Machine Learning-Assisted DFT Screening of Nitrogen-Doped Graphene Diatomic Catalysts for Nitrogen Reduction Reaction
Abstract
1. Introduction
2. Structure and Discussion
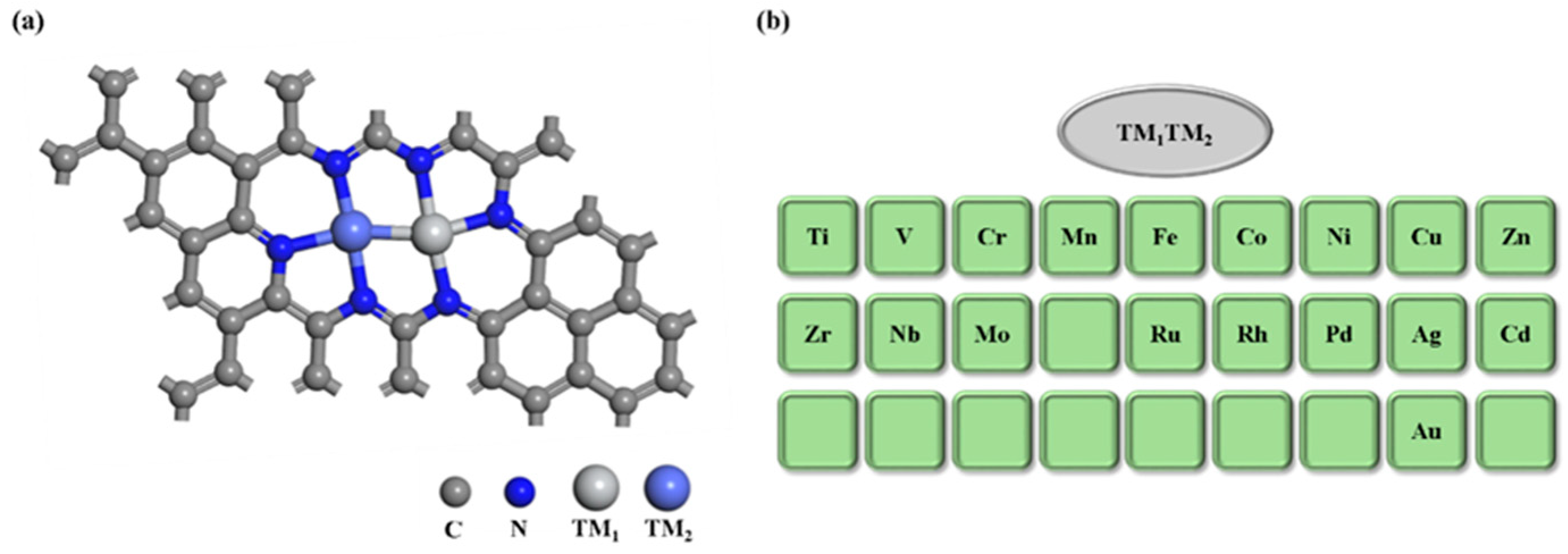
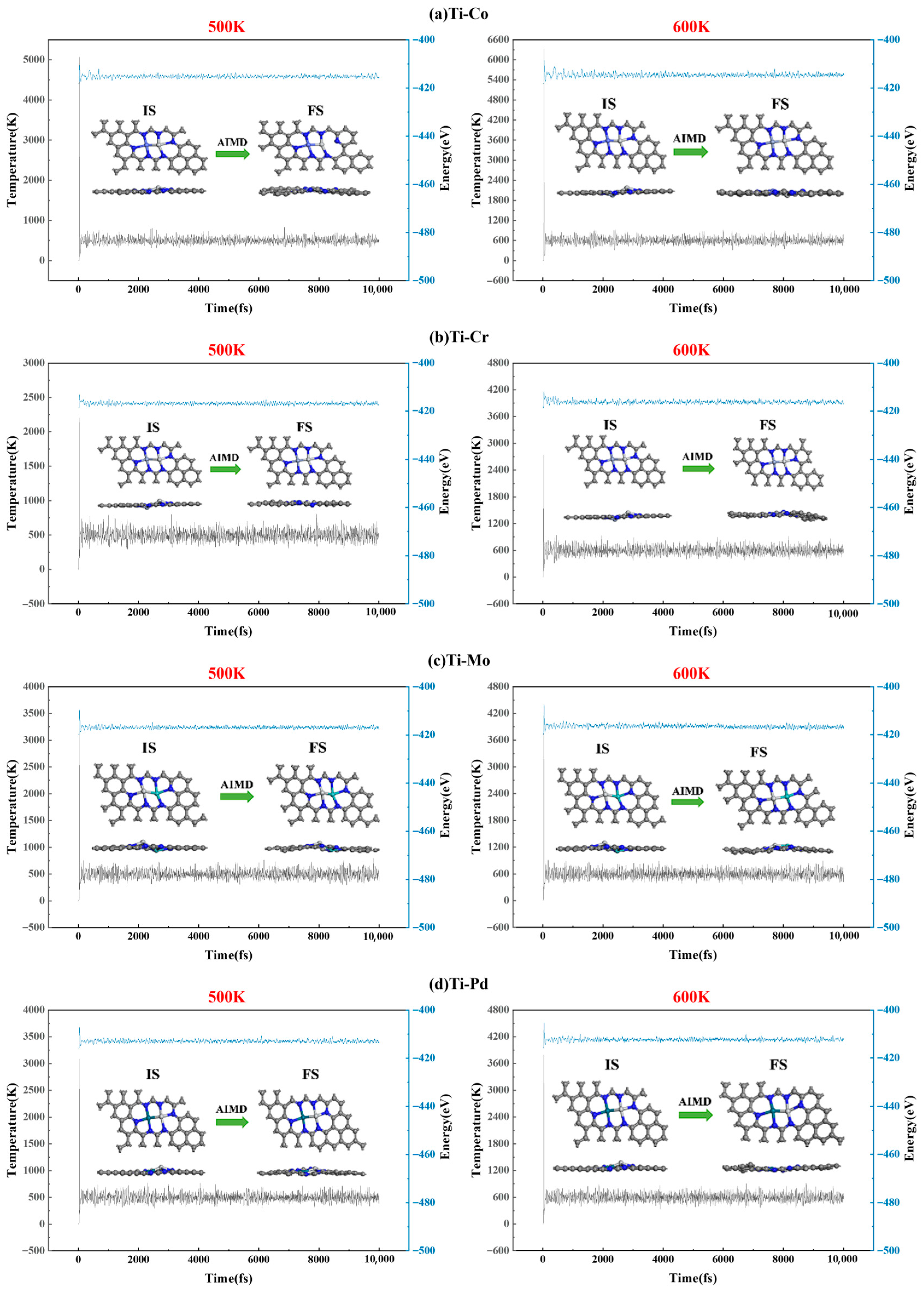
2.1. TM1-TM2@N6G Catalyst Structure and Stability
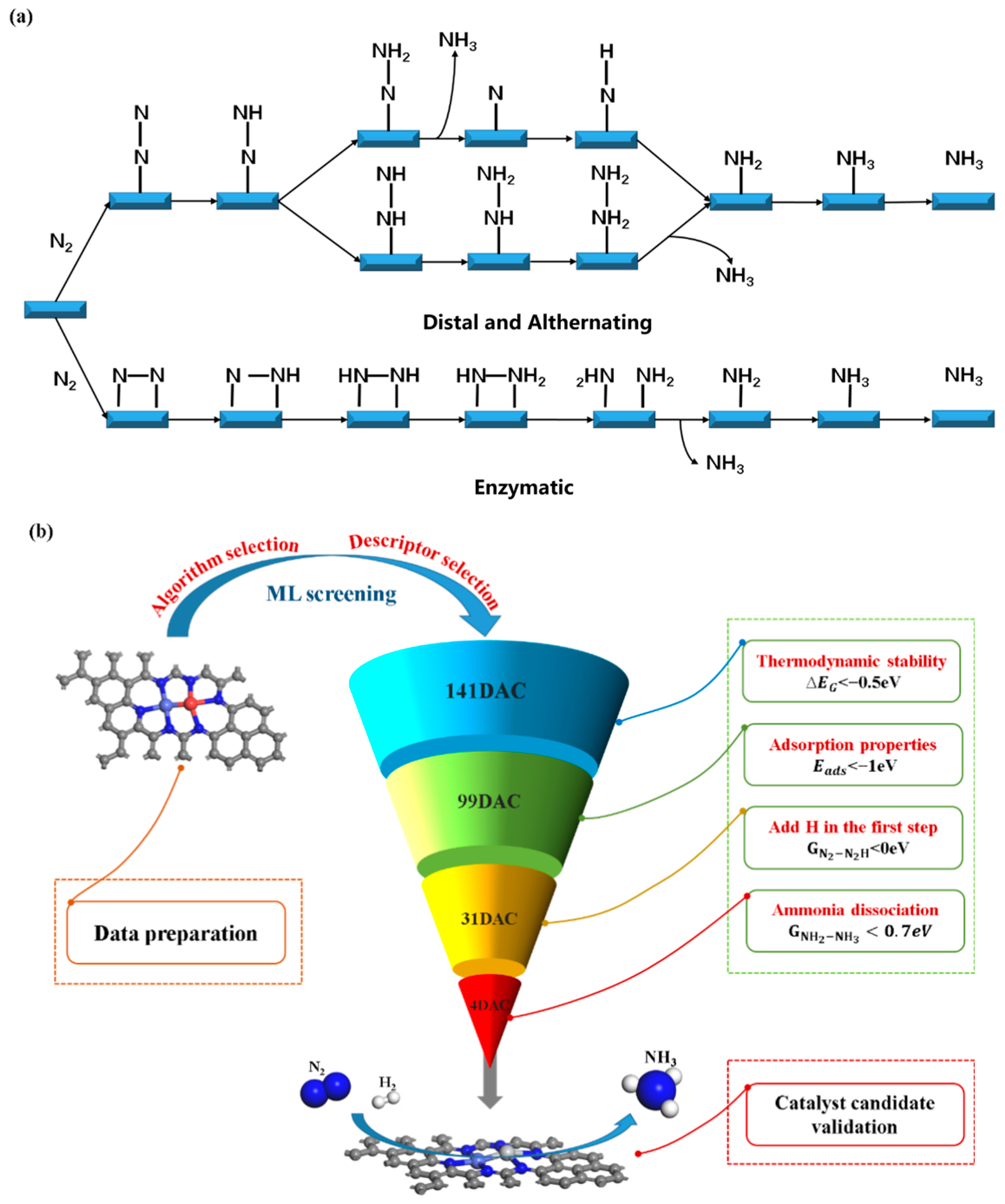
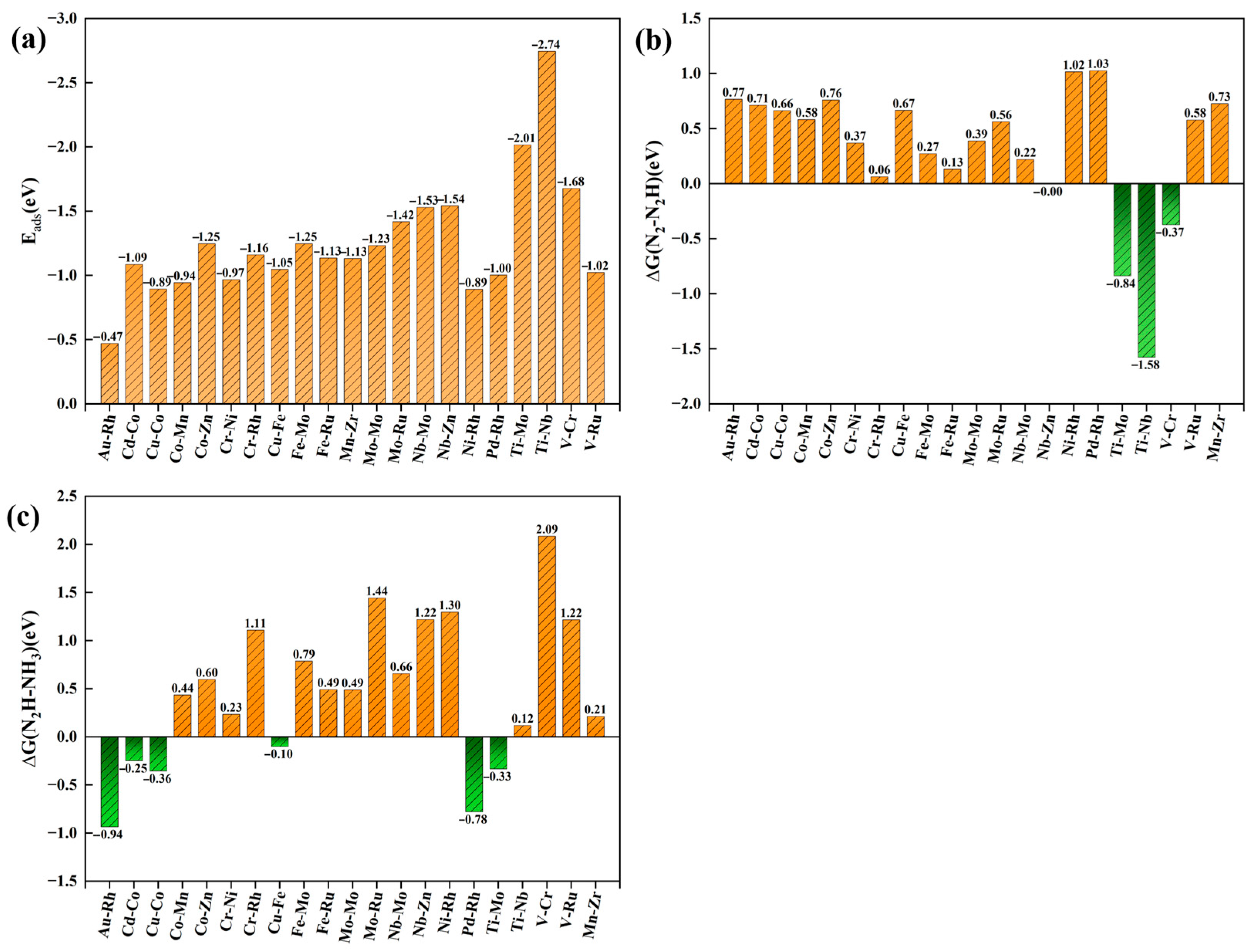
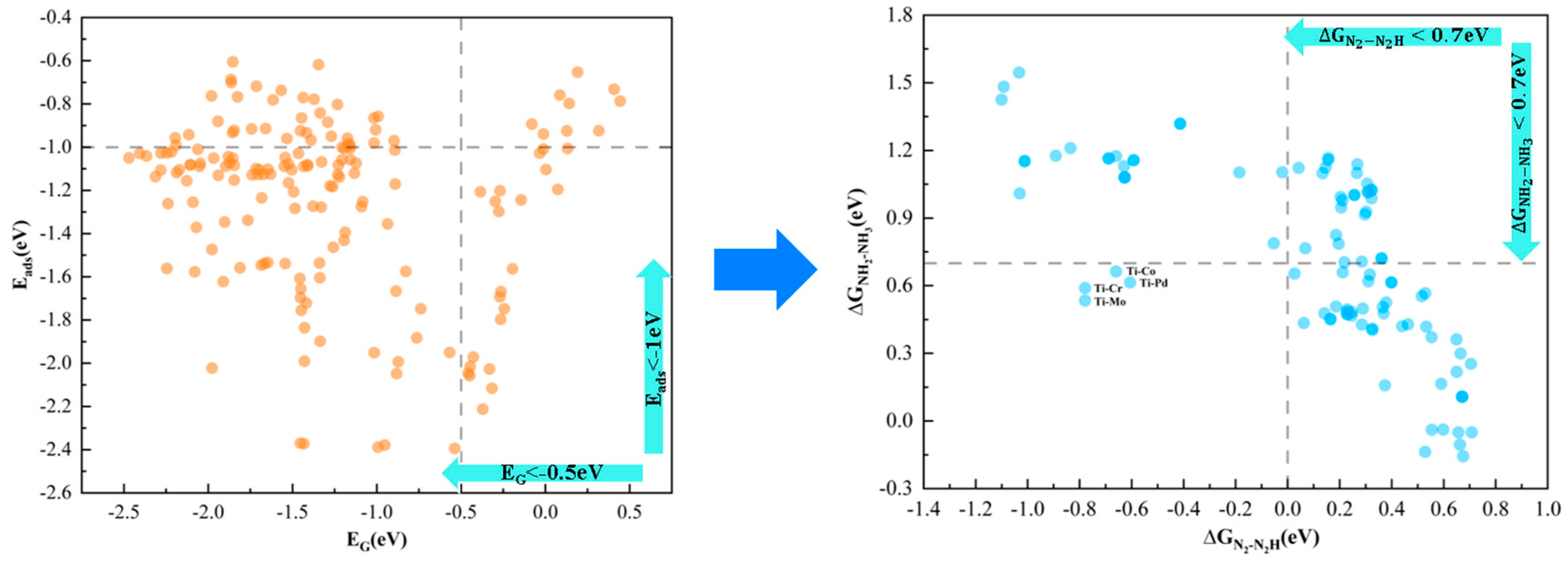
2.2. Computational Screening of NRR Catalytic Candidates
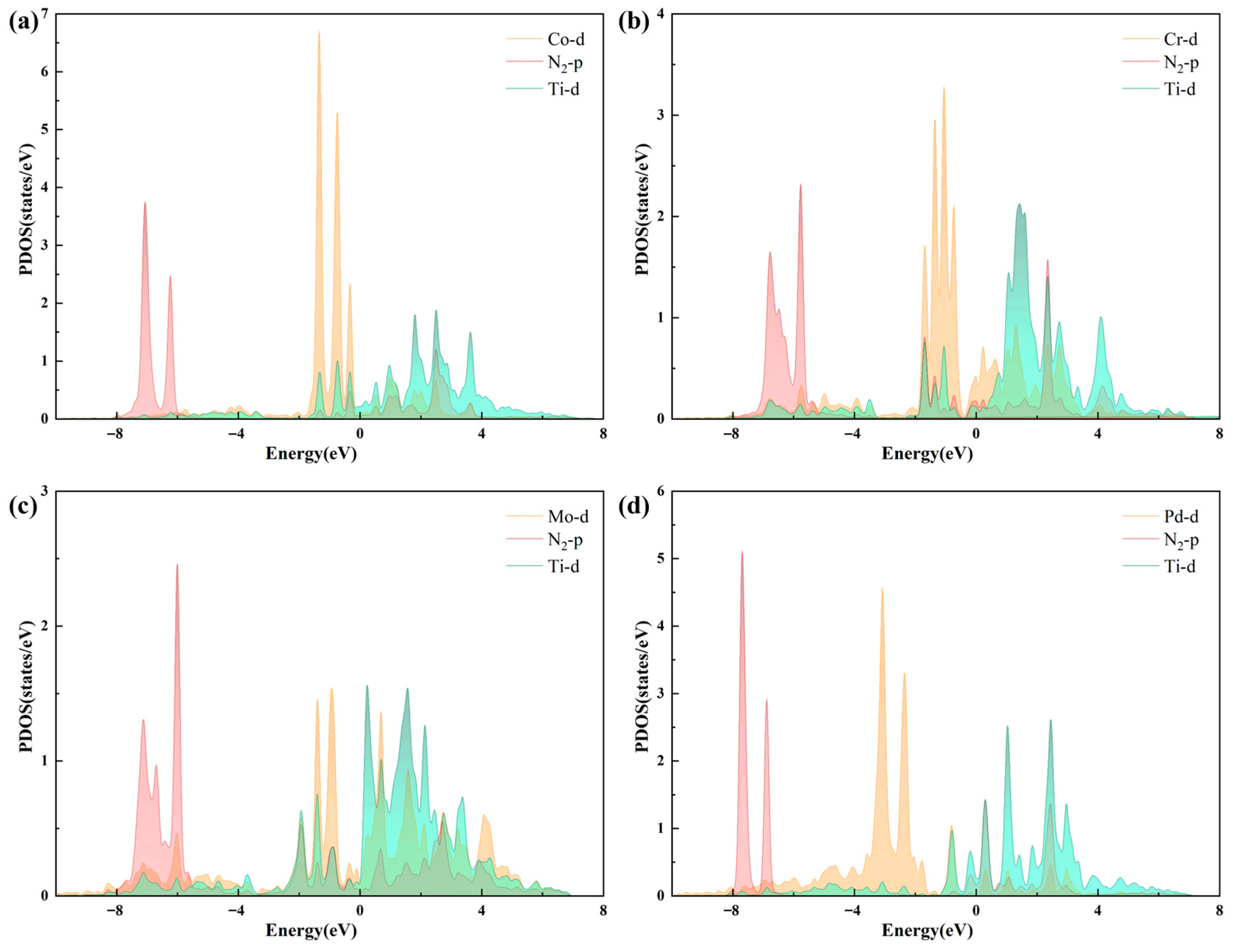
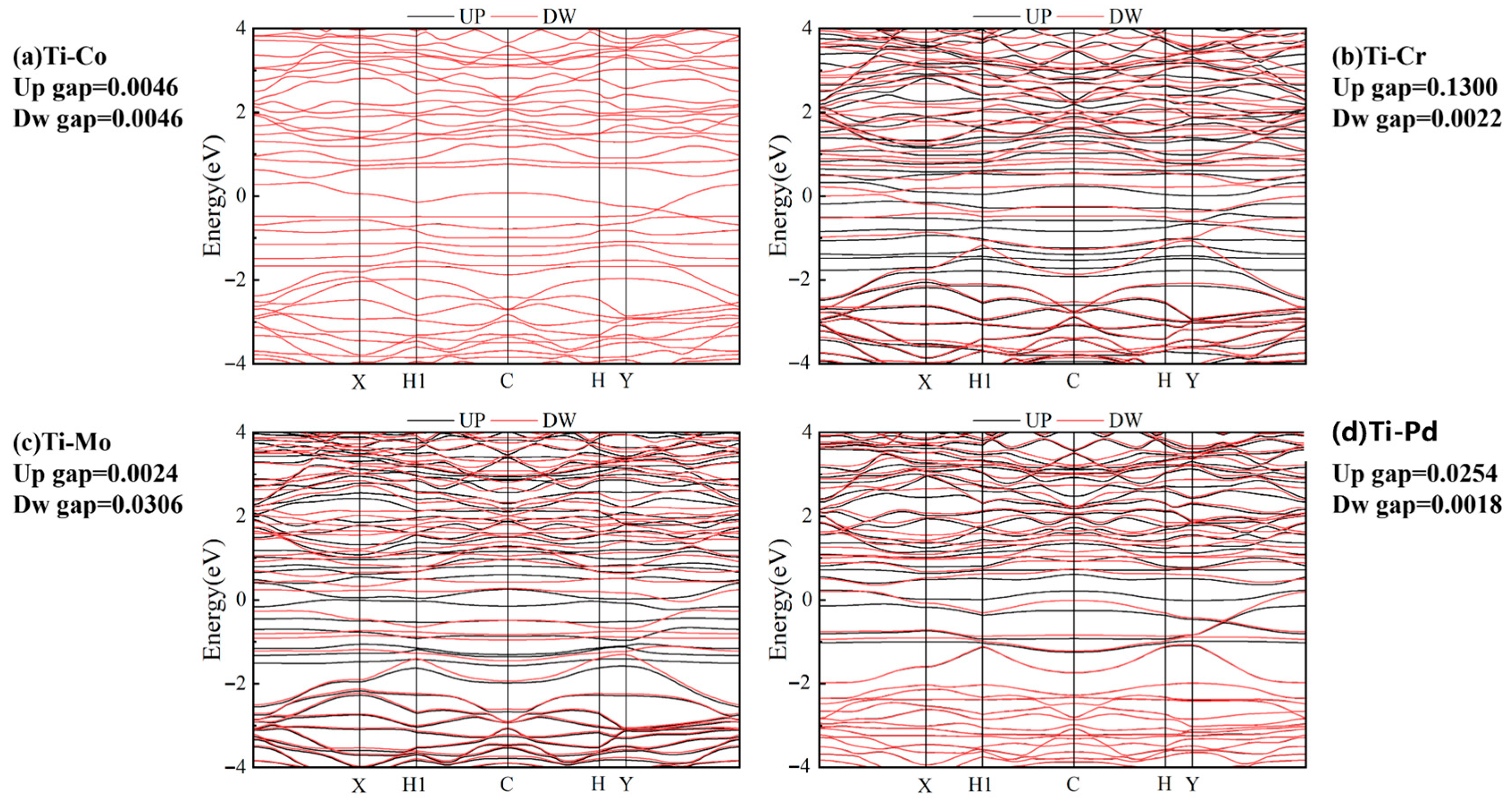
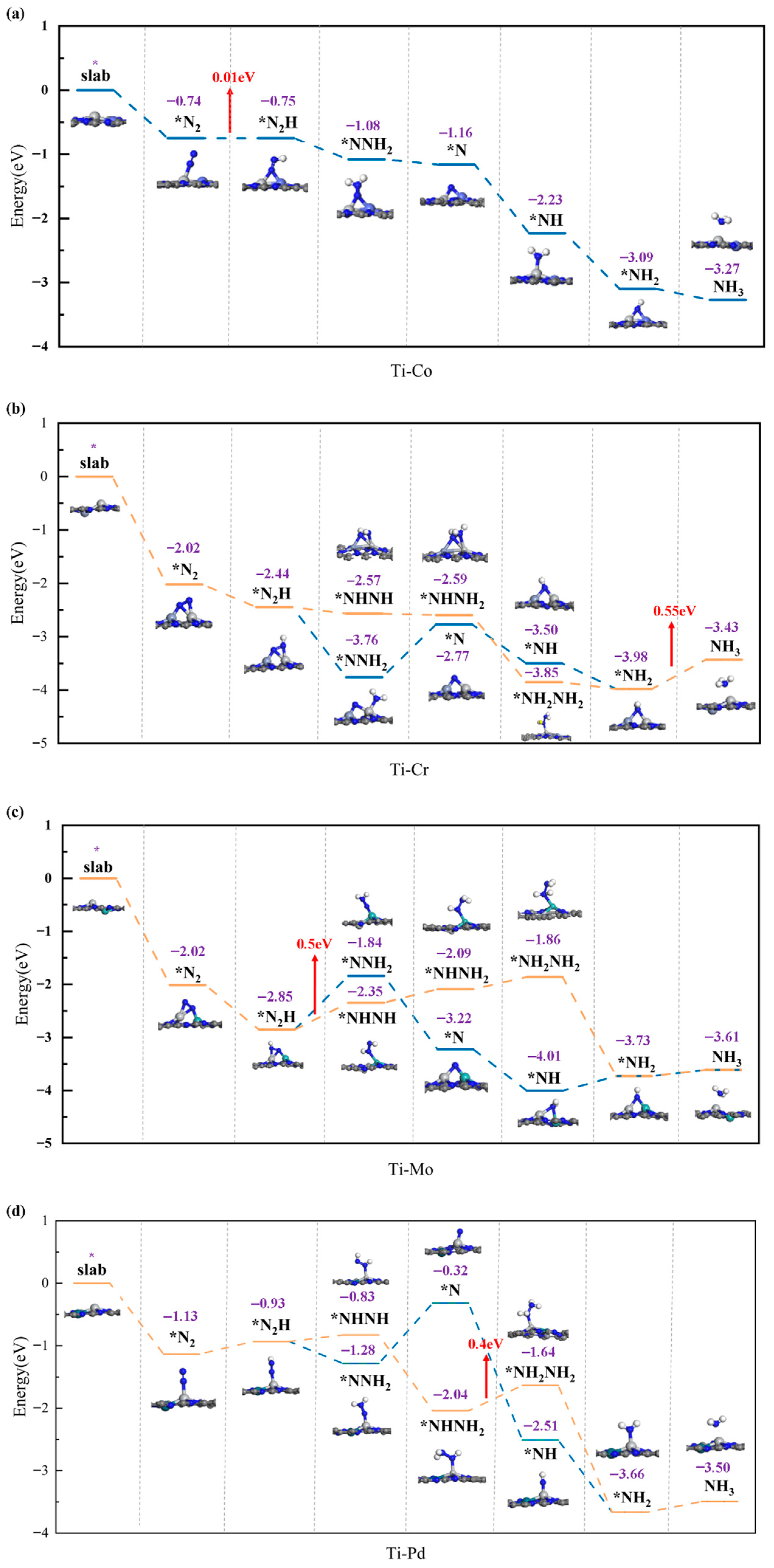
2.3. Performance Exploration of TM1-TM2@N6G Candidates
3. Computational Details
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NRR | Nitrogen reduction reaction |
| ML | Machine learning |
| DFT | Density functional theory |
| SACs | Single-atom catalysts |
| DACs | Dual-atom catalysts |
| N6G | 6-nitrogen-doped graphene |
| AIMD | Ab initio molecular dynamics |
| PDOS | Projected density of states |
| VASP | Vienna Ab initio Simulation Package |
| PAW | Projector Augmented Wave |
| GGA | Generalized Gradient Approximation |
| PBE | Perdew–Burke–Ernzerhof |
| RFR | Random Forest Algorithm |
| KNN | K-nearest Neighbor Regression |
| DT | Decision Tree |
| RMSE | Root mean square error |
| R2 | Coefficient of determination |
References
- Klerke, A.; Christensen, C.H.; Nørskov, J.K.; Vegge, T. Ammonia for hydrogen storage: Challenges and opportunities. J. Mater. Chem. 2008, 18, 2304–2310. [Google Scholar] [CrossRef]
- Schlögl, R. Catalytic synthesis of ammonia—A “never-ending story”? Angew. Chem. Int. Ed. 2003, 42, 2004–2008. [Google Scholar] [CrossRef]
- Smil, V. Detonator of the population explosion. Nature 1999, 400, 415. [Google Scholar] [CrossRef]
- Chen, X.; Li, N.; Kong, Z.; Ong, W.; Zhao, X. Photocatalytic fixation of nitrogen to ammonia: State-of-the-art advancements and future prospects. Mater. Horiz. 2018, 5, 9–27. [Google Scholar] [CrossRef]
- Saadatjou, N.; Jafari, A.; Sahebdelfar, S. Ruthenium nanocatalysts for ammonia synthesis: A review. Chem. Eng. Commun. 2015, 202, 420–448. [Google Scholar] [CrossRef]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef]
- Shi, M.M.; Bao, D.; Li, S.J.; Wulan, B.; Jiang, Q. Anchoring Pd-Cu amorphous nanocluster on graphene for electrochemical reduction of N2 to NH3 under ambient conditions in aqueous solution. Adv. Energy Mater. 2018, 8, 1800124. [Google Scholar] [CrossRef]
- Shi, M.M.; Bao, D.; Wulan, B.R.; Li, Y.H.; Zhang, Y.F.; Yan, J.M.; Jiang, Q. Au sub-nanoclusters on TiO2 toward highly efficient and selective electro catalyst for N2 conversion to NH3 at ambient conditions. Adv. Mater. 2017, 29, 1606550. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, S.; Chen, C.; Peng, Q.; Wang, D. Single-atom catalysts: Synthetic strategies and electrochemical applications. Joule 2018, 2, 1242–1264. [Google Scholar] [CrossRef]
- Zhu, C.; Fu, S.; Shi, Q.; Du, D.; Lin, Y. Single-atom electro catalysts. Angew. Chem. Int. Ed. 2017, 56, 13944–13960. [Google Scholar] [CrossRef]
- Li, X.F.; Li, Q.K.; Cheng, J.; Liu, L.L.; Yan, Q.; Wu, Y.C.; Zhang, X.H.; Wang, Z.Y.; Qi, Q.; Luo, Y. Conversion of dinitrogen to ammonia by FeN3-embedded graphene. J. Am. Chem. Soc. 2016, 138, 8706–8709. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Li, H.; Xiao, J.; Tu, Y. Triggering the electro catalytic hydrogen evolution activity of the inert two-dimensional MoS2 surface via single-atom metal doping. Energy Environ. Sci. 2015, 8, 1594–1601. [Google Scholar] [CrossRef]
- Wang, Y.; Arandiyan, H.; Scott, J.; Auguey-Zinsou, K.; Amal, R. Single atom and nanocluster Pt catalysts for selective CO2 reduction. ACS Appl. Energy Mater. 2018, 1, 6781–6789. [Google Scholar] [CrossRef]
- Wang, S.; Yu, W.; Xu, S.; Han, K.; Wang, F. Ammonia from photothermal N2 hydrogenation over Ni/TiO2 catalysts under mild conditions. ACS Sustain. Chem. Eng. 2021, 10, 115–123. [Google Scholar] [CrossRef]
- Cao, X.; Xing, S.; Ma, D.; Tan, Y.; Zhu, Y.; Hu, J. Design of high-performance ion-doped Co-P systems for hydrogen evolution: From multi-level screening calculations to experiment. J. Energy Chem. 2023, 82, 307–316. [Google Scholar] [CrossRef]
- Wan, X.; Zhang, Z.; Niu, H.; Yin, Y.; Kuai, C.; Wang, J.; Shao, C.; Guo, Y. Machine-learning-accelerated catalytic activity predictions of transition metal phthalocyanine dual-metal-site catalysts for CO2 reduction. J. Phys. Chem. Lett. 2021, 12, 6111–6118. [Google Scholar] [CrossRef]
- Sun, M.; Dougherty, A.W.; Huang, B.; Li, Y.; Yan, C. Accelerating atomic catalyst discovery by theoretical calculations-machine learning strategy. Adv. Energy Mater. 2020, 10, 1903949. [Google Scholar] [CrossRef]
- Zafari, M.; Kumar, D.; Umer, M.; Kim, K. Machine learning-based high throughput screening for nitrogen fixation on boron-doped single atom catalysts. J. Mater. Chem. A 2020, 8, 5209–5216. [Google Scholar] [CrossRef]
- Van Santen, R.A. Complementary structure sensitive and insensitive catalytic relationships. Acc. Chem. Res. 2009, 42, 57–66. [Google Scholar] [CrossRef]
- Li, Z.; Wang, S.; Xin, H. Toward artificial intelligence in catalysis. Nat. Catal. 2018, 1, 641–642. [Google Scholar] [CrossRef]
- Ma, S.; Liu, Z.P. Machine learning for atomic simulation and activity prediction in heterogeneous catalysis: Current status and future. ACS Catal. 2020, 10, 13213–13226. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, X.; Chen, A.; Yan, S.; Hu, X.; Zhou, Z. Targeted design of advanced electrocatalysts by machine learning. Chin. J. Catal. 2022, 43, 11–32. [Google Scholar] [CrossRef]
- Liu, J.; Luo, W.; Wang, L.; Zhang, J.; Fu, X.; Luo, J. Toward excellence of electrocatalyst design by emerging descriptor-oriented machine learning. Adv. Funct. Mater. 2022, 32, 2110748. [Google Scholar] [CrossRef]
- Tabor, D.P.; Roch, L.M.; Saikin, S.K.; Kreisbeck, C.; Sheberla, D.; Montoya, J.; Dwaraknath, S.; Aykol, M.; Ortiz, C.; Tribukait, H.; et al. Accelerating the discovery of materials for clean energy in the era of smart automation. Nat. Rev. Mater. 2018, 3, 5–20. [Google Scholar] [CrossRef]
- Tran, K.; Ulissi, Z.W. Active learning across intermetallics to guide discovery of electrocatalysts for CO2 reduction and H2 evolution. Nat. Catal. 2018, 1, 696–703. [Google Scholar] [CrossRef]
- Zhong, M.; Tran, K.; Min, Y.; Wang, C.; Wang, Z.; Dinh, C.; Luna, P.; Yu, Z.; Rasouli, A.; Brodersen, P.; et al. Accelerated discovery of CO2 electrocatalysts using active machine learning. Nature 2020, 581, 178–183. [Google Scholar] [CrossRef]
- O’Connor, N.J.; Jonayat, A.S.M.; Janik, M.J.; Senftle, T. Interaction trends between single metal atoms and oxide supports identified with density functional theory and statistical learning. Nat. Catal. 2018, 1, 531–539. [Google Scholar] [CrossRef]
- Panapitiya, G.; Avendaño-Franco, G.; Ren, P.; Wen, X.; Li, Y.; Lewis, J.P. Machine-learning prediction of CO adsorption in thiolated, Ag-alloyed Au nanoclusters. J. Am. Chem. Soc. 2018, 140, 17508–17514. [Google Scholar] [CrossRef]
- Wexler, R.B.; Martirez, J.M.P.; Rappe, A.M. Chemical pressure-driven enhancement of the hydrogen evolving activity of Ni2P from nonmetal surface doping interpreted via machine learning. J. Am. Chem. Soc. 2018, 140, 4678–4683. [Google Scholar] [CrossRef]
- Wu, L.; Guo, T.; Li, T. Rational design of transition metal single-atom electrocatalysts: A simulation-based, machine learning-accelerated study. J. Mater. Chem. A 2020, 8, 19290–19299. [Google Scholar] [CrossRef]
- Ma, X.; Li, Z.; Achenie, L.E.K.; Xin, H. Machine-learning-augmented chemisorption model for CO2 electroreduction catalyst screening. J. Phys. Chem. Lett. 2015, 6, 3528–3533. [Google Scholar] [PubMed]
- Boonpalit, K.; Wongnongwa, Y.; Prommin, C.; Nutanong, S.; Numuangruk, S. Data-driven discovery of graphene-based dual-atom catalysts for hydrogen evolution reaction with graph neural network and DFT calculations. ACS Appl. Mater. Interfaces 2023, 15, 12936–12945. [Google Scholar]
- Wang, S.; Qian, C.; Zhou, S. Machine learning design of single-atom catalysts for nitrogen fixation. ACS Appl. Mater. Interfaces 2023, 15, 40656–40664. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sun, K.; Duan, X.; Tian, S.; Sun, X. Construction of dual-atom Fe via face-to-face assembly of molecular phthalocyanine for superior oxygen reduction reaction. Chem. Mater. 2022, 34, 5598–5606. [Google Scholar] [CrossRef]
- Wang, J.; Liu, W.; Luo, G.; Li, Z.; Zhao, C.; Zhang, H.; Zhu, M.; Xu, Q.; Wang, X.; Zhao, C.; et al. Synergistic effect of well-defined dual sites boosting the oxygen reduction reaction. Energy Environ. Sci. 2018, 11, 3375–3379. [Google Scholar] [CrossRef]
- Liu, X.; Qi, L.; Song, E.; Gao, W. Effective descriptor for nitrogen reduction on atomic catalysts. Catal. Lett. 2023, 153, 300–310. [Google Scholar]
- Hu, X.; Su, N.Q.; Fang, W.H. Metallated graphynes as efficient single-atom electrocatalysts for nitric oxide reduction to ammonia. J. Phys. Chem. C 2023, 127, 11026–11039. [Google Scholar] [CrossRef]
- Zhao, Z.; Hu, M.; Nie, T.; Zhou, W.; Pan, B.; Xing, B.; Zhu, L. Improved electronic structure from spin-state reconstruction of a heteronuclear Fe-Co diatomic pair to boost the Fenton-like reaction. Environ. Sci. Technol. 2023, 57, 4556–4567. [Google Scholar] [CrossRef]
- Ling, C.; Ouyang, Y.; Li, Q.; Bai, X.; Mao, X.; Du, A.; Wang, J. A general two-step strategy–based high-throughput screening of single atom catalysts for nitrogen fixation. Small Methods 2019, 3, 1800376. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.S.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef]
- Skulason, E.; Bligaard, T.; Gudmundsdóttir, S.; Studt, F.; Rossmeisl, J.; Abild-Pedersen, F.; Vegge, T.; Jónsson, H.; Nørskov, J. A theoretical evaluation of possible transition metal electro-catalysts for N2 reduction. Phys. Chem. Chem. Phys. 2012, 14, 1235–1245. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]
- Wang, V.; Xu, N.; Liu, J.C.; Tang, G.; Geng, W. VASPKIT: A user-friendly interface facilitating high-throughput computing and analysis using VASP code. Comput. Phys. Commun. 2021, 267, 108033. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Devroye, L.; Gyorfi, L.; Krzyzak, A.; Lugosi, G. On the strong universal consistency of nearest neighbor regression function estimates. Ann. Stat. 1994, 22, 1371–1385. [Google Scholar] [CrossRef]
- Aksöz, Z.Y.; Günay, M.E.; Aziz, M.; Tunc, K. Machine Learning Analysis of Thermal Performance Indicator of Heat Exchangers with Delta Wing Vortex Generators. Energies 2024, 17, 1380. [Google Scholar] [CrossRef]
- Pedregosa, F. Scikit-learn: Machine learning in python Fabian. J. Mach. Learn. Res. 2011, 12, 2825. [Google Scholar]

| Input Feature Value | Symbol | Input Feature Value | Symbol |
|---|---|---|---|
| The number of d orbital electrons of metal A | NdA | The first ionization energy of metal B | IEB |
| The number of d orbital electrons of metal B | NdB | The absolute value of the first ionization energy between metals | IE |
| The absolute value of the number of d orbital electrons between metals | Nd | The electron affinity energy of metal A | EAA |
| The sum of the number of d orbital electrons between metals | NdAB | The electron affinity energy of metal B | EAB |
| Electronegativity of metal A | pA | The absolute value of the electron affinity between metals | EA |
| Electronegativity of metal B | pB | The atomic radius of metal A | RA |
| The absolute value of the difference in electronegativity between metals | pA−B | The atomic radius of metal B | RB |
| The sum of electronegativity between metals | pA+B | The sum of the atomic radii of the center of the metal | RA+B |
| The number of s orbital electrons of metal A | NsA | The atomic mass of metal A | MA |
| The number of s orbital electrons of metal B | NsB | The atomic mass of metal B | MB |
| The absolute value of the number of s orbital electrons between metals | Ns | The atomic mass of metals A, B | MA+B |
| The first ionization energy of metal A | IEA |
| ML Algorithm | Evaluation Criterion | /eV | /eV | /eV | /eV |
|---|---|---|---|---|---|
| RFR | R2 | 0.9054 | 0.9129 | 0.9233 | 0.9095 |
| RMSE | 0.1698 | 0.1219 | 0.1664 | 0.1644 | |
| DT | R2 | 0.8171 | 0.7071 | 0.6310 | 0.8417 |
| RMSE | 0.4475 | 0.4613 | 0.5314 | 0.3739 | |
| KNR | R2 | 0.7777 | 0.7655 | 0.6920 | 0.8164 |
| RMSE | 0.3504 | 0.2134 | 0.3033 | 0.2752 |
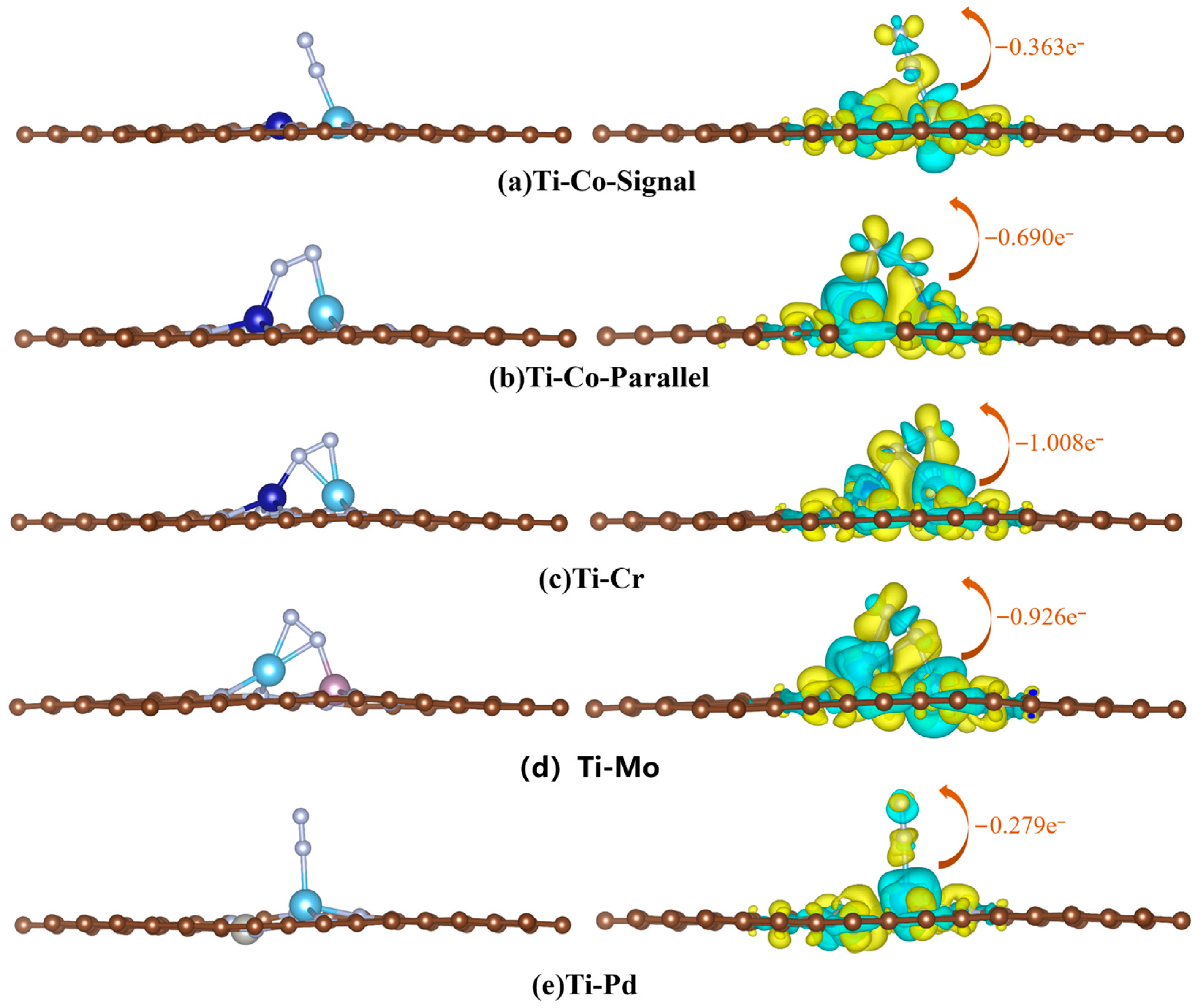
| N≡N/Å | Bader/e− | Eads/eV | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Bridge | Parallel | Single | Bridge | Parallel | Single | Bridge | Parallel | Single | |
| Ti-Co | — | 1.214 | 1.145 | — | −0.690 | −0.363 | — | −0.733 | −0.748 |
| Ti-Mo | — | 1.245 | — | — | −1.008 | — | — | −2.014 | — |
| Ti-Cr | — | 1.249 | — | — | −0.926 | — | — | −2.020 | — |
| Ti-Pd | — | — | −1.138 | — | — | −0.279 | — | — | −1.134 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Nie, S.; Yao, H.; Wu, S.; Li, Y.; Feng, J.; Sui, Y.; Zhang, Y.; Wang, X.; Zhang, X. Machine Learning-Assisted DFT Screening of Nitrogen-Doped Graphene Diatomic Catalysts for Nitrogen Reduction Reaction. Molecules 2025, 30, 4131. https://doi.org/10.3390/molecules30204131
Wang X, Nie S, Yao H, Wu S, Li Y, Feng J, Sui Y, Zhang Y, Wang X, Zhang X. Machine Learning-Assisted DFT Screening of Nitrogen-Doped Graphene Diatomic Catalysts for Nitrogen Reduction Reaction. Molecules. 2025; 30(20):4131. https://doi.org/10.3390/molecules30204131
Chicago/Turabian StyleWang, Xiulin, Suofu Nie, Huichao Yao, Sida Wu, Yanze Li, Junli Feng, Yiyan Sui, Yuqing Zhang, Xinwei Wang, and Xiuxia Zhang. 2025. "Machine Learning-Assisted DFT Screening of Nitrogen-Doped Graphene Diatomic Catalysts for Nitrogen Reduction Reaction" Molecules 30, no. 20: 4131. https://doi.org/10.3390/molecules30204131
APA StyleWang, X., Nie, S., Yao, H., Wu, S., Li, Y., Feng, J., Sui, Y., Zhang, Y., Wang, X., & Zhang, X. (2025). Machine Learning-Assisted DFT Screening of Nitrogen-Doped Graphene Diatomic Catalysts for Nitrogen Reduction Reaction. Molecules, 30(20), 4131. https://doi.org/10.3390/molecules30204131







