Phytochemical Characterization and In Vitro Biological Activities of Macleania rupestris (Ericaceae): Insights into Nutraceutical Potential
Abstract
1. Introduction
2. Results
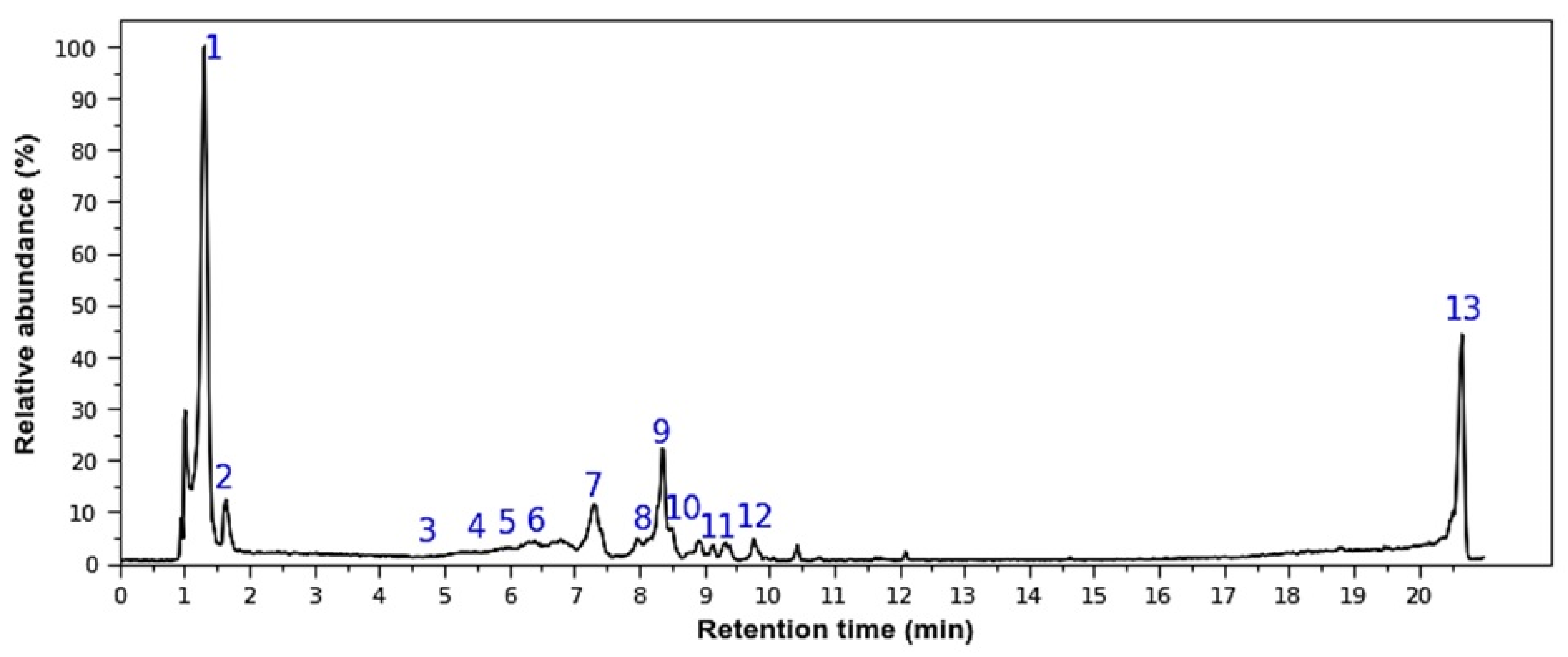
2.1. Chemical Characterization
| ID | Positive/ Negative | RT | m/z | MS/MS | Tentative Identification | Reference |
|---|---|---|---|---|---|---|
| 1 | P | 1.38 | 381 | 219 (100), 201 (35), 363 (15) | Disaccharide (putative) | [8] |
| 2 | P | 1.41 | 403 | 325 (100), 289 (15), 271 (5) 325→289 (100), 271 (30), 307 (25), 163 (20) | Disaccharide-like ion (putative) | MzCloud 82.4% |
| 3 | P | 4.75 | 435 | 303 (100) | Delphinidin-3-arabinoside | [10] |
| 4 | P | 5.47 | 479 | 317 (100) | Petunidin-3-glucoside | [11,16] |
| 5 | P | 5.98 | 449 | 287 (100), 317 (46) | Cyanidin 3-glucoside | (**) |
| 6 | P | 6.40 | 493 | 331 (100) | Malvidin-3-glucoside | [11,16] |
| 7 | P | 7.31 | 481 | 319 (100) 319→273 (100), 301 (45), 165 (35), 245 (30), 153 (30) | Myricetin 3-o-hexoside | MzCloud 94.9% |
| N | 479 | 317 (100), 271 (10) | ||||
| 8 | P | 8.29 | 465 | 303 (100), 319 (20), 333 (15) 303→257 (100), 285 (70), 229 (65), 165 (65), 247 (35) | Quercetin-hexoside | (**) |
| N | 463 | 301 (100), 151 (10) | ||||
| 9 | P | 8.36 | 519 | 339 (100), 357 (90), 175 (50), 193 (25), 309 (20), 165 (10) 357→147 (100), 193 (30), 165 (10) | Acylated iridoid glycoside (vaccinoside) (putative) | |
| N | 535 | 371 (100), 191 (95), 448 (80), 241 (60), 373 (40), 491 (20), 163 (15) | ||||
| 10 | P | 8.50 | 495 | 333 (100), 477 (5) | O-methyl-myricetin O-hexoside | GNPS 83% |
| N | 493 | 331 (100), 330 (45), 315 (15), 413 (8), 179 (5) | ||||
| 11 | P | 8.92 | 435 | 303 (100), 361 (6) 303→257 (100), 285 (70), 229 (65), 165 (65), 247 (35) | Quercetin O-pentoside | MzCloud 92.9% |
| N | 433 | 301 (100) | ||||
| 12 | P | 9.76 | 509 | 347 (100), 491 (5) | O-methylated flavonol O-hexoside (syringetin-type) (putative) | [12] |
| N | 507 | 329 (100), 344 (70), 345 (60) | ||||
| 13 | P | 20.66 | 288 | 227 (100), 106 (50), 87 (20), 270 (10) | UI | (molecular ion not identified) |
2.2. Hemolytic Activity
2.3. Antitumoral Activity
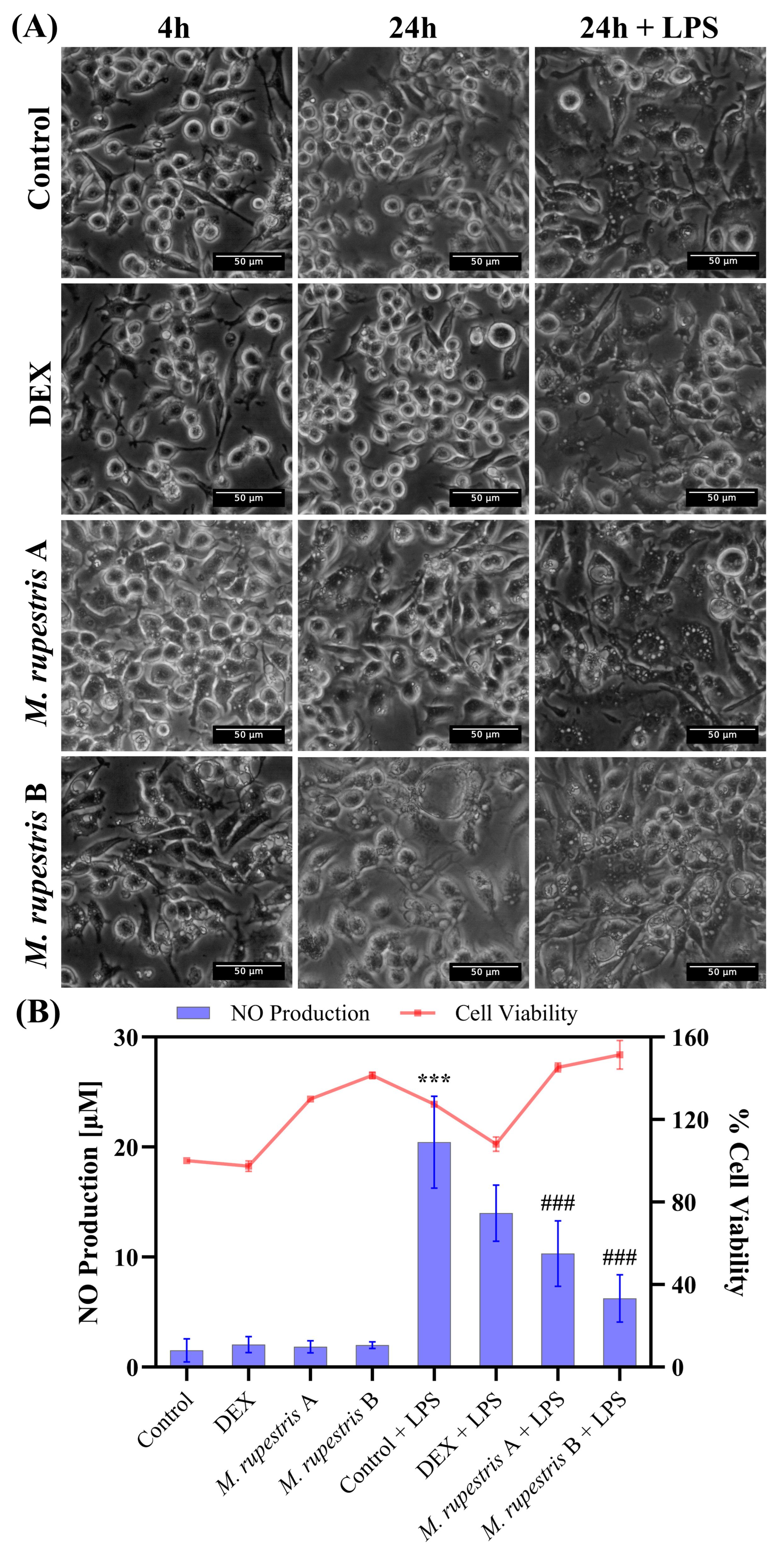
2.4. Anti-Inflammatory Activity
2.5. Leishmanicidal and Cytotoxic Activity
3. Discussion
Limitations and Future Perspectives
4. Materials and Methods
4.1. Plant Material and Physico-Chemical Analysis
4.2. Plant Extract
4.3. HPLC-MS/MS Analysis
4.4. Hemolytic Activity Evaluation Assay
4.5. Anti-Tumoral Activity Evaluation Assay
4.6. Anti-Inflammatory Activity Evaluation Assay
4.7. Leishmanicidal and Cytotoxic Activity Evaluation Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Martău, G.A.; Bernadette-Emőke, T.; Odocheanu, R.; Soporan, D.A.; Bochiș, M.; Simon, E.; Vodnar, D.C. Vaccinium species (Ericaceae): Phytochemistry and biological properties of medicinal plants. Molecules 2023, 28, 1533. [Google Scholar] [CrossRef]
- Barba-Ostria, C.; Carrera-Pacheco, S.E.; Gonzalez-Pastor, R.; Zuñiga-Miranda, J.; Mayorga-Ramos, A.; Tejera, E.; Guamán, L.P. Exploring the Multifaceted Biological Activities of Anthocyanins Isolated from Two Andean Berries. Foods 2024, 13, 2625. [Google Scholar] [CrossRef]
- Esquivel-Chirino, C.; Bolaños-Carrillo, M.A.; Carmona-Ruiz, D.; Lopéz-Macay, A.; Hernández-Sánchez, F.; Montés-Sánchez, D.; Escuadra-Landeros, M.; Gaitán-Cepeda, L.A.; Maldonado-Frías, S.; Yáñez-Ocampo, B.R.; et al. The protective role of cranberries and blueberries in oral cancer. Plants 2023, 12, 2330. [Google Scholar] [CrossRef]
- Mayorga-Ramos, A.; Zúñiga-Miranda, J.; Coyago-Cruz, E.; Heredia-Moya, J.; Guamán-Bautista, J.; Guamán, L.P. Phytochemical Composition and Biological Properties of Macleania rupestris Fruit Extract: Insights into Its Antimicrobial and Antioxidant Activity. Antioxidants 2025, 14, 394. [Google Scholar] [CrossRef]
- Tundis, R.; Tenuta, M.C.; Loizzo, M.R.; Bonesi, M.; Finetti, F.; Trabalzini, L.; Deguin, B. Vaccinium Species (Ericaceae): From Chemical Composition to Bio-Functional Activities. Appl. Sci. 2021, 11, 5655. [Google Scholar] [CrossRef]
- Pacheco Flores de Valgaz, A.; Barcos-Arias, M.; Naranjo-Morán, J.; Peña Tapia, D.; Moreira-Gómez, R. Ericaceous Plants: A Review for the Bioprospecting of Ericoid Mycorrhizae from Ecuador. Diversity 2022, 14, 648. [Google Scholar] [CrossRef]
- Ștefănescu, B.E.; Szabo, K.; Mocan, A.; Crişan, G. Phenolic Compounds from Five Ericaceae Species Leaves and Their Related Bioavailability and Health Benefits. Molecules 2019, 24, 2046. [Google Scholar] [CrossRef] [PubMed]
- Overy, D.P.; Enot, D.P.; Tailliart, K.; Jenkins, H.; Parker, D.; Beckmann, M.; Draper, J. Explanatory signal interpretation and metabolite identification strategies for nominal mass FIE-MS metabolite fingerprints. Nat. Protoc. 2008, 3, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; Yang, H.; Yan, C.; Song, F.; Liu, Z.; Liu, S. Differentiation of glucose-containing disaccharides isomers by fragmentation of the deprotonated non-covalent dimers using negative electrospray ionization tandem mass spectrometry. Talanta 2013, 115, 870–875. [Google Scholar] [CrossRef]
- Guevara-Terán, M.; Padilla-Arias, K.; Beltrán-Novoa, A.; González-Paramás, A.M.; Giampieri, F.; Battino, M.; Vásquez-Castillo, W.; Fernandez-Soto, P.; Tejera, E.; Alvarez-Suarez, J.M. Influence of Altitudes and Development Stages on the Chemical Composition, Antioxidant, and Antimicrobial Capacity of the Wild Andean Blueberry (Vaccinium floribundum Kunth). Molecules 2022, 27, 7525. [Google Scholar] [CrossRef]
- Alcalde-Eon, C.; Saavedra, G.; de Pascual-Teresa, S.; Rivas-Gonzalo, J.C. Liquid chromatography-mass spectrometry identification of anthocyanins of isla oca (Oxalis tuberosa, Mol.) tubers. J. Chromatogr. A 2004, 1054, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Dastmalchi, K.; Flores, G.; Wu, S.-B.; Pedraza-Peñalosa, P.; Long, C.; Kennelly, E.J. Antioxidant and metabolite profiling of North American and neotropical blueberries using LC-TOF-MS and multivariate analyses. J. Agric. Food Chem. 2013, 61, 3548–3559. [Google Scholar] [CrossRef]
- Hokkanen, J.; Mattila, S.; Jaakola, L.; Pirttilä, A.M.; Tolonen, A. Identification of Phenolic Compounds from Lingonberry (Vaccinium vitis-idaea L.), Bilberry (Vaccinium myrtillus L.) and Hybrid Bilberry (Vaccinium x intermedium Ruthe L.) Leaves. J. Agric. Food Chem. 2009, 57, 9437–9447. [Google Scholar] [CrossRef]
- Mieres-Castro, D.; Schmeda-Hirschmann, G.; Theoduloz, C.; Gómez-Alonso, S.; Pérez-Navarro, J.; Márquez, K.; Jiménez-Aspee, F. Antioxidant activity and the isolation of polyphenols and new iridoids from Chilean Gaultheria phillyreifolia and G. poeppigii berries. Food Chem. 2019, 291, 167–179. [Google Scholar] [CrossRef]
- Geng, C.A.; Chen, H.; Chen, X.L.; Zhang, X.M.; Lei, L.G.; Chen, J.J. Rapid characterization of chemical constituents in Saniculiphyllum guangxiense by ultra fast liquid chromatography with diode array detection and electrospray ionization tandem mass spectrometry. Int. J. Mass Spectrom. 2014, 361, 9–22. [Google Scholar] [CrossRef]
- Ha, T.J.; Lee, M.H.; Park, C.H.; Pae, S.B.; Shim, K.B.; Ko, J.M.; Shin, S.O.; Baek, I.Y.; Park, K.Y. Identification and Characterization of Anthocyanins in Yard-Long Beans (Vigna unguiculata ssp. sesquipedalis L.) by High-Performance Liquid Chromatography with Diode Array Detection and Electrospray Ionization/Mass Spectrometry (HPLC−DAD−ESI/MS) Analysis. J. Agric. Food Chem. 2010, 58, 2571–2576. [Google Scholar] [CrossRef] [PubMed]
- Gawlikowski, M.; El Fray, M.; Janiczak, K.; Zawidlak-Węgrzyńska, B.; Kustosz, R. In-Vitro Biocompatibility and Hemocompatibility Study of New PET Copolyesters Intended for Heart Assist Devices. Polymers 2020, 12, 2857. [Google Scholar] [CrossRef]
- van Oeveren, W. Obstacles in haemocompatibility testing. Scientifica 2013, 2013, 392584. [Google Scholar] [CrossRef]
- Silva, G.C.C.; Machado, M.d.A.; Sakumoto, K.; Inumaro, R.S.; Gonçalves, J.E.; Mandim, F.; Vaz, J.; do Valle, J.S.; Faria, M.G.I.; Ruiz, S.P.; et al. Cellular Antioxidant, Anti-Inflammatory, and Antiproliferative Activities from the Flowers, Leaves and Fruits of Gallesia integrifolia Spreng Harms. Molecules 2023, 28, 5406. [Google Scholar] [CrossRef]
- Remigante, A.; Spinelli, S.; Basile, N.; Caruso, D.; Falliti, G.; Dossena, S.; Marino, A.; Morabito, R. Oxidation stress as a mechanism of aging in human erythrocytes: Protective effect of quercetin. Int. J. Mol. Sci. 2022, 23, 7781. [Google Scholar] [CrossRef]
- Bebek Markovinović, A.; Brdar, D.; Putnik, P.; Bosiljkov, T.; Durgo, K.; Huđek Turković, A.; Brčić Karačonji, I.; Jurica, K.; Pavlić, B.; Granato, D.; et al. Strawberry tree fruits (Arbutus unedo L.): Bioactive composition, cellular antioxidant activity, and 3D printing of functional foods. Food Chem. 2024, 433, 137287. [Google Scholar] [CrossRef]
- Agraharam, G.; Girigoswami, A.; Girigoswami, K. Myricetin: A multifunctional flavonol in biomedicine. Curr. Pharmacol. Rep. 2022, 8, 48–61. [Google Scholar] [CrossRef]
- Quispe-Díaz, I.M.; Ybañez-Julca, R.O.; Pino-Ríos, R.; Quispe-Rodríguez, J.D.; Asunción-Alvarez, D.; Mantilla-Rodríguez, E.; Rengifo-Penadillos, R.A.; Vásquez-Corales, E.; de Albuquerque, R.D.D.G.; Gutiérrez-Alvarado, W.O.; et al. Chemical composition, antioxidant activities, antidepressant effect, and lipid peroxidation of peruvian blueberry: Molecular docking studies on targets involved in oxidative stress and depression. Plants 2024, 13, 1643. [Google Scholar] [CrossRef]
- Luteyn, J. The plant family ericaceae (“blueberries”) in ecuador: Ecology, diversity, economic importance, and conservation. REMCB 2021, 42, 1–32. [Google Scholar] [CrossRef]
- Ashique, S.; Mukherjee, T.; Mohanty, S.; Garg, A.; Mishra, N.; Kaushik, M.; Bhowmick, M.; Chattaraj, B.; Mohanto, S.; Srivastava, S.; et al. Blueberries in focus: Exploring the phytochemical potentials and therapeutic applications. J. Agric. Food Res. 2024, 18, 101300. [Google Scholar] [CrossRef]
- Hara, S.; Morita, R.; Ogawa, T.; Segawa, R.; Takimoto, N.; Suzuki, K.; Hamadate, N.; Hayashi, S.-M.; Odachi, A.; Ogiwara, I.; et al. Tumor suppression effects of bilberry extracts and enzymatically modified isoquercitrin in early preneoplastic liver cell lesions induced by piperonyl butoxide promotion in a two-stage rat hepatocarcinogenesis model. Exp. Toxicol. Pathol. 2014, 66, 225–234. [Google Scholar] [CrossRef]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Varela-López, A.; Quiles, J.L.; Mezzetti, B.; Battino, M. Chemopreventive and therapeutic effects of edible berries: A focus on colon cancer prevention and treatment. Molecules 2016, 21, 169. [Google Scholar] [CrossRef] [PubMed]
- Onali, T.; Kivimäki, A.; Mauramo, M.; Salo, T.; Korpela, R. Anticancer effects of lingonberry and bilberry on digestive tract cancers. Antioxidants 2021, 10, 850. [Google Scholar] [CrossRef] [PubMed]
- Hattiholi, A.; Tendulkar, S.; Kumbar, V.; Rao, M.; Kugaji, M.; Muddapur, U.; Bhat, K. Evaluation of Anti-cancer Activities of Cranberries Juice Concentrate in Osteosarcoma Cell Lines (MG-63). IJPER Indian J. Pharm. Educ. Res. 2022, 56, 1141–1149. [Google Scholar] [CrossRef]
- Lamdan, H.; Garcia-Lazaro, R.S.; Lorenzo, N.; Caligiuri, L.G.; Alonso, D.F.; Farina, H.G. Anti-proliferative effects of a blueberry extract on a panel of tumor cell lines of different origin. Exp. Oncol. 2020, 42, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Damodaran, B.; Nagaraja, P.; Jain, V.; Manuja Viraj Wimalasiri, M.P.; Sankolli, G.M.; Kumar, G.; Prabhu, V. Phytochemical screening and evaluation of cytotoxic activity of Calotropis gigantea leaf extract on MCF7, HeLa, and A549 cancer cell lines. J. Nat. Sci. Biol. Med. 2019, 10, 131. [Google Scholar] [CrossRef]
- Park, S.-H.; Kim, M.; Lee, S.; Jung, W.; Kim, B. Therapeutic potential of natural products in treatment of cervical cancer: A review. Nutrients 2021, 13, 154. [Google Scholar] [CrossRef]
- Peng, F.; Liao, M.; Qin, R.; Zhu, S.; Peng, C.; Fu, L.; Chen, Y.; Han, B. Regulated cell death (RCD) in cancer: Key pathways and targeted therapies. Signal Transduct. Target. Ther. 2022, 7, 286. [Google Scholar] [CrossRef]
- Machuca, A.; Peñalver, G.A.; Garcia, R.A.-F.; Martinez-Lopez, A.; Castillo-Lluva, S.; Garcia-Calvo, E.; Luque-Garcia, J.L. Advancing rhodium nanoparticle-based photodynamic cancer therapy: Quantitative proteomics and in vivo assessment reveal mechanisms targeting tumor metabolism, progression and drug resistance. J. Mater. Chem. B Mater. Biol. Med. 2024, 12, 12073–12086. [Google Scholar] [CrossRef]
- Ndongwe, T.; Witika, B.A.; Mncwangi, N.P.; Poka, M.S.; Skosana, P.P.; Demana, P.H.; Summers, B.; Siwe-Noundou, X. Iridoid Derivatives as Anticancer Agents: An Updated Review from 1970–2022. Cancers 2023, 15, 770. [Google Scholar] [CrossRef] [PubMed]
- Camero, C.M.; Germanò, M.P.; Rapisarda, A.; D’Angelo, V.; Amira, S.; Benchikh, F.; Braca, A.; De Leo, M. Anti-angiogenic activity of iridoids from Galium tunetanum. Rev. Bras. Farmacogn. 2018, 28, 374–377. [Google Scholar] [CrossRef]
- Ha, T.K.; Jung, I.; Kim, M.E.; Bae, S.K.; Lee, J.S. Anti-cancer activity of myricetin against human papillary thyroid cancer cells involves mitochondrial dysfunction-mediated apoptosis. Biomed. Pharmacother. 2017, 91, 378–384. [Google Scholar] [CrossRef]
- Devi, K.P.; Rajavel, T.; Habtemariam, S.; Nabavi, S.F.; Nabavi, S.M. Molecular mechanisms underlying anticancer effects of myricetin. Life Sci. 2015, 142, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zheng, Y.M.; Ho, W.S. Effect of quercetin glucosides from Allium extracts on HepG2, PC-3 and HT-29 cancer cell lines. Oncol. Lett. 2018, 15, 4657–4661. [Google Scholar] [CrossRef] [PubMed]
- Karadeniz, F.; Oh, J.H.; Seo, Y.; Yang, J.; Lee, H.; Kong, C.-S. Quercetin 3-O-Galactoside Isolated from Limonium tetragonum Inhibits Melanogenesis by Regulating PKA/MITF Signaling and ERK Activation. Int. J. Mol. Sci. 2023, 24, 3064. [Google Scholar] [CrossRef]
- El Gendy, S.N.; Elmotayam, A.K.; Samir, R.; Ezzat, M.I.; Abo-Elfadl, M.T.; El Sayed, A.M. Biotransformation of quercetin by Bacillus subtilis and anticancer activity evaluation: In vitro and in Silico. AMB Express 2025, 15, 58. [Google Scholar] [CrossRef]
- Tang, S.; Wang, B.; Liu, X.; Xi, W.; Yue, Y.; Tan, X.; Bai, J.; Huang, L. Structural insights and biological activities of flavonoids: Implications for novel applications. Food Front. 2025, 6, 218–247. [Google Scholar] [CrossRef]
- Radziejewska, I.; Supruniuk, K.; Tomczyk, M.; Izdebska, W.; Borzym-Kluczyk, M.; Bielawska, A.; Bielawski, K.; Galicka, A. p-Coumaric acid, Kaempferol, Astragalin and Tiliroside Influence the Expression of Glycoforms in AGS Gastric Cancer Cells. Int. J. Mol. Sci. 2022, 23, 8602. [Google Scholar] [CrossRef]
- Carlos-Reyes, Á.; López-González, J.S.; Meneses-Flores, M.; Gallardo-Rincón, D.; Ruíz-García, E.; Marchat, L.A.; Astudillo-de la Vega, H.; Hernández de la Cruz, O.N.; López-Camarillo, C. Dietary compounds as epigenetic modulating agents in cancer. Front. Genet. 2019, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Arslan, M.E. Anticarcinogenic properties of malic acid on glioblastoma cell line through necrotic cell death mechanism. MANAS J. Eng. 2021, 9, 22–29. [Google Scholar] [CrossRef]
- Burbidge, C.A.; Ford, C.M.; Melino, V.J.; Wong, D.C.J.; Jia, Y.; Jenkins, C.L.D.; Soole, K.L.; Castellarin, S.D.; Darriet, P.; Rienth, M.; et al. Biosynthesis and cellular functions of tartaric acid in grapevines. Front. Plant Sci. 2021, 12, 643024. [Google Scholar] [CrossRef]
- Li, M.; Su, J.; Yang, H.; Feng, L.; Wang, M.; Xu, G.; Shao, J.; Ma, C. Grape tartaric acid: Chemistry, function, metabolism, and regulation. Horticulturae 2023, 9, 1173. [Google Scholar] [CrossRef]
- Shahbaz, M.; Naeem, H.; Momal, U.; Imran, M.; Alsagaby, S.A.; Al Abdulmonem, W.; Waqar, A.B.; El-Ghorab, A.H.; Ghoneim, M.M.; Abdelgawad, M.A.; et al. Anticancer and apoptosis inducing potential of quercetin against a wide range of human malignancies. Int. J. Food Prop. 2023, 26, 2590–2626. [Google Scholar] [CrossRef]
- Sadeghi Ekbatan, S.; Iskandar, M.M.; Sleno, L.; Sabally, K.; Khairallah, J.; Prakash, S.; Kubow, S. Absorption and Metabolism of Phenolics from Digests of Polyphenol-Rich Potato Extracts Using the Caco-2/HepG2 Co-Culture System. Foods 2018, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, S.M.; Kim, G.S. Functions of polyphenols and its anticancer properties in biomedical research: A narrative review. Transl. Cancer Res. 2020, 9, 7619–7631. [Google Scholar] [CrossRef]
- Ng, R.F.L.; Zainal Abidin, N.; Shuib, A.S.; Israf Ali, D.A. Inhibition of nitric oxide production by Solanum melongena and Solanum macrocarpon on RAW 264.7 cells. Front. Life Sci. 2015, 8, 241–248. [Google Scholar] [CrossRef]
- Li, L.-C.; Pan, Z.-H.; Ning, D.-S.; Fu, Y.-X. Anti-Inflammatory Effect of Simonsinol on Lipopolysaccharide Stimulated RAW 264.7 Cells through Inactivation of NF-κB Signaling Pathway. Molecules 2020, 25, 3573. [Google Scholar] [CrossRef]
- Yu, G.-J.; Choi, I.-W.; Kim, G.-Y.; Kim, B.-W.; Park, C.; Hong, S.-H.; Moon, S.-K.; Cha, H.-J.; Chang, Y.-C.; Paek, K.Y.; et al. Anti-inflammatory potential of saponins derived from cultured wild ginseng roots in lipopolysaccharide-stimulated RAW 264.7 macrophages. Int. J. Mol. Med. 2015, 35, 1690–1698. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Wu, Q.; Wang, J.; Li, M.; Qian, J.; Li, S. Quercetin inhibits LPS-induced macrophage migration by suppressing the iNOS/FAK/paxillin pathway and modulating the cytoskeleton. Cell Adhes. Migr. 2019, 13, 1–12. [Google Scholar] [CrossRef]
- Zhuang, J.C.; Wogan, G.N. Growth and viability of macrophages continuously stimulated to produce nitric oxide. Proc. Natl. Acad. Sci. USA 1997, 94, 11875–11880. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, R.; Han, L.; Kuerban, K.; Ye, L.; Pan, S.; Li, S.; Yuan, Y. Activation of autophagy reverses gemcitabine-induced immune inhibition of RAW 264.7 macrophages by promoting TNF-α, IL-6 and MHC-II expression. Immunol. Res. 2021, 69, 352–362. [Google Scholar] [CrossRef] [PubMed]
- Sims, K.; Haynes, C.A.; Kelly, S.; Allegood, J.C.; Wang, E.; Momin, A.; Leipelt, M.; Reichart, D.; Glass, C.K.; Sullards, M.C.; et al. Kdo2-lipid A, a TLR4-specific agonist, induces de novo sphingolipid biosynthesis in RAW 264.7 macrophages, which is essential for induction of autophagy. J. Biol. Chem. 2010, 285, 38568–38579. [Google Scholar] [CrossRef]
- Ryu, J.H.; Sung, J.; Xie, C.; Shin, M.-K.; Kim, C.-W.; Kim, N.-G.; Choi, Y.J.; Choi, B.D.; Kang, S.S.; Kang, D. Aplysia kurodai-derived glycosaminoglycans increase the phagocytic ability of macrophages via the activation of AMP-activated protein kinase and cytoskeletal reorganization in RAW 264.7 cells. J. Funct. Foods 2016, 27, 122–130. [Google Scholar] [CrossRef]
- Cao, D.L.; Woo, M.-S.; Kim, E.-J.; Ahn, B.; Prayoga, A.H.; Kang, S.S.; Cho, K.M.; Kang, D. Anti-Inflammatory Effects of Fermented and Aged Mountain-Cultivated Ginseng Sprouts via Suppression of MAPK-NF-κB Pathway in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. Food Sci. Nutr. 2024, 12, 9566–9576. [Google Scholar] [CrossRef]
- Xu, Y.; Jagannath, C.; Liu, X.-D.; Sharafkhaneh, A.; Kolodziejska, K.E.; Eissa, N.T. Toll-like receptor 4 is a sensor for autophagy associated with innate immunity. Immunity 2007, 27, 135–144. [Google Scholar] [CrossRef]
- Hou, W.; Hu, S.; Su, Z.; Wang, Q.; Meng, G.; Guo, T.; Zhang, J.; Gao, P. Myricetin attenuates LPS-induced inflammation in RAW 264.7 macrophages and mouse models. Future Med. Chem. 2018, 10, 2253–2264. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Kwak, H.-K.; Hwang, K.T. Antioxidant and antiinflammatory activities of cyanidin-3-glucoside and cyanidin-3-rutinoside in hydrogen peroxide and lipopolysaccharide-treated RAW 264.7 cells. Food Sci. Biotechnol. 2014, 23, 2053–2062. [Google Scholar] [CrossRef]
- Vasco, C.; Riihinen, K.; Ruales, J.; Kamal-Eldin, A. Chemical composition and phenolic compound profile of mortiño (Vaccinium floribundum Kunth). J. Agric. Food Chem. 2009, 57, 8274–8281. [Google Scholar] [CrossRef]
- Cerrato, A.; Piovesana, S.; Aita, S.E.; Cavaliere, C.; Felletti, S.; Laganà, A.; Montone, C.M.; Vargas-de-la-Cruz, C.; Capriotti, A.L. Detailed investigation of the composition and transformations of phenolic compounds in fresh and fermented Vaccinium floribundum berry extracts by high-resolution mass spectrometry and bioinformatics. Phytochem. Anal. 2022, 33, 507–516. [Google Scholar] [CrossRef]
- Marracino, L.; Punzo, A.; Severi, P.; Nganwouo Tchoutang, R.; Vargas-De-la-Cruz, C.; Fortini, F.; Vieceli Dalla Sega, F.; Silla, A.; Porru, E.; Simoni, P.; et al. Fermentation of Vaccinium floribundum Berries with Lactiplantibacillus plantarum Reduces Oxidative Stress in Endothelial Cells and Modulates Macrophages Function. Nutrients 2022, 14, 1560. [Google Scholar] [CrossRef]
- Esposito, D.; Overall, J.; Grace, M.H.; Komarnytsky, S.; Lila, M.A. Alaskan berry extracts promote dermal wound repair through modulation of bioenergetics and integrin signaling. Front. Pharmacol. 2019, 10, 1058. [Google Scholar] [CrossRef]
- Marín, C.; Clares, M.P.; Ramírez-Macías, I.; Blasco, S.; Olmo, F.; Soriano, C.; Verdejo, B.; Rosales, M.J.; Gomez-Herrera, D.; García-España, E.; et al. In vitro activity of scorpiand-like azamacrocycle derivatives in promastigotes and intracellular amastigotes of Leishmania infantum and Leishmania braziliensis. Eur. J. Med. Chem. 2013, 62, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, J.V.A.; de Andrade, M.T.; Rafael, D.D.; Zhu, F.; Martins, S.V.C.; Nunes-Nesi, A.; Benedito, V.; Fernie, A.R.; Zsögön, A. Anthocyanins and reactive oxygen species: A team of rivals regulating plant development? Plant Mol. Biol. 2023, 112, 213–223. [Google Scholar] [CrossRef]
- Patterson, S.; Wyllie, S.; Norval, S.; Stojanovski, L.; Simeons, F.R.; Auer, J.L.; Osuna-Cabello, M.; Read, K.D.; Fairlamb, A.H. The anti-tubercular drug delamanid as a potential oral treatment for visceral leishmaniasis. eLife 2016, 5, e09744. [Google Scholar] [CrossRef]
- Islamuddin, M.; Chouhan, G.; Tyagi, M.; Abdin, M.Z.; Sahal, D.; Afrin, F. Leishmanicidal activities of Artemisia annua leaf essential oil against Visceral Leishmaniasis. Front. Microbiol. 2014, 5, 626. [Google Scholar] [CrossRef]
- Van Assche, T.; Deschacht, M.; da Luz, R.A.I.; Maes, L.; Cos, P. Leishmania-macrophage interactions: Insights into the redox biology. Free Radic. Biol. Med. 2011, 51, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Brendler, T.; Chua, L.S.; Abdullah, L.C.; Yam, M.F. Editorial: Developing medicinal plant extracts commercially: The importance of extraction and fractionation. Front. Pharmacol. 2023, 14, 1140783. [Google Scholar] [CrossRef] [PubMed]
- Coelho, G.D.P.; Martins, V.S.; do Amaral, L.V.; Novaes, R.D.; Sarandy, M.M.; Gonçalves, R.V. Applicability of isolates and fractions of plant extracts in murine models in type II diabetes: A systematic review. Evid. Based Complement. Alternat. Med. 2016, 2016, 3537163. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Chaudhary, P.; Patel, S. Genomics, transcriptomics, proteomics and metabolomics approaches. In Fenugreek: Biology and Applications; Naeem, M., Aftab, T., Khan, M.M.A., Eds.; Springer: Singapore, 2021; pp. 355–373. ISBN 978-981-16-1196-4. [Google Scholar]
- Hashiguchi, A.; Tian, J.; Komatsu, S. Proteomic contributions to medicinal plant research: From plant metabolism to pharmacological action. Proteomes 2017, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Lalarukh; Hussain, S.M.; Ali, S.; Yilmaz, E.; Zahoor, A.F.; Javid, A.; Alshehri, M.A.; Shahzad, M.M.; Naeem, A.; Mahrukh. Microencapsulation: An innovative technology in modern science. Polym. Adv. Technol. 2025, 36, e70066. [Google Scholar] [CrossRef]
- Junaid, P.M.; Dar, A.H.; Dash, K.K.; Rohilla, S.; Islam, R.; Shams, R.; Pandey, V.K.; Srivastava, S.; Panesar, P.S.; Zaidi, S. Polysaccharide-based hydrogels for microencapsulation of bioactive compounds: A review. J. Agric. Food Res. 2024, 15, 101038. [Google Scholar] [CrossRef]
- Donkor, M.N.; Donkor, A.-M.; Mosobil, R. Combination therapy: Synergism among three plant extracts against selected pathogens. BMC Res. Notes 2023, 16, 83. [Google Scholar] [CrossRef]
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing New Antimicrobial Therapies: Are Synergistic Combinations of Plant Extracts/Compounds with Conventional Antibiotics the Solution? Pharmacogn. Rev. 2017, 11, 57–72. [Google Scholar] [CrossRef]
- Celi, D.; Quiroz, E.; Beltrán-Noboa, A.; Machado, A.; Tejera, E.; Fernandez-Soto, P. A chemical analysis of the Pelargonium species: P. odoratissimum, P. graveolens, and P. zonale identifies secondary metabolites with activity against gram-positive bacteria with multidrug-resistance. PLoS ONE 2024, 19, e0306637. [Google Scholar] [CrossRef]
- Sæbø, I.P.; Bjørås, M.; Franzyk, H.; Helgesen, E.; Booth, J.A. Optimization of the hemolysis assay for the assessment of cytotoxicity. Int. J. Mol. Sci. 2023, 24, 2914. [Google Scholar] [CrossRef]
- F756-00; Standard Practice for Assessment of Hemolytic Properties of Materials. ASTM Int.: West Conshohocken, PA, USA, 2010. [CrossRef]
- Hussain, S.; Liufang, H.; Shah, S.M.; Ali, F.; Khan, S.A.; Shah, F.A.; Li, J.B.; Li, S. Cytotoxic effects of extracts and isolated compounds from Ifloga spicata (forssk.) sch. bip against HepG-2 cancer cell line: Supported by ADMET analysis and molecular docking. Front. Pharmacol. 2022, 13, 986456. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Li, Z. Blueberries inhibit cyclooxygenase-1 and cyclooxygenase-2 activity in human epithelial ovarian cancer. Oncol. Lett. 2017, 13, 4897–4904. [Google Scholar] [CrossRef] [PubMed]
- Wallert, M.; Schmölz, L.; Koeberle, A.; Krauth, V.; Glei, M.; Galli, F.; Werz, O.; Birringer, M.; Lorkowski, S. α-Tocopherol long-chain metabolite α-13′-COOH affects the inflammatory response of lipopolysaccharide-activated murine RAW 264.7 macrophages. Mol. Nutr. Food Res. 2015, 59, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
| Extract Concentration (mg/mL) | %HR (Mean ± SD) |
|---|---|
| C- | 0.0 ± 0.08 |
| C+ | 100 ± 0.76 |
| 10 | 2.27 ± 0.21 |
| 5 | 2.23 ± 0.42 |
| 2.5 | 1.83 ± 0.80 |
| 1.25 | 1.73 ± 0.59 |
| 0.625 | 0.23 ± 0.2 |
| HeLa | HCT116 | MDAMB231 | THJ29T | HepG2 | NIH3T3 | |
|---|---|---|---|---|---|---|
| IC50 | 22.5 ± 0.7 | 11.1 ± 1.1 | 13.2 ± 0.5 | 11.7 ± 1.9 | 10.4 ± 1.3 | 11.1 ± 1.1 |
| TI a | 0.8 | 1.7 | 1.4 | 1.6 | 1.8 | - |
| Extract Concentration (µg/mL) | Viability (% ± SD) | Cell Type |
|---|---|---|
| Amphotericin B (inhibition control C+) | 12.83 ± 0.74 | Leishmania mexicana promastigote |
| DMSO 2%, (vehicle control, C−) | 103.38 ± 9.77 | |
| RPMI (untreated control, C−) | 100 | |
| 100 | 107.98 ± 9.70 | |
| 10 | 82.56 ± 10.81 | |
| 1 | 81.02 ± 4.98 | |
| 0.1 | 84.85 ± 8.50 | |
| 0.01 | 80.89 ± 5.34 | |
| Saponin (inhibition control, C+) | 3.44 ± 0.14 | RAW 264.7 macrophages |
| DMSO 2%, (vehicle control, C−) | 93.41 ± 5.49 | |
| DMEM (untreated control, C−) | 100 | |
| 100 | 75.64 ± 5.08 | |
| 10 | 83.73 ± 3.37 | |
| 1 | 84.82 ± 5.49 | |
| 0.1 | 87.28 ± 8.11 | |
| 0.01 | 101.62 ± 8.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayorga-Ramos, A.; Gonzalez-Pastor, R.; Puente-Pineda, J.A.; Barba-Ostria, C.; Tejera, E.; Celi, D.; Rojas-Silva, P.; Peñaherrera-Pazmiño, A.B.; Guamán, L.P. Phytochemical Characterization and In Vitro Biological Activities of Macleania rupestris (Ericaceae): Insights into Nutraceutical Potential. Molecules 2025, 30, 4251. https://doi.org/10.3390/molecules30214251
Mayorga-Ramos A, Gonzalez-Pastor R, Puente-Pineda JA, Barba-Ostria C, Tejera E, Celi D, Rojas-Silva P, Peñaherrera-Pazmiño AB, Guamán LP. Phytochemical Characterization and In Vitro Biological Activities of Macleania rupestris (Ericaceae): Insights into Nutraceutical Potential. Molecules. 2025; 30(21):4251. https://doi.org/10.3390/molecules30214251
Chicago/Turabian StyleMayorga-Ramos, Arianna, Rebeca Gonzalez-Pastor, Juan A. Puente-Pineda, Carlos Barba-Ostria, Eduardo Tejera, Diana Celi, Patricio Rojas-Silva, Ana Belén Peñaherrera-Pazmiño, and Linda P. Guamán. 2025. "Phytochemical Characterization and In Vitro Biological Activities of Macleania rupestris (Ericaceae): Insights into Nutraceutical Potential" Molecules 30, no. 21: 4251. https://doi.org/10.3390/molecules30214251
APA StyleMayorga-Ramos, A., Gonzalez-Pastor, R., Puente-Pineda, J. A., Barba-Ostria, C., Tejera, E., Celi, D., Rojas-Silva, P., Peñaherrera-Pazmiño, A. B., & Guamán, L. P. (2025). Phytochemical Characterization and In Vitro Biological Activities of Macleania rupestris (Ericaceae): Insights into Nutraceutical Potential. Molecules, 30(21), 4251. https://doi.org/10.3390/molecules30214251











