Quality Management and Sustainability in the Design of Active Biocomposites: Evaluation of Double-Layer Protein–Polysaccharide Complexes Enriched with Plant Extracts
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical and Structural Characterization of the Films
2.2. Water Properties
2.3. Mechanical Properties
2.4. Bioactive Properties of the Films
2.5. Packaging Performance Evaluation
2.6. Assessment of Environmental Impact
3. Materials and Methods
3.1. Materials Used for Active Double-Layer Film Preparation
3.2. Preparation of Double-Layer Active Films
3.3. Physicochemical and Structural Characterization of Films
3.3.1. FTIR Spectroscopy
3.3.2. UV–Vis Spectroscopy Analysis
3.3.3. Water Vapor Transmission Rate (WVTR)
3.3.4. Water Content and Solubility
3.3.5. Mechanical Properties
3.4. Bioactive Properties of Films
3.4.1. Analysis of Antioxidant Properties
3.4.2. Antimicrobial Properties of Films
3.5. Packaging Performance Evaluation—Experimental Setup for Salmon Storage
3.5.1. Experimental Setup for Salmon Storage
3.5.2. Microbiological Analyses of Atlantic Salmon
3.5.3. Thiobarbituric Acid Reactive Substances (TBARS)
3.5.4. pH Measurement
3.6. Assessment of Environmental Impact
3.6.1. Biodegradation Assessment of Films
3.6.2. Ecotoxicity Testing
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials. Food Res. Int. 2020, 137, 109625. [Google Scholar] [CrossRef] [PubMed]
- D’Almeida, A.P.; de Albuquerque, T.L. Innovations in Food Packaging: From Bio-Based Materials to Smart Packaging Systems. Processes 2024, 12, 2085. [Google Scholar] [CrossRef]
- Nowak, N.; Cholewa-Wójcik, A.; Tkaczewska, J.; Grzebieniarz, W.; Tkacz, K.; Modzelewska-Kapituła, M.; Zduńczyk, W.; Kopeć, M.; Jamróz, E. The use of active compounds to shape the quality of active double-layer films based on furcellaran intended for packaging salad-dressing—Assessment of utilitarian and storage properties. Food Chem. 2024, 438, 137957. [Google Scholar] [CrossRef]
- Abdullah; Cai, J.; Hafeez, M.A.; Wang, Q.; Farooq, S.; Huang, Q.; Tian, W.; Xiao, J. Biopolymer-based functional films for packaging applications: A review. Front. Nutr. 2022, 9, 1000116. [Google Scholar] [CrossRef]
- Westlake, J.R.; Tran, M.W.; Jiang, Y.; Zhang, X.; Burrows, A.D.; Xie, M. Biodegradable biopolymers for active packaging: Demand, development and directions. Sustain. Food Technol. 2023, 1, 50–72. [Google Scholar] [CrossRef]
- Shamsuri, A.A.; Md. Jamil, S.N.A. Functional Properties of Biopolymer-Based Films Modified with Surfactants: A Brief Review. Processes 2020, 8, 1039. [Google Scholar] [CrossRef]
- Cha, D.S.; Chinnan, M.S. Biopolymer-Based Antimicrobial Packaging: A Review. Crit. Rev. Food Sci. Nutr. 2004, 44, 223–237. [Google Scholar] [CrossRef]
- Musa, Y.; Bwatanglang, I.B. Chapter 6—Current role and future developments of biopolymers in green and sustainable chemistry and catalysis. In Sustainable Nanocellulose and Nanohydrogels from Natural Sources; Mohammad, F., Al-Lohedan, H.A., Jawaid, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 131–154. [Google Scholar]
- Bhaskar, R.; Zo, S.M.; Narayanan, K.B.; Purohit, S.D.; Gupta, M.K.; Han, S.S. Recent development of protein-based biopolymers in food packaging applications: A review. Polym. Test. 2023, 124, 108097. [Google Scholar] [CrossRef]
- Wang, L.Z.; Liu, L.; Holmes, J.; Kerry, J.F.; Kerry, J.P. Assessment of film-forming potential and properties of protein and polysaccharide-based biopolymer films. Int. J. Food Sci. Technol. 2007, 42, 1128–1138. [Google Scholar] [CrossRef]
- Nowak, N.; Grzebieniarz, W.; Jamróz, E.; Ozogul, F. Sustainable Encapsulation Materials Derived from Seaweed. In Seaweeds and Seaweed-Derived Compounds: Meeting the Growing Need for Healthy Biologically Active Compounds; Ozogul, F., Trif, M., Rusu, A., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 459–487. [Google Scholar]
- Jamróz, E.; Janik, M.; Juszczak, L.; Kruk, T.; Kulawik, P.; Szuwarzyński, M.; Kawecka, A.; Khachatryan, K. Composite biopolymer films based on a polyelectrolyte complex of furcellaran and chitosan. Carbohydr. Polym. 2021, 274, 118627. [Google Scholar] [CrossRef]
- Negi, P.S. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. Int. J. Food Microbiol. 2012, 156, 7–17. [Google Scholar] [CrossRef]
- Bolouri, P.; Salami, R.; Kouhi, S.; Kordi, M.; Asgari Lajayer, B.; Hadian, J.; Astatkie, T. Applications of Essential Oils and Plant Extracts in Different Industries. Molecules 2022, 27, 8999. [Google Scholar] [CrossRef] [PubMed]
- Dirpan, A.; Ainani, A.F.; Djalal, M. A Review on Biopolymer-Based Biodegradable Film for Food Packaging: Trends over the Last Decade and Future Research. Polymers 2023, 15, 2781. [Google Scholar] [CrossRef]
- Dutta, D.; Sit, N. Application of natural extracts as active ingredient in biopolymer based packaging systems. J. Food Sci. Technol. 2023, 60, 1888–1902. [Google Scholar] [CrossRef]
- Nowak, N.; Grzebieniarz, W.; Cholewa-Wójcik, A.; Juszczak, L.; Konieczna-Molenda, A.; Dryzek, E.; Sarnek, M.; Szuwarzyński, M.; Mazur, T.; Jamróz, E. Effects of Selected Plant Extracts on the Quality and Functional Properties of Gelatin and Furcellaran-Based Double-Layer Films. Food Bioprocess Technol. 2024, 17, 1201–1214. [Google Scholar] [CrossRef]
- Azeredo, H.M.C.; Waldron, K.W. Crosslinking in polysaccharide and protein films and coatings for food contact—A review. Trends Food Sci. Technol. 2016, 52, 109–122. [Google Scholar] [CrossRef]
- Almeida, P.; Lannes, S.; Calarge, F.; Farias, T.M.B.; Santana, J. FTIR characterization of gelatin from chicken feet. J. Chem. Chem. Eng. 2012, 6, 1029–1032. [Google Scholar]
- Łopusiewicz, Ł.; Jędra, F.; Bartkowiak, A. New Active Packaging Films Made from Gelatin Modified with Fungal Melanin. World Sci. News 2018, 101, 1–30. [Google Scholar]
- Ramli, N.A.; Adam, F.; Mohd Amin, K.N.; Abu Bakar, N.F.; Ries, M.E. Mechanical and Thermal Evaluation of Carrageenan/Hydroxypropyl Methyl Cellulose Biocomposite Incorporated with Modified Starch Corroborated by Molecular Interaction Recognition. ACS Appl. Polym. Mater. 2023, 5, 182–192. [Google Scholar] [CrossRef]
- Tran, T.V.; Nguyen, N.-N.; Nguyen, Q.-D.; Nguyen, T.-P.; Lien, T.-N. Gelatin/carboxymethyl cellulose edible films: Modification of physical properties by different hydrocolloids and application in beef preservation in combination with shallot waste powder. RSC Adv. 2023, 13, 10005–10014. [Google Scholar] [CrossRef]
- Wang, B.; Pham, L.B.; Adhikari, B. Complexation and conjugation between phenolic compounds and proteins: Mechanisms, characterisation and applications as novel encapsulants. Sustain. Food Technol. 2024, 2, 1206–1227. [Google Scholar] [CrossRef]
- Takahara, Y.; Beni, Y.; Sekine, Y.; Nankawa, T.; Ikeda-Fukazawa, T. Structural Changes of Water in Carboxymethyl Cellulose Nanofiber Hydrogels during Vapor Swelling and Drying. ACS Omega 2024, 9, 45554–45563. [Google Scholar] [CrossRef] [PubMed]
- Yuwono, S.; Wahyuningsih, E.; Noviany, N.; Kiswandono, A.; Simanjuntak, W.; Hadi, S. Characterization of Carboxymethyl Cellulose (CMC) Synthesized from Microcellulose of Cassava Peel. Mater. Plast. 2021, 57, 225–235. [Google Scholar] [CrossRef]
- Hoult, R.; Perston, B.; Spragg, R. Polystyrene Film as a Standard for Testing FT-IR Spectrometers. Spectrosc.-Springf. Then Eugene Then Duluth 2013, 28, 38–43. [Google Scholar]
- Kola, V.; Carvalho, I.S. Plant extracts as additives in biodegradable films and coatings in active food packaging. Food Biosci. 2023, 54, 102860. [Google Scholar] [CrossRef]
- Nowak-Nazarkiewicz, N.; Grzebieniarz, W.; Synkiewicz-Musialska, B.; Juszczak, L.; Cholewa-Wójcik, A.; Jamróz, E. Functional Multilayer Biopolymer Films with Botanical Additives for Sustainable Printed Electronics. Materials 2025, 18, 4328. [Google Scholar] [CrossRef]
- Tijani, A.T.; Ayodele, T.; Liadi, M.; Sarker, N.C.; Hammed, A. Mechanical and Thermal Characteristics of Films from Glycerol Mixed Emulsified Carnauba Wax/Polyvinyl Alcohol. Polymers 2024, 16, 3024. [Google Scholar] [CrossRef]
- Li, K.; Jin, S.; Zhou, Y.; Luo, J.; Li, J.; Li, X.; Shi, S.Q.; Li, J. Bioinspired interface design of multifunctional soy protein-based biomaterials with excellent mechanical strength and UV-blocking performance. Compos. Part B Eng. 2021, 224, 109187. [Google Scholar] [CrossRef]
- Gennaro, M.; Büyüktaş, D.; Carullo, D.; Pinto, A.; Dallavalle, S.; Farris, S. UV-Shielding Biopolymer Coatings Loaded with Bioactive Compounds for Food Packaging Applications. Coatings 2025, 15, 741. [Google Scholar] [CrossRef]
- Gore, A.H.; Prajapat, A.L. Biopolymer Nanocomposites for Sustainable UV Protective Packaging. Front. Mater. 2022, 9, 855727. [Google Scholar] [CrossRef]
- Oliveira, H.; Correia, P.; Pereira, A.R.; Araújo, P.; Mateus, N.; de Freitas, V.; Oliveira, J.; Fernandes, I. Exploring the Applications of the Photoprotective Properties of Anthocyanins in Biological Systems. Int. J. Mol. Sci. 2020, 21, 7464. [Google Scholar] [CrossRef] [PubMed]
- Bandoniene, D.; Murkovic, M.; Venskutonis, P.R. Determination of rosmarinic acid in sage and borage leaves by high-performance liquid chromatography with different detection methods. J. Chromatogr. Sci. 2005, 43, 372–376. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pavić, V.; Jakovljević, M.; Molnar, M.; Jokić, S. Extraction of Carnosic Acid and Carnosol from Sage (Salvia officinalis L.) Leaves by Supercritical Fluid Extraction and Their Antioxidant and Antibacterial Activity. Plants 2019, 8, 16. [Google Scholar] [CrossRef]
- Vartiainen, J.; V?h?-Nissi, M.; Harlin, A. Biopolymer Films and Coatings in Packaging Applications¡ªA Review of Recent Developments. Mater. Sci. Appl. 2014, 5, 11. [Google Scholar] [CrossRef]
- Jamróz, E.; Kulawik, P.; Kopel, P. The Effect of Nanofillers on the Functional Properties of Biopolymer-Based Films: A Review. Polymers 2019, 11, 675. [Google Scholar] [CrossRef]
- Mir, S.A.; Dar, B.N.; Wani, A.A.; Shah, M.A. Effect of plant extracts on the techno-functional properties of biodegradable packaging films. Trends Fdood Sci. Technol. 2018, 80, 141–154. [Google Scholar] [CrossRef]
- Fu, K.; Li, J.; Ge, J.; Zheng, Y.; Sang, Y.; Tian, G. Structural modification of gelatin with four flavonoids for improving its properties. Food Chem. 2025, 492, 145518. [Google Scholar] [CrossRef]
- Pan, J.; Li, C.; Liu, J.; Jiao, Z.; Zhang, Q.; Lv, Z.; Yang, W.; Chen, D.; Liu, H. Polysaccharide-Based Packaging Coatings and Films with Phenolic Compounds in Preservation of Fruits and Vegetables—A Review. Foods 2024, 13, 3896. [Google Scholar] [CrossRef]
- Suput, D.; Lazic, V.; Popović, S.; Hromis, N.; Bulut, S. Biopolymer films synthesis and characterisation. J. Process. Energy Agric. 2017, 21, 9–12. [Google Scholar] [CrossRef]
- Mujtaba, M.; Lipponen, J.; Ojanen, M.; Puttonen, S.; Vaittinen, H. Trends and challenges in the development of bio-based barrier coating materials for paper/cardboard food packaging; a review. Sci. Total Environ. 2022, 851, 158328. [Google Scholar] [CrossRef]
- Nowak, N.; Tkaczewska, J.; Grzebieniarz, W.; Juszczak, L.; Mazur, T.; Szuwarzyński, M.; Guzik, P.; Jamróz, E. Active and Intelligent Four-Layer Films Based on Chitosan, Gelatin, Furcellaran and Active Ingredients—Preparation, Characterisation and Application on Salmon. Food Bioprocess Technol. 2024, 17, 1862–1875. [Google Scholar] [CrossRef]
- Bhatia, S.; Abbas Shah, Y.; Al-Harrasi, A.; Jawad, M.; Koca, E.; Aydemir, L.Y. Enhancing Tensile Strength, Thermal Stability, and Antioxidant Characteristics of Transparent Kappa Carrageenan Films Using Grapefruit Essential Oil for Food Packaging Applications. ACS Omega 2024, 9, 9003–9012. [Google Scholar] [CrossRef]
- Eslami, Z.; Elkoun, S.; Robert, M.; Adjallé, K. A Review of the Effect of Plasticizers on the Physical and Mechanical Properties of Alginate-Based Films. Molecules 2023, 28, 6637. [Google Scholar] [CrossRef]
- Zhang, Y.; Han, J. Sorption Isotherm and Plasticization Effect of Moisture and Plasticizers in Pea Starch Film. J. Food Sci. 2008, 73, E313–E324. [Google Scholar] [CrossRef]
- Suderman, N.; Isa, M.I.N.; Sarbon, N.M. The effect of plasticizers on the functional properties of biodegradable gelatin-based film: A review. Food Biosci. 2018, 24, 111–119. [Google Scholar] [CrossRef]
- Cvetković, K.; Lazarević, A.; Stamenković Stojanović, S.; Dordevic, S.; Dordevic, D.; Karabegović, I.; Danilović, B. pH-Sensitive Starch-Based Packaging Films Enhanced with Wild Blackberry Extract. Processes 2025, 13, 1148. [Google Scholar] [CrossRef]
- Albert, C.; Codină, G.G.; Héjja, M.; András, C.D.; Chetrariu, A.; Dabija, A. Study of Antioxidant Activity of Garden Blackberries (Rubus fruticosus L.) Extracts Obtained with Different Extraction Solvents. Appl. Sci. 2022, 12, 4004. [Google Scholar] [CrossRef]
- Francik, S.; Francik, R.; Sadowska, U.; Bystrowska, B.; Zawiślak, A.; Knapczyk, A.; Nzeyimana, A. Identification of Phenolic Compounds and Determination of Antioxidant Activity in Extracts and Infusions of Salvia Leaves. Materials 2020, 13, 5811. [Google Scholar] [CrossRef]
- Rashidi, A.; Mousavi, B.; Rahmani, M.; Rezaee, M.; Hosaini, W.; Motaharinia, Y.; Davari, B.; Zamini, G. Evaluation of antifungal effect of Lavandula officinalis, Salvia officinalis L., Sumac, Glycyrrhiza glabra and Althoae officinalis extracts on Aspergillus Niger, Aspergillus Fumigatus and Aspergillus Flavus species. J. Med. Plant Res. 2011, 6, 309–313. [Google Scholar] [CrossRef]
- Salević, A.; Stojanović, D.; Lević, S.; Pantić, M.; Đorđević, V.; Pešić, R.; Bugarski, B.; Pavlović, V.; Uskoković, P.; Nedović, V. The Structuring of Sage (Salvia officinalis L.) Extract-Incorporating Edible Zein-Based Materials with Antioxidant and Antibacterial Functionality by Solvent Casting versus Electrospinning. Foods 2022, 11, 390. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Q.; Ning, Z.; Ma, Y.; Huang, Y.; Wu, Y.; Yang, Y.; Xiao, M.; Ye, J. Preparation, antibacterial activity, and structure-activity relationship of low molecular weight κ-carrageenan. Int. J. Biol. Macromol. 2024, 266, 131021. [Google Scholar] [CrossRef]
- Chedea, V.S.; Tomoiagǎ, L.L.; Macovei, Ş.O.; Mǎgureanu, D.C.; Iliescu, M.L.; Bocsan, I.C.; Buzoianu, A.D.; Voşloban, C.M.; Pop, R.M. Antioxidant/Pro-Oxidant Actions of Polyphenols From Grapevine and Wine By-Products-Base for Complementary Therapy in Ischemic Heart Diseases. Front. Cardiovasc. Med. 2021, 8, 750508. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Park, S.-W.; Shin, H.-S. Fish Freshness Indicator for Sensing Fish Quality during Storage. Foods 2023, 12, 1801. [Google Scholar] [CrossRef] [PubMed]
- Nair, N.R.; Sekhar, V.C.; Nampoothiri, K.M.; Pandey, A. 32—Biodegradation of Biopolymers. In Current Developments in Biotechnology and Bioengineering; Pandey, A., Negi, S., Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 739–755. [Google Scholar]
- Tkaczewska, J.; Jamróz, E.; Guzik, P.; Kopeć, M. Attempt to Extend the Shelf-Life of Fish Products by Means of Innovative Double-Layer Active Biodegradable Films. Polymers 2022, 14, 1717. [Google Scholar] [CrossRef]
- Yue, H.; Li, X.; Mai, L.; Wu, Q.; He, M.; Yin, G.; Peng, J.; Yang, C.; Guo, J. Sustainable cottonseed protein bioplastics: Physical and chemical reinforcement, and plant seedling growth application. Chem. Eng. J. 2024, 497, 154794. [Google Scholar] [CrossRef]
- Rigobello, E.; de Campos, S.; Azevedo, E.; Dantas, A.; Vieira, E. Comparative characterization of humic substances extracted from freshwater and peat of different apparent molecular sizes. Ambiente E Agua—Interdiscip. J. Appl. Sci. 2017, 12, 774. [Google Scholar] [CrossRef][Green Version]
- Trevisan, S.; Francioso, O.; Quaggiotti, S.; Nardi, S. Humic substances biological activity at the plant-soil interface: From environmental aspects to molecular factors. Plant Signal. Behav. 2010, 5, 635–643. [Google Scholar] [CrossRef]
- Nowak, N.; Grzebieniarz, W.; Juszczak, L.; Cholewa-Wójcik, A.; Synkiewicz-Musialska, B.; Huber, V.; Touraud, D.; Kunz, W.; Jamróz, E. Influence of Curcuma Longa extract in citral addition on functional properties of thin films with triple-layer structure based on furcellaran and gelatin. Int. J. Biol. Macromol. 2024, 266, 131344. [Google Scholar] [CrossRef]
- ISO 2528:2017; Sheet Materials—Determination of Water Vapour Transmission Rate (WVTR)—Gravimetric (Dish) Method. ISO: Geneva, Switzerland, 2017.
- Pastor, C.; Sánchez-González, L.; Chiralt, A.; Cháfer, M.; González-Martínez, C. Physical and antioxidant properties of chitosan and methylcellulose based films containing resveratrol. Food Hydrocoll. 2013, 30, 272–280. [Google Scholar] [CrossRef]
- Irissin-Mangata, J.; Bauduin, G.; Boutevin, B.; Gontard, N. New plasticizers for wheat gluten films. Eur. Polym. J. 2001, 37, 1533–1541. [Google Scholar] [CrossRef]
- ISO 527-1:2019; Plastics—Determination of Tensile Properties—Part 1: General Principles. ISO: Geneva, Switzerland, 2019.
- Khantaphant, S.; Benjakul, S. Comparative study on the proteases from fish pyloric caeca and the use for production of gelatin hydrolysate with antioxidative activity. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 151, 410–419. [Google Scholar] [CrossRef]
- Xie, Z.; Huang, J.; Xu, X.; Jin, Z. Antioxidant activity of peptides isolated from alfalfa leaf protein hydrolysate. Food Chem. 2008, 111, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-C.; Chen, H.-M.; Shiau, C.-Y. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus). Food Res. Int. 2003, 36, 949–957. [Google Scholar] [CrossRef]
- Grzebieniarz, W.; Tkaczewska, J.; Juszczak, L.; Kawecka, A.; Krzyściak, P.; Nowak, N.; Guzik, P.; Kasprzak, M.; Janik, M.; Jamróz, E. The influence of aqueous butterfly pea (Clitoria ternatea) flower extract on active and intelligent properties of furcellaran Double-Layered films—In vitro and in vivo research. Food Chem. 2023, 413, 135612. [Google Scholar] [CrossRef]
- ISO 6887-1:2017; Microbiology of the Food Chain—Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination—Part 1: General Rules for the Preparation of the Initial Suspension and Decimal Dilutions. ISO: Geneva, Switzerland, 2017.
- ISO 4833-1:2013; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique. ISO: Geneva, Switzerland, 2013.
- ISO 17410:2019; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Psychrotrophic Microorganisms. ISO: Geneva, Switzerland, 2019.
- ISO 14855-1:2012; Determination of the Ultimate Aerobic Biodegradability of Plastic Materials Under Controlled Composting Conditions—Method by Analysis of Evolved Carbon Dioxide—Part 1: General Method. ISO: Geneva, Switzerland, 2012.
- Griffith, S.M.; Schnitzer, M. The Isolation and Characterization of Stable Metal-Organic Complexes from Tropical Volcanic Soils. Soil Sci. 1975, 120, 126–131. [Google Scholar] [CrossRef]
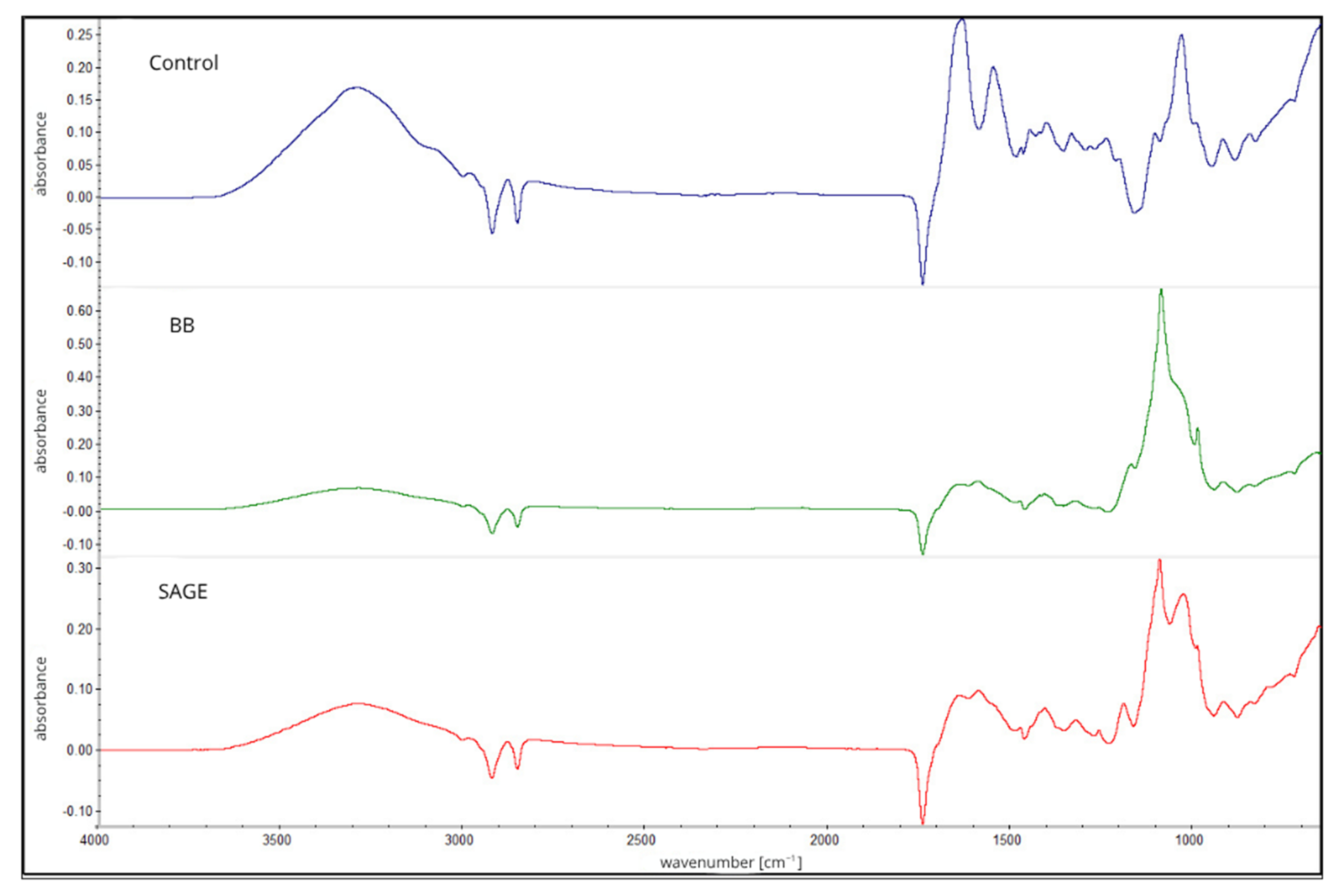
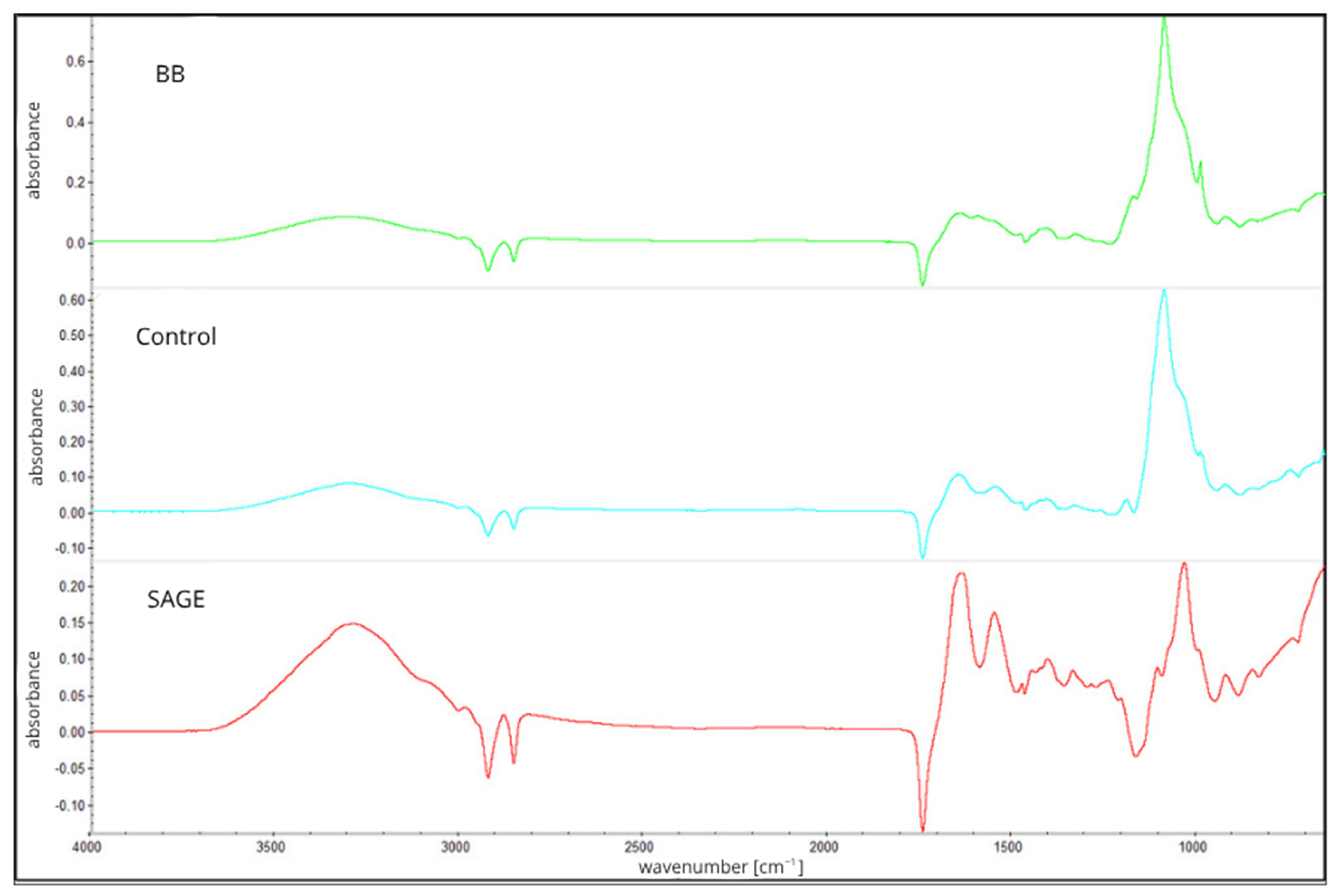


| Type of Film/Parameter | Water Content [%] | Solubility [%] | WVTR [g/m2 × d] |
|---|---|---|---|
| Control | 7.89 ± 0.38 a | 53.45 ± 1.26 a | 543.02 ± 17.64 a |
| BB | 9.54 ± 1.39 b | 41.73 ± 1.73 b | 588.62 ± 33.07 a |
| SAGE | 10.67 ± 0.26 ab | 55.99 ± 1.14 a | 548.49 ± 38.06 a |
| Type of Film/Parameter | Max Breaking Load [N] | Tensile Strength [MPa] | Elongation at Break [%] | Young’s Modulus [N/mm2] |
|---|---|---|---|---|
| Control | 83.09 ± 4.97 c | 5.54 ± 0.33 c | 20.00 ± 2.42 a | 477.58 ± 50.03 b |
| BB | 51.92 ± 2.87 b | 3.46 ± 0.19 b | 35.35 ± 2.11 b | 127.33 ± 24.74 a |
| SAGE | 38.27 ± 0.96 a | 2.55 ± 0.06 a | 39.29 ± 0.84 b | 103.23 ± 7.56 a |
| Type of Film/Parameter | FRAP [µTrolox/mg of Dried Films] | DPPH [%] | Metal Chelating Activity [%] |
|---|---|---|---|
| Control | 0.38 ± 0.22 a | 6.38 ± 0.74 a | 0 a |
| BB | 1.97 ± 0.17 b | 85.68 ± 8.25 b | 0 a |
| SAGE | 4.48 ± 0.23 c | 78.25 ± 0.23 b | 0 a |
| Microorganism | Control | BB | SAGE |
|---|---|---|---|
| Candida albicans | No effect | No effect | No effect |
| Candida krusei | No effect | No effect | No effect |
| Aspergillus brasiliensis | No effect | No effect | No effect |
| Aspergillus flavus | 20 ± 0.0 | 20 ± 0.0 | 20 ± 0.0 |
| Escherichia coli | No effect | No effect | No effect |
| Enterococcus faecalis | No effect | No effect | No effect |
| Pseudomonas aeruginosa | No effect | No effect | No effect |
| Staphylococcus aureus | No effect | No effect | No effect |
| Salmonella enterica | No effect | No effect | No effect |
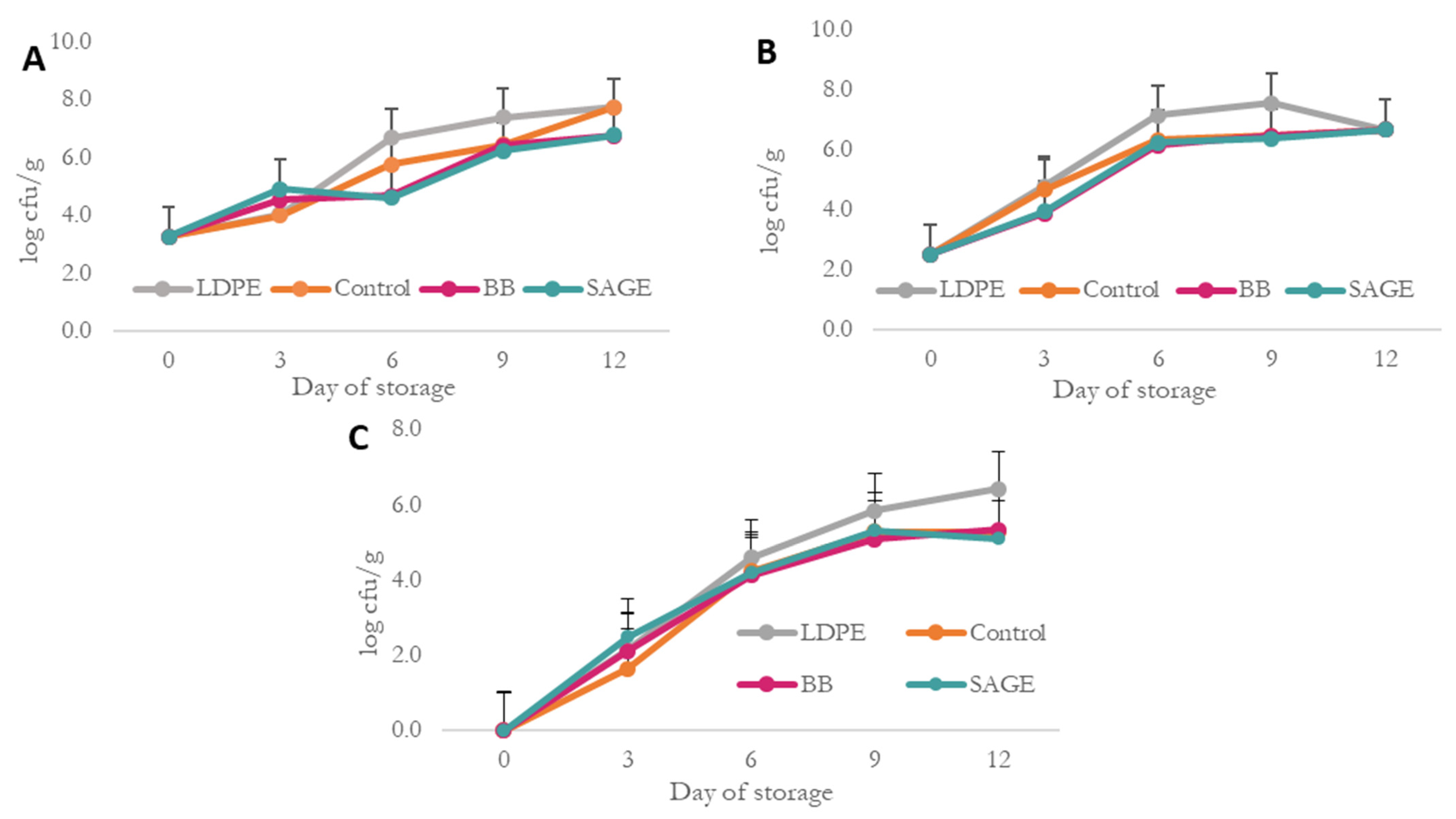
| TBARS [mg MDA/kg] | |||||
| Type of Film/Day | D0 | D3 | D6 | D9 | D12 |
| Control | 0.08 ± 0.03 A | 0.14 ± 0.03 aA | 0.66 ± 0.27 aBC | 0.51 ± 0.21 aB | 0.77 ± 0.18 aC |
| BB | 0.21 ± 0.02 bB | 0.64 ± 0.21 aCE | 0.92 ± 0.19 cD | 0.63 ± 0.19 aEC | |
| SAGE | 0.19 ± 0.04 bA | 0.41 ± 0.19 abB | 0.51 ± 0.18 aBC | 0.63 ± 0.10 aC | |
| LDPE | 0.19 ± 0.01 aB | 0.30 ± 0.05 bC | 0.30 ± 0.05 bC | 0.28 ± 0.05 bC | |
| pH | |||||
| Control | 6.34 ± 0.06 A | 6.35 ± 0.06 aA | 6.30 ± 0.13 aBAD | 6.60 ± 0.16 aCA | 6.27 ± 0.11 aDAB |
| BB | 6.31 ± 0.06 aAB | 6.34 ± 0.08 aAB | 6.28 ± 0.08 bAB | 6.21 ± 0.03 aB | |
| SAGE | 6.32 ± 0.04 aA | 6.30 ± 0.04 aA | 6.32 ± 0.03 bA | 6.30 ± 0.09 aA | |
| LDPE | 6.38 ± 0.09 aA | 6.39 ± 0.12 aA | 6.36 ± 0.07 abA | 6.73 ± 0.04 bB | |
| Respiratory Activity | |||
|---|---|---|---|
| Control | BB | SAGE | |
| 1–48 h | |||
| y | 1.7291x − 1.4105 | 1.6714x − 2.6477 | 1.6881x + 1.304 |
| R2 | 0.9453 | 0.9353 | 0.9496 |
| 49–672 h | |||
| y | 0.0994x + 78.636 | 0.1047x + 71.41 | 0.1123x + 83.335 |
| R2 | 0.984 | 0.985 | 0.991 |
| RA28 (mg O2/g d.m.) | 140.9 ± 8.8 a | 137.2 ± 7.4 a | 156.3 ± 10.1 a |
| Toxicity test | |||
| RA8 [mg O2 × (100 seeds)−1] | 114.6 ± 10.2 a | 66.9 ± 4.2 b | 59.4 ± 5.2 b |
| Type of Film/Day | C Extract | C Humic Acids | C Fulvic Acids | Ckh/Ckf Ratio |
|---|---|---|---|---|
| Control | 73.3 ± 1.3 a | 31.8 ± 0.3 a | 41.5 ± 2.3 b | 0.77 ± 0.18 a |
| BB | 78.0 ± 1.1 c | 37.6 ± 2.5 c | 40.3 ± 0.6 a | 0.93 ± 0.86 c |
| SAGE | 75.8 ± 1.6 b | 34.0 ± 3.1 b | 41.8 ± 1.51 b | 0.81 ± 0.61 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak-Nazarkiewicz, N.; Grzebieniarz, W.; Tkaczewska, J.; Cholewa-Wójcik, A.; Kopeć, M.; Gondek, K.; Derechowska, H.; Jamróz, E. Quality Management and Sustainability in the Design of Active Biocomposites: Evaluation of Double-Layer Protein–Polysaccharide Complexes Enriched with Plant Extracts. Molecules 2025, 30, 4259. https://doi.org/10.3390/molecules30214259
Nowak-Nazarkiewicz N, Grzebieniarz W, Tkaczewska J, Cholewa-Wójcik A, Kopeć M, Gondek K, Derechowska H, Jamróz E. Quality Management and Sustainability in the Design of Active Biocomposites: Evaluation of Double-Layer Protein–Polysaccharide Complexes Enriched with Plant Extracts. Molecules. 2025; 30(21):4259. https://doi.org/10.3390/molecules30214259
Chicago/Turabian StyleNowak-Nazarkiewicz, Nikola, Wiktoria Grzebieniarz, Joanna Tkaczewska, Agnieszka Cholewa-Wójcik, Michał Kopeć, Krzysztof Gondek, Hanna Derechowska, and Ewelina Jamróz. 2025. "Quality Management and Sustainability in the Design of Active Biocomposites: Evaluation of Double-Layer Protein–Polysaccharide Complexes Enriched with Plant Extracts" Molecules 30, no. 21: 4259. https://doi.org/10.3390/molecules30214259
APA StyleNowak-Nazarkiewicz, N., Grzebieniarz, W., Tkaczewska, J., Cholewa-Wójcik, A., Kopeć, M., Gondek, K., Derechowska, H., & Jamróz, E. (2025). Quality Management and Sustainability in the Design of Active Biocomposites: Evaluation of Double-Layer Protein–Polysaccharide Complexes Enriched with Plant Extracts. Molecules, 30(21), 4259. https://doi.org/10.3390/molecules30214259








