Metabolomic Profiling, Box–Behnken Design-Based Optimization of Ultrasonic Extraction, and Skin Anti-Aging Potential of the Green Husk of Juglans regia L. as a Sustainable Natural Waste
Abstract
1. Introduction
2. Results and Discussion
2.1. Results of Elastase Inhibition
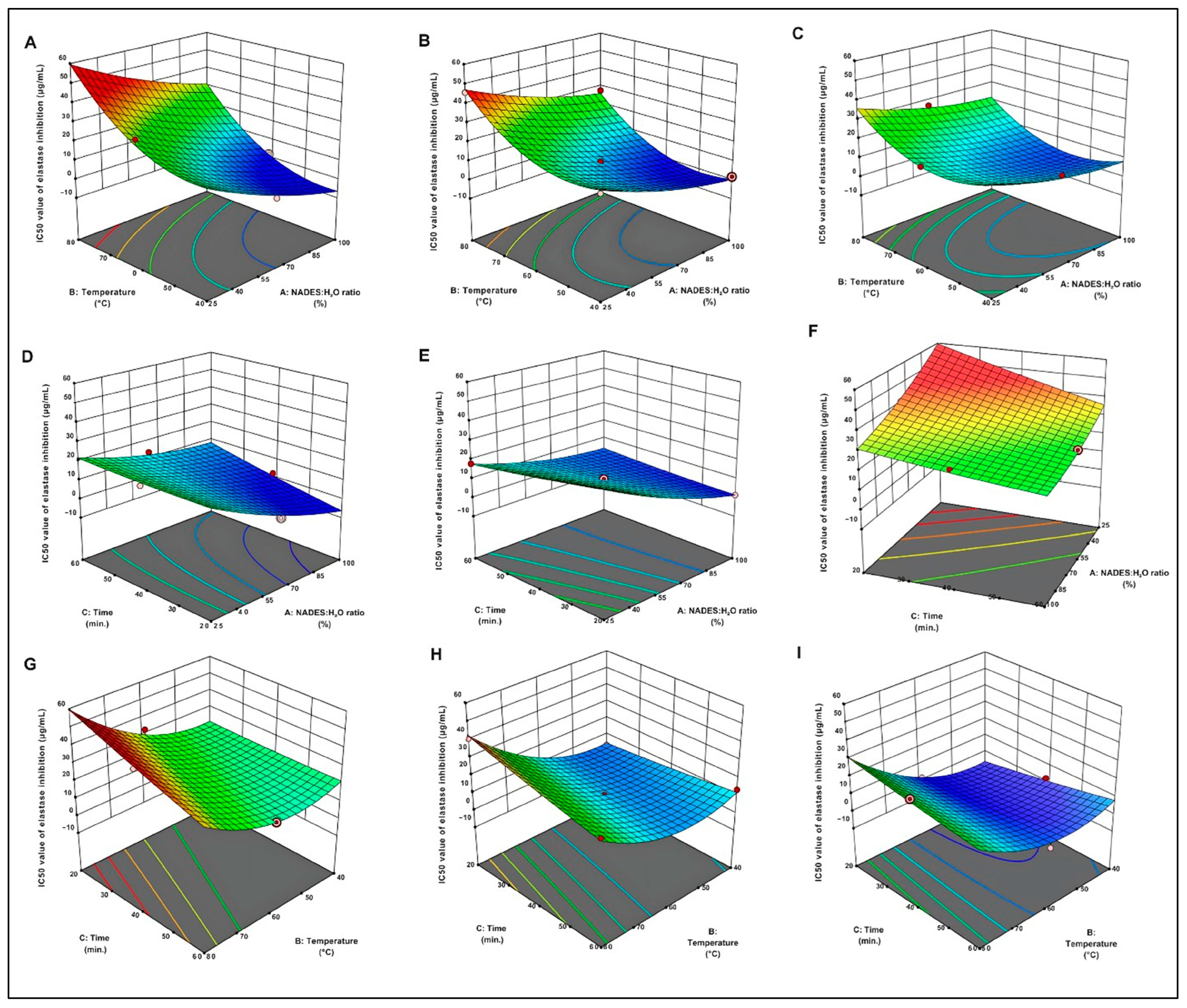
2.2. Analysis of Box–Behnken Design
2.2.1. Effect of Extraction Temperature
2.2.2. Effect of Extraction Time
2.2.3. Effect of NADES/H2O Ratio
2.3. Validation of the Box–Behnken Design’s Accuracy
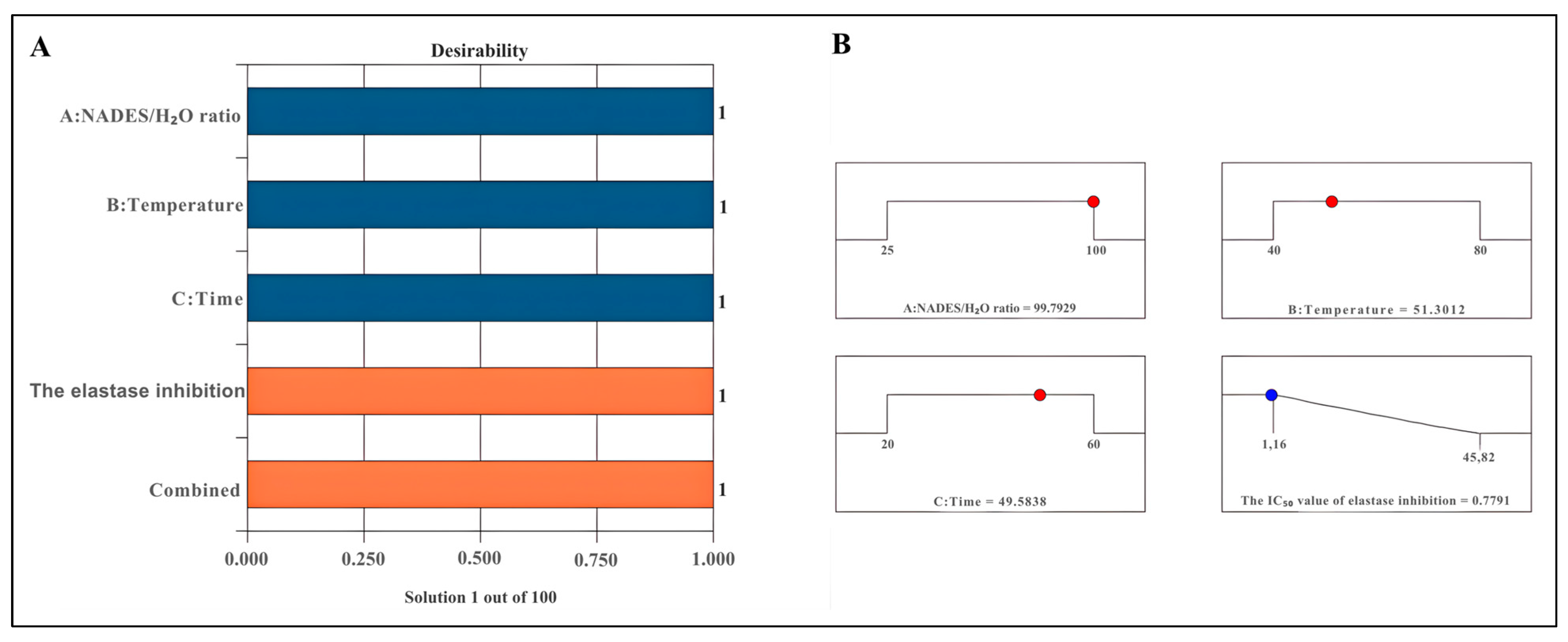
2.4. Optimisation of Extraction Procedures
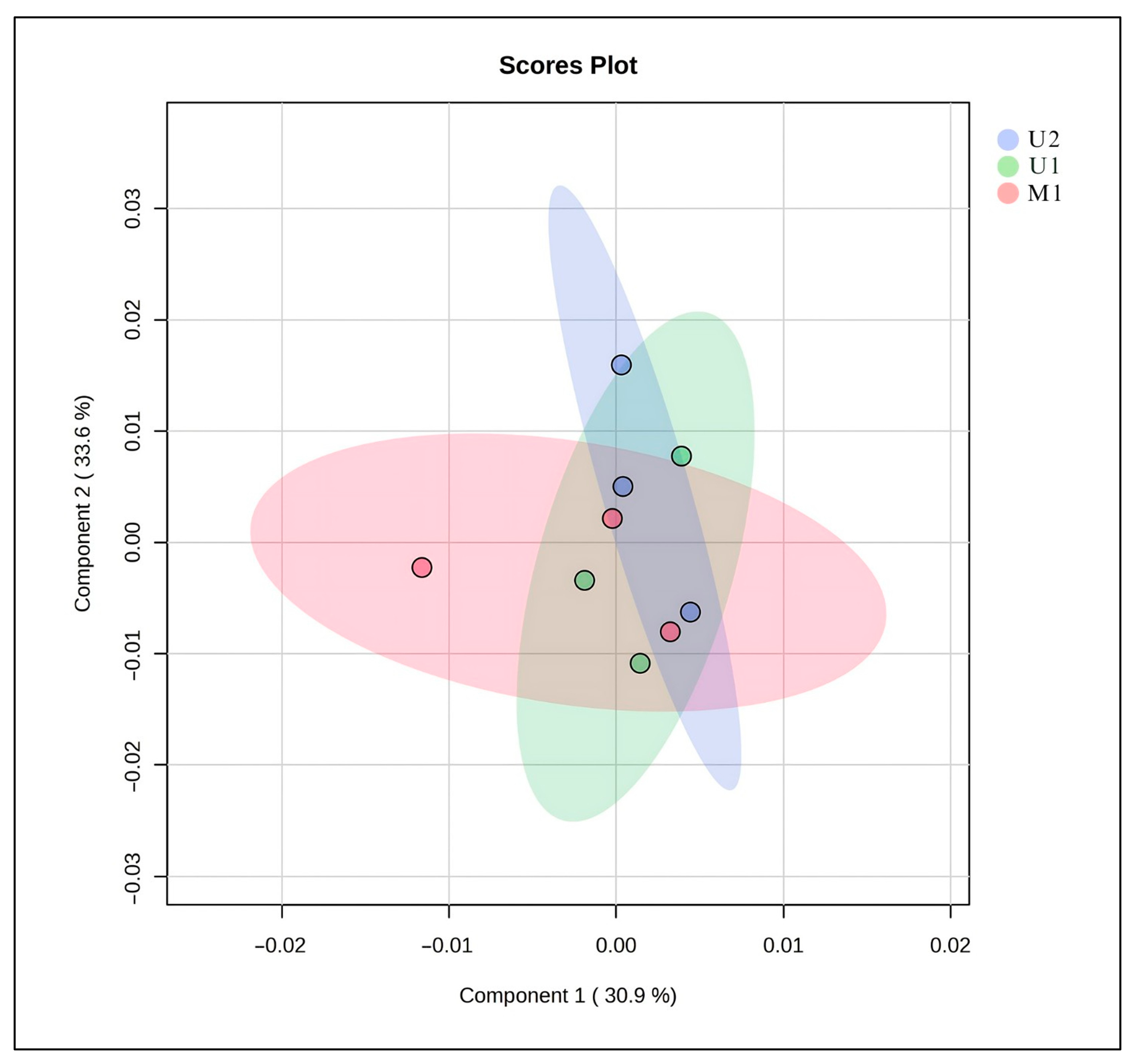
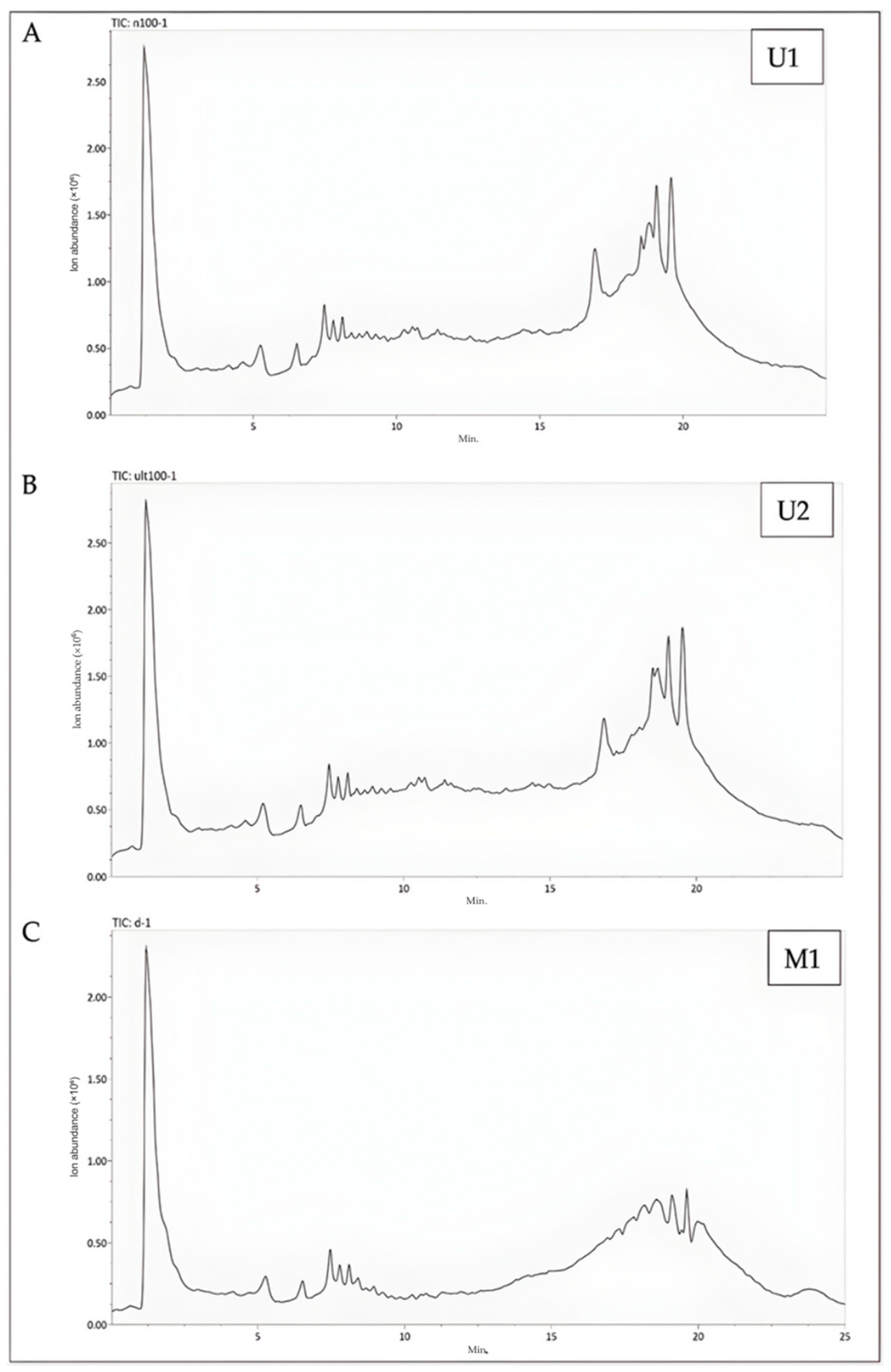
2.5. Results of Metabolomics Analysis
3. Materials and Methods
3.1. Raw Materials
3.2. Preparation of NADES
3.3. Preparations of Extracts with NADES
3.4. Evaluation of Elastase Inhibition
3.5. Optimization of Extraction Using Response Surface Methodology (RSM)
3.6. Statistical Analysis
3.7. Metabolomics Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WHO | World Health Organization |
| USA | United States of America |
| UV | Ultraviolet |
| ILs | Ionic liquids |
| DES | Deep eutectic solvents |
| NADES | Natural Deep Eutectic Solvents |
| HBD | Hydrogen bond donor |
| BBD | Box–Behnken design |
| UAE | Ultrasound-assisted extraction |
| IC50 | Half maximal inhibitory concentration |
| U1 | The BBD-optimized extract |
| M1 | The Maceration extract |
| U2 | The Optimized extract at maceration temperature |
| 2FI | Two-factor interaction |
| R2 | Coefficient of determination |
| PRE | Percent relative error |
| ANOVA | Analysis of variance |
| CV% | Coefficient of variance |
| R2a | Adjusted coefficient of determination |
| GAE | Gallic acid equivalent |
| dw | Dry weight |
| RE | Rutin equivalent |
| ChCl | Choline chloride |
| RSM | Response surface methodology |
| PCA | Principal Component Analysis |
| ROS | Reactive oxygen species |
| ERK1/2 | Extracellular signal-regulated kinase 1/2 |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| NO | Nitric oxide |
| HNE | Human neutrophil elastase |
| PGE2 | Prostaglandin E2 |
| IL | Interleukin |
| Dct | Dopachrome tatamerase |
| TRP-1 | Tyrosinase-related protein-1 |
| MITF | Microphthalmia-associated transcription factor |
| cAMP | Cyclic adenosine monophosphate |
| CREB | cAMP-responsive binding protein |
| dH2O | Deionized water |
| HBA | Hydrogen bond acceptor |
| DMSO | Dimethyl sulfoxide |
| STANA | N-succinyl-ala-ala-ala-p-nitroanilide |
| LC-qTOF-MS | Liquid chromatography–quadrupole time-of-flight mass spectrometry |
| UNPD | Universal Natural Products Database |
References
- World Health Organization. Ageing: Healthy Ageing and Functional Ability. 2020. Available online: https://www.who.int/news-room/questions-and-answers/item/healthy-ageing-and-functional-ability (accessed on 21 October 2025).
- Ganceviciene, R.; Liakou, A.I.; Theodoridis, A.; Makrantonaki, E.; Zouboulis, C.C. Skin anti-aging strategies. Dermato-Endocrinology 2012, 4, 308–319. [Google Scholar] [CrossRef]
- Öztürkcan, S.; Kayhan, T.Ç. Deri yaşlanmasına karşı medikal önlemler. Dermatoz 2010, 1, 29–34. [Google Scholar]
- Global Industry Analysts. Anti-Aging Products-Global Market Trajectory & Analytics. Dublin. 2020. Available online: https://www.researchandmarkets.com/r/jnrvp0 (accessed on 23 June 2022).
- Vijayakumar, R.; Gani, S.S.A.; Mokhtar, N. Anti-elastase, anti-collagenase and antimicrobial activities of the underutilized red pitaya peel: An in vitro study for anti-aging applications. Asian J. Pharm. Clin. Res. 2017, 10, 251–255. [Google Scholar] [CrossRef]
- Imokawa, G.; Ishida, K. Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging I: Reduced skin elasticity, highly associated with enhanced dermal elastase activity, triggers wrinkling and sagging. Int. J. Mol. Sci. 2015, 16, 7753–7775. [Google Scholar] [CrossRef]
- Tsuji, N.; Moriwaki, S.; Suzuki, Y.; Takema, Y.; Imokawa, G. The role of elastases secreted by fibroblasts in wrinkle formation: Implication through selective ınhibition of elastase activity. Photochem. Photobiol. 2001, 74, 283–290. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Amarowicz, R. Walnut (Juglans regia L.) shell pyroligneous acid: Chemical constituents and functional applications. RSC Adv. 2018, 8, 22376–22391. [Google Scholar] [CrossRef]
- Przekora, A.; Belcarz, A.; Kowalczyk, K.; Wójcik, M.; Wojciechowska, K.; Ginalska, G. UVB protective, anti-aging, and anti-inflammatory properties of aqueous extract of walnut (Juglans regia L.) seeds. Acta Pol. Pharm. 2018, 75, 1167–1176. [Google Scholar] [CrossRef]
- Li, B.; Cui, C.; Zhang, C.; Liu, J.; Hao, F.; Han, L.; Bai, C.; Wei, S. Traditional applications, ethnopharmacology, and phytochemistry of walnut green husk (Juglans regia L.): A review. Nat. Prod. Commun. 2024, 19, 1934578X241262156. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Tabibiazar, M.; Amarowicz, R. A comprehensive review on the chemical constituents and functional uses of walnut (Juglans spp.) husk. Int. J. Mol. Sci. 2019, 20, 3920. [Google Scholar] [CrossRef]
- Liu, M.; Li, C.; Cao, C.; Wang, L.; Li, X.; Che, J.; Yang, H.; Zhang, X.; Zhao, H.; He, G.; et al. Walnut fruit processing equipment: Academic insights and perspectives. Food Eng. Rev. 2021, 13, 822–857. [Google Scholar] [CrossRef]
- Barekat, S.; Nasirpour, A.; Keramat, J.; Dinari, M.; Meziane-Kaci, M.; Paris, C.; Desobry, S. Phytochemical composition, antimicrobial, anticancer properties, and antioxidant potential of green husk from several walnut varieties (Juglans regia L.). Antioxidants. 2023, 12, 52. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Abert-Vian, M.; Fabiano-Tixier, A.S.; Strube, J.; Uhlenbrock, L.; Gunjevic, V.; Cravotto, G. Green extraction of natural products. Origins, current status, and future challenges. Trend. Anal. Chem. 2019, 118, 248–263. [Google Scholar] [CrossRef]
- Demirdoven, A.D.; Tokatli, K.; Korkmaz, Y. Geleneksel ve ultrasonik yöntemlerle vişne posası antosiyaninlerinin ekstraksiyonu. Gıda 2020, 46, 168–179. [Google Scholar] [CrossRef]
- Çağındı, Ö. Mikrodalga uygulamasının kırmızı üzüm suyunun antosiyanin içeriği ile bazı fizikokimyasal özellikleri üzerine etkisi. Akademik Gıda 2016, 14, 356–361. [Google Scholar]
- Picot-Allain, C.; Mahomoodally, M.F.; Ak, G.; Zengin, G. Conventional versus green extraction techniques—A comparative perspective. Curr. Opin. Food Sci. 2021, 40, 144–156. [Google Scholar] [CrossRef]
- Sakti, A.S.; Saputri, F.C.; Mun’im, A. Optimization of choline chloride-glycerol based natural deep eutectic solvent for extraction bioactive substances from Cinnamomum burmannii barks and Caesalpinia sappan heartwoods. Heliyon 2019, 5, e02915. [Google Scholar] [CrossRef]
- Kutlu, N.; Kamiloglu, A.; Elbir, T. Optimization of ultrasound extraction of phenolic compounds from Tarragon (Artemisia dracunculus L.) using Box–Behnken design. Biomass Conv. Bioref. 2022, 12, 5397–5408. [Google Scholar] [CrossRef]
- Ferreira, S.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; Souza, A.S.; Dos Santos, W.N.L. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef]
- Khajeh, M. Optimization of process variables for essential oil components from Satureja hortensis by supercritical fluid extraction using Box-Behnken experimental design. J. Supercrit. Fluid. 2011, 55, 944–948. [Google Scholar] [CrossRef]
- Eshawu, A.B.; Ghalsasi, V.V. Metabolomics of natural samples: A tutorial review on the latest technologies. J. Sep. Sci. 2024, 47, 2300588. [Google Scholar] [CrossRef] [PubMed]
- Chitiva, L.C.; Rezende-Teixeira, P.; Leão, T.F.; Lozano-Puentes, H.S.; Londoño, X.; Díaz-Ariza, L.A.; Costa-Lotufo, L.V.; Prieto-Rodríguez, J.A.; Costa, G.M.; Castro-Gamboa, I. Metabolomic profiling of Guadua species and its correlation with antioxidant and cytotoxic activities. ACS Omega 2024, 9, 36939–36960. [Google Scholar] [CrossRef]
- Gonulalan, E.M.; Nemutlu, E.; Bayazeid, O.; Kocak, E.; Yalçın, F.N.; Demirezer, L.O. Metabolomics and proteomics profiles of some medicinal plants and correlation with BDNF activity. Phytomedicine 2020, 74, 152920. [Google Scholar] [CrossRef] [PubMed]
- Csekes, E.; Račková, L. Skin aging, cellular senescence and natural polyphenols. Int. J. Mol. Sci. 2021, 22, 12641. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.C.; Chin, N.L.; Yusof, Y.A. A Box–Behnken design for determining the optimum experimental condition of cake batter mixing. Food Bioprocess Technol. 2012, 5, 972–982. [Google Scholar] [CrossRef]
- Sankalia, M.G.; Mashru, R.C.; Sankalia, J.M.; Sutariya, V.B. Reversed chitosan–alginate polyelectrolyte complex for stability improvement of alpha-amylase: Optimization and physicochemical characterization. Eur. J. Pharm. Biopharm. 2007, 65, 215–232. [Google Scholar] [CrossRef]
- Maran, J.P.; Mekala, V.; Manikandan, S. Modeling and optimization of ultrasound-assisted extraction of polysaccharide from Cucurbita moschata. Carbohydr. Polym. 2013, 92, 2018–2026. [Google Scholar] [CrossRef]
- Shakeel, F.; Haq, N.; Raish, M.; Anwer, M.K.; Al-Shdefat, R. Solubility and thermodynamic analysis of sinapic acid in various neat solvents at different temperatures. J. Mol. Liq. 2016, 222, 167–171. [Google Scholar] [CrossRef]
- Shen, L.; Pang, S.; Zhong, M.; Sun, Y.; Qayum, A.; Liu, Y.; Rashid, A.; Xu, B.; Liang, Q.; Ma, H.; et al. A comprehensive review of ultrasonic assisted extraction (UAE) for bioactive components: Principles, advantages, equipment, and combined technologies. Ultrason. Sonochem. 2023, 101, 106646. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Wang, G.H.; Kuo, J.T.; Lin, Y.T.; Huang, H.J.; Chang, Y.C.; Chung, Y.C. Ultrasound-assisted extraction of Peucedanum ostruthium leaves: A feasible alternative to rhizomes for industrial applications. Front. Pharmacol. 2025, 16, 1636312. [Google Scholar] [CrossRef]
- Bimakr, M.; Rahman, R.A.; Taip, F.S.; Adzahan, N.M.; Sarker, M.Z.I.; Ganjloo, A. Optimization of ultrasound-assisted extraction of crude oil from winter melon (Benincasa hispida) seed using response surface methodology and evaluation of its antioxidant activity, total phenolic content and fatty acid composition. Molecules 2012, 17, 11748–11762. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Cui, L.; Ma, Z.; Wang, D.; Niu, Y. Ultrasound-assisted extraction, optimization, isolation, and antioxidant activity analysis of flavonoids from Astragalus membranaceus stems and leaves. Ultrason. Sonochem. 2022, 90, 106190. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.B.; Xue, R.; Zhuang, X.C.; Zhang, X.S.; Shi, S.L. Ultrasound-assisted extraction optimization of polyphenols from Boletus bicolor and evaluation of its antioxidant activity. Front. Nutr. 2023, 10, 1135712. [Google Scholar] [CrossRef]
- Slimani, C.; Rais, C.; Mansouri, F.; Rais, S.; Benjelloun, M.; Ullah, R.; Lazraq, A. Optimization of ultrasound-assisted extraction of phenols from Crocus sativus by-products using sunflower oil as a sustainable solvent alternative. Food Chem. X 2024, 23, 101579. [Google Scholar] [CrossRef]
- Jiang, C.; Li, X.; Jiao, Y.; Jiang, D.; Zhang, L.; Fan, B.; Zhang, Q. Optimization for ultrasound-assisted extraction of polysaccharides with antioxidant activity in vitro from the aerial root of Ficus microcarpa. Carbohydr. Polym. 2014, 110, 10–17. [Google Scholar] [CrossRef]
- Thao My, P.L.; Sung, V.V.; Dat, T.D.; Nam, H.M.; Phong, M.T.; Hieu, N.H. Ultrasound-assisted extraction of fucoidan from Vietnamese brown seaweed Sargassum mcclurei and testing bioactivities of the extract. ChemistrySelect 2020, 5, 4371–4380. [Google Scholar] [CrossRef]
- Drouet, S.; Leclerc, E.A.; Garros, L.; Tungmunnithum, D.; Kabra, A.; Abbasi, B.H.; Hano, C. A green ultrasound-assisted extraction optimization of the natural antioxidant and anti-aging flavonolignans from milk thistle Silybum marianum (L.) Gaertn. fruits for cosmetic applications. Antioxidants 2019, 8, 304. [Google Scholar] [CrossRef]
- Landa-Cansigno, C.; Serviere-Zaragoza, E.; Reyes, A.G.; Monribot-Villanueva, J.L.; Guerrero-Analco, J.A.; Sandoval-Gil, J.M.; Zertuche-González, J.A. Untargeted metabolomics-driven insights into the anti-aging potential of brown seaweed Eisenia arborea: Ultrasound-assisted extraction for sustainable cosmetics. J. Appl. Phycol. 2025, 37, 1–13. [Google Scholar] [CrossRef]
- Alshammari, F.; Alam, M.B.; Naznin, M.; Kim, S.; Lee, S.H. Optimization, metabolomic analysis, antioxidant potential and depigmenting activity of polyphenolic compounds from unmature ajwa date seeds (Phoenix dactylifera L.) using ultrasonic-assisted extraction. Antioxidants 2024, 13, 238. [Google Scholar] [CrossRef] [PubMed]
- Gouvinhas, I.; Saavedra, M.J.; Alves, M.J.; Garcia, J. Exploring the impact of ultrasound-assisted extraction on the phytochemical composition and bioactivity of Tamus communis L. fruits. Pharmaceuticals 2025, 18, 1342. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents—Solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Chanioti, S.; Tzia, C. Extraction of phenolic compounds from olive pomace by using natural deep eutectic solvents and innovative extraction techniques. Innov. Food Sci. Emerg. Technol. 2018, 48, 228–239. [Google Scholar] [CrossRef]
- Pavlić, B.; Mrkonjić, Ž.; Teslić, N.; Kljakić, A.C.; Pojić, M.; Mandić, A.; Mišan, A. Natural deep eutectic solvent (NADES) extraction improves polyphenol yield and antioxidant activity of wild thyme (Thymus serpyllum L.) extracts. Molecules 2022, 27, 1508. [Google Scholar] [CrossRef]
- Ferreira, A.S.; Craveiro, R.; Duarte, A.R.; Barreiros, S.; Cabrita, E.J.; Paiva, A. Effect of water on the structure and dynamics of choline chloride/glycerol eutectic systems. J. Mol. Liq. 2021, 342, 117463. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Bildik, F. Application of choline chloride as natural deep eutectic solvents for the green extraction of phenolic compounds from Rheum ribes leaves. Eur. J. Tech. 2021, 11, 140–143. [Google Scholar] [CrossRef]
- Frontini, A.; Tarentini, G.; Negro, C.; Luvisi, A.; Apollonio, M.; De Bellis, L. Effective liquid–liquid extraction for the recovery of grape pomace polyphenols from natural deep eutectic solvents (NaDES). Separations 2025, 12, 148. [Google Scholar] [CrossRef]
- García-Roldán, A.; Piriou, L.; Jauregi, P. Natural deep eutectic solvents as a green extraction of polyphenols from spent coffee ground with enhanced bioactivities. Front. Plant Sci. 2023, 13, 1072592. [Google Scholar] [CrossRef] [PubMed]
- Swamy, G.J.; Sangamithra, A.; Chandrasekar, V. Response surface modeling and process optimization of aqueous extraction of natural pigments from Beta vulgaris using Box–Behnken design of experiments. Dyes Pigm. 2014, 111, 64–74. [Google Scholar] [CrossRef]
- Stoica, F.; Constantin, O.E.; Stănciuc, N.; Aprodu, I.; Bahrim, G.E.; Râpeanu, G. Optimization of the parameters influencing the antioxidant activity and concentration of anthocyanins extracted from red onion skins using a central composite design. Inventions 2022, 7, 89. [Google Scholar] [CrossRef]
- Radhakrishnan, N.; Prabhakaran, V.S.; Wadaan, M.A.; Baabbad, A.; Vinayagam, R.; Kang, S.G. STITCH, physicochemical, ADMET, and in silico analysis of selected Mikania constituents as anti-inflammatory agents. Processes 2023, 11, 1722. [Google Scholar] [CrossRef]
- Deniz, F.S.S.; Salmas, R.E.; Emerce, E.; Cankaya, I.I.T.; Yusufoglu, H.S.; Orhan, I.E. Evaluation of collagenase, elastase and tyrosinase inhibitory activities of Cotinus coggygria Scop. through in vitro and in silico approaches. S. Afr. J. Bot. 2020, 132, 277–288. [Google Scholar] [CrossRef]
- Wittenauer, J.; Mäckle, S.; Sußmann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef]
- Wu, Y.; Ding, C.; Zhang, Z.; Zhang, J.; Li, Y.; Song, X.; Zhang, D. Sesquilignans: Current research and potential prospective. Eur. J. Med. Chem. 2024, 271, 116445. [Google Scholar] [CrossRef] [PubMed]
- Aydın, T.; Gümüştaş, M.; Sancı, T.Ö.; Çakır, A. Herniarin and skimmin coumarins in spice and edible plants and their benefits for health. In Studies in Natural Products Chemistry; Atta-Ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2024; Volume 81, pp. 339–365. [Google Scholar] [CrossRef]
- Chang, K.C.; Laffin, B.; Ponder, J.; Énzsöly, A.; Németh, J.; LaBarbera, D.V.; Petrash, J.M. Beta-glucogallin reduces the expression of lipopolysaccharide-induced inflammatory markers by inhibition of aldose reductase in murine macrophages and ocular tissues. Chem. Biol. Interact. 2013, 202, 283–287. [Google Scholar] [CrossRef]
- Kim, H.G.; Kim, K.S.; Kim, M.; Shin, S.H.; Lee, Y.G.; Bang, M.H.; Lee, D.G.; Baek, N.I. β-Glucogallin isolated from Fusidium coccineum and its enhancement of skin barrier effects. Appl. Biol. Chem. 2020, 63, 77. [Google Scholar] [CrossRef]
- Liang, H.; Huang, Q.; Zou, L.; Wei, P.; Lu, J.; Zhang, Y. Methyl gallate: Review of pharmacological activity. Pharmacol. Res. 2023, 194, 106849. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Vani, M.G.; Wang, S.Y.; Liao, J.W.; Hsu, L.S.; Yang, H.L.; Hseu, Y.C. In vitro and in vivo studies disclosed the depigmenting effects of gallic acid: A novel skin lightening agent for hyperpigmentary skin diseases. Biofactors 2013, 39, 259–270. [Google Scholar] [CrossRef]
- Lee, I.K.; Yun, B.S. Styrylpyrone-class compounds from medicinal fungi Phellinus and Inonotus spp., and their medicinal importance. J. Antibiot. 2011, 64, 349–359. [Google Scholar] [CrossRef]
- Kim, M.; Kim, J.; Shin, Y.K.; Kim, K.Y. Gentisic acid stimulates keratinocyte proliferation through ERK1/2 phosphorylation. Int. J. Med. Sci. 2020, 17, 626. [Google Scholar] [CrossRef]
- Ma, N.S.; Abraham, M.; Choi, M.J.; Kim, J.S. Skin permeation and comparative evaluation of gentisic acid ester derivatives as skin-lightening agents. J. Drug Deliv. Sci. Technol. 2014, 24, 212–217. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, M.; Zhang, Y.; Zheng, Y.; Li, J.; Cai, Q.; Qu, Y. Synergistic and antagonistic mechanisms of Arctium lappa L. polyphenols on human neutrophil elastase inhibition: Insights from molecular docking and enzymatic kinetics. Molecules 2025, 30, 2764. [Google Scholar] [CrossRef]
- Tóth, B.; Chang, F.R.; Hwang, T.L.; Szappanos, Á.; Mándi, A.; Hunyadi, A.; Vasas, A. Screening of Luzula species native to the Carpathian Basin for anti-inflammatory activity and bioactivity-guided isolation of compounds from Luzula luzuloides (Lam.) Dandy & Wilmott. Fitoterapia 2017, 116, 131–138. [Google Scholar] [CrossRef]
- Girsang, E.; Lister, I.N.E.; Ginting, C.N.; Bethasari, M.; Amalia, A.; Widowati, W. Comparison of antiaging and antioxidant activities of protocatechuic and ferulic acids. Mol. Cell. Biomed. Sci. 2020, 4, 68–75. [Google Scholar] [CrossRef]
- Xing, X.; Yang, X.; Cao, Y. Study of ellagic acid as a natural elastase inhibitor by spectroscopic methods. J. Appl. Spectrosc. 2016, 83, 149–155. [Google Scholar] [CrossRef]
- Maity, N.; Nema, N.K.; Abedy, M.K.; Sarkar, B.K.; Mukherjee, P.K. Exploring Tagetes erecta Linn flower for the elastase, hyaluronidase and MMP-1 inhibitory activity. J. Ethnopharmacol. 2011, 137, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Vieira, V.; Pereira, C.; Abreu, R.M.; Calhelha, R.C.; Alves, M.J.; Coutinho, J.A.P.; Ferreira, O.; Barros, L.; Ferreira, I.C. Hydroethanolic extract of Juglans regia L. green husks: A source of bioactive phytochemicals. Food Chem. Toxicol. 2020, 137, 111189. [Google Scholar] [CrossRef] [PubMed]
- Skarpalezos, D.; Detsi, A. Deep eutectic solvents as extraction media for valuable flavonoids from natural sources. Appl. Sci. 2019, 9, 4169. [Google Scholar] [CrossRef]
- Rolińska, K.; Jakubowska, E.; Żmieńko, M.; Łęczycka-Wilk, K. Choline chloride-based deep eutectic solvents as plasticizer and active agent in chitosan films. Food Chem. 2024, 444, 138375. [Google Scholar] [CrossRef]
- Huang, M.M.; Yiin, C.L.; Lock, S.S.M.; Chin, B.L.F.; Othman, I.; Chan, Y.H. Natural deep eutectic solvents (NADES) for sustainable extraction of bioactive compounds from medicinal plants: Recent advances, challenges, and future directions. J. Mol. Liq. 2025, 425, 127202. [Google Scholar] [CrossRef]
- Freitas, D.S.; Rocha, D.; Castro, T.G.; Noro, J.; Castro, V.I.; Teixeira, M.A.; Silva, C. Green extraction of cork bioactive compounds using natural deep eutectic mixtures. ACS Sustain. Chem. Eng. 2022, 10, 7974–7989. [Google Scholar] [CrossRef]
- Moon, J.Y.; Yim, E.Y.; Song, G.; Lee, N.H.; Hyun, C.G. Screening of elastase and tyrosinase inhibitory activity from Jeju Island plants. Eurasia J. Biosci. 2010, 4, 41–53. [Google Scholar] [CrossRef]
- Abd-El-Aziz, N.M.; Hifnawy, M.S.; El-Ashmawy, A.A.; Lotfy, R.A.; Younis, I.Y. Application of Box–Behnken design for optimization of phenolics extraction from Leontodon hispidulus in relation to its antioxidant, anti-inflammatory and cytotoxic activities. Sci. Rep. 2022, 12, 8829. [Google Scholar] [CrossRef]
- Ahmad, A.; Rehman, M.U.; Wali, A.F.; El-Serehy, H.A.; Al-Misned, F.A.; Maodaa, S.N.; Aljawdah, H.M.; Mir, T.M.; Ahmad, P. Box–Behnken response surface design of polysaccharide extraction from Rhododendron arboreum and the evaluation of its antioxidant potential. Molecules 2020, 25, 3835. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, S.; Verza, S.G.; Moraes, R.C.; Pittol, V.; Peñaloza, E.M.C.; Pavei, C.; Ortega, G.G. Extraction optimization of polyphenols, oxindole alkaloids and quinovic acid glycosides from cat’s claw bark by Box–Behnken design. Ind. Crops Prod. 2013, 48, 153–161. [Google Scholar] [CrossRef]
- Gomes, S.V.; Portugal, L.A.; dos Anjos, J.P.; de Jesus, O.N.; de Oliveira, E.J.; David, J.P.; David, J.M. Accelerated solvent extraction of phenolic compounds exploiting a Box–Behnken design and quantification of five flavonoids by HPLC-DAD in Passiflora species. Microchem. J. 2017, 132, 28–35. [Google Scholar] [CrossRef]
- Guo, L.; Zhu, W.; Xu, F.; Liu, M.; Xie, Y.; Zhang, J. Optimized ultrasonic-assisted extraction of polysaccharides from Cyclina sinensis and evaluation of antioxidant activities in vitro. CyTA J. Food 2014, 12, 32–39. [Google Scholar] [CrossRef]
- Waris, M.; Kocak, E.; Gonulalan, E.M.; Demirezer, L.O.; Kır, S.; Nemutlu, E. Metabolomics analysis insight into medicinal plant science. TrAC Trends Anal. Chem. 2022, 157, 116795. [Google Scholar] [CrossRef]
- Lee, J.; Ji, J.; Park, S.H. Antiwrinkle and antimelanogenesis activity of the ethanol extracts of Lespedeza cuneata G. Don for development of the cosmeceutical ingredients. Food Sci. Nutr. 2018, 6, 1307–1316. [Google Scholar] [CrossRef]
- Lai, Z.; Tsugawa, H.; Wohlgemuth, G.; Mehta, S.; Mueller, M.; Zheng, Y.; Ogiwara, A.; Meissen, J.; Showalter, M.; Takeuchi, K.; et al. Identifying metabolites by integrating metabolome databases with mass spectrometry cheminformatics. Nat. Methods 2018, 15, 53–56. [Google Scholar] [CrossRef] [PubMed]
| A | B | C | R1 (Elastase Inhibition) | |||
|---|---|---|---|---|---|---|
| % | °C | min. | Exp. (µg/mL) | Pre. (µg/mL) | PRE % | |
| 1 | 0 | −1 | −1 | 3.39 | 4.70 | 27.87 |
| 2 | 0 | 0 | 0 | 9.99 | 9.83 | −1.63 |
| 3 | 0 | 0 | 0 | 9.96 | 9.83 | −1.32 |
| 4 | +1 | −1 | 0 | 1.78 | 0.39 | −346.34 |
| 5 | −1 | −1 | 0 | 19.61 | 21.24 | 7.67 |
| 6 | −1 | 0 | +1 | 18.06 | 17.99 | −0.39 |
| 7 | +1 | 0 | −1 | 1.41 | 1.48 | 4.73 |
| 8 | 0 | 0 | 0 | 9.62 | 9.83 | 2.14 |
| 9 | 0 | −1 | +1 | 13.68 | 12.12 | −12.87 |
| 10 | 0 | 0 | 0 | 9.72 | 9.83 | 1.12 |
| 11 | 0 | +1 | −1 | 40.85 | 42.41 | 3.68 |
| 12 | −1 | 0 | −1 | 32.67 | 29.73 | −9.89 |
| 13 | +1 | 0 | +1 | 1.16 | 4.10 | 71.71 |
| 14 | −1 | +1 | 0 | 45.82 | 47.20 | 2.92 |
| 15 | 0 | +1 | +1 | 27.19 | 25.88 | −5.06 |
| 16 | +1 | +1 | 0 | 27.52 | 25.89 | −6.30 |
| 17 | 0 | 0 | 0 | 9.86 | 9.83 | −0.31 |
| Elastase Inhibition | |||||
|---|---|---|---|---|---|
| p Value | Coefficient Estimate | Standard Error | 95% CI Low | 95% CI High | |
| Model | <0.0001 | ||||
| Intercept | 9.83 | 0.9973 | 7.47 | 12.19 | |
| A-Solvent Ratio | <0.0001 | −10.54 | 0.7884 | −12.40 | −8.67 |
| B-Temperature | <0.0001 | 12.87 | 0.7884 | 11.00 | 14.73 |
| C-Time | 0.0233 | −2.28 | 0.7884 | −4.14 | −0.4144 |
| AB | 0.9190 | −0.1175 | 1.12 | −2.75 | 2.52 |
| AC | 0.0147 | 3.59 | 1.12 | 0.9534 | 6.23 |
| BC | 0.0010 | −5.99 | 1.12 | −8.62 | −3.35 |
| A2 | 0.0300 | 2.95 | 1.09 | 0.3801 | 5.52 |
| B2 | <0.0001 | 10.90 | 1.09 | 8.33 | 13.47 |
| C2 | 0.6314 | 0.5450 | 1.09 | −2.02 | 3.11 |
| Residual | |||||
| Lack of fit | <0.0001 | ||||
| U1 | U2 | M1 | |
|---|---|---|---|
| NADES/H2O ratio (w/w) → 99.79% Temperature (°C) → 51.30 Time (min.) → 49.58 | NADES/H2O ratio (w/w) →99.79% Temperature (°C) → 25 Time (min.) → 49.58 | NADES/H2O ratio (w/w) → 99.79% Temperature (°C) → 25 Time (min.) → 24 h | |
| IC50 values of elastase inhibition | |||
| Predicted | Experimental | Experimental | Experimental |
| 0.7791 ± 0.0275 μg/mL | 0.7735 ± 0.0182 μg/mL | 1.8213 ± 0.3173 μg/mL | 12.6377 ± 0.5275 μg/mL |
| Independent Variables | |||||
|---|---|---|---|---|---|
| Factors | Name | Units | Coded Low | Coded Mean | Coded High |
| A | NADES/H2O ratio * | % | −1 ↔ 25.00 | 0 ↔ 62.50 | +1 ↔ 100.00 |
| B | Temperature | °C | −1 ↔ 40.00 | 0 ↔ 60.00 | +1 ↔ 80.00 |
| C | Time | min. | −1 ↔ 20.00 | 0 ↔ 40.00 | +1 ↔ 60.00 |
| Dependent Variables | |||||
| Responses | Name | Units | |||
| R1 | Elastaz inhibition (IC50) | μg/mL | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Şener, S.Ö.; Shaha, S.; Barazandeh, S.S.; Şen, Ö.; Koçak, E.; Subaş, T.; Kıraç, Ş.N.; Nemutlu, E. Metabolomic Profiling, Box–Behnken Design-Based Optimization of Ultrasonic Extraction, and Skin Anti-Aging Potential of the Green Husk of Juglans regia L. as a Sustainable Natural Waste. Molecules 2025, 30, 4191. https://doi.org/10.3390/molecules30214191
Şener SÖ, Shaha S, Barazandeh SS, Şen Ö, Koçak E, Subaş T, Kıraç ŞN, Nemutlu E. Metabolomic Profiling, Box–Behnken Design-Based Optimization of Ultrasonic Extraction, and Skin Anti-Aging Potential of the Green Husk of Juglans regia L. as a Sustainable Natural Waste. Molecules. 2025; 30(21):4191. https://doi.org/10.3390/molecules30214191
Chicago/Turabian StyleŞener, Sıla Özlem, Sabita Shaha, Sahar Sadigh Barazandeh, Ömer Şen, Engin Koçak, Tuğba Subaş, Şerife Nur Kıraç, and Emirhan Nemutlu. 2025. "Metabolomic Profiling, Box–Behnken Design-Based Optimization of Ultrasonic Extraction, and Skin Anti-Aging Potential of the Green Husk of Juglans regia L. as a Sustainable Natural Waste" Molecules 30, no. 21: 4191. https://doi.org/10.3390/molecules30214191
APA StyleŞener, S. Ö., Shaha, S., Barazandeh, S. S., Şen, Ö., Koçak, E., Subaş, T., Kıraç, Ş. N., & Nemutlu, E. (2025). Metabolomic Profiling, Box–Behnken Design-Based Optimization of Ultrasonic Extraction, and Skin Anti-Aging Potential of the Green Husk of Juglans regia L. as a Sustainable Natural Waste. Molecules, 30(21), 4191. https://doi.org/10.3390/molecules30214191