Doping of Magnéli Phase—New Direction in Pollutant Degradation and Electrochemistry
Abstract
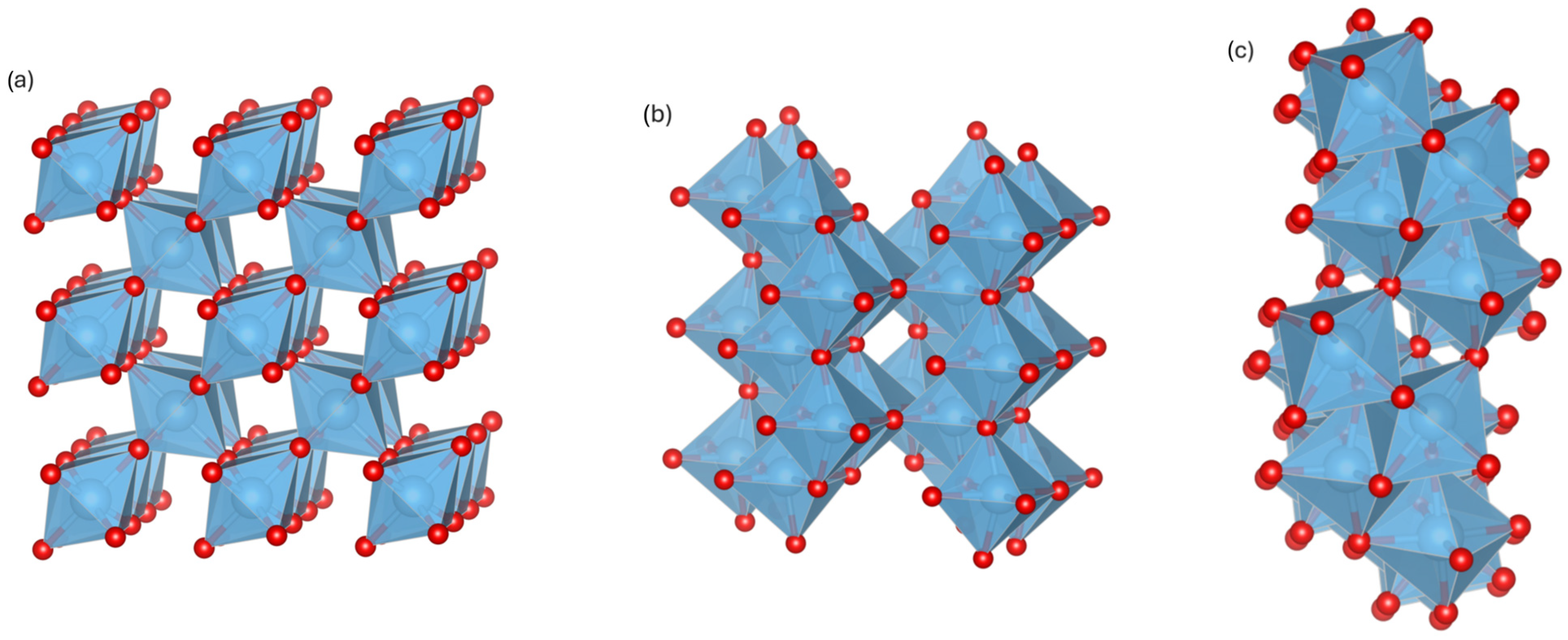
1. Introduction
2. Synthesis of Doped Magnéli Phases
3. Electrocatalytic Applications
4. Theoretical Insights
5. Perspectives and Outlook
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saputra, E.; Muhammad, S.; Sun, H.; Ang, H.-M.; Tadé, M.O.; Wang, S. Shape-Controlled Activation of Peroxymonosulfate by Single Crystal α-Mn2O3 for Catalytic Phenol Degradation in Aqueous Solution. Appl. Catal. B 2014, 154–155, 246–251. [Google Scholar] [CrossRef]
- Kitada, A.; Hasegawa, G.; Kobayashi, Y.; Kanamori, K.; Nakanishi, K.; Kageyama, H. Selective Preparation of Macroporous Monoliths of Conductive Titanium Oxides TinO2n−1 (n = 2, 3, 4, 6). J. Am. Chem. Soc. 2012, 134, 10894–10898. [Google Scholar] [CrossRef]
- Ghicov, A.; Schmuki, P. Self-Ordering Electrochemistry: A Review on Growth and Functionality of TiO2 Nanotubes and Other Self-Aligned MOx Structures. Chem. Commun. 2009, 45, 2791–2808. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Cumbal, L. Light Harvesting Titanium Nanocatalyst for Remediation of Methyl Orange. Int. J. Chem. Mol. Eng. 2014, 8, 196–199. [Google Scholar] [CrossRef]
- Theron, J.; Walker, J.A.; Cloete, T.E. Nanotechnology and Water Treatment: Applications and Emerging Opportunities. Crit. Rev. Microbiol. 2008, 34, 43–69. [Google Scholar] [CrossRef] [PubMed]
- Quétel, C.R.; Vassileva, E.; Petrov, I.; Chakarova, K.; Hadjiivanov, K.I. First Results on Fe Solid-Phase Extraction from Coastal Seawater Using Anatase TiO2 Nano-Particles. Anal. Bioanal. Chem. 2010, 396, 2349–2361. [Google Scholar] [CrossRef]
- Bai, J.; Zhou, B. Titanium Dioxide Nanomaterials for Sensor Applications. Chem. Rev. 2014, 114, 10131–10176. [Google Scholar] [CrossRef]
- Yang, F.; Hong, F.; You, W.; Liu, C.; Gao, F.; Wu, C.; Yang, P. Influences of Nano-Anatase TiO2 on the Nitrogen Metabolism of Growing Spinach. Biol. Trace Elem. Res. 2006, 110, 179–190. [Google Scholar] [CrossRef]
- Slimen, H.; Ochiai, T.; Nakata, K.; Murakami, T.; Houas, A.; Morito, Y.; Fujishima, A. Photocatalytic Decomposition of Cigarette Smoke Using a TiO2-Impregnated Titanium Mesh Filter. Ind. Eng. Chem. Res. 2012, 51, 587–590. [Google Scholar] [CrossRef]
- Mbonyiryivuze, A.; Zongo, S.; Diallo, A.; Bertrand, S.; Minani, E.; Lal Yadav, L.; Mwakikunga, B.; Dhlamini, S.M.; Maaza, M.; Yadav, L.L. Titanium Dioxide Nanoparticles Biosynthesis for Dye Sensitized Solar Cells Application: Review. Phys. Mater. Chem. 2015, 3, 12–17. [Google Scholar] [CrossRef]
- Spasiano, D.; Marotta, R.; Malato, S.; Fernandez-Ibañez, P.; Di Somma, I. Solar Photocatalysis: Materials, Reactors, Some Commercial, and Pre-Industrialized Applications. A Comprehensive Approach. Appl. Catal. B 2015, 170–171, 90–123. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-Assisted Photocatalytic Degradation of Azo Dyes in Aqueous Solution: Kinetic and Mechanistic Investigations. Appl. Catal. B 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Smith, J.R.; Walsh, F.C.; Clarke, R.L. Electrodes Based on Magnéli Phase Titanium Oxides: The Properties and Applications of Ebonex® Materials. J. Appl. Electrochem. 1998, 28, 1021–1033. [Google Scholar] [CrossRef]
- Miller-Folk, R.R.; Noftle, R.E.; Pletcher, D. Electron Transfer Reactions at Ebonex Ceramic Electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1989, 274, 257–261. [Google Scholar] [CrossRef]
- Walsh, F.C.; Wills, R.G.A. The Continuing Development of Magnéli Phase Titanium Sub-Oxides and Ebonex® Electrodes. Electrochim. Acta 2010, 55, 6342–6351. [Google Scholar] [CrossRef]
- Toyoda, M.; Yano, T.; Tryba, B.; Mozia, S.; Tsumura, T.; Inagaki, M. Preparation of Carbon-Coated Magneli Phases TinO2n−1 and Their Photocatalytic Activity under Visible Light. Appl. Catal. B 2009, 88, 160–164. [Google Scholar] [CrossRef]
- Ellis, K.; Hill, A.; Hill, J.; Loyns, A.; Partington, T. The Performance of Ebonex® Electrodes in Bipolar Lead-Acid Batteries. J. Power Sources 2004, 136, 366–371. [Google Scholar] [CrossRef]
- Pang, Q.; Kundu, D.; Cuisinier, M.; Nazar, L.F. Surface-Enhanced Redox Chemistry of Polysulphides on a Metallic and Polar Host for Lithium-Sulphur Batteries. Nat. Commun. 2014, 5, 4759. [Google Scholar] [CrossRef]
- Rashkova, V.; Kitova, S.; Vitanov, T. Electrocatalytic Behavior of Thin Co–Te–O Films in Oxygen Evolution and Reduction Reactions. Electrochim. Acta 2007, 52, 3794–3803. [Google Scholar] [CrossRef]
- Arif, A.F.; Balgis, R.; Ogi, T.; Iskandar, F.; Kinoshita, A.; Nakamura, K.; Okuyama, K. Highly Conductive Nano-Sized Magnéli Phases Titanium Oxide (TiOx). Sci. Rep. 2017, 7, 3646. [Google Scholar] [CrossRef]
- Gusev, A.A.; Avvakumov, E.G.; Medvedev, A.Z.H.; Masliy, A.I. Ceramic Electrodes Based on Magneli Phases of Titanium Oxides. Sci. Sinter. 2007, 39, 51–57. [Google Scholar] [CrossRef]
- Ioroi, T.; Senoh, H.; Yamazaki, S.; Siroma, Z.; Fujiwara, N.; Yasuda, K. Stability of Corrosion-Resistant Magnéli-Phase Ti4O7-Supported PEMFC Catalysts at High Potentials. J. Electrochem. Soc. 2008, 155, B321. [Google Scholar] [CrossRef]
- Tao, X.; Wang, J.; Ying, Z.; Cai, Q.; Zheng, G.; Gan, Y.; Huang, H.; Xia, Y.; Liang, C.; Zhang, W.; et al. Strong Sulfur Binding with Conducting Magnéli-Phase TinO2n−1 Nanomaterials for Improving Lithium–Sulfur Batteries. Nano Lett. 2014, 14, 5288–5294. [Google Scholar] [CrossRef] [PubMed]
- Harada, S.; Tanaka, K.; Inui, H. Thermoelectric Properties and Crystallographic Shear Structures in Titanium Oxides of the Magnèli Phases. J. Appl. Phys. 2010, 108, 083703. [Google Scholar] [CrossRef]
- Tsuyumoto, I.; Hosono, T.; Murata, M. Thermoelectric Power in Nonstoichiometric Orthorhombic Titanium Oxides. J. Am. Ceram. Soc. 2006, 89, 2301–2303. [Google Scholar] [CrossRef]
- Wang, G.; Liu, Y.; Ye, J.; Qiu, W. Synthesis, Microstructural Characterization, and Electrochemical Performance of Novel Rod-like Ti4O7 Powders. J. Alloys Compd. 2017, 704, 18–25. [Google Scholar] [CrossRef]
- Bursill, L.A.; Hyde, B.G. Crystal Structures in the {l32} CS Family of Higher Titanium Oxides TinO2n−1. Acta Crystallogr. B 1971, 27, 210–215. [Google Scholar] [CrossRef]
- Padilha, A.C.M.; Osorio-Guillén, J.M.; Rocha, A.R.; Dalpian, G.M. TinO2n−1 Magnéli Phases Studied Using Density Functional Theory. Phys. Rev. B 2014, 90, 035213. [Google Scholar] [CrossRef]
- Sensoy, M.G.; Carpick, R.W.; Srolovitz, D.J.; Rappe, A.M. Manipulating the Electrical Properties of Conductive Substoichiometric Titanium Oxides. Phys. Rev. B 2024, 109, 064106. [Google Scholar] [CrossRef]
- Le Page, Y.; Strobel, P. Structural Chemistry of Magnéli Phases TinO2n−1 (4 ≤ n ≤ 9). I. Cell and Structure Comparisons. J. Solid State Chem. 1982, 43, 314–319. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Ye, J. Investigation of Fabrication of Ti4O7 by Carbothermal Reduction in Argon Atmosphere and Vacuum. J. Mater. Sci. Mater. Electron. 2016, 27, 3683–3692. [Google Scholar] [CrossRef]
- Malik, H.; Sarkar, S.; Mohanty, S.; Carlson, K. Modelling and Synthesis of Magnéli Phases in Ordered Titanium Oxide Nanotubes with Preserved Morphology. Sci. Rep. 2020, 10, 8050. [Google Scholar] [CrossRef]
- Portehault, D.; Maneeratana, V.; Candolfi, C.; Oeschler, N.; Veremchuk, I.; Grin, Y.; Sanchez, C.; Antonietti, M. Facile General Route toward Tunable Magnéli Nanostructures and Their Use As Thermoelectric Metal Oxide/Carbon Nanocomposites. ACS Nano 2011, 5, 9052–9061. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Y.; Ye, J.; Zhu, R. Fabrication of Ti4O7 Electrodes by Spark Plasma Sintering. Mater. Lett. 2014, 114, 34–36. [Google Scholar] [CrossRef]
- Yu, M.; Saunders, T.; Grasso, S.; Mahajan, A.; Zhang, H.; Reece, M.J. Magnéli Phase Titanium Suboxides by Flash Spark Plasma Sintering. Scr. Mater. 2018, 146, 241–245. [Google Scholar] [CrossRef]
- Fahem, M.Q.; Hassan, T.A.A. Magnéli Phase Titanium Sub-Oxide Production Using a Hydrothermal Process. Karbala Int. J. Mod. Sci. 2022, 8, 651–656. [Google Scholar] [CrossRef]
- Liu, H.J.; Luo, M.Q.; Yang, L.X.; Zeng, C.L.; Fu, C. A High Strength and Conductivity Bulk Magnéli Phase Ti4O7 with Superior Electrochemical Performance. Ceram. Int. 2022, 48, 25538–25546. [Google Scholar] [CrossRef]
- Geng, X.; Xia, Y.; Liang, H.; Yao, D.; Zeng, Y.-P. Fabrication of High-Performance Magnéli Phase Ti4O7 Ceramics by in-Situ Hot-Pressed Sintering in a Single Step. Mater. Today Commun. 2023, 37, 107058. [Google Scholar] [CrossRef]
- Mito, M.; Mokutani, N.; Tang, Y.; Matsumoto, K.; Tajiri, T.; Horita, Z. Achieving High-Tc Superconductivity in Magnéli Phase Based on Ti Oxides: Prediction by Machine Learning and Material Synthesis by High-Pressure Torsion Processing. J. Mater. Sci. 2024, 59, 5981–5994. [Google Scholar] [CrossRef]
- Kao, W.; Patel, P.; Haberichter, S.L. Formation Enhancement of a Lead/Acid Battery Positive Plate by Barium Metaplumbate and Ebonex®. J. Electrochem. Soc. 1997, 144, 1907–1911. [Google Scholar] [CrossRef]
- English, J.T.; Wilkinson, D.P. The Thermal-Oxidation Behavior of Pristine and Doped Magnéli Phase Titanium Oxides. ECS J. Solid State Sci. Technol. 2021, 10, 034004. [Google Scholar] [CrossRef]
- George, J.; Waroquiers, D.; Di Stefano, D.; Petretto, G.; Rignanese, G.-M.; Hautier, G. The Limited Predictive Power of the Pauling Rules. Angew. Chem. Int. Ed. 2020, 59, 7569. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, H.; Wang, Y. A Review: Synthesis and Applications of Titanium Sub-Oxides. Materials 2023, 16, 6874. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, T.; Jia, H.; Li, J.; Liu, B. Preparation and Electrochemical Applications of Magnéli Phase Titanium Suboxides: A Review. Chem. Eur. J. 2024, 30, e202402188. [Google Scholar] [CrossRef]
- Kumar, A.; Barbhuiya, N.H.; Singh, S.P. Magnéli Phase Titanium Sub-Oxides Synthesis, Fabrication and Its Application for Environmental Remediation: Current Status and Prospect. Chemosphere 2022, 307, 135878. [Google Scholar] [CrossRef]
- Calestani, D.; Licci, F.; Kopnin, E.; Calestani, G.; Gauzzi, A.; Bolzoni, F.; Besagni, T.; Boffa, V.; Marezio, M. Preparation and Characterization of Powders and Crystals of Vn−xTixO2n−1 Magneli Oxides. Cryst. Res. Technol. 2005, 40, 1067–1071. [Google Scholar] [CrossRef]
- Yuan, Z.; Gong, J.; Xu, S.; Li, Z.; Tang, G. Investigation of the Thermoelectric Properties of Reduced Nb-Doped TiO2-Ceramics. J. Alloys Compd. 2017, 710, 778–783. [Google Scholar] [CrossRef]
- English, J.T.; Wilkinson, D.P. The Thermal-Oxidation Behavior of Pristine and Doped Magnéli Phase Titanium Oxides. ECS Trans. 2020, 98, 11–22. [Google Scholar] [CrossRef]
- Gardos, M.N. Magnéli Phases of Anion-Deficient Rutile as Lubricious Oxides. Part II. Tribological Behavior of Cu-Doped Polycrystalline Rutile (TinO2n−1). Tribol. Lett. 2000, 8, 79–96. [Google Scholar] [CrossRef]
- Teramoto, T.; Takai, Y.; Hashiguchi, H.; Okunishi, E.; Tanaka, K. Distribution of Alloying Quadrivalent Zirconium in TiO2−x Magnèli Phase. Mater. Trans. 2019, 60, 2199–2203. [Google Scholar] [CrossRef]
- Wei, W.; Jin, N.; Ye, J. Structural and Electronic Properties of Ce-doped Ti4O7 Based on Experiments and First-principles Calculations. J. Am. Ceram. Soc. 2023, 106, 7556–7566. [Google Scholar] [CrossRef]
- Sun, Y.; Luo, Y.; Dai, L.; Zheng, Y.; Zhang, H.; Wang, Y. Sn Bulk Phase Doping and Surface Modification on Ti4O7 for Oxygen Reduction to Hydrogen Peroxide. Chem. Eur. J. 2024, 30, e202303602. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka, E.; Domaschke, M.; Denisov, N.; Fehn, D.; Hwang, I.; Kaufmann, M.; Kunstmann, B.; Schmidt, J.; Meyer, K.; Peukert, W.; et al. Magnéli Phases Doped with Pt for Photocatalytic Hydrogen Evolution. ACS Appl. Energy Mater. 2019, 2, 8399–8404. [Google Scholar] [CrossRef]
- Lin, H.; Xiao, R.; Xie, R.; Yang, L.; Tang, C.; Wang, R.; Chen, J.; Lv, S.; Huang, Q. Defect Engineering on a Ti4O7 Electrode by Ce3+ Doping for the Efficient Electrooxidation of Perfluorooctanesulfonate. Environ. Sci. Technol. 2021, 55, 2597–2607. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Zhu, X.; Li, L.; Wang, Y.; Li, G.; Dong, S.; Wang, Y.; Lin, H.; Li, K.; Huang, Q. Robust Titanium Suboxide Anodes Doped by Sintering Enhance PFOS Degradation in Water. Chemosphere 2025, 379, 144438. [Google Scholar] [CrossRef]
- English, J.T.; Wilkinson, D.P. Vanadium-Doped Ti4O7 Porous Transport Layers for Efficient Electrochemical Oxidation of Industrial Wastewater Contaminants. J. Electrochem. Soc. 2023, 170, 083501. [Google Scholar] [CrossRef]
- Tao, N.; Tian, Y.; Wang, J.; Teng, J. Enhanced Electrochemical Removal of Sulfamethazine on Sm-Doped Ti4O7 Anode: Mechanisms and Toxicity Evaluation. J. Hazard. Mater. Adv. 2025, 17, 100607. [Google Scholar] [CrossRef]
- Jing, J.; Li, S.; Wu, H.; Li, S.; Zhang, Y.; Song, G.; Li, W.; Liang, R.; Zhou, M. Energy-Efficient Wastewater Treatment Using Enhanced Fenton-like Electroactive Ceramic Membrane with Atomic-Level Engineered Cobalt on Magnéli Phase Supports. Appl. Catal. B Environ. Energy 2025, 377, 125484. [Google Scholar] [CrossRef]
- Jia, H.; Jiang, W.; Chen, T.; Yang, W.; Lu, W.; Ma, D.; Lang, A.; Li, J.; Liu, B. Magnéli-Phase Sn-Ti4O7-Coated Electrodes via Plasma Electrolytic Oxidation: Enhanced Charge Transfer and Electrochemical Degradation of Tetracycline. J. Environ. Chem. Eng. 2025, 13, 117719. [Google Scholar] [CrossRef]
- Chen, J.; He, X.; Lei, C.; Li, W.; Yang, Z.; Zhou, Q. Research on Carbon Black and Cerium Co-Doped Ti4O7-CB-Ce Electrocatalytic Oxidation of Tetracycline-Based Antibiotics. Environ. Sci. Pollut. Res. 2024, 31, 44983–44994. [Google Scholar] [CrossRef]
- Xu, J.; Liu, Y.; Li, D.; Li, L.; Zhang, Y.; Chen, S.; Wu, Q.; Wang, P.; Zhang, C.; Sun, J. Insights into the Electrooxidation of Florfenicol by a Highly Active La-Doped Ti4O7 Anode. Sep. Purif. Technol. 2022, 291, 120904. [Google Scholar] [CrossRef]
- Lee, W.; Choung, S.; Kim, S.; Hong, J.; Kim, D.; Tarpeh, W.A.; Han, J.W.; Cho, K. Atomically Dispersed Ru-doped Ti4O7 Electrocatalysts for Chlorine Evolution Reaction with a Universal Activity. Small 2024, 20, 2401248. [Google Scholar] [CrossRef]
- Zhang, K.; Han, Y.; Wang, P.; Bu, Z.; Wang, B.; Shi, H.; Wang, H.; Zhang, W.; Gao, S.; Huang, Q. 3D Printed Lanthanide-Doped Ti4O7 Reactive Membrane for Efficient Electrochemical Disinfection and Degradation of Antibiotic Resistance Genes. Chem. Eng. J. 2024, 502, 157829. [Google Scholar] [CrossRef]
- Misal, S.N.; Lin, M.-H.; Mehraeen, S.; Chaplin, B.P. Modeling Electrochemical Oxidation and Reduction of Sulfamethoxazole Using Electrocatalytic Reactive Electrochemical Membranes. J. Hazard. Mater. 2020, 384, 121420. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.; Xia, Y.; Zhu, M.; Zhao, J.; Yao, D.; Zeng, Y. Effect of Al2O3 and Ta2O5 on Mechanical and Electrical Properties of Ti4O7 Ceramics. Adv. Eng. Mater. 2025, 27, 2500876. [Google Scholar] [CrossRef]
- Yuan, T.; Wei, W.; Wang, Y.; Jin, N.; Ye, J. Effect of V-Doping on Structure and Electrical Conductivity of Magnéli Phase Ti4O7. J. Alloys Compd. 2024, 978, 173492. [Google Scholar] [CrossRef]
- Yuan, T.; Wei, W.; Yun, Y.; Jin, N.; Ye, J. The Formation of V-Doped Ti4O7 Hollow Magnéli Phase with Improved Electrical Conductivity and Thermal Stability. Ceram. Int. 2023, 49, 40668–40675. [Google Scholar] [CrossRef]
- Kao, H.-Y. The Development of M3+-Doped Mesoporous Ti4O7 Electrode for Pharmaceutical Degradation in Fresh Human Urine. Master’s Thesis, University of Washington, Washington, DC, USA, 2022. [Google Scholar]
- Yuan, T.; Jin, N.; Cheng, W.; Yun, Y.; Tian, X.; Wang, L.; Ye, J. Insight into the Stability in Cation Substitution of Magnéli Phase Ti4O7. Appl. Phys. Lett. 2022, 121, 214101. [Google Scholar] [CrossRef]
| Dopant | Application/Characteristics | Performance Metric | Author/ Reference | |
|---|---|---|---|---|
| Ion/Atom | Conc. | |||
| Pt | 290 ppm | Photocatalytic hydrogen evolution reaction | 50–100 times higher H2 evolution than plain anatase loaded with a similar amount of Pt | Wierzbicka et al., 2019. [53] |
| Ce3+ | 1–3 at% | Electrooxidation of perfluorooctane sulfonate (PFOS) | Oxidation rate 2.4× greater than that of the pristine Ti4O7 electrode | Lin et al., 2021. [54] |
| Ce3+ Nb5+ | 2.6 at% Ce 5.5 at% Nb | Electrooxidation of PFOS | Nb-TSO anode 1.8 times lower and Ce-TSO anode higher PFOS degradation rate than that of the Ti4O7 anode | Sui et al. 2025. [55] |
| V | nominally 1, 2, and 5 at% | Electrochemical oxidation of industrial wastewater | Decolorization >99 and chemical oxygen demand removal >98% of 100 mg L−1 of methyl orange | English et al., 2023. [56] |
| Sm3+ | 0.25, 0.5, 1% | Electrochemical removal of sulfamethazine | 91.2% removal efficiency for sulfamethazine (0.25% Sm-Ti4O7 anode) | Tao et al., 2025. [57] |
| Co2+ Co3+ | 3.05 wt% | Sulfamethoxazole (SMX) removal | 100% SMX (10 mg/L) removal with a high rate constant (34.07 min−1) | Jing et al. [58] |
| Sn4+/Sn2+ | 0.88% | Electrochemical oxidation of tetracycline | 92% removal rate within 120 min (compared to 71.4% for pristine Ti4O7) and complete removal within 180 min | Jia et al., 2025. [59] |
| Ce3+ | 10 at% Ce | Monocycline (MNC) degradation within wastewater | 100% MNC removal within 20 min; removal rate reduces from 100 to 98.5% after five cycles | Chen et al., 2024. [60] |
| Sn4+ | 0.5–5 at%, (best at 1 at%) | ORR for producing H2O2 | H2O2 selectivity of 95.7% | Sun et al., 2024. [52] |
| La | 1.60% | Electrooxidation of florfenicol (FLO) | Removal efficiency of FLO (>93.5%) within 20 degradation cycles | Xu et al., 2022. [61] |
| Ru2+ | 0.13 wt% | Electrochemical chlorine evolution reaction (ClER) | Active ClER both in 5 M NaCl (pH 2.3) and 0.1 M NaCl (pH 6.5) electrolytes. (Ru1-Ti4O7 catalysts outperform existing DSA materials—ClER TOF 17-fold and mass activity 23-fold greater) | Lee et al., 2024. [62] |
| Nd | 1 wt% | Electrochemical disinfection and degradation of antibiotic resistance genes | Complete inactivation (>8.0-log inactivation) of antibiotic-resistant Escherichia coli | Zhang et al., 2024. [63] |
| Pd, Cu | Pd:Cu = 2:1 2 wt% Pd | Electrochemical oxidation and reduction of sulfamethoxazole (SMX) | Electrochemical reduction using the Pd-Cu/Ti4O7 achieved up to 96.1 ± 3.9% removal of SMX at a potential of −1.14 V/SHE and a permeate flux of 300 L m−2 h−1 | Misal et al., 2020. [64] |
| Al3+ and Ta5+ | Ti4O7 with 1 mol% Al2O3, Ti4O7 with 1 mol% Ta2O5 and Ti4O7 co-doped with 1 mol% Al2O3 and 1 mol% Ta2O5 | Effect of doping on mechanical and electrical properties—mixed oxides | Co-doping simultaneously reduced the carrier concentration and enhanced carrier mobility (higher conductivity), but mechanical properties were not significantly improved | Geng et al., 2025. [65] |
| V, Cr, Fe | 2 at% | Modification of the oxidation stability of MP in air | V-, Fe-Ti4O7 anodes—improved oxidation stability; Cr-Ti4O7—reduced lifetime | English et al., 2021. [41] |
| mixed V3+/V4+/V5+ | 6, 10, 18, 22 at% | Effects on structure and electrical conductivity | The synergistic interaction between carrier mobility and concentration leads to V-enhanced Ti4O7 conductivity. | Yuan et al., 2024. [66] |
| V | added amounts of V were 23, 27, 31, and 33 at% | Hollow V-doped MP, improved electrical conductivity and thermal stability | Improved the electrical conductivity 0.67 times, and the thermal stability (by increasing the reaction energy required to oxidize Ti4O7 to TiO2) of Ti4O7 | Yuan et al., 2023. [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vojnović, V.; Ranković, M.; Jevremović, A.; Mijailović, N.R.; Nedić Vasiljević, B.; Milojević-Rakić, M.; Bajuk-Bogdanović, D.; Gavrilov, N. Doping of Magnéli Phase—New Direction in Pollutant Degradation and Electrochemistry. Molecules 2025, 30, 4282. https://doi.org/10.3390/molecules30214282
Vojnović V, Ranković M, Jevremović A, Mijailović NR, Nedić Vasiljević B, Milojević-Rakić M, Bajuk-Bogdanović D, Gavrilov N. Doping of Magnéli Phase—New Direction in Pollutant Degradation and Electrochemistry. Molecules. 2025; 30(21):4282. https://doi.org/10.3390/molecules30214282
Chicago/Turabian StyleVojnović, Vanja, Maja Ranković, Anka Jevremović, Nataša R. Mijailović, Bojana Nedić Vasiljević, Maja Milojević-Rakić, Danica Bajuk-Bogdanović, and Nemanja Gavrilov. 2025. "Doping of Magnéli Phase—New Direction in Pollutant Degradation and Electrochemistry" Molecules 30, no. 21: 4282. https://doi.org/10.3390/molecules30214282
APA StyleVojnović, V., Ranković, M., Jevremović, A., Mijailović, N. R., Nedić Vasiljević, B., Milojević-Rakić, M., Bajuk-Bogdanović, D., & Gavrilov, N. (2025). Doping of Magnéli Phase—New Direction in Pollutant Degradation and Electrochemistry. Molecules, 30(21), 4282. https://doi.org/10.3390/molecules30214282










