Phytochemical Analysis and In-Vitro Biological Activities of Three Wild Eryngium Species: E. beecheyanum, E. heterophyllum, and E. mexiae
Abstract
1. Introduction
2. Results
2.1. Phytochemical Analysis of the EB, EH, and EM
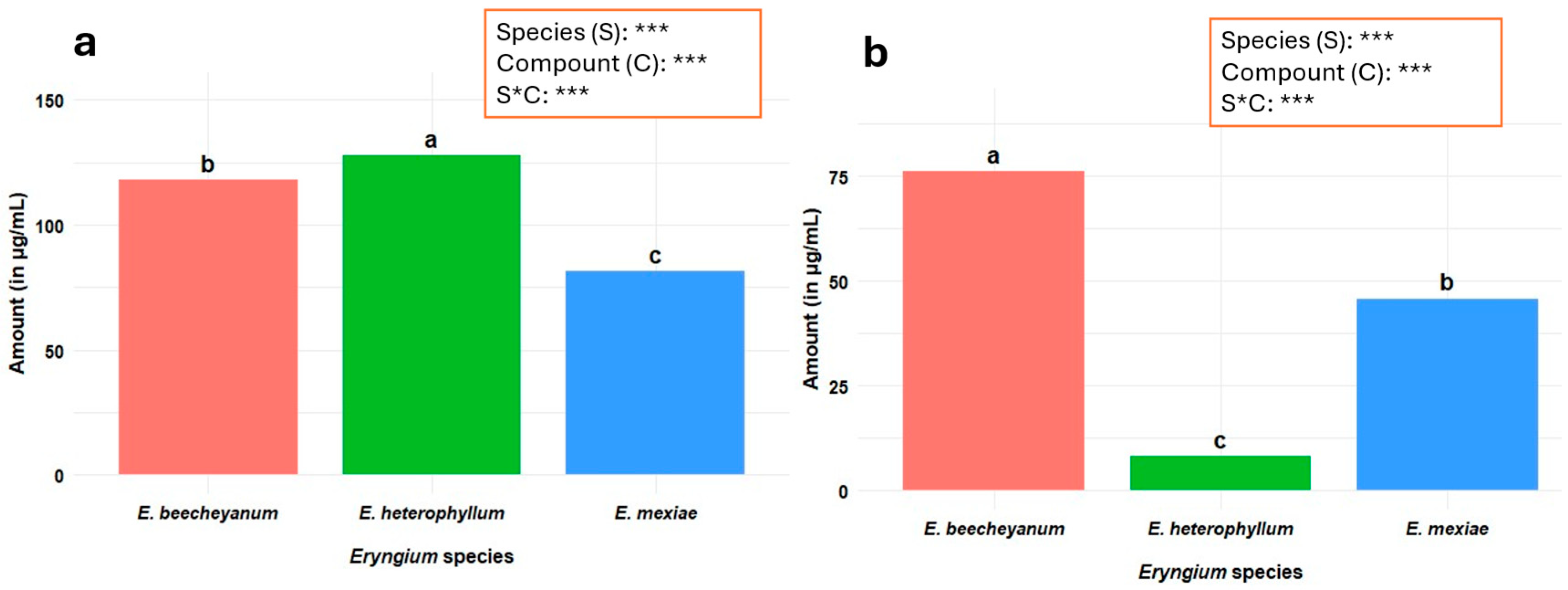
2.2. Quantification of Rutin in EB, EH, and EM
2.3. Quantification of Catechin in EB, EH, and EM
2.4. Quantification of Ferulic Acid in EB, EH, and EM
2.5. Quantification of Gallic Acid in EB, EH, and EM
2.6. Quantification of Caffeic Acid in EB, EH, and EM
2.7. Quantification of β-Sitosterol in EB, EH, and EM
2.8. Antioxidant Activity
Antioxidant Activity Based on ABTS●+ and DPPH• Assays
2.9. Hypoglycemic Activity of EB, EH, and EM Extracts
2.10. Pearson Correlation
3. Discussion
4. Materials and Methods
4.1. Plant Samples
4.2. Preparation of EB, EH, and EM Extracts
4.3. Determination and Quantification of Metabolites by High-Performance Thin-Layer Chromatography (HPTLC)
4.4. Antioxidant Activity by ABTS•+ and DPPH• Microdulution Method
4.4.1. Antioxidant Activity via ABTS•+ Assay
4.4.2. Antioxidant Activity via the DPPH• Assay
4.5. Antihyperglycemic Activity
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agidew, M.G. Phytochemical Analysis of Some Selected Traditional Medicinal Plants in Ethiopia. Bull. Natl. Res. Cent. 2022, 46, 87. [Google Scholar] [CrossRef]
- Noor, F.; Tahir ul Qamar, M.; Ashfaq, U.A.; Albutti, A.; Alwashmi, A.S.S.; Aljasir, M.A. Network Pharmacology Approach for Medicinal Plants: Review and Assessment. Pharmaceuticals 2022, 15, 572. [Google Scholar] [CrossRef]
- Cedillo-Cortezano, M.; Martinez-Cuevas, L.R.; López, J.A.M.; Barrera López, I.L.; Escutia-Perez, S.; Petricevich, V.L. Use of Medicinal Plants in the Process of Wound Healing: A Literature Review. Pharmaceuticals 2024, 17, 303. [Google Scholar] [CrossRef]
- Cárdenas-Valdovinos, J.G.; García-Ruiz, I.; Angoa-Pérez, M.V.; Mena-Violante, H.G. Ethnobotany, Biological Activities and Phytochemical Compounds of Some Species of the Genus Eryngium (Apiaceae), from the Central-Western Region of Mexico. Molecules 2023, 28, 4094. [Google Scholar] [CrossRef] [PubMed]
- Gómez Álvarez, R. Plantas medicinales en una aldea del estado de Tabasco, México. Rev. Fitotec. Mex. 2012, 35, 43–49. [Google Scholar] [CrossRef]
- Vukic, M.D.; Vukovic, N.L.; Djelic, G.T.; Obradovic, A.; Kacaniova, M.M.; Markovic, S.; Popović, S.; Baskić, D. Phytochemical Analysis, Antioxidant, Antibacterial and Cytotoxic Activity of Different Plant Organs of Eryngium serbicum L. Ind. Crops Prod. 2018, 115, 88–97. [Google Scholar] [CrossRef]
- Erdem, S.A.; Nabavi, S.F.; Orhan, I.E.; Daglia, M.; Izadi, M.; Nabavi, S.M. Blessings in Disguise: A Review of Phytochemical Composition and Antimicrobial Activity of Plants Belonging to the Genus Eryngium. DARU J. Pharm. Sci. 2015, 23, 53. [Google Scholar] [CrossRef]
- Pasayeva, L.; Safak, E.K.; Arigun, T.; Fatullayev, H.; Tugay, O. In Vitro Antioxidant Capacity and Phytochemical Characterization of Eryngium kotschyi Boiss. J. Pharm. Pharmacogn. Res. 2020, 8, 18–31. [Google Scholar] [CrossRef]
- Paul, J.H.A.; Seaforth, C.E.; Tikasingh, T. Eryngium Foetidum L.: Una Revisión. Fitoterapia 2011, 82, 302–308. [Google Scholar] [CrossRef]
- Nejati, M.; Masoudi, S.; Dastan, D.; Masnabadi, N.; Nejati, M.; Masoudi, S.; Dastan, D.; Masnabadi, N. Phytochemical Analysis and Antibacterial Activity of Eryngium pyramidale Boiss. & HAUSSKN. J. Chil. Chem. Soc. 2021, 66, 5230–5236. [Google Scholar] [CrossRef]
- Wu, J.; Lv, S.; Zhao, L.; Gao, T.; Yu, C.; Hu, J.; Ma, F. Advances in the Study of the Function and Mechanism of the Action of Flavonoids in Plants Under Environmental Stresses. Planta 2023, 257, 108. [Google Scholar] [CrossRef]
- Bag, S.; Mondal, A.; Majumder, A.; Mondal, S.K.; Banik, A. Flavonoid Mediated Selective Cross-Talk Between Plants and Beneficial Soil Microbiome. Phytochem. Rev. 2022, 21, 1739–1760. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, B.R.; Heleno, S.A.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Phenolic Compounds: Current Industrial Applications, Limitations and Future Challenges. Food Funct. 2021, 12, 14–29. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant Activity of Caffeic Acid (3,4-Dihydroxycinnamic Acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef]
- Lu, Z.; Nie, G.; Belton, P.S.; Tang, H.; Zhao, B. Structure–Activity Relationship Analysis of Antioxidant Ability and Neuroprotective Effect of Gallic Acid Derivatives. Neurochem. Int. 2006, 48, 263–274. [Google Scholar] [CrossRef]
- Monteiro Espíndola, K.M.; Ferreira, R.G.; Mosquera Narvaez, L.E.; Rocha Silva Rosario, A.C.; Machado Da Silva, A.H.; Bispo Silva, A.G.; Oliveira Vieira, A.P.; Chagas Monteiro, M. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- Kianersi, F.; Abdollahi, M.R.; Mirzaie-asl, A.; Dastan, D.; Rasheed, F. Biosynthesis of Rutin Changes in Capparis Spinosa Due to Altered Expression of Its Pathway Genes under Elicitors’ Supplementation. Plant Cell Tiss. Organ Cult. (PCTOC) 2020, 141, 619–631. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An Overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Pérez Trueba, G. Los Flavonoides: Antioxidantes o Prooxidantes. Rev. Cuba. Investig. Biomédicas 2003, 22, 48–57. [Google Scholar]
- Latos-Brozio, M.; Masek, A. Structure-Activity Relationships Analysis of Monomeric and Polymeric Polyphenols (Quercetin, Rutin and Catechin) Obtained by Various Polymerization Methods. Chem. Biodivers. 2019, 16, e1900426. [Google Scholar] [CrossRef]
- Pivec, T.; Kargl, R.; Maver, U.; Bračič, M.; Elschner, T.; Žagar, E.; Gradišnik, L.; Kleinschek, K.S. Chemical Structure–Antioxidant Activity Relationship of Water–Based Enzymatic Polymerized Rutin and Its Wound Healing Potential. Polymers 2019, 11, 1566. [Google Scholar] [CrossRef]
- Valenzuela, A.; Ronco, A.M. Fitoesteroles y Fitoestanoles: Aliados Naturales para la Protección de la Salud Cardiovascular. Rev. Chil. Nutr. 2004, 31, 161–169. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, B.; Yong, B.; Liu, T.; Peng, Y.; Zhang, X.; Ma, X.; Huang, L.; Liu, W.; Nie, G. Metabolomics and Physiological Analyses Reveal β-Sitosterol as an Important Plant Growth Regulator Inducing Tolerance to Water Stress in White Clover. Planta 2019, 250, 2033–2046. [Google Scholar] [CrossRef]
- Babu, S.; Jayaraman, S. An Update on β-Sitosterol: A Potential Herbal Nutraceutical for Diabetic Management. Biomed. Pharmacother. 2020, 131, 110702. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.H.; Bang, S.I.; Shin, H.; Cho, E.J.; Lee, S. Antioxidant Activity of Edible Sprouts and Phytosterol Contents by HPLC/UV Analysis. Hortic. Environ. Biotechnol. 2022, 63, 769–778. [Google Scholar] [CrossRef]
- Wang, P.; Su, Z.; Yuan, W.; Deng, G.; Li, S. Phytochemical Constituents and Pharmacological Activities of Eryngium L. (Apiaceae). Pharm. Crop 2012, 3, 99–120. [Google Scholar] [CrossRef]
- Marčetić, M.; Petrović, S.; Milenković, M.; Niketić, M. Composition, Antimicrobial and Antioxidant Activity of the Extracts of Eryngium palmatum Pančić and Vis. (Apiaceae). Open Life Sci. 2014, 9, 149–155. [Google Scholar] [CrossRef]
- Noriega-Cisneros, R.; Ortiz-Ávila, O.; Esquivel-Gutiérrez, E.; Clemente-Guerrero, M.; Manzo-Avalos, S.; Salgado-Garciglia, R.; Cortés-Rojo, C.; Boldogh, I.; Saavedra-Molina, A. Hypolipidemic Activity of Eryngium carlinae on Streptozotocin-Induced Diabetic Rats. Biochem. Res. Int. 2012, 2012, 603501. [Google Scholar] [CrossRef]
- Heidari, A.; Meftahi, G.; Bahrami, F.; Bahari, Z.; Zarei Mahmoudabadi, A.; Fallah Huseini, H.; Jangravi, Z. Anti-Adipogenic Effects of Eryngium Billardieri Extract during 3T3-L1 Adipocyte Differentiation. J. Adv. Med. Bio. Res. 2023, 31, 391–397. [Google Scholar] [CrossRef]
- Thiem, B.; Goslinska, O.; Kikowska, M.; Budzianowski, J. Antimicrobial Activity of Three Eryngium L. Species (Apiaceae). Herba Pol. 2010, 56, 52–59. [Google Scholar]
- Al-Askar, A.A.; Bashir, S.; Mohamed, A.E.; Sharaf, O.A.; Nabil, R.; Su, Y.; Abdelkhalek, A.; Behiry, S.I. Antimicrobial Efficacy and HPLC Analysis of Polyphenolic Compounds in a Whole-Plant Extract of Eryngium campestre. Separations 2023, 10, 362. [Google Scholar] [CrossRef]
- Pereira, C.G.; Locatelli, M.; Innosa, D.; Cacciagrano, F.; Polesná, L.; Santos, T.F.; Rodrigues, M.J.; Custódio, L. Unravelling the Potential of the Medicinal Halophyte Eryngium maritimum L.: In Vitro Inhibition of Diabetes-Related Enzymes, Antioxidant Potential, Polyphenolic Profile and Mineral Composition. S. Afr. J. Bot. 2019, 120, 204–212. [Google Scholar] [CrossRef]
- Pérez-Ramírez, I.F.; Enciso-Moreno, J.A.; Guevara-González, R.G.; Gallegos-Corona, M.A.; Loarca-Piña, G.; Reynoso-Camacho, R. Modulation of Renal Dysfunction by Smilax cordifolia and Eryngium carlinae, and Their Effect on Kidney Proteome in Obese Rats. J. Funct. Foods 2016, 20, 545–555. [Google Scholar] [CrossRef]
- Zengin, G.; Yagi, S.; Eldahshan, O.A.; Singab, A.N.; Selvi, S.; Rodrigues, M.J.; Custodio, L.; Dall’Acqua, S.; Kumar M Ponnaiya, S.; Aly, S.H. Decoding Chemical Profiles and Biological Activities of Aerial Parts and Roots of Eryngium thorifolium Boiss by HPLC-MS/MS, GC-MS and In Vitro Chemical Assays. Food Biosci. 2024, 61, 104556. [Google Scholar] [CrossRef]
- García, M.D.; Sáenz, M.T.; Gómez, M.A.; Fernández, M.A. Topical Antiinflammatory Activity of Phytosterols Isolated from Eryngium Foetidum on Chronic and Acute Inflammation Models. Phytother. Res. 1999, 13, 78–80. [Google Scholar] [CrossRef]
- Thi, N.Q.N.; An, T.N.T.; Nguyen, O.B.; Dung, L.T.; Minh, L.V.; Nhan, L.T.H. Phytochemical Content and Antioxidant Activity in Aqueous and Ethanolic Extracts of Eryngium foetidum L. IOP Conf. Ser. Mater. Sci. Eng. 2020, 991, 012026. [Google Scholar] [CrossRef]
- Nijveldt, R.J.; Van Nood, E.; Van Hoorn, D.E.C.; Boelens, P.G.; Van Norren, K.; Van Leeuwen, P.A.M. Flavonoids: A Review of Probable Mechanisms of Action and Potential Applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.Z.; Long, J.T.; Gong, Z.F.; Nong, K.Y.; Liang, X.M.; Qin, T.; Huang, W.; Yang, L. Current State of Knowledge on the Antioxidant Effects and Mechanisms of Action of Polyphenolic Compounds. Nat. Prod. Commun. 2021, 16. [Google Scholar] [CrossRef]
- Cizmarova, B.; Hubkova, B.; Bolerazska, B.; Marekova, M.; Birkova, A. Caffeic Acid: A Brief Overview of Its Presence, Metabolism, and Bioactivity. Bioact. Compd. Health Dis. 2020, 3, 74–81. [Google Scholar] [CrossRef]
- Cádiz-Gurrea, M.L.; Fernández-Arroyo, S.; Joven, J.; Segura-Carretero, A. Comprehensive Characterization by UHPLC-ESI-Q-TOF-MS from an Eryngium Bourgatii Extract and Their Antioxidant and Anti-Inflammatory Activities. Food Res. Int. 2013, 50, 197–204. [Google Scholar] [CrossRef]
- Dalar, A.; Türker, M.; Zabaras, D.; Konczak, I. Phenolic Composition, Antioxidant and Enzyme Inhibitory Activities of Eryngium bornmuelleri Leaf. Plant Foods Hum. Nutr. 2014, 69, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, N.; Xue, M.; Zhang, M.; Liu, W.; Xu, C.; Fan, Y.; Meng, Y.; Zhang, Q.; Zhou, Y. Anti-Inflammatory and Antioxidant Properties of β-Sitosterol in Copper Sulfate-Induced Inflammation in Zebrafish (Danio rerio). Antioxidants 2023, 12, 391. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas-Valdovinos, J.G.; Mena-Violante, H.G.; Rosas-Cárdenas, F.D.F.; Angoa-Pérez, M.V.; Luna-Suárez, S. Phytochemistry and Biological Activities of Hedeoma piperita Benth. (Quiensabe). Plant Foods for Hum. Nutr. 2025, 26, 1640. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, A.; Rashid, U.; Ahmad, S.; Zahoor, M.; AlAjmi, M.F.; Ullah, R.; Noman, O.M.; Ullah, F.; Ayaz, M.; Khan, I.; et al. Treating Hyperglycemia from Eryngium caeruleum M. Bieb: In-Vitro α-Glucosidase, Antioxidant, in-Vivo Antidiabetic and Molecular Docking-Based Approaches. Front. Chem. 2020, 8, 558641. [Google Scholar] [CrossRef]
- Espinoza-Hernández, F.; Andrade-Cetto, A.; Escandón-Rivera, S.; Mata-Torres, G.; Mata, R. Contribution of Fasting and Postprandial Glucose-Lowering Mechanisms to the Acute Hypoglycemic Effect of Traditionally Used Eryngium cymosum F. Delaroche. J. Ethnopharmacol. 2021, 279, 114339. [Google Scholar] [CrossRef]
- Romo-Pérez, A.; Escandón-Rivera, S.M.; Miranda, L.D.; Andrade-Cetto, A. Phytochemical Study of Eryngium cymosum F. Delaroche and the Inhibitory Capacity of Its Main Compounds on Two Glucose-Producing Pathway Enzymes. Plants 2022, 11, 992. [Google Scholar] [CrossRef]
- Un, J.J.; Lee, M.K.; Yong, B.P.; Jeon, S.M.; Choi, M.S. Antihyperglycemic and Antioxidant Properties of Caffeic Acid in Db/Db Mice. J. Pharmacol. Exp. Ther. 2006, 318, 476–483. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, Z. Preparation and Characterization of Catechin-Grafted Chitosan with Antioxidant and Antidiabetic Potential. Int. J. Biol. Macromol. 2014, 70, 150–155. [Google Scholar] [CrossRef]
- Riyaphan, J.; Pham, D.C.; Leong, M.K.; Weng, C.F. In Silico Approaches to Identify Polyphenol Compounds as α-Glucosidase and α-Amylase Inhibitors against Type-II Diabetes. Biomolecules 2021, 11, 1877. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, X.; Zhang, Y.; Li, R.; Bai, J.; Xiao, H.; Zhang, Y.; Yan, Y.; Jia, F.; Xu, H.; et al. Potential of Different Fractions of Polyphenols in Persimmon Peels: Phenolic Profiles, Bio-Activities, and Mechanism of Inhibition Against α-Glucosidase. Food Chem. 2025, 492, 145373. [Google Scholar] [CrossRef]
- Paun, G.; Neagu, E.; Moroeanu, V.; Albu, C.; Savin, S.; Lucian Radu, G. Chemical and Bioactivity Evaluation of Eryngium planum and Cnicus benedictus Polyphenolic-Rich Extracts. BioMed. Res. Int. 2019, 2019, 3692605. [Google Scholar] [CrossRef] [PubMed]
- Gomathi, S.; Maneemegalai, S. Isolation, Characterization of Β-Sitosterol from the Herbal Extracts and Evaluation of in Vitro Anti-Oxidant and Anti-Diabetic Activities. J. Microbiol. Biotechnol. Food Sci. 2024, 13, e10236. [Google Scholar] [CrossRef]
- Bernal-Gallardo, J.O.; Mena-Violante, H.G.; Luna-Suárez, S. Study of the Phenolic Compounds and Biological Activities of the Wild Fruits of Vaccinium leucanthum Schltdl. Horticulturae 2024, 10, 1091. [Google Scholar] [CrossRef]

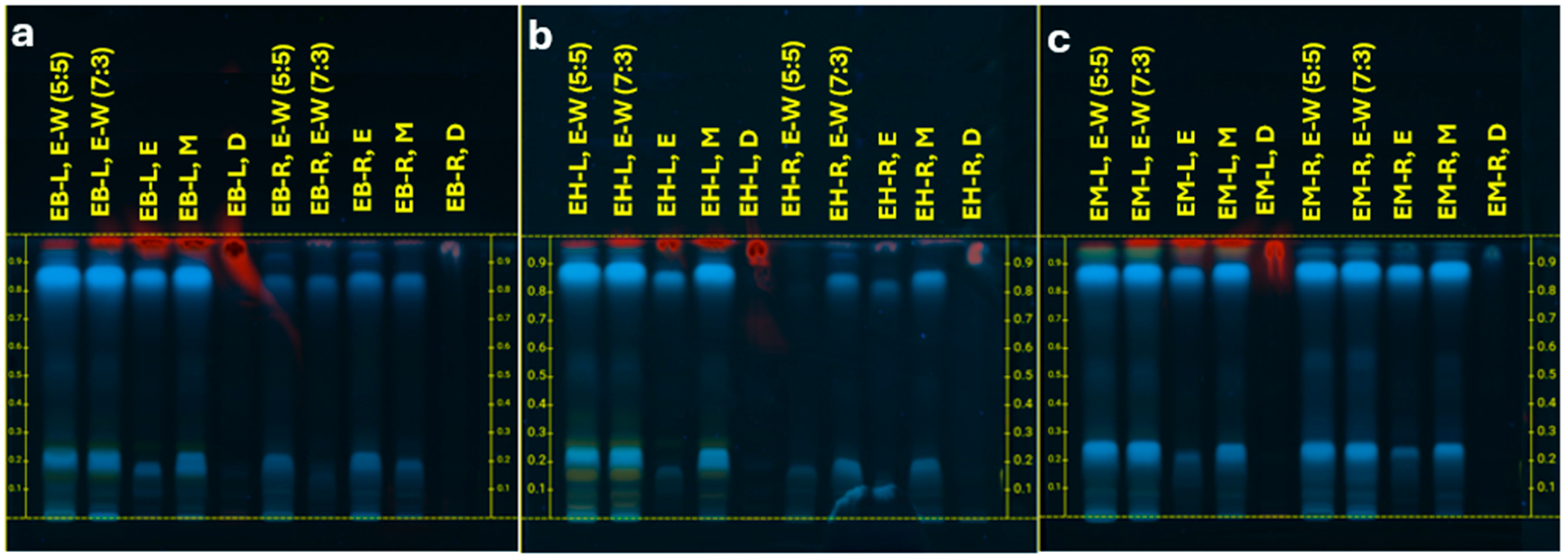

| Species | Rutin | Catechin | ||
|---|---|---|---|---|
| Leaf | Root | Leaf | Root | |
| E. beecheyanum | 241.3 ± 6.3 a | ND | 94.2 ± 0.5 b | 61.3 ± 1.4 a |
| E. heterophyllum | 189.1 ± 5.3 b | ND | 88.3 ± 2.2 c | 41.8 ± 2.4 b |
| E. mexiae | NQ | ND | 137.3 ± 0.3 a | 64.4 ± 2.0 a |
| Species | Ferulic Acid | Gallic Acid | Caffeic Acid | |||
|---|---|---|---|---|---|---|
| Leaf | Root | Leaf | Root | Leaf | Root | |
| E. beecheyanum | 71.1 ± 1.9 b | NQ | 32.5 ± 1.6 a | NQ | 71.3 ± 1.9 a | NQ |
| E. heterophyllum | 105.5 ± 1.3 a | NQ | 31.6 ± 1.0 a | NQ | 37.6 ± 1.3 b | 7.4 ± 0.4 b |
| E. mexiae | 108.7 ± 1.7 a | NQ | 31. 8 ± 1.2 a | 11.3 ± 0.8 a | 68.5 ± 1.7 a | 105.3 ± 0.8 a |
| Species | β-Sitosterol | |
|---|---|---|
| Leaf | Root | |
| E. beecheyanum | 197.5 ± 4.5 b | 394.7 ± 6.5 a |
| E. heterophyllum | 315.9 ± 2.3 a | NQ |
| E. mexiae | 173.9 ± 5.8 c | 93.0 ± 1.4 b |
| Specie | ABTS ●+ 1 | Inhibition % 2 | IC50 3 | DPPH ● 1 | Inhibition % 2 | IC50 3 |
|---|---|---|---|---|---|---|
| E. beecheyanum | 220.0 ± 5.5 a | 71.3 | 20.0 | 101.2 ± 3.0 a | 85.5 | 19.7 |
| E. heterophyllum | 187.0 ± 2.7 b | 64.4 | 21.5 | 46.8 ± 2.8 b | 64.9 | 20.1 |
| E. mexiae | 215.0 ± 6.2 a | 69.5 | 20.7 | 52.6 ± 1.1 b | 67.4 | 22.4 |
| Specie | ABTS ●+ 1 | Inhibition % 2 | IC50 3 | DPPH ● 1 | Inhibition % 2 | IC50 3 |
|---|---|---|---|---|---|---|
| E. beecheyanum | 28.6 ± 3.0 c | 10.0 | 23.2 | 58.0 ± 1.4 b | 68.4 | 23.1 |
| E. heterophyllum | 266 ± 3.1 a | 89.4 | 21.8 | 84.3 ± 3.0 a | 78.2 | 20.7 |
| E. mexiae | 64.4 ± 5.0 b | 21.4 | 22.4 | 88.6 ± 1.4 a | 81.4 | 18.0 |
| Specie | Inhibition in leaf (%) 1 | IC50 2 | Inhibition in root (%) 1 | IC50 2 |
|---|---|---|---|---|
| E. beecheyanum | 37.4 ± 2.1 c | 7.1 | 41.7 ± 1.6 b | 5.8 |
| E. heterophyllum | 26.8 ± 2.9 d | 9.6 | 42.8 ± 2.0 b | 5.8 |
| E. mexiae | 49.1 ± 1.2 b | 5.1 | 42.3 ± 1.6 b | 5.9 |
| Acarbose (CTL+) | 80.4 ± 1.3 a | 2.7 | 80.4 ± 1.3 a | 2.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villa-Santiago, M.; Camacho-Díaz, B.H.; López-Bonilla, A.; Mena-Violante, H.G.; Cárdenas-Valdovinos, J.G.; Ochoa-Cruz, Z.; Angoa-Pérez, M.V. Phytochemical Analysis and In-Vitro Biological Activities of Three Wild Eryngium Species: E. beecheyanum, E. heterophyllum, and E. mexiae. Molecules 2025, 30, 4250. https://doi.org/10.3390/molecules30214250
Villa-Santiago M, Camacho-Díaz BH, López-Bonilla A, Mena-Violante HG, Cárdenas-Valdovinos JG, Ochoa-Cruz Z, Angoa-Pérez MV. Phytochemical Analysis and In-Vitro Biological Activities of Three Wild Eryngium Species: E. beecheyanum, E. heterophyllum, and E. mexiae. Molecules. 2025; 30(21):4250. https://doi.org/10.3390/molecules30214250
Chicago/Turabian StyleVilla-Santiago, Mariana, Brenda Hildeliza Camacho-Díaz, Argelia López-Bonilla, Hortencia Gabriela Mena-Violante, Jeanette Guadalupe Cárdenas-Valdovinos, Zaida Ochoa-Cruz, and María Valentina Angoa-Pérez. 2025. "Phytochemical Analysis and In-Vitro Biological Activities of Three Wild Eryngium Species: E. beecheyanum, E. heterophyllum, and E. mexiae" Molecules 30, no. 21: 4250. https://doi.org/10.3390/molecules30214250
APA StyleVilla-Santiago, M., Camacho-Díaz, B. H., López-Bonilla, A., Mena-Violante, H. G., Cárdenas-Valdovinos, J. G., Ochoa-Cruz, Z., & Angoa-Pérez, M. V. (2025). Phytochemical Analysis and In-Vitro Biological Activities of Three Wild Eryngium Species: E. beecheyanum, E. heterophyllum, and E. mexiae. Molecules, 30(21), 4250. https://doi.org/10.3390/molecules30214250








