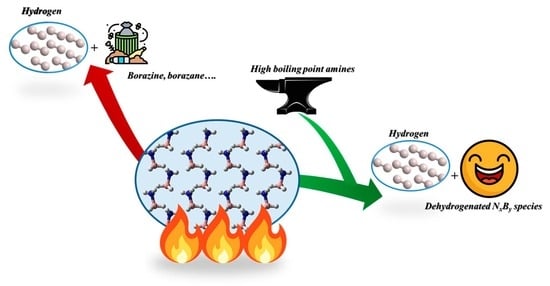
Tuning the Solid-State Hydrogen Release of Ammonia Borane by Entrapping the Intermediates: The Role of High-Boiling-Point Amines
Abstract
1. Introduction
2. Results and Discussion
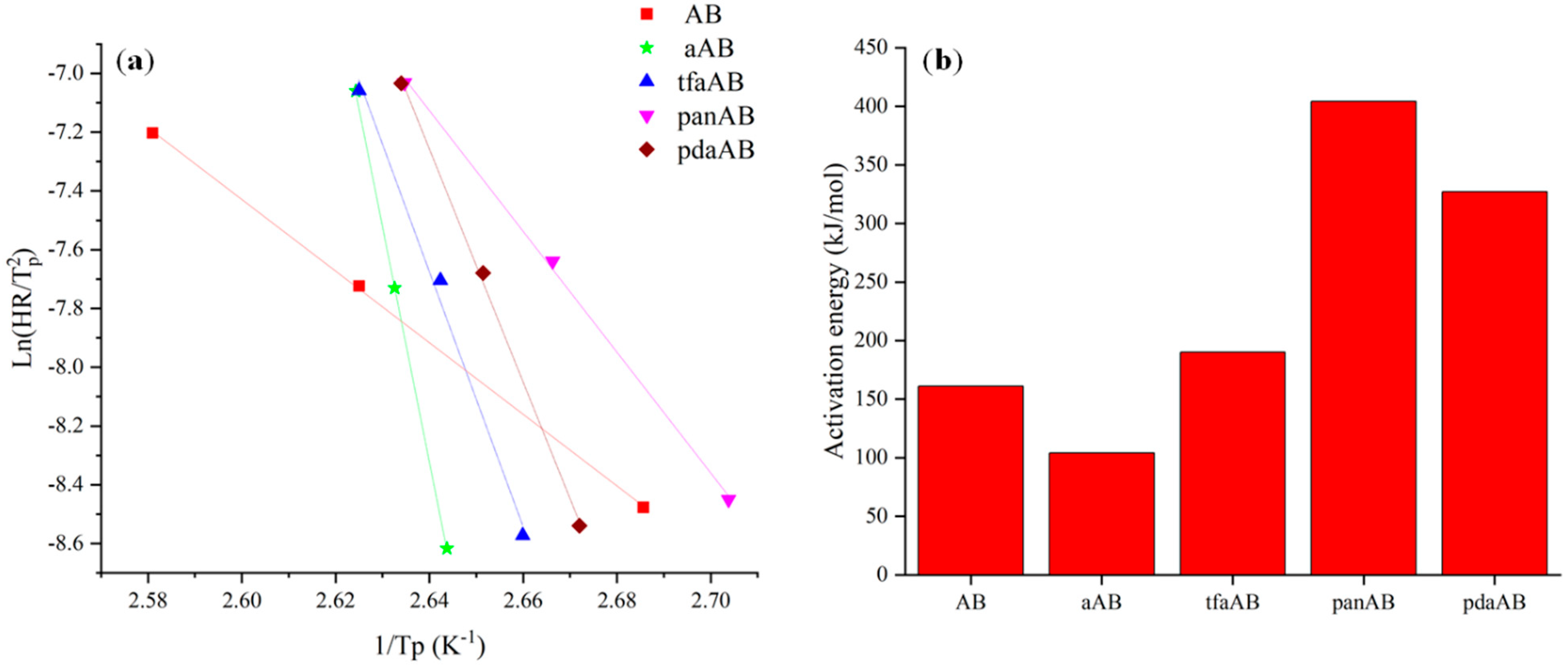
2.1. Evaluation of Activation Energy of Thermal Degradation of AB in Presence of High-Boiling-Point Amines Through Non-Isothermal Kissinger Equation
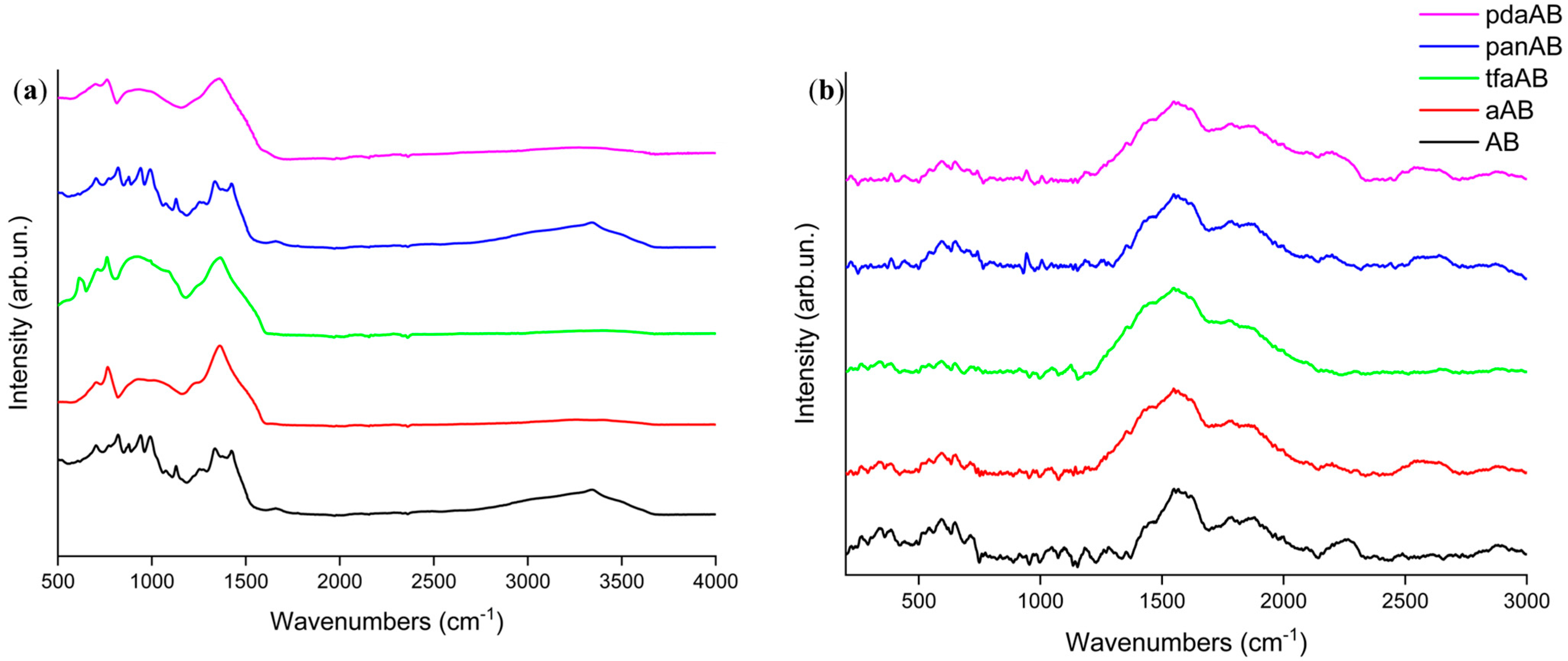
2.2. Solid-State Thermal Degradation of AB in Presence of High-Boiling-Point Amines
| Specie | Nitrogen (%) | Boron (%) | |||
|---|---|---|---|---|---|
| N-B | NH/NH2-B | NH3-B | NB | B-O | |
| AB | 80.7 | 18.8 | 0.5 | 25.6 | 74.4 |
| aAB | 85.8 | 12.2 | 1.9 | 29.8 | 70.2 |
| tfaAB | 96.4 | 3.6 | 0.0 | 36.4 | 63.6 |
| panAB | 79.1 | 19.8 | 1.2 | 12.9 | 87.1 |
| pdaAB | 34.0 | 54.9 | 11.1 | 11.6 | 88.4 |
3. Materials and Methods
3.1. Materials
3.2. Preparation of High-Boiling-Point Amine/AB Mixtures
3.3. Physicochemical Characterization of High-Boiling-Point Amine/AB Mixtures
3.4. Calculation of Formation Energy of Intermediates Formed During Solid-State Thermal Degradative of AB in Presence of High-Boiling-Point Amines
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Usman, M.R. Hydrogen storage methods: Review and current status. Renew. Sustain. Energy Rev. 2022, 167, 112743. [Google Scholar] [CrossRef]
- Aziz, M. Liquid hydrogen: A review on liquefaction, storage, transportation, and safety. Energies 2021, 14, 5917. [Google Scholar] [CrossRef]
- Michler, T.; Lindner, M.; Eberle, U.; Meusinger, J. Assessing hydrogen embrittlement in automotive hydrogen tanks. In Gaseous Hydrogen Embrittlement of Materials in Energy Technologies; Elsevier: Amsterdam, The Netherlands, 2012; pp. 94–125. [Google Scholar]
- Hosseini, S.E.; Butler, B. An overview of development and challenges in hydrogen powered vehicles. Int. J. Green Energy 2020, 17, 13–37. [Google Scholar] [CrossRef]
- Dash, S.K.; Chakraborty, S.; Roccotelli, M.; Sahu, U.K. Hydrogen fuel for future mobility: Challenges and future aspects. Sustainability 2022, 14, 8285. [Google Scholar] [CrossRef]
- Filippov, S.P.; Yaroslavtsev, A.B. Hydrogen energy: Development prospects and materials. Russ. Chem. Rev. 2021, 90, 627. [Google Scholar] [CrossRef]
- Najjar, Y.S. Hydrogen safety: The road toward green technology. Int. J. Hydrogen Energy 2013, 38, 10716–10728. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Eberle, U.; Felderhoff, M.; Schueth, F. Chemical and physical solutions for hydrogen storage. Angew. Chem. Int. Ed. 2009, 48, 6608–6630. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.M.; Croiset, E.; Epling, W. Review of methane catalytic cracking for hydrogen production. Int. J. Hydrogen Energy 2011, 36, 2904–2935. [Google Scholar] [CrossRef]
- LeValley, T.L.; Richard, A.R.; Fan, M. The progress in water gas shift and steam reforming hydrogen production technologies–A review. Int. J. Hydrogen Energy 2014, 39, 16983–17000. [Google Scholar] [CrossRef]
- Ding, Z.; Li, S.; Zhou, Y.; Chen, Z.; Yang, W.; Ma, W.; Shaw, L. LiBH4 for hydrogen storage-new perspectives. Nano Mater. Sci. 2020, 2, 109–119. [Google Scholar] [CrossRef]
- Webb, C. A review of catalyst-enhanced magnesium hydride as a hydrogen storage material. J. Phys. Chem. Solids 2015, 84, 96–106. [Google Scholar] [CrossRef]
- Ley, M.B.; Jepsen, L.H.; Lee, Y.-S.; Cho, Y.W.; Von Colbe, J.M.B.; Dornheim, M.; Rokni, M.; Jensen, J.O.; Sloth, M.; Filinchuk, Y. Complex hydrides for hydrogen storage–new perspectives. Mater. Today 2014, 17, 122–128. [Google Scholar] [CrossRef]
- Geanangel, R.; Wendlandt, W. The thermal light emission of NH3· BH3. Thermochim. Acta 1987, 113, 383–385. [Google Scholar] [CrossRef]
- Sit, V.; Geanangel, R.; Wendlandt, W. The thermal dissociation of NH3BH3. Thermochim. Acta 1987, 113, 379–382. [Google Scholar] [CrossRef]
- Chandra, M.; Xu, Q. A high-performance hydrogen generation system: Transition metal-catalyzed dissociation and hydrolysis of ammonia–borane. J. Power Sources 2006, 156, 190–194. [Google Scholar] [CrossRef]
- Shimizu, Y.; Tamaki, R.; Tripathi, A.K.; Takamine, S.; Tian, Y.; Kishimoto, K.; Teruya, T.; Unten, Y.; Shinjo, H.; Nakagawa, T. Fundamental properties of ammonia borane aqueous solution: Dissolution enthalpy of solution, freezing points and solubility curve, thermal analysis, stability and phase diagram. J. Jpn. Inst. Energy 2023, 102, 65–76. [Google Scholar] [CrossRef]
- Demirci, U.B. Ammonia borane, a material with exceptional properties for chemical hydrogen storage. Int. J. Hydrogen Energy 2017, 42, 9978–10013. [Google Scholar] [CrossRef]
- Zhao, W.; Li, H.; Zhang, H.; Yang, S.; Riisager, A. Ammonia borane-enabled hydrogen transfer processes: Insights into catalytic strategies and mechanisms. Green Energy Environ. 2023, 8, 948–971. [Google Scholar] [CrossRef]
- Hu, M.; Geanangel, R.; Wendlandt, W. The thermal decomposition of ammonia borane. Thermochim. Acta 1978, 23, 249–255. [Google Scholar] [CrossRef]
- Demirci, U.B. Mechanistic insights into the thermal decomposition of ammonia borane, a material studied for chemical hydrogen storage. Inorg. Chem. Front. 2021, 8, 1900–1930. [Google Scholar] [CrossRef]
- Bartoli, M.; Etzi, M.; Lettieri, S.; Ferraro, G.; Pirri, C.F.; Chiodoni, A.M.; Bocchini, S. Complex Waste Stream Utilization for Hydrogen Evolution: Ammonia Borane Hydrolysis Over Red Mud Catalyst Under Mild Conditions. Catal. Lett. 2025, 155, 273. [Google Scholar] [CrossRef]
- Gianola, G.; Bartoli, M.; Pirri, C.F.; Bocchini, S. Hydrogen evolution through ammonia borane hydrolysis over iron tailored pig manure catalyst. Int. J. Hydrogen Energy 2024, 51, 21–28. [Google Scholar] [CrossRef]
- Bartoli, M.; Etzi, M.; Lettieri, S.; Ferraro, G.; Pirri, C.F.; Bocchini, S. Ultrasound induced hydrogen release from ammonia borane mediated by oxidized multiwalled carbon nanotube under hydrolytic conditions. Appl. Surf. Sci. 2025; 164800, in press. [Google Scholar] [CrossRef]
- Astorino, C.; De Nardo, E.; Lettieri, S.; Ferraro, G.; Bartoli, M.; Etzi, M.; Chiodoni, A.M.; Pirri, C.F.; Bocchini, S. Investigation of Solid-State Thermal Decomposition of Ammonia Borane Mix with Sulphonated Poly (ellagic Acid) for Hydrogen Release. Polymers 2024, 16, 3471. [Google Scholar] [CrossRef]
- Bartoli, M.; Pirri, C.F.; Bocchini, S. Unraveling the Effect of Carbon Nanotube Oxidation on Solid-State Decomposition of Ammonia Borane/Carbon Nanotube Composites. J. Phys. Chem. C 2022, 126, 16587–16594. [Google Scholar] [CrossRef]
- Aum, H.; Kim, J.; Kang, H.; Baik, K.; Jung, J. Silanol-Enhanced Dehydrogenation of Ammonia Borane with Silicic Acid Catalyst. Korean J. Chem. Eng. 2025, 42, 679–687. [Google Scholar] [CrossRef]
- Castilla-Martinez, C.A.; Gaveau, P.; Semsarilar, M.; Alonso, B.; Demirci, U.B. Isothermal Dehydrogenation of Ammonia Borane: Insights into BNH Polymers and Challenges in Regeneration. Chem. Asian J. 2025, 20, e202500140. [Google Scholar] [CrossRef]
- Nakamura, Y.; Nakayama, J.; Miyake, A.; Izato, Y.-i. Thermal stability evaluation of ammonia borane as an onboard hydrogen carrier based on kinetic analysis employing thermal analysis. Int. J. Hydrogen Energy 2025, 106, 1258–1266. [Google Scholar] [CrossRef]
- Gangal, A.C.; Sharma, P. Kinetic analysis and modeling of thermal decomposition of ammonia borane. Int. J. Chem. Kinet. 2013, 45, 452–461. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Gutowska, A.; Li, L.; Shin, Y.; Wang, C.M.; Li, X.S.; Linehan, J.C.; Smith, R.S.; Kay, B.D.; Schmid, B.; Shaw, W. Nanoscaffold mediates hydrogen release and the reactivity of ammonia borane. Angew. Chem. 2005, 117, 3644–3648. [Google Scholar] [CrossRef]
- Kim, Y.; Baek, H.; Lee, J.H.; Yeo, S.; Kim, K.; Hwang, S.-J.; Eun, B.; Nam, S.W.; Lim, T.-H.; Yoon, C.W. Metal-free, polyether-mediated H 2-release from ammonia borane: Roles of hydrogen bonding interactions in promoting dehydrogenation. Phys. Chem. Chem. Phys. 2013, 15, 19584–19594. [Google Scholar] [CrossRef]
- McBride, J.; Nicholls, R. The vibration-rotation spectrum of ammonia gas. I. J. Phys. B At. Mol. Phys. (1968–1987) 1972, 5, 408. [Google Scholar] [CrossRef]
- Anderson, W.E.; Barker, E. The Infra-Red Absorption Spectrum of Diborane. J. Chem. Phys. 1950, 18, 698–705. [Google Scholar] [CrossRef]
- Gerry, M.; Lewis-Bevan, W.; Merer, A.; Westwood, N. The infrared spectrum of gaseous aminoborane, H2N=BH2: Location of the fundamentals and rotational structure in the 401 band (BN stretching vibration at 1337 cm−1). J. Mol. Spectrosc. 1985, 110, 153–163. [Google Scholar] [CrossRef]
- Kaldor, A.; Porter, R.F. Matrix isolation study of borazine and boroxine. Vibrational analysis. Inorg. Chem. 1971, 10, 775–785. [Google Scholar] [CrossRef]
- Heldebrant, D.J.; Karkamkar, A.; Hess, N.J.; Bowden, M.; Rassat, S.; Zheng, F.; Rappe, K.; Autrey, T. The Effects of Chemical Additives on the Induction Phase in Solid-State Thermal Decomposition of Ammonia Borane. Chem. Mater. 2008, 20, 5332–5336. [Google Scholar] [CrossRef]
- Mukherjee, M.; Bandyopadhyay, B.; Biswas, P.; Chakraborty, T. Amine inversion effects on the IR spectra of aniline in the gas phase and cold inert gas matrixes. Indian J. Phys. 2012, 86, 201–208. [Google Scholar] [CrossRef]
- Komm, R.; Geanangel, R.; Liepins, R. Synthesis and Studies of Poly (aminoborane),(H2NBH2) x. Inorg. Chem. 1983, 22, 1684–1686. [Google Scholar] [CrossRef]
- Parker, S.F. Complete assignment of the vibrational spectra of borazine: The inorganic benzene. RSC Adv. 2018, 8, 23875–23880. [Google Scholar] [CrossRef]
- Impens, N.E.; Vansant, E. Synthesis of a covalently bound polymeric borazine structure on a mesoporous silica gel. J. Chem. Soc. Faraday Trans. 1997, 93, 2309–2314. [Google Scholar] [CrossRef]
- Orlando, A.; Franceschini, F.; Muscas, C.; Pidkova, S.; Bartoli, M.; Rovere, M.; Tagliaferro, A. A comprehensive review on Raman spectroscopy applications. Chemosensors 2021, 9, 262. [Google Scholar] [CrossRef]
- Weismiller, M.; Wang, S.; Chowdhury, A.; Thynell, S.; Yetter, R. Confined rapid thermolysis studies of ammonia borane. Thermochim. Acta 2013, 551, 110–117. [Google Scholar] [CrossRef]
- Taylor, R.C. Vibrational Frequencies, Assignments, and Force Constants for Some Compounds Containing Boron-Nitrogen Dative Bonds; ACS Publications: Washington, DC, USA, 1964. [Google Scholar]
- Yaala, M.B.; Marot, L.; Steiner, R.; Moser, L.; De Temmerman, G.; Porosnicu, C.; Lungu, C.; Oberkofler, M.; Meyer, E. Quartz micro-balance and in situ XPS study of the adsorption and decomposition of ammonia on gold, tungsten, boron, beryllium and stainless steel surfaces. Nucl. Fusion 2018, 58, 106012. [Google Scholar] [CrossRef]
- Prakash, A.; Sundaram, K.B. Deposition and XPS studies of dual sputtered BCN thin films. Diam. Relat. Mater. 2016, 64, 80–88. [Google Scholar] [CrossRef]
- Chabanne, P.; Tighzert, L.; Pascault, J.-P. Monoepoxy polymerization initiated by BF3-amine complexes in bulk. II. Influence of water and by-products on polymer formation. J. Appl. Polym. Sci. 1994, 53, 769–785. [Google Scholar] [CrossRef]
- Lewars, E.G. Semiempirical calculations. In Computational Chemistry: Introduction to the Theory and Applications of Molecular and Quantum Mechanics; Springer: Berlin/Heidelberg, Germany, 2024; pp. 433–492. [Google Scholar]
- Hu, Q.; Zhang, Y.; Li, R.; Zhu, Z. A modified Polak-Ribière-Polyak type conjugate gradient method for vector optimization. Optim. Methods Softw. 2025, 40, 725–754. [Google Scholar] [CrossRef]
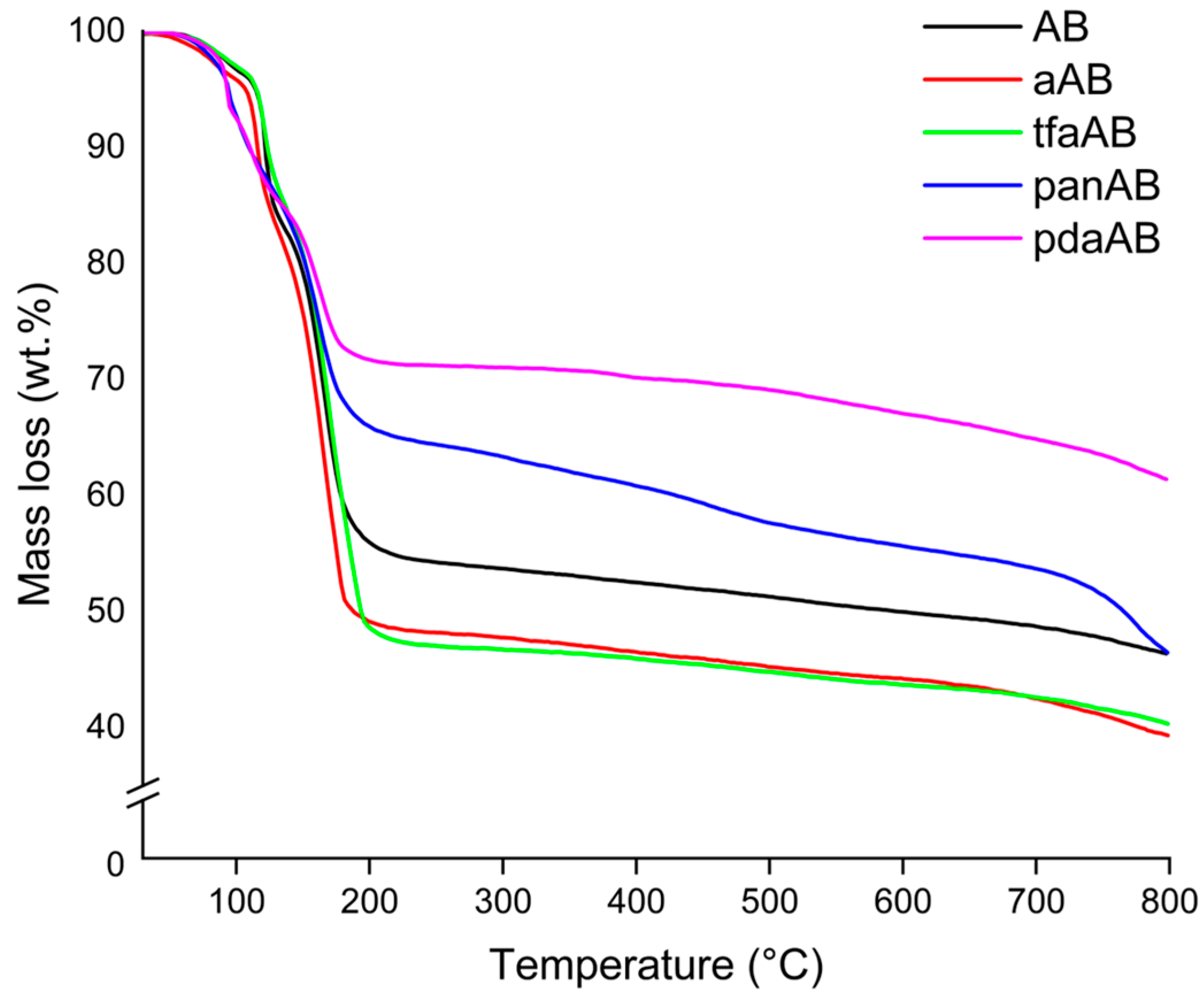



| Species | Tonset (°C) | Tmax1 (°C) | Residue @ Tmax1 (%) | Tmax2 (°C) | Residue @ Tmax2 (%) | Tmax3 (°C) | Residue @ Tmax3 (%) | Residue @ 800 °C (%) | H2 Solo Production (wt.%) a |
|---|---|---|---|---|---|---|---|---|---|
| AB | 105 | 106 | 93.4 | 162 | 75.1 | Not observed | Not observed | 43.1 | 0.2 |
| aAB | 112 | 119 | 79.1 | 167 | 71.7 | 172 | 58.3 | 40.2 | 2.4 |
| tfaAB | 115 | 120 | 84.7 | 168 | 74.1 | 180 | 62.7 | 36.5 | 1.5 |
| panAB | 92.1 | 104 | 86.7 | 162 | 79.5 | Not observed | Not observed | 43.8 | 1.0 |
| pdaAB | 92.0 | 111 | 87.9 | 162 | 73.1 | Not observed | Not observed | 58.2 | 0.9 |
| Amine | Melting Point (°C) | Boiling Point (°C) | pKa | Amine/AB Acronym |
|---|---|---|---|---|
| Aniline | −6 | 184 | 4.1 | aAB |
| triphenylamine | 127 | 347 | 3.04 | tfaAB |
| benzene-1,4-diamine | 113 | 267 | 4.5 | panAB |
| 4-methoxyaniline | 57 | 243 | 5.34 | pdaAB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartoli, M.; Ferraro, G.; Etzi, M.; Lettieri, S.; Pirri, C.F.; Bocchini, S. Tuning the Solid-State Hydrogen Release of Ammonia Borane by Entrapping the Intermediates: The Role of High-Boiling-Point Amines. Molecules 2025, 30, 4057. https://doi.org/10.3390/molecules30204057
Bartoli M, Ferraro G, Etzi M, Lettieri S, Pirri CF, Bocchini S. Tuning the Solid-State Hydrogen Release of Ammonia Borane by Entrapping the Intermediates: The Role of High-Boiling-Point Amines. Molecules. 2025; 30(20):4057. https://doi.org/10.3390/molecules30204057
Chicago/Turabian StyleBartoli, Mattia, Giuseppe Ferraro, Marco Etzi, Stefania Lettieri, Candido Fabrizio Pirri, and Sergio Bocchini. 2025. "Tuning the Solid-State Hydrogen Release of Ammonia Borane by Entrapping the Intermediates: The Role of High-Boiling-Point Amines" Molecules 30, no. 20: 4057. https://doi.org/10.3390/molecules30204057
APA StyleBartoli, M., Ferraro, G., Etzi, M., Lettieri, S., Pirri, C. F., & Bocchini, S. (2025). Tuning the Solid-State Hydrogen Release of Ammonia Borane by Entrapping the Intermediates: The Role of High-Boiling-Point Amines. Molecules, 30(20), 4057. https://doi.org/10.3390/molecules30204057