2.1. Research Sequence
The study of the impact of irradiation on the chemical yield and reaction rate of volatile organic compound transformations involved six stages (
Figure 1) which are described in the materials and methods section.
At the first stage, 1-hexanol alcohol was diluted in a 0.9% saline solution to the initial concentrations of 50 mg/L and placed in polypropylene 2 mL Eppendorf tubes. At the second stage, a part of the 1-hexanol solution was irradiated with 1 MeV electrons and the other part was exposed to 80 keV bremsstrahlung irradiation. The irradiation dose and the dose rate applied to the hexanol solution varied to observe the dynamics of 1-hexanol decomposition and accumulation of VOCs resulting from 1-hexanol decomposition. The 50 mg/L 1-hexanol solutions were irradiated with accelerated electrons with the doses ranging from 100 Gy to 1200 Gy at the dose rates of 1 Gy/s and 10 Gy/s, while X-ray irradiation was performed with the doses ranging from 100 Gy to 2000 Gy at the dose rate of 0.2 Gy/s and with the doses ranging from 100 Gy to 8000 Gy at the dose rate of 2 Gy/s.
The third stage involved measuring the doses absorbed by the 1-hexanol solution during electron beam irradiation and X-ray irradiation using Fricke dosimetry solution. At the fourth stage, we performed Geant4 computer simulation to control the dose uniformity in the irradiated solutions. Geant4-DNA computer simulation was carried out to compare the contribution of direct ionization and indirect action through free radicals during e-beam irradiation and X-ray irradiation.
Stage five, which followed immediately after irradiation with e-beam or X-rays, involved GC-MS analysis to measure the concentration of 1-hexanol and its decomposition products in irradiated and non-irradiated 1-hexanol solutions. The impact of the irradiation dose and the dose rate on the hexanol decomposition rate and accumulation rate of 1-hexanol decomposition products was estimated separately for e-beam and X-ray irradiated solutions. We also compared the 1-hexanol decomposition rate after the 1-hexanol solution was irradiated with e-beam and X-rays at similar dose rate values. At the sixth stage, we found the key quantitative indicator of the extent of 1-hexanol decomposition using a mathematical model of the dynamics of VOCs and mechanisms behind radiation-induced VOCs transformations in the irradiated solutions calculated based on density functional theory (DFT) using Priroda04 software.
2.2. The Impact of Irradiation Dose and the Dose Rate on the Transformation of 1-Hexanol into Its Degradation Products
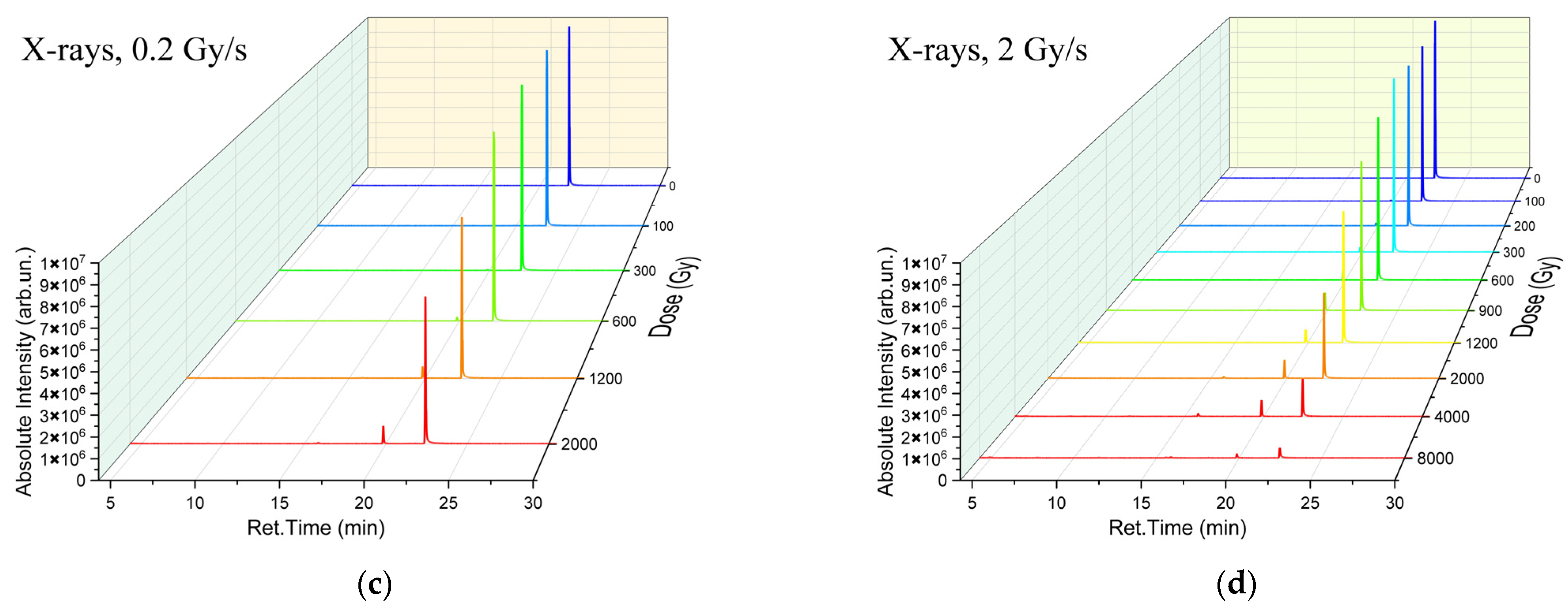
To illustrate the impact of accelerated electrons and X-rays on the concentration of 1-hexanol and VOCs which appeared as a result of 1-hexanol degradation in the irradiated suspensions we have provided the chromatograms of the non-irradiated 1-hexanol suspension as well as the suspensions irradiated with accelerated electrons (
Figure 2a,b) and X-rays (
Figure 2c,d) at different doses and dose rates. As it can be seen from
Figure 2a–d, both irradiation types decreased the concentration of 1-hexanol and led to the formation of 1-hexanol degradation products.
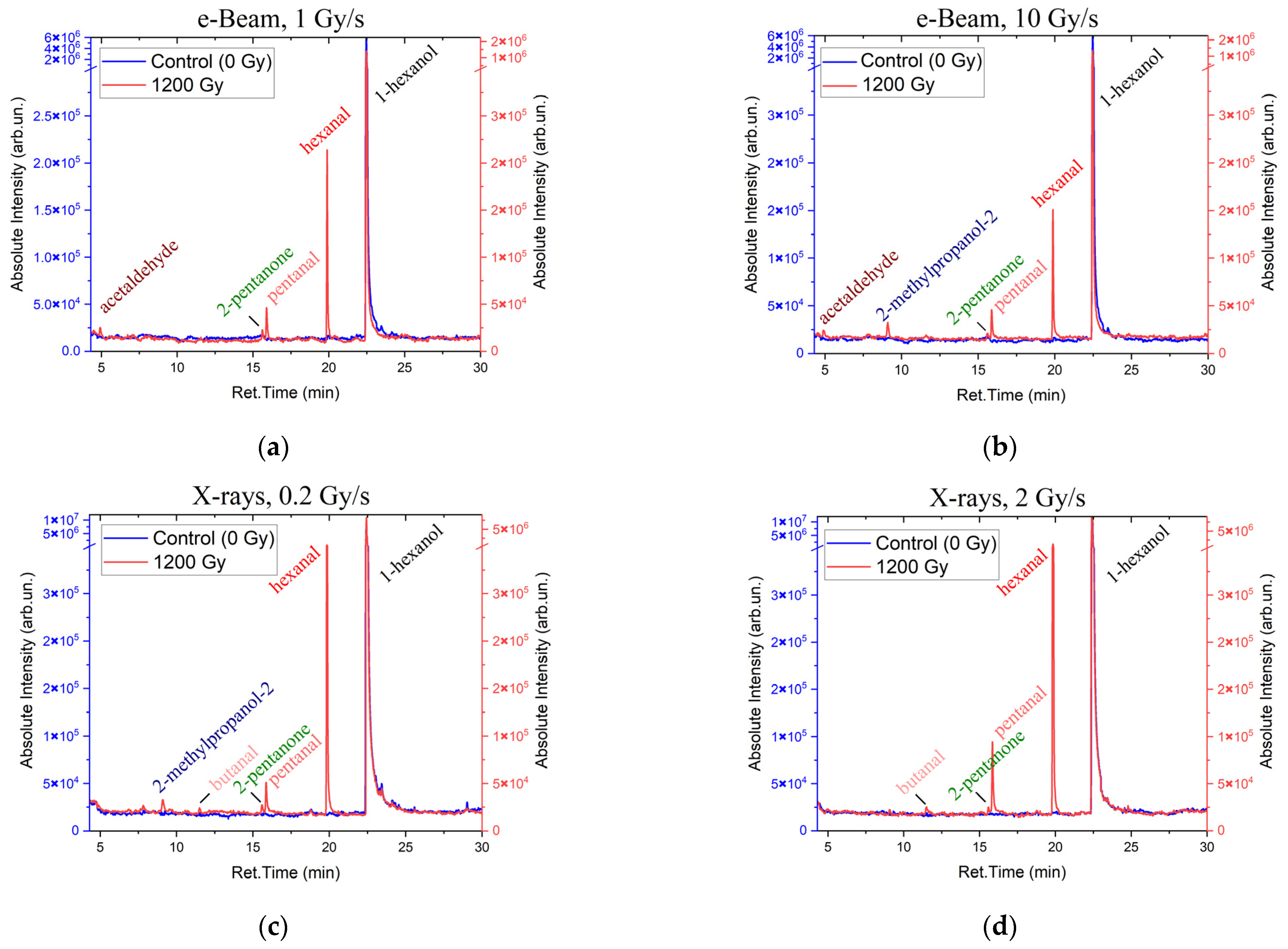
To compare the concentrations of 1-hexanol and the VOCs which appeared as a result of 1-hexanol degradation in the suspensions irradiated at different dose rates,
Figure 3 shows the 1-hexanol suspension irradiated with the dose of 1200 Gy at the dose rates 1 Gy/s (
Figure 3a) and 10 Gy/s (
Figure 3b) for electrons and 0.2 Gy/s (
Figure 3c) and 2 Gy/s (
Figure 3d) for X-ray irradiation as well as the non-irradiated 1-hexanol suspensions.
A total of six compounds were identified in the 1-hexanol suspension irradiated with accelerated electrons; alcohols: 1-hexanol and 2-methylpropanol-2; a ketone: 2-pentanone; and aldehydes: acetaldehyde, pentanal, and hexanal. In the samples irradiated with X-rays, six compounds were also identified in the 1200 Gy suspensions, and ten compounds were detected at the dose of 8000 Gy; alcohols: 1-hexanol and 2-methylpropanol-2; ketones: acetone, 2-butanone, and 2-pentanone; and aldehydes: acetaldehyde, propanal, butanal, pentanal, and hexanal. All identified compounds, their molecular weights, retention times, and concentrations are given in
Table A1,
Table A2,
Table A3 and
Table A4 in
Appendix A. It can be assumed that the higher the dose the more VOCs appearing as a result of 1-hexanol degradation were identified in the 1-hexanol solution.
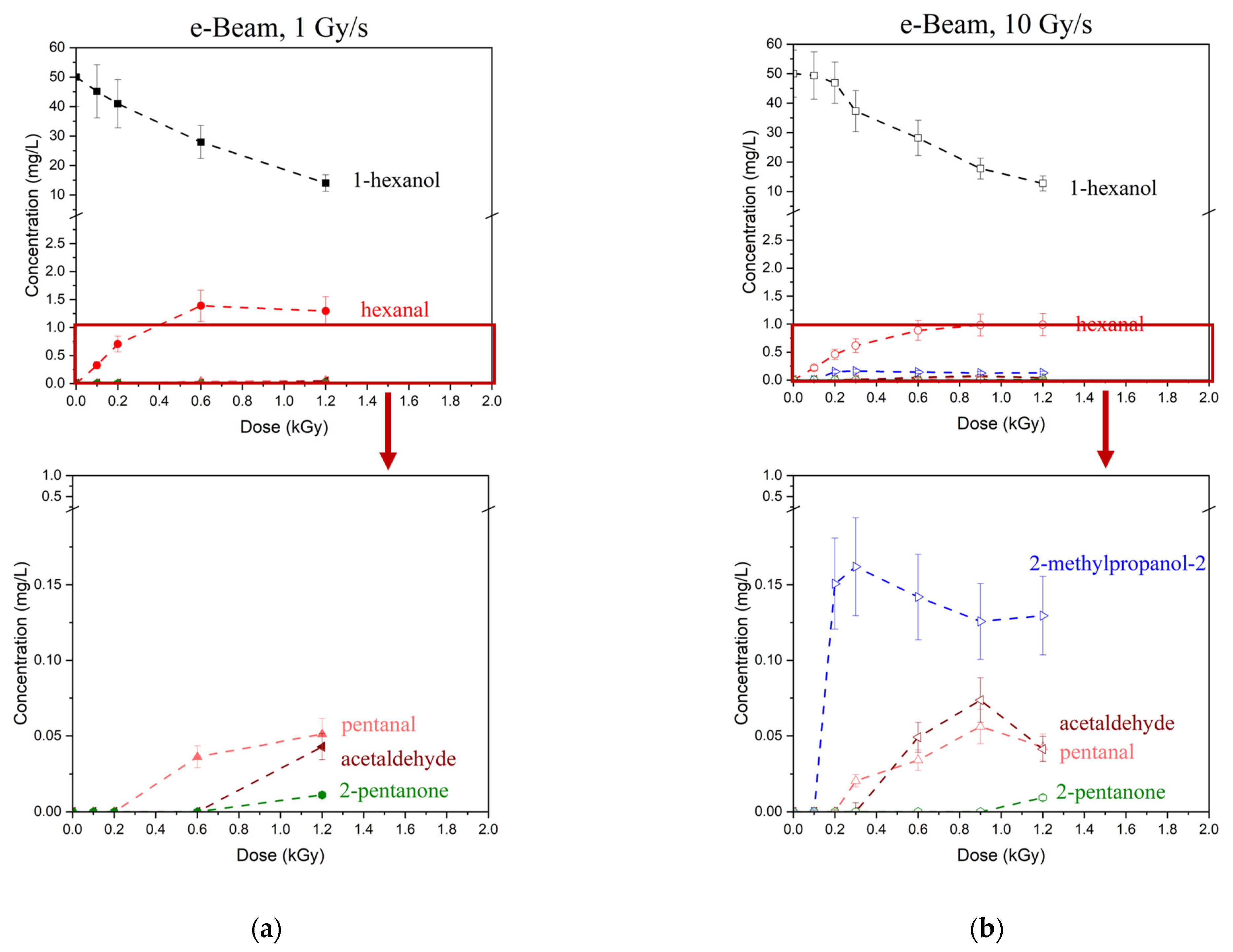
Figure 4a–d shows the dependencies of 1-hexanol concentration on the irradiation dose and the dependencies of the concentrations of VOCs formed due to the decomposition of 1-hexanol. While most VOCs were present in irradiated 1-hexanol solutions in the marginal concentration of less than 1 mg/L, hexanal was the main contributor to the radiation–chemical yield of 1-hexanol molecules. It should be noted that the irradiation with doses up to 2000 Gy increased the hexanal concentration; a further increase in the dose, however, decreased the hexanal concentration. An initial increase in the concentration of 1-hexanol degradation products with a higher dose range followed by the decrease in their concentration is an indication of two competing processes: the accumulation of degradation products due to 1-hexanol decomposition as well as the decomposition of degradation products as a result of irradiation.
After irradiation most VOCs were detected with a certain threshold dose D
T exceeding 100 Gy, while hexanal was identified at 100 Gy and higher. An increase in the dose rate decreased the threshold dose D
T for most VOCs identified in 1-hexanol after irradiation with both accelerated electrons and X-rays. These results align with the study showing that the degradation pathways of benzene and dienes under the action of irradiation strongly depend on the dose rate [
54].
To compare the impact of accelerated electrons and X-rays on the decomposition rate of hexanol and hexanal accumulation rate in hexanol solutions irradiated with the doses ranging from 100 Gy to 2000 Gy at dose rates ranging from 0.2 Gy/s to 10 Gy/s we performed a two-way and a three-way ANOVA test. The test showed a significant difference between the 1-hexanol decomposition rates in the solutions irradiated with X-ray irradiation and electron irradiation (p-value = 0.00027). The comparison of the 1-hexanol decomposition rate under the action of e-beam and X-ray irradiation at close dose rates, equal to 1 Gy/s for electrons and 2 Gy/s for X-rays, showed that the 1-hexanol decomposition rate was significantly higher (p-value = 0.011) for e-beam irradiation compared to X-rays at the doses ranging from 300 Gy to 2000 Gy.
The accumulation rate of hexanal was significantly higher (p-value = 0.006) when the 1-hexanol solution was irradiated with X-rays compared to e-beam and depended on the dose rate for doses ranging from 300 Gy to 2000 Gy. Interestingly, while a ten-fold increase in the X-ray dose rate led to a marginal increase in the hexanal accumulation rate (p-value = 0.10), no significant difference was observed for electron beam irradiation between the dose rates of 1 and 10 Gy/s (p-value = 0.49) in the dose range of 300 Gy to 1000 Gy.
2.3. Absorbed Dose Distribution, Linear Energy Transfer (LET) Distribution and Radiation–Chemical Yield of Free Radicals in Water Irradiated with Electron Beam and X-Rays
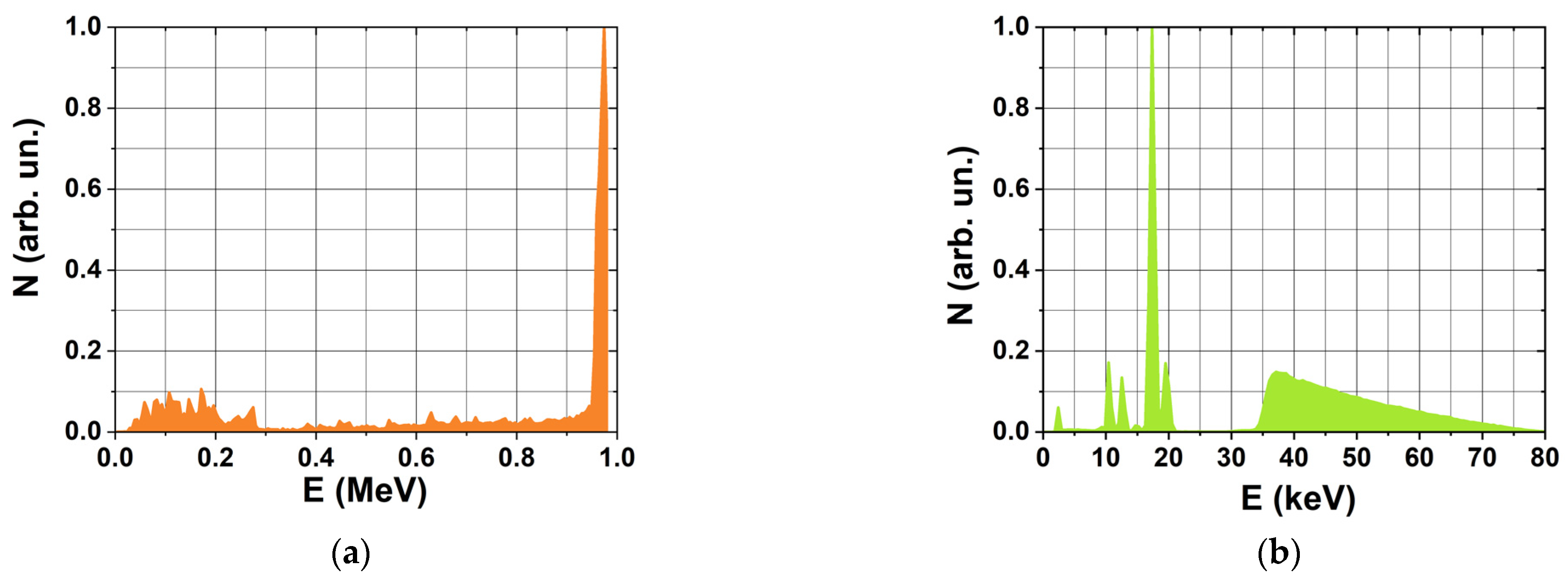
To assess the contribution of direct ionization and indirect action of irradiation to radiation-induced transformations of hexanol, we performed Geant4 and Geant4-DNA computer simulation which is used to calculate distributions of dosimetry values and radiation–chemical yield of free radicals for e-beam irradiation and X-ray irradiation. The simulation uses energy spectra of accelerated electrons (
Section 3.2) and X-rays (
Section 3.2) as well as the linear dimensions of polypropylene tubes filled with hexanol solutions, specifically for each irradiation method.
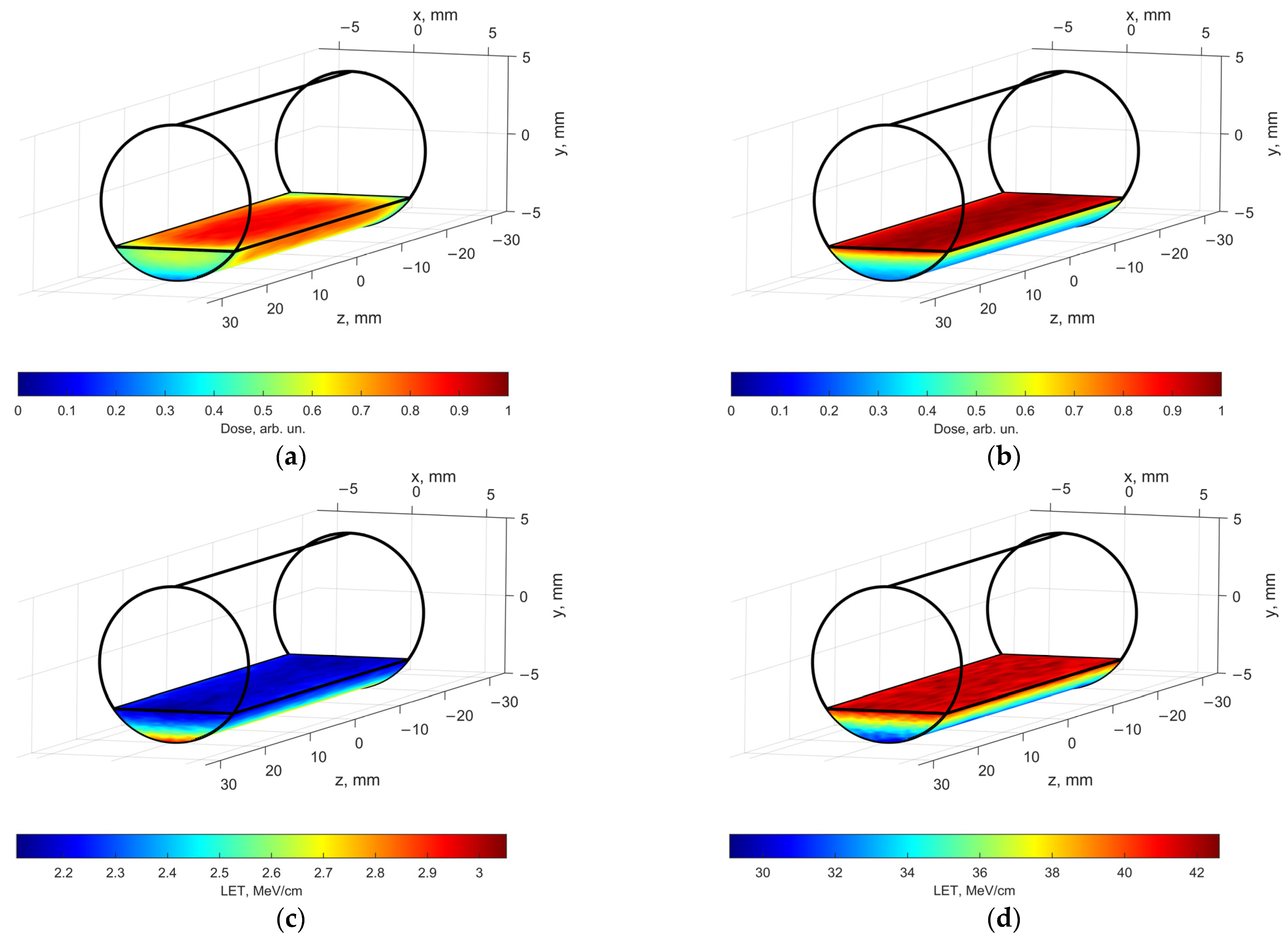
Figure 5 shows the calculated spatial absorbed dose distributions generated by a 1 MeV electron beam (
Figure 5a) and by 80 keV X-rays (
Figure 5b) in a 2 mm thick water phantom simulating a solution placed in a polypropylene cylindrical Eppendorf tube. The geometry of the phantom corresponds to the geometry of the solution in a 2 mL Eppendorf tube.
When the water phantom is irradiated with 1 MeV accelerated electrons (
Figure 5a), the dose uniformity, which is the ratio of the minimum to the maximum dose, is 0.3 in the entire volume of the phantom with most of the volume having the dose uniformity of 0.6. Similarly, when the water phantom is irradiated with 80 keV X-ray photons (
Figure 5b), the dose uniformity is slightly under 0.3. Compared with accelerated electrons, the absorbed dose generated by X-rays decreases more sharply with depth, which is associated with an exponential weakening of the radiation fluence when passing through the water phantom.
Linear energy transfer (LET) values averaged over the absorbed energy in the water phantom irradiated with accelerated electrons (
Figure 5c) are much lower (~2.2–3.0 MeV/cm) compared with LET of secondary electrons formed as a result of the interaction of X-rays (
Figure 5d) with the water phantom (up to 42 MeV/cm). In the case of X-ray irradiation, more ionization events are formed per unit path of secondary electrons passing in the water than per unit path of 1 MeV primary electrons.
Despite the dose non-uniformity and the different nature of the interaction of electrons and X-rays with water, the use of water solutions provides partial equalization of concentrations of free radicals occurring as a result of water radiolysis due to diffusion and convection, especially when linear dimensions of the volume are only several milliliters.
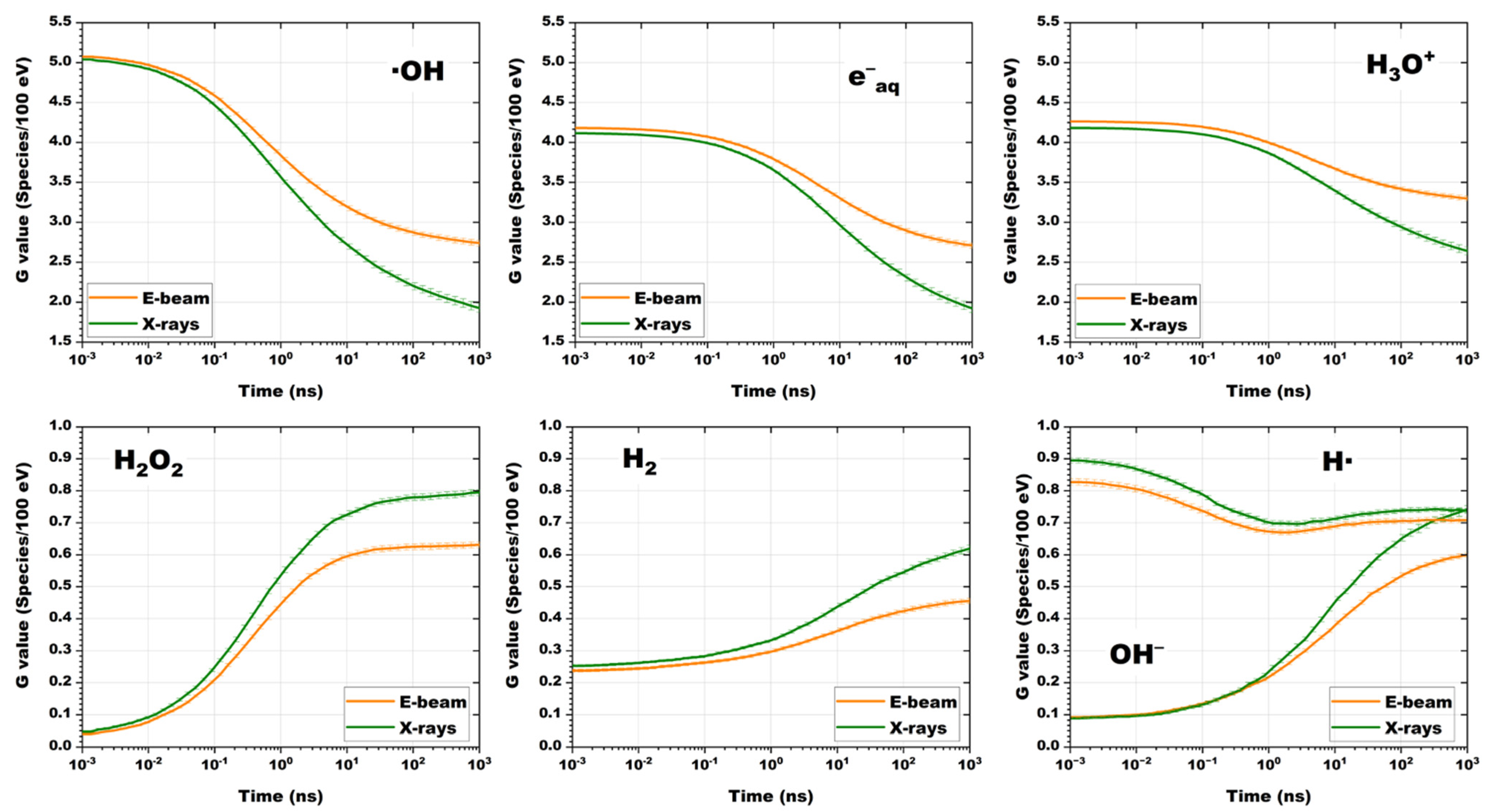
The calculated time dependencies of the radiation–chemical yield of free radicals, G (species/100 eV), caused by the exposure to 1 MeV accelerated electrons and 80 keV X-ray photons are shown in
Figure 6.
It can be seen from
Figure 6 that when the water is exposed to 1 MeV accelerated electrons and 80 keV bremsstrahlung photons, the changes in the radiation–chemical yield of radicals over time have similar dynamics. The radiation–chemical yield of •OH, e
−aq, H
3O
+ and H• radicals for both types of irradiations have close values after exposure during 10
−3–1 ns. As the time passes, from 1 ns to 10
3 ns, the G-values in X-ray irradiated water decrease faster than in the water irradiated with accelerated electrons, which is probably due to the rapid recombination of radicals in areas of high ionization density. The rate of accumulation of H
2O
2, H
2 and OH
− during X-ray irradiation is higher, especially during the time exceeding 10 ns, which can be due to high LET values which can lead to frequent “radical-radical reactions”, such as •OH + •OH → H
2O
2 and H• + H• → H
2.
According to
Figure 6, the calculated G-values (species/100 eV) for all radicals registered at 10
3 ns are:
The calculated G-values of various radicals when the water is exposed to accelerated electrons fully correspond to similar experimental values, practically do not depend on the dose rate, and are within the measurement errors described in [
58], which indicates the reliability of the simulation. It can be concluded that the type of irradiation and its energy spectrum determine not only the spatial dose distribution, but also the spatial distribution of water radiolysis products. These differences should be taken into account when interpreting the results of radiation–chemical transformations of 1-hexanol and its decomposition products presented in this work.
The formation of radicals is a very rapid process occurring during irradiation and ranging from a few nanoseconds to a few microseconds. Since degradation products of hexanol were detected in hexanol solution immediately after irradiation, it can be concluded that all transformations of hexanol and its degradation products occurred during irradiation.
2.4. Comparison of Radiation-Induced Degradation Efficiency of 1-Hexanol After Electron Beam Irradiation and X-Ray Irradiation
To compare the impact of 1 MeV accelerated electrons and 80 keV X-rays at different dose rates on the decomposition rate of hexanol, the degradation efficiency of 1-hexanol ε was calculated as follows:
where D (Gy) is the irradiation dose, C
H(0) and C
H(D) (mg/L) are the initial concentration of 1-hexanol and the concentration of 1-hexanol after irradiation with the dose D, respectively.
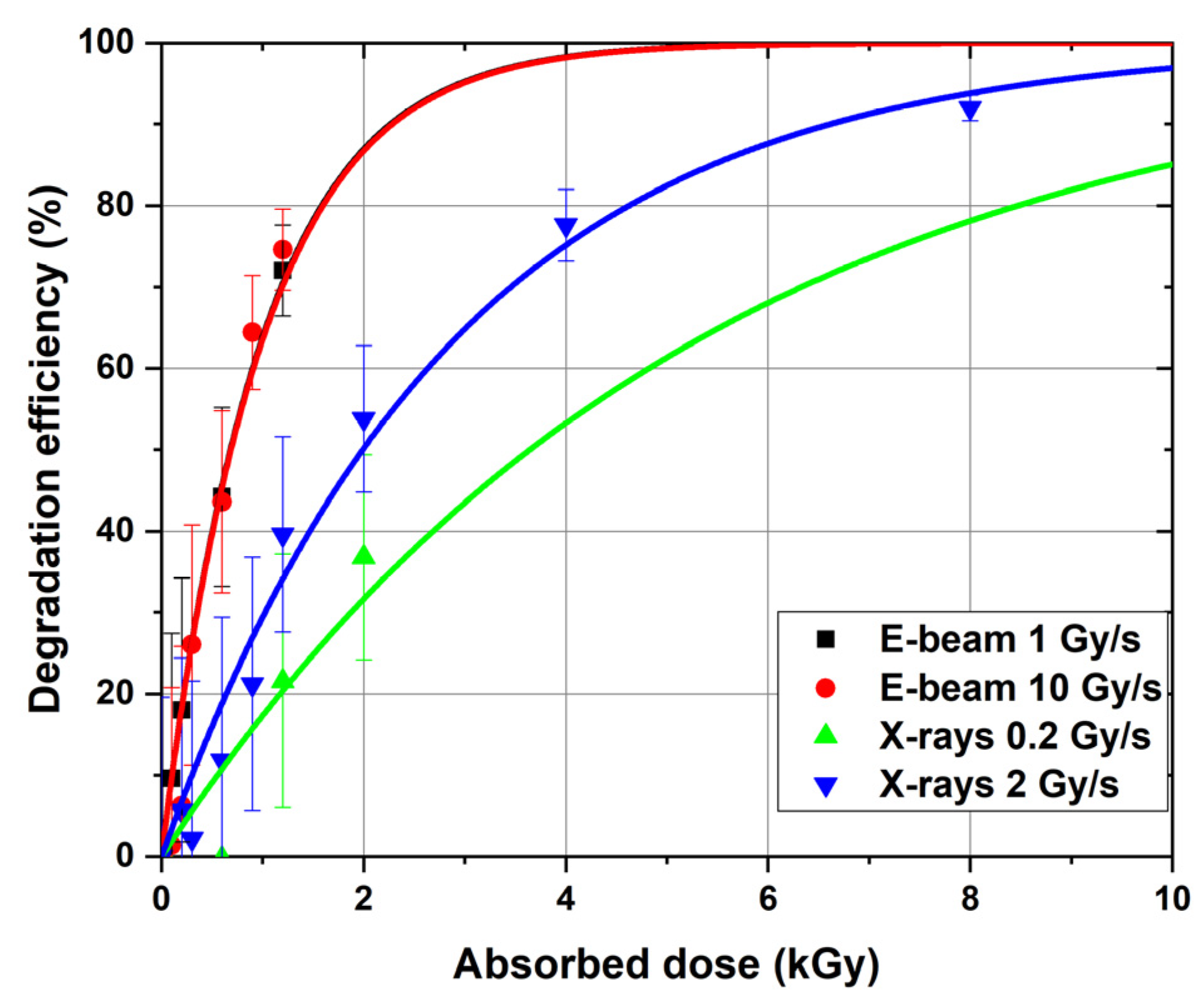
Figure 7 compares the degradation efficiency of 1-hexanol irradiated with accelerated electrons and X-rays at different dose rates.
Figure 7 shows that when 1-hexanol is irradiated with accelerated electrons, high degradation efficiency (>70%) is achieved at the dose of 1200 Gy. At the same time, there is practically no difference (
p-value = 0.49) between the dose rates of 1 and 10 Gy/s. In the case of X-ray irradiation at the dose rate of 2 Gy/s, the degradation efficiency of 1-hexanol is more than 70% only at 4 kGy, which is three times less efficient than that observed for accelerated electrons. It should be noted that there is a marginal effect (
p-value = 0.10) of dose rate on hexanol degradation.
The fact that practically no difference between the hexanol degradation efficiency in the solution irradiated at the dose rates ranging from 0.2 Gy/s to 10 Gy/s was observed is consistent with the observations of other authors stating that the radiation–chemical yield of reactive oxygen species in 4% albumin solutions under the action of e-beam irradiation at the dose rate ranging from 1.4 Gy/s to 8.7 Gy/s practically does not depend on the dose rate and is within the measurement error [
59]. On the other hand, the study of the effect of dose rate on the release of radicals e
−aq, H
2O
2, etc. in water irradiated 230 MeV protons shows a decrease in G-value with an increase in the dose rate from 0.1 to 50 Gy/s [
60]. On the contrary, another recent study of the effect of gamma irradiation dose rate on ethanol, methanol, and NaBr solutions shows an increase in the radiation yield of H
2O
2 with an increase in the dose rate from 3.2 Gy/s to 0.2 Gy/s [
42]. Therefore, the effect of the dose rate on degradation of chemical compounds in water solutions as a result of irradiation depends not only on the type of irradiation but also on the extent to which the dose rates are different.
To quantify the effect of electrons and X-rays on hexanol degradation, the experimental dependencies of degradation efficiency on the dose can be approximated by the following function:
where
k is the hexanol decomposition rate, indicating how much hexanol is decomposed per unit of the absorbed dose. The G-values are calculated after irradiation of the hexanol solution with the dose of 1200 Gy and the decomposition rate
k for e-beam and X-rays at different dose rates are shown in
Table 1.
As it can be seen, irradiation of 1-hexanol with accelerated electrons showed a higher radiation–chemical yield compared to X-ray irradiation: G
1200Gy = 2.81–2.91 molecules/100 eV (e-beam) and G
1200Gy = 0.84–1.54 molecules/100 eV (X-rays). The comparison of radiation–chemical yield of VOCs during 1 MeV electron beam irradiation and 80 keV photons at the close dose rates shows that the degradation efficiency of 1-hexanol is higher for electrons than that for X-ray irradiation which can be explained by higher radiation–chemical yield for some radicals, such as OH●, e
−aq and H
3O
+ as well as the different nature of the interaction of electrons and X-rays with water, different dose distribution, and linear energy transfer distribution. These results generally align with G-values for aerated Fricke dosimetry solution showing that the G-value 15.5 ± 0.2 ions/100 eV, which was measured in the dosimetry solution irradiated with electrons, is higher than the G-value 14.7 ± 0.3 ions/100 eV for photons [
61,
62].
The analysis of studies on the radiation–chemical yield of aliphatic alcohols and their derivatives shows that G values vary from 0.1 to 3.2 molecules/100 eV, depending on the type of irradiation, dose rate initial concentrations of alcohols in aqueous solutions, and aeration with oxygen [
63,
64,
65,
66,
67]. For instance, in [
67], the authors irradiated ethanol in the water solution and revealed that radiation–chemical yield (G) of acetaldehyde, which is the main decomposition product of ethanol, increased from 1.2 to 3.2 molecules/100 eV with the increase in the initial ethanol concentration from 1% to 95%, which generally coincides with the findings presented in this article.
2.5. 1-Hexanol Decomposition Mechanisms Triggered by Irradiation
Since irradiation transfers energy in discrete portions, volatile molecules break down into smaller structures as they rearrange as a result of the breaking of chemical bonds. The extent of molecular transformations depends on the bonding energy and molecular structure of the compound as well as the physical parameters of irradiation, such as particle flux density, energy spectrum, dose uniformity, the linear energy transfer values, as well as the spatial distribution of water radiolysis products. The difference in irradiation parameters between e-beam and X-rays, including energy spectrum, explains why the same VOCs identified in 1-hexanol solution behave differently when the same irradiation dose is applied.
During a 1 MeV electron beam irradiation primary electrons and secondary electrons ejected from the matter can produce direct ionization of the matter. Direct ionization of 1-hexanol molecules, further referred to as the Ionizing Reaction (IR), caused by primary and secondary electrons when irradiated with accelerated electrons (e−), initiate the breaking of chemical bonds. 1 MeV accelerated electrons can also trigger electron–photon cascades causing secondary electrons to occur and contribute to direct ionization of the matter. Similarly, when exposed to X-rays (γ), secondary electrons occurring as a result of the Compton effect break chemical bonds causing IR. Secondary electrons can cause bremsstrahlung photons to occur; thus, triggering electron–photon cascades. All secondary electrons contribute to direct ionization of the matter. The presence of oxygen causes additional oxidation of 1-hexanol molecules, further referred to as the Oxidation Reaction (OR).
Another mechanism of 1-hexanol degradation in water solutions is indirect action of irradiation occurring in water and involving radiolysis products. The intensity of indirect action of irradiation going through water radiolysis products, such as hydroxyl radical •OH, hydrated e
−aq, and hydrogen atom H• with 1-hexanol molecules or its degradation products, known as Radical Reaction (RR), is determined by the number of free radicals as well as the diffusion coefficients of radicals and the molecular structure of the compounds. Radical reactions play a major role in the transformations of molecules in aqueous environments [
68]. Both IR with/without OR and RR lead to the formation of new low-molecular-weight compounds, which are probabilistic in nature and can be described using logistic functions [
5].
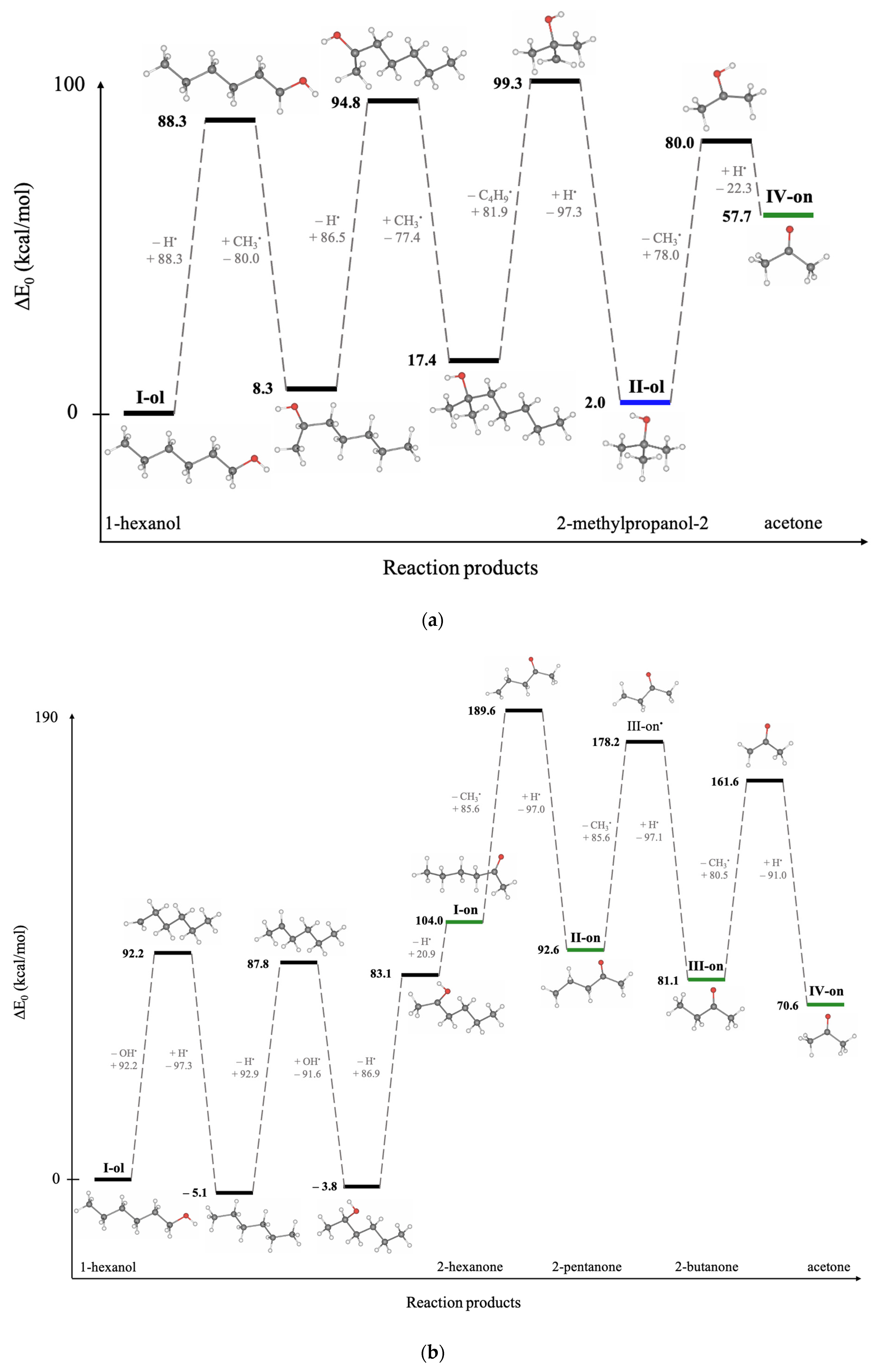
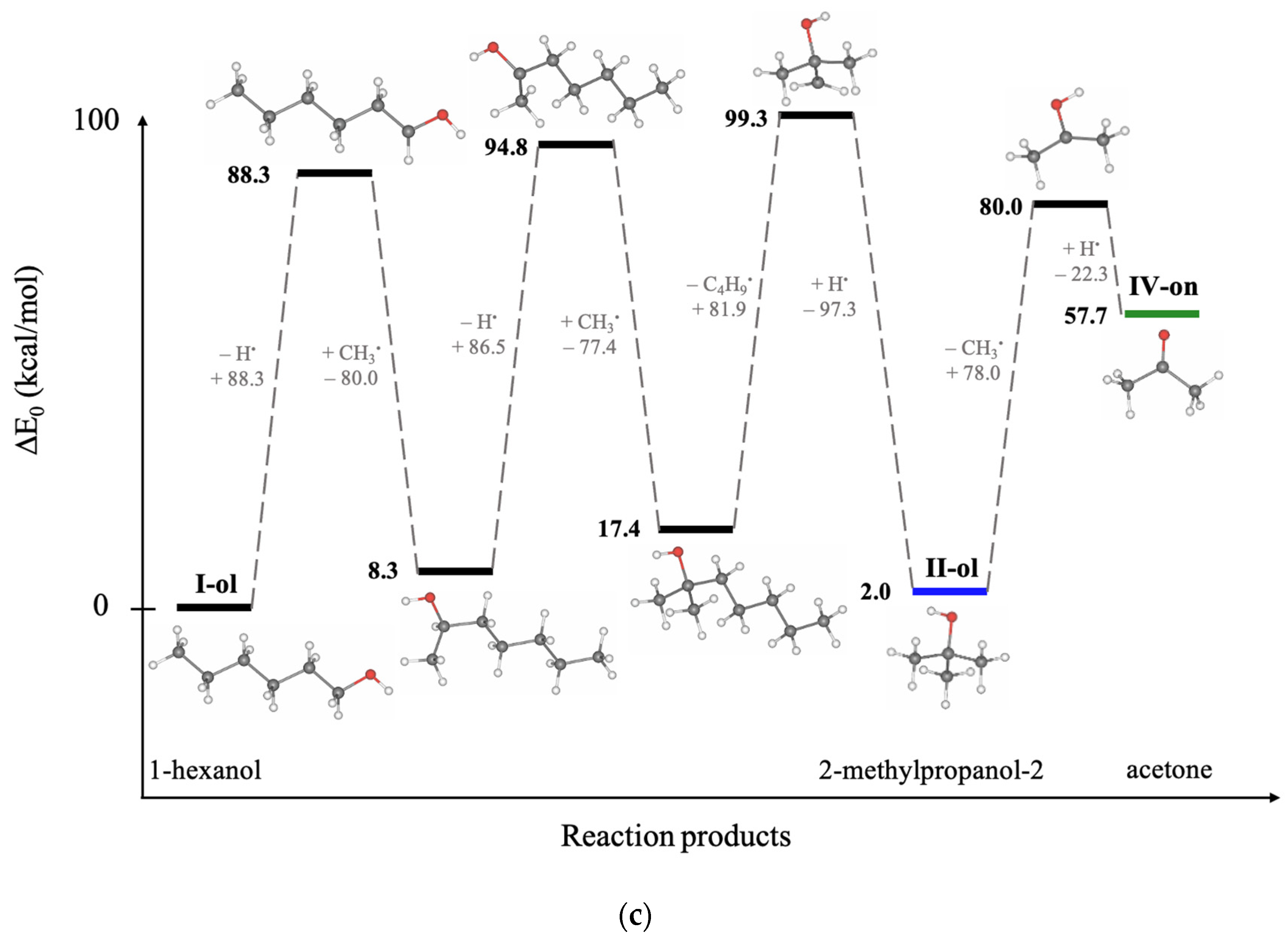
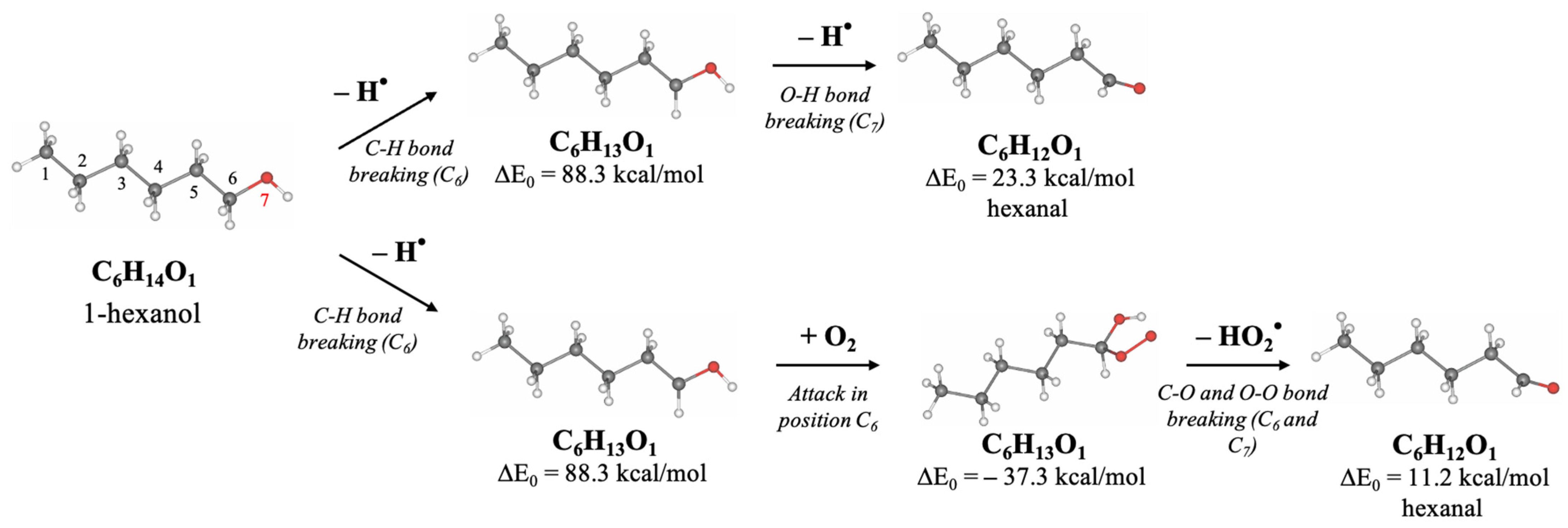
Since hexanol undergoes different radiation-induced chemical reactions resulting in various decomposition products, Density Functional Theory (DFT) involving thermodynamic calculations of radical reactions, oxidation reactions, and direct ionization was used to explore the most probable mechanisms behind the formation of 1-hexanol decomposition products. To find the degradation products which are most likely to occur in the 1-hexanol solution as a result of irradiation, we have examined the thermodynamic values of the bond energies E (kcal/mol) and the bond energies involving the energies of molecular vibrational motions E
0 (kcal/mol), whose rupture leads to the formation of intermediate and final decomposition products identified in the hexanol solution, as well as the Gibbs energies G (kcal/mol) and the structures of all identified volatile organic compounds. All energies and the molecular structures of the 1-hexanol decomposition products calculated using DFT method are given in
Table A5, in
Appendix A.
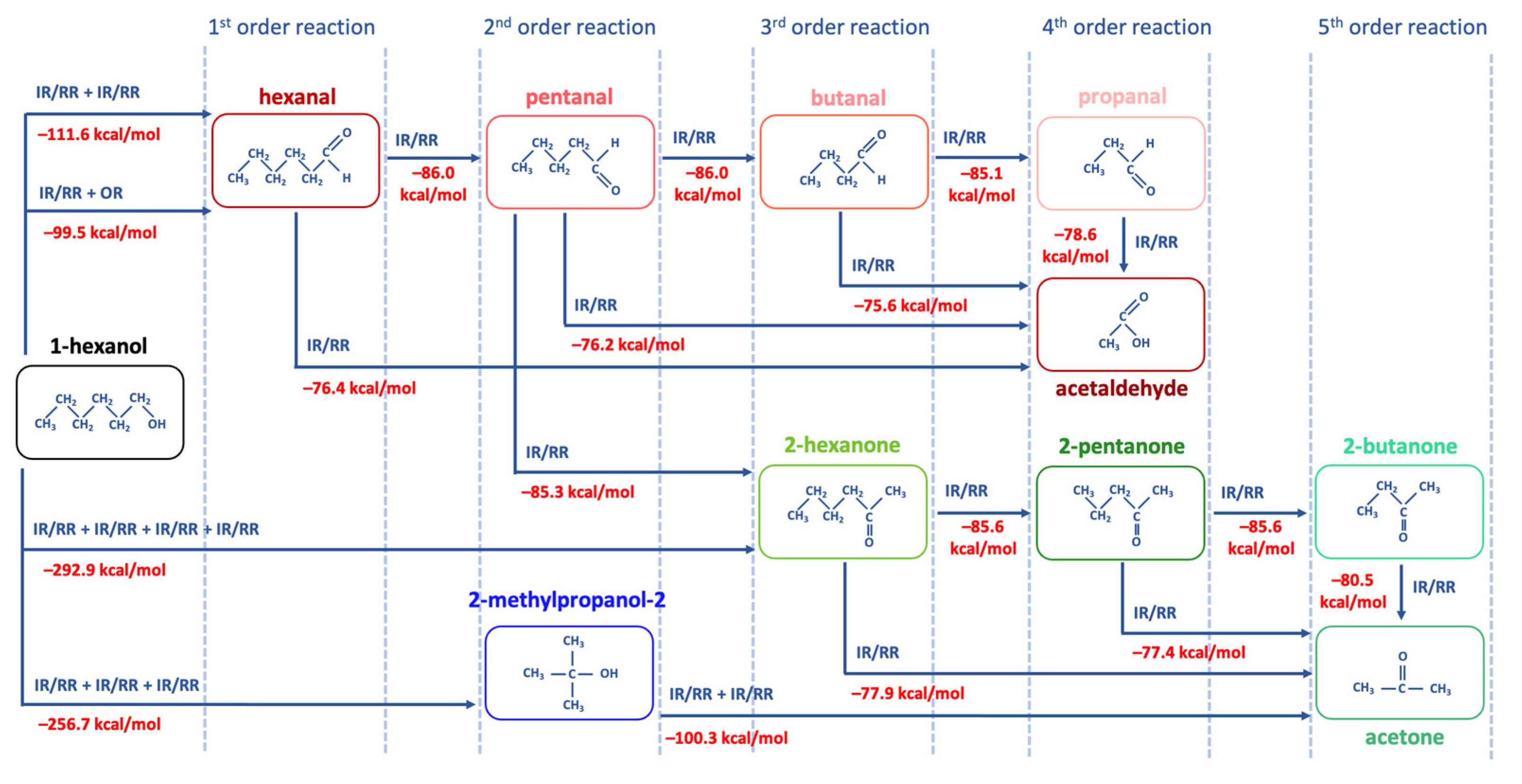
Figure 8 below illustrates the structures of 1-hexanol secondary decomposition products identified in the 1-hexanol solution and the energy ∆E
0 used to form secondary decomposition products; ∆E
0 is the difference between the energy of the identified compound and the one preceding it taking into account all potential intermediate compounds. The detailed DFT calculations of the radiation-induced reactions for the conversion of 1-hexanol into degradation products are shown in
Figure A1, in
Appendix A.
Since hexanal is the main contributor to the radiation–chemical yield of 1-hexanol decomposition, the other VOCs, whose formation is more complex in nature as they undergo multiple transformations due to direct and indirect ionization, are secondary oxidation products of 1-hexanol.
DFT calculations performed to determine the most probable ways of the 1-hexanol transformations to decomposition products identified in the 1-hexanol solution after irradiation show that the probable transformation of 1-hexanol to hexanal occurs by breaking C–H and C–O bonds involving different ionization types:
- 2.
Indirect Ionizing Reaction—Radical Reaction (RR):
- 3.
IR/RR and subsequent Oxidation Reaction (OR):
Figure 9 illustrates the proposed mechanism of hexanal formation from 1-hexanol due to irradiation. The initial compound 1-hexanol uses a structural formula where carbon atoms are numbered from 1 to 6. At first, the cleavage of C–H bonds occurs either through direct ionization or radiation-induced hydrogen atom (H•) abstraction. After that, as the concentrations of ions or radicals in the medium increase, further abstraction of a hydrogen atom can occur, leading to the formation of hexanal. An alternative pathway involves a radical reaction with molecular oxygen (O
2), followed by conversion to hexanal through the formation of a peroxyl radical and a hydroperoxide intermediate.
This mechanism corresponds to the typical process of radiation-induced peroxidation of saturated alcohols, fatty acids, and alkanes in aqueous or oxygen-rich environments. The mechanism includes the initiation of radical reactions, their propagation through chain processes, and the formation of stable oxidation products, particularly aldehydes such as hexanal, which are often used as markers of oxidative degradation [
69,
70,
71].
The energies and the formation pathways of other degradation products of 1-hexanol are presented in
Figure A1, in
Appendix A.
IR or RR involves the detachment of two hydrogen atoms H● one after the other, whereby ∆E0 = 111.6 kcal/mol is expended. In the presence of oxygen O2, the IR/RR reactions are followed by the OR, which lowers the energy to ∆E0 = 99.5 kcal/mol, increasing the probability of this reaction. Secondary oxidation products of 1-hexanol, however, require more energy due to a greater number of ionization events. Thus, to obtain a 2-hexanone ketone, it is necessary to break four bonds, expending ∆E0 = 292.9 kcal/mol, whereas the formation of alcohol 2-methypropanol-2 requires ∆E0 = 256.7 kcal/mol.
The transformation pathways outlined in
Figure 8 show that each compound formed can be categorized into groups based on the order of their formation. The first group includes reactions of the first kind, which involve the formation of hexanal from 1-hexanol through OR and/or RR/IR. The second group consists of compounds formed by the radical or ionizing reaction of hexanal (e.g., pentanal) or those that have more complex production pathways directly from 1-hexanol (e.g., 2-methylpropanol-2). The VOCs of the third group, such as butanal and 2-hexanone and the fourth and fifth group, such as acetaldehyde, propanal, 2-pentanone, 2-butanone, and acetone can be a daughter product of the first and the second groups or the decomposition product of primary 1-hexanol molecules. The analysis of mechanisms behind irradiation-induced transformations of 1-hexanol reveals that hexanal is a high-probability decomposition marker of 1-hexanol since, unlike other VOCs, hexanal requires only two ionization events, either two direct ionizations or two radicals H
●/OH
●, or one direct ionization or a radical reaction combined with oxidation.
2.6. Mathematical Model Describing VOCs Transformations Under Irradiation
For quantitative estimation of the radiation–chemical yield of low-molecular-weight compounds and molecule transformations under irradiation, we propose a mathematical model describing the dynamics of the transformation of 1-hexanol into hexanal. Since irradiation of the 1-hexanol solution caused 1-hexanol concentration to decrease while forming other secondary VOCs, the summary concentration of the resultant low-molecular-weight compounds can be expressed as:
where D (Gy) is the irradiation dose, C
H(0) and C
H(D) (mg/L) are the initial concentration of 1-hexanol, and the concentration of 1-hexanol after irradiation with the dose D, respectively, ∑C
VOCs (mg/L) is the sum of the VOCs concentrations formed as a result of 1-hexanol degradation.
Since secondary VOCs were found in different concentrations in 1-hexanol solutions irradiated with different doses, the share of VOCs appearing as a result of 1-hexanol degradation in the total amount of 1-hexanol molecules was estimated as shown in
Figure 10 using the following formula:
where
A(D) is the share of 1-hexanol degradation VOCs identified in the 1-hexanol irradiated with the dose D; C
i(D) (mg/L) are the concentrations of VOCs
i = 1 (hexanal),
i = 2 (2-methylpropanol-2),
i = 3 (pentanal), etc., in the 1-hexanol irradiated with dose D.
As can be seen from
Figure 10, the share of VOCs decreases exponentially with an increase in the irradiation dose, which can be expressed using the following formula:
where
A0 is the initial relative concentration of 1-hexanol molecules in the non-irradiated suspension,
B is the rate at which the share of 1-hexanol degradation VOCs decrease with a higher dose.
The fact that the share of total VOCs identified as a result of 1-hexanol degradation in the total amount of 1-hexanol molecules is under 100% suggests that some VOCs, such as oxygen, water, carbon dioxide, formaldehyde, etc., could not be identified since their molecular weight is beyond the range of m/z values 33–350 which can be registered using the given scan mode:
where ∑C
VOCs and ∑C′
VOCs are the sum of the concentrations of the identified and non-identified VOCs, respectively.
The exponential decrease in the concentration of 1-hexanol
CH with an increase in the dose (
Figure 4a–d) can be described by the following differential Equation (7):
where
kH (Gy
−1) is the decomposition rate of 1-hexanol molecules and
= 50 mg/L is the 1-hexanol concentration in the non-irradiated suspension. The solution of Equation (7) shows that
kH reflects the dose at which the concentration of 1-hexanol decreases by
e times:
The non-linear dependency of hexanal concentration on the irradiation dose after X-ray irradiation with a wide dose range (
Figure 4d) is a clear sign of two competing processes—the accumulation of hexanal as a result of 1-hexanol decomposition and the decomposition of hexanal as a result of irradiation. The differential equation which describes a change in the hexanal concentration with an increase in the irradiation dose can be expressed as follows:
where
Ch (mg/L) is the concentration of hexanal identified in the irradiated 1-hexanol suspension,
kh (Gy
−1) is the decomposition rate of hexanal,
AHh(D) is the share of 1-hexanol molecules which transform to hexanal in the total amount of 1-hexanol molecules and
Ch(0) is the concentration of hexanal in the non-irradiated 1-hexanol solution.
Taking into account that the share of hexanal
AHh(D) formed due to 1-hexanol degradation in the total amount of decomposed 1-hexanol molecules decreased exponentially with an increase in the irradiation dose Equation (5), the following dose dependency of the hexanal concentration was obtained from Equation (9):
Figure 11a,b shows the experimental dependencies and dependencies of the concentrations of 1-hexanol and hexanal in the 1-hexanol solutions irradiated with e-beam and X-rays at different dose rates calculated using Equations (8) and (10).
As can be seen from
Figure 11, accelerated electrons led to a more considerable degradation of 1-hexanol and its main degradation product hexanal compared to X-ray irradiation, which can be attributed to a higher ionization density throughout the whole volume of 1-hexanol solution and a higher dose rate during e-beam irradiation.
Table 2 shows the values of the coefficients
,
kH,
kh,
A, and
B calculated using Equations (8) and (10) for 1-hexanol and hexanal identified in the 1-hexanol solutions irradiated with accelerated electrons and X-rays at different dose rates. The order of magnitude differences in the decomposition rate of both compounds under different types of irradiations indicate a more significant influence of electron irradiation on volatile compounds compared to X-ray irradiation. The fact that the decomposition rates of 1-hexanol and hexanal are similar in order of magnitude is a clear sign that the volatile organic compounds from different classes with similar chemical structure and molecular weight have a similar decomposition mechanism as a result of irradiation.
The formation of the other VOCs, consisting of multiple transformations of 1-hexanol secondary decomposition products with concentration C
j can be expressed as follows:
In this formula C
j (mg/L) is the concentration of compound
j, C
i (mg/L) is the concentration of compound
i formed from compound
j;
kj and
ki (Gy
−1) are the decomposition rates of compound
i and
j, respectively, and
Aij(D) is the share of transition of compound
j to compound
i in the total amount of compound
j molecules taking into account the threshold dose D
T at which compound
i was identified. The solution to Equation (11) can be represented as:
Considering that the compound i can not only occur as a result of 1-hexanol decomposition but can also be a daughter product of 1-hexanol decomposition VOCs and solving the equation requires a great number of input parameters which are hard to identify, further research of the behavior of these compounds is not deemed feasible. Hexanal dose-dependent behavior, however, has proved to be easy to trace and estimate which makes hexanal a quantitative marker of irradiation dose-induced decomposition of 1-hexanol. The application of Equation (10) approximating the experimental dose dependency for hexanal allows to determine the quantitative parameters of dose-dependent 1-hexanol. Knowing the initial concentration of 1-hexanol, it is possible to determine the extent at which 1-hexanol molecules decompose after irradiation with a certain dose.
Having analyzed the results of DFT calculations, we elaborated an alternative approach based on radiobiological target theory which is believed to be a more efficient for describing the dose-dependent behavior of the secondary 1-hexanol decomposition products. DFT calculations show that the formation of decomposition products requires two to five ionization events depending on the type of decomposition product ensuing the appearance of certain compounds which can be identified at the dose exceeding the threshold dose D
T. The dose behavior of these compounds can thus be described using a multi-hit dose–response model [
72,
73,
74]. According to this model, the probability of the appearance of a certain compound can be estimated as follows:
where
n is the number of ionizing events, further referred to as hits, triggering the formation of a certain compound, whereas λ is the formation rate of a certain compound per unit dose. Using this approach, dose dependencies for the 1-hexanol decomposition products can be represented as shown in
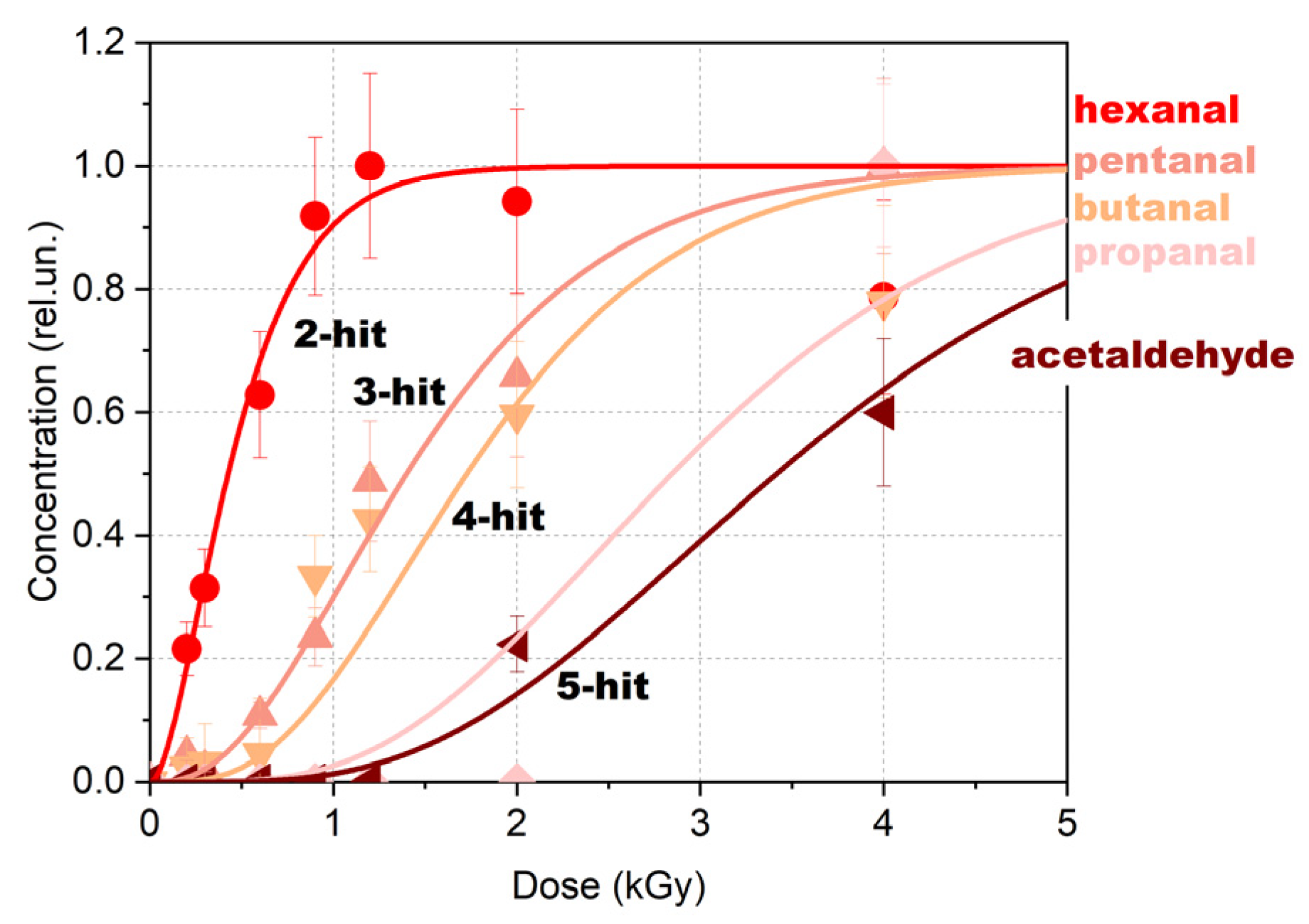
Figure 12, which shows the number of hits required for the formation of a certain compound as per DFT calculations.
As can be seen from
Figure 12, an increase in the number of hits causes a transition from an exponential dose dependency to a sigmoidal one that highlights the threshold dose at which a certain compound is identified in the 1-hexanol solution. For example, while hexanal formation requires two hits leading to an exponential dependency of the hexanal concentration on the irradiation dose, acetaldehyde requires five hits which determines a sigmoidal dose dependency. The multi-hit dose response-based approach described in this section has proved the validity of the mathematical model provided below.