Preparation of Lignin Nanoparticles from Thlaspi arvense L. Rhizomes via Ultrasound-Assisted Antisolvent Precipitation: Nanostructural Characterization and Evaluation of Their Radical Scavenging Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. Single-Factor Experiments
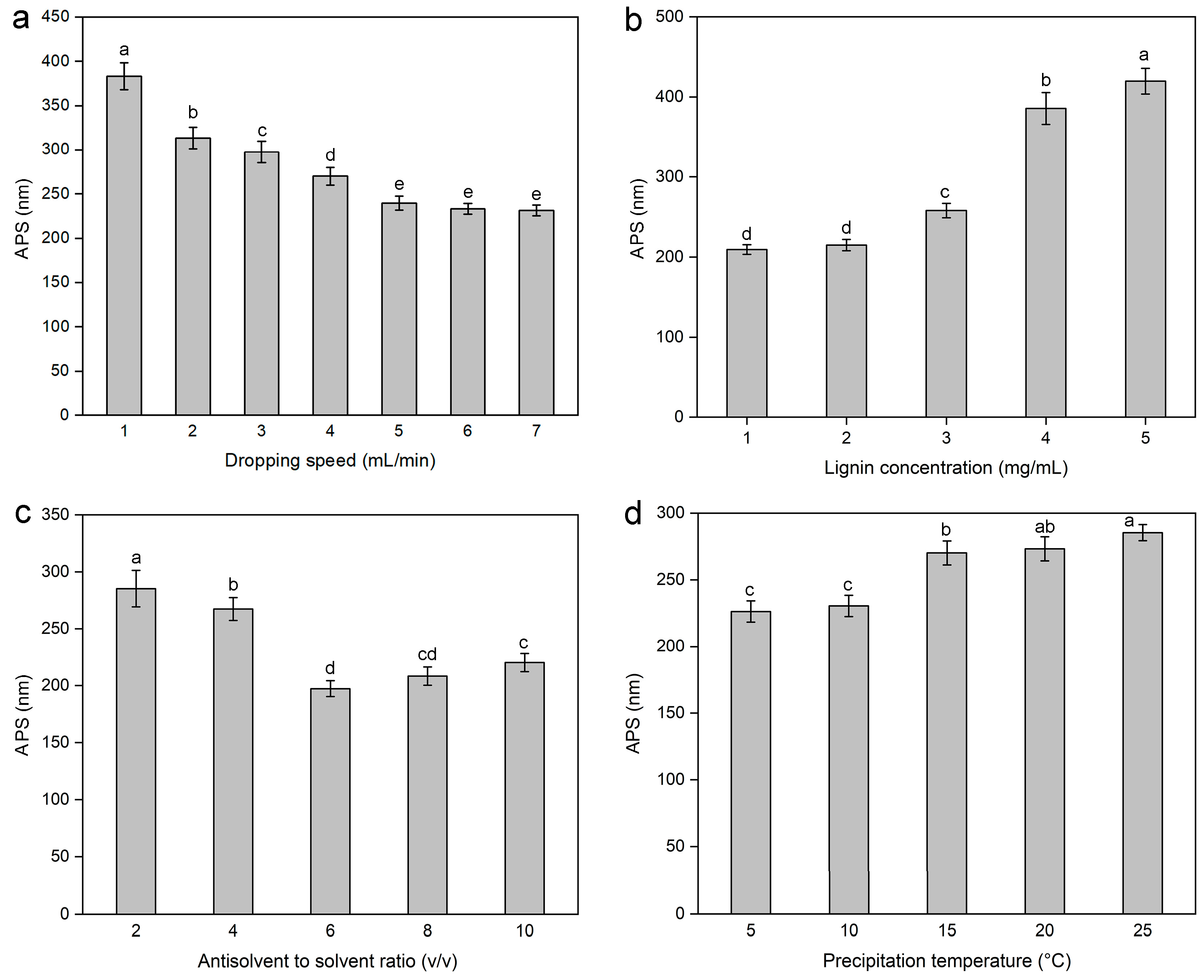
2.1.1. Effect of the Dropping Speed on the Average Particle Size of Lignin Nanoparticles
2.1.2. Effect of Lignin Concentration on the Average Particle Size of Lignin Nanoparticles
2.1.3. Effects of the Antisolvent-to-Solvent Ratio on the Average Particle Size of Lignin Nanoparticles
2.1.4. Effect of Precipitation Temperature on the Average Particle Size of Lignin Nanoparticles
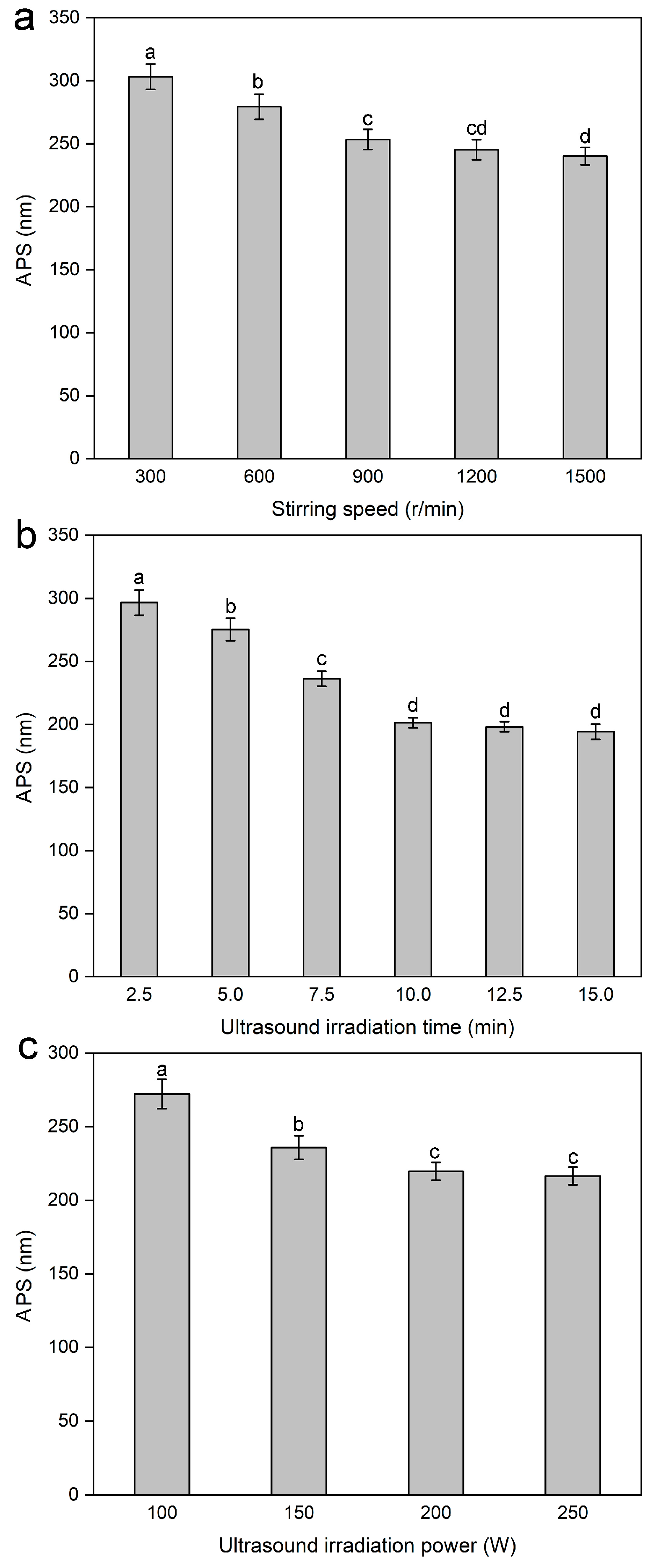
2.1.5. Effect of Stirring Speed on the Average Particle Size of Lignin Nanoparticles
2.1.6. Effect of Ultrasound Irradiation Time on the Average Particle Size of Lignin Nanoparticles
2.1.7. Effect of Ultrasound Irradiation Power on the Average Particle Size of Lignin Nanoparticles
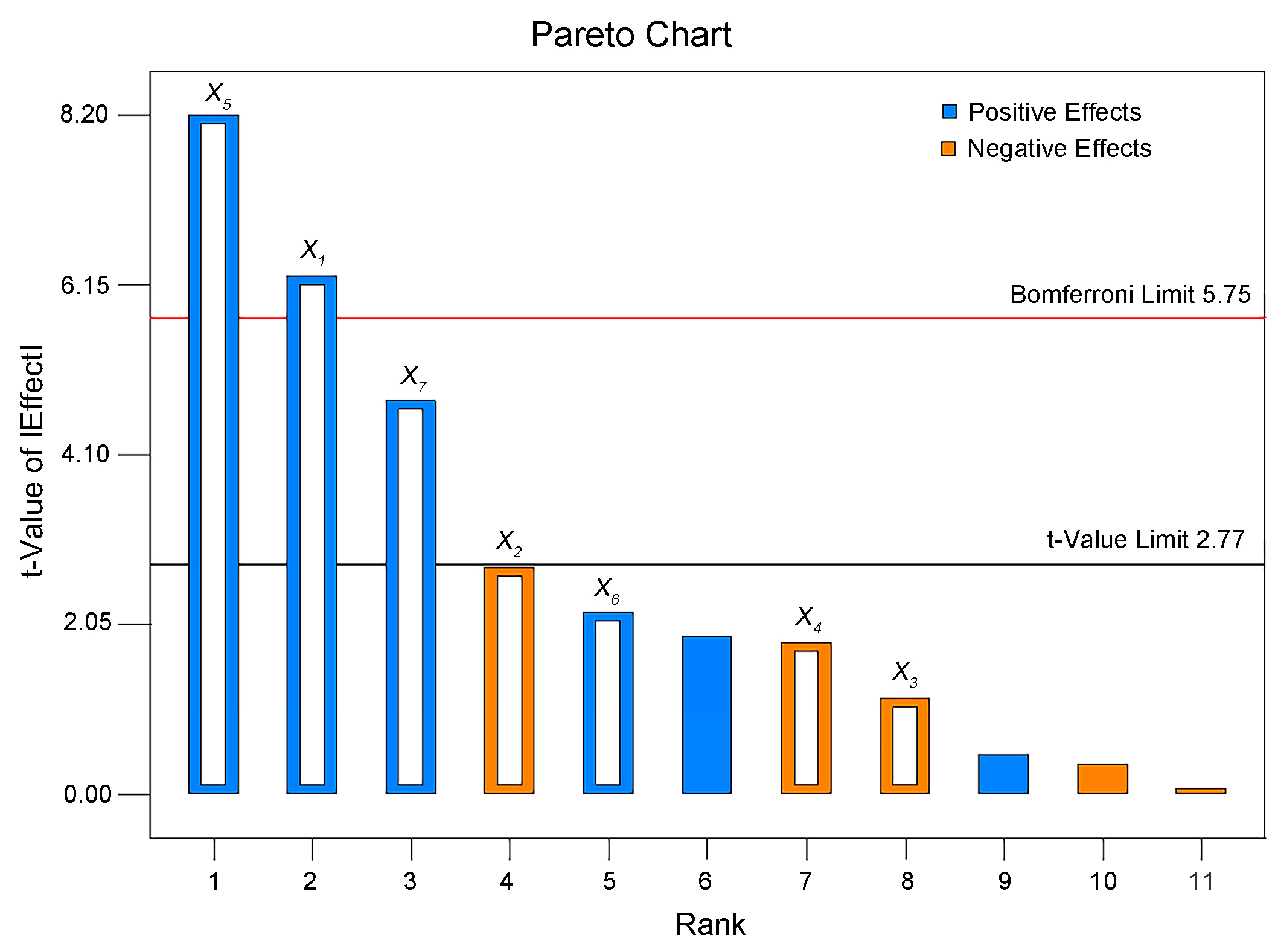
2.2. Screening of Significant Factors Affecting Lignin Nanoparticle Preparation via PBD
2.3. Optimization of the Optimal Conditions for Lignin Nanoparticle Preparation via BBD
2.3.1. Model Establishment and Analysis
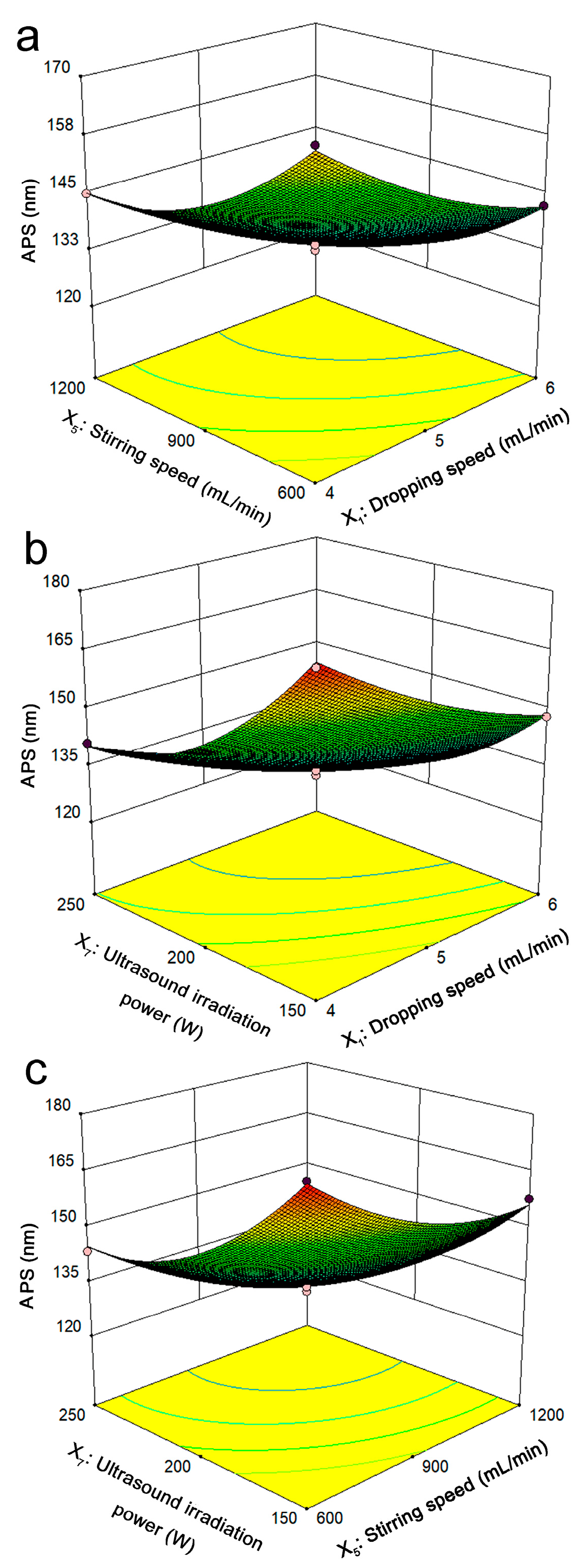
2.3.2. Analysis of Response Surface Plots
2.3.3. Verification Experiments
2.4. Model Adequacy Survey
2.5. Characterization of Lignin Nanoparticles
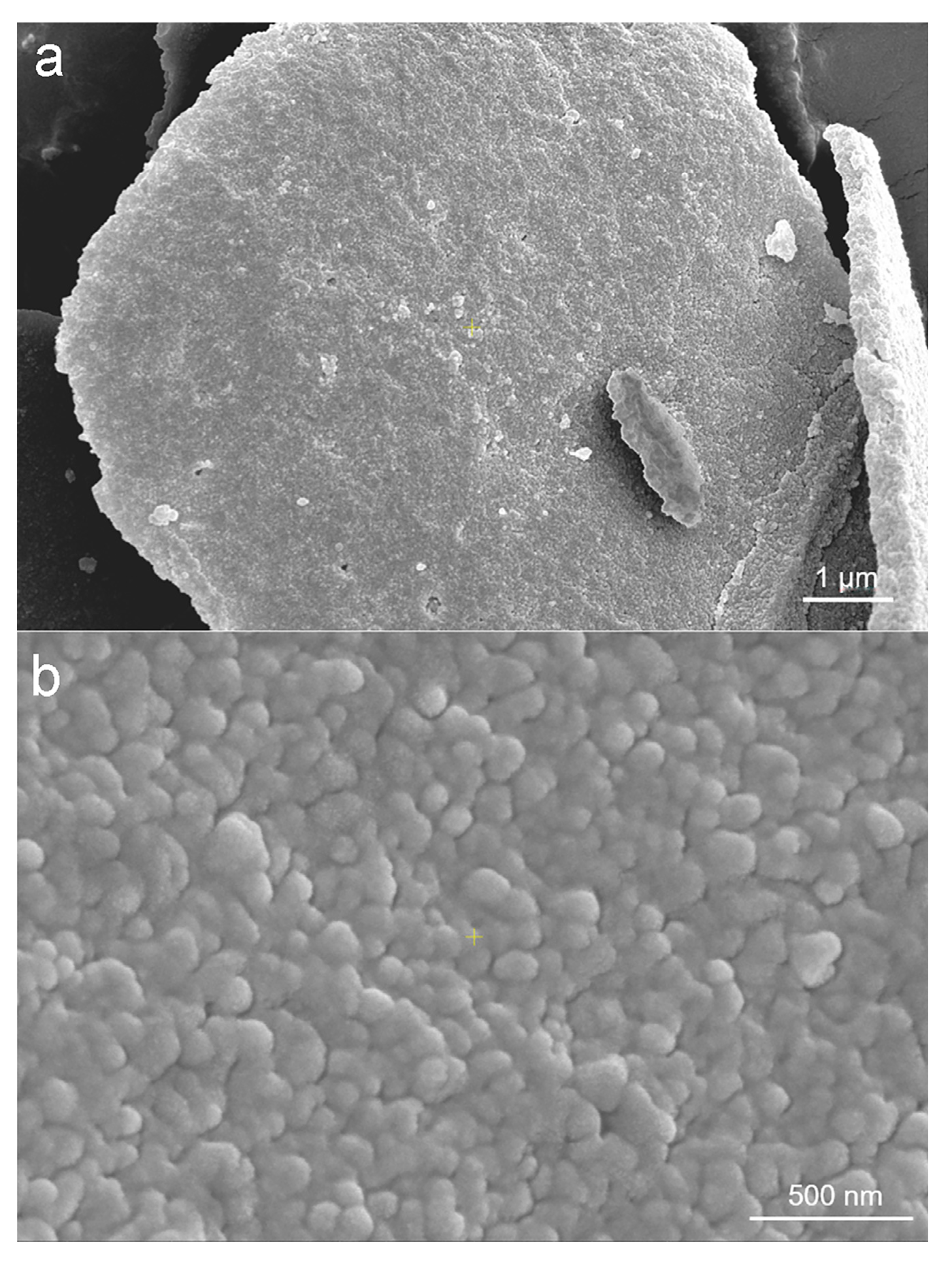
2.5.1. Scanning Electron Microscopy
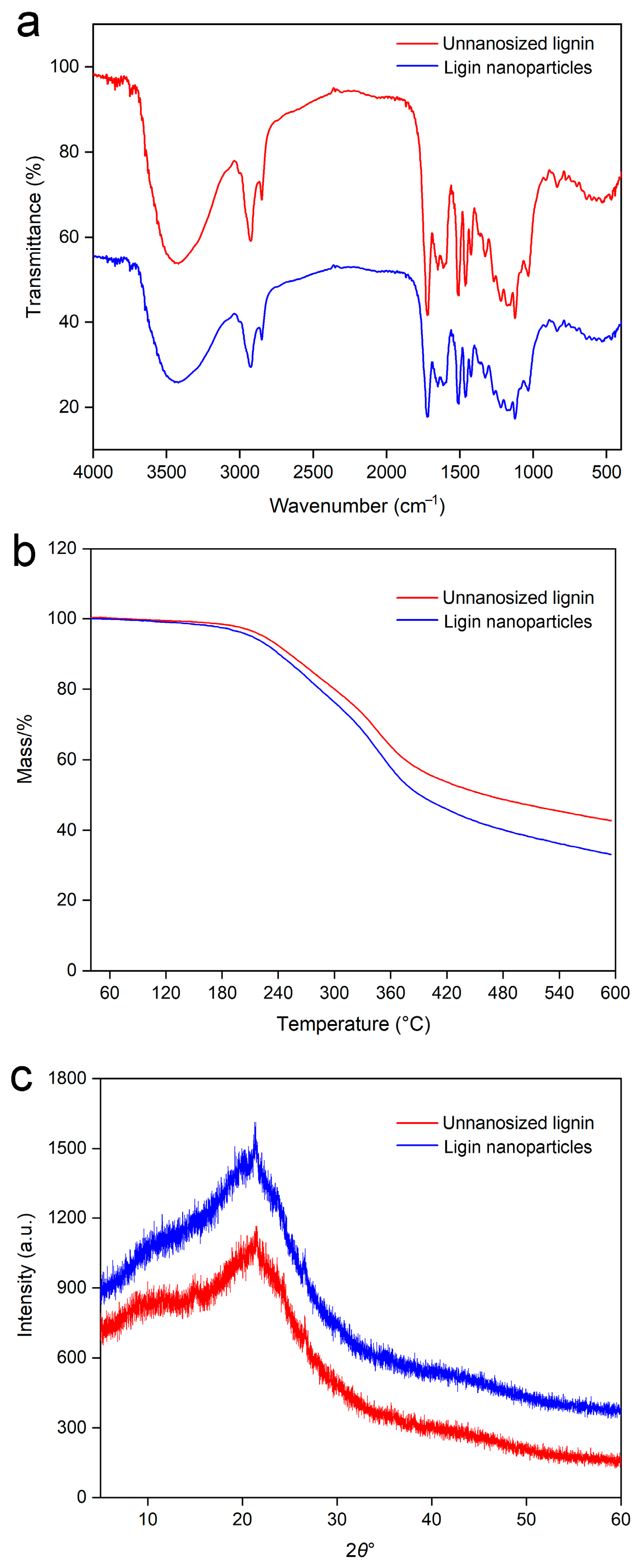
2.5.2. FTIR Analysis
2.5.3. Thermogravimetric Analysis
2.5.4. X-Ray Diffraction Analysis
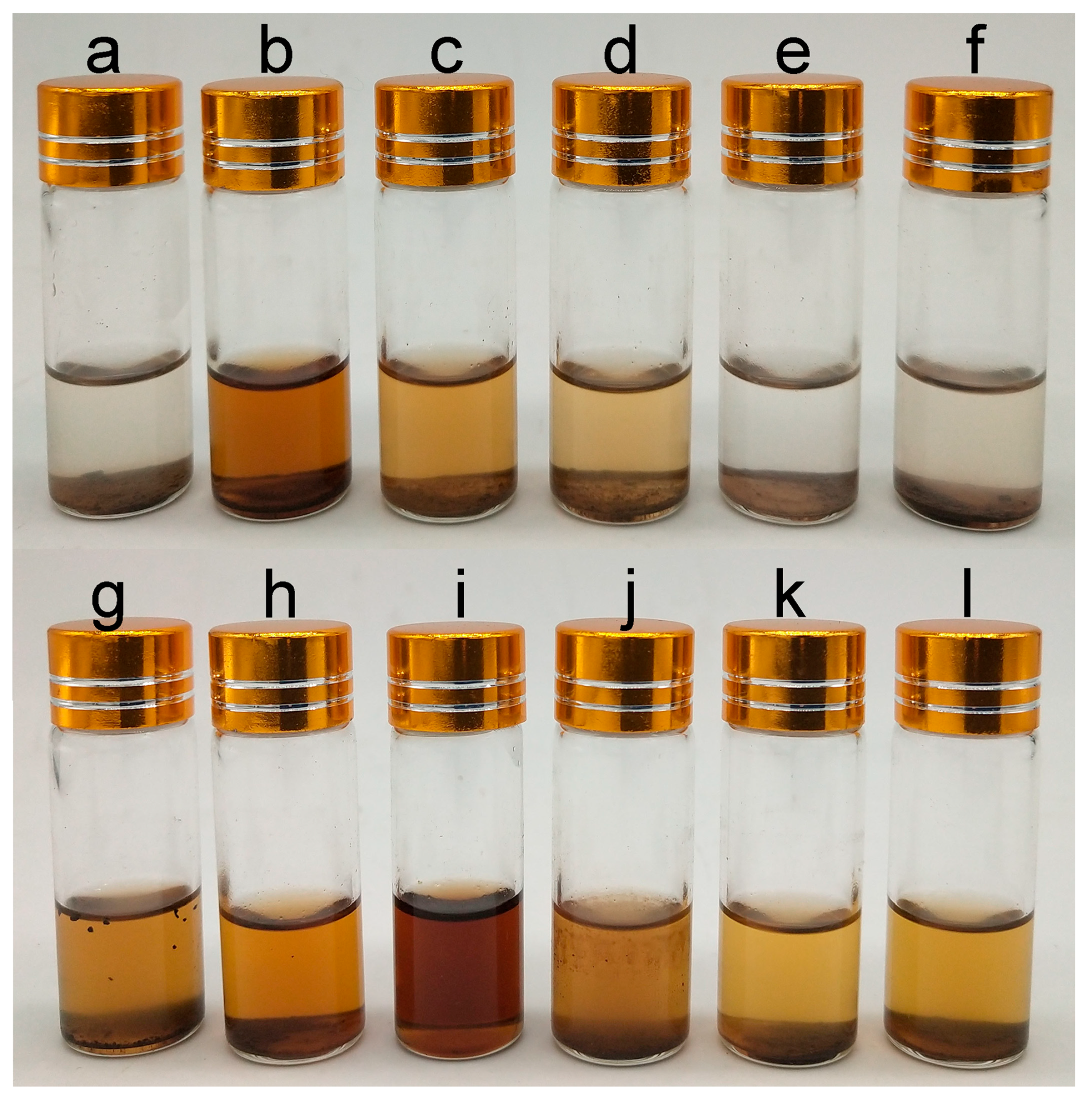
2.6. Solubility Determination of Lignin Samples
2.7. Evaluation of the Radical Scavenging Activity of Lignin Samples
2.8. Analysis of the Possible Mechanism of Ultrasound in the Preparation of Lignin Nanoparticles
3. Conclusions
4. Materials and Methods
4.1. Raw Materials and Reagents
4.2. Extraction Process of Lignin from T. arvense Rhizomes
4.3. Selection of the Lignin Solvent and Antisolvent
4.4. Ultrasound-Assisted Antisolvent Precipitation of Lignin Nanoparticles
4.5. Experimental Optimization Design
4.5.1. Plackett–Burman Design
4.5.2. Box—Behnken Design
4.6. Characterization of Lignin Physicochemical Properties
4.6.1. Scanning Electron Microscopy
4.6.2. Fourier Transform Infrared Spectroscopy
4.6.3. X-Ray Diffraction Analysis
4.6.4. Thermogravimetric Analysis
4.6.5. Solubility Test
4.6.6. Determination of Antioxidant Activity
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abbas, S.C.; Alam, A.; Mian, M.M.; Walker, C.; Ni, Y. Hydrothermal liquefaction of sewage sludge for circular bioeconomy: Focus on lignocellulose wastes, microplastics, and pharmaceuticals. J. Bioresour. Bioprod. 2025, in press. [CrossRef]
- Kumar, V. A comprehensive review on biomass-derived biodiesel additives with hydrogen enrichment and condition monitoring techniques for enhanced engine performance and emission control. Biomass Bioenergy 2025, 202, 108200. [Google Scholar] [CrossRef]
- Gao, D.; Shipman, W.D.; Sun, Y.; Glahn, J.Z.; Beraki, L.; Hsia, H.C. Macroporous scaffolds based on biomass polymers and their applications in wound healing. J. Bioresour. Bioprod. 2025, 10, 14–31. [Google Scholar] [CrossRef]
- Wu, T.; Sugiarto, S.; Chee, P.L.; Wang, C.; Ong, P.J.; Yang, R.; Liu, G.; Zhu, Q.; Li, Z.; Loh, X.J.; et al. Bio-based design and synthesis of lignin-graft-lignin copolymers and their nanomaterials. Int. J. Biol. Macromol. 2025, 320, 146134. [Google Scholar] [CrossRef]
- Ivaldi, C.; Chabbert, B.; Molinié, R.; Crônier, D.; Mathiron, D.; Fontaine, J.X.; Mesnard, F.; Velard, F.; Rakotoarivonina, H. Phenolic compounds fingerprints show distinct ligninolytic bacteria behaviours during technical lignins conversion. New Biotechnol. 2025, 89, 60–72. [Google Scholar] [CrossRef]
- Jeong, S.; Zhang, M.; Meng, X.; Wang, Y.; Scheidemantle, B.; Zhang, B.; Min, H.; Ji, A.; Crovella, P.; Cai, C.M.; et al. Properties and performance of lignin-based polyurethane foams from lignin and castor oil as synergistic bio-polyols. Chem. Eng. J. 2025, 520, 166159. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, Y.; Zeng, X.; Lu, L.; Jiang, J. Research progress in catalytic conversion of lignin to produce liquid fuels. J. Fuel Chem. Technol. 2025, 53, 994–1008. [Google Scholar] [CrossRef]
- He, Z.; Li, Y.; Liu, C.; Yang, J.; Qian, M.; Zhu, Y.; Wang, X. Turning lignin into treasure: An innovative filler comparable to commercial carbon black for the green development of the rubber industry. Int. J. Biol. Macromol. 2022, 218, 891–899. [Google Scholar] [CrossRef]
- Bjurström, A.; Hedenqvist, M.S.; Prade, T.; Mensah, R.A.; Das, O.; Åhrlin, A.; Matsson, A.; Helgesson, D.; Carrick, C.; Roulin, T.; et al. Synergistic enhancement of fire performance and carbon footprint reduction in polymer biocomposites through combined use of lignin and biochar. Ind. Crops Prod. 2025, 233, 121402. [Google Scholar] [CrossRef]
- Li, N.; Zhu, S.; Bai, L.; Li, B.; Liu, Z. Revolutionizing lignin valorization: Key advances in demethylation, methylation, and methyl metabolism. Biotechnol. Adv. 2025, 83, 108634. [Google Scholar] [CrossRef]
- Jacumazo, J.; do Amaral, L.F.M.; Huamani, P.A.M.; da Silva, N.M.; Costa, N.B.P.; de Freitas, R.A. Lignin nanoparticles as sustainable stabilizers for pickering emulsions. J. Mol. Liq. 2025, 427, 127393. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, S.; Yang, H.; Lu, F.; Yue, F. Investigating the Structure–Size Relationship between Organosolv Lignin and Its Colloidal Particles. Biomacromolecules 2025, in press. [CrossRef] [PubMed]
- Nie, K.; Liu, S.; Zhao, T.; Tan, Z.; Zhang, Y.; Song, Y.; Li, B.; Li, L.; Lv, W.; Han, G.; et al. Efficient fractionation of biomass by acid deep eutectic solvent (DES) and rapid preparation of lignin nanoparticles. Biomass Convers. Biorefinery 2024, 14, 28755–28765. [Google Scholar] [CrossRef]
- Li, W.; Liu, K.; Yang, H.; Xu, Y.; Zhang, M.; Pang, T.; Zhu, L.; Xu, T.; Wang, G.; Shen, F.; et al. Precision-engineered lignin-based food packaging materials. Trends Food Sci. Technol. 2025, 163, 105168. [Google Scholar] [CrossRef]
- Afrakomah, G.S.; Afedzi, A.E.K.; Gyan, K.; Puthson, P.; Wanmolee, W.; Parakulsuksatid, P. Potential for integrating enzymatic and conventional approaches in lignin nanoparticle production from oil palm biomass. Bioresour. Technol. Rep. 2025, 31, 102189. [Google Scholar] [CrossRef]
- Wan, Z.; Zhang, H.; Niu, M.; Zhang, W.; Guo, Y.; Li, H. Preparation of lignin nanoparticles by ultrasonication and its incorporation in DLP 3D printing UV-curable resin as bio-filler. Ind. Crops Prod. 2025, 224, 120394. [Google Scholar] [CrossRef]
- Lobato-Peralta, D.R.; Duque-Brito, E.; Villafán-Vidales, H.I.; Longoria, A.; Sebastian, P.J.; Cuentas-Gallegos, A.K.; Arancibia-Bulnes, C.A.; Okoye, P.U. A review on trends in lignin extraction and valorization of lignocellulosic biomass for energy applications. J. Clean. Prod. 2021, 293, 126123. [Google Scholar] [CrossRef]
- Saleem, A.; Wu, L.; Shi, H.; Wasim, M.; Huang, L.; Jia, W.; Arbab, A.; Tazeen, H. Cutting-edge innovations in lignin-based nanoparticles: A review of synthesis techniques, characterization, and diverse applications. Int. J. Biol. Macromol. 2025, 307, 142123. [Google Scholar] [CrossRef]
- Babaeipour, S.; Nousiainen, P.; Garcia, E.M.; Mohammadi, P.; Vuoriluoto, M.; Kimiaei, E.; Koivula, H.; Österberg, M. Oxidative crosslinking for the development of barrier coatings utilizing lignin-containing cellulose nanofibrils and lignin nanoparticles. Food Packag. Shelf 2025, 49, 101538. [Google Scholar] [CrossRef]
- Moser, B.R.; Cermak, S.C.; Evangelista, R.L. Fully biobased epoxy resins from ring opening polymerization of epoxidized pennycress (Thlaspi arvense L.) oil with itaconic and citric acids. Ind. Crops Prod. 2024, 208, 117914. [Google Scholar] [CrossRef]
- Pan, Y.; Li, R.; Jing, N.; Yi, J.; Liu, X.; Lu, J.; Zhu, J. Isorhamnetin-lignin nanoparticles: Green preparation, characterization, remarkable bioavailability and long-lasting bioactivity in vivo. Food Biosci. 2025, 69, 106931. [Google Scholar] [CrossRef]
- García-Fuentevilla, L.L.; Martín-Sampedro, R.; Darder, M.; Ibarra, D.; Eugenio, M.E. Bioactive nanocellulose films by incorporation of enzymatically polymerized lignin nanoparticles. Int. J. Biol. Macromol. 2025, 299, 140051. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Luo, X.; Su, Y.; Zhang, Z.; Wang, L.; Jiang, J.; Zhang, Y. High strength silk fibroin films induced by lignin nanoparticles for wireless mechanical sensing module. Chem. Eng. J. 2025, 518, 164712. [Google Scholar] [CrossRef]
- Gilca, I.A.; Popa, V.I.; Crestini, C. Obtaining lignin nanoparticles by sonication. Ultrason. Sonochem. 2015, 23, 369–375. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, L. Antisolvent precipitation for the preparation of high polymeric procyanidin nanoparticles under ultrasonication and evaluation of their antioxidant activity in vitro. Ultrason. Sonochem. 2018, 43, 208–218. [Google Scholar] [CrossRef]
- Peters, D. Ultrasound in materials chemistry. J. Mater. Chem. 1996, 6, 1605–1618. [Google Scholar] [CrossRef]
- Manjunatha, J.R.; Bettadaiah, B.K.; Negi, P.S.; Srinivas, P. Synthesis of amino acid conjugates of tetrahydrocurcumin and evaluation of their antibacterial and anti-mutagenic properties. Food Chem. 2013, 139, 332–338. [Google Scholar] [CrossRef]
- Morcillo-Martín, R.; Borrega, M.; Bertella, S.; Widsten, P.; Rincón, E.; Espinosa, E.; Rodríguez, A. Lignin micro/nanoparticles from alternative biomass sources for neutral-pH Pickering emulsions and quercetin encapsulation. Int. J. Biol. Macromol. 2025, 310, 143223. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Z.; Tang, S.; Yu, Z.; Zhang, Y.; Zheng, B. Ultrasound-assisted production of biodiesel from field pennycress (Thlaspi arvense L.) seeds: Process optimization and quality evaluation. Ind. Crops Prod. 2023, 203, 117224. [Google Scholar] [CrossRef]
- Dose, H.L.; Eberle, C.A.; Forcella, F.; Gesch, R.W. Early planting dates maximize winter annual field pennycress (Thlaspi arvense L.) yield and oil content. Ind. Crops Prod. 2017, 97, 477–483. [Google Scholar] [CrossRef]
- Boateng, A.A.; Mullen, C.A.; Goldberg, N.M. Producing Stable Pyrolysis Liquids from the Oil-Seed Presscakes of Mustard Family Plants: Pennycress (Thlaspi arvense L.) and Camelina (Camelina sativa). Energy Fuels 2010, 24, 6624–6632. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, X.; Li, M.; Peng, X.; Wei, M.; Zhang, X.; Yang, L.; Li, J. Biodiesel preparation from Thlaspi arvense L. seed oil utilizing a novel ionic liquid core-shell magnetic catalyst. Ind. Crops Prod. 2021, 162, 113316. [Google Scholar] [CrossRef]
- Zanetti, F.; Isbell, T.A.; Gesch, R.W.; Evangelista, R.L.; Alexopoulou, E.; Moser, B.; Monti, A. Turning a burden into an opportunity: Pennycress (Thlaspi arvense L.) a new oilseed crop for biofuel production. Biomass Bioenergy 2019, 130, 105354. [Google Scholar] [CrossRef]
- Zhao, R.; Ben, A.; Wei, M.; Ruan, M.; Gu, H.; Yang, L. Essential oil obtained from Thlaspi arvense L. leaves and seeds using microwave-assisted hydrodistillation and extraction in situ by vegetable oil and its antifungal activity against Penicillium expansum. LWT 2022, 165, 113718. [Google Scholar] [CrossRef]
- Kakran, M.; Sahoo, N.G.; Li, L.; Judeh, Z. Fabrication of quercetin nanoparticles by anti-solvent precipitation method for enhanced dissolution. Powder Technol. 2012, 223, 59–64. [Google Scholar] [CrossRef]
- Kakran, M.; Sahoo, N.G.; Li, L.; Judeh, Z.; Wang, Y.; Chong, K.; Loh, L. Fabrication of drug nanoparticles by evaporative precipitation of nanosuspension. Int. J. Pharm. 2010, 383, 285–292. [Google Scholar] [CrossRef]
- Reverchon, E.; Antonacci, A. Polymer microparticles production by supercritical assisted atomization. J. Supercrit. Fluid. 2007, 39, 444–452. [Google Scholar] [CrossRef]
- Wang, Y.; Pfeffer, R.; Dave, R.; Enick, R. Polymer encapsulation of fine particles by a supercritical antisolvent process. AIChE J. 2005, 51, 440–455. [Google Scholar] [CrossRef]
- Chen, A.; Pu, X.; Kang, Y.; Liao, L.; Yao, Y.; Yin, G. Study of poly(L-lactide) microparticles based on supercritical CO2. J. Mater. Sci. Mater. Med. 2007, 18, 2339–2345. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, Z.; Zhong, J.; Hu, T.; Chen, J.; Ma, Z.; Yun, J. Preparation of amorphous cefuroxime axetil nanoparticles by controlled nanoprecipitation method without surfactants. Int. J. Pharm. 2006, 323, 153–160. [Google Scholar] [CrossRef]
- Vinatoru, M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001, 8, 303–313. [Google Scholar] [CrossRef]
- Zhao, R.; Wei, M.; Shi, G.; Wang, X.; Gao, H.; Zhang, L.; Gu, H. One-pot process for simultaneously obtaining oil and sinigrin from field pennycress (Thlaspi arvense) seeds using microwave-assisted biphasic extraction. Ind. Crops Prod. 2021, 166, 113483. [Google Scholar] [CrossRef]
- Domínguez, J.C.; Oliet, M.; Alonso, M.V.; Gilarranz, M.A.; Rodríguez, F. Thermal stability and pyrolysis kinetics of organosolv lignins obtained from Eucalyptus globulus. Ind. Crops Prod. 2008, 27, 150–156. [Google Scholar] [CrossRef]
- Freitas, F.M.C.; Cerqueira, M.A.; Gonçalves, C.; Azinheiro, S.; Garrido-Maestu, A.; Vicente, A.A.; Pastrana, L.M.; Teixeira, J.A.; Michelin, M. Green synthesis of lignin nano- and micro-particles: Physicochemical characterization, bioactive properties and cytotoxicity assessment. Int. J. Biol. Macromol. 2020, 163, 1798–1809. [Google Scholar] [CrossRef] [PubMed]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Tejado, A.; Peña, C.; Labidi, J.; Echeverria, J.M.; Mondragon, I. Physico-chemical characterization of lignins from different sources for use in phenol-formaldehyde resin synthesis. Bioresour. Technol. 2007, 98, 1655–1663. [Google Scholar] [CrossRef]
- Wen, J.; Sun, S.; Xue, B.; Sun, R. Quantitative structures and thermal properties of birch lignins after ionic liquid pretreatment. J. Agric. Food Chem. 2013, 61, 635–645. [Google Scholar] [CrossRef]
- Chen, A.; Li, Y.; Chau, F.; Lau, T.; Hu, J.; Zhao, Z.; Mok, D.K. Application of organic nonsolvent in the process of solution-enhanced dispersion by supercritical CO2 to prepare puerarin fine particles. J. Supercrit. Fluid. 2009, 49, 394–402. [Google Scholar] [CrossRef]
- Tran, P.H.L.; Tran, H.T.T.; Lee, B.J. Modulation of microenvironmental pH and crystallinity of ionizable telmisartan using alkalizers in solid dispersions for controlled release. J. Control. Release 2008, 129, 59–65. [Google Scholar] [CrossRef]
- An, L.; Wang, G.; Jia, H.; Liu, C.; Sui, W.; Si, C. Fractionation of enzymatic hydrolysis lignin by sequential extraction for enhancing antioxidant performance. Int. J. Biol. Macromol. 2017, 99, 674–681. [Google Scholar] [CrossRef]
- Michelin, M.; Liebentritt, S.; Vicente, A.A.; Teixeira, J.A. Lignin from an integrated process consisting of liquid hot water and ethanol organosolv: Physicochemical and antioxidant properties. Int. J. Biol. Macromol. 2018, 120, 159–169. [Google Scholar] [CrossRef]
- Bahramian, B.; Kiani-Salmi, N.; Abedi-Firoozjah, R.; Tavassoli, M.; Dehnad, D.; Azizian, A.; Rezaie, M.; Hasani, R.; Ghaderi, S.; Majlesi, M. Ali Ehsani Innovative applications of lignin nanoparticles in food packaging: A comprehensive review. Future Foods 2025, 12, 100749. [Google Scholar] [CrossRef]
- Argenziano, R.; Viggiano, S.; Laezza, A.; Scalia, A.C.; Aprea, P.; Bochicchio, B.; Pepe, A.; Panzella, L.; Cochis, A.; Rimondini, L.; et al. Highly Cytocompatible Polylactic Acid Based Electrospun Microfibers Loaded with Silver Nanoparticles Generated onto Chestnut Shell Lignin for Targeted Antibacterial Activity and Antioxidant Action. ACS. Appl. Mater. Interfaces 2024, 16, 28230–28244. [Google Scholar] [CrossRef]
- Liu, C.; Liang, B.; Zhang, J.; Liu, Y.; Yi, S.; Li, J.; Ye, B.; Li, X. Rheological characteristics, microstructures and stability of soy protein isolate nanoparticles stabilized silver carp oil pickering emulsions via mechanical-ultrasonic shearing. Ultrason. Sonochem. 2025, in press. [Google Scholar] [CrossRef]
- Lu, Q.; Zhu, M.; Zu, Y.; Liu, W.; Yang, L.; Zhang, Y.; Zhao, X.; Zhang, X.; Zhang, X.; Li, W. Comparative antioxidant activity of nanoscale lignin prepared by a supercritical antisolvent (SAS) process with non-nanoscale lignin. Food Chem. 2012, 135, 63–67. [Google Scholar] [CrossRef]
- Zhou, S.; Lu, L.; Wang, B.; Xu, F.; Sun, R. Microwave-enhanced extraction of lignin from birch in formic acid: Structural characterization and antioxidant activity study. Process Biochem. 2012, 47, 1799–1806. [Google Scholar] [CrossRef]
- Jeffery, E.H.; Araya, M. Physiological effects of broccoli consumption. Phytochem. Rev. 2009, 8, 283–298. [Google Scholar] [CrossRef]

| No. | X1 a | X2 | X3 | X4 | X5 | X6 | X7 | APS (nm) |
|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 3 | 8 | 5 | 600 | 12.5 | 250 | 145 |
| 2 | 6 | 3 | 6 | 15 | 1200 | 12.5 | 150 | 143 |
| 3 | 6 | 3 | 6 | 5 | 600 | 12.5 | 150 | 161 |
| 4 | 6 | 1 | 8 | 5 | 1200 | 7.5 | 150 | 148 |
| 5 | 4 | 3 | 8 | 15 | 60 | 7.5 | 150 | 153 |
| 6 | b | 1 | 6 | 5 | 1200 | 12.5 | 250 | 153 |
| 7 | 4 | 1 | 8 | 15 | 1200 | 12.5 | 150 | 142 |
| 8 | 6 | 1 | 6 | 15 | 600 | 7.5 | 250 | 199 |
| 9 | 6 | 1 | 8 | 15 | 600 | 12.5 | 250 | 168 |
| 10 | 4 | 1 | 6 | 5 | 600 | 7.5 | 150 | 175 |
| 11 | 4 | 3 | 6 | 15 | 1200 | 7.5 | 250 | 141 |
| 12 | 6 | 3 | 8 | 5 | 1200 | 7.5 | 250 | 135 |
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value | Inference |
|---|---|---|---|---|---|---|
| Model | 3586.15 | 7 | 512.31 | 20.85 | 0.0054 | * |
| Residual | 98.30 | 4 | 24.57 | |||
| Cor total | 3684.45 | 11 | ||||
| Regression data | ||||||
| Term a | Effect | Coefficient | Standard error | F-value | p-value | |
| X1 | −17.90 | −8.95 | 1.43 | 39.12 | 0.0033 | * |
| X2 | 7.89 | 3.92 | 1.43 | 7.49 | 0.0522 | |
| X3 | 3.30 | 1.65 | 1.43 | 1.33 | 0.3131 | |
| X4 | 5.24 | 2.62 | 1.43 | 3.34 | 0.1415 | |
| X5 | −23.46 | −11.73 | 1.43 | 67.23 | 0.0012 | * |
| X6 | −6.30 | −3.15 | 1.43 | 4.85 | 0.0925 | |
| X7 | −13.60 | −6.80 | 1.43 | 22.58 | 0.0090 | * |
| No. | X1: Dropping Speed (mL/min) | X5: Stirring Speed (r/min) | X7: Ultrasound Irradiation Power (W) | APS (nm) | |
|---|---|---|---|---|---|
| Predicted Value | Actual Value | ||||
| 1 | 4 | 900 | 150 | 178.3 | 179.6 |
| 2 | 4 | 600 | 200 | 170.0 | 169.1 |
| 3 | 6 | 900 | 250 | 123.5 | 122.2 |
| 4 | 5 | 900 | 200 | 135.4 | 134.4 |
| 5 | 5 | 600 | 250 | 143.5 | 144.9 |
| 6 | 5 | 900 | 200 | 133.7 | 134.4 |
| 7 | 5 | 900 | 200 | 132.5 | 134.4 |
| 8 | 4 | 1200 | 200 | 145.0 | 145.2 |
| 9 | 5 | 900 | 200 | 135.5 | 134.4 |
| 10 | 6 | 600 | 200 | 142.3 | 142.1 |
| 11 | 5 | 600 | 150 | 179.8 | 179.4 |
| 12 | 6 | 900 | 150 | 148.0 | 148.6 |
| 13 | 5 | 1200 | 250 | 124.6 | 125.0 |
| 14 | 5 | 1200 | 150 | 157.7 | 156.2 |
| 15 | 4 | 900 | 250 | 141.0 | 140.4 |
| 16 | 5 | 900 | 200 | 134.9 | 134.4 |
| 17 | 5 | 1200 | 200 | 122.0 | 122.9 |
| ANOVA | ||||||||
|---|---|---|---|---|---|---|---|---|
| Source | Sum of Squares | Degree of Freedom | Mean Square | F-Value | p-Value | |||
| Model a | 5081.78 | 9 | 564.64 | 230.57 | <0.0001 * | |||
| X1 | 1212.78 | 1 | 1212.78 | 495.23 | <0.0001 * | |||
| X5 | 930.96 | 1 | 930.96 | 380.15 | <0.0001 * | |||
| X7 | 2151.68 | 1 | 2151.68 | 878.62 | <0.0001 * | |||
| X1X5 | 5.52 | 1 | 5.52 | 2.26 | 0.1769 | |||
| X1X7 | 40.96 | 1 | 40.96 | 16.73 | 0.0046 * | |||
| X5X7 | 2.56 | 1 | 2.56 | 1.05 | 0.3406 | |||
| X12 | 47.61 | 1 | 47.61 | 19.44 | 0.0031 * | |||
| X52 | 210.02 | 1 | 210.02 | 85.76 | <0.0001 * | |||
| X2 | 415.81 | 1 | 415.81 | 169.79 | <0.0001 * | |||
| Residual | 17.14 | 7 | 2.45 | |||||
| Lack of fit | 10.58 | 3 | 3.53 | 2.15 | 0.2366 | |||
| Pure error | 6.56 | 4 | 1.64 | |||||
| Corrected total | 5098.92 | 16 | ||||||
| Credibility analysis of the regression equations | ||||||||
| Index mark | Standard deviation | Mean | CV % | Press | R2 | Adjust R2 | Predicted R2 | Adequacy precision |
| APS b | 1.56 | 143.98 | 1.09 | 179.57 | 0.9966 | 0.9923 | 0.9648 | 47.845 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, R.; Xu, W.; Tang, Y.; Liu, J.; Li, X.; Tan, L.; Ben, A.; Liu, T.; Yang, L. Preparation of Lignin Nanoparticles from Thlaspi arvense L. Rhizomes via Ultrasound-Assisted Antisolvent Precipitation: Nanostructural Characterization and Evaluation of Their Radical Scavenging Activity. Molecules 2025, 30, 4070. https://doi.org/10.3390/molecules30204070
Zhao R, Xu W, Tang Y, Liu J, Li X, Tan L, Ben A, Liu T, Yang L. Preparation of Lignin Nanoparticles from Thlaspi arvense L. Rhizomes via Ultrasound-Assisted Antisolvent Precipitation: Nanostructural Characterization and Evaluation of Their Radical Scavenging Activity. Molecules. 2025; 30(20):4070. https://doi.org/10.3390/molecules30204070
Chicago/Turabian StyleZhao, Ru, Wenjun Xu, Yuxiang Tang, Jinwen Liu, Xiaoli Li, Liangui Tan, Ailing Ben, Tingli Liu, and Lei Yang. 2025. "Preparation of Lignin Nanoparticles from Thlaspi arvense L. Rhizomes via Ultrasound-Assisted Antisolvent Precipitation: Nanostructural Characterization and Evaluation of Their Radical Scavenging Activity" Molecules 30, no. 20: 4070. https://doi.org/10.3390/molecules30204070
APA StyleZhao, R., Xu, W., Tang, Y., Liu, J., Li, X., Tan, L., Ben, A., Liu, T., & Yang, L. (2025). Preparation of Lignin Nanoparticles from Thlaspi arvense L. Rhizomes via Ultrasound-Assisted Antisolvent Precipitation: Nanostructural Characterization and Evaluation of Their Radical Scavenging Activity. Molecules, 30(20), 4070. https://doi.org/10.3390/molecules30204070







