3.2. Hemi-Synthesis Reactions
All reactions were followed by TLC or FTIR.
3.2.1. Procedure for Obtaining Compound 1
Previously dried, commercially obtained (+)-catechin (4 g, 13.78 mmol, 1 equivalent) was dissolved in anhydrous acetone (80 mL). Acetic anhydride (5.63 g, 55.12 mmol, 4 equivalents) and then Et3N (5.58 g, 55.12 mmol, 4 equivalents) were added dropwise. The mixture was stirred at room temperature overnight. Acetone was evaporated under low pressure and CH2Cl2 (100 mL) was added. The organic phase was washed with saturated NaHCO3 (25 mL) until neutral pH of the aqueous phase obtained. Organic phase was dried on anhydrous MgSO4 and the solvent was evaporated under low pressure. The residue has been purified by recrystallization in water.
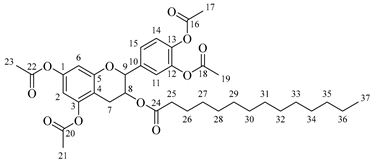
![Molecules 30 04223 i001 Molecules 30 04223 i001]()
4-(5,7-Diacetoxy-3-hydroxychroman-2-yl)-1,2-phenylene diacetate (1). C23H22O10. Light orange solid. Yield: 96% (6.06 g). 1H-NMR (400 MHz, DMSO-d6): δ (ppm) = 2.20 (s, 3H, H-17), 2.24 (s, 6H, H-19, H-23), 2.26 (s, 3H, H-21), 2.48 (dd, J = 16, 9 Hz, 1H, H-7α), 2.74 (dd, J = 16, 5 Hz, 1H, H-7β), 3.92–4.00 (m, 1H, H-8), 4.83 (d, J = 8 Hz, 1H, H-9), 5.37 (d, J = 5 Hz, H-24), 6.56 (d, J = 2 Hz, 1H, H-2), 6.58 (d, J = 2 Hz, 1H, H-6), 7.23–7.33 (m, 3H, H-11, H-14, H15). 13C-NMR (100 MHz, DMSO-d6): δ (ppm) = 20.7 (C-17), 20.9 (C-19, C-23), 21.2 (C-21), 28.5 (C-7), 65.5 (C-8), 80.9 (C-9), 107.8 (C-6), 109.1 (C-2), 112.5 (C-4), 122.8 (C-15), 123.8 (C-14), 126.0 (C-11), 138.0 (C-10), 142.1 (C-13), 142.2 (C-12), 149.7 (C-1, C-3), 155.0 (C-5), 168.8 (C-16), 169.1 (C-18, C-22), 169.5 (C-20). IR: ν (cm−1) = 1760 (C=O acetyl), 3512 (-OH aliphatic).
3.2.2. Procedure for Obtaining Compounds 2a–2d
Compound 1 (0.5 g, 1.09 mmol, 1 equivalent) and one equivalent of fatty acid were dissolved in 10 mL of anhydrous THF or anhydrous Me-THF. Concentrated H2SO4 (50 μL, 0.92 mmol) has been added and the mixture has been agitated in a microwave at 120 °C for 10 min using a power of 100 W. Ethyl acetate (100 mL) has been added and washed successively with 25 mL of saturated NaHCO3 until neutral pH of the aqueous phase and with 25 mL of saturated NaCl. The organic phase was dried over anhydrous MgSO4, filtered and evaporated.
![Molecules 30 04223 i002 Molecules 30 04223 i002]()
4-(5,7-Diacetoxy-3-(octanoyloxy)chroman-2-yl)-1,2-phenylene diacetate (2a). C31H36O11. Whitish solid. Yield: 78% (0.497 g). 1H-NMR (400 MHz, acetone-d6): δ (ppm) = 0.88 (t, J = 7 Hz, 3H, H-31), 1.26–1.38 (ma, 8H, H-27-30), 1.59 (q, J = 7 Hz, 2H, H-26), 2.23 (s, 3H, H-17), 2.27 (s, 6H, H-19, H-23), 2.28 (t, J = 7 Hz, 2H, H-25), 2.30 (s, 3H, H-21), 2.57–2.65 (m, 1H, H-7α), 2.88–2.95 (dd, J = 16, 5 Hz, 1H, H-7β), 4.06–4.14 (m, 1H, H-8), 4.85 (d, J = 8 Hz, 1H, H-9), 6.55 (d, J = 2 Hz, 1H, H-2), 6.57 (d, J = 2Hz, 1H, H-6), 7.23–7.41 (ma, 3H, H-11, H-14, H-15). 13C-NMR (100 MHz, CDCl3): δ (ppm) = 14.0 (C-31), 20.6 (C-17), 20.7 (C-19, C-23), 21.0 (C-21), 22.6 (C-30), 24.7 (C-7), 28.2 (C-29), 28.9 (C-28), 29.0 (C-27), 31.6 (C-26), 33.9 (C-25), 67.6 (C-8), 81.2 (C-9), 107.8 (C-2), 108.7 (C-6), 111.7 (C-4), 122.4 (C-15), 123.7 (C-14), 125.6 (C-11), 136.7 (C-10), 149.5 (C-13), 149.7 (C-12), 155.0 (C-1, C-3), 168.4 (C-16), 168.5 (C-18, C-22), 168.6 (C-20), 170.3 (C-24). IR: ν (cm−1) = 1764 (C=O acetyl), 1740 (C=O ester).
![Molecules 30 04223 i003 Molecules 30 04223 i003]()
4-(5,7-Diacetoxy-3-(dodecanoyloxy)chroman-2-yl)-1,2-phenylene diacetate (2b). C35H44O11. Whitish solid. Yield: 84% (0.586 g). 1H-NMR (400 MHz, CDCl3): δ (ppm) = 0.81 (t, J = 7 Hz, 3H, H-35), 1.17–1.31 (ma, 8H, H-27-34), 1.56 (q, J = 7 Hz, 2H, H-26), 2.19 (s, 3H, H-17), 2.21 (s, 6H, H-19, H-23), 2.23 (s, 3H, H-21), 2.27 (t, J = 7 Hz, 2H, H-25), 2.52–2.59 (m, 1H, H-7α), 2.86–2.94 (dd, J = 16, 6 Hz, 1H, H-7β), 3.84–3.92 (m, 1H, H-8), 4.48 (d, J = 8 Hz, 1H, H-9), 6.53 (d, J = 2 Hz, 1H, H-2), 6.57 (d, J = 2Hz, 1H, H-6), 7.09–7.28 (ma, 3H, H-11, H-14, H-15). 13C-NMR (100 MHz, CDCl3): δ (ppm) = 14.1 (C-35), 20.6 (C-17), 20.7 (C-19, C-23), 21.0 (C-21), 22.6 (C-34), 24.7 (C-7), 28.1 (C-33), 29.0 (C-32), 29.2 (C-31), 29.3 (C-30), 29.4 (C-29), 29.6 (C28-27), 31.9 (C-26), 33.8 (C-25), 67.6 (C-8), 81.1 (C-9), 107.8 (C-2), 108.7 (C-6), 111.7 (C-4), 122.3 (C-15), 123.4 (C-14), 125.5 (C-11), 136.6 (C-10), 142.2 (C-13), 142.3 (C-12), 149.7 (C-1), 155.0 (C-3), 168.3 (C-16), 168.4 (C-18), 168.5 (C-22), 169.0 (C-20), 170.1 (C-24). IR: ν (cm−1) = 1763 (C=O acetyl), 1740 (C=O ester).
![Molecules 30 04223 i004 Molecules 30 04223 i004]()
4-(5,7-Diacetoxy-3-(tetradecanoyloxy)chroman-2-yl)-1,2-phenylene diacetate (2c). C37H48O11. Whitish solid. Yield: 85% (0.619 g). 1H-NMR (400 MHz, CDCl3): δ (ppm) = 0.80 (t, J = 7 Hz, 3H, H-37), 1.16–1.31 (ma, 10H, H-27-36), 1.55 (q, J = 7 Hz, 2H, H-26), 2.20 (s, 3H, H-17), 2.22 (s, 6H, H-19, H-23), 2.23 (s, 3H, H-21), 2.28 (t, J = 7 Hz, 2H, H-25), 2.52–2.60 (m, 1H, H-7α), 2.86–2.94 (dd, J = 16, 6 Hz, 1H, H-7β), 3.85–3.93 (m, 1H, H-8), 4.49 (d, J = 8 Hz, 1H, H-9), 6.54 (d, J = 2 Hz, 1H, H-2), 6.58 (d, J = 2Hz, 1H, H-6), 7.10–7.27 (ma, 3H, H-11, H-14, H-15). 13C-NMR (100 MHz, CDCl3): δ (ppm) = 14.0 (C-37), 20.7 (C-17), 20.9 (C-19, C-23), 21.0 (C-21), 22.4 (C-36), 24.6 (C-7), 28.2 (C-35), 29.1 (C-34), 29.2 (C-33), 29.3 (C-32), 29.4 (C-31), 29.5 (C-30), 29.6 (C29-27), 31.9 (C-26), 33.7 (C-25), 67.5 (C-8), 81.3 (C-9), 107.9 (C-2), 108.6 (C-6), 111.6 (C-4), 122.4 (C-15), 123.4 (C-14), 125.6 (C-11), 136.7 (C-10), 142.1 (C-13), 142.3 (C-12), 149.6 (C-1), 155.1 (C-3), 168.2 (C-16), 168.4 (C-18), 168.5 (C-22), 169.0 (C-20), 170.3 (C-24). IR: ν (cm−1) = 1763 (C=O acetyl), 1741 (C=O ester).
![Molecules 30 04223 i005 Molecules 30 04223 i005]()
4-(5,7-Diacetoxy-3-(hexadecanoyloxy)chroman-2-yl)-1,2-phenylene diacetate (2d). C39H52O11. Whitish solid. Yield: 83% (0.630 g). 1H-NMR (400 MHz, CDCl3): δ (ppm) = 0.81 (t, J = 7 Hz, 3H, H-39), 1.12–1.31 (ma, 2H, H-27-38), 1.56 (q, J = 7 Hz, 2H, H-26), 2.21 (s, 3H, H-17), 2.22 (s, 6H, H-19, H-23), 2.24 (s, 3H, H-21), 2.28 (t, J = 7 Hz, 2H, H-25), 2.51–2.64 (m, 1H, H-7α), 2.89–2.96 (dd, J = 16, 5 Hz, 1H, H-7β), 3.86–3.94 (m, 1H, H-8), 4.65 (d, J = 8 Hz, 1H, H-9), 6.48 (d, J = 2 Hz, 1H, H-2), 6.53 (d, J = 2Hz, 1H, H-6), 7.09–7.30 (ma, 3H, H-11, H-14, H-15). 13C-NMR (100 MHz, CDCl3): δ (ppm) = 14.1 (C-39), 20.6 (C-17), 20.7 (C-19, C-23), 21.0 (C-21), 22.7 (C-38), 24.7 (C-7), 29.0 (C-37), 29.2 (C-36), 29.3 (C-35), 29.4 (C-34), 29.5 (C-33), 29.6 (C-32), 29.7 (C27-31), 31.9 (C-26), 33.7 (C-25), 67.7 (C-8), 81.1 (C-9), 107.8 (C-2), 108.8 (C-6), 111.7 (C-4), 122.4 (C-15), 123.7 (C-14), 125.6 (C-11), 136.7 (C-10), 149.5 (C-13), 149.7 (C-12), 155.0 (C-1, C-3), 168.4 (C-16), 168.5 (C-18, C-22), 168.6 (C-20), 178.0 (C-24). IR: ν (cm−1) = 1764 (C=O acetyl), 1741 (C=O ester).
3.2.3. Procedure for Obtaining Compounds 3a–3d
l-alanine ethyl ester (1 g, 6.51 mmol, 1 equivalent) and one equivalent of fatty acid have been dissolved in 15 mL of anhydrous THF or anhydrous Me-THF. The mixture has been stirred in a microwave at 120 °C for 10 min using a power of 100 W. A white solid has then been filtrated and washed with 20 mL of anhydrous THF or MeTHF.
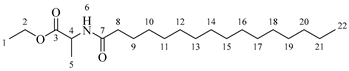
Ethyl octanoylalaninate (3a). C13H25NO3. White solid. Yield: 93% (1.472 g). 1H-NMR (400 MHz, CDCl3): δ (ppm) = 0.82 (t, J = 7 Hz, 3H, H-14), 1.11–1.29 (ma, 11H, H-10-13, H-1), 1.32 (d, J = 7 Hz, 3H, H-5), 1.48–1.63 (ma, 2H, H-9), 2.13 (t, J = 7 Hz, 2H, H-8), 4.13 (q, J = 7 Hz, 2H, H-2), 4.51 (m, J = 7 Hz, 1H, H-4), 6.03 (s, 1H, H-6). 13C-NMR (100 MHz, DMSO-d6): δ (ppm) = 14.1 (C-1, C-14), 17.3 (C-5), 22.7 (C-13), 25.6 (C-9), 28.6 (C-10, C-11), 31.8 (C-12), 36.5 (C-8), 52.1 (C-4), 61.3 (C-2), 171.5 (C-3), 173.9 (C-7). IR: ν (cm−1) = 1649 (C=O amide), 1738 (C=O ester), 3317 (-NH- amide).
![Molecules 30 04223 i007 Molecules 30 04223 i007]()
Ethyl dodecanoylalaninate (3b). C17H33NO3. White solid. Yield: 92% (1.792 g). 1H-NMR (400 MHz, CDCl3): δ (ppm) = 0.74 (t, J = 7 Hz, 3H, H-18), 1.05–1.17 (ma, 19H, H-10-17, H-1), 1.19 (d, J = 7 Hz, 3H, H-5), 1.38–1.51 (ma, 2H, H-9), 2.05 (t, J = 7 Hz, 2H, H-8), 3.98 (q, J = 7 Hz, 2H, H-2), 4.26 (m, J = 7 Hz, 1H, H-4), 7.13 (s, 1H, H-6). 13C-NMR (100 MHz, DMSO-d6): δ (ppm) = 14.3 (C-1, C-14), 17.5 (C-5), 23.1 (C-17), 25.2 (C-9), 28.6 (C-10), 28.9 (C-11), 29.3 (C-15), 29.6 (C-12-14), 31.9 (C-16), 36.6 (C-8), 52.0 (C-4), 61.3 (C-2), 172.0 (C-3), 173.9 (C-7). IR: ν (cm−1) = 1647 (C=O amide), 1738 (C=O ester), 3315 (-NH- amide).
![Molecules 30 04223 i008 Molecules 30 04223 i008]()
Ethyl tetradecanoylalaninate (3c). C19H37NO3. White solid. Yield: 95% (2.024 g). 1H-NMR (400 MHz, CDCl3): δ (ppm) = 0.81 (t, J = 7 Hz, 3H, H-20), 1.12–1.29 (ma, 23H, H-10-19, H-1), 1.32 (d, J = 7 Hz, 3H, H-5), 1.49–1.64 (ma, 2H, H-9), 2.13 (t, J = 7 Hz, 2H, H-8), 4.13 (q, J = 7 Hz, 2H, H-2), 4.51 (m, J = 7 Hz, 1H, H-4), 5.96 (s, 1H, H-6). 13C-NMR (100 MHz, DMSO-d6): δ (ppm) = 14.0 (C-1, C-20), 17.1 (C-5), 22.5 (C-19), 25.9 (C-9), 28.6 (C-10), 28.9 (C-11), 29.5 (C-17), 29.9 (C-12-16), 32.5 (C-18), 37.5 (C-8), 52.2 (C-4), 62.7 (C-2), 170.2 (C-3), 174.2 (C-7). IR: ν (cm−1) = 1648 (C=O amide), 1738 (C=O ester), 3319 (-NH- amide).
![Molecules 30 04223 i009 Molecules 30 04223 i009]()
Ethyl hexadecanoylalaninate (3d). C21H41NO3. White solid. Yield: 94% (2.174 g). 1H-NMR (400 MHz, CDCl3): δ (ppm) = 0.83 (t, J = 7 Hz, 3H, H-22), 1.10–1.34 (ma, 27H, H-10-21, H-1), 1.35 (d, J = 7 Hz, 3H, H-5), 1.35–1.49 (ma, 2H, H-9), 2.09 (t, J = 7 Hz, 2H, H-8), 4.25 (q, J = 7 Hz, 2H, H-2), 4.43 (m, J = 7 Hz, 1H, H-4), 6.54 (s, 1H, H-6). 13C-NMR (100 MHz, DMSO-d6): δ (ppm) = 13.9 (C-1, C-22), 17.5 (C-5), 23.1 (C-21), 25.2 (C-9), 28.8 (C-10), 29.3 (C-11), 29.5 (C-19), 29.8 (C-10-18), 31.5 (C-20), 36.7 (C-8), 53.6 (C-4), 61.7 (C-2), 171.2 (C-3), 173.7 (C-7). IR: ν (cm−1) = 1644 (C=O amide), 1735 (C=O ester), 3315 (-NH- amide).
3.2.4. Procedure for Obtaining Compounds 4a–4d
Alanine–fatty acid coupling (1 g) has been dissolved in a solution of five equivalents of NaOH in 90 mL of a mixture of MeOH/H2O (9:1). After two hours of magnetic agitation at room temperature, a white precipitate has been formed. The round bottom flask has been placed in an ice bath and five equivalents of HCl 1 M solution has been added until the white precipitate has formed again. The methanol has been evaporated and the rest of the solution has been lyophilized to obtain the saponification product.
![Molecules 30 04223 i010 Molecules 30 04223 i010]()
Octanoylalanine (4a). C11H21NO3. Yellowish liquid. Yield: 93% (0.822 g). 1H-NMR (400 MHz, CDCl3): δ (ppm) = 0.80 (t, J = 7 Hz, 3H, H-13), 1.11–1.33 (ma, 8H, H-9-12), 1.39 (d, J = 7 Hz, 3H, H-4), 1.51–1.62 (ma, 2H, H-8), 2.17 (t, J = 7 Hz, 2H, H-7), 4.51 (m, J = 7 Hz, 1H, H-3), 6.01 (s, 1H, H-5). 13C-NMR (100 MHz, DMSO-d6): δ (ppm) = 14.1 (C-13), 17.0 (C-4), 22.7 (C-12), 25.6 (C-8), 28.6 (C-9-10), 31.9 (C-11), 36.5 (C-7), 51.3 (C-3), 173.9 (C-6), 174.7 (C-2). IR: ν (cm−1) 1644 (C=O amide), 1703 (C=O acid), 3227 (-NH- amide), 3308 (-OH acid).
![Molecules 30 04223 i011 Molecules 30 04223 i011]()
Dodecanoylalanine (4b). C15H29NO3. White solid. Yield: 92% (0.833 g). 1H-NMR (400, MHz, CDCl3): δ (ppm) = 0.80 (t, J = 7 Hz, 3H, H-17), 1.12–1.33 (ma, 16H, H-9-16), 1.40 (d, J = 7 Hz, 3H, H-4), 1.51–1.62 (ma, 2H, H-8), 2.16 (t, J = 7 Hz, 2H, H-7), 4.50 (m, J = 7 Hz, 1H, H-3), 6.25 (s, 1H, H-5). 13C-NMR (100 MHz, DMSO-d6): δ (ppm) = 14.1 (C-17), 17.0 (C-4), 22.7 (C-16), 25.6 (C-8), 28.6 (C-9), 28.9 (C-10), 29.6 (C-11-13), 29.3 (C-14), 31.9 (C-15), 36.5 (C-7), 51.3 (C-3), 173.9 (C-6), 174.7 (C-2). IR: ν (cm−1) = 1643 (C=O amide), 1702 (C=O acid), 3229 (-NH- amide), 3311 (-OH acid).
![Molecules 30 04223 i012 Molecules 30 04223 i012]()
Tetradecanoylalanine (4c). C17H33NO3. White solid. Yield: 94% (0.861 g). 1H-NMR (400 MHz, CDCl3): δ (ppm) = 0.82 (t, J = 7 Hz, 3H, H-19), 1.1–1.33 (ma, 20H, H-9-18), 1.39 (d, J = 7 Hz, 3H, H-4), 1.52–1.62 (ma, 2H, H-8), 2.17 (t, J = 7 Hz, 2H, H-7), 4.49 (m, J = 7 Hz, 1H, H-3), 6.12 (s, 1H, H-5). 13C-NMR (100 MHz, DMSO-d6): δ (ppm) = 14 (C-19), 17.2 (C-4), 22.5 (C-18), 25.6 (C-18), 28.6 (C-9), 28.9 (C-10), 29.6 (C-11-15), 29.3 (C-16), 31.9 (C-17), 36.6 (C-7), 51.3 (C-3), 174.0 (C-6), 174.7 (C-2). IR: ν (cm−1) = 1644 (C=O amide), 1705 (C=O acid), 3227 (-NH- amide), 3315 (-OH acid).
![Molecules 30 04223 i013 Molecules 30 04223 i013]()
Hexadecanoylalanine (4d). C19H37NO3. White solid. Yield: 95% (0.874 g). 1H-NMR (400 MHz, CDCl3): δ (ppm) = 0.79 (t, J = 7 Hz, 3H, H-21), 1.12–1.31 (ma, 24H, H-9-20), 1.41 (d, J = 7 Hz, 3H, H-4), 1.52–1.62 (ma, 2H, H-8), 2.15 (t, J = 7 Hz, 2H, H-7), 4.51 (m, J = 7 Hz, 1H, H-3), 6.07 (s, 1H, H-5). 13C-NMR (100 MHz, DMSO-d6): δ (ppm) = 14.2 (C-21), 17.1 (C-4), 22.7 (C-20), 25.6 (C-8), 28.6 (C-9), 28.9 (C-10), 29.6 (C-11-17), 29.4 (C-18), 32.0 (C-19), 36.2 (C-7), 51.4 (C-3), 174.0 (C-6), 174.6 (C-2). IR: ν (cm−1) = 1644 (C=O amide), 1703 (C=O acid), 3222 (-NH- amide), 3317 (-OH acid)
3.2.5. Procedure for Obtaining Compounds 5a–5d
Compound 1 (0.5 g, 1.09 mmol, 1 equivalent) and one equivalent of alanine–fatty acid coupling have been dissolved in 10 mL of anhydrous THF or anhydrous Me-THF. Concentrated H2SO4 (50 μL, 0.92 mmol) has been added and the mixture was stirred in a microwave at 120 °C for 10 min using a power of 100 W. An amount of 100 mL of ethyl acetate has been added and the organic phase has been washed successively with 25 mL of saturated NaHCO3 (several times if necessary until neutral pH of the aqueous phase) and with 25 mL of saturated NaCl. The organic phase has been dried over anhydrous MgSO4 and the solvent has been evaporated under low pressure.
![Molecules 30 04223 i014 Molecules 30 04223 i014]()
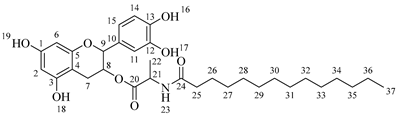
4-(5,7-Diacetoxy-3-((octanoylalanyl)oxy)chroman-2-yl)-1,2-phenylene diacetate (5a). C34H41NO12. Whitish solid. Yield: 82% (0.585 g). 1H-NMR (400 MHz, CDCl3): δ (ppm) = 0.81 (t, J = 7 Hz, 3H, H-35), 1.18–1.28 (ma, 8H, H-31-34), 1.36 (d, J = 7 Hz, 3H, H-26), 1.55 (q, J = 7 Hz, 2H, H-30), 2.20 (s, 3H, H-17), 2.21 (s, 6H, H-19, H-23), 2.23 (s, 3H, H-21), 2.26 (t, J = 7 Hz, 2H, H-29), 2.53–2.64 (m, 1H, H-7α), 2.87–2.95 (dd, J = 16, 6 Hz, 1H, H-7β), 3.85–3.94 (m, 1H, H-8), 4.28 (q, J = 7 Hz, 1H, H-25), 4.66 (d, J = 8 Hz, 1H, H-9), 6.49 (d, J = 2 Hz, 1H, H-2), 6.54 (d, J = 2 Hz, 1H, H-6), 6.60 (d, J = 6 Hz, 1H, H-27), 7.10–7.29 (ma, 3H, H-11, H-14, H-15). 13C-NMR (100 MHz, CDCl3): δ (ppm) = 14.0 (C-35), 17.8 (C-26), 20.6 (C-17), 20.7 (C-19, C-23), 21.0 (C-21), 22.7 (C-34), 25.5 (C-7), 28.1 (C-33), 29.1 (C-32), 29.2 (C-31), 31.9 (C-30), 36.3 (C-29), 36.8 (C-25), 67.4 (C-8), 81.1 (C-9), 107.7 (C-2), 108.7 (C-6), 111.6 (C-4), 122.3 (C-15), 123.7 (C-14), 125.5 (C-11), 136.5 (C-10), 142.2 (C-12), 142.3 (C-13), 149.7 (C-1), 155.0 (C-3), 168.0 (C-16), 168.2 (C-18-22), 168.5 (C-20), 170.1 (C-24), 175.0 (C-28). IR: ν (cm−1) = 3315 (N-H amide), 1763 (C=O acetyl), 1743 (C=O ester), 1642 (C=O amide).
![Molecules 30 04223 i015 Molecules 30 04223 i015]()
4-(5,7-Diacetoxy-3-((dodecanoylalanyl)oxy)chroman-2-yl)-1,2-phenylene diacetate (5b). C38H49NO12. Whitish solid. Yield: 78% (0.544 g). 1H-NMR (400 MHz, CDCl3): δ (ppm) = 0.81 (t, J = 7 Hz, 3H, H-39), 1.12–1.29 (ma, 16H, H-31-38), 1.38 (d, J = 7 Hz, 3H, H-26), 1.56 (q, J = 7 Hz, 2H, H-30), 2.17 (t, J = 7 Hz, 2H, H-29), 2.20 (s, 3H, H-17), 2.21 (s, 6H, H-19, H-23), 2.23 (s, 3H, H-21), 2.51–2.63 (m, 1H, H-7α), 2.87–2.95 (dd, J = 16, 6 Hz, 1H, H-7β), 3.85–3.94 (m, 1H, H-8), 4.49 (q, J = 7 Hz, 1H, H-25), 4.66 (d, J = 8 Hz, 1H, H-9), 6.01 (d, J = 6 Hz, 1H, H-27), 6.48 (d, J = 2 Hz, 1H, H-2), 6.53 (d, J = 2 Hz, 1H, H-6), 7.07–7.28 (ma, 3H, H-11, H-14, H-15). 13C-NMR (100 MHz, CDCl3): δ (ppm) = 14.1 (C-39), 17.8 (C-26), 20.6 (C-17), 20.7 (C-19, C-23), 21.0 (C-21), 22.6 (C-38), 25.5 (C-7), 28.1 (C-37), 29.1 (C-36), 29.2 (C-35), 29.3 (C-34), 29.4, (C-33), 29.6 (C-32-31), 31.9 (C-30), 36.3 (C-29), 48.3 (C-25), 67.6 (C-8), 81.1 (C-9), 107.8 (C-2), 108.7 (C-6), 111.6 (C-4), 122.3 (C-15), 123.7 (C-14), 125.5 (C-11), 136.5 (C-10), 142.3 (C-12-13), 149.5 (C-1), 155.0 (C-3), 168.3 (C-16), 168.4 (C-18-22), 168.6 (C-20), 174.2 (C-24), 175.0 (C-28). IR: ν (cm−1) = 3315 (N-H amide), 1766 (C=O acetyl), 1740 (C=O ester), 1642 (C=O amide).
![Molecules 30 04223 i016 Molecules 30 04223 i016]()
4-(5,7-Diacetoxy-3-((tetradecanoylalanyl)oxy)chroman-2-yl)-1,2-phenylene diacetate (5c). C40H53NO12. Whitish solid. Yield: 85% (0.619 g). 1H-NMR (400 MHz, CDCl3): δ (ppm) = 0.82 (t, J = 7 Hz, 3H, H-41), 1.14–1.29 (ma, 20H, H-31-40), 1.40 (d, J = 7 Hz, 3H, H-26), 1.57 (q, J = 7 Hz, 2H, H-30), 2.18 (t, J = 7 Hz, 2H, H-29), 2.20 (s, 3H, H-17), 2.22 (s, 6H, H-19, H-23), 2.24 (s, 3H, H-21), 2.54–2.63 (m, 1H, H-7α), 2.88–2.96 (dd, J = 16, 6 Hz, 1H, H-7β), 3.88–3.95 (m, 1H, H-8), 4.50 (q, J = 7 Hz, 1H, H-25), 4.68 (d, J = 8 Hz, 1H, H-9), 5.99 (d, J = 7 Hz, 1H, H-27), 6.49 (d, J = 2 Hz, 1H, H-2), 6.54 (d, J = 2 Hz, 1H, H-6), 7.08–7.30 (ma, 3H, H-11, H-14, H-15). 13C-NMR (100 MHz, CDCl3): δ (ppm) = 14.1 (C-41), 17.8 (C-26), 20.6 (C-17), 20.7 (C-19, C-23), 21.0 (C-21), 22.7 (C-40), 25.5 (C-7), 28.1 (C-39), 29.1 (C-38), 29.2 (C-37), 29.3 (C-36), 29.4 (C-35), 29.5 (C-34), 29.6 (C-33), 29.7 (C-32-31), 31.9 (C-30), 36.4 (C-29), 48.3 (C-25), 67.6 (C-8), 81.1 (C-9), 107.8 (C-2), 108.8 (C-6), 111.6 (C-4), 122.3 (C-15), 123.7 (C-14), 125.4 (C-11), 136.5 (C-10), 142.2 (C-12), 142.3 (C-13), 149.5 (C-1), 155.0 (C-3), 168.2 (C-16), 168.3 (C-18-22), 168.5 (C-20), 174.2 (C-24), 175.0 (C-28). IR: ν (cm−1) = 3312 (N-H amide), 1766 (C=O acetyl), 1741 (C=O ester), 1645 (C=O amide).
![Molecules 30 04223 i017 Molecules 30 04223 i017]()
4-(5,7-Diacetoxy-3-((hexadecanoylalanyl)oxy)chroman-2-yl)-1,2-phenylene diacetate (5d). C42H57NO12. Whitish solid. Yield: 76% (0.577 g). 1H-NMR (400 MHz, CDCl3): δ (ppm) = 0.82 (t, J = 7 Hz, 3H, H-43), 1.13–1.28 (ma, 24H, H-31-42), 1.38 (d, J = 7 Hz, 3H, H-26), 1.56 (q, J = 7 Hz, 2H, H-30), 2.16 (t, J = 7 Hz, 2H, H-29), 2.20 (s, 3H, H-17), 2.21 (s, 6H, H-19, H-23), 2.24 (s, 3H, H-21), 2.52–2.60 (m, 1H, H-7α), 2.87–2.94 (dd, J = 16, 6 Hz, 1H, H-7β), 3.86–3.93 (m, 1H, H-8), 4.50 (q, J = 7 Hz, 1H, H-25), 4.66 (d, J = 8 Hz, 1H, H-9), 6.02 (d, J = 7 Hz, 1H, H-27), 6.48 (d, J = 2 Hz, 1H, H-2), 6.53 (d, J = 2 Hz, 1H, H-6), 7.13–7.28 (ma, 3H, H-11, H-14, H-15). 13C-NMR (100 MHz, CDCl3): δ (ppm) = 14.1 (C-43), 17.8 (C-26), 20.6 (C-17), 20.7 (C-19, C-23), 21.0 (C-21), 22.6 (C-42), 25.5 (C-7), 28.1 (C-41), 29.1 (C-40), 29.2 (C-39), 29.3 (C-38), 29.4 (C-37), 29.5 (C-36), 29.6 (C-35-34), 29.7 (C33-32), 31.9 (C-31), 33.7 (C-30), 36.4 (C-29), 48.2 (C-25), 67.5 (C-8), 81.1 (C-9), 107.7 (C-2), 108.7 (C-6), 111.6 (C-4), 122.3 (C-15), 123.7 (C-14), 125.5 (C-11), 136.5 (C-10), 142.3 (C-12-13), 149.5 (C-1), 155.0 (C-3), 168.3 (C-16), 168.5 (C-18-22), 168.9 (C-20), 174.0 (C-24), 175.1 (C-28). IR: ν (cm−1) = 3313 (N-H amide), 1764 (C=O acetyl), 1740 (C=O ester), 1645 (C=O amide).
3.2.6. Procedure for Obtaining Compounds 6a–6d and 7a–7d
Acetylated compound (0.65 mmol, 1 equivalent) has been solubilized in 100 mL of a mixture of MeOH/CH2Cl2 (1:1). Acetyl chloride (0.102 g, 1.30 mmol, 2 equivalents) has been added dropwise. The mixture has been stirred for 48 h at room temperature. The reaction has been followed by FTIR by evaluation of the disappearance of the band at 1760 cm−1. Once there was no longer any starting product, the solvents evaporated. Water was added to precipitate the final product. The purification has been performed by recrystallization in water.
![Molecules 30 04223 i018 Molecules 30 04223 i018]()
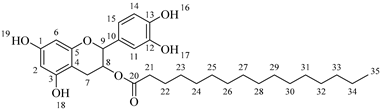
2-(3,4-Dihydroxyphenyl)-5,7-dihydroxychroman-3-yl octanoate (
6a) [
36]. Whitish solid. Yield: 77% (0.208 g). m.p. 105 °C. Anal. Calculated for C
23H
28O
7: C, 66.33; H, 6.78; Found: C, 66.78; H, 6.60.
1H-NMR (400 MHz, DMSO-
d6): δ (ppm) = 0.84 (t,
J = 7 Hz, 3H, H-27), 1.20–1.28 (ma, 8H, H-23-26), 1.49 (q,
J = 7 Hz, 2H, H-22), 2.27 (t,
J = 7 Hz, 2H, H-21), 2.30–2.38 (m, 1H, H-7α), 2.60–2.68 (dd,
J = 16, 5 Hz, 1H, H-7β), 3.76–3.86 (m, 1H, H-8), 4.89 (d,
J = 5 Hz, 1H, H-9), 5.68 (d,
J = 2 Hz, 1H, H-2), 5.87 (d,
J = 2 Hz, 1H, H-6), 6.51–6.73 (ma, 3H, H-11, H-14, H-15), 8.84 (s, 1H, H-16), 8.87 (s, 1H, H-17), 8.98 (s, 1H, H-19), 9.22 (s, 1H, H-18).
13C-NMR (100 MHz, MeOD): δ (ppm) = 14.4 (C-27), 23.6 (C-26), 26.0 (C-7), 28.5 (C-25), 30.0 (C-24), 30.1 (C-23), 32.8 (C-22), 34.8 (C-21), 68.7 (C-8), 82.8 (C-9), 95.4 (C-2), 96.2 (C-6), 100.7 (C-4), 115.2 (C-15), 116.6 (C-14), 120.0 (C-11), 132.1 (C-10), 145.1 (C-13), 145.2 (C-12), 156.6 (C-1), 157.5 (C-3), 157.7 (C-5), 174.8 (C-20). IR: ν (cm
−1) = 3000–3676 (-OH), 1742 (C=O ester).
![Molecules 30 04223 i019 Molecules 30 04223 i019]()
2-(3,4-Dihydroxyphenyl)-5,7-dihydroxychroman-3-yl dodecanoate (
6b) [
36]. Whitish solid. Yield: 75% (0.230 g). m.p. 120 °C. Anal. Calculated for C
27H
36O
7: C, 68.62; H, 7.68; Found: C, 68.65; H, 7.48.
1H-NMR (400 MHz, DMSO-
d6): δ (ppm) = 0.84 (t,
J = 7 Hz, 3H, H-31), 1.19–1.29 (ma, 16H, H-23-30), 1.49 (q,
J = 7 Hz, 2H, H-22), 2.26 (t,
J = 7 Hz, 2H, H-21), 2.31–2.39 (m, 1H, H-7α), 2.60–2.68 (dd,
J = 16, 5 Hz, 1H, H-7β), 3.76–3.86 (m, 1H, H-8), 4.89 (d,
J = 5 Hz, 1H, H-9), 5.68 (d,
J = 2 Hz, 1H, H-2), 5.87 (d,
J = 2 Hz, 1H, H-6), 6.51–6.73 (ma, 3H, H-11, H-14, H-15), 8.88 (s, 1H, H-16), 8.98 (s, 1H, H-17), 9.03 (s, 1H, H-19), 9.22 (s, 1H, H-18).
13C-NMR (100 MHz, MeOD): δ (ppm) = 14.4 (C-31), 23.7 (C-30), 26.0 (C-7), 28.5 (C-29), 30.1 (C-28), 30.3 (C-27), 30.4 (C-26), 30.5 (C-25), 30.7 (C-24), 30.8 (C-23), 33.0 (C-22), 34.8 (C-21), 68.8 (C-8), 82.8 (C-9), 95.5 (C-2), 96.3 (C-6), 100.8 (C-4), 115.3 (C-15), 116.2 (C-14), 120.0 (C-11), 132.2 (C-10), 144.3 (C-13), 146.4 (C-12), 156.9 (C-1), 158.0 (C-3), 158.5 (C-5), 174.5 (C-20). IR: ν (cm
−1) = 2994–3700 (-OH), 1742 (C=O ester).
![Molecules 30 04223 i020 Molecules 30 04223 i020]()
2-(3,4-Dihydroxyphenyl)-5,7-dihydroxychroman-3-yl tetradecanoate (
6c) [
36]. Whitish solid. Yield: 78% (0.254 g). m.p. 130 °C. Anal. Calculated for C
29H
40O
7: C, 69.58; H, 8.05; Found: C, 69.52; H, 8.10.
1H-NMR (400 MHz, DMSO-
d6): δ (ppm) = 0.84 (t,
J = 7 Hz, 3H, H-33), 1.19–1.27 (ma, 20H, H-23-32), 1.49 (q,
J = 7 Hz, 2H, H-22), 2.27 (t,
J = 7 Hz, 2H, H-21), 2.30–2.38 (m, 1H, H-7α), 2.61–2.68 (dd,
J = 16, 5 Hz, 1H, H-7β), 3.78–3.83 (m, 1H, H-8), 4.89 (d,
J = 5 Hz, 1H, H-9), 5.68 (d,
J = 2 Hz, 1H, H-2), 5.87 (d,
J = 2 Hz, 1H, H-6), 6.51–6.73 (ma, 3H, H-11, H-14, H-15), 8.84 (s, 1H, H-16), 8.87 (s, 1H, H-17), 8.98 (s, 1H, H-19), 9.22 (s, 1H, H-18).
13C-NMR (100 MHz, MeOD): δ (ppm) = 14.5 (C-33), 23.7 (C-32), 26.0 (C-7), 28.5 (C-31), 30.1 (C-30), 30.3 (C-29), 30.4 (C-28), 30.5 (C-27), 30.6 (C-26), 30.7 (C-24-25), 30.8 (C-23), 33.0 (C-22), 34.8 (C-21), 68.7 (C-8), 82.7 (C-9), 95.4 (C-2), 96.2 (C-6), 100.7 (C-4), 115.2 (C-15), 116.0 (C-14), 120.0 (C-11), 132.1 (C-10), 146.1 (C-13), 146.2 (C-12), 156.8 (C-1), 157.5 (C-3), 157.7 (C-5), 176.2 (C-20). IR: ν (cm
−1) = 2998–3678 (-OH), 1743 (C=O ester).
![Molecules 30 04223 i021 Molecules 30 04223 i021]()
2-(3,4-Dihydroxyphenyl)-5,7-dihydroxychroman-3-yl hexadecanoate (
6d) [
36]. Whitish solid. Yield: 77% (0.264 g). m.p. 150 °C. Anal. Calculated for C
31H
44O
7: C, 70.43; H, 8.39; Found: C, 70.47; H, 8.47.
1H-NMR (400 MHz, DMSO-
d6): δ (ppm) = 0.84 (t,
J = 7 Hz, 3H, H-35), 1.19–1.28 (ma, 24H, H-23-34), 1.49 (q,
J = 7 Hz, 2H, H-22), 2.28 (t,
J = 7 Hz, 2H, H-21), 2.30–2.39 (m, 1H, H-7α), 2.60–2.67 (dd,
J = 16, 5 Hz, 1H, H-7β), 3.77–3.83 (m, 1H, H-8), 4.89 (d,
J = 5 Hz, 1H, H-9), 5.68 (d,
J = 2 Hz, 1H, H-2), 5.87 (d,
J = 2 Hz, 1H, H-6), 6.51–6.73 (ma, 3H, H-11, H-14, H-15), 8.84 (s, 1H, H-16), 8.86 (s, 1H, H-17), 8.98 (s, 1H, H-19), 9.21 (s, 1H, H-18).
13C-NMR (100 MHz, MeOD): δ (ppm) = 14.4 (C-35), 23.7 (C-34), 26.0 (C-7), 30.1 (C-33), 30.3 (C-32), 30.4 (C-31), 30.5 (C-30), 30.6 (C-29), 30.7 (C-28), 30.8 (C24-27), 30.9 (C-23), 33.0 (C-22), 34.8 (C-21), 61.5 (C-8), 82.7 (C-9), 95.4 (C-2), 96.2 (C-6), 100.7 (C-4), 116.0 (C-15), 116.3 (C-14), 120.0 (C-11), 132.1 (C-10), 146.1 (C-13), 146.2 (C-12), 156.8 (C-1), 157.5 (C-3), 157.7 (C-5), 176.0 (C-20). IR: ν (cm
−1) = 3013–3700 (-OH), 1743 (C=O ester).
![Molecules 30 04223 i022 Molecules 30 04223 i022]()
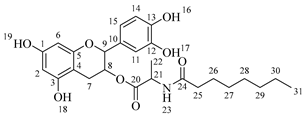
2-(3,4-Dihydroxyphenyl)-5,7-dihydroxychroman-3-yl octanoylalaninate (7a). Whitish solid. Yield: 81% (0.256 g). m.p. 120 °C. Anal. Calculated for C26H33NO8: C, 64.05; H, 6.82; N, 2.87; Found: C, 64.17; H, 6.80; N, 2.84. 1H-NMR (400 MHz, DMSO-d6): δ (ppm) = 0.94 (t, J = 7 Hz, 3H, H-31), 1.31–1.39 (ma, 8H, H-27-30), 1.40 (d, J = 7 Hz, 3H, H-22), 1.65 (q, J = 7 Hz, 2H, H-26), 2.26 (t, J = 7 Hz, 2H, H-25), 2.51–2.59 (m, 1H, H-7α), 2.85–2.94 (dd, J = 16, 6 Hz, 1H, H-7β), 3.98–4.05 (m, 1H, H-8), 4.43 (q, J = 7 Hz, 1H, H-21), 4.61 (d, J = 8 Hz, 1H, H-9), 5.90 (d, J = 2 Hz, 1H, H-2), 5.97 (d, J = 2 Hz, 1H, H-6), 6.74–6.84 (ma, 3H, H-11, H-14, H-15) 6.88 (d, J = 6Hz, 1H, H-23), 8.84 (s, 1H, H-16), 8.86 (s, 1H, H-17), 8.98 (s, 1H, H-19), 9.21 (s, 1H, H-18). 13C-NMR (100 MHz, MeOD): δ (ppm) = 14.4 (C-31), 17.3 (C-22), 23.7 (C-30), 26.9 (C-7), 28.5 (C-29), 30.2 (C-28), 30.4 (C-27), 33.0 (C-26), 36.6 (C-25), 52.5 (C-21), 68.8 (C-8), 82.9 (C-9), 94.9 (C-2), 95.5 (C-6), 100.0 (C-4), 115.2 (C-14), 115.8 (C-15), 120.0 (C-11), 131.5 (C-10), 146.2 (C-12-13), 156.9 (C-1), 157.9 (C-3), 170.2 (C-20), 174.9 (C-24). IR: ν (cm−1) = 2996–3677 (-OH), 3315 (N-H amide), 1725 (C=O ester), 1642 (C=O amide).
![Molecules 30 04223 i023 Molecules 30 04223 i023]()
2-(3,4-Dihydroxyphenyl)-5,7-dihydroxychroman-3-yl dodecanoylalaninate (7b). Whitish solid. Yield: 78% (0.275 g). m.p. 140 °C. Anal. Calculated for C30H41NO8: C, 66.28; H, 7.60; N, 2.58; Found: C, 66.30; H, 7.61; N, 2.54. 1H-NMR (400 MHz, DMSO-d6): δ (ppm) = 0.94 (t, J = 7 Hz, 3H, H-35), 1.31–1.39 (ma, 16H, H-27-34), 1.40 (d, J = 7Hz, 3H, H-22), 1.65 (q, J = 7 Hz, 2H, H-26), 2.26 (t, J = 7 Hz, 2H, H-25), 2.51–2.59 (m, 1H, H-7α), 2.85–2.94 (dd, J = 16, 6 Hz, 1H, H-7β), 3.98–4.05 (m, 1H, H-8), 4.43 (q, J = 7 Hz, 1H, H-21), 4.61 (d, J = 8 Hz, 1H, H-9), 5.90 (d, J = 2 Hz, 1H, H-2), 5.97 (d, J = 2 Hz, 1H, H-6), 6.74–6.84 (ma, 3H, H-11, H-14, H-15) 6.88 (d, J = 6Hz, 1H, H-23), 8.84 (s, 1H, H-16), 8.86 (s, 1H, H-17), 8.98 (s, 1H, H-19), 9.21 (s, 1H, H-18). 13C-NMR (100 MHz, MeOD): δ (ppm) = 14.4 (C-35), 17.3 (C-22), 23.7 (C-34), 26.9 (C-7), 28.5 (C-33), 30.2 (C-32), 30.4 (C30-31), 30.6 (C-29), 30.7 (C27-28), 33.0 (C-26), 36.6 (C-25), 52.7 (C-21), 68.8 (C-8), 82.9 (C-9), 95.5 (C-2), 96.3 (C-6), 100.8 (C-4), 115.3 (C-14), 116.0 (C-15), 120.0 (C-11), 132.2 (C-10), 146.2 (C-12-13), 156.9 (C-1), 157.9 (C-3), 170.2 (C-20), 175.0 (C-24). IR: ν (cm−1) = 3000–3677 (-OH), 3320 (N-H amide), 1725 (C=O ester), 1642 (C=O amide).
![Molecules 30 04223 i024 Molecules 30 04223 i024]()
2-(3,4-Dihydroxyphenyl)-5,7-dihydroxychroman-3-yl tetradecanoylalaninate (7c). Whitish solid. Yield: 82% (0.304 g). m.p. 135 °C. Anal. Calculated for C32H45NO8: C, 67.23; H, 7.93; N, 2.45; Found: C, 67.22; H, 7.96; N, 2.43. 1H-NMR (400 MHz, DMSO-d6): δ (ppm) = 0.94 (t, J = 7 Hz, 3H, H-37), 1.30–1.39 (ma, 20H, H-27-36), 1.40 (d, J = 7 Hz, 3H, H-22), 1.64 (q, J = 7 Hz, 2H, H-26), 2.35 (t, J = 7 Hz, 2H, H-25), 2.51–2.59 (m, 1H, H-7α), 2.86–2.94 (dd, J = 16, 6 Hz, 1H, H-7β), 3.98–4.05 (m, 1H, H-8), 4.43 (q, J = 7 Hz, 1H, H-21), 4.59 (d, J = 8 Hz, 1H, H-9), 5.90 (d, J = 2 Hz, 1H, H-2), 5.97 (d, J = 2 Hz, 1H, H-6), 6.74–6.84 (ma, 3H, H-11, H-14, H-15) 6.88 (d, J = 6 Hz, 1H, H-23), 8.84 (s, 1H, H-16), 8.87 (s, 1H, H-17), 8.98 (s, 1H, H-19), 9.22 (s, 1H, H-18). 13C-NMR (100 MHz, MeOD): δ (ppm) = 14.4 (C-37), 17.4 (C-22), 23.7 (C-36), 26.0 (C-35), 26.3 (C-34), 26.9 (C-7), 28.5 (C-33), 30.2 (C-32), 30.3 (C30) 30.4 (C-31), 30.5 (C-30), 30.6 (C-29), 30.7 (C27-28), 33.0 (C-26), 36.6 (C-25), 52.0 (C-21), 68.8 (C-8), 82.9 (C-9), 95.5 (C-2), 96.5 (C-6), 100.8 (C-4), 115.3 (C-14), 116.0 (C-15), 120.0 (C-11), 132.2 (C-10), 146.2 (C-12-13), 156.9 (C-1), 157.9 (C-3), 170.2 (C-20), 174.9 (C-24). IR: ν (cm−1) = 3998–3694 (-OH), 3320 (N-H amide), 1730 (C=O ester), 1645 (C=O amide).
![Molecules 30 04223 i025 Molecules 30 04223 i025]()
2-(3,4-Dihydroxyphenyl)-5,7-dihydroxychroman-3-yl hexadecanoylalaninate (7d). Whitish solid. Yield: 79% (0.308 g). m.p. 150 °C. Anal. Calculated for C34H49NO8: C, 68.09; H, 8.24; N, 2.34; Found: C, 68.12; H, 8.27; N, 2.39. 1H-NMR (400 MHz, DMSO-d6): δ (ppm) = 0.94 (t, J = 7 Hz, 3H, H-39), 1.27–1.39 (ma, 24H, H-27-38), 1.40 (d, J = 7 Hz, 3H, H-22), 1.65 (q, J = 7 Hz, 2H, H-26), 2.26 (t, J = 7 Hz, 2H, H-25), 2.32–2.43 (m, 1H, H-7α), 2.86–2.94 (dd, J = 16, 6 Hz, 1H, H-7β), 3.98–4.05 (m, 1H, H-8), 4.43 (q, J = 7 Hz, 1H, H-21), 4.59 (d, J = 8 Hz, 1H, H-9), 5.91 (d, J = 2 Hz, 1H, H-2), 5.98 (d, J = 2 Hz, 1H, H-6), 6.74–6.84 (ma, 3H, H-11, H-14, H-15) 6.90 (d, J = 6Hz, 1H, H-23), 8.84 (s, 1H, H-16), 8.87 (s, 1H, H-17), 8.98 (s, 1H, H-19), 9.22 (s, 1H, H-18). 13C-NMR (100 MHz, MeOD): δ (ppm) = 14.4 (C-39), 17.3 (C-22), 23.7 (C-38), 26.9 (C-7), 30.2 (C-37), 30.3 (C-36), 30.4 (C-35), 30.6 (C-34), 30.8 (C-33), 30.8 (C-27-32), 33.1 (C-26), 36.6 (C-25), 52.8 (C-21), 68.8 (C-8), 82.9 (C-9), 95.5 (C-2), 96.5 (C-6), 100.8 (C-4), 115.3 (C-14), 116.0 (C-15), 120.0 (C-11), 132.2 (C-10), 146.2 (C-12-13), 156.9 (C-1), 157.9 (C-3), 170.3 (C-20), 175.0 (C-24). IR: ν (cm−1) = 3998–3700 (-OH), 3315 (N-H amide), 1730 (C=O ester), 1645 (C=O amide).