Abstract
Serpentinite rocks and their processing waste represent a valuable source of magnesium and silicon; however, their complex composition complicates the efficient recovery of individual components. This study investigates the combined acid–alkali processing of serpentinite waste from the Zhitikara deposit (Kazakhstan). In the acid leaching stage, sulfuric acid enables magnesium extraction, while subsequent treatment with sodium hydroxide (NaOH) facilitates the selective recovery of silica gel formed during acid attack. At the final neutralization step, amorphous silica is precipitated with a yield exceeding 60% of its initial content. The obtained silica was characterized using FTIR spectroscopy, X-ray diffraction (XRD), scanning electron microscopy with energy-dispersive spectroscopy (SEM-EDS), inductively coupled plasma mass spectrometry (ICP-MS, Thermo iCAP-Q), and nitrogen adsorption measurements via the BET method. It was established that the synthesized silica gel, according to the IUPAC classification, belongs to mesoporous materials, possesses a well-developed specific surface area (400 m2·g−1), and is suitable for adsorption and catalytic applications.
1. Introduction
Serpentinite rocks and their processing waste represent a valuable source of magnesium (up to 43 wt.% MgO) and silica (up to 45 wt.% SiO2). They have long attracted the attention of researchers as a potential raw material for the production of industrially important magnesium- and silica-based compounds. However, despite extensive research into the integrated processing of serpentinite, its practical implementation remains limited. This is primarily due to both technological and economic constraints that hinder the development and application of efficient recovery methods.
From a technological perspective, the challenge lies in the structural and chemical complexity of serpentinite. These rocks are mainly composed of minerals from the serpentine group—such as chrysotile, antigorite, and lizardite—with the general formula Mg3Si2O5(OH)4 or [Mg(OH)2(MgOH)2Si2O5]. While the magnesium-bearing phases are readily soluble in acids, they are unreactive in alkaline media. Consequently, most research has focused on acid-based leaching methods [1,2,3,4,5,6]. However, although these methods offer certain advantages, they also present serious limitations from both technological and environmental standpoints.
One of the major issues associated with acid leaching (e.g., using H2SO4, HCl, or HNO3) is the formation of insoluble polysilicic acids [7], which convert into hydrated silica gel (SiO2·nH2O) during the leaching process. This gel layer forms on the surface of the particles, impeding further dissolution, complicating solid–liquid separation, and reducing the extraction efficiency of magnesium. In particular, it severely affects filtration and the removal of metal impurities from the pregnant solutions. Extracting high amounts of magnesium from acid-resistant serpentinite (80–90%) generally requires intensified conditions—namely, leaching for ≥1 h at 70–90 °C in concentrated mineral acids (H2SO4, HCl, HNO3) [2,8]. Various technologies have been proposed to optimize the acid leaching of magnesium, aimed at improving process conditions as well as enhancing the technological and economic performance of acid-based methods. In particular, the amount of aggressive reagent consumed during leaching can be reduced if serpentinites are subjected to preliminary thermal treatment [9]. This approach to acid processing facilitates the dissolution of serpentine, ensuring higher yields of magnesium compounds within shorter leaching times under mildly acidic conditions. Thermal activation of serpentinites also makes it possible to reveal structural features inherent to the silicate layers of their polymeric structure, as well as the characteristics of their thermal decomposition accompanied by the formation of a certain amount of free MgO phases in the system [10]. However, concerns have been raised that the beneficial effects of thermal activation are likely to be offset by increased production and energy costs of magnesium products derived from serpentinite. In order to overcome these shortcomings, the use of low concentrations of acids (up to 30–40% of the stoichiometric amount of H2SO4 required) has been proposed, under which the amount of SiO2·nH2O gel formed is insufficient to block the surface of serpentinite particles. This method makes it possible to achieve a high acid utilization coefficient (up to 98–100%), while the degree of magnesium extraction amounts to 30–40% [11]. The technological and economic aspects of developing processing technologies for serpentinites and the large-tonnage chrysotile waste accumulated worldwide remain highly relevant. Therefore, further research on serpentine dissolution is required to develop alternative approaches to serpentinite processing that can address the existing technological, economic, and environmental challenges. In the present study, another approach to acid processing of serpentinite was applied, combining acid–alkali leaching for the simultaneous recovery of magnesium and silicon. The development of this method makes it possible to minimize the above-mentioned problems. Reports on such processes in the literature are scarce and appear only sporadically. Matus et al. (2020) [12] demonstrated the effective extraction of magnesium from olivine using sequential leaching. Ulum et al. (2023) [13] developed an optimized process for obtaining high-purity silica from slags through alkaline precipitation. Other studies have shown the potential for using serpentine-based residues to produce functional materials such as adsorbents and construction additives [14]. One of the features of obtaining amorphous silica from layered magnesium silicates is that silica produced through acid treatment retains the morphology of the original minerals [15]. Typical values of the specific surface area of silica obtained from layer silicates are as follows: from talc, SBET ≈ 130 m2·g−1 [16]; from olivine/serpentinites, SBET up to 1000 m2·g−1 [17,18]; and from serpentinite, SBET up to 50–400 m2·g1 [19]. Through multistage purification of serpentinite, high-purity SiO2 (>99%) can be obtained while preserving the microstructure. The adsorption properties of amorphous silica derived from layered magnesium silicates largely depend on the morphology of the serpentinite raw material (from different deposits). The morphology of the resulting silica is also influenced by the mechanism of gel formation and evolution under acid treatment (removal of magnesium → release of H4SiO4, and/or formation of silica-rich layers). Acids ([H+]) simultaneously accelerate the dissolution of magnesium and modify the rates of polymerization and flocculation of silica. In some cases, the reaction rate at the initial stage approximates first-order kinetics with respect to [H2SO4]. Consequently, the rate constant increases with increasing [H+]. In turn, the rate of polycondensation of monosilicic acid is also a function of the pH of the medium. Thus, under different acid treatment conditions, various types of amorphous silica with different pore structures and parameters may be formed, which define its functional applicability in diverse fields. Therefore, it is necessary in each case to investigate their adsorption properties in order to identify potential areas of application. The search for new approaches and alternative pathways for the utilization of serpentinite to obtain valuable products is highly relevant for Kazakhstan, which possesses significant reserves of serpentinite rocks and large volumes of chrysotile asbestos waste. In the context of increasing interest in technologies for processing industrial waste, this approach may serve as a basis for the production of useful magnesium- and silica-based products from serpentinite tailings in Kazakhstan and in other countries rich in chrysotile raw materials.
The aim of this study is to investigate the processes of combined acid–alkali treatment of serpentinite waste from the Zhitikara deposit (Kazakhstan). Physicochemical analysis methods (XRD, FTIR spectroscopy, BET adsorption) were employed to study the mechanisms of serpentinite dissolution in sulfuric acid, as well as the adsorption characteristics of amorphous silica obtained through acid and combined acid–alkali treatment of serpentinite.
2. Results and Discussion
The combined method involving acid and alkali treatment is based on sequential processes occurring during the dissolution of serpentinite in acid and subsequent treatment of the acid-insoluble residues in alkaline media:
During the acid leaching stage, the destruction of the crystalline lattice of serpentinite occurs, particularly in the regions of Mg–OH bonds and partially in Si–O–Mg bonds:
Mg(OH)2(MgOH)2Si2O5 + 6H+ → 3Mg2+ + 2SiO2 + 5H2O
Mg2+ ions enter the solution, while the formed amorphous silica (SiO2·nH2O) creates a gel-like phase, which causes technological complications during the filtration and purification stages.
The amorphous SiO2·nH2O reacts well with alkalis, transforming into soluble silicates:
SiO2·nH2O + 2NaOH → Na2SiO3 + (n + 1) H2O
2.1. Interactions in the “Serpentinite–H2SO4” System
The acid treatment of the initial serpentinite waste (SP0) was performed following the procedure outlined in the Section 3. Based on chemical analysis, the compositions of the filtrate and the insoluble residue were determined, along with the leaching efficiencies of individual elements from SP0. The results are summarized in Table 1.

Table 1.
Distribution of elements in the filtrate and insoluble residue after acid leaching of the initial serpentinite waste SP0.
According to Table 1, during the initial acid leaching of the serpentine waste SP0 with sulfuric acid solution, a significant portion of magnesium, manganese, chromium, calcium, and aluminum (over 50%) is transferred into the sulfate solution.
At the same time, iron was leached only weakly (27%), and silicon to an insignificant extent (2%). The insoluble residue after leaching was substantially enriched with silicon (up to 98%) and iron (up to 73%). The general trend of changes in the composition of acid-insoluble residues depending on the concentration of H2SO4 used is shown in Figure 1, Figure 2 and Figure 3. Figure 1 illustrates the consumption of sulfuric acid and the change in the amount of Mg extracted from serpentinite (SP). As can be seen, when SP (Mg) and H2SO4 are taken at a molar ratio of 1:1, the maximum consumption of H2SO4 for Mg extraction is about 0.6, and the amount of acid not involved in the reaction is comparable to the value of free acid in the solution after the interaction, as determined through titration with NaOH.
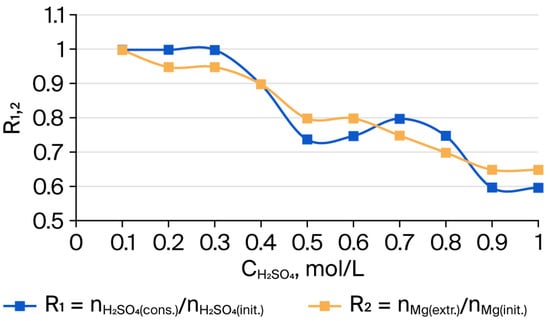
Figure 1.
Dependence of the molar ratios R1 =
and R2 = on the treatment of 10 g of serpentinite with sulfuric acid solutions (0.1–1.0 mol/L), corresponding to the stoichiometrically required amount, and with τ = 10 min and t = 98 °C.
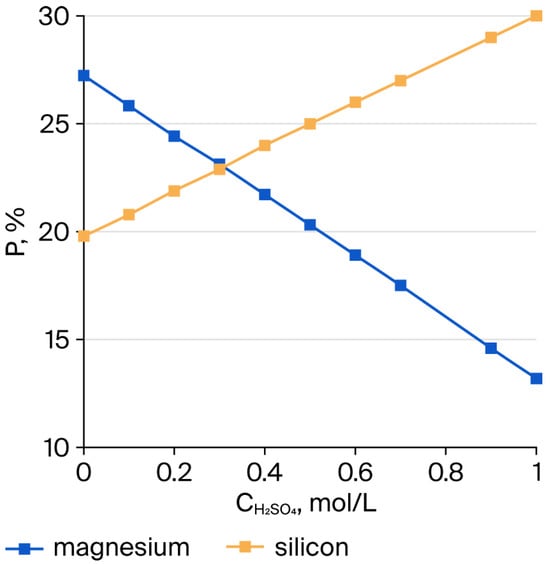
Figure 2.
Variation in magnesium and silicon concentrations in the insoluble residue after the interaction of serpentinite with H2SO4, at a ratio of 10 g of serpentinite Mg (1.1 mol/L)/H2SO4 (0.1–1.0 mol/L), using the stoichiometrically required amount, for τ = 10 min and t = 98 °C.
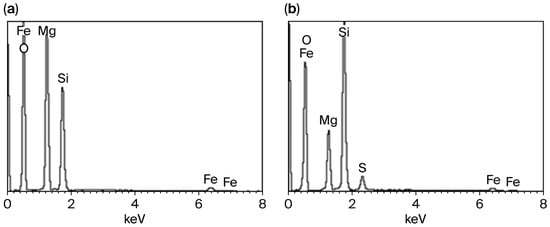
Figure 3.
Energy-dispersive spectra of the initial serpentinite (a) and after treatment with H2SO4 solution at SP:H2SO4 = 1:1 (stoichiometrically required amount) (b).
The observed fact that a certain amount of free H+ remains in the solution indicates incomplete progress of the reaction of SP with sulfuric acid.
The dissolution of magnesium from the crystalline framework of SP as a result of its interaction with H+ ions leads to the formation of silicon dioxide accompanied by the complete destruction of the molecular structure of the outer layers of SP fibers, which consist of tubes coiled into bundles. The dissolution of these tubular layers, in general, appears to proceed in a predominantly stepwise manner. As shown in Figure 1, the variations of and exhibit approximately the same trend. The changes in the amounts of Si and Mg in the insoluble residue after treatment of serpentinite with H2SO4 are presented in Figure 2. The simultaneous decrease in Mg content and increase in Si content in the residue indicate that the interaction of sulfuric acid with SP proceeds in a predominantly stepwise fashion.
Comparative energy-dispersive spectra of SP before and after treatment with 1.0 mol/L H2SO4 (Figure 3) show that the Mg content in the residue decreases by approximately 60–65%. The residue still contains about 12% magnesium, which is most likely only located in the unreacted inner layers of the SP fibers.
The unreacted layer-packages retain their molecular–structural framework, which is protected from further destruction by a denser hydrated silica surface layer that limits the subsequent diffusion of H+ ions into the deeper parts of the SP particles.
2.2. XRD and FTIR Spectroscopy
Phase transformations occurring during the acid–alkali treatment of the initial serpentinite are shown in Figure 4a–c. In the XRD pattern of the raw serpentinite (a), the identified phases are chrysotile (SP), antigorite (AN) Mg3Si2O5(OH)4, and magnetite Fe3O4. In the XRD pattern of the acid-treated serpentinite SPI (b), the identified phases are chrysotile (SP), antigorite (AN) Mg3Si2O5(OH)4, and magnetite Fe3O4. The main changes in the phase composition after acid treatment include the disappearance of brucite [Mg(OH)2] (BR) reflections at interplanar spacings of d/n = 4.77, 2.365, and 1.794 Å. The phase transformation associated with the formation of amorphous silica during sulfuric acid treatment of serpentinite is not manifested in the XRD pattern of the acid-insoluble residue. However, the twofold decrease in the intensity of the main serpentinite (SP) peaks at d/n = 7.38, 3.661, 2.487, and 1.53 Å may be attributed to the formation of an amorphous Si-rich layer on the surface of the serpentinite particles.
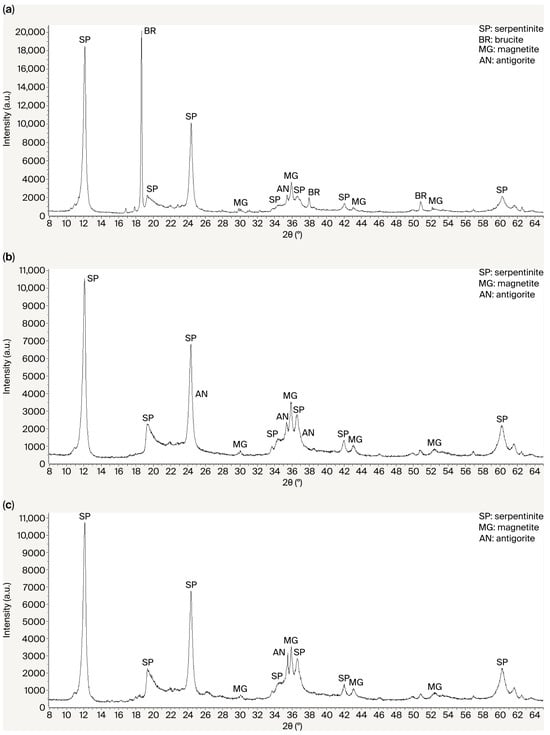
Figure 4.
X-ray diffraction patterns of the initial serpentine waste SP0 (a), the acid-insoluble residue SPI (b), and the alkali-insoluble residue SPII (c).
During the alkaline treatment of the acid-insoluble residue (SPI) (Figure 4c), the characteristic X-ray diffraction reflections of the serpentinite minerals (SP0) reappeared with their initial intensity. The original texture of serpentinite was restored. Upon repeated treatment (additional verification) of the alkaline residue of the acid-treated serpentinite (SPIII), the formation of hydrated amorphous silica was again recorded, along with SiO2·nH2O phases. The serpentinite peaks regained their previous intensity. The changes observed in the diffraction patterns after the acid–alkali treatment of serpentinite demonstrate that acid exposure resulted in the formation of a multilayer silica coating enriched with magnesium disilicate. The acid simultaneously accelerated magnesium dissolution and modified the rate of silica polymerization and flocculation. As a result, a gel/Si-rich layer was formed.
The mechanism of the transformation of silicate fragments of serpentinite into amorphous silica during acid treatment has been reported previously [7]. Specific characteristic absorption bands of amorphous silica (νas(Si–O–Si)) and hydroxyl groups (Si–OH) were detected in the FTIR spectra (Figure 5) of acid-treated serpentinite samples after treatment with H2SO4 solutions at concentrations close to C = 0.7–1.0 M (stoichiometrically required amount of H2SO4). These absorption bands simultaneously indicated a significant disruption of the tetrahedral–octahedral connectivity of the crystalline lattice of serpentinite. At the same time, the presence of vibrations associated with iron was not detected in the FTIR spectrum. Apparently, during dissolution, the isomorphically incorporated iron atoms do not participate in the formation of the Si-rich layer.
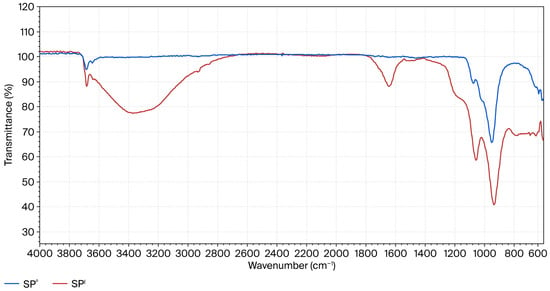
Figure 5.
FTIR spectra of the initial sample SP0 and the acid-insoluble residue SPI after treatment [7].
Broad intense peaks at 1160–2040 cm−1 indicate the presence of various acidic silanol (–Si–OH) groups, while the wide band at 3600–3200 cm−1 is associated with the formation of different siloxane (–Si–O–Si–OH) and hydrated groups of silica monomers, dimers, and tri- and tetra-polymers, as well as branched silica networks on the surface of serpentinite particles, marking the onset of silica gel formation on the surface layer of SiO2·nH2O [20].
The results obtained from experimental XRD and FTIR studies suggest the possibility of extracting amorphous silica formed in the SP–H2SO4 system as a separate product by applying the combined acid–alkali treatment method.
2.3. Production of Amorphous Silica via the Combined Acid–Alkali Method and Its Properties
According to the elemental analysis of the acid-insoluble residue (SPI) obtained after leaching the initial sample SP0 with sulfuric acid solution, the following mass fractions were determined: Mg—15.0%; Si—21.2%; Fe—3.0%; Cr—0.19%; and S—3.18%. Calculations based on the magnesium, silicon, and oxygen contents indicated that the composition approximately corresponds to the formula 1.25 MgO·1.5 SiO2·3H2O [SPI]. The insoluble residue weighing 83.6 g (SPI obtained after acid treatment) was subjected to alkaline treatment (stage 2). Calculations performed to establish the stoichiometry of the interaction of this composition with NaOH solution and the conditions for silica precipitation were carried out according to Reactions (1) and (2), presented in Table 2.

Table 2.
Reactions and stoichiometric calculations for the interaction of the acid-insoluble residue (SPI) with NaOH for silica precipitation.
According to Reaction (1) (Table 2), 325 cm3 of 4.0 M NaOH solution (ρ = 1.155 g/cm3) was used for the treatment.
Elemental analysis of the initial sample (SP0), the acid-leached residue (SPI), and the alkaline residue (SPII) showed that after the two-step treatment SP0 (acid and alkaline), the final residue SPII became comparable in composition to the original SP0 in terms of the main elements (Mg and Si). In SP0, the molar Mg/Si ratio was 1.68, while in SPII it was 1.56. These data indicate that after alkaline treatment, i.e., the removal of the silica layer from the surface of SPI particles, the inner layers retain the serpentine structure [Mg3Si2O5(OH)4]. To confirm this assumption, the alkaline residue (SPII, 36 g) was subjected to repeated acid leaching using a 2.0 M H2SO4 solution (stage 3). The resulting acid-insoluble residue (SPIII) was subsequently treated with alkali to obtain the alkaline residue (SPIV). The experimental procedures applied at stages 3 (H2SO4 treatment) and 4 (NaOH treatment) were analogous to those used at stages 1 and 2. The obtained SPIII and SPIV residues were also analyzed following the same approach as for SPI and SPII. The results of the elemental analysis of the insoluble residues (the average value of three parallel experiments) after sequential acid–alkaline treatment (H2SO4 and NaOH) are summarized in Table 3.

Table 3.
Elemental composition (wt.%) of insoluble residues after sequential acid–alkali treatment of serpentinite waste (SP0) with H2SO4 and NaOH solutions.
From the data in Table 3, it should be noted that the contents of the main elements, magnesium and silica, after alkaline treatment (stages 2 and 4) are close to those in the acid-insoluble residue of the initial serpentinite (SP0). In the conditional compositions of the acid-insoluble residues at various stages of acid–alkali extraction of magnesium and silica, enrichment in silica occurs, with a change in the MgO/SiO2 ratio in the series SP0 → SPI → SPIII equal to 1.5 → 0.83 → 0.46, respectively.
The yield of amorphous silica (after two acid and two alkaline stages) is as follows: (1) 95.78% from 83.6 g of the acid-insoluble residue (SPI); (2) 60.3% from the initial serpentinite (SP0). Thus, the results demonstrate that the acid–alkali method makes it possible to process serpentinite waste with selective silicon extraction at sufficiently high yields by regulating the number of sequential acid and alkali leaching stages.
2.4. Study of the Properties of Amorphous Silica
The amorphous silica obtained by the combined acid–alkali method was investigated using ICP–MS (Thermo iCAP-Q), FTIR spectroscopy, and BET adsorption analysis.
The composition of the amorphous SiO2 determined via ICP–MS (Thermo iCAP-Q) showed the presence of the following impurities (wt.%): Al—0.1696; Ca—0.2025; Na—0.7010; the results showed a total impurity content of 1.08%, and 8.0% adsorbed H2O. The formal composition corresponds to SiO2·0.36H2O. The FTIR spectrum of the amorphous silica of this composition is shown in Figure 6.
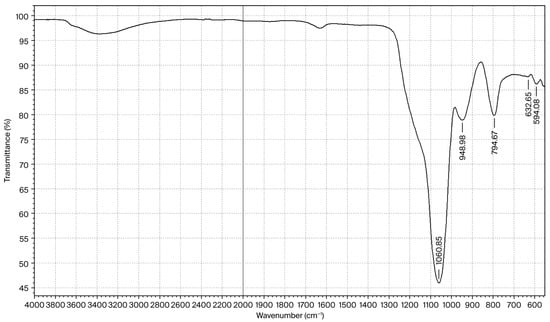
Figure 6.
FTIR spectrum of silica with the composition SiO2·0.36H2O.
In the FTIR spectrum of silica, valence and deformation vibrations of water are observed at the following frequencies: νas(O–H)—3200–3600 cm−1; δ(H–O–H)—1642 cm−1, while asymmetric and symmetric stretching vibrations are observed for the siloxane group (νasSi–O–Si) at 1064 cm−1 and for νsSi–O–Si at 794 cm−1. Vibrations of the silanol group (νSi–OH) are observed at 948 cm−1, which is characteristic of amorphous silica [20].
The shape of the nitrogen adsorption–desorption isotherm hysteresis loop for the synthesized silica indicates a type IV isotherm with an H3 hysteresis loop (Figure 7a), which confirms the presence of a micro- and mesoporous structure with partial condensation of nitrogen in the pores of the solid. The pronounced rise in adsorption observed from 0 to 90 cm3·g−1 in the relative pressure range of P/P0 = 0–0.2 corresponds to the presence of micropores with diameters ranging from 0.35 to 2 nm. In addition, a small step on the desorption branch further confirms the slit-shaped nature of the pores. In the relative pressure range of P/P0 = 0.2–0.96, a significant increase in adsorption capacity from 90 cm3·g−1 to 204 cm3·g−1 was observed, which is characteristic of micro- and mesopores. At P/P0 > 0.96, the adsorption capacity increases further to 65–68 cm3·g−1, indicating the presence of macropores (50–200 nm). At the same time, in the case of this silica, a significant contribution of micro- and mesopores is evident, which is typical for microporous sorbents, as also supported by the steep rise in the adsorption curve at low relative pressures and at P/P0 ≈ 0.96.
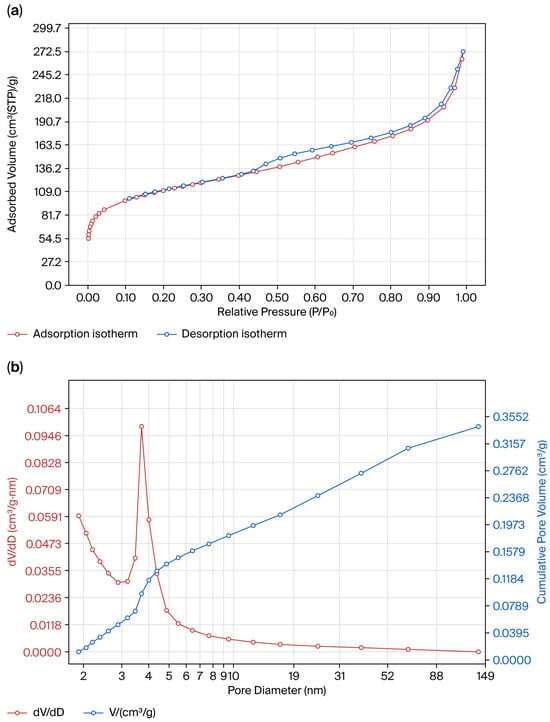
Figure 7.
Nitrogen adsorption–desorption isotherms at 77 K (BET) (a) and pore size distribution (BJH) (b) of the synthesized amorphous silica.
The results of the pore distribution analysis conducted via the BJH method (desorption branch) (Figure 7b) revealed a pronounced peak at ~3.7 nm, indicating the predominance of mesopores and a uniform pore structure of the material. Additionally, the presence of micropores (1.7–2.1 nm), confirmed via the DR and DA methods, was observed. The pore size distribution confirmed that the obtained silica is a micro- and mesoporous material. Micropores (0.35–2 nm) accounted for 46% of the surface area and 21% of the pore volume, while mesopores (2–10 nm) comprised 48% of the surface area and 42% of the pore volume. Thus, micropores and mesopores together provided the largest contribution, 95% of the surface area and 63% of the pore volume, which supports the structural uniformity of the synthesized amorphous silica. The total pore volume of 0.42 cm3·g−1 indicates the developed porous structure, making this material promising for adsorption and catalytic applications. The main parameters characterizing the textural properties of silica are summarized in Table 4.

Table 4.
Main parameters characterizing the textural properties of amorphous silica (SiO2).
The analysis of the textural characteristics of silica showed that the material possesses a high specific surface area of 400 m2·g−1 (BET method) and 559.13 m2·g−1 (Langmuir method), which is consistent with the data on the developed mesoporous structure. The total pore volume was 0.42 cm3·g−1, while the contribution of micropores (T-plot) was 0.09 cm3·g−1, confirming the combined micro- and mesoporous nature of the material. The average pore diameter calculated using the BET method (4V/S) was 4.18 nm, whereas the BJH method (desorption branch) yielded larger values of 5.68 nm due to the features of mesopore distribution. The most probable pore diameter (dmode) from BJH desorption was 3.73 nm, indicating the predominance of relatively narrow slit-like mesopores.
Additional calculations by the DR and DA methods revealed micropores with diameters of 1.57–2.14 nm, and the Horvath–Kawazoe (H–K) method also confirmed the presence of micropores in the range of 1.6–1.9 nm. Thus, the material demonstrates a bimodal pore distribution, with micropores <2 nm and mesopores around 3–6 nm. The main contribution to the textural properties is from mesopores with a diameter of 3–6 nm, accounting for ~64% of the surface area and ~52% of the pore volume. The contribution of micropores (<2 nm) was ~21% of the surface area and ~46% of the pore volume. The presence of a narrow pore size distribution and the combined micro- and mesoporous structure confirm the structural uniformity of the synthesized silica. According to the IUPAC classification, the nitrogen adsorption isotherm of silica corresponds to type IV with an H3 hysteresis loop, which is characteristic of mesoporous materials with slit-shaped pores formed by aggregates of plate-like particles. The high specific surface area (400 m2·g−1) and significant total pore volume (0.42 cm3·g−1) are described in the updated IUPAC recommendations (2015) [21] as a ‘well-developed mesoporous structure’, which corresponds to industrial adsorbents (desiccants, catalyst supports, chromatography).
In this study, other parameters influencing the extraction of silica from the acid-insoluble residue (such as temperature, stoichiometry of reagents, and others) that could affect the resulting textural properties of amorphous silica were not specifically considered. However, the obtained results indicate that amorphous silica with favorable textural characteristics can be achieved through the combined acid–alkali method of serpentinite processing.
3. Materials and Methods
Serpentinite waste with a particle size of ≤0.14 mm from the Zhitikara deposit (Kazakhstan) was used as the raw material. The elemental composition of the sample was as follows (wt.%): Mg—25.0; Si—17.45; Fe—2.93; Al—0.54; Ca—0.50.
X-ray diffraction (XRD) patterns were recorded using a D8 Advance diffractometer (Bruker AXS GmbH, Karlsruhe, Germany) operating with Cu-Kα radiation at 40 kV and 40 mA. Diffraction data were processed using the EVA software (version 4.2) package, and phase identification was carried out using the Search/Match function based on the PDF-2 Powder Diffraction File (JCPDS-ICDD). The phases were identified according to the JCPDS database: serpentine—16-0613, Mg(OH)2—07-0239, MgO—45-0946.
Fourier-transform infrared (FTIR) spectra were recorded using a Shimadzu IR Prestige-21 spectrometer (Shimadzu Corporation, Kyoto, Japan) equipped with an ATR (attenuated total reflectance) Miracle accessory (Pike Technologies, Madison, WI, USA).
The specific surface area and pore size distribution were determined through the static capacity method using a BSD-660S A3 instrument (BSD Instrument Technology, Beijing, China).
Elemental distribution during leaching and purification was analyzed using a JSM-6490LV scanning electron microscope (JEOL Ltd., Tokyo, Japan) equipped with an INCA Energy 350 energy-dispersive X-ray spectroscopy (EDS) system and an inductively coupled plasma mass spectrometer (ICP-MS, Thermo iCAP-Q, Thermo Fisher Scientific, Bremen, Germany).
Amorphous silica (SiO2) was produced through a combined acid–alkali method. For the experiment, 100 g of serpentine waste (SP0) containing 25.75 g or 1.073 mol of Mg was used. According to reaction Equation (1), complete leaching of Mg from 100 g of SP0 requires 1.073 mol of H2SO4.
The reactor was charged with 455 cm3 of a solution containing 1.073 mol of H2SO4, and 100.0 g of SP0 was gradually added to the solution under stirring, with time monitoring initiated simultaneously. During the addition, intensive boiling was observed, caused by the exothermic reaction. The suspension was maintained under heating for 2 h, after which it was filtered hot via vacuum suction through a blue-ribbon filter. The filtration time was 2 h. The total volume of the obtained filtrate together with the wash water was 470 cm3, with a pH of 0.64, while the mass of the insoluble residue after drying at 105 °C was 83.6 g. The transparent pale-green filtrate represented a solution of metal sulfates—MgSO4, CaSO4, Al2(SO4)3, CrSO4, and Fe2(SO4)3.
To obtain amorphous silica, the acid-insoluble residue of SP0 was treated with alkali according to reaction (3):
1.25MgO·1.5SiO2·3H2O + NaOH → 1.25MgO·0.075SiO2·3H2O+ 1.425Na2SiO3
An amount of 83.6 g of the acid-insoluble residue was treated with 325 cm3 of a 4.0 M NaOH solution under stirring for 0.5 h, after which it was filtered under vacuum, and the precipitate was washed with distilled water. Filtration proceeded slowly but satisfactorily. The volume of the alkaline filtrate was V = 1000 cm3, with a pH of 11.46. The filtrate was neutralized with 20% H2SO4 solution to a pH of 4.5–5.0 and then left to allow silica precipitation (Table 2, reaction 2). After 12–14 h, a finely dispersed white precipitate of silica was formed. The precipitate was filtered and washed with distilled water until no SO42− ions were detected in the wash water.
After drying at 105 °C, the obtained silica was analyzed using ICP-MS (Thermo iCAP-Q).
4. Conclusions
The combined acid–alkali processing method overcomes the limitations of traditional one-stage approaches by ensuring efficient dissolution of magnesium and selective extraction of silicon from serpentinite waste. The dissolution of the surface silica-enriched layer in an alkaline medium, formed as a result of acid treatment, promotes subsequent access of H3O+ ions to the inner layers of the mineral. The obtained amorphous silica is characterized by high purity (>95% SiO2) and a developed mesoporous structure, which makes it a promising material for use as an adsorbent or catalyst support. The obtained results, along with further development of the combined acid–alkali method for extracting magnesium and silicon from serpentinite, may strengthen the feasibility of this approach for designing new technological schemes for serpentinite processing.
Author Contributions
Conceptualization, A.A. and C.Y.; methodology, A.A. and C.Y.; software, E.D.; validation, A.I. and K.A.; formal analysis, C.Y.; investigation, A.I. and E.D.; resources, K.A. and A.I.; data curation, E.D.; writing—original draft preparation, C.Y.; writing—review and editing, A.A.; visualization, K.A.; supervision, A.A.; project administration, A.A.; funding acquisition, A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by the Science Committee of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant No. BR21882242).
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that they have no known competing interests.
References
- Beglaryan, H.; Isahakyan, A.; Zulumyan, N.; Melikyan, S.; Terzyan, A. A study of magnesium dissolution from serpentinites composed of different serpentine group minerals. Miner. Eng. 2023, 201, 108171. [Google Scholar] [CrossRef]
- Liu, W.; Peng, X.; Liu, W.; Zhang, N.; Wang, X. A cost-effective approach to recycle serpentine tailings: Destruction of stable layered structure and solvent displacement crystallization. Int. J. Min. Sci. Technol. 2022, 32, 595–603. [Google Scholar] [CrossRef]
- Vieira, K.R.M.; Arce, G.L.A.F.; Luna, C.M.R.; Facio, V.O.; Carvalho, J.A., Jr.; Soares Neto, T.G.; Ávila, I. Understanding the acid dissolution of Serpentinites (Tailings and waste rock) for use in indirect mineral carbonation. S. Afr. J. Chem. Eng. 2022, 40, 154–164. [Google Scholar] [CrossRef]
- Zhao, D.; Sun, H.; Peng, T.; Zeng, L.; Wu, M. The acid-leaching process and structural changes of lizardite, chlorite and talc in sulfuric acid medium. Clay Miner. 2024, 59, 298–309. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, X.; Wu, L.; Tong, L.; Zhu, J. Recovery of Mg from H2SO4 Leaching Solution of Serpentine to Precipitation of High-Purity Mg(OH)2 and 4MgCO3·Mg(OH)2·4H2O. Minerals 2023, 13, 318. [Google Scholar] [CrossRef]
- Yoo, K.; Kim, B.-S.; Kim, M.-S.; Lee, J.-C.; Jeong, J. Dissolution of Magnesium from Serpentine Mineral in Sulfuric Acid Solution. Mater. Trans. 2009, 50, 1225–1230. [Google Scholar] [CrossRef]
- Auyeshov, A.; Arynov, K.; Yeskibayeva, C.; Dikanbayeva, A.; Auyeshov, D.; Raiymbekov, Y. Transformation of Silicate Ions into Silica under the Influence of Acid on the Structure of Serpentinite. Molecules 2024, 29, 2502. [Google Scholar] [CrossRef] [PubMed]
- Sirota, V.; Selemenev, V.; Kovaleva, M.; Pavlenko, I.; Mamunin, K.; Dokalov, V.; Yapryntsev, M. Preparation of crystalline Mg(OH)2 nanopowder from serpentinite mineral. Int. J. Min. Sci. Technol. 2018, 28, 499–503. [Google Scholar] [CrossRef]
- Fedoročková, A.; Hreus, M.; Raschman, P.; Sučik, G. Dissolution of magnesium from calcined serpentinite in hydrochloric acid. Min. Eng. 2012, 32, 1–4. [Google Scholar] [CrossRef]
- Zulumyan, N.; Mirgorodski, A.; Isaakyan, A.; Beglaryan, H. The mechanism of decomposition of serpentines from peridotites on heating. J. Therm. Anal. Calorim. 2014, 115, 1003–1012. [Google Scholar] [CrossRef]
- Yeskibayeva, C.; Auyeshov, A.; Arynov, K.; Dikanbayeva, A.; Satimbekova, A. Nature of serpentinite interactions with low- concentration sulfuric acid solutions. Green Process. Synth. 2024, 13, 20240034. [Google Scholar] [CrossRef]
- Matus, M.; Korbicz, A.; Pietrzyk, S. Mechanism of Nickel, Magnesium, and Iron Recovery from Olivine-Bearing Ore during Leaching with Hydrochloric Acid Including a Carbonation Pre-Treatment. Metals 2020, 10, 811. [Google Scholar] [CrossRef]
- Ulum, R.; Natalin, R.; Ahmad, M. Pyro-Hydrometallurgy Routes to Recover Silica from Indonesian Ferronickel Slag. Recycling 2023, 8, 13. [Google Scholar] [CrossRef]
- Peng, X.; Liu, W.; Liu, W.; Zhao, P.; Yu, X.; Wang, Y. Fabrication of eco-friendly adsorbent derived from serpentine tailings for removal of organic dyes. Colloids Surf. A Physicochem. Eng. Asp. 2022, 643, 128761. [Google Scholar] [CrossRef]
- Zulumyan, N.O.; Hovhannisyan, E.B.; Hovhannisyan, Z.G.; Karakhanyan, S.S. On thermoacid treatment of serpentinites of the northeastern coast of Lake Sevan. Rep. NAS RA. Inorg. Chem. 2002, 102, 238–242. (In Russian) [Google Scholar]
- Yang, H.; Du, C.; Hu, Y.; Jin, S.; Yang, W.; Tang, A.; Avvakumov, E.G. Preparation of porous material from talc by mechanochemical treatment and subsequent leaching. Appl. Clay Sci. 2006, 31, 290–297. [Google Scholar] [CrossRef]
- Lazaro, A.; Chen, Y.; Verhoeven, C.; Hendrix, Y. One-step synthesis of ordered mesoporous silica from olivine and its pore size tailoring. J. Clean. Prod. 2019, 238, 117951. [Google Scholar] [CrossRef]
- Raza, N.; Raza, W.; Madeddu, S.; Agbe, H.; Kumar, R.V.; Kim, K.-H. Synthesis and characterization of amorphous precipitated silica from alkaline dissolution of olivine. RSC Adv. 2018, 8, 32651–32658. [Google Scholar] [CrossRef] [PubMed]
- Çakan, Y.; Gönen, M. Amorphous Silica Production from Serpentine and Its Techno-Economic Analysis. J. Turk. Chem. Soc. Sect. B Chem. Eng. 2024, 7, 61–68. [Google Scholar] [CrossRef]
- Chukin, G.D. Surface Chemistry and Structure of Dispersed Silica; Paladin Printa LLC: Moscow, Russia, 2008; p. 172. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).