Kaempferol and Kaempferin Alleviate MRSA Virulence by Suppressing β-Lactamase and Inflammation
Abstract
1. Introduction
2. Results
2.1. Analysis of the Results
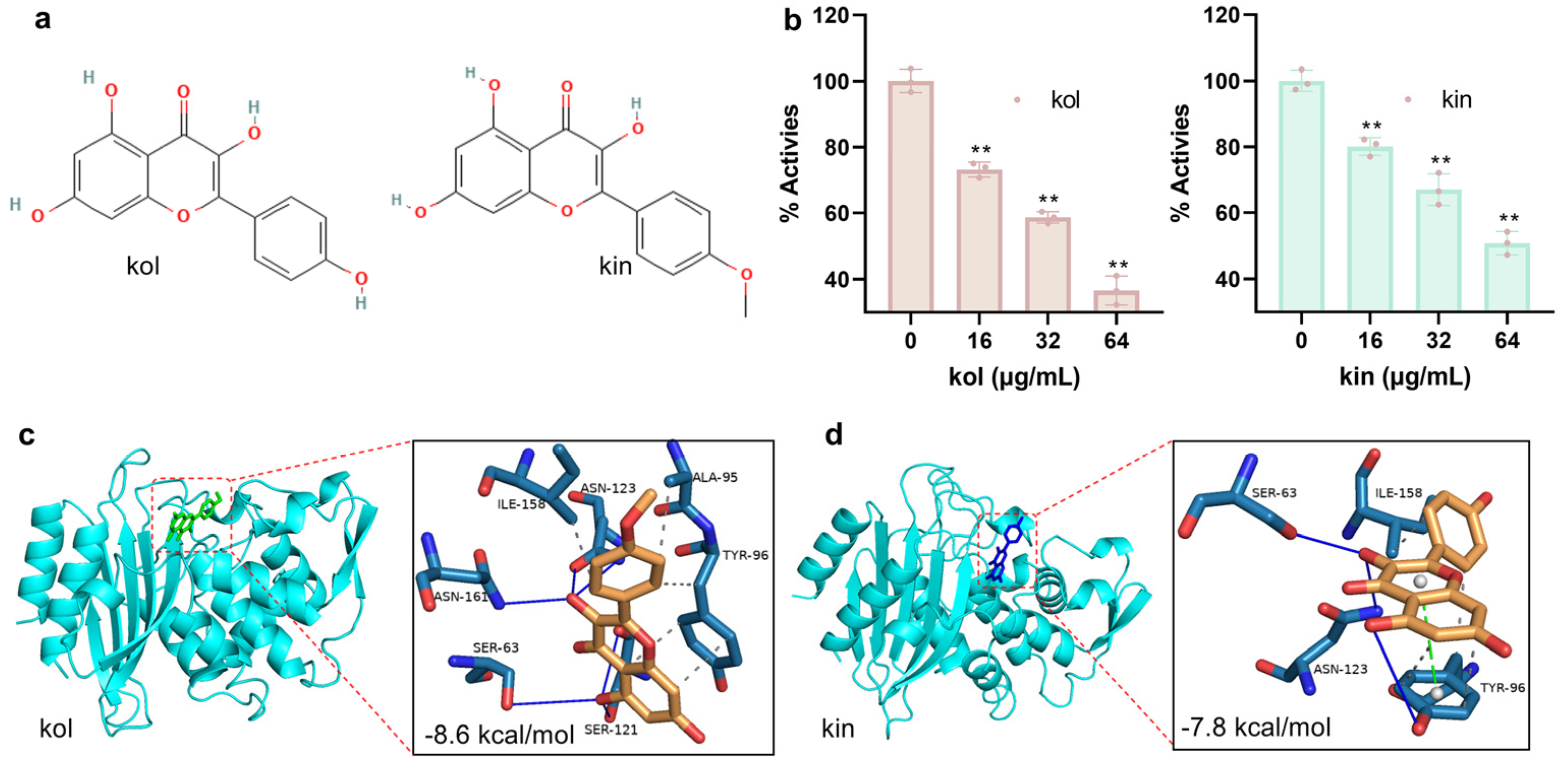
2.1.1. Kol and Kin Inhibited the Activity of S. aureus β-Lactamase by Binding to the Active Center
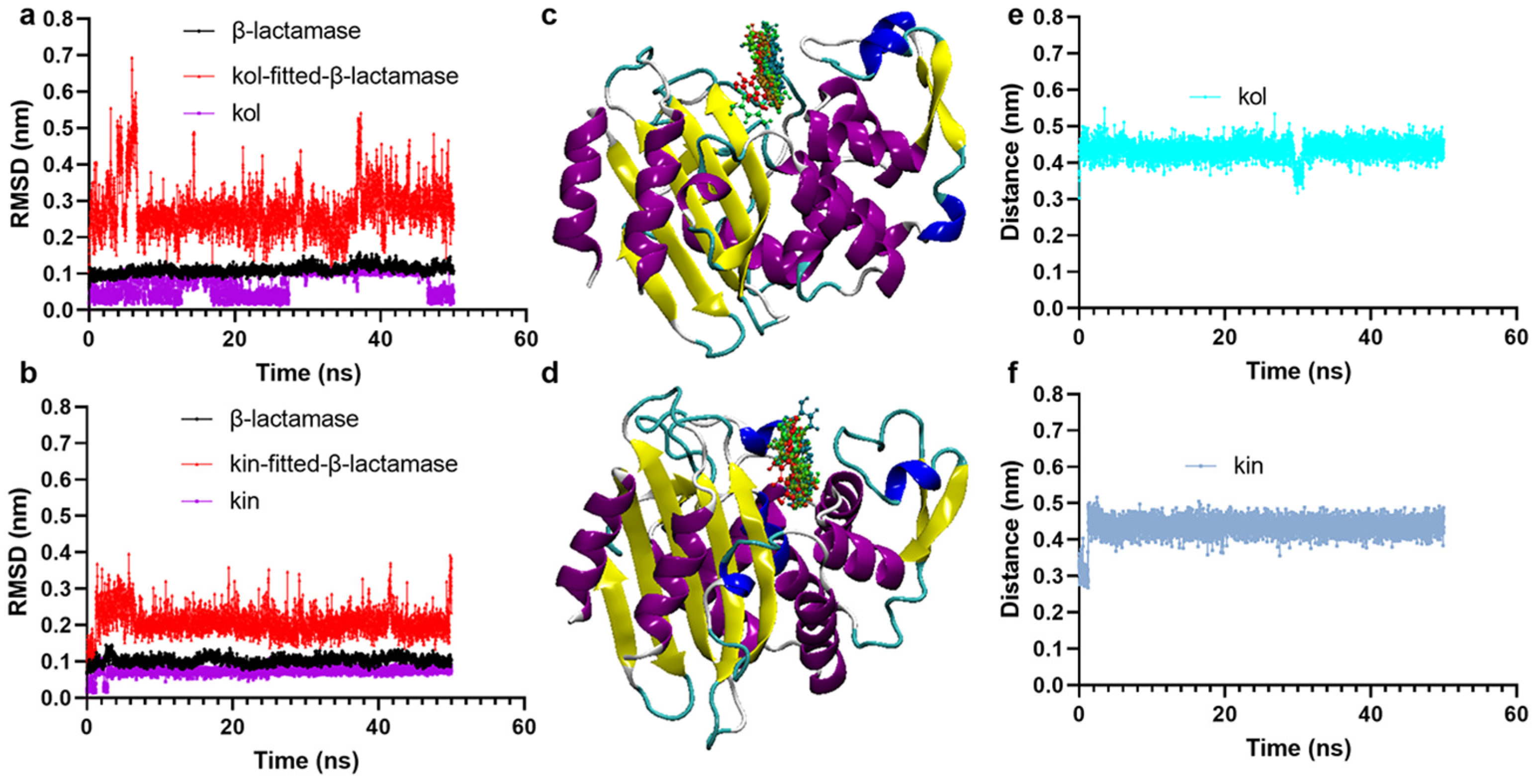
2.1.2. Kol and Kin Maintained Stable Binding with β-Lactamase
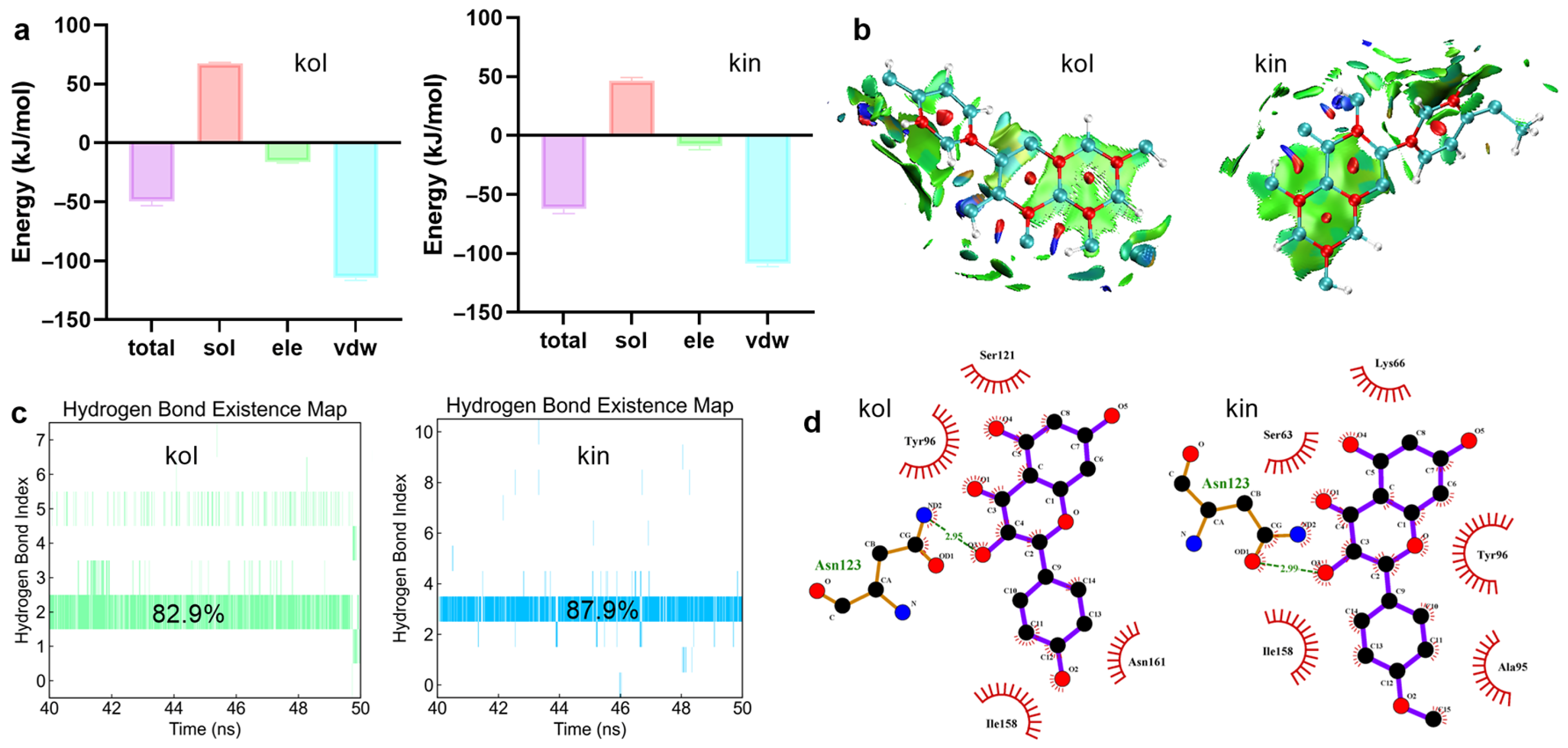
2.1.3. Hydrogen Bonds and van der Waals Interactions Were Critical for Promoting the Binding Between β-Lactamase and Kol or Kin
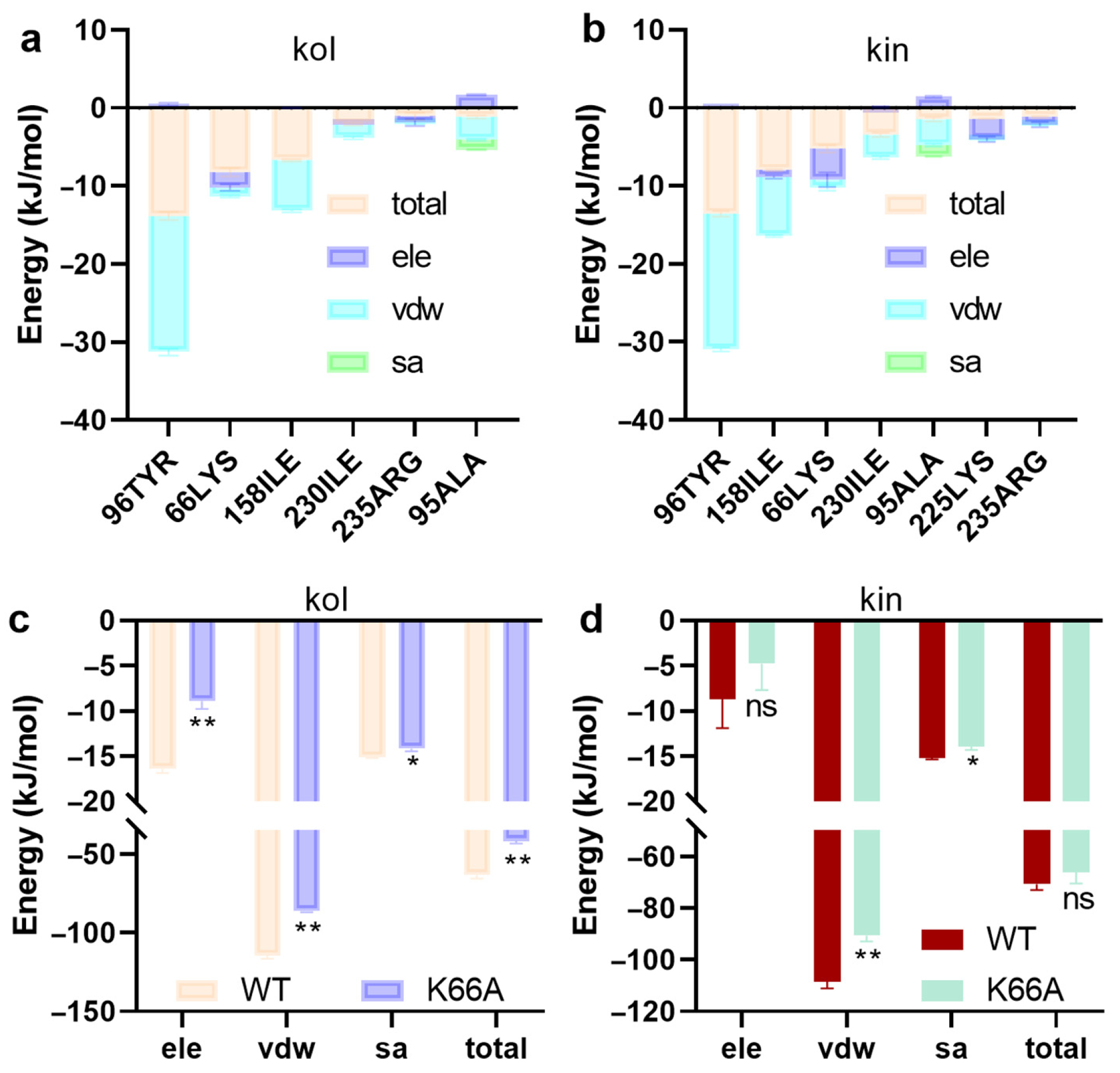
2.1.4. LYS66 Is Critical for the Binding Between β-Lactamase and Kol or Kin
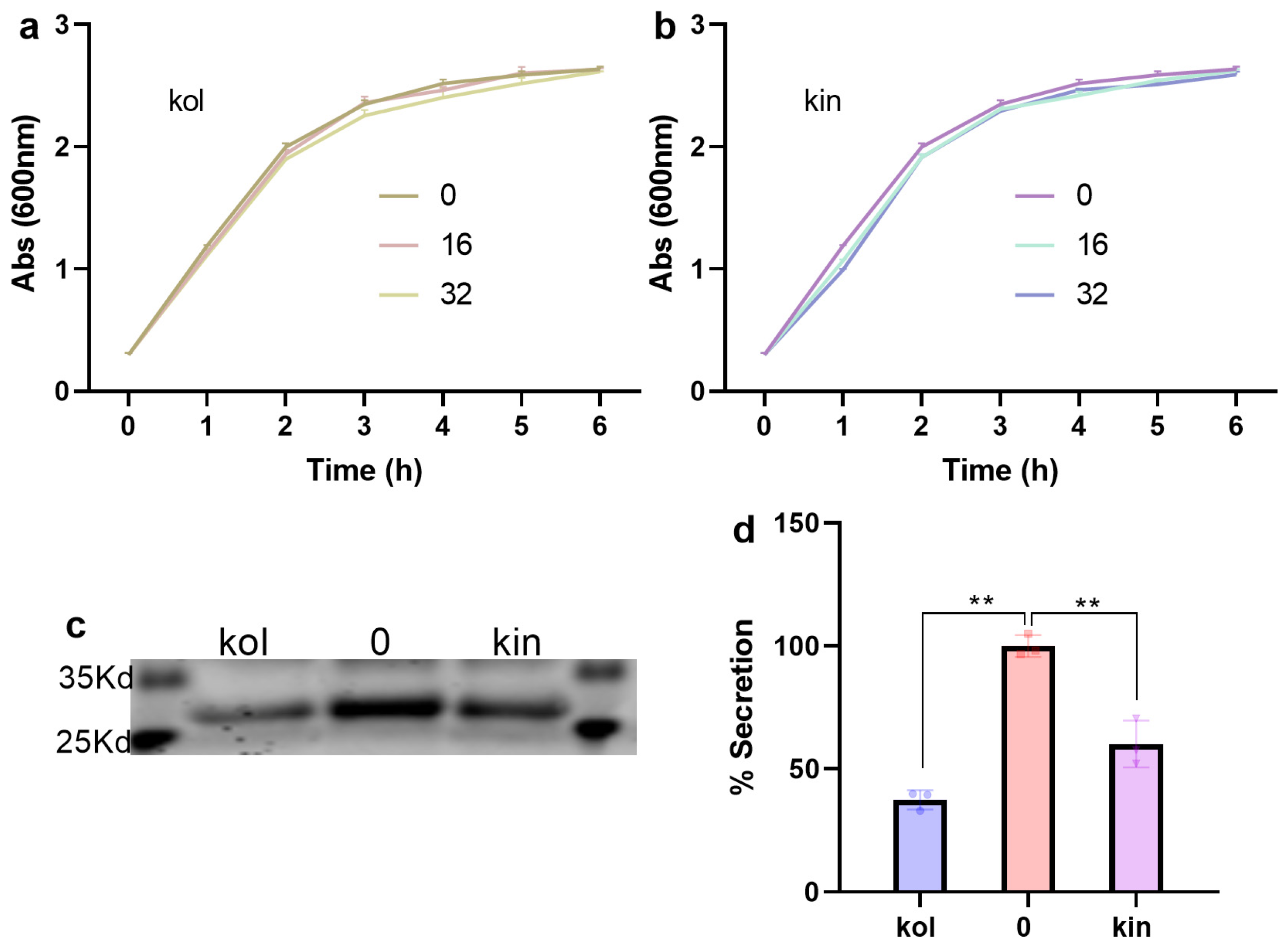
2.1.5. Kol and Kin Did Not Show Direct Antibacterial Activity Against S. aureus USA300
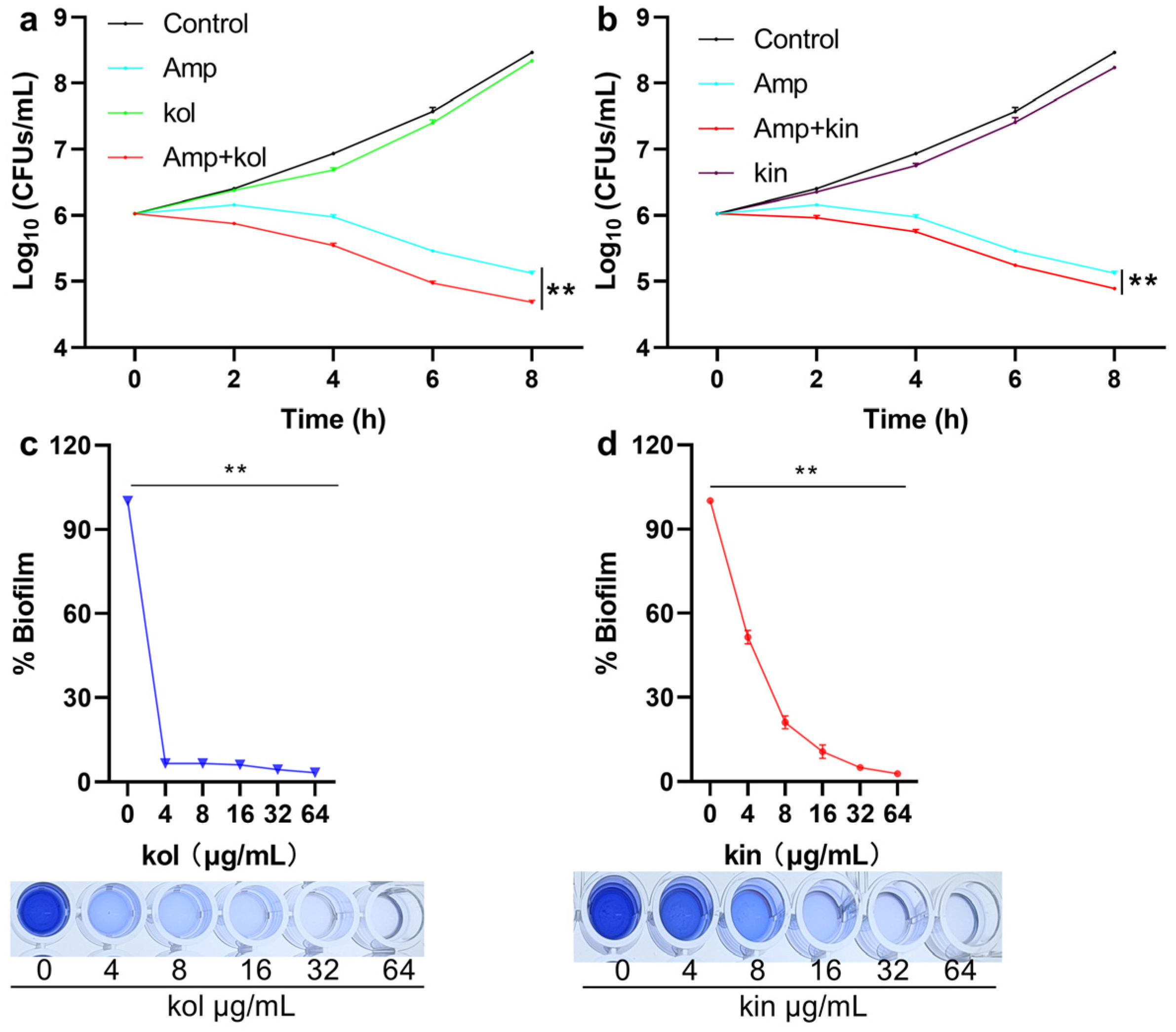
2.1.6. Kol and Kin Enhanced the Bactericidal Activity of Amp and Inhibited the Biofilm Formation of S. aureus USA300
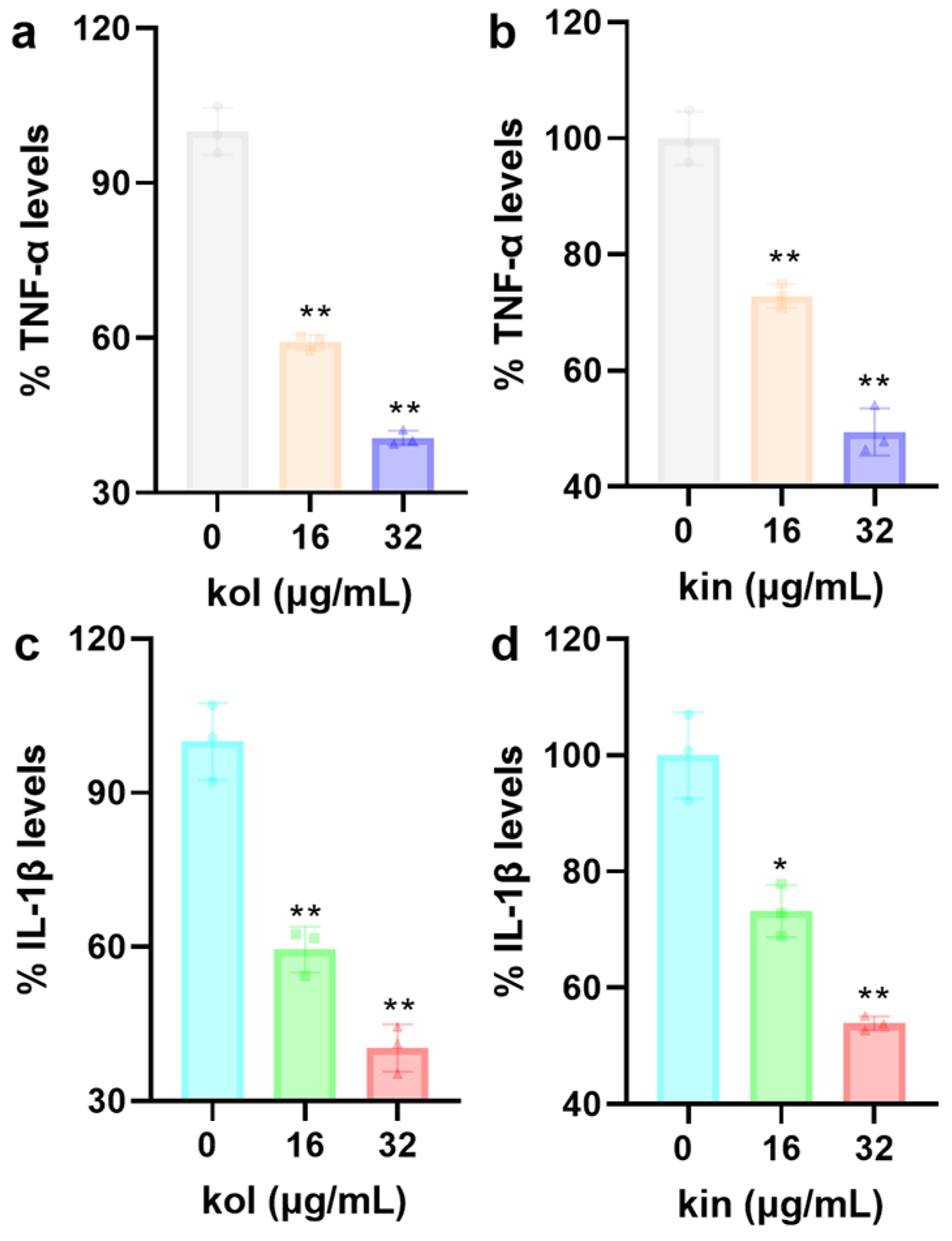
2.1.7. Kol and Kin Alleviated the Inflammatory Response of Mouse Macrophages
Induced by S. aureus USA300
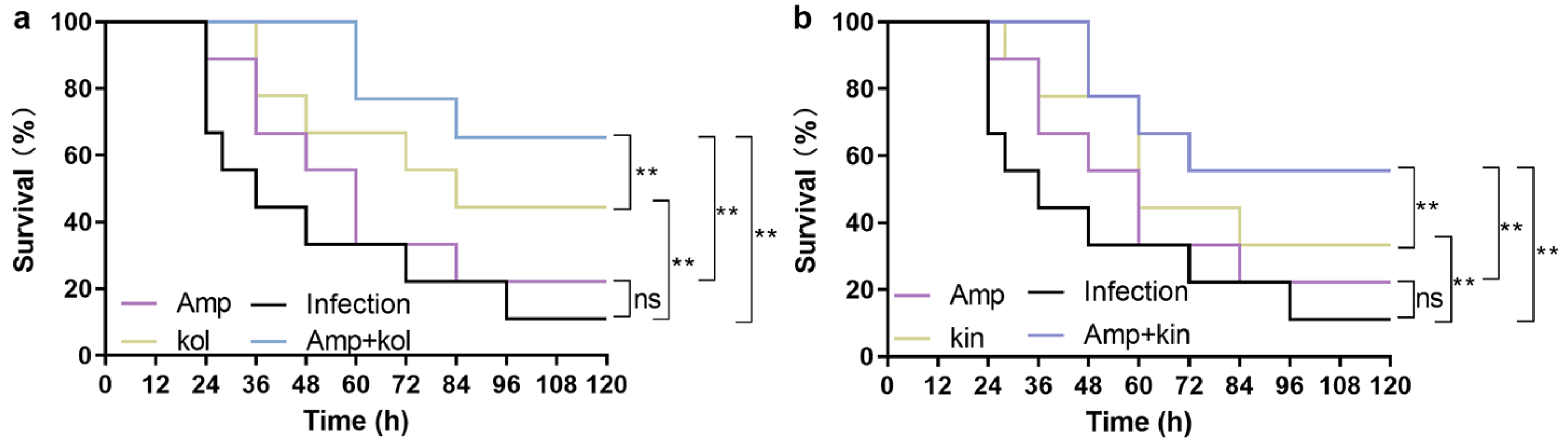
2.1.8. Kol and Kin Combined with Amp Protect G. Mellonella from S. Aureus USA300
Infection
3. Discussion
4. Materials and Methods
4.1. Reagents, Strains, and Cultural Conditions
4.2. β-Lactamase Activity Inhibition Assay
4.3. Docking and Calculation Assay
4.4. Anti-Bacterial Properties and β-Lactamase Secretion Assay
4.5. Biofilm Inhibition Assay
4.6. Time-Dependent Bacterial Killing Assay
4.7. Cytokines Detection
4.8. G. mellonella Protection Assay
4.9. Data Statistics and Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thomas, A.; Palathoti, N.; Azam, M.A. Methicillin-resistant Staphylococcus aureus Infection and its Health Perspective: A Review. Curr. Pharm. Biotechnol. 2025, 26, 1331–1347. [Google Scholar] [CrossRef]
- Miller, W.R.; Arias, C.A. ESKAPE pathogens: Antimicrobial resistance, epidemiology, clinical impact and therapeutics. Nat. Rev. Microbiol. 2024, 22, 598–616. [Google Scholar] [CrossRef]
- Guo, Y.; Song, G.; Sun, M.; Wang, J.; Wang, Y. Prevalence and Therapies of Antibiotic-Resistance in Staphylococcus aureus. Front. Cell Infect. Microbiol. 2020, 10, 107. [Google Scholar] [CrossRef]
- Flores, G.A.; Cusumano, G.; Venanzoni, R.; Angelini, P. Advancements in Antibacterial Therapy: Feature Papers. Microorganisms 2025, 13, 557. [Google Scholar] [CrossRef]
- Alexander, J.A.N.; Worrall, L.J.; Hu, J.; Vuckovic, M.; Satishkumar, N.; Poon, R.; Sobhanifar, S.; Rosell, F.I.; Jenkins, J.; Chiang, D. Structural basis of broad-spectrum β-lactam resistance in Staphylococcus aureus. Nature 2023, 613, 375–382. [Google Scholar] [CrossRef]
- McNeil, J.C.; Sommer, L.M.; Joseph, M.; Hulten, K.G.; Kaplan, S.L. Penicillin susceptibility among Staphylococcus aureus skin and soft tissue infections at a children’s hospital. Microbiol. Spectr. 2024, 12, e0086924. [Google Scholar] [CrossRef] [PubMed]
- Brdová, D.; Ruml, T.; Viktorová, J. Mechanism of staphylococcal resistance to clinically relevant antibiotics. Drug. Resist. Updat. 2024, 77, 101147. [Google Scholar] [CrossRef] [PubMed]
- Drawz, S.M.; Bonomo, R.A. Three decades of beta-lactamase inhibitors. Clin. Microbiol. Rev. 2010, 23, 160–201. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, J.; Tan, L.; Liu, X.; Zheng, Y.; Cui, Z.; Li, C.; Yeung, K.W.K.; Li, Z.; Liang, Y.; et al. Using tea nanoclusters as β-lactamase inhibitors to cure multidrug-resistant bacterial pneumonia: A promising therapeutic strategy by Chinese materioherbology. Fundam. Res. 2022, 2, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Guo, Y.; Sun, X.; Ding, R.; Wang, Y.; Niu, X.; Wang, J.; Deng, X. Application of Oleanolic Acid and Its Analogues in Combating Pathogenic Bacteria In Vitro/Vivo by a Two-Pronged Strategy of β-Lactamases and Hemolysins. ACS Omega 2020, 5, 11424–11438. [Google Scholar] [CrossRef]
- Kline, S.N.; Orlando, N.A.; Lee, A.J.; Wu, M.-J.; Zhang, J.; Youn, C.; Feller, L.E.; Pontaza, C.; Dikeman, D.; Limjunyawong, N.; et al. Staphylococcus aureus proteases trigger eosinophil-mediated skin inflammation. Proc. Natl. Acad. Sci. USA 2024, 121, e2309243121. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, J.; He, Y.; Lv, Z.; Liang, Z.; Chen, J.; Li, P.; Liu, J.; Yang, H.; Tao, A.; et al. Exploring the Role of Staphylococcus aureus in Inflammatory Diseases. Toxins 2022, 14, 464. [Google Scholar] [CrossRef]
- Tang, C.; Li, Q.; Lin, T. Lycopene attenuates Staphylococcus aureus-induced inflammation via inhibiting α-hemolysin expression. Microbes. Infect. 2021, 23, 104853. [Google Scholar] [CrossRef] [PubMed]
- Joyner, J.A.; Daly, S.M.; Peabody, J.; Triplett, K.D.; Pokhrel, S.; Elmore, B.O.; Adebanjo, D.; Peabody, D.S.; Chackerian, B.; Hall, P.R. Vaccination with VLPs Presenting a Linear Neutralizing Domain of S. aureus Hla Elicits Protective Immunity. Toxins 2020, 12, 450. [Google Scholar] [CrossRef] [PubMed]
- Jordan, P.M.; Gerstmeier, J.; Pace, S.; Bilancia, R.; Rao, Z.; Börner, F.; Miek, L.; Gutiérrez-Gutiérrez, Ó.; Arakandy, V.; Rossi, A.; et al. Staphylococcus aureus-Derived α-Hemolysin Evokes Generation of Specialized Pro-resolving Mediators Promoting Inflammation Resolution. Cell Rep. 2020, 33, 108247. [Google Scholar] [CrossRef]
- Rabes, A.; Suttorp, N.; Opitz, B. Inflammasomes in Pneumococcal Infection: Innate Immune Sensing and Bacterial Evasion Strategies. Curr. Top. Microbiol. Immunol. 2016, 397, 215–227. [Google Scholar] [PubMed]
- Grousd, J.A.; Rich, H.E.; Alcorn, J.F. Host-Pathogen Interactions in Gram-Positive Bacterial Pneumonia. Clin. Microbiol. Rev. 2019, 32, e00107-18. [Google Scholar] [CrossRef]
- Jung, S.H.; Kim, B.J.; Lee, E.H.; Osborne, N.N. Isoquercitrin is the most effective antioxidant in the plant Thuja orientalis and able to counteract oxidative-induced damage to a transformed cell line (RGC-5 cells). Neurochem. Int. 2010, 57, 713–721. [Google Scholar] [CrossRef]
- Kaur, S.; Mendonca, P.; Soliman, K.F.A. The Anticancer Effects and Therapeutic Potential of Kaempferol in Triple-Negative Breast Cancer. Nutrients 2024, 16, 2392. [Google Scholar] [CrossRef]
- Ren, J.; Lu, Y.; Qian, Y.; Chen, B.; Wu, T.; Ji, G. Recent progress regarding kaempferol for the treatment of various diseases. Exp. Ther. Med. 2019, 18, 2759–2776. [Google Scholar] [CrossRef]
- Hua, F.; Li, J.Y.; Zhang, M.; Zhou, P.; Wang, L.; Ling, T.J.; Bao, G.H. Kaempferol-3-O-rutinoside exerts cardioprotective effects through NF-κB/NLRP3/Caspase-1 pathway in ventricular remodeling after acute myocardial infarction. J. Food Biochem. 2022, 46, e14305. [Google Scholar] [CrossRef]
- Yin, N.; Yang, X.; Wang, L.; Zhang, C.; Guan, J.; Tao, Y.; Guo, X.; Zhao, Y.; Song, W.; Wang, B.; et al. Kaempferol inhibits the expression of α-hemolysin and protects mice from methicillin-resistant Staphylococcus aureus-induced lethal pneumonia. Microb. Pathog. 2022, 162, 105336. [Google Scholar] [CrossRef] [PubMed]
- Ming, D.; Wang, D.; Cao, F.; Xiang, H.; Mu, D.; Cao, J.; Li, B.; Zhong, L.; Dong, X.; Zhong, X.; et al. Kaempferol Inhibits the Primary Attachment Phase of Biofilm Formation in Staphylococcus aureus. Front. Microbiol. 2017, 8, 2263. [Google Scholar] [CrossRef]
- Mary, T.R.J.; Kannan, R.R.; Iniyan, A.M.; Ranjith, W.A.C.; Nandhagopal, S.; Vishwakarma, V.; Vincent, S.G.P. β-lactamase inhibitory potential of kalafungin from marine Streptomyces in Staphylococcus aureus infected zebrafish. Microbiol. Res. 2021, 244, 126666. [Google Scholar]
- Miklasińska-Majdanik, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Wąsik, T.J. Phenolic Compounds Diminish Antibiotic Resistance of Staphylococcus Aureus Clinical Strains. Int. J. Environ. Res. Public Health 2018, 15, 2321. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Herzberg, O. Structures of the acyl-enzyme complexes of the Staphylococcus aureus beta-lactamase mutant Glu166Asp:Asn170Gln with benzylpenicillin and cephaloridine. Biochemistry 2001, 40, 2351–2358. [Google Scholar] [CrossRef]
- Hosseini, M.; Shapouri Moghaddam, A.; Derakhshan, S.; Hashemipour, S.M.A.; Hadadi-Fishani, M.; Pirouzi, A.; Khaledi, A. Correlation Between Biofilm Formation and Antibiotic Resistance in MRSA and MSSA Isolated from Clinical Samples in Iran: A Systematic Review and Meta-Analysis. Microb. Drug. Resist. 2020, 26, 1071–1080. [Google Scholar] [CrossRef]
- Ricciardi, B.F.; Muthukrishnan, G.; Masters, E.; Ninomiya, M.; Lee, C.C.; Schwarz, E.M. Staphylococcus aureus Evasion of Host Immunity in the Setting of Prosthetic Joint Infection: Biofilm and Beyond. Curr. Rev. Musculoskelet. Med. 2018, 11, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, A.; Kest, H.; Sood, M.; Steussy, B.W.; Thieman, C.; Gupta, S. Biofilm Producing Methicillin-Resistant Staphylococcus aureus (MRSA) Infections in Humans: Clinical Implications and Management. Pathogens 2024, 13, 76. [Google Scholar] [CrossRef]
- Ahmed, G.E.; Elshahid, Z.A.; El-Sawy, E.R.; Abdel-Aziz, M.S.; Abdel-Aziem, A. Synthesis, biofilm formation inhibitory, and inflammation inhibitory activities of new coumarin derivatives. Sci. Rep. 2024, 14, 9106. [Google Scholar] [CrossRef]
- Chiu, K.-C.; Shih, Y.-H.; Wang, T.-H.; Lan, W.-C.; Li, P.-J.; Jhuang, H.-S.; Hsia, S.-M.; Shen, Y.-W.; Chen, M.Y.-C.; Shieh, T.-M. In Vitro antimicrobial and antipro-inflammation potential of honokiol and magnolol against oral pathogens and macrophages. J. Formos. Med. Assoc. 2021, 120, 827–837. [Google Scholar] [CrossRef]
- Bhatia, E.; Sharma, S.; Jadhav, K.; Banerjee, R. Combinatorial liposomes of berberine and curcumin inhibit biofilm formation and intracellular methicillin resistant Staphylococcus aureus infections and associated inflammation. J. Mater. Chem. B 2021, 9, 864–875. [Google Scholar] [CrossRef]
- Yu, Y.-L.; Wu, J.-J.; Lin, C.-C.; Qin, X.; Tay, F.R.; Miao, L.; Tao, B.-L.; Jiao, Y. Elimination of methicillin-resistant Staphylococcus aureus biofilms on titanium implants via photothermally-triggered nitric oxide and immunotherapy for enhanced osseointegration. Mil. Med. Res. 2023, 10, 21. [Google Scholar] [CrossRef]
- Yan, N.; Zhou, H.; Jin, P.; Li, T.; Liu, Q.; Ning, H.; Ma, Z.; Feng, L.; Jin, T.; Deng, Y.; et al. A Multifunctional Cobalt-Containing Implant for Treating Biofilm Infections and Promoting Osteointegration in Infected Bone Defects Through Macrophage-Mediated Immunomodulation. Adv. Sci. 2025, 12, e2409200. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, X.; Gao, Y.; Niu, X. Insight into the Dual Inhibition Mechanism of Corilagin against MRSA Serine/Threonine Phosphatase (Stp1) by Molecular Modeling. ACS Omega 2020, 5, 32959–32968. [Google Scholar] [CrossRef]
- Xie, P.; Gao, Y.; Wu, C.; Li, X.; Yang, Y. The inhibitory mechanism of echinacoside against Staphylococcus aureus Ser/Thr phosphatase Stp1 by virtual screening and molecular modeling. J. Mol. Model. 2023, 29, 320. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Homeyer, N.; Gohlke, H. Free Energy Calculations by the Molecular Mechanics Poisson-Boltzmann Surface Area Method. Mol. Inform. 2012, 31, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, Q. Visualization Analysis of Weak Interactions in Chemical Systems. Compr. Comput. Chem. 2024, 2, 240–264. [Google Scholar]
- Lu, T. Visualization Analysis of Covalent and Noncovalent Interactions in Real Space. Angew. Chem. Int. Ed. Engl. 2025, 64, e202504895. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, W.; Littlejohn, T.G. Swiss-PDB Viewer (Deep View). Brief. Bioinform. 2001, 2, 195–197. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Wen, J.; Lu, J.; Zhou, H.; Wang, G. Kaempferol and Kaempferin Alleviate MRSA Virulence by Suppressing β-Lactamase and Inflammation. Molecules 2025, 30, 4132. https://doi.org/10.3390/molecules30204132
Liu J, Wen J, Lu J, Zhou H, Wang G. Kaempferol and Kaempferin Alleviate MRSA Virulence by Suppressing β-Lactamase and Inflammation. Molecules. 2025; 30(20):4132. https://doi.org/10.3390/molecules30204132
Chicago/Turabian StyleLiu, Junlu, Jingyao Wen, Jiahui Lu, Hanbing Zhou, and Guizhen Wang. 2025. "Kaempferol and Kaempferin Alleviate MRSA Virulence by Suppressing β-Lactamase and Inflammation" Molecules 30, no. 20: 4132. https://doi.org/10.3390/molecules30204132
APA StyleLiu, J., Wen, J., Lu, J., Zhou, H., & Wang, G. (2025). Kaempferol and Kaempferin Alleviate MRSA Virulence by Suppressing β-Lactamase and Inflammation. Molecules, 30(20), 4132. https://doi.org/10.3390/molecules30204132






