Synthesis and Characterization of 4-Indolylcyanamide: A Potential IR Probe for Local Environment
Abstract
1. Introduction
2. Results and Discussion
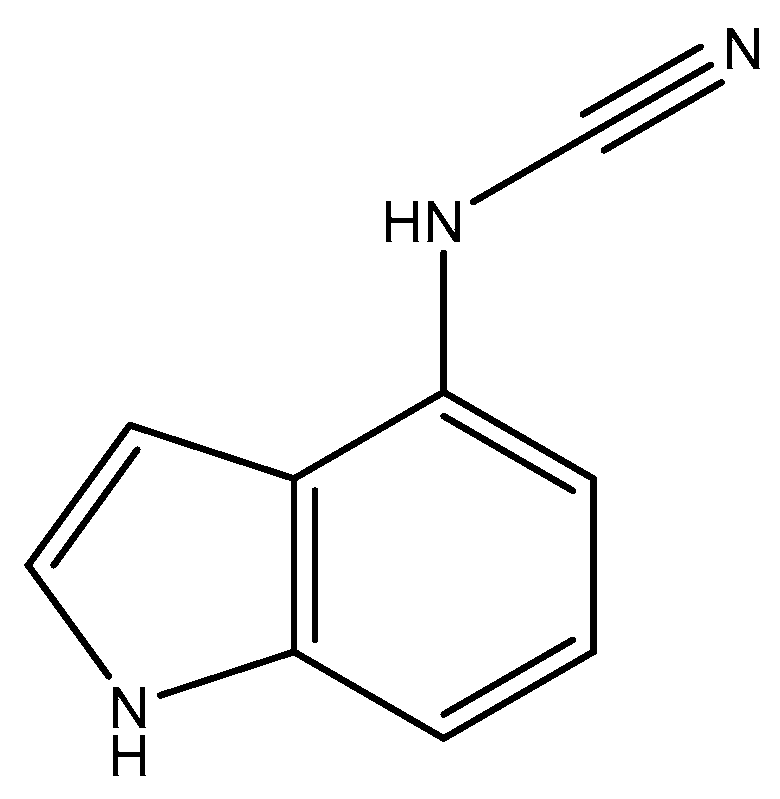
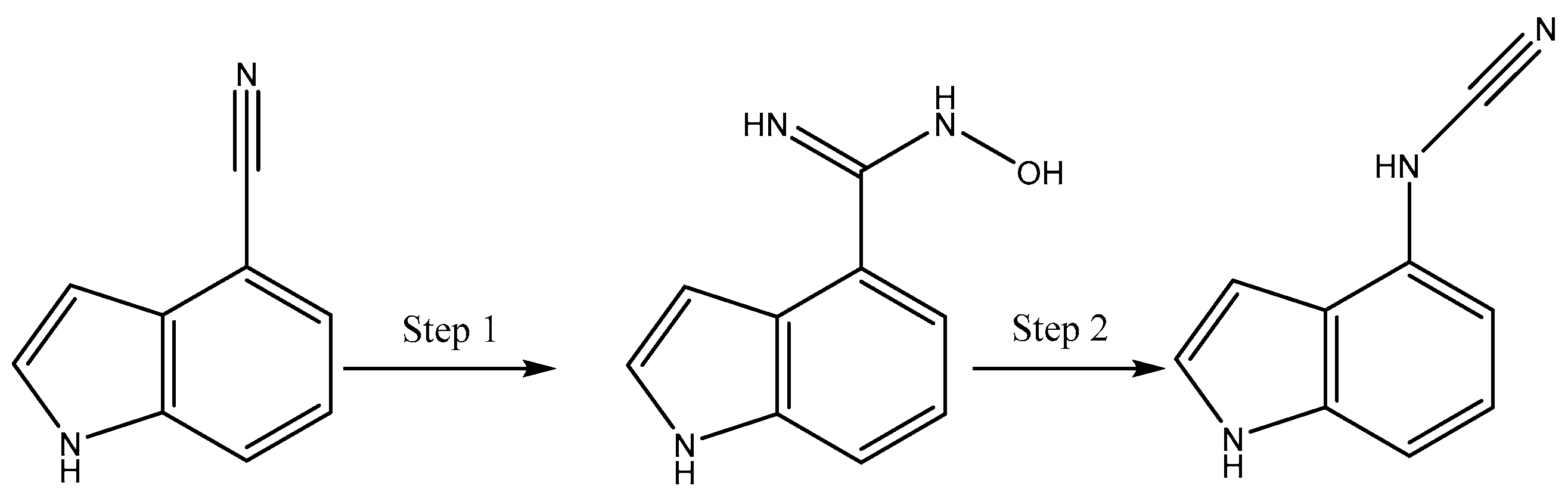
2.1. Chemistry
2.2. FTIR Spectroscopy
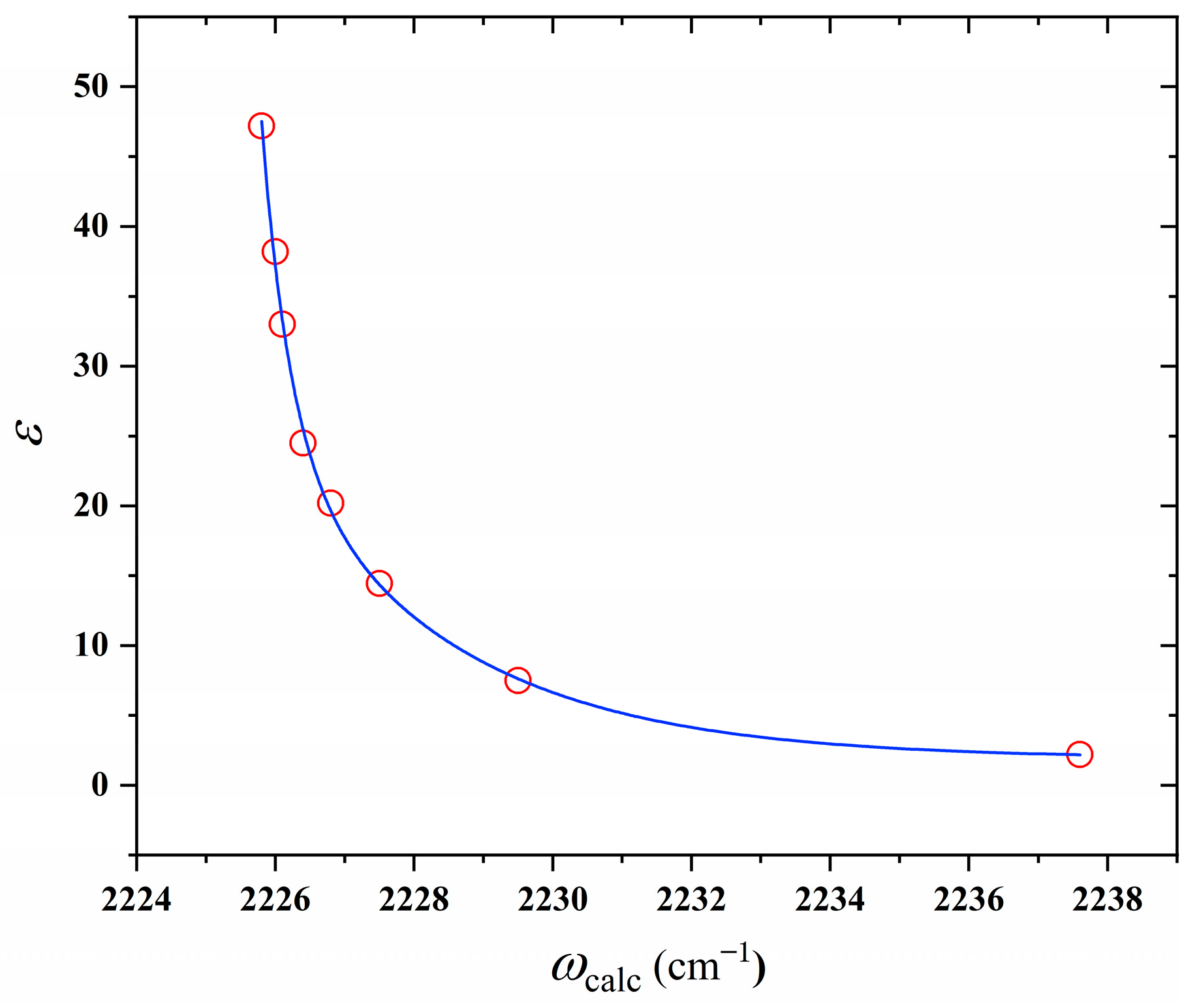
2.3. Quantum Chemical Calculations
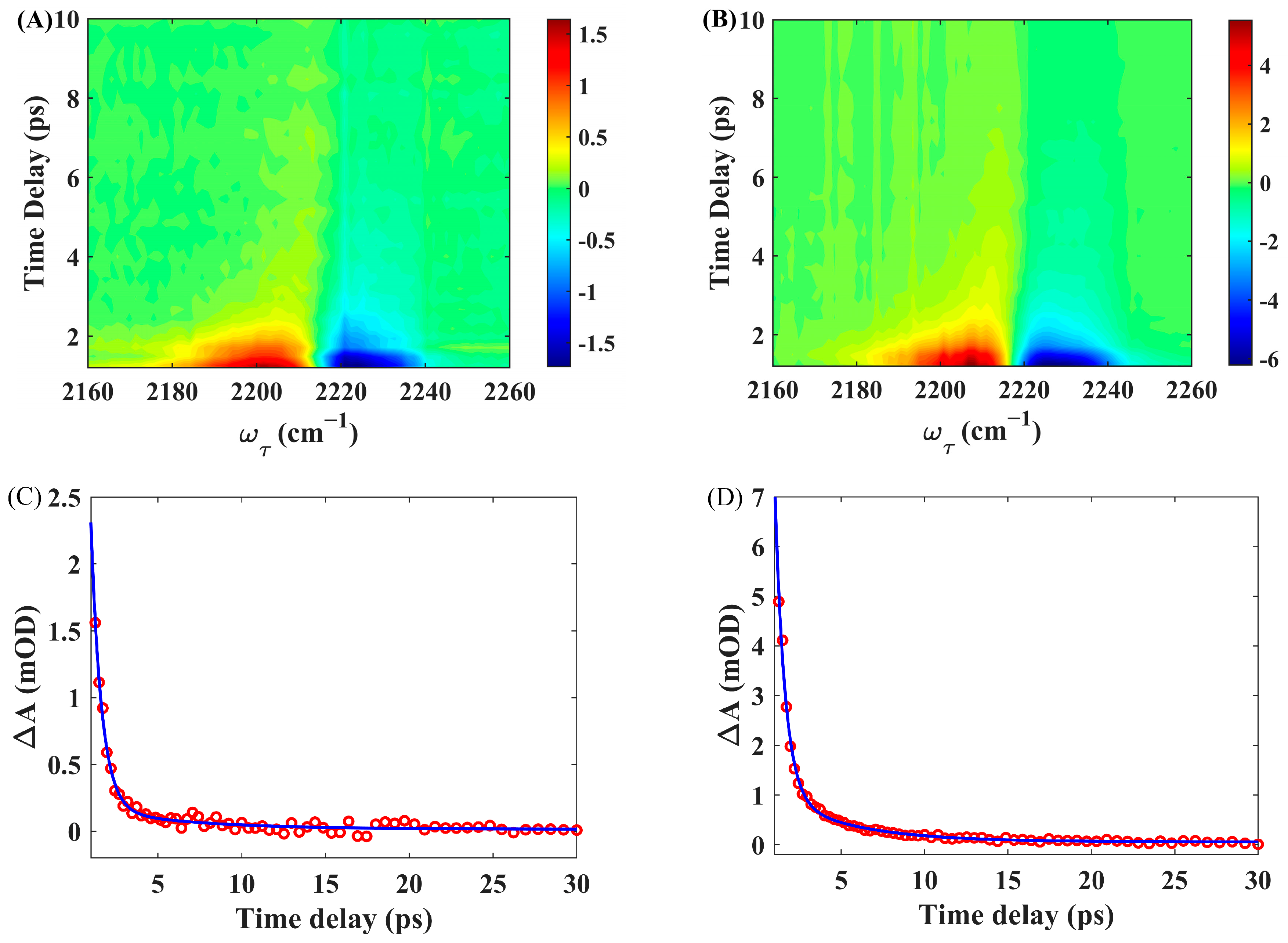
2.4. IR Pump–Probe Spectroscopy
3. Materials and Methods
3.1. Materials and Sample Preparation
3.2. Spectroscopic Measurements
3.3. Computational
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhuang, S.-H.; Lin, Y.-C.; Chou, L.-C.; Hsu, M.-H.; Lin, H.-Y.; Huang, C.-H.; Lien, J.-C.; Kuo, S.-C.; Huang, L.-J. Synthesis and anticancer activity of 2,4-disubstituted furo [3,2-b] indole derivatives. Eur. J. Med. Chem. 2013, 66, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Rzepka, Z.; Bębenek, E.; Chrobak, E.; Wrześniok, D. Synthesis and anticancer activity of indole-functionalized derivatives of betulin. Pharmaceutics 2022, 14, 2372. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Singh, D.; Singh, J.; Yadav, V.; Pathak, A.K.; Dabur, R.; Chhillar, A.K.; Singh, R.; Sharma, G.; Chandra, R.; et al. Synthesis and antibacterial activity of substituted 1,2,3,4-tetrahydropyrazino [1,2-a] indoles. Bioorg. Med. Chem. Lett. 2006, 16, 413–416. [Google Scholar] [CrossRef]
- Gomha, S.M.; Riyadh, S.M. Synthesis under microwave irradiation of [1,2,4] triazolo [3,4-b][1,3,4] thiadiazoles and other diazoles bearing indole moieties and their antimicrobial evaluation. Molecules 2011, 16, 8244–8256. [Google Scholar] [CrossRef]
- Rani, P.; Srivastava, V.; Kumar, A. Synthesis and antiinflammatory activity of heterocyclic indole derivatives. Eur. J. Med. Chem. 2004, 39, 449–452. [Google Scholar] [CrossRef]
- Zhang, M.-Z.; Chen, Q.; Yang, G.-F. A review on recent developments of indole-containing antiviral agents. Eur. J. Med. Chem. 2015, 89, 421–441. [Google Scholar] [CrossRef]
- Xue, S.; Ma, L.; Gao, R.; Li, Y.; Li, Z. Synthesis and antiviral activity of some novel indole-2-carboxylate derivatives. Acta Pharm. Sin. B 2014, 4, 313–321. [Google Scholar] [CrossRef]
- Dorababu, A. Indole–a promising pharmacophore in recent antiviral drug discovery. RSC Med. Chem. 2020, 11, 1335–1353. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Yamamoto, Y.; Matsumura, Y.; Ohba, K.; Sakamaki, S.; Kimata, H.; Nakayama, K.; Kuriyama, C.; Matsushita, Y.; Ueta, K. Novel indole-N-glucoside, TA-1887 as a sodium glucose cotransporter 2 inhibitor for treatment of type 2 diabetes. ACS Med. Chem. Lett. 2014, 5, 51–55. [Google Scholar] [CrossRef]
- Taha, M.; Rahim, F.; Imran, S.; Ismail, N.H.; Ullah, H.; Selvaraj, M.; Javid, M.T.; Salar, U.; Ali, M.; Khan, K.M. Synthesis, α-glucosidase inhibitory activity and in silico study of tris-indole hybrid scaffold with oxadiazole ring: As potential leads for the management of type-II diabetes mellitus. Bioorg. Chem. 2017, 74, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Praveen, C.; Ayyanar, A.; Perumal, P.T. Practical synthesis, anticonvulsant, and antimicrobial activity of N-allyl and N-propargyl di (indolyl) indolin-2-ones. Bioorg. Med. Chem. Lett. 2011, 21, 4072–4077. [Google Scholar] [CrossRef]
- Tan, C.; Yang, S.-J.; Zhao, D.-H.; Li, J.; Yin, L.-Q. Antihypertensive activity of indole and indazole analogues: A review. Arab. J. Chem. 2022, 15, 103756. [Google Scholar] [CrossRef]
- Pappolla, M.A.; Perry, G.; Fang, X.; Zagorski, M.; Sambamurti, K.; Poeggeler, B. Indoles as essential mediators in the gut-brain axis. Their role in Alzheimer’s disease. Neurobiol. Dis. 2021, 156, 105403. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.R.; Khan, S.I.; Singh, S.; Khan, I.A.; Vishwakarma, R.A.; Bharate, S.B. Synthesis, antimalarial and antitubercular activities of meridianin derivatives. Eur. J. Med. Chem. 2015, 98, 160–169. [Google Scholar] [CrossRef]
- Helm, R.; Clara, M.; Grebner, T.L.; Neusser, H.J. Hydrogen Bonding in the Indole− Water Complex: A High Resolution UV Study of the Hydrogen Donor Conformer. J. Phys. Chem. A 1998, 102, 3268–3272. [Google Scholar] [CrossRef]
- Biswal, H.S.; Wategaonkar, S. Nature of the n− h··· s hydrogen bond. J. Phys. Chem. A 2009, 113, 12763–12773. [Google Scholar] [CrossRef]
- Oshita, H.; Suzuki, T.; Kawashima, K.; Abe, H.; Tani, F.; Mori, S.; Yajima, T.; Shimazaki, Y. π–π Stacking Interaction in an Oxidized CuII–Salen Complex with a Side-Chain Indole Ring: An Approach to the Function of the Tryptophan in the Active Site of Galactose Oxidase. Chem.–A Eur. J. 2019, 25, 7649–7658. [Google Scholar] [CrossRef]
- Geng, Y.; Takatani, T.; Hohenstein, E.G.; Sherrill, C.D. Accurately characterizing the π−π interaction energies of indole−benzene complexes. J. Phys. Chem. A 2010, 114, 3576–3582. [Google Scholar] [CrossRef]
- Norman, K.E.; Nymeyer, H. Indole localization in lipid membranes revealed by molecular simulation. Biophys. J. 2006, 91, 2046–2054. [Google Scholar] [CrossRef] [PubMed]
- Gaede, H.C.; Yau, W.-M.; Gawrisch, K. Electrostatic contributions to indole− lipid interactions. J. Phys. Chem. B 2005, 109, 13014–13023. [Google Scholar] [CrossRef]
- Talukder, P.; Chen, S.; Roy, B.; Yakovchuk, P.; Spiering, M.M.; Alam, M.P.; Madathil, M.M.; Bhattacharya, C.; Benkovic, S.J.; Hecht, S.M. Cyanotryptophans as Novel Fluorescent Probes for Studying Protein Conformational Changes and DNA-Protein Interaction. Biochemistry 2015, 54, 7457–7469. [Google Scholar] [CrossRef]
- Hilaire, M.R.; Ahmed, I.A.; Lin, C.W.; Jo, H.; DeGrado, W.F.; Gai, F. Blue fluorescent amino acid for biological spectroscopy and microscopy. Proc. Natl. Acad. Sci. USA 2017, 114, 6005–6009. [Google Scholar] [CrossRef] [PubMed]
- Hilaire, M.R.; Mukherjee, D.; Troxler, T.; Gai, F. Solvent dependence of cyanoindole fluorescence lifetime. Chem. Phys. Lett. 2017, 685, 133–138. [Google Scholar] [CrossRef]
- Markiewicz, B.N.; Mukherjee, D.; Troxler, T.; Gai, F. Utility of 5-cyanotryptophan fluorescence as a sensitive probe of protein hydration. J. Phys. Chem. B 2016, 120, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Markiewicz, B.N.; Doerksen, R.S.; Smith III, A.B.; Gai, F. C [triple bond, length as m-dash] N stretching vibration of 5-cyanotryptophan as an infrared probe of protein local environment: What determines its frequency? Phys. Chem. Chem. Phys. 2016, 18, 7027–7034. [Google Scholar] [CrossRef]
- Markiewicz, B.N.; Lemmin, T.; Zhang, W.; Ahmed, I.A.; Jo, H.; Fiorin, G.; Troxler, T.; DeGrado, W.F.; Gai, F. Infrared and fluorescence assessment of the hydration status of the tryptophan gate in the influenza A M2 proton channel. Phys. Chem. Chem. Phys. 2016, 18, 28939–28950. [Google Scholar] [CrossRef]
- Rodgers, J.M.; Abaskharon, R.M.; Ding, B.; Chen, J.; Zhang, W.; Gai, F. Fermi resonance as a means to determine the hydrogen-bonding status of two infrared probes. Phys. Chem. Chem. Phys. 2017, 19, 16144–16150. [Google Scholar] [CrossRef] [PubMed]
- Waegele, M.M.; Tucker, M.J.; Gai, F. 5-Cyanotryptophan as an infrared probe of local hydration status of proteins. Chem. Phys. Lett. 2009, 478, 249–253. [Google Scholar] [CrossRef]
- Huang, X.-y.; You, M.; Ran, G.-l.; Fan, H.-r.; Zhang, W.-k. Ester-Derivatized indoles as fluorescent infrared probes for hydration environments. Chin. J. Chem. Phys. 2018, 31, 477. [Google Scholar] [CrossRef]
- You, M.; Zhou, L.; Huang, X.; Wang, Y.; Zhang, W. Isonitrile-Derivatized Indole as an Infrared Probe for Hydrogen-Bonding Environments. Molecules 2019, 24, 1379. [Google Scholar] [CrossRef]
- You, M.; Gao, Z.; Zhou, L.; Guo, C.; Guo, Q. Investigation of the Vibrational Characteristics of 6-Isocyano-1-Methyl-1H-Indole: Utilizing the Isonitrile Group as an Infrared Probe. Molecues 2023, 28, 6939. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Dickinson, C.; Taft, R.W. Linear solvation energy relationships-solvent effects on some fluorescence probes. Chem. Phys. Lett. 1981, 77, 69–72. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Abboud, J.L.M.; Abraham, M.H.; Taft, R. Linear solvation energy relationships. 23. A comprehensive collection of the solvatochromic parameters,. pi.*,. alpha., and. beta., and some methods for simplifying the generalized solvatochromic equation. J. Org. Chem. 1983, 48, 2877–2887. [Google Scholar] [CrossRef]
- Gutmann, V.J.C.C.R. Solvent effects on the reactivities of organometallic compounds. Coord. Chem. Rev. 1976, 18, 225–255. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, G.; Chen, L.; Wang, Q.; Wang, G.; Zhang, H.; Li, Y.; Liu, C.J.U.S. A Review: Principles and Applications of High-Pressure In Situ Time-Resolved Transient Absorption Spectroscopy. Ultrafast Sci. 2024, 4, 44. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, B.; Zhang, L.; Yu, J.J.C. Femtosecond transient absorption spectroscopy investigation into the electron transfer mechanism in photocatalysis. Chem. Commun. 2023, 59, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zeng, P.; Chen, S.; Smith, T.A.; Liu, M.J.L.; Reviews, P. Charge Transfer Dynamics at the Interface of CsPbX3 Perovskite Nanocrystal-Acceptor Complexes: A Femtosecond Transient Absorption Spectroscopy Study. Laser Photonics Rev. 2022, 16, 2200280. [Google Scholar] [CrossRef]
- Moilanen, D.E.; Piletic, I.R.; Fayer, M.D.J.T.J. Water dynamics in Nafion fuel cell membranes: The effects of confinement and structural changes on the hydrogen bond network. J. Phys. Chem. C 2007, 111, 8884–8891. [Google Scholar] [CrossRef]
- Fayer, M.D. Ultrafast Infrared Vibrational Spectroscopy; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Fox, Gaussian 16, Revision A.02; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Barone, V.; Cossi, M. Quantum calculation of molecular energies energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
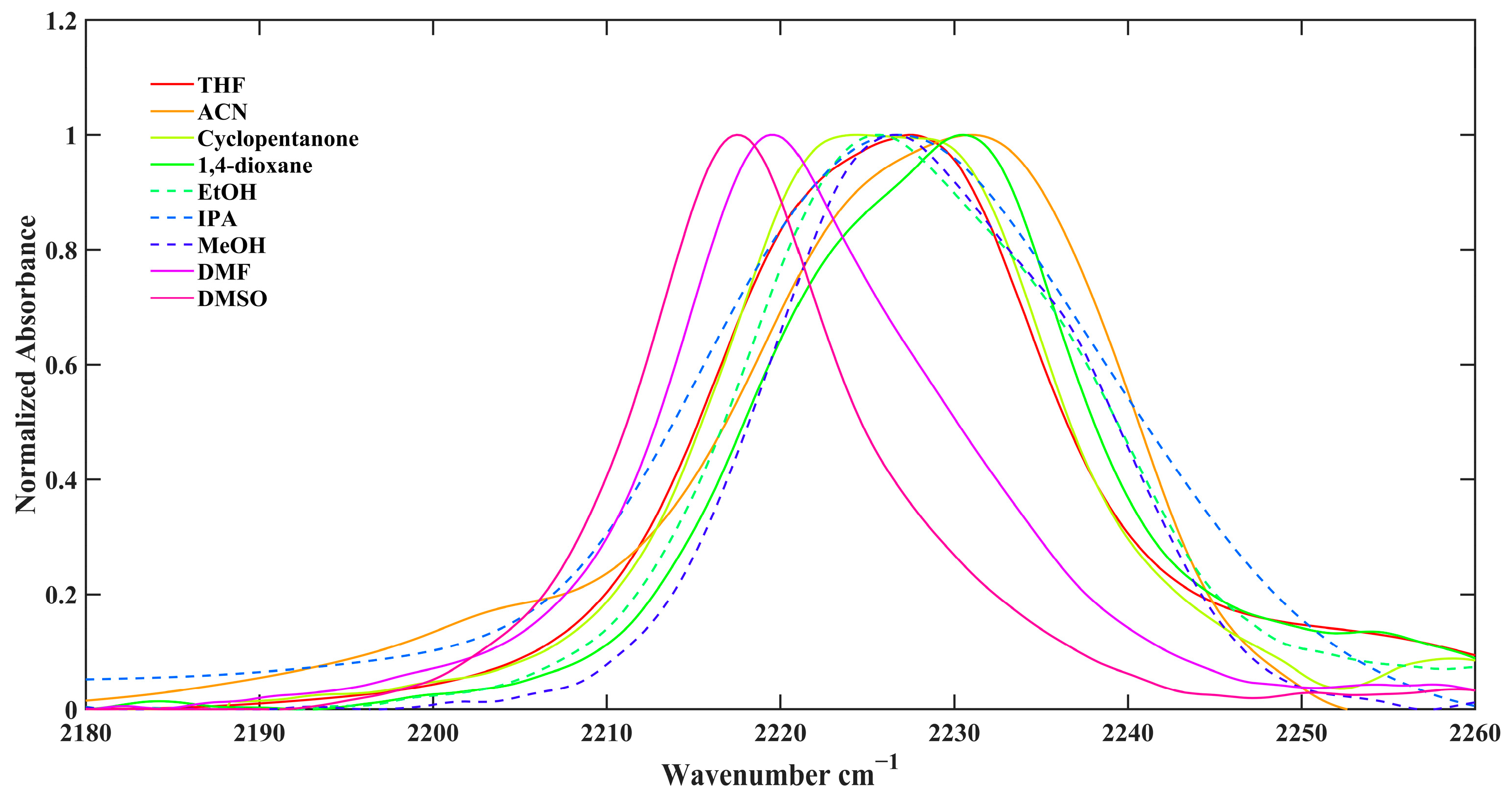
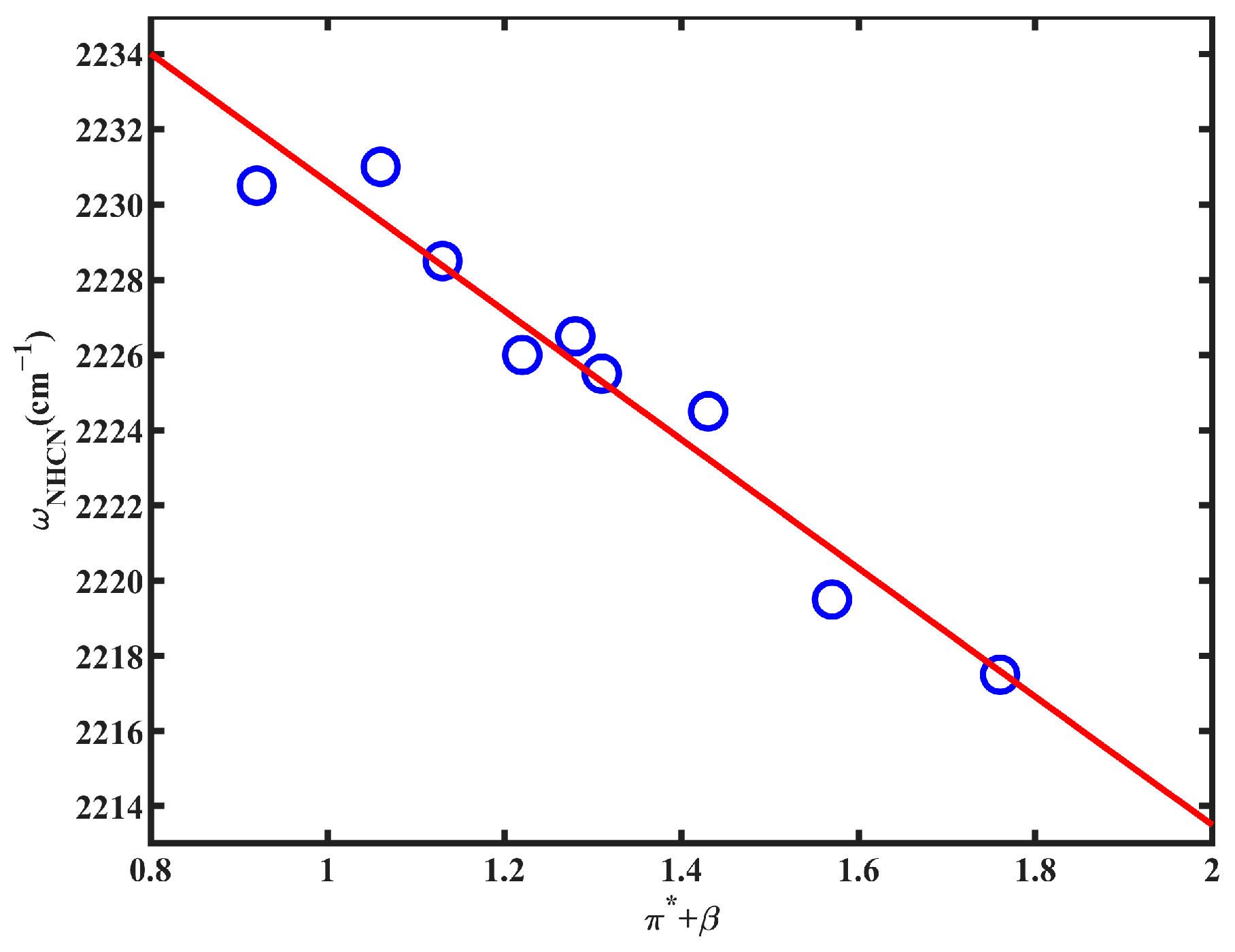
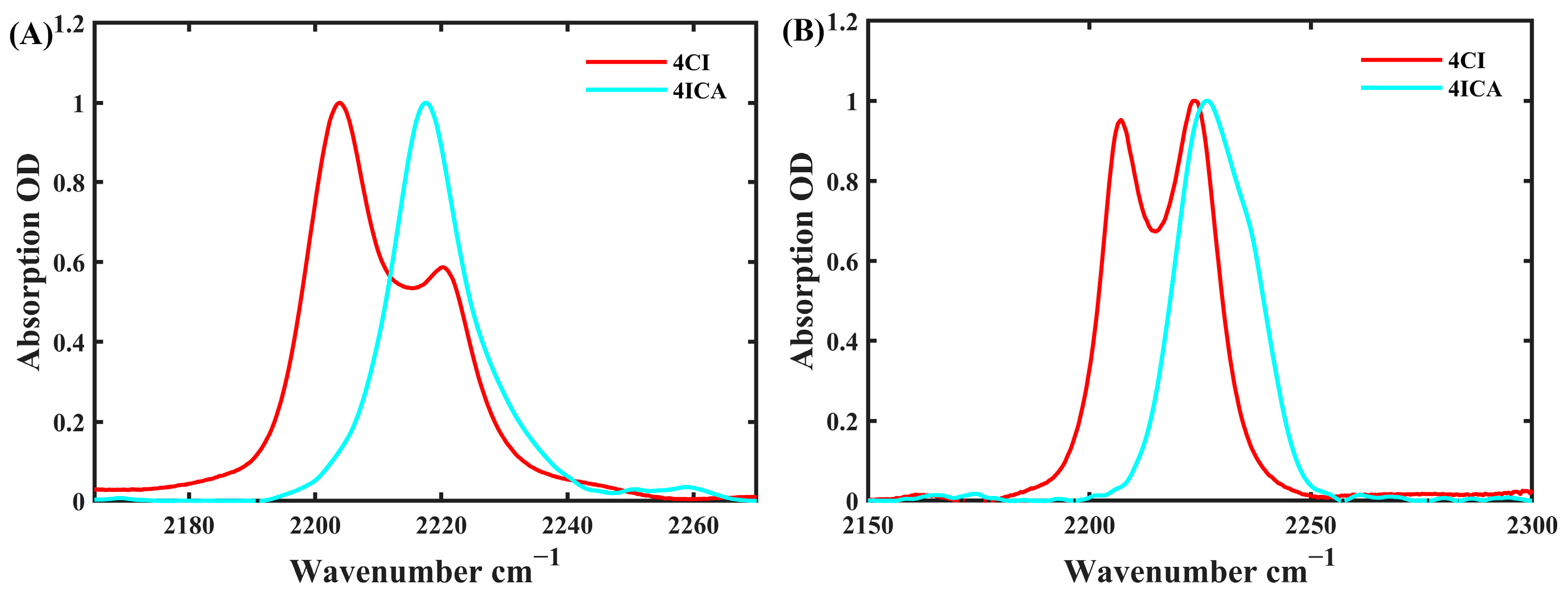
| Solvent | ω(NHCN) | FWHM | π* | β | α |
|---|---|---|---|---|---|
| dimethyl sulfoxide (DMSO) | 2217.5 | 14.0 | 1 | 0.76 | 0 |
| N,N-Dimethylformamide (DMF) | 2219.5 | 18.0 | 0.88 | 0.69 | 0 |
| isopropanol (IPA) | 2224.5 | 26.7 | 0.48 | 0.95 | 0.76 |
| ethanol (EtOH) | 2225.5 | 22.2 | 0.54 | 0.77 | 0.83 |
| methanol (MeOH) | 2226.0 | 20.5 | 0.6 | 0.62 | 0.93 |
| cyclopentanone | 2226.5 | 19.8 | 0.76 | 0.52 | 0 |
| tetrahydrofuran (THF) | 2228.5 | 20.2 | 0.58 | 0.55 | 0 |
| 1,4-dioxane | 2230.5 | 20.4 | 0.55 | 0.37 | 0 |
| Acetonitrile (ACN) | 2231.0 | 21.2 | 0.75 | 0.31 | 0.19 |
| Compound | Solvent | |||
|---|---|---|---|---|
| Ethanol | DMSO | DMF | MeOH | |
| 4ICA | 205.0 | 242.5 | 231.5 | 241.3 |
| 5ICI [30] | 85.3 | 125.2 | 117.3 | 97.5 |
| 6MICI [31] | 79.8 | 103.4 | 100.1 | 81.5 |
| 4CI | 77.6 | 125.4 | 120.9 | 81.1 |
| Solvents | Esolvation | μ | Δμ | ωcalc | ωexp | Δω | LCN | ΔLCN | fKBM | ε |
|---|---|---|---|---|---|---|---|---|---|---|
| Vacuum | 4.7969 | 2246.7 | 1.1685 | |||||||
| DMSO | −41.3466 | 6.4699 | 1.6731 | 2225.8 | 2217.5 | −8.31 | 1.1705 | 0.00201 | 0.484 | 47.2 |
| DMF | −41.0657 | 6.4565 | 1.6596 | 2226.0 | 2219.5 | −6.50 | 1.1705 | 0.00199 | 0.481 | 38.2 |
| MeOH | −40.8714 | 6.4471 | 1.6503 | 2226.1 | 2226.0 | −0.13 | 1.1705 | 0.00198 | 0.478 | 33 |
| EtOH | −40.3857 | 6.4242 | 1.6273 | 2226.4 | 2225.5 | −0.95 | 1.1705 | 0.00195 | 0.470 | 24.5 |
| IPA | −39.7976 | 6.3963 | 1.5994 | 2226.8 | 2224.5 | −2.34 | 1.1704 | 0.00191 | 0.464 | 20.2 |
| cyclopentanone | −38.7054 | 6.3451 | 1.5482 | 2227.5 | 2226.5 | −1.04 | 1.1704 | 0.00184 | 0.450 | 14.45 |
| THF | −35.6992 | 6.2067 | 1.4098 | 2229.5 | 2228.5 | −1.31 | 1.1702 | 0.00166 | 0.406 | 7.5 |
| 1,4-dioxane | −21.2248 | 5.5861 | 0.7892 | 2237.6 | 2230.5 | −0.95 | 1.1694 | 0.00087 | 0.222 | 2.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, M.; Li, Q.; Gao, Z.; Guo, C.; Zhou, L. Synthesis and Characterization of 4-Indolylcyanamide: A Potential IR Probe for Local Environment. Molecules 2025, 30, 4063. https://doi.org/10.3390/molecules30204063
You M, Li Q, Gao Z, Guo C, Zhou L. Synthesis and Characterization of 4-Indolylcyanamide: A Potential IR Probe for Local Environment. Molecules. 2025; 30(20):4063. https://doi.org/10.3390/molecules30204063
Chicago/Turabian StyleYou, Min, Qingxue Li, Zilin Gao, Changyuan Guo, and Liang Zhou. 2025. "Synthesis and Characterization of 4-Indolylcyanamide: A Potential IR Probe for Local Environment" Molecules 30, no. 20: 4063. https://doi.org/10.3390/molecules30204063
APA StyleYou, M., Li, Q., Gao, Z., Guo, C., & Zhou, L. (2025). Synthesis and Characterization of 4-Indolylcyanamide: A Potential IR Probe for Local Environment. Molecules, 30(20), 4063. https://doi.org/10.3390/molecules30204063







