Rethinking Synthetic Berberine in Nutraceuticals: Nitrosamine Risks, Regulatory Oversight, and Safer Alternatives
Abstract
1. Introduction
2. Molecular Basis of Nitrosamine Formation
3. Nitrosamine Risk in Synthetic and Natural Berberine Hydrochloride: Routes, Inte Mediates, and Regulatory Implications
| Route/Author | Key Reagents/Intermediates | Toxic or GHS-Classified Reagents | Solvents (ICH Class) | Nitrosamine Risk * |
|---|---|---|---|---|
| Kametani [39] | Phosphorus oxychloride, diazomethane, formaldehyde | Diazomethane (Acute Tox. 1), POCl3 (Skin Corr. 1B) | DCM (Class 2) | Moderate–secondary amine under acid |
| Guangxi Nanning [23] | Safrole, sodium dichromate, glyoxal | Na2Cr2O7 (Carc. 1B, Muta. 1B), HCl | Aqueous/DCM (Class 2) | High–secondary amine + acid + nitrites |
| Hangzhou [45] | Catechol, paraformaldehyde, sodium cyanide → β-amino alcohol | NaCN (Acute Tox. 2), Paraformaldehyde, DCM (Carc. 2) | DCM (Class 2) | High–persistent secondary amine + acid |
| Northeast Pharma [25] | Phenol, paraformaldehyde, methyl chloroacetate | Paraformaldehyde (Acute Tox. 4) | DCM (Class 2) | Moderate–amine intermediates |
| Gatland [46] | Iodomethane, Pd catalyst, glycol acetal | Iodomethane (Acute Tox. 3), Pd salts | MeOH (Class 2) | Low–late-stage quaternization |
| Anand [40] | TMS-arylalkyne, silver nitrite, TBAF | AgNO2 (Env. Tox. 1), TBAF (Corr.), Pd catalyst | THF (Class 2) | Moderate–nitrite present, no persistent amine |
| Tong [41] | Copper iodide, TPAP, DPPA | CuI (Aquatic Acute 1), DPPA (Expl. 1.1, Tox. 2) | Acetonitrile (Class 2) | Moderate–amine intermediates |
| Chen 1 [50] | Catechol, diethyl malonate | – | DCM (Class 2) | Moderate–amine intermediates |
| Chen 2 [51] | 1,2-Methylenedioxybenzene, CuBr·DMS, DMS | CuBr–DMS complex (Irritant), DMS (flammable) | DMS (Class 3) | Moderate–amine intermediates |
| Clift [43] | Intermediate 14, triflic acid, methanol | TfOH (Corr.), MeOH (Class 2) | Methanol (Class 2) | Low–quaternary ammonium end product |
| Konno [52] | Boron tribromide, aryl aldehyde | BBr3 (Tox. 3, water reactive) | MeOH (Class 2) | Moderate–demethylation + amine |
| Dong Z. Li [53] | TFAA, benzyl chloride | TFAA (Tox. 3), Benzyl chloride (Carc. 2) | DCM (Class 2) | Moderate–amidation route |
4. Residual Solvent Risks in Synthetic Berberine Hydrochloride
5. Natural vs. Synthetic Berberine
6. Discussion
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Grycová, L.; Dostál, J.; Marek, R. Quaternary Protoberberine Alkaloids. Phytochemistry 2007, 68, 150–175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Zou, D.; Liu, W.; Yang, J.; Zhu, N.; Huo, L.; Wang, M.; Hong, J.; Wu, P.; et al. Treatment of Type 2 Diabetes and Dyslipidemia with the Natural Plant Alkaloid Berberine. J. Clin. Endocrinol. Metab. 2008, 93, 2559–2565. [Google Scholar] [CrossRef] [PubMed]
- Imanshahidi, M.; Hosseinzadeh, H. Pharmacological and Therapeutic Effects of Berberis spp. and Its Active Constituent, Berberine. Phytother. Res. 2008, 22, 999–1012. [Google Scholar] [CrossRef]
- Yin, J.; Xing, H.; Ye, J. Efficacy of Berberine in Patients with Type 2 Diabetes Mellitus. Metabolism 2008, 57, 712–717. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, W.S.; Kim, K.H.; Yoon, M.J.; Cho, H.J.; Shen, Y.; Ye, J.M.; Lee, Y.L.; Kim, J.W.; Kim, J.B. Berberine, a Natural Plant Product, Activates AMP-Activated Protein Kinase with Beneficial Metabolic Effects in Diabetic and Insulin-Resistant States. Diabetes 2006, 55, 2256–2264. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, J.; Xue, R.; Wu, J.D.; Zhao, W.; Wang, Z.Z.; Wang, S.K.; Zhou, Z.X.; Song, D.Q.; Wang, Y.M.; et al. Berberine Lowers Blood Glucose in Type 2 Diabetes Mellitus Patients through Increasing Insulin Receptor Expression. Metabolism 2010, 59, 285–292. [Google Scholar] [CrossRef]
- Tian, E.; Sharma, G.; Dai, C. Neuroprotective Properties of Berberine: Molecular Mechanisms and Clinical Implications. Antioxidants 2023, 12, 1883. [Google Scholar] [CrossRef]
- Li, Z.; Geng, Y.-N.; Jiang, J.-D.; Kong, W.-J. Antioxidant and Anti-Inflammatory Activities of Berberine in the Treatment of Diabetes Mellitus. Evid. Based Complement. Alternat. Med. 2014, 2014, 289264. [Google Scholar] [CrossRef]
- Ma, H.; Xing, C.; Wei, H.; Li, Y.; Wang, L.; Liu, S.; Wu, Q.; Sun, C.; Ning, G. Berberine Attenuates Neuronal Ferroptosis via the AMPK–NRF2–HO-1-Signaling Pathway in Spinal Cord-Injured Rats. Int. Immunopharmacol. 2024, 142, 113227. [Google Scholar] [CrossRef]
- García-Muñoz, A.M.; Victoria-Montesinos, D.; Ballester, P.; Cerdá, B.; Zafrilla, P. A Descriptive Review of the Antioxidant Effects and Mechanisms of Action of Berberine and Silymarin. Molecules 2024, 29, 4576. [Google Scholar] [CrossRef] [PubMed]
- Siliman, M.M.; Destrumelle, S.; Le Jan, D.; Mansour, N.M.; Fizanne, L.; Ouguerram, K.; Desfontis, J.C.; Mallem, M.Y. Preventive Effects of a Nutraceutical Mixture of Berberine, Citrus and Apple Extracts on Metabolic Disturbances in Zucker Fatty Rats. PLoS ONE 2024, 19, e0306783. [Google Scholar] [CrossRef]
- Alnuqaydan, A.M.; Almutary, A.G.; Azam, M.; Manandhar, B.; De Rubis, G.; Madheswaran, T.; Paudel, K.R.; Hansbro, P.M.; Chellappan, D.K.; Dua, K. Phytantriol-Based Berberine-Loaded Liquid Crystalline Nanoparticles Attenuate Inflammation and Oxidative Stress in Lipopolysaccharide-Induced RAW264.7 Macrophages. Nanomaterials 2022, 12, 4312. [Google Scholar] [CrossRef]
- Mostafa, M.; Abd El-Emam, M.M.; Mansour, M.F.; Behairy, A.; Khamis, T.; Mahmoud, S.M.; Alsemeh, A.E.; El Sayed, M.M.; Mady, F.M.; Qelliny, M.R. Berberine Hydrochloride-Loaded Lipid-Based Nanoparticles Ameliorate β-Cell Function by Targeting Nrf2/NF-κB Signaling Pathway in Alloxan-Induced Diabetes Using a Murine Model: Optimization through Full Factorial Design. J. Drug Deliv. Sci. Technol. 2024, 93, 106076. [Google Scholar] [CrossRef]
- Makeiff, D.A.; Smith, B.; Azyat, K.; Xia, M.; Alam, S.B. Development of “Gelled-Oil” Nanocarriers for Controlled Release and Enhanced Bioavailability of Berberine. ACS Omega 2023, 8, 37, 33774. [Google Scholar] [CrossRef] [PubMed]
- Heidarzadeh, M.; Amininasab, M.; Rezayat, S.M.; Mousavi, S.E. Investigation of Antioxidant and Anti-Inflammatory Properties of Berberine Nanomicelles: In Vitro and In Vivo Studies. Curr. Drug Deliv. 2024, 21, 1273–1283. [Google Scholar] [CrossRef]
- Tucker, J.; Fischer, T.; Upjohn, L.; Mazzera, D.; Kumar, M. Unapproved Pharmaceutical Ingredients Included in Dietary Supplements Associated with US Food and Drug Administration Warnings. JAMA Netw. Open 2018, 1, e183337. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Che, S.; Li, J.; Luo, J.; Zhang, W.; Chen, Y.; Zhao, Z.; Wei, H.; Xie, W. Synthesis of Berberine and Canagliflozin Chimera and Investigation into New Antibacterial Activity and Mechanisms. Molecules 2022, 27, 2848. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Some N-Nitroso Compounds. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 1978; Volume 17, Available online: https://publications.iarc.fr/48 (accessed on 24 July 2025).
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). M7(R2): Assessment and Control of DNA Reactive (Mutagenic) Impurities in Pharmaceuticals to Limit Potential Carcinogenic Risk; ICH Harmonised Guideline: Geneva, Switzerland, 2023; Available online: https://www.ich.org/page/multidisciplinary-guidelines (accessed on 24 July 2025).
- U.S. Food and Drug Administration (FDA). Control of Nitrosamine Impurities in Human Drugs: Guidance for Industry; U.S. Department of Health and Human Services: Silver Spring, MD, USA, 2020. Available online: https://www.fda.gov/media/141720/download (accessed on 24 July 2025).
- China National Intellectual Property Administration (CNIPA). A Kind of Berberine Synthesis Technique. Patent CN106543171A, 29 March 2017. Available online: https://patents.google.com/patent/CN106543171A/en (accessed on 24 July 2025).
- China National Intellectual Property Administration (CNIPA). Synthetic Preparation Method of Berberine Hydrochloride. Patent CN113735847A, 7 June 2022. Available online: https://patents.google.com/patent/CN113735847A/en (accessed on 24 July 2025).
- Guangxi Nanning Pharmaceutical Factory. Process Method for Atmospheric Pressure Synthesis of Berberine. J. Pharm. Ind. 1973, 7, 1–4. [Google Scholar]
- Mitch, W.A.; Sharp, J.O.; Trussell, R.R.; Valentine, R.L.; Alvarez-Cohen, L.; Sedlak, D.L. N-Nitrosodimethylamine (NDMA) as a Drinking Water Contaminant: A Review. Environ. Eng. Sci. 2003, 20, 389–404. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). Information About Nitrosamine Impurities in Medications. 2024. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/information-about-nitrosamine-impurities-medications (accessed on 24 July 2025).
- Bharate, S.S. Critical Analysis of Drug Product Recalls Due to Nitrosamine Impurities. J. Med. Chem. 2021, 64, 2923–2936. [Google Scholar] [CrossRef]
- Shephard, E.A.; Nawarskas, J.J. Nitrosamine Impurities in Angiotensin Receptor Blockers. Cardiol. Rev. 2020, 28, 262–265. [Google Scholar] [CrossRef]
- Zeng, Y.; Teo, J.; Lim, J.Q.; Hoi, W.K.; Kee, C.L.; Low, M.Y.; Ge, X. Determination of N-Nitroso Folic Acid in Folic Acid and Multivitamin Supplements by LC-MS/MS. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2024, 41, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, N.R.; Nagappan, K.; Singh, M.T.; Dhanabal, S.P. Nitrosamine Impurities in Herbal Formulations: A Review of Risks and Mitigation Strategies. Drug Res. 2023, 73, 431–440. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Nitrosamines EMEA-H-A53-1490—Questions and Answers for Marketing Authorisation Holders/Applicants on the CHMP Opinion for the Article 5(3) of Regulation (EC) No 726/2004 Referral: Nitrosamine Impurities in Human Medicinal Products. Available online: https://www.ema.europa.eu/en/documents/opinion-any-scientific-matter/nitrosamines-emea-h-a53-1490-questions-answers-marketing-authorisation-holders-applicants-chmp-opinion-article-53-regulation-ec-no-726-2004-referral-nitrosamine-impurities-human-medicinal-products_en.pdf (accessed on 23 October 2025).
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). Q3C (R8): Impurities: Guideline for Residual Solvents. 2021. Available online: https://www.ich.org/page/quality-guidelines (accessed on 24 July 2025).
- Teasdale, A.; Elder, D.; Harvey, J. Residual Solvents in Pharmaceuticals: A Practical Guide to ICH Q3C. Pharm. Technol. 2018, 42, 28–34. [Google Scholar]
- U.S. Food and Drug Administration (FDA). Dietary Supplement Health and Education Act of 1994. 1994. Available online: https://ods.od.nih.gov/About/DSHEA_Wording.aspx (accessed on 24 July 2025).
- Pawar, R.S.; Grundel, E. Overview of Regulation of Dietary Supplements in the USA and Issues of Adulteration with Phenethylamines (PEAs). Drug Test. Anal. 2017, 9, 500–517. [Google Scholar] [CrossRef]
- Liu, X. Summary of Total Synthesis of Berberine. Hans J. Chem. Eng. Technol. 2020, 10, 306–313. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Some Chemicals Used as Solvents and in Polymer Manufacture. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2016; Volume 110, Available online: https://publications.iarc.who.int/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Some-Chemicals-Used-As-Solvents-And-In-Polymer-Manufacture-2016 (accessed on 24 July 2025).
- Beta Analytic. Turmeric Case Study: Verifying “Natural” Claims by Carbon-14 and HPLC Analyses. Beta Lab Services. 2021. Available online: https://www.betalabservices.com/turmeric-hplc-carbon-14-testing/ (accessed on 24 July 2025).
- Pictet, A.; Gams, A. Synthese des Berberins. Ber. Dtsch. Chem. Ges. 1911, 44, 2480–2485. [Google Scholar] [CrossRef]
- Kametani, T.; Noguchi, I. Studies on the Syntheses of Heterocyclic Compounds. Part CCCII. Alternative Total Syntheses of (±)-Nandinine, (±)-Canadine, and Berberine Iodide. J. Chem. Soc. C 1969, 15, 2036–2038. [Google Scholar] [CrossRef]
- Reddy, V.; Jadhav, A.S.; Anand, R.V. A Room-Temperature Protocol to Access Isoquinolines through Ag(I) Catalysed Annulation of O-(1-Alkynyl) Arylaldehydes and Ketones with NH4OAc: Elaboration to Berberine and Palmatine. Org. Biomol. Chem. 2015, 13, 3732–3741. [Google Scholar] [CrossRef]
- Zhou, S.; Tong, R. A General, Concise Strategy That Enables Collective Total Syntheses of over 50 Protoberberine and Five Aporhoeadane Alkaloids within Four to Eight Steps. Chem. Eur. J. 2016, 22, 7084–7089. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration (FDA). FDA Requests Removal of All Ranitidine Products (Zantac) from the Market. 2020. Available online: https://www.fda.gov/news-events/press-announcements/fda-requests-removal-all-ranitidine-products-zantac-market (accessed on 24 July 2025).
- Mori-Quiroz, L.M.; Hedrick, S.L.; De Los Santos, A.R.; Clift, M.D. A Unified Strategy for the Syntheses of the Isoquinolinium Alkaloids Berberine, Coptisine, and Jatrorrhizine. Org. Lett. 2018, 20, 4281–4284. [Google Scholar] [CrossRef] [PubMed]
- China National Intellectual Property Administration (CNIPA). The Preparation Method of Berberine Hydrochloride. Patent CN101245064A, 2 May 2012. Available online: https://patents.google.com/patent/CN101245064A/en (accessed on 23 October 2025).
- Reagent Laboratory of Hangzhou First Pharmaceutical Factory. Reagent Process for the Synthesis of Berberine Hydrochloride from o-Dichlorobenzene. J. Pharm. Ind. 1974, 8, 6–8. [Google Scholar]
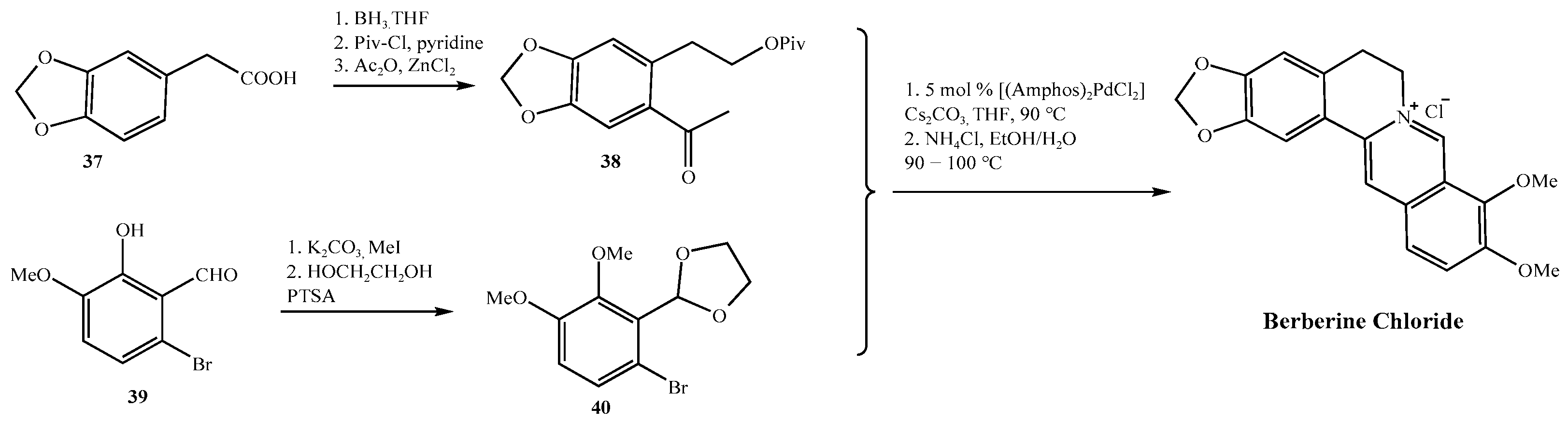
- Gatland, A.E.; Pilgrim, B.S.; Procopiou, P.A.; Donohoe, T.J. Short and Efficient Syntheses of Protoberberine Alkaloids Using Palladium-Catalyzed Enolate Arylation. Angew. Chem. Int. Ed. 2014, 53, 14555–14558. [Google Scholar] [CrossRef]
- Huang, S.; Huang, G. A Comprehensive Review of Recent Advances in the Extraction and Therapeutic Potential of Berberine. RSC Adv. 2025, 15, 24596–24611. [Google Scholar] [CrossRef]
- Neag, M.A.; Mocan, A.; Echeverría, J.; Pop, R.M.; Bocsan, C.I.; Crişan, G.; Buzoianu, A.D. Berberine: Botanical Occurrence, Traditional Uses, Extraction Methods, and Relevance in Cardiovascular, Metabolic, Hepatic, and Renal Disorders. Front. Pharmacol. 2018, 9, 557. [Google Scholar] [CrossRef]
- Mokgadi, J.; Turner, C.; Torto, N. Pressurized Hot Water Extraction of Alkaloids in Goldenseal. Am. J. Anal. Chem. 2013, 4, 398–403. [Google Scholar] [CrossRef]
- Chen, C.; Luo, Z.; Yang, H.; Feng, Y. Study on the Synthesis of Berberine Hydrochloride. Chin. J. Org. Chem. 2016, 36, 1426–1430. [Google Scholar] [CrossRef]
- Chen, C.; Xu, M.; Zhao, Q.; Liu, C.; Yang, H.; Feng, Y. Convergent Synthesis of Berberine Hydrochloride. Chin. J. Org. Chem. 2017, 37, 503–507. [Google Scholar] [CrossRef]
- Tajiri, M.; Yamada, R.; Hotsumi, M.; Makabe, K.; Konno, H. The Total Synthesis of Berberine and Selected Analogues, and Their Evaluation as Amyloid Beta Aggregation Inhibitors. Eur. J. Med. Chem. 2021, 215, 113289. [Google Scholar] [CrossRef]
- Yan, X.; Zheng, J.; Li, W.-D.Z. Concise Total Syntheses of Berberine and Its Analogues Enabled by Trifluoroacetic Anhydride-Promoted Decarbonylative-Elimination Reaction. Tetrahedron Lett. 2023, 132, 154826. [Google Scholar] [CrossRef]
- Zeng, T.; Li, R.J.; Mitch, W.A. Structural Modifications to Quaternary Ammonium Polymer Coagulants to Inhibit N-Nitrosamine Formation. Environ. Sci. Technol. 2016, 50, 4778–4787. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, A.; Mohanty, S. Efficacy and Safety of HIMABERB® Berberine on Glycemic Control in Patients with Prediabetes: Double-Blind, Placebo-Controlled, and Randomized Pilot Trial. BMC Endocr. Disord. 2023, 23, 190. [Google Scholar] [CrossRef] [PubMed]
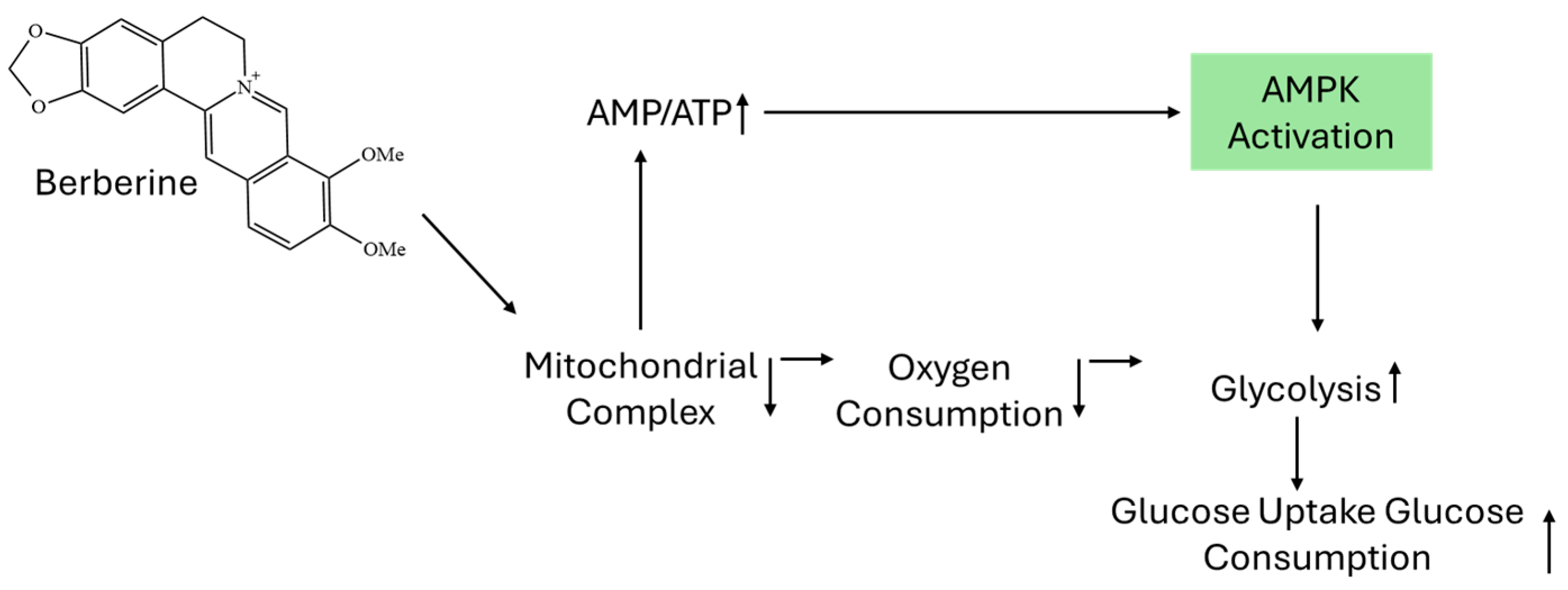
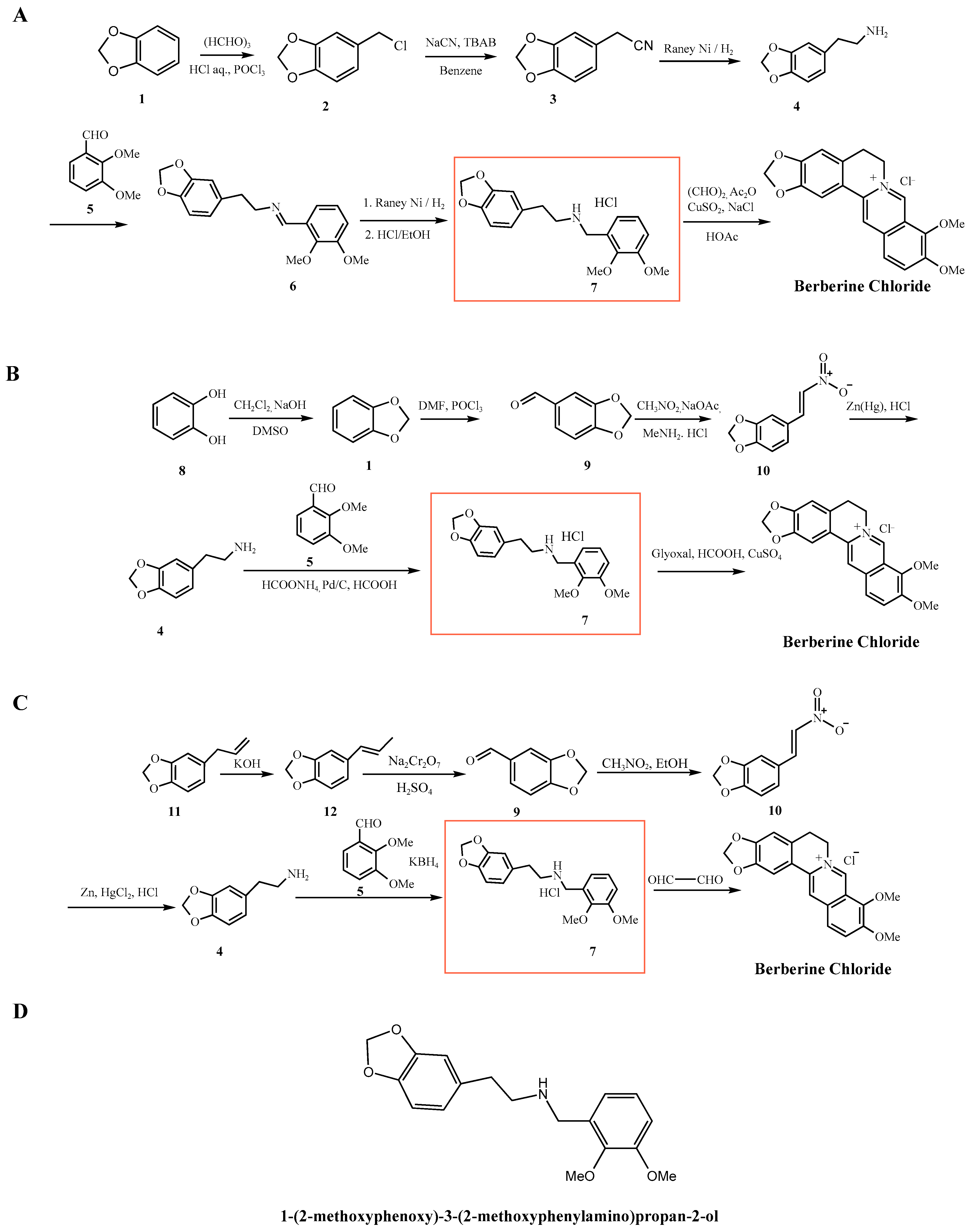
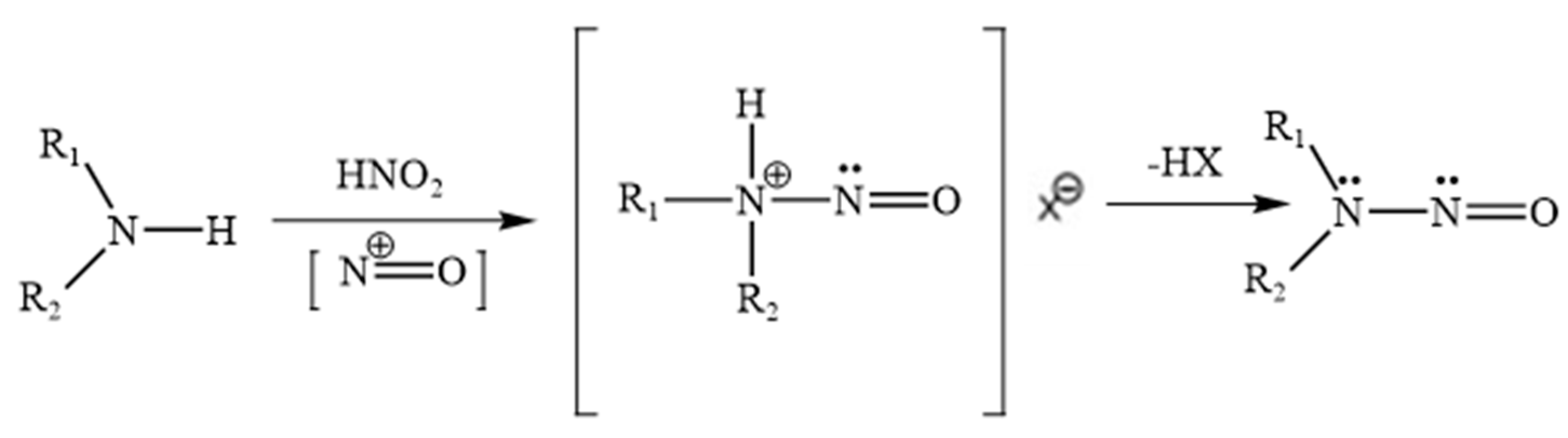

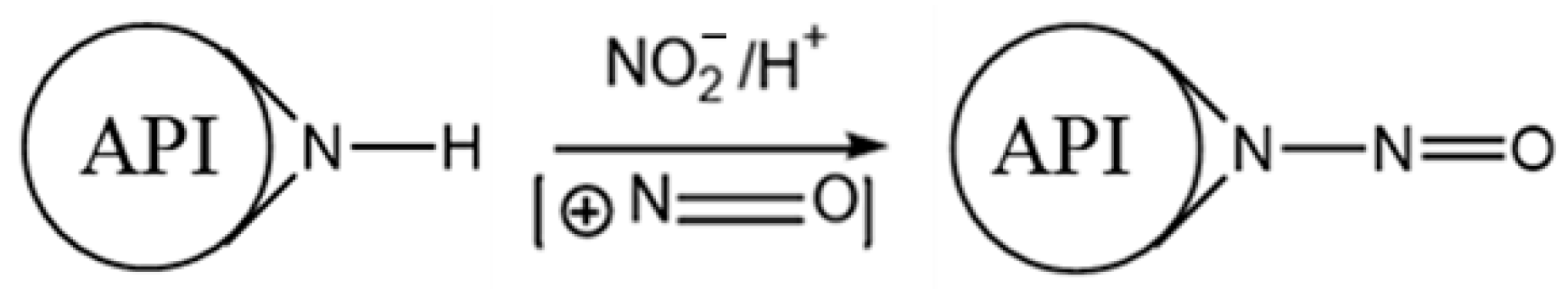
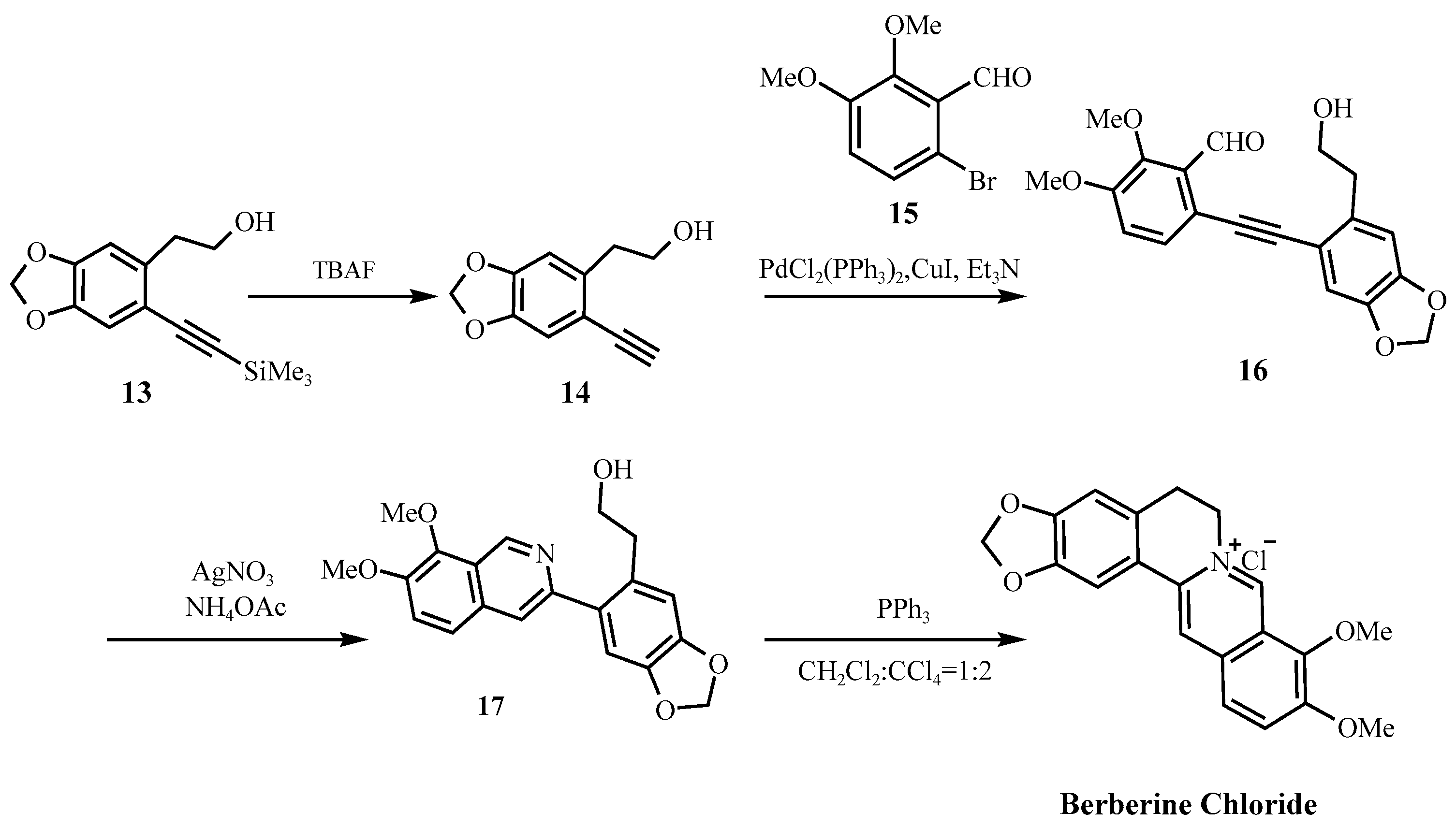

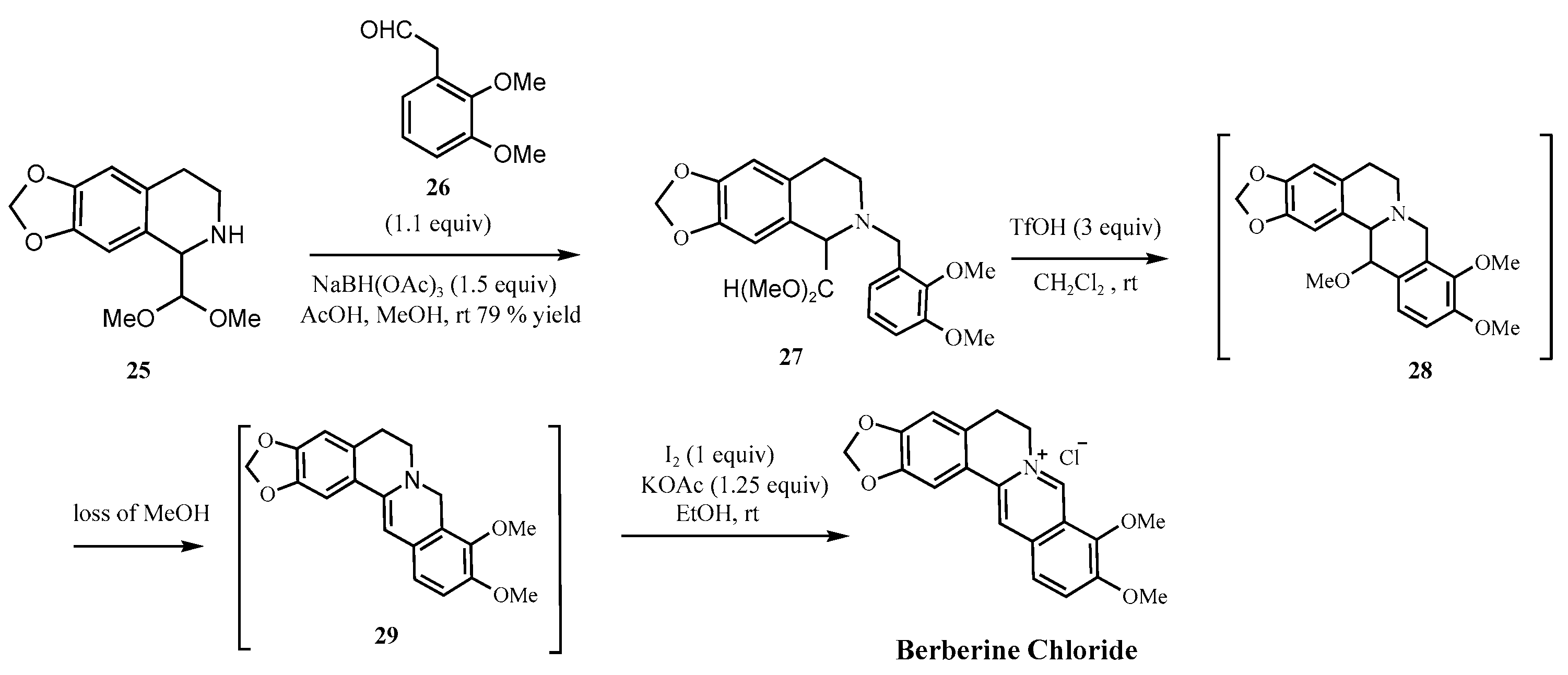
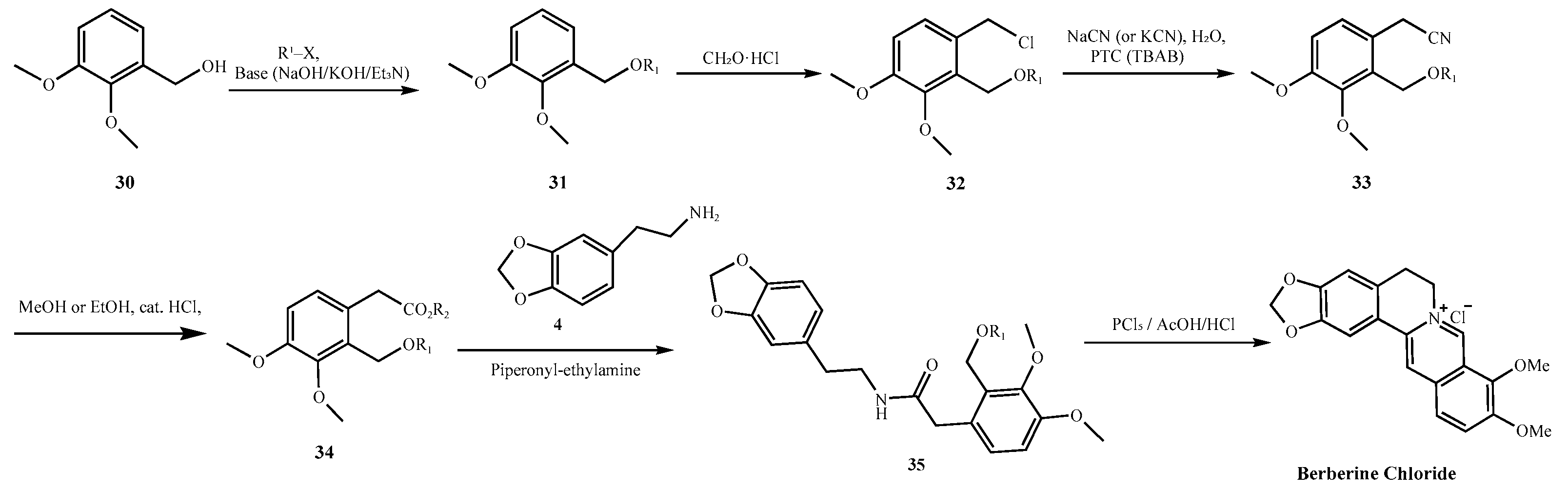
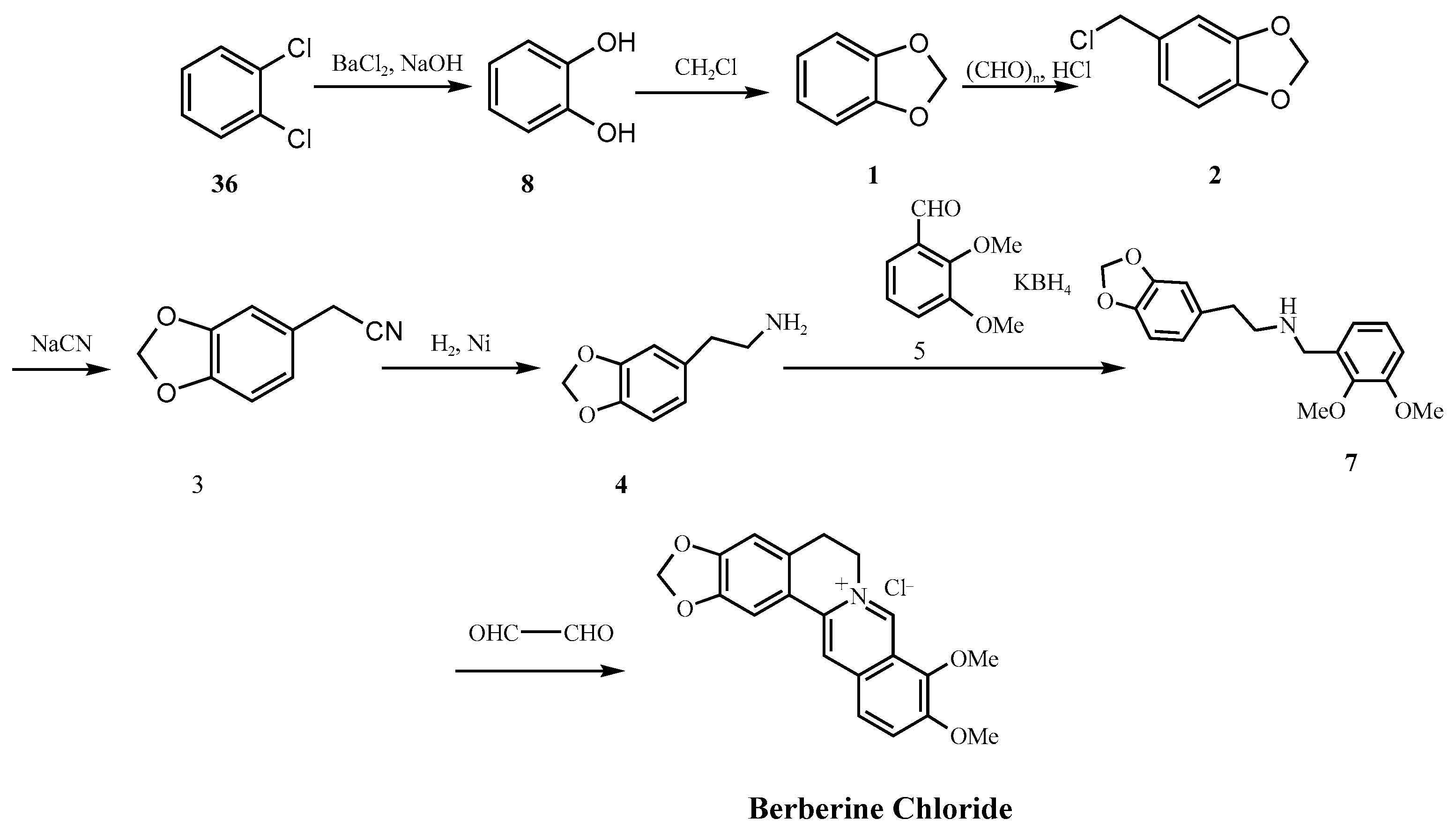
| Parameter | Natural Berberine (Plant-Derived) | Synthetic Berberine (Chemically Synthesized) |
|---|---|---|
| Source | Extracted from medicinal plants (Berberis, Coptis) with centuries of traditional use | Produced via multistep chemical synthesis using petrochemical-derived precursors |
| Apparatus | Stainless-steel extraction vessels, percolators, or Soxhlet apparatus; concentration typically by rotary evaporator or spray dryer | Glass-lined or stainless-steel reactors with reflux condensers; purification through crystallization or chromatography |
| Yield | Low, derived from renewable, biogenic sources | High, dependent on synthetic efficiency and raw material availability |
| Purity | Requires tedious purification, but typically free from synthetic byproducts | High assay purity, but may harbor trace-level synthetic impurities such as nitrosamines |
| Toxic Impurity Risk | Minimal; aqueous or alcoholic extraction avoids nitrosamine formation | Elevated; potential for nitrosamines, residual solvents, or unreacted intermediates |
| Nitrosamine Risk (FDA 2024 Guidance) | Negligible under aqueous, alcoholic and acid-base extraction conditions | Notable concern: many synthetic routes can lead to nitrosamine formation unless mitigated |
| Process Solvents | Water, Ethanol, methanol, environmentally benign (Except methanol) and food-grade | Involves organic solvents (e.g., dichloromethane, toluene), which may require stringent residue control |
| Consumer Perception | Viewed as holistic, natural, and safer for long-term use | Increasing concern over “lab-made” compounds and hidden risks among informed consumers |
| Environmental Impact | Lower carbon and chemical footprint if sustainably harvested | Higher impact unless green chemistry principles and solvent recovery are employed |
| Cost Efficiency | Higher per-gram cost, but offset by holistic composition and perceived safety | Lower cost per gram, but potential trade-offs in safety and public trust |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meher, A.K.; Zarouri, A.; Kumar, M. Rethinking Synthetic Berberine in Nutraceuticals: Nitrosamine Risks, Regulatory Oversight, and Safer Alternatives. Molecules 2025, 30, 4274. https://doi.org/10.3390/molecules30214274
Meher AK, Zarouri A, Kumar M. Rethinking Synthetic Berberine in Nutraceuticals: Nitrosamine Risks, Regulatory Oversight, and Safer Alternatives. Molecules. 2025; 30(21):4274. https://doi.org/10.3390/molecules30214274
Chicago/Turabian StyleMeher, Anil Kumar, Akli Zarouri, and Manish Kumar. 2025. "Rethinking Synthetic Berberine in Nutraceuticals: Nitrosamine Risks, Regulatory Oversight, and Safer Alternatives" Molecules 30, no. 21: 4274. https://doi.org/10.3390/molecules30214274
APA StyleMeher, A. K., Zarouri, A., & Kumar, M. (2025). Rethinking Synthetic Berberine in Nutraceuticals: Nitrosamine Risks, Regulatory Oversight, and Safer Alternatives. Molecules, 30(21), 4274. https://doi.org/10.3390/molecules30214274








