Modern Pro-Health Applications of Medicinal Mushrooms: Insights into the Polyporaceae Family, with a Focus on Cerrena unicolor
Abstract
1. Introduction
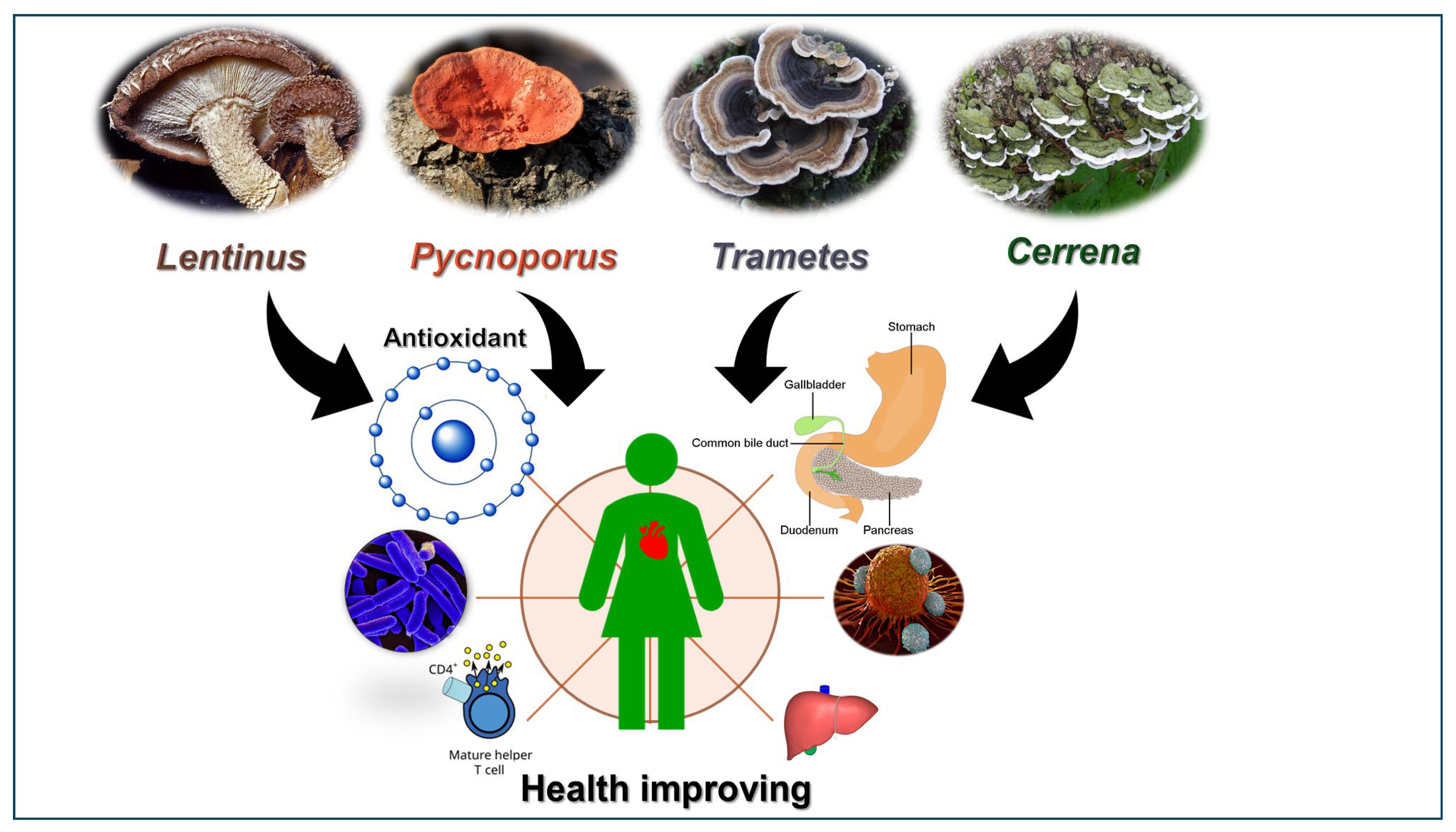
2. Traditional and Current Utilization of the Health-Promoting Properties of Selected Fungi from the Polyporaceae Family
2.1. The Polyporaceae Family
2.2. The Lentinus
2.3. The Pycnoporus
2.4. The Trametes
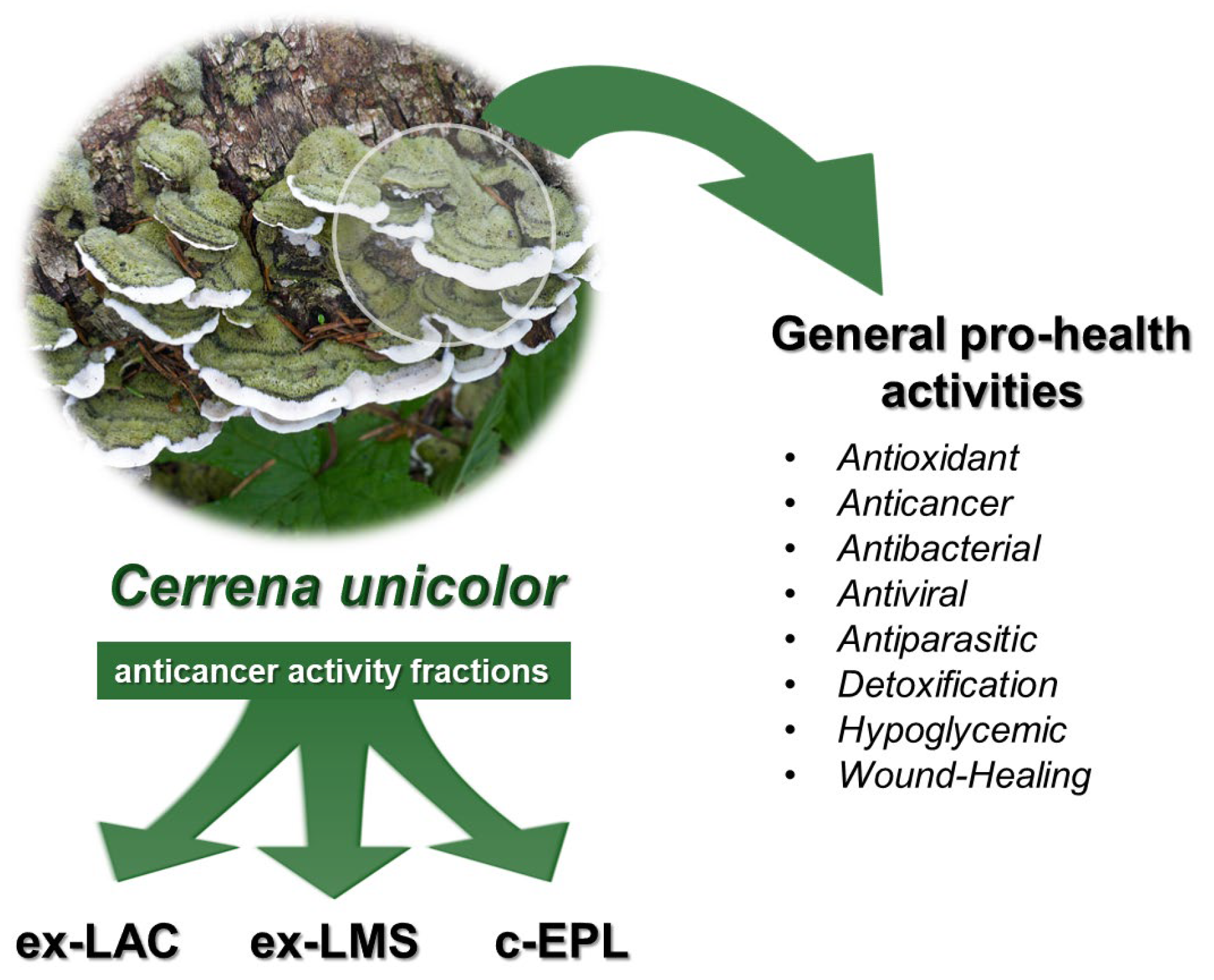
3. Cerrena unicolor and the Modern Examples of Its Usage as a Pro-Health Material
3.1. Characteristic of Cerrena unicolor
3.2. Antioxidant Activity of Cerrena unicolor
3.3. Anticancer Activity of Cerrena unicolor
3.4. Antimicrobial and Antiviral Activity of the Cerrena unicolor
3.5. Antiparasitic Properties of Cerrena unicolor
3.6. Detoxification Activity of Cerrena unicolor
3.7. Hypoglycemic Effect of Cerrena unicolor
3.8. Wound-Healing Activity of Cerrena unicolor
3.9. Challenges and Future Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid) radical-scavenging test |
| AFB1 | aflatoxin B1 |
| ALT | alanine transaminase |
| AS | acetosyringone |
| c-EPL | endopolysaccharides |
| DPPH | free radical 1,1-diphenyl-2-picryl-hydrazyl-scavenging test |
| EC50 | half maximal effective concentration |
| ECIS | electric cell–substrate impedance sensing technique |
| EMCV | encephalomyocarditis virus |
| EPS | exopolysaccharides |
| ex-LAC | extracellular laccase |
| ex-LMS | low-molecular-weight secondary metabolite subfractions |
| HHV-1 | human herpes virus type 1 |
| IC50- | half maximal inhibitory concentration |
| IL-6 | interleukin-6 |
| LDH | lactate dehydrogenase |
| MTT-3 | (4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide test |
| NGF | nerve growth factor |
| NR | neutral red assay |
| OH | hydroxyl radical-scavenging activity assay |
| OSI | oxidative stress index |
| RL-Ps | ribotoxin-like proteins |
| ROS | reactive oxygen species |
| SA | syringaldehyde |
| SEM | scanning electron microscope |
| SOD | superoxide dismutase |
| SRL | ricin–sarcin loop |
| SSC | solid-state cultivation |
| TAS | total antioxidant status |
| TNF | total antioxidant status |
| TOS | total oxidant status |
| WHO | World Health Organization |
| XTT | (2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) test |
References
- Venturella, G.; Ferraro, V.; Cirlincione, F.; Gargano, M.L. Medicinal mushrooms: Bioactive compounds, use, and clinical trials. Int. J. Mol. Sci. 2021, 22, 634. [Google Scholar] [CrossRef] [PubMed]
- Elkhateeb, W.A.; Elkhateeb, W.A. What Medicinal Mushroom Can Do? Chem. Res. J. 2020, 5, 106–118. [Google Scholar]
- Jeitler, M.; Michalsen, A.; Frings, D.; Hübner, M.; Fischer, M.; Koppold-Liebscher, D.A.; Murthy, V.; Kessler, C.S. Significance of Medicinal Mushrooms in Integrative Oncology: A Narrative Review. Front. Pharmacol. 2020, 11, 580656. [Google Scholar] [CrossRef]
- Elsayed, E.A.; El Enshasy, H.; Wadaan, M.A.M.; Aziz, R. Mushrooms: A potential natural source of anti-inflammatory compounds for medical applications. Mediators Inflamm. 2014, 2014, 805841. [Google Scholar] [CrossRef]
- Ślusarczyk, J.; Adamska, E.; Czerwik-Marcinkowska, J. Fungi and algae as sources of medicinal and other biologically active compounds: A review. Nutrients 2021, 13, 3178. [Google Scholar] [CrossRef]
- Citores, L.; Ragucci, S.; Russo, R.; Gay, C.C.; Chambery, A.; Di Maro, A.; Iglesias, R.; Ferreras, J.M. Structural and functional characterization of the cytotoxic protein ledodin, an atypical ribosome-inactivating protein from shiitake mushroom (Lentinula edodes). Protein Sci. 2023, 32, e4621. [Google Scholar] [CrossRef]
- Joshi, M.; Bhargava, P.; Bhatt, M.; Kadri, S.; Shri, M.; Joshi, C.G. (Eds.) Polyporaceae BT—Mushrooms of Gujarat; Springer Nature Singapore: Singapore, 2021; pp. 101–113. ISBN 978-981-16-4999-8. [Google Scholar]
- Guo, Y.; Chen, X.; Gong, P. Classification, structure and mechanism of antiviral polysaccharides derived from edible and medicinal fungus. Int. J. Biol. Macromol. 2021, 183, 1753–1773. [Google Scholar] [CrossRef] [PubMed]
- Kirk, P.; Cannon, P.; Stalpers, J.; Minter, D.W. Dictionary of the Fungi, 10th ed.; CABI Publishing in Great Britain: Wallingford, UK, 2008. [Google Scholar]
- Citores, L.; Ragucci, S.; Gay, C.C.; Russo, R.; Chambery, A.; Di Maro, A.; Iglesias, R.; Ferreras, J.M. Edodin: A New Type of Toxin from Shiitake Mushroom (Lentinula edodes) That Inactivates Mammalian Ribosomes. Toxins 2024, 16, 185. [Google Scholar] [CrossRef]
- Gaitán-Hernández, R.; López-Peña, D.; Esqueda, M.; Gutiérrez, A. Review of Bioactive Molecules Production, Biomass, and Basidiomata of Shiitake Culinary-Medicinal Mushrooms, Lentinus edodes (Agaricomycetes). Int. J. Med. Mushrooms 2019, 21, 841–850. [Google Scholar] [CrossRef]
- Seelan, J.S.S.; Justo, A.; Nagy, L.G.; Grand, E.A.; Redhead, S.A.; Hibbett, D. Phylogenetic relationships and morphological evolution in Lentinus, Polyporellus and Neofavolus, emphasizing southeastern Asian taxa. Mycologia 2015, 107, 460–474. [Google Scholar] [CrossRef]
- Sadi, G.; Emsen, B.; Kaya, A.; Kocabaş, A.; Çınar, S.; Kartal, D.İ. Cytotoxicity of some edible mushrooms extracts over liver hepatocellular carcinoma cells in conjunction with their antioxidant and antibacterial properties. Pharmacogn. Mag. 2015, 11, S6–S18. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, H.; Ng, T. A laccase with HIV-1 reverse transcriptase inhibitory activity from the broth of mycelial culture of the mushroom Lentinus tigrinus. J. Biomed. Biotechnol. 2012, 2012, 536725. [Google Scholar] [CrossRef]
- Dulay, R.M.R.; Arenas, M.C.; Kalaw, S.P.; Reyes, R.G.; Cabrera, E.C. Proximate composition and functionality of the culinary-medicinal tiger sawgill mushroom, Lentinus tigrinus (higher Basidiomycetes), from the Philippines. Int. J. Med. Mushrooms 2014, 16, 85–94. [Google Scholar] [CrossRef]
- He, P.; Wu, S.; Pan, L.; Sun, S.; Mao, D.; Xu, C. Effect of tween 80 and acetone on the secretion, structure and antioxidant activities of exopolysaccharides from Lentinus tigrinus. Food Technol. Biotechnol. 2016, 54, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Ragasa, C.Y.; Tan, M.C.S.; De Castro, M.E.; De Los Reyes, M.M.; Oyong, G.G.; Shen, C.C. Sterols from lentinus tigrinus. Pharmacogn. J. 2018, 10, 1079–1081. [Google Scholar] [CrossRef]
- Mohammadnejad, S.; Pourianfar, H.R.; Drakhshan, A.; Jabaleh, I.; Rezayi, M. Potent antiproliferative and pro-apoptotic effects of a soluble protein fraction from culinary-medicinal mushroom Lentinus tigrinus on cancer cells. J. Food Meas. Charact. 2019, 13, 3015–3024. [Google Scholar] [CrossRef]
- Kumar, M.; Kaviyarasan, V. Distribution of Lentinus tuberregium (Fr.), an indigenous edible medicinal mushroom in Tamil Nadu, South India. J. Acad. Indus. Res. 2012, 1, 296–300. [Google Scholar]
- Mossebo, D.C.; Metsebing, B.-P.; Oba, R.; Tsigaing Tsigaing, F.; Ryvarden, L.; Fonkui, T.Y.; Mungoh Tata, C.; Ndinteh, D.T. Comparative evaluation of antifungal and antibacterial activities of crude extracts of Pleurotus sajor-caju, Pleurotus tuber-regium and Lentinus squarrosulus (Basidiomycota, Pleurotaceae, Lentinaceae) from Cameroon. Eur. J. Biol. Biotechnol. 2020, 1, 1–7. [Google Scholar] [CrossRef]
- Téllez-Téllez, M.; Villegas, E.; Rodríguez, A.; Acosta-Urdapilleta, M.L.; O’Donovan, A.; Díaz-Godínez, G. Mycosphere Essay 11: Fungi of Pycnoporus: Morphological and molecular identification, worldwide distribution and biotechnological potential. Mycosphere 2016, 7, 1500–1525. [Google Scholar] [CrossRef]
- Bourdette, J.O.O.; Calixte, N.E.H.; Misso, R.-L.N.M.; Obiang, C.S.; Prudence, Y.Y.; Ondo, J.P.; Roger, N.A.G.; Engonga, L.-C.O. Phytochemical Screening, Antioxidant and Antiangiogenic activities of Daedaleopsis nitida, Pycnoporus sanguineus and Phellinus gilvus Medicinal Mushrooms from Gabon. Pharm. Chem. J. 2019, 6, 71–80. [Google Scholar]
- Mounguengui, S.; Saha Tchinda, J.B.; Ndikontar, M.K.; Dumarçay, S.; Attéké, C.; Perrin, D.; Gelhaye, E.; Gérardin, P. Total phenolic and lignin contents, phytochemical screening, antioxidant and fungal inhibition properties of the heartwood extractives of ten Congo Basin tree species. Ann. For. Sci. 2016, 73, 287–296. [Google Scholar] [CrossRef]
- Bourdette, J.O.O.; Ndong, H.C.E.; Bourobou, H.P.B.; Engonga, L.C.O. Mycochemical analysis, anti-inflammatory and cytotoxic activities of Pycnoporus sanguineus (L.) Murrill, a medicinal mushroom from Gabon. World J. Biol. Pharm. Res. 2022, 3, 001–008. [Google Scholar] [CrossRef]
- Jaszek, M.; Osinska-Jaroszuk, M.; Sulej, J.; Matuszewska, A.; Stefaniuk, D.; Maciag, K.; Polak, J.; Matuszewski, L.; Grzywnowicz, K. Stimulation of the antioxidative and antimicrobial potential of the blood red bracket mushroom Pycnoporus sanguineus (higher Basidiomycetes). Int. J. Med. Mushrooms 2015, 17, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Bhardwaj, G.; Nayik, G.A. Edible and Medicinal Mushrooms of the Himalayas: Climate Change, Critically Endangered Species, and the Call for Sustainable Development; CRC Press: Boca Raton, FL, USA, 2023; ISBN 9781000926835. [Google Scholar]
- Díaz-Godínez, G.; Téllez-Téllez, M.; Rodríguez, A.; Obregón-Barbosa, V.; De Lourdes Acosta-Urdapilleta, M.; Villegas, E. Enzymatic, antioxidant, antimicrobial, and insecticidal activities of Pleurotus pulmonarius and Pycnoporus cinnabarinus grown separately in an airlift reactor. BioResources 2016, 11, 4186–4200. [Google Scholar] [CrossRef]
- Soković, M.; Glamočlija, J.; Ćirić, A.; Petrović, J.; Stojković, D. Chapter 5- Mushrooms as Sources of Therapeutic Foods. Ther. Foods 2018, 141–178. [Google Scholar] [CrossRef]
- Olou, B.A.; Krah, F.S.; Piepenbring, M.; Yorou, N.S.; Langer, E. Diversity of Trametes (Polyporales, Basidiomycota) in tropical Benin and description of new species Trametes parvispora. MycoKeys 2020, 65, 25–47. [Google Scholar] [CrossRef]
- Sharma, D.; Singh, V.P.; Singh, N.K. A Review on Phytochemistry and Pharmacology of Medicinal as well as Poisonous Mushrooms. Mini Rev. Med. Chem. 2018, 18, 1095–1109. [Google Scholar] [CrossRef]
- Muñoz-Castiblanco, T.; Mejia-Giraldo, J.C.; Puertas-Mejia, M.A. Trametes genus, a source of chemical compounds with anticancer activity in human osteosarcoma: A systematic review. J. Appl. Pharm. Sci. 2020, 10, 121–129. [Google Scholar] [CrossRef]
- Tišma, M.; Žnidaršič-Plazl, P.; Šelo, G.; Tolj, I.; Šperanda, M.; Bucić-Kojić, A.; Planinić, M. Trametes versicolor in lignocellulose-based bioeconomy: State of the art, challenges and opportunities. Bioresour. Technol. 2021, 330, 124997. [Google Scholar] [CrossRef]
- Shokrzadeh, M.; Azdo, S.; Amirahmadi, M.; Habibi, E. Anti-diabetic effect of methanol extract of Trametes versicolor on male mice. J. Maz. Univ. Med. Sci. 2017, 26, 165–175. [Google Scholar]
- Janjušević, L.; Pejin, B.; Kaišarević, S.; Gorjanović, S.; Pastor, F.; Tešanović, K.; Karaman, M. Trametes versicolor ethanol extract, a promising candidate for health-promoting food supplement. Nat. Prod. Res. 2018, 32, 963–967. [Google Scholar] [CrossRef]
- Shnyreva, A.V.; Shnyreva, A.A.; Espinoza, C.; Padrón, J.M.; Trigos, Á. Antiproliferative Activity and Cytotoxicity of Some Medicinal Wood-Destroying Mushrooms from Russia. Int. J. Med. Mushrooms 2018, 20, 1–11. [Google Scholar] [CrossRef]
- Pan, J.; Yang, C.; Jiang, Z.; Huang, J. Trametes robiniophila murr: A traditional Chinese medicine with potent anti-tumor effects. Cancer Manag. Res. 2019, 11, 1541–1549. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Chen, T.; Jiang, L.; Yang, Q. Huaier Extract Inhibits Breast Cancer Progression Through a LncRNA-H19/MiR-675-5p Pathway. Cell. Physiol. Biochem. 2017, 44, 581–593. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, H.; Wang, X.; Wang, C.; Gan, L.; Zhu, J.; Tong, J.; Li, Z. Huaier Granule extract inhibit the proliferation and metastasis of lung cancer cells through down-regulation of MTDH, JAK2/STAT3 and MAPK signaling pathways. Biomed. Pharmacother. 2018, 101, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yang, A.; Su, G.; Zhao, Y.; Wang, Y.; Chai, X.; Tu, P. Huaier restrains proliferative and invasive potential of human hepatoma SKHEP-1 cells partially through decreased Lamin B1 and elevated NOV. Sci. Rep. 2016, 6, 31298. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Liu, P.; Jiang, K.; Zhang, X.; Xie, L.; Wang, Z.; Gong, P. Huaier polysaccharide induces apoptosis in hepatocellular carcinoma cells through p38 MAPK. Oncol. Lett. 2016, 12, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, Z.; Liu, Z. Effects of Huaier aqueous extract on proliferation and apoptosis in the melanoma cell line A875. Acta Histochem. 2013, 115, 705–711. [Google Scholar] [CrossRef]
- Li, C.; Wu, X.; Zhang, H.; Yang, G.; Hao, M.; Sheng, S.; Sun, Y.; Long, J.; Hu, C.; Sun, X.; et al. A Huaier polysaccharide inhibits hepatocellular carcinoma growth and metastasis. Tumour Biol. 2015, 36, 1739–1745. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, S.; Liu, N.; Liu, J.; Wang, W. A polysaccharide from Trametes robiniophila inhibits human osteosarcoma xenograft tumor growth in vivo. Carbohydr. Polym. 2015, 124, 157–163. [Google Scholar] [CrossRef]
- Zhao, X.; Ma, S.; Liu, N.; Liu, J.; Wang, W. A polysaccharide from Trametes robiniophila Murrill induces apoptosis through intrinsic mitochondrial pathway in human osteosarcoma (U-2 OS) cells. Tumour Biol. 2015, 36, 5255–5263. [Google Scholar] [CrossRef]
- Jouybari, H.B.; Bekhradnia, A.; Mirzaee, F.; Hosseinzadeh, M.H.; Habibi, E. Chemical Composition of the Lumpy Bracket Mushroom (Trametes gibbosa). Res. J. Pharmacogn. 2022, 9, 19–27. [Google Scholar] [CrossRef]
- Knežević, A.; Stajić, M.; Sofrenić, I.; Stanojković, T.; Milovanović, I.; Tešević, V.; Vukojević, J. Antioxidative, antifungal, cytotoxic and antineurodegenerative activity of selected Trametes species from Serbia. PLoS ONE 2018, 13, e0203064. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Karanfil, A.; Uren, M.C.; Kocak, M.S.; Sarikurkcu, C.; Gungor, H.; Nancy Picot, C.M.; Mahomoodally, M.F. Phenolic content, antioxidant and enzyme inhibitory capacity of two: Trametes species. RSC Adv. 2016, 6, 73351–73357. [Google Scholar] [CrossRef]
- Begum, H.A. Exploring the pharmacological potential of Trametes hirsuta (White Rot Fungi): Analgesic, anti-Inflammatory, antispasmodic and antimicrobial activities. Pure Appl. Biol. 2023, 12, 1183–1193. [Google Scholar] [CrossRef]
- He, N.; Tian, L.; Zhai, X.; Zhang, X.; Zhao, Y. Composition characterization, antioxidant capacities and anti-proliferative effects of the polysaccharides isolated from Trametes lactinea (Berk.) Pat. Int. J. Biol. Macromol. 2018, 115, 114–123. [Google Scholar] [CrossRef]
- Xie, J.; Lin, D.; Li, J.; Zhou, T.; Lin, S.; Lin, Z. Effects of Ganoderma lucidum polysaccharide peptide ameliorating cyclophosphamide-induced immune dysfunctions based on metabolomics analysis. Front. Nutr. 2023, 10, 1179749. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Alsayegh, A.A.; Ahmad, F.A.; Akhtar, M.S.; Alavudeen, S.S.; Bantun, F.; Wahab, S.; Ahmed, A.; Ali, M.; Elbendary, E.Y.; et al. Ganoderma lucidum: Insight into antimicrobial and antioxidant properties with development of secondary metabolites. Heliyon 2024, 10, e25607. [Google Scholar] [CrossRef]
- Cui, F.J.; Yang, Y.M.; Sun, L.; Zan, X.Y.; Sun, W.J.; Zeb, U. Grifola frondosa polysaccharides: A review on structure/activity, biosynthesis and engineering strategies. Int. J. Biol. Macromol. 2024, 257, 128584. [Google Scholar] [CrossRef]
- Gravina, A.G.; Pellegrino, R.; Auletta, S.; Palladino, G.; Brandimarte, G.; D’Onofrio, R.; Arboretto, G.; Imperio, G.; Ventura, A.; Cipullo, M.; et al. Hericium erinaceus, a medicinal fungus with a centuries-old history: Evidence in gastrointestinal diseases. World J. Gastroenterol. 2023, 29, 3048–3065. [Google Scholar] [CrossRef]
- Sharma, A.; Bhardwaj, G.; Nayik, G.A. Edible and Medicinal Mushrooms of the Himalayas; Taylor & Francis: London, UK, 2023; ISBN 9781032195568. [Google Scholar]
- Pawlik, A.; Jaszek, M.; Stefaniuk, D.; Świderska-Burek, U.; Mazur, A.; Wielbo, J.; Koper, P.; Żebracki, K.; Janusz, G. Combined effect of light and nutrients on the micromorphology of the white rot fungus Cerrena unicolor. Int. J. Mol. Sci. 2020, 21, 1678. [Google Scholar] [CrossRef]
- Bernicchia, A.; Gorjón, S. Polypores of the Mediterranean Region; Romar SRI: Segrate, Italy, 2020. [Google Scholar]
- Michniewicz, A.; Ullrich, R.; Ledakowicz, S.; Hofrichter, M. The white-rot fungus Cerrena unicolor strain 137 produces two laccase isoforms with different physico-chemical and catalytic properties. Appl. Microbiol. Biotechnol. 2006, 69, 682–688. [Google Scholar] [CrossRef]
- Pawlik, A.; Ruminowicz-Stefaniuk, M.; Frac, M.; Mazur, A.; Wielbo, J.; Janusz, G. The wood decay fungus Cerrena unicolor adjusts its metabolism to grow on various types of wood and light conditions. PLoS ONE 2019, 14, e0211744. [Google Scholar] [CrossRef] [PubMed]
- Belova, O.V.; Lisov, A.V.; Vinokurova, N.G.; Kostenevich, A.A.; Sapunova, L.I.; Lobanok, A.G.; Leont’evskiĭ, A.A. [Xylanase and cellulase of fungus Cerrena unicolor VKM F-3196: Production, properties, and applications for the saccharification of plant material]. Prikl. Biokhim. Mikrobiol. 2014, 50, 171–176. [Google Scholar] [CrossRef]
- Pawlik, A.; Ciołek, B.; Sulej, J.; Mazur, A.; Grela, P.; Staszczak, M.; Niścior, M.; Jaszek, M.; Matuszewska, A.; Janusz, G.; et al. Cerrena unicolor Laccases, Genes Expression and Regulation of Activity. Biomolecules 2021, 11, 468. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, K.; Gong, W.; Wang, Q.; Xu, D.; Liu, M.; Bi, K.; Song, Y. Anticancer, antioxidant, and antimicrobial activities of anemone (Anemone cathayensis). Food Sci. Biotechnol. 2012, 21, 551–557. [Google Scholar] [CrossRef]
- Jaszek, M.; Osińska-Jaroszuk, M.; Janusz, G.; Matuszewska, A.; Stefaniuk, D.; Sulej, J.; Polak, J.; Ruminowicz, M.; Grzywnowicz, K.; Jarosz-Wilkołazka, A. New bioactive fungal molecules with high antioxidant and antimicrobial capacity isolated from Cerrena unicolor idiophasic cultures. Biomed Res. Int. 2013, 2013, 497492. [Google Scholar] [CrossRef]
- Zhang, L.B.; Yang, W.W.J.; Qiu, T.T. Genome-wide study of Cerrena unicolor 87613 laccase gene family and their mode prediction in association with substrate oxidation. BMC Genom. 2023, 24, 504. [Google Scholar] [CrossRef] [PubMed]
- Sevindik, M. Antioxidant and antimicrobial activity of Cerrena unicolor. Mycopath 2018, 16, 11–14. [Google Scholar]
- Matuszewska, A.; Jaszek, M.; Stefaniuk, D.; Ciszewski, T.; Matuszewski, Ł. Anticancer, antioxidant, and antibacterial activities of low molecular weight bioactive subfractions isolated from cultures of wood degrading fungus Cerrena unicolor. PLoS ONE 2018, 13, e0197044. [Google Scholar] [CrossRef]
- Matuszewska, A.; Stefaniuk, D.; Jaszek, M.; Pięt, M.; Zając, A.; Matuszewski, Ł.; Cios, I.; Grąz, M.; Paduch, R.; Bancerz, R. Antitumor potential of new low molecular weight antioxidative preparations from the white rot fungus Cerrena unicolor against human colon cancer cells. Sci. Rep. 2019, 9, 1975. [Google Scholar] [CrossRef] [PubMed]
- Pięt, M.; Zając, A.; Paduch, R.; Jaszek, M.; Frant, M.; Stefaniuk, D.; Matuszewska, A.; Grzywnowicz, K. Chemopreventive activity of bioactive fungal fractions isolated from milk-supplemented cultures of Cerrena unicolor and Pycnoporus sanguineus on colon cancer cells. 3 Biotech 2021, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; Dandona, L.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [CrossRef] [PubMed]
- Zając, A.; Pięt, M.; Stefaniuk, D.; Chojnacki, M.; Jakubowicz-Gil, J.; Paduch, R.; Matuszewska, A.; Jaszek, M. Pro-health and anti-cancer activity of fungal fractions isolated from milk-supplemented cultures of lentinus (Pleurotus) sajor-caju. Biomolecules 2021, 11, 1089. [Google Scholar] [CrossRef]
- Joseph, T.P.; Chanda, W.; Padhiar, A.A.; Batool, S.; LiQun, S.; Zhong, M.; Huang, M. A Preclinical Evaluation of the Antitumor Activities of Edible and Medicinal Mushrooms: A Molecular Insight. Integr. Cancer Ther. 2018, 17, 200–209. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Jiang, Y.; Li, X.; He, Y.; Zeng, P.; Guo, Z.; Chang, Y.; Luo, H.; Liu, Y.; et al. Lentinan as an immunotherapeutic for treating lung cancer: A review of 12 years clinical studies in China. J. Cancer Res. Clin. Oncol. 2018, 144, 2177–2186. [Google Scholar] [CrossRef]
- Matuszewska, A.; Karp, M.; Jaszek, M.; Janusz, G.; Osińska-Jaroszuk, M.; Sulej, J.; Stefaniuk, D.; Tomczak, W.; Giannopoulos, K. Laccase purified from Cerrena unicolor exerts antitumor activity against leukemic cells. Oncol. Lett. 2016, 11, 2009–2018. [Google Scholar] [CrossRef]
- Mizerska-Dudka, M.; Jaszek, M.; Błachowicz, A.; Rejczak, T.P.; Matuszewska, A.; Osińska-Jaroszuk, M.; Stefaniuk, D.; Janusz, G.; Sulej, J.; Kandefer-Szerszeń, M. Fungus Cerrena unicolor as an effective source of new antiviral, immunomodulatory, and anticancer compounds. Int. J. Biol. Macromol. 2015, 79, 459–468. [Google Scholar] [CrossRef]
- Prendecka-Wróbel, M.; Pigoń-Zając, D.; Jaszek, M.; Matuszewska, A.; Stefaniuk, D.; Opielak, G.; Piotrowska, K.; Rahnama-Hezavah, M.; Małecka-Massalska, T. Electric Cell-Substrate Impedance Sensing (ECIS) as a Convenient Tool to Assess the Potential of Low Molecular Fraction Derived from Medicinal Fungus Cerrena unicolor in Action on L929 and CT-26 Cell Lines. Molecules 2022, 27, 6251. [Google Scholar] [CrossRef]
- Pigoń-Zając, D.; Derlatka, K.; Chuchmacz, W.; Kulczycka, M.; Grzelczak, K.; Stefaniuk, D.; Matuszewska, A.; Homa-Mlak, I.; Prendecka-Wróbel, M.; Małecka-Massalska, T.; et al. The anticancer activity of laccase from white rot fungus Cerrena unicolor on the example of its action on Caov-3 and NIH:OVCAR-3 ovarian cancer cells. Ann. Agric. Environ. Med. 2024, 32, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Sondej, D.; Pigoń-Zając, D.; Jaszek, M.; Stefaniuk, D.; Matuszewska, A.; Bielak, K.; Opielak, G.; Małecka-Massalska, T.; Rahnama-Hezavah, M.; Prendecka-Wróbel, M. Is laccase from medicinal mushroom Cerrena unicolor cytotoxic to colon cancer cell line CT-26? PLoS ONE 2025, 20, e0322211. [Google Scholar] [CrossRef] [PubMed]
- Chaiharn, M.; Phutdhawong, W.S.; Amornlerdpison, D.; Phutdhawong, W. Antibacterial, antioxidant properties and bioactive compounds of thai cultivated mushroom extracts against food-borne bacterial strains. Chiang Mai J. Sci. 2018, 45, 1713–1727. [Google Scholar]
- Demir, M.S.; Yamac, M. Antimicrobial activities of basidiocarp, submerged mycelium and exopolysaccharide of some native Basidiomycetes strains. J. Appl. Biol. Sci. 2008, 2, 89–93. [Google Scholar]
- Fong, D.; Chan, M.M. Soil-Transmitted Helminth Infections. In Human Parasites; World Scientific Publishing: Singapore, 2022; pp. 502–527. [Google Scholar]
- Ziaja-Sołtys, M.; Kołodziej, P.; Stefaniuk, D.; Matuszewska, A.; Jaszek, M.; Bogucka-Kocka, A. Low-Molecular-Weight Secondary Metabolites from Fungi: Cerrena unicolor as a New Proposal of an Effective Preparation against Rhabditis Nematodes. Molecules 2022, 27, 1660. [Google Scholar] [CrossRef]
- Uyanoglu, M.; Yamac, M.; Canbek, M.; Senturk, H.; Kartkaya, K.; Oglakci, A.; Turgak, O.; Kanbak, G. Curative effect of crude exopolysaccharides of some macrofungi on alcohol-induced liver damage. Ultrastruct. Pathol. 2013, 37, 218–226. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, R.; Ng, T.B.; Lai, Y.; Yang, J.; Ye, X. A New Laccase of Lac 2 from the White Rot Fungus Cerrena unicolor 6884 and Lac 2-Mediated Degradation of Aflatoxin B(1). Toxins 2020, 12, 476. [Google Scholar] [CrossRef]
- Yamaç, M.; Zeytinoglu, M.; Kanbak, G.; Bayramoglu, G.; Senturk, H. Hypoglycemic effect of crude exopolysaccharides produced by Cerrena unicolor, Coprinus comatus, and Lenzites betulina isolates in streptozotocin- induced diabetic rats. Pharm. Biol. 2009, 47, 168–174. [Google Scholar] [CrossRef]
- Deng, X.; Gould, M.; Ali, M.A. A review of current advancements for wound healing: Biomaterial applications and medical devices. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 2542–2573. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Butnariu, M.; Ezzat, S.M.; Adetunji, C.O.; Imran, M.; Sobhani, S.R.; Tufail, T.; Hosseinabadi, T.; Ramírez-Alarcón, K.; Martorell, M.; et al. Mushrooms-Rich Preparations on Wound Healing: From Nutritional to Medicinal Attributes. Front. Pharmacol. 2020, 11, 567518. [Google Scholar] [CrossRef]
- Stefaniuk, D.; Misztal, T.; Pięt, M.; Zając, A.; Kopycińska, M.; Matuszewska, A.; Ruminowicz-Stefaniuk, M.; Matuszewski, Ł.; Marcińczyk, N.; Belcarz, A.; et al. Thromboelastometric analysis of anticancer cerrena unicolor subfractions reveal their potential as fibrin glue drug carrier enhancers. Biomolecules 2021, 11, 1263. [Google Scholar] [CrossRef] [PubMed]
- Berovic, M.; Podgornik, B.B.; Gregori, A. Cultivation Technologies for Production of Medicinal Mushroom Biomass: Review. Int. J. Med. Mushrooms 2022, 24, 1–22. [Google Scholar] [CrossRef] [PubMed]
| Mushroom Species | Bioactive Fraction/Compound | Medicinal Properties | Source |
|---|---|---|---|
| Lentinus edodes | lentinan, lectins, β-(1-3)-glucan, ledodin, and edodin | anticancer, antioxidant, antibacterial, antifungal, cytostatic immunomodulatory activities, and rRNA N-glycosylase activity inhibits protein synthesis and leads to cell death | [6,10,11] |
| Lentinus tigrinus | laccase, n-hexane extracts, ethanolic extracts, and exopolysaccharides | antioxidant, antiviral, antibacterial, and anticancer activities | [13,14,15,16,17,18] |
| Lentinus tuberregium | extracts | therapeutic properties to treat coughs, indigestion, dysentery, and diarrhea | [19] |
| Lentinus squarrosulus | extracts | treat heart problems and mumps and antibacterial activities | [20] |
| Pycnoporus sanguineus | crude dichloromethane–methanol extract and low-molecular weight (ex-LMS) metabolites | antibacterial, antifungal, anti-radical, antiviral, and cytotoxic properties and anti-inflammatory activity | [24,25] |
| Pycnoporus cinnabarinus | cinnabarine | antiviral and antibacterial properties and insecticidal potential | [27,54] |
| Trametes versicolor | extracts | antioxidant, antidiabetic, antibacterial, and anti-inflammatory activities | [28,33,34,35] |
| Trametes robiniophilaMurr | proteoglycan and polysaccharides | therapeutic effect on tuberous sclerosis nephrosis, colitis, and anticancer activities | [36,37,38,39,40,41] |
| Trametes gibbosa | polysaccharides, steroids, and phenolics | anticancer, immunomodulatory, antiviral, anti-inflammatory, antioxidant, antibacterial, and neuroprotective properties | [45,46,47] |
| Trametes hirsuta | extracts | pharmacological, antioxidant, antibacterial, antispasmodic, anti-inflammatory, and antipyretic activities | [48] |
| Trametes lactinea | heteropolysaccharides | antiproliferation and anticancer activities | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pigoń-Zając, D.; Małecka-Massalska, T.; Łapiński, J.; Prendecka-Wróbel, M. Modern Pro-Health Applications of Medicinal Mushrooms: Insights into the Polyporaceae Family, with a Focus on Cerrena unicolor. Molecules 2025, 30, 4089. https://doi.org/10.3390/molecules30204089
Pigoń-Zając D, Małecka-Massalska T, Łapiński J, Prendecka-Wróbel M. Modern Pro-Health Applications of Medicinal Mushrooms: Insights into the Polyporaceae Family, with a Focus on Cerrena unicolor. Molecules. 2025; 30(20):4089. https://doi.org/10.3390/molecules30204089
Chicago/Turabian StylePigoń-Zając, Dominika, Teresa Małecka-Massalska, Jacek Łapiński, and Monika Prendecka-Wróbel. 2025. "Modern Pro-Health Applications of Medicinal Mushrooms: Insights into the Polyporaceae Family, with a Focus on Cerrena unicolor" Molecules 30, no. 20: 4089. https://doi.org/10.3390/molecules30204089
APA StylePigoń-Zając, D., Małecka-Massalska, T., Łapiński, J., & Prendecka-Wróbel, M. (2025). Modern Pro-Health Applications of Medicinal Mushrooms: Insights into the Polyporaceae Family, with a Focus on Cerrena unicolor. Molecules, 30(20), 4089. https://doi.org/10.3390/molecules30204089







