177Lu-Labeled Magnetic Nano-Formulations: Synthesis, Radio- and Physico-Chemical Characterization, Biological Applications, Current Challenges, and Future Perspectives
Abstract
1. Introduction
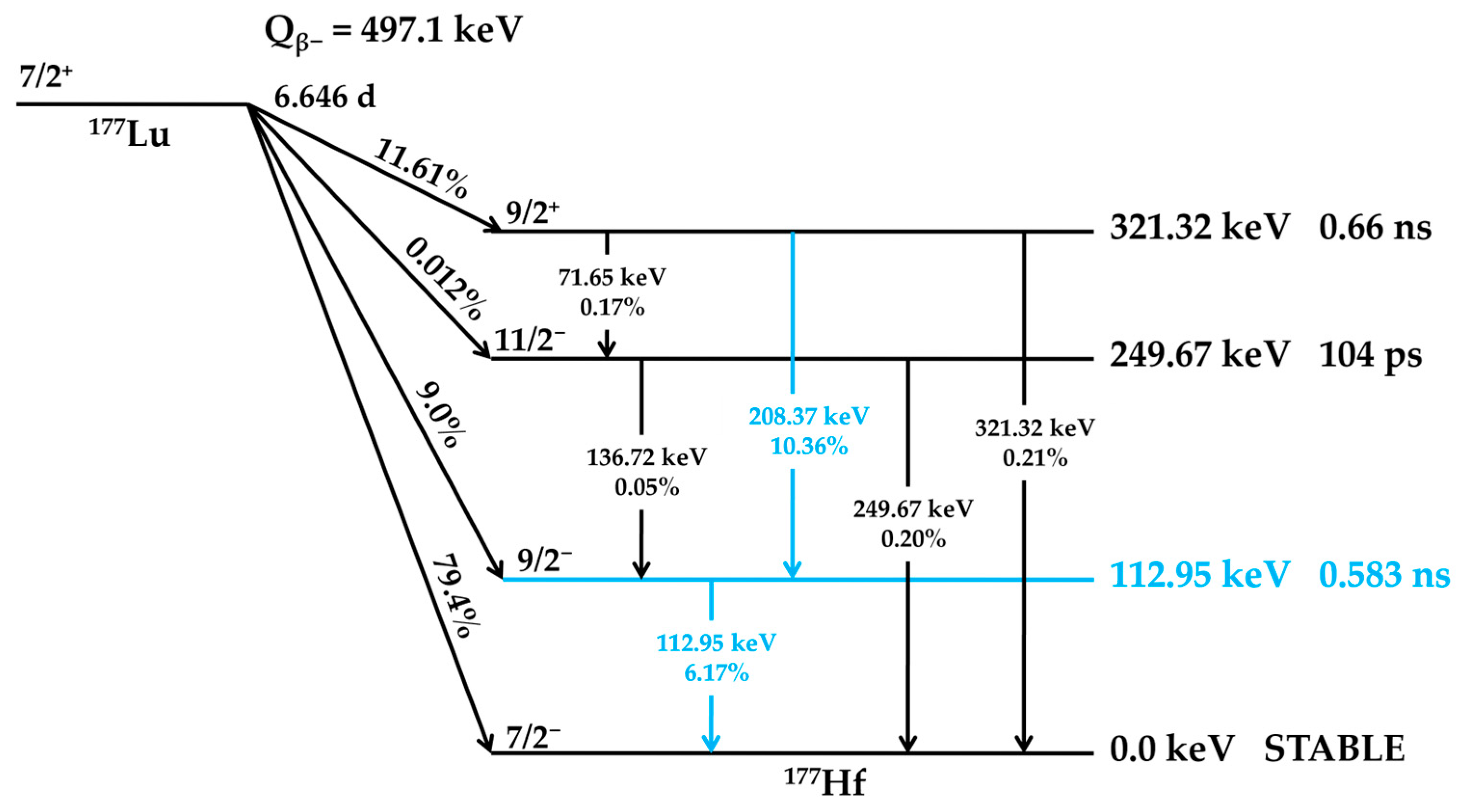
2. 177Lutetium
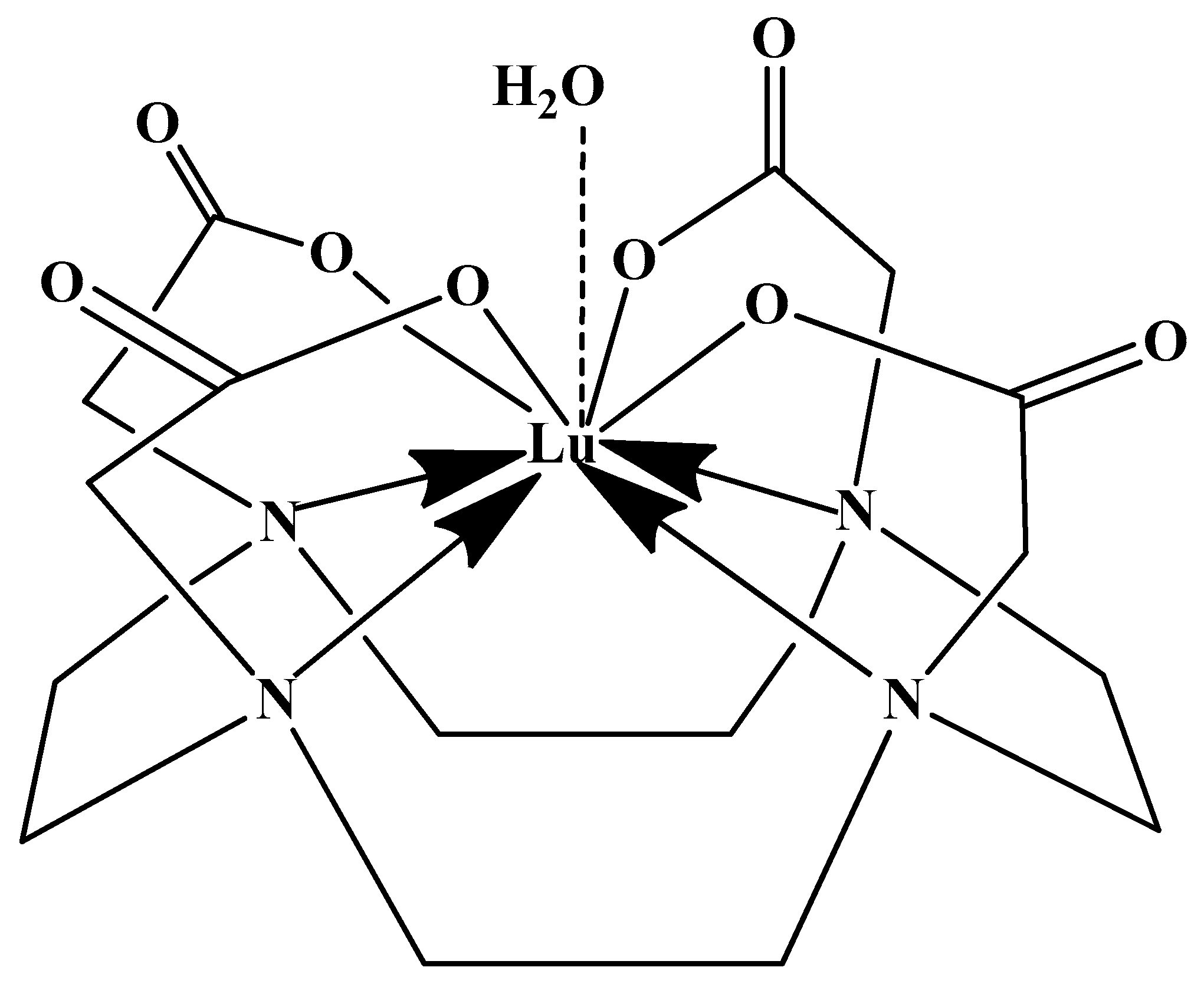
2.1. 177Lutetium Chemistry
| Radionuclides for SPECT Imaging | |||||
| Radionuclide | Haf-Life | Max Energy (keV) | Decay | Production | Common Production Reaction |
| Au-198 | 2.7 days | 960 | β−, γ | Cyclotron | 197Au(n,g)198Au |
| Au-199 | 3.1 days | 452.6 | β−, γ | Cyclotron | 198Au(n,g)199Au |
| Co-57 | 270 days | 692 | Electron capture, γ | Cyclotron | 56Fe(d,n)57Co |
| Fe-59 | 44.5 days | 1291 | β−, γ | Cyclotron | 59Co(p,n)59Fe |
| Ga-67 | 78.3 h | 300 | Auger e−, γ | Cyclotron | 68Zn(p,2n)67Ga |
| Gd-153 | 240.4 days | 103 | Electron capture, γ | Cyclotron | 152Gd(n,g)153Gd |
| In-111 | 2.81 days | 245 | γ | Cyclotron | 111Cd(p,n)111In |
| I-123 | 13.3 h | 159 | Auger e−, γ | Cyclotron | 127I(p,5n)123Xe |
| Re-186 | 91 h | 1080 | β−, γ | Cyclotron | 186W(p,n)186Re |
| Tc-99m | 6.0 h | 140 | γ | Generator | 99Mo/99mTc |
| Tl-201 | 3.0 days | 71 | γ | Cyclotron | 203Tl(p,3n)201Pb |
| Radionuclides for PET imaging | |||||
| As-72 | 25.9 h | 3320 | β+ | Cyclotron | 72Ge(p,n)72As |
| Br-76 | 16 h | 3980 | β+ | Cyclotron | 76Se(p,n)76Br |
| C-11 | 20.4 min | 961 | β+ | Cyclotron | 14N(p,a)11C |
| Cu-62 | 9.7 min | 2926 | β+ | Generator | 62Zn/62Cu |
| Cu-64 | 12.7 h | 656 | Electron capture, β+, β− | Cyclotron | 64Ni(p,n)64Cu |
| F-18 | 109.7 min | 634 | Electron capture, β+ | Cyclotron | 18F(F−):18O(p,n)18F |
| Ga-68 | 67.6 min | 1899 | Electron capture, β+ | Generator/ Cyclotron | 68Ge/68Ga |
| Ge-69 | 39.1 h | 1205 | β+ | Cyclotron | 69Ga(p,n)69Ge |
| I-124 | 4.2 days | 2100 | Electron capture, β+ | Cyclotron | 124Te(p,n)124I |
| Mn-52 | 5.6 days | 1434 | β+ | Cyclotron | 52Cr(p,n)52Mn |
| N-13 | 9.9 min | 1199 | β+ | Cyclotron | 16O(p,a)13N |
| O-15 | 2.1 min | 1732 | β+ | Cyclotron | 15N(p,n)15O |
| Rb-82 | 1.3 min | 3378 | Electron capture, β+ | Generator | 82Sr/82Rb |
| Y-86 | 14.7 h | 3150 | β+ | Cyclotron | 86Sr(p,n)86Y |
| Zr-89 | 78.4 h | 900 | Electron capture, β+ | Cyclotron | 89Y(p,n)89Zr |
| Radionuclides for therapy applications (β-emission, Linear energy transfer ~0.2 keV.μm−1) | |||||
| Radionuclide | Haf-life | Maximum energy (keV) | Decay | Production | Maximum particle range |
| Au-198 | 2.7 days | 960 | β−, γ | Cyclotron | 4.0 mm |
| Y-90 | 64.0 h | 2280 | β− | Generator | 12.0 mm |
| Lu-177 | 6.7 days | 500 | β−, γ | Cyclotron | 1.5 mm |
| I-131 | 8.0 days | 610 | β−, γ | Fission | 2.0 mm |
| Cu-67 | 62 h | 577 | β−, γ | Cyclotron | 1.8 mm |
| Re-186 | 91 h | 1080 | β−, γ | Cyclotron | 5.0 mm |
| Re-188 | 16.9 h | 2120 | β−, γ | Generator | 10.0 mm |
| Radionuclides for therapy applications (α-emission, Linear energy transfer ~80 keV.μm−1) | |||||
| At-211 | 7.2 h | 6000 | α | Cyclotron | 0.08 mm |
| Ac-225 | 10 days | 8000 | α, β− | Cyclotron | 0.1 mm |
| Bi-212 | 60.6 min | 6000 | α, β− | Cyclotron | 0.09 mm |
| Bi-213 | 46 min | 6000 | α, β− | Cyclotron | <0.1 mm |
| Ra-223 | 11.4 days | 7000 | α, β− | Cyclotron | <0.1 mm |
| Pb-212 | 10.6 h | 7800 | α, β− | Cyclotron | <0.1 mm |
| Tb-149 | 4.2 h | 400 | α | Cyclotron | <0.1 mm |
| Radionuclides for therapy applications (Auger-emission, Linear energy transfer ~4–26 keV.μm−1) | |||||
| Ga-67 | 78.3 h | 300 | Auger e−, γ | Cyclotron | 10 nm |
| I-123 | 13.3 h | 159 | Auger e−, γ | Cyclotron | 10 nm |
| I-125 | 60.5 days | 27 | Auger e−, γ | Neutron activation | 10 nm |
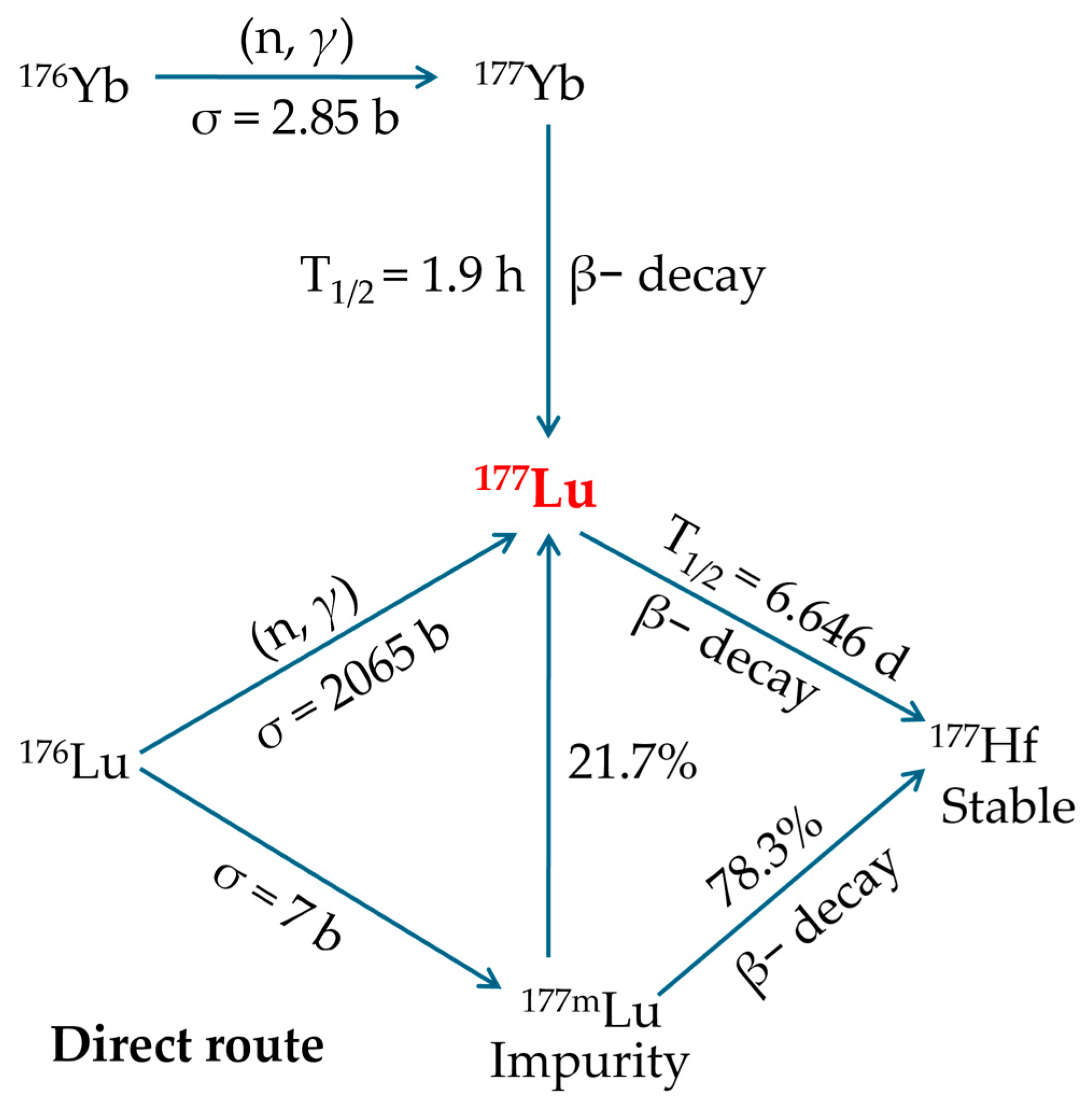
2.2. 177Lutetium Production
- This is the most straightforward production method, requiring only minor adjustments to existing reactor and processing facilities.
- It employs a 176Lu2O3 target, which maintains excellent chemical and thermal stability during reactor irradiation.
- This route represents the most inexpensive option for obtaining 177Lu with the required purity.
- The nuclear reaction has a high cross-section (~2065 barn) and a neutron capture resonance integral of ~1087 barn, resulting in enhanced production yields and elevated specific activity, meaning just 1 mg of enriched 176Lu can yield roughly 50 patient doses.
- Significant specific activities of 177Lu are attainable by irradiating highly enriched targets in research reactors with flux levels in the medium-high range.
- Enables production scaling by simply modifying target dimensions to meet fluctuating demand.
- Post-irradiation processing of the target is straightforward, requiring only the conversion to 177LuCl3 and without the need for further refinement or preparation prior to clinical administration.
- Only about 30% of the irradiated 176Lu becomes the desired 177Lu, and this proportion decreases further due to radioactive decay before administration. Therefore, the highest achievable specific activity requires irradiation in high-flux reactor facilities.
- To significantly increase both the production yield and specific activity of 177Lu, it is essential to use targets enriched in the 176Lu isotope, as its natural abundance is only 2.6%.
- In practice, the specific activity of 177Lu from this route is 740–1110 GBq·mg−1, only about 18–27% of the theoretical 4.07 TBq/mg. This is because the product mixture contains just 25% radioactive 177Lu atoms, with the rest being stable lutetium isotopes. Therefore, the maximum achievable specific activity, even under ideal conditions in high-flux reactors, caps at around 70% of the theoretical limit.
- Directly produced 177Lu achieves an initial specific activity of 740–1110 GBq·mg−1 (20–30 Ci·mg−1), which may be suitable for PPRT. However, the continual decrease in specific activity due to radioactive decay limits its shelf-life, making it less ideal for procedures requiring consistently high specific activity.
- The process also generates 177mLu, an unwanted beta-emitting byproduct with a 160-day half-life, complicating waste handling and disposal.
- After radiochemical separation from 177Yb, the final product contains only pure 177Lu with exceptionally high specific activity.
- The production pathway avoids creation of the problematic 177mLu byproduct.
- SA does not depend on neutron flux.
- The radiochemical performance is sufficient.
- This production method yields 177Lu with extended viability (approximately 14 days) due to minimal SA loss over time.
- The nuclear reaction has a low cross-section (2.85 barn), requiring approximately 1 g of enriched 176Yb to generate activity equivalent to that produced from just 1 mg of enriched 176Lu in the carrier-added route. Therefore, to achieve sufficient yields and ensure efficient use of the enriched target material, neutron irradiation of the 176Yb2O3 target must be performed in a high-flux nuclear reactor.
- Using enriched targets with low neutron-capture cross-sections proves economically inefficient, as much of the material remains unactivated and requires expensive recovery and rigorous radiochemical processing to ensure complete 176Yb removal, further increasing complexity and cost.
- Extracting 177Lu from 177Yb is highly complex due to its extremely low concentration (~1 177Lu atom per 5000 177Yb atoms), necessitating an exceptionally efficient combination of purification methods, such as column chromatography, solvent extraction, supported liquid membrane extraction, extraction chromatography, or even electrochemical separation, cementation, and electro-amalgamation processes.
- The maximum achievable yield (saturation yield) of 177Lu requires irradiation lasting approximately 5–6 half-lives, typically necessitating several weeks of continuous neutron exposure. As a result, several factors contribute to substantially higher production costs, such as the requirement of isotopically enriched target material, larger irradiation volumes, extended irradiation periods, and complex post-irradiation radiochemical separation processes.
- This production method is significantly more expensive than alternative routes for obtaining 177Lu of the required purity.
2.3. Cost-Effectiveness and Distribution Efficiency of 177Lu
2.4. 177Lutetium Clinical Advancements
3. Magnetic Nano-Formulations
3.1. Magnetic Nano-Formulations as Theranostic Agents
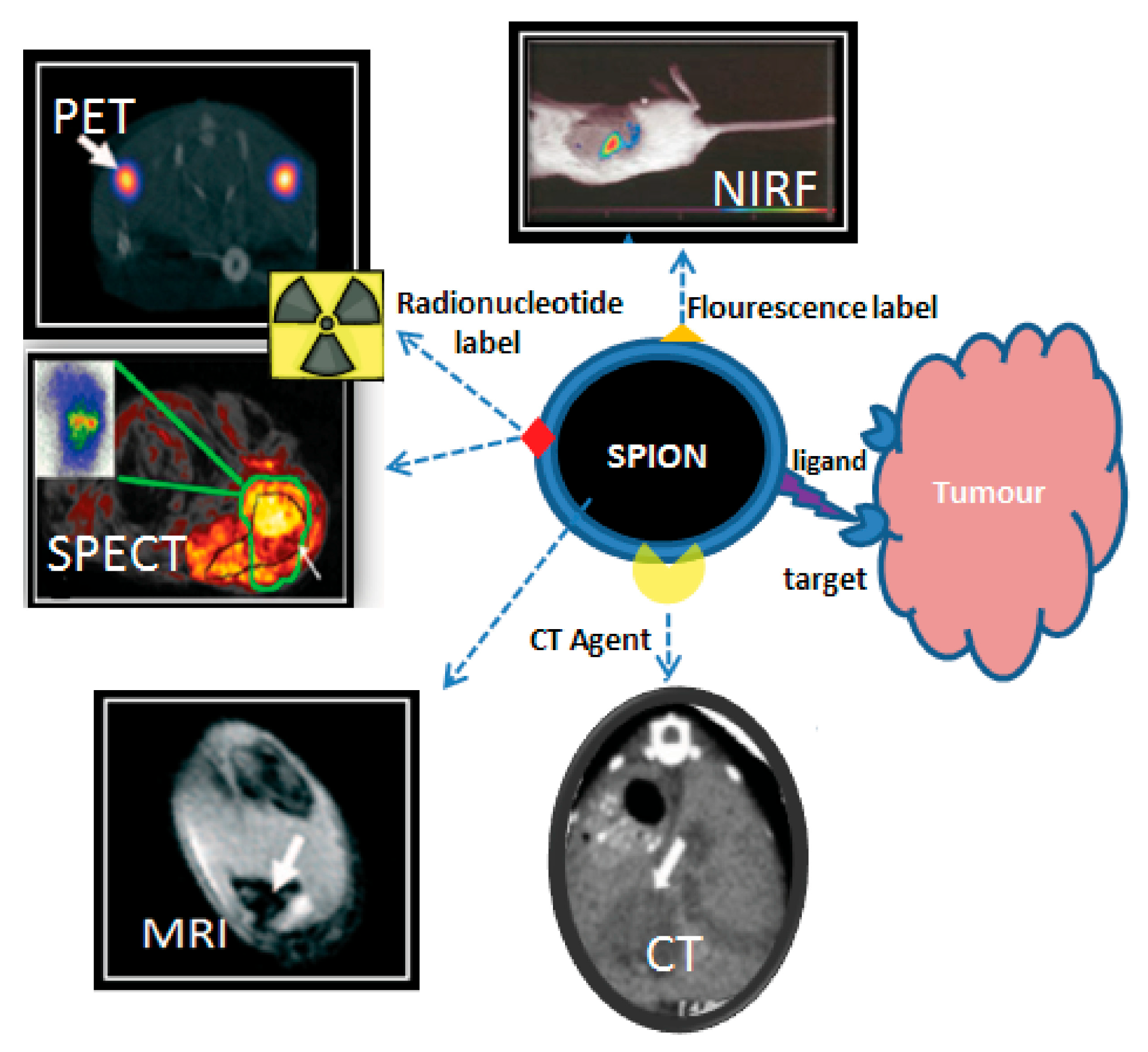
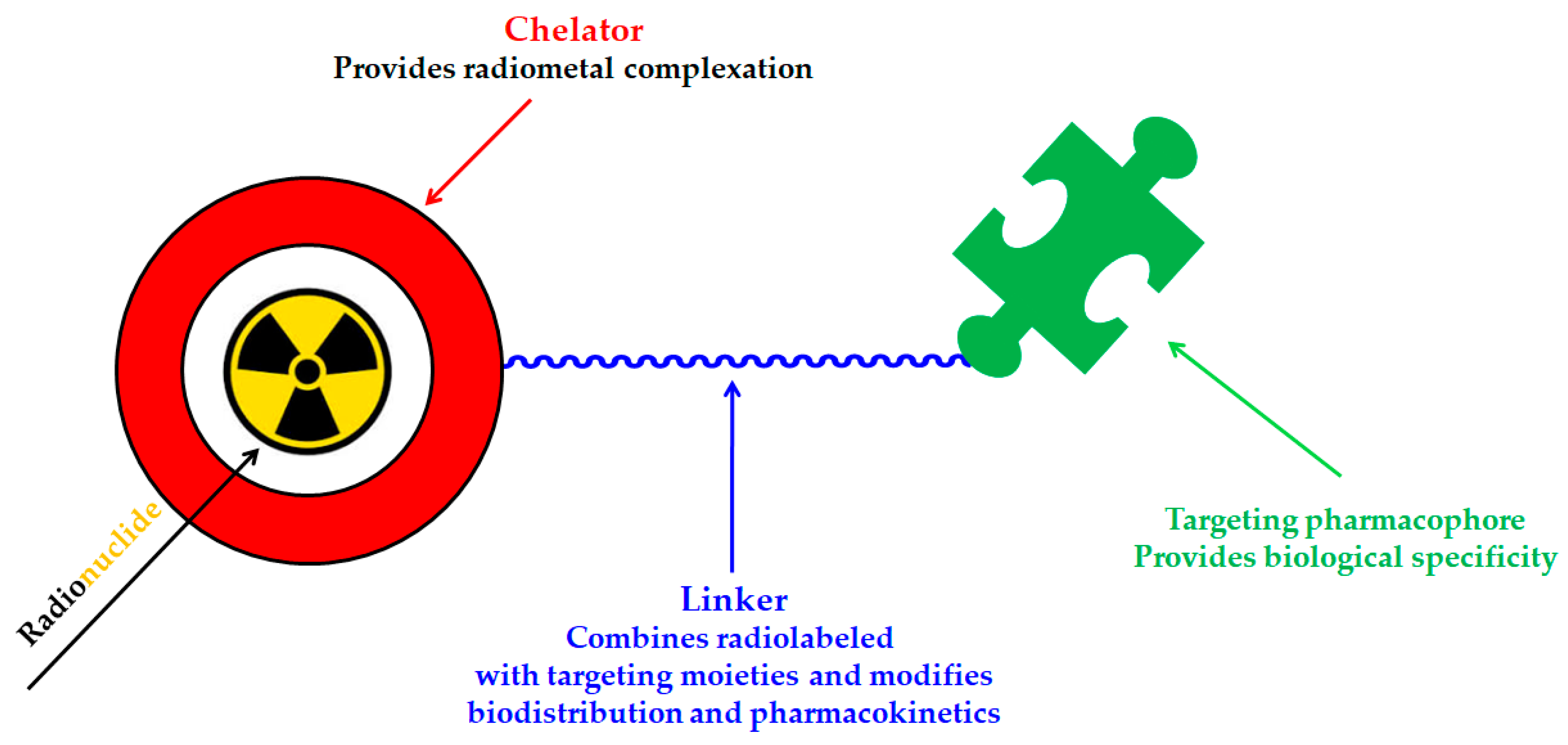
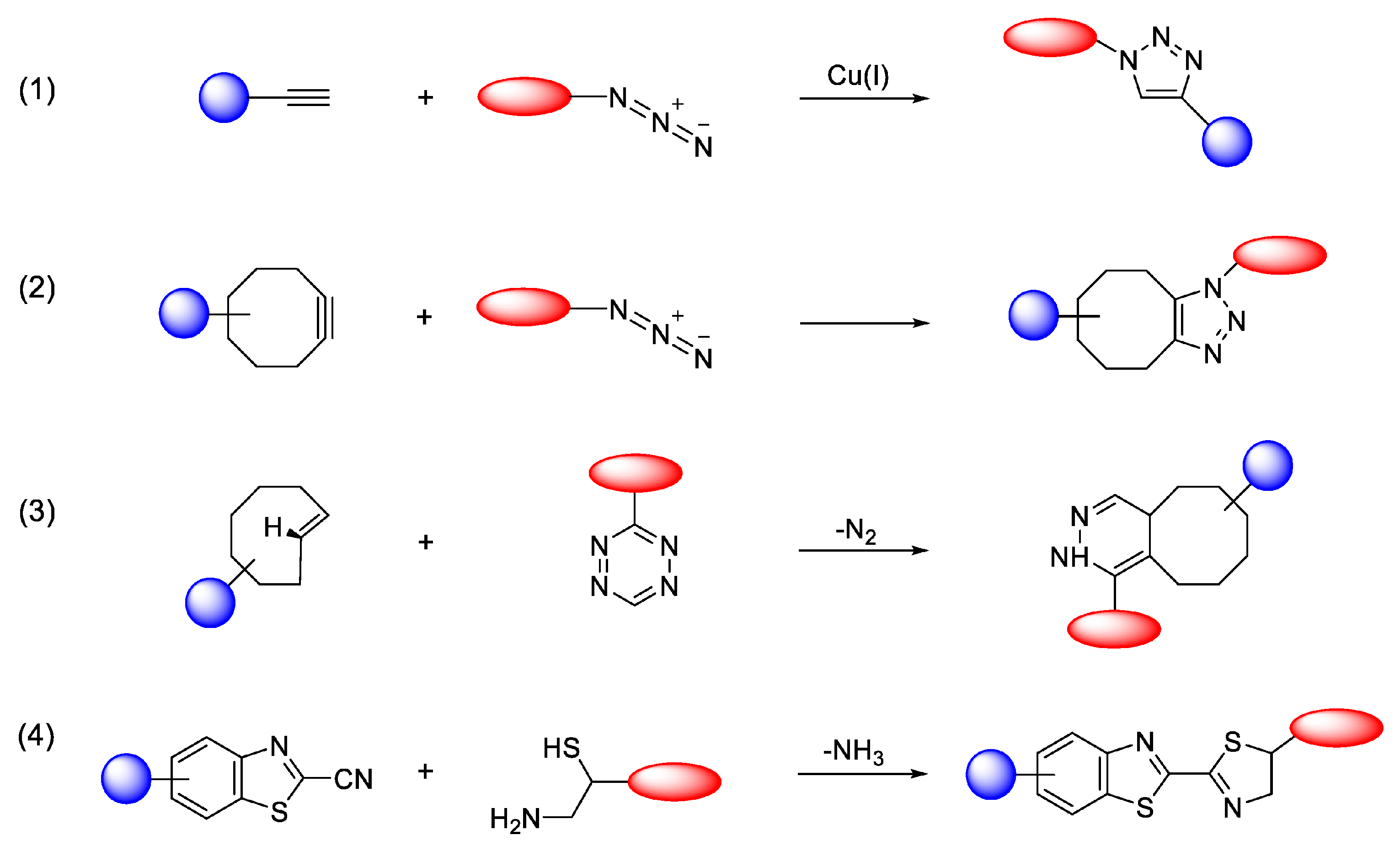
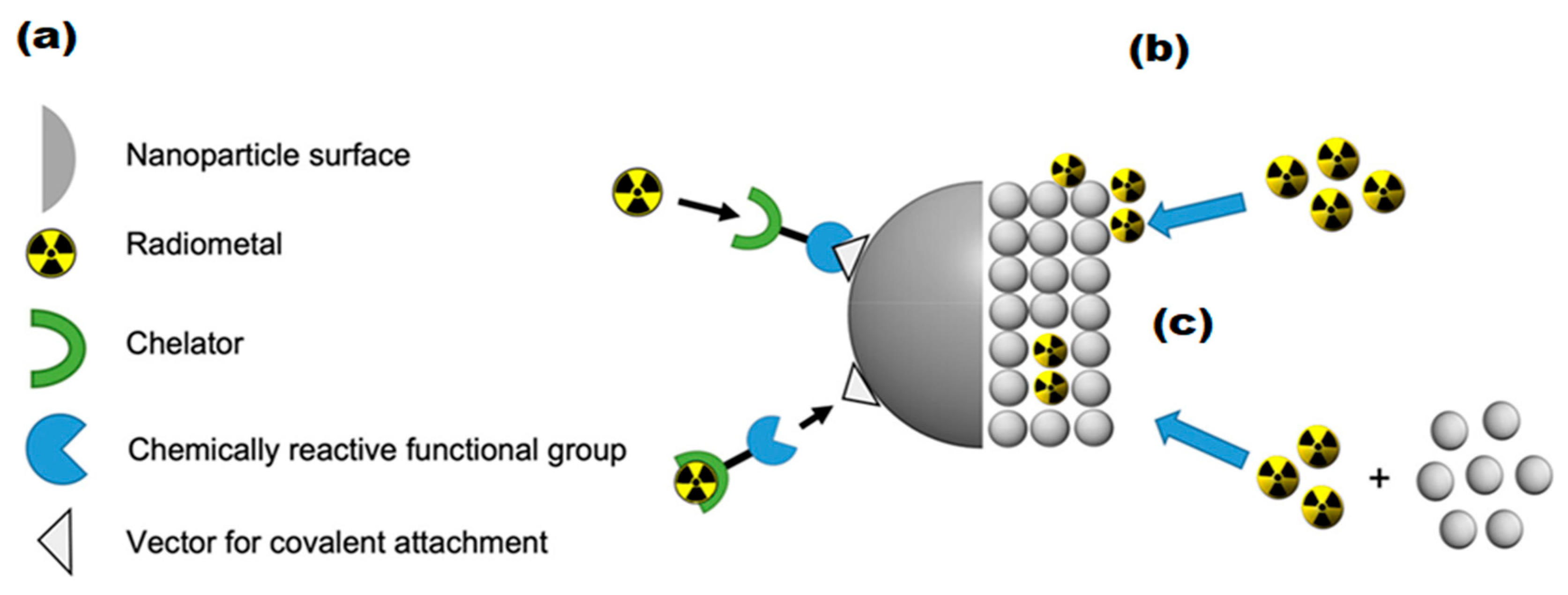
3.2. Radiolabeling of Magnetic Nano-Formulations
4. 177Lu-Labeled Magnetic Nano-Formulations
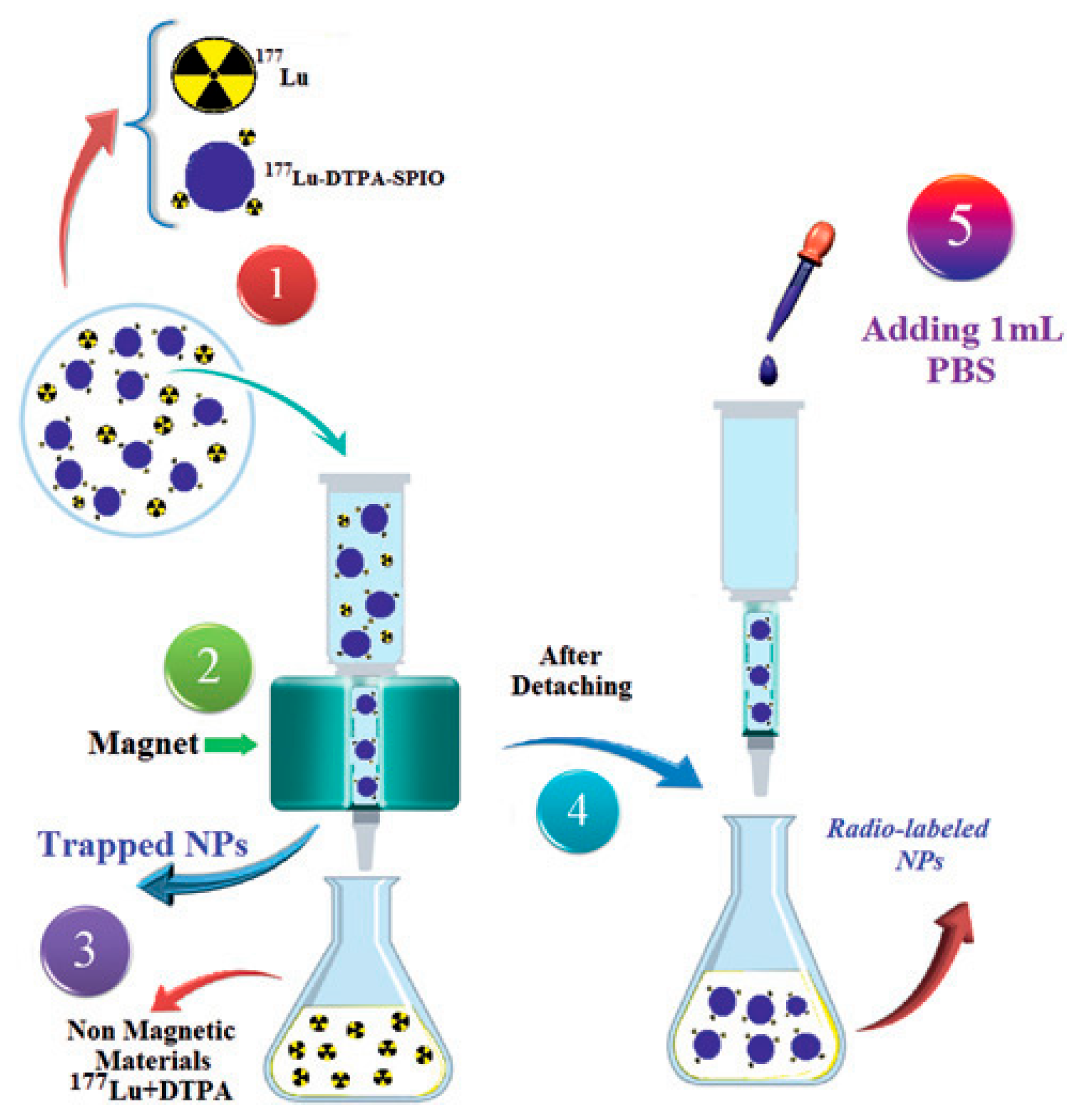
4.1. Synthesis, Radio- and Physico-Chemical Characterization
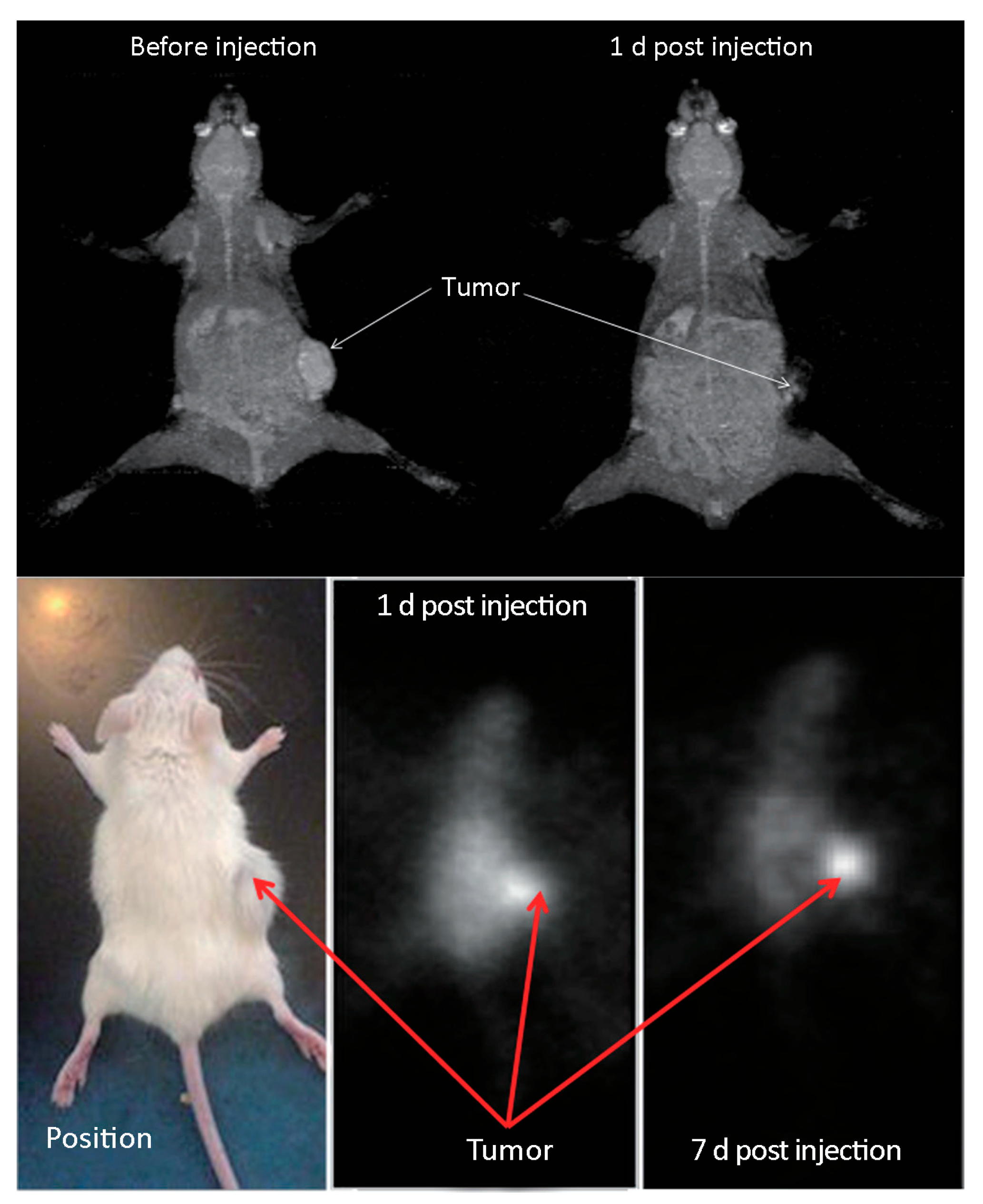
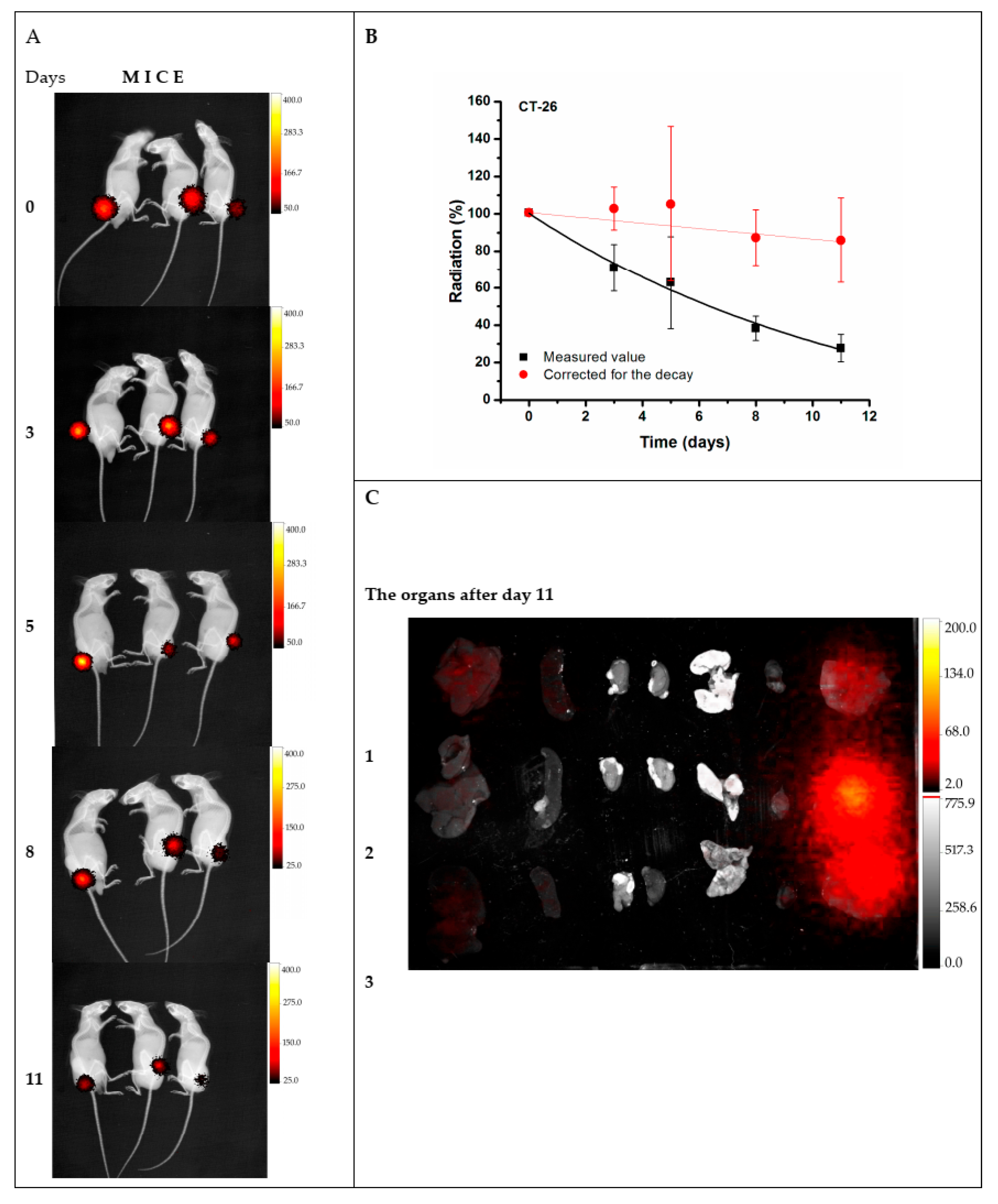
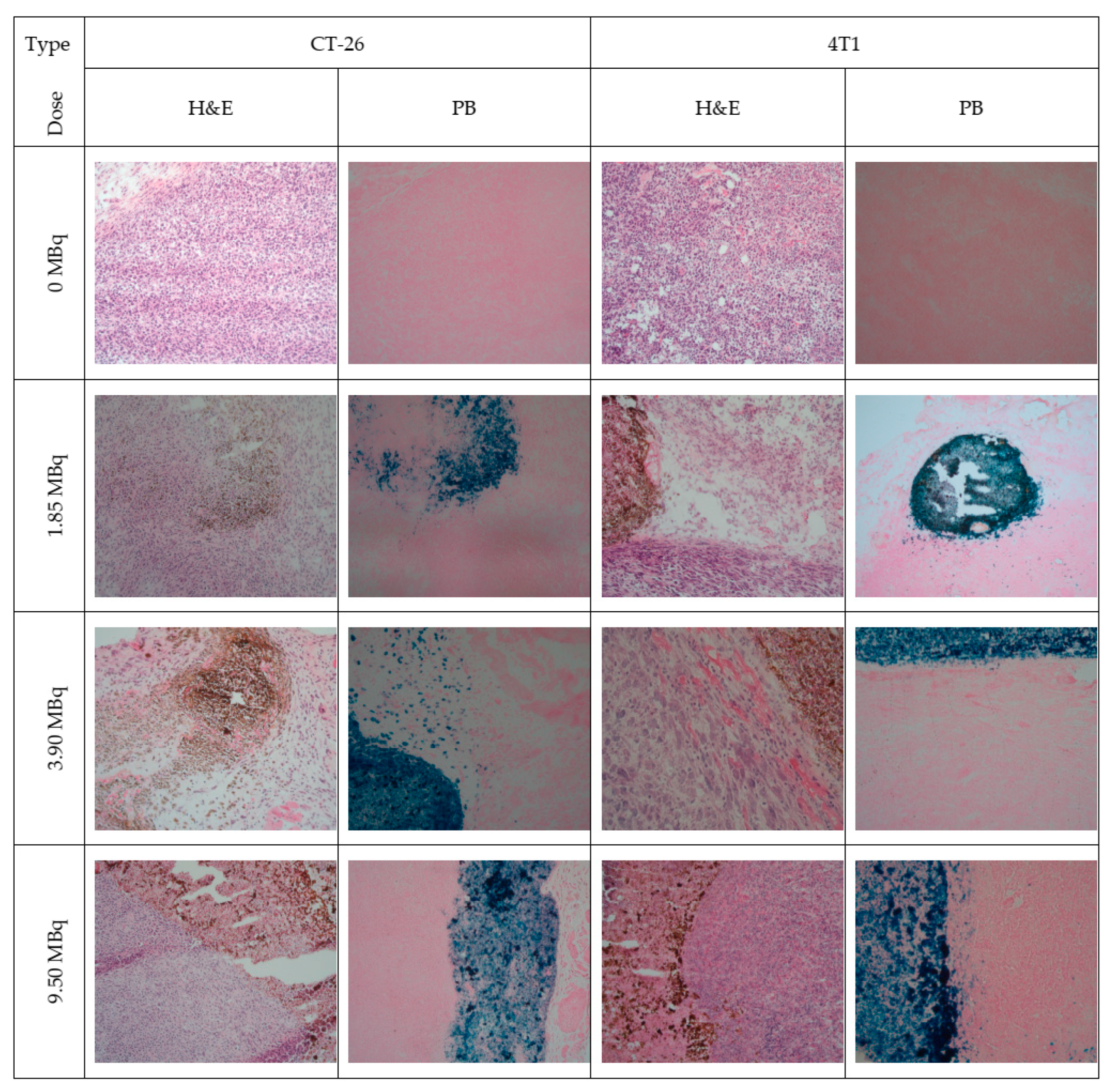
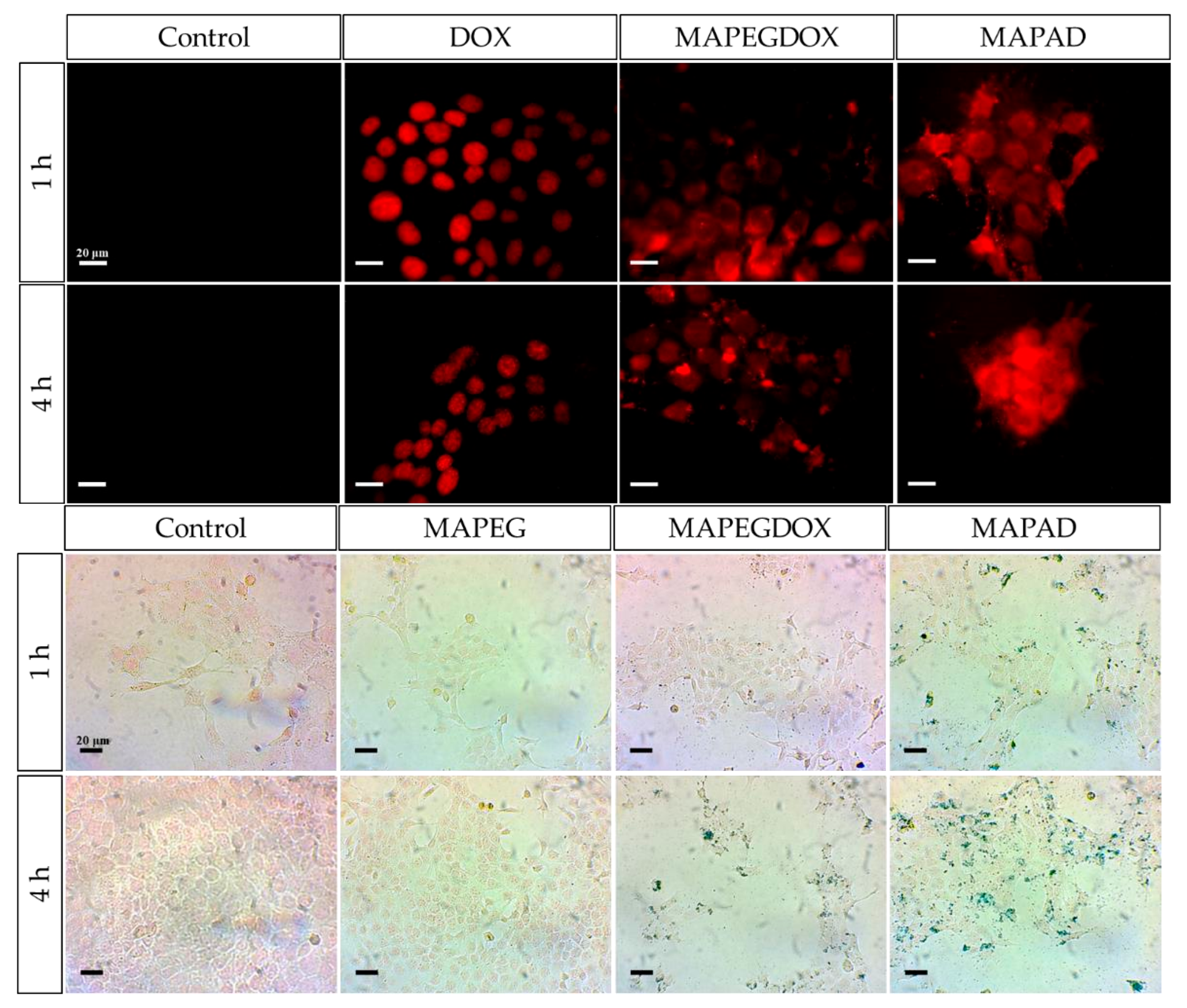
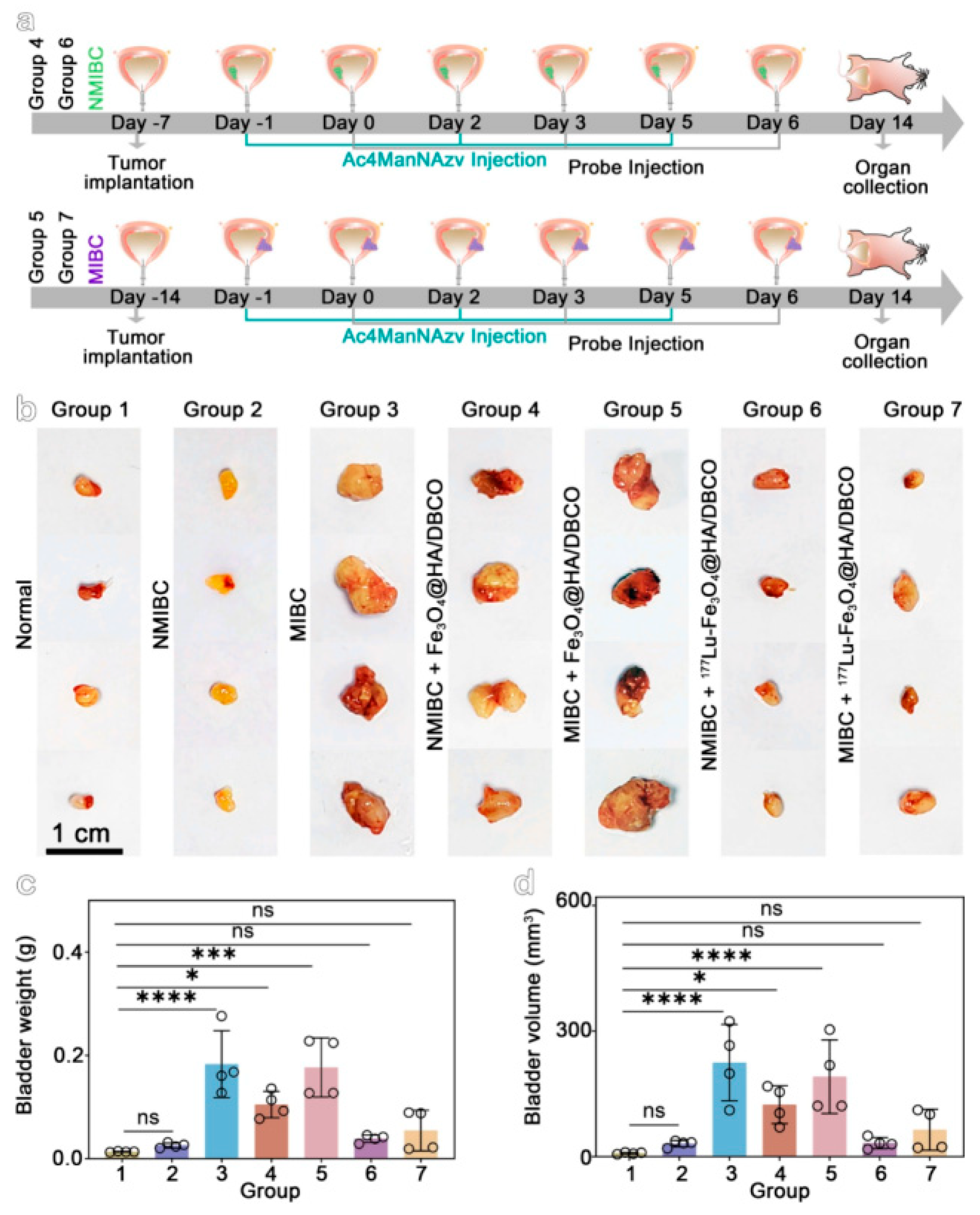
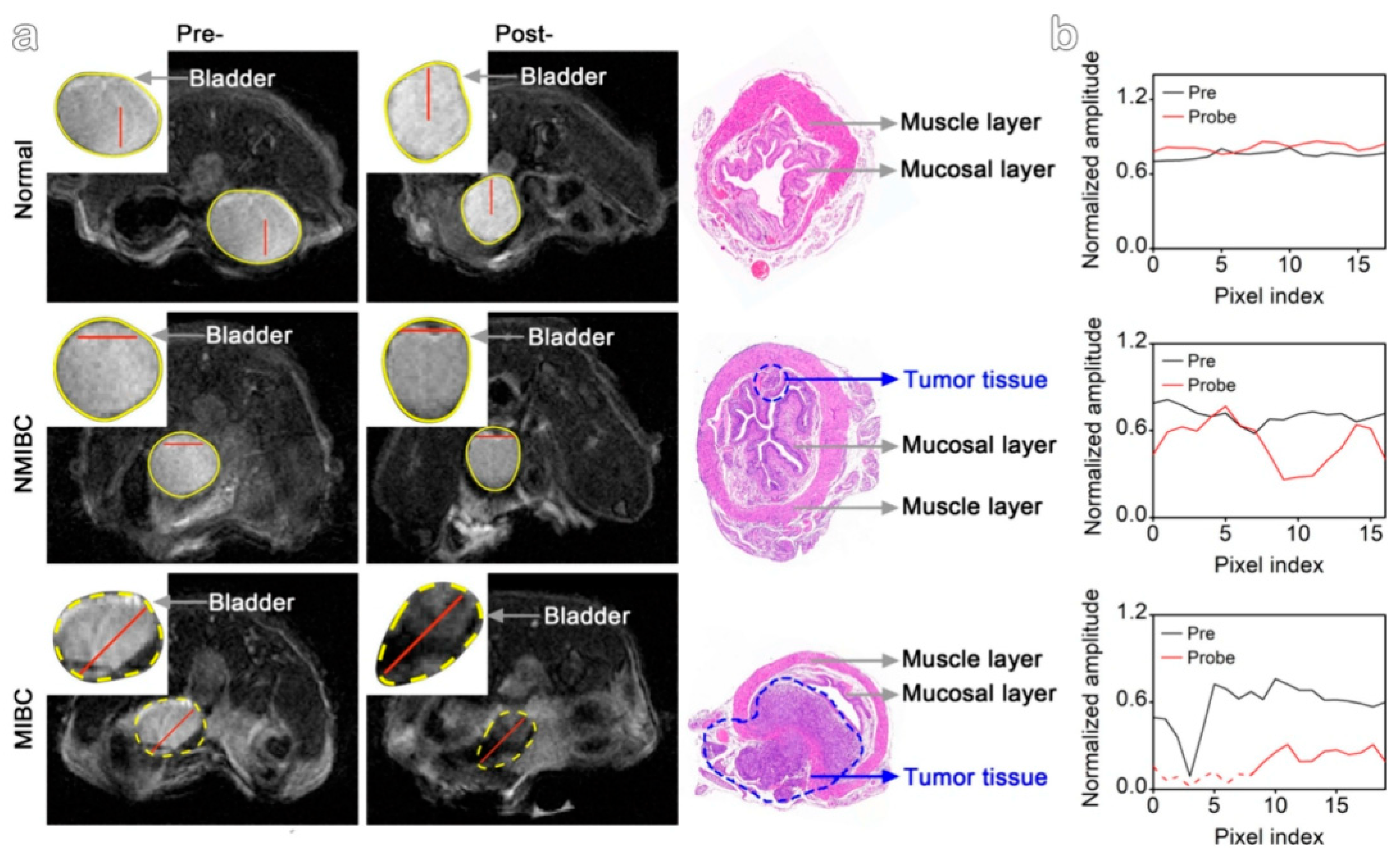
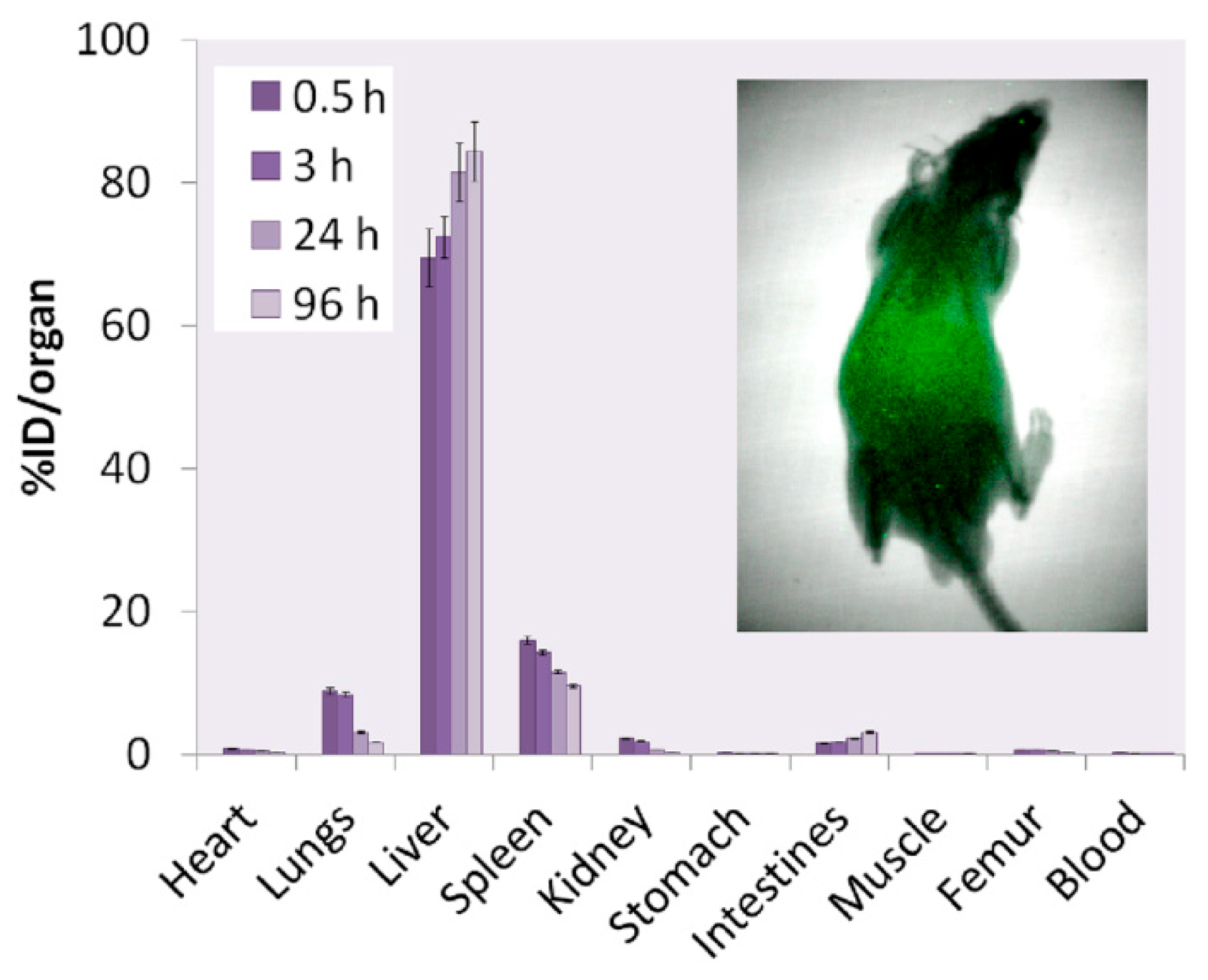
4.2. Biological Applications
4.3. Analysis of Multimodal Functionality
5. Current Challenges
6. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 177Lu | Lutetium-177 |
| TRT | Targeted radiation therapy |
| MNPs | Magnetic nanoparticles |
| NPs | Nanoparticles |
| MRI | Magnetic resonance imaging |
| SPECT | Single Photon Emission Computed Tomography |
| PET | Positron Emission Tomography |
| ICRP | International Commission on Radiological Protection |
| Lutathera® | [177Lu]Lu-DOTATATE |
| Pluvicto® | [177Lu]Lu-Vivipotide tetraxetan |
| PRRT | Peptide receptor radionuclide therapy |
| CuAAC | Copper(I)-catalyzed azide-alkyne cycloaddition |
| SPAAC | Strain-promoted azide-alkyne cycloaddition |
| IEDDA | Inverse-electron-demand Diels–Alder |
| CBT | 2-cyanobenzothiazole |
| NTA | Nitrilotriacetic acid |
| EDTA | Ethylenediaminetetraacetic acid |
| DTPA | Diethylenetriaminepentaacetic acid |
| DOTA | 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid |
| DO3A | 1,4,7,10-Tetraazacyclododecane-1,4,7-triacetic acid |
| NOTA | 1,4,7-Triazacyclononane-1,4,7-triacetic acid |
| NOTAGA | (NOTA (1,4,7-triazacyclononane-1,4,7-triacetic acid) + GA (glutaric acid)) |
| NETA | {4-[2-(bis-carboxymethylamino)-ethyl]-7-carboxymethyl-[1,4,7]-triazonan-1-yl}-acetic acid |
| DOTRP | 1,4,7,10-tetraazacyclodecane-1,4,7,10-tetra(R)ester phosphinic acid |
| NORP | 1,4,7-teriazacyclononane-1,4,7-tri(R)esterphosphinic acid |
| 177Hf | Hafnium-177 |
| 176Yb | Ytterbium-176 |
| 176Lu | Lutetium-176 |
| 169Yb | Ytterbium-169 |
| 175Yb | Ytterbium-175 |
| 177Yb | Ytterbium-177 |
| SA | Specific activity |
| GEP-NETs | Gastroenteropancreatic neuroendocrine tumors |
| PPGLs | Pheochromocytomas and paragangliomas |
| LAR | Long-acting repeatable |
| SSTR2 | Somatostatin Receptor 2 |
| mCRPC | Metastatic castration-resistant prostate cancer |
| PI(s) | Principal investigator(s) |
| PSMA | Prostate-specific membrane antigen |
| PET/CT | Positron emission tomography/Computed tomography |
| NETs | Neuroendocrine tumors |
| EPR | Enhanced permeability and retention |
| MIONPs | Magnetic iron oxide NPs |
| IONPs | Iron oxide NPs |
| SPIONPs | Superparamagnetic iron oxide NPs |
| SPIO | Superparamagnetic iron oxide |
| Fe3O4 | Magnetite |
| γ-Fe2O3 | Maghemite |
| PEG | Polyethylene glycol |
| co-CNCs | Condensed colloidal nanocrystal clusters |
| MA | Alginate-coated nanocrystallites |
| MAPEG | PEGylated MA |
| Dh | Hydrodynamic diameter |
| DLS | Dynamic light scattering |
| ITLC-SG | Instant Thin Layer Chromatography-Silica Gel |
| NBT | Nanobrachytherapy |
| DMSA | Meso-1,2-dimercaptosuccinic acid |
| TEM | Transmission electron microscopy |
| PDI | Polydispersity index |
| XRD | X-ray diffraction |
| FT-IR | Fourier-transform infrared |
| CA | Citric acid |
| PAA | Poly(acrylic acid) |
| SAED | Selected-area electron diffraction |
| MS | Saturation magnetization |
| EDC | N-ethyl-N′-(3-(dimethylamino)propyl)carbodiimide |
| NHS | N-hydroxysuccinimide |
| AA | Alginic acid |
| DOX | Doxorubicin |
| BVCZ | Bevacizumab |
| HPLC | High-performance liquid chromatography |
| TGA | Thermogravimetric analysis |
| RP-HPLC | Reverse-phase high-performance liquid chromatography |
| PBS | Phosphate-buffered saline |
| HIR | Heat-induced radiolabeling |
| FH | Feraheme |
| CMD | Carboxymethyldextran |
| ICP-MS | Inductively coupled plasma mass spectrometry |
| SEC | Size exclusion chromatography |
| LAGMERAL | Ligand Anchoring Group-Mediated Radiolabeling |
| DP-PEG | Diphosphonate-polyethylene glycol |
| FBS | Fetal bovine serum |
| ccDTPA | Cyclohexane-1,4-diyldinitrilo)tetraacetic acid dianhydride |
| MACS | Magnetic-assorting cell separation |
| PCS | Photon correlation spectroscopy |
| PLL | Poly-L-lysine |
| SAR | Specific absorption rate |
| Pro | Proline |
| Trp | Tryptophan |
| UV-Vis | Ultraviolet-Visible |
| DCS | Differential centrifugal sedimentation |
| HA | Hyaluronic acid |
| DBCO | Dibenzocyclooctyne |
| 177Lu-TCL-SPIONPs | 177Lu-labeled thermally cross-linked SPIONPs |
| RES | Reticuloendothelial system |
| VEGF | Vascular Endothelial Growth Factor |
| NMIBC | Nonmuscle-invasivebladder cancer |
| MIBC | Muscle-invasive bladder cancer |
| 177Lu-MNFs | 177Lu-labeled magnetic nano-formulations |
| GMP | Good Manufacturing Practice |
| RCY | Radiochemical yield |
| RCP | Radiochemical purity |
References
- Ferlay, J.; Ervik, M.; Lam, F.; Laversanne, M.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2024; Available online: https://gco.iarc.who.int/today (accessed on 5 August 2025).
- World Health Organization. Global Cancer Burden Growing, Amidst Mounting Need for Services. Available online: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services (accessed on 5 August 2025).
- Zafar, A.; Khatoon, S.; Khan, M.J.; Abu, J.; Naeem, A. Advancements and limitations in traditional anti-cancer therapies: A comprehensive review of surgery, chemotherapy, radiation therapy, and hormonal therapy. Discov. Oncol. 2025, 16, 607. [Google Scholar] [CrossRef]
- Gill, M.R.; Falzone, N.; Du, Y.; Vallis, K.A. Targeted radionuclide therapy in combined-modality regimens. Lancet Oncol. 2017, 18, e414–e423. [Google Scholar] [CrossRef]
- Chakraborty, K.; Mondal, J.; An, J.M.; Park, J.; Lee, Y.K. Advances in radionuclides and radiolabelled peptides for cancer therapeutics. Pharmaceutics 2023, 15, 971. [Google Scholar] [CrossRef] [PubMed]
- Ferro-Flores, G.; Azorín-Vega, E.; Ocampo-García, B.; Luna-Gutiérrez, M.; Cruz-Nova, P.; Meléndez-Alafort, L. Effects of targeted radionuclide therapy on cancer cells beyond the ablative radiation dose. Int. J. Mol. Sci. 2025, 26, 6968. [Google Scholar] [CrossRef]
- Niu, T.; Fan, M.; Lin, B.; Gao, F.; Tan, B.; Du, X. Current clinical application of lutetium-177 in solid tumors (Review). Exp. Ther. Med. 2024, 27, 225. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, A.; Qi, X.; Han, Z.; Song, L.; Zhou, J.; Wang, G.; Zhu, R.; Li, J. Radionuclide-Labeled Biomaterials: A Novel Strategy for Tumor-Targeted Therapy. Biomimetics 2025, 10, 394. [Google Scholar] [CrossRef] [PubMed]
- Cieslik, P.; Kubeil, M.; Zarschler, K.; Ullrich, M.; Brandt, F.; Anger, K.; Wadepohl, H.; Kopka, K.; Bachmann, M.; Pietzsch, J.; et al. Toward Personalized Medicine: One Chelator for Imaging and Therapy with Lutetium-177 and Actinium-225. J. Am. Chem. Soc. 2022, 144, 21555–21567. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Al-Toubah, T.; El-Haddad, G.; Strosberg, J. 177Lu-DOTATATE for the treatment of gastroenteropancreatic neuroendocrine tumors. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 1023–1031. [Google Scholar] [CrossRef]
- Hennrich, U.; Eder, M. [177Lu]Lu-PSMA-617 (PluvictoTM): The First FDA-approved radiotherapeutical for treatment of prostate cancer. Pharmaceuticals 2022, 15, 1292. [Google Scholar] [CrossRef]
- Karimi, A.; Bogdani, C.; O’Dwyer, E.; Siolas, D. Emerging innovations in theranostics for pancreatic neuroendocrine tumors. NPJ Precis. Oncol. 2025, 9, 146. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; et al. Progressing nanotechnology to improve targeted cancer treatment: Overcoming hurdles in its clinical implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef]
- Fahim, Y.A.; Hasani, I.W.; Mahmoud, R.W. Promising biomedical applications using superparamagnetic nanoparticles. Eur. J. Med. Res. 2025, 30, 441. [Google Scholar] [CrossRef]
- Zhu, L.; Zhou, Z.; Mao, H.; Yang, L. Magnetic nanoparticles for precision oncology: Theranostic magnetic iron oxide nanoparticles for image-guided and targeted cancer therapy. Nanomedicine 2016, 12, 73–87. [Google Scholar] [CrossRef]
- Bentivoglio, V.; Nayak, P.; Varani, M.; Lauri, C.; Signore, A. Methods for radiolabeling nanoparticles (Part 3): Therapeutic use. Biomolecules 2023, 13, 1241. [Google Scholar] [CrossRef]
- Chakravarty, R.; Goel, S.; Dash, A.; Cai, W. Radiolabeled inorganic nanoparticles for positron emission tomography imaging of cancer: An overview. Q. J. Nucl. Med. Mol. Imaging 2017, 61, 181–204. [Google Scholar] [CrossRef]
- Imtiaz, S.; Ferdous, U.T.; Nizela, A.; Hasan, A.; Shakoor, A.; Zia, A.W.; Uddin, S. Mechanistic study of cancer drug delivery: Current techniques, limitations, and future prospects. Eur. J. Med. Chem. 2025, 290, 117535. [Google Scholar] [CrossRef]
- Banerjee, S.; Pillai, M.R.; Knapp, F.F. Lutetium-177 therapeutic radiopharmaceuticals: Linking chemistry, radiochemistry, and practical applications. Chem. Rev. 2015, 115, 2934–2974. [Google Scholar] [CrossRef]
- Eckerman, K.; Endo, A. ICRP Publication 107. Nuclear decay data for dosimetric calculations. Ann. ICRP 2008, 38, 7–96. [Google Scholar] [PubMed]
- Lyra, M.E.; Andreou, M.; Georgantzoglou, A.; Kordolaimi, S.; Lagopati, N.; Ploussi, A.; Salvara, A.L.; Vamvakas, I. Radionuclides used in nuclear medicine therapy–From production to dosimetry. Curr. Med. Imaging 2013, 9, 51–75. [Google Scholar] [CrossRef]
- Yeong, C.H.; Cheng, M.H.; Ng, K.H. Therapeutic radionuclides in nuclear medicine: Current and future prospects. J. Zhejiang Univ. Sci. B 2014, 15, 845–863. [Google Scholar] [CrossRef]
- Stokke, C.; Kvassheim, M.; Blakkisrud, J. Radionuclides for targeted therapy: Physical properties. Molecules 2022, 27, 5429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, X.; Gao, X.; Chen, X.; Li, L.; Li, G.; Liu, C.; Miao, Y.; Wang, R.; Hu, K. Radiopharmaceuticals and their applications in medicine. Sig. Transduct. Target. Ther. 2025, 10, 1. [Google Scholar] [CrossRef]
- Pellico, J.; Ruiz-Cabello, J.; Herranz, F. Radiolabeled iron oxide nanomaterials for multimodal nuclear imaging and positive contrast magnetic resonance imaging (MRI): A review. ACS Appl. Nano Mater. 2023, 6, 20523–20538. [Google Scholar] [CrossRef]
- Hamoudeh, M.; Kamleh, M.A.; Diab, R.; Fessi, H. Radionuclides delivery systems for nuclear imaging and radiotherapy of cancer. Adv. Drug Deliv. Rev. 2008, 60, 1329–1346. [Google Scholar] [CrossRef] [PubMed]
- Qaim, S.M. Therapeutic radionuclides and nuclear data. Radiochim. Acta 2001, 89, 297–304. [Google Scholar] [CrossRef]
- Ersahin, D.; Doddamane, I.; Cheng, D. Targeted radionuclide therapy. Cancers 2011, 3, 3838–3855. [Google Scholar] [CrossRef]
- Salih, S.; Alkatheeri, A.; Alomaim, W.; Elliyanti, A. Radiopharmaceutical treatments for cancer therapy, Radionuclides characteristics, applications, and challenges. Molecules 2022, 27, 5231. [Google Scholar] [CrossRef]
- Browne, E. Nuclear Data Sheets for A=177. Nucl. Data Sheets 1993, 68, 747. [Google Scholar] [CrossRef]
- Welsh, J.S. Beta decay in science and medicine. Am. J. Clin. Oncol. Cancer Clin. Trials 2007, 30, 437–439. [Google Scholar] [CrossRef]
- Ferdinandus, J.; Violet, J.; Sandhu, S.; Hofman, M.S. Prostate-specific membrane antigen theranostics: Therapy with Lutetium-177. Curr. Opin. Urol. 2018, 28, 197–204. [Google Scholar] [CrossRef]
- Read, E.D.; Eu, P.; Little, P.J.; Piva, T.J. The status of radioimmunotherapy in CD20+ Non-Hodgkin’s lymphoma. Target. Oncol. 2015, 10, 15–26. [Google Scholar] [CrossRef]
- Schötzig, U.; Schrader, H.; Schönfeld, E.; Günther, E.; Klein, R. Standardisation and decay data of 177Lu and 188Re. Appl. Radiat. Isot. 2001, 55, 89–96. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Cheng, L.; Wu, J.; Bao, T.; Yao, R.; Liu, Y. A 3-dimensional stationary cascade gamma-ray coincidence imager. Phys. Med. Biol. 2021, 66, 225001. [Google Scholar] [CrossRef] [PubMed]
- Cutler, C.S.; Hennkens, H.M.; Sisay, N.; Huclier-Markai, S.; Jurisson, S.S. Radiometals for combined imaging and therapy. Chem. Rev. 2013, 113, 858–883. [Google Scholar] [CrossRef]
- Dash, A.; Knapp, F.F.; Pillai, M.R.A. Targeted radionuclide therapy—An overview. Curr. Radiopharm. 2013, 6, 152–180. [Google Scholar] [CrossRef]
- Ramogida, C.F.; Orvig, C. Tumour targeting with radiometals for diagnosis and therapy. Chem. Commun. 2013, 49, 4720–4739. [Google Scholar] [CrossRef]
- Volkert, W.A.; Goeckeler, W.F.; Ehrhardt, G.J.; Ketring, A.R. Therapeutic radionuclides: Production and decay property considerations. J. Nucl. Med. 1991, 32, 174–185. [Google Scholar]
- Ercan, M.T.; Caglar, M. Therapeutic radiopharmaceuticals. Curr. Pharm. Des. 2000, 6, 1085–1121. [Google Scholar]
- Guo, H.; Miao, Y. Melanoma targeting property of a Lu-177-labeled lactam bridge-cyclized alpha-MSH peptide. Bioorg. Med. Chem. Lett. 2013, 23, 2319–2323. [Google Scholar] [CrossRef] [PubMed]
- Yousefnia, H.; Jalilian, A.R.; Zolghadri, S.; Bahrami-Samani, A.; Shirvani-Arani, S.; Ghannadi-Maragheh, M. Preparation and quality control of 177Lu-[tris(1,10-phenanthroline) lutetium(III)] complex for therapy. Nucl. Med. Rev. Cent. East. Eur. 2010, 13, 49–54. [Google Scholar] [PubMed]
- Bakker, W.H.; Breeman, W.A.; Kwekkeboom, D.J.; De Jong, L.C.; Krenning, E.P. Practical aspects of peptide receptor radionuclide therapy with [177Lu][DOTA0, Tyr3]octreotate. Q. J. Nucl. Med. Mol. Imaging. 2006, 50, 265–271. [Google Scholar]
- Dash, A.; Pillai, M.R.; Knapp, F.F., Jr. Production of 177Lu for targeted radionuclide therapy: Available options. Nucl. Med. Mol. Imaging 2015, 49, 85–107. [Google Scholar] [CrossRef]
- Dadgar, H.; Jafari, E.; Ahmadzadehfar, H.; Rekabpour, S.J.; Ravanbod, M.R.; Kalantarhormozi, M.; Nabipour, I.; Assadi, M. Feasibility and therapeutic potential of the 68Ga/177Lu-DOTATATE theranostic pair in patients with metastatic medullary thyroid carcinoma. Ann. Endocrinol. 2023, 84, 45–51. [Google Scholar] [CrossRef]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef]
- Parus, J.; Pawlak, D.; Mikolajczak, R.; Duatti, A. Chemistry and bifunctional chelating agents for binding 177Lu. Curr. Radiopharm. 2015, 8, 86–94. [Google Scholar] [CrossRef]
- Shi, D.; Beasock, D.; Fessler, A.; Szebeni, J.; Ljubimova, J.Y.; Afonin, K.A.; Dobrovolskaia, M.A. To PEGylate or not to PEGylate: Immunological properties of nanomedicine’s most popular component, polyethylene glycol and its alternatives. Adv. Drug Deliv. Rev. 2022, 180, 114079. [Google Scholar] [CrossRef]
- Wu, J.R.; Canjels, A.; Miyauchi, R.; Kwon, E.J. Impact of conjugation chemistry on the pharmacokinetics of peptide-polymer conjugates in a model of traumatic brain injury. Bioconjug. Chem. 2025, 36, 1483–1493. [Google Scholar] [CrossRef] [PubMed]
- Zeglis, B.M.; Lewis, J.S. A practical guide to the construction of radiometallated bioconjugates for positron emission tomography. Dalton Trans. 2011, 40, 6168–6195. [Google Scholar] [CrossRef] [PubMed]
- George, S.C.; Samuel, E.J.J. Developments in 177Lu-based radiopharmaceutical therapy and dosimetry. Front. Chem. 2023, 11, 1218670. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, S.; Yun, S.-J.; Jeon, J. Recent advances in bioorthogonal click chemistry for efficient synthesis of radiotracers and radiopharmaceuticals. Molecules 2019, 24, 3567. [Google Scholar] [CrossRef]
- Nair, D.P.; Podgorski, M.; Chatani, S.; Gong, T.; Xi, W.; Fenoli, C.R.; Bowman, C.N. The thiol-Michael addition click reaction: A powerful and widely used tool in materials chemistry. Chem. Mater. 2013, 26, 724–744. [Google Scholar] [CrossRef]
- Crisalli, P.; Kool, E.T. Water-soluble organocatalysts for hydrazone and oxime formation. J. Org. Chem. 2013, 78, 1184–1189. [Google Scholar] [CrossRef]
- Wang, Q.; Chan, T.R.; Hilgraf, R.; Fokin, V.V.; Sharpless, K.B.; Finn, M.G. Bioconjugation by copper (I)-catalyzed azide-alkyne [3+2] cycloaddition. J. Am. Chem. Soc. 2003, 125, 3192–3193. [Google Scholar] [CrossRef] [PubMed]
- Jewett, J.C.; Bertozzi, C.R. Cu-free click cycloaddition reactions in chemical biology. Chem. Soc. Rev. 2010, 39, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Šečkutė, J.; Devaraj, N.K. Expanding room for tetrazine ligations in the in vivo chemistry toolbox. Curr. Opin. Chem. Biol. 2013, 17, 761–767. [Google Scholar] [CrossRef]
- Walsh, J.C.; Kolb, H.C. Applications of click chemistry in radiopharmaceutical development. CHIMIA Int. J. Chem. 2010, 64, 29–33. [Google Scholar]
- Li, Z.; Conti, P.S. Radiopharmaceutical chemistry for positron emission tomography. Adv. Drug Deliv. Rev. 2010, 62, 1031–1051. [Google Scholar] [CrossRef]
- Price, E.W.; Orvig, C. Matching chelators to radiometals for radiopharmaceuticals. Chem. Soc. Rev. 2014, 43, 260–290. [Google Scholar] [CrossRef]
- Liu, S. Bifunctional coupling agents for radiolabeling of biomolecules and target-specific delivery of metallic radionuclides. Adv. Drug Deliv. Rev. 2008, 60, 1347–1370. [Google Scholar] [CrossRef]
- Kang, C.S.; Sun, X.; Jia, F.; Song, H.A.; Chen, Y.; Lewis, M.; Chong, H.S. Synthesis and preclinical evaluation of bifunctional ligands for improved chelation chemistry of 90Y and 177Lu for targeted radioimmunotherapy. Bioconjug. Chem. 2012, 23, 1775–1782. [Google Scholar] [CrossRef]
- Milenic, D.E.; Brady, E.D.; Brechbiel, M.W. Antibody-targeted radiation cancer therapy. Nat. Rev. Drug Discov. 2004, 3, 488–499. [Google Scholar] [CrossRef]
- Ando, A.; Ando, I.; Tonami, N.; Kinuya, S.; Kazuma, K.; Kataiwa, A.; Nakagawa, M.; Fujita, N. 177Lu-EDTMP: A potential therapeutic bone agent. Nucl. Med. Commun. 1998, 19, 587–591. [Google Scholar] [CrossRef]
- Solá, G.A.R.; Argüelles, M.G.; Bottazzini, D.L.; Furnari, J.C.; Parada, I.G.; Rojo, A.; Ruiz, H.V. Lutetium-177-EDTMP for bone pain palliation. Preparation, biodistribution and pre-clinical studies. Radiochim. Acta 2000, 88, 157–162. [Google Scholar] [CrossRef]
- Chakraborty, S.; Das, T.; Unni, P.R.; Sarma, H.D.; Samuel, G.; Banerjee, S.; Venkatesh, M.; Ramamoorthy, N.; Pillai, M.R. 177Lu labelled polyaminophosphonates as potential agents for bone pain palliation. Nucl. Med. Commun. 2002, 23, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Chakraborty, S.; Unni, P.R.; Banerjee, S.; Samuel, G.; Sarma, H.D.; Venkatesh, M.; Pillai, M.R.A. 177Lu-labeled cyclic polyaminophosphonates as potential agents for bone pain palliation. Appl. Radiat. Isot. 2002, 57, 177–184. [Google Scholar] [CrossRef]
- Chakraborty, S.; Das, T.; Sarma, H.D.; Venkatesh, M.; Banerjee, S. Comparative studies of 177Lu-EDTMP and 177Lu-DOTMP as potential agents for palliative radiotherapy of bone metastasis. Appl. Radiat. Isot. 2008, 66, 1196–1205. [Google Scholar] [CrossRef]
- Bodei, L.; Mueller-Brand, J.; Baum, R.P.; Pavel, M.E.; Hörsch, D.; O’Dorisio, M.S.; O’Dorisio, T.M.; Howe, J.R.; Cremonesi, M.; Kwekkeboom, D.J.; et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 800–816. [Google Scholar] [CrossRef]
- Kwekkeboom, D.J.; Bakker, W.H.; Kooij, P.P.; Konijnenberg, M.W.; Srinivasan, A.; Erion, J.L.; Schmidt, M.A.; Bugaj, J.L.; de Jong, M.; Krenning, E.P. [177Lu-DOTAOTyr3]octreotate: Comparison with [111In-DTPAo]octreotide in patients. Eur. J. Nucl. Med. 2001, 28, 1319–1325. [Google Scholar] [CrossRef]
- Vogel, W.V.; van der Marck, S.C.; Versleijen, M.W.J. Challenges and future options for the production of lutetium-177. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2329–2335. [Google Scholar] [CrossRef] [PubMed]
- Carollo, A.; Papi, S.; Chinol, M. Lutetium-177 labeled peptides: The European Institute of Oncology Experience. Curr. Radiopharm. 2016, 9, 19–32. [Google Scholar] [CrossRef]
- Global Lutetium-177 (Lu-177) Market Research Report 2025. Available online: https://reports.valuates.com/market-reports/QYRE-Auto-4H13190/global-lutetium-177-lu-177 (accessed on 26 October 2025).
- Lutetium-177 Market Size, Share & Industry Analysis, By Drug (LUTATHERA {lutetium Lu 177 dotatate}, PLUVICTO {lutetium Lu 177 vipivotide tetraxetan}, and Others) By Age Group (Adults and Pediatrics), By Application (Neuroendocrine Tumor, Prostate Cancer, and Others) By End-User (Hospitals, Specialty Clinics, and Others), and Regional Forecast, 2025–2032. Available online: https://www.fortunebusinessinsights.com/lutetium-177-market-112740 (accessed on 26 October 2025).
- Trials, C. Clinicaltrials.gov Search Results for 177Lu. 2025. Available online: https://www.clinicaltrials.gov/search?term=177Lu&viewType=Table&aggFilters=results:with,status:com%20act%20ter&limit=50&page=1 (accessed on 26 October 2025).
- Aggarwal, R.; Starzinski, S.; de Kouchkovsky, I.; Koshkin, V.; Bose, R.; Chou, J.; Desai, A.; Kwon, D.; Kaushal, S.; Trihy, L.; et al. Single-dose 177Lu-PSMA-617 followed by maintenance pembrolizumab in patients with metastatic castration-resistant prostate cancer: An open-label, dose-expansion, phase 1 trial. Lancet Oncol. 2023, 24, 1266–1276. [Google Scholar] [CrossRef]
- Vlachostergios, P.J.; Niaz, M.J.; Skafida, M.; Mosallaie, S.A.; Thomas, C.; Christos, P.J.; Osborne, J.R.; Molina, A.M.; Nanus, D.M.; Bander, N.H.; et al. Imaging expression of prostate-specific membrane antigen and response to PSMA-targeted β-emitting radionuclide therapies in metastatic castration-resistant prostate cancer. Prostate 2021, 81, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Iravani, A.; Violet, J.; Azad, A.; Hofman, M.S. Lutetium-177 prostate-specific membrane antigen (PSMA) theranostics: Practical nuances and intricacies. PCAN 2020, 23, 38–52. [Google Scholar] [CrossRef]
- Chi, K.N.; Yip, S.M.; Bauman, G.; Probst, S.; Emmenegger, U.; Kollmannsberger, C.K.; Martineau, P.; Niazi, T.; Pouliot, F.; Rendon, R.; et al. 177Lu-PSMA-617 in metastatic castration-resistant prostate cancer: A review of the evidence and implications for Canadian clinical practice. Curr. Oncol. 2024, 31, 1400–1415. [Google Scholar] [CrossRef]
- Fizazi, K.; Herrmann, K.; Krause, B.J.; Rahbar, K.; Chi, K.N.; Morris, M.J.; Sartor, O.; Tagawa, S.T.; Kendi, A.T.; Vogelzang, N.; et al. Health-related quality of life and pain outcomes with [177Lu]Lu-PSMA-617 plus standard of care versus standard of care in patients with metastatic castration-resistant prostate cancer (VISION): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2023, 24, 597–610. [Google Scholar] [CrossRef]
- Herrmann, K.; Rahbar, K.; Eiber, M.; Sparks, R.; Baca, N.; Krause, B.J.; Lassmann, M.; Jentzen, W.; Tang, J.; Chicco, D.; et al. Renal and multiorgan safety of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer in the VISION dosimetry substudy. J. Nucl. Med. 2024, 65, 71–78. [Google Scholar] [CrossRef]
- Kuo, P.H.; Yoo, D.C.; Avery, R.; Seltzer, M.; Calais, J.; Nagarajah, J.; Weber, W.A.; Fendler, W.P.; Hofman, M.S.; Krause, B.J.; et al. A VISION substudy of reader agreement on 68Ga-PSMA-11 PET/CT scan interpretation to determine patient eligibility for 177Lu-PSMA-617 radioligand therapy. J. Nucl. Med. 2023, 64, 1259–1265. [Google Scholar] [CrossRef]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–PSMA-617 for metastatic castration-resistant prostate cancer. N. Engl. J. Med. 2021, 385, 1091–1103. [Google Scholar] [CrossRef]
- Armstrong, A.J.; Sartor, O.; de Bono, J.; Chi, K.; Fizazi, K.; Krause, B.J.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Saad, F.; et al. Association of declining prostate-specific antigen levels with clinical outcomes in patients with metastatic castration-resistant prostate cancer receiving [177Lu]Lu-PSMA-617 in the phase 3 VISION trial. Eur. Urol. 2024, 86, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.N.; Armstrong, A.J.; Krause, B.J.; Herrmann, K.; Rahbar, K.; de Bono, J.S.; Adra, N.; Garje, R.; Michalski, J.M.; Kempel, M.M.; et al. Safety analyses of the phase 3 VISION trial of [177Lu]Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer. Eur. Urol. 2024, 85, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.; Gafita, A.; de Bono, J.S.; Sartor, O.; Chi, K.N.; Krause, B.J.; Rahbar, K.; Tagawa, S.T.; Czernin, J.; El-Haddad, G.; et al. Multivariable models of outcomes with [177Lu]Lu-PSMA-617: Analysis of the phase 3 VISION trial. EClinicalMedicine 2024, 77, 102862. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.H.; Morris, M.J.; Hesterman, J.; Kendi, A.T.; Rahbar, K.; Wei, X.X.; Fang, B.; Adra, N.; Garje, R.; Michalski, J.M.; et al. Quantitative 68Ga-PSMA-11 PET and clinical outcomes in metastatic castration-resistant prostate cancer following 177Lu-PSMA-617 (VISION trial). Radiology 2024, 312, e233460. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.J.; de Bono, J.; Nagarajah, J.; Sartor, O.; Wei, X.X.; Nordquist, L.T.; Koshkin, V.S.; Chi, K.N.; Krause, B.J.; Herrmann, K.; et al. Correlation analyses of radiographic progression-free survival with clinical and health-related quality of life outcomes in metastatic castration-resistant prostate cancer: Analysis of the phase 3 VISION trial. Cancer 2024, 130, 3426–3435. [Google Scholar] [CrossRef]
- Kim, C.; Liu, S.V.; Subramaniam, D.S.; Torres, T.; Loda, M.; Esposito, G.; Giaccone, G. Phase I study of the 177Lu-DOTA0-Tyr3-Octreotate (lutathera) in combination with nivolumab in patients with neuroendocrine tumors of the lung. J. Immunother. Cancer 2020, 8, e000980. [Google Scholar] [CrossRef] [PubMed]
- Dahle, J.; Repetto-Llamazares, A.H.; Mollatt, C.S.; Melhus, K.B.; Bruland, O.S.; Kolstad, A.; Larsen, R.H. Evaluating antigen targeting and anti-tumor activity of a new anti-CD37 radioimmunoconjugate against non-Hodgkin’s lymphoma. Anticancer Res. 2013, 33, 85–95. [Google Scholar]
- Repetto-Llamazares, A.H.; Larsen, R.H.; Mollatt, C.; Lassmann, M.; Dahle, J. Biodistribution and dosimetry of (177)Lu-tetulomab, a new radioimmunoconjugate for treatment of non-Hodgkin lymphoma. Curr. Radiopharm. 2013, 6, 20–27. [Google Scholar] [CrossRef]
- Cheson, B.D.; Pfistner, B.; Juweid, M.E.; Gascoyne, R.D.; Specht, L.; Horning, S.J.; Coiffier, B.; Fisher, R.I.; Hagenbeek, A.; Zucca, E.; et al. International Harmonization Project on Lymphoma. Revised response criteria for malignant lymphoma. J. Clin. Oncol. 2007, 25, 579–586. [Google Scholar] [CrossRef]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A.; Alliance, Australasian Leukaemia and Lymphoma Group; Eastern Cooperative Oncology Group; European Mantle Cell Lymphoma Consortium; et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014, 32, 3059–3068. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Caplin, M.E.; Kunz, P.L.; Ruszniewski, P.B.; Bodei, L.; Hendifar, A.; Mittra, E.; Wolin, E.M.; Yao, J.C.; Pavel, M.E.; et al. 177Lu-Dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): Final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1752–1763. [Google Scholar] [CrossRef]
- Strosberg, J.; Kunz, P.L.; Hendifar, A.; Yao, J.; Bushnell, D.; Kulke, M.H.; Baum, R.P.; Caplin, M.; Ruszniewski, P.; Delpassand, E.; et al. Impact of liver tumour burden, alkaline phosphatase elevation, and target lesion size on treatment outcomes with 177Lu-Dotatate: An analysis of the NETTER-1 study. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2372–2382. [Google Scholar] [CrossRef]
- Strosberg, J.; Wolin, E.; Chasen, B.; Kulke, M.; Bushnell, D.; Caplin, M.; Baum, R.P.; Kunz, P.; Hobday, T.; Hendifar, A.; et al. Health-related quality of life in patients with progressive midgut neuroendocrine tumors treated with 177Lu-Dotatate in the Phase III NETTER-1 trial. J. Clin. Oncol. 2018, 36, 2578–2584. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef]
- Tagawa, S.T.; Milowsky, M.I.; Morris, M.J.; Vallabhajosula, S.; Goldsmith, S.; Matulich, D.; Kaplan, J.; Berger, F.; Scher, H.I.; Bander, N.H.; et al. Phase II trial of 177Lutetium radiolabeled anti-prostate-specific membrane antigen (PSMA) monoclonal antibody J591 in patients with metastatic castrate-resistant prostate cancer. J. Clin. Oncol. 2008, 26 (Suppl. 15), 5140. [Google Scholar] [CrossRef]
- Tagawa, S.T.; Milowsky, M.I.; Morris, M.; Vallabhajosula, S.; Christos, P.; Akhtar, N.H.; Osborne, J.; Goldsmith, S.J.; Larson, S.; Taskar, N.P.; et al. Phase II study of Lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2013, 19, 5182–5191. [Google Scholar] [CrossRef]
- Stillebroer, A.B.; Boerman, O.C.; Desar, I.M.; Boers-Sonderen, M.J.; van Herpen, C.M.; Langenhuijsen, J.F.; Smith-Jones, P.M.; Oosterwijk, E.; Oyen, W.J.; Mulders, P.F. Phase 1 radioimmunotherapy study with lutetium 177-labeled anti-carbonic anhydrase IX monoclonal antibody girentuximab in patients with advanced renal cell carcinoma. Eur. Urol. 2013, 64, 478–485. [Google Scholar] [CrossRef] [PubMed]
- Stillebroer, A.B.; Zegers, C.M.; Boerman, O.C.; Oosterwijk, E.; Mulders, P.F.; O’Donoghue, J.A.; Visser, E.P.; Oyen, W.J. Dosimetric analysis of 177Lu-cG250 radioimmunotherapy in renal cell carcinoma patients: Correlation with myelotoxicity and pretherapeutic absorbed dose predictions based on 111In-cG250 imaging. J. Nucl. Med. 2012, 53, 82–89. [Google Scholar] [CrossRef]
- Pelaz, B.; Alexiou, C.; Alvarez-Puebla, R.A.; Alves, F.; Andrews, A.M.; Ashraf, S.; Balogh, L.P.; Ballerini, L.; Bestetti, A.; Brendel, C.; et al. Diverse applications of nanomedicine. ACS Nano 2017, 11, 2313–2381. [Google Scholar] [CrossRef]
- Szczyglewska, P.; Feliczak-Guzik, A.; Nowak, I. Nanotechnology–General aspects: A chemical reduction approach to the synthesis of nanoparticles. Molecules 2023, 28, 4932. [Google Scholar] [CrossRef] [PubMed]
- Puri, A.; Loomis, K.; Smith, B.; Lee, J.H.; Yavlovich, A.; Heldman, E.; Blumenthal, R. Lipid-based nanoparticles as pharmaceutical drug carriers: From concepts to clinic. Crit. Rev. Ther. Drug Carr. Syst. 2009, 26, 523–580. [Google Scholar] [CrossRef]
- Perez-Herrero, E.; Fernandez-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef]
- Zhang, L.W.; Gao, S.; Zhang, F.; Yang, K.; Ma, Q.J.; Zhu, L. Activatable hyaluronic acid nanoparticle as a theranostic agent for optical/photoacoustic image-guided photothermal therapy. ACS Nano 2014, 8, 12250–12258. [Google Scholar] [CrossRef]
- Zhou, H.; Fan, Z.; Deng, J.; Lemons, P.K.; Arhontoulis, D.C.; Bowne, W.B.; Cheng, H. Hyaluronidase embedded in nanocarrier PEG shell for enhanced tumor penetration and highly efficient antitumor efficacy. Nano Lett. 2016, 16, 3268–3277. [Google Scholar] [CrossRef]
- Fu, G.; Zhu, L.; Yang, K.; Zhuang, R.; Xie, J.; Zhang, F. Diffusion weighted magnetic resonance imaging for therapy response monitoring and early treatment prediction of photothermal therapy. ACS Appl. Mater. Interfaces 2016, 8, 5137–5147. [Google Scholar] [CrossRef]
- Makino, A.; Kimura, S. Solid tumor-targeting theranostic polymer nanoparticle in nuclear medicinal fields. Sci. World J. 2014, 2014, 424513. [Google Scholar] [CrossRef]
- Al-Thani, A.N.; Jan, A.G.; Abbas, M.; Geetha, M.; Sadasivuni, K.K. Nanoparticles in cancer theragnostic and drug delivery: A comprehensive review. Life Sci. 2024, 352, 122899. [Google Scholar] [CrossRef]
- Giner-Casares, J.J.; Henriksen-Lacey, M.; Coronado-Puchau, M.; Liz-Marzán, L.M. Inorganic nanoparticles for biomedicine: Where materials scientists meet medical research. Mater. Today 2016, 19, 19–28. [Google Scholar] [CrossRef]
- Raheem, M.A.; Rahim, M.A.; Gul, I.; Zhong, X.; Xiao, C.; Zhang, H.; Wei, J.; He, Q.; Hassan, M.; Zhang, C.Y.; et al. Advances in nanoparticles-based approaches in cancer theranostics. OpenNano 2023, 12, 100152. [Google Scholar] [CrossRef]
- Cho, E.C.; Glaus, C.; Chen, J.; Welch, M.J.; Xia, Y. Inorganic nanoparticle-based contrast agents for molecular imaging. Trends Mol. Med. 2010, 16, 561–573. [Google Scholar] [CrossRef]
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Wang, T.; Zhang, S. Radionuclide-labelled nanoparticles for cancer combination therapy: A review. J. Nanobiotechnol. 2024, 22, 728. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.K.; Park, J.; Jon, S. Targeting strategies for multifunctional nanoparticles in cancer imaging and therapy. Theranostics 2012, 2, 3–44. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Elst, V.L.; Muller, R.N. Magnetic iron oxide nanoparticles: Synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Nie, D.; Liu, Y.; Yu, M.; Gan, Y. Advances in particle shape engineering for improved drug delivery. Drug Discov. Today 2019, 24, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, J.; Chen, H.; Wu, H.; Xu, Y.; Li, Y.; Yi, H.; Wang, Y.A.; Yang, L.; Mao, H. Exerting enhanced permeability and retention effect driven delivery by ultrafine iron oxide nanoparticles with T1–T2 switchable magnetic resonance imaging contrast. ACS Nano 2017, 11, 4582–4592. [Google Scholar] [CrossRef]
- Laurent, S.; Boutry, S.; Mahieu, I.; Vander, E.L.; Muller, R.N. Iron oxide based MR contrast agents: From chemistry to cell labeling. Curr. Med. Chem. 2009, 16, 4712–4727. [Google Scholar] [CrossRef]
- Thomas, R.; Park, I.-K.; Jeong, Y.Y. Magnetic iron oxide nanoparticles for multimodal imaging and therapy of cancer. Int. J. Mol. Sci. 2013, 14, 15910–15930. [Google Scholar] [CrossRef]
- Núñez-Salinas, A.; Parra-Garretón, C.; Acuña, D.; Peñaloza, S.; Günther, G.; Bollo, S.; Arriagada, F.; Morales, J. Nanoradiopharmaceuticals: Design principles, radiolabeling strategies, and biomedicine applications. Pharmaceutics 2025, 17, 912. [Google Scholar] [CrossRef]
- Salehirozveh, M.; Dehghani, P.; Mijakovic, I. Synthesis, Functionalization, and Biomedical Applications of Iron Oxide Nanoparticles (IONPs). J. Funct. Biomater. 2024, 15, 340. [Google Scholar] [CrossRef]
- Sperling, R.A.; Parak, W.J. Surface modification, functionalization and bioconjugation of colloidal inorganic nanoparticles. Philos. Trans. A Math. Phys. Eng. Sci. 2010, 368, 1333–1383. [Google Scholar] [CrossRef]
- Elias, D.R.; Poloukhtine, A.; Popik, V.; Tsourkas, A. Effect of ligand density, receptor density, and nanoparticle size on cell targeting. Nanomedicine 2013, 9, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Adrover, J.M.; Pellico, J.; Fernandez-Barahona, I.; Martin-Salamanca, S.; Ruiz-Cabello, J.; Hidalgo, A.; Herranz, F. Thrombo-tag, an in vivo formed nanotracer for the detection of thrombi in mice by fast pre-targeted molecular imaging. Nanoscale 2020, 12, 22978–22987. [Google Scholar] [CrossRef]
- Pellico, J.; Fernández-Barahona, I.; Benito, M.; Gaitán-Simón, A.; Gutiérrez, L.; Ruiz-Cabello, J.; Herranz, F. Unambiguous detection of atherosclerosis using bioorthogonal nanomaterials. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 26–35. [Google Scholar] [CrossRef]
- Venrooij, K.R.; de Bondt, L.; Bonger, K.M. Mutually orthogonal bioorthogonal reactions: Selective chemistries for labeling multiple biomolecules simultaneously. Top. Curr. Chem. 2024, 382, 24. [Google Scholar] [CrossRef]
- Juhl, K.; Jørgensen, K.A. The First Organocatalytic enantioselective inverse-electron-demand hetero-Diels–Alder reaction. Angew. Chem. Int. Ed. 2003, 42, 1498–1501. [Google Scholar] [CrossRef] [PubMed]
- Wilbur, D.S. Chemical and radiochemical considerations in radiolabeling with α-emitting radionuclides. Curr. Radiopharm. 2011, 4, 214–247. [Google Scholar] [CrossRef]
- Hu, A.; Wilson, J.J. Advancing chelation strategies for large metal ions for nuclear medicine applications. Acc. Chem. Res. 2022, 55, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, A.J.; Fallis, I.A.; Pope, S.J.A. Chelating agents for radiolanthanides: Applications to imaging and therapy. Coord. Chem. Rev. 2017, 340, 198–219. [Google Scholar] [CrossRef]
- Patrick, P.S.; Bogart, L.K.; Macdonald, T.J.; Southern, P.; Powell, M.J.; Zaw-Thin, M.; Voelcker, N.H.; Parkin, I.P.; Pankhurst, Q.A.; Lythgoe, M.F.; et al. Surface radio-mineralisation mediates chelate-free radiolabelling of iron oxide nanoparticles. Chem. Sci. 2019, 10, 2592–2597. [Google Scholar] [CrossRef]
- Pellico, J.; Gawne, P.J.; de Rosales, R.T.M. Radiolabelling of nanomaterials for medical imaging and therapy. Chem. Soc. Rev. 2021, 50, 3355–3423. [Google Scholar] [CrossRef]
- Salvanou, E.-A.; Kolokithas-Ntoukas, A.; Liolios, C.; Xanthopoulos, S.; Paravatou-Petsotas, M.; Tsoukalas, C.; Avgoustakis, K.; Bouziotis, P. Preliminary evaluation of iron oxide nanoparticles radiolabeled with 68Ga and 177Lu as potential theranostic agents. Nanomaterials 2022, 12, 2490. [Google Scholar] [CrossRef]
- Stanković, D.; Radović, M.; Stanković, A.; Mirković, M.; Vukadinović, A.; Mijović, M.; Milanović, Z.; Ognjanović, M.; Janković, D.; Antić, B.; et al. Synthesis, characterization, and therapeutic efficacy of 177Lu-DMSA@SPIONs in nanobrachytherapy of solid tumors. Pharmaceutics 2023, 15, 1943. [Google Scholar] [CrossRef]
- Ognjanović, M.; Radović, M.; Mirković, M.; Prijović, Z.; del Puerto Morales, M.; Čeh, M.; Vranješ-Đurić, S.; Antić, B. 99mTc-, 90Y-, and 177Lu-labeled iron oxide nanoflowers designed for potential use in dual magnetic hyperthermia/radionuclide cancer therapy and diagnosis. ACS Appl. Mater. Interfaces 2019, 11, 41109–41117. [Google Scholar] [CrossRef]
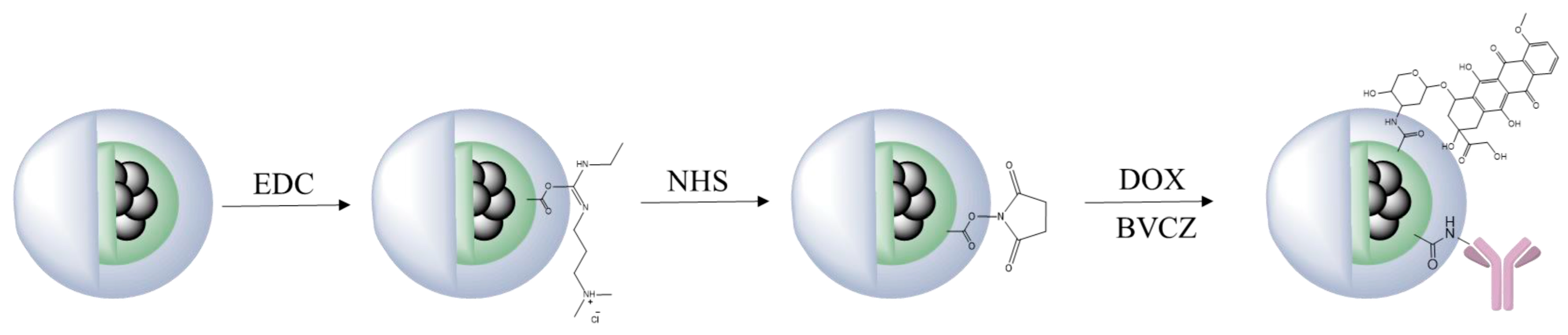
- Salvanou, E.-A.; Kolokithas-Ntoukas, A.; Prokopiou, D.; Theodosiou, M.; Efthimiadou, E.; Koźmiński, P.; Xanthopoulos, S.; Avgoustakis, K.; Bouziotis, P. 177Lu-labeled iron oxide nanoparticles functionalized with doxorubicin and nevacizumab as nanobrachytherapy agents against breast cancer. Molecules 2024, 29, 1030. [Google Scholar] [CrossRef]
- Gholami, Y.H.; Josephson, L.; Akam, E.A.; Caravan, P.; Wilks, M.Q.; Pan, X.Z.; Maschmeyer, R.; Kolnick, A.; El Fakhri, G.; Normandin, M.D.; et al. A chelate-free nano-platform for incorporation of diagnostic and therapeutic isotopes. Int. J. Nanomed. 2020, 15, 31–47. [Google Scholar] [CrossRef]
- Ge, J.; Chen, L.; Huang, B.; Gao, Y.; Zhou, D.; Zhou, Y.; Chen, C.; Wen, L.; Li, Q.; Zeng, J.; et al. Anchoring group-mediated radiolabeling of inorganic nanoparticles-A universal method for constructing nuclear medicine imaging nanoprobes. ACS Appl. Mater. Interfaces 2022, 14, 8838–8846. [Google Scholar] [CrossRef] [PubMed]
- Rasaneh, S.; Rajabi, H.; Daha, F.J. Activity estimation in radioimmunotherapy using magnetic nanoparticles. Chin. J. Cancer Res. 2015, 27, 203–208. [Google Scholar] [PubMed]
- Shanehsazzadeh, S.; Grüttner, C.; Yousefnia, H.; Lahooti, A.; Gholami, A.; Nosrati, S.; Zolghadri, S.; Anijdan, S.H.M.; Lotfabadi, A.; Varnamkhasti, B.S.; et al. Development of 177Lu-DTPA-SPIO conjugates for potential use as a dual contrast SPECT/MRI imaging agent. Radiochim. Acta 2016, 104, 337–344. [Google Scholar] [CrossRef]
- Mirković, M.; Milanović, Z.; Perić, M.; Vranješ-Đurić, S.; Ognjanović, M.; Antić, B.; Kuraica, M.; Krstić, I.; Kubovcikova, M.; Antal, I.; et al. Design and preparation of proline, tryptophan and poly-L-lysine functionalized magnetic nanoparticles and their radiolabeling with 131I and 177Lu for potential theranostic use. Int. J. Pharm. 2022, 628, 122288. [Google Scholar] [CrossRef]
- Li, Y.; Shan, S.; Zhang, R.; Sun, C.; Hu, X.; Fan, J.; Wang, Y.; Duan, R.; Gao, M. Imaging and downstaging bladder cancer with the 177Lu-labeled bioorthogonal nanoprobe. ACS Nano 2024, 18, 17209–17217. [Google Scholar] [CrossRef] [PubMed]
- Goel, M.; Mackeyev, Y.; Krishnan, S. Radiolabeled nanomaterial for cancer diagnostics and therapeutics: Principles and concepts. Cancer Nanotechnol. 2023, 14, 15. [Google Scholar] [CrossRef]
- Hue, J.J.; Lee, H.J.; Nam, S.Y.; Kim, J.S.; Lee, B.J.; Yun, Y.W. Distribution and accumulation of 177Lu-labeled thermally cross-linked superparamagnetic iron oxide nanoparticles in the tissues of ICR mice. Korean J. Vet. Res. 2015, 55, 57–60. [Google Scholar] [CrossRef]
- Weissleder, R.; Bogdanov, A.; Neuwelt, E.A.; Papisov, M. Long-circulating iron oxides for MR imaging. Adv. Drug Deliv. Rev. 1995, 16, 321–334. [Google Scholar] [CrossRef]

| Alpha Particle | Beta Particle | Auger Electron | |
|---|---|---|---|
| Type of particles | 4He nucleus | Energetic electron | Low energy electron; electron capture and/or internal conversion |
| Particle energy | 4–9 MeV | 50–2300 keV | 25–80 keV |
| Particle path length | 40–100 μm | 0.05–12 mm | Nanometers |
| Linear energy transfer | ~80 keV/μm | ~0.2 keV/μm | 4–26 keV/μm |
| Hypoxic tumors | Effective | Less effective | Effective |
| Toxicity | Effective in creating double-strand breaks in DNA | High dose rates (tumor survival rates close to linear exponential). Low dose rates (single-strand breaks), repairable with shouldering the dose–response curve | Potential creation of double-strand breaks DNA and cell membrane |
| Bystander effect/crossfire | Yes/low | Yes | Yes |
| Tumor size | Micro/small | Higher volume solid tumor | Micro |
| 177Lu Bifunctional Radioligand-Based Radiopharmaceuticals | Treatment Conditions |
|---|---|
| 177Lu-DOTATATE (Lutathera®) | Gastroenteropancreatic neuroendocrine tumors |
| 177Lu-PSMA-617 (Pluvicto®) | Prostate-specific membrane antigen–positive metastatic castration-resistant prostate cancer |
| 177Lu-J591 | Prostate-specific membrane antigen–positive metastatic castration-resistant prostate cancer |
| 177Lu-DOTATOC | Neuroendocrine tumors |
| 177Lu-PSMA-I&T | Prostate-specific membrane antigen–positive metastatic castration-resistant prostate cancer |
| 177Lu-DOTA-HH1 (Betalutin) | B-cell non-Hodgkin lymphomas |
| 177Lu-PNT2002 | Metastatic castration-resistant prostate cancer |
| 177Lu-DOTA-ABM-5G | Advanced/metastatic pancreatic ductal adenocarcinoma |
| 177Lu-DOTA-EB-TATE | Metastatic neuroendocrine tumors |
| 177Lu-CC49 | Prostate-specific membrane antigen–positive metastatic castration-resistant prostate cancer |
| CTT1403 | Prostate-specific membrane antigen–positive metastatic castration-resistant prostate cancer |
| 177Lu-DOTA-JR11 | Neuroendocrine tumors |
| 177Lu-NeoB | Gastrin-releasing peptide receptor-positive tumors |
| 177Lu-girentuximab | Metastatic clear cell renal cell carcinoma |
| GD2-SADA:177Lu-DOTA | GD2-positive solid tumors |
| 177Lu-FAP-2286 | Fibroblast activation protein-positive solid tumors |
| 177Lu-Ludotadipep | Metastatic castration-resistant prostate cancer |
| 177Lu-rhPSMA-10.1 | Prostate-specific membrane antigen–positive metastatic castration-resistant prostate cancer |
| 177Lu-DOTA.SA.FAPi | Solid tumors |
| 177Lu-DOTAGA.(SA.FAPi)2 | Solid tumors |
| 177Lu-FAPi-46 | Solid tumors |
| 177Lu-trastuzumab | Breast cancer |
| 177Lu-brentuximab | Hypoxic tumors |
| 177Lu-PP-F11N | Medullary thyroid cancer |
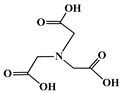 | Nitrilotriacetic acid (NTA) |
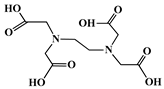 | Ethylenediaminetetraacetic acid (EDTA) |
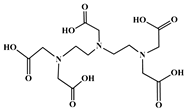 | Diethylenetriaminepentaacetic acid (DTPA) |
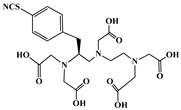 | p-SCN-Bn-1B-DTPA |
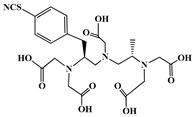 | p-SCN-Bn-1B4M-DTPA |
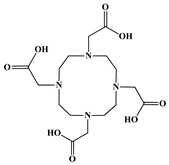 | 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) |
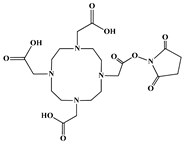 | DOTA-NHS-ester |
 | p-SCN-Bn-DOTA |
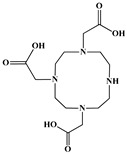 | 1,4,7,10-Tetraazacyclododecane-1,4,7-triacetic acid (DO3A) |
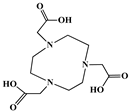 | 1,4,7-Triazacyclononane-1,4,7-triacetic acid (NOTA) |
 | p-SCN-Bn-NOTA |
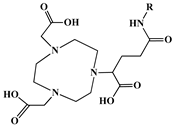 | (NOTA (1,4,7-triazacyclononane-1,4,7-triacetic acid) + GA (glutaric acid)) NOTAGA (R = NHS-ester, amide) |
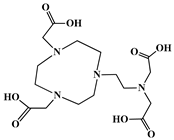 | {4-[2-(bis-carboxymethylamino)-ethyl]-7- carboxymethyl-[1,4,7]-triazonan-1-yl}-acetic acid (NETA) |
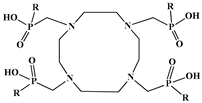 | 1,4,7,10-tetraazacyclodecane-1,4,7,10- tetra(R)ester phosphinic acid (DOTRP) |
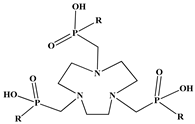 | 1,4,7-teriazacyclononane-1,4,7-tri(R)ester phosphinic acid (NORP) |
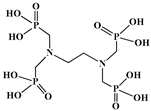 | Ethylenediamine-tetra(methylene-phosphonic acid), (EDTMP) |
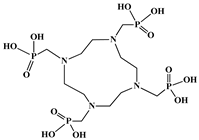 | 1,4,7,10-tetraazacyclododecane-1,4,7,10- tetramethylene-phosphonic acid (DOTMP) |
| Ion | NTA | EDTA | DTPA | NOTA | DOTA | DO3A | DOTMP |
|---|---|---|---|---|---|---|---|
| Lu(III) | 12.49 | 19.83 | 22.44 | 15.3 | 25.4 | 23.0 | 29.6 |
| Metrics | Details |
|---|---|
| Accounted market size in the year 2025 | US$ 2.73 billion |
| Forecasted market size in 2032 | US$ 10.84 billion |
| Compound annual growth rate from 2025 to 2032 | 21.8% |
| Production by type | Non-carrier-added Carrier-added |
| Production by application | Nuclear Therapy Others |
| Production by region | North America Europe Australia South Africa |
| Consumption by region | North America (United States, Canada) Europe (Germany, France, UK, Italy, Russia) Asia-Pacific (China, Japan, South Korea, Taiwan) Southeast Asia (India) Latin America (Mexico, Brazil) |
| Production by company | Advanced Accelerator Applications (Novartis) Saint-Genis-Pouilly, France Eckert & Ziegler Strahlen, Berlin, Germany SHINE Technologies, Janesville, WI, USA ANSTO, Sydney and Melbourne, Australia NTP Radioisotopes, Pelindaba, South Africa |
| Study Title | Status | Conditions | Interventions | Study Type | Adverse Events and Outcomes |
|---|---|---|---|---|---|
| Evaluation of safety and dosimetry of Lutathera in adolescent patients with gastroenteropancreatic neuroendocrine tumors (GEP-NETs) and pheochromocytomas and paragangliomas (PPGLs) | Active |
|
| Interventional | |
| 177Lu-PSMA-617 vs. androgen receptor-directed therapy in the treatment of progressive metastatic castrate resistant prostate cancer | Active |
|
| ||
| Telotristat with Lutathera in neuroendocrine tumors (NETs) | Terminated |
|
| Zero adverse events reported. However, only 1 participant was enrolled in this study. Based on the low enrolment number, no data is reported here in order to protect and maintain participant privacy/confidentiality. | |
| Post-authorization safety study of LysaKare® in adult GEP-NET patients | Completed |
|
| All participants enrolled in the study received one dose of arginine/lysine solution administered intravenously over a 4 h period. Zero serious adverse events were reported. There is an agreement between principal investigators (PIs) and the sponsor (or its agents) that restricts the PI’s rights to discuss or publish trial results after the trial’s completion. | |
| 177Lu-DTPA-Omburtamab radioimmunotherapy for recurrent or refractory medulloblastoma | Terminated |
|
| From 1st dose to 5 weeks after last dose, up to 10 weeks (2 cycles). Partial seizures were reported as serious adverse events for all participants. | |
| Evaluation of efficacy and safety of Lutathera in patients with grade 2 and grade 3 advanced GEP-NET | Active |
|
| ||
| A trial of CTT1403 for metastatic castration resistant prostate cancer (mCRPC) | Completed |
|
| Information on adverse events was collected throughout the course of the study and up to 6 months after the last administered dose of the study drug, up to a total of 7 months. The highest administered dose of CTT1403 was 9.0 GBq. There is an agreement between PIs and the sponsor (or its agents) that restricts the PI’s rights to discuss or publish trial results after the trial’s completion. | |
| Evaluation of safety and efficacy of Betalutin and Rituximab in patients with follicular lymphoma | Completed |
|
| All adverse events were collected from the time of informed consent until 4 weeks after the last rituximab dose, up to 25 months. Thereafter, treatment-related adverse events were collected up to 36 months for the first two participants only. Erysipelas, bronchitis viral, thrombocytopenia, palpitations, and other gastrointestinal and general disorders were reported as serious and less serious adverse events. | |
| 177Lu-PSMA-617 and Pembrolizumab in treating patients with mCRPC | Completed |
|
| The additional efficacy endpoints, including median duration of response, PSA50 response rate, rPFS rate at 6 months, median PSA progression-free survival, median OS, and median time to SSRE, were planned to be analyzed in the overall study cohort (n = 43). Post hoc determination of efficacy outcomes for schedules 1,2, 3 analyzed separately is precluded by the limited sample size of patients enrolled on schedules 2 and 3 (n = 6 each). More information can be found in [76]. | |
| Evaluation of safety, tolerability, biodistribution, and anti-tumor activity of 177Lu-OPS201 with companion imaging 68Ga-OPS202 PET/CT in previously treated subjects with locally advanced or metastatic cancers expressing Somatostatin Receptor 2 (SSTR2) | Terminated |
|
| This study was terminated early due to unsuccessful screening and not due to subjects’ safety. No outcomes measures were evaluated as no subjects received the therapeutic 177Lu-satoreotide tetraxetan. There is an agreement between PIs and the sponsor (or its agents) that restricts the PI’s rights to discuss or publish trial results after the trial’s completion. | |
| 177Lu-J591 and 177Lu-PSMA-617 combination for mCRPC | Terminated |
|
| The study was terminated early due to sponsor withdrawal (PSMA-617 no longer available for purchase). More information can be found in [77]. | |
| Evaluation of safety and activity (including distribution) of 177Lu-3BP-227 in subjects with solid tumors expressing Neurotensin Receptor Type 1. | Terminated |
|
| The sponsor terminated the study early and phase 1 dose expansion and phase 2 were not started. The decision to terminate the study was not due to any safety or tolerability concern, or any event associated with the use of 177Lu-3BP-227. | |
| Study of 177Lu-PSMA-617 in mCRPC | Completed |
|
| There is an agreement between PIs and the sponsor (or its agents) that restricts the PI’s rights to discuss or publish trial results after the trial’s completion. However, the following publications are provided voluntarily by the person who enters information about the study and are about the study results [78,79,80,81,82,83,84,85,86,87,88]. | |
| Nivolumab and 177Lu-DOTA0-Tyr3-Octreotate for patients with extensive-stage small cell lung cancer | Completed |
|
| There is an agreement between PIs and the sponsor (or its agents) that restricts the PI’s rights to discuss or publish trial results after the trial’s completion. More information can be found in [89]. | |
| Phase I dose-escalation study of fractionated 177Lu-PSMA-617 for progressive mCRPC | Active |
|
| ||
| 177Lu prostate-specific membrane antigen (PSMA)-directed endoradiotherapy | Terminated |
|
| The study began on 5 July 2017as an Investigator Initiated Trial and sponsorship was transferred to Endocyte on 1 June 2018. Recruitment was stopped before the target sample size was achieved based on strategic considerations. | |
| Study of Betalutin for treatment of relapsed or refractory Non-Hodgkin lymphoma (LYMRIT-37-05) | Completed |
|
| There is an agreement between PIs and the sponsor (or its agents) that restricts the PI’s rights to discuss or publish trial results after the trial’s completion. More information can be found in [90,91]. | |
| Evaluation of safety and preliminary efficacy of 177Lu-OPS201 in NETs | Terminated |
|
| Due to strategic reasons, the Ipsen management team decided to early terminate the D-FR-01072-00 /OPS-C-001 study. This decision was not due to any safety or tolerability concern, or any event associated with the use of the study drug. | |
| A phase 1/2 study of Betalutin for treatment of relapsed Non-Hodgkin lymphoma | Completed |
|
| There is an agreement between PIs and the sponsor (or its agents) that restricts the PI’s rights to discuss or publish trial results after the trial’s completion. More information can be found in [90,91,92,93]. | |
| A study comparing treatment with 177Lu-DOTA0-Tyr3-Octreotate to Octreotide LAR in patients with inoperable, progressive, SSTR-positive midgut carcinoid tumors | Completed |
|
| There is an agreement between PIs and the sponsor (or its agents) that restricts the PI’s rights to discuss or publish trial results after the trial’s completion. More information can be found in [94,95,96,97]. | |
| 177Lu radiolabeled monoclonal antibody HuJ591 (177Lu-J591) and Ketoconazole in patients with prostate cancer | Active |
|
| ||
| Treatment with radiolabeled monoclonal antibody HuJ591-GS (177Lu-J591) in patients with metastatic prostate cancer | Completed |
|
| These publications are provided voluntarily by the person who enters information about the study and are about the study results [98,99]. | |
| Phase I/II dose-escalation study of 177Lu-labeled cG250 in patients with advanced renal cancer | Completed |
|
| There is an agreement between PIs and the sponsor (or its agents) that restricts the PI’s rights to discuss or publish trial results after the trial’s completion. More information can be found in [100,101]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halevas, E.; Varna, D. 177Lu-Labeled Magnetic Nano-Formulations: Synthesis, Radio- and Physico-Chemical Characterization, Biological Applications, Current Challenges, and Future Perspectives. Molecules 2025, 30, 4290. https://doi.org/10.3390/molecules30214290
Halevas E, Varna D. 177Lu-Labeled Magnetic Nano-Formulations: Synthesis, Radio- and Physico-Chemical Characterization, Biological Applications, Current Challenges, and Future Perspectives. Molecules. 2025; 30(21):4290. https://doi.org/10.3390/molecules30214290
Chicago/Turabian StyleHalevas, Eleftherios, and Despoina Varna. 2025. "177Lu-Labeled Magnetic Nano-Formulations: Synthesis, Radio- and Physico-Chemical Characterization, Biological Applications, Current Challenges, and Future Perspectives" Molecules 30, no. 21: 4290. https://doi.org/10.3390/molecules30214290
APA StyleHalevas, E., & Varna, D. (2025). 177Lu-Labeled Magnetic Nano-Formulations: Synthesis, Radio- and Physico-Chemical Characterization, Biological Applications, Current Challenges, and Future Perspectives. Molecules, 30(21), 4290. https://doi.org/10.3390/molecules30214290










