals for CO2 methanation. Int. J. Hydrogen Energy 2024, 53, 468–481. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Liu, Y.; Chen, J.; Wang, H.; Huang, J.; Wang, J. Hierarchical Porous Zeolite ZSM-5/Graphene Nanosheets as Robust Heterogeneous Acid Catalysts. Chem. Sel. 2017, 2, 5810–5815. [Google Scholar] [CrossRef]
- Wrasman, C.J.; Wilson, A.N.; Mante, O.D.; Iisa, K.; Dutta, A.; Talmadge, M.S.; Dayton, D.C.; Uppili, S.; Watson, M.J.; Xu, X.; et al. Catalytic pyrolysis as a platform technology for supporting the circular carbon economy. Nat. Catal. 2023, 6, 563–573. [Google Scholar] [CrossRef]
- Zhang, L.; Bao, Z.; Xia, S.; Lu, Q.; Walters, K.B. Catalytic Pyrolysis of Biomass and Polymer Wastes. Catalysts 2018, 8, 659. [Google Scholar] [CrossRef]
- Sun, W.; Yan, Y.; Wei, Y.; Ma, J.; Niu, Z.; Hu, G. Catalytic Pyrolysis of Biomass: A Review of Zeolite, Carbonaceous, and Metal Oxide Catalysts. Nanomaterials 2025, 15, 493. [Google Scholar] [CrossRef]
- Song, P.; Guo, S.; Zuo, M.; Wang, X.; Qiu, H.; Shen, B. A review on the catalytic pyrolysis of lipids to produce alternative aromatic hydrocarbons: Synthesis of hierarchically porous zeolites and aromatization mechanism. Circ. Econ. 2025, 4, 100128. [Google Scholar] [CrossRef]
- Cai, R.; Pei, X.; Pan, H.; Wan, K.; Chen, H.; Zhang, Z.; Zhang, Y. Biomass Catalytic Pyrolysis over Zeolite Catalysts with an Emphasis on Porosity and Acidity: A State-of-the-Art Review. Energy Fuels 2020, 34, 11771–11790. [Google Scholar] [CrossRef]
- Gamliel, D.P.; Cho, H.J.; Fan, W.; Valla, J.A. On the effectiveness of tailored mesoporous MFI zeolites for biomass catalytic fast pyrolysis. Appl. Cataly. A Gen. 2016, 522, 109–119. [Google Scholar] [CrossRef]
- Jia, L.Y.; Raad, M.; Hamieh, S.; Toufaily, J.; Hamieh, T.; Bettahar, M.; Mauviel, G.; Tarrighi, M.; Pinard, L.; Dufour, A. Catalytic fast pyrolysis of biomass: Superior selectivity of hierarchical zeolite to aromatics. Green Chem. 2017, 19, 5442–5459. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, H.; Chen, H.; Chen, K.; Lu, X.; Ouyang, P.; Fu, J. Enhancement in the aromatic yield from the catalytic fast pyrolysis of rice straw over hexadecyl trimethyl ammonium bromide modified hierarchical HZSM-5. Bioresour. Technol. 2018, 256, 241–246. [Google Scholar] [CrossRef]
- Bi, Y.; Lei, X.; Xu, G.; Chen, H.; Hu, J. Catalytic Fast Pyrolysis of Kraft Lignin over Hierarchical HZSM-5 and H Zeolites. Catalysts 2018, 8, 82. [Google Scholar] [CrossRef]
- Khan, W.; Jia, X.; Wu, Z.; Choi, J.; Yip, A.C.K. Incorporating Hierarchy into Conventional Zeolites for Catalytic Biomass Conversions: A Review. Catalysts 2019, 9, 127. [Google Scholar] [CrossRef]
- Soltanian, S.; Lee, C.L.; Lam, S.S. A review on the role of hierarchical zeolites in the production of transportation fuels through catalytic fast pyrolysis of biomass. Biofuel Res. J. 2020, 27, 1217–1234. [Google Scholar] [CrossRef]
- Wu, L.; Xin, J.; Xia, D.; Sun, J.; Liang, J. Enhanced production of hydrocarbons from the catalytic pyrolysis of maize straw over hierarchical ZSM-11 zeolites. Appl. Catal. B Environ. 2022, 317, 121775. [Google Scholar] [CrossRef]
- Pinard, L.; Jia, L.; Pichot, N.; Astafan, A.; Dufour, A. Catalytic Fast Pyrolysis of Biomass over Hierarchical Zeolites: Comparison of Mordenite, Beta, and ZSM-5. Energy Fuels 2024, 38, 14351–14364. [Google Scholar] [CrossRef]
- Wu, L.; Liu, J.; Chen, L.; Wang, X.; Zhou, Q.; Yu, F.; Liang, J. Hierarchical beta zeolites assisted aromatics production from lignin via catalytic fast pyrolysis. Chem. Eng. J. 2024, 484, 149618. [Google Scholar] [CrossRef]
- Butt, W.; Cabello, J.H.; Hedlund, J.; Shafaghat, H. Structure-Modified Zeolites for an Enhanced Production of Bio Jet Fuel Components via Catalytic Pyrolysis of Forestry Residues. Catal. Lett. 2025, 155, 116. [Google Scholar] [CrossRef]
- Liang, J.; Shan, G.; Sun, Y. Catalytic fast pyrolysis of lignocellulosic biomass: Critical role of zeolite catalysts. Renew. Sustain. Energy Rev. 2021, 139, 110707. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, C.; Xue, X.; Pan, Z.; Wang, D.; Zhang, R. Catalytic pyrolysis of lignin over hierarchical HZSM-5 zeolites prepared by post-treatment with alkaline solutions. J. Anal. Appl. Pyrolysis 2019, 137, 86–95. [Google Scholar] [CrossRef]
- Ávila, M.I.; Alonso-Doncel, M.M.; Cueto, J.; Briones, L.; Gómez-Pozuelo, G.; Escola, J.M.; Serrano, D.P.; Peral, A.; Botas, J.A. Production of high value-added phenolic compounds through lignin catalytic pyrolysis over ion-exchanged hierarchical ZSM-5 and Beta zeolites. Catal. Today 2025, 456, 115343. [Google Scholar] [CrossRef]
- Hertzog, J.; Carré, V.; Jia, L.; Mackay, C.L.; Pinard, L.; Dufour, A.; Mašek, O.; Aubriet, F. Catalytic Fast Pyrolysis of Biomass over Microporous and Hierarchical Zeolites: Characterization of Heavy Products. ACS Sustain. Chem. Eng. 2018, 6, 4717–4728. [Google Scholar] [CrossRef]
- Zhou, H.; Lin, W.; Chen, C.; Liu, C.; Wu, J.; Wang, J.; Fu, J. Anchoring Effect of Organosilanes on Hierarchical ZSM-5 Zeolite for Catalytic Fast Pyrolysis of Cellulose to Aromatics. ACS Omega 2022, 7, 15870–15879. [Google Scholar] [CrossRef]
- He, Q.; Akin, O.; Ureel, Y.; Yazdani, P.; Li, L.; Varghese, R.J.; Van Geem, K.M. Enhancing catalytic pyrolysis of polypropylene using mesopore-modified HZSM-5 catalysts: Insights and strategies for improved performance. Front. Chem. Eng. 2024, 6, 1439400. [Google Scholar] [CrossRef]
- Qie, Z.; Xiang, H.; Xiang, H.; Zou, R.; Alhelali, A.; Alhassawi, H.; Ding, S.; Jiao, Y.; Holmes, S.M.; Garforth, A.A.; et al. Catalytic pyrolysis of high-density polyethylene (HDPE) over hierarchical ZSM-5 zeolites produced by microwave-assisted chelation-alkaline treatment. Fuel 2024, 368, 131532. [Google Scholar] [CrossRef]
- Lin, X.; Kong, L.; Ren, X.; Zhang, D.; Cai, H.; Lei, H. Catalytic co-pyrolysis of torrefied poplar wood and high-density polyethylene over hierarchical HZSM-5 for mono-aromatics production. Renew. Energy 2021, 164, 87–95. [Google Scholar] [CrossRef]
- Hu, J.; Jiang, H.; Guo, Y.; Liu, J.; Xu, D.; Wu, G.; Yi, Y.; Zhao, Z.; Guo, H. Efficient synthesis of smaller crystal ZSM-5 zeolite in low TPAOH-to-silica ratio dry-gel hydrothermal system. Microporous Mesoporous Mater. 2025, 390, 113596. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, Z.; Zhang, B.; Gu, J.; Shi, K. Catalytic Fast Pyrolysis of Bamboo over Micro-mesoporous Composite Molecular Sieves. Energy Technol. 2018, 6, 728–736. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, Z.; Zhang, B.; Wang, W.; Seufitelli, G.V.S.; Resende, F.L.P. Catalytic fast co-pyrolysis of waste greenhouse plastic films and rice husk using hierarchical micro-mesoporous composite molecular sieve. Waste Manag. 2020, 102, 561–568. [Google Scholar] [CrossRef]
- Li, Z.; Zhong, Z.; Zhang, B.; Wang, W.; Zhao, H.; Seufitelli, G.V.S.; Resende, F.L.P. Microwave-assisted catalytic fast pyrolysis of rice husk over a hierarchical HZSM-5/MCM-41 catalyst prepared by organic base alkaline solutions. Sci. Total Environ. 2021, 750, 141215. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Li, Y.; Zhou, Y.; Deng, S.; Zhang, H. Efficient Pyrolysis of Low-Density Polyethylene for Regulatable Oil and Gas Products by ZSM-5, HY and MCM-41 Catalysts. Catalysts 2023, 13, 382. [Google Scholar] [CrossRef]
- Ratnasari, D.K.; Bijl, A.; Yang, W.; Jönsson, P.G. Effect of H-ZSM-5 and Al-MCM-41 Proportions in Catalyst Mixtures on the Composition of Bio-Oil in Ex-Situ Catalytic Pyrolysis of Lignocellulose Biomass. Catalysts 2020, 10, 868. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, C.; Zhuo, J.; Yao, Q. Investigation of a Ni-Modified MCM-41 Catalyst for the Reduction of Oxygenates and Carbon Deposits during the Co-Pyrolysis of Cellulose and Polypropylene. ACS Omega 2020, 5, 20299–20310. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Wang, B.; Bolatibieke, D.; Nan, D.-H.; Zhang, Z.-X.; Cui, M.-S.; Lu, Q. Catalytic fast pyrolysis of biomass with Ni-P-MCM-41 to selectively produce levoglucosenone. J. Anal. Appl. Pyrol. 2020, 148, 104824. [Google Scholar] [CrossRef]
- De Sousa Castro, K.; de Medeiros Costa, L.F.; Fernandes, V.J.; de Oliveira Lima, R.; de Morais Araújo, A.M.; de Sant’Anna, M.C.S.; dos Santos, N.A.; Gondim, A.D. Catalytic pyrolysis (Ni/Al-MCM-41) of palm (Elaeis guineensis) oil to obtain renewable hydrocarbons. RSC Adv. 2021, 11, 555–564. [Google Scholar] [CrossRef]
- Genel, S.; Durak, H.; Genel, Y. Catalytic effect of metal powder and MCM-41/metal catalysts on the pyrolysis of cellulose. Environ. Prog. Sustain. Energy 2024, 43, e14225. [Google Scholar] [CrossRef]
- Yu, L.; Farinmade, A.; Ajumobi, O.; Su, Y.; John, V.T.; Valla, J.A. MCM-41/ZSM-5 composite particles for the catalytic fast pyrolysis of biomass. Appl. Catal. A Gen. 2020, 602, 117727. [Google Scholar] [CrossRef]
- Lu, Q.; Gong, Y.; Lu, J.; Liu, J.; Wu, Y. Hydrothermal Catalytic Upgrading of Model Compounds of Algae Based Bio-Oil to Monocyclic Aromatic Hydrocarbons over Hierarchical HZSM-5. Ind. Eng. Chem. Res. 2020, 59, 20551–20560. [Google Scholar] [CrossRef]
- Puértolas, B.; Veses, A.; Callén, M.S.; Mitchell, S.; García, T.; Pérez-Ramrez, J. Porosity–Acidity Interplay in HierarchicalZSM-5 Zeolites for Pyrolysis Oil Valorization to Aromatics. ChemSusChem 2015, 8, 3283–3293. [Google Scholar] [CrossRef]
- Palizdar, A.; Sadrameli, S.M. Catalytic upgrading of biomass pyrolysis oil over tailored hierarchical MFI zeolite: Effect of porosity enhancement and porosity-acidity interaction on deoxygenation reactions. Renew. Energy 2020, 148, 674–688. [Google Scholar] [CrossRef]
- Shao, S.S.; Zhang, H.Y.; Shen, D.K.; Xiao, R. Enhancement of hydrocarbon production and catalyst stability during catalytic conversion of biomass pyrolysis-derived compounds over hierarchical HZSM-5. RSC Adv. 2016, 6, 44313–44320. [Google Scholar] [CrossRef]
- Che, Q.; Yi, W.; Liu, Y.; Wang, X.; Yang, H.; Chen, H. Effect of Mesopores in ZSM-5 on the Catalytic Conversion of Acetic Acid, Furfural, and Guaiacol. Fuels 2021, 35, 6022–6029. [Google Scholar] [CrossRef]
- Venegas-Vásconez, D.; Orejuela-Escobar, L.; Valarezo-Garcés, A.; Guerrero, V.H.; Tipanluisa-Sarchi, L.; Alejandro-Martín, S. Biomass Valorization through Catalytic Pyrolysis Using Metal-Impregnated Natural Zeolites: From Waste to Resources. Polymers 2024, 16, 1912. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Shi, C.; Jiang, L.; Li, P.; Bai, J.; Chang, C. Influence of metal (Fe/Zn) modified ZSM-5 catalysts on product characteristics based on the bench-scale pyrolysis and Py-GC/MS of biomass. Int. J. Energy Res. 2020, 44, 5455–5467. [Google Scholar] [CrossRef]
- Persson, H.; Duman, I.; Wang, S.; Pettersson, L.J.; Yang, W. Catalytic pyrolysis over transition metal-modified zeolites: A comparative study between catalyst activity and deactivation. J. Anal. Appl. Pyrol. 2019, 138, 54–61. [Google Scholar] [CrossRef]
- Hernandez-Gimenez, A.M.; Heracleous, E.; Pachatouridou, E.; Horvat, A.; Hernando, H.; Serrano, D.P.; Lappas, A.A.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Effect of Mesoporosity, Acidity and Crystal Size of Zeolite ZSM-5on Catalytic Performance during the Ex-situ Catalytic Fast Pyrolysis of Biomass. ChemCatChem 2021, 13, 1207–1219. [Google Scholar] [CrossRef]
- Wong, S.L.; Armenise, S.; Nyakuma, B.B.; Bogush, A.; Towers, S.; Lee, C.H.; Wong, K.Y.; Lee, T.H.; Rebrov, E.; Muñoz, M. Plastic Pyrolysis over HZSM-5 Zeolite and Fluid Catalytic Cracking Catalyst under Ultra-Fast Heating. J. Anal. Appl. Pyrol. 2022, 169, 105793. [Google Scholar] [CrossRef]
- Afraz, M.; Nisar, J.; Shah, A.; Ali, G.; Muhammad, F.; Anwar, F.; Ghani, W.A.W.A.K. Thermo-catalytic decomposition of cotton seed press cake over nickel doped zeolite Y, hydrogen: Enhanced yield of bio-oil with highly selective fuel-range hydrocarbons. RSC Adv. 2024, 14, 31549–31559. [Google Scholar] [CrossRef] [PubMed]
- Paucar-Sánchez, M.F.; Calero, M.; Blázquez, G.; Solís, R.R.; Munoz-Batista, M.J.; Martín-Lara, M.Á. Impact of Metal Impregnation of Commercial Zeolites in the Catalytic Pyrolysis of Real Mixture of Post-Consumer Plastic Waste. Catalysts 2024, 14, 168. [Google Scholar] [CrossRef]
- Ulloa-Murillo, L.M.; Ortíz i Roca, V.; Gholizadeh, M.; Fabrega, S.M.; Clarens, F.; Tlustoš, P. Pyrolysis of municipal waste catalyzed with synthesized zeolite from steel making factory waste. Sci. Rep. 2025, 15, 10556. [Google Scholar] [CrossRef]
- Bronstein, L.M.; Matveeva, V.G. Multifunctional Catalysts for Cascade Reactions in Biomass Processing. Nanomaterials 2024, 14, 1937. [Google Scholar] [CrossRef]
- Hernando, H.; Moreno, I.; Fermoso, J.; Ochoa-Hernández, C.; Pizarro, P.; Coronado, J.M.; Čejka, J.; Serrano, D.P. Biomass catalytic fast pyrolysis over hierarchical ZSM-5 and Beta zeolites modified with Mg and Zn oxides. Biomass Convers. Biorefin. 2017, 7, 289–304. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Shao, S.; Dong, L.; Zhang, J.; Hu, C.; Cai, Y. Catalytic upgrading of pyrolysis vapor from rape straw in a vacuum pyrolysis system over La/HZSM-5 with hierarchical structure. Bioresour. Technol. 2018, 259, 191–197. [Google Scholar] [CrossRef]
- Dai, G.; Wang, S.; Zou, Q.; Huang, S. Improvement of aromatics production from catalytic pyrolysis of cellulose over metal-modified hierarchical HZSM-5. Fuel Process. Technol. 2018, 179, 319–323. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, H.; Zhou, F.; Chen, K.; Qiao, K.; Lu, X.; Ouyang, P.; Fu, J. Catalytic fast pyrolysis of rice straw to aromatic compounds over hierarchical HZSM-5 produced by alkali treatment and metal-modification. J. Anal. Appl. Pyrol. 2018, 131, 76–84. [Google Scholar] [CrossRef]
- Dai, L.; Wang, Y.; Liu, Y.; Ruan, R.; Duan, D.; Zhao, Y.; Yu, Z.; Jiang, L. Catalytic fast pyrolysis of torrefied corn cob to aromatic hydrocarbons over Ni-modified hierarchical ZSM-5 catalyst. Bioresour. Technol. 2019, 272, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Nishu; Yellezuome, D.; Chai, M.; Li, C.; Liu, R. Catalytic pyrolysis of biomass over Fe-modified hierarchical ZSM-5: Insights into mono-aromatics selectivity and pyrolysis behavior using Py-GC/MS and TG-FTIR. J. Energy Inst. 2021, 99, 218–228. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Yuan, H.; Chen, Y. Enhancement of aromatics production via cellulose fast pyrolysis over Ru modified hierarchical zeolites. Renew. Energy 2022, 184, 280–290. [Google Scholar] [CrossRef]
- Veses, A.; Puértolas, B.; López, J.M.; Callén, M.S.; Solsona, B.; García, T. Promoting Deoxygenation of Bio-Oil by Metal-Loaded Hierarchical ZSM-5 Zeolites. ACS Sustain. Chem. Eng. 2016, 4, 1653–1660. [Google Scholar] [CrossRef]
- Olsbye, U.; Svelle, S.; Bjørgen, M.; Beato, P.; Janssens, T.V.W.; Joensen, F.; Bordiga, S.; Lillerud, K.P. Conversion of Methanol to Hydrocarbons: How Zeolite Cavity and Pore Size Controls Product Selectivity. Angew. Chem. Int. Ed. 2012, 51, 5810–5831. [Google Scholar] [CrossRef]
- Tian, P.; Wei, Y.; Ye, M.; Liu, Z. Methanol to Olefins (MTO): From Fundamentals to Commercialization. ACS Catal. 2015, 5, 1922–1938. [Google Scholar] [CrossRef]
- Yarulina, I.; Chowdhury, A.D.; Meirer, F.; Weckhuysen, B.M.; Gascon, J. Recent trends and fundamental insights in the methanol-to-hydrocarbons process. Nat. Catal. 2018, 1, 398–411. [Google Scholar] [CrossRef]
- Lee, S.; Choi, M. Unveiling coke formation mechanism in MFI zeolites during methanol-to-hydrocarbons conversion. J. Catal. 2019, 375, 183–192. [Google Scholar] [CrossRef]
- Standl, S.; Hinrichsen, O. Kinetic Modeling of Catalytic Olefin Cracking and Methanol-to-Olefins (MTO) over Zeolites: A Review. Catalysts 2018, 8, 626. [Google Scholar] [CrossRef]
- Wu, X.; Wei, Y.; Liu, Z. Dynamic Catalytic Mechanism of the Methanol-to-Hydrocarbons Reaction over Zeolites. Acc. Chem. Res. 2023, 56, 2001–2014. [Google Scholar] [CrossRef]
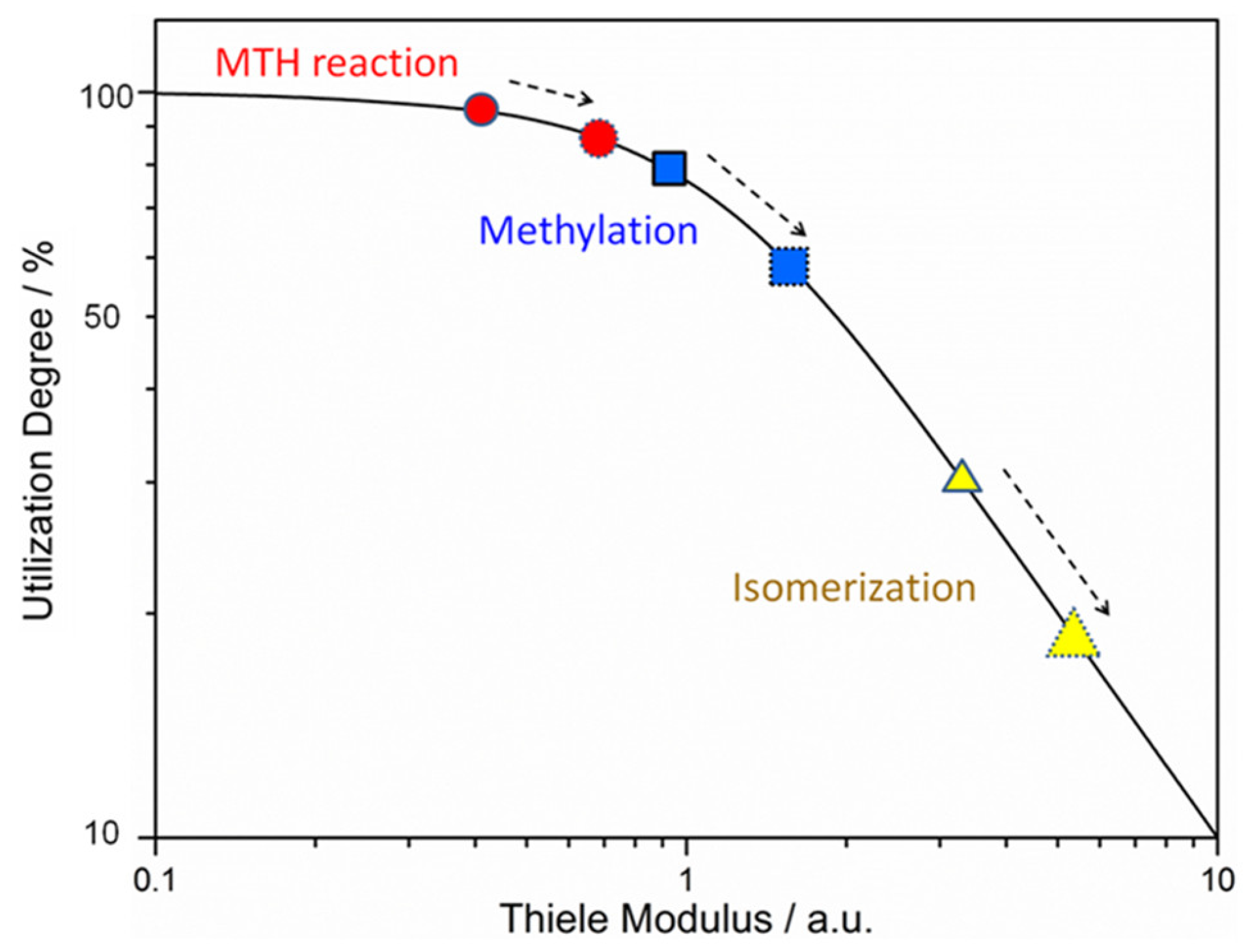
- Zhou, J.; Wang, Y.; Zou, W.; Wang, C.; Li, L.; Liu, Z.; Zheng, A.; Kong, D.; Yang, W.; Xie, Z. Mass Transfer Advantage of Hierarchical Zeolites Promotes Methanol Converting into para-Methyl Group in Toluene Methylation. Ind. Eng. Chem. Res. 2017, 56, 9310–9321. [Google Scholar] [CrossRef]
- Adawi, H.I.; Saraze, M.L. Kinetic implications of (hierarchical) zeolite fouling during liquid-phase aromatics upgrading. J. Catal. 2024, 433, 115456. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Meng, F.; Gao, F.; Sun, C.; Sun, L.; Wang, S.; Wang, L.; Wang, Y. CTAB resulted direct synthesis and properties of hierarchical ZSM-11/5 composite zeolite in the absence of template. Microporous Mesoporous Mater. 2017, 243, 271–280. [Google Scholar] [CrossRef]
- Bingre, R.; Li, R.; Wang, Q.; Nguyen, P.; Onfroy, T.; Louis, B. Porosity Design of Shaped Zeolites for Improved Catalyst Lifetime in the Methanol-to-Hydrocarbons Reaction. Catalysts 2019, 9, 545. [Google Scholar] [CrossRef]
- Lu, P.; Ghosh, S.; de Mello, M.D.; Kamaluddin, H.S.; Li, X.; Kumar, G.; Duan, X.; Abeykoon, M.; Boscoboinik, J.A.; Qi, L.; et al. Few-Unit-Cell MFI Zeolite Synthesized using a Simple Di-quaternary Ammonium Structure-Directing Agent. Angew. Chem. Int. Ed. 2021, 60, 19214–19221. [Google Scholar] [CrossRef] [PubMed]
- Anekwe, I.M.S.; Oboirien, B.; Isa, Y.M. Performance evaluation of a newly developed transition metal-doped HZSM-5 zeolite catalyst for single-step conversion of C1–C3 alcohols to fuel-range hydrocarbons. Energy Adv. 2024, 3, 1314–1328. [Google Scholar] [CrossRef]
- Anekwe, I.M.S.; Oboirien, B.; Isa, Y.M. Effects of transition metal doping on the properties and catalytic performance of ZSM-5 zeolite catalyst on ethanol-to-hydrocarbons conversion. Fuel Commun. 2024, 18, 100101. [Google Scholar] [CrossRef]
- Anekwe, I.M.S.; Maharaj, L.; Chetty, M.; Oboirien, B.; Khotseng, L.; Isa, Y.M. Stability, deactivation and regeneration study of a newly developed HZSM-5 and Ni-doped HZSM-5 zeolite catalysts for ethanol-to-hydrocarbon conversion. Catal. Commun. 2024, 186, 106802. [Google Scholar] [CrossRef]
- Anekwe, I.M.S.; Isa, Y.M. Influence of metal doping on the coke formation of a novel hierarchical HZSM-5 zeolite catalyst in the conversion of 1-propanol to fuel blendstock. RSC Adv. 2025, 15, 3988–3999. [Google Scholar] [CrossRef]
- Shannon, M.D.; Cox, P.A.; Mayoral, A.; Hsieh, M.-F.; Turrina, A. The Real Structure of Intergrown ZSM-5/ZSM-11 Industrial Zeolite Catalysts. Small Struct. 2025, 2500184. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, H.; Li, X.; Xie, S.; Wang, Y.; Xin, W.; Liu, S.; Xu, L. Differences between ZSM-5 and ZSM-11 zeolite catalysts in 1-hexene aromatization and isomerization. Fuel Process. Technol. 2010, 91, 449–455. [Google Scholar] [CrossRef]
- Wang, S.; Wang, P.; Qin, Z.; Chen, Y.; Dong, M.; Li, J.; Zhang, K.; Liu, P.; Wang, J.; Fan, W. Relation of Catalytic Performance to the Aluminum Siting of Acidic Zeolites in the Conversion of Methanol to Olefins, Viewed via a Comparison between ZSM-5 and ZSM-11. ACS Catal. 2018, 8, 5485–5505. [Google Scholar] [CrossRef]
- Li, T.; Shoinkhorova, T.; Gascon, J.; Ruiz-Martínez, J. Aromatics Production via Methanol-Mediated Transformation Routes. ACS Catal. 2021, 11, 7780–7819. [Google Scholar] [CrossRef]
- Ma, Z.; Shi, D.; Wang, S.; Dong, M.; Fan, W. Control of aluminum distribution in ZSM-5 zeolite for enhancement of its catalytic performance for propane aromatization. Front. Chem. Sci. Eng. 2024, 18, 86. [Google Scholar] [CrossRef]
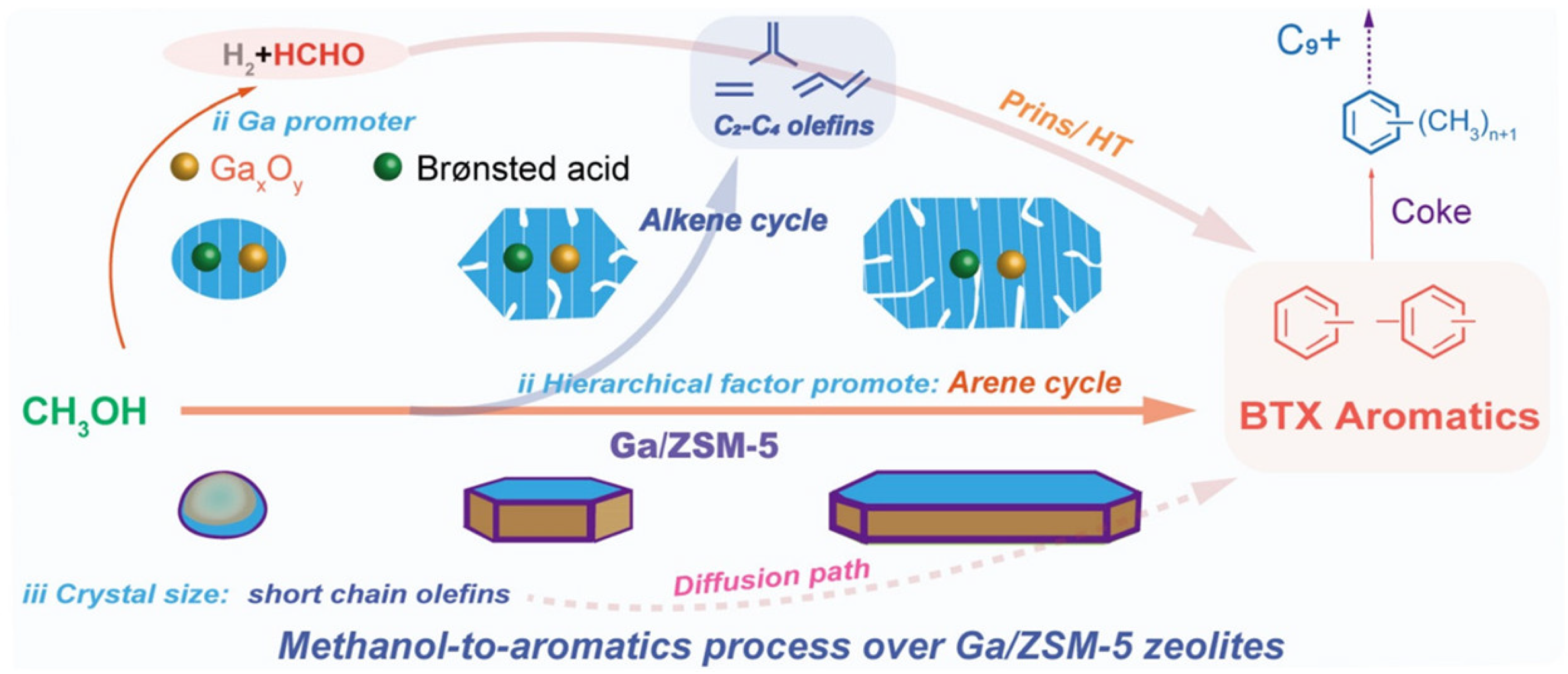
- Liu, K.; Shoinkhorova, T.; You, X.; Gong, X.; Zhang, X.; Chung, S.-H.; Ruiz-Martínez, J.; Gascon, J.; Chowdhury, A.D. The synergistic interplay of hierarchy, crystal size, and Ga-promotion in the methanol-to-aromatics process over ZSM-5 zeolites. Dalton Trans. 2024, 53, 11344–11353. [Google Scholar] [CrossRef]
- Ni, Y.; Zhu, W.; Liu, Z. Formaldehyde intermediate participating in the conversion of methanol to aromatics over zinc modified H-ZSM-5. J. Energy Chem. 2021, 54, 174–178. [Google Scholar] [CrossRef]
- Shen, K.; Qian, W.; Wang, N.; Su, C.; Wei, F. Centrifugation-free and high yield synthesis of nanosized H-ZSM-5 and its structure-guided aromatization of methanol to 1,2,4 trimethylbenzene. J. Mater. Chem. A 2014, 2, 19797–19808. [Google Scholar] [CrossRef]
- Shen, K.; Wang, N.; Qian, W.; Cui, Y.; Wei, F. Atmospheric pressure synthesis of nanosized ZSM-5 with enhanced catalytic performance for methanol to aromatics reaction. Catal. Sci. Technol. 2014, 4, 3840–3844. [Google Scholar] [CrossRef]
- Wang, N.; Qian, W.; Wei, F. Fabrication and catalytic properties of three dimensional ordered zeolite arrays with interconnected micro-meso-macroporous structure. J. Mater. Chem. A 2016, 4, 10834–10841. [Google Scholar] [CrossRef]
- Shen, X.; Kang, J.; Niu, W.; Wang, M.; Zhang, Q.; Wang, Y. Impact of hierarchical pore structure on the catalytic performances of MFI zeolites modified by ZnO for the conversion of methanol to aromatics. Catal. Sci. Technol. 2017, 7, 3598–3612. [Google Scholar] [CrossRef]
- Wang, F.; Liu, B.; Li, Q.; Wu, F.; Xiao, G. Catalytic upgradation of crude glycerol to produce bio-based aromatics over hierarchical MFI zeolite: Effect of bimodal hierarchical porosity enhancement and porosity-acidity interaction. Mol. Catal. 2023, 535, 112858. [Google Scholar] [CrossRef]
- Martino, M.; Meloni, E.; Festa, G.; Palma, V. Propylene Synthesis: Recent Advances in the Use of Pt-Based Catalysts for Propane Dehydrogenation Reaction. Catalysts 2021, 11, 1070. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, K.; Liu, T.; Bo, F.; Song, W.; Gao, X.; Zhang, J. A review on understanding the structure-performance relationships of metal-zeolite catalysts for direct dehydrogenation of propane to propylene. Fuel 2025, 380, 133181. [Google Scholar] [CrossRef]
- Huang, Z.; Cao, X.; Wang, H.; Chen, D.; Chen, X.; He, D.; Huang, S.; Luo, Y. Ni-doped Co/SPP zeolite catalyst with optimized metal oxide-support interaction for propane dehydrogenation to propylene. Chem. Eng. J. 2025, 511, 162074. [Google Scholar] [CrossRef]
- Liu, Y.; Kamata, H.; Ohara, H.; Izumi, Y.; Ong, D.S.W.; Chang, J.; Poh, C.K.; Chen, L.; Borgna, A. Low-Olefin Production Process Based on Fischer–Tropsch Synthesis: Process Synthesis, Optimization, and Techno-Economic Analysis. Ind. Eng. Chem. Res. 2020, 59, 8728–8739. [Google Scholar] [CrossRef]
- Yahyazadeh, A.; Dalai, A.K.; Ma, W.; Zhang, L. Fischer–Tropsch Synthesis for Light Olefins from Syngas: A Review of Catalyst Development. Reactions 2021, 2, 227–257. [Google Scholar] [CrossRef]
- Wang, C.; Fang, W.; Liu, Z.; Wang, L.; Liao, Z.; Yang, Y.; Li, H.; Liu, L.; Zhou, H.; Qin, X.; et al. Fischer-Tropsch synthesis to olefins boosted by MFI zeolite nanosheets. Nat. Nanotechnol. 2022, 17, 714–720. [Google Scholar] [CrossRef]
- Lin, S.; Li, H.; Tian, P.; Wei, Y.; Ye, M.; Liu, Z. Methanol to Olefins (MTO): Understanding and Regulating Dynamic Complex Catalysis. JACS 2025, 147, 11585–11607. [Google Scholar] [CrossRef]
- Zhou, J.; Gao, M.; Zhang, J.; Liu, W.; Zhang, T.; Li, H.; Xu, Z.; Ye, M.; Liu, Z. Directed transforming of coke to active intermediates in methanol-to-olefins catalyst to boost light olefins selectivity. Nat. Commun. 2021, 12, 17. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, W.; Ma, X.; Liu, Y.; Zhang, L.; Zheng, J.; Wang, Y.; Li, W.; Fan, B.; Li, R. A highly efficient SAPO-34 catalyst for improving light olefins in methanol conversion: Insight into the role of hierarchical porosities and tailoring acid properties based on in situ NH3-poisoning. Fuel 2023, 331, 125935. [Google Scholar] [CrossRef]
- Wu, J.; Dai, M.; Yang, B.; Li, P.; Wang, C.; Wu, G.; Jiang, X.; Yu, S.; Li, W.; Li, X.; et al. The effect of hierarchical pore structure SAPO-34 catalyst on the diffusion and reaction behavior in MTO reaction. Chem. Eng. J. 2024, 482, 148947. [Google Scholar] [CrossRef]
- Shen, X.; Ding, J.; Ye, Y.; Liu, H.; Xie, Z. Ingrown Leading to Hierarchical SAPO-34 with High Catalytic Activity. Chem.-Methods 2025, 5, e202400027. [Google Scholar] [CrossRef]
- Zhu, X.; Kosinov, N.; Kubarev, A.V.; Bolshakov, A.; Mezari, B.; Valastyan, I.; Hofmann, J.P.; Roeffaers, M.B.J.; Sarkadi-Pribóczki, E.; Hensen, E.J.M. Probing the Influence of SSZ-13 Zeolite Pore Hierarchy in Methanol-to-Olefins Catalysis by Using Nanometer Accuracy by Stochastic Chemical Reactions Fluorescence Microscopy and Positron Emission Profiling. ChemCatChem 2017, 9, 3470–3477. [Google Scholar] [CrossRef]
- Kedia, A.O.; Anand, N.; Shah, V.; Kumar, D. Production of light olefins from methanol using catalytic fixed bed reactor with different catalytic bed composition. S. Afr. J. Chem. Eng. 2024, 47, 262–269. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Fan, C.; Sun, C.; Wang, X.; Wang, C.; Zhang, X.; Wang, S. Facile synthesis of a superior MTP catalyst: Hierarchical micro-mesomacroporous ZSM-5 zeolites. Appl. Catal. A Gen. 2018, 551, 31–48. [Google Scholar] [CrossRef]
- Tarach, K.A.; Góra-Marek, K.; Martinez-Triguero, J.; Melián-Cabrera, I. Acidity and accessibility studies of desilicated ZSM-5 zeolites in terms of their effectiveness as catalysts in acid-catalyzed cracking processes. Catal. Sci. Technol. 2017, 7, 858–873. [Google Scholar] [CrossRef]
- Tarach, K.A.; Pyra, K.; Góra-Marek, K. Opening up ZSM-5 Hierarchical Zeolite’s Porosity through Sequential Treatments for Improved Low-Density Polyethylene Cracking. Molecules 2020, 25, 2878. [Google Scholar] [CrossRef]
- Costa, C.S.; Fernandes, A.; Munoz, M.; Ribeiro, M.R.; Silva, J.M. Analyzing HDPE Thermal and Catalytic Degradation in Hydrogen Atmosphere: A Model-Free Approach to the Activation Energy. Catalysts 2024, 14, 514. [Google Scholar] [CrossRef]
- Armenise, S.; Costa, C.S.; Luing, W.S.; Ribeiro, M.R.; Silva, J.M.; Onfroy, T.; Valentin, L.; Casale, S.; Muñoz, M.; Launay, F. Evaluation of two approaches for the synthesis of hierarchical micro-/mesoporous catalysts for HDPE hydrocracking. Microporous Mesoporous Mater. 2023, 356, 112605. [Google Scholar] [CrossRef]
- Azam, M.U.; Fernandes, A.; Ferreira, M.J.; Afzal, W.; Graça, I. Pore-Structure Engineering of Hierarchical β Zeolites for the Enhanced Hydrocracking of Waste Plastics to Liquid Fuels. ACS Catal. 2024, 14, 16148–16165. [Google Scholar] [CrossRef]
- Azam, M.U.; Fernandes, A.; Ferreira, M.J.; McCue, A.J.; Graça, I.; Afzal, W. Unlocking the structure-activity relationship of hierarchical MFI zeolites towards the hydrocracking of HDPE. Fuel 2025, 379, 132990. [Google Scholar] [CrossRef]
- Zhao, P.; Guo, W.; Gui, Z.; Jiang, J.; Zhu, Z.; Li, J.-J.; Zhao, L.; Zhou, J.; Xi, Z. Selective Hydrocracking of Waste Polyolefins toward Gasoline-Range Liquid Fuels via Tandem Catalysis over a Cerium-Promoted Pt/HY Catalyst. ACS Sustain. Chem. Eng. 2024, 12, 5738–5752. [Google Scholar] [CrossRef]
- Han, X.; Tang, Z.; Zhou, X.; Ji, T.; Zeng, F.; Chen, R. Boosting the catalytic performance of metal–zeolite catalysts in the hydrocracking of polyolefin wastes by optimizing the nanoscale proximity. EES Catal. 2024, 2, 300–310. [Google Scholar] [CrossRef]
- Li, W.; Ma, T.; Zhang, Y.; Gong, Y.; Wu, Z.; Dou, T. Facile control of inter-crystalline porosity in the synthesis of size-controlled mesoporous MFI zeolites via in-situ converting silica gel into zeolite nanocrystals for catalytic cracking. CrystEngComm 2015, 17, 5680–5689. [Google Scholar] [CrossRef]
- Ding, J.; Xue, T.; Wu, H.; He, M. One-step post-synthesis treatment for preparing hydrothermally stable hierarchically porous ZSM-5. Chin. J. Catal. 2017, 37, 48–57. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, B.; Liang, H.; Hou, X.; Wang, L.; Zhang, X.; Liu, G. Synthesis and performance of pillared HZSM-5 nanosheet zeolites for n-decane catalytic cracking to produce light olefins. App. Catal. A Gen. 2019, 572, 24–33. [Google Scholar] [CrossRef]
- Ye, L.; Yang, W.; Fan, J.; Pu, X.; Han, X.; Qin, X.; Ma, M.; Huang, Z.; Wang, T.; Zhu, K.; et al. The influence of pore-architecture over n-heptane cracking using MFI, *BEA and MSE zeolite catalysts: Multipore versus hierarchical porosity. Chem. Eng. J. 2024, 482, 148705. [Google Scholar] [CrossRef]
- Na, J.; Liu, G.; Zhou, T.; Ding, G.; Hu, S.; Wang, L. Synthesis and Catalytic Performance of ZSM-5/MCM-41 Zeolites With Varying Mesopore Size by Surfactant-Directed Recrystallization. Catal. Lett. 2013, 143, 267–275. [Google Scholar] [CrossRef]
- Parsapur, R.K.; Hengne, A.M.; Melinte, G.; Koseoglu, O.R.; Hodgkins, R.P.; Bendjeriou-Sedjerari, A.; Lai, Z.; Huang, K.-W. Post-Synthetic Ensembling Design of Hierarchically Ordered FAU type Zeolite Frameworks for Vacuum Gas Oil Hydrocracking. Angew. Chem. Int. Ed. 2024, 63, e202314217. [Google Scholar] [CrossRef]
- Souza, J.C.; Mello, M.I.S.; Barbosa, F.F.; Souza, I.M.S.; Sachse, A.; Pergher, S.B.C. Influence of Secondary Porosity Introduction via Top-Down Methods on MOR, ZSM-5, and Y Zeolites on Their Cumene Cracking Performance. Catalysts 2025, 15, 146. [Google Scholar] [CrossRef]
- Wang, L.; Yao, Q.; Liu, Y.; Ma, D.; He, L.; Hao, Q.; Chen, H.; Sun, M.; Ma, X. Modeling the catalytic conversion of anthracene oil fraction to light aromatics over mesoporous HZSM-5. Fuel 2022, 319, 123825. [Google Scholar] [CrossRef]
- Ali, S.A.; Almulla, F.M.; Jermy, B.R.; Aitani, A.M.; Abudawoud, R.H.; AlAmer, M.; Qureshi, Z.S.; Mohammad, T.; Alasiri, H.S. Hierarchical composite catalysts of MCM-41 on zeolite Beta for conversion of heavy reformate to xylenes. J. Ind. Eng. Chem. 2021, 98, 189–199. [Google Scholar] [CrossRef]
- Hu, Z.; Wu, T.; Xie, H.; Zhang, Y.; Ge, S.; Wu, Z. Synthesis and consequence of three-dimensionally ordered macroporous Beta zeolite supported NiW catalyst for efficient hydrocracking of 1-methylnaphthalene to BTX. Chem. Eng. J. 2024, 499, 155761. [Google Scholar] [CrossRef]
- Sontisawate, T.; Nasu, H.; Hashimoto, T.; Ishihara, A. Catalytic Cracking of Soybean Oil Using Zeolite-containing Microporous and Mesoporous Mixed Catalysts with Curie Point Pyrolyzer. J. Jpn. Pet. Inst. 2016, 59, 184–196. [Google Scholar] [CrossRef][Green Version]
- Yang, X.; Liu, J.; Fan, K.; Rong, L. Hydrocracking of Jatropha Oil over non-sulfided PTA-NiMo/ZSM-5 Catalyst. Sci. Rep. 2017, 7, 41654. [Google Scholar] [CrossRef]
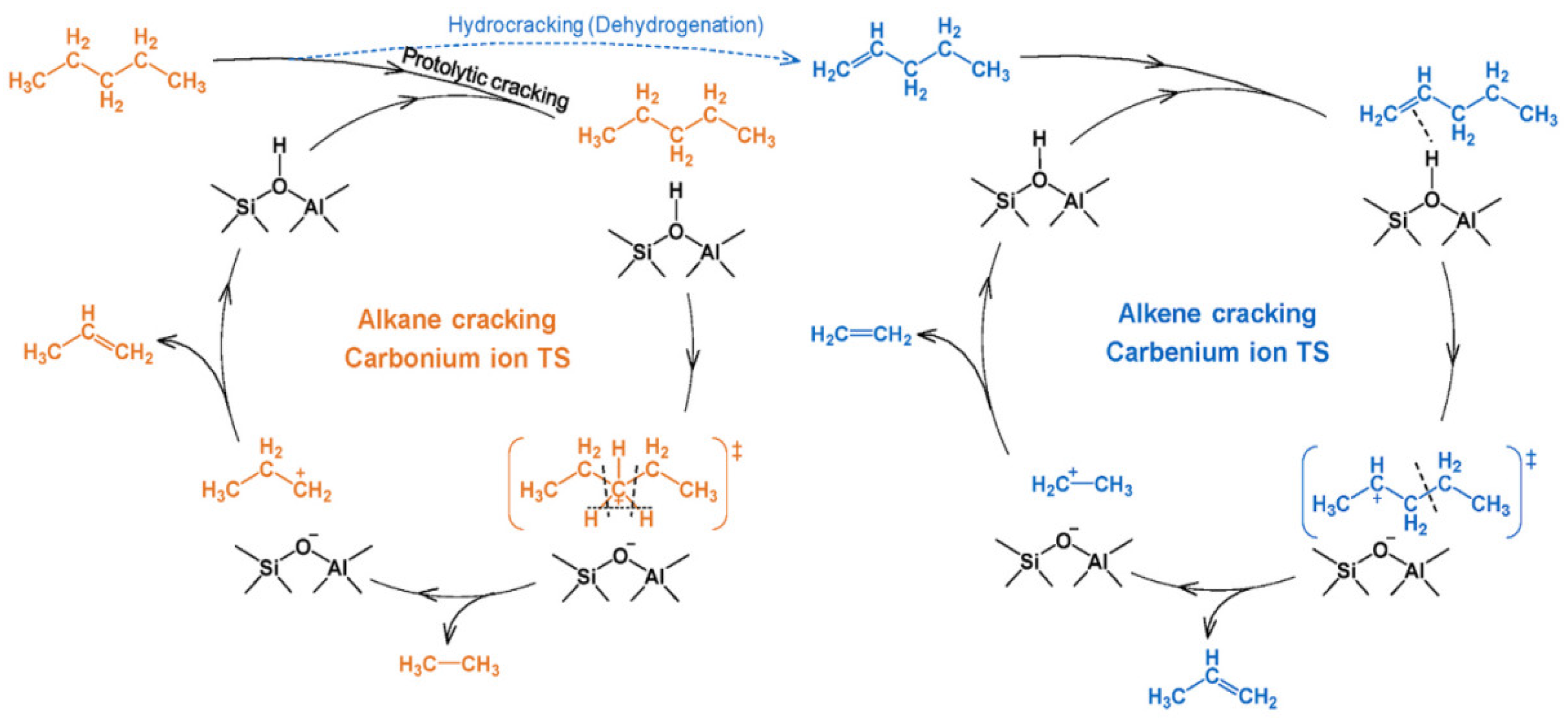
- Zhao, R.; Haller, G.L.; Lercher, J.A. Alkene adsorption and cracking on acidic zeolites—A gradual process of understanding. Microporous Mesoporous Mater. 2023, 358, 112390. [Google Scholar] [CrossRef]
- Wei, L.; Wang, H.; Dong, Q.; Li, Y.; Xiang, H. A Review on the Research Progress of Zeolite Catalysts for Heavy Oil Cracking. Catalysts 2025, 15, 401. [Google Scholar] [CrossRef]
- Ma, P.; Zhou, H.; Li, Y.; Wang, M.; Nastase, S.A.F.; Zhu, M.; Cui, J.; Cavallo, L.; Cheng, K.; Chowdhury, A.D. Selectivity descriptors of the catalytic n-hexane cracking process over 10-membered ring zeolites. Chem. Sci. 2024, 15, 11937–11945. [Google Scholar] [CrossRef] [PubMed]
- Peng, P.; Gao, X.-H.; Yan, Z.-F.; Mintova, S. Diffusion and catalyst efficiency in hierarchical zeolite catalysts. Natl. Sci. Rev. 2020, 7, 1726–1742. [Google Scholar] [CrossRef]
- He, M.; Qie, G.; Ali, M.F.; Rizwan, M.; Song, Y.; Zhou, X. Study of Coke Formation Mechanism on HZSM-5 Zeolite During Co-cracking of n-Hexane and Alcohols. Catal. Lett. 2024, 154, 3628–3644. [Google Scholar] [CrossRef]
- Lee, K.; Lee, S.; Jun, Y.; Choi, M. Cooperative effects of zeolite mesoporosity and defect sites on the amount and location of coke formation and its consequence in deactivation. J. Catal. 2017, 347, 222–230. [Google Scholar] [CrossRef]
- Sree, S.P.; Van der Donck, T.; Vanbutsele, G.; Breynaert, E.; Radhakrishnan, S.; Seo, J.W.; Martens, J.A. Hierarchical ISI-1 zeolite catalyst for hydroconversion of long-chain paraffins. Catal. Sci. Technol. 2021, 11, 1519–1525. [Google Scholar] [CrossRef]
- Qie, G.; Zhai, M.; Zhu, K.; Zhu, X. Generating Disk-Shaped MTWZeolite with Reduced Channel Length Using Polycation Structure-Directing Agent for Hydroisomerization of N-Dodecane. Catalysts 2024, 14, 925. [Google Scholar] [CrossRef]
- Wu, Q.; Jia, G.; Zhang, Y.; Liu, Z.; Li, X.; Wang, W.; Wu, W. Bifunctional catalysts based on hierarchical SAPO-41 nanosheet for Highly-efficient hydroisomerization of n-Hexadecane. Fuel 2023, 352, 129066. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, C.; Wang, W.; Xu, C.; Wu, W. Effect of diffusion and metal-acid synergy on catalytic behavior of the Pd/Hierarchical SAPO-31 nanoparticles for hydroisomerization of n-hexadecane. Fuel Process. Technol. 2024, 256, 108076. [Google Scholar] [CrossRef]
- Al-Taweel, S.K.; Aljendeel, H.A.; Al-Tabbakh, B.A. Synthesis of sulfated zirconia-HY zeolite catalysts doped by platinum metal for hydroisomerization reaction. J. Ecol. Eng. 2025, 26, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Al-Rawi, U.A.; Sher, F.; Hazafa, A.; Bilal, M.; Lima, E.C.; Al-Shara, N.K.; Jubeen, F.; Shanshool, J. Synthesis of Zeolite supported bimetallic catalyst and application in n-hexane hydro-isomerization using supercritical CO2. J. Environ. Chem. Eng. 2021, 9, 105206. [Google Scholar] [CrossRef]
- Glotov, A.; Vutolkina, A.; Artemova, M.; Demikhova, N.; Smirnova, E.; Roldugina, E.; Stavitskaya, A.; Ivanov, E.; Egazar’yants, S.; Vinokurov, V. Micro-mesoporous MCM-41/ZSM-5 supported Pt and Pd catalysts for hydroisomerization of C-8 aromatic fraction. Appl. Catal. AGen. 2020, 603, 117764. [Google Scholar] [CrossRef]
- Popova, M.; Dimitrov, M.; Boycheva, S.; Dimitrov, I.; Ublekov, F.; Koseva, N.; Atanasova, G.; Karashanova, D.; Szegedi, Á. Ni-Cu and Ni-Co-Modified Fly Ash Zeolite Catalysts for Hydrodeoxygenation of Levulinic Acid to γ-Valerolactone. Molecules 2024, 29, 5753. [Google Scholar] [CrossRef]
- Petcuta, O.A.; Guzo, N.C.; Bordeiasu, M.; Nicolaev, A.; Parvulescu, V.I.; Coman, S.M. Ru/Beta Zeolite Catalysts for Levulinic Acid Hydrogenation: The Importance of Catalyst Synthesis Methodology. Catalysts 2025, 15, 80. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Gao, G.; Zhao, Z.; Sun, P.; Li, F. Enhanced stability of hierarchical zeolite-metal bifunctional catalysts for conversion of levulinic acid to valeric biofuels. Chem. Eng. J. 2024, 483, 149405. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, D.; Tao, S.; Lyu, Y.; Wang, X.; Liu, X.; Liu, S.; Li, M.; Zhao, R.; Yu, S. Deoxygenation of stearic acids using alkaline treated beta molecular sieves assisted by microwave irradiation. Catal. Sci. Technol. 2021, 11, 4812–4822. [Google Scholar] [CrossRef]
- Ding, S.; Fernandez Ainaga, D.L.; Hu, M.; Qiu, B.; Khalid, U.; D’Agostino, C.; Ou, X.; Spencer, B.; Zhong, X.; Peng, Y.; et al. Spatial segregation of catalytic sites within Pd doped H-ZSM-5 for fatty acid hydrodeoxygenation to alkanes. Nat. Commun. 2024, 15, 7718. [Google Scholar] [CrossRef]
- Meng, Q.; Yin, X.; Liu, C.; Li, X.; Kong, X. Construction of hierarchical Ga-promoted MFI zeolite supported Ni catalyst for lignin derivatives hydrodeoxygenation. Mol. Catal. 2025, 573, 114848. [Google Scholar] [CrossRef]
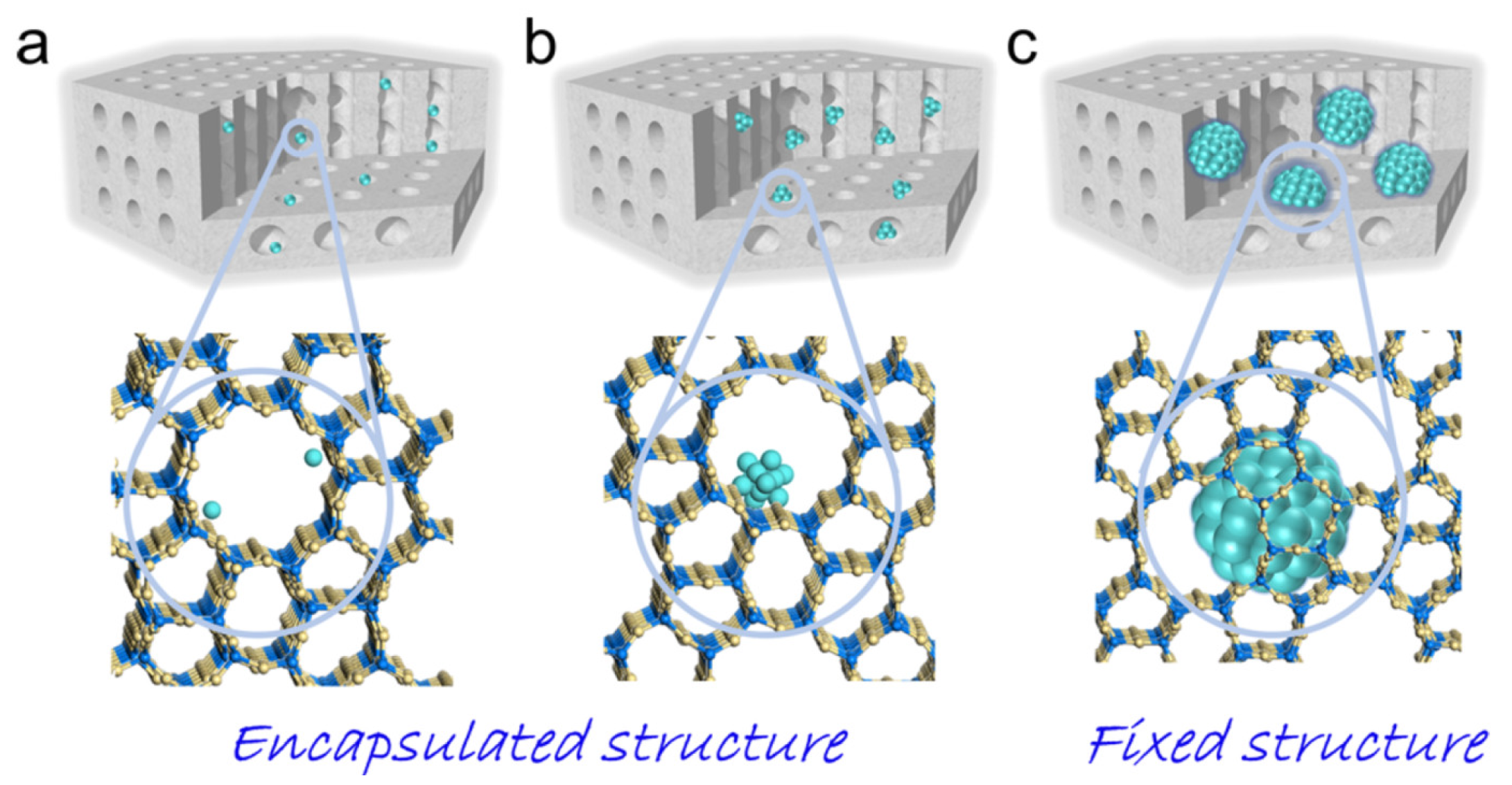
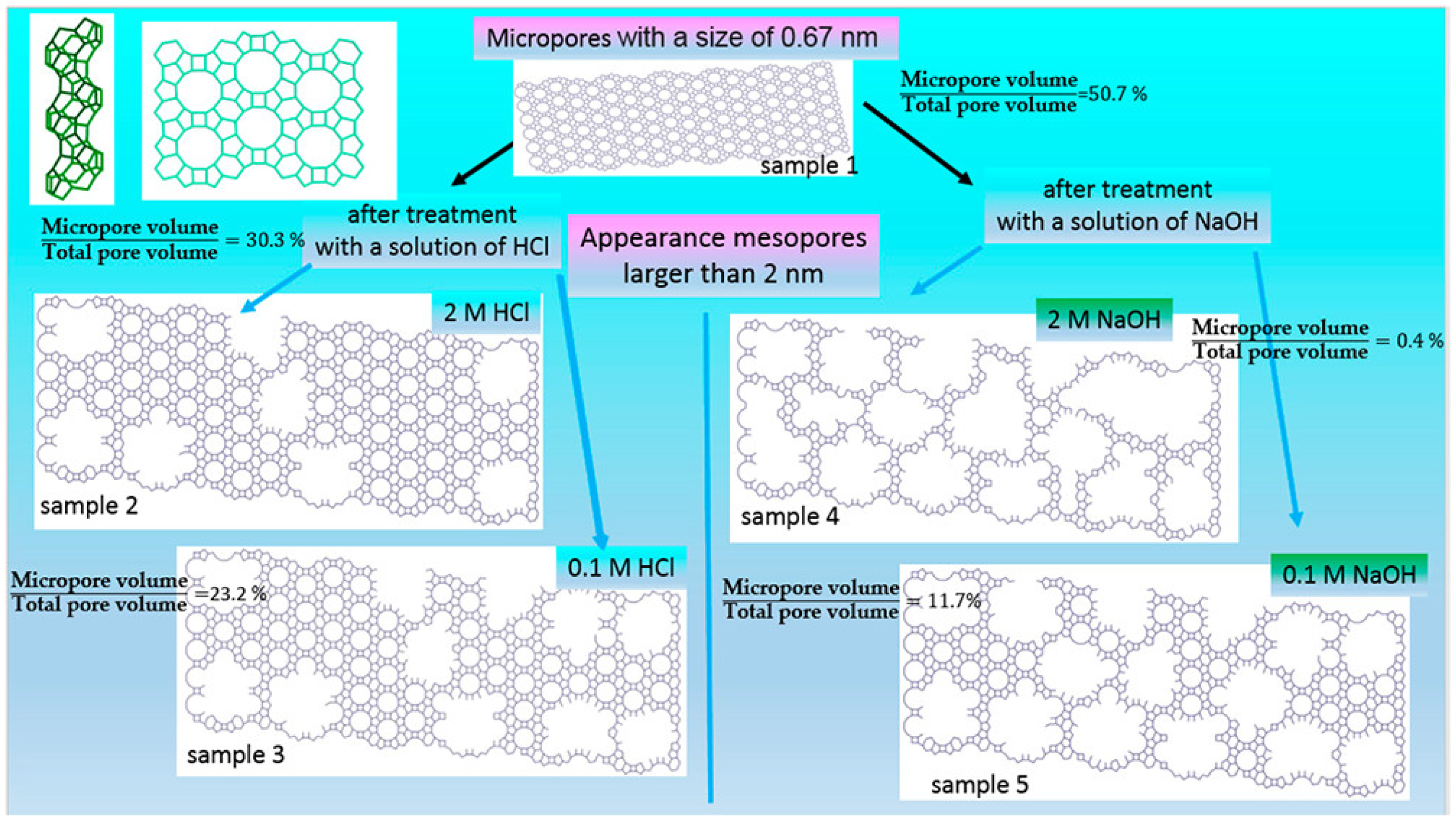
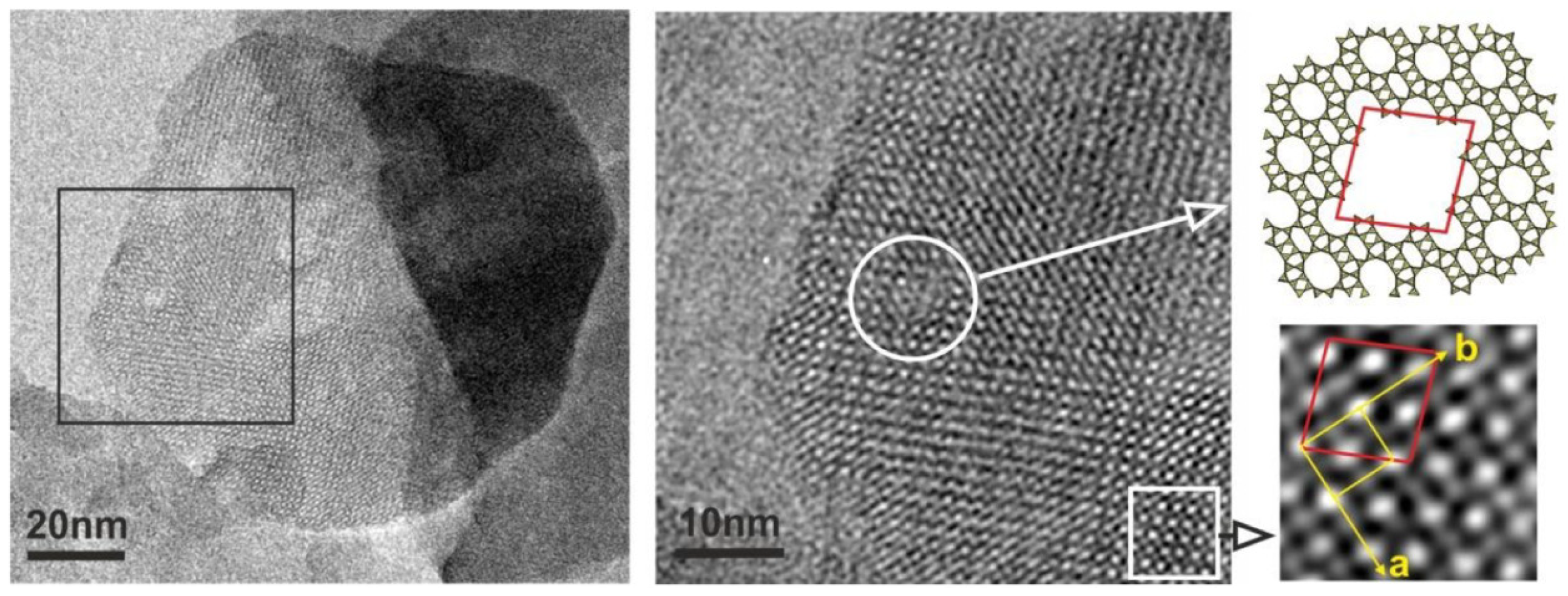
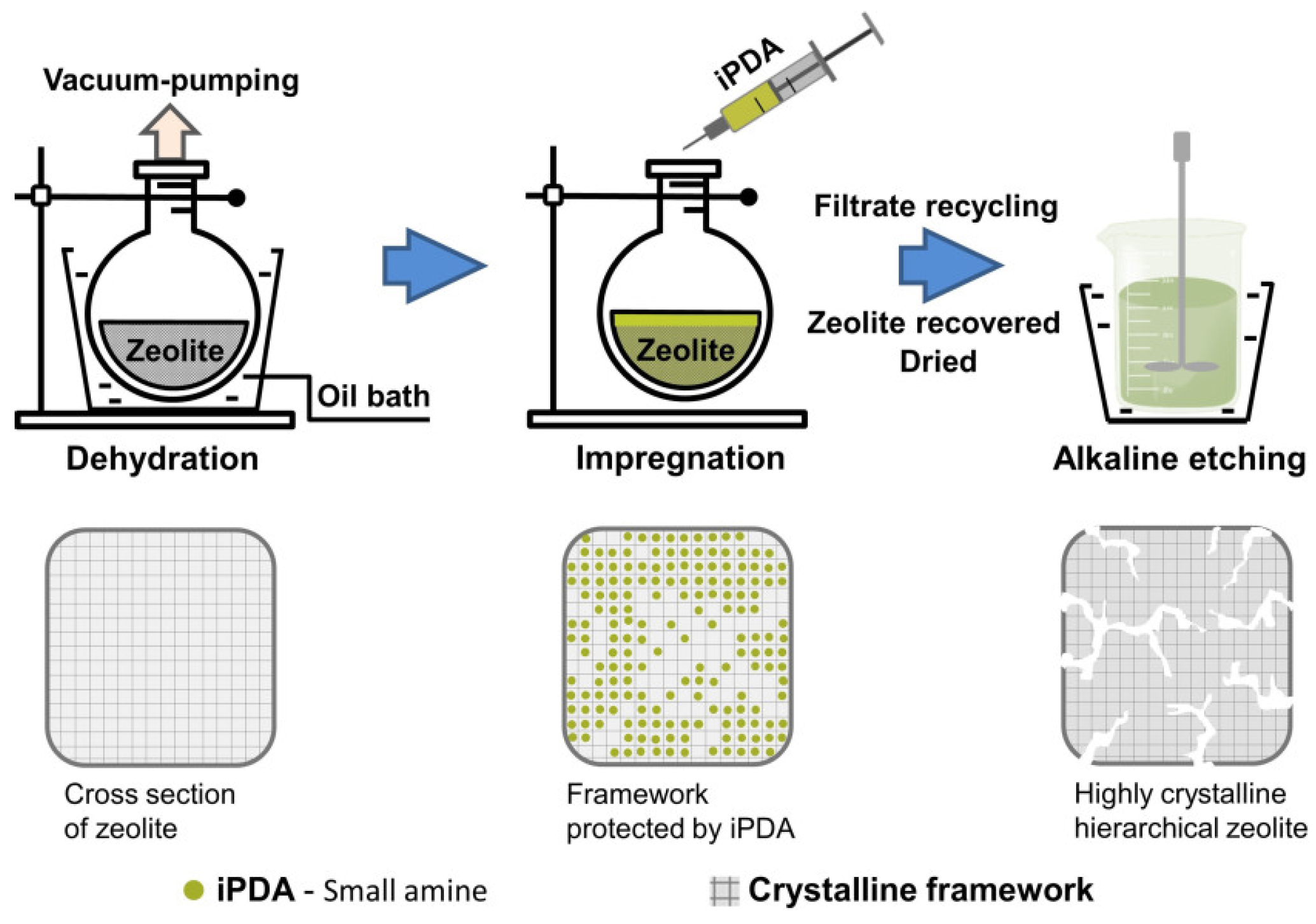
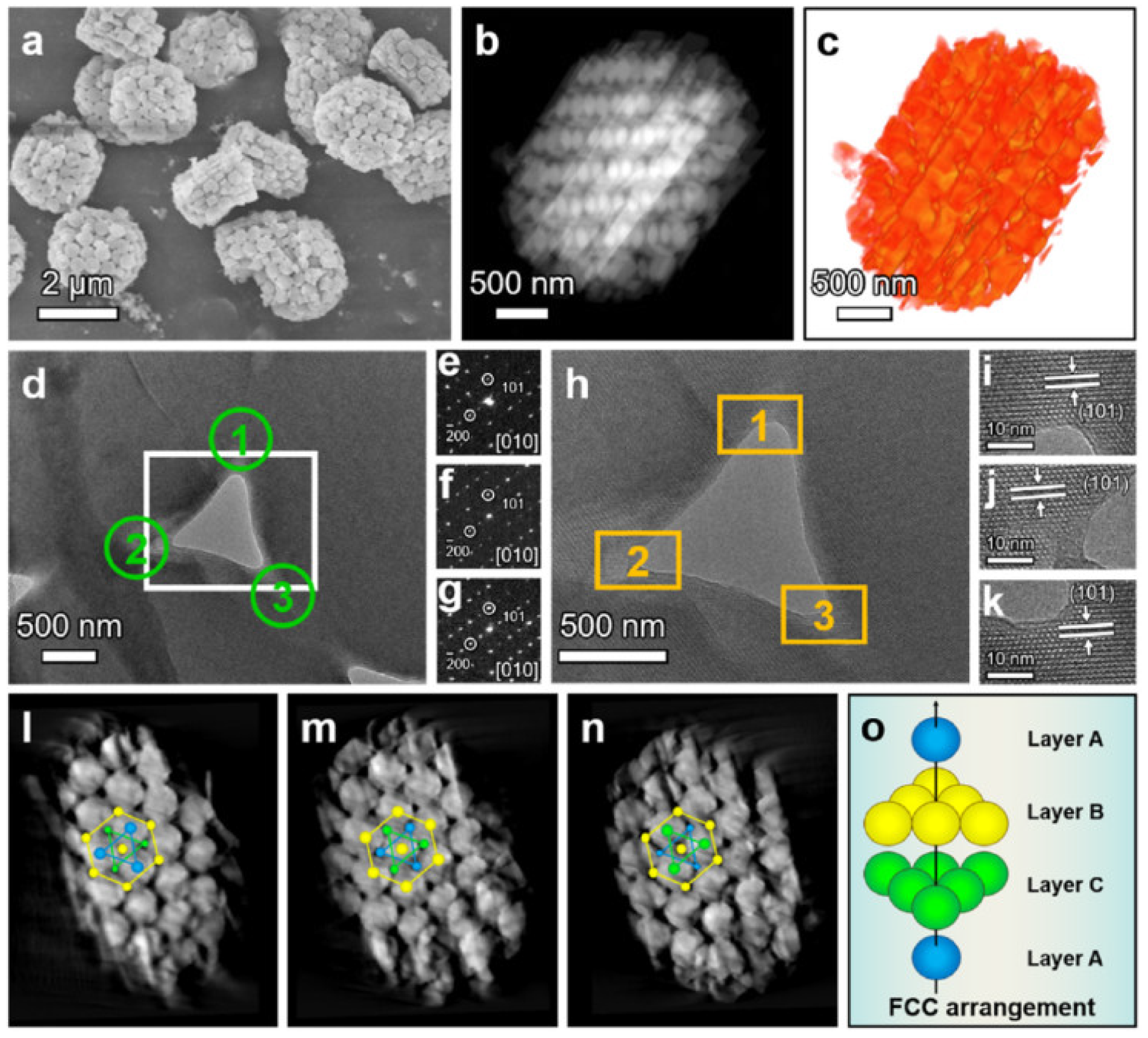
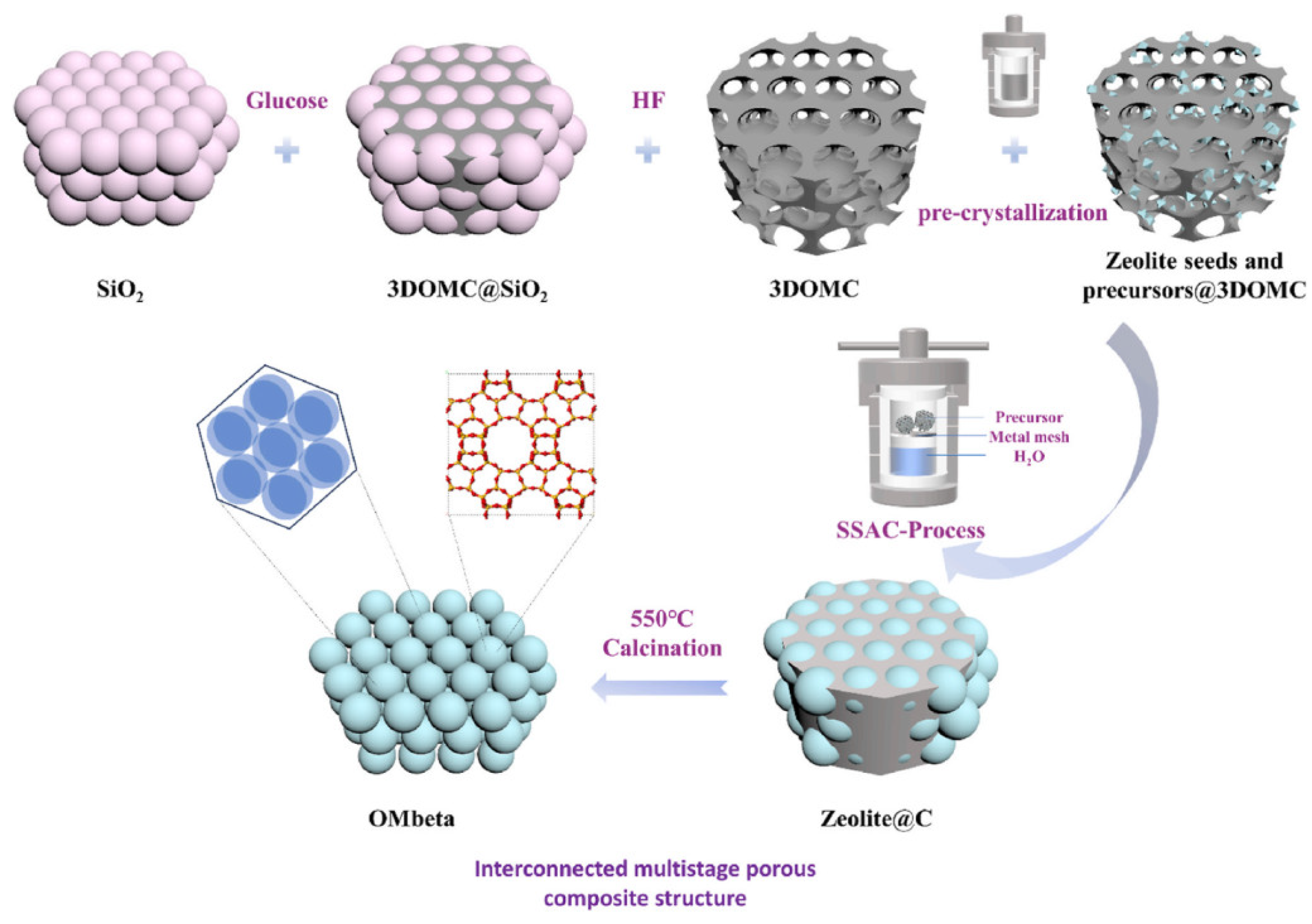
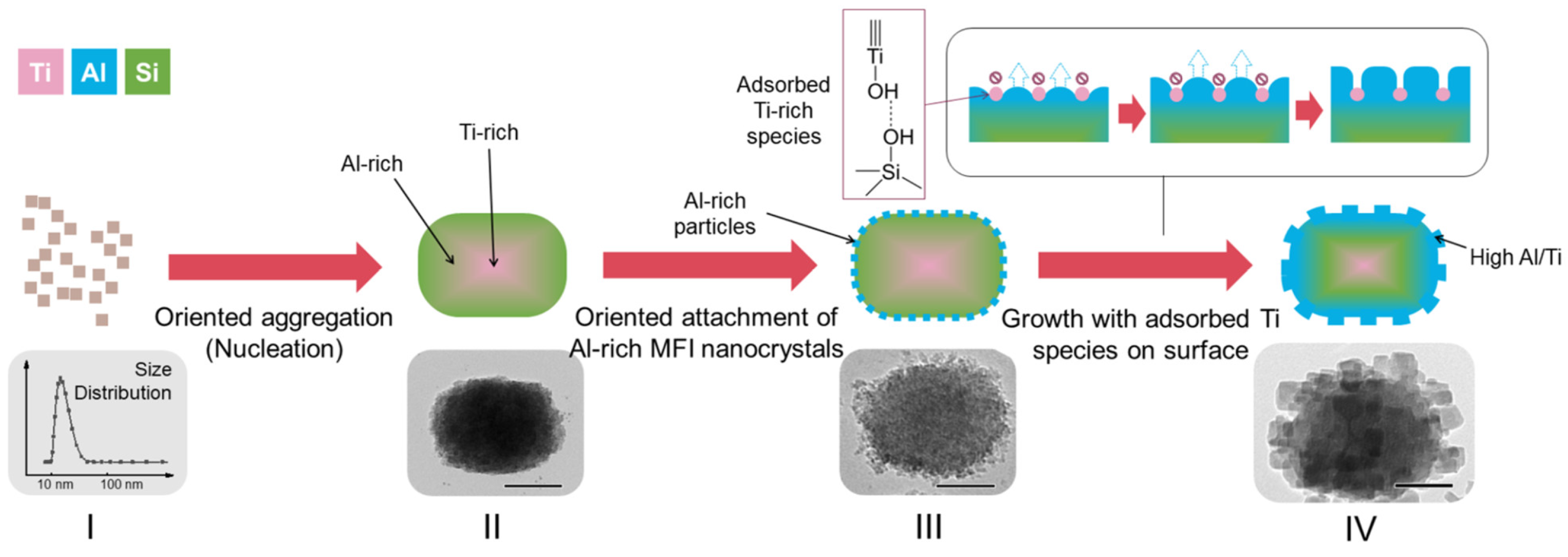
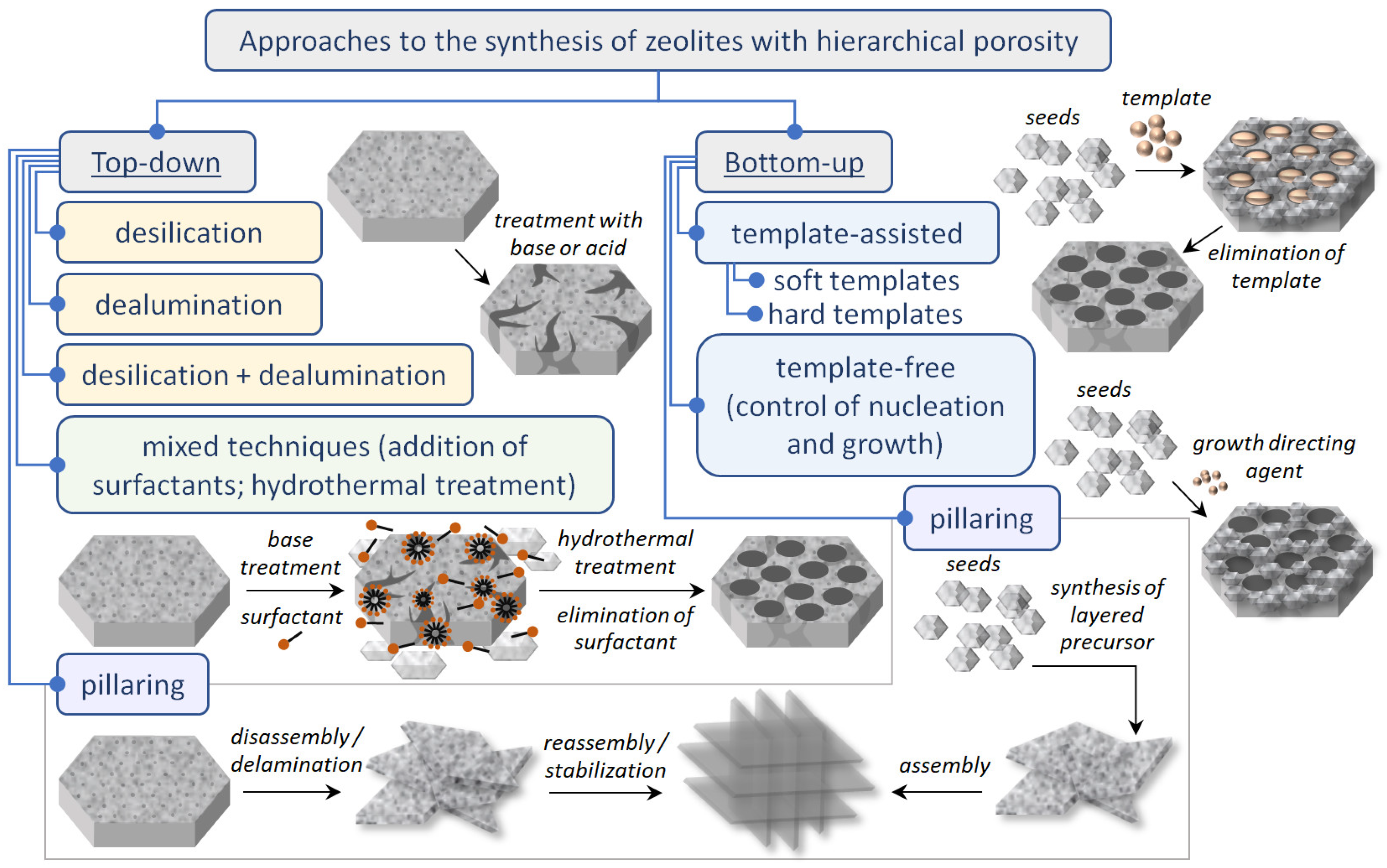
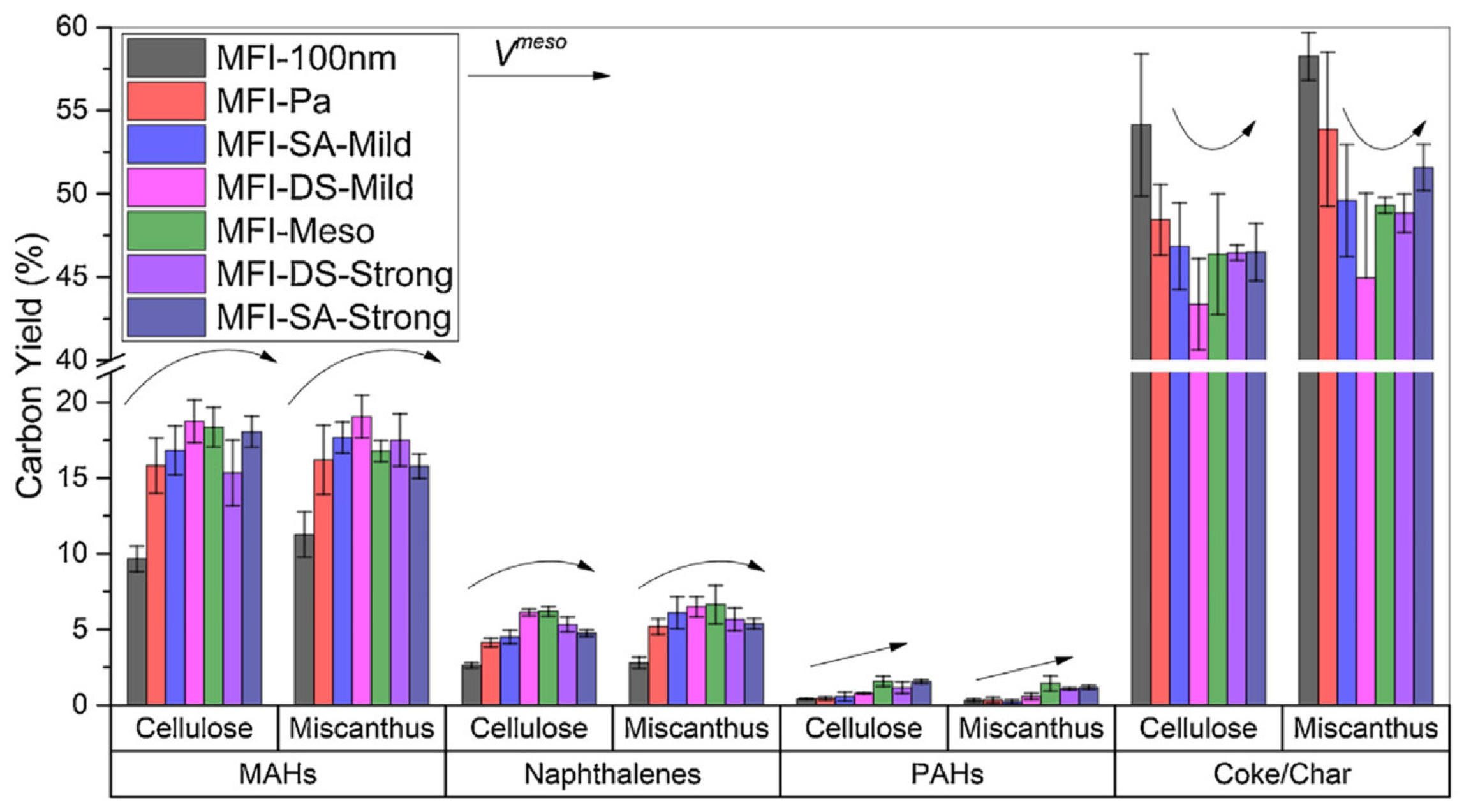
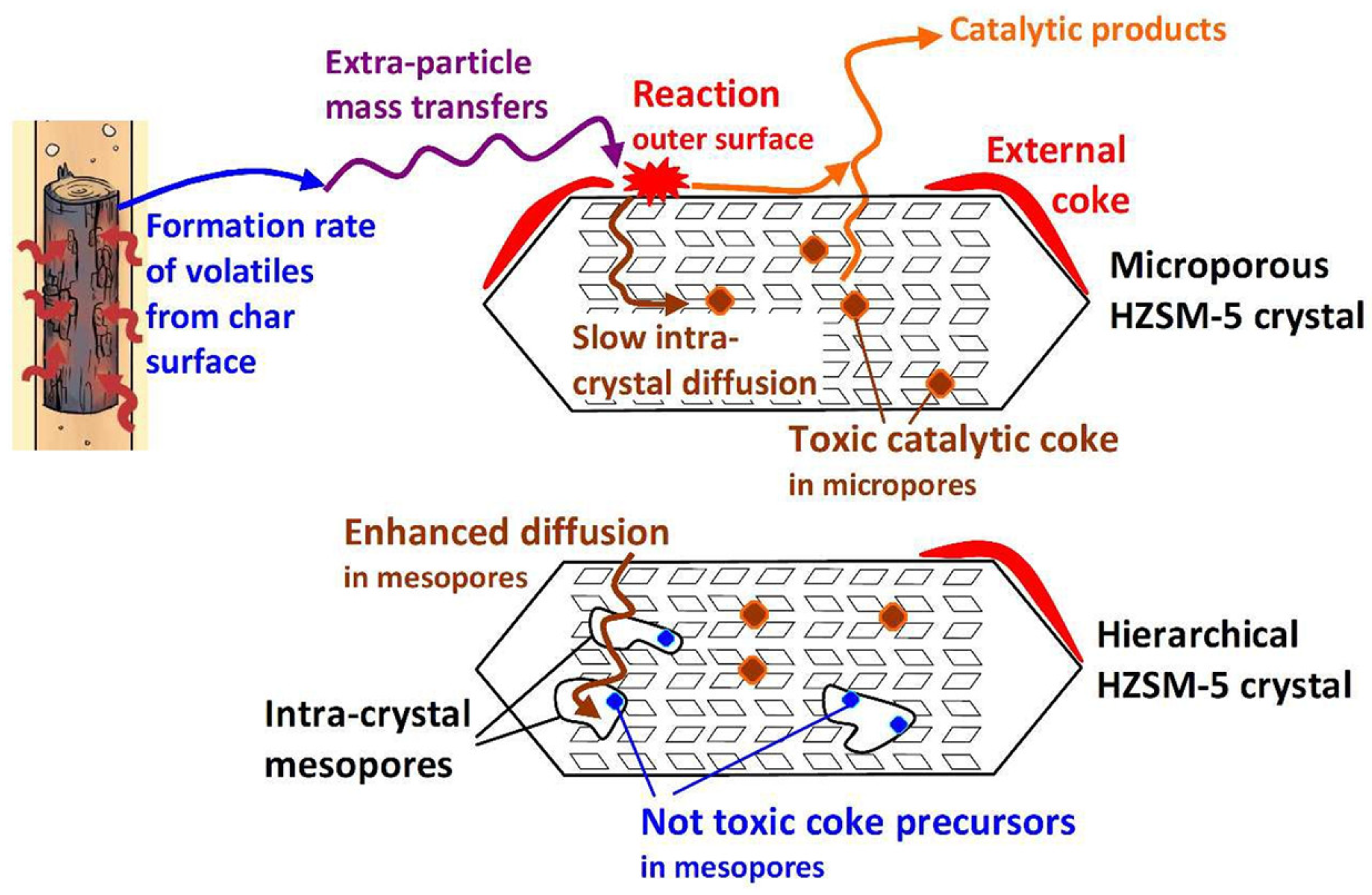
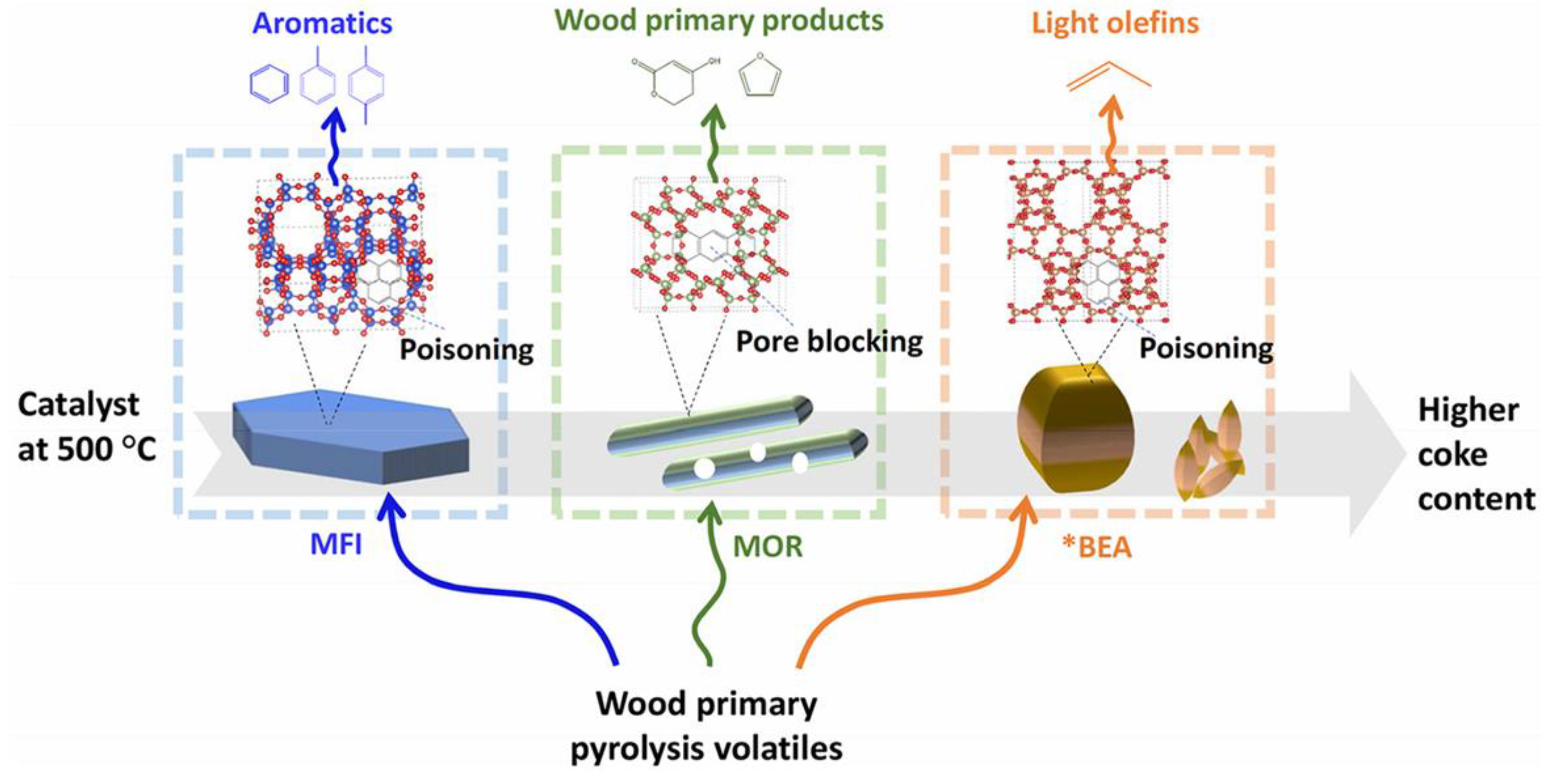

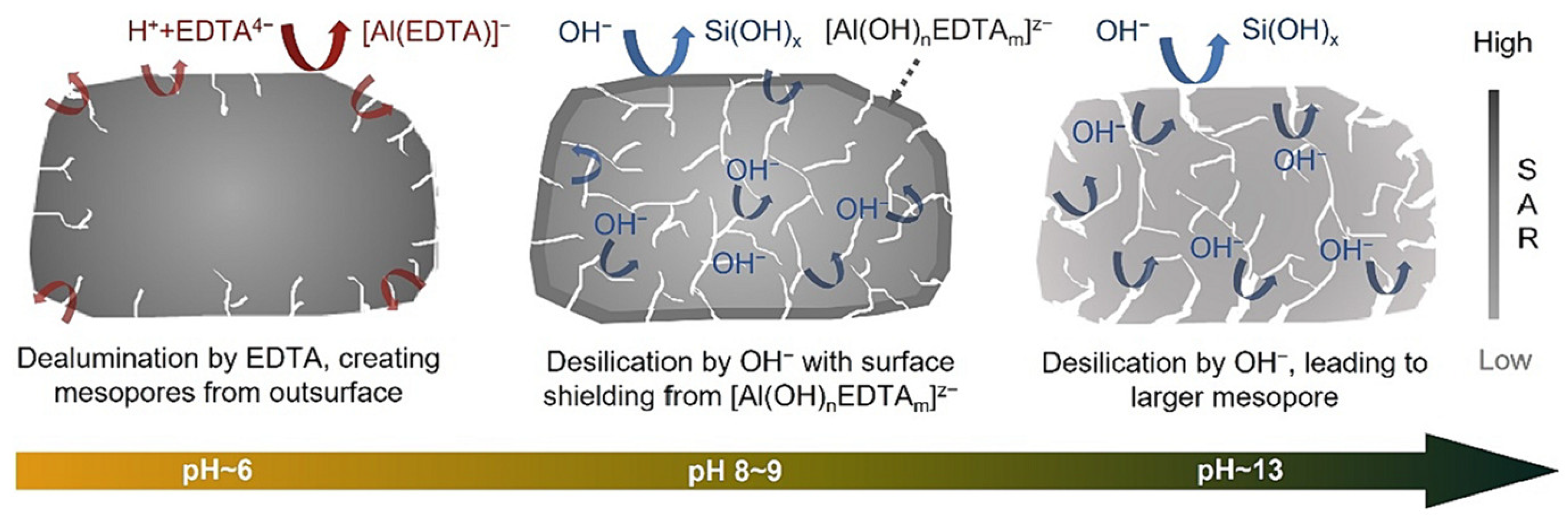
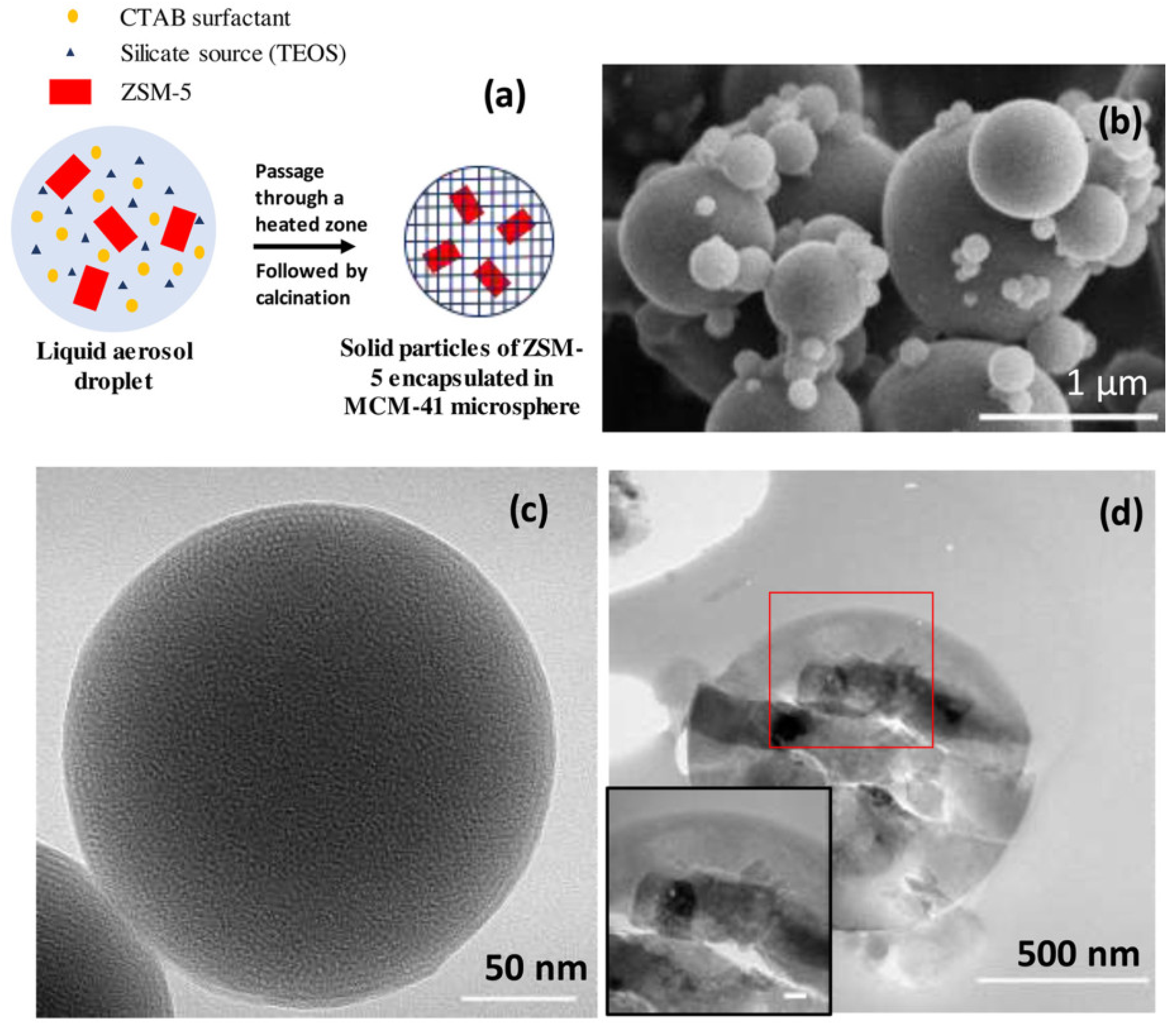
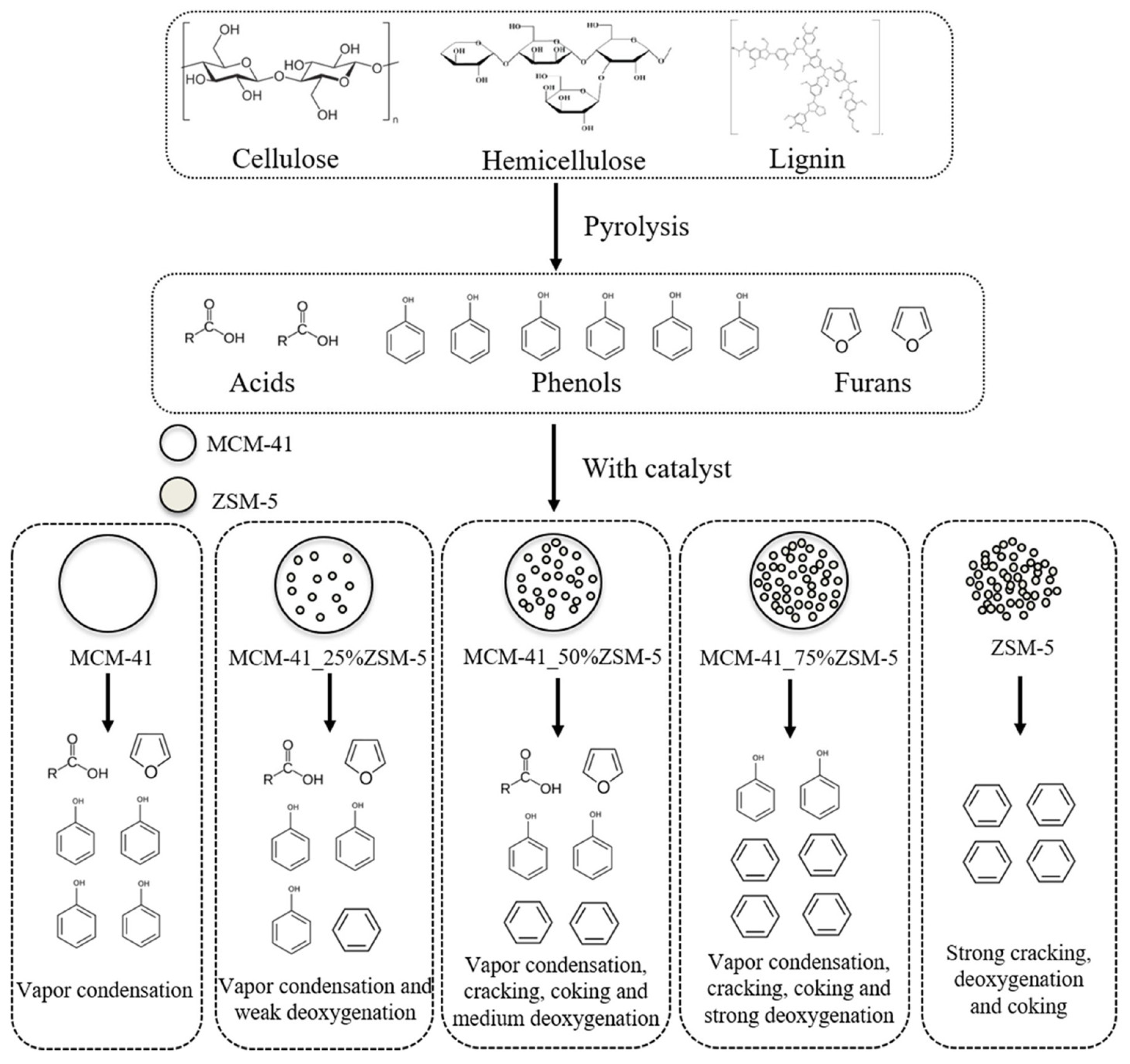
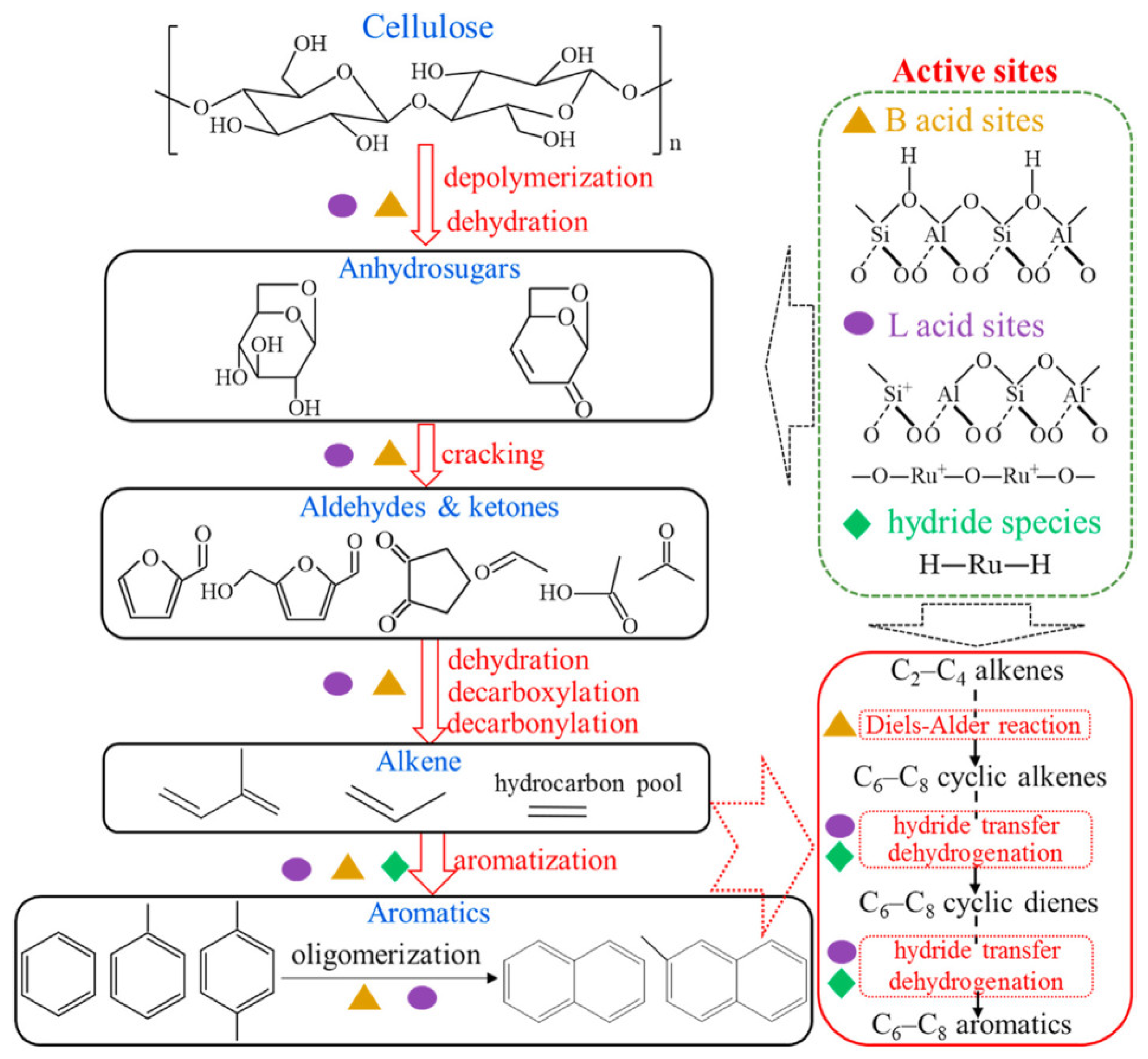
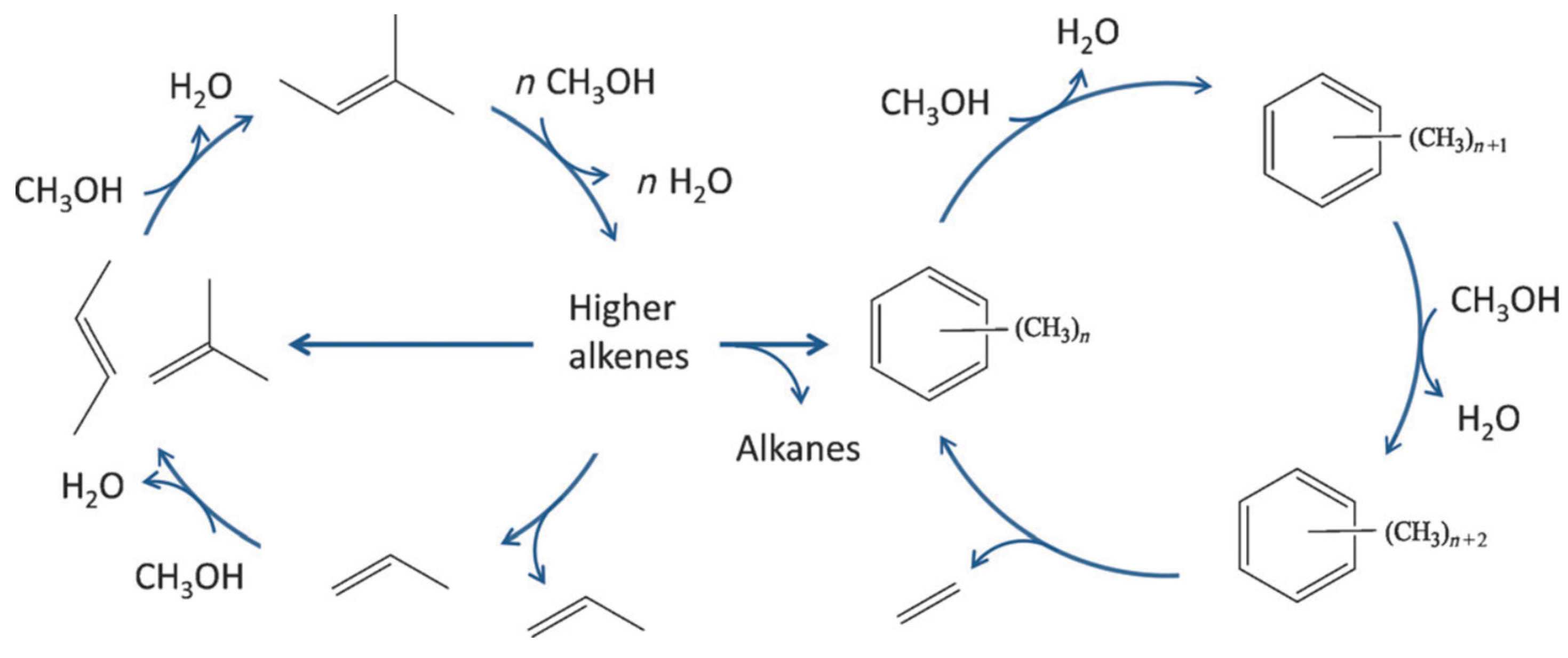


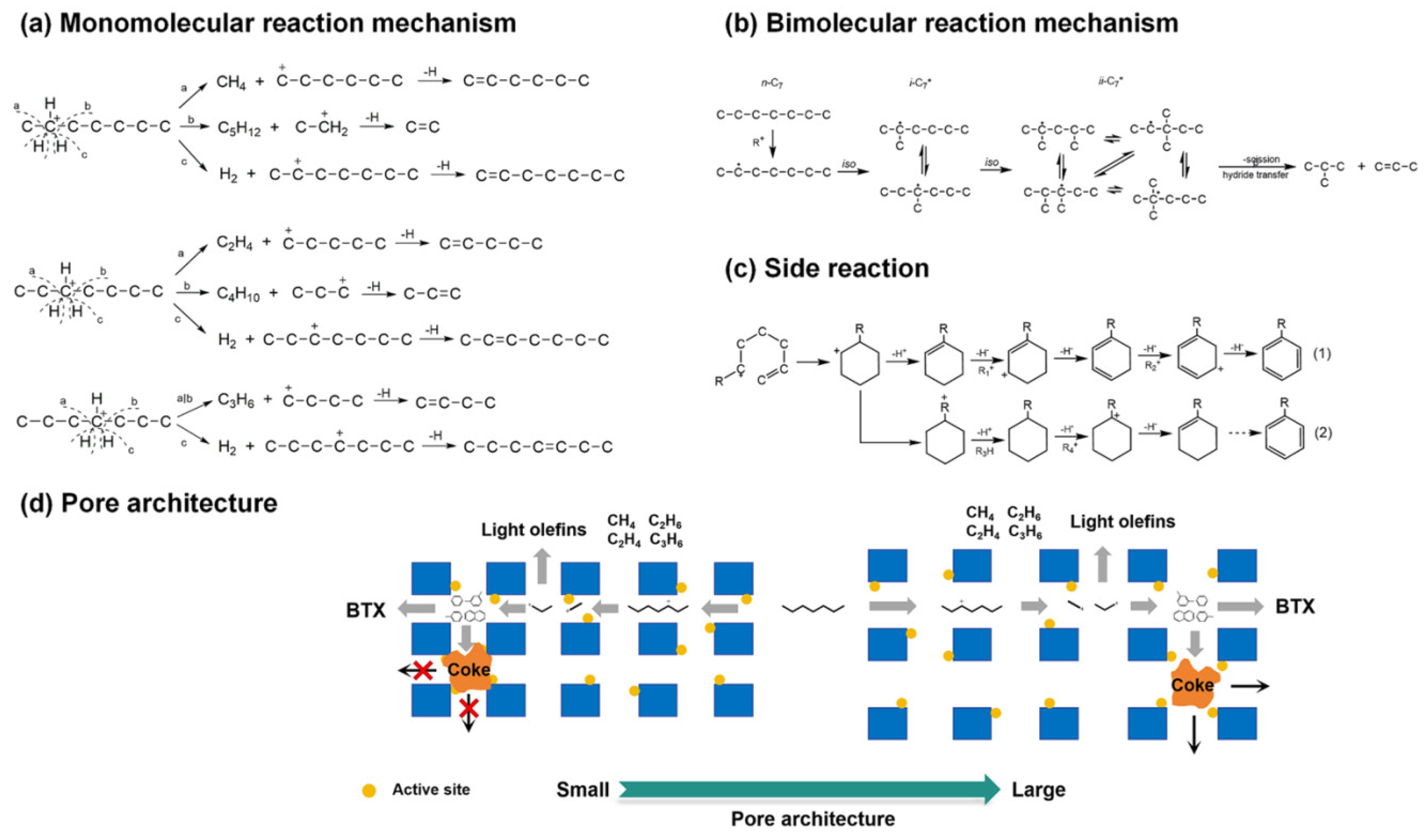
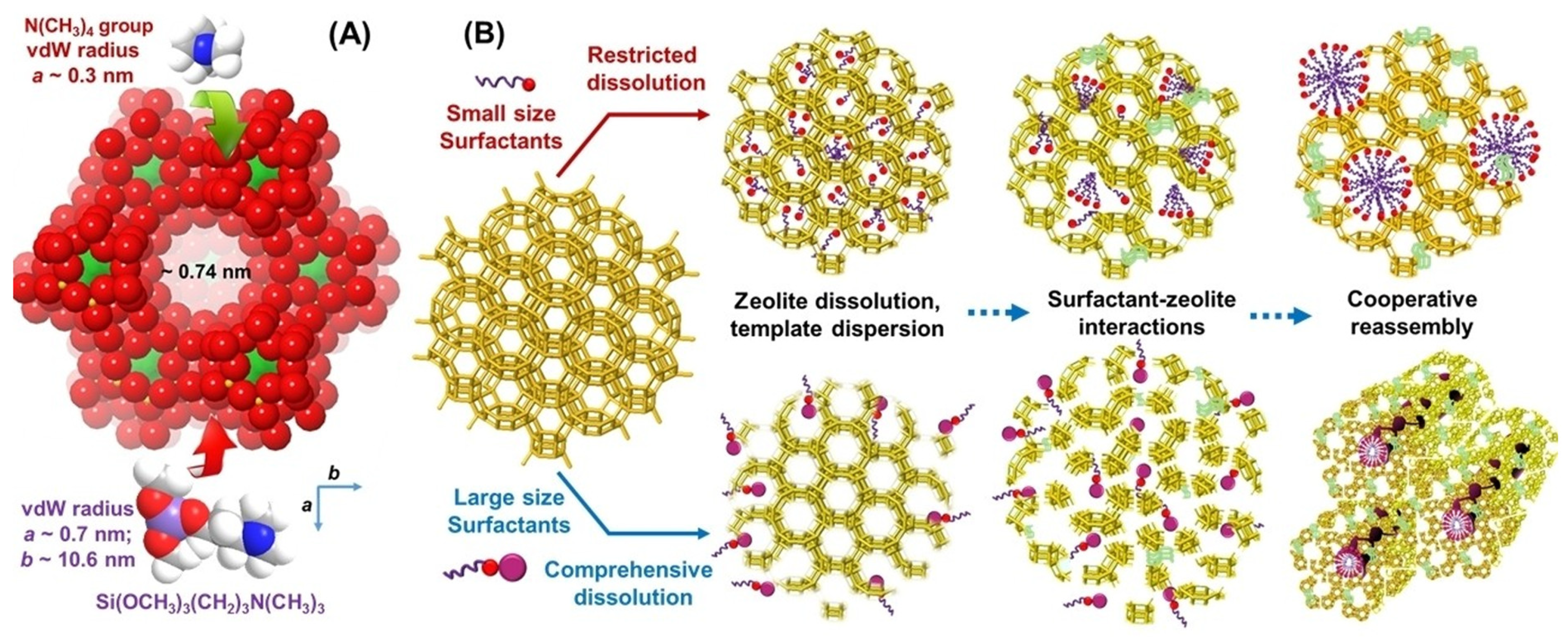

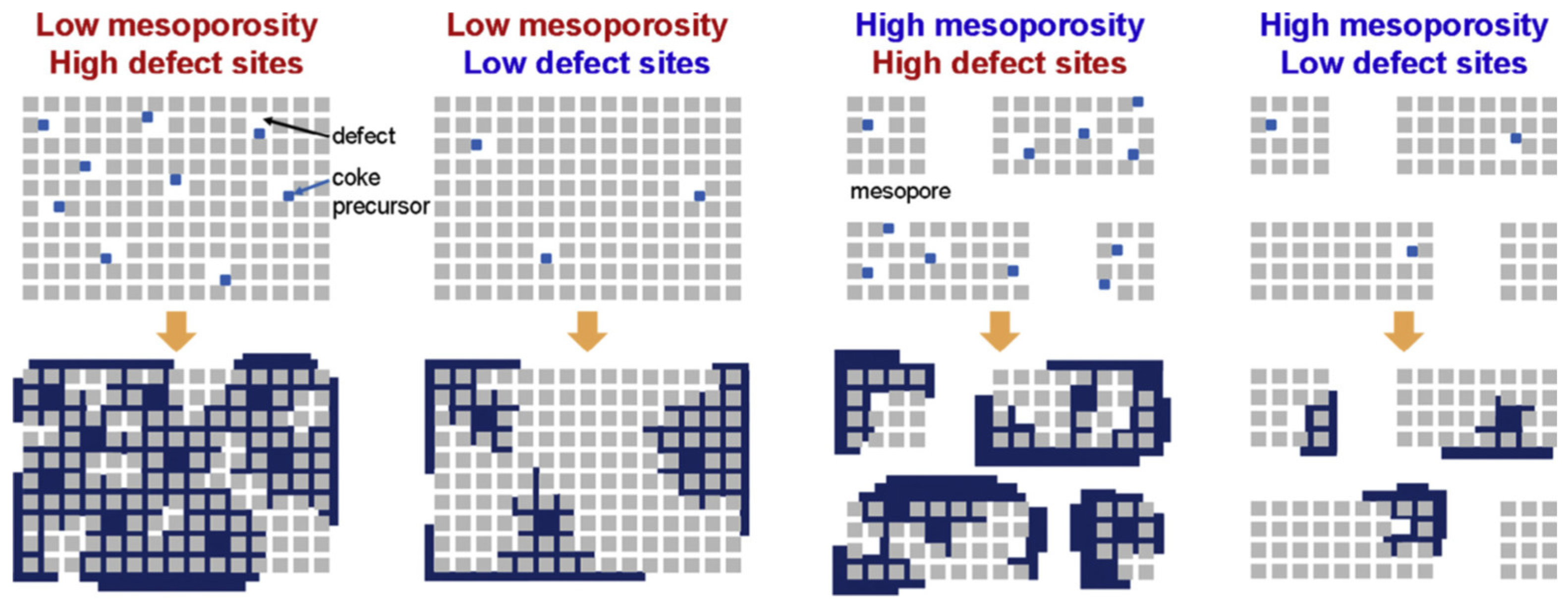
| Zeolite (Designation) | Synthesis Method of Hierarchical Zeolite | Feedstock | Pyrolysis Conditions | Yield or Selectivity to Aromatics | Ref. |
|---|---|---|---|---|---|
| HZSM-5 (MFI-Meso) | bottom-up (TPAOH + NaOH + hydrothermal teatment) | miscanthus | in situ, quartz microreactor, 600 °C, residence time (r.t.) 20 s, total loading 5 mg, BCR 0.2 | yield of MAHs is 18.0% | [75] |
| HZSM-5 (MFI-SA-Mild) | top-down (NaOH) | miscanthus | in situ, quartz microreactor, 600 °C, r.t. 20 s, total loading 5 mg, BCR 0.2 | yield of MAHs is 17.4% | [75] |
| HZSM-5 (MFI-DS-Mild) | top-down (NaOH + CTAB) | miscanthus | in situ, quartz microreactor, 600 °C, r.t. 20 s, total loading 5 mg, BCR 0.2 | yield of MAHs is 19.0% | [75] |
| MCM-41_75%ZSM-5 | spray drying of ZSM-5 suspension with CTAB and TEOS | miscanthus | in situ, quartz microreactor, 600 °C, r.t. 20 s, total loading 5 mg, BCR 0.2 | yield of aromatics is ~20% | [103] |
| HZSM-5 | top-down (NaOH) | Oak | MFBR, 500 °C, height of the fluidized bed 5 cm, BCR 0.85 | yield of aromatics is 6.2% | [76] |
| HZSM-5 (HZ-0.01) | bottom-up (TPAOH + CTAB) | rice straw | in situ, tandem μ-reactor system, 600 °C, total loading 5 mg, BCR 0.05 | yield of aromatics is 26.8% | [77] |
| HZSM-5 | top-down (NaOH) | kraft lignin | in situ, micro-furnace system, 500 °C, kraft lignin 0.3 mg, BCR 0.1 | selectivity to BTX is ~27% | [78] |
| Hβ | top-down (NaOH) | kraft lignin | in situ, micro-furnace system, 500 °C, kraft lignin 0.3 mg, BCR 0.1 | selectivity to BTX is ~27% | [78] |
| HZSM-11 (NS-HZSM-11(20)-0.3) | bottom-up (TBAOH) | maize straw | in situ, fixed-bed reactor, 500 °C, maize straw 1 g, BCR 1 | N/A (23.6% of bio-oil, hydrocarbons 63.6%) | [81] |
| HZSM-5 | top-down (NaOH) | oakwood | in situ, double fixed bed microreactor, 773 K, oakwood 85 mg, catalyst 100 mg, BCR 0.8 | yield of MAHs is ~4–5% | [82] |
| Beta(36) (B36-0.3-24) | top-down (TEAOH + hydrothermal teatment) | lignin | in situ, fixed-bed reactor, 600 °C, lignin 1.0 g, catalyst 1.0 g | yield of bio-oil is 13.1%, 56.0%MAHs | [83] |
| ZSM-5-F | bottom-up (TPAOH, TEAOH, HF) | stem wood | in situ, micro-pyrolyser, 500 °C, feedstock 0.4 mg, catalyst 2.0 mg | selectivity to BTX is 20.6% | [84] |
| ZSM-5 (ODDMMS (0.05)-Z5) | bottom-up (TPAOH + OSA) | cellulose | in situ, tandem μ-reactor system, 600 °C, cellulose 4 mg, BCR 0.05 | yield of aromatics is 42.2% | [89] |
| CBV80-ZM (ZM is HZSM-5/MCM-41) | top-down (NaOH + CTAB) | PP | ex situ, micro-pyrolyzer, 550 °C, feedstock 0.4 mg, catalyst 32 mg | total light olefins and MAHs yield is 92% | [90] |
| ZSM-5 (Z-HT-0.3-3) | top-down (MW, Na2H2EDTA + NaOH) | HDPE | ex situ, two-stage pyrolysis-catalysis reactor, 500 °C, HDPE 4 g, catalyst 0.4 g | ~12% yield of oil, aromatics is78.7% | [91] |
| HZSM-5 | top-down (NaOH) | TPW + HDPE | in situ, 550 °C, TPW: HDPE = 1, catalyst 3 g, BCR 1 | yield of MAHs is 71.75% | [92] |
| HZSM-5 covered with MCM-41 layer (HM-10%CTAB) | top-down (NaOH + CTAB) | bamboo | ex situ, Py-GC/MS, 600 °C, bamboo 1 mg, BCR 0.5 | N/A (yield of hydro- carbons is 53.23%) | [94] |
| HZSM-5/MCM-41 | top-down (NaOH + CTAB) | rice husk (R) + greenhouse plastic films (W) | ex situ, Py-GC/MS, 600 °C, feedstock 1 mg, R/W = 1:1.5, BCR 0.5 | 71.1% of hydro- carbons, >43% MAHs | [95] |
| HZSM-5/MCM-41 | top-down (TPAOH + CTAB) | rice husk | in situ, MACFP, 550 °C, rice husk 8 g, BCR 1 | 60.5% of hydro- carbons, 43.5% MAHs | [96] |
| Catalyst (Metal Content) | Synthesis Method of Hierarchical Zeolite | Feedstock | Pyrolysis Conditions | Yield or Selectivity to Aromatics | Ref. |
|---|---|---|---|---|---|
| MgO/h-ZSM-5 (8.4 wt.% of Mg) | bottom-up (TPAOH + PHAPTMS) | eucalyptus woodchips | ex situ, two-zone fixed-bed reactor, 500 °C, catalyst 1 g, BCR 5 | aromatics is ~6% of bio-oil | [118] |
| MgO/h-Beta (8.7 wt.% of Mg) | bottom-up (TEAOH + PHAPTMS) | eucalyptus woodchips | ex situ, two-zone fixed-bed reactor) 500 °C, catalyst 1 g, BCR 5 | aromatics is ~1.5% of bio-oil | [118] |
| ZnO/h-ZSM-5 (9.7 wt.% of Zn) | bottom-up (TPAOH + PHAPTMS (silanization agent)) | eucalyptus woodchips | ex situ, two-zone fixed-bed reactor, 500 °C, catalyst 1 g, BCR 5 | aromatics is ~5% of bio-oil | [118] |
| ZnO/h-Beta (10 wt.% of Zn) | bottom-up (TEAOH + PHAPTMS) | eucalyptus woodchips | ex situ, two-zone fixed-bed reactor, 500 °C, catalyst 1 g, BCR 5 | aromatics is ~1.5% of bio-oil | [118] |
| La/Hi-ZSM-5 (5 wt.% of La) | top-down (Na2CO3) | rape straw | ex situ, two-stage fixed- bed reactor, 500 °C, rape straw 150 g, catalyst 30 g | aromatics is 49.86% of bio-oil organic fraction | [119] |
| Ga/HZSM-5-0.3M (1 wt.% of Ga) | top-down (NaOH) | cellulose | in situ, micro-pyrolyzer, 500 °C, total loading 10 mg, r.t. 30 s, BCR 0.05 | selectivity to BTXis 53.70% | [120] |
| Fe/HZSM-5-0.3M (1 wt.% of Fe) | top-down (NaOH) | cellulose | in situ, micro-pyrolyzer, 500 °C, total loading 10 mg, r.t. 30 s, BCR 0.05 | selectivity to BTX is 58.13% | [120] |
| 0.1%Cu/AHZ-0.2 | top-down (NaOH) | rice straw | in situ, tandem μ-reactor system, 600 °C, total loading 5 mg, BCR 0.05 | yield of aromatics is 29.2% | [121] |
| 0.1%Ni/AHZ-0.2 | top-down (NaOH) | rice straw | in situ, tandem μ-reactor system, 600 °C, total loading 5 mg, BCR 0.05 | yield of aromatics is 28.0% | [121] |
| 0.5%Ga/AHZ-0.2 | top-down (NaOH) | rice straw | in situ, tandem μ-reactor system, 600 °C, total loading 5 mg, BCR 0.05 | yield of aromatics is 28.1% | [121] |
| 8 wt.% Ni- hierarchical ZSM-5 | top-down (NaOH) | torrefied corn cob | in situ, double-shot pyrolyzer, 550 °C, biomass 2 mg, BCR 0.5 | aromatics is 54.42% of bio-oil | [122] |
| Fe(4)/Hie-ZSM-5 (4 wt.% of Fe) | top-down (NaOH) | poplar sawdust | in situ, Py-GC/MS reactor, 550 °C, total loading 0.1 g, BCR 1 | aromatics is 19.92% | [123] |
| 2Ru/MZSM (2 wt.% of Ru) | top-down (NaOH) | cellulose | in situ, analytical pyroprobe reactor, 650 °C, cellulose 0.2 mg, BCR 0.1 | yield of aromatics is 16.8% | [124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikoshvili, L.Z.; Bronstein, L.M.; Matveeva, V.G.; Sulman, M.G. Enhancing the Catalytic Performance of Zeolites via Metal Doping and Porosity Control. Molecules 2025, 30, 3798. https://doi.org/10.3390/molecules30183798
Nikoshvili LZ, Bronstein LM, Matveeva VG, Sulman MG. Enhancing the Catalytic Performance of Zeolites via Metal Doping and Porosity Control. Molecules. 2025; 30(18):3798. https://doi.org/10.3390/molecules30183798
Chicago/Turabian StyleNikoshvili, Linda Zh., Lyudmila M. Bronstein, Valentina G. Matveeva, and Mikhail G. Sulman. 2025. "Enhancing the Catalytic Performance of Zeolites via Metal Doping and Porosity Control" Molecules 30, no. 18: 3798. https://doi.org/10.3390/molecules30183798
APA StyleNikoshvili, L. Z., Bronstein, L. M., Matveeva, V. G., & Sulman, M. G. (2025). Enhancing the Catalytic Performance of Zeolites via Metal Doping and Porosity Control. Molecules, 30(18), 3798. https://doi.org/10.3390/molecules30183798









