Expanding the Terpene Universe: Synthetic Biology and Non-Natural Chemistry in Engineered Microorganisms
Abstract
1. Introduction
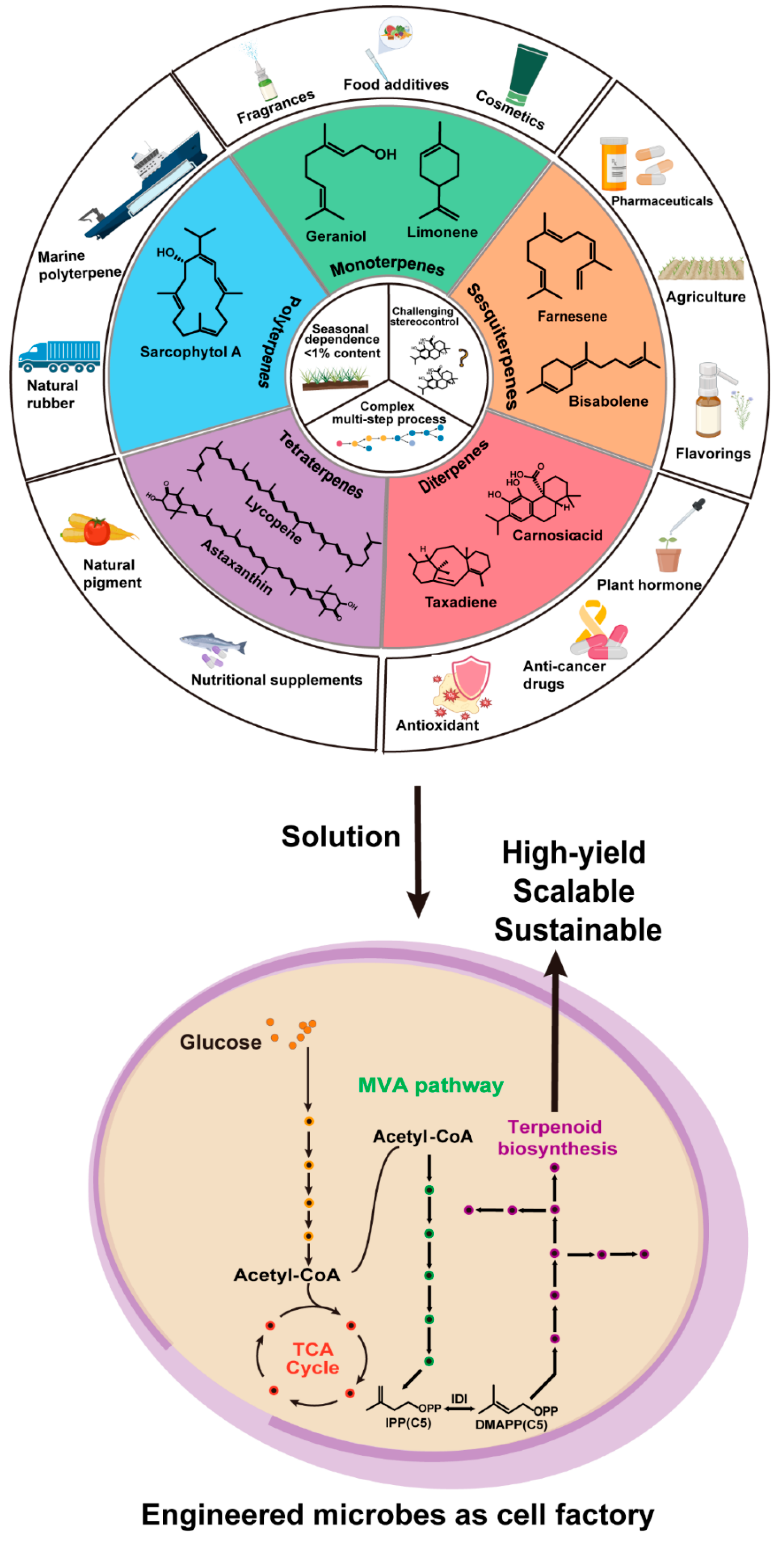
1.1. Terpenes: Biological Ubiquity and Industrial Significance
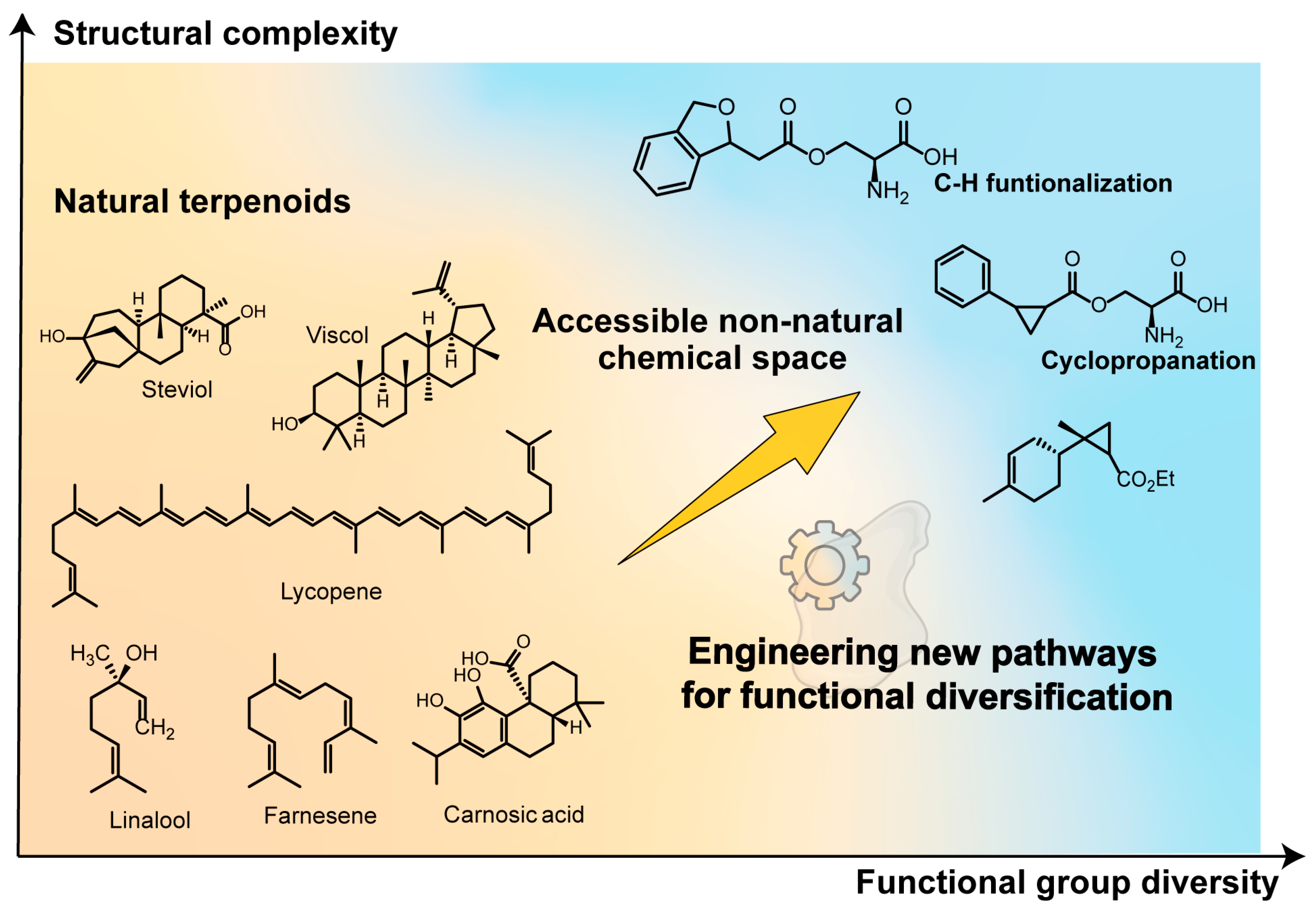
1.2. Expanding Beyond Nature’s Biosynthetic Toolkit
1.3. Toward Programmable Biosynthesis of Terpenes
2. Foundation: Native Terpene Biosynthetic Pathways in an Engineering Context
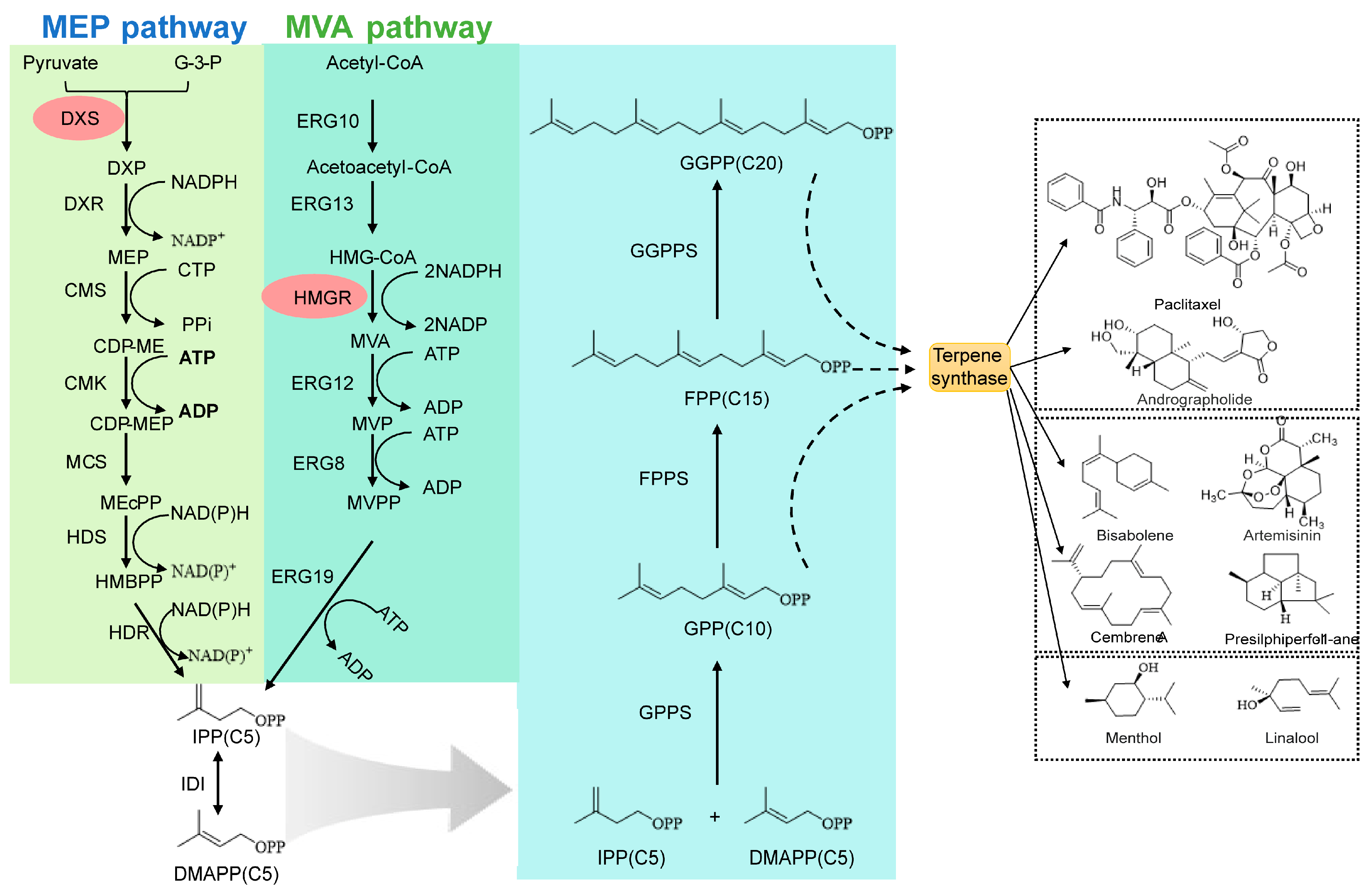
2.1. The Two Source Pathways: MVA vs. MEP—An Engineering Perspective
| Product | Host | Pathway | Bottleneck | Engineering Strategy | Maximum Titer |
|---|---|---|---|---|---|
| Sclareol | Y. lipolytica | MVA | Flux imbalance; unsuitable chassis; low enzyme activity | Enzyme engineering; increasing GGPPS supply | 12.9 g/L [32] |
| Amorpha-4,11-diene | E. coli | MEP | Low growth-coupled production | Semi-continuous biomanufacturing | 8.32 g/L [33] |
| Artemisinic acid | S. cerevisiae | MVA | Low expression of enzyme | Plant dehydrogenase introduction; additional cytochrome | 25 g/L [34] |
| Bisabolene | S. cerevisiae | MVA | Growth limitations; insufficient precursors | MVA pathway enhancement; temperature-sensitive regulation | 18.6 g/L [35] |
| β-Farnesene | Y. lipolytica | MVA | Insufficient precursors; flux imbalance | Acetyl-CoA boosting; large-scale optimization | 35.2 g/L [36] |
| (S)-linalool | Pantoea ananatis | MVA | Poor enzyme compatibility; insufficient precursors | Increasing protein solubility; elevating precursor or supply; dual-phase fed-batch fermentation | 10.9 g/L [37] |
| Geranylgeraniol | Y. lipolytica | MVA | Flux imbalance; several rate-limiting enzymes | Overexpressing bottleneck enzymes; expanding acetyl-CoA pool; downregulating FPP flux | 3.3 g/L [38] |
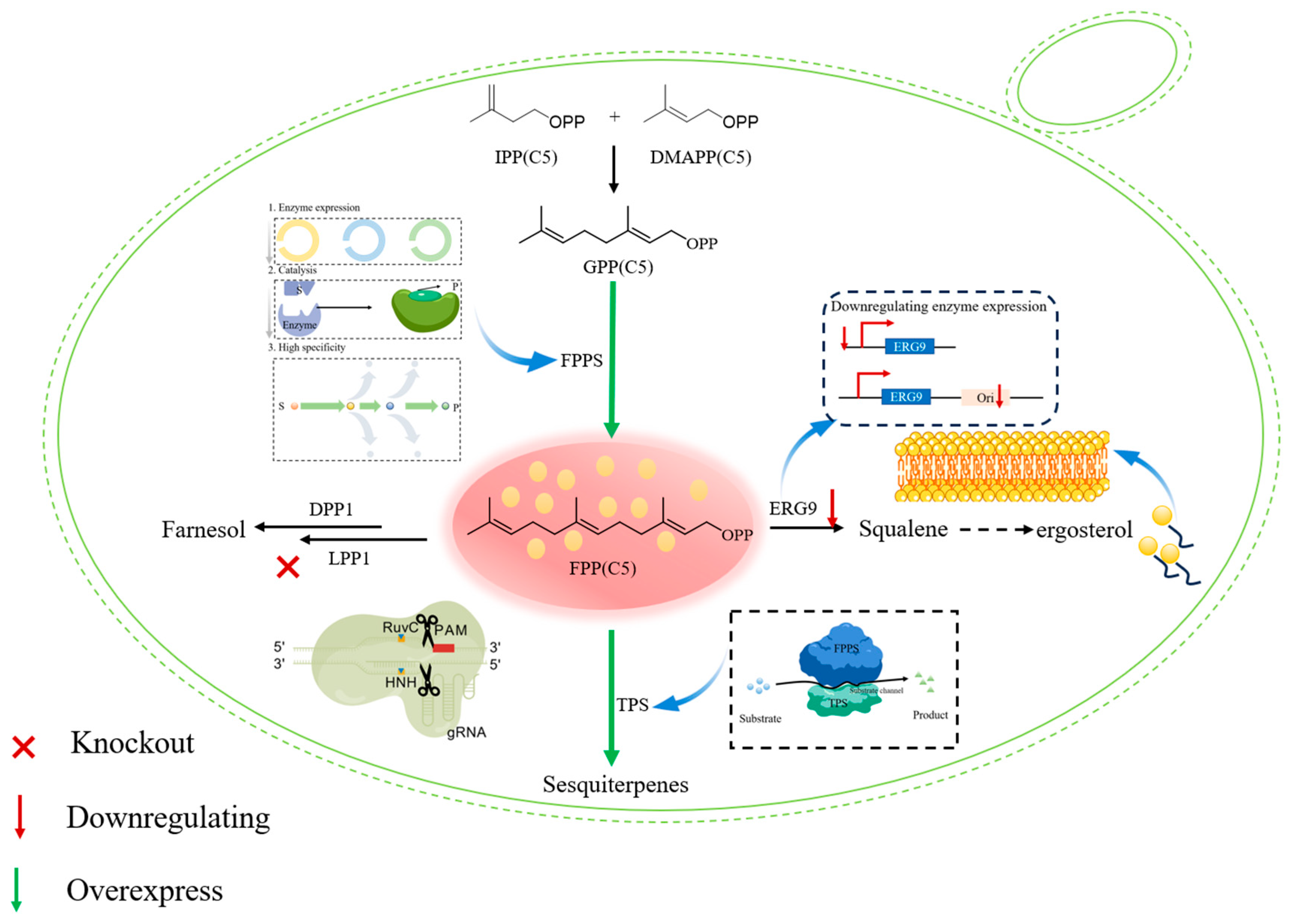
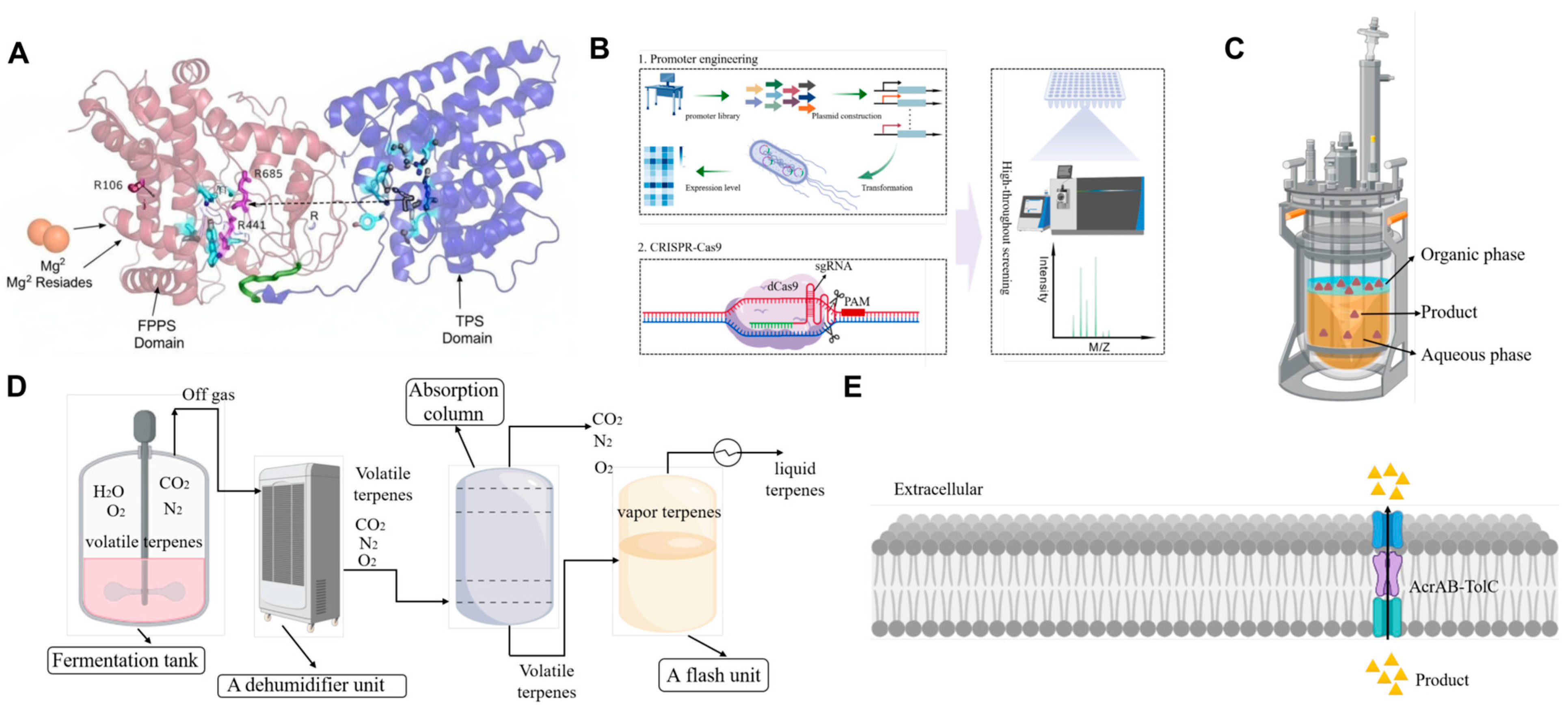
2.2. Building Key Precursors: Prenyltransferases as Nodes for Flux Control
2.3. Generating Carbon Skeletons: Terpene Synthases as Modular Plug-And-Play Units
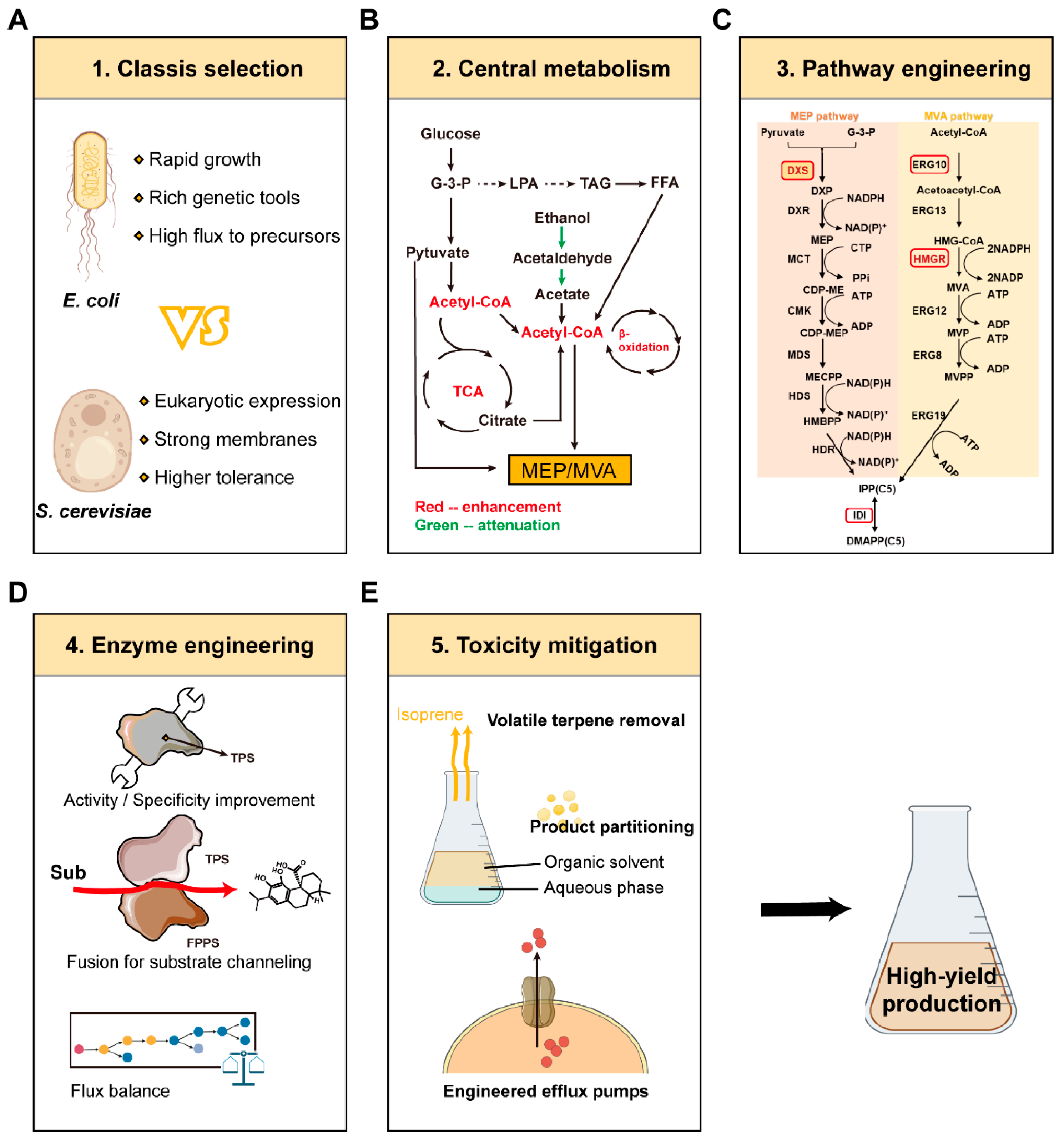
3. Metabolic Engineering Strategies for Microbial Terpene Production
3.1. Foundational Platform Design: Chassis Selection and Central Metabolism Optimization
3.2. Pathway Engineering and Optimization Strategies
3.3. Advanced System Integration and Toxicity Mitigation
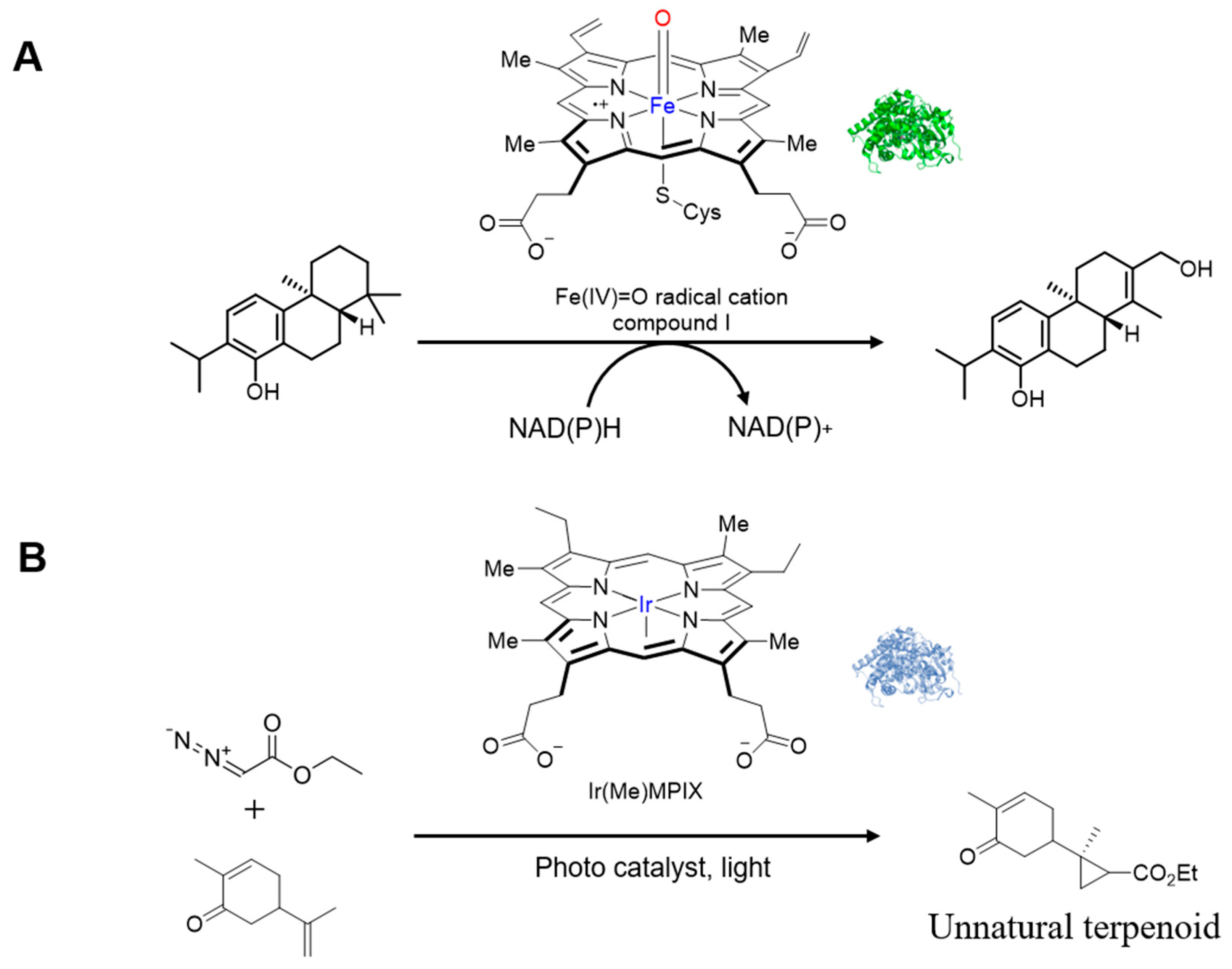
4. Expanding the Enzymatic Toolkit: Diversification Reactions
4.1. Natural Modifying Enzymes and Their Functional Expression
4.2. The Engineering Challenge of Natural Diversification
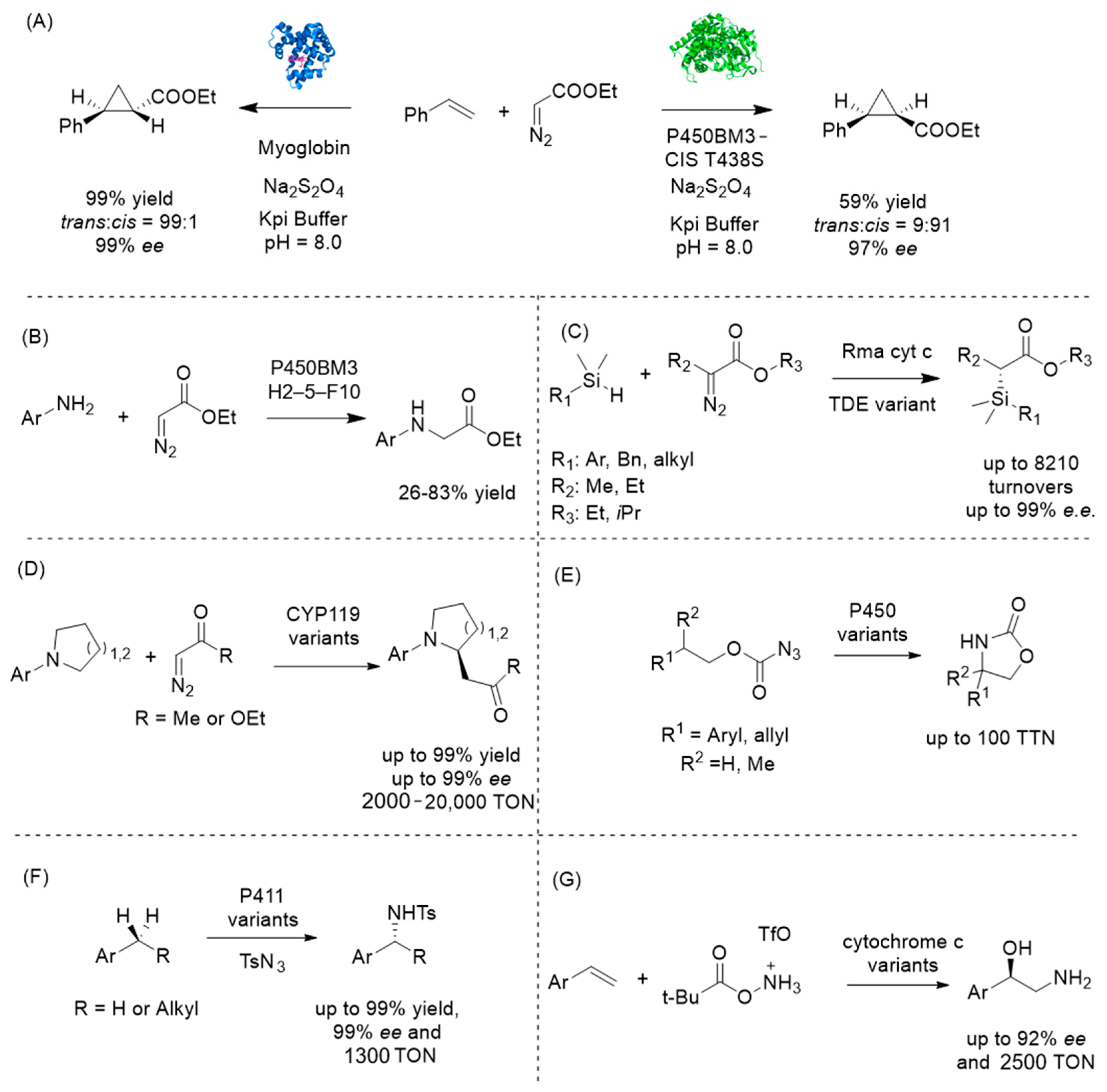
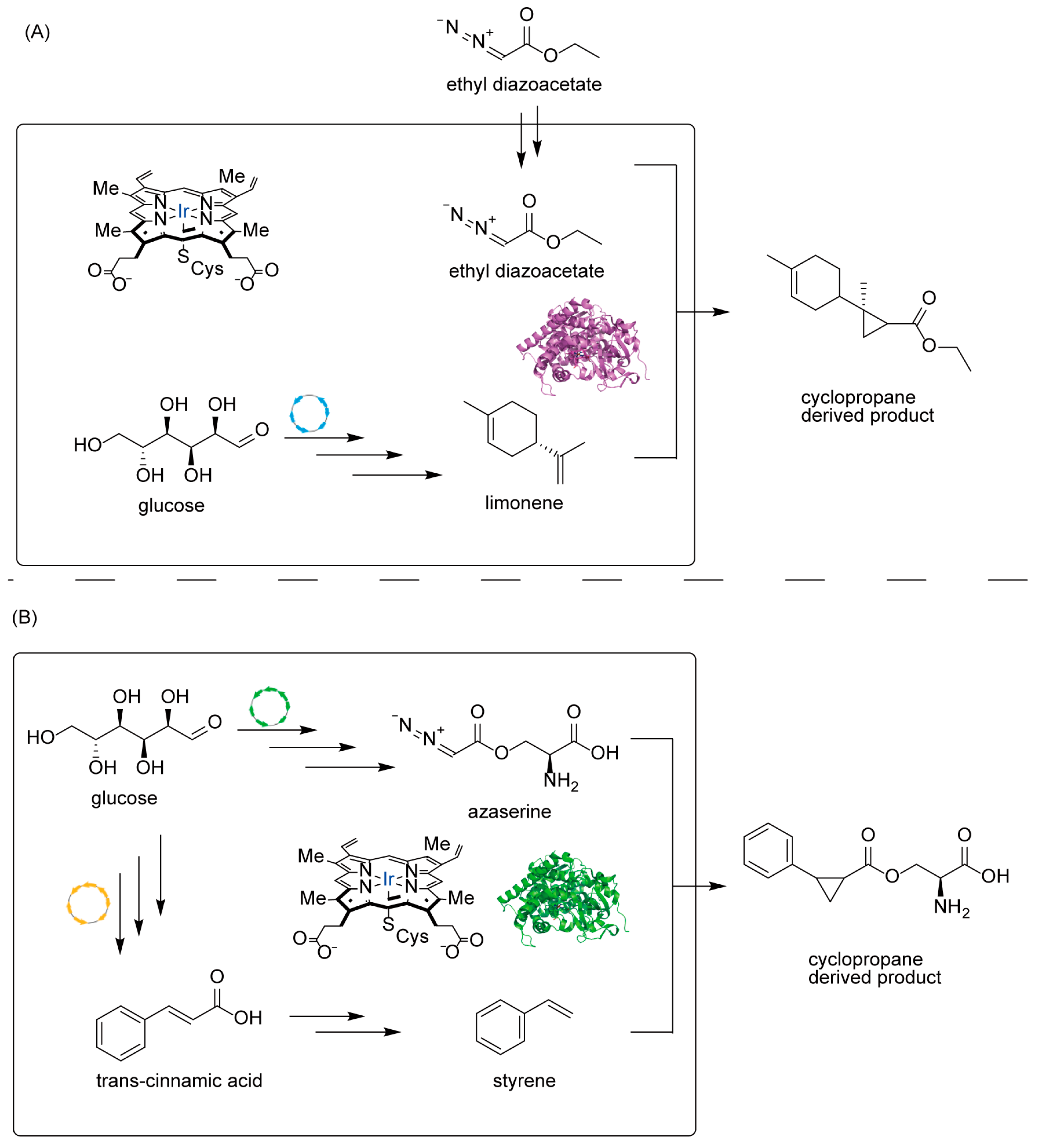
4.3. Introducing Abiological Chemistry: Expanding Nature’s Repertoire
5. Applications and Case Studies of Engineered Terpenes
5.1. Advanced Biofuels and Bulk Chemicals from Short-Chain Terpenes
5.2. Pharmaceutical and Nutraceutical Applications
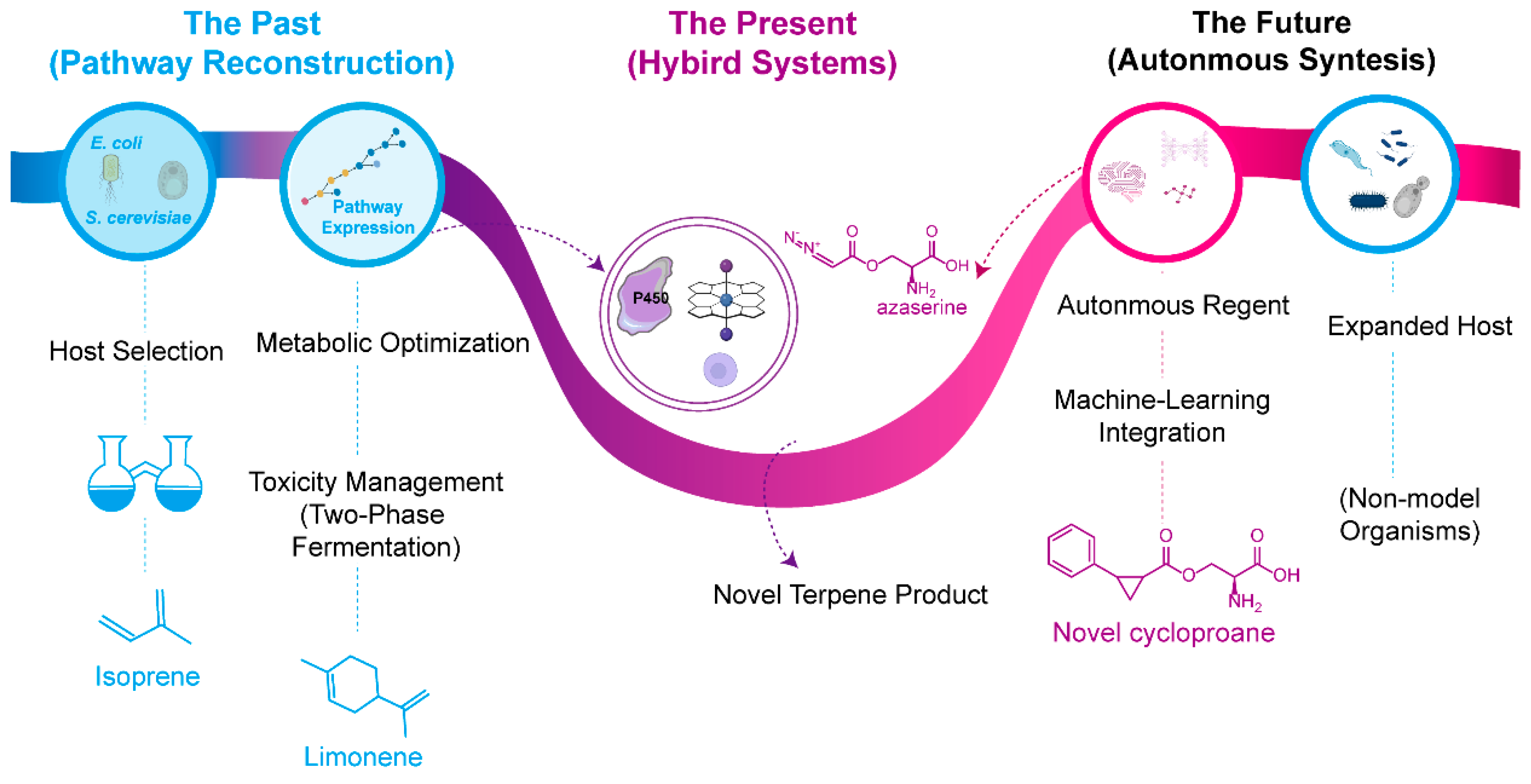
5.3. Frontier Applications of Non-Natural Terpenes
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Chen, R.; Wang, M.; Keasling, J.D.; Hu, T.; Yin, X. Expanding the structural diversity of terpenes by synthetic biology approaches. Trends Biotechnol. 2024, 42, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Wang, Y.Z.; Xu, Y.S.; Shi, T.Q.; Liu, W.Z.; Sun, X.M.; Huang, H. Biotechnological production of lipid and terpenoid from thraustochytrids. Biotechnol. Adv. 2021, 48, 107725. [Google Scholar] [CrossRef]
- Zhang, Q.; Zeng, W.; Xu, S.; Zhou, J. Metabolism and strategies for enhanced supply of acetyl-CoA in Saccharomyces cerevisiae. Bioresour. Technol. 2021, 342, 125978. [Google Scholar] [CrossRef]
- Jeong, B.R.; Jang, J.; Jin, E. Genome engineering via gene editing technologies in microalgae. Bioresour. Technol. 2023, 373, 128701. [Google Scholar] [CrossRef]
- Chai, L.; Che, J.; Qi, Q.; Hou, J. Metabolic Engineering for Squalene Production: Advances and Perspectives. J. Agric. Food Chem. 2024, 72, 27715–27725. [Google Scholar] [CrossRef]
- Reetz, M.T.; Qu, G.; Sun, Z. Engineered enzymes for the synthesis of pharmaceuticals and other high-value products. Nat. Synth. 2024, 3, 19–32. [Google Scholar] [CrossRef]
- Papada, E. The effects of terpenes on metabolism: A comprehensive review on recent updates. Curr. Opin. Clin. Nutr. Metab. Care 2025, 28, 323–329. [Google Scholar] [CrossRef]
- Sha, Y.; Ge, M.; Lu, M.; Xu, Z.; Zhai, R.; Jin, M. Advances in metabolic engineering for enhanced acetyl-CoA availability in yeast. Crit. Rev. Biotechnol. 2025, 45, 904–922. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Zhang, J.; Zhou, Y.; Wang, F.; Wang, Z.; Li, X. Advances in microbial production of geraniol: From metabolic engineering to potential industrial applications. Crit. Rev. Biotechnol. 2025, 45, 727–742. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, X.; Deng, Z.; Liu, T. Innovative approaches in the discovery of terpenoid natural products. Curr. Opin. Microbiol. 2025, 83, 102575. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Rosenfeldt, M.; Koutsaviti, A.; Harizani, M.; Zhao, Y.; Leelahakorn, N.; Frachon, A.; Raadam, M.H.; Miettinen, K.; Pateraki, I.; et al. Systematic biotechnological production of isoprenoid analogs with bespoke carbon skeletons. Nat. Commun. 2025, 16, 2098. [Google Scholar] [CrossRef]
- Eisenreich, W.; Bacher, A.; Arigoni, D.; Rohdich, F. Biosynthesis of isoprenoids via the non-mevalonate pathway. Cell Mol. Life Sci. 2004, 61, 1401–1426. [Google Scholar] [CrossRef] [PubMed]
- Bureau, J.A.; Oliva, M.E.; Dong, Y.; Ignea, C. Engineering yeast for the production of plant terpenoids using synthetic biology approaches. Nat. Prod. Rep. 2023, 40, 1822–1848. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, J.N.; Leferink, N.G.H.; Johannissen, L.O.; Hay, S.; Scrutton, N.S. Decoding Catalysis by Terpene Synthases. ACS Catal. 2023, 13, 12774–12802. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, A.; Gupta, N.; Mutturi, S. Engineering of Saccharomyces cerevisiae towards synthesis of linalool using linalool synthase from Magnolia champaca. Biochem. Eng. J. 2024, 211, 109477. [Google Scholar] [CrossRef]
- Tan, J.C.; Hu, Q.; Scrutton, N.S. A growth-coupling strategy for improving the stability of terpenoid bioproduction in Escherichia coli. Microb. Cell Factories 2024, 23, 279. [Google Scholar] [CrossRef]
- Bach, T.J.; Boronat, A.; Campos, N.; Ferrer, A.; Vollack, K.U. Mevalonate biosynthesis in plants. Crit. Rev. Biochem. Mol. Biol. 1999, 34, 107–122. [Google Scholar] [CrossRef]
- Rohmer, M.; Rohmer, M. The discovery of a mevalonate-independent pathway for isoprenoid biosynthesis in bacteria, algae and higher plants. Nat. Prod. Rep. 1999, 16, 565–574. [Google Scholar] [CrossRef]
- Dewick, P.M. The biosynthesis of C5–C25 terpenoid compounds. Nat. Prod. Rep. 2002, 19, 181–222. [Google Scholar] [CrossRef]
- Kuzuyama, T.; Seto, H. Diversity of the biosynthesis of the isoprene units. Nat. Prod. Rep. 2003, 20, 171–183. [Google Scholar] [CrossRef]
- van der Meer, J.Y.; Hirsch, A.K.H. The isoprenoid-precursor dependence of Plasmodium spp. Nat. Prod. Rep. 2012, 29, 721–728. [Google Scholar] [CrossRef]
- Gao, M.; Sun, J.; Xiao, Q.; Zhai, Y.; Tian, Y.; Zhang, Z.; Xu, F.; Zhang, P. Sensitive quantification of mevalonate pathway intermediates and prediction of relative novel analogs by chemical derivatization-based LC-MS/MS. J. Chromatogr. A 2024, 1731, 465163. [Google Scholar] [CrossRef]
- Liao, P.; Hemmerlin, A.; Bach, T.J.; Chye, M.L. The potential of the mevalonate pathway for enhanced isoprenoid production. Biotechnol. Adv. 2016, 34, 697–713. [Google Scholar] [CrossRef]
- Matsumoto, T.; Tanaka, T.; Kondo, A. Engineering metabolic pathways in Escherichia coli for constructing a “microbial chassis” for biochemical production. Bioresour. Technol. 2017, 245, 1362–1368. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Vagner, S. Boosting Immunity by Targeting Post-translational Prenylation of Small GTPases. Cell 2018, 175, 901–902. [Google Scholar] [CrossRef]
- Ma, Y.R.; Wang, K.F.; Wang, W.J.; Ding, Y.; Shi, T.Q.; Huang, H.; Ji, X.J. Advances in the metabolic engineering of Yarrowia lipolytica for the production of terpenoids. Bioresour. Technol. 2019, 281, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Leichner, G.S.; Avner, R.; Harats, D.; Roitelman, J. Metabolically regulated endoplasmic reticulum-associated degradation of 3-hydroxy-3-methylglutaryl-CoA reductase: Evidence for requirement of a geranylgeranylated protein. J. Biol. Chem. 2011, 286, 32150–32161. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.; Bauchart, P.; Gold, N.D.; Zhu, Y.; De Luca, V.; Martin, V.J.J. Engineering of a Nepetalactol-Producing Platform Strain of Saccharomyces cerevisiae for the Production of Plant Seco-Iridoids. ACS Synth. Biol. 2016, 5, 405–414. [Google Scholar] [CrossRef]
- Qi, M.; Liu, T.; Zhang, W.; Wan, H.; Wang, M.; Kang, W.; Xue, C. Enhancing cannabichromenic acid biosynthesis in Saccharomyces cerevisiae. ACS Synth. Biol. 2025, 14, 531–541. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, C.; Zhang, H.; Zhu, M.; Yang, H.; Xia, Y.; Shen, W.; Chen, X. Cytoplasmic-peroxisomal spatial combination engineering in Candida tropicalis for enhanced terpenoid production. Green Chem. 2025, 27, 3693–3705. [Google Scholar] [CrossRef]
- Perez-Gil, J.; Behrendorff, J.; Douw, A.; Vickers, C.E. The methylerythritol phosphate pathway as an oxidative stress sense and response system. Nat. Commun. 2024, 15, 5303. [Google Scholar] [CrossRef]
- Sun, M.L.; Han, Y.; Yu, X.; Wang, K.; Lin, L.; Ledesma-Amaro, R.; Ji, X.J. Constructing a green oleaginous yeast cell factory for sustainable production of the plant-derived diterpenoid sclareol. Green Chem. 2024, 26, 5202–5210. [Google Scholar] [CrossRef]
- Castillo-Saldarriaga, C.; Sarria, S.; Santos, C.N.S.; Ajikumar, P.K.; Takors, R. Semi-continuous biomanufacturing for maximizing the production of complex chemicals and fuels: A case study of amorpha-4,11-diene. Trends Biotechnol. 2024, 42, 1777–1794. [Google Scholar] [CrossRef]
- Paddon, C.J.; Westfall, P.J.; Pitera, D.J.; Benjamin, K.; Fisher, K.; McPhee, D.; Leavell, M.D.; Tai, A.; Main, A.; Eng, D.; et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature 2013, 496, 528–532. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Sun, B.; Bi, H.; Bao, Y.; Wang, M.; Zhang, H.; Fang, Y. Developing Thermosensitive Metabolic Regulation Strategies in the Fermentation Process of Saccharomyces cerevisiae to Enhance α-Bisabolene Production. ACS Synth. Biol. 2025, 14, 1129–1141. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Li, Q.; Wang, Z.; Cui, Z.; Su, T.; Lu, X.; Qi, Q.; Hou, J. Engineering Yarrowia lipolytica for the sustainable production of β-farnesene from waste oil feedstock. Biotechnol. Biofuels 2022, 15, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Nitta, N.; Tajima, Y.; Yamamoto, Y.; Moriya, M.; Matsudaira, A.; Hoshino, Y.; Nishio, Y.; Usuda, Y. Fermentative production of enantiopure (S)-linalool using a metabolically engineered Pantoea ananatis. Microb. Cell Fact. 2021, 20, 54. [Google Scholar] [CrossRef]
- Wang, K.; Yin, M.; Sun, M.L.; Zhao, Q.; Ledesma-Amaro, R.; Ji, X.J.; Lin, L. Engineering Yarrowia lipolytica for Efficient Synthesis of Geranylgeraniol. J. Agric. Food Chem. 2024, 72, 20568–20581. [Google Scholar] [CrossRef]
- Cheah, L.C.; Liu, L.; Stark, T.; Plan, M.R.; Peng, B.; Lu, Z.; Schenk, G.; Sainsbury, F.; Vickers, C.E. Metabolic flux enhancement from the translational fusion of terpene synthases is linked to terpene synthase accumulation. Metab. Eng. 2023, 77, 143–151. [Google Scholar] [CrossRef]
- Ma, Y.; Zu, Y.; Huang, S.; Stephanopoulos, G. Engineering a universal and efficient platform for terpenoid synthesis in yeast. Proc. Natl. Acad. Sci. USA 2023, 120, e2207680120. [Google Scholar] [CrossRef]
- Waller, D.D.; Park, J.; Tsantrizos, Y.S. Inhibition of farnesyl pyrophosphate (FPP) and/or geranylgeranyl pyrophosphate (GGPP) biosynthesis and its implication in the treatment of cancers. Crit. Rev. Biochem. Mol. Biol. 2019, 54, 41–60. [Google Scholar] [CrossRef]
- Li, M.; Hou, F.; Wu, T.; Jiang, X.; Li, F.; Liu, H.; Xian, M.; Zhang, H. Recent advances of metabolic engineering strategies in natural isoprenoid production using cell factories. Nat. Prod. Rep. 2020, 37, 80–99. [Google Scholar] [CrossRef]
- Rinaldi, M.A.; Ferraz, C.A.; Scrutton, N.S. Alternative metabolic pathways and strategies to high-titre terpenoid production in Escherichia coli. Nat. Prod. Rep. 2022, 39, 90–118. [Google Scholar] [CrossRef]
- Dinday, S.; Ghosh, S. Recent advances in triterpenoid pathway elucidation and engineering. Biotechnol. Adv. 2023, 68, 108214. [Google Scholar] [CrossRef]
- Zhao, J.; Matsunaga, Y.; Fujita, K.; Sakai, K. Signal transduction and metabolic flux of β-thujaplicin and monoterpene biosynthesis in elicited Cupressus lusitanica cell cultures. Metab. Eng. 2006, 8, 14–29. [Google Scholar] [CrossRef]
- Liu, D.; Wang, L.; Gou, L.; Ma, Y.; Lu, Y.; Yao, S.; Fan, T.P.; Deng, H.; Cai, Y. Engineering an Extremely Monoterpene-Tolerant Serratia marcescens for High-Yield Geraniol Production via a Rationally Modified Insect Phosphatase. ACS Sustain. Chem. Eng. 2025, 13, 6197–6208. [Google Scholar] [CrossRef]
- Fan, J.; Wei, P.L.; Li, Y.; Zhang, S.; Ren, Z.; Li, W.; Yin, W.B. Developing filamentous fungal chassis for natural product production. Bioresour. Technol. 2025, 415, 131703. [Google Scholar]
- Liu, Z.Y.; Yu, X.Z. Engineering Bacillus subtilis for high-value bioproduction: Recent advances and applications. Microb Cell Fact 2025, 24, 182. [Google Scholar]
- Madsen, C.S.; Kimbrel, J.A.; Diep, P.; Ricci, D.P. Synthetic communities as a model for determining interactions between a biofertilizer chassis organism and native microbial consortia. ISME J. 2025, 19, wraf170. [Google Scholar]
- Tong, B.; Yu, Y.; Shi, S. Rhodotorula sp. as a promising host for microbial cell factories. Metab. Eng. 2025, 90, 178–196. [Google Scholar] [CrossRef]
- Xing, S.; Kang, X.; Wang, R.; Wang, C.; Wang, Y.; Bao, X.; Zhao, J. Microbial Production of Nicotinamide Mononucleotide: Key Enzymes Discovery, Host Cells Selection, and Pathways Design and Optimization. ACS Synth. Biol. 2025, 14, 1352–1366. [Google Scholar] [CrossRef]
- Zhang, Y.; Yun, J.; Zhang, G.; Zabed, H.M.; Tian, Y.; Tang, X.; Li, J.; Qi, X. A Refined Adaptive Laboratory Evolution Strategy With Biosensor-Assisted Selection Resolves the Tolerance-Efficiency Trade-Off in Toxic Chemical Biosynthesis. Adv. Sci. 2025, e07740. [Google Scholar]
- Zhu, Y.; Yogiswara, S.; Willekens, A.; Gerardin, A.; Lavigne, R.; Goossens, A.; Pinheiro, V.B.; Dai, Z.; Verstrepen, K.J. Beyond CEN.PK—Parallel engineering of selected S. cerevisiae strains reveals that superior chassis strains require different engineering approaches for limonene production. Metab. Eng. 2025, 91, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Bakanas, I.; Lusi, R.F.; Wiesler, S.; Hayward Cooke, J.; Sarpong, R. Strategic application of C–H oxidation in natural product total synthesis. Nat. Rev. Chem. 2023, 7, 783–799. [Google Scholar] [CrossRef]
- Asadollahi, M.A.; Maury, J.; Moeller, K.; Nielsen, K.F.; Schalk, M.; Clark, A.; Nielsen, J. Production of plant sesquiterpenes in Saccharomyces cerevisiae: Effect of ERG9 repression on sesquiterpene biosynthesis. Biotechnol. Bioeng. 2007, 99, 666–677. [Google Scholar] [CrossRef]
- Gonzalez-Pacanowska, D.; Arison, B.; Havel, C.M.; Watson, J.A. Isopentenoid synthesis in isolated embryonic Drosophila cells. Farnesol catabolism and ω-oxidation. J. Biol. Chem. 1988, 263, 1301. [Google Scholar]
- Morley, K.L.; Kazlauskas, R.J. Improving enzyme properties: When are closer mutations better? Trends Biotechnol. 2005, 23, 231–237. [Google Scholar] [CrossRef]
- Al-Karablieh, N.; Weingart, H.; Ullrich, M.S. The outer membrane protein TolC is required for phytoalexin resistance and virulence of the fire blight pathogen Erwinia amylovora. Microb. Biotechnol. 2009, 2, 465–475. [Google Scholar] [CrossRef]
- Ren, X.; O’Hanlon, J.A.; Morris, M.; Robertson, J.; Wong, L.L. Synthesis of Imidazolidin-4-ones via a Cytochrome P450-Catalyzed Intramolecular C-H Amination. ACS Catal. 2016, 6, 6833–6837. [Google Scholar] [CrossRef]
- Fasan, R. Enzymatic catalysis New functional twists for P450s. Nat. Chem. 2017, 9, 609–611. [Google Scholar] [CrossRef]
- Ren, X.; Yorke, J.A.; Taylor, E.; Zhang, T.; Zhou, W.; Wong, L.L. Drug Oxidation by Cytochrome P450BM3: Metabolite Synthesis and Discovering New P450 Reaction Types. Chem. Eur. J. 2015, 21, 15039–15047. [Google Scholar] [CrossRef]
- Coelho, P.S.; Brustad, E.M.; Kannan, A.; Arnold, F.H. Olefin Cyclopropanation via Carbene Transfer Catalyzed by Engineered Cytochrome P450 Enzymes. Science 2013, 339, 307–310. [Google Scholar] [CrossRef]
- Coelho, P.S.; Wang, Z.J.; Ener, M.E.; Baril, S.A.; Kannan, A.; Arnold, F.H.; Brustad, E.M. A serine-substituted P450 catalyzes highly efficient carbene transfer to olefins in vivo. Nat. Chem. Biol. 2013, 9, 485–487. [Google Scholar] [CrossRef]
- Bajaj, P.; Sreenilayam, G.; Tyagi, V.; Fasan, R. Gram-Scale Synthesis of Chiral Cyclopropane-Containing Drugs and Drug Precursors with Engineered Myoglobin Catalysts Featuring Complementary Stereoselectivity. Angew. Chem. Int. Ed. Engl. 2016, 55, 16110–16114. [Google Scholar] [CrossRef]
- Bordeaux, M.; Singh, R.; Fasan, R. Intramolecular C(sp3)-H amination of arylsulfonyl azides with engineered and artificial myoglobin-based catalysts. Bioorg. Med. Chem. 2014, 22, 5697–5704. [Google Scholar]
- Bordeaux, M.; Tyagi, V.; Fasan, R. Highly Diastereoselective and Enantioselective Olefin Cyclopropanation Using Engineered Myoglobin-Based Catalysts. Angew. Chem. Int. Ed. Engl. 2015, 54, 1744–1748. [Google Scholar] [CrossRef][Green Version]
- Farwell, C.C.; McIntosh, J.A.; Hyster, T.K.; Wang, Z.J.; Arnold, F.H. Enantioselective Imidation of Sulfides via Enzyme-Catalyzed Intermolecular Nitrogen-Atom Transfer. J. Am. Chem. Soc. 2014, 136, 8766–8771. [Google Scholar] [CrossRef]
- Farwell, C.C.; Zhang, R.K.; McIntosh, J.A.; Hyster, T.K.; Arnold, F.H. Enantioselective Enzyme-Catalyzed Aziridination Enabled by Active-Site Evolution of a Cytochrome P450. ACS Cent. Sci. 2015, 1, 89–93. [Google Scholar] [CrossRef]
- Kan, S.B.J.; Lewis, R.D.; Chen, K.; Arnold, F.H. Directed evolution of cytochrome c for carbon-silicon bond formation: Bringing silicon to life. Science 2016, 354, 1048–1051. [Google Scholar] [CrossRef]
- Key, H.M.; Dydio, P.; Clark, D.S.; Hartwig, J.F. Abiological catalysis by artificial haem proteins containing noble metals in place of iron. Nature 2016, 534, 534–537. [Google Scholar] [CrossRef]
- McIntosh, J.A.; Coelho, P.S.; Farwell, C.C.; Wang, Z.J.; Lewis, J.C.; Brown, T.R.; Arnold, F.H. Enantioselective Intramolecular C-H Amination Catalyzed by Engineered Cytochrome P450 Enzymes In Vitro and In Vivo. Angew. Angew. Chem. Int. Ed. Engl. 2013, 52, 9309–9312. [Google Scholar] [CrossRef]
- Chandgude, A.L.; Ren, X.; Fasan, R. Stereodivergent Intramolecular Cyclopropanation Enabled by Engineered Carbene Transferases. J. Am. Chem. Soc. 2019, 141, 9145–9150. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Chandgude, A.L.; Fasan, R. Highly Stereoselective Synthesis of Fused Cyclopropane-γ-Lactams via Biocatalytic Iron-Catalyzed Intramolecular Cyclopropanation. ACS Catal. 2020, 10, 2308–2313. [Google Scholar] [CrossRef]
- Ren, X.; Fasan, R. Engineered and artificial metalloenzymes for selective C-H functionalization. Curr. Opin. Green Sustain. Chem. 2021, 31, 100494. [Google Scholar] [PubMed]
- Ren, X.; Liu, N.; Chandgude, A.L.; Fasan, R. An Enzymatic Platform for the Highly Enantioselective and Stereodivergent Construction of Cyclopropyl-δ-lactones. Angew. Chem. Int. Ed. Engl. 2020, 59, 21634–21639. [Google Scholar] [CrossRef]
- Steck, V.; Carminati, D.M.; Johnson, N.R.; Fasan, R. Enantioselective synthesis of chiral amines via biocatalytic carbene N-H insertion. ACS Catal. 2020, 10, 10967–10977. [Google Scholar] [CrossRef]
- Ren, X.; Chandgude, A.L.; Carminati, D.M.; Shen, Z.; Khare, S.D.; Fasan, R. Highly stereoselective and enantiodivergent synthesis of cyclopropylphosphonates with engineered carbene transferases. Chem. Sci. 2022, 13, 8550–8556. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Couture, B.M.; Liu, N.; Lall, M.S.; Kohrt, J.T.; Fasan, R. Enantioselective Single and Dual α-C-H Bond Functionalization of Cyclic Amines via Enzymatic Carbene Transfer. J. Am. Chem. Soc. 2023, 145, 537–550. [Google Scholar] [CrossRef]
- Bloomer, B.J.; Natoli, S.N.; Garcia-Borras, M.; Pereira, J.H.; Hu, D.B.; Adams, P.D.; Houk, K.N.; Clark, D.S.; Hartwig, J.F. Mechanistic and structural characterization of an iridium-containing cytochrome reveals kinetically relevant cofactor dynamics. Nat. Catal. 2023, 6, 39–51. [Google Scholar] [CrossRef]
- Dydio, P.; Key, H.M.; Nazarenko, A.; Rha, J.Y.E.; Seyedkazemi, V.; Clark, D.S.; Hartwig, J.F. An artificial metalloenzyme with the kinetics of native enzymes. Science 2016, 354, 102–106. [Google Scholar] [CrossRef]
- Gu, Y.; Bloomer, B.J.; Liu, Z.; Chen, R.; Clark, D.S.; Hartwig, J.F. Directed Evolution of Artificial Metalloenzymes in Whole Cells. Angew. Chem. Int. Ed. Engl. 2022, 61, e202110519. [Google Scholar] [CrossRef]
- Gu, Y.; Natoli, S.N.; Liu, Z.; Clark, D.S.; Hartwig, J.F. Site-Selective Functionalization of (sp3)C-H Bonds Catalyzed by Artificial Metalloenzymes Containing an Iridium-Porphyrin Cofactor. Angew. Chem. Int. Ed. 2019, 58, 13954–13960. [Google Scholar]
- Van Cura, D.; Ng, T.L.; Huang, J.; Hager, H.; Hartwig, J.F.; Keasling, J.D.; Balskus, E.P. Discovery of the Azaserine Biosynthetic Pathway Uncovers a Biological Route for α-Diazoester Production. Angew. Chem. Int. Ed. 2023, 62, e202304646. [Google Scholar] [CrossRef]
- Huang, J.; Quest, A.; Cruz-Morales, P.; Deng, K.; Pereira, J.H.; Van Cura, D.; Kakumanu, R.; Baidoo, E.E.K.; Dan, Q.; Chen, Y.; et al. Complete integration of carbene-transfer chemistry into biosynthesis. Nature 2023, 617, 403–408. [Google Scholar] [CrossRef]
- Scipion, C.P.M.; Esque, J.; Borkar, S.; Ong, J.S.; Seah, C.; Ong, L.; Ng, P.; Kanagasundaram, Y.; Remaud-Simeon, M.; Bozonnet, S.; et al. Enhanced Production of (+)-Limonene through Targeted Engineering of Citrus sinensis Limonene Synthase. ACS Synth. Biol. 2025, 14, 2151–2161. [Google Scholar] [CrossRef]
- Hao, Y.; Liu, M.; Fordjour, E.; Yu, P.; Yang, Y.; Liu, X.; Li, Y.; Liu, C.L.; Bai, Z. Engineering Escherichia coli for Perillyl Alcohol Production with Reduced Endogenous Dehydrogenation. ACS Synth. Biol. 2025, 14, 1594–1605. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Z.; Bloomer, B.J.; Clark, D.S.; Mukhopadhyay, A.; Keasling, J.D.; Hartwig, J.F. Unnatural biosynthesis by an engineered microorganism with heterologously expressed natural enzymes and an artificial metalloenzyme. Nat. Chem. 2021, 13, 1186–1191. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Hou, R.; Cai, P.; Yao, L.; Wu, X.; Li, Y.; Zhang, L.; Zhou, Y.J. Engineering Yeast Peroxisomes for α-Bisabolene Production from Sole Methanol with the Aid of Proteomic Analysis. JACS Au 2024, 4, 2474–2483. [Google Scholar] [CrossRef]
- Fuentes, P.; Armarego-Marriott, T.; Bock, R. Plastid transformation and its application in metabolic engineering. Curr. Opin. Biotechnol. 2018, 49, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhu, Y.; Jia, H.; Han, Y.; Zheng, X.; Wang, M.; Feng, W. From Plant to Yeast-Advances in Biosynthesis of Artemisinin. Molecules 2022, 27, 6888. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.B.; Chekan, J.R. Engineering yeast for industrial-level production of the antimalarial drug artemisinin. Trends Biotechnol. 2023, 41, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, X.; Xiang, H.; Zhu, H.; Lu, X.; Feng, B. Two-Phase Fermentation Systems for Microbial Production of Plant-Derived Terpenes. Molecules 2024, 29, 1127. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.; Tang, Y. Natural and engineered production of taxadiene with taxadiene synthase. Biotechnol. Bioeng. 2015, 112, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Qiang, G.; Lv, J.; Zhou, B.; Li, G.; Guo, J. Opportunities and Challenges of in vitro Synthetic Biosystem for Terpenoids Production. Biotechnol. Bioprocess Eng. 2022, 27, 697–705. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Y.; Yuan, Z.; Wang, Q.; Wang, Z.; Cao, J.; Wu, J.; Ren, X. Expanding the Terpene Universe: Synthetic Biology and Non-Natural Chemistry in Engineered Microorganisms. Molecules 2025, 30, 4065. https://doi.org/10.3390/molecules30204065
Hu Y, Yuan Z, Wang Q, Wang Z, Cao J, Wu J, Ren X. Expanding the Terpene Universe: Synthetic Biology and Non-Natural Chemistry in Engineered Microorganisms. Molecules. 2025; 30(20):4065. https://doi.org/10.3390/molecules30204065
Chicago/Turabian StyleHu, Yueli, Ziyan Yuan, Qian Wang, Ziyan Wang, Jianan Cao, Jiaxin Wu, and Xinkun Ren. 2025. "Expanding the Terpene Universe: Synthetic Biology and Non-Natural Chemistry in Engineered Microorganisms" Molecules 30, no. 20: 4065. https://doi.org/10.3390/molecules30204065
APA StyleHu, Y., Yuan, Z., Wang, Q., Wang, Z., Cao, J., Wu, J., & Ren, X. (2025). Expanding the Terpene Universe: Synthetic Biology and Non-Natural Chemistry in Engineered Microorganisms. Molecules, 30(20), 4065. https://doi.org/10.3390/molecules30204065







