2.1. Structure and Texture Properties
The Bi
3TiNbO
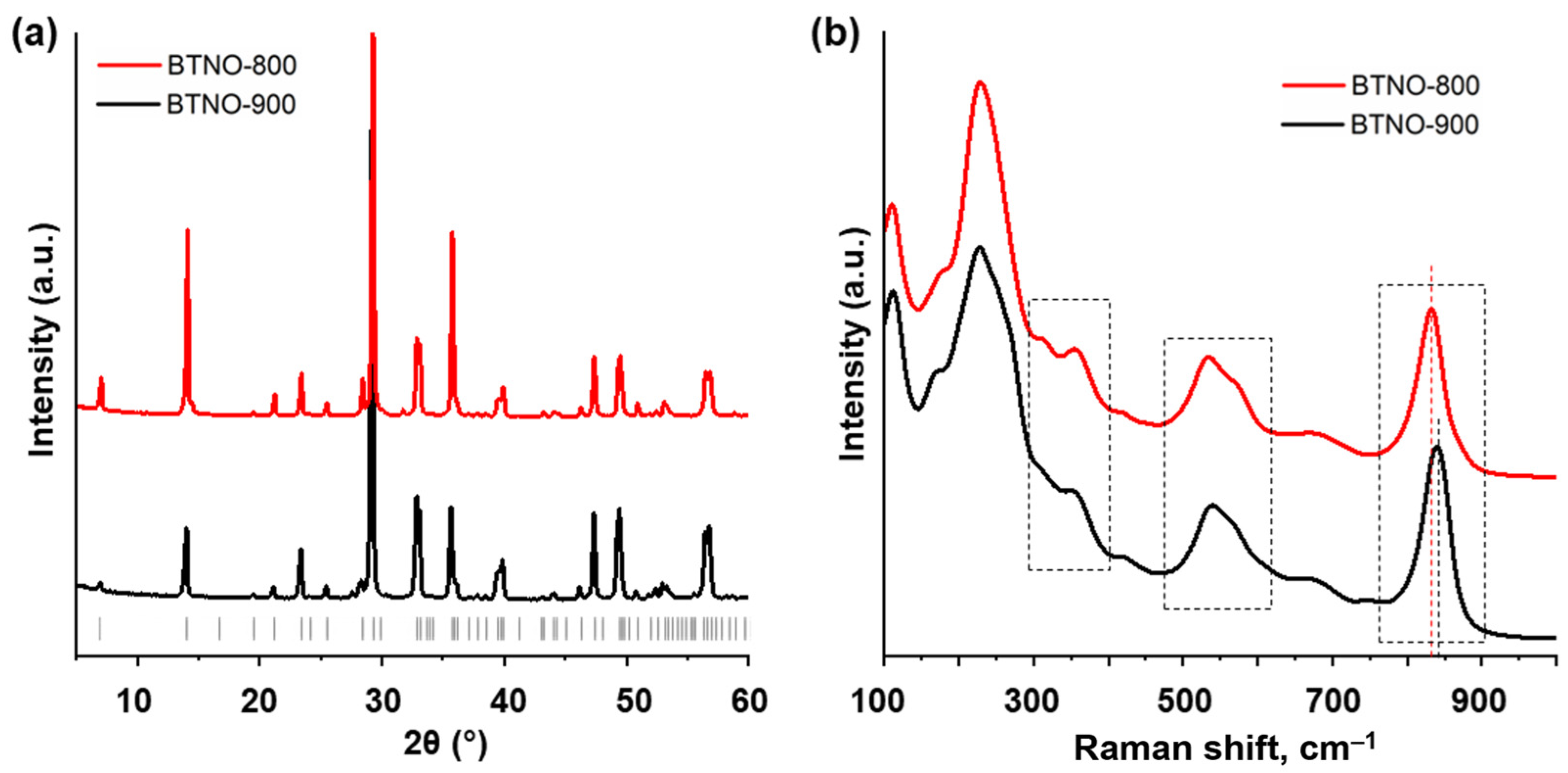
9 materials prepared by two different approaches were primarily identified by the means of powder XRD analysis (
Figure 1a). Both ceramic (BTNO-900) and melt-synthesized (BTNO-800) oxides represent well crystallized samples giving distinct diffraction maxima and not containing significant amounts of crystalline impurity phases. XRD patterns of the materials obtained are consistent with those from the ICDD database and best match the card № 01-072-7962 corresponding to orthorhombic space group A21am. typical for ferroelectric Aurivillius-phase oxide. The lattice parameters of ceramic and melt-synthesized Bi
3TiNbO
9 (
Table 1) are close in
a and
b parameters and slightly different in
c parameter. It is general that
c parameter stronger depends on method of synthesis as it could be visible in comparison with data obtained for single crystal [
34] and sample synthesized by hydrothermal method [
35].
Raman spectra of the ceramic and melt-synthesized Bi
3TiNbO
9 (
Figure 1b) are generally consistent with those presented in the available literature and show clearly distinguishable characteristic peaks corresponding to the O–M–O bending (300–400 cm
−1), opposing excursions of the external apical oxygens in the MO
6 octahedra (500–600 cm
−1) and symmetric stretching of the axial M–O bonds directed towards the interlayer space (800–900 cm
−1) (M = Ti, Nb) [
35]. At the same time, the vibrational modes of the two Bi
3TiNbO
9 samples are not completely identical. Particularly, the aforementioned M–O stretching in the ceramic material (840 cm
−1) is shifted towards greater frequencies as compared to that of the melt-synthesized one (832 cm
−1), which might indicate a somewhat higher distortion of the octahedra (elongation) in sample’s lattice [
34] that results in the increasing of the c parameter (
Table 1).
Figure 2 presents the SEM micrographs of Bi
3TiNbO
9 samples synthesized via solid-state reaction (
Figure 2a) and molten salt method (
Figure 2b).
The ceramic sample (BTNO-900,
Figure 2a) exhibits an aggregated microstructure composed of irregularly shaped particles, showing pronounced agglomeration and limited growth of individual crystallites. Such morphology is indicative of a low degree of texturing and poor control over grain growth, which is typical of conventional solid-state synthesis. In contrast, the sample obtained via the molten salt route (BTNO-800,
Figure 2b) displays well-defined, plate-like grains with pronounced facets and moderate agglomeration. At higher magnification (
Figure 2c,d), these differences become even more pronounced. The BTNO-900 sample (
Figure 2c) shows coarse particles with uneven, defect-rich surfaces, whereas the BTNO-800 sample (
Figure 2d) exhibits uniform and clean faceted grains with sharp edges and well-developed planes, reflecting a more ordered layered structure and enhanced crystal growth control. This suggests a more oriented grain growth and a well-ordered layered structure characteristic of Aurivillius-type phases. The observed morphology is consistent with the XPS results, which reveal that BTNO-800 possesses a more structurally ordered surface enriched in lattice oxygen, with minimal contributions from surface defects and adsorbed species.
The elemental composition and distribution of the main components in Bi
3TiNbO
9 were further confirmed by EDX analysis (see
Table S1 and Figures S1 and S2 in the Supplementary Materials). The spectra reveal the presence of Bi, Ti, Nb, and O as the major elements, in agreement with the expected stoichiometry. Trace signals of Na, K, and Cl (<0.5 at.%) were detected only for the BTNO-800 sample, which can be attributed to residual precursor salts or adsorbed ionic species. The uniform distribution of the main elements supports the formation of a single-phase Bi3TiNbO9 structure.
These morphological distinctions are further supported by nitrogen adsorption measurements (
Table 2 see also
Figure S3 in the Supplementary Materials). The melt-synthesized BTNO-800 sample demonstrates a significantly higher specific surface area (5.9 m
2/g) compared to the ceramic BTNO-900 (1.4 m
2/g), which correlates with its pronounced texturing observed in the SEM images. The average pore diameters for both samples are similar (2.8 nm and 2.6 nm, respectively), indicating a retained mesoporous structure in both cases. However, the lower total pore volume observed for BTNO-800 (0.005 cm
3/g) relative to BTNO-900 (0.008 cm
3/g) suggests denser packing and lower overall porosity in the melt-synthesized material.
Taken together, the morphological and textural differences revealed by SEM and nitrogen physisorption analyses clearly demonstrate the substantial impact of the synthesis route on the surface architecture of Bi3TiNbO9. The more ordered morphology and increased specific surface area of BTNO-800 indicate a greater degree of structural integrity, which likely influences the distribution and nature of surface-active centers and oxygen-containing species.
2.2. Surface Chemistry
To further elucidate the differences in surface chemistry between the Bi3TiNbO9 samples synthesized by molten salt (BTNO-800) and solid-state reaction (BTNO-900), high-resolution X-ray photoelectron spectroscopy (XPS) analysis was conducted.
The survey XPS spectrum (
Figure 3a) displays well-defined peaks corresponding to the main constituent elements of Bi
3TiNbO
9, namely O 1s, Ti 2p, Nb 3d, and Bi 4f. No significant contamination signals were detected, confirming the chemical purity of the sample surfaces. However, the relative intensities of specific peaks vary depending on the synthesis route. In the melt-synthesized sample (BTNO-800), the Bi 4f peak is notably more intense, whereas the O 1s signal is reduced, which may indicate differences in the density of oxygen-related surface species and the coordination environment of cations.
High-resolution O 1s spectra (
Figure 3b) reveal pronounced differences in oxygen coordination. For BTNO-800, the O 1s signal is dominated by lattice oxygen, accounting for 92.8% of the total oxygen species at a binding energy of 529.5 eV, with only a minor contribution (7.2%) from adsorbed oxygen species. In contrast, BTNO-900 exhibits a shift in the O 1s binding energy to 529.7 eV, accompanied by a substantial increase in the proportion of surface-adsorbed oxygen and hydroxyl groups—collectively exceeding 57%. These changes are indicative of a significantly higher concentration of oxygen vacancies and surface defects, most likely resulting from limited ion diffusion and uneven crystallite growth during solid-state synthesis.
The high-resolution XPS spectra of Ti 2p for the BTNO samples (
Figure 3c) exhibit Ti 2p
3/2 peaks centered at 457.9 eV, which confirms the presence of Ti in the +4 oxidation state, in agreement with previous reports [
33]. It should be noted that the spectral region from 460 to 472 eV contains overlapping contributions from Ti 2p
1/2, Nb 3s, and Bi 4d
3/2 levels. Accordingly, the composite peak was deconvoluted into three distinct components. For BTNO-900, these components were identified at 463.7 eV (Ti 2p
1/2), 466.0 eV (Nb 3s), and 466.5 eV (Bi 4d
3/2). The observed shifts in these peaks to higher binding energies in BTNO-900 may be attributed to changes in the local electronic environment of the cations and have previously been associated with enhanced local polarization effects and coordination distortions in defect-rich Aurivillius-type structures [
35].
The Bi 4f spectra (
Figure 3d) of BTNO-800 exhibit well-defined peaks at 158.9 eV and 164.3 eV, corresponding to Bi 4f
7/2 and Bi 4f
5/2, respectively. These binding energies are characteristic of Bi
3+ in a symmetric coordination environment. In contrast, BTNO-900 shows a noticeable shift in the Bi 4f peaks toward higher binding energies (159.2 eV and 164.5 eV), accompanied by the appearance of shoulder components, indicating a modified local coordination of bismuth and the influence of polarizable structural defects, as previously reported for related Aurivillius-type phases [
38]. The shift in peak positions without a change in oxidation state suggests a redistribution of electron density around Bi ions, likely due to an increased concentration of oxygen vacancies.
In the Nb 3d spectra (
Figure 3e), BTNO-800 displays characteristic Nb 3d
5/2 and Nb 3d
3/2 peaks at 206.5 eV and 209.2 eV, respectively, consistent with the Nb
5+ oxidation state. For BTNO-900, these peaks are shifted to 206.7 eV and 209.5 eV, which may be attributed to changes in the local coordination environment and electronic structure, driven by a higher density of oxygen vacancies and surface defects induced by the solid-state synthesis route.
These findings suggest that solid-state synthesis leads to a more defect-rich surface with elevated concentrations of oxygen vacancies and chemisorbed oxygen species. Such defects can facilitate the formation of reactive oxygen species (ROS), contributing to enhanced piezocatalytic activity. However, their excessive presence may also promote charge carrier recombination, thereby reducing the efficiency of photocatalytic processes.
2.3. Optical Properties
To investigate the optical properties BTNO-900 and BTNO-800 Bi
3TiNbO
9 samples, diffuse reflectance spectroscopy (DRS) combined with the Kubelka–Munk transformation was employed (
Figure 4a,b). Both materials exhibit a pronounced absorption edge in the near-UV region, associated with interband electronic transitions. Notably, the absorption edge of the ceramic BTNO-900 sample is red-shifted toward longer wavelengths (λ
max = 386 nm) compared to BTNO-800 (λ
max = 371 nm), indicating a reduction in the effective optical band gap for BTNO-900 (see
Table 3). The obtained values of the bandgap energy are in good agreement with the data available in the literature: 3.31–3.33 eV for synthesis in molten salts and 3.27 eV for solidstate synthesis [
33,
36,
37,
39].
The optical band gap energies (E
g) were determined by extrapolating the linear portion of the (F(R)hν)
2–hν plots to the energy axis. For BTNO-900, Eg was found to be 3.21 eV, whereas for BTNO-800 it was 3.34 eV. It should be emphasized that the reduction in the optical band gap observed for BTNO-900 is most likely not associated with a fundamental shift in the conduction or valence band edges, but rather with the formation of defect-induced sub-band states within the forbidden gap, caused by the higher concentration of oxygen vacancies and surface states revealed by XPS analysis. These defect-related energy levels facilitate optical transitions at lower photon energies, leading to the red shift in the absorption edge. The apparent narrowing of the band gap and the increased density of defect states characteristic of BTNO-900 are expected to directly influence the recombination dynamics of photogenerated charge carriers. To verify this assumption, photoluminescence (PL) and time-resolved PL (TRPL) studies were performed (
Figure 5) to evaluate the recombination kinetics of electron–hole pairs and correlate them with the morphological and defect-chemical differences between the samples.
Upon excitation at 340 nm, both samples exhibit pronounced emission bands in the 405–410 nm region, consistent with the position of the optical absorption edge (see
Table 3). However, the PL intensity for BTNO-800 is significantly lower than that for BTNO-900. Analysis of the photoluminescence decay kinetics revealed that the average lifetime of photogenerated carriers in BTNO-900 (τ = 30 ns) is approximately 2.5 times longer than in BTNO-800 (τ = 12 ns). The longer carrier lifetime in BTNO-900 correlates with its higher concentration of oxygen vacancies and surface hydroxyl groups, which facilitate spatial separation of electrons and holes via local internal electric fields.
At first glance, the combination of high PL intensity and prolonged lifetime in BTNO-900 might appear contradictory, since strong luminescence is typically associated with fast radiative recombination and thus shorter carrier lifetimes. However, a more detailed analysis of the recombination processes shows that such behavior is characteristic of systems in which defects and internal electric fields simultaneously retard total recombination—leading to longer τ—while suppressing nonradiative channels, thus ensuring a high quantum efficiency of emission. In BTNO-900, the high concentration of oxygen vacancies and surface hydroxyl groups revealed by XPS analysis creates localized states within the band gap and internal electric fields that hinder the rapid spatial annihilation of electron–hole pairs. These fields enable spatial separation of carriers, reducing the probability of nonradiative recombination and increasing the fraction of slower radiative processes, which manifests as both higher PL intensity and longer carrier lifetimes. In contrast, in BTNO-800, where the concentration of defects and internal electric fields is lower, carriers undergo rapid recombination, with a substantial fraction lost through nonradiative pathways, leading to both reduced lifetimes and lower PL intensity.
Thus, the combination of enhanced PL intensity and longer carrier lifetimes in BTNO-900 reflects the key role of defect-induced internal fields and carrier localization in suppressing nonradiative recombination and enhancing radiative efficiency. These processes enable prolonged survival of separated electron–hole pairs, providing a mechanistic basis for the enhanced photocatalytic activity of the ceramic sample, which will be discussed in the following section.
2.4. Catalytic Activity
The catalytic activity of the samples was investigated via the degradation of MB dye. In order to accurately assess the effect of the catalysts on MB decomposition, it was first necessary to study the impact of ultrasound (US) and light separately, in the absence of catalysts. The results are presented in
Figure 6. It is evident that sonolysis of the MB solution resulted in approximately 47% degradation within 60 min. This result is attributed to the cavitation effect, as it is well known that rapid pressure fluctuations in water during periodic compression and expansion lead to the formation and growth of voids or microbubbles [
7]. Both theoretical and experimental studies have demonstrated that adiabatic collapse of microbubbles creates local regions with high pressure (~1000 atm) and temperature (~5000 K). These conditions provide sufficient energy for the dissociation of water molecules, especially at the gas–liquid interface, resulting in the formation of highly reactive ·OH and ·H radicals [
40]. In a similar control experiment with only light exposure (photolysis) (
Figure 6a), the degree of MB degradation after 60 min was also 47%. To eliminate the influence of dye adsorption on the assessment of photo-, piezo-, and piezophotocatalytic activities, prior to each experiment, the samples were stirred in MB solution in the dark until adsorption–desorption equilibrium was reached. According to the results, equilibrium was achieved after approximately 30 min, with a decrease in MB concentration of about 30% for both samples. The samples exhibited different activities in the photocatalytic experiments. For the BTNO-900 the MB concentration decreased by approximately 84%, indicating good photocatalytic activity, whereas for the BTNO-800, the result did not exceed that of photolysis (
Figure 6c), at about 45%. This is likely due to rapid recombination of charge carriers, as demonstrated by the time-resolved photoluminescence experiments. The charge carrier lifetime for BTNO-800 was 2.5 times shorter than for BTNO-900: 12 ns and 30 ns.
Subsequently, the piezocatalytic activity of the samples was studied using an ultrasonic bath as a source of mechanical stimulation. The results of MB degradation under piezocatalytic conditions showed that both samples exhibited similar catalytic activity, with a reduction in methylene blue concentration of about 78%. The activity observed under ultrasonic irradiation can be interpreted based on mechanisms described in [
41], with the following being the most probable:
Indirect sonolysis and sonocatalysis: These processes occur due to the cavitational pyrolysis of water, where hydroxyl radical generation is caused by the formation and collapse of cavitation bubbles. The contribution of sonolysis is well-established and can be controlled. According to sonocatalytic theory, enhancement of the effect upon addition of nanoparticles is attributed to their ability to either enhance or suppress cavitation, depending on their morphology and size. However, despite differences in size and morphology between the studied nanoparticles, their piezocatalytic activities were comparable. Therefore, this mechanism does not explain the observed results.
Photocatalytic and thermocatalytic mechanisms (PCM, TCM): According to these theories, sonoluminescence or local temperature increase during cavitation can initiate the generation of electron-hole pairs in the semiconductor catalyst, enhancing the catalytic process. However, since the samples exhibit different photocatalytic activities, these mechanisms also cannot explain the identical results obtained under piezocatalytic conditions.
Thus, the most probable explanation for the observed experimental data is the presence of the piezoelectric effect in the studied materials. According to this hypothesis, ultrasonic irradiation induces piezoelectric polarization and an internal electric field at the particle surface, which promote charge separation and interfacial charge transfer, thereby initiating a cascade of redox reactions.
It is known that materials exhibiting both photo- and piezocatalytic activities are capable of demonstrating the piezophototronic effect, resulting in enhanced catalytic activity under simultaneous action of both effects. To verify this, a piezophotocatalytic experiment was conducted. As shown in
Figure 6a,c, the degree of MB degradation after 60 min under these conditions reached approximately 93% for both samples, indicating a pronounced synergistic effect. Particularly noteworthy is the result for the BTNO-800, which initially did not exhibit significant photocatalytic activity. The substantial increase in reaction rate under piezophotocatalytic conditions, 4.3 and 1.8 times higher compared to photocatalysis and piezocatalysis, respectively, can be attributed to the generation of an electric field via the piezoelectric effect. This electric field promotes spatial separation of electron-hole pairs, leading to increased charge carrier lifetimes and, consequently, higher catalytic efficiency. The reaction rate constants calculated using the pseudo-first-order Langmuir-Hinshelwood model from the slope of –ln(C/C
0) versus time (
Figure S4), support these conclusions and are presented in the diagrams in
Figure 6b,d. Under ultrasound irradiation without catalyst, the MB degradation rate constant was relatively low at k = 0.012 min
−1, while in the presence of the catalyst, k = 0.032 min
−1, indicating that the catalyst is an integral component of the piezocatalytic reaction. The rate of MB degradation via piezocatalysis (k = 0.032 min
−1) exceeded that of sonolysis by 2.6 times for BTNO-900 and by 2.4 times for BTNO-800.
Under piezophotocatalytic conditions, the rate constants were k = 0.049 min−1 for BTNO-900 and k = 0.047 min−1 for BTNO-800, nearly four times higher than the rates for sonolysis and photolysis, thus confirming the synergistic effect of the combined piezo- and photocatalytic processes.
In order to confirm the validity of the adopted kinetic model, pseudo-first-order (Langmuir–Hinshelwood type) and second-order equations were tested for all degradation modes using BTNO-800 as an example. A comparison of the correlation coefficients (R
2) and rate constants (
Table S2, Supplementary Materials) demonstrates that the pseudo-first-order model yields R
2 values that are marginally higher or equivalent to those of the second-order model (0.97–0.99), thereby substantiating its adequacy within the confines of our experimental setup. Consequently, the pseudo-first-order kinetics was choosen for the purpose of further analysis and discussion.
The morphological and compositional stability of the BTNO-800 catalyst after the piezophotocatalytic process was further verified by SEM and EDX analyses. As shown in the
Supplementary Materials (Figure S5), the Bi
3TiNbO
9 particles retain their original plate-like morphology and chemical composition after repeated catalytic cycles. The impurity peaks of Na, K, and Cl observed in the pristine BTNO-800 sample completely disappear after catalysis, indicating a surface self-cleaning effect and high structural stability of the material under piezophotocatalytic conditions.
2.5. Reaction Mechanism/Trapping Experiments
To elucidate the piezocatalytic mechanism and provide a deeper analysis of MB degradation processes, trapping experiments were conducted to identify the key reactive species involved in the piezophotocatalytic degradation using Bi3TiNbO9 under ultrasonic irradiation. The experimental methodology was similar to the procedure used for the evaluation of piezocatalytic activity, except for the addition of specific scavengers to selectively inhibit certain reactive species.
The following scavengers were used: isopropyl alcohol (IPA) to capture hydroxyl radicals (·OH), benzoquinone (BQ) to trap superoxide radicals (·O
2−), disodium ethylenediaminetetraacetate (EDTA-2Na) as a hole (h
+) scavenger, and silver nitrate (AgNO
3) as an electron scavenger. The results are presented in
Figure 7.
After the introduction of isopropyl alcohol (IPA), used as a scavenger for hydroxyl radicals (·OH), the degradation efficiency of MB was significantly reduced, reaching 28.4% for the BTNO-900 and 30.8% for the BTNO-800. This indicates that ·OH are the dominant reactive species in the piezophotocatalytic process.
The effect of adding benzoquinone (BQ), used as a scavenger for superoxide radicals (·O2−), was ambiguous. For the BTNO-900, inhibition of the degradation process to 73.3% was observed, indicating a significant role of superoxide radicals in the degradation mechanism. In contrast, for the BTNO-800 sample, the addition of BQ resulted in only a slight acceleration of the reaction, suggesting either the absence of superoxide radicals (·O2−) or their negligible formation in this case. The addition of disodium ethylenediaminetetraacetate (EDTA-2Na), used as a hole (h+) scavenger, led to a similar inhibition of the process for both catalysts, reducing the degradation efficiency to 81–82%. This highlights the importance of holes (h+) as active species in the piezophotocatalytic reaction for both samples. Thus, the results of radical trapping experiments demonstrate the differing roles of reactive species depending on the synthesis conditions and properties of the catalysts.
The addition of silver nitrate (AgNO3), which acts as an electron scavenger, led to an acceleration of MB degradation for both catalysts. This phenomenon can be explained by the occurrence of redox reactions, which enhance catalytic activity. Silver nitrate (Ag+) captures electrons, thereby preventing electron-hole recombination and increasing the concentration of active holes. The deposition of metallic silver (Ag) on the catalyst surface may further catalyze oxidation reactions.
Based on the results of the trapping experiments, a reaction mechanism for the piezophotocatalytic degradation occurring on the surface of Bi
3TiNbO
9 under ultrasonic vibration and light irradiation is proposed. The main processes start with the photo-generation of electron–hole pairs under illumination:
Concurrently, ultrasonic deformation induces a piezoelectric field that enhances spatial separation and interfacial transport of these carriers (piezo-phototronic effect), thereby facilitating the formation of reactive species.
Holes (h
+) interact with water molecules or hydroxide ions to generate hydroxyl radicals (·OH), which are powerful oxidizing agents that play a key role in the degradation of organic pollutants such as MB:
Superoxide radicals (·O2−) are formed as a result of the reaction between electrons (e−) and dissolved oxygen, also contributing to redox processes. The resulting superoxide radicals can undergo further reactions with protons or water to form hydroperoxyl radicals (·HO2) and hydrogen peroxide (H2O2), which subsequently decompose into hydroxyl radicals:
e
− + O
2 → ·O
2− (For the ceramic sample, the amount of adsorbed oxygen is higher, and consequently, ·O
2− is also higher.)
The addition of silver nitrate (AgNO
3) as an electron scavenger leads to reaction acceleration by preventing electron-hole recombination. Ag
+ captures electrons and is reduced to metallic silver (Ag), which can further catalyze oxidation reactions. As a result, the concentration of active holes (h
+) increases, enhancing the degradation process:
In addition, ultrasonic vibration induces cavitation effects, accompanied by local increases in temperature and pressure, which also promote the formation of active radicals, including ·OH and ·H. These radicals contribute further to MB degradation, providing a comprehensive mechanism involving multiple redox processes:
Following the scavenger tests, the electronic band-edge positions of BTNO-800 were estimated using the Mulliken electronegativity theory to clarify the origin of the observed radical selectivity and the role of piezoelectric polarization in charge dynamics.
The absolute electronegativity (χ) of Bi3TiNbO9 was calculated as the geometric mean of its constituent atoms (Bi = 4.69 eV, Ti = 3.45 eV, Nb = 4.00 eV, O = 7.54 eV), yielding χ = 6.156 eV. According to the Mulliken formalism: ECB = χ − Ee − 2Eg, EVB = ECB + Eg, where eV is the energy of a free electron on the NHE scale, and eV is the optical band gap determined from the Tauc plot. The resulting conduction and valence band potentials of BTNO-800 are approximately ECB = −0.01 eV, EVB = +3.33 eV. This configuration indicates that the valence band is sufficiently positive to oxidize H2O or OH− to hydroxyl radicals (E(H2O/·OH) = +1.99 eV), while the conduction band is not negative enough to reduce O2 to superoxide (E(O2/·O2−) = −0.33 eV). Consequently, ·OH radicals dominate the oxidation pathway, in full agreement with the inhibition pattern: strong suppression by IPA and EDTA-2Na, negligible effect of BQ, and acceleration by AgNO3, which removes conduction-band electrons and further promotes the hole-mediated oxidation cycle. Under ultrasonic vibration, mechanical deformation of the Aurivillius-type BTNO lattice generates a piezopotential that spatially separates photogenerated charges, reducing recombination and inducing band bending at the solid–liquid interface. This piezoelectric field drives holes toward the catalyst surface, facilitating ·OH generation, while electrons migrate in the opposite direction to defect sites or electron acceptors (e.g., Ag+). The synergistic coupling of optical excitation and piezoelectric polarization (L + U mode) therefore results in enhanced charge separation, higher ·OH yield, and a superadditive rate constant (kL+U = 0.047 min−1 > kL + kU = 0.037 min−1).
A schematic representation of the proposed piezophotocatalytic mechanism of BTNO-800 is shown in
Figure 8.
2.6. Synergistic Effect in the Piezophotocatalisis
The obtained data indicate a fundamentally different nature of the synergistic effects in the piezophotocatalytic reactions of BTNO-900 and BTNO-800. Let us denote kL as the photocatalytic rate constant, kU as the piezocatalytic rate constant, and kL+U as the piezophotocatalytic rate constant.
For the ceramic BTNO-900 sample, the following values were observed: k
L = 0.025 min
−1, k
U = 0.032 min
−1, k
L + k
U = 0.057 min
−1. However, the experimentally determined constant for the synergistic regime was k
L+U = 0.049 min
−1, which is significantly lower than the sum of the individual contributions. This subadditive effect can be attributed to overlapping reaction pathways, competition for active surface sites, and saturation of intermediate states, particularly those associated with the generation of superoxide radicals (·O
2−). According to XPS analysis, the surface of BTNO-900 is characterized by a high fraction of adsorbed oxygen-hydroxyl species, which lowers the barrier for O
2 reduction and promotes the formation of ·O
2−. Furthermore, an extended carrier lifetime and a reduced bandgap were observed, both of which contribute to enhanced photocatalytic activity. Nevertheless, the excessive concentration of defects and increased photoconductivity lead to partial screening of the piezoelectric field by mobile charges, thereby diminishing the efficiency of the piezocatalytic mechanism. As a consequence, the application of mechanical stimulation does not result in a linear enhancement of the overall process but instead leads to partial duplication of the already active photocatalytic pathway, which manifests as a subadditive behavior [
15,
42,
43].
For the melt-derived BTNO-800 sample, the kinetic constants are as follows: kL = 0.011 min−1, kU = 0.026 min−1, kL + kU = 0.037 min−1. In contrast, the experimentally determined value of kL+U = 0.047 min−1, considerably exceeds the sum of the individual contributions. This superadditive effect indicates that the combined stimulation activates additional mechanisms that are inaccessible under single-mode excitation. In the case of BTNO-800, the intrinsically low photocatalytic activity is associated with the predominance of charge carrier recombination. However, ultrasonic excitation generates a piezoelectric field that spatially separates electrons and holes, effectively suppressing their recombination and thereby enabling the formation of superoxide radicals ·O2−. In the absence of defect-related conductivity, the piezoelectric field is preserved with greater stability and efficiently unlocks a previously inaccessible reaction pathway. This process accounts for the superadditive behavior, in which the observed reaction rate exceeds the simple sum of the photo- and piezo-induced contributions.
In summary, BTNO-900 and BTNO-800 exhibit contrasting scenarios of synergy: BTNO-900 demonstrates high photocatalytic activity due to abundant defects and surface oxygen-containing groups. However, these same factors increase conductivity and induce screening of the piezoelectric field, thereby limiting the contribution of the piezostimulus. The combined activation results in partial overlap of pathways and a subadditive effect. BTNO-800, in contrast, shows low intrinsic photocatalytic activity due to rapid carrier recombination. The piezoelectric field effectively mitigates this limiting factor, maintains charge separation, and activates additional pathways, leading to superadditivity.
The attempt to combine high photocatalytic activity and efficient piezocatalytic performance within a single material is associated with several fundamental contradictions arising from the different nature of charge transport and energy conversion processes. Structural, electronic, and morphological features that enhance photocatalysis often attenuate the piezocatalytic response, and vice versa.
For efficient piezocatalysis, wide-bandgap, low-conductivity materials are generally required, since under these conditions the induced piezoelectric field is maintained for longer periods and is not readily screened by mobile charge carriers. Photocatalysis, in contrast, benefits from bandgap narrowing and enhanced conductivity achieved through doping or defect engineering; however, the increase in carrier concentration accelerates the screening of polarization and reduces the efficiency of the piezoelectric effect [
44].
Defects, primarily oxygen vacancies, play a decisive role. A moderate concentration of such defects promotes the adsorption of O
2 and H
2O, facilitates the generation of reactive radicals, and partially suppresses charge carrier recombination, thereby enhancing photocatalysis. However, an excessive concentration of vacancies increases defect-induced conductivity and creates centers for rapid recombination, which results in the screening of the piezoelectric field and a subsequent weakening of the piezocatalytic response [
45,
46].
Ferroelectric polarization is also of critical importance, as it generates internal electric fields and induces band bending, thereby facilitating the separation of photogenerated charge carriers. At the same time, in liquid media, surface adsorbates and ions can effectively screen this polarization, reducing both the beneficial role of the ferroelectric field in photocatalysis and the contribution of the piezoelectric effect [
47]. Morphological characteristics also impose significant constraints: a high specific surface area, nanocrystallinity, and porosity enhance photocatalytic activity by increasing the number of active sites. However, excessive porosity and low mechanical strength reduce the mechanical quality factor of vibrations and, consequently, diminish the amplitude of the piezoelectric response [
16]. Particular attention should be paid to the influence of illumination: the increase in the concentration of electrons and holes enhances photocatalysis but simultaneously accelerates the screening of the piezopotential, thereby limiting the contribution of mechanical activation. In contemporary literature, this compromise is described within the framework of the
piezophototronics concept, which emphasizes the necessity of optimizing charge carrier extraction and introducing co-catalysts in order to stabilize the piezoelectric field under illumination conditions [
48,
49].
In Aurivillius-phase layered oxides, such as Bi
4Ti
3O
12 and Bi
3TiNbO
9, the high spontaneous polarization is favorable for photocatalysis and photoelectrochemistry. However, the anisotropy of transport properties and the tendency toward leakage currents under defect modification significantly limit the piezocatalytic response. Contemporary approaches in this field are focused on differentiated defect control, the orientation of crystallographic domains, and the design of heterostructures, which together enable the simultaneous enhancement of both mechanisms [
50,
51,
52].
Thus, achieving high efficiency of both photocatalysis and piezocatalysis within a single material requires a delicate balance among defect engineering, morphological design, domain structure control, and regulation of electrical conductivity. Shifting the material parameters in favor of one mechanism almost inevitably reduces the efficiency of the other, making the search for optimized composites and tailored architectures one of the key challenges in the advancement of piezophotocatalysis.