The Effect of SO2 on C3H8 Oxidation over Ru@CoMn2O4 Spinel
Abstract
1. Introduction
2. Results and Discussion
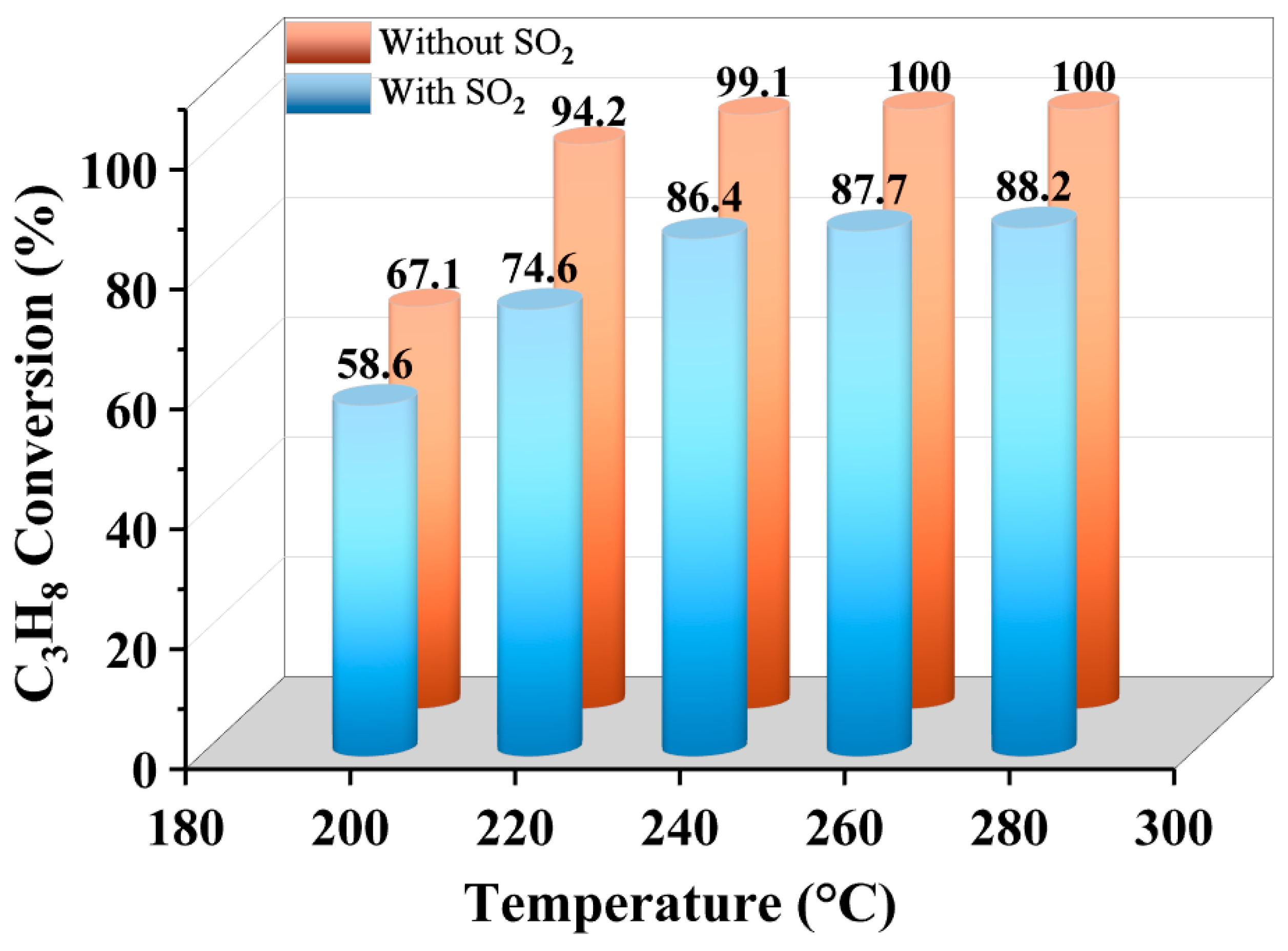
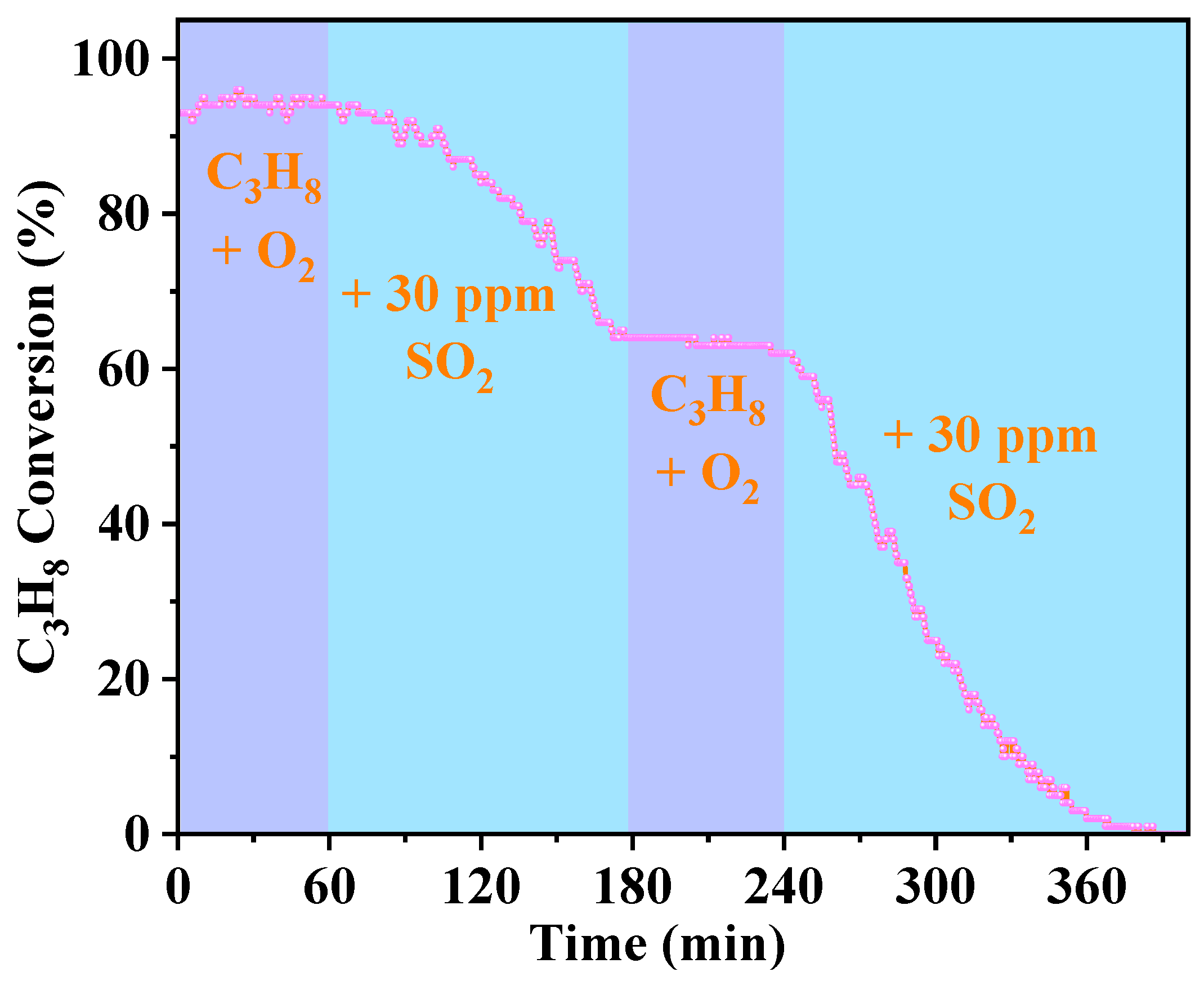
2.1. Catalytic Performance
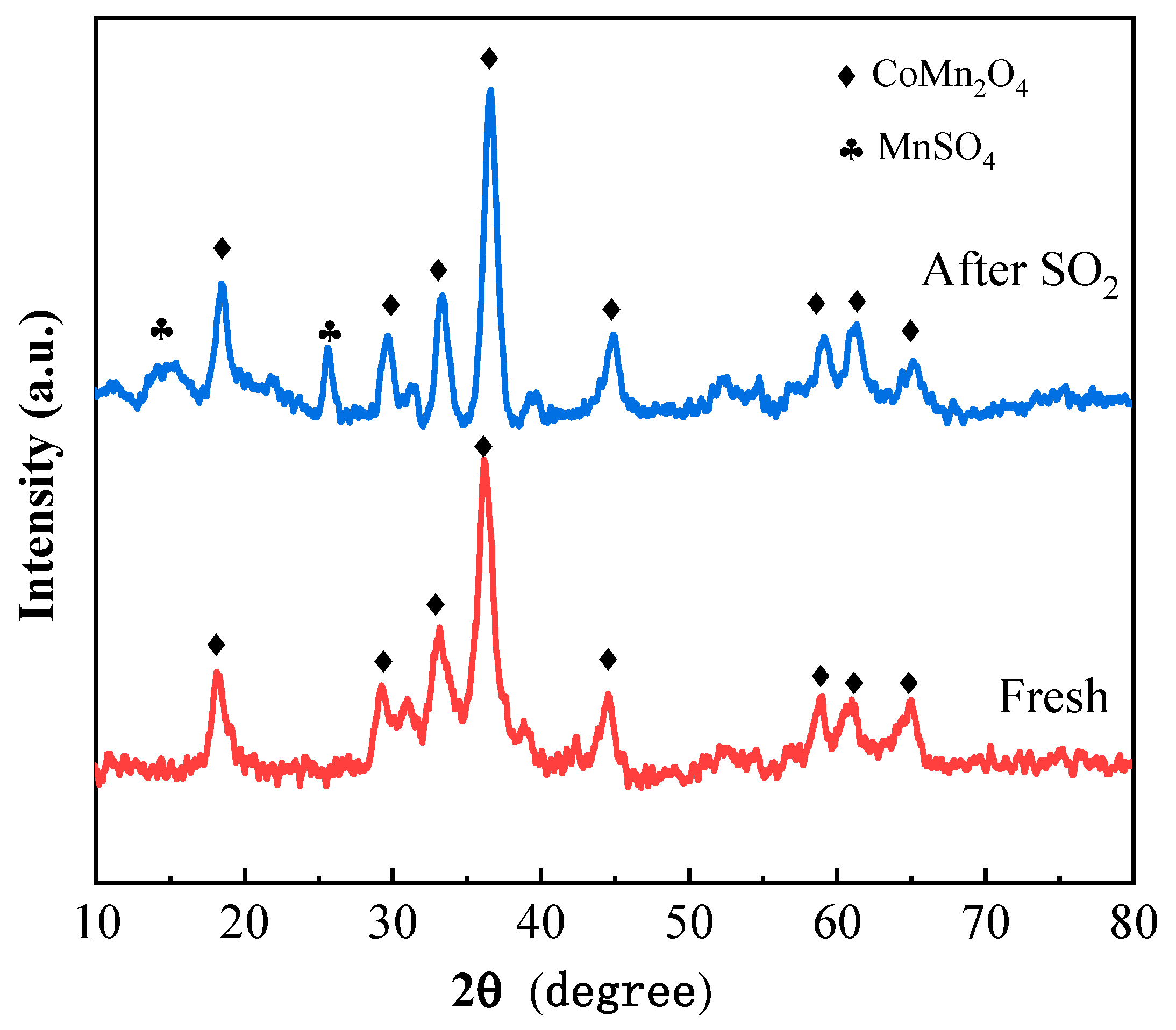
2.2. Physical Characteristics
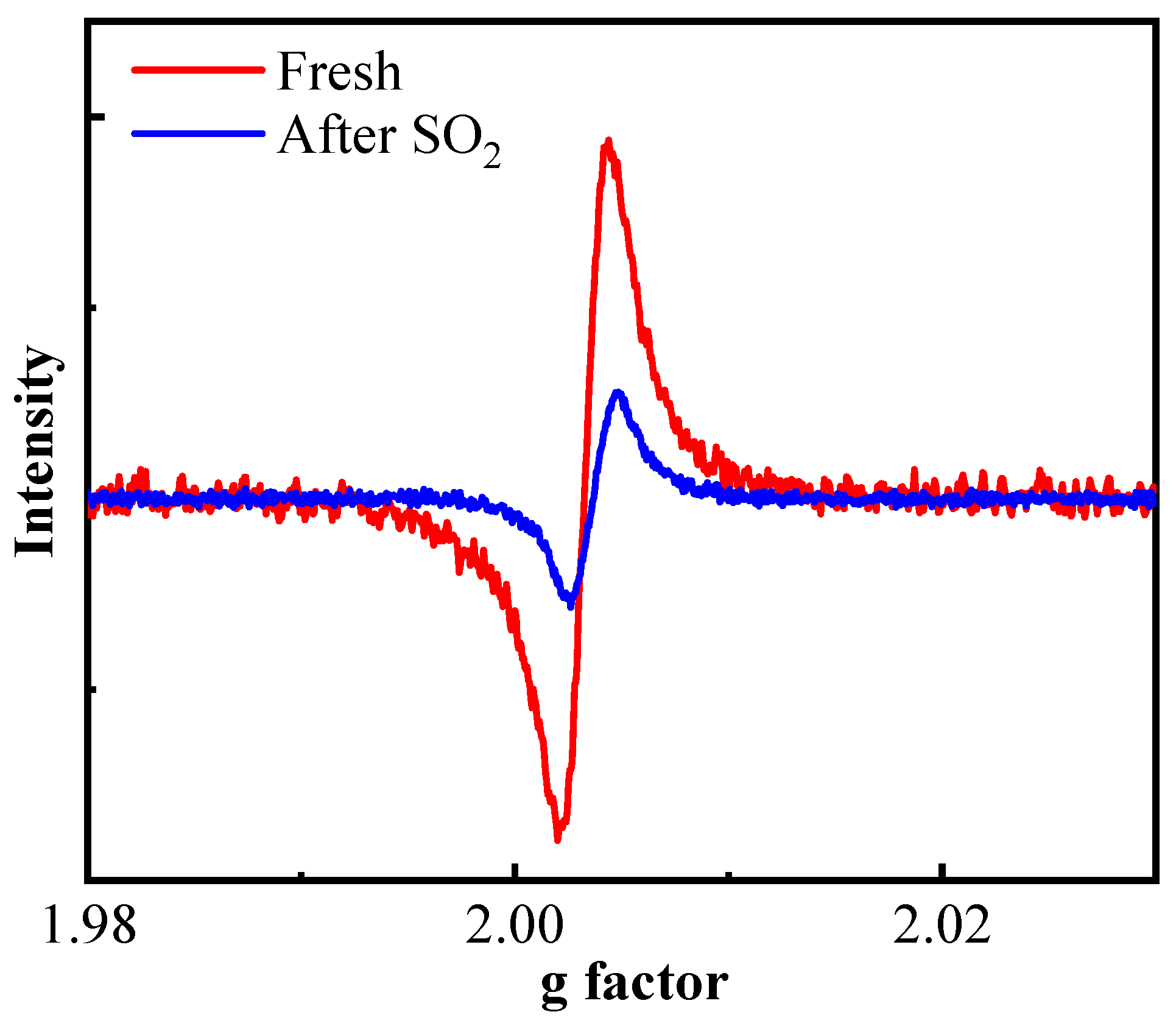
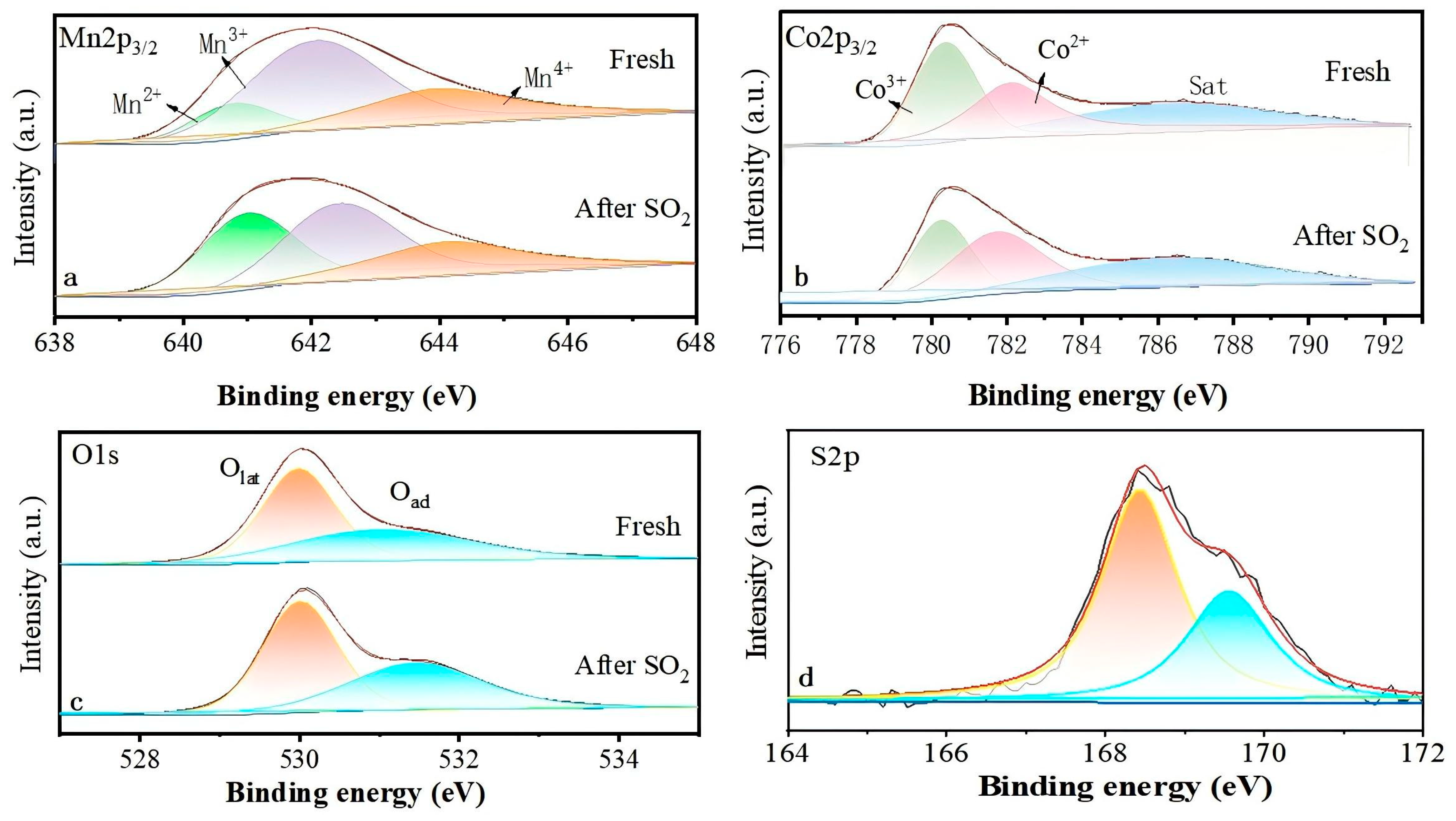
2.3. Surface Chemical Properties
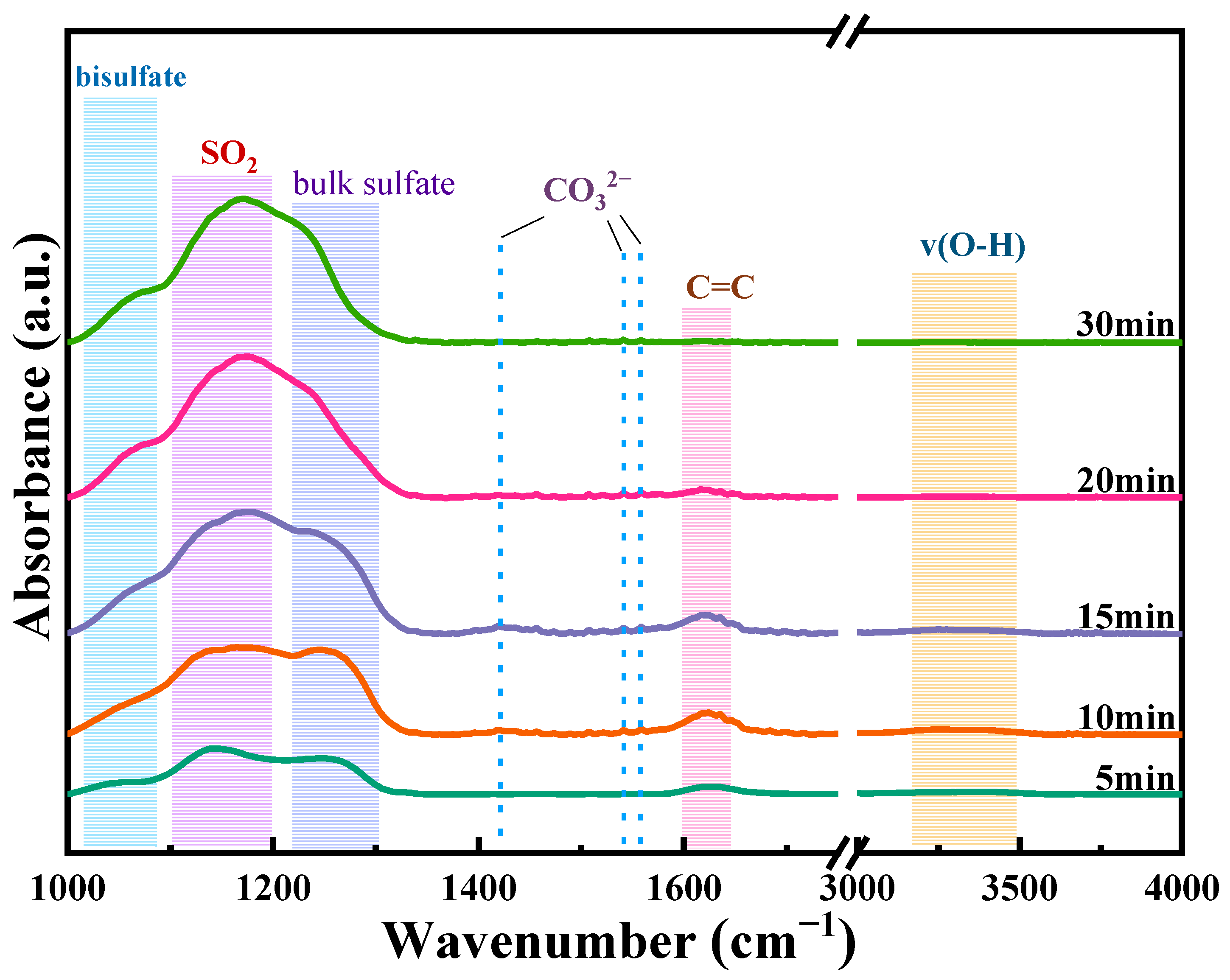
2.4. Poisoning Mechanism
3. Experiment
3.1. Catalyst Preparation
3.2. Catalyst Characterization
3.3. Catalytic Performance Evaluations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xian, Y.W.; Li, B.; Wen, C.H.; Xu, L.Y.; Lu, W.X.; Zhou, Q.; Jia, W.Z.; Luo, M.F.; Chen, J. Boosting propane combustion on dual active sites of Pt/WO3 through regulating Pt sites and activating propane on WO3 surface. Fuel 2025, 385, 134172. [Google Scholar] [CrossRef]
- Xu, W.F.; Zhou, L.M.; Liu, L.N.; Duan, H.M.; Ben, H.X.; Chen, S.; Li, X.Y. Less Is More: Selective-Atom-Removal-Derived Defective MnOx Catalyst for Efficient Propane Oxidation. Nanomaterials 2024, 14, 907. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, S.; Zhang, L.; Lv, L.; Xu, D.; Liu, W.; Wang, S. Investigation of supported palladium catalysts for combustion of methane: The activation effect caused by SO2. Chem. Eng. J. 2020, 382, 122969. [Google Scholar] [CrossRef]
- Timergazin, K.K.; Khursan, S.L. Quantum-chemical calculations of the dissociation energy of the C-H bond in hydrocarbons, alcohols, and ethers. Russ. Chem. Bull. 1996, 45, 2709–2712. [Google Scholar] [CrossRef]
- Dinhova, T.N.; Bezkrovnyi, O.; Piliai, L.; Khalakhan, I.; Chakraborty, S.; Ptak, M.; Kraszkiewicz, P.; Vaidulych, M.; Mazur, M.; Vajda, S.; et al. Unraveling the Effects of Reducing and Oxidizing Pretreatments and Humidity on the Surface Chemistry of the Ru/CeO2 Catalyst during Propane Oxidation. J. Phys. Chem. C 2025, 129, 1746–1757. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wang, Y.; Ding, M.; Zheng, X.; Wang, L.; Guo, Y.; Guo, Y.; Dai, Q.; Zhan, W.; Wang, A. Highly efficient Ru/CeO2 catalyst using colloidal chemical method for propane oxidation. Chem. Eng. J. 2024, 500, 156936. [Google Scholar] [CrossRef]
- Wang, A.; Ding, J.; Li, M.; Song, P.; Zhao, Z.; Guo, Y.; Guo, Y.; Wang, L.; Dai, Q.; Zhan, W. Robust Ru/Ce@Co Catalyst with an Optimized Support Structure for Propane Oxidation. Environ. Sci. Technol. 2024, 58, 12742–12753. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Feng, C.; Chen, H.-C.; Li, J.; Jiang, F.; Tao, S.; Wang, Y.; Li, Y.; Pan, Y. Atomically Dispersed Pt-Doped Co3O4 Spinel Nanoparticles Embedded in Polyhedron Frames for Robust Propane Oxidation at Low Temperature. Small Methods 2023, 7, 2300121. [Google Scholar] [CrossRef]
- Shan, C.; Zhang, Y.; Zhao, Q.; Li, J.; Wang, Y.; Han, R.; Liu, C.; Liu, Q. New insight into opposite oxidation behavior in acetone and propane catalytic oxidation over CoMn based spinel oxides. Chem. Eng. J. 2023, 476, 146550. [Google Scholar] [CrossRef]
- Feng, C.; Chen, C.; Xiong, G.; Yang, D.; Wang, Z.; Pan, Y.; Fei, Z.; Lu, Y.; Liu, Y.; Zhang, R.; et al. Cr-doping regulates Mn3O4 spinel structure for efficient total oxidation of propane: Structural effects and reaction mechanism determination. Appl. Catal. B-Environ. 2023, 328, 122528. [Google Scholar] [CrossRef]
- Feng, C.; Wang, Y.; Chen, C.; Fu, X.; Pan, Y.; Xin, H.; Wang, Z.; Lu, Y.; Li, X.; Zhang, R.; et al. Fabrication of highly dispersed Pd-Mn3O4 catalyst for efficient catalytic propane total oxidation. J. Colloid. Interf. Sci. 2023, 650, 1415–1423. [Google Scholar] [CrossRef]
- Chai, G.T.; Zhang, W.D.; Liotta, L.F.; Li, M.Q.; Guo, Y.L.; Giroir-Fendler, A. Total oxidation of propane over Co3O4-based catalysts: Elucidating the influence of Zr dopant. Appl. Catal. B-Environ. 2021, 298, 120606. [Google Scholar] [CrossRef]
- Chai, Q.Q.; Li, C.Q.; Song, L.Y.; Liu, C.; Peng, T.; Lin, C.C.; Zhang, Y.Y.; Li, S.M.; Guo, Q.; Sun, S.R.; et al. The influence of crystal facet on the catalytic performance of MOFs-derived NiO with different morphologies for the total oxidation of propane: The defect engineering dominated by solvent regulation effect. J. Hazard. Mater. 2024, 475, 134917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.C.; Sui, X.; Zhang, X.; Niu, M.; Li, C.Q.; Wan, H.Q.; Qiao, Z.A.; Xie, H.J.; Li, X.Y. Multi-defects engineering of NiCo2O4 for catalytic propane oxidation. Appl. Surf. Sci. 2022, 600, 154040. [Google Scholar] [CrossRef]
- Ruth, K.; Hayes, M.; Burch, R.; Tsubota, S.; Haruta, M. The effects of SO2 on the oxidation of CO and propane on supported Pt and Au catalysts. Appl. Catal. B-Environ. 2000, 24, 133–138. [Google Scholar] [CrossRef]
- Burch, R.; Halpin, E.; Hayes, M.; Ruth, K.; Sullivan, J.A. The nature of activity enhancement for propane oxidation over supported Pt catalysts exposed to sulphur dioxide. Appl. Catal. B-Environ. 1998, 19, 199–207. [Google Scholar] [CrossRef]
- Corro, G.; Montiel, R.; Vazquez, C. Promoting and inhibiting effect of SO2 on propane oxidation over Pt/Al2O3. Catal. Commun. 2002, 3, 533–539. [Google Scholar] [CrossRef]
- Gremminger, A.; Lott, P.; Merts, M.; Casapu, M.; Grunwaldt, J.D.; Deutschmann, O. Sulfur poisoning and regeneration of bimetallic Pd-Pt methane oxidation catalysts. Appl. Catal. B-Environ. 2017, 218, 833–843. [Google Scholar] [CrossRef]
- Arevalo, R.L.; Aspera, S.M.; Nakanishi, H. Sulfation of a PdO(101) methane oxidation catalyst: Mechanism revealed by first principles calculations. Catal. Sci. Technol. 2019, 9, 232–240. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, J.; Fang, Y.R.; Wang, Y.T.; Hu, J.P.; Zhu, Y.H.; Zhu, X.X.; Li, W.H.; Zhang, Q.; Pan, C.Q.; et al. Role of the In-Situ-Formed Surface (Pt-S-O)-Ti Active Structure in SO2-Promoted C3H8 Combustion over a Pt/TiO2 Catalyst. Environ. Sci. Technol. 2024, 58, 3041–3053. [Google Scholar] [CrossRef]
- Cui, Y.; Zeng, Z.Q.; Hou, Y.Q.; Ma, S.; Shen, W.Z.; Huang, Z.G. A Low-Noble-Metal Ru@CoMn2O4 Spinel Catalyst for the Efficient Oxidation of Propane. Molecules 2024, 29, 2255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, G.; Wu, P.; Zhuang, K.; Shen, K.; Wang, S.; Huang, T. Effect of SO2 on the low-temperature denitrification performance of Ho-modified Mn/Ti catalyst. Chem. Eng. J. 2020, 400, 122597. [Google Scholar] [CrossRef]
- Li, X.; Ren, S.; Liu, L.; Jiang, Y.; Chen, T.; Wang, L.; Liu, M. Study on simultaneous removal of SO2 and NO from sintering flue gas over Fe-Mn/AC catalyst. Catal. Today 2024, 425, 114304. [Google Scholar] [CrossRef]
- Fan, Z.; Shi, J.-W.; Niu, C.; Wang, B.; He, C.; Cheng, Y. The insight into the role of Al2O3 in promoting the SO2 tolerance of MnOx for low-temperature selective catalytic reduction of NOx with NH3. Chem. Eng. J. 2020, 398, 125572. [Google Scholar] [CrossRef]
- Zhang, A.C.; Zhang, Z.H.; Lu, H.; Liu, Z.C.; Xiang, J.; Zhou, C.S.; Xing, W.B.; Sun, L.S. Effect of Promotion with Ru Addition on the Activity and SO2 Resistance of MnOx-TiO2 Adsorbent for Hg0 Removal. Ind. Eng. Chem. Res. 2015, 54, 2930–2939. [Google Scholar] [CrossRef]
- Mao, L.; T-Raissi, A.; Huang, C.; Muradov, N.Z. Thermal decomposition of (NH4)2SO4 in presence of Mn3O4. Int. J. Hydrogen Energy 2011, 36, 5822–5827. [Google Scholar] [CrossRef]
- Xu, R.; Zhou, Z.; Zeng, P.; Liu, X.; Xu, M. Mn-Co binary core–shell nanospheres with boosted low-temperature catalytic activity for NO oxidation. Fuel 2025, 386, 134229. [Google Scholar] [CrossRef]
- Dong, C.; Qu, Z.P.; Qin, Y.; Fu, Q.; Sun, H.C.; Duan, X.X. Revealing the Highly Catalytic Performance of Spinel CoMn2O4 for Toluene Oxidation: Involvement and Replenishment of Oxygen Species Using In Situ Designed-TP Techniques. Acs Catal. 2019, 9, 6698–6710. [Google Scholar] [CrossRef]
- Ren, A.; Hu, X.; Feng, R.; Li, T.; Yang, F.; Zhao, G.; Yan, X. Enhanced Mn-Co synergies of manganese-cobalt spinel oxide for efficient aerobic oxidation of benzyl alcohol. Mol. Catal. 2024, 560, 114121. [Google Scholar] [CrossRef]
- Chen, G.Y.; Zhang, D.; Zhang, A.C.; Zhang, Z.H.; Liu, Z.C.; Hou, L.A. CrOx-MnOx-TiO2 adsorbent with high resistance to SO2 poisoning for Hg0 removal at low temperature. J. Ind. Eng. Chem. 2017, 55, 119–127. [Google Scholar] [CrossRef]
- Kong, M.; Liao, H.P.; Song, L.J.; Zhang, S.C.; Wang, Y.C.; Feng, W.; Liu, Z.F.; Deng, X.L.; Yao, L.; Zhang, H.D. Insight into SO2 poisoning mechanism of MnOx-CeO2/Ti-bearing blast furnace slag catalyst for low temperature NH3-SCR reaction. Mol. Catal. 2024, 569, 114609. [Google Scholar] [CrossRef]
- Tian, H.; He, J.; Liu, L.; Wang, D.; Hao, Z.; Ma, C. Highly active manganese oxide catalysts for low-temperature oxidation of formaldehyde. Microporous Mesoporous Mater. 2012, 151, 397–402. [Google Scholar] [CrossRef]
- Ma, S.; Hou, Y.Q.; Yang, Y.T.; Li, Y.F.; Li, B.; Cui, Y.; Chen, H.J.; Huang, Z.G. Mechanism of SO2 effect on the simultaneous removal of NOx and propane over bifunctional NiMn2O4-CeO2 catalysts. J. Hazard. Mater. 2024, 480, 136473. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zheng, F.; Zhou, Y.; Li, X.; Wang, Y.; Xiao, J.; Li, Y.; Chen, D.; Lu, J. Catalytic oxidation of VOCs over 3D@2D Pd/CoMn2O4 nanosheets supported on hollow Al2O3 microspheres. J. Colloid. Interf. Sci. 2022, 613, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Zhang, Z.; Fang, W.; Yao, X.; Zou, G.; Shangguan, W. Low-temperature catalytic oxidation of formaldehyde over Co3O4 catalysts prepared using various precipitants. Chin. J. Catal. 2016, 37, 947–954. [Google Scholar] [CrossRef]
- Chen, H.; He, J.; Zhang, C.; He, H. Self-assembly of novel mesoporous manganese oxide nanostructures and their application in oxidative decomposition of formaldehyde. J. Phys. Chem. C 2007, 111, 18033–18038. [Google Scholar] [CrossRef]
- Lin, H.F.; Zhao, S.Y.; Li, J.T.; Yao, Z.T.A.; Li, C.M.; Gaoa, S.Q.; Yu, J. The effect of SO2 on the structural evolution of a supported Mn2V2O7 catalyst and its DeNOx performance. Catal. Sci. Technol. 2021, 11, 5598–5605. [Google Scholar] [CrossRef]
- Sun, X.; Li, J.; Fan, J.; Fan, J.; Deng, Q.; Xu, M.; Huang, H.; Wu, J.; Ye, D. Study on sulfur-resistant Pd-based catalysts for high-efficiency oxidation of VOCs. J. Hazard. Mater. 2025, 491, 137958. [Google Scholar] [CrossRef]
- Li, G.; Li, N.; Sun, Y.; Qu, Y.; Jiang, Z.; Zhao, Z.; Zhang, Z.; Cheng, J.; Hao, Z. Efficient defect engineering in Co-Mn binary oxides for low-temperature propane oxidation. Appl. Catal. B-Environ. 2021, 282, 119512. [Google Scholar] [CrossRef]
- Xu, R.; Lan, X.; Jing, Y.; Niu, X.; Zhao, L.; Hao, H. Mechanism of Ni-Modified CeCuCoAlO catalyst in promoting SO2 tolerance for CO-SCR: Experimental and DFT studies. Fuel 2025, 390, 134274. [Google Scholar] [CrossRef]



| Catalyst | SBET (m2/g) | Average Pore Size (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| Fresh | 82.97 | 17.22 | 0.36 |
| Poisoned (After SO2) | 62.69 | 18.65 | 0.29 |
| Catalyst | Mn2+/Mn | Mn3+/Mn | Mn4+/Mn | Co2+/Co | Co3+/Co | Olat/Oad | SO42−/SO32− |
|---|---|---|---|---|---|---|---|
| Fresh | 0.15 | 0.58 | 0.27 | 0.48 | 0.52 | 1.44 | -- |
| Poisoned (After SO2) | 0.36 | 0.40 | 0.24 | 0.58 | 0.42 | 1.33 | 1.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Zeng, Z.; Hou, Y.; Ma, S.; Yang, J.; Zheng, J.; Shen, W.; Huang, Z. The Effect of SO2 on C3H8 Oxidation over Ru@CoMn2O4 Spinel. Molecules 2025, 30, 4253. https://doi.org/10.3390/molecules30214253
Cui Y, Zeng Z, Hou Y, Ma S, Yang J, Zheng J, Shen W, Huang Z. The Effect of SO2 on C3H8 Oxidation over Ru@CoMn2O4 Spinel. Molecules. 2025; 30(21):4253. https://doi.org/10.3390/molecules30214253
Chicago/Turabian StyleCui, Yan, Zequan Zeng, Yaqin Hou, Shuang Ma, Jieyang Yang, Jianfeng Zheng, Wenzhong Shen, and Zhanggen Huang. 2025. "The Effect of SO2 on C3H8 Oxidation over Ru@CoMn2O4 Spinel" Molecules 30, no. 21: 4253. https://doi.org/10.3390/molecules30214253
APA StyleCui, Y., Zeng, Z., Hou, Y., Ma, S., Yang, J., Zheng, J., Shen, W., & Huang, Z. (2025). The Effect of SO2 on C3H8 Oxidation over Ru@CoMn2O4 Spinel. Molecules, 30(21), 4253. https://doi.org/10.3390/molecules30214253










