The Role of Probiotics and Their Postbiotic Metabolites in Post-COVID-19 Syndrome
Abstract
1. Introduction
2. Methodology of the Review—Literature Search Strategy
3. COVID-19 Pandemic and Its Impact on the Natural Human Microbiota
3.1. Main Aspects of COVID-19 Incidence
3.2. Gut Microbiota in COVID-19
3.2.1. Role of Microbiota in Maintaining Health
3.2.2. Microbiota in the Course of SARS-CoV-2 Infection
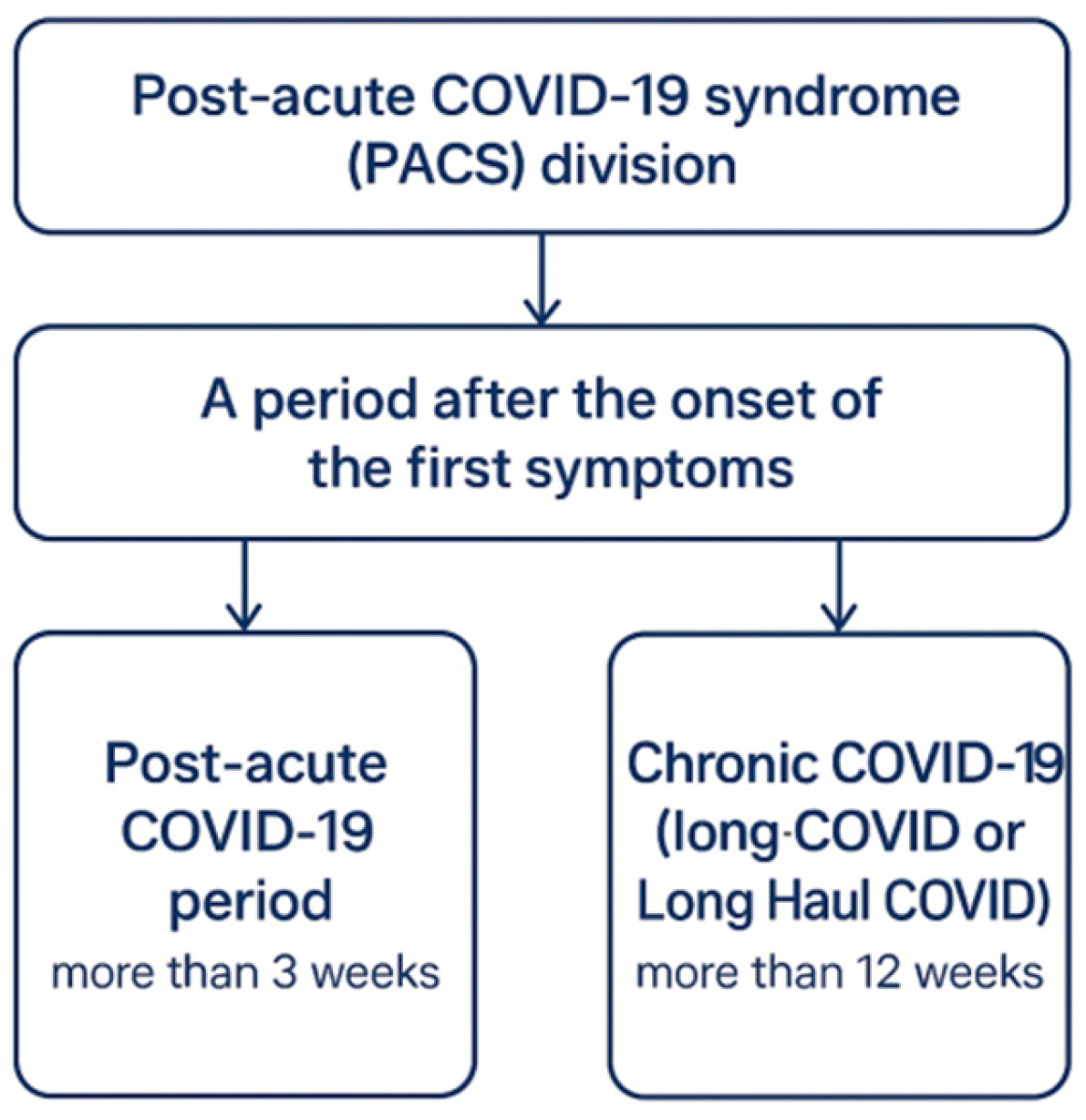
3.3. Post-COVID: Long-Term Consequences of SARS-CoV-2 Infection
- Those who had acute respiratory distress syndrome and were initially hospitalized but now have long-term breathing problems;
- Those who did not require initial hospitalization but now show signs of damage to organs and various systems, such as the respiratory, cardiovascular, or nervous systems;
- Those who have not been hospitalized but still have persistent symptoms, often accompanied by fatigue without obvious signs of respiratory damage.
3.3.1. Post-COVID Patient-Specific Factors
3.3.2. Mechanistic Insights into the Role of Microbiota in Post-COVID
Gut-Immune Axis and Inflammatory Regulation
Gut–Brain Axis and Neuropsychological Symptoms
3.3.3. Cardiovascular and Muscle Problems in Post-COVID
4. Probiotics Versus COVID-19
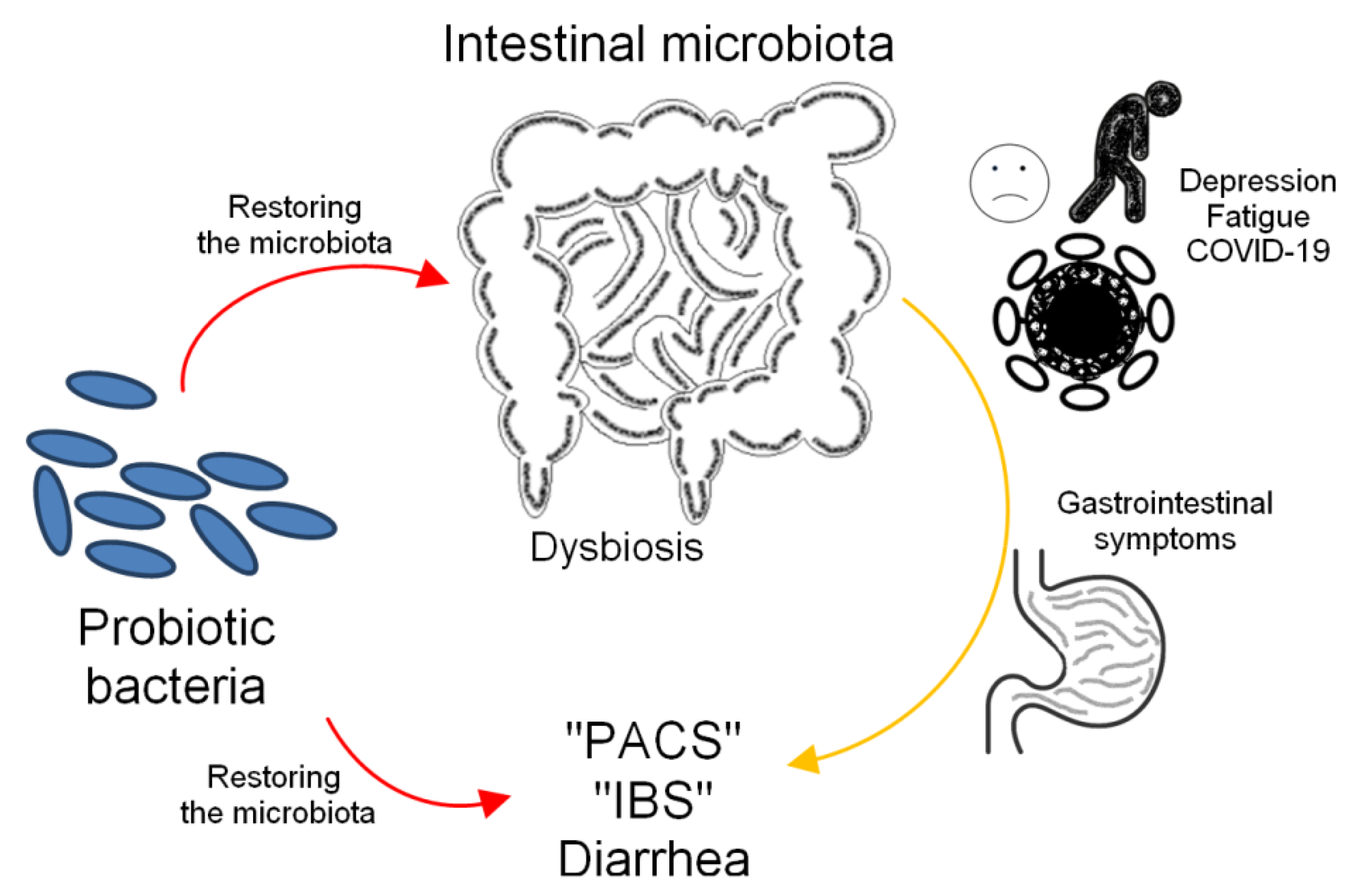
4.1. Probiotics as a Way to Rebuild a Healthy Microbiota
4.2. Antiviral Effects of Probiotics
4.2.1. Molecular Mechanisms of Microbiota Modulation in Post-COVID
Tight Junction Modulation and Barrier Function
Inhibition of Viral Fusion
Bioactive Postbiotics and Specific Microenvironment
Antimicrobial Peptides and Selective Pathogen Targeting
Cytokine and Chemokine Regulation
4.2.2. Efficacy of Probiotics Against SARS-CoV-2
5. Use of Probiotics to Alleviate Post-Acute COVID-19 Syndrome
5.1. Neuropsychological Manifestations of Post-COVID
5.1.1. Effect of Probiotics on Fatigue
5.1.2. Effect of Probiotics on Depression
5.2. Gastrointestinal Symptoms of Post-COVID
Effect of Probiotics on Irritable Bowel Syndrome (IBS)
6. Conclusions
Future Direction
- Identify optimal probiotic and postbiotic formulations based on specific post-COVID phenotypes;
- Standardize clinical trial endpoints and stratify outcomes according to age, comorbidities, baseline microbiota profiles, and COVID-19 severity;
- Evaluate synbiotic approaches combining probiotics with targeted prebiotics or bioactive compounds;
- Examine the regulatory environment and safety profiles of novel postbiotic therapies.
- Employ omics technologies (metabolomics, proteomics, transcriptomics) to better understand host-microbiota interactions;
- Investigate long-term outcomes and sustainability of microbiota changes after recovery from COVID-19;
- Incorporate microbiome-based interventions into broader rehabilitation programs that include elements of nutritional, psychological, and physical therapy;
- Recent findings suggest a potential role for bacteriophages in modulating the composition of the gut microbiota and the host immune response in the context of post-COVID conditions [250]. However, this is still a new field that requires further mechanistic and clinical research. Bacteriophages are being studied as a separate, microbiota-targeting strategy, which may complement or serve as a parallel method to probiotic/postbiotic interventions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAD | Antibiotic-associated diarrhea |
| ACE2 | Angiotensin-converting enzyme 2 |
| AMPs | Antimicrobial peptides |
| APCs | Antigen-presenting cells |
| CD | Crohn’s disease |
| CFS | Chalder Fatigue Scale |
| CFU | Colony forming unit |
| CNS | Central nervous system |
| FAO | Food and Agriculture Organization |
| GI | Gastrointestinal |
| GPR | G protein-coupled receptors |
| HAM-D | Hamilton Rating Scale for Depression |
| HDAC | Histone deacetylases |
| HIV | Human immunodeficiency virus |
| HPA | Hypothalamic–pituitary–adrenal |
| HSV | Human simplex virus |
| IBD | Inflammatory Bowel Disease |
| IBS | Irritable bowel syndrome |
| IFN | Interferon |
| IgA | Immunoglobulin class A |
| IL | Interleukin |
| IRF | Interferon regulatory factor |
| LAB | Lactic acid bacteria |
| LPS | Lipopolysaccharides |
| MV | Measles virus |
| NF-κB | Nuclear factor kappa B |
| NK | Natural killer |
| NLRs | NOD-like receptors |
| PACS | Post-acute COVID-19 syndrome |
| PCS | Post-COVID-19 syndrome |
| PGF | Placental growth factor |
| POTS | Postural orthostatic tachycardia syndrome |
| PRRs | Pattern recognition receptors |
| PV | Poliomyelitis virus |
| RBD | Receptor binding protein |
| S | Spike |
| s.l. | Sensu lato |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome Coronavirus 2 |
| SCAFs | Short-chain fatty acids |
| SIP | sphingosine-1-phosphate |
| TJ | Tight junction |
| TLRs | Toll-like receptors |
| UC | Ulcerative colitis |
| VOCs | Variants of concern |
| VSV | Vesicular stomatitis virus |
| VUM | Variant under monitoring |
| WHO | World Health Organization |
| ZO | Zonula occludens |
References
- Martín Giménez, V.M.; Modrego, J.; Gómez-Garre, D.; Manucha, W.; De Las Heras, N. Gut Microbiota Dysbiosis in COVID-19: Modulation and Approaches for Prevention and Therapy. Int. J. Mol. Sci. 2023, 24, 12249. [Google Scholar] [CrossRef] [PubMed]
- Frentzen, E.; Fegert, J.M.; Martin, A.; Witt, A. Child and Adolescent Mental Health during the Covid-19 Pandemic: An Overview of Key Findings from a Thematic Series. Child Adolesc. Psychiatry Ment. Health 2025, 19, 57. [Google Scholar] [CrossRef]
- WHO. COVID-19 Cases | WHO COVID-19 Dashboard. Datadot. Available online: https://data.who.int/dashboards/covid19/cases (accessed on 29 May 2025).
- Boni, M.F.; Lemey, P.; Jiang, X.; Lam, T.T.-Y.; Perry, B.W.; Castoe, T.A.; Rambaut, A.; Robertson, D.L. Evolutionary Origins of the SARS-CoV-2 Sarbecovirus Lineage Responsible for the COVID-19 Pandemic. Nat. Microbiol. 2020, 5, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Pink, I.; Welte, T. Häufigkeit, Spektrum und Risikofaktoren von Long-COVID. Inn. Med. 2022, 63, 813–818. [Google Scholar] [CrossRef]
- Nalbandian, A.; Desai, A.D.; Wan, E.Y. Post-COVID-19 Condition. Annu. Rev. Med. 2023, 74, 55–64. [Google Scholar] [CrossRef]
- Pintos-Pascual, I.; Moreno-Torres, V.; Ibánez-Estéllez, F.; Corrales-Rodriguez, P.; Treviño, A.; Corpas, M.; Corral, O.; Soriano, V.; De Mendoza, C. Is SARS-CoV-2 the Only Cause of Long-COVID? AIDSRev 2024, 24, 9863. [Google Scholar] [CrossRef]
- Czechowska-Bieluga, M.; Lewicka -Zelent, A.; Zielińska, P. The Psychosocial Effects of the Pandemic COVID-19 between Poles in Early, Middle and Late Adulthood. Probacja 2023, 2, 31–49. [Google Scholar] [CrossRef]
- Campos, M.D.S.B.; Brito, G.M.G.D.; Santos, K.S.D.C.; Santos, M.A.A.; Martins-Filho, P.R.; Sousa, A.C.S. Chronotropic Incompetence Is Associated with Reduced Aerobic Conditioning and Sedentary Behavior in Patients with Post-Acute COVID-19 Syndrome. Rev. Inst. Med. Trop. São Paulo 2024, 66, e32. [Google Scholar] [CrossRef]
- Parker, A.M.; Brigham, E.; Connolly, B.; McPeake, J.; Agranovich, A.V.; Kenes, M.T.; Casey, K.; Reynolds, C.; Schmidt, K.F.R.; Kim, S.Y.; et al. Addressing the Post-Acute Sequelae of SARS-CoV-2 Infection: A Multidisciplinary Model of Care. Lancet Respir. Med. 2021, 9, 1328–1341. [Google Scholar] [CrossRef]
- Parotto, M.; Gyöngyösi, M.; Howe, K.; Myatra, S.N.; Ranzani, O.; Shankar-Hari, M.; Herridge, M.S. Post-Acute Sequelae of COVID-19: Understanding and Addressing the Burden of Multisystem Manifestations. Lancet Respir. Med. 2023, 11, 739–754. [Google Scholar] [CrossRef]
- Kim, H.J.; Song, J.; Park, S.M. Healthcare Response Strategies for the Long-COVID Era. J. Korean Med. Assoc. 2023, 66, 50–59. [Google Scholar] [CrossRef]
- Chee, Y.J.; Fan, B.E.; Young, B.E.; Dalan, R.; Lye, D.C. Clinical Trials on the Pharmacological Treatment of Long COVID: A Systematic Review. J. Med. Virol. 2023, 95, e28289. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Mu, M.; Yang, P.; Sun, Y.; Wang, R.; Yan, J.; Li, P.; Hu, B.; Wang, J.; Hu, C.; et al. Clinical Characteristics of COVID-19 Patients with Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. Am. J. Gastroenterol. 2020, 115, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.C.S.; Rego, M.S.; Silva, R.C.C.D.; Silva, R.D.A.; Arruda, I.E.S.; Paiva-Júnior, S.D.S.L.; Balbino, V.D.Q. Gut Microbiota and COVID-19: Unraveling the Gut–Lung Axis and Immunomodulatory Therapies. ACS Infect. Dis. 2025, 11, 1844–1853. [Google Scholar] [CrossRef]
- Blankestijn, J.M.; Baalbaki, N.; Beijers, R.J.H.C.G.; Cornelissen, M.E.B.; Wiersinga, W.J.; Abdel-Aziz, M.I.; Maitland-van Der Zee, A.H. Exploring Heterogeneity of Fecal Microbiome in Long COVID Patients at 3 to 6 Months After Infection. Int. J. Mol. Sci. 2025, 26, 1781. [Google Scholar] [CrossRef]
- Liu, Q.; Mak, J.W.Y.; Su, Q.; Yeoh, Y.K.; Lui, G.C.-Y.; Ng, S.S.S.; Zhang, F.; Li, A.Y.L.; Lu, W.; Hui, D.S.-C.; et al. Gut Microbiota Dynamics in a Prospective Cohort of Patients with Post-Acute COVID-19 Syndrome. Gut 2022, 71, 544–552. [Google Scholar] [CrossRef]
- Petakh, P.; Kobyliak, N.; Kamyshnyi, A. Gut Microbiota in Patients with COVID-19 and Type 2 Diabetes: A Culture-Based Method. Front. Cell. Infect. Microbiol. 2023, 13, 1142578. [Google Scholar] [CrossRef]
- Righi, E.; Dalla Vecchia, I.; Auerbach, N.; Morra, M.; Górska, A.; Sciammarella, C.; Lambertenghi, L.; Gentilotti, E.; Mirandola, M.; Tacconelli, E.; et al. Gut Microbiome Disruption Following SARS-CoV-2: A Review. Microorganisms 2024, 12, 131. [Google Scholar] [CrossRef]
- Oh, S.E.; Parikh, N.S. Recent Advances in the Impact of Infection and Inflammation on Stroke Risk and Outcomes. Curr. Neurol. Neurosci. Rep. 2022, 22, 161–170. [Google Scholar] [CrossRef]
- Davison, J.M.; Wischmeyer, P.E. Probiotic and Synbiotic Therapy in the Critically Ill: State of the Art. Nutrition 2019, 59, 29–36. [Google Scholar] [CrossRef]
- Umair, M.; Jabbar, S.; Zhaoxin, L.; Jianhao, Z.; Abid, M.; Khan, K.-U.R.; Korma, S.A.; Alghamdi, M.A.; El-Saadony, M.T.; Abd El-Hack, M.E.; et al. Probiotic-Based Bacteriocin: Immunity Supplementation Against Viruses. An Updated Review. Front. Microbiol. 2022, 13, 876058. [Google Scholar] [CrossRef]
- Balendra, V.; Rosenfeld, R.; Amoroso, C.; Castagnone, C.; Rossino, M.G.; Garrone, O.; Ghidini, M. Postbiotics as Adjuvant Therapy in Cancer Care. Nutrients 2024, 16, 2400. [Google Scholar] [CrossRef]
- Karuvelan, M.; Raj, S.; Chelliah, R.; Barathikannan, K.; Vijayalakshmi, S.; Rubab, M.; Oh, D.-H.; Sultan, G. Postbiotics and Host–Microbe Interactions. In Postbiotics; Elsevier: Amsterdam, The Netherlands, 2025; pp. 3–16. [Google Scholar] [CrossRef]
- Barathikannan, K.; Chelliah, R.; David, H.A.; Kulandairaj, E.P.; Vijayalakshmi, S.; Rubab, M.; Oh, D.-H. Integrative Multiomics: Understanding Postbiotics and Their Role in Human Physiology. In Postbiotics; Elsevier: Amsterdam, The Netherlands, 2025; pp. 397–415. [Google Scholar] [CrossRef]
- Aggarwal, S.; Sabharwal, V.; Kaushik, P.; Joshi, A.; Aayushi, A.; Suri, M. Postbiotics: From Emerging Concept to Application. Front. Sustain. Food Syst. 2022, 6, 887642. [Google Scholar] [CrossRef]
- Łoniewski, I.; Skonieczna-Żydecka, K.; Sołek-Pastuszka, J.; Marlicz, W. Probiotics in the Management of Mental and Gastrointestinal Post-COVID Symptomes. J. Clin. Med. 2022, 11, 5155. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Ahmad Farouk, I.; Lal, S.K. COVID-19: A Review on the Novel Coronavirus Disease Evolution, Transmission, Detection, Control and Prevention. Viruses 2021, 13, 202. [Google Scholar] [CrossRef] [PubMed]
- Devaux, C.A.; Fantini, J. Possible Contribution of Rare Alleles of Human ACE2 in the Emergence of SARS-CoV-2 Variants Escaping the Immune Response. Front. Immunol. 2023, 14, 1252367. [Google Scholar] [CrossRef]
- McCarthy, K.R.; Rennick, L.J.; Nambulli, S.; Robinson-McCarthy, L.R.; Bain, W.G.; Haidar, G.; Duprex, W.P. Recurrent Deletions in the SARS-CoV-2 Spike Glycoprotein Drive Antibody Escape. Science 2021, 371, 1139–1142. [Google Scholar] [CrossRef]
- Fan, H.; Tian, M.; Liu, S.; Ye, C.; Li, Z.; Wu, K.; Zhu, C. Strategies Used by SARS-CoV-2 to Evade the Innate Immune System in an Evolutionary Perspective. Pathogens 2024, 13, 1117. [Google Scholar] [CrossRef]
- Oliver, J.L.; Bernaola-Galván, P.; Carpena, P.; Perfectti, F.; Gómez-Martín, C.; Castiglione, S.; Raia, P.; Verdú, M.; Moya, A. Strong Evidence for the Evolution of Decreasing Compositional Heterogeneity in SARS-CoV-2 Genomes during the Pandemic. Sci. Rep. 2025, 15, 12246. [Google Scholar] [CrossRef]
- Almehdi, A.M.; Khoder, G.; Alchakee, A.S.; Alsayyid, A.T.; Sarg, N.H.; Soliman, S.S.M. SARS-CoV-2 Spike Protein: Pathogenesis, Vaccines, and Potential Therapies. Infection 2021, 49, 855–876. [Google Scholar] [CrossRef]
- Kim, S.-J.; Nguyen, V.-G.; Park, Y.-H.; Park, B.-K.; Chung, H.-C. A Novel Synonymous Mutation of SARS-CoV-2: Is This Possible to Affect Their Antigenicity and Immunogenicity? Vaccines 2020, 8, 220. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Lin, X.; Chen, B.; Hou, Z.; Zhang, Q.; Lin, S.; Geng, L.; Sun, Z.; Cao, C.; Shi, Y.; et al. The Mutation Features and Geographical Distributions of the Surface Glycoprotein (S Gene) in SARS-CoV-2 Strains: A Comparative Analysis of the Early and Current Strains. J. Med. Virol. 2022, 94, 5363–5374. [Google Scholar] [CrossRef] [PubMed]
- Hoteit, R.; Yassine, H.M. Biological Properties of SARS-CoV-2 Variants: Epidemiological Impact and Clinical Consequences. Vaccines 2022, 10, 919. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Parums, D.V. Editorial: A Rapid Global Increase in COVID-19 Is Due to the Emergence of the EG.5 (Eris) Subvariant of Omicron SARS-CoV-2. Med. Sci. Monit. 2023, 29, e942244. [Google Scholar] [CrossRef]
- Khan, S.; Yahiro, T.; Kimitsuki, K.; Hashimoto, T.; Matsuura, K.; Yano, S.; Noguchi, K.; Sonezaki, A.; Yoshizawa, K.; Kumasako, Y.; et al. Exploring the Replication and Pathogenic Characteristics of Alpha, Delta, and Omicron Variants of SARS-CoV-2. Int. J. Mol. Sci. 2024, 25, 12641. [Google Scholar] [CrossRef]
- Tandel, D.; Sah, V.; Singh, N.K.; Potharaju, P.S.; Gupta, D.; Shrivastava, S.; Sowpati, D.T.; Harshan, K.H. SARS-CoV-2 Variant Delta Potently Suppresses Innate Immune Response and Evades Interferon-Activated Antiviral Responses in Human Colon Epithelial Cells. Microbiol. Spectr. 2022, 10, e01604-22. [Google Scholar] [CrossRef]
- Hussain, M.S.; Gupta, G. The Rise of FLiRT Variants in the COVID-19 Pandemic: What We Know so Far. Curr. Pharm. Des. 2025, 31, 659–662. [Google Scholar] [CrossRef]
- Chen, L.; Kaku, Y.; Okumura, K.; Uriu, K.; Zhu, Y.; Ito, J.; Sato, K. Virological Characteristics of the SARS-CoV-2 LP.8.1 Variant. Lancet Infect. Dis. 2025, 25, e193. [Google Scholar] [CrossRef]
- WHO. WHO TAG-VE Risk Evaluation for SARS-CoV-2 Variant Under Monitoring: NB.1.8.1. 2025. Available online: https://cdn.who.int/media/docs/default-source/documents/epp/tracking-sars-cov-2/23052025_nb.1.8.1_ire.pdf (accessed on 1 September 2025).
- Wu, Z.; McGoogan, J.M. Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases from the Chinese Center for Disease Control and Prevention. JAMA 2020, 323, 1239. [Google Scholar] [CrossRef]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061. [Google Scholar] [CrossRef]
- Hu, B.; Huang, S.; Yin, L. The Cytokine Storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef]
- Pelaia, C.; Tinello, C.; Vatrella, A.; De Sarro, G.; Pelaia, G. Lung under Attack by COVID-19-Induced Cytokine Storm: Pathogenic Mechanisms and Therapeutic Implications. Ther. Adv. Respir. Dis. 2020, 14, 1753466620933508. [Google Scholar] [CrossRef]
- Zhang, Y.; Geng, X.; Tan, Y.; Li, Q.; Xu, C.; Xu, J.; Hao, L.; Zeng, Z.; Luo, X.; Liu, F.; et al. New Understanding of the Damage of SARS-CoV-2 Infection Outside the Respiratory System. Biomed. Pharmacother. 2020, 127, 110195. [Google Scholar] [CrossRef]
- Raman, B.; Ramasamy, M.N. Synbiotics in Post-Acute COVID-19 Syndrome—A Potential New Treatment Framework? Lancet Infect. Dis. 2024, 24, 219–221. [Google Scholar] [CrossRef]
- Tamariz, L.; Bast, E.; Klimas, N.; Palacio, A. Low-Dose Naltrexone Improves Post–COVID-19 Condition Symptoms. Clin. Ther. 2024, 46, e101–e106. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.; Abo Omirah, M.; Hussein, A.; Saeed, H. Assessment and Characterisation of post-COVID-19 Manifestations. Int. J. Clin. Pract. 2021, 75, e13746. [Google Scholar] [CrossRef] [PubMed]
- The Lancet. Long COVID: 3 Years In. Lancet 2023, 401, 795. [Google Scholar] [CrossRef]
- De Sordi, L.; Lourenço, M.; Debarbieux, L. The Battle Within: Interactions of Bacteriophages and Bacteria in the Gastrointestinal Tract. Cell Host Microbe 2019, 25, 210–218. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome Definition Re-Visited: Old Concepts and New Challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef]
- Shreiner, A.B.; Kao, J.Y.; Young, V.B. The Gut Microbiome in Health and in Disease. Curr. Opin. Gastroenterol. 2015, 31, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the Human Intestinal Microbial Flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Hamady, M.; Yatsunenko, T.; Cantarel, B.L.; Duncan, A.; Ley, R.E.; Sogin, M.L.; Jones, W.J.; Roe, B.A.; Affourtit, J.P.; et al. A Core Gut Microbiome in Obese and Lean Twins. Nature 2009, 457, 480–484. [Google Scholar] [CrossRef] [PubMed]
- Karkman, A.; Lehtimäki, J.; Ruokolainen, L. The Ecology of Human Microbiota: Dynamics and Diversity in Health and Disease. Ann. N. Y. Acad. Sci. 2017, 1399, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703. [Google Scholar] [CrossRef]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery Mode Shapes the Acquisition and Structure of the Initial Microbiota across Multiple Body Habitats in Newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef]
- Ying, S.; Zeng, D.-N.; Chi, L.; Tan, Y.; Galzote, C.; Cardona, C.; Lax, S.; Gilbert, J.; Quan, Z.-X. The Influence of Age and Gender on Skin-Associated Microbial Communities in Urban and Rural Human Populations. PLoS ONE 2015, 10, e0141842. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.-H.; Lakshmanan, A.; Wade, W.G. The Human Oral Microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef]
- Ruan, W.; Engevik, M.A.; Spinler, J.K.; Versalovic, J. Healthy Human Gastrointestinal Microbiome: Composition and Function After a Decade of Exploration. Dig. Dis. Sci. 2020, 65, 695–705. [Google Scholar] [CrossRef]
- Lopetuso, L.R.; Scaldaferri, F.; Franceschi, F.; Gasbarrini, A. The Gastrointestinal Microbiome—Functional Interference between Stomach and Intestine. Best Pract. Res. Clin. Gastroenterol. 2014, 28, 995–1002. [Google Scholar] [CrossRef]
- Jach, M.E.; Serefko, A.; Szopa, A.; Sajnaga, E.; Golczyk, H.; Santos, L.S.; Borowicz-Reutt, K.; Sieniawska, E. The Role of Probiotics and Their Metabolites in the Treatment of Depression. Molecules 2023, 28, 3213. [Google Scholar] [CrossRef]
- Goulet, O. Potential Role of the Intestinal Microbiota in Programming Health and Disease. Nutr. Rev. 2015, 73 (Suppl. S1), 32–40. [Google Scholar] [CrossRef] [PubMed]
- Fallah, A.; Sedighian, H.; Kachuei, R.; Fooladi, A.A.I. Human Microbiome in Post-Acute COVID-19 Syndrome (PACS). Curr. Res. Microb. Sci. 2025, 8, 100324. [Google Scholar] [CrossRef]
- Dumas, A.; Bernard, L.; Poquet, Y.; Lugo-Villarino, G.; Neyrolles, O. The Role of the Lung Microbiota and the Gut-Lung Axis in Respiratory Infectious Diseases. Cell. Microbiol. 2018, 20, e12966. [Google Scholar] [CrossRef] [PubMed]
- Groves, H.T.; Higham, S.L.; Moffatt, M.F.; Cox, M.J.; Tregoning, J.S. Respiratory Viral Infection Alters the Gut Microbiota by Inducing Inappetence. mBio 2020, 11, e03236-19. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, G.L.V.; Oliveira, C.N.S.; Pinzan, C.F.; De Salis, L.V.V.; Cardoso, C.R.D.B. Microbiota Modulation of the Gut-Lung Axis in COVID-19. Front. Immunol. 2021, 12, 635471. [Google Scholar] [CrossRef]
- Mahooti, M.; Miri, S.M.; Abdolalipour, E.; Ghaemi, A. The Immunomodulatory Effects of Probiotics on Respiratory Viral Infections: A Hint for COVID-19 Treatment? Microb. Pathogenes. 2020, 148, 104452. [Google Scholar] [CrossRef]
- Buttó, L.F.; Haller, D. Dysbiosis in Intestinal Inflammation: Cause or Consequence. Int. J. Med. Microbiol. 2016, 306, 302–309. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, G.; Zhao, L.; Wang, W. Nutritional Modulation of Gut Microbiota Alleviates Severe Gastrointestinal Symptoms in a Patient with Post-Acute COVID-19 Syndrome. mBio 2022, 13, e03801-21. [Google Scholar] [CrossRef]
- Zuo, T.; Zhang, F.; Lui, G.C.Y.; Yeoh, Y.K.; Li, A.Y.L.; Zhan, H.; Wan, Y.; Chung, A.C.K.; Cheung, C.P.; Chen, N.; et al. Alterations in Gut Microbiota of Patients with COVID-19 During Time of Hospitalization. Gastroenterology 2020, 159, 944–955.e8. [Google Scholar] [CrossRef]
- Reinold, J.; Farahpour, F.; Fehring, C.; Dolff, S.; Konik, M.; Korth, J.; Van Baal, L.; Hoffmann, D.; Buer, J.; Witzke, O.; et al. A Pro-Inflammatory Gut Microbiome Characterizes SARS-CoV-2 Infected Patients and a Reduction in the Connectivity of an Anti-Inflammatory Bacterial Network Associates with Severe COVID-19. Front. Cell. Infect. Microbiol. 2021, 11, 747816. [Google Scholar] [CrossRef]
- Rafiqul Islam, S.M.; Foysal, M.J.; Hoque, M.N.; Mehedi, H.M.H.; Rob, M.A.; Salauddin, A.; Tanzina, A.Y.; Biswas, S.; Noyon, S.H.; Siddiki, A.M.A.M.Z.; et al. Dysbiosis of Oral and Gut Microbiomes in SARS-CoV-2 Infected Patients in Bangladesh: Elucidating the Role of Opportunistic Gut Microbes. Front. Med. 2022, 9, 821777. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Khan, Z. COVID-2019-Associated Overexpressed Prevotella Proteins Mediated Host–Pathogen Interactions and Their Role in Coronavirus Outbreak. Bioinformatics 2020, 36, 4065–4069. [Google Scholar] [CrossRef]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A New Coronavirus Associated with Human Respiratory Disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, M.; Takeshita, T.; Furuta, M.; Kageyama, S.; Takeuchi, K.; Hata, J.; Ninomiya, T.; Yamashita, Y. Tongue Microbiota and Oral Health Status in Community-Dwelling Elderly Adults. mSphere 2018, 3, e00332-18. [Google Scholar] [CrossRef] [PubMed]
- Bucci, V.; Ward, D.V.; Bhattarai, S.; Rojas-Correa, M.; Purkayastha, A.; Holler, D.; Qu, M.D.; Mitchell, W.G.; Yang, J.; Fountain, S.; et al. The Intestinal Microbiota Predicts COVID-19 Severity and Fatality Regardless of Hospital Feeding Method. mSystems 2023, 8, e00310-23. [Google Scholar] [CrossRef]
- Cui, G.-Y.; Rao, B.-C.; Zeng, Z.-H.; Wang, X.-M.; Ren, T.; Wang, H.-Y.; Luo, H.; Ren, H.-Y.; Liu, C.; Ding, S.-Y.; et al. Characterization of Oral and Gut Microbiome and Plasma Metabolomics in COVID-19 Patients after 1-Year Follow-Up. Mil. Med. Res. 2022, 9, 32. [Google Scholar] [CrossRef]
- Khan, S.A.; Goliwas, K.F.; Deshane, J.S. Sphingolipids in Lung Pathology in the Coronavirus Disease Era: A Review of Sphingolipid Involvement in the Pathogenesis of Lung Damage. Front. Physiol. 2021, 12, 760638. [Google Scholar] [CrossRef]
- Hach, T.; Shakeri-Nejad, K.; Bigaud, M.; Dahlke, F.; De Micco, M.; Petricoul, O.; Graham, G.; Piani-Meier, D.; Turrini, R.; Brinkmann, V.; et al. Rationale for Use of Sphingosine-1-Phosphate Receptor Modulators in COVID-19 Patients: Overview of Scientific Evidence. J. Interferon Cytokine Res. 2023, 43, 246–256. [Google Scholar] [CrossRef]
- Winkler, M.S.; Claus, R.A.; Schilder, M.; Pöhlmann, S.; Coldewey, S.M.; Grundmann, J.; Fricke, T.; Moerer, O.; Meissner, K.; Bauer, M.; et al. Erythrocytes Increase Endogenous Sphingosine 1-Phosphate Levels as an Adaptive Response to SARS-CoV-2 Infection. Clin. Sci. 2021, 135, 2781–2791. [Google Scholar] [CrossRef]
- Hromić-Jahjefendić, A.; Mahmutović, L.; Sezer, A.; Bećirević, T.; Rubio-Casillas, A.; Redwan, E.M.; Uversky, V.N. The Intersection of Microbiome and Autoimmunity in Long COVID-19: Current Insights and Future Directions. Cytokine Growth Factor Rev. 2025, 82, 43–54. [Google Scholar] [CrossRef]
- Benitez-Baez, M.J.; Angulo-Varela, E.A.; Aispuro-Heredia, J.D.R.; Vázquez-Noriega, E.G.; Karam-León, A.; Angulo-Zamudio, U.A.; Canizalez-Roman, A.; León-Sicairos, N. The Microbiota and Microbiome in COVID-19 in Adults and Children and Potential Therapeutic Interventions: A Review. Trends Sci. 2024, 21, 8702. [Google Scholar] [CrossRef]
- Robalino, J.G.; Salazar, P.A.; Muñóz, N.E.; Tene, D.M.; Pedreáñez, A.B. The Gut Microbiome and Severity of SARS-CoV-2 Infection: What Is the Link? Nov. Res. Microbiol. J. 2022, 6, 1635–1658. [Google Scholar] [CrossRef]
- Bredon, M.; Hausfater, P.; Khalki, L.; Tijani, Y.; Cheikh, A.; Brot, L.; Creusot, L.; Rolhion, N.; Trottein, F.; Lambeau, G.; et al. Gut Microbiota Alterations Are Linked to COVID-19 Severity in North African and European Populations. npj Biofilms Microbiomes 2025, 11, 106. [Google Scholar] [CrossRef] [PubMed]
- Straume, Z.; Krūmiņa, N.; Elbere, I.; Rozenberga, M.; Erts, R.; Rudzīte, D.; Proskurina, A.; Krumina, A. Impact of Vitamins, Antibiotics, Probiotics, and History of COVID-19 on the Gut Microbiome in Ulcerative Colitis Patients: A Cross-Sectional Study. Medicina 2025, 61, 284. [Google Scholar] [CrossRef]
- Chippa, V.; Aleem, A.; Anjum, F. Postacute Coronavirus (COVID-19) Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major Findings, Mechanisms and Recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Knight, M.; A’Court, C.; Buxton, M.; Husain, L. Management of Post-Acute Covid-19 in Primary Care. BMJ 2020, 370, m3026. [Google Scholar] [CrossRef]
- Bonilla, H.; Peluso, M.J.; Rodgers, K.; Aberg, J.A.; Patterson, T.F.; Tamburro, R.; Baizer, L.; Goldman, J.D.; Rouphael, N.; Deitchman, A.; et al. Therapeutic Trials for Long COVID-19: A Call to Action from the Interventions Taskforce of the RECOVER Initiative. Front. Immunol. 2023, 14, 1129459. [Google Scholar] [CrossRef]
- Ladds, E.; Rushforth, A.; Wieringa, S.; Taylor, S.; Rayner, C.; Husain, L.; Greenhalgh, T. Persistent Symptoms after Covid-19: Qualitative Study of 114 “Long Covid” Patients and Draft Quality Principles for Services. BMC Health Serv. Res. 2020, 20, 1144. [Google Scholar] [CrossRef]
- O’Mahoney, L.L.; Routen, A.; Gillies, C.; Ekezie, W.; Welford, A.; Zhang, A.; Karamchandani, U.; Simms-Williams, N.; Cassambai, S.; Ardavani, A.; et al. The Prevalence and Long-Term Health Effects of Long COVID among Hospitalised and Non-Hospitalised Populations: A Systematic Review and Meta-Analysis. eClinicalMedicine 2023, 55, 101762. [Google Scholar] [CrossRef]
- Lim, H.X.; Khalid, K.; Abdullah, A.D.I.; Lee, L.-H.; Raja Ali, R.A. Subphenotypes of Long COVID and the Clinical Applications of Probiotics. Biomed. Pharmacother. 2025, 183, 117855. [Google Scholar] [CrossRef]
- Smail, S.W.; Albarzinji, N.; Salih, R.H.; Taha, K.O.; Hirmiz, S.M.; Ismael, H.M.; Noori, M.F.; Azeez, S.S.; Janson, C. Microbiome Dysbiosis in SARS-CoV-2 Infection: Implication for Pathophysiology and Management Strategies of COVID-19. Front. Cell. Infect. Microbiol. 2025, 15, 1537456. [Google Scholar] [CrossRef]
- Blackett, J.W.; Li, J.; Jodorkovsky, D.; Freedberg, D.E. Prevalence and Risk Factors for Gastrointestinal Symptoms after Recovery from COVID-19. Neurogastroenterol. Motil. 2022, 34, e14251. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Tariq, R.; Jena, A.; Vesely, E.K.; Singh, S.; Khanna, S.; Sharma, V. Gastrointestinal Manifestations of Long COVID: A Systematic Review and Meta-Analysis. Ther. Adv. Gastroenterol. 2022, 15, 17562848221118403. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 Long-Term Effects of COVID-19: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef] [PubMed]
- Townsend, L.; Dyer, A.H.; Jones, K.; Dunne, J.; Mooney, A.; Gaffney, F.; O’Connor, L.; Leavy, D.; O’Brien, K.; Dowds, J.; et al. Persistent Fatigue Following SARS-CoV-2 Infection Is Common and Independent of Severity of Initial Infection. PLoS ONE 2020, 15, e0240784. [Google Scholar] [CrossRef]
- Kariyawasam, J.C.; Jayarajah, U.; Riza, R.; Abeysuriya, V.; Seneviratne, S.L. Gastrointestinal Manifestations in COVID-19. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 1362–1388. [Google Scholar] [CrossRef]
- Basaca, D.-G.; Jugănaru, I.; Belei, O.; Nicoară, D.-M.; Asproniu, R.; Stoicescu, E.R.; Mărginean, O. Long COVID in Children and Adolescents: Mechanisms, Symptoms, and Long-Term Impact on Health—A Comprehensive Review. J. Clin. Med. 2025, 14, 378. [Google Scholar] [CrossRef]
- Verma, V.K.; Yadav, R.; Beevi, S.S.; Mohod, A.S.; Mancharla, S.; Damodar, N.; Darapuneni, R.C.; Reddy, S.G.; Upendram, P.; Salt, M.N.; et al. Differential Host Responses to COVID-19: Unraveling the Complexity. Diagn. Microbiol. Infect. Dis. 2024, 109, 116281. [Google Scholar] [CrossRef]
- Ando, K.; Suzuki, A.; Yoshida, H. Clinical Impact of COVID-19 Omicron Variant on Patients in Home Health Care. Home Health Care Manag. Pract. 2024, 36, 326–333. [Google Scholar] [CrossRef]
- González Iruma, A.; Guamán Guamán, M.I.; Cruz Castillo, Y.M.; Bastidas Tello, G. Predictores Clínicos de Severidad En Pacientes de COVID-19. Boletín Malariol. Salud Ambient. 2022, 62, 376–382. [Google Scholar] [CrossRef]
- Nair, P.; Nair, C.V.; Kulirankal, K.G.; Corley, E.M.; Edathadathil, F.; Gutjahr, G.; Moni, M.; Sathyapalan, D.T. Characterization and Predictive Risk Scoring of Long COVID in a South Indian Cohort after Breakthrough COVID Infection; a Prospective Single Centre Study. BMC Infect. Dis. 2023, 23, 670. [Google Scholar] [CrossRef]
- Egger, M.; Vogelgesang, L.; Reitelbach, J.; Bergmann, J.; Müller, F.; Jahn, K. Severe Post-COVID-19 Condition after Mild Infection: Physical and Mental Health Eight Months Post Infection: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2023, 21, 21. [Google Scholar] [CrossRef]
- Badinlou, F.; Rahimian, F.; Hedman-Lagerlöf, M.; Lundgren, T.; Abzhandadze, T.; Jansson-Fröjmark, M. Trajectories of Mental Health Outcomes Following COVID-19 Infection: A Prospective Longitudinal Study. BMC Public Health 2024, 24, 452. [Google Scholar] [CrossRef] [PubMed]
- Hejazian, S.S.; Sadr, A.V.; Shahjouei, S.; Vemuri, A.; Abedi, V.; Zand, R. Prevalence and Determinants of Long-Term Post-COVID Conditions in the United States: 2022 Behavioral Risk Factor Surveillance System. Am. J. Med. 2025, 138, 513–523.e10. [Google Scholar] [CrossRef] [PubMed]
- Boland, B.; Gale, T. Mental and Behavioural Disorders and COVID-19-Associated Death in Older People. BJPsych Open 2020, 6, e101. [Google Scholar] [CrossRef] [PubMed]
- Yousif, M.G.; Zeiny, L.S.; Al-Amran, F.G.; Sadeq, A.M.; Rawaf, S.; Al-Jumeily, D. Demographics, Risk Factors, and Post-COVID-19 Syndrome Among Patients in the Middle Euphrates Region of Iraq. Adv. Life Sci. 2023, 10, 41. [Google Scholar] [CrossRef]
- Stölting, A.; Schröder, D.; Schmachtenberg, T.; Schimansky, I.; Yaqubi-Naqizadah, M.; Klemann, C.; Rebmann, F.; Mikuteit, M.; Steffens, S.; Behrens, G.M.N.; et al. Childhood Trauma and Mental Health Outcomes in Post-COVID Syndrome: Results from a Cross-Sectional Study in Germany. Brain Behav. Immun.-Health 2025, 48, 101069. [Google Scholar] [CrossRef]
- Shaver, J.L.F.; Woods, N.F.; Von Ah, D.; Alexander, I.M. Persistent Post COVID-19: Implications for Women’s Health Research and Policy from Members of the Women’s Health Expert Panel of the American Academy of Nursing. Nurs. Outlook 2025, 73, 102341. [Google Scholar] [CrossRef]
- Hwang, S.; Shin, H. Gender Gap in Mental Health during the COVID-19 Pandemic in South Korea: A Decomposition Analysis. Int. J. Environ. Res. Public Health 2023, 20, 2250. [Google Scholar] [CrossRef]
- Yatskov, I.A.; Beloglazov, V.A.; Ageeva, E.S.; Ablaeva, R.N.; Zhukova, A.A.; Onuchina, I.G. Effect of IL-2 T-330G Polymorphism on Markers of Systemic Inflammation, Intestinal Permeability and Vascular Regulation in Post-COVID Patients. Med. Immunol. 2024, 27, 417–422. [Google Scholar] [CrossRef]
- Bates, L.M. Explaining Binary Sex and Gender Patterns in the Direct and Indirect Health Effects of COVID-19: Biologic and Social Constructions of Difference. In The Social Epidemiology of the COVID-19 Pandemic; Duncan, D.T., Kawachi, I., Morse, S.S., Eds.; Oxford University Press: New York, NY, USA, 2024; pp. 147–177. [Google Scholar] [CrossRef]
- Tejpal, A.; Gianos, E.; Cerise, J.; Hirsch, J.S.; Rosen, S.; Kohn, N.; Lesser, M.; Weinberg, C.; Majure, D.; Satapathy, S.K.; et al. Sex-Based Differences in COVID-19 Outcomes. J. Womens Health 2021, 30, 492–501. [Google Scholar] [CrossRef]
- Mangion, K.; Morrow, A.J.; Sykes, R.; Kamdar, A.; Bagot, C.; Bruce, G.; Connelly, P.; Delles, C.; Gibson, V.B.; Gillespie, L.; et al. Post-COVID-19 Illness and Associations with Sex and Gender. BMC Cardiovasc. Disord. 2023, 23, 389. [Google Scholar] [CrossRef] [PubMed]
- Petito, A.; Loconsole, A.; Severo, M.; Ricciardi, E.; Angelillo, M.; Iuso, S.; Ferrante, S.; Scioscia, G.; Pierucci, P.; Portacci, A.; et al. Female Gender and Psychological Profile of Outpatients Attending Post-COVID-19 Follow-up: Some Preliminary Results. Mediterr. J. Clin. Psychol. 2023, 11, 1–23. [Google Scholar] [CrossRef]
- Naslavsky, M.S.; Vidigal, M.; Matos, L.D.R.B.; Cória, V.R.; Batista Junior, P.B.; Razuk, Á.; Saldiva, P.H.N.; Dolhnikoff, M.; Schidlowski, L.; Prando, C.; et al. Extreme Phenotypes Approach to Investigate Host Genetics and COVID-19 Outcomes. Genet. Mol. Biol. 2021, 44 (Suppl. S1), e20200302. [Google Scholar] [CrossRef] [PubMed]
- Martins, B.A.A.; Garcia, A.L.H.; Borges, M.S.; Picinini, J.; Serpa, E.T.; Nobles, D.D.R.; Silva, L.L.; Dalberto, D.; Hansen, A.W.; Spilki, F.R.; et al. Exploring the Relationship between Genetic Instability and Health Outcomes in Acute and Chronic Post-COVID Syndrome. Mutagenesis 2024, 39, 287–300. [Google Scholar] [CrossRef]
- Westerman, K.E.; Lin, J.; Sevilla-Gonzalez, M.D.R.; Tadess, B.; Marchek, C.; Manning, A.K. Gene-Environment Interaction Analysis Incorporating Sex, Cardiometabolic Diseases, and Multiple Deprivation Index Reveals Novel Genetic Associations with COVID-19 Severity. Front. Genet. 2022, 12, 782172. [Google Scholar] [CrossRef]
- Li, A.Y.; Li, W.X.; Li, J. Emerging Trends in Management of Long COVID with a Focus on Pulmonary Rehabilitation: A Review. Clin. Respir. J. 2024, 18, e13777. [Google Scholar] [CrossRef]
- Pagliano, P.; Salzano, F.; D’Amore, C.; Spera, A.; Conti, V.; Folliero, V.; Franci, G.; Ascione, T. How Do Drug Discovery Scientists Address the Unmet Need of Long COVID Syndrome Therapeutics and What More Can Be Done? Expert Opin. Drug Discov. 2025, 20, 1251–1265. [Google Scholar] [CrossRef]
- Reyes, Z.; Stovall, M.C.; Punyamurthula, S.; Longo, M.; Maraganore, D.; Solch-Ottaiano, R.J. The Impact of Gut Microbiome and Diet on Post-Acute Sequelae of SARS-CoV-2 Infection. J. Neurol. Sci. 2024, 467, 123295. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Zuo, T.; Lui, G.C.-Y.; Zhang, F.; Liu, Q.; Li, A.Y.; Chung, A.C.; Cheung, C.P.; Tso, E.Y.; Fung, K.S.; et al. Gut Microbiota Composition Reflects Disease Severity and Dysfunctional Immune Responses in Patients with COVID-19. Gut 2021, 70, 698–706. [Google Scholar] [CrossRef]
- Mussabay, K.; Kozhakhmetov, S.; Dusmagambetov, M.; Mynzhanova, A.; Nurgaziyev, M.; Jarmukhanov, Z.; Vinogradova, E.; Dusmagambetova, A.; Daulbaeva, A.; Chulenbayeva, L.; et al. Gut Microbiome and Cytokine Profiles in Post-COVID Syndrome. Viruses 2024, 16, 722. [Google Scholar] [CrossRef] [PubMed]
- Dinakis, E.; O’Donnell, J.A.; Marques, F.Z. The Gut–Immune Axis during Hypertension and Cardiovascular Diseases. Acta Physiol. 2024, 240, e14193. [Google Scholar] [CrossRef] [PubMed]
- Eom, J.A.; Kwon, G.H.; Kim, N.Y.; Park, E.J.; Won, S.M.; Jeong, J.J.; Raja, G.; Gupta, H.; Asmelash Gebru, Y.; Sharma, S.; et al. Diet-Regulating Microbiota and Host Immune System in Liver Disease. Int. J. Mol. Sci. 2021, 22, 6326. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; He, Y.; Xie, K.; Feng, L.; Gao, S.; Cai, L. Review of Microbiota Gut Brain Axis and Innate Immunity in Inflammatory and Infective Diseases. Front. Cell. Infect. Microbiol. 2023, 13, 1282431. [Google Scholar] [CrossRef]
- Basting, C.M.; Langat, R.; Broedlow, C.A.; Guerrero, C.R.; Bold, T.D.; Bailey, M.; Velez, A.; Schroeder, T.; Short-Miller, J.; Cromarty, R.; et al. SARS-CoV-2 Infection Is Associated with Intestinal Permeability, Systemic Inflammation, and Microbial Dysbiosis in Hospitalized Patients. Microbiol. Spectr. 2024, 12, e00680-24. [Google Scholar] [CrossRef]
- Teixeira, P.C.; Dorneles, G.P.; Santana Filho, P.C.; Da Silva, I.M.; Schipper, L.L.; Postiga, I.A.L.; Neves, C.A.M.; Rodrigues Junior, L.C.; Peres, A.; Souto, J.T.D.; et al. Increased LPS Levels Coexist with Systemic Inflammation and Result in Monocyte Activation in Severe COVID-19 Patients. Int. Immunopharmacol. 2021, 100, 108125. [Google Scholar] [CrossRef]
- Barichello, T.; Kluwe-Schiavon, B.; Borba, L.A.; Pedro, L.C.; Niero, F.S.; Dos Santos, L.N.; Leonardo, L.M.; Ignácio, Z.M.; Morales, R.; Ceretta, L.B.; et al. Alterations in Gut Microbiome Composition and Increased Inflammatory Markers in Post-COVID-19 Individuals. Mol. Neurobiol. 2025, 62, 8038–8047. [Google Scholar] [CrossRef]
- Zhou, B.; Pang, X.; Wu, J.; Liu, T.; Wang, B.; Cao, H. Gut Microbiota in COVID-19: New Insights from Inside. Gut Microbes 2023, 15, 2201157. [Google Scholar] [CrossRef]
- Dawson, S.L.; Todd, E.; Ward, A.C. The Interplay of Nutrition, the Gut Microbiota and Immunity and Its Contribution to Human Disease. Biomedicines 2025, 13, 329. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Xu, L.; Zhang, X. Maintaining the Balance of Intestinal Flora through the Diet: Effective Prevention of Illness. Foods 2021, 10, 2312. [Google Scholar] [CrossRef]
- Austhof, E.; Bell, M.L.; Riddle, M.S.; Catalfamo, C.; McFadden, C.; Cooper, K.; Scallan Walter, E.; Jacobs, E.; Pogreba-Brown, K. Persisting Gastrointestinal Symptoms and Post-Infectious Irritable Bowel Syndrome Following SARS-CoV-2 Infection: Results from the Arizona CoVHORT. Epidemiol. Infect. 2022, 150, e136. [Google Scholar] [CrossRef]
- Durairajan, S.S.K.; Singh, A.K.; Saravanan, U.B.; Namachivayam, M.; Radhakrishnan, M.; Huang, J.-D.; Dhodapkar, R.; Zhang, H. Gastrointestinal Manifestations of SARS-CoV-2: Transmission, Pathogenesis, Immunomodulation, Microflora Dysbiosis, and Clinical Implications. Viruses 2023, 15, 1231. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, L.; Zheng, Y.; An, J.; Wen, G.; Jin, H.; Tuo, B. Digestive System Infection by SARS-CoV-2: Entry Mechanism, Clinical Symptoms and Expression of Major Receptors (Review). Int. J. Mol. Med. 2023, 51, 19. [Google Scholar] [CrossRef]
- Puoti, M.G.; Rybak, A.; Kiparissi, F.; Gaynor, E.; Borrelli, O. SARS-CoV-2 and the Gastrointestinal Tract in Children. Front. Pediatr. 2021, 9, 617980. [Google Scholar] [CrossRef]
- Saviano, A.; Brigida, M.; Petruzziello, C.; Zanza, C.; Candelli, M.; Morabito Loprete, M.R.; Saleem, F.; Ojetti, V. Intestinal Damage, Inflammation and Microbiota Alteration during COVID-19 Infection. Biomedicines 2023, 11, 1014. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.T.; Khan, H.; Khalid, A.; Mahmood, S.F.; Nasir, N.; Khanum, I.; De Siqueira, I.; Van Voorhis, W. Chronic Inflammation in Post-Acute Sequelae of COVID-19 Modulates Gut Microbiome: A Review of Literature on COVID-19 Sequelae and Gut Dysbiosis. Mol. Med. 2025, 31, 22. [Google Scholar] [CrossRef] [PubMed]
- Aktas, B.; Aslim, B. Neuropathy in COVID-19 Associated with Dysbiosis-Related Inflammation. Turk. J. Biol. 2021, 45, 390–403. [Google Scholar] [CrossRef]
- King, L.R. Gastrointestinal Manifestations of Long COVID. Life Sci. 2024, 357, 123100. [Google Scholar] [CrossRef]
- Haduch, A.; Bromek, E.; Kuban, W.; Daniel, W.A. The Engagement of Cytochrome P450 Enzymes in Tryptophan Metabolism. Metabolites 2023, 13, 629. [Google Scholar] [CrossRef]
- Ruddick, J.P.; Evans, A.K.; Nutt, D.J.; Lightman, S.L.; Rook, G.A.W.; Lowry, C.A. Tryptophan Metabolism in the Central Nervous System: Medical Implications. Expert Rev. Mol. Med. 2006, 8, 1–27. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, Tryptophan Metabolism and the Brain-Gut-Microbiome Axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, T.J.; Gates, E.J.; Ranger, A.L.; Klegeris, A. Short-Chain Fatty Acids (SCFAs) Alone or in Combination Regulate Select Immune Functions of Microglia-like Cells. Mol. Cell. Neurosci. 2020, 105, 103493. [Google Scholar] [CrossRef] [PubMed]
- Knox, E.G.; Aburto, M.R.; Tessier, C.; Nagpal, J.; Clarke, G.; O’Driscoll, C.M.; Cryan, J.F. Microbial-Derived Metabolites Induce Actin Cytoskeletal Rearrangement and Protect Blood-Brain Barrier Function. iScience 2022, 25, 105648. [Google Scholar] [CrossRef] [PubMed]
- Kalyan, M.; Tousif, A.H.; Sonali, S.; Vichitra, C.; Sunanda, T.; Praveenraj, S.S.; Ray, B.; Gorantla, V.R.; Rungratanawanich, W.; Mahalakshmi, A.M.; et al. Role of Endogenous Lipopolysaccharides in Neurological Disorders. Cells 2022, 11, 4038. [Google Scholar] [CrossRef]
- Cortes, G.M.; Marcialis, M.A.; Bardanzellu, F.; Corrias, A.; Fanos, V.; Mussap, M. Inflammatory Bowel Disease and COVID-19: How Microbiomics and Metabolomics Depict Two Sides of the Same Coin. Front. Microbiol. 2022, 13, 856165. [Google Scholar] [CrossRef]
- Liang, Z.; Wang, N.; Fan, C.; Shang, L.; Zhang, Y.; Gao, C.; Luo, J. Disturbance of Adaptive Immunity System Was Accompanied by a Decrease in Plasma Short-Chain Fatty Acid for Patients Hospitalized During SARS-CoV-2 Infection After COVID-19 Vaccination. J. Inflamm. Res. 2023, 16, 5261–5272. [Google Scholar] [CrossRef]
- Mendes De Almeida, V.; Engel, D.F.; Ricci, M.F.; Cruz, C.S.; Lopes, Í.S.; Alves, D.A.; D’ Auriol, M.; Magalhães, J.; Machado, E.C.; Rocha, V.M.; et al. Gut Microbiota from Patients with COVID-19 Cause Alterations in Mice That Resemble Post-COVID Symptoms. Gut Microbes 2023, 15, 2249146. [Google Scholar] [CrossRef]
- Khetpal, V.; Berkowitz, J.; Vijayakumar, S.; Choudhary, G.; Mukand, J.A.; Rudolph, J.L.; Wu, W.-C.; Erqou, S. Long-Term Cardiovascular Manifestations and Complications of COVID-19: Spectrum and Approach to Diagnosis and Management. R. I. Med. J. 2022, 105, 16–22. [Google Scholar]
- Ryu, T.; Adler, B.L.; Jeong, S.J.; Lee, D.C.; Hoke, A.; Na, C.H.; Chung, T. Quantitative Serum Proteomic Analysis for Biomarker Discovery in Post-COVID-19 Postural Orthostatic Tachycardia Syndrome (PC-POTS) Patients. Auton. Neurosci. 2025, 258, 103247. [Google Scholar] [CrossRef]
- Silva, C.C.; Bichara, C.N.C.; Carneiro, F.R.O.; Palacios, V.R.D.C.M.; Berg, A.V.S.V.D.; Quaresma, J.A.S.; Magno Falcão, L.F. Muscle Dysfunction in the Long Coronavirus Disease 2019 Syndrome: Pathogenesis and Clinical Approach. Rev. Med. Virol. 2022, 32, e2355. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Rodrigues, C.F.; Stojanović-Radić, Z.; Dimitrijević, M.; Aleksić, A.; Neffe-Skocińska, K.; Zielińska, D.; Kołożyn-Krajewska, D.; Salehi, B.; Milton Prabu, S.; et al. Probiotics: Versatile Bioactive Components in Promoting Human Health. Medicina 2020, 56, 433. [Google Scholar] [CrossRef] [PubMed]
- Lara-Villoslada, F.; Olivares, M.; Xaus, J. Safety of Probiotic Bacteria. In Bioactive Foods in Promoting Health; Elsevier: Amsterdam, The Netherlands, 2010; pp. 47–58. [Google Scholar] [CrossRef]
- Abdollahi, M.; Abdolghaffari, A.H.; Gooshe, M.; Ghasemi-Niri, F. Safety of Probiotic Bacteria. In Probiotics, Prebiotics, and Synbiotics; Elsevier: Amsterdam, The Netherlands, 2016; pp. 227–243. [Google Scholar] [CrossRef]
- Sharma, P.; Tomar, S.K.; Goswami, P.; Sangwan, V.; Singh, R. Antibiotic Resistance among Commercially Available Probiotics. Food Res. Int. 2014, 57, 176–195. [Google Scholar] [CrossRef]
- Doron, S.; Snydman, D.R. Risk and Safety of Probiotics. Clin. Infect. Dis. 2015, 60 (Suppl. S2), S129–S134. [Google Scholar] [CrossRef]
- Sarita, B.; Samadhan, D.; Hassan, M.Z.; Kovaleva, E.G. A Comprehensive Review of Probiotics and Human Health-Current Prospective and Applications. Front. Microbiol. 2025, 15, 1487641. [Google Scholar] [CrossRef]
- Dixit, K.; Chaudhari, D.; Dhotre, D.; Shouche, Y.; Saroj, S. Restoration of Dysbiotic Human Gut Microbiome for Homeostasis. Life Sci. 2021, 278, 119622. [Google Scholar] [CrossRef]
- Kim, S.-K.; Guevarra, R.B.; Kim, Y.-T.; Kwon, J.; Kim, H.; Cho, J.H.; Kim, H.B.; Lee, J.-H. Role of Probiotics in Human Gut Microbiome-Associated Diseases. J. Microbiol. Biotechnol. 2019, 29, 1335–1340. [Google Scholar] [CrossRef]
- Jakubczyk, D.; Leszczyńska, K.; Górska, S. The Effectiveness of Probiotics in the Treatment of Inflammatory Bowel Disease (IBD)—A Critical Review. Nutrients 2020, 12, 1973. [Google Scholar] [CrossRef]
- Tian, Y.; Ran, H.; Wen, X.; Fu, G.; Zhou, X.; Liu, R.; Pan, T. Probiotics Improve Symptoms of Patients with COVID-19 through Gut-Lung Axis: A Systematic Review and Meta-Analysis. Front. Nutr. 2023, 10, 1179432. [Google Scholar] [CrossRef]
- Blaabjerg, S.; Artzi, D.; Aabenhus, R. Probiotics for the Prevention of Antibiotic-Associated Diarrhea in Outpatients—A Systematic Review and Meta-Analysis. Antibiotics 2017, 6, 21. [Google Scholar] [CrossRef]
- Kang, E.-J.; Kim, S.Y.; Hwang, I.-H.; Ji, Y.-J. The Effect of Probiotics on Prevention of Common Cold: A Meta-Analysis of Randomized Controlled Trial Studies. Korean J. Fam. Med. 2013, 34, 2. [Google Scholar] [CrossRef] [PubMed]
- Kanauchi, O.; Andoh, A.; AbuBakar, S.; Yamamoto, N. Probiotics and Paraprobiotics in Viral Infection: Clinical Application and Effects on the Innate and Acquired Immune Systems. Curr. Pharm. Des. 2018, 24, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Brahma, S.; Naik, A.; Lordan, R. Probiotics: A Gut Response to the COVID-19 Pandemic but What Does the Evidence Show? Clin. Nutr. ESPEN 2022, 51, 17–27. [Google Scholar] [CrossRef]
- Prescott, D.; Lee, J.; Philpott, D.J. An Epithelial Armamentarium to Sense the Microbiota. Semin. Immunol. 2013, 25, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Kayama, H.; Takeda, K. Manipulation of Epithelial Integrity and Mucosal Immunity by Host and Microbiota-derived Metabolites. Eur. J. Immunol. 2020, 50, 921–931. [Google Scholar] [CrossRef]
- Ferreira-Junior, A.S.; Borgonovi, T.F.; De Salis, L.V.V.; Leite, A.Z.; Dantas, A.S.; De Salis, G.V.V.; Cruz, G.N.F.; De Oliveira, L.F.V.; Gomes, E.; Penna, A.L.B.; et al. Detection of Intestinal Dysbiosis in Post-COVID-19 Patients One to Eight Months after Acute Disease Resolution. Int. J. Environ. Res. Public Health 2022, 19, 10189. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, T.; Wang, Y.; Li, Y.; Sun, Y.; Qiu, H.-J. Short-Chain Fatty Acids: Key Antiviral Mediators of Gut Microbiota. Front. Immunol. 2025, 16, 1614879. [Google Scholar] [CrossRef]
- Wang, Y.; Moon, A.; Huang, J.; Sun, Y.; Qiu, H.-J. Antiviral Effects and Underlying Mechanisms of Probiotics as Promising Antivirals. Front. Cell. Infect. Microbiol. 2022, 12, 928050. [Google Scholar] [CrossRef]
- Al Kassaa, I.; Hober, D.; Hamze, M.; Chihib, N.E.; Drider, D. Antiviral Potential of Lactic Acid Bacteria and Their Bacteriocins. Probiotics Antimicrob. Proteins 2014, 6, 177–185. [Google Scholar] [CrossRef]
- Klebanoff, S.J.; Coombs, R.W. Viricidal Effect of Lactobacillus Acidophilus on Human Immunodeficiency Virus Type 1: Possible Role in Heterosexual Transmission. J. Exp. Med. 1991, 174, 289–292. [Google Scholar] [CrossRef]
- Wachsman, M.B.; Castilla, V.; de Ruiz Holgado, A.P.; de Torres, R.A.; Sesma, F.; Coto, C.E. Enterocin CRL35 Inhibits Late Stages of HSV-1 and HSV-2 Replication in Vitro. Antivir. Res. 2003, 58, 17–24. [Google Scholar] [CrossRef]
- Todorov, S.D.; Wachsman, M.B.; Knoetze, H.; Meincken, M.; Dicks, L.M.T. An Antibacterial and Antiviral Peptide Produced by Enterococcus mundtii ST4V Isolated from Soya Beans. Int. J. Antimicrob. Agents 2005, 25, 508–513. [Google Scholar] [CrossRef]
- Kanmani, P.; Kim, H. Immunobiotic Strains Modulate Toll-Like Receptor 3 Agonist Induced Innate Antiviral Immune Response in Human Intestinal Epithelial Cells by Modulating IFN Regulatory Factor 3 and NF-κB Signaling. Front. Immunol. 2019, 10, 1536. [Google Scholar] [CrossRef] [PubMed]
- Farkas, A.E.; Capaldo, C.T.; Nusrat, A. Regulation of Epithelial Proliferation by Tight Junction Proteins. Ann. N. Y. Acad. Sci. 2012, 1258, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Abbas, Z.; Wu, S.; Zhang, J.; Mo, P.; Wang, J.; Li, Z.; Zhang, H.; Hu, X.; Yao, M.; et al. Postbiotics from Bacillus amyloliquefaciens J and Lactiplantibacillus plantarum SN4 (PWE) Alleviates E. coli-Induced Enteritis via Gut Barrier Enhancement, Microbiota Modulation, and Liver Protection. Food Biosci. 2025, 68, 106732. [Google Scholar] [CrossRef]
- Wang, J.; Ji, H.; Wang, S.; Liu, H.; Zhang, W.; Zhang, D.; Wang, Y. Probiotic Lactobacillus plantarum Promotes Intestinal Barrier Function by Strengthening the Epithelium and Modulating Gut Microbiota. Front. Microbiol. 2018, 9, 1953. [Google Scholar] [CrossRef]
- Rose, E.C.; Odle, J.; Blikslager, A.T.; Ziegler, A.L. Probiotics, Prebiotics and Epithelial Tight Junctions: A Promising Approach to Modulate Intestinal Barrier Function. Int. J. Mol. Sci. 2021, 22, 6729. [Google Scholar] [CrossRef]
- Sun, W.; Chen, W.; Meng, K.; Cai, L.; Li, G.; Li, X.; Jiang, X. Dietary Supplementation with Probiotic Bacillus licheniformis S6 Improves Intestinal Integrity via Modulating Intestinal Barrier Function and Microbial Diversity in Weaned Piglets. Biology 2023, 12, 238. [Google Scholar] [CrossRef]
- Kalkan, A.E.; BinMowyna, M.N.; Raposo, A.; Ahmad, M.F.; Ahmed, F.; Otayf, A.Y.; Carrascosa, C.; Saraiva, A.; Karav, S. Beyond the Gut: Unveiling Butyrate’s Global Health Impact Through Gut Health and Dysbiosis-Related Conditions: A Narrative Review. Nutrients 2025, 17, 1305. [Google Scholar] [CrossRef]
- Nagai, M.; Yaoita, E.; Yoshida, Y.; Kuwano, R.; Nameta, M.; Ohshiro, K.; Isome, M.; Fujinaka, H.; Suzuki, S.; Suzuki, J.; et al. Coxsackievirus and Adenovirus Receptor, a Tight Junction Membrane Protein, Is Expressed in Glomerular Podocytes in the Kidney. Lab. Investig. 2003, 83, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Botic, T.; Klingberg, T.; Weingartl, H.; Cencic, A. A Novel Eukaryotic Cell Culture Model to Study Antiviral Activity of Potential Probiotic Bacteria. Int. J. Food Microbiol. 2007, 115, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chai, W.; Burwinkel, M.; Twardziok, S.; Wrede, P.; Palissa, C.; Esch, B.; Schmidt, M.F.G. Inhibitory Influence of Enterococcus faecium on the Propagation of Swine Influenza A Virus In Vitro. PLoS ONE 2013, 8, e53043. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.G.; Sencio, V.; Trottein, F. Short-Chain Fatty Acids as a Potential Treatment for Infections: A Closer Look at the Lungs. Infect. Immun. 2021, 89, e00188-21. [Google Scholar] [CrossRef]
- Ñahui Palomino, R.A.; Zicari, S.; Vanpouille, C.; Vitali, B.; Margolis, L. Vaginal Lactobacillus Inhibits HIV-1 Replication in Human Tissues Ex Vivo. Front. Microbiol. 2017, 8, 906. [Google Scholar] [CrossRef]
- Conti, C.; Malacrino, C.; Mastromarino, P. Inhibition of Herpes Simplex Virus Type 2 by Vaginal Lactobacilli. J. Physiol. Pharmacol. 2009, 60 (Suppl. S6), 19–26. [Google Scholar]
- Hegarty, J.W.; Guinane, C.M.; Ross, R.P.; Hill, C.; Cotter, P.D. Bacteriocin Production: A Relatively Unharnessed Probiotic Trait? F1000Research 2016, 5, 2587. [Google Scholar] [CrossRef]
- Wachsman, M.B.; Farías, M.E.; Takeda, E.; Sesma, F.; De Ruiz Holgado, A.P.; De Torres, R.A.; Coto, C.E. Antiviral Activity of Enterocin CRL35 against Herpesviruses. Int. J. Antimicrob. Agents 1999, 12, 293–299. [Google Scholar] [CrossRef]
- Raheem, A.; Liang, L.; Zhang, G.; Cui, S. Modulatory Effects of Probiotics During Pathogenic Infections with Emphasis on Immune Regulation. Front. Immunol. 2021, 12, 616713. [Google Scholar] [CrossRef]
- Thomas, C.M.; Versalovic, J. Probiotics-Host Communication: Modulation of Signaling Pathways in the Intestine. Gut Microbes 2010, 1, 148–163. [Google Scholar] [CrossRef]
- Abraham, C.; Abreu, M.T.; Turner, J.R. Pattern Recognition Receptor Signaling and Cytokine Networks in Microbial Defenses and Regulation of Intestinal Barriers: Implications for Inflammatory Bowel Disease. Gastroenterology 2022, 162, 1602–1616.e6. [Google Scholar] [CrossRef]
- Chiba, E.; Tomosada, Y.; Vizoso-Pinto, M.G.; Salva, S.; Takahashi, T.; Tsukida, K.; Kitazawa, H.; Alvarez, S.; Villena, J. Immunobiotic Lactobacillus rhamnosus Improves Resistance of Infant Mice against Respiratory Syncytial Virus Infection. Int. Immunopharmacol. 2013, 17, 373–382. [Google Scholar] [CrossRef]
- Villena, J.; Chiba, E.; Vizoso-Pinto, M.; Tomosada, Y.; Takahashi, T.; Ishizuka, T.; Aso, H.; Salva, S.; Alvarez, S.; Kitazawa, H. Immunobiotic Lactobacillus rhamnosus Strains Differentially Modulate Antiviral Immune Response in Porcine Intestinal Epithelial and Antigen Presenting Cells. BMC Microbiol. 2014, 14, 126. [Google Scholar] [CrossRef]
- Kawashima, T.; Hayashi, K.; Kosaka, A.; Kawashima, M.; Igarashi, T.; Tsutsui, H.; Tsuji, N.M.; Nishimura, I.; Hayashi, T.; Obata, A. Lactobacillus plantarum Strain YU from Fermented Foods Activates Th1 and Protective Immune Responses. Int. Immunopharmacol. 2011, 11, 2017–2024. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Lan, T.; Li, Q.; Li, B.; Yuan, Y.; Xu, F.; Wang, W. A Comprehensive Perspective on the Interaction between Gut Microbiota and COVID-19 Vaccines. Gut Microbes 2023, 15, 2233146. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, C.; Romero, E.; Garrido-Sanchez, L.; Alcaín-Martínez, G.; Andrade, R.J.; Taminiau, B.; Daube, G.; García-Fuentes, E. Microbiota insights in clostridium difficile infection and inflammatory bowel disease. Gut Microbes 2020, 12, 1725220. [Google Scholar] [CrossRef] [PubMed]
- d’Ettorre, G.; Ceccarelli, G.; Marazzato, M.; Campagna, G.; Pinacchio, C.; Alessandri, F.; Ruberto, F.; Rossi, G.; Celani, L.; Scagnolari, C.; et al. Challenges in the Management of SARS-CoV2 Infection: The Role of Oral Bacteriotherapy as Complementary Therapeutic Strategy to Avoid the Progression of COVID-19. Front. Med. 2020, 7, 389. [Google Scholar] [CrossRef]
- Bozkurt, H.S.; Bilen, Ö. Oral Booster Probiotic Bifidobacteria in SARS-COV-2 Patients. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211059677. [Google Scholar] [CrossRef]
- Gutiérrez-Castrellón, P.; Gandara-Martí, T.; Abreu Y Abreu, A.T.; Nieto-Rufino, C.D.; López-Orduña, E.; Jiménez-Escobar, I.; Jiménez-Gutiérrez, C.; López-Velazquez, G.; Espadaler-Mazo, J. Probiotic Improves Symptomatic and Viral Clearance in COVID-19 Outpatients: A Randomized, Quadruple-Blinded, Placebo-Controlled Trial. Gut Microbes 2022, 14, 2018899. [Google Scholar] [CrossRef]
- Di Pierro, F.; Iqtadar, S.; Mumtaz, S.U.; Bertuccioli, A.; Recchia, M.; Zerbinati, N.; Khan, A. Clinical Effects of Streptococcus salivarius K12 in Hospitalized COVID-19 Patients: Results of a Preliminary Study. Microorganisms 2022, 10, 1926. [Google Scholar] [CrossRef] [PubMed]
- Forsgård, R.A.; Rode, J.; Lobenius-Palmér, K.; Kamm, A.; Patil, S.; Tacken, M.G.J.; Lentjes, M.A.H.; Axelsson, J.; Grompone, G.; Montgomery, S.; et al. Limosilactobacillus reuteri DSM 17938 Supplementation and SARS-CoV-2 Specific Antibody Response in Healthy Adults: A Randomized, Triple-Blinded, Placebo-Controlled Trial. Gut Microbes 2023, 15, 2229938. [Google Scholar] [CrossRef] [PubMed]
- Wischmeyer, P.E.; Tang, H.; Ren, Y.; Bohannon, L.; Jiang, D.; Bergens, M.; Ramirez, Z.E.; Andermann, T.M.; Messina, J.A.; Sung, J.A.; et al. Efficacy of Probiotic Treatment as Post-Exposure Prophylaxis for COVID-19: A Double-Blind, Placebo-Controlled Randomized Trial. Clin. Nutr. 2024, 43, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Vaezi, M.; Ravanshad, S.; Akbari Rad, M.; Zarrinfar, H.; Kabiri, M. The Effect of Synbiotic Adjunct Therapy on Clinical and Paraclinical Outcomes in Hospitalized COVID-19 Patients: A Randomized Placebo-controlled Trial. J. Med. Virol. 2023, 95, e28463. [Google Scholar] [CrossRef]
- Wu, J.-Y.; Huang, P.-Y.; Liu, T.-H.; Kuo, C.-Y.; Tsai, Y.-W.; Tang, H.-J.; Lai, C.-C. Clinical Efficacy of Probiotics in the Treatment of Patients with COVID-19: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Expert Rev. Anti-Infect. Ther. 2023, 21, 667–674. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, Y.; Kwong, J.S.; Li, X.; Zheng, W.; He, R. Quality Assessment of the Chinese Clinical Trial Protocols Regarding Treatments for Coronavirus Disease 2019. Front. Pharmacol. 2020, 11, 1330. [Google Scholar] [CrossRef]
- Xavier-Santos, D.; Padilha, M.; Fabiano, G.A.; Vinderola, G.; Gomes Cruz, A.; Sivieri, K.; Costa Antunes, A.E. Evidences and Perspectives of the Use of Probiotics, Prebiotics, Synbiotics, and Postbiotics as Adjuvants for Prevention and Treatment of COVID-19: A Bibliometric Analysis and Systematic Review. Trends Food Sci. Technol. 2022, 120, 174–192. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, F.; Xu, Q.; Su, Y.; Cai, X.; Zeng, F.; Zhang, Y. The Role of Probiotics in Coronavirus Disease-19 Infection in Wuhan: A Retrospective Study of 311 Severe Patients. Int. Immunopharmacol. 2021, 95, 107531. [Google Scholar] [CrossRef]
- Rabiei, S.; Kamali, Z.; Jamilian, P.; Jamilian, P. Beneficial Effects of Probiotics to Flatten the Curve of COVID-19 Pandemic: A Review. Clin. Nutr. Open Sci. 2024, 58, 348–360. [Google Scholar] [CrossRef]
- Hilpert, K. Is the Gut Microbiome a Target for Adjuvant Treatment of COVID-19? Biologics 2021, 1, 285–299. [Google Scholar] [CrossRef]
- SeyedAlinaghi, S.; Shahidi, R.; Afzalian, A.; Paranjkhoo, P.; Ghorbanzadeh, K.; Mojdeganlou, H.; Razi, A.; Mojdeganlou, P.; Dashti, M.; Ghasemzadeh, A.; et al. Probiotics in Prevention and Treatment of COVID-19: A Systematic Review of Current Evidence. Russ. J. Infect. Immun. 2023, 13, 709–722. [Google Scholar] [CrossRef]
- Kow, C.S.; Ramachandram, D.S.; Hasan, S.S. The Effect of Probiotics on the Risk of Mortality in Patients with COVID-19: Systematic Review and Meta-Analysis of Randomized Trials. Inflammopharmacology 2023, 31, 3327–3332. [Google Scholar] [CrossRef] [PubMed]
- Amrouche, T.; Lammi, S.; Drider, D. Probiotics and Prebiotics Intervention in Respiratory and Digestive Infections Linked to COVID-19. Probiotics Antimicrob. Proteins 2025, 17, 1356–1367. [Google Scholar] [CrossRef] [PubMed]
- Lau, R.I.; Su, Q.; Lau, I.S.F.; Ching, J.Y.L.; Wong, M.C.S.; Lau, L.H.S.; Tun, H.M.; Mok, C.K.P.; Chau, S.W.H.; Tse, Y.K.; et al. A Synbiotic Preparation (SIM01) for Post-Acute COVID-19 Syndrome in Hong Kong (RECOVERY): A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Infect. Dis. 2024, 24, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Ranisavljev, M.; Stajer, V.; Todorovic, N.; Ostojic, J.; Cvejic, J.H.; Steinert, R.E.; Ostojic, S.M. The Effects of 3-Month Supplementation with Synbiotic on Patient-Reported Outcomes, Exercise Tolerance, and Brain and Muscle Metabolism in Adult Patients with Post-COVID-19 Chronic Fatigue Syndrome (STOP-FATIGUE): A Randomized Placebo-Controlled Clinical Trial. Eur. J. Nutr. 2025, 64, 28. [Google Scholar] [CrossRef]
- Marinoni, B.; Rimondi, A.; Bottaro, F.; Ciafardini, C.; Amoroso, C.; Muià, M.; Caridi, B.; Noviello, D.; Bandera, A.; Gori, A.; et al. The Role of VSL#3® in the Treatment of Fatigue and Other Symptoms in Long COVID-19 Syndrome: A Randomized, Double-Blind, Placebo-Controlled Pilot Study (DELong#3). medRxiv 2023. [Google Scholar] [CrossRef]
- Santinelli, L.; Laghi, L.; Innocenti, G.P.; Pinacchio, C.; Vassalini, P.; Celani, L.; Lazzaro, A.; Borrazzo, C.; Marazzato, M.; Tarsitani, L.; et al. Oral Bacteriotherapy Reduces the Occurrence of Chronic Fatigue in COVID-19 Patients. Front. Nutr. 2022, 8, 756177. [Google Scholar] [CrossRef]
- Thomas, R.; Williams, M.; Aldous, J.; Yanagisawa, Y.; Kumar, R.; Forsyth, R.; Chater, A. A Randomised, Double-Blind, Placebo-Controlled Trial Evaluating Concentrated Phytochemical-Rich Nutritional Capsule in Addition to a Probiotic Capsule on Clinical Outcomes among Individuals with COVID-19—The UK Phyto-V Study. COVID 2022, 2, 433–449. [Google Scholar] [CrossRef]
- Blomberg, B.; Mohn, K.G.-I.; Brokstad, K.A.; Zhou, F.; Linchausen, D.W.; Hansen, B.-A.; Lartey, S.; Onyango, T.B.; Kuwelker, K.; Sævik, M.; et al. Long COVID in a Prospective Cohort of Home-Isolated Patients. Nat. Med. 2021, 27, 1607–1613. [Google Scholar] [CrossRef]
- Mazza, M.G.; Palladini, M.; Villa, G.; De Lorenzo, R.; Rovere Querini, P.; Benedetti, F. Prevalence, Trajectory over Time, and Risk Factor of Post-COVID-19 Fatigue. J. Psychiatr. Res. 2022, 155, 112–119. [Google Scholar] [CrossRef]
- Joli, J.; Buck, P.; Zipfel, S.; Stengel, A. Post-COVID-19 Fatigue: A Systematic Review. Front. Psychiatry 2022, 13, 947973. [Google Scholar] [CrossRef]
- Chasco, E.E.; Dukes, K.; Jones, D.; Comellas, A.P.; Hoffman, R.M.; Garg, A. Brain Fog and Fatigue Following COVID-19 Infection: An Exploratory Study of Patient Experiences of Long COVID. Int. J. Environ. Res. Public Health 2022, 19, 15499. [Google Scholar] [CrossRef]
- Miszczak, K.; Łukowiak, J.; Tuz-Hrycyna, N. Symptoms and Complications of SARS-CoV-2virus Infection in the Aspect of Speech Therapyrehabilitation—Literature Review. Pol. Przegląd Otorynolaryngologiczny 2023, 12, 39–45. [Google Scholar] [CrossRef]
- Jennings, G.; Monaghan, A.; Xue, F.; Duggan, E.; Romero-Ortuño, R. Comprehensive Clinical Characterisation of Brain Fog in Adults Reporting Long COVID Symptoms. J. Clin. Med. 2022, 11, 3440. [Google Scholar] [CrossRef] [PubMed]
- Rathi, A.; Jadhav, S.B.; Shah, N. A Randomized Controlled Trial of the Efficacy of Systemic Enzymes and Probiotics in the Resolution of Post-COVID Fatigue. Medicines 2021, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Mazza, M.G.; Palladini, M.; Poletti, S.; Benedetti, F. Post-COVID-19 Depressive Symptoms: Epidemiology, Pathophysiology, and Pharmacological Treatment. CNS Drugs 2022, 36, 681–702. [Google Scholar] [CrossRef]
- Deng, J.; Zhou, F.; Hou, W.; Silver, Z.; Wong, C.Y.; Chang, O.; Huang, E.; Zuo, Q.K. The Prevalence of Depression, Anxiety, and Sleep Disturbances in COVID-19 Patients: A Meta-analysis. Ann. N. Y. Acad. Sci. 2021, 1486, 90–111. [Google Scholar] [CrossRef]
- Dong, F.; Liu, H.; Dai, N.; Yang, M.; Liu, J. A Living Systematic Review of the Psychological Problems in People Suffering from COVID-19. J. Affect. Disord. 2021, 292, 172–188. [Google Scholar] [CrossRef]
- Barandouzi, Z.A.; Starkweather, A.R.; Henderson, W.A.; Gyamfi, A.; Cong, X.S. Altered Composition of Gut Microbiota in Depression: A Systematic Review. Front. Psychiatry 2020, 11, 541. [Google Scholar] [CrossRef]
- Wong, M.-L.; Inserra, A.; Lewis, M.D.; Mastronardi, C.A.; Leong, L.; Choo, J.; Kentish, S.; Xie, P.; Morrison, M.; Wesselingh, S.L.; et al. Inflammasome Signaling Affects Anxiety- and Depressive-like Behavior and Gut Microbiome Composition. Mol. Psychiatry 2016, 21, 797–805. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Wallace, C.J.K.; Milev, R.V. The Efficacy, Safety, and Tolerability of Probiotics on Depression: Clinical Results from an Open-Label Pilot Study. Front. Psychiatry 2021, 12, 618279. [Google Scholar] [CrossRef]
- Ghorbani, Z.; Nazari, S.; Etesam, F.; Nourimajd, S.; Ahmadpanah, M.; Razeghi Jahromi, S. The Effect of Synbiotic as an Adjuvant Therapy to Fluoxetine in Moderate Depression: A Randomized Multicenter Trial. Arch. Neurosci. 2018, 5, e60507. [Google Scholar] [CrossRef]
- Barrea, L.; Grant, W.B.; Frias-Toral, E.; Vetrani, C.; Verde, L.; De Alteriis, G.; Docimo, A.; Savastano, S.; Colao, A.; Muscogiuri, G. Dietary Recommendations for Post-COVID-19 Syndrome. Nutrients 2022, 14, 1305. [Google Scholar] [CrossRef] [PubMed]
- Paramythiotis, D.; Karlafti, E.; Didagelos, M.; Fafouti, M.; Veroplidou, K.; Protopapas, A.; Kaiafa, G.; Netta, S.; Michalopoulos, A.; Savopoulos, C. Post-COVID-19 and Irritable Bowel Syndrome: A Literature Review. Medicina 2023, 59, 1961. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.; Shams, U.; Mishra, P.; Sahu, S.; Goenka, M.K.; Ghoshal, U.; Ghoshal, U.C. Post-Infection Irritable Bowel Syndrome Following Coronavirus Disease-19: A Systematic Review and Meta-Analysis. Indian J. Gastroenterol. 2024, 43, 557–566. [Google Scholar] [CrossRef]
- Dale, H.F.; Rasmussen, S.H.; Asiller, Ö.Ö.; Lied, G.A. Probiotics in Irritable Bowel Syndrome: An Up-to-Date Systematic Review. Nutrients 2019, 11, 2048. [Google Scholar] [CrossRef]
- Majeed, M.; Nagabhushanam, K.; Natarajan, S.; Sivakumar, A.; Ali, F.; Pande, A.; Majeed, S.; Karri, S.K. Bacillus coagulans MTCC 5856 Supplementation in the Management of Diarrhea Predominant Irritable Bowel Syndrome: A Double Blind Randomized Placebo Controlled Pilot Clinical Study. Nutr. J. 2015, 15, 21. [Google Scholar] [CrossRef]
- Mezzasalma, V.; Manfrini, E.; Ferri, E.; Sandionigi, A.; La Ferla, B.; Schiano, I.; Michelotti, A.; Nobile, V.; Labra, M.; Di Gennaro, P. A Randomized, Double-Blind, Placebo-Controlled Trial: The Efficacy of Multispecies Probiotic Supplementation in Alleviating Symptoms of Irritable Bowel Syndrome Associated with Constipation. BioMed Res. Int. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Krammer, H.; Storr, M.; Madisch, A.; Riffel, J. Reizdarmbehandlung mit Lactobacillus plantarum 299v: Längere Einnahme verstärkt Behandlungserfolg—Ergebnisse einer nichtinterventionellen Studie. Z. Gastroenterol. 2021, 59, 125–134. [Google Scholar] [CrossRef]
- Zurabov, F.M.; Chernevskaya, E.A.; Beloborodova, N.V.; Zurabov, A.Y.; Petrova, M.V.; Yadgarov, M.Y.; Popova, V.M.; Fatuev, O.E.; Zakharchenko, V.E.; Gurkova, M.M.; et al. Bacteriophage Cocktails in the Post-COVID Rehabilitation. Viruses 2022, 14, 2614. [Google Scholar] [CrossRef]

| Symptoms of Post-COVID | Prevalence (%) |
|---|---|
| Fatigue | 40–58 |
| Headache | 44 |
| Concentration disturbances (attention deficits) | 27 |
| Hair loss | 25 |
| Dyspnea | 24 |
| Gastrointestinal disorders | 22 |
| Loss of appetite | 20 |
| Irritable bowel syndrome (IBS) | 17–39 |
| Loss of taste | 17 |
| Abdominal pain | 14 |
| Post-COVID Patient-Specific Factors | Impact on Post-COVID Conditions |
|---|---|
| Older age | Higher risk of severe outcomes and post-COVID symptoms, such as fatigue and cognitive impairments |
| Younger age | Significant mental health issues, such as anxiety and depression |
| Female sex | Higher levels of persistent post-COVID symptoms, such as anxiety, depression, and post-traumatic stress disorder |
| Diabetes | Increased risk of severe outcomes and persistent symptoms |
| Hypertension | Higher incidence of post-COVID |
| Obesity | Worse post-COVID outcomes and prolonged symptoms |
| Mental health | Exacerbation of post-COVID symptoms, higher rates of depression and anxiety |
| Other comorbidities | Chronic conditions, such as cardiovascular and kidney diseases, contribute to severe outcomes |
| Inflammatory markers | Elevated IL-6 levels linked to post-COVID |
| Microbiota profiles | Potential influence on immune response and inflammation |
| Genetic factors | Significant influence on post-COVID health outcomes, affecting both the severity of the initial infection and long-term consequences |
| Functions of Probiotics | Mechanism of Action of Probiotics |
|---|---|
| Restoration of normal intestinal microbiota | Restoration of a healthy intestinal microbiota by colonizing the epithelium and competing with pathogens thus ensuring microbial balance. |
| Production of antimicrobial agents | Production of bacteriocins and organic acids inhibiting the growth and reproduction of pathogens. |
| Modulation of enzymatic activity and toxin metabolism | Participation in the metabolism of toxic substances and production of volatile fatty acids contributing to energy balance. |
| Increased intestinal cell adhesion and mucin production | Strengthening the intestinal barrier by increasing cell adhesion and producing a protective layer of mucus (mucins). |
| Regulation of the activity of the immune system and lymphoid tissue | Participation in regulating the immune system and maintaining healthy intestinal lymphatic tissue. |
| Strains Used | Daily Dose in CFU | Number of Patients (n) | Test Results | Reference |
|---|---|---|---|---|
| Streptococcus thermophilus DSM 32345; Lactobacillus acidophilus DSM 32241; Lactobacillus helveticus DSM 32242; Lacticaseibacillus paracasei DSM 32243; Lactiplantibacillus plantarum DSM 32244; Lactobacillus brevis DSM 27961; Bifidobacterium animalis subsp. lactis DSM 32246; Bifidobacterium lactis DSM 32247 | 2.4 × 1012 CFU for 7 days | n = 70: probiotic n = 28; placebo n = 42 | Reduction in diarrhea, shortness of breath, cough, and fever, and the risk of respiratory failure was 8 times lower in patients taking the probiotic | [207] |
| Bifidobacterium animalis subsp. Lactis BB-12 | 1 × 1012 CFU for 3 days | n = 44: probiotic n = 20; without probiotic n = 24 | In 95% of patients who received the probiotic, the average hospital stay was reduced by an average of 7.6 days compared to the control group (an average of 13.6 days); the decrease in the mortality rate was 5% in the probiotic group versus 20.83% in the non-probiotic group. | [208] |
| Lactiplantibacillus plantarum KABP022, Lactiplantibacillus plantarum KABP023, Lactiplantibacillus plantarum KAPB033; Pediococcus acidilactici KABP021 | 2 × 109 CFU for 30 days | n = 293: probiotic n = 147; placebo n = 146 | 53.1% of patients taking the probiotic experienced remission (elimination of symptoms and virus); 28.1% of patients in the placebo group experienced remission. Patients taking the probiotic experienced a reduction in the number of days with fever, headache, cough, shortness of breath, and body aches | [209] |
| Streptococcus salivarius K12 | 1 × 109 CFU for 14 days | n = 50: probiotic n = 25; placebo n = 25 | Probiotic administration contributed to colonization of the oral environment, improved blood parameters, and reduced mortality in patients with COVID-19 from 32% in the non-probiotic group to 8% in the probiotic group | [210] |
| Limosilactobacillus reuteri DSM 17938 Vitamin D3 | 2 × 108 CFU 20 μg for 6 months | n = 132: Probiotic with D3 n = 70, including n = 15 vaccinated; Placebo with D3 n = 62, including n = 15 vaccinated; | Long-term enhancement of the immune response, especially in those fully vaccinated with the mRNA vaccine (at 28 days post-vaccination), who showed higher levels of anti-RBD IgA | [211] |
| Lacticaseibacillus rhamnosus GG (LGG) as post-exposure prophylaxis for COVID-19 | 10 × 109 CFU for 28 days | n = 182: LGG n = 91; placebo n = 91 | LGG was associated with a prolonged time to COVID-19 infection and reduced the incidence of illness symptoms and gut microbiome changes when used as prophylaxis for more than 7 days post-COVID-19 exposure, but not the overall incidence | [212] |
| Lactobacillus (L.) rhamnosus, L. helveticus, L. casei, Bifidobacterium (B.) lactis, L. acidophilus, B. breve, L. bulgaricus, B. longum, L. plantarum, B. bifidum, L. gasseri, and Streptococcus (S.) thermophilus, fructooligosaccharides-prebiotic agent | 2 × 109 CFU for 2 weeks | n = 78: probiotic n = 39; placebo n = 39 | A significant reduction in pro-inflammatory markers like IL-6 and improvements in white blood cell counts in hospitalized COVID-19 patients; other findings showed no statistical differences between groups | [213] |
| Probiotic Strain(s) | Daily Dose/Metabolites/Postbiotic Compounds | Number of Patients (n) | Targeted Symptoms | Outcomes | Reference |
|---|---|---|---|---|---|
| Synbiotic blend SIM01: Bifidobacterium adolescentis, Bifidobacterium longum, Bifidobacterium bifidum, prebiotics such as galacto-oligosaccharides, xylooligosaccharides, resistant dextrin | 10 × 109 CFU in sachets twice daily for 30 days/SCFAs, tryptophan metabolites | n = 463: SIM01: n = 232 Placebo: n = 231 | Fatigue, memory loss, hair loss, GI symptoms, | Significant improvement in fatigue, memory, and GI symptoms; significant increase in SCFA-producing strains | [223] |
| Synbiotic blend (DSM-Firmenich): Lacticaseibacillus rhamnosus DSM 32550, Lactiplantibacillus plantarum DSM 34532 (Humiome®), Bifdobacterium lactis DSM 32269, Bifdobacterium longum DSM 32946, 2.5 g of prebiotic fiber fructooligosaccharides, 5 mg of zinc | The dosage of probiotics was not specified, for 3 months/ creatine, SCFAs | n = 26: probiotics n = 13; placebo n = 13 | Post-exertional malaise, cognitive function | Improved post-exercise recovery and increased brain creatine levels | [224] |
| VSL#3®, a combination of 3 Bifidobacterium strains (B. breve, B. longum, B. infantis), 4 strains of Lactobacillus s.l. (L. acidophilus, L. plantarum, L. casei, L. bulgaricus), Streptococcus thermophilus | 450 × 109 CFU in sachets twice daily for 28 days/no measurement of any probiotic-derived metabolites or postbiotics | n = 38: probiotics n = 19; placebo n = 19 | Fatigue, physical functioning, GI symptoms | Significant decrease in fatigue, significant amelioration in physical functioning, and improvement in GI symptoms; VSL#3® treatment was not associated with improvements in symptoms of anxiety, depression, performance, or somatization | [225] |
| SLAB51 (Sivomixx800®)-a probiotic mixture: 5 strains of Lactobacilli s.l. (L. acidophilus, L. helveticus, L. paracasei, L. plantarum, L. brevis), 2 strains of Bifidobacterium (B. lactis DSM 32246®, B. lactis DSM 32247®), Streptococcus thermophilus | 2400 × 109 CFU daily for 6 months/arginine, asparagine, lactate, and 3-hydroxyisobutyrate | n = 58: probiotics + antibiotics n = 24; placebo + antibiotics n = 34 | Fatigue | Decrease in chronic fatigue; higher levels of serum lactate, asparagine, and arginine in the probiotic group; 3-Hydroxyisobutyrate levels were significantly lower in the probiotic-treated participants compared to controls | [226] |
| Synbiotic capsule: 5 strains of Lactobacillus s.l. (L. plantarum, L. rhamnosus, L. bulgaricus, L. lactis and L. paracasei) +200 mg of prebiotic Inulin daily; phytochemical rich capsule (PC): Citrus Sinensis fruit (400 mg from 200 mg of 2:1 extract, standardized to contain 70 mg of Bioflavonoids); Chamomile, Matricaria recutita L. flower) (1000 mg from 22 mg of 10:1 extract and 65 mg of 12:1 extract). Curcuma longa rhizome in Curcumin Complex (1600 mg of curcumin from 25 mg of 64:1 extract, standardised to contain 23.8 mg of curcuminoid). Pomegranate (Punica granatum L. rinds and seeds) (1000 mg from 25 mg of 40:1 extract, standardised to contain 10 mg of Ellagic Acid). Polygonum cuspidatum root containing 100 mg of resveratrol | 10 × 109 CFU daily for 30 days/no measurement of any probiotic-derived metabolites or postbiotics, herb substances | n = 147: synbiotic + PC n = 74 Placebo n = 73 | Fatigue, physical functioning, common toxicity symptoms, cough scores, subjective well-being scores, duration of pyrexia | Decrease in cough score and decrease in the fatigue score in the synbiotic group; improvement in the overall well-being score; mild bloating in two participants. | [227] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jach, M.E.; Sajnaga, E.; Bumbul, M.; Serefko, A.; Borowicz, K.K.; Golczyk, H.; Kieliszek, M.; Wiater, A. The Role of Probiotics and Their Postbiotic Metabolites in Post-COVID-19 Syndrome. Molecules 2025, 30, 4130. https://doi.org/10.3390/molecules30204130
Jach ME, Sajnaga E, Bumbul M, Serefko A, Borowicz KK, Golczyk H, Kieliszek M, Wiater A. The Role of Probiotics and Their Postbiotic Metabolites in Post-COVID-19 Syndrome. Molecules. 2025; 30(20):4130. https://doi.org/10.3390/molecules30204130
Chicago/Turabian StyleJach, Monika E., Ewa Sajnaga, Marharyta Bumbul, Anna Serefko, Kinga K. Borowicz, Hieronim Golczyk, Marek Kieliszek, and Adrian Wiater. 2025. "The Role of Probiotics and Their Postbiotic Metabolites in Post-COVID-19 Syndrome" Molecules 30, no. 20: 4130. https://doi.org/10.3390/molecules30204130
APA StyleJach, M. E., Sajnaga, E., Bumbul, M., Serefko, A., Borowicz, K. K., Golczyk, H., Kieliszek, M., & Wiater, A. (2025). The Role of Probiotics and Their Postbiotic Metabolites in Post-COVID-19 Syndrome. Molecules, 30(20), 4130. https://doi.org/10.3390/molecules30204130