Recent Applications of Albumin as a Green and Versatile Catalyst in Organic Synthesis
Abstract
1. Introduction
2. Single Transformations
2.1. C-C Bond and C-N Formation Reactions
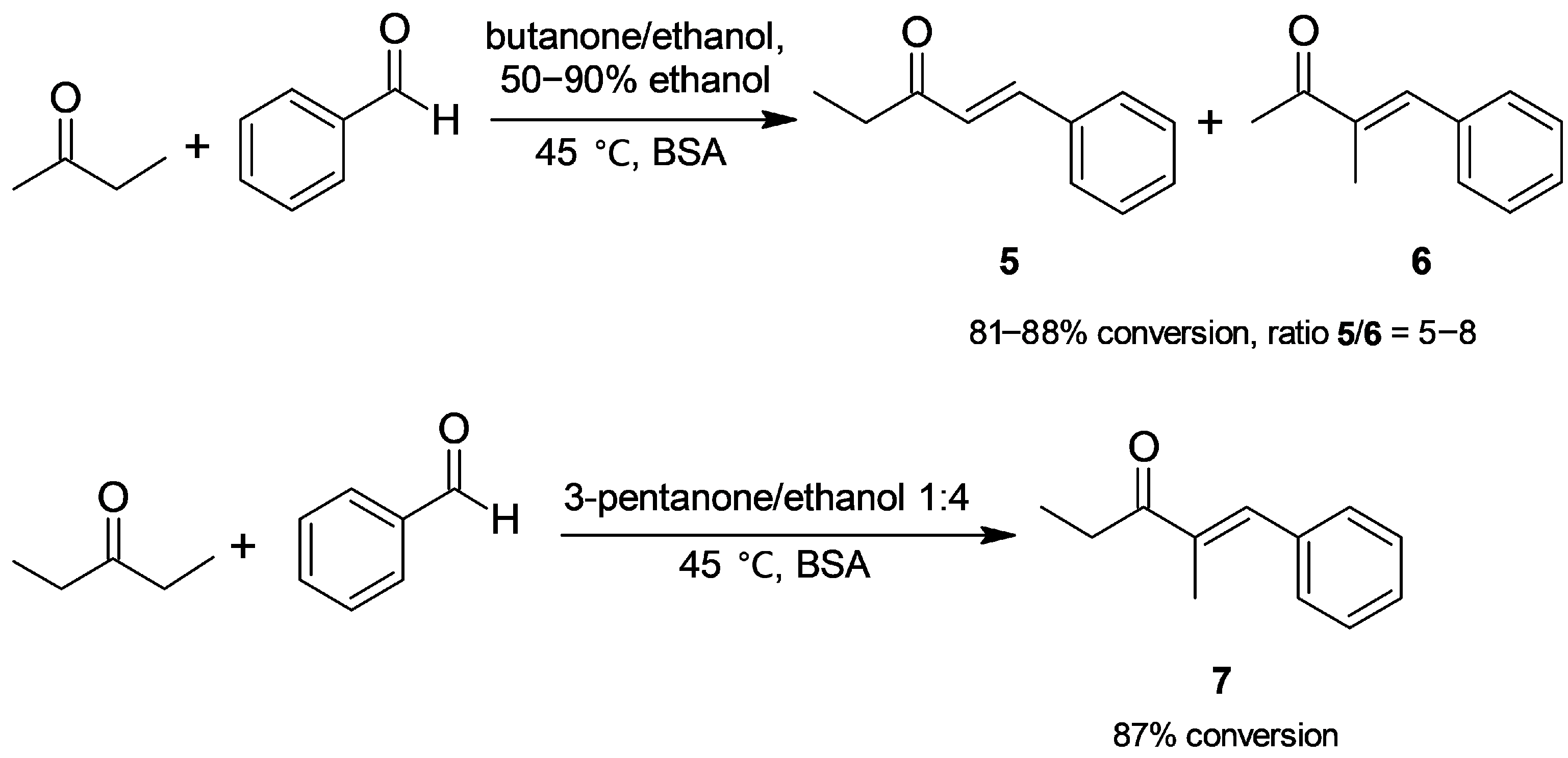
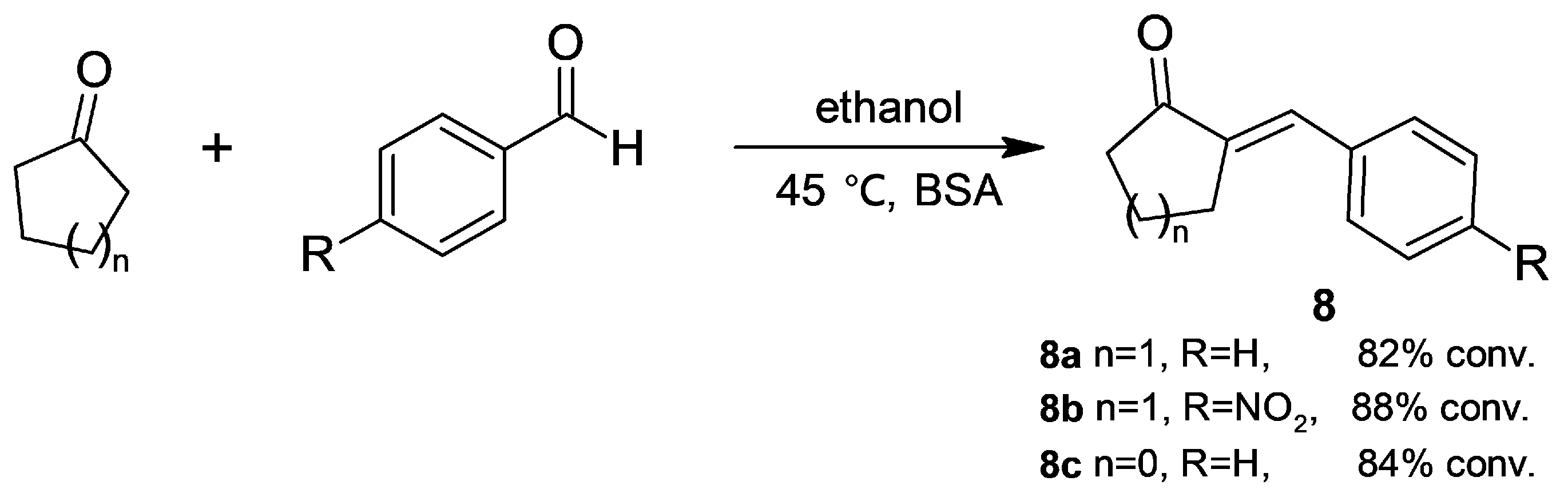
2.1.1. Cross-Aldol Condensation
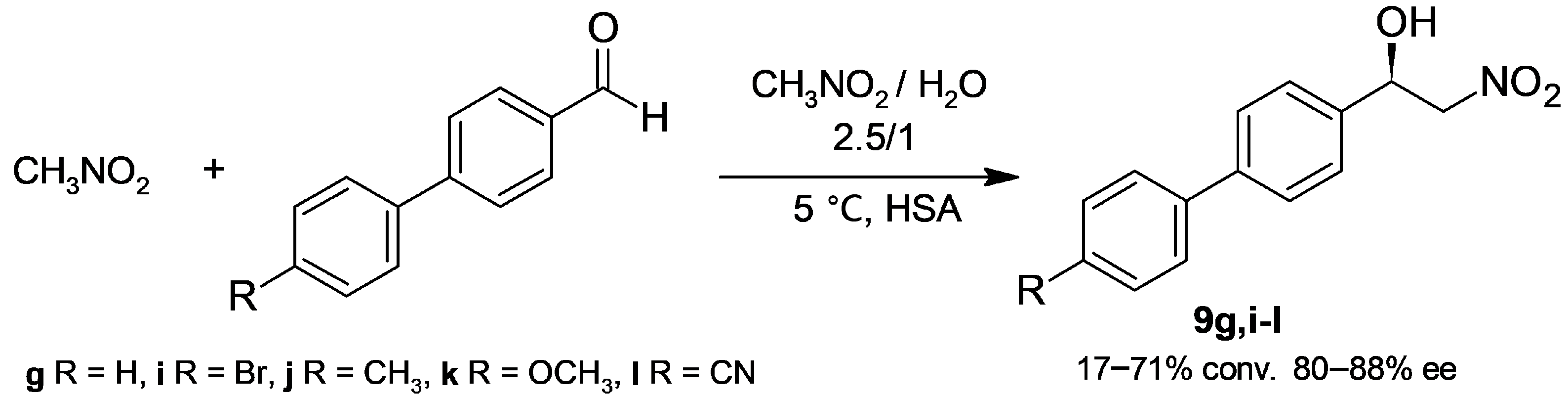
2.1.2. Henry Reaction
2.1.3. Knoevenagel Condensation
2.1.4. Imine Formation
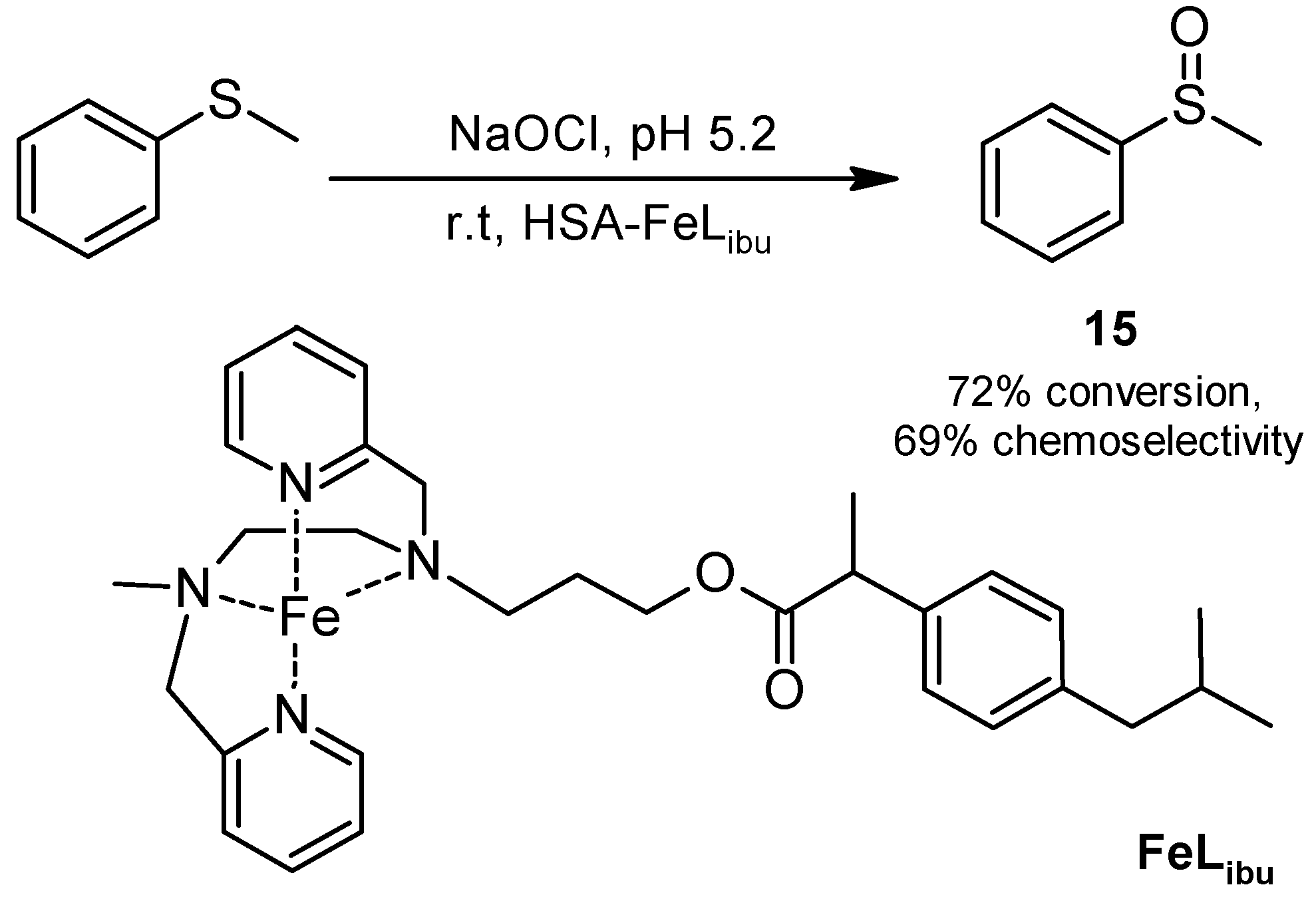
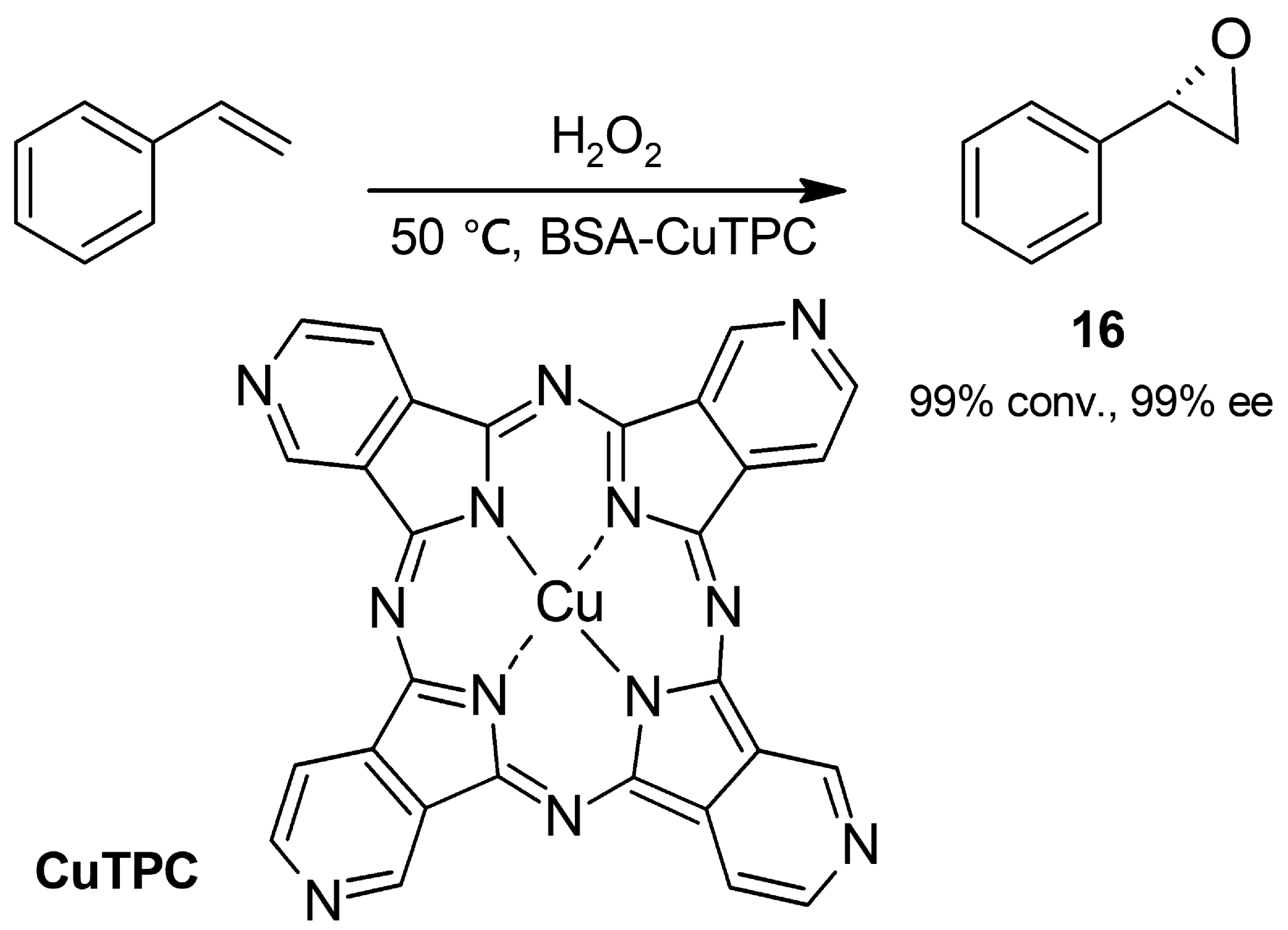
2.2. Oxidations
2.2.1. Oxidation of Thiols into Disulfides
2.2.2. Sulfoxidation
2.2.3. Epoxidation
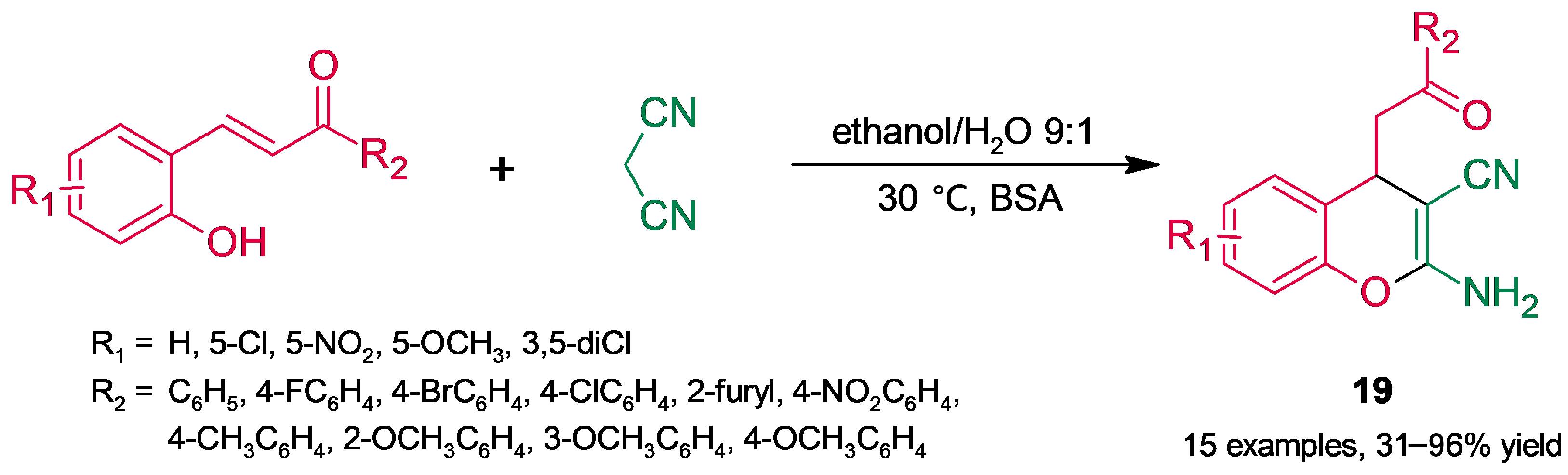
3. Tandem and Multicomponent Reactions
3.1. C-S Bond Formation Reactions
3.2. Heterocycle Synthesis
3.2.1. Oxygenated Heterocycles
3.2.2. Nitrogenated Heterocycles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BHT | Butylated hydroxytoluene |
| DFT | Discrete Fourier transform |
| EPR | Electron paramagnetic resonance |
| ESI-MS | Electrospray ionization mass spectrometry |
| FESEM | Field emission scanning electron microscope |
| FT-IR | Fourier-transform infrared spectroscopy |
| MALDI-TOF | Matrix-assisted laser desorption/ionization time-of-flight |
| NMR | Nuclear magnetic resonance |
| TEM | Transmission electron microscopy |
| TEMPO | 2,2,6,6-Tetramethylpiperidine-1-oxyl |
| UV–vis | Ultraviolet–visible |
| XRD | X-ray diffraction |
References
- Sheldon, R.A. Biocatalysis and Green Chemistry. In Green Biocatalysis; Wiley: Hoboken, NJ, USA, 2016; pp. 1–15. [Google Scholar]
- O’Connell, A.; Barry, A.; Burke, A.J.; Hutton, A.E.; Bell, E.L.; Green, A.P.; O’Reilly, E. Biocatalysis: Tandmark Discoveries and Applications in Chemical Synthesis. Chem. Soc. Rev. 2024, 53, 2828–2850. [Google Scholar] [CrossRef]
- Roy, I.; Gupta, M.N. White and Grey Biotechnologies for Shaping a Sustainable Future. RSC Sustain. 2023, 1, 1722–1736. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Brady, D.; Bode, M.L. The Hitchhiker’s Guide to Biocatalysis: Recent Advances in the Use of Enzymes in Organic Synthesis. Chem. Sci. 2020, 11, 2587–2605. [Google Scholar] [CrossRef]
- Reisenbauer, J.C.; Sicinski, K.M.; Arnold, F.H. Catalyzing the Future: Recent Advances in Chemical Synthesis Using Enzymes. Curr. Opin. Chem. Biol. 2024, 83, 102536. [Google Scholar] [CrossRef]
- Wu, S.; Snajdrova, R.; Moore, J.C.; Baldenius, K.; Bornscheuer, U.T. Biocatalysis: Enzymatic Synthesis for Industrial Applications. Angew. Chem. Int. Ed. 2021, 60, 88–119. [Google Scholar] [CrossRef]
- Humble, M.S.; Berglund, P. Biocatalytic Promiscuity. Eur. J. Org. Chem. 2011, 3391–3401. [Google Scholar] [CrossRef]
- Singla, P.; Bhardwaj, R.D. Enzyme Promiscuity—A Light on the “Darker” Side of Enzyme Specificity. Biocatal. Biotransformation 2020, 38, 81–92. [Google Scholar] [CrossRef]
- Khersonsky, O.; Tawfik, D.S. Enzyme Promiscuity: A Mechanistic and Evolutionary Perspective. Annu. Rev. Biochem. 2010, 79, 471–505. [Google Scholar] [CrossRef] [PubMed]
- Copley, S.D. Shining a Light on Enzyme Promiscuity. Curr. Opin. Struct. Biol. 2017, 47, 167–175. [Google Scholar] [CrossRef]
- Tawfik, D.S. Messy Biology and the Origins of Evolutionary Innovations. Nat. Chem. Biol. 2010, 6, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Leveson-Gower, R.B.; Mayer, C.; Roelfes, G. The Importance of Catalytic Promiscuity for Enzyme Design and Evolution. Nat. Rev. Chem. 2019, 3, 687–705. [Google Scholar] [CrossRef]
- Bornscheuer, U.T.; Kazlauskas, R. Hydrolases in Organic Chemistry. Regio- and Stereoselective Transformations, 2nd ed.; Wiley: Weinheim, Germany, 2006. [Google Scholar]
- Busto, E.; Gotor-Fernández, V.; Gotor, V. Hydrolases: Catalytically Promiscuous Enzymes for Non-Conventional Reactions in Organic Synthesis. Chem. Soc. Rev. 2010, 39, 4504. [Google Scholar] [CrossRef]
- López-Iglesias, M.; Gotor-Fernández, V. Recent Advances in Biocatalytic Promiscuity: Hydrolase—Catalyzed Reactions for Nonconventional Transformations. Chem. Rec. 2015, 15, 743–759. [Google Scholar] [CrossRef]
- Dwivedee, B.P.; Soni, S.; Sharma, M.; Bhaumik, J.; Laha, J.K.; Banerjee, U.C. Promiscuity of Lipase—Catalyzed Reactions for Organic Synthesis: A Recent Update. ChemistrySelect 2018, 3, 2441–2466. [Google Scholar] [CrossRef]
- Orozco, E.V.M.; Porto, A.L.M. Promiscuous Activity of Hydrolases. In Biocatalysis for Practitioners; De Gonzalo, G., Lavandera, I., Eds.; Wiley: Hoboken, NJ, USA, 2021; pp. 113–142. [Google Scholar] [CrossRef]
- Patti, A.; Sanfilippo, C. Stereoselective Promiscuous Reactions Catalyzed by Lipases. Int. J. Mol. Sci. 2022, 23, 2675. [Google Scholar] [CrossRef]
- Zhao, H. Recent Advances in Enzymatic Carbon–Carbon Bond Formation. RSC Adv. 2024, 14, 25932–25974. [Google Scholar] [CrossRef]
- Zhao, H.; Roy, A.; Samaranayake, A.; Ishtaweera, P.; Baker, G.A.; Duong, N.; Markmann, L.G.; Fernando, N.S.; Mitchell-Koch, K.R. Lipase-Catalyzed Michael Addition in ‘Water-like’ Ionic Liquids and Tertiary Amides: What Is the Role of the Enzymes? Lagmuir 2025, 41, 12718–12730. [Google Scholar] [CrossRef]
- Fanali, G.; Di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human Serum Albumin: From Bench to Bedside. Mol. Aspects Med. 2012, 33, 209–290. [Google Scholar] [CrossRef]
- Albanese, D.C.M.; Gaggero, N. Albumin as a Promiscuous Biocatalyst in Organic Synthesis. RSC Adv. 2015, 5, 10588–10598. [Google Scholar] [CrossRef]
- Hollfelder, F.; Kirby, A.J.; Tawfik, D.S. Off-the-Shelf Proteins That Rival Tailor-Made Antibodies as Catalysts. Nature 1996, 383, 60–63. [Google Scholar] [CrossRef]
- Kikuchi, K.; Thorn, S.N.; Hilvert, D. Albumin-Catalyzed Proton Transfer. J. Am. Chem. Soc. 1996, 118, 8184–8185. [Google Scholar] [CrossRef]
- Hollfelder, F.; Kirby, A.J.; Tawfik, D.S.; Kikuchi, K.; Hilvert, D. Characterization of Proton-Transfer Catalysis by Serum Albumins. J. Am. Chem. Soc. 2000, 122, 1022–1029. [Google Scholar] [CrossRef]
- Benedetti, F.; Berti, F.; Bidoggia, S. Aldolase Activity of Serum Albumins. Org. Biomol. Chem. 2011, 9, 4417. [Google Scholar] [CrossRef] [PubMed]
- Luisi, I.; Pavan, S.; Fontanive, G.; Tossi, A.; Benedetti, F.; Savoini, A.; Maurizio, E.; Sgarra, R.; Sblattero, D.; Berti, F. An Albumin-Derived Peptide Scaffold Capable of Binding and Catalysis. PLoS ONE 2013, 8, e56469. [Google Scholar] [CrossRef] [PubMed]
- Kragh-Hansen, U. Molecular and Practical Aspects of the Enzymatic Properties of Human Serum Albumin and of Albumin–Ligand Complexes. Biochim. Biophys. Acta BBA—Gen. Subj. 2013, 1830, 5535–5544. [Google Scholar] [CrossRef] [PubMed]
- Ardanaz, S.M.; Velez Rueda, A.J.; Parisi, G.; Iribarren, A.M.; Iglesias, L.E. A Mild Procedure for Enone Preparation Catalysed by Bovine Serum Albumin in a Green and Easily Available Medium. Catal. Lett. 2018, 148, 1750–1757. [Google Scholar] [CrossRef]
- Milker, S.; Pätzold, M.; Bloh, J.Z.; Holtmann, D. Comparison of Deep Eutectic Solvents and Solvent-Free Reaction Conditions for Aldol Production. Mol. Catal. 2019, 466, 70–74. [Google Scholar] [CrossRef]
- Velez Rueda, A.J.; Benítez, G.I.; Sommese, L.M.; Ardanaz, S.M.; Borucki, E.L.; Palopoli, N.; Iglesias, L.E.; Parisi, G. Structural and Evolutionary Analysis Unveil Functional Adaptations in the Promiscuous Behavior of Serum Albumins. Biochimie 2022, 197, 113–120. [Google Scholar] [CrossRef]
- Ning, N.; Zhao, Z.; Li, X.; Liu, Y.; Zhang, Y. A Proline-Based Artificial Enzyme That Favors Aldol Condensation Enables Facile Synthesis of Aliphatic Ketones via Tandem Catalysis. ACS Synth. Biol. 2024, 13, 1100–1104. [Google Scholar] [CrossRef]
- Ardanaz, S.M.; Borucki, E.L.; Velez Rueda, A.J.; Parisi, G.; Iribarren, A.M.; Iglesias, L.E. Bovine Serum Albumin-Catalysed Cross Aldol Condensation: Influence of Ketone Structure. Process Biochem. 2019, 86, 50–57. [Google Scholar] [CrossRef]
- Matsumoto, K.; Asakura, S. Albumin-Mediated Asymmetric Nitroaldol Reaction of Aromatic Aldehydes with Nitromethane in Water. Tetrahedron Lett. 2014, 55, 6919–6921. [Google Scholar] [CrossRef]
- Matsumoto, K.; Kitabayashi, R.; Fukuchi, N.; Suka, N. Preparation of Optically Active Biphenyl Compounds via an Albumin-Mediated Asymmetric Nitroaldol Reaction. Lett. Org. Chem. 2022, 19, 118–125. [Google Scholar] [CrossRef]
- Koszelewski, D.; Ostaszewski, R. Enzyme Promiscuity as a Remedy for the Common Problems with Knoevenagel Condensation. Chem.—Eur. J. 2019, 25, 10156–10164. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Campbell, C.D. Enzymatic Knoevenagel Condensation in Dual-Functionalized Water-Mimic Ionic Liquids and Tertiary Amide Solvents. J. Chem. Technol. Biotechnol. 2024, 99, 780–787. [Google Scholar] [CrossRef]
- Li, W.; Li, R.; Yu, X.; Xu, X.; Guo, Z.; Tan, T.; Fedosov, S.N. Lipase-Catalyzed Knoevenagel Condensation in Water–Ethanol Solvent System. Does the Enzyme Possess the Substrate Promiscuity? Biochem. Eng. J. 2015, 101, 99–107. [Google Scholar] [CrossRef]
- Jafari, A.; Shahedi, M.; Habibi, Z. One-Pot Synthesis of Benzothiazole Derivatives Using Bovine Serum Albumin and Novozym435. Tetrahedron 2024, 160, 134051. [Google Scholar] [CrossRef]
- Saima; Kumar, L.; Lavekar, A.G.; Sharma, T.; Shamsuzzama; Equbal, D.; Siddiqi, M.I.; Sinha, A.K.; Nazir, A. Chemo—Biocatalytic Oxidative Condensation of Natural Arylpropene with 2—Aminobenzothiazole into Schiff—Bases as Potent Anti—Amyloid Agents: Studies Employing Transgenic C. Elegans. ChemistrySelect 2018, 3, 3539–3547. [Google Scholar] [CrossRef]
- Saima; Lavekar, A.G.; Kumar, R.; Sinha, A.K. Bovine Serum Albumin Triggered Waste-Free Aerobic Oxidative Coupling of Thiols into Disulphides on Water: An Extended Synthesis of Bioactive Dithiobis(Phenylene)Bis(Benzylideneimine) via Sequential Oxidative Coupling–Condensation Reactions in One Pot from Aminothiophenol and Benzaldehyde. J. Mol. Catal. B Enzym. 2015, 116, 113–123. [Google Scholar] [CrossRef]
- Lin, Y.-W. Rational Design of Metalloenzymes: From Single to Multiple Active Sites. Coord. Chem. Rev. 2017, 336, 1–27. [Google Scholar] [CrossRef]
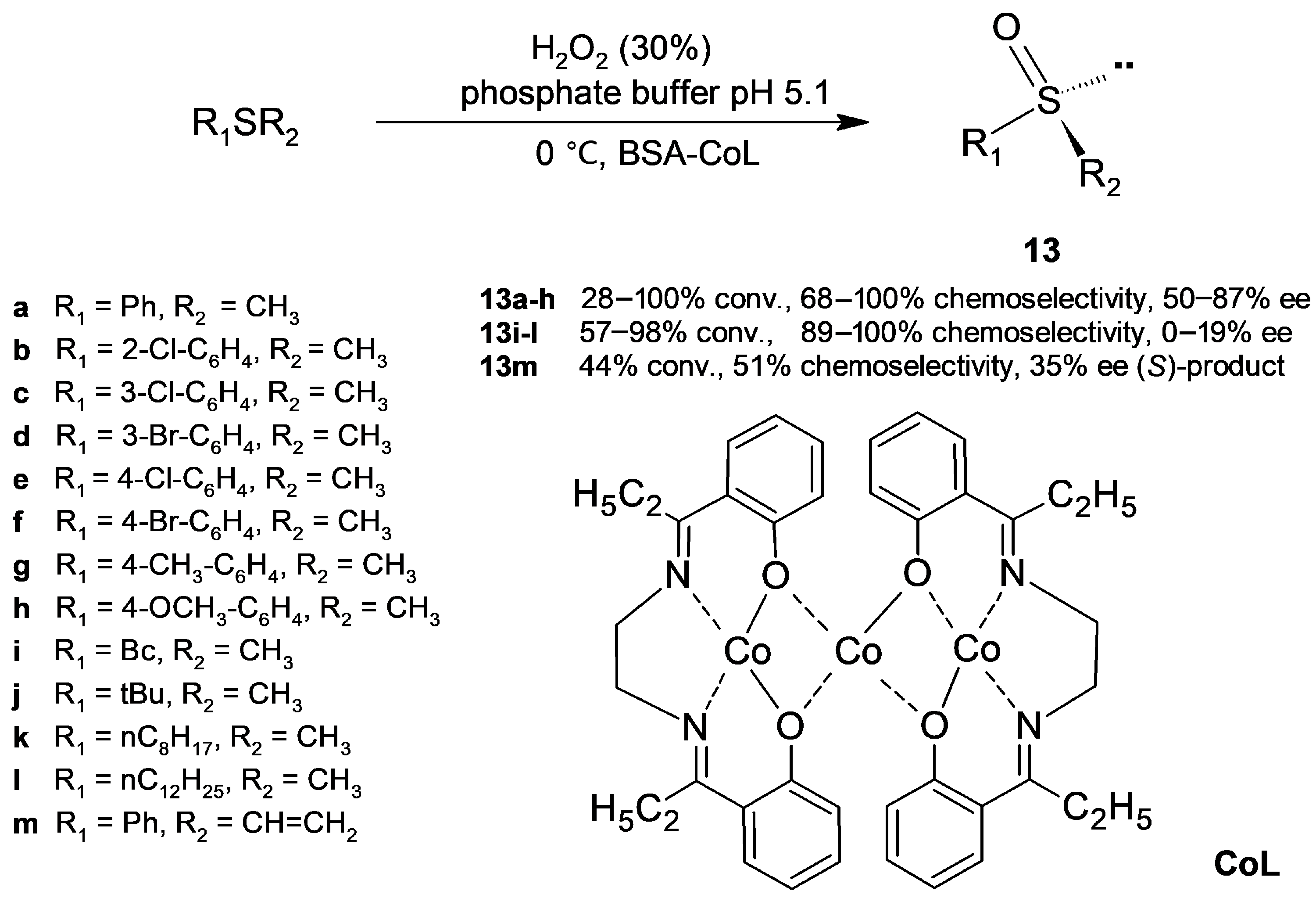
- Tang, J.; Huang, F.; Wei, Y.; Bian, H.; Zhang, W.; Liang, H. Bovine Serum Albumin–Cobalt(ii) Schiff Base Complex Hybrid: An Efficient Artificial Metalloenzyme for Enantioselective Sulfoxidation Using Hydrogen Peroxide. Dalton Trans. 2016, 45, 8061–8072. [Google Scholar] [CrossRef]
- Tang, J.; Yao, P.; Huang, F.; Luo, M.; Wei, Y.; Bian, H. Stereoselective Sulfoxidation Catalyzed by Achiral Schiff Base Complexes in the Presence of Serum Albumin in Aqueous Media. Tetrahedron Asymmetry 2017, 28, 1700–1707. [Google Scholar] [CrossRef]
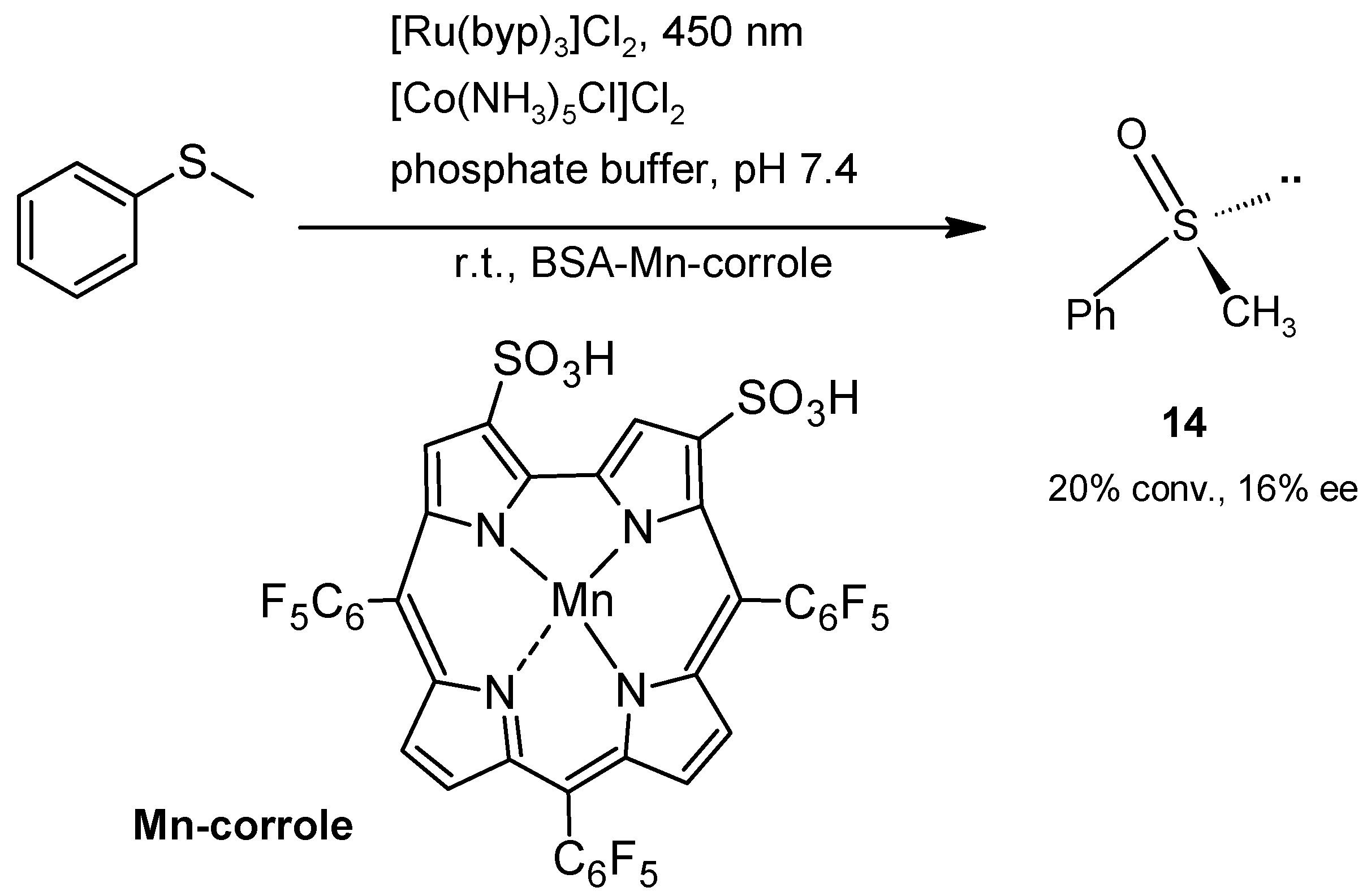
- Herrero, C.; Quaranta, A.; Ricoux, R.; Trehoux, A.; Mahammed, A.; Gross, Z.; Banse, F.; Mahy, J.-P. Oxidation Catalysis via Visible-Light Water Activation of a [Ru(Bpy)3]2+ Chromophore BSA–Metallocorrole Couple. Dalton Trans. 2016, 45, 706–710. [Google Scholar] [CrossRef]
- Leboffe, L.; di Masi, A.; Polticelli, F.; Trezza, V.; Ascenzi, P. Structural Basis of Drug Recognition by Human Serum Albumin. Curr. Med. Chem. 2020, 27, 4907–4931. [Google Scholar] [CrossRef] [PubMed]
- De Simone, G.; Di Masi, A.; Ascenzi, P. Serum Albumin: A Multifaced Enzyme. Int. J. Mol. Sci. 2021, 22, 10086. [Google Scholar] [CrossRef]
- Rondot, L.; Girgenti, E.; Oddon, F.; Marchi-Delapierre, C.; Jorge-Robin, A.; Ménage, S. Catalysis without a Headache: Modification of Ibuprofen for the Design of Artificial Metalloenzyme for Sulfide Oxidation. J. Mol. Catal. Chem. 2016, 416, 20–28. [Google Scholar] [CrossRef]
- Liaqat, M.; McDonald, E.; Ortega, R.J.V.; Lopes, A.; Codreanu, F.; Carlisle, H.; Kumar, C.V.; Yao, X.; Rusling, J.F.; He, J. Cu-Albumin Artificial Enzymes with Peroxidase and Oxidase Activity for Stereoselective Oxidations. ACS Catal. 2024, 14, 16344–16352. [Google Scholar] [CrossRef]
- Mulina, O.M.; Ilovaisky, A.I.; Terent’ev, A.O. Sulfenylation of Indoles Mediated by Iodine and Its Compounds. ChemistrySelect 2021, 6, 10369–10378. [Google Scholar] [CrossRef]
- Saima, S.; Equbal, D.; Lavekar, A.G.; Sinha, A.K. Cooperative Catalysis by Bovine Serum Albumin–Iodine towards Cascade Oxidative Coupling-C(Sp2)–H Sulfenylation of Indoles/Hydroxyaryls with Thiophenols on Water. Org. Biomol. Chem. 2016, 14, 6111–6118. [Google Scholar] [CrossRef]
- Thopate, Y.; Singh, R.; Sinha, A.K.; Kumar, V.; Siddiqi, M.I. NMR and DFT Insight into the Synergistic Role of Bovine Serum Albumin–Ionic Liquid for Multicomponent Cascade Aldol/Knoevenagel—thia—Michael/Michael Reactions in One Pot. ChemCatChem 2016, 8, 3050–3056. [Google Scholar] [CrossRef]
- Hashmi, S.Z.; Bareth, D.; Dwivedi, J.; Kishore, D.; Alvi, P.A. Green Advancements towards the Electrochemical Synthesis of Heterocycles. RSC Adv. 2024, 14, 18192–18246. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Hong, G.; Wang, L. Recent Advances in Green Multi-Component Reactions for Heterocyclic Compound Construction. Org. Biomol. Chem. 2025, 23, 2059–2078. [Google Scholar] [CrossRef]
- Lechner, H.; Pressnitz, D.; Kroutil, W. Biocatalysts for the Formation of Three- to Six-Membered Carbo- and Heterocycles. Biotechnol. Adv. 2015, 33, 457–480. [Google Scholar] [CrossRef]
- Castagnolo, D.; Zhao, F.; Masci, D.; Tomarelli, E. Biocatalytic and Chemo-Enzymatic Approaches for the Synthesis of Heterocycles. Synthesis 2020, 52, 2948–2961. [Google Scholar] [CrossRef]
- Rathore, D.; Geetanjali; Singh, R. Enzyme-Mediated Synthesis of Heterocyclic Compounds. In Advances in Green Synthesis: Avenues and Sustainability; Springer: Cham, Switzerland, 2021; pp. 277–288. [Google Scholar]
- Budhiraja, M.; Ali, A.; Tyagi, V. Recent Advances in Lipase Catalyzed Multicomponent Reactions to Synthesize N—Heterocycles. Asian J. Org. Chem. 2023, 12, e202300427. [Google Scholar] [CrossRef]
- Jumbam, N.D.; Masamba, W. Bio-Catalysis in Multicomponent Reactions. Molecules 2020, 25, 5935. [Google Scholar] [CrossRef]
- Voskressensky, L.G.; Festa, A.A.; Varlamov, A.V. Domino Reactions Based on Knoevenagel Condensation in the Synthesis of Heterocyclic Compounds. Recent Advances. Tetrahedron 2014, 70, 551–572. [Google Scholar] [CrossRef]
- Patil, S.A.; Patil, R.; Pfeffer, L.M.; Miller, D.D. Chromenes: Potential New Chemotherapeutic Agents for Cancer. Future Med. Chem. 2013, 5, 1647–1660. [Google Scholar] [CrossRef]
- Li, L.-Y.; Zeng, Q.-Q.; Yang, Y.-X.; Hu, H.-F.; Xu, M.; Guan, Z.; He, Y.-H. A Domino Reaction for the Synthesis of 2-Amino-4 H -Chromene Derivatives Using Bovine Serum Albumin as a Catalyst. J. Mol. Catal. B Enzym. 2015, 122, 1–7. [Google Scholar] [CrossRef]
- Zhou, L.H.; Huang, W.H.; Ma, L.F.; Jin, Y.J.; Chen, F.; Zhu, Y.L. Protein-Promoted Synthesis of Chromene Derivatives via Biocatalytic Domino Reaction. Heterocycles 2018, 96, 1740–1749. [Google Scholar] [CrossRef]
- Ahmad, A.; Rao, S.; Shetty, N.S. Green Multicomponent Synthesis of Pyrano[2,3- c]Pyrazole Derivatives: Current Insights and Future Directions. RSC Adv. 2023, 13, 28798–28833. [Google Scholar] [CrossRef]
- Dalal, K.S.; Tayade, Y.A.; Wagh, Y.B.; Trivedi, D.R.; Dalal, D.S.; Chaudhari, B.L. Bovine Serum Albumin Catalyzed One-Pot, Three-Component Synthesis of Dihydropyrano[2,3-c]Pyrazole Derivatives in Aqueous Ethanol. RSC Adv. 2016, 6, 14868–14879. [Google Scholar] [CrossRef]
- Huang, X.; Li, Z.; Wang, D.; Li, Y. Bovine Serum Albumin: An Efficient and Green Biocatalyst for the One-Pot Four-Component Synthesis of Pyrano[2,3-c]Pyrazoles. Chin. J. Catal. 2016, 37, 1461–1467. [Google Scholar] [CrossRef]
- Khan, M.K.; Huma, Z.E.; Dangles, O. A Comprehensive Review on Flavanones, the Major Citrus Polyphenols. J. Food Compos. Anal. 2014, 33, 85–104. [Google Scholar] [CrossRef]
- Borucki, E.L.; Iribarren, A.M.; Iglesias, L.E. Albumin-Catalysed Synthesis of Flavanones. Process Biochem. 2023, 125, 1–6. [Google Scholar] [CrossRef]
- Farahanipour, A.; Bavandi, H.; Shahedi, M.; Habibi, Z. Synthesis of 1H-Pyrazolo[1,2-b]Phthalazine-5,10-Dione and 1H-Pyrazolo[1,2-a]Pyridazine-5,8-Dione Derivatives by Bovine Serum Albumin in Water. Polycycl. Aromat. Compd. 2023, 43, 7042–7051. [Google Scholar] [CrossRef]
- Siddiqui, S.; Rather, R.A.; Siddiqui, Z.N. Bovine Serum Albumin—silica Functionalized γ—Fe2 O3 Nanoparticles (BSA—Si@Fe2 O3): A Highly Efficient and Magnetically Recoverable Heterogeneous Catalyst for the Synthesis of Substituted Pyrrole Derivatives. Appl. Organomet. Chem. 2021, 35, e6232. [Google Scholar] [CrossRef]
- Rostamabadi, H.; Chaudhary, V.; Chhikara, N.; Sharma, N.; Nowacka, M.; Demirkesen, I.; Rathnakumar, K.; Falsafi, S.R. Ovalbumin, an Outstanding Food Hydrocolloid: Applications, Technofunctional Attributes, and Nutritional Facts, A Systematic Review. Food Hydrocoll. 2023, 139, 108514. [Google Scholar] [CrossRef]
- Salehi, N.; Mirjalili, B.B.F. Nano-Ovalbumin: A Green Biocatalyst for Biomimetic Synthesis of Tetrahydrodipyrazolo Pyridines in Water. Res. Chem. Intermed. 2018, 44, 7065–7077. [Google Scholar] [CrossRef]
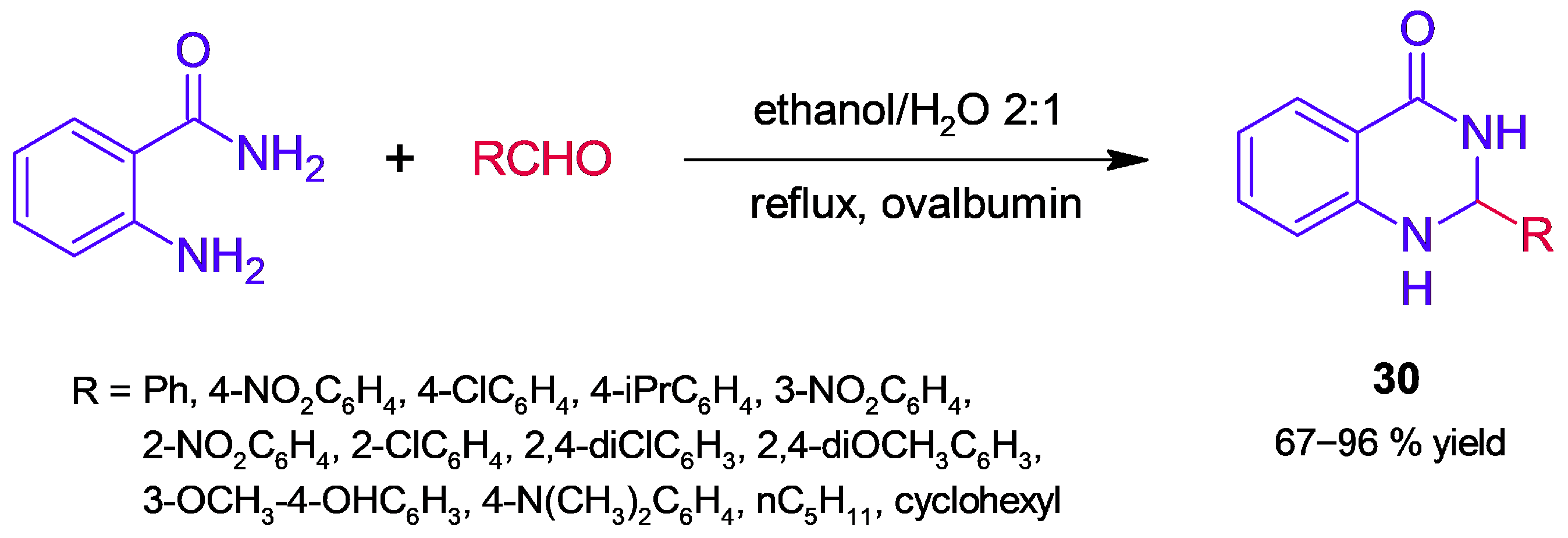
- Mirjalili, B.B.F.; Eliin, S.D.; Fazeli-Attar, S.A. Synthesis of 2,3-Dihydroquinazolinones Using Nano-Ovalbumin as a Non-Toxic Biocatalyst. SN Appl. Sci. 2020, 2, 830. [Google Scholar] [CrossRef]


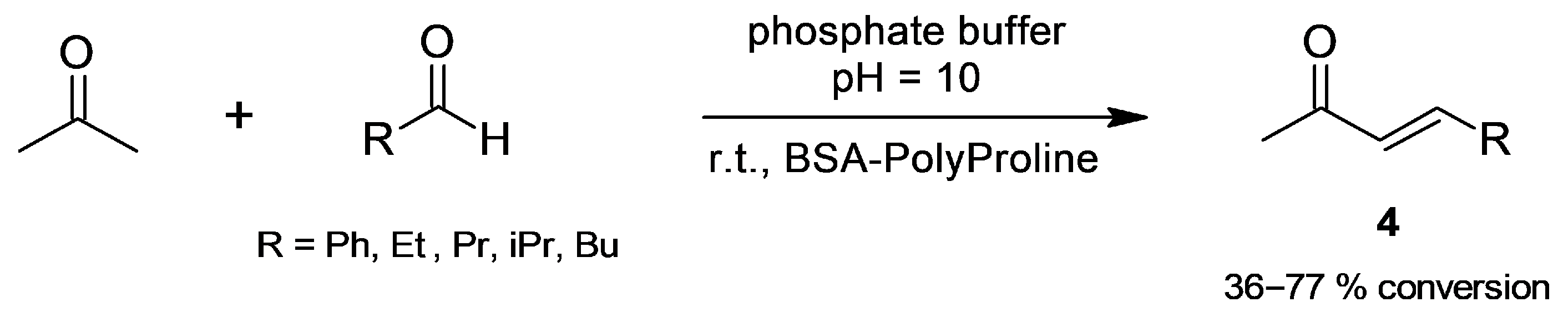



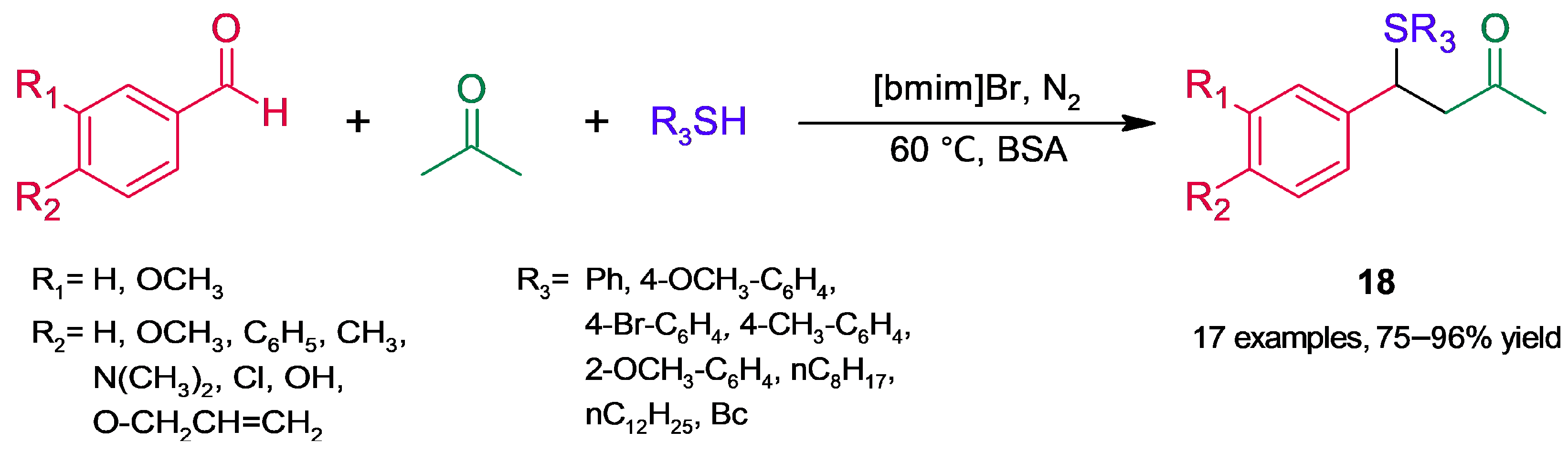





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borucki, E.L.; Iglesias, L.E. Recent Applications of Albumin as a Green and Versatile Catalyst in Organic Synthesis. Molecules 2025, 30, 4168. https://doi.org/10.3390/molecules30214168
Borucki EL, Iglesias LE. Recent Applications of Albumin as a Green and Versatile Catalyst in Organic Synthesis. Molecules. 2025; 30(21):4168. https://doi.org/10.3390/molecules30214168
Chicago/Turabian StyleBorucki, Estefanía L., and Luis E. Iglesias. 2025. "Recent Applications of Albumin as a Green and Versatile Catalyst in Organic Synthesis" Molecules 30, no. 21: 4168. https://doi.org/10.3390/molecules30214168
APA StyleBorucki, E. L., & Iglesias, L. E. (2025). Recent Applications of Albumin as a Green and Versatile Catalyst in Organic Synthesis. Molecules, 30(21), 4168. https://doi.org/10.3390/molecules30214168




