Comparative Studies on Leachability of Zinc and Iron from High-Energy Milled Waste of Scrap-Based EAF Steelmaking
Abstract
1. Introduction
2. Results
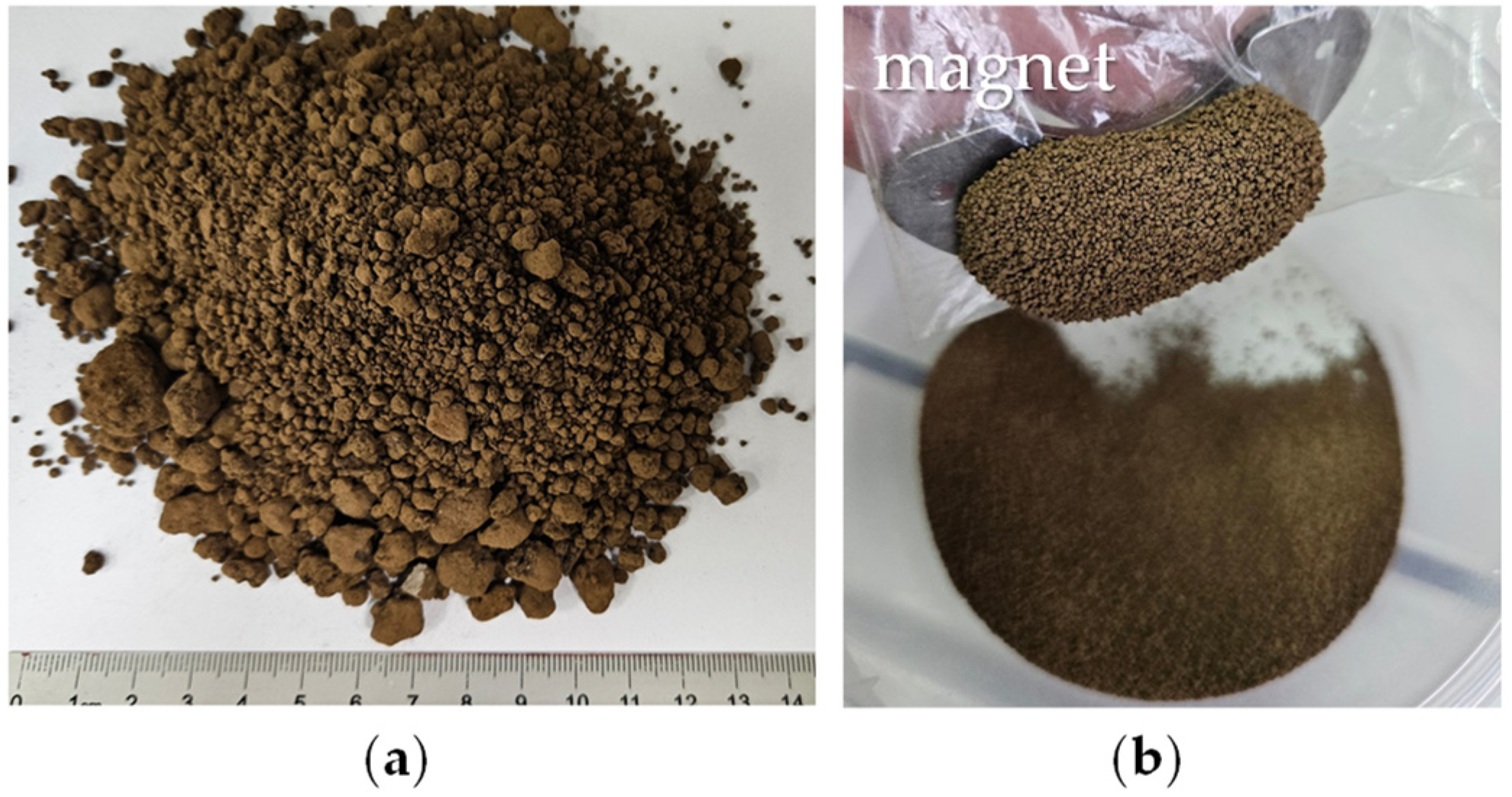
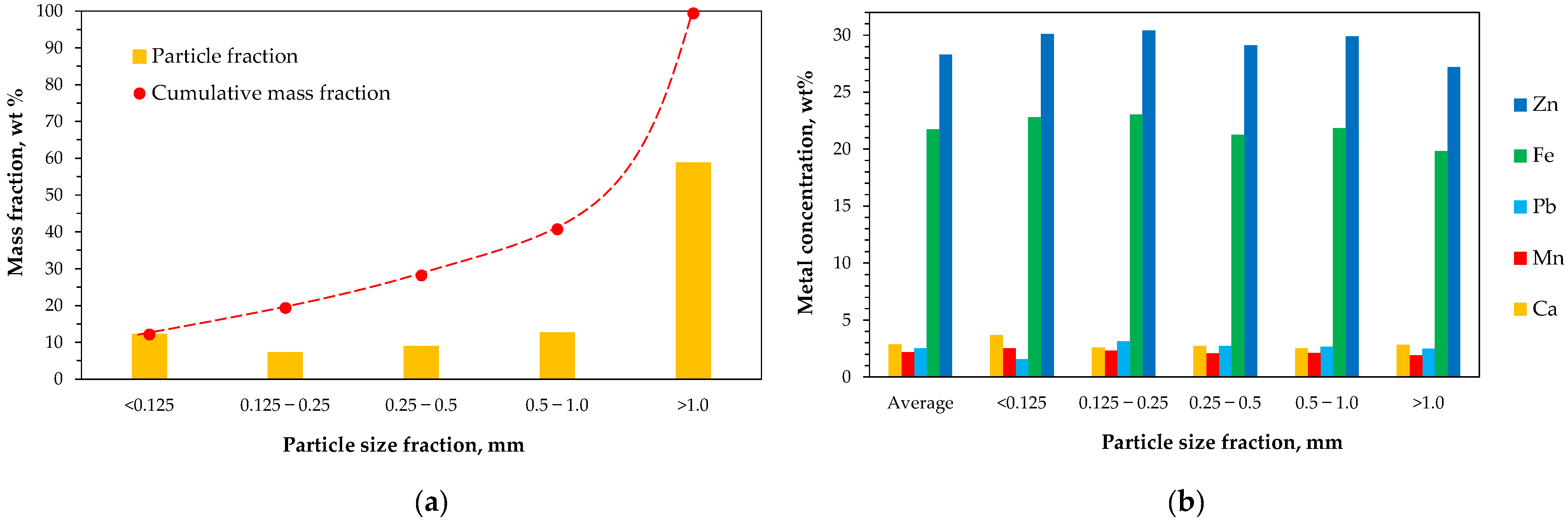
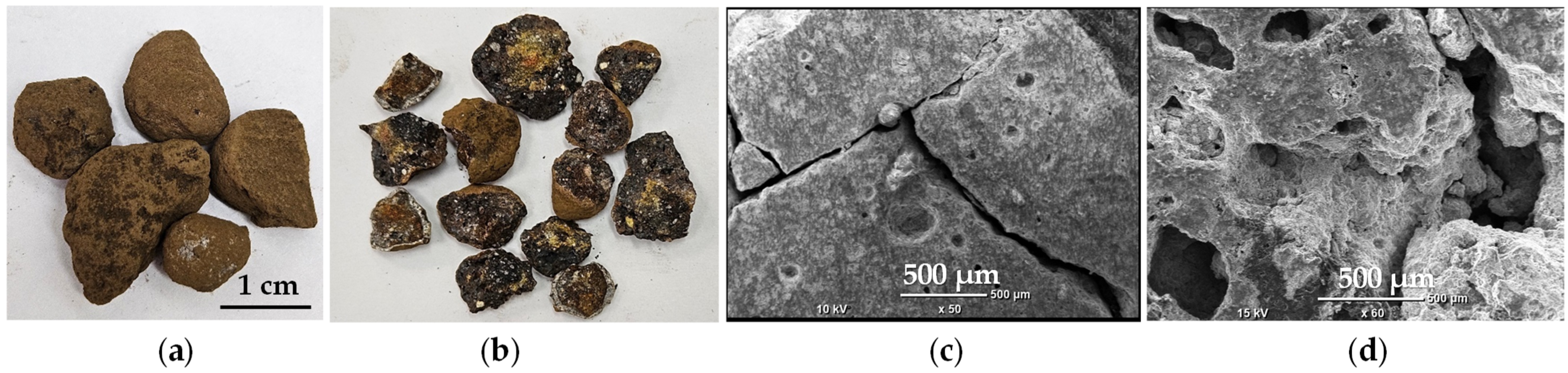
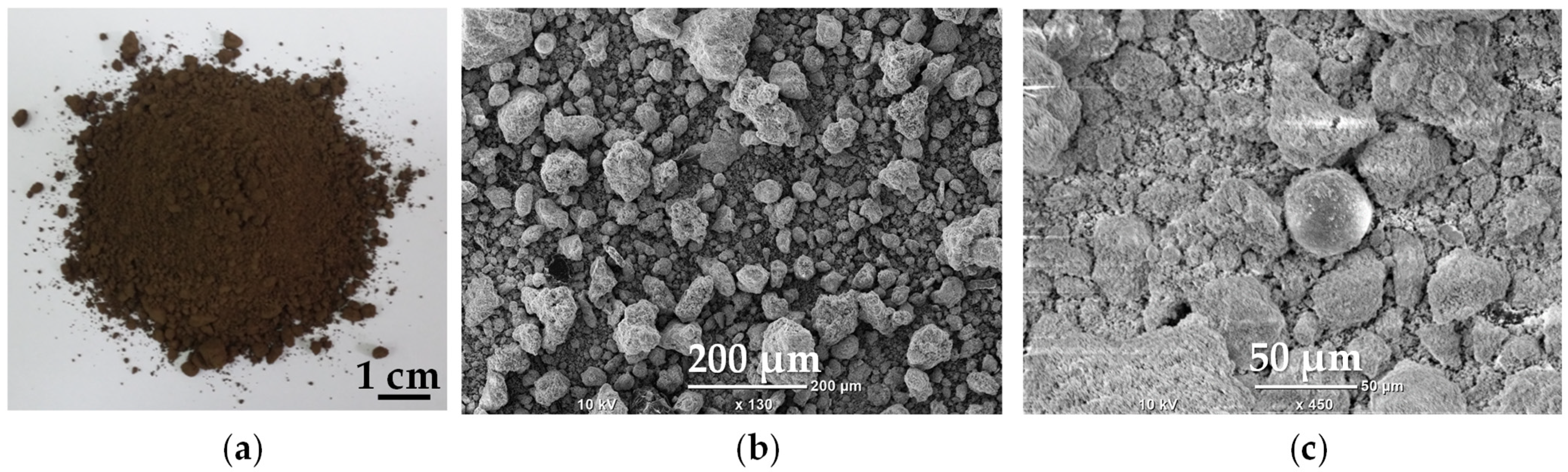
2.1. Raw Material
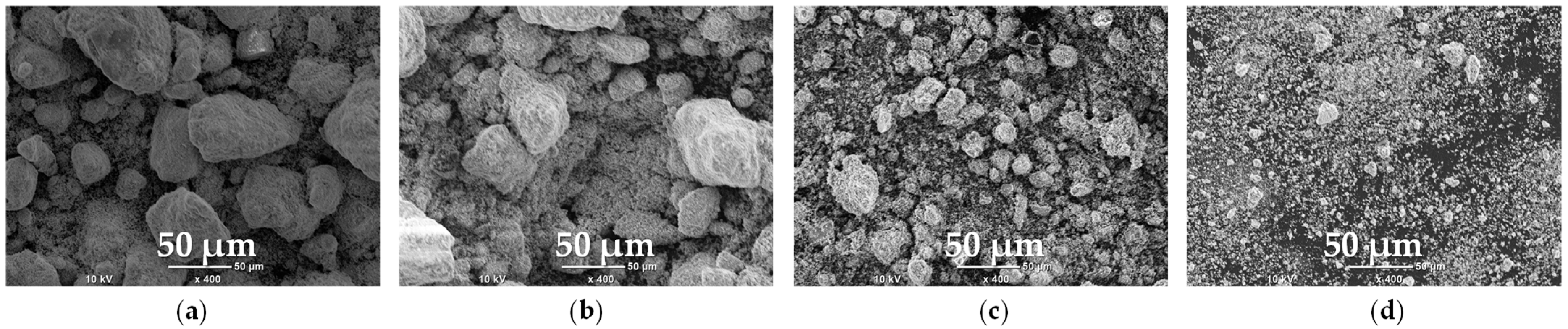
2.2. High-Energy Milled Materials
2.2.1. Morphology
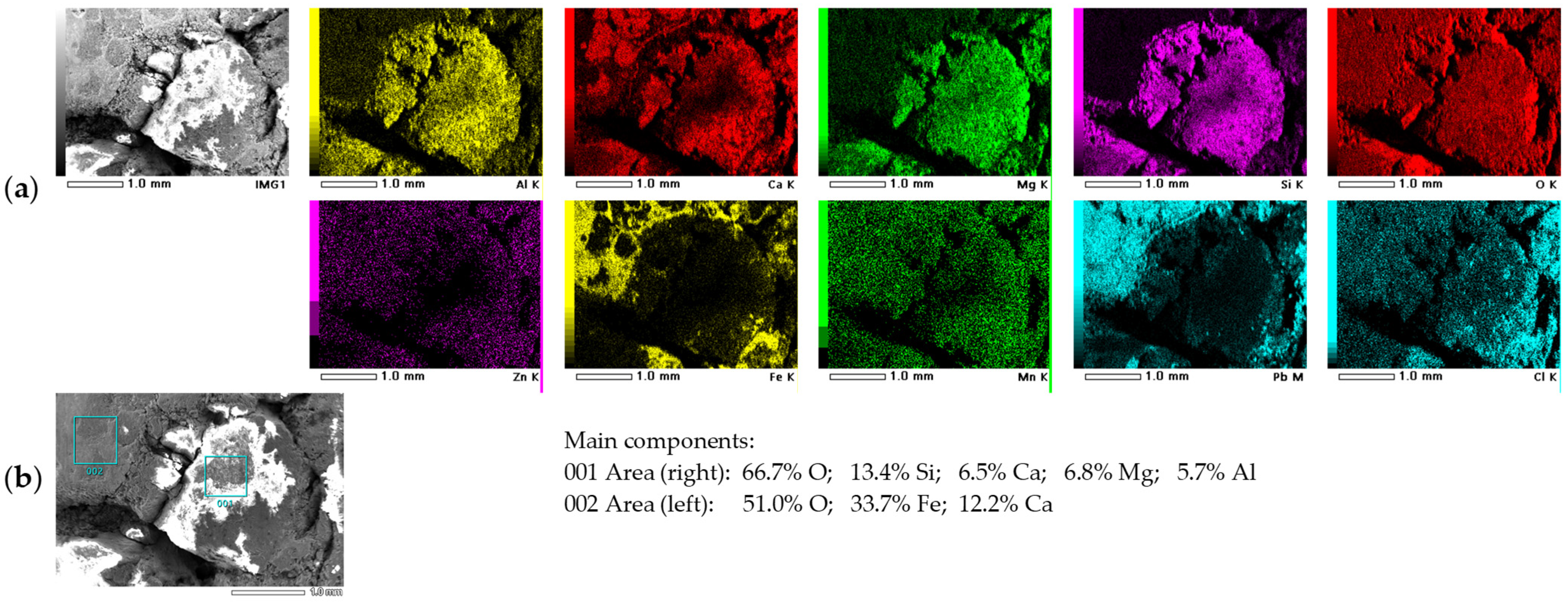
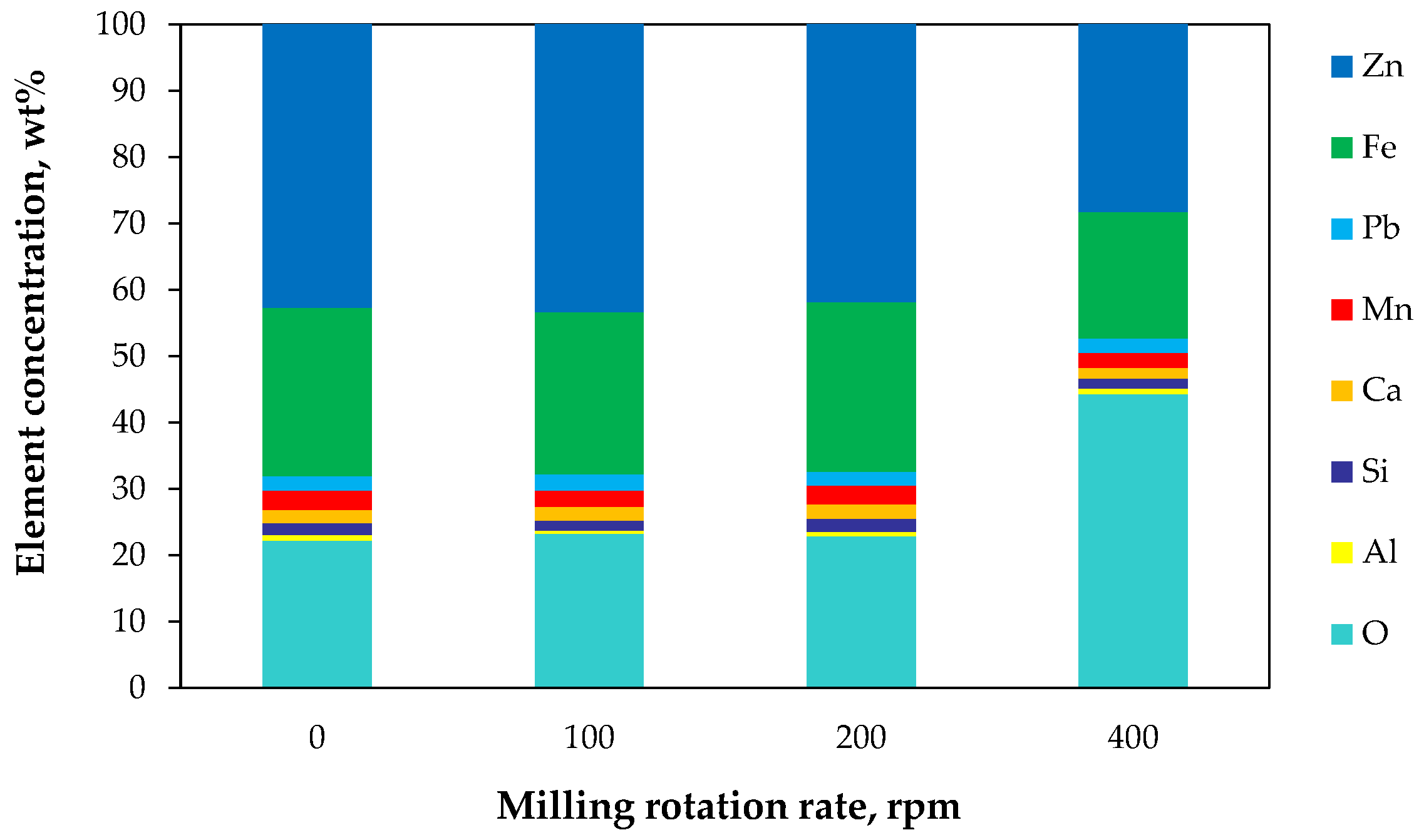
2.2.2. Elemental Composition
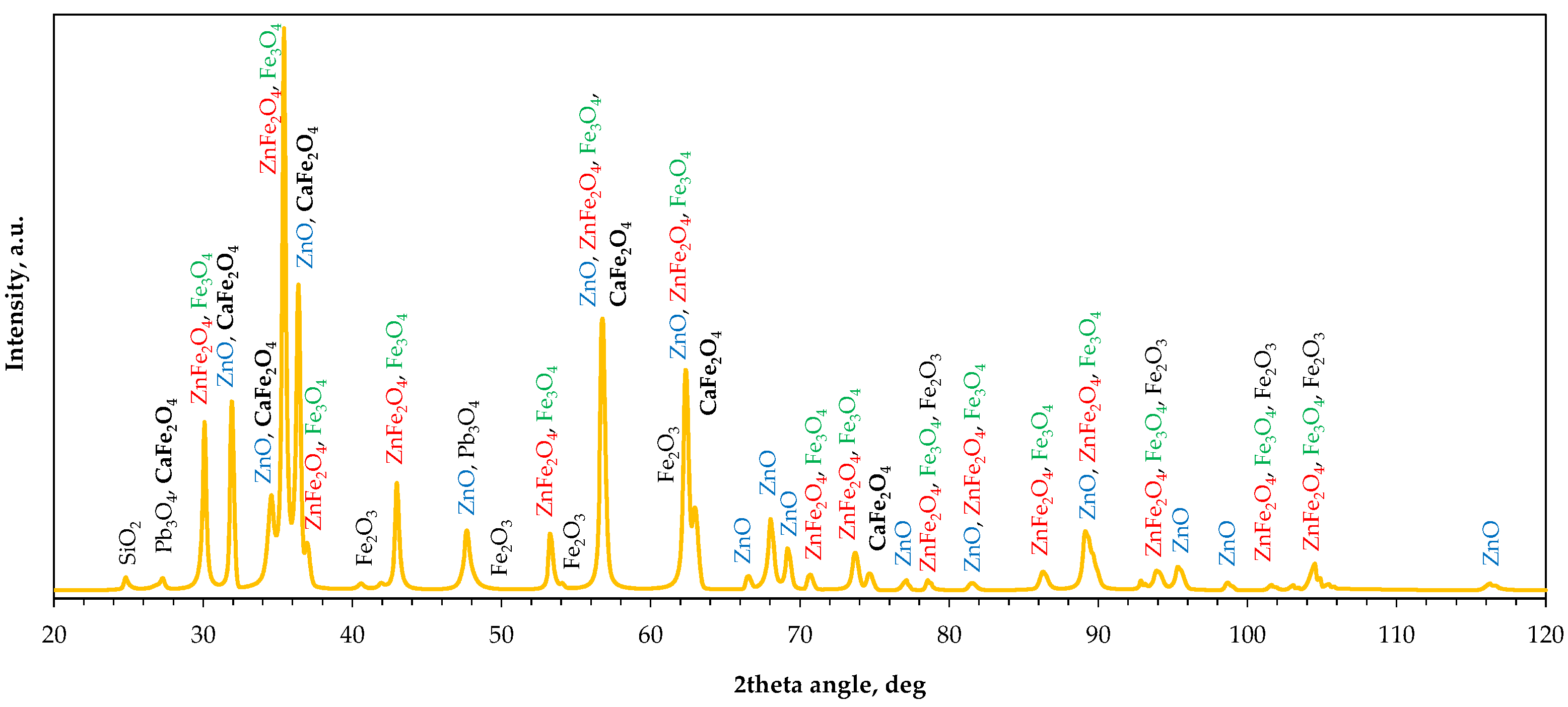
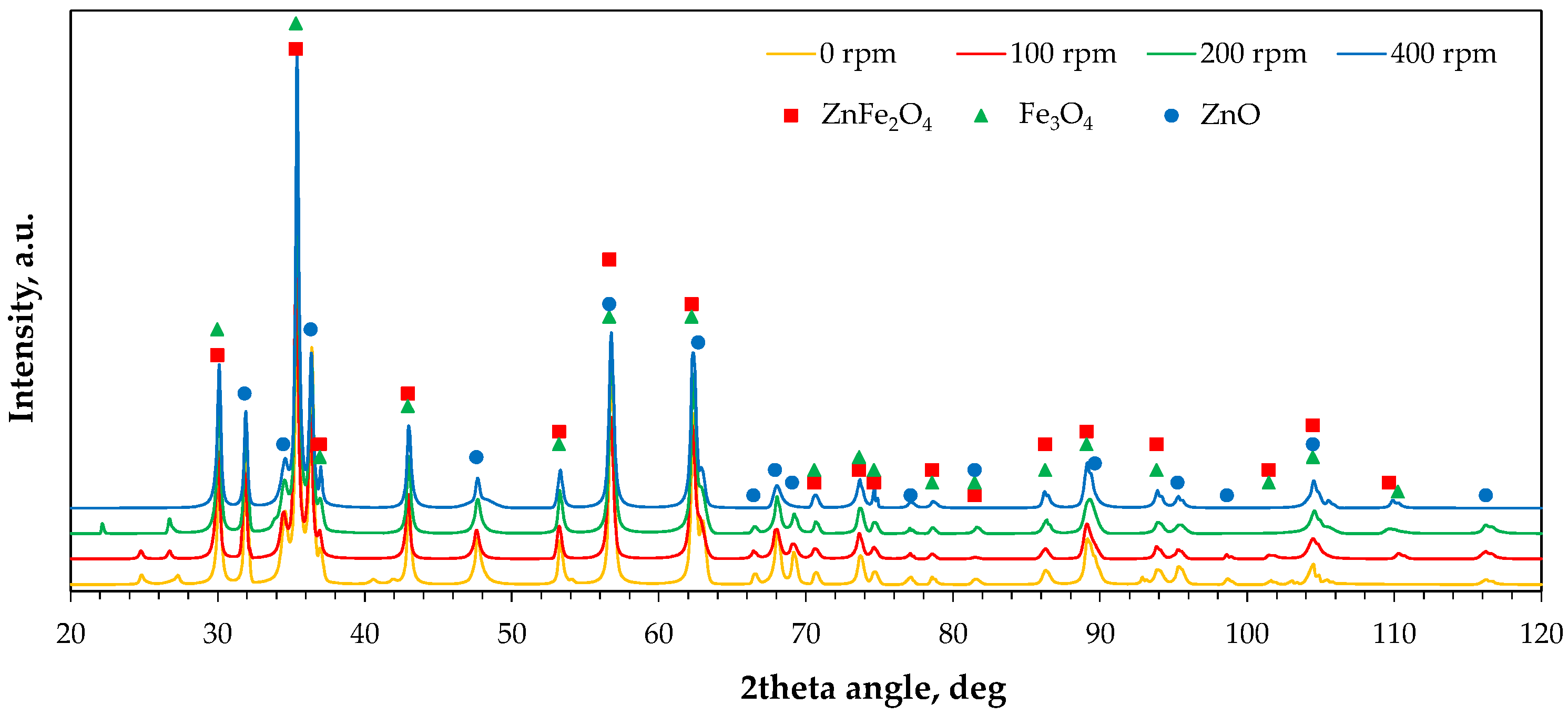
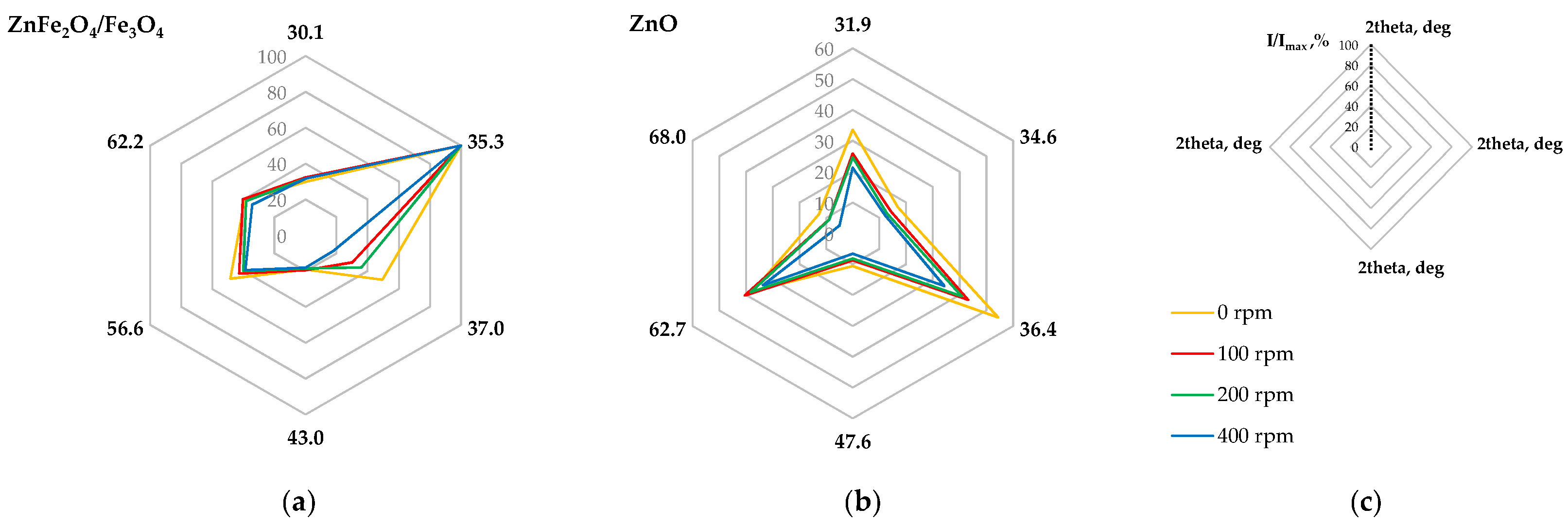
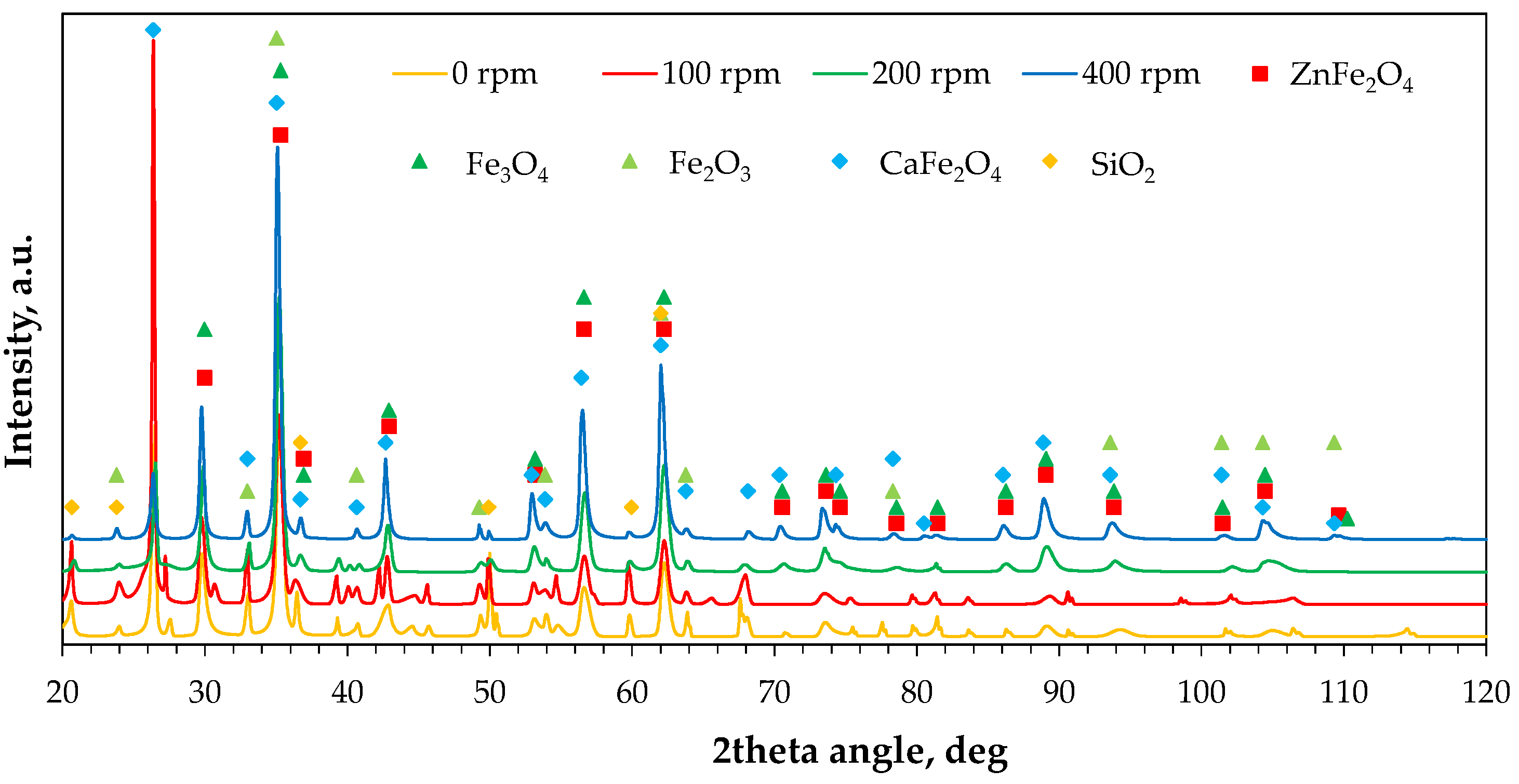
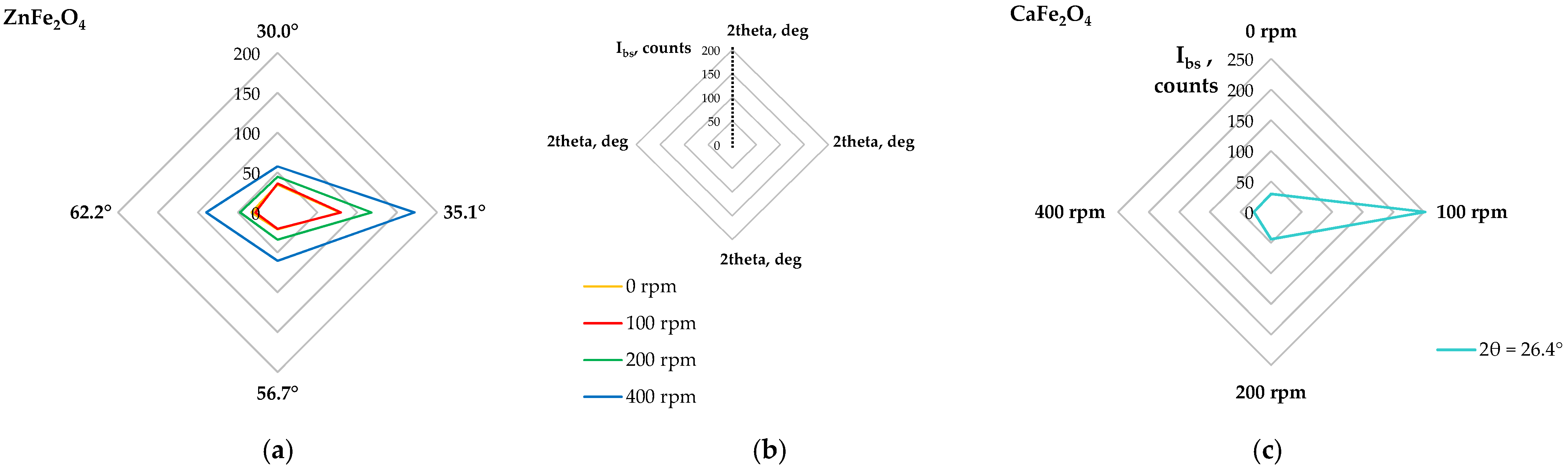
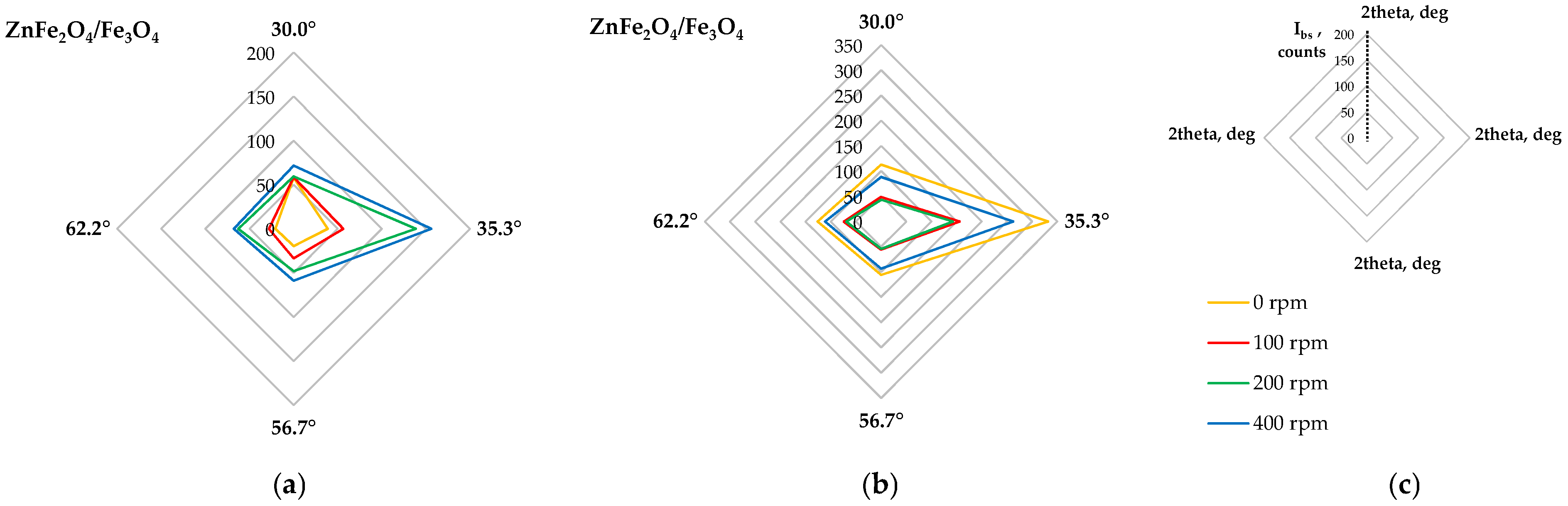
2.2.3. Phase Composition
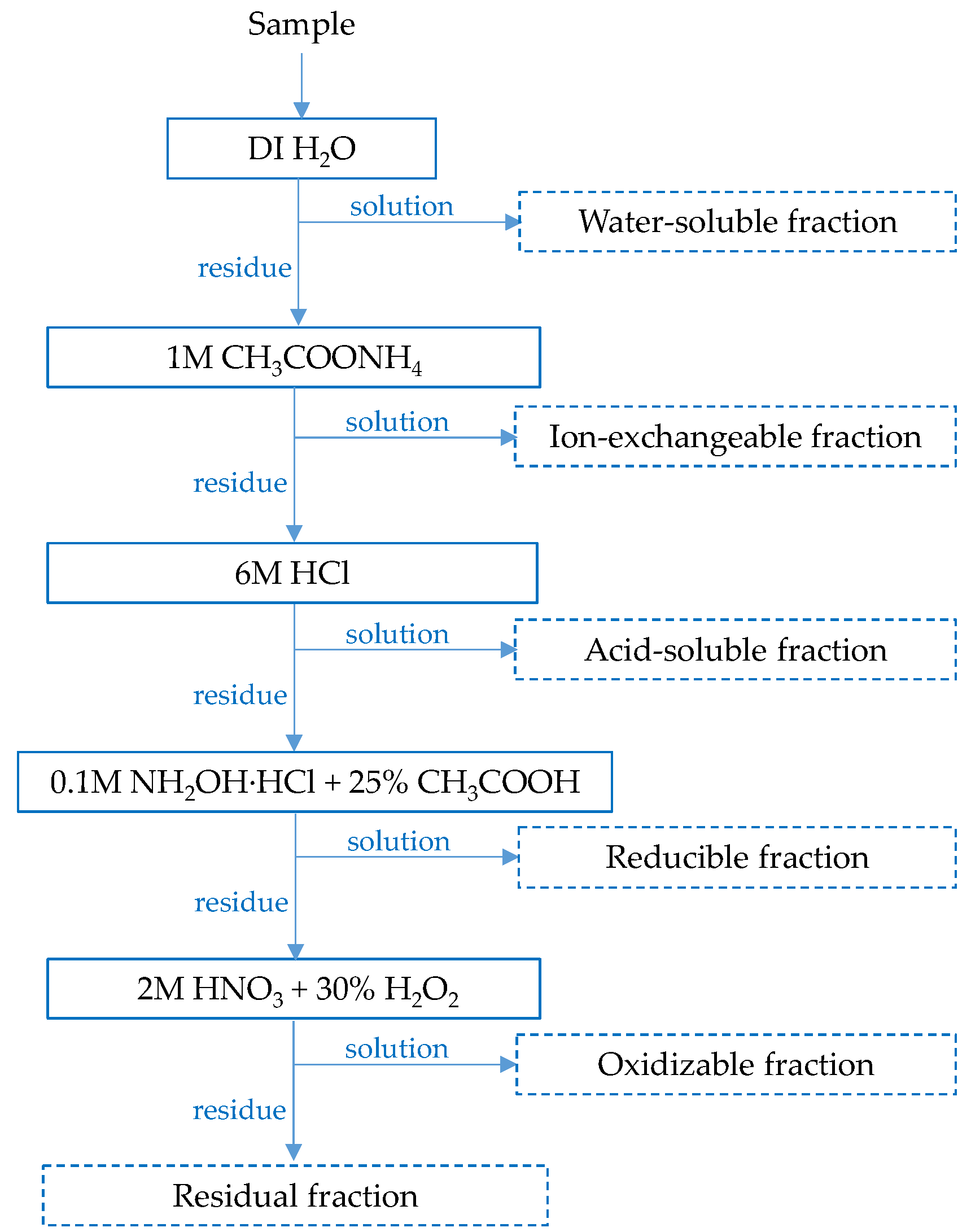
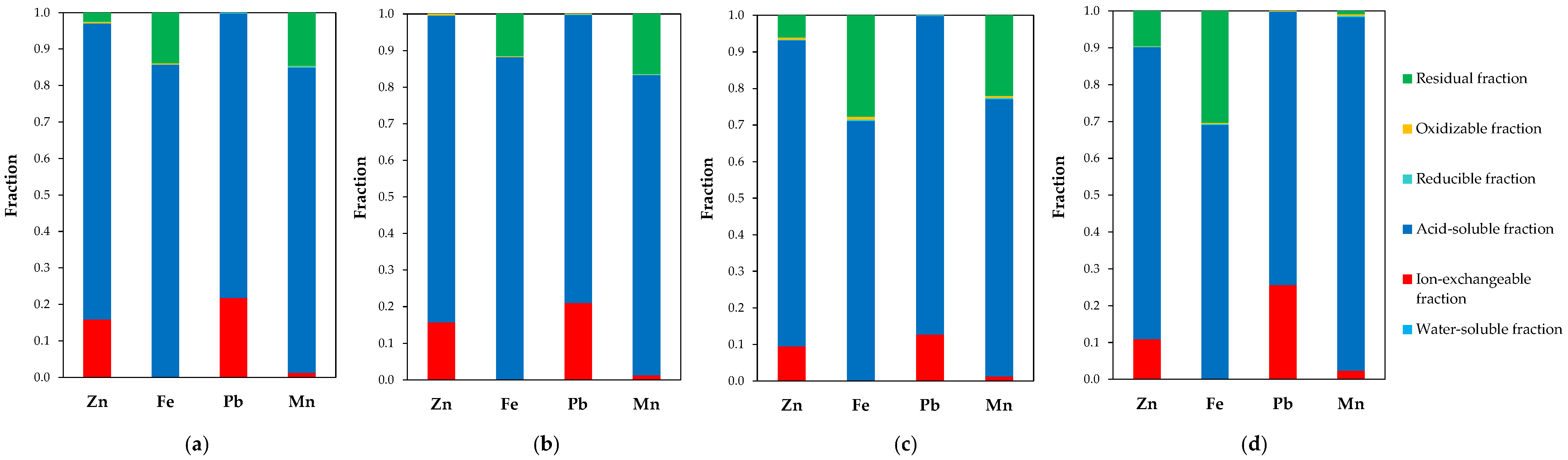
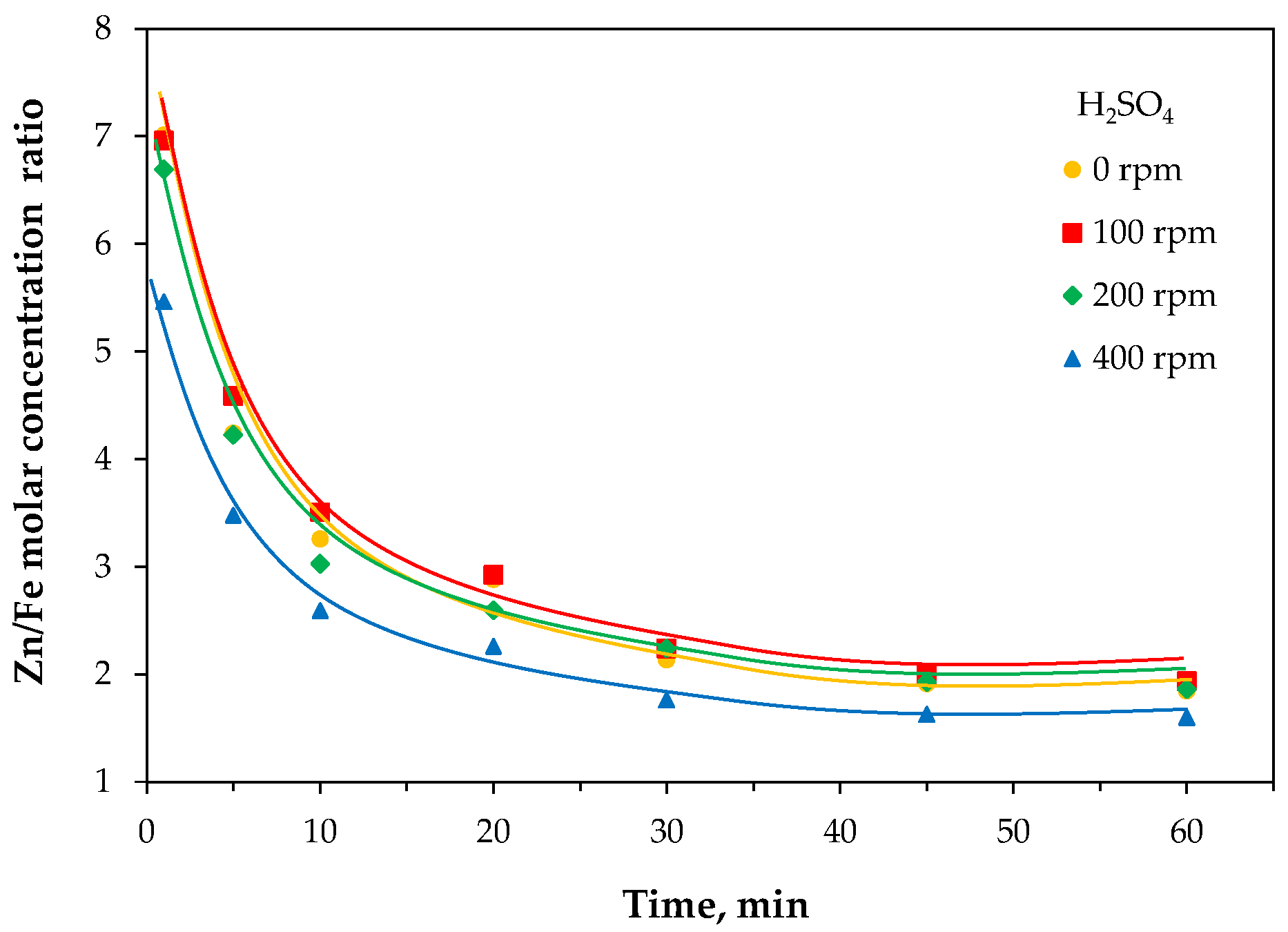
2.2.4. Sequential Leaching
2.3. Acid and Alkaline Leaching
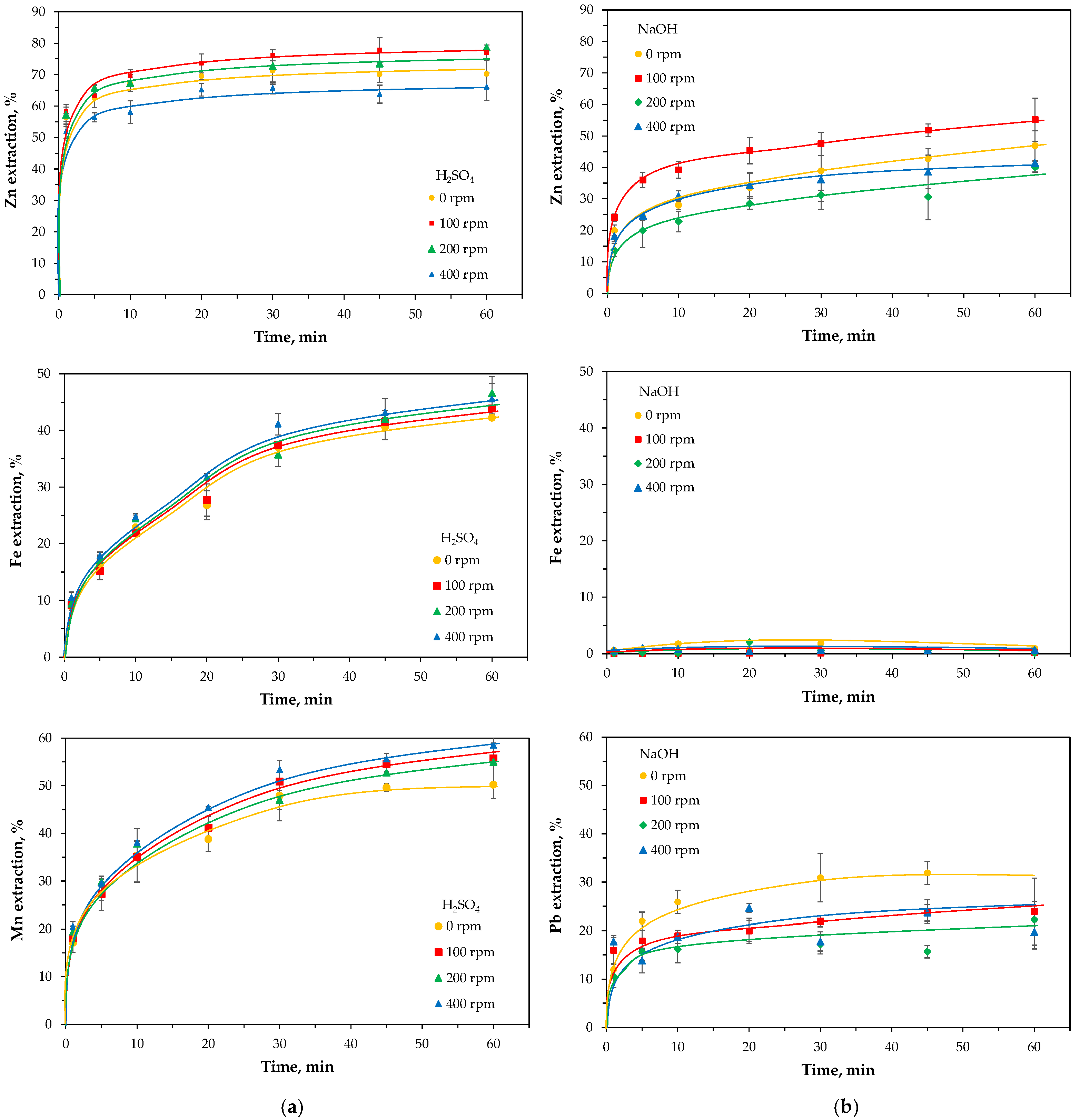
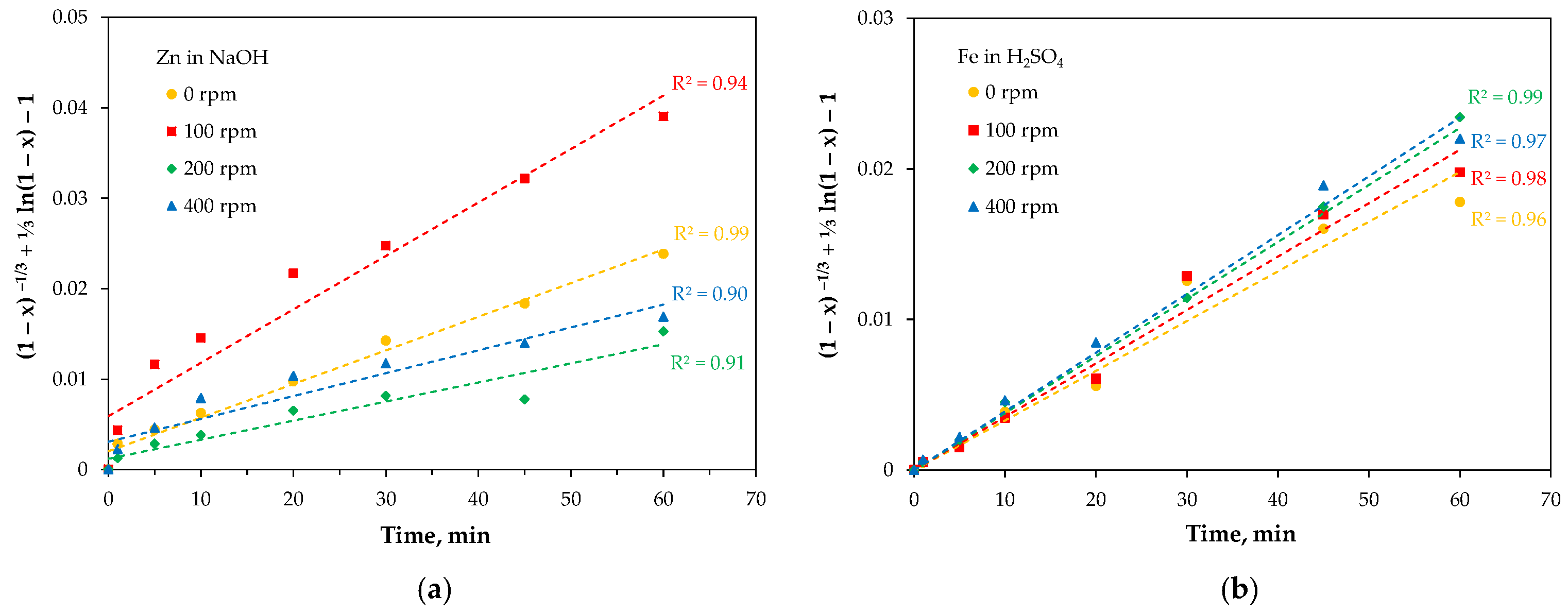
2.3.1. Leaching
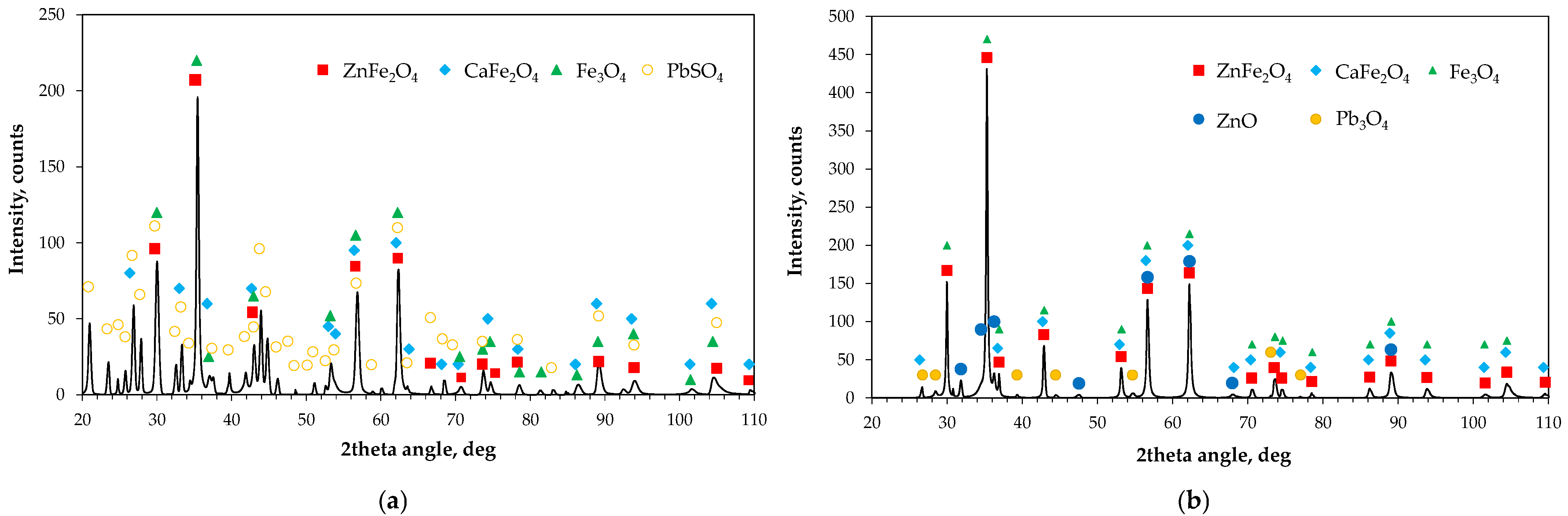
2.3.2. Solid Residues
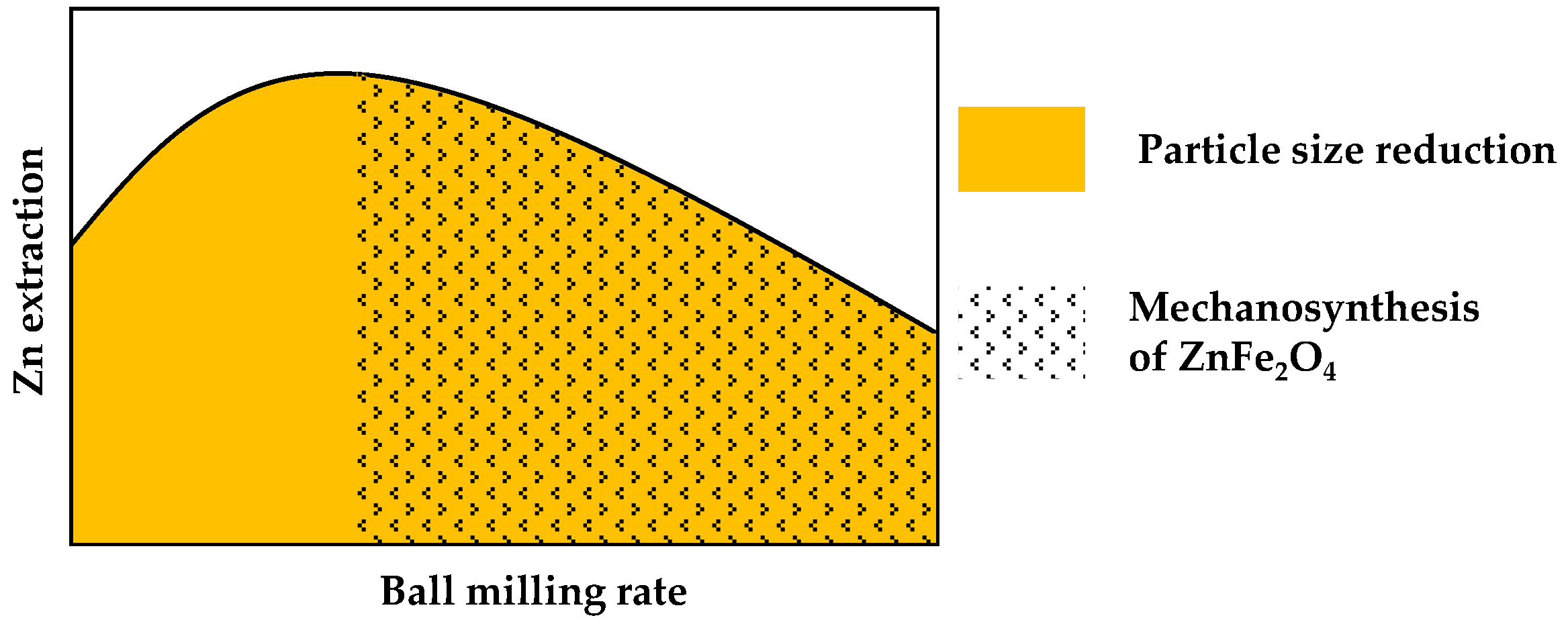
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EAF | Electric arc furnace |
| EAFD | Electric arc furnace dust |
| EDS | Energy dispersive X-ray spectroscopy |
References
- International Lead and Zinc Study Group. The World Zinc Factbook. International Lead and Zinc Study Group: Lisbon, Portugal, 2024; Available online: https://www.ilzsg.org/ (accessed on 1 August 2025).
- Watari, T.; Nansai, K.; Nakijama, K. Major metals demand, supply, and environmental impacts to 2100: A critical review. Res. Conserv. Recycl. 2021, 164, 105107. [Google Scholar] [CrossRef]
- The Canadian Critical Minerals Strategy. Available online: https://www.canada.ca/en/campaign/critical-minerals-in-canada/canadian-critical-minerals-strategy.html (accessed on 1 August 2025).
- U.S. Geological Survey Releases 2022 List of Critical Minerals. Available online: https://www.usgs.gov/news/national-news-release/us-geological-survey-releases-2022-list-critical-minerals (accessed on 1 August 2024).
- Updates to Australia’s Critical Mineral List. Available online: https://www.industry.gov.au/news/updates-australias-critical-minerals-list (accessed on 1 August 2025).
- Statista. Forecast Market Value of Selected Critical Materials Worldwide from 2027 to 2030. Available online: https://www.statista.com/statistics/1361230/forecast-global-market-value-of-critical-materials/ (accessed on 1 August 2025).
- DebRoy, T.; Elmer, J.W. Metals beyond tomorrow: Balancing supply, demand, sustainability, substitution, and innovations. Mater. Today 2024, 80, 737–757. [Google Scholar] [CrossRef]
- Kania, H.; Saternus, M. Evaluation and current state of primary and secondary zinc production—A review. Appl. Sci. 2023, 13, 2003. [Google Scholar] [CrossRef]
- Corneille, A.; Agarwal, A.; Seguineaud, C.; Ventricelli, V.; Conesa, R. Unlocking Potential in the Global Scrap Steel Market: Opportunities and Challenges; OECD Science, Technology and Industry Policy Papers, No. 170; OECD publishing: Paris, France, 2024. [Google Scholar] [CrossRef]
- Karbowniczek, M. Electric Arc Furnace Steelmaking; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- World Steel Recycling in Figures 2019–2023, 15th ed.; Bureau of International Recycling: Brussels, Belgium, 2024; Available online: https://www.bir.org/images/uploads/publications/Ferrous_report_2019-2023.pdf (accessed on 29 June 2025).
- Hasanbeigi, A. Steel Climate Impact—An International Benchmarking of Energy and CO2 Intensities; Global Efficiency Intelligence: St. Petersburg, FL, USA, 2022. [Google Scholar]
- Remus, R.; Aguado-Monsobet, M.A.; Roudier, S.; Sancho, L.D. Best Available Techniques (BAT) Reference Document for Iron and Steel Production. Industrial Emissions Directive 2010/75/EU; EC Joint Research Centre: Seville, Spain, 2013. [Google Scholar]
- Wang, J.; Zhang, Y.; Cui, K.; Fu, T.; Gao, J.; Hussain, S.; AlGarni, T.S. Pyrometallurgical recovery of zinc and valuable metals from electric arc furnace dust—A review. J. Clean. Prod. 2021, 298, 126788. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Fernandez, A.M.; Torres, V.M. Hydrometallurgical processes for the recovery of metals from steel industry by-products: A critical review. J. Sustain. Metall. 2020, 6, 505–540. [Google Scholar] [CrossRef]
- Rudnik, E. Hydrometallurgical treatment of EAF by-products for metal’s recovery: Opportunities and challenges. Metals 2025, 15, 914. [Google Scholar] [CrossRef]
- Gamutan, J.; Koide, S.; Sasaki, Y.; Nagasaka, T. Selective dissolution of leaching zinc from lime treated electric arc furnace dust by alkaline media. J. Environ. Chem. Eng. 2024, 12, 111789. [Google Scholar] [CrossRef]
- Chairaksa-Fujimoto, R.; Maruyama, K.; Miki, T.; Nagasaka, T. The selective alkaline leaching of zinc oxide from electric arc furnace dust pre-treated with calcium oxide. Hydrometallurgy 2016, 159, 120–125. [Google Scholar] [CrossRef]
- Miki, T.; Chairaksa-Fujimoto, R.; Maruyama, K.; Nagasaka, T. Hydrometallurgical extraction of zinc from CaO treated EAF dust in ammonium chloride solution. J. Hazard. Mater. 2016, 146, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Bayoumi, R.A.; Abdelmonem, N.M.; Soliman, M.A.; Ismail, I.M.; Refaat, A.A. Hybrid hydro-pyrometallurgical process for Zn recovery from electric arc furnace dust. J. Phys. Conf. Ser. 2022, 2305, 012009. [Google Scholar] [CrossRef]
- Wu, H.; Li, J.; Teng, W.; Chen, Y.; Liu, W.; Ren, S.; Yang, J.; Liu, Q. One-step extraction of zinc and separation of iron from hazardous electric arc furnace dust via sulphating roasting-water leaching. J. Environ. Chem. Eng. 2023, 11, 11155. [Google Scholar] [CrossRef]
- Ju, J.; Yang, W.; Long, T.; Deng, S.; Yang, C. High-efficiency separation and recovery of Zn and Fe from EAF dust via ammonium salt roasting-water leaching: Process optimization and mechanistic insights. Process Saf. Environ. Prot. 2025, 199, 107276. [Google Scholar] [CrossRef]
- Sajal, W.R.; Ahmad, S.; Gulshan, F. Optimisation of hybrid process to recover zinc from electric furnace dust using design of experiment approach. Can. Metall. Quart. 2025, 64, 664–679. [Google Scholar] [CrossRef]
- Youcai, Z.; Stanforth, R. Integrated hydrometallurgical process for production of zinc from electric furnace dust in alkaline medium. J. Hazard. Mater. 2000, 80, 223–240. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.; Altarawneh, S.; Al-Omari, M. Selective dissolution of zinc and lead from electric arc furnace dust via oxidative thermolysis with polyvinyl chloride and water-leaching process. Hydrometallurgy 2022, 212, 105898. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.; Kingman, S.; Hamilton, I. Microwave treatment of electric arc furnace dust with tetrabromobisphenol A: Dielectric characterization and pyrolysis-leaching. J. Anal. Appl. Pyrol. 2017, 128, 168–175. [Google Scholar] [CrossRef]
- Baláž, P. Mechanical activation on hydrometallurgy. Int. J. Miner. Process. 2003, 72, 341–354. [Google Scholar] [CrossRef]
- Ou, Z.; Li, J.; Wang, Z. Application of mechanochemistry to metal recovery from second-hand resources: A technical overview. Environ. Sci. Process. Impacts 2015, 17, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Zoz, H.; Kaupp, G.; Ren, H.; Goepel, K.; Naimi-Jamal, M.R. Recycling of EAF dust by semi-continuous high kinetic process. In Proceedings of the 2006 Powder Metallurgy World Congress and Exhibition, Busan, Republic of Korea, 24–28 September 2006. [Google Scholar]
- Zhang, C.; Wang, J.; Bai, J.; Zhao, Y. Recovering of zinc from solid waste bearing sphalerite or zinc ferrite by mechano-chemical extraction in alkaline solution. Proc. Environ. Sci. 2012, 16, 786–790. [Google Scholar] [CrossRef]
- Zhang, C.; Zhuang, L.; Wang, J.; Bai, J.; Yuan, W. Extraction of zinc from ferrites by alkaline leaching: Enhancing recovery by mechanochemical reduction with metallic iron. J. S. Afr. Inst. Min. Metall. 2016, 116, 1111–1114. [Google Scholar] [CrossRef]
- Yildirim, I.Z.; Prezzi, M. Chemical, mineralogical, and morphological properties of steel slag. Adv. Civ. Eng. 2011, 2011, 463638. [Google Scholar] [CrossRef]
- Machado, J.G.M.S.; Brehm, F.A.; Moraes, C.A.M.; dos Santos, C.A.; Vilela, A.C.F.; da Cunha, J.B.M. Chemical, physical, structural and morphological characterization of the electric arc furnace dust. J. Hazard. Mater. 2006, 136, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Simonyan, L.M.; Alpatova, A.A.; Demidova, N.V. The EAF dust chemical and phase composition research techniques. J. Mater. Res. Technol. 2019, 8, 1601–1607. [Google Scholar] [CrossRef]
- Roy, T.K. Assessing hardness and fracture toughness in sintered zinc oxide ceramics through indentation technique. Mater. Sci. Eng. A 2015, 640, 267–274. [Google Scholar] [CrossRef]
- Tran, D.H.; Pham, A.T.; Nam, N.H.; Thuy, K.X.; Truong, A.D.; Le, T.; Le, V.C.; Hong, N.T.M.; Hai, P.; Hop, D.T.B.; Dogruer, M. Effect of ZnFe2O4 addition on the phase stability, hardness and superconducting properties of Bi-2223 phase. Ceram. Inter. 2025, 51, 30974–30983. [Google Scholar] [CrossRef]
- Asada, M.; Omori, Y.; Sanbongi, K. Study on the properties of calcium ferrites in Fe-Ca-O system—Study on the properties of self-fluxing sinters. Trans. ISIJ 1968, 8, 245–250. [Google Scholar] [CrossRef]
- Suetens, T.; Guo, M.; Van Acker, K.; Blanpain, B. Formation of the ZnFe2O4 phase in an electric arc furnace off-gas treatment system. J. Hazard. Mater. 2015, 287, 180–187. [Google Scholar] [CrossRef]
- Lumongsod, M.N.; Gamutan, J.; Hara, K.; Sasaki, Y.; Nagasaka, T. Enhanced leaching of zinc ferrite by the formation of a solid solution with magnetite in hydrochloric acid solution. Metall. Mater. Trans. B 2023, 54B, 2291–2301. [Google Scholar] [CrossRef]
- Tkáčová, K.; Šepelák, V.; Števulová, N.; Boldyrev, V.V. Structure-reactivity study of mechanically activated zinc ferrite. J. Solid State Chem. 1996, 123, 100–108. [Google Scholar] [CrossRef]
- Harris, V.G.; Šepelák, V. Mechanochemically processed zinc ferrite nanoparticles: Evolution of structure and impact of induced cation inversion. J. Magn. Magn. Mater. 2018, 465, 603–610. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Davidson, C.M.; Thomas, R.P.; McVey, S.E.; Perala, R.; Littlejohn, D.; Ure, A.M. Evaluation of a sequential extraction procedure for the speciation of heavy metals in sediments. Anal. Chim. Acta. 1994, 291, 277–286. [Google Scholar] [CrossRef]
- Sammut, M.L.; Rose, J.; Masion, A.; Fiani, E.; Depoux, M.; Ziebel, A.; Hazeman, J.L.; Proux, O.; Borschneck, D.; Noack, Y. Determination of zinc speciation in basic oxygen furnace flying dust by chemical extractions and X-ray spectroscopy. Chemosphere 2008, 70, 1945–1951. [Google Scholar] [CrossRef]
- Lanzerstorfer, C.; Preitschopf, W. Steelmaking dust: Speciation of zinc by sequential leaching. Inż Min. 2020, 1, 79–82. [Google Scholar] [CrossRef]
- Kang, C.; Yang, S.; Qiao, J.; Zhao, Y.; Dong, S.; Wang, Y.; Duan, C.; Liu, J. Extraction of valuable critical metals from coal gangue by roasting activation–sulfuric acid leaching. Int. J. Coal Prep. Util. 2024, 44, 1810–1827. [Google Scholar] [CrossRef]
- Núñez, C.; Viñals, J. Kinetics of leaching of zinc ferrite in aqueous hydrochloric acid solutions. Metall. Trans. B 1984, 15B, 221–228. [Google Scholar] [CrossRef]
- Druska, P.; Steinike, U.; Šepelák, V. Surface structure of mechanically activated and mechanosynthesized zinc ferrite. J. Solid State Chem. 1999, 146, 13–21. [Google Scholar] [CrossRef]
- Zoraga, M.; Ilhan, S.; Kalpakli, A.O. Leaching kinetics of electric arc furnace dust in nitric acid solutions. Int. J. Chem. Kinet. 2020, 52, 933–942. [Google Scholar] [CrossRef]
- Bingöl, D.; Canbazoğlu, M.; Aydoğan, S. Dissolution kinetics of malachite in ammonia/ammonium carbonate leaching. Hydrometallurgy 2005, 76, 55–62. [Google Scholar] [CrossRef]
- Kukurugya, F.; Vindt, T.; Havlik, T. Behavior of zinc, iron and calcium from electric arc furnace (EAF) dust in hydrometallurgical processing in sulfuric acid solutions: Thermodynamic and kinetic aspects. Hydrometallurgy 2015, 154, 30–32. [Google Scholar] [CrossRef]
- Jarupisitthorn, C.; Pimtong, T.; Lothongkum, G. Investigation kinetics of zinc leaching from electric arc furnace dust by sodium hydroxide. Mater. Chem. Phys. 2002, 77, 531–535. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rudnik, E.; Stępień, M.; Palimąka, P. Comparative Studies on Leachability of Zinc and Iron from High-Energy Milled Waste of Scrap-Based EAF Steelmaking. Molecules 2025, 30, 4055. https://doi.org/10.3390/molecules30204055
Rudnik E, Stępień M, Palimąka P. Comparative Studies on Leachability of Zinc and Iron from High-Energy Milled Waste of Scrap-Based EAF Steelmaking. Molecules. 2025; 30(20):4055. https://doi.org/10.3390/molecules30204055
Chicago/Turabian StyleRudnik, Ewa, Michał Stępień, and Piotr Palimąka. 2025. "Comparative Studies on Leachability of Zinc and Iron from High-Energy Milled Waste of Scrap-Based EAF Steelmaking" Molecules 30, no. 20: 4055. https://doi.org/10.3390/molecules30204055
APA StyleRudnik, E., Stępień, M., & Palimąka, P. (2025). Comparative Studies on Leachability of Zinc and Iron from High-Energy Milled Waste of Scrap-Based EAF Steelmaking. Molecules, 30(20), 4055. https://doi.org/10.3390/molecules30204055







