The Effect of Ozone on the Behavior of Systemic and Non-Systemic Pesticides in Cereal Grains
Abstract
1. Introduction
2. Results
2.1. Method Validation Results
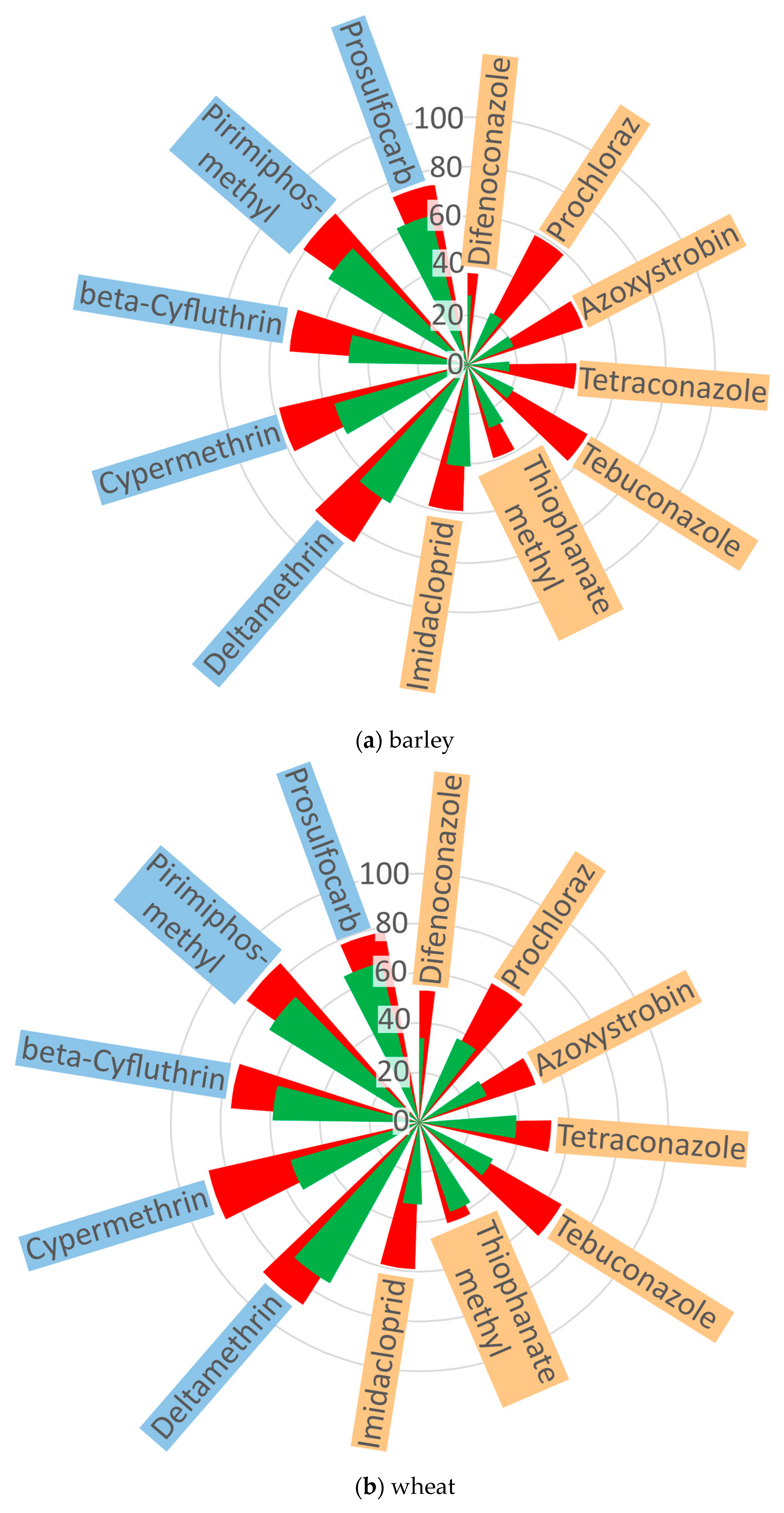
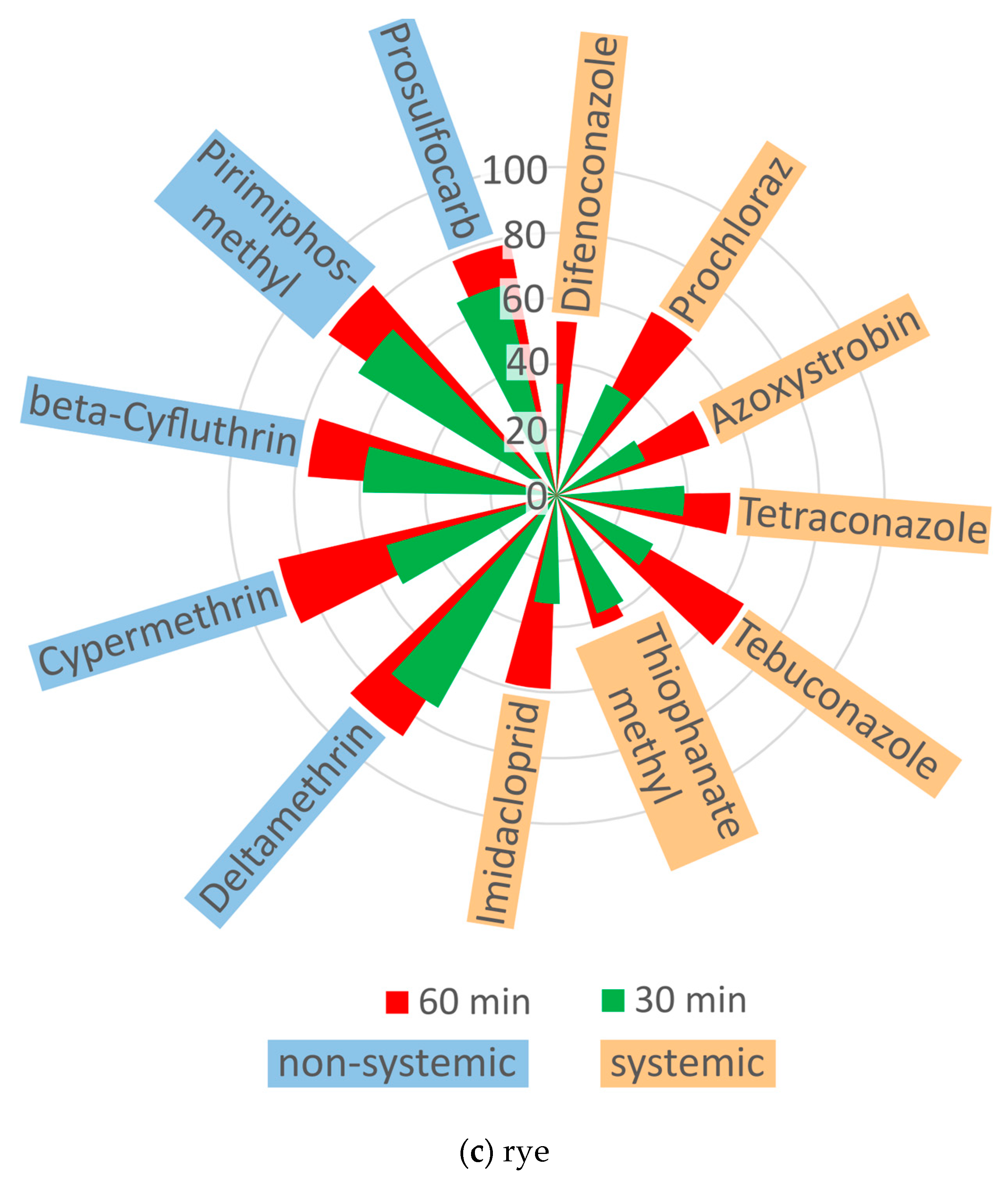
2.2. The Effect of Ozone on the Behavior of Pesticides in Cereal Grains
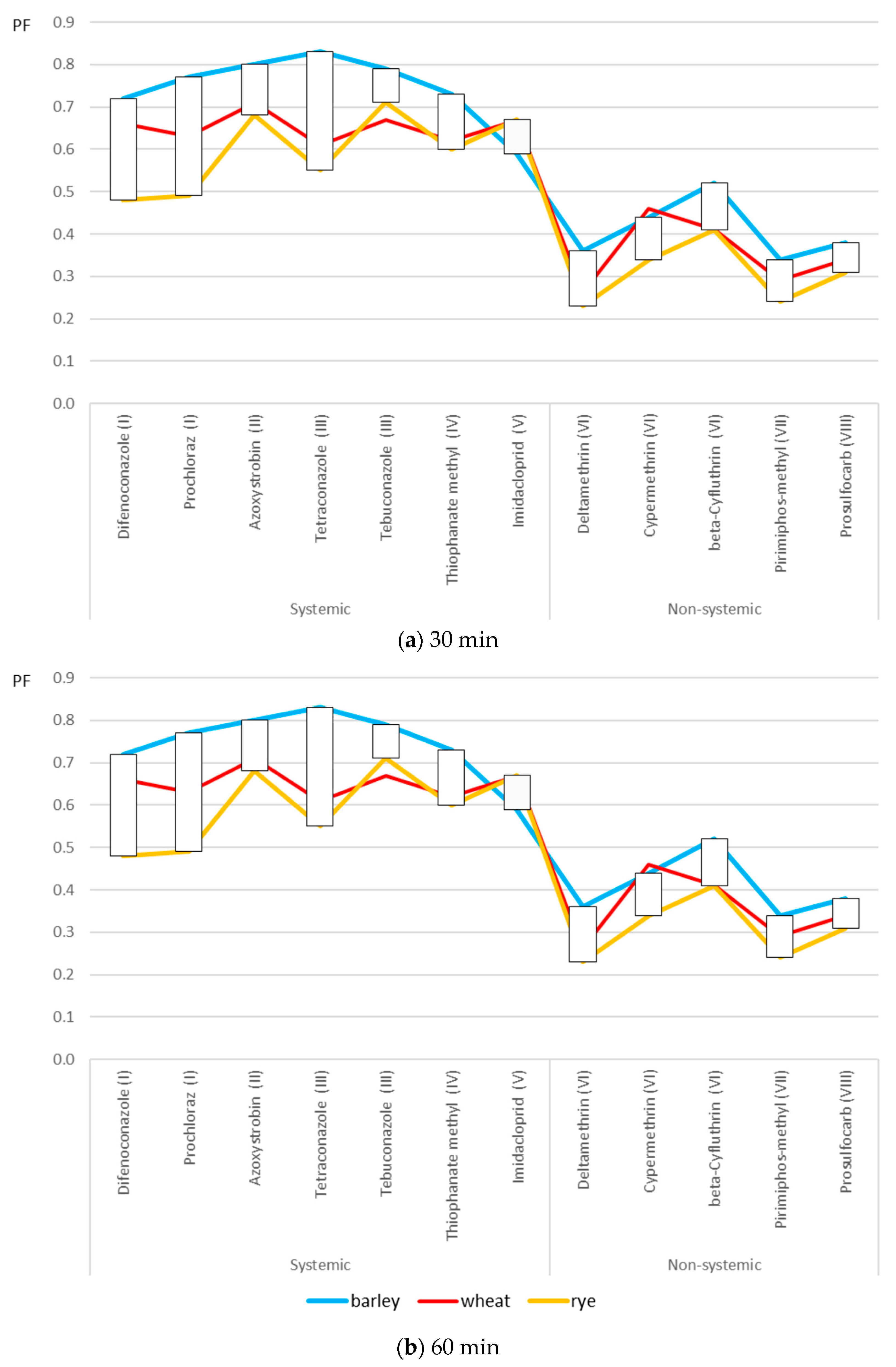
2.3. Processing Factors
3. Discussion
| Pesticide | Group | Chemical Group | Formula | Structure | M (g mol−1) | log P |
|---|---|---|---|---|---|---|
| systemic | ||||||
| Difenoconazole | I | Conazole | C19H17Cl2N3O3 | 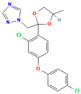 | 406.26 | 4.36 |
| Prochloraz | I | Conazole | C15H16Cl3N3O2 | 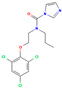 | 376.7 | 3.5 |
| Azoxystrobin | II | Strobilurin | C22H17N3O5 | 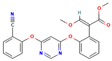 | 403.4 | 2.5 |
| Tetraconazole | III | Triazole | C13H11Cl2F4N3O | 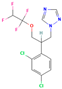 | 372.15 | 3.56 |
| Tebuconazole | III | Triazole | C16H22ClN3O | 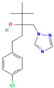 | 307.82 | 3.7 |
| Thiophanate methyl | IV | Carbamate | C12H14N4O4S2 | 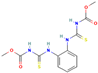 | 342.39 | 1.4 |
| Imidacloprid | V | Neonicotinoid | C9H10ClN5O2 | 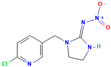 | 255.66 | 0.57 |
| non-systemic | ||||||
| Deltamethrin | VI | Pyrethroid | C22H19Br2NO3 |  | 505.2 | 4.6 |
| Cypermethrin | VI | Pyrethroid | C22H19Cl2NO3 | 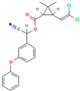 | 416.3 | 5.55 |
| beta-Cyfluthrin | VI | Pyrethroid | C22H18Cl2FNO3 |  | 416.3 | 5.8 |
| Pirimiphos-methyl | VII | Organophosphate | C11H20N3O3PS |  | 305.33 | 4.2 |
| Prosulfocarb | VIII | Thiocarbamate | C14H21NOS |  | 251.39 | 4.48 |
4. Materials and Methods
4.1. Chemicals
4.2. Spiking of Cereal Grain Samples
4.3. Ozonation
4.4. Sample Preparation
4.5. Instrumentation Conditions
4.6. Method Validation
4.7. Data Analysis
4.8. Statistical Analysis
5. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| R | Recovery |
| RSD | Relative standard deviation |
| ME | Matrix effect |
| U | Uncertainty |
| PF | Processing factor |
| TPP | Triphenyl phosphate |
| ISs | Internal standards |
| d-SPE | Dispersive solid-phase extraction |
| MgSO4 | Magnesium Sulfate |
| PSA | Primary Secondary Amine |
| C18 | Octadecylsilane |
| M | Molar mass |
| P | Logarithm of the octanol-water partition coefficient |
| MRM | Multiple Reaction Monitoring |
| Cpc | Concentration of pesticide residues in the processed cereal |
| Crac | Concentration in the raw agricultural commodity |
References
- Vielba-Fernández, A.; Polonio, Á.; Ruiz-Jiménez, L.; de Vicente, A.; Pérez-García, A.; Fernández-Ortuño, D. Fungicide Resistance in Powdery Mildew Fungi. Microorganisms 2020, 8, 1431. [Google Scholar] [CrossRef]
- Iwaniuk, P.; Konecki, R.; Kaczynski, P.; Rysbekova, A.; Lozowicka, B. Influence of seven levels of chemical/biostimulator protection on amino acid profile and yield traits in wheat. Crop J. 2022, 10, 1198–1206. [Google Scholar] [CrossRef]
- Bayarsaikhan, U.; Im, K.S.; Bae, Y.S. Six new recorded species of Lithosiini (Lepidoptera: Erebidae, Arctiinae) in Cambodia. J. Asia Pac. Biodives 2020, 13, 384–390. [Google Scholar] [CrossRef]
- Rubenstein, J.M.; Hulme, P.E.; Rolston, M.P.; Stewart, A.V.; Hampton, J.G. A century of weed change in New Zealand’s forage seed multiplication industry. Neobiota 2023, 85, 167–195. [Google Scholar] [CrossRef]
- Willis, C.E.; Foster, S.P.; Zimmer, C.T.; Elias, J.; Chang, X.; Field, L.M.; Williamson, M.S.; Davies, T.G. Investigating the status of pyrethroid resistance in UK populations of the cabbage stem flea beetle (Psylliodes chrysocephala). Crop Prot. 2020, 138, 105316. [Google Scholar] [CrossRef] [PubMed]
- Łozowicka, B.; Kaczyński, P.; Jankowska, M.; Rutkowska, E.; Iwaniuk, P.; Konecki, R.; Rogowska, W.; Zhagyparova, A.; Absatarova, D.; Łuniewski, S.; et al. Evaluation of Broad-Spectrum Pesticides Based on Unified Multi-Analytical Procedure in Fruits and Vegetables for Acute Health Risk Assessment. Foods 2025, 14, 2528. [Google Scholar] [CrossRef]
- Rutkowska, E.; Kaczyński, P.; Iwaniuk, P.; Łozowicka, B.; Hrynko, I.; Jankowska, M.; Konecki, R.; Rogowska, W.; Rusiłowska, J.; Pietkun, M.; et al. An extensive pesticide residue study in minor polish vegetables based on critical consumer diets. Food Control 2025, 176, 111383. [Google Scholar] [CrossRef]
- Jankowska, M.; Hrynko, I.; Łozowicka, B. Human Health Risk Assessment of Pesticides Residues in Fruit, Vegetable and Cereal Samples from Poland—5-Year Survey. J. Plant Prot. Res. 2022, 62, 385–392. Available online: https://www.plantprotection.pl/Human-health-risk-assessment-of-pesticide-residues-in-fruit-vegetable-and-cereal,156073,0,2.html (accessed on 15 September 2025).
- Ahmad, M.F.; Ahmad, F.A.; Alsayegh, A.A.; Zeyaullah, M.; AlShahrani, A.M.; Muzammil, K.; Saati, A.A.; Wahab, S.; Elbendary, E.Y.; Kambal, N.; et al. Pesticides impacts on human health and the environment with their mechanisms of action and possible countermeasures. Heliyon 2024, 10, e29128. [Google Scholar] [CrossRef]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2021, 283, 124657. [Google Scholar] [CrossRef]
- Perrin, L.; Spinosi, J.; Chaperon, L.; Kab, S.; Moisan, F.; Ebaz, A. Pesticides expenditures by farming type and incidence of Parkinson disease in farmers: A French nationwide study. Environ. Res. 2021, 197, 111161. [Google Scholar] [CrossRef]
- Osman, K.A.; Reda, A.; Tarek, N.; Mohamed, A.; Ismaeil, R.; Adel, N.; Soliman, S.A.; Nabila, S.A.; Salama, A.K.; Salama, M.S. Ozone treatment as a green technology for removing acetamiprid residues from some vegetables and its impact on the quality of vegetables. J. Food Compos. Anal. 2025, 146, 107975. [Google Scholar] [CrossRef]
- Botondi, R.; Barone, M.; Grasso, C. A Review into the Effectiveness of Ozone Technology for Improving the Safety and Preserving the Quality of Fresh-Cut Fruits and Vegetables. Foods 2021, 10, 748. [Google Scholar] [CrossRef]
- Kocsis, G.; Szabó-Bárdos, E.; Fónagy, O.; Farsang, E.; Juzsakova, T.; Jakab, M.; Pekker, P.; Kovács, M.; Horváth, O. Characterization of Various Titanium-Dioxide-Based Catalysts Regarding Photocatalytic Mineralization of Carbamazepine also Combined with Ozonation. Molecules 2022, 27, 8041. [Google Scholar] [CrossRef]
- Epelle, E.I.; Macfarlane, A.; Cusack, M.; Burns, A.; Okolie, J.A.; Mackay, W.; Rateb, M.; Yaseen, M. Ozone application in different industries: A review of recent developments. Chem. Eng. J. 2023, 454, 140188. [Google Scholar] [CrossRef]
- Lemic, D.; Galešić, M.A.; Bjeliš, M.; Viric Gasparic, H. Ozone Treatment as a Sustainable Alternative for Suppressing Blue Mold in Mandarins and Extending Shelf Life. Agriculture 2024, 14, 1196. [Google Scholar] [CrossRef]
- Chamnan, S.; Varith, J.; Jaturonglumlert, S.; Phimphimol, J.; Sujinda, N. Effects of High Concentration Ozone Gas Fumigation on the Quality and Shelf-life of Longan Fruit. Ozone Sci. Eng. 2021, 44, 105–116. [Google Scholar] [CrossRef]
- de Souza, L.P.; Faroni, L.R.D.A.; Heleno, F.F.; Pinto, F.G.; de Queiroz, M.E.L.R.; Prates, L.H.F. Ozone treatment for pesticide removal from carrots: Optimization by response surface methodology. Food Chem. 2018, 243, 435–441. [Google Scholar] [CrossRef]
- Bahchevnikov, O.; Braginets, A.V. Ozone in Grain Storage and Processing: Review. Food Process. Tech. Technol. 2024, 54, 483–494. [Google Scholar] [CrossRef]
- Mishra, G.; Palle, A.A.; Srivastava, S.; Mishra, H.N. Disinfestation of stored wheat grain infested with Rhyzopertha dominica by ozone treatment: Process optimization and impact on grain properties. J. Sci. Food Agric. 2019, 99, 5008–5018. [Google Scholar] [CrossRef] [PubMed]
- Rozas, O.; Baeza, C.; Núñez, K.; Rossner, A.; Urrutia, R.; Mansilla, H.D. Organic micropollutants (OMPs) oxidation by ozone: Effect of activated carbon on toxicity abatement. Sci. Total Environ. 2017, 590, 430–439. [Google Scholar] [CrossRef]
- Sivaranjani, S.; Prasath, V.A.; Pandiselvam, R.; Kothakota, A.; Khaneghah, M.A. Recent advances in applications of ozone in the cereal industry. LWT 2021, 146, 111412. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Wang, T.; Li, C.; Wu, Z. Effects of ozone treatment on pesticide residues in food: A review. Int. J. Food Sci. Technol. 2019, 54, 301–312. [Google Scholar] [CrossRef]
- Horvitz, S.; Cantalejo, M.J. Application of ozone for the postharvest treatment of fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2014, 54, 312–339. [Google Scholar] [CrossRef]
- Kusvuran, E.; Yildirim, D.; Mavruk, F.; Ceyhan, M. Removal of chlorpyrifos ethyl, tetradifon and chlorothalonil pesticide residues from citrus by using ozone. J. Hazard. Mater. 2012, 241–242, 287–300. [Google Scholar] [CrossRef]
- Özen, T.; Koyuncu, M.A.; Erbaş, D. Effect of ozone treatments on the removal of pesticide residues and postharvest quality in green pepper. J. Food Sci. Technol. 2021, 58, 2186–2196. [Google Scholar] [CrossRef]
- Ikeura, H.; Kobayashi, F.; Tamaki, M. Removal of residual pesticide, fenitrothion, in vegetables by using ozone microbubbles generated by different methods. J. Food Eng. 2011, 103, 345–349. [Google Scholar] [CrossRef]
- Al-Antary, T.M.; Shaderma, A.M.; Al-Dabbas, M.B. Effect of ozonation treatment on spiked chlorfenapyr pesticide on lettuce. Feb-Fresenius Environ. Bull. 2019, 28, 370–375. [Google Scholar]
- Al-Antary, T.M.; Shaderma, A.M.; Al-Dabbas, M.B. Ozonation treatment effect on spiked chlorfenapyr pesticide on tomato fruits. Feb-Fresenius Environ. Bull. 2018, 27, 7822–7826. [Google Scholar]
- Al-Dabbas, M.M.; Shaderma, A.A.; Al-Antary, T.M.; Ghazzawi, H.A.; Hamad, H.J. Effect of ozonation on cypermethrin and chlorpyrifos pesticides residues degradation in tomato fruits. Feb-Fresenius Environ. Bull. 2018, 27, 6628–6633. [Google Scholar]
- Antos, P.; Piechowicz, B.; Gorzelany, J.; Matłok, N.; Migut, D.; Józefczyk, R.; Balawejder, M. Effect of ozone on fruit quality and fungicide residue degradation in apples during cold storage. Ozone Sci. Eng. 2018, 40, 482–486. [Google Scholar] [CrossRef]
- Sadło, S.; Szpyrka, E.; Piechowicz, B.; Antos, P.; Józefczyk, R.; Balawejder, M. Reduction of captan, boscalid and pyraclostrobin residues on apples using water only, gaseous ozone, and ozone aqueous solution. Ozone Sci. Eng. 2017, 39, 97–103. [Google Scholar] [CrossRef]
- Heleno, F.F.; de Queiroz, M.E.; Neves, A.A.; Faroni, L.R.; Sousa, F.A.D.; Oliveira, A.F.D. Ozone treatment for the removal of residual chlorothalonil and effects on the quality of table grapes. J. Braz. Chem. Soc. 2015, 26, 687–694. [Google Scholar] [CrossRef]
- Gabler, F.M.; Smilanick, J.L.; Mansour, M.F.; Karaca, H. Influence of fumigation with high concentrations of ozone gas on postharvest gray mold and fungicide residues on table grapes. Postharvest Biol. Technol. 2010, 55, 85–90. [Google Scholar] [CrossRef]
- Amjad, A.; Sohaib, M.; Suleman, R.; Javed, M.S.; Ahmad, S.; Amjad, M.R.; Shah, M.; Ali, S.A.; Nawaz, N. Dissipation of selected pesticide residues in grapes by using ozone enriched atmosphere. J. Saudi Soc. Agric. Sci. 2024, in press. [CrossRef]
- Łozowicka, B.; Jankowska, M.; Hrynko, I.; Kaczynski, P. Removal of 16 pesticide residues from strawberries by washing with tap and ozone water, ultrasonic cleaning and boiling. Environ. Monit. Assess. 2016, 188, 51. [Google Scholar] [CrossRef]
- Antos, P.; Kurdziel, A.; Sadło, S.; Balawejder, M. Preliminary study on the use of ozonation for the degradation of dithiocarbamate residues in the fruit drying process: Mancozeb residue in blackcurrant is the example used. J. Plant Prot. 2013, 53, 48–52. [Google Scholar] [CrossRef]
- Freitas, R.S.; Faroni, L.R.D.A.; de Queiroz, M.E.L.R.; Heleno, F.F.; Prates, L.H.F. Degradation kinetics of pirimiphos-methyl residues in maize grains exposed to ozone gas. J. Stored Prod. Res. 2017, 74, 1–5. [Google Scholar] [CrossRef]
- Hrynko, I.; Kaczyński, P.; Iwaniuk, P.; Konecki, R.; Jankowska, M.; Rutkowska, E.; Borusiewicz, A.; Toyzhigitova, B.; Łozowicka, B. The effect of temperature and microwave radiation on the behavior of pesticides during the drying of cereal grains. Food Control 2025, 76, 111399. [Google Scholar] [CrossRef]
- Cruz-Alcalde, A.; Sans, C.; Esplugas, S. Priority pesticide dichlorvos removal from water by ozonation process: Reactivity, transformation products and associated toxicity. Sep. Purif. Technol. 2018, 192, 123–129. [Google Scholar] [CrossRef]
- Nazari, M.; Sabahi, M.; Salehipour, A.; Ahmadi, S.A.; Kazemi, A.; Razipour, S.; Faraji, N.; Komaki, A. Effects of cypermethrin exposure on learning and memory functions and anxiety-like behavior in rats. BMC Pharmacol. Toxicol. 2025, 26, 12. [Google Scholar] [CrossRef]
- Savi, G.D.; Piacentini, K.C.; Scussel, V.M. Reduction in residues of deltamethrin and fenitrothion on stored wheat grains by ozone gas. J. Stored Prod. Res. 2015, 61, 65–69. [Google Scholar] [CrossRef]
- de Ávila, M.B.R.; Faroni, L.R.A.; Heleno, F.F.; de Queiroz, M.E.L.R.; Costa, L.P. Ozone as degradation agent of pesticide residues in stored rice grains. J. Food Sci. Technol. 2017, 54, 4092–4099. [Google Scholar] [CrossRef] [PubMed]
- Kavallieratos, N.G.; Michail, E.J.; Boukouvala, M.C.; Nika, E.P.; Skourti, A. Efficacy of pirimiphos-methyl, deltamethrin, spinosad and silicoSec against adults and larvae of Tenebrio molitor L. on wheat, barley and maize. J. Stored Prod. Res. 2019, 83, 161–167. [Google Scholar] [CrossRef]
- Kaushal, J.; Khatri, M.; Arya, S.K. A treatise on Organophosphate pesticide pollution: Current strategies and advancements in their environmental degradation and elimination. Ecotoxicol. Environ. Saf. 2021, 207, 111483. [Google Scholar] [CrossRef]
- Savi, G.D.; Piacentini, K.C.; Bortolotto, T.; Scussel, V.M. Degradation of bifenthrin and pirimiphos-methyl residues in stored wheat grains (Triticum aestivum L.) by ozonation. Food Chem. 2016, 203, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Sintuya, P.; Narkprasom, K.; Jaturonglumlert, S.; Whangchai, N.; Peng-Ont, D.; Varith, J. Effect of gaseous ozone fumigation on organophosphate pesticide degradation of dried chilies. Ozone Sci. Eng. 2018, 40, 473–481. [Google Scholar] [CrossRef]
- Matin, M.M.; Matin, P.; Rahman, M.R.; Hadda, T.B.; Almalki, F.A.; Mahmud, S.; Ghoneim, M.M.; Alruwaily, M.; Alshehri, S. Triazoles and Their Derivatives: Chemistry, Synthesis, and Therapeutic Applications. Front. Mol. Biosci. 2022, 9, 864286. [Google Scholar] [CrossRef]
- Rokbani, O.; Fattouch, S.; Chakir, A.; Roth, E. Heterogeneous oxidation of two triazole pesticides (diniconazole and tebuconazole) by OH-radicals and ozone. Sci. Total Environ. 2019, 694, 133745. [Google Scholar] [CrossRef]
- Dagnew, M.; Xue, Q.; Zhang, J.; Wang, Z.; Zhou, A.; Li, M.; Zhao, C. A Review of Various Advanced Oxidation Techniques for Pesticide Degradation for Practical Application in Aqueous Environments. Sustainability 2025, 17, 4710. [Google Scholar] [CrossRef]
- Rodrigues, A.A.Z.; de Queiroz, M.E.L.R.; Neves, A.A.; de Oliveira, A.F.; Prates, L.H.F.; de Freitas, J.F.; Heleno, F.F.; D’Antonino Faroni, L.R. Use of ozone and detergent for removal of pesticides and improving storage quality of tomato. Food Res. Int. 2019, 125, 108626. [Google Scholar] [CrossRef]
- Chen, J.Y.; Lin, Y.J.; Kuo, W.C. Pesticide residue removal from vegetables by ozonation. J. Food Eng. 2013, 114, 404–411. [Google Scholar] [CrossRef]
- PPDB. The FOOTPRINT Pesticide Properties Database; Pesticide Properties DataBase; University of Hertfordshire: Hatfield, UK, 2025; Available online: https://sitem.herts.ac.uk/aeru/ppdb/ (accessed on 4 August 2025).
- Hrynko, I.; Kaczyński, P.; Wołejko, E.; Łozowicka, B. Impact of technological processes on tebuconazole reduction in selected cereal species and the primary cereal product, and dietary exposure assessment. Food Chem. 2023, 422, 136249. [Google Scholar] [CrossRef]
- Kaczyński, P.; Iwaniuk, P.; Hrynko, I.; Łuniewski, S.; Łozowicka, B. The effect of the multi-stage process of wheat beer brewing on the behavior of pesticides according to their physicochemical properties. Food Control 2024, 160, 110356. [Google Scholar] [CrossRef]
- SANTE 11312/2021. Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed. 2021. Available online: https://www.eurl-pesticides.eu/docs/public/tmplt_article.asp?CntID=727 (accessed on 4 August 2025).
- Hrynko, I.; Kaczyński, P.; Pietruszyńska, M.; Łozowicka, B. The effect of food thermal processes on the residue concentration of systemic and non-systemic pesticides in apples. Food Control 2023, 143, 109267. [Google Scholar] [CrossRef]
- Jankowska, M.; Łozowicka, B.; Kaczyński, P. Comprehensive toxicological study over 160 processing factors of pesticides in selected fruit and vegetables after water, mechanical and thermal processing treatments and their application to human health risk assessment. Sci. Total Environ. 2019, 652, 1156–1167. [Google Scholar] [CrossRef] [PubMed]

| Compound | Wheat | Rye | Barley | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R, % (RSD, %) | ME, % | U, % | R, % (RSD, %) | ME, % | U, % | R, % (RSD, %) | ME, % | U, % | ||
| systemic | ||||||||||
| Difenoconazole | 0.001 | 113 (4) | −2 | 13 | 98 (8) | 12 | 4 | 86 (12) | 7 | 6 |
| 0.010 | 86 (5) | −5 | 12 | 78 (8) | −11 | 9 | 75 (7) | −7 | 7 | |
| 0.100 | 73 (7) | 8 | 7 | 100 (7) | −5 | 8 | 94 (2) | 8 | 6 | |
| Prochloraz | 0.001 | 75 (2) | 5 | 8 | 81 (3) | 5 | 9 | 88 (5) | 16 | 10 |
| 0.010 | 85 (7) | 4 | 11 | 83 (8) | −2 | 10 | 92 (4) | −8 | 11 | |
| 0.100 | 73 (6) | 4 | 16 | 78 (5) | 10 | 17 | 70 (12) | 15 | 10 | |
| Azoxystrobin | 0.001 | 105 (6) | 2 | 10 | 86 (9) | 5 | 10 | 88 (2) | −10 | 12 |
| 0.010 | 98 (8) | 5 | 15 | 96 (5) | 7 | 12 | 89 (7) | 9 | 14 | |
| 0.100 | 78 (5) | 8 | 8 | 105 (2) | 10 | 6 | 78 (12) | 4 | 10 | |
| Tetraconazole | 0.001 | 83 (7) | 8 | 11 | 82 (4) | 7 | 7 | 78 (10) | 10 | 8 |
| 0.010 | 82 (7) | 10 | 12 | 86 (5) | 12 | 18 | 78 (8) | 13 | 14 | |
| 0.100 | 112 (4) | 4 | 7 | 105 (7) | −8 | 13 | 110 (15) | −7 | 9 | |
| Tebuconazole | 0.001 | 74 (5) | 6 | 5 | 115 (6) | 8 | 12 | 77 (10) | −5 | 8 |
| 0.010 | 78 (7) | 8 | 4 | 90 (7) | 6 | 8 | 85 (5) | 7 | 6 | |
| 0.100 | 91 (3) | 8 | 4 | 94 (4) | 7 | 9 | 98 (6) | −2 | 9 | |
| Thiophanate methyl | 0.001 | 78 (4) | 15 | 11 | 85 (5) | −5 | 11 | 100 (3) | 8 | 8 |
| 0.010 | 71 (8) | −6 | 13 | 83 (7) | −7 | 17 | 76 (6) | −10 | 10 | |
| 0.100 | 98 (2) | 5 | 10 | 92 (12) | 8 | 6 | 99 (4) | −9 | 12 | |
| Imidacloprid | 0.001 | 115 (2) | −3 | 14 | 95 (5) | 11 | 5 | 118 (12) | 6 | 8 |
| 0.010 | 87 (5) | −7 | 10 | 88 (8) | −12 | 9 | 75 (7) | −7 | 9 | |
| 0.100 | 75 (4) | 2 | 8 | 104 (3) | −4 | 7 | 99 (3) | 8 | 6 | |
| non-systemic | ||||||||||
| Deltamethrin | 0.001 | 78 (8) | 4 | 8 | 116 (6) | 8 | 8 | 88 (10) | −5 | 8 |
| 0.010 | 88 (7) | 6 | 9 | 92 (7) | 4 | 11 | 86 (9) | 10 | 8 | |
| 0.100 | 92 (5) | 10 | 6 | 102 (4) | 10 | 9 | 98 (6) | −6 | 10 | |
| Cypermethrin | 0.001 | 89 (7) | 10 | 12 | 82 (5) | −8 | 11 | 105 (4) | 19 | 6 |
| 0.010 | 45 (8) | −3 | 10 | 78 (7) | −3 | 12 | 116 (6) | −10 | 8 | |
| 0.100 | 96 (7) | 2 | 8 | 86 (12) | 5 | 6 | 97 (2) | −9 | 5 | |
| beta-Cyfluthrin | 0.001 | 77 (4) | 5 | 11 | 85 (5) | −5 | 11 | 100 (3) | 8 | 8 |
| 0.010 | 71 (8) | −6 | 13 | 83 (7) | −7 | 17 | 76 (6) | −10 | 10 | |
| 0.100 | 98 (2) | 5 | 10 | 92 (12) | 8 | 6 | 99 (4) | −9 | 12 | |
| Pirimiphos-methyl | 0.001 | 100 (7) | 6 | 14 | 112 (6) | 10 | 12 | 104 (8) | 12 | 14 |
| 0.010 | 76 (11) | 11 | 16 | 79 (10) | 9 | 14 | 71 (13) | 17 | 18 | |
| 0.100 | 73 (5) | 9 | 10 | 74 (5) | 4 | 10 | 85 (13) | 8 | 5 | |
| Prosulfocarb | 0.001 | 109 (5) | −5 | 12 | 99 (5) | 8 | 3 | 88 (10) | −5 | 6 |
| 0.010 | 75 (2) | −7 | 12 | 79 (9) | −11 | 8 | 75 (7) | 6 | 8 | |
| 0.100 | 72 (4) | 8 | 5 | 102 (5) | −4 | 7 | 94 (2) | 9 | 13 | |
| Pesticide | Group | Ozone Treatment | Concentration (mg kg−1) | Means ± SD (mg kg−1) | ||
|---|---|---|---|---|---|---|
| Raw | 30 min | 60 min | ||||
| systemic | ||||||
| Difenoconazole | I | barley | 0.7148 | 0.2977 | 0.2632 | 0.2805 ± 0.024 bc |
| wheat | 0.5252 | 0.3484 | 0.2451 | 0.2968 ± 0.073 b | ||
| rye | 0.4256 | 0.2022 | 0.1662 | 0.1842 ± 0.025 g | ||
| Prochloraz | I | barley | 0.5492 | 0.2532 | 0.1235 | 0.1884 ± 0.092 g |
| wheat | 0.5776 | 0.2344 | 0.1164 | 0.1754 ± 0.083 g | ||
| rye | 0.5792 | 0.1962 | 0.0841 | 0.1402 ± 0.079 h | ||
| Azoxystrobin | II | barley | 0.3224 | 0.2576 | 0.1651 | 0.2114 ± 0.065 e |
| wheat | 0.4480 | 0.3199 | 0.2264 | 0.2732 ± 0.066 c | ||
| rye | 0.4320 | 0.2935 | 0.1741 | 0.2338 ± 0.084 d | ||
| Tetraconazole | III | barley | 0.4412 | 0.3662 | 0.2465 | 0.3064 ± 0.085 b |
| wheat | 0.5278 | 0.3232 | 0.2502 | 0.2867 ± 0.052 bc | ||
| rye | 0.4032 | 0.2221 | 0.1673 | 0.1947 ± 0.039 fg | ||
| Tebuconazole | III | barley | 0.4012 | 0.3165 | 0.1765 | 0.2465 ± 0.099 d |
| wheat | 0.4776 | 0.3183 | 0.1621 | 0.2402 ± 0.110 d | ||
| rye | 0.3692 | 0.2588 | 0.1115 | 0.1852 ± 0.104 g | ||
| Thiophanate methyl | IV | barley | 0.6216 | 0.4519 | 0.3812 | 0.4166 ± 0.050 a |
| wheat | 0.4608 | 0.2851 | 0.2656 | 0.2754 ± 0.014 c | ||
| rye | 0.4212 | 0.2515 | 0.1971 | 0.2243 ± 0.038 e | ||
| Imidacloprid | V | barley | 0.1996 | 0.1178 | 0.0821 | 0.1000 ± 0.025 j |
| wheat | 0.1888 | 0.1256 | 0.0966 | 0.1111 ± 0.021 i | ||
| rye | 0.2044 | 0.1365 | 0.0378 | 0.0872 ± 0.070 k | ||
| non-systemic | ||||||
| Deltamethrin | VI | barley | 0.1864 | 0.0668 | 0.0281 | 0.0475 ± 0.027 m |
| wheat | 0.3072 | 0.0807 | 0.405 | 0.2429 ± 0.229 d | ||
| rye | 0.4236 | 0.0955 | 0.0232 | 0.0594 ± 0.051 m | ||
| Cypermethrin | VI | barley | 0.2888 | 0.1066 | 0.0644 | 0.0855 ± 0.030 kl |
| wheat | 0.9656 | 0.4362 | 0.1261 | 0.2812 ± 0.219 bc | ||
| rye | 0.8312 | 0.2826 | 0.1252 | 0.2039 ± 0.111 e | ||
| beta-Cyfluthrin | VI | barley | 0.2928 | 0.1523 | 0.0821 | 0.1172 ± 0.050 i |
| wheat | 0.6407 | 0.2615 | 0.1561 | 0.2088 ± 0.075 ef | ||
| rye | 0.6836 | 0.2782 | 0.1577 | 0.2180 ± 0.085 e | ||
| Pirimiphos-methyl | VII | barley | 0.3708 | 0.1255 | 0.0723 | 0.0989 ± 0.038 k |
| wheat | 0.3624 | 0.1038 | 0.0556 | 0.0797 ± 0.034 l | ||
| rye | 0.3258 | 0.0865 | 0.0186 | 0.0526 ± 0.048 m | ||
| Prosulfocarb | VIII | barley | 0.2464 | 0.1531 | 0.0931 | 0.1231 ± 0.042 i |
| wheat | 0.3318 | 0.2044 | 0.1141 | 0.1593 ± 0.064 h | ||
| rye | 0.2488 | 0.1228 | 0.0782 | 0.1005 ± 0.032 j | ||
| Instrument | Eksigent Ultra LC-100 with MS/MS 6500 QTRAP | |||
|---|---|---|---|---|
| Column | KINETEX C18 column (2.6 μm, 2.1 × 100 mm) | |||
| Mobile phase | A: water with 0.2% formic acid and 5 mM ammonium formate B: methanol with 0.2% formic acid and 5 mM ammonium formate | |||
| Gradient table | Time (min) | A (%) | B (%) | |
| 0.0 | 99 | 1 | ||
| 0.5 | 99 | 1 | ||
| 5.0 | 10 | 90 | ||
| 7.0 | 10 | 90 | ||
| 8.0 | 99 | 1 | ||
| 10.0 | 99 | 1 | ||
| Flow rate | 0.5 mL min−1 | |||
| Column temp. | 40 °C | |||
| Injection volume | 10 μL | |||
| MRM condition | MRM Pair | MRM 1 (quantitative) | MRM 2 (qualitative) | Retention time (min) |
| Difenoconazole | 406 > 251 | 406 > 188 | 5.80 | |
| Prochloraz | 376 > 307.9 | 376 > 70 | 5.40 | |
| Azoxystrobin | 404.1 > 371.9 | 404.1 > 344 | 5.05 | |
| Tetraconazole | 372 > 159 | 372 > 70 | 5.35 | |
| Tebuconazole | 308.1 > 70 | 308.1 > 125.1 | 5.55 | |
| Thiophanate methyl | 343 > 151 | 343 > 192 | 4.20 | |
| Imidacloprid | 256 > 209.1 | 256 > 175.1 | 3.20 | |
| Deltamethrin * | 525 > 507.8 | 522.9 > 280.8 | 6.80; 6.90 | |
| Cypermethrin * | 433.2 > 191 | 433.2 > 416.1 | 6.70; 6.75; 6.90 | |
| beta-Cyfluthrin * | 451 > 206 | 451 > 191 | 6,65 | |
| Pirimiphos-methyl | 306.9 > 164.1 | 306.9 > 108 | 5.05; 5.50 | |
| Prosulfocarb | 252.1 > 91; 252.1 | 252.1 > 128.1 | 5.95 | |
| Ionization mode | ESI Positive | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hrynko, I. The Effect of Ozone on the Behavior of Systemic and Non-Systemic Pesticides in Cereal Grains. Molecules 2025, 30, 4087. https://doi.org/10.3390/molecules30204087
Hrynko I. The Effect of Ozone on the Behavior of Systemic and Non-Systemic Pesticides in Cereal Grains. Molecules. 2025; 30(20):4087. https://doi.org/10.3390/molecules30204087
Chicago/Turabian StyleHrynko, Izabela. 2025. "The Effect of Ozone on the Behavior of Systemic and Non-Systemic Pesticides in Cereal Grains" Molecules 30, no. 20: 4087. https://doi.org/10.3390/molecules30204087
APA StyleHrynko, I. (2025). The Effect of Ozone on the Behavior of Systemic and Non-Systemic Pesticides in Cereal Grains. Molecules, 30(20), 4087. https://doi.org/10.3390/molecules30204087






