Overview of Ayurveda and Ashwagandha: Bioactive Phytochemicals and Potential Applications to Gliomas
Abstract
1. Introduction
1.1. Ayurveda: Overview of Concepts and Philosophy
1.2. Medicinal Plants and Ethnopharmacology
2. Analysis of Ashwagandha and Bioactive Phytochemicals
2.1. Steroidal Lactones and Withanolides
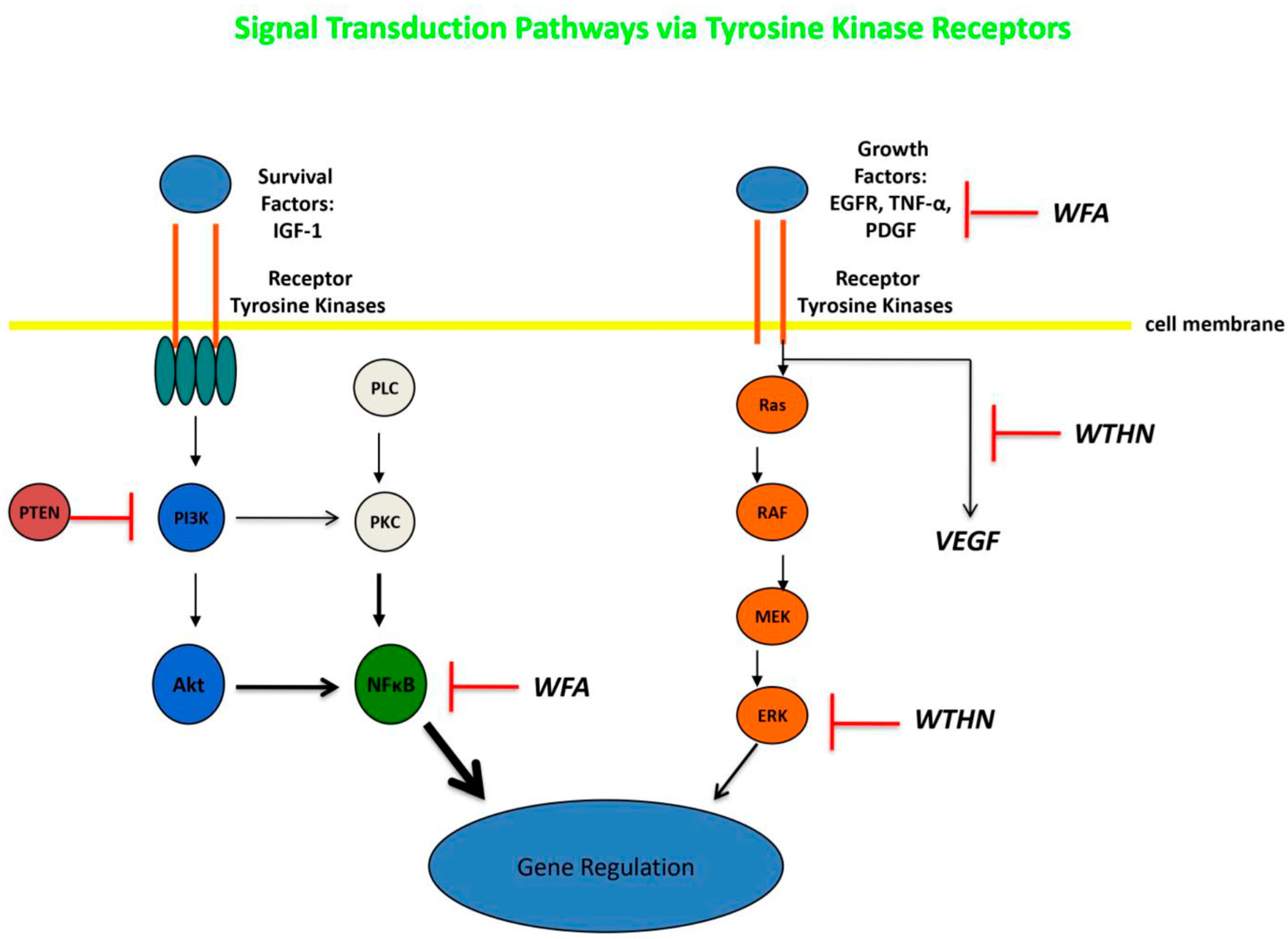
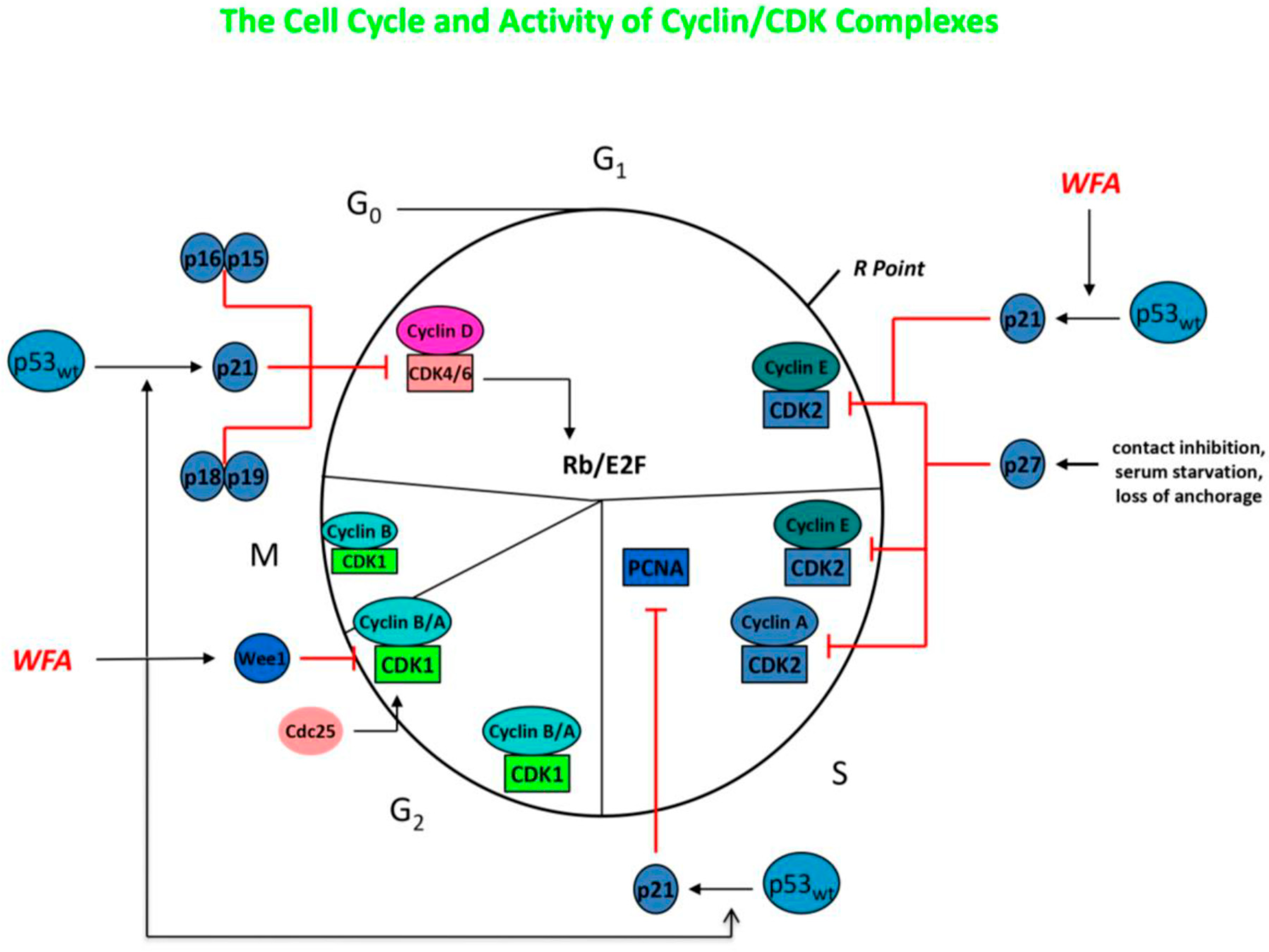
2.2. Withaferin-A: Overview and Application to Systemic Tumors
2.3. Ashwagandha and Withaferin-A: Application to Gliomas
2.4. Withanone: Overview and Application to Systemic Tumors
2.5. Withanone: Application to Gliomas
2.6. Other Withanolides/Bioactive Compounds: Application to Systemic Cancer and Gliomas
3. Conclusions and Future Considerations
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Macozzi, M.S. Traditional medicines of India and Nepal: Ayurveda and Siddha. In Fundamentals of Complementary, Alternative, and Integrative Medicine, 6th ed.; Micozzi, M.S., Ed.; Elsevier: St. Louis, MO, USA, 2018; Volume 36, pp. 591–615. [Google Scholar]
- Lad, V. Textbook of Ayurveda, Volume 1: Fundamental Principles; The Ayurveda Press: Kuala Lumpur, Malaysia, 2002. [Google Scholar]
- Mukherjee, P.K.; Harwansh, R.K.; Bahadur, S.; Banerjee, S.; Kar, A.; Chanda, J.; Biswas, S.; Ahmmed, S.M.; Katiyar, C.K. Development of Ayurveda—Tradition to trend. J. Ethnopharmacol. 2017, 197, 10–24. [Google Scholar] [CrossRef]
- Arnold, J.T. Integrating ayurvedic medicine into cancer research programs part 1: Ayurveda background and applications. J. Ayurveda Integ. Med. 2023, 14, 100676. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.; Gibbons, S. Ethnopharmacology in drug discovery: An analysis of its role and potential contribution. J. Pharm. Pharmacol. 2001, 53, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.; Bremmer, P. Ethnobotany and ethnopharmacy—Their role for anti-cancer drug development. Curr. Drug Targets 2006, 7, 239–245. [Google Scholar] [CrossRef]
- Tariq, A.; Sadia, S.; Pan, K.; Ullah, I.; Mussarat, S.; Sun, F.; Abiodun, O.O.; Batbaatar, A.; Li, Z.; Song, D.; et al. A systematic review on ethnomedicines of anti-cancer plants. Phytother. Res. 2017, 31, 202–264. [Google Scholar] [CrossRef]
- Arnold, J.T. Integrating ayurvedic medicine into cancer research programs part 2: Ayurvedic herbs and research opportunities. J. Ayurveda Integr. Med. 2023, 14, 100677. [Google Scholar] [CrossRef]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef]
- Paul, S.; Chakraborty, S.; Anand, U.; Dey, S.; Nandy, S.; Ghorai, M.; Saha, S.C.; Patil, M.T.; Kandimalla, R.; Prockow, J.; et al. Withania somnifera (L.) Dunal (Ashwagandha): A comprehensive review on ethnopharmacology, pharmacotherapeutics, biomedicinal and toxicological aspects. Biomed. Pharmacother. 2021, 143, 112175. [Google Scholar] [CrossRef] [PubMed]
- Gaurav, H.; Yadav, D.; Maurya, A.; Yadav, H.; Yadav, R.; Shukla, A.C.; Sharma, M.; Gupta, V.K.; Palazon, J. Biodiversity, biochemical profiling, and pharmaco-commercial applications of Withania somnifera: A review. Molecules 2023, 28, 1208. [Google Scholar] [CrossRef]
- Ingawale, D.S.M.; Namdeo, A.G. Pharmacological evaluation of Ashwagandha highlighting its healthcare claims, safety, and toxicity aspects. J. Diet. Suppl. 2021, 18, 183–226. [Google Scholar]
- Bashir, A.; Nabi, M.; Tabassum, N.; Afzal, S.; Ayoub, M. An updated review on phytochemistry and molecular targets of Withania somnifera (L.) Dunal (Ashwagandha). Front. Pharmacol. 2023, 14, 1049334. [Google Scholar] [CrossRef]
- Gannon, J.M.; Brar, J.; Rai, A.; Chengappa, K.N.R. Effects of a standardized extract of Withania somnifera (Ashwagandha) on depression and anxiety symptoms in persons with schizophrenia participating in a randomized, placebo-controlled clinical trial. Ann. Clin. Psychiat. 2019, 31, 123–129. [Google Scholar] [CrossRef]
- Ziegenfuss, T.N.; Kedia, A.W.; Sandrock, J.E.; Raub, B.J.; Kerksick, C.M.; Lopez, H.L. Effects of an aqueous extract of Withania somnifera on Strength Training Adaptations and Recovery: The STAR Trial. Nutrients 2018, 10, 1807. [Google Scholar] [CrossRef]
- Nasimi Doost Azgomi, R.; Nazemiyeh, H.; Sadeghi Bazaargai, H.; Fazljou, S.M.B.; Nejatbakhsh, F.; Moini Jazani, A.; Ahmai Asrbadr, Y.; Zomorrodi, A. Comparative evaluation of the effects of Withania somnifera with pentoxifylline on the sperm paramaters of idiopathic male infertility: A triple-blind randomized clinical trial. Andrologia 2018, 50, e13041. [Google Scholar] [CrossRef]
- Sharma, A.K.; Basu, I.; Singh, S. Efficacy and safety of ashwagandha root extract in subclinical hypothyroid patients: A double-blind, randomized placebo-controlled trial. J. Altern. Complement. Med. 2018, 24, 243–248. [Google Scholar] [CrossRef]
- Choudhary, D.; Bhattacharyya, S.; Bose, S. Efficacy and safety of ashwagandha (Withania somnifera (L.) Dunal) root extract in improving memory and cognitive function. J. Diet. Suppl. 2017, 14, 599–612. [Google Scholar] [CrossRef]
- Choudhary, D.; Bhattacharyya, S.; Joshi, K. Body weight management in adults under chronic stress through treatment with ashwagandha root extract: A double-blind, randomized, placebo-controlled trial. J. Evid. Based Complement. Altern. Med. 2017, 22, 96–106. [Google Scholar] [CrossRef]
- Kohlmuenzer, S.; Krupinska, J. Chemotherapeutic properties of substances isolated from leaves of Withania somnifera Dunal. Acta Physiol. Pol. 1960, 11, 778–780. [Google Scholar]
- Menssen, H.G.; Stapel, G.A. C28-steroidlacton from the roots of Withania somnifera. Planta Med. 1973, 24, 8–12. [Google Scholar] [CrossRef]
- Singh, N.; Yadav, S.S.; Rao, A.S.; Nandal, A.; Kumar, S.; Ganale, S.S.; Narasihman, B. Review on anticancerous therapeutic potential of Withania somnifera (L.) Dunal. J. Ethnopharmacol. 2021, 270, 113704. [Google Scholar] [CrossRef]
- Newton, H.B. Indian Ayurvedic medicine: Overview and application to brain cancer. J. Ayurveda Integr. Med. 2024, 15, 101013. [Google Scholar] [CrossRef]
- Zhang, Q.; Yuan, Y.K.; Cao, S.; Kang, N.; Qui, F. Withanolides: Promising candidates for cancer therapy. Phytother. Res. 2024, 38, 1104–1158. [Google Scholar] [CrossRef]
- Singh, A.; Raza, A.; Amin, S.; Damodaran, C.; Sharma, A.K. Recent advances in the chemistry and therapeutic evaluation of naturally occurring and synthetic withanolides. Molecules 2022, 27, 886. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Y.; Xu, Y.; Liu, Y.; Li, H.; Chen, L. Molecular targets and mechanisms of anti-cancer effects of withanolides. Chem.-Biol. Interact. 2023, 384, 110698. [Google Scholar] [CrossRef]
- Grover, A.; Singh, R.; Shandilya, A.; Priyandoko, D.; Agrawal, V.; Bisaria, V.S.; Wadhwa, R.; Kaul, S.; Sundar, D. Ashwagandha derived withanone targets TPX2-Aurora A complex: Computational and experimental evidence to its anticancer activity. PLoS ONE 2012, 7, e30890. [Google Scholar] [CrossRef]
- Ichikawa, H.; Takada, Y.; Shishodia, S.; Jayaprakasam, B.; Nair, M.G.; Aggarwal, B.B. Withanolides potentiate apoptosis, inhibit invasion, and abolish osteoclastogenesis through suppression of nuclear factor-κB (NF-κB) activation and NF-κB-regulated gene expression. Mol. Cancer Ther. 2006, 5, 1431–1445. [Google Scholar] [CrossRef]
- Biswal, B.M.; Sulaiman, A.M.; Ismail, H.C.; Zakaria, H.; Jalil Abdul, M.I.; Muhammad, K.I. AOS14 phase II clinical study of combination chemotherapy with herb Withania somnifera (ashwagandha) in breast cancer. Eur. J. Cancer 2012, 48, S8–S9. [Google Scholar] [CrossRef]
- Biswal, B.M.; Sulaiman, A.M.; Ismail, H.C.; Zakaria, H.; Musa, K.I. Effect of Withania somnifera (ashwagandha) on the development of chemotherapy-induced fatigue and quality of life in breast cancer patients. Integr. Cancer Ther. 2013, 12, 312–322. [Google Scholar] [CrossRef]
- Pires, N.; Gota, V.; Hingorani, L.; Agarwal, M.; Puri, A. Safety and pharmacokinetics of Withaferin-A in advanced stage high grade osteosarcoma: A phase I trial. Ayurveda Integr. Med. 2020, 11, 68–72. [Google Scholar] [CrossRef]
- Vyas, A.R.; Singh, S.V. Molecular targets and mechanisms of cancer prevention and treatment by withaferin A, a naturally occurring steroidal lactone. AAPS J. 2014, 16, 1–10. [Google Scholar] [CrossRef]
- Dutta, R.; Khalil, R.; Green, R.; Mohapatra, S.S.; Mohapatra, S. Withania somnifera (Ashwagandha) and Withaferin A: Potential in Integrative Oncology. Int. J. Mol. Sci. 2019, 20, 5310. [Google Scholar] [CrossRef]
- Hassannia, B.; Logie, E.; Vandenabeele, P.; Berghe, T.V.; Berghe, W.V. Withaferin A: From ayurvedic folk medicine to preclinical anti-cancer drug. Biochem. Pharmacol. 2020, 173, 113602. [Google Scholar] [CrossRef]
- Sultana, T.; Okla, M.K.; Ahmed, M.; Akhtar, N.; Al-Hashimi, A.; Abdelgawad, H.; Haq, I. Withaferin A: From ancient remedy to potential drug candidate. Molecules 2021, 26, 7696. [Google Scholar] [CrossRef]
- Atteeq, M. Evaluating anticancer properties of withaferin A—A potent phytochemical. Front. Pharmacol. 2022, 13, 975320. [Google Scholar] [CrossRef]
- Xing, Z.; Su, A.; Mi, L.; Zhang, Y.; He, T.; Qui, Y.; Wei, T.; Li, Z.; Zhu, J.; Wu, W. Withaferi A: A dietary supplement with promising potential as an anti-tumor therapeutic for cancer treatment—Pharmacology and mechanisms. Drug Des. Dev. Ther. 2023, 17, 2909–2929. [Google Scholar] [CrossRef]
- Abeesh, P.; Guruvayoorappan, C. The therapeutic effects of Withaferin A against cancer: Overview and update. Curr. Mol. Med. 2024, 24, 404–419. [Google Scholar] [CrossRef]
- Devabattula, G.; Panda, B.; Yadav, R.; Gudugu, C. The potential pharmacological effects of natural product Withaferin A in cancer: Opportunities and challenges for clinical translation. Planta Med. 2024, 90, 440–453. [Google Scholar] [CrossRef]
- Lin, C.C.; Yang, T.Y.; Lu, H.J.; Wan, C.K.; Hsu, S.L.; Wu, C.C. Attenuating role of withaferin A in the proliferation and migration of lung cancer cells via a p53-miR-27a/miR-10b pathway. Oncol. Lett. 2021, 21, 232. [Google Scholar] [CrossRef]
- Kyakulaga, A.H.; Aqil, F.; Munagala, R.; Gupta, R.C. Withaferin A inhibits epithelial to mesenchymal transition in non-small cell lung cancer cells. Sci. Rep. 2018, 8, 15737. [Google Scholar] [CrossRef]
- Hsu, J.H.M.; Chang, P.M.H.; Cheng, T.S.; Kuo, Y.L.; Wu, A.T.H.; Tran, T.H.; Yang, Y.H.; Chen, J.M.; Tsai, Y.C.; Chu, Y.S.; et al. Identification of withaferin A as a potential candidate for anti-cancer therapy in non-small cell lung cancer. Cancers 2019, 11, 1003. [Google Scholar] [CrossRef]
- vel Szic, S.K.; de Beeck, K.; Ratman, D.; Wouters, A.; Beck, I.M.; Ceclerck, K.; Heyninck, K.; Fransen, E.; Bracke, M.; De Bosscher, K.; et al. Pharmacological levels of withaferin A (Withania somnifera) trigger clinically relevant anticancer effects specific to triple negative breast cancer cells. PLoS ONE 2014, 9, e87850. [Google Scholar] [CrossRef]
- Hahm, E.R.; Kim, S.H.; Singh, K.B.; Singh, K.; Singh, S.V. A comprehensive review and perspective on anticancer mechanisms of withaferin A in breast cancer. Cancer Prev. Res. 2020, 13, 721–734. [Google Scholar] [CrossRef]
- Shah, N.; Kataria, H.; Kaul, S.C.; Ishii, T.; Kaur, G.; Wadhwa, R. Effect of the alcoholic extract of Ashwagandha leaves and its components on proliferation, migration, and differentiation of glioblastoma cells: Combinational approach for enhanced differentiation. Cancer Sci. 2009, 100, 1740–1747. [Google Scholar] [CrossRef]
- Kataria, H.; Shah, N.; Kaul, S.C.; Wadhwa, R.; Kaur, G. Water extract of Ashwagandha leaves limits proliferation and migration, and induces differentiation in glioma cells. Evid. Based Comp. Alt. Med. 2011, 2011, 267614. [Google Scholar] [CrossRef]
- Kataria, H.; Kumar, S.; Chaudhary, H.; Kaur, G. Withania somnifera suppresses tumor growth of intracranial allograft of glioma cells. Mol. Neurobiol. 2016, 53, 4143–4158. [Google Scholar] [CrossRef]
- Grogan, P.T.; Samadi, A.D.; Cohen, M.S. A novel cytotoxic agent induced apoptosis in malignant gliomas. J. Surg. Res. 2010, 158, 341–342. [Google Scholar] [CrossRef]
- Grogan, P.T.; Sleder, K.D.; Stecklein, S.R.; Cohen, M.S. Vassobia Brevifloria root-extract Withaferin A as a novel cytotoxic and synergistic agent against malignant gliomas. J. Surg. Res. 2011, 165, 311. [Google Scholar] [CrossRef]
- Grogan, P.T.; Sleder, K.D.; Samadi, A.K.; Timmermann, B.N.; Cohen, M.S. Cytotoxicity of withaferin A in glioblastomas involves induction of an oxidative stress-mediated heat shock response while altering Akt/mTOR and MAPK signaling pathways. Invest. New Drugs 2013, 31, 545–557. [Google Scholar] [CrossRef]
- Grogan, P.T.; Sarkaria, J.N.; Timmermann, B.N.; Cohen, M.S. Oxidative cytotoxic agent withaferin A resensitizes temozolomide-resistant glioblastomas via MGMT depletion and induces apoptosis through Akt/mTOR pathway inhibitory modulation. Invest. New Drugs 2014, 32, 604–617. [Google Scholar] [CrossRef]
- Hou, W.C.; Miao, X.H.; Ma, L.J.; Bai, X.X.; Liu, Q.; Song, L. Withaferin A induces apoptosis in rat C6 glioma cells through regulating NF-κB nuclear translocation and activation of caspase cascade. Afr. J. Complement. Altern. Med. 2016, 14, 319–324. [Google Scholar] [CrossRef]
- Dhami, J.; Chang, E.; Gambhir, S.S. Withaferin A and its potential role in glioblastoma (GBM). J. Neuro-Oncol. 2017, 131, 201–211. [Google Scholar] [CrossRef] [PubMed]
- Widodo, N.; Priyandoko, D.; Shah, N.; Wadhwa, R.; Kaul, S.C. Selective killing of cancer cells by ashwagandha leaf extract and its component withanone involves ROS signaling. PLoS ONE 2010, 5, e13536. [Google Scholar] [CrossRef]
- Chang, E.; Pohling CNatarajan, A.; Witney, T.H.; Kaur, J.; Xu, L.; Gowrishankar, G.; D’Souza, A.L.; Murty, S.; Schick SChen, L.; Wu, N.; et al. AshwaMAX and withaferin A inhibits gliomas in cellular and murine orthotopic models. J. Neuro-Oncol. 2016, 126, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Pohling, C.; Beygul, N.; Patel, C.B.; Rosenberg, J.; Ha, D.H.; Gambhir, S.S. Synergistic inhibition of glioma cell proliferation by withaferin A and tumor treating fields. J. Neuro-Oncol. 2017, 134, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.; Young, R.J.; Miranda, P.D.; Wenger, C.; Lantos, J.; Wong, E.T. Tumor treating fields: Therapy preclinical and clinical data. In Handbook of Neuro-Oncology Neuroimaging, 3rd ed.; Newton, H.B., Ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: London, UK, 2022; Volume 25, pp. 269–284. [Google Scholar]
- Tang, Q.; Ren, L.; Liu, J.; Li, W.; Zheng, X.; Wang, J.; Du, G. Withaferin A triggers G2/M arrest and intrinsic apoptosis in glioblastoma cells via ATF-ATF3-CHOP axis. Cell Prolif. 2020, 53, e12706. [Google Scholar] [CrossRef]
- Marlow, M.M.; Shah, S.S.; Veliz, E.A.; Ivan, M.E.; Graham, R.M. Treatment of adult and pediatric high-grade gliomas with withaferin A: Antitumor mechanisms and future perspectives. J. Mat. Med. 2017, 71, 16–26. [Google Scholar] [CrossRef]
- Stephen, S.; Tune, B.X.J.; Wu, Y.S.; Batumalaie, K.; Sekar, M.; Sarker, M.M.R.; Subramaniyan, V.; Fuloria, N.K.; Fuloria, S.; Gopinath, S.C.B. Withanone as an emerging anticancer agent and understanding its molecular mechanisms: Experimental and computational evidence. Curr. Cancer Drug Targets 2024, 25, 574–585. [Google Scholar] [CrossRef]
- Wadegaonkar, V.P.; Wadegaonkar, P.A. Withanone as an inhibitor of survivin: A potential drug candidate for cancer therapy. J. Biotechnol. 2013, 168, 229–233. [Google Scholar] [CrossRef]
- Vaishnavi KSaxena, N.; Shah, N.; Singh, R.; Manjunath, K.; Uthayakumar, M.; Kanauhjia, S.P.; Kaul, S.C.; Sekar, K.; Wadhwa, R. Differential activities of the two closely related withanolides, withaferin A and withanone: Bioinformatics and experimental evidences. PLoS ONE 2012, 7, e44419. [Google Scholar] [CrossRef]
- Grover, A.; Priyandoko, D.; Gao, R.; Shandilya, A.; Widodo, N.; Bisaria, V.S.; Kaul, S.C.; Wadhwa, R.; Sundar, D. Withanone binds to mortalin and abrogates mortalin-p53 complex: Computational and experimental evidence. Int. J. Biochem. Cell Biol. 2012, 44, 496–504. [Google Scholar] [CrossRef]
- Gao, R.; Shah, N.; Lee, J.S.; Katiyar, S.P.; Li, L.; Oh, E.; Sundar, D.; Yun, C.O.; Wadhwa, R.; Kaul, S.C. Withanone-rich combination of ashwagandha withanolides restricts metastasis and angiogenesis through hnRNP-K. Mol. Cancer Ther. 2014, 13, 2930–2940. [Google Scholar] [CrossRef]
- Malik, V.; Kumar, V.; Kaul, S.C.; Wadhwa, R.; Sundar, D. Computational insights into the potential of withaferin-A, withanone and caffeic acid phenyl ester for treatment of aberrant-EGFR driven lung cancers. Biomolecules 2021, 11, 160. [Google Scholar] [CrossRef]
- Malik, V.; Radharkrishnan, N.; Kaul, S.C.; Wadhwa, R.; Sundar, D. Computational identification of BCR-ABL oncogenic signaling as a candidate target of withaferin A and withanone. Biomolecules 2022, 12, 212. [Google Scholar] [CrossRef]
- Kumar, V.; Dhanjal, J.K.; Saari, A.N.; Khurana, M.; Kaul, S.C.; Wadhwa, R.; Sundar, D. Effect of withaferin-A, withanone, and caffeic acid phenethyl ester on DNA methyltransfereases: Potential in epigenetic cancer therapy. Curr. Top. Med. Chem. 2024, 24, 379–391. [Google Scholar] [CrossRef]
- Hankey, A. Establishing the scientific validity of Tridosha. Part 1: Doshas, Subdoshas, and Dosha Prakritis. Ancient Sci. Life 2010, 29, 6–18. [Google Scholar]
- Prasher, B.; Gibson, G.; Mukerji, M. Genomic insights into ayurvedic and western approaches to personalized medicine. J. Genet. 2016, 95, 209–228. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Singh, V.; Chandra, S.; Garg, R. Towards standardization of Prakriti evaluation: A scoping review of modern assessment tools and their psychometric properties in Ayurvedic medicine. J. Ayurveda Integr. Med. 2025, 16, 101157. [Google Scholar] [CrossRef]
- Wallace, R.K. Ayurgenomics and modern medicine. Medicina 2020, 56, 661. [Google Scholar] [CrossRef]
- Sharma, H.; Wallace, R.K. Ayurveda and epigenetics. Medicina 2020, 56, 687. [Google Scholar] [CrossRef]
- Travis, F.T.; Wallace, R.K. Dosha brain-types: A neural model of individual differences. J. Ayurveda Integr. Med. 2015, 6, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, V.G.; Naik, N.N.; Ganu, G.; Parmar, V.; Jagtap, S.; Saste, G.; Bhatt, A.; Mulay, V.; Girme, A.; Modi, S.J.; et al. Clinical pharmacokinetic evaluation of Withania somnifera (L.) Dunal root extract in healthy human volunteers: A non-randomized, single dose study utilizing UHPLC-MS/MS analysis. J. Ethnopharmacol. 2024, 322, 117603. [Google Scholar] [CrossRef] [PubMed]
- Newton, H.B.; Fonkem, E. Overview of pathology and treatment of primary brain tumors. In Handbook of Neuro-Oncology Neuroimaging, 3rd ed.; Newton, H.B., Ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: London, UK, 2022; Volume 2, pp. 9–24. [Google Scholar]
- Reardon, D.A. The use of temozolomide for chemoradiation and adjuvant therapy of high-grade gliomas. In Handbook of Brain Tumor Chemotherapy, Molecular Therapeutics, and Immunotherapy, 3rd ed.; Newton, H.B., Ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: London, UK, 2018; Volume 30, pp. 411–418. [Google Scholar]
- Newton, H.B. Bevacizumab: Review of development, pharmacology, and application to brain tumors. Clin. Med. Ther. 2009, 1, 1577–1597. [Google Scholar] [CrossRef]
- Sundar, S.J.; Shakya, S.; Barnett, A.; Wallace, L.C.; Jeon, H.; Sloan, A.; Recinos, V.; Hubert, C.G. Three-dimensional organoid culture unveils resistance to clinical therapies in adult and pediatric glioblastoma. Transl. Oncol. 2022, 15, 101251. [Google Scholar] [CrossRef] [PubMed]



| Plant Parts | Ethnomedicinal Use | Preparation | Route |
|---|---|---|---|
| Root | Anemia | Root powder | Oral |
| Whole plant | Arthritis, anxiety, insomnia | Whole plant | Oral |
| Leaves | Weight loss | Raw leaves | Oral |
| Leaves and roots | Diabetes | Leaf infusion and root powder | Oral |
| Leaves | Memory enhancer | Fresh leaves | Oral |
| Root | Joint pain | Stem juice | Oral |
| Root | Anti-tumor | Root powder | Oral |
| Root | Aphrodisiac, impotency | Root powder | Oral |
| Whole plant | Menstruation | Powder | Oral |
| Root tuber | Skin cancers | Paste | Topical |
| Bark | Piles | Decoction | Topical |
| Tubers | Tumors | Paste | Topical |
| Rhizome | Nervous disorders | Decoction | Oral |
| Tuber | Healing wounds | Paste | Topical |
| Root | Fatigue, poor appetite | Decoction | Oral |
| Leaves | Wounds and burns | Paste | Topical |
| Root | Seizure activity | Root juice | Oral |
| Roots and leaves | Female sterility | Root/leaf decoction | Oral |
| Whole plant and flowers and fruits | Breast cancer | Powder | Oral |
| Leaves and fruits | Digestive problems | Powder | Oral |
| Whole plant and roots | Male sterility | Plant/root powder | Oral |
| Steroidal Lactones | Alkaloids | Flavonoids | Salts | Steroids | Nitrogen-Containing Compounds |
|---|---|---|---|---|---|
| Withaferin-A | Withanine | Kaempferol | Cuscohygrine | Cholesterol | Withanol |
| Withanone | Withananine | Quercetin | Tropine | Sitoinosides | Aomnitol |
| Withanolide-A | Withasomnine | Pseudotropin | Diosgenin | Somnisol | |
| E, F, G, H, I | Somniferine | Anahygrine | Sigmasterol | ||
| J, K, L, M | Somniferinine | Anaferine | β-sitosterol | ||
| Nicotine | Stigmastadien | ||||
| Tropeltigloate |
| ASHWAGANDHA/WITHAFERIN-A (WFA) | ||
|---|---|---|
| Herb Preparation | Tumor Type | Anti-Cancer Effect |
| Alcoholic extract leaves, WFA, withanolide-A, withanone [43] | C6 rat glioma and YKG1 human glioma cell lines | Induce growth arrest; induce apoptosis; cell cycle arrest; WFA lowest IC50 |
| Aqueous extract leaves [44] | C6 rat glioma and YKG1, U118MG, A172 human glioma cell lines | Reduce proliferation; induce apoptosis; inhibit cell cycle; induce differentiation |
| ASH-WEX aqueous extract [45] | C6 rat glioma intracranial xenografts | Reduced pNF-κB, pAkt, VEGF, cyclin D1, and Bcl-xl; enhanced apoptosis; cell cycle arrest G1/G0; tumor shrinkage |
| WFA [48] | GL26 murine and U87, U251 human glioma cell lines | Dose-dependent shift cell cycle; G2/M arrest: WFA—0.5–1.5 µM; induce apoptosis; reduce Akt and mTOR; reduce EGFR and cMET |
| WFA [49] | TMZ-resistant U251, U87 human glioma cell lines | Reduce proliferation; cell cycle arrest G2/M; induce apoptosis; reduce EGFR and cMET; reduce MGMT |
| WFA [50] | C6 rat glioma cell lines | Induce apoptosis; induce caspase-3, -9; increase Bax; reduce Bcl2; reduce TNF-α and NF-κB |
| WFA and AshwaMAX [51] | U87, GBM2, GBM39 human glioma cell lines; mouse xenograft model | Reduce proliferation, reduce nanospheres—WFA 0.25–0.31 µM, AshwaMAX 2.1–14.8 µg/mL; xenografts inhibited x 3–4 weeks |
| WFA and AshwaMAX, tumor treating fields (TTFs) [52] | U87, GBM2, GBM39 human glioma cell lines | Synergistic effect of WFA and TTFs; reduce growth cell lines and neurospheres |
| WFA [53] | U251, U87 human glioma cell lines and nude mouse xenografts | Inhibit cell growth; increase apoptosis; increase Bim, Bad; cell cycle arrest G2/M; shrink xenografts |
| WITHANONE | ||
| Aqueous extract leaves [44] | C6 rat glioma and YKG1, U118MG, A172 human glioma cell lines | Reduce proliferation; induce apoptosis; inhibit cell cycle; induce differentiation |
| Withanone [54] | C6 rat glioma and YKG1, A172 human glioma cell lines | Reduce hnRNP-6, mortalin, and ezrin; reduce VEGF |
| WITHANOLIDE-A | ||
| Aqueous extract leaves [44] | C6 rat glioma and YKG1, U118MG, A172 human glioma cell lines | Reduce proliferation; induce apoptosis; inhibit cell cycle; induce differentiation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Newton, H.B. Overview of Ayurveda and Ashwagandha: Bioactive Phytochemicals and Potential Applications to Gliomas. Molecules 2025, 30, 4272. https://doi.org/10.3390/molecules30214272
Newton HB. Overview of Ayurveda and Ashwagandha: Bioactive Phytochemicals and Potential Applications to Gliomas. Molecules. 2025; 30(21):4272. https://doi.org/10.3390/molecules30214272
Chicago/Turabian StyleNewton, Herbert B. 2025. "Overview of Ayurveda and Ashwagandha: Bioactive Phytochemicals and Potential Applications to Gliomas" Molecules 30, no. 21: 4272. https://doi.org/10.3390/molecules30214272
APA StyleNewton, H. B. (2025). Overview of Ayurveda and Ashwagandha: Bioactive Phytochemicals and Potential Applications to Gliomas. Molecules, 30(21), 4272. https://doi.org/10.3390/molecules30214272







