Phenolic Derivatives of Astragalus aitosensis with Selective MAO-B Inhibition and Mitochondrial Protection
Abstract
1. Introduction
2. Results
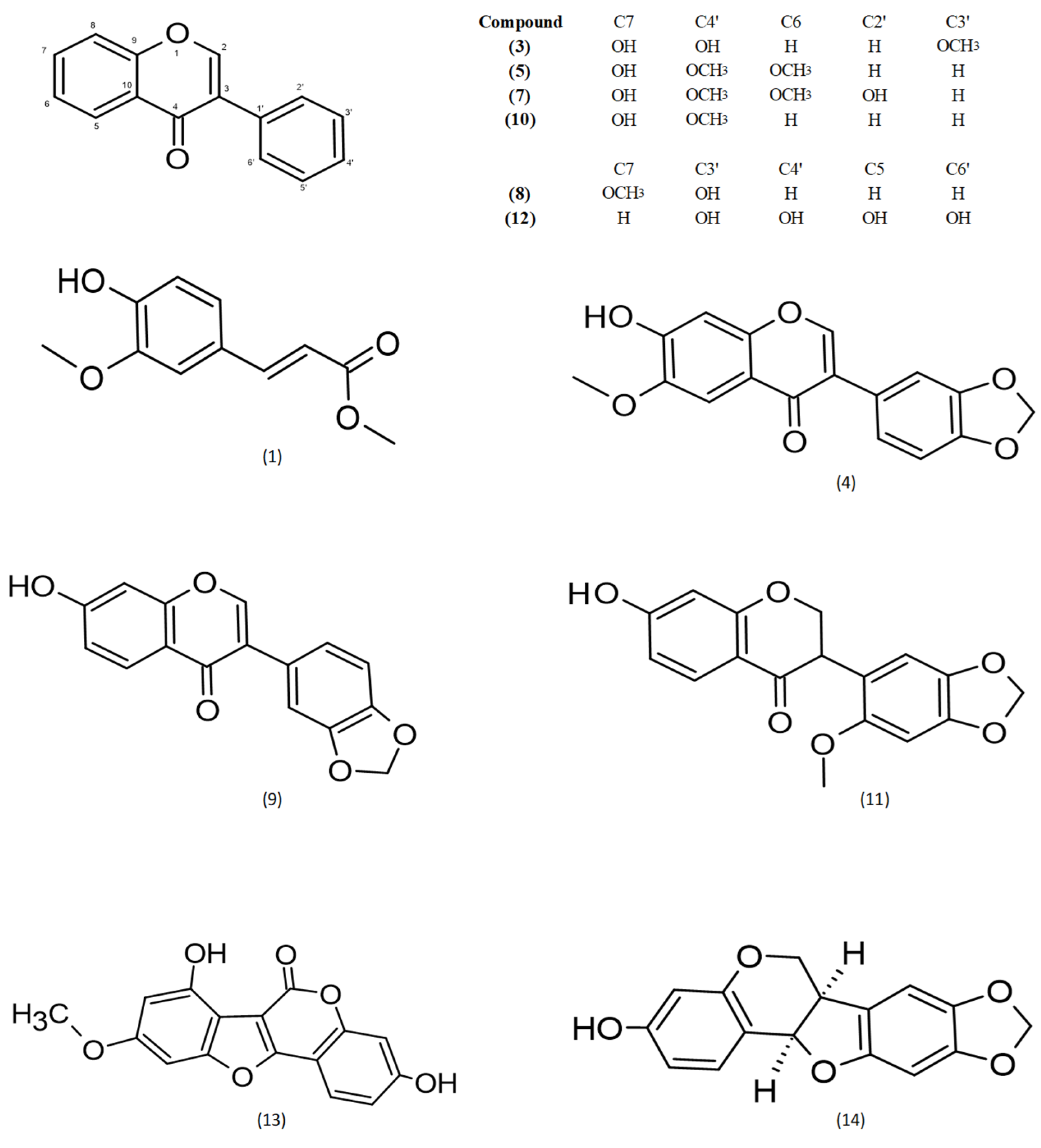
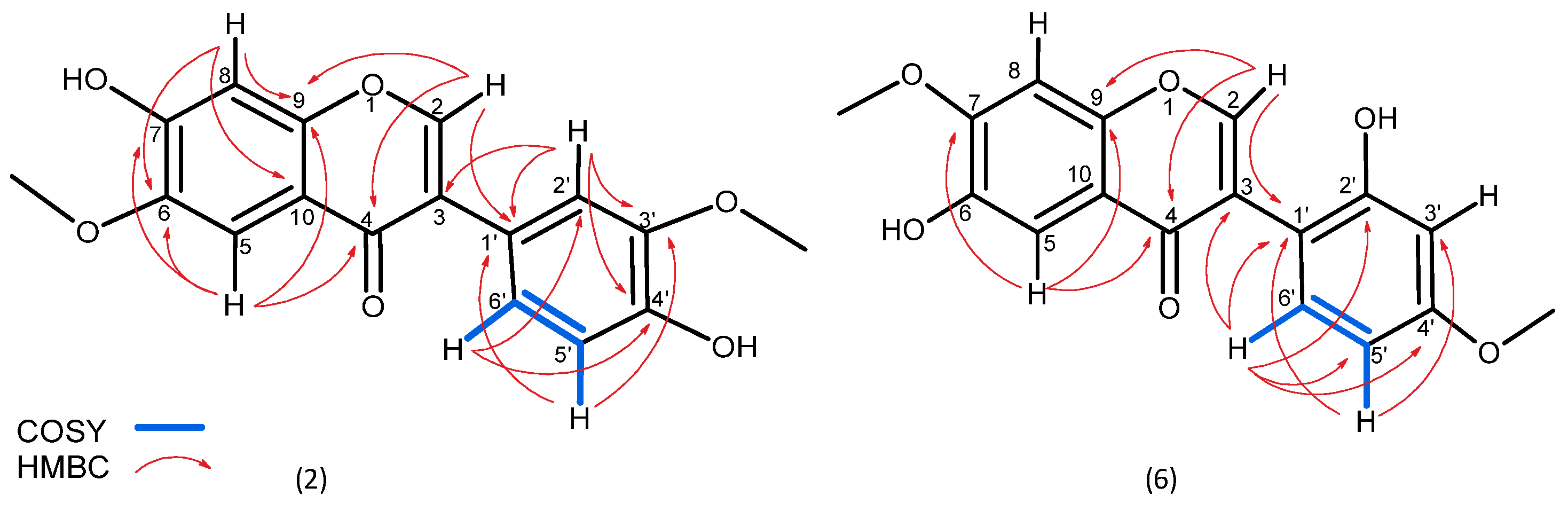
2.1. Identification of Isolated Substances
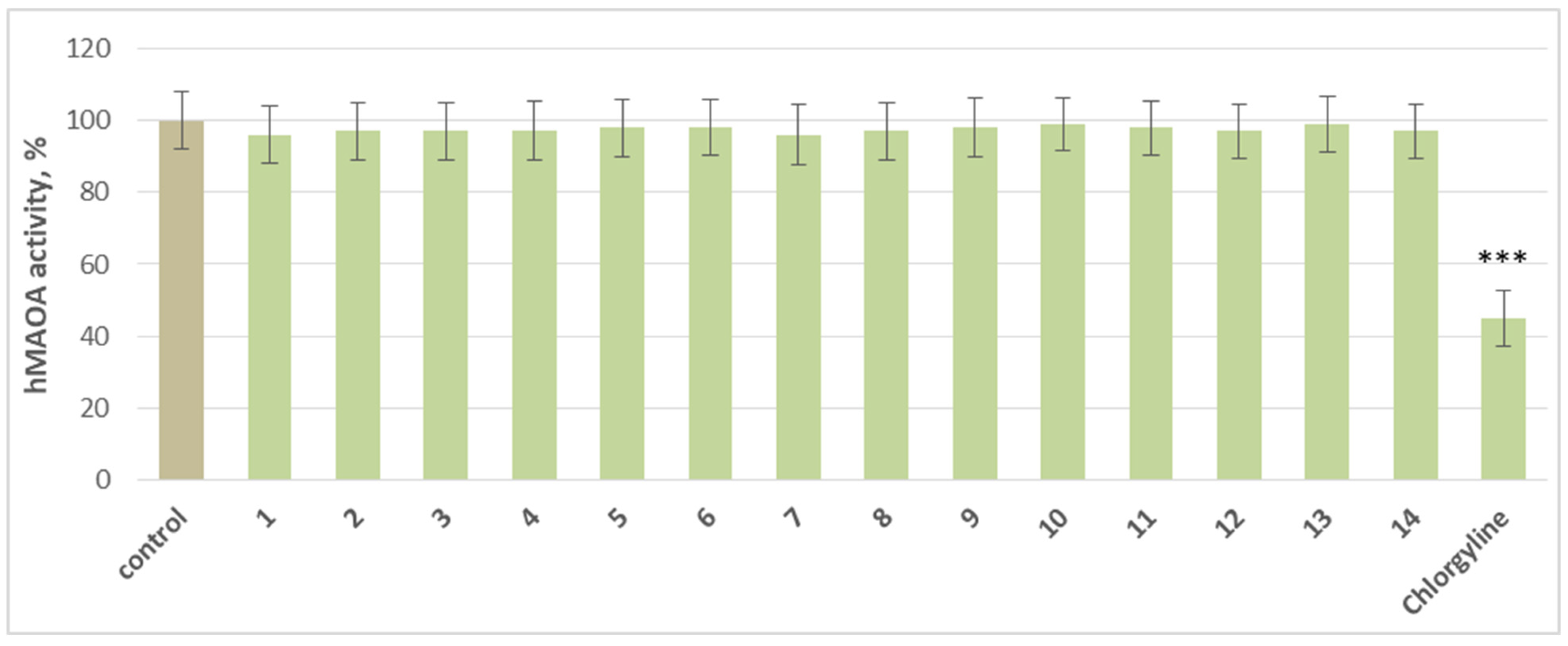
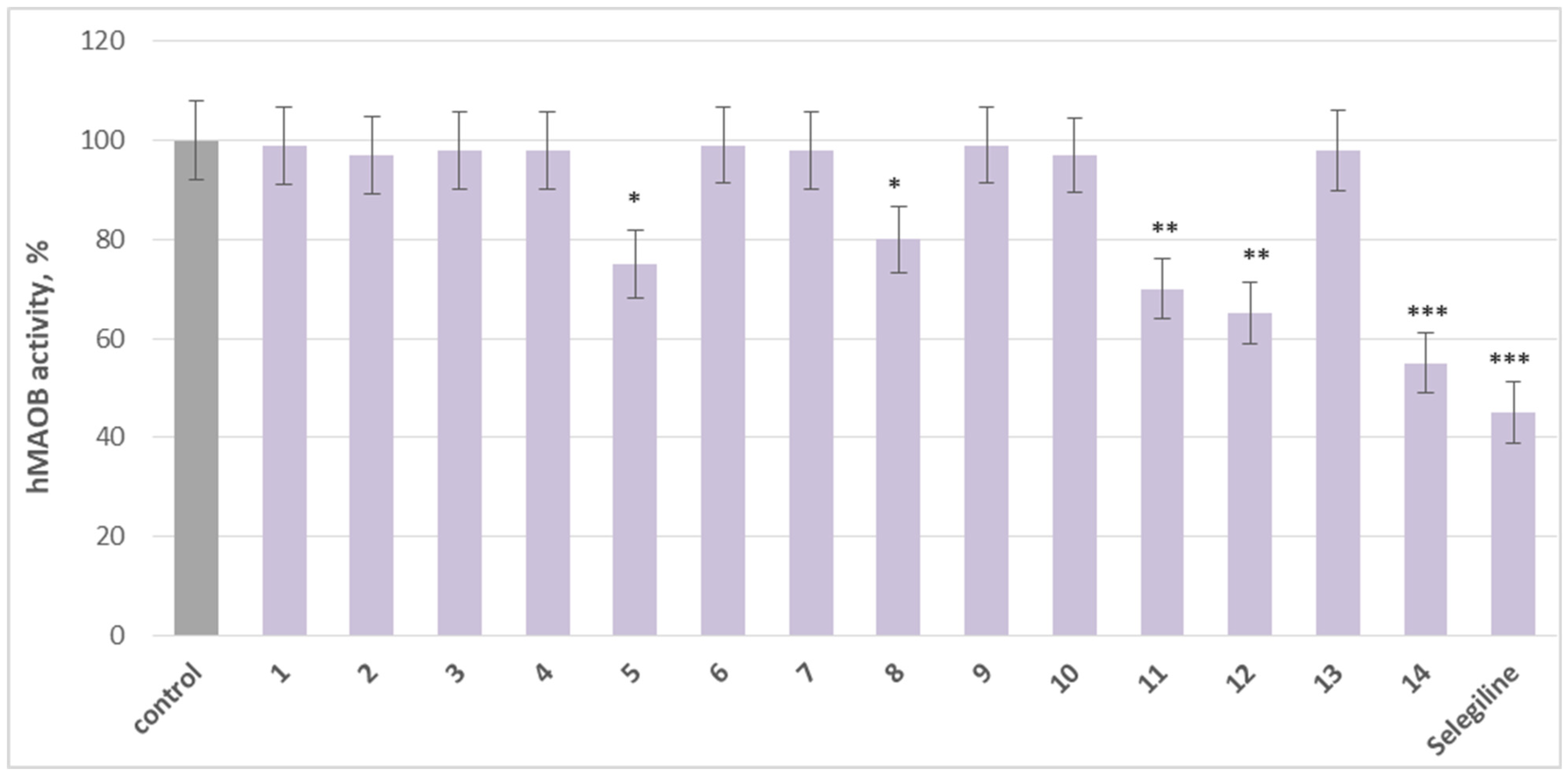
2.2. Effects on Human Recombinant MAO-A/MAO-B Enzyme (hMAO-A/hMAO-B)
2.3. Effects on Isolated Rat Brain Synaptosomes
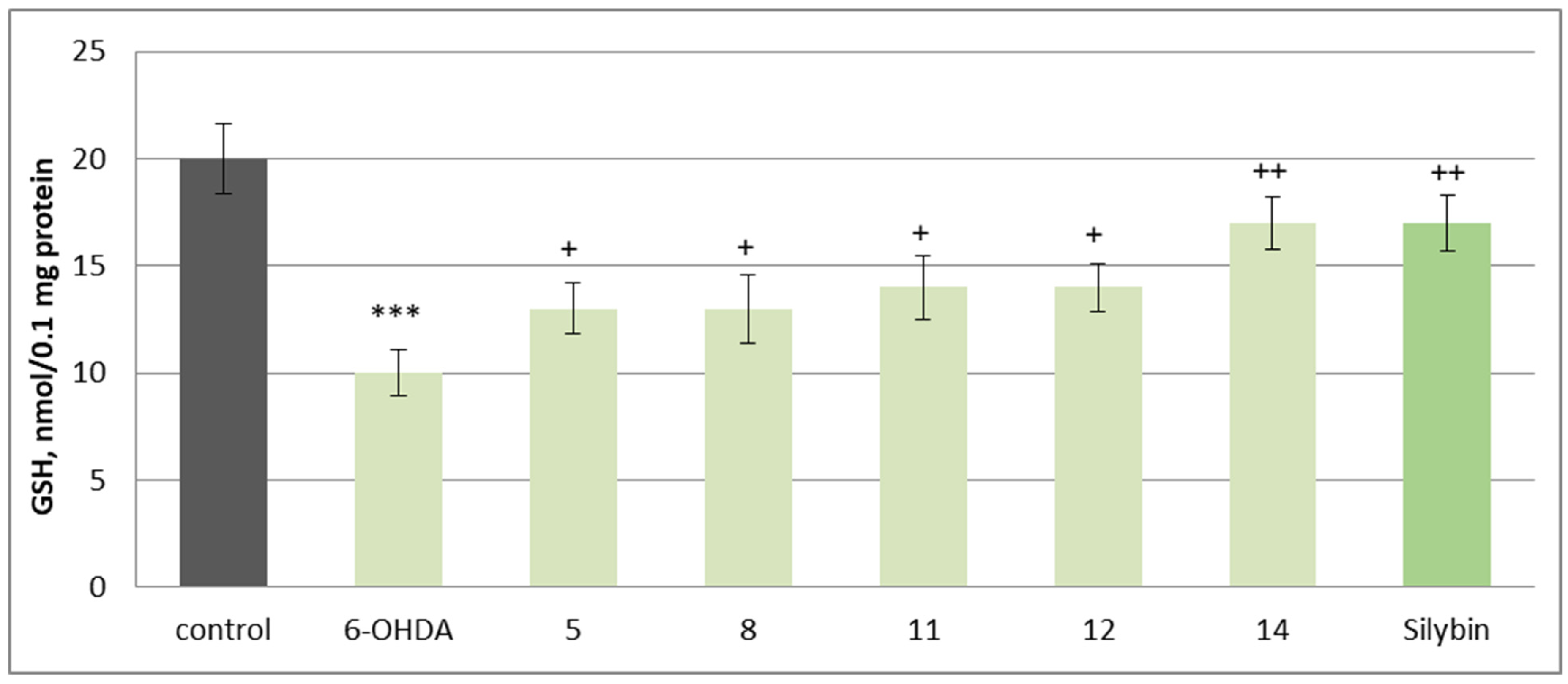
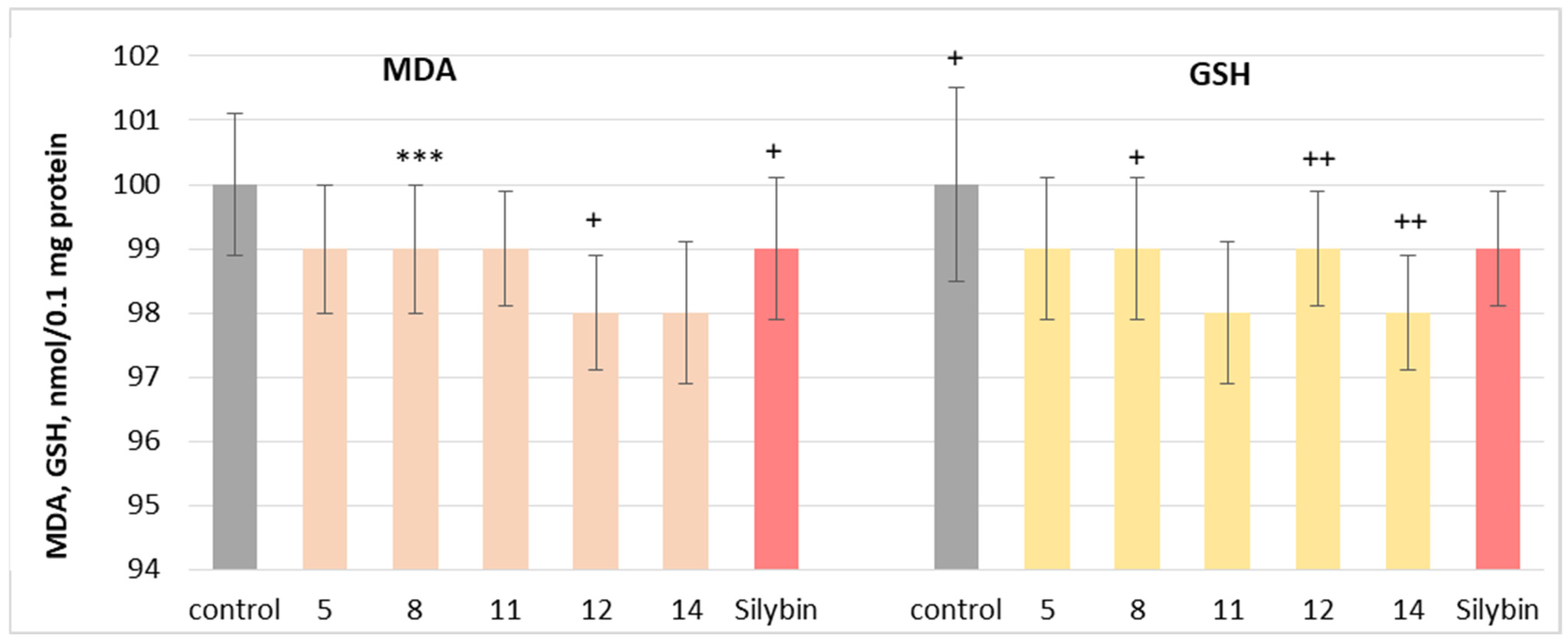
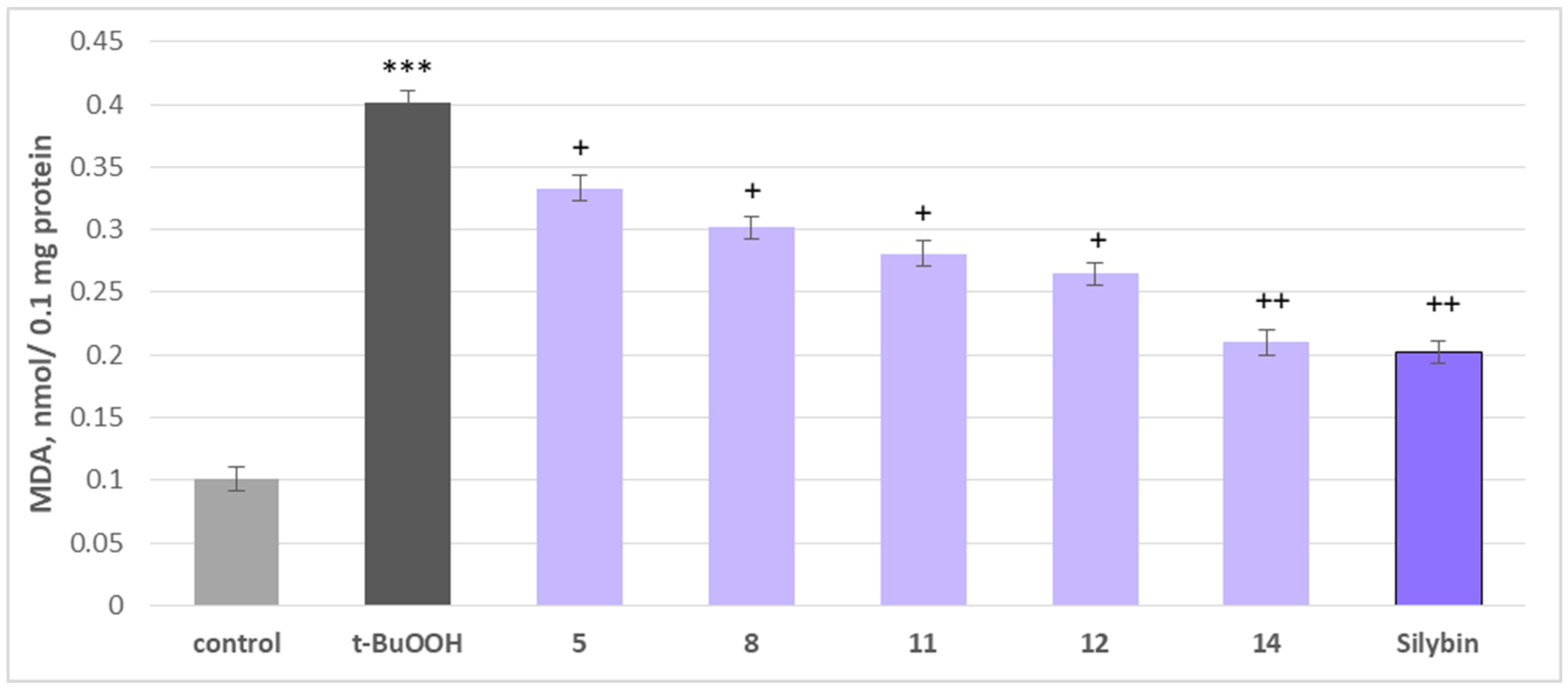
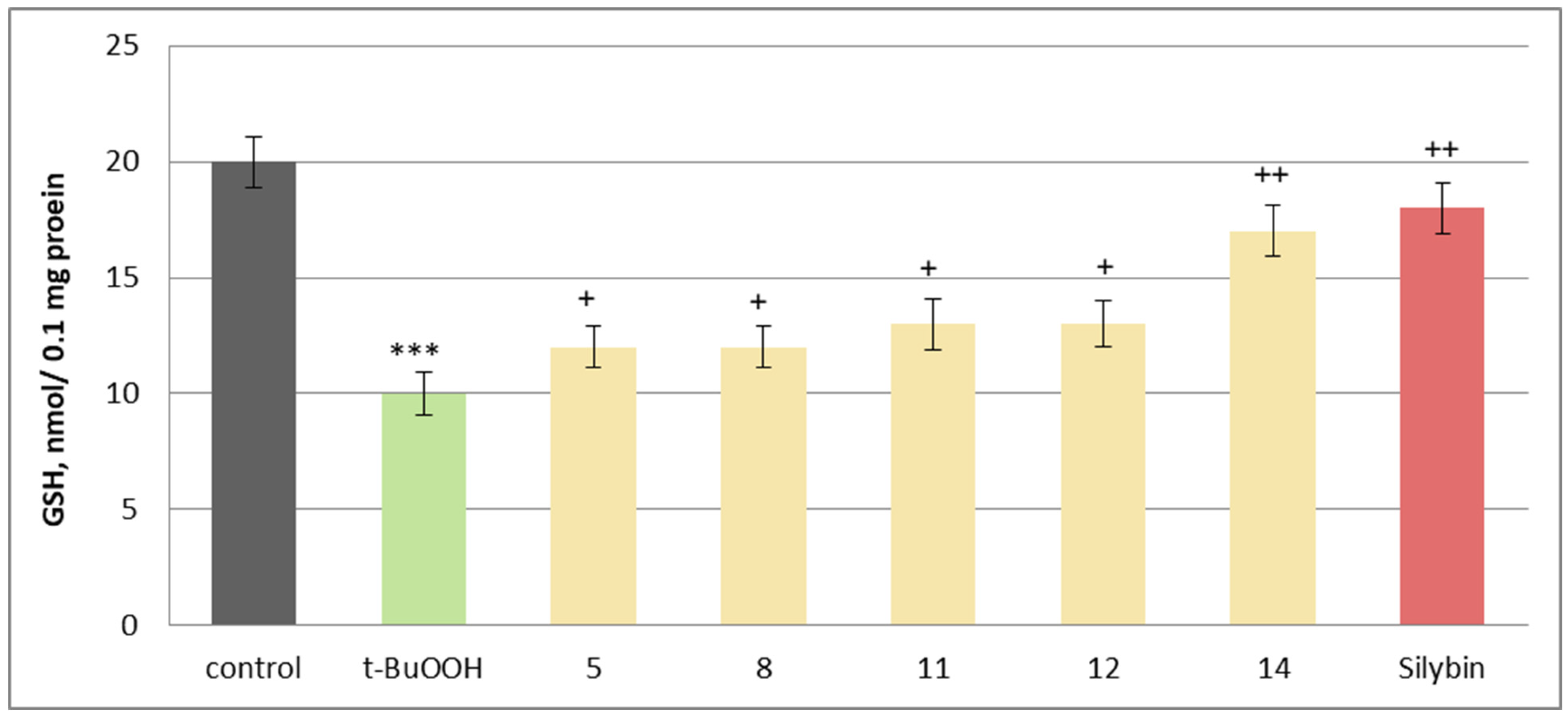
2.4. Neuroprotective and Antioxidant Effects
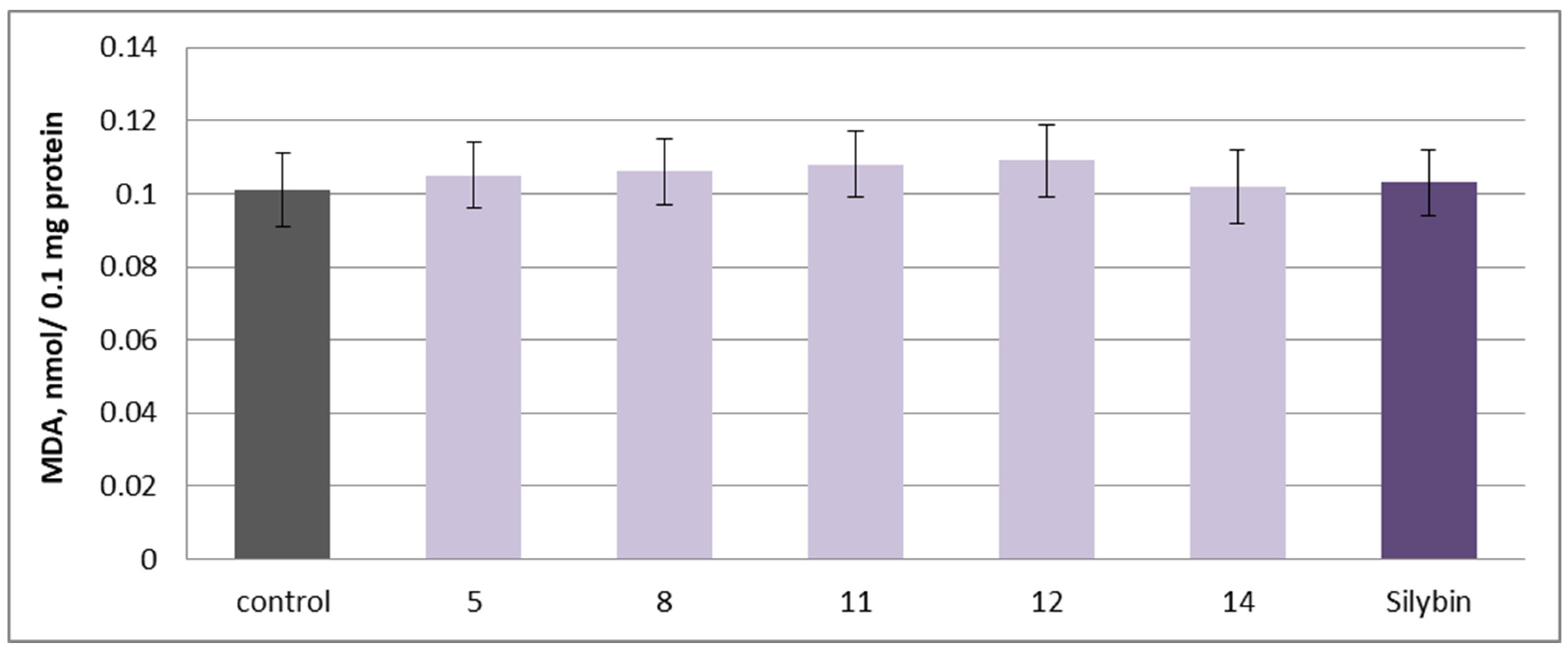
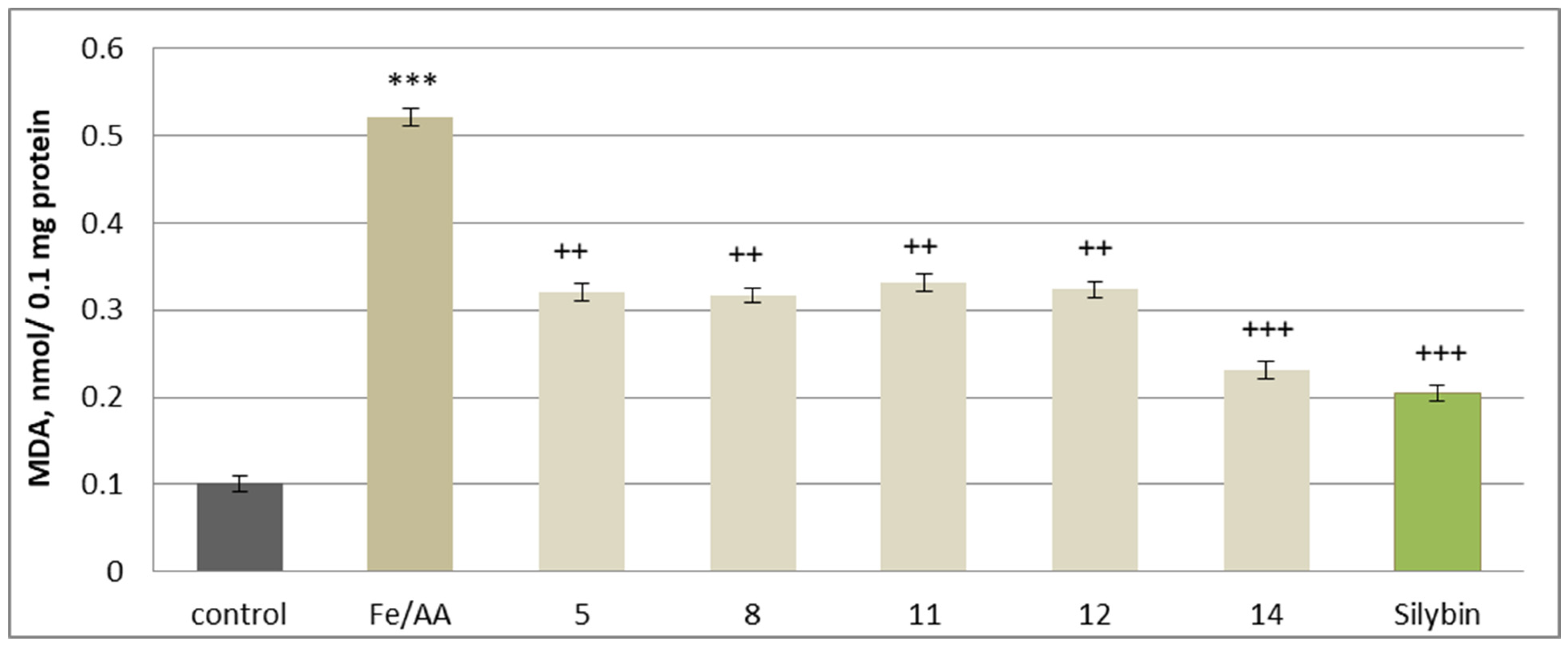
2.5. Effects on Isolated Rat Brain Mitochondria
2.6. Effects on Isolated Rat Brain Microsomes
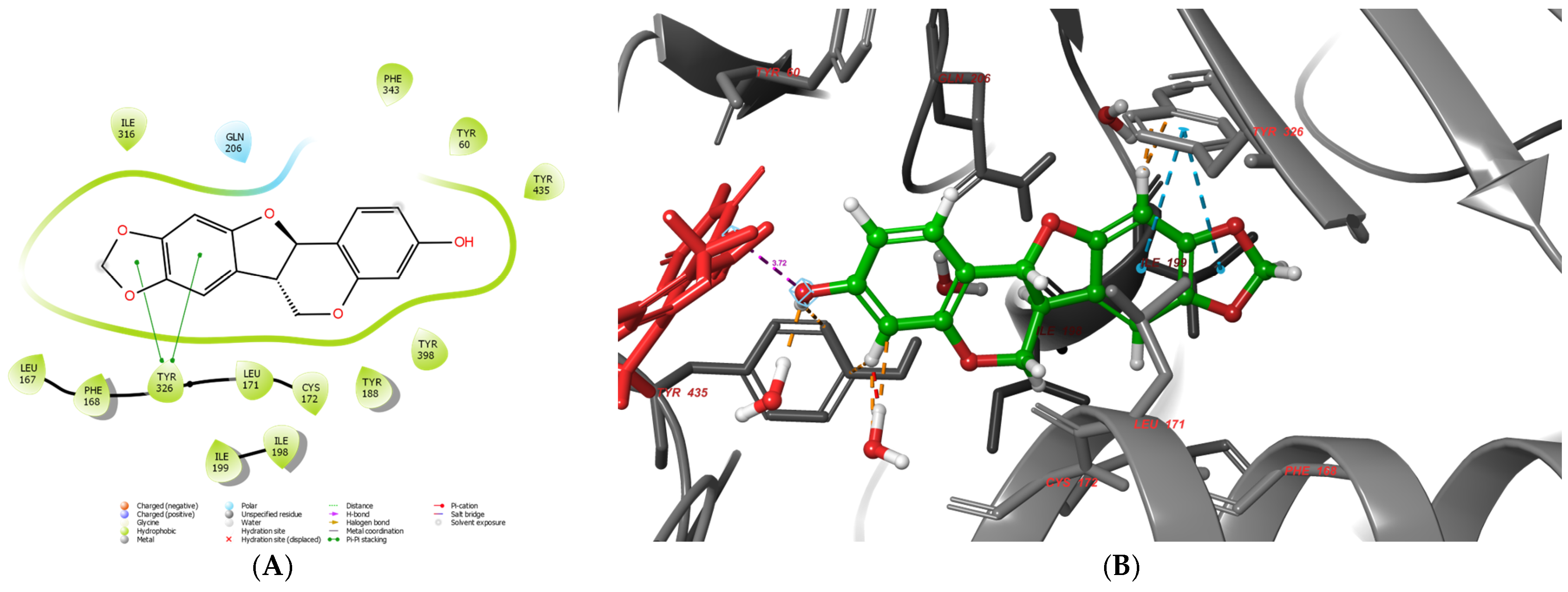
2.7. Molecular Docking Results
3. Discussion
3.1. Occurrence of the Isolated Phytochemicals
3.2. Pharmacological Evaluation
3.3. Structure–Activity Relationship
4. Materials and Methods
4.1. Plant Material
4.2. General Experimental Procedures
4.3. Extraction and Isolation
4.4. Pharmacology Procedures
4.5. Molecular Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amiri, M.S.; Joharchi, M.R.; Nadaf, M.; Nasseh, Y. Ethnobotanical Knowledge of Astragalus Spp.: The World’s Largest Genus of Vascular Plants. Avicenna J. Phytomed. 2020, 10, 128–142. [Google Scholar]
- Li, X.; Qu, L.; Dong, Y.; Han, L.; Liu, E.; Fang, S.; Zhang, Y.; Wang, T. A Review of Recent Research Progress on the Astragalus Genus. Molecules 2014, 19, 18850–18880. [Google Scholar] [CrossRef]
- Walter, K.S.; Gillett, H.J. 1997 IUCN Red List of Threatened Plants; International Union for Conservation of Nature and Natural Resources, Ed.; IUCN, World Conservation Union: Cambridge, UK, 1998.
- Astracantha arnacantha: Red Data Book of Bulgaria. Available online: http://www.e-ecodb.bas.bg/rdb/en/vol1/Astarnac.html (accessed on 20 January 2025).
- Petrova, A.; Vladimirov, V.; Dimitrova, D.; Ivanova, D. Current State of the Bulgarian Fern and Seed Plant Biodiversity; Bulgarian Biodiversity Platform: Sofia, Bulgaria, 2005. (In Bulgarian) [Google Scholar]
- Communities of Astracantha aitosensis: Red Data Book of Bulgaria. Available online: http://e-ecodb.bas.bg/rdb/en/vol3/30F7.html (accessed on 20 January 2025).
- Shkondrov, A.; Krasteva, I.; Bucar, F.; Kunert, O.; Kondeva-Burdina, M.; Ionkova, I. Flavonoids and Saponins from Two Bulgarian Astragalus Species and Their Neuroprotective Activity. Phytochem. Lett. 2018, 26, 44–49. [Google Scholar] [CrossRef]
- Bratkov, V.M.; Shkondrov, A.M.; Zdraveva, P.K.; Krasteva, I.N. Flavonoids from the Genus Astragalus: Phytochemistry and Biological Activity. Pharmacogn. Rev. 2016, 10, 11–32. [Google Scholar] [CrossRef] [PubMed]
- Ionkova, I.; Shkondrov, A.; Zarev, Y.; Kozuharova, E.; Krasteva, I. Anticancer Secondary Metabolites: From Ethnopharmacology and Identification in Native Complexes to Biotechnological Studies in Species of Genus Astragalus L. and Gloriosa L. Curr. Issues Mol. Biol. 2022, 44, 3884–3904. [Google Scholar] [CrossRef] [PubMed]
- Stambolov, I.; Shkondrov, A.; Krasteva, I. Astragalus glycyphyllos L.: Phytochemical Constituents, Pharmacology, and Biotechnology. Pharmacia 2023, 70, 635–641. [Google Scholar] [CrossRef]
- Dong, N.; Lin, H. Contribution of Phenylpropanoid Metabolism to Plant Development and Plant–Environment Interactions. J. Integr. Plant Biol. 2021, 63, 180–209. [Google Scholar] [CrossRef]
- Liang, C.; Wang, P.; Li, M.; Li, R.; Lai, K.P.; Chen, J. Anti-Cancer Mechanisms of Natural Isoflavones against Melanoma. Heliyon 2024, 10, e28616. [Google Scholar] [CrossRef]
- Khan, A.N.; Jawarkar, R.D.; Zaki, M.E.A.; Mutairi, A.A.A.; Mali, S.N. Targeting PI3K/AKT/mTOR Signalling Pathway in Non-Small-Cell Lung Carcinoma: Exploring Promising Bioactive Natural Compounds as Anti-Cancer Agents. Chem. Phys. Impact 2025, 10, 100793. [Google Scholar] [CrossRef]
- Luo, H.; Yang, S.; Deng, P.; Peng, Y.; Chen, Z.; Yang, C.; Wang, M.; Qin, R.; Yuan, L.; Chen, X.; et al. Network Pharmacology Combined with Transcriptomics Reveals That Formononetin, a Biologically Component of Astragalus Membranaceus (Fisch.) Bunge, Inhibits the PI3K/AKT Signaling Pathway to Improve Chronic Renal Failure. J. Ethnopharmacol. 2025, 338, 119041. [Google Scholar] [CrossRef]
- Joshi, A.; Kathuria, D.; Paul, M.; Singh, N. An Overview on the Potential Application of Nanotechnology in Enhancing the Therapeutic Efficacy of Phytoestrogens. Food Chem. 2025, 464, 141779. [Google Scholar] [CrossRef]
- Shi, Y.; Shi, X.; Zhao, M.; Ma, S.; Zhang, Y. Pharmacological Potential of Astragali Radix for the Treatment of Kidney Diseases. Phytomedicine 2024, 123, 155196. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Ryu, H.W.; Kang, M.-G.; Park, D.; Oh, S.-R.; Kim, H. Potent Selective Monoamine Oxidase B Inhibition by Maackiain, a Pterocarpan from the Roots of Sophora flavescens. Bioorg. Med. Chem. Lett. 2016, 26, 4714–4719. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-S.; Lee, S.A.; Hong, S.S.; Lee, K.S.; Lee, M.K.; Hwang, B.Y.; Ro, J.S. Monoamine Oxidase Inhibitory Components from the Roots of Sophora flavescens. Arch. Pharm. Res. 2005, 28, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.M.; Jang, H.-J.; Kang, M.-G.; Mun, S.-K.; Park, D.; Hong, S.-J.; Kim, M.H.; Kim, S.-Y.; Yee, S.-T.; Kim, H. Medicarpin and Homopterocarpin Isolated from Canavalia lineata as Potent and Competitive Reversible Inhibitors of Human Monoamine Oxidase-B. Molecules 2022, 28, 258. [Google Scholar] [CrossRef]
- Chaurasiya, N.; Leon, F.; Muhammad, I.; Tekwani, B. Natural Products Inhibitors of Monoamine Oxidases—Potential New Drug Leads for Neuroprotection, Neurological Disorders, and Neuroblastoma. Molecules 2022, 27, 4297. [Google Scholar] [CrossRef]
- Gampe, N.; Dávid, D.N.; Takács-Novák, K.; Backlund, A.; Béni, S. In Vitro and In Silico Evaluation of Ononis Isoflavonoids as Molecules Targeting the Central Nervous System. PLoS ONE 2022, 17, e0265639. [Google Scholar] [CrossRef]
- Pu, W.; Wang, D.; Zhou, D. Structural Characterization and Evaluation of the Antioxidant Activity of Phenolic Compounds from Astragalus taipaishanensis and Their Structure-Activity Relationship. Sci. Rep. 2015, 5, 13914. [Google Scholar] [CrossRef]
- Abd El-Latif, R.R.; Shabana, M.H.; El-Gandour, A.H.; Mansour, R.M.; Sharaf, M. A New Isoflavone from Astragalus peregrinus. Chem. Nat. Compd. 2003, 39, 536–537. [Google Scholar] [CrossRef]
- Lenssen, A.W.; Townsend, C.E.; Martin, S.S. Clonal-by-Environment Interactions Influence Isoflavonoid Accumulation in Cicer milkvetch. Crop Sci. 1995, 35, 756–763. [Google Scholar] [CrossRef]
- Fraser, C.M.; Chapple, C. The Phenylpropanoid Pathway in Arabidopsis. Arab. Book 2011, 9, e0152. [Google Scholar] [CrossRef]
- Martin, S.S.; Townsend, C.E.; Lenssen, A.W. Induced Isoflavonoids in Diverse Populations of Astragalus cicer. Biochem. Syst. Ecol. 1994, 22, 657–661. [Google Scholar] [CrossRef]
- El-Sebakhy, N.A.; Asaad, A.M.; Abdallah, R.M.; Toaima, S.M.; Abdel-Kader, M.S.; Stermitz, F.R. Antimicrobial Isoflavans from Astragalus species. Phytochemistry 1994, 36, 1387–1389. [Google Scholar] [CrossRef] [PubMed]
- Ingham, J.L.; Dewick, P.M. Astraciceran: A New Isoflavan Phytoalexin from Astragalus cicer. Phytochemistry 1980, 19, 1767–1770. [Google Scholar] [CrossRef]
- Vasilev, H.; Ross, S.; Šmejkal, K.; Maršík, P.; Jankovská, D.; Havlík, J.; Veselý, O. Flavonoid Glycosides from Endemic Bulgarian Astragalus aitosensis (Ivanisch.). Molecules 2019, 24, 1419. [Google Scholar] [CrossRef]
- Ionkova, I. Isolation and HPLC-TLC Analysis of the Major Flavonoids from Astragalus aitosensis. Planta Med. 1990, 56, 581. [Google Scholar] [CrossRef]
- Taupin, P.; Zini, S.; Cesselin, F.; Ben-Ari, Y.; Roisin, M. Subcellular Fractionation on Percoll Gradient of Mossy Fiber Synaptosomes: Morphological and Biochemical Characterization in Control and Degranulated Rat Hippocampus. J. Neurochem. 1994, 62, 1586–1595. [Google Scholar] [CrossRef]
- Sims, N.R.; Anderson, M.F. Isolation of Mitochondria from Rat Brain Using Percoll Density Gradient Centrifugation. Nat. Protoc. 2008, 3, 1228–1239. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Stokes, A.H.; Freeman, W.M.; Mitchell, S.G.; Burnette, T.A.; Hellmann, G.M.; Vrana, K.E. Induction of GADD45 and GADD153 in Neuroblastoma Cells by Dopamine-Induced Toxicity. NeuroToxicology 2002, 23, 675–684. [Google Scholar] [CrossRef]
- Karlsson, J.; Emgard, M.; Brundin, P.; Burkitt, M.J. Trans -Resveratrol Protects Embryonic Mesencephalic Cells from tert -Butyl Hydroperoxide: Electron Param. J. Neurochem. 2000, 75, 141–150. [Google Scholar] [CrossRef]
- Ravindranath, V.; Anandatheerthavarada, H.K. Preparation of Brain Microsomes with Cytochrome P450 Activity Using Calcium Aggregation Method. Anal. Biochem. 1990, 187, 310–313. [Google Scholar] [CrossRef]
- Mungarro-Menchaca, X.; Ferrera, P.; Morán, J.; Arias, C. β-Amyloid Peptide Induces Ultrastructural Changes in Synaptosomes and Potentiates Mitochondrial Dysfunction in the Presence of Ryanodine. J. Neurosci. Res. 2002, 68, 89–96. [Google Scholar] [CrossRef]
- Robyt, J.F.; Ackerman, R.J.; Chittenden, C.G. Reaction of Protein Disulfide Groups with Ellman’s Reagent: A Case Study of the Number of Sulfhydryl and Disulfide Groups in Aspergillus Oryzae α-Amylase, Papain, and Lysozyme. Arch. Biochem. Biophys. 1971, 147, 262–269. [Google Scholar] [CrossRef]
- Shirani, M.; Alizadeh, S.; Mahdavinia, M.; Dehghani, M.A. The Ameliorative Effect of Quercetin on Bisphenol A-Induced Toxicity in Mitochondria Isolated from Rats. Environ. Sci. Pollut. Res. 2019, 26, 7688–7696. [Google Scholar] [CrossRef]
- Mansuy, D.; Sassi, A.; Dansette, P.M.; Plat, M. A New Potent Inhibitor of Lipid Peroxidation In Vitro and In Vivo, the Hepatoprotective Drug Anisyldithiolthione. Biochem. Biophys. Res. Commun. 1986, 135, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Aguilera, O.M.; Esteban, G.; Bolea, I.; Nikolic, K.; Agbaba, D.; Moraleda, I.; Iriepa, I.; Samadi, A.; Soriano, E.; Unzeta, M.; et al. Design, Synthesis, Pharmacological Evaluation, QSAR Analysis, Molecular Modeling and ADMET of Novel Donepezil–Indolyl Hybrids as Multipotent Cholinesterase/Monoamine Oxidase Inhibitors for the Potential Treatment of Alzheimer’s Disease. Eur. J. Med. Chem. 2014, 75, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Binda, C.; Wang, J.; Pisani, L.; Caccia, C.; Carotti, A.; Salvati, P.; Edmondson, D.E.; Mattevi, A. Structures of Human Monoamine Oxidase B Complexes with Selective Noncovalent Inhibitors: Safinamide and Coumarin Analogs. J. Med. Chem. 2007, 50, 5848–5852. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger Release Notes—Release 2024-1. Schrödinger. Available online: https://www.schrodinger.com/life-science/download/release-notes/release-2024-1/ (accessed on 15 April 2025).
- Botti, G.; Bianchi, A.; Pavan, B.; Tedeschi, P.; Albanese, V.; Ferraro, L.; Spizzo, F.; Del Bianco, L.; Dalpiaz, A. Effects of Microencapsulated Ferulic Acid or Its Prodrug Methyl Ferulate on Neuroinflammation Induced by Muramyl Dipeptide. Int. J. Environ. Res. Public Health 2022, 19, 10609. [Google Scholar] [CrossRef]
- Yahara, S.; Ogata, T.; Saijo, R.; Konishi, R.; Yamahara, J.; Miyahara, K.; Nohara, T. Isoflavan and Related Compounds from Dalbergia odorifera. I. Chem. Pharm. Bull. 1989, 37, 979–987. [Google Scholar] [CrossRef]
- Rong, H.; Stevens, J.; Deinzer, M.; Cooman, L.; Keukeleire, D. Identification of Isoflavones in the Roots of Pueraria Lobata. Planta Med. 1998, 64, 620–627. [Google Scholar] [CrossRef]
- Velozo, L.S.M.; Da Silva, B.P.; Da Silva, E.M.B.; Parente, J.P. Constituents from the Roots of Bowdichia virgilioides. Fitoterapia 1999, 70, 532–535. [Google Scholar] [CrossRef]
- Puebla, P.; Oshima-Franco, Y.; Franco, L.M.; Santos, M.G.D.; Silva, R.V.D.; Rubem-Mauro, L.; Feliciano, A.S. Chemical Constituents of the Bark of Dipteryx alata Vogel, an Active Species against Bothrops jararacussu Venom. Molecules 2010, 15, 8193–8204. [Google Scholar] [CrossRef] [PubMed]
- Belofsky, G.; Carreno, R.; Lewis, K.; Ball, A.; Casadei, G.; Tegos, G.P. Metabolites of the “Smoke Tree”, Dalea spinosa, Potentiate Antibiotic Activity against Multidrug-Resistant Staphylococcus aureus. J. Nat. Prod. 2006, 69, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Isogai, Y.; Komoda, Y.; Okamoto, T. Plant Growth Regulators in the Pea Plant (Pisum Sativum L.). Chem. Pharm. Bull. 1970, 18, 1872–1879. [Google Scholar] [CrossRef]
- Marzouk, M.M.; Ragheb, A.Y.; Youssef, E.M.; Ragab, N.A.; El-Taher, E.M.; Garf, I.A.E.; Kassem, M.E.S. Isoflavone-Rich Extract of Trifolium resupinatum: Anti-Obesity Attributes with In Silico Investigation of Its Constituents. Rev. Bras. Farmacogn. 2024, 34, 522–535. [Google Scholar] [CrossRef]
- Srivastava, A.; Mishra, R.; Kumar, S.; Dev, K.; Tandon, P.; Maurya, R. Molecular Structure, Spectral Investigation (1H NMR, 13C NMR, UV–Visible, FT-IR, FT-Raman), NBO, Intramolecular Hydrogen Bonding, Chemical Reactivity and First Hyperpolarizability Analysis of Formononetin [7-Hydroxy-3(4-Methoxyphenyl)Chromone]: A Quantum Chemical Study. J. Mol. Struct. 2015, 1084, 55–73. [Google Scholar] [CrossRef]
- Gampe, N.; Darcsi, A.; Lohner, S.; Béni, S.; Kursinszki, L. Characterization and Identification of Isoflavonoid Glycosides in the Root of Spiny Restharrow (Ononis Spinosa L.) by HPLC-QTOF-MS, HPLC–MS/MS and NMR. J. Pharm. Biomed. Anal. 2016, 123, 74–81. [Google Scholar] [CrossRef]
- Kovalev, V.N.; Spiridonov, V.N.; Borisov, M.I.; Kovalev, I.P.; Gordienko, V.G.; Kolesnikov, D.D. Phenolic Compounds of Ononis arvensis the Structure of Onogenin. Chem. Nat. Compd. 1975, 11, 367–369. [Google Scholar] [CrossRef]
- Dahiya, J.S.; Strange, R.N.; Bilyard, K.G.; Cooksey, C.J.; Garratt, P.J. Two Isoprenylated Isoflavone Phytoalexins from Cajanus cajan. Phytochemistry 1984, 23, 871–873. [Google Scholar] [CrossRef]
- Ingham, J.L. Induced Isoflavonoids from Fungus-Infected Stems of Pigeon Pea (Cajanus cajan). Z. Naturforsch. C 1976, 31, 504–508. [Google Scholar] [CrossRef]
- Livingston, A.L.; Bickoff, E.M.; Lundin, R.E.; Jurd, L. Trifoliol, a New Coumestan from Ladino Clover. Tetrahedron 1964, 20, 1963–1970. [Google Scholar] [CrossRef]
- Huh, J.; Lee, J.; Jeon, E.; Ryu, H.W.; Oh, S.; Ahn, K.; Jun, H.S.; Ha, U. Maackiain, a Compound Derived from Sophora flavescens, Increases IL-1β Production by Amplifying Nigericin-mediated Inflammasome Activation. FEBS Open Bio 2020, 10, 1482–1491. [Google Scholar] [CrossRef] [PubMed]
- Ghribi, L.; Waffo-Téguo, P.; Cluzet, S.; Marchal, A.; Marques, J.; Mérillon, J.-M.; Ben Jannet, H. Isolation and Structure Elucidation of Bioactive Compounds from the Roots of the Tunisian Ononis angustissima L. Bioorg. Med. Chem. Lett. 2015, 25, 3825–3830. [Google Scholar] [CrossRef] [PubMed]
- Drahota, Z.; Křiváková, P.; Červinková, Z.; Kmoníčková, E.; Lotková, H.; Kučera, O.; Houštěk, J. Tert-Butyl Hydroperoxide Selectively Inhibits Mitochondrial Respiratory-Chain Enzymes in Isolated Rat Hepatocytes. Physiol. Res. 2005, 54, 67–72. [Google Scholar] [CrossRef]
- Saikia, S.; Bordoloi, M. Molecular Docking: Challenges, Advances and Its Use in Drug Discovery Perspective. Curr. Drug Targets 2019, 20, 501–521. [Google Scholar] [CrossRef]
- Geha, R.M.; Chen, K.; Wouters, J.; Ooms, F.; Shih, J.C. Analysis of Conserved Active Site Residues in Monoamine Oxidase A and B and Their Three-Dimensional Molecular Modeling. J. Biol. Chem. 2002, 277, 17209–17216. [Google Scholar] [CrossRef]
- Bonomo, S.; Jørgensen, F.S.; Olsen, L. Dissecting the Cytochrome P450 1A2- and 3A4-Mediated Metabolism of Aflatoxin B1 in Ligand and Protein Contributions. Chem. A Eur. J. 2017, 23, 2884–2893. [Google Scholar] [CrossRef]
- Hubálek, F.; Binda, C.; Khalil, A.; Li, M.; Mattevi, A.; Castagnoli, N.; Edmondson, D.E. Demonstration of Isoleucine 199 as a Structural Determinant for the Selective Inhibition of Human Monoamine Oxidase B by Specific Reversible Inhibitors. J. Biol. Chem. 2005, 280, 15761–15766. [Google Scholar] [CrossRef]
- Mateev, E.; Georgieva, M.; Mateeva, A.; Zlatkov, A.; Ahmad, S.; Raza, K.; Azevedo, V.; Barh, D. Structure-Based Design of Novel MAO-B Inhibitors: A Review. Molecules 2023, 28, 4814. [Google Scholar] [CrossRef]
- Yang, R.-Y.; Lan, Y.-S.; Huang, Z.-J.; Shao, C.-L.; Liang, H.; Chen, Z.-F.; Li, J. Isoflavonoids from Sophora tonkinensis. Chem. Nat. Compd. 2012, 48, 674–676. [Google Scholar] [CrossRef]
- Guohui, L. Chemical Constituents from Roots of Pueraria lobata. China J. Chin. Mater. Med. 2010, 35, 3156–3160. [Google Scholar] [CrossRef]
- Beldjoudi, N.; Mambu, L.; Labaïed, M.; Grellier, P.; Ramanitrahasimbola, D.; Rasoanaivo, P.; Martin, M.T.; Frappier, F. Flavonoids from Dalbergia louvelii and Their Antiplasmodial Activity. J. Nat. Prod. 2003, 66, 1447–1450. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.A.; Avery, M.A.; Burandt, C.L.; Goins, D.K.; Mikell, J.R.; Nash, T.E.; Azadegan, A.; Walker, L.A. Antigiardial Activity of Isoflavones from Dalbergia frutescens Bark. J. Nat. Prod. 2000, 63, 1414–1416. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.; Kamara, B.I.; Brandt, E.V.; Joubert, E. Phenolic Compounds from Cyclopia Intermedia (Honeybush Tea). 1. J. Agric. Food Chem. 1998, 46, 3406–3410. [Google Scholar] [CrossRef]
- Xu, M.; Jin, Z.; Ohm, J.-B.; Schwarz, P.; Rao, J.; Chen, B. Effect of Germination Time on Antioxidative Activity and Composition of Yellow Pea Soluble Free and Polar Soluble Bound Phenolic Compounds. Food Funct. 2019, 10, 6840–6850. [Google Scholar] [CrossRef]
- Máximo, P.; Lourenço, A.; Feio, S.S.; Roseiro, J.C. Flavonoids from Ulex airensis and Ulex europaeus ssp. europaeus. J. Nat. Prod. 2002, 65, 175–178. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid Biosynthesis. Mol. Plant 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Sajid, M.; Stone, S.R.; Kaur, P. Recent Advances in Heterologous Synthesis Paving Way for Future Green-Modular Bioindustries: A Review With Special Reference to Isoflavonoids. Front. Bioeng. Biotechnol. 2021, 9, 673270. [Google Scholar] [CrossRef]
- Tibenda, J.J.; Du, Y.; Nan, Y.; Huang, S.; Chen, G.; Ning, N.; Li, X.; Yang, Y.; Yuan, L. Astragalus mongholicus: A Review of Its Pharmacological Mechanisms Against Gastric Cancer. J. Herb. Med. 2024, 45, 100881. [Google Scholar] [CrossRef]
- Gong, G.; Zheng, Y.; Yang, Y.; Sui, Y.; Wen, Z. Pharmaceutical Values of Calycosin: One Type of Flavonoid Isolated from Astragalus. Evid. Based Complement. Altern. Med. 2021, 2021, 9952578. [Google Scholar] [CrossRef]
- Zhang, L.-J.; Liu, H.-K.; Hsiao, P.-C.; Kuo, L.-M.Y.; Lee, I.-J.; Wu, T.-S.; Chiou, W.-F.; Kuo, Y.-H. New Isoflavonoid Glycosides and Related Constituents from Astragali Radix (Astragalus membranaceus) and Their Inhibitory Activity on Nitric Oxide Production. J. Agric. Food Chem. 2011, 59, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Gai, Q.-Y.; Jiao, J.; Luo, M.; Wang, W.; Gu, C.-B.; Fu, Y.-J.; Ma, W. Tremendous Enhancements of Isoflavonoid Biosynthesis, Associated Gene Expression and Antioxidant Capacity in Astragalus membranaceus Hairy Root Cultures Elicited by Methyl Jasmonate. Process Biochem. 2016, 51, 642–649. [Google Scholar] [CrossRef]
- Tang, D.; Shen, Y.-B.; Wang, Z.-H.; He, B.; Xu, Y.-H.; Nie, H.; Zhu, Q. Rapid Analysis and Guided Isolation of Astragalus Isoflavonoids by UHPLC–DAD–MS n and Their Cellular Antioxidant Defense on High-Glucose-Induced Mesangial Cell Dysfunction. J. Agric. Food Chem. 2018, 66, 1105–1113. [Google Scholar] [CrossRef] [PubMed]


| 2 | 6 | |||
|---|---|---|---|---|
| Position | δC | δH | δC | δH |
| 2 | 152.7, s | 8.36, s | 152.5, s | 8.23, s |
| 3 | n.o. | – | 125.1, s | – |
| 4 | 174.4, s | 174.1, s | – | |
| 5 | 107.3, d | 7.38, s | 104.5, d | 7.39, s |
| 6 | 153.8, s | – | n.o. | – |
| 7 | 145.7, s | – | 147.5, s | – |
| 8 | 100.1, d | 7.15, s | n.o. | 6.86, s |
| 9 | 150.8, s | – | 152.6, s | – |
| 10 | 117.5, s | – | n.o. | |
| 1′ | 123.1, s | – | 122.9, s | – |
| 2′ | 113.0, d | 7.16, d (2.0 Hz) | 146.1, s | – |
| 3′ | 147.4, s | – | 119.8, d | 6.94, s |
| 4′ | 146.5, s | – | 147.5, s | – |
| 5′ | 114.9, d | 6.80, d (8.2 Hz) | 116.6, d | 7.03, s |
| 6′ | 121.3, d | 6.99, dd (8.18, 2.05 Hz) | 112.0, d | 6.94, s |
| -OCH3- | 55.95, q (C-6) | 3.91, s | 55.75, q (C-7) | 3.86, s |
| -OCH3- | 55.48, q (C-3′) | 3.78, s | 55.67, q (C-4′) | 3.78, s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enchev, P.; Kondeva-Burdina, M.; Mateev, E.; Ionkova, I.; Zarev, Y. Phenolic Derivatives of Astragalus aitosensis with Selective MAO-B Inhibition and Mitochondrial Protection. Molecules 2025, 30, 4069. https://doi.org/10.3390/molecules30204069
Enchev P, Kondeva-Burdina M, Mateev E, Ionkova I, Zarev Y. Phenolic Derivatives of Astragalus aitosensis with Selective MAO-B Inhibition and Mitochondrial Protection. Molecules. 2025; 30(20):4069. https://doi.org/10.3390/molecules30204069
Chicago/Turabian StyleEnchev, Preslav, Magdalena Kondeva-Burdina, Emilio Mateev, Iliana Ionkova, and Yancho Zarev. 2025. "Phenolic Derivatives of Astragalus aitosensis with Selective MAO-B Inhibition and Mitochondrial Protection" Molecules 30, no. 20: 4069. https://doi.org/10.3390/molecules30204069
APA StyleEnchev, P., Kondeva-Burdina, M., Mateev, E., Ionkova, I., & Zarev, Y. (2025). Phenolic Derivatives of Astragalus aitosensis with Selective MAO-B Inhibition and Mitochondrial Protection. Molecules, 30(20), 4069. https://doi.org/10.3390/molecules30204069








