Silicon@Carbon Composite with Bioinspired Root-Nodule Nanostructures as Anode for High-Performance Lithium-Ion Batteries
Abstract
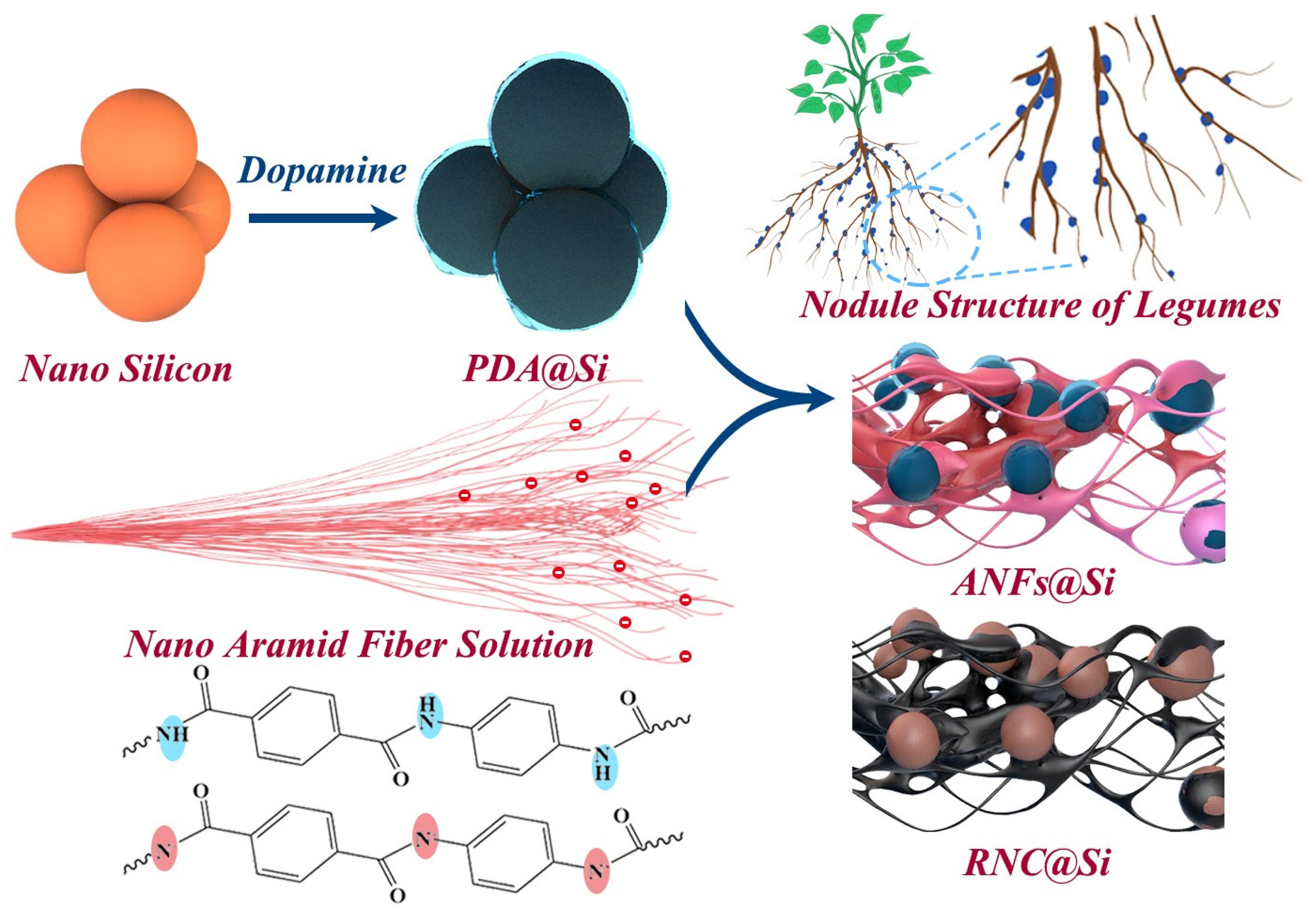
1. Introduction
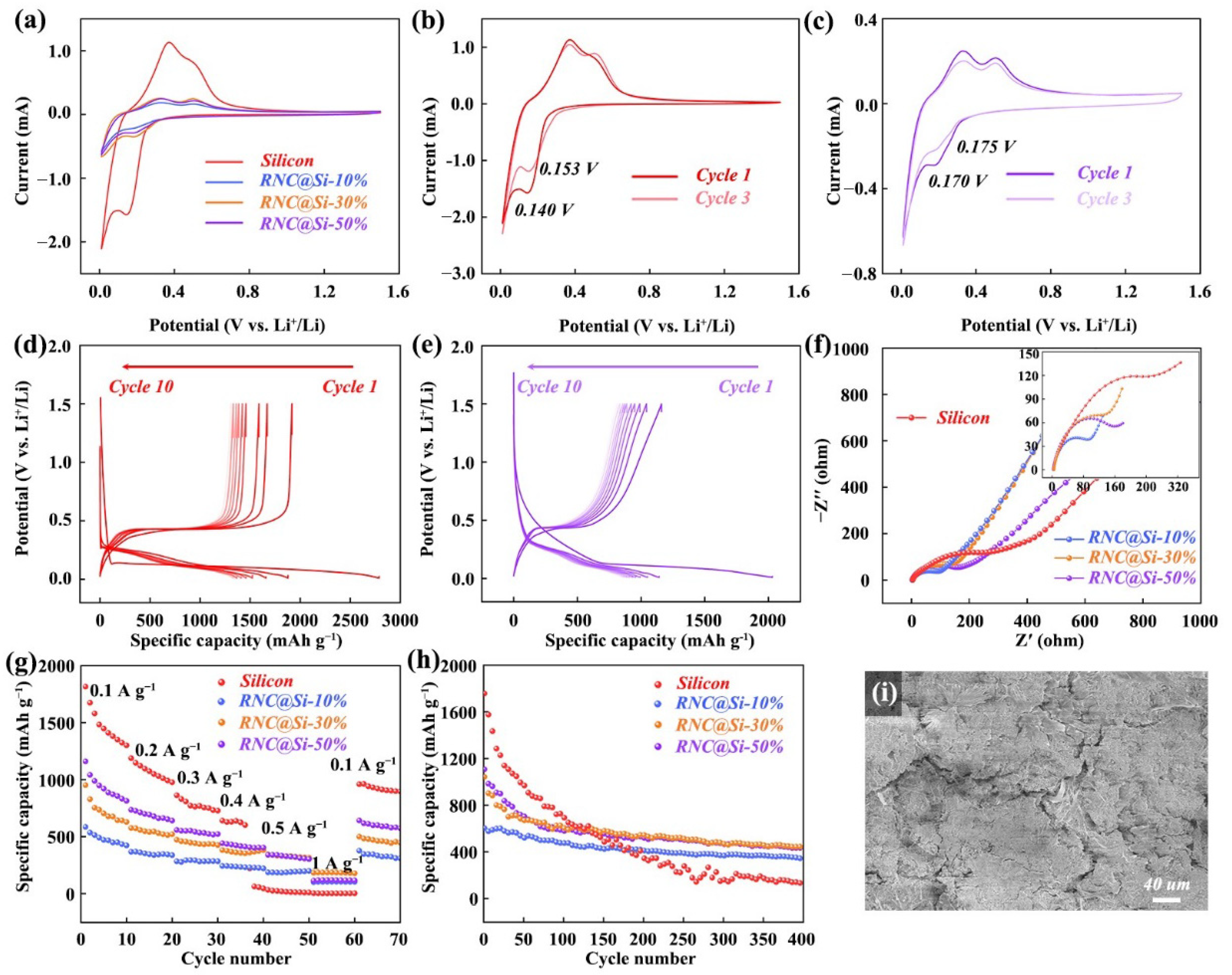
2. Results and Discussion
3. Experimental Section
3.1. Materials
3.2. Materials Characterizations
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crabtree, G. The coming electric vehicle transformation. Science 2019, 366, 422–424. [Google Scholar] [CrossRef]
- Viswanathan, V.; Epstein, A.H.; Chiang, Y.M.; Takeuchi, E.; Bradley, M.; Langford, J.; Winter, M. The challenges and opportunities of battery-powered flight. Nature 2022, 603, 519–525. [Google Scholar] [CrossRef]
- Zhao, L.; Li, Y.; Yu, M.M.; Peng, Y.Y.; Ran, F. Electrolyte-wettability issues and challenges of electrode materials in electrochemical energy storage, energy conversion, and beyond. Adv. Sci. 2023, 10, 2300283. [Google Scholar] [CrossRef]
- Zhao, L.; Tan, Y.T.; Sun, Y.T.; Liu, H.R.; Yang, N.N.; Mi, N. Vanadium-based compounds for aqueous zinc ion batteries with excellent rate capability and cyclic stability. J. Electroanal. Chem. 2023, 944, 117636. [Google Scholar] [CrossRef]
- Li, J.L.; Fleetwood, J.; Hawley, W.B.; Kays, W. From materials to cell: State-of-the-art and prospective technologies for lithium-ion battery electrode processing. Chem. Rev. 2022, 122, 903–956. [Google Scholar] [CrossRef]
- Sheng, Y.; Wang, Y.S.; Yin, S.J.; Zhao, L.Y.; Zhang, X.Q.; Liu, D.D.; Wen, G.W. Niobium-based oxide for anode materials for lithium-ion batteries. Chem.—A Eur. J. 2024, 30, e202302865. [Google Scholar] [CrossRef] [PubMed]
- Feng, K.; Li, M.; Liu, W.W.; Kashkooli, A.G.; Xiao, X.C.; Cai, M.; Chen, Z.W. Silicon-based anodes for lithium-ion batteries: From fundamentals to practical applications. Small 2018, 14, 1702737. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.F.; Yang, N.H.; Liu, R.S. Silicon anode design for lithium-ion batteries: Progress and perspectives. J. Phys. Chem. C 2017, 121, 27775–27787. [Google Scholar] [CrossRef]
- Wang, Y.P.; Attam, A.; Fan, H.G.; Zheng, W.S.; Liu, W. Engineering of siloxanes for stabilizing silicon anode materials. Small 2023, 19, 2303804. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, L.; Yang, L.S.; Chen, T.; Li, J.; Pan, H.; Yang, Y.H.; Wang, Z.C. Carbon free silicon/polyaniline hybrid anodes with 3D conductive structures for superior lithium-ion batteries. Chem. Commun. 2020, 56, 2328–2331. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Tang, W.; Yang, C.; Cai, W.L.; Chen, F.; Fu, Q. Reducing volume expansion in micro silicon anodes via aramid nanofibers for stable lithium-ion batteries. Chem. Commun. 2023, 59, 7435–7438. [Google Scholar] [CrossRef]
- Zhao, J.K.; Wang, B.; Zhan, Z.H.; Hu, M.Y.; Cai, F.P.; Swierczek, K.; Yang, K.M.; Ren, J.N.; Guo, Z.H.; Wang, Z.L. Boron-doped three-dimensional porous carbon framework/carbon shell encapsulated silicon composites for high-performance lithium-ion battery anodes. J. Colloid Interface Sci. 2024, 664, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, B.; Bi, J.; Zhang, X.; Mu, D.; Zhang, X.-Y.; Zhang, L.; Xiao, Y.; Wu, F. Facilitating prelithiation of silicon carbon anode by localized high-concentration electrolyte for high-rate and long-cycle lithium storage. Carbon Energy 2024, 6, 2637–9368. [Google Scholar] [CrossRef]
- Deng, Y.; Feng, X.; Qian, Z.; Ma, J.; Ouyang, Y.; Li, W.; Han, C. Silicon anode modification strategies in solid-state lithium-ion batteries. Mater. Horiz. 2025, 12, 5513–5538. [Google Scholar] [CrossRef]
- Kim, G.T.; Kennedy, T.; Brandon, M.; Geaney, H.; Ryan, K.M.; Passerini, S.; Appetecchi, G.B. Behavior of germanium and silicon nanowire anodes with ionic liquid electrolytes. ACS Nano 2017, 11, 5933–5943. [Google Scholar] [CrossRef]
- Dewees, R.; Thapa, A.; Sunkara, M. Silicon nanotube anode on copper foils for Li-ion batteries. ACS Appl. Mater. Interfaces 2024, 17, 3742–3748. [Google Scholar] [CrossRef]
- Jimenez, N.P.; Balogh, M.P.; Halalay, I.C. High porosity single-phase silicon negative electrode made with phase-inversion. J. Electrochem. Soc. 2021, 168, 040507. [Google Scholar] [CrossRef]
- Shang, H.; Zuo, Z.C.; Yu, L.; Wang, F.; He, F.; Li, Y.L. Low-temperature growth of all-carbon graphdiyne on a silicon anode for high-performance lithium-ion batteries. Adv. Mater. 2018, 30, 1801459. [Google Scholar] [CrossRef]
- Lou, D.; Chen, S.; Langrud, S.; Razzaq, A.A.; Mao, M.; Younes, H.; Xing, W.; Lin, T.; Hong, H. Scalable Fabrication of Si-Graphene Composite as Anode for Li-ion Batteries. Appl. Sci. 2022, 12, 10926. [Google Scholar] [CrossRef]
- Tian, H.; Tian, H.J.; Yang, W.; Zhang, F.; Yang, W.; Zhang, Q.B.; Wang, Y.; Liu, J.; Silva, S.R.P.; Liu, H. Stable Hollow-structured silicon suboxide-based anodes toward high-performance lithium-ion batteries. Adv. Funct. Mater. 2021, 31, 2101796. [Google Scholar] [CrossRef]
- Mae, T.; Kaneko, K.; Li, M.C.; Noda, S. Stable and high-capacity SiO negative electrode held in reversibly deformable sponge-like matrix of carbon nanotubes. Carbon 2023, 209, 118014. [Google Scholar] [CrossRef]
- Chae, S.; Choi, S.H.; Kim, N.; Sung, J.; Cho, J. Integration of graphite and silicon anodes for the commercialization of high-energy lithium-ion batteries. Angew. Chem.-Int. Ed. 2020, 59, 110–135. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Zeng, J.R.; Sun, W.L.; Qian, H.; Fu, F.; Bai, H.C.; Kong, H.B.; Chen, H.W. Robust dual-cross-linked networks enable stable silicon anodes. Chem. Commun. 2023, 59, 12855–12858. [Google Scholar] [CrossRef]
- He, W.; Luo, H.; Jing, P.; Wang, H.M.; Xu, C.H.Y.; Wu, H.; Wang, Q.; Zhang, Y. Embedding silicon in biomass-derived porous carbon framework as high-performance anode of lithium-ion batteries. J. Alloys Compd. 2022, 918, 165364. [Google Scholar] [CrossRef]
- Casimir, A.; Zhang, H.G.; Ogoke, O.; Amine, J.C.; Lu, J.; Wu, G. Silicon-based anodes for lithium-ion batteries: Effectiveness of materials synthesis and electrode preparation. Nano Energy 2016, 27, 359–376. [Google Scholar] [CrossRef]
- Zuo, X.X.; Zhu, J.; Müller-Buschbaum, P.; Cheng, Y.J. Silicon based lithium-ion battery anodes: A chronicle perspective review. Nano Energy 2017, 31, 113–143. [Google Scholar] [CrossRef]
- Kamikawa, Y.; Amezawa, K.; Terada, K. Chemomechanical simulation of LiF-Rich solid-electrolyte interphase formed from fluoroethylene carbonate on a silicon anode. ACS Appl. Energy Mater. 2021, 4, 3231–3239. [Google Scholar] [CrossRef]
- Pan, J.; Peng, H.L.; Yan, Y.A.; Bai, Y.Z.; Yang, J.; Wang, N.N.; Dou, S.X.; Huang, F.Q. Solid-state batteries designed with high ion conductive composite polymer electrolyte and silicon anode. Energy Storage Mater. 2021, 43, 165–171. [Google Scholar] [CrossRef]
- Liu, X.Z.; Wang, D.; Wang, X.T.; Wang, D.Y.; Li, Y.; Fu, J.; Zhang, R.; Liu, Z.Y.; Zhou, Y.Z.; Wen, G.W. Designing compatible ceramic/polymer composite solid-state electrolyte for stable silicon nanosheet anodes. Small 2024, 20, 2309724. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Gao, H.; Li, J.Y.; Sun, Y.W.; Deng, Z.S.; Yuan, X.Y.; Li, C.C.; Chen, T.X.; Peng, X.W.; Wang, C. Plasma-enhanced vacancy engineering for sustainable high-performance recycled silicon in lithium-ion batteries. Energy Storage Mater. 2025, 77, 104231. [Google Scholar] [CrossRef]
- Dou, F.; Shi, L.; Chen, G.; Zhang, D. Silicon/carbon composite anode materials for lithium-ion batteries. Electrochem. Energy Rev. 2019, 2, 149–198. [Google Scholar] [CrossRef]
- Pei, Y.X.; Wang, Y.X.; Chang, A.Y.; Liao, Y.X.; Zhang, S.; Wen, X.F.; Wang, S.N. Nanofiber-in-microfiber carbon/silicon composite anode with high silicon content for lithium-ion batteries. Carbon 2023, 203, 436–444. [Google Scholar] [CrossRef]
- Zhou, N.; Li, X.; Zheng, Z.; Liu, J.; Downie, J.A.; Xie, F. RinRK1 enhances NF receptors accumulation in nanodomain-like structures at root-hair tip. Nat. Commun. 2024, 15, 3568. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, Y.; Ren, Z.; Zhang, X.; Ren, J.; Su, J.; Zhang, C.; Tian, J.; Yu, Y.; Gao, G.F.; et al. Transfer cells mediate nitrate uptake to control root nodule symbiosis. Nat. Plants 2020, 6, 800–808. [Google Scholar] [CrossRef]
- Yang, B.; Wang, L.; Zhang, M.Y.; Luo, J.J.; Lu, Z.Q.; Ding, X.Y. Fabrication, applications, and prospects of aramid nanofiber. Adv. Funct. Mater. 2020, 30, 2000186. [Google Scholar] [CrossRef]
- Lin, L.S.; Cong, Z.X.; Cao, J.B.; Ke, K.M.; Peng, Q.L.; Gao, J.H.; Yang, H.H.; Liu, G.; Chen, X.Y. Multifunctional Fe3O4@polydopamine core-shell nanocomposites for intracellular mRNA detection and imaging-guided photothermal therapy. ACS Nano 2014, 8, 3876–3883. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Y.; Thaiboonrod, S.; Fang, J.H.; Cao, S.M.; Miao, M.; Feng, X. In-situ growth of polypyrrole on aramid nanofibers for electromagnetic interference shielding films with high stability. Nano Res. 2022, 15, 8536–8545. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Zhao, L.; Mi, N.; He, J.; Xu, J. Silicon@Carbon Composite with Bioinspired Root-Nodule Nanostructures as Anode for High-Performance Lithium-Ion Batteries. Molecules 2025, 30, 4157. https://doi.org/10.3390/molecules30214157
Sun Y, Zhao L, Mi N, He J, Xu J. Silicon@Carbon Composite with Bioinspired Root-Nodule Nanostructures as Anode for High-Performance Lithium-Ion Batteries. Molecules. 2025; 30(21):4157. https://doi.org/10.3390/molecules30214157
Chicago/Turabian StyleSun, Yitong, Lei Zhao, Ning Mi, Jiahao He, and Jiantie Xu. 2025. "Silicon@Carbon Composite with Bioinspired Root-Nodule Nanostructures as Anode for High-Performance Lithium-Ion Batteries" Molecules 30, no. 21: 4157. https://doi.org/10.3390/molecules30214157
APA StyleSun, Y., Zhao, L., Mi, N., He, J., & Xu, J. (2025). Silicon@Carbon Composite with Bioinspired Root-Nodule Nanostructures as Anode for High-Performance Lithium-Ion Batteries. Molecules, 30(21), 4157. https://doi.org/10.3390/molecules30214157





