4.1. Chemistry
4.1.1. General Methods
All reactions involving air- or moisture-sensitive compounds were performed with atmospheric N
2. Solvents and reagents were obtained from commercial suppliers and were used without further purification. Compound
22 [
6] was synthesized and identified according to and by comparison with literature. (
S)-(−)-2-(aminomethyl)-1-ethylpyrrolidine, bromoalkyl-phtalimides (
40 and
41), and aryl piperazines (
42–
50) were purchased.
Microwave-assisted reactions were performed with Microwave Initiator Eight 2.5 (Biotage®, Uppsala, Sweden) in the standard configuration as delivered, including proprietary software. All experiments were carried out in sealed microwave process vials under normal absorption. At the end of the reaction, the vial was cooled to 25 °C by air jet cooling before opening. Reaction temperatures were monitored by an IR sensor on the outside wall of the reaction.
Flash column chromatography purification (FC) was performed automatically on Flash-master (Biotage®, Uppsala, Sweden) with pre-packed Biotage® SNAP silica gel cartridges or manually on silica gel (Kieselgel 60, 0.040−0.063 mm, Merck®, Darmstadt, Germany). Thin-layer chromatography (TLC) was performed with Polygram SIL N-HR/HV254 pre-coated plastic sheets (0.2 mm) on aluminum sheets (Kieselgel 60 F254, Merck®, Darmstadt, Germany). Melting points were obtained on a Köfler melting point apparatus and are uncorrected.
IR spectra were recorded as KBr pellets with a Jasco
® (Cremella, Italy) FT/IR 460 plus spectrophotometer after 64 scans at 4 cm
−1 resolution and are expressed in
ν (cm
−1). NMR experiments were recorded at room temperature, and all spectra were recorded on a Varian
® (Irvine, CA, USA) XL-200 NMR spectrometer (200 MHz for
1H and 50 MHz for
13C). Spectra were acquired in deuterated chloroform (chloroform-d) as a solvent. Chemical shifts (δ) for
1H-NMR spectra are reported in parts per million (ppm) using the residual non-deuterated solvent resonance as the internal standard (chloroform-d: 7.26 ppm,
1H, and 77.16 ppm,
13C). Data are reported as follows: chemical shift (sorted in descending order), multiplicity (s for singlet, br s for broad singlet, d for doublet, t for triplet, dd for double doublet, q for quadruplet, qu for quintuplet, and m for multiplet), and integration and coupling constants
(J) in Hertz (Hz) (
Figures S3–S22). Spectroscopic data are in accordance with the structures. LC/MS analyses were run on an Agilent
® (Santa Clara, CA, USA) 1100 LC/MSD system consisting of a single quadrupole detector (SQD) mass spectrometer (MS) equipped with an electrospray ionization (ESI) interface and a photodiode array (PDA) detector, range 120−550 nm. ESI in positive mode was applied. Mobile phases: (A) MeOH in H
2O (8:2) (
Figures S23–S42). Analyses were performed with a flow rate of 0.9 mL/min and a temperature of 350 °C. The purity of all final compounds was determined by elemental analysis on a PerkinElmer
® (Waltham, MA, USA) 240B analyzer (C, H, and N). All the final compounds were found to be >95% when analyzed.
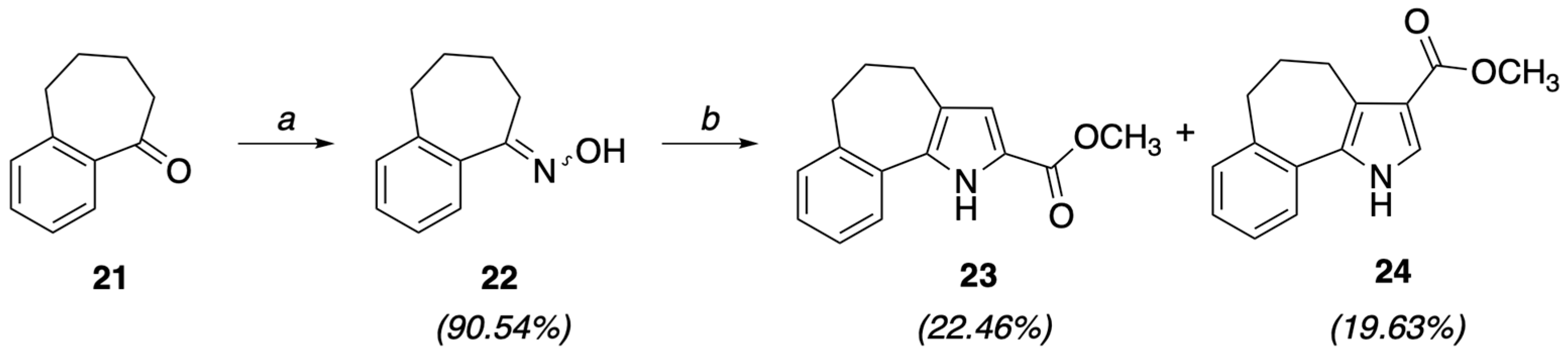
4.1.2. Synthesis of Methyl-5,6-dihydro-4H-benzo[6,7]cyclohepta[1,2-b]pyrrole-2-carboxylate (23) and Methyl-5,6-dihydro-4H-benzo[6,7]cyclohepta[1,2-b]pyrrole-3-carboxylate (24)
To a solution of oxime 22 (10.43 mmol) and DABCO (1.04 mmol, 0.1 eq) in toluene (18 mL), methyl propiolate (10.43 mmol, 1 eq) was dropwise added at −5 °C. The solution was brought back to room temperature, then underwent a double microwave irradiation (normal abs) cycle: the first at 80 °C for 10 min, followed by the second at 170 °C for another 10 min. After solvent evaporation by rotavapor, the crude product was purified by FC (petroleum ether/EtOAc 9/1) to give ester 23 with a retention factor (Rf) of 0.237 as a yellow solid (0.56 g, 22.46%), mp = 126–128 °C, 1H NMR (CDCl3) ppm: 2.03–2.08 (m, 2H), 2.78–2.83 (m, 4H), 3.86 (s, 3H), 6.80 (d, 1H, J = 2.8 Hz), 7.18–7.19 (m, 2H), 7.22–7.28 (m, 2H), 7.46 (d, 1H, J = 7 Hz), 9.11 (br s, 1H, NH exch. with D2O); Anal. calcd for C15H15NO2: C, 74.67; H, 6.27; N, 5.81. Found: C, 74.89; H, 6.29; N, 5.83; and 24 with a lower Rf (0.106) as a yellow solid (0.494 g, 19.63%), mp = 166–168 °C, 1H NMR (200 MHz, CDCl3) ppm: 2.02–2.04 (m, 2H), 2.79–2.82 (m, 2H), 3.14 (t, 2H, J = 6.8 Hz), 3.81 (s, 3H), 7.13–7.18 (m, 2H), 7.20–7.25 (m, 1H), 7.34 (d, 1H, J = 7.6 Hz), 7.50 (d, 1H, J = 3.2 Hz), 8.59 (br s, 1H, NH exch. with D2O). Anal. calcd for C15H15NO2: C, 74.67; H, 6.27; N, 5.81. Found: C, 74.93; H, 6.29; N, 5.83.
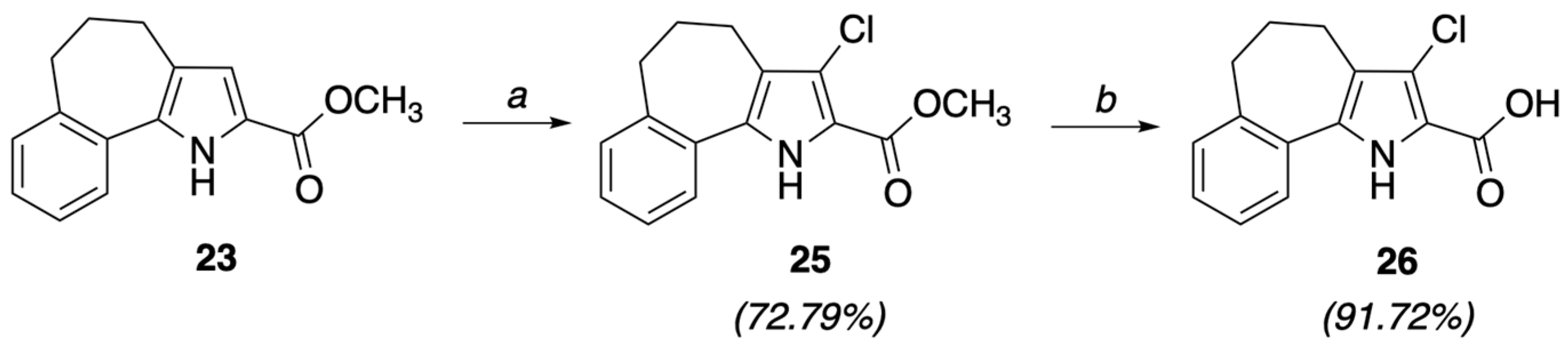
4.1.3. Methyl 3-chloro-5,6-dihydro-4H-benzo[6,7]cyclohepta[b]pyrrole-2-carboxylate (25)
A solution of 23 (1.76 mmol) and N-chlorosuccinimide (1.94 mmol, 1.1 eq) in chloroform (8 mL) was refluxed for 3 h, then allowed to cool to room temperature and washed with saturated aqueous NaHCO3. The organic phase was dried (Na2SO4) and concentrated to yield a crude product, which trituration with ether gave 25 as a white solid (0.353 g, 72.79%), mp = 160–162 °C, 1H NMR (CDCl3) ppm: 1.98–2.12 (m, 2H), 2.70–2.90 (m, 4H), 3.92 (s, 3H), 7.18–7.50 (m, 4H), 9.01 (br s, 1H, NH exch. with D2O). Anal. calcd for C15H14ClNO2: C, 65.34; H, 5.12; Cl, 12.86; N, 5.08. Found: C, 65.50; H, 5.13; Cl, 12.89; N, 5.09.
4.1.4. 3-Chloro-5,6-dihydro-4H-benzo[6,7]cyclohepta[b]pyrrole-2-carboxylic acid (26)
A mixture of ester 25 (1.09 mmol) in hydro-alcoholic solution (H2O/EtOH 1/2, 10.5 mL) of 2.5 M NaOH was refluxed for 12 h, then poured onto ice. The solution was acidified with concd. HCl and the solid precipitate were filtered, taken up in 5% aqueous NaHCO3 and filtered off. Concentrated HCl was dropwise added to the filtrate to give a white solid, which was filtered and air-dried to give acid 26 as a white solid (0.260 g, 91.72%), mp = 171–173 °C, 1H NMR (CDCl3) ppm: 2.28–2.98 (m, 2H), 2.60–2.90 (m, 4H), 3.89 (br s, 1H, OH exch. with D2O), 7.12–7.60 (m, 4H), 9.16 (br s, 1H, NH exch. with D2O). Anal. calcd for C14H12ClNO2: C, 64.24; H, 4.62; Cl, 13.55; N, 5.35. Found: C, 64.37; H, 4.63; Cl, 13.58; N, 5.36.
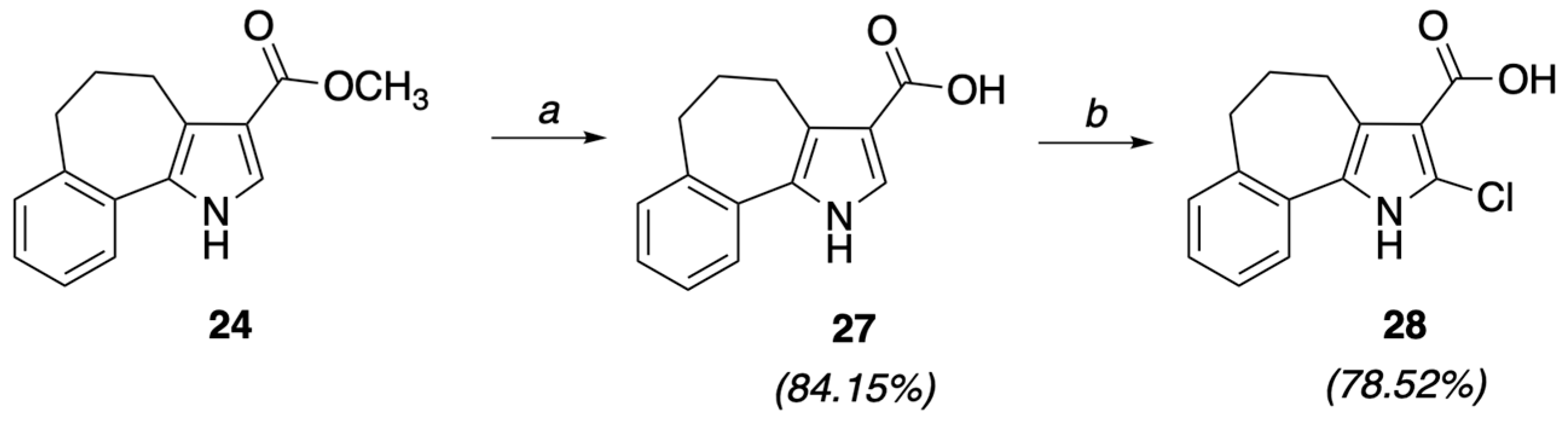
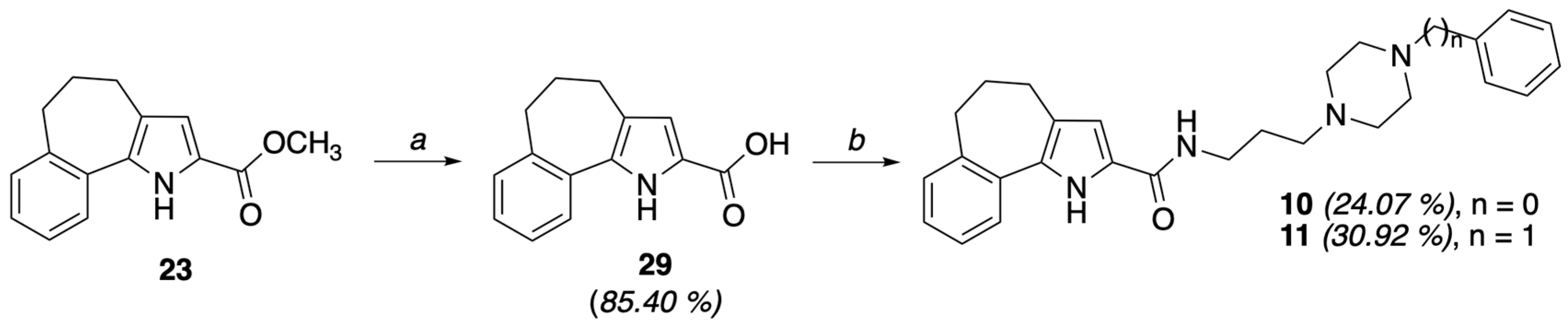
4.1.5. General Procedure I: Synthesis of 5,6-dihydro-4H-benzo[6,7]cyclohepta[b]pyrrole-3-carboxylic acid (27) and 5,6-dihydro-4H-benzo[6,7]cyclohepta[b]pyrrole-2-carboxylic acid (29)
To a solution of the appropriate ester 23 or 24 (0.83 mmol) in CH3OH (10 mL), an aqueous 20% solution of NaOH (10 mL) was added, and the mixture was refluxed for 9 h. The resulting solution was poured onto ice and concentrated HCl was added. The resulting precipitate was filtered and air-dried to give the corresponding acid. Acid 27 (from 24) as a beige solid (0.159 g, 84.15%), mp = 218–220 °C, 1H NMR (CDCl3) ppm: 1.58 (br. s, 1H, OH exch. with D2O), 1.99–2.10 (m, 2H), 2.80 (t, 2H, J = 5.0 Hz), 3.11 (t, 2H, J = 7.2 Hz), 7.16–7.20 (m, 2H), 7.33–7.38 (m, 2H), 7.59–7.63 (m, 1H), 8.53 (br. s, 1H, NH exch. with D2O). Anal. calcd for C14H13NO2: C, 73.99; H, 5.77; N, 6.16. Found: C, 74.25; H, 5.79; N, 6.18. Acid 29 (from 23) as a white solid (0.161 g, 84.15%), mp = 198–200 °C, 1H NMR (CDCl3) ppm: 2.02–2.04 (m, 2H), 2.75–2.80 (m, 4H), 3.80 (br. s, 1H, OH exch. with D2O), 6.90 (d, 1H, J = 1.6 Hz), 7.17–7.24 (m, 3H), 7.43 (d, 1H, J = 7.6 Hz), 9.14 (br. s, 1H, NH exch. with D2O). Anal. calcd for C14H13NO2: C, 73.99; H, 5.77; N, 6.16. Found: C, 74.24; H, 5.79; N, 6.17.
4.1.6. 2-Chloro-5,6-dihydro-4H-benzo[6,7]cyclohepta[b]pyrrole-3-carboxylic acid (28)
A solution of 27 (2.07 mmol) and N-chlorosuccinimide (2.48 mmol, 1.2 eq) in chloroform (12 mL) was refluxed for 4 h, then allowed to cool to room temperature and extracted with saturated aqueous NaHCO3. The aqueous solution was acidified with concentrated HCl. And the precipitate was filtered, washed (H2O), and air-dried to yield 25 as a grey solid (0.424 g, 78.52%), mp = 188–190 °C, 1H NMR (CDCl3) ppm: 1.68 (br. s, 1H, OH exch. with D2O), 1.98–2.10 (m, 2H), 2.80 (t, 2H, J = 5.0 Hz); 3.11 (t, 2H, J = 7.2 Hz), 7.16–7.20 (m, 2H); 7.26–7.31 (m, 2H); 8.48 (br. s, 1H, NH exch. with D2O). Anal. calcd for C14H12ClNO2: C, 64.24; H, 4.62; Cl, 13.55; N, 5.35. Found: C, 64.10; H, 4.61; Cl, 13.51; N, 5.33.
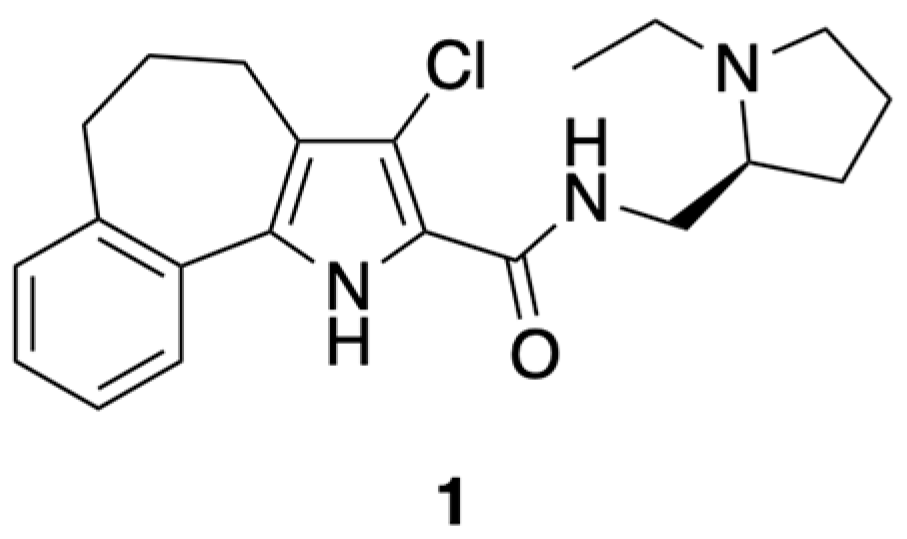
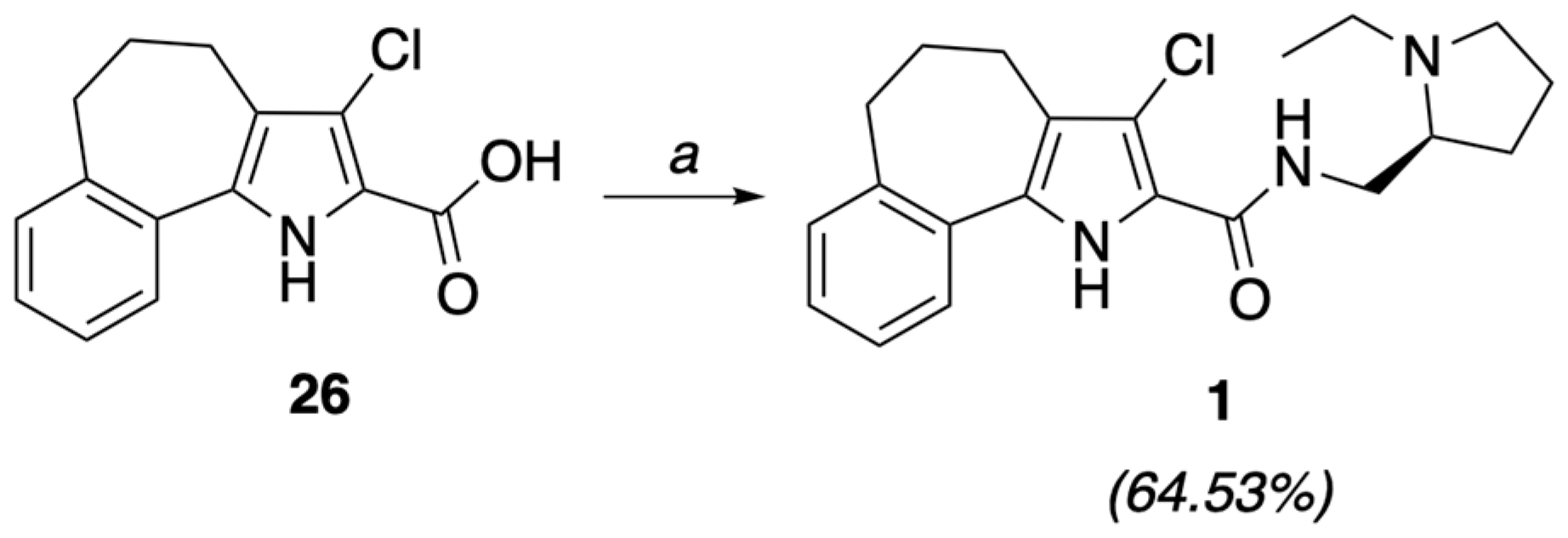
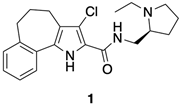
4.1.7. (S)-3-Chloro-N-(1-ethyl-2-pyrrolidinylmethyl)-5,6-dihydro-4H-benzo[6,7]cyclohepta[1,2-b]pyrrol-2-carboxamide (1)
To a stirred solution of 26 (0.38 mmol) in CH2Cl2 (1 mL) were added CDI (0.41 mmol, 1.1 eq) and HOBt (0.41 mmol, 1.1 eq). After stirring the reaction mixture at room temperature for 1.5 h, (S)-(−)-2-(aminomethyl)-1-ethylpyrrolidine (0.38 mmol, 1 eq) was added and stirring continued for 12 h. The reaction mixture was filtered on Celite® and the filtrate evaporated. The crude residue was taken up with EtOAc and washed (saturated aqueous NaCl). The organic phase was dried (Na2SO4) and evaporated to give a crude product, which was purified by flash chromatography (CHCl3/CH3OH 9/1) to give pure title compound 1 as a beige solid (0.091 g, 64.53%), mp = 136–139 °C, 1H NMR (CDCl3) ppm: 1.15 (t, 3H, J = 7.2 Hz), 1.60–1.90 (m, 4H), 1.99–2.15 (m, 2H), 2.16–2.38 (m, 2H), 2.72–2.95 (m, 6H), 3.22–3.411 (m, 2H), 3.66–3.80 (m, 1H), 7.17–7.26 (m, 3H), 7.50 (d, 1H, J = 7.6 Hz), 7.58 (br s, 1H, NH exch. with D2O), 9.43 (br s, 1H, exch. with D2O). 13C NMR (CDCl3) ppm: 14.20 (CH3), 23.07 (CH2), 25.31 (CH2 × 2), 26.42 (CH2), 28.47 (CH2), 34.76 (CH2), 40.95 (CH2), 48.18 (CH2), 53.56 (CH), 113.75 (C), 118.03 (C), 121.04 (CH), 124.66 (CH), 126.06 (CH), 127.27 (C), 129.27 (C), 129.69 (CH), 130.25 (C), 141.38 (C), 160.10 (C). MS (ESI): C21H26ClN3O requires m/z 371.18, found 372.18 [M+H]+. C21H26ClN3O: C, 67.82; H, 7.05; Cl, 9.53; N, 11.30. Found: C, 67.58; H, 6.96; Cl, 9.51; N, 11.27.
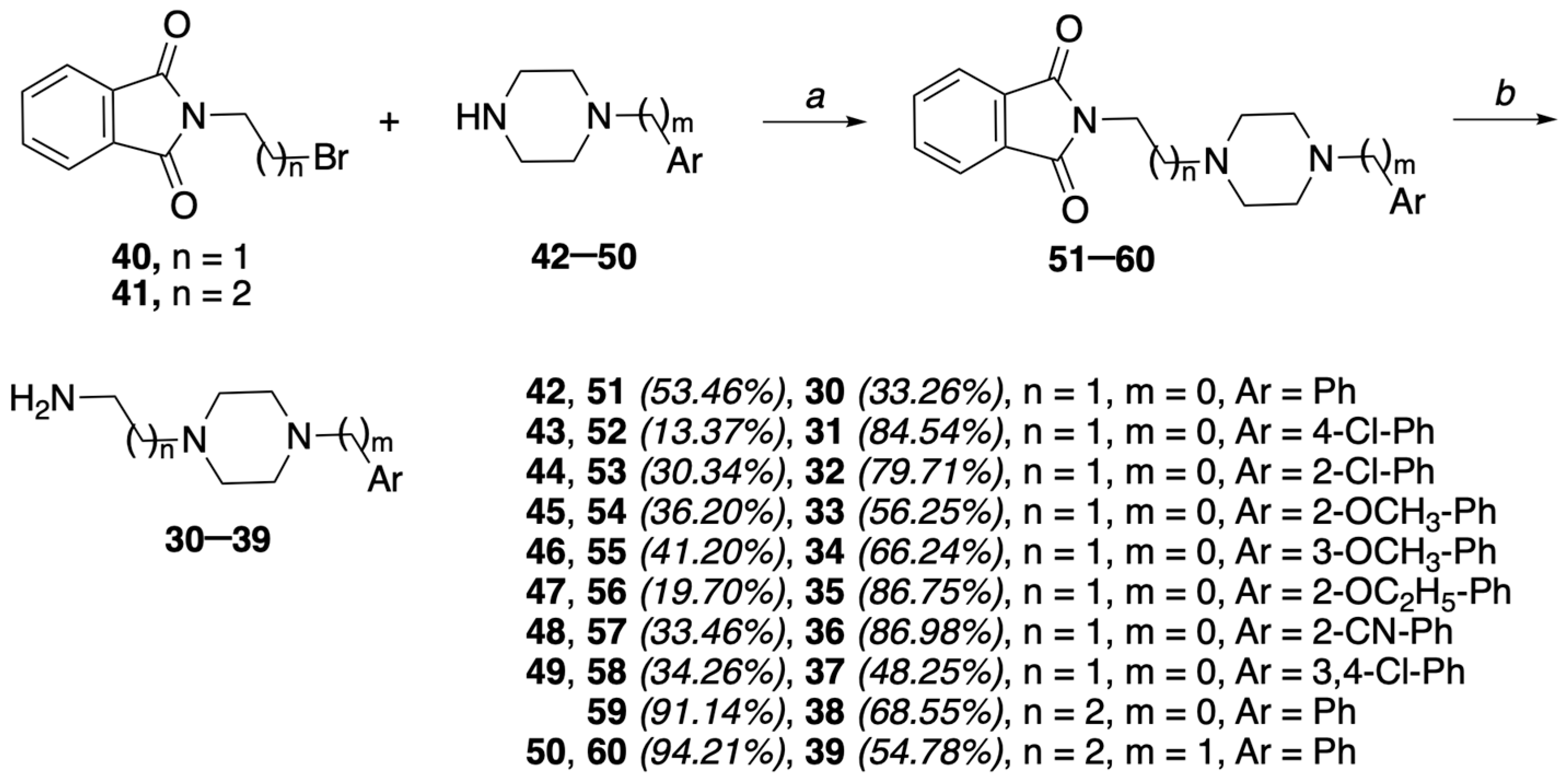
4.1.8. General Procedure II: Synthesis of N-(2-(4-arylpiperazin-1-yl)ethyl)phtalimides 51–58 and N-(3-(4-arylpiperazin-1-yl)propyl) phtalimides 59 and 60
To a solution of N-bromoalkylphtalimide 40 or 41 (1.23 mmol) in anhydrous DMF (3.8 mL) were added the appropriate aryl-piperazine (42–50) (1 eq) and K2CO3 (2.5 eq): the suspension underwent microwave irradiation (normal abs) at 55 °C for 2 h, then was poured onto water, and the crude precipitate was taken up in EtOAc. The solution was washed (H2O), dried (Na2SO4), and concentrated to give a crude product, which was purified by FC to yield the N-(arylpiperazin-1-yl)alkyl)phtalimides 51–60.
4.1.9. N-(2-(4-Phenylpiperazin-1-yl)ethyl)phtalimide (51)
The title compound was prepared from 40 and 1-phenylpiperazine (42) using the general procedure II to afford 51 as a pale yellow solid (0.317 g, 53.46%), after FC purification (petroleum ether/EtOAc 8/2), mp = 152–155 °C, 1H NMR (CDCl3) ppm: 2.60–2.80 (m, 6H), 3.13 (t, 4H, J = 5.0 Hz), 3.87 (t, 2H, J = 6.6 Hz), 6.78–6.93 (m, 3H), 7.19–7.28 (m, 2H), 7.67–7.75 (m, 2H), 7.80–7.88 (m, 2H). Anal. calcd for C20H21N3O2: C, 71.62; H, 6.31; N, 12.53. Found: C, 71.44; H, 6.29; N, 12.50.
4.1.10. N-(2-(4-(4-Chlorophenyl)piperazin-1-yl)ethyl)phtalimide (52)
The title compound was prepared from 40 and 1-(4-chlorophenyl)piperazine (43) using the general procedure II to afford 52 as a pale yellow solid (0.061 g, 13.37%), after FC purification (petroleum ether/EtOAc 8/2, then CHCl3/CH3OH 9.8/0.2), mp = 165–167 °C, 1H NMR (CDCl3) ppm: 2.63–2.74 (m, 6H), 3.09 (t, 4H, J = 5.2 Hz), 3.86 (t, 2H, J = 6.6 Hz), 6.81 (d, 2H, J = 9.2 Hz), 7.17 (d, 2H, J = 9.0 Hz), 7.68–7.73 (m, 2H), 7.80–7.88 (m, 2H). Anal. calcd for C20H20ClN3O2: C, 64.95; H, 5.45; Cl, 9.85; N, 11.36. Found: C, 65.14; H, 5.47; Cl, 9.88; N, 11.39.
4.1.11. N-(2-(4-(2-Chlorophenyl)piperazin-1-yl)ethyl)phtalimide (53)
The title compound was prepared from 40 and 1-(2-chlorophenyl)piperazine (44) using the general procedure II to afford 53 as a white solid (0.138 g, 30.34%), after FC purification (petroleum ether/EtOAc 7/3), mp = 106–109 °C, 1H NMR (CDCl3) ppm: 2.63–2.74 (m, 6H), 2.96–3.10 (m, 4H), 3.87 (t, 2H, J = 6.6 Hz), 6.89–7.31 (m, 2H), 7.18 (dd, 1H, Jo = 7.4 Hz, Jm = 1.6 Hz), 7.34 (dd, 1H, Jo = 7.6 Hz, Jm = 1.6 Hz), 7.65–7.77 (m, 2H), 7.82–7.90 (m, 2H). Anal. calcd for C20H20ClN3O2: C, 64.95; H, 5.45; Cl, 9.85; N, 11.36. Found: C, 64.82; H, 5.44; Cl, 9.82; N, 11.34.
4.1.12. N-(2-(4-(2-Methoxyphenyl)piperazin-1-yl)ethyl)phtalimide (54)
The title compound was prepared from 40 and 1-(2-methoxyphenyl)piperazine (45) using the general procedure II to afford 54 as a white solid (0.163 g, 36.20%), after FC purification (petroleum ether/EtOAc 1/1), mp = 115–117 °C, 1H NMR (CDCl3) ppm: 2.56–2.76 (m, 2H), 2.99–3.10 (m, 4H), 3.85 (s, 3H), 3.86–3.91 (m, 2H), 6.84–6.96 (m, 4H), 7.67–7.74 (m, 2H), 7.80–7.90 (m, 2H). Anal. calcd for C21H23N3O3: C, 69.02; H, 6.34; N, 11.50. Found: C, 61.90; H, 6.33; N, 11.48.
4.1.13. N-(2-(4-(3-Methoxyphenyl)piperazin-1-yl)ethyl)phtalimide (55)
The title compound was prepared from 40 and 1-(3-methoxyphenyl)piperazine (46) using the general procedure II to afford 55 as a yellow solid (0.186 g, 41.20%), after FC purification (petroleum ether/EtOAc 1/1), mp = 110–112 °C, 1H NMR (CDCl3) ppm: 2.62–2.75 (m, 6H), 3.13 (t, 4H, J = 4.8 Hz), 3.78 (s, 3H), 3.86 (t, 2H, J = 6.6 Hz), 6.33–6.45 (m, 2H), 6.46–6.54 (m, 1H), 7.15 (t, 1H, J = 8.2 Hz), 7.66–7.76 (m, 2H), 7.80–7.90 (m, 2H). Anal. calcd for C21H23N3O3: C, 69.02; H, 6.34; N, 11.50. Found: C, 61.83; H, 6.32; N, 11.46.
4.1.14. N-(2-(4-(2-Ethoxyphenyl)piperazin-1-yl)ethyl)phtalimide (56)
The title compound was prepared from 40 and 1-(2-ethoxyphenyl)piperazine (47) using the general procedure II to afford 56 as a yellow solid (0.100 g, 19.70%), after FC purification (CHCl3/CH3OH 98/2), mp = 102–105 °C, 1H NMR (CDCl3) ppm: 1.45 (t, 3H, J = 6.8 Hz), 2.65–2.76 (m, 6H), 3.00–3.20 (m, 4H), 3.87 (t, 2H, J = 6.6 Hz), 4.05 (q, 2H, J = 7.2 Hz), 6.80–6.97 (m, 4H), 7.66–7.79 (m, 2H), 7.84–7.92 (m, 2H). Anal. calcd for C22H25N3O3: C, 69.64; H, 6.64; N, 11.07. Found: C, 69.85; H, 6.66; N, 11.10.
4.1.15. 4-(4-(2-(Phtalimido)ethyl)piperazin-1-yl)benzonitrile (57)
The title compound was prepared from 40 and 2-(piperazin-1-yl)benzonitrile (48) using the general procedure II to afford 57 as an orange solid (0.171 g, 33.46%), after FC purification (petroleum ether/EtOAC 6/4), mp = 111–113 °C, 1H NMR (CDCl3) ppm: 2.69–2.77 (m, 6H), 3.14–3.19 (m, 4H), 3.86 (t, 2H, J = 6.6 Hz), 6.90–7.10 (m, 2H), 7.44 (dd, 1H, Jo = 9.6 Hz, Jm = 1.6 Hz), 7.54 (dd, 1H, Jo = 8.0 Hz, Jm = 1.6 Hz), 7.68–7.78 (m, 2H), 7.80–7.90 (m, 2H). Anal. calcd for C21H20N4O2: C, 69.98; H, 5.59; N, 15.55. Found: C, 69.84; H, 5.58; N, 15.52.
4.1.16. N-(2-(4-(3,4-Dichlorophenyl)piperazin-1-yl)ethyl)phtalimide (58)
The title compound was prepared from 40 and 1-(3,4-dichlorophenyl)piperazine (49) using the general procedure II to afford 58 as a white solid (0.170 g, 34.26%), after FC purification (petroleum ether/EtOAC 7/3), mp = 165–168 °C, 1H NMR (CDCl3) ppm: 2.68 (dt, 6H, J = 5.2 Hz), 3.10 (dt, 4H, J = 5.2 Hz), 3.86 (t, 2H, J = 6.1 Hz), 6.71 (dd, 1H, Jo = 9 Hz, Jm = 3.0 Hz), 6.92 (d, 1H, J = 3.0 Hz), 7.22 (s, 1H), 7.67–7.76 (m, 2H), 7.80–7.90 (m, 2H). Anal. calcd for C20H19Cl2N3O2: C, 59.42; H, 4.74; Cl, 17.54; N, 10.39. Found: C, 59.54; H, 4.83; Cl, 17.57; N, 10.41.
4.1.17. N-(2-(4-Phenylpiperazin-1-yl)propyl)phtalimide (59)
The title compound was prepared from 41 and 1-phenylpiperazine (42) using the general procedure II to afford 59 as a pale yellow solid (0.392 g, 91.14%), after trituration with petroleum ether, mp = 117–119 °C, 1H NMR (CDCl3) ppm: 1.91 (qu, 2H, J = 6.8 Hz), 2.48 (t, 2H, J = 6.8 Hz), 2.54 (t, 4H, J = 4.8 Hz), 3.04 (t, 4H, J = 4.8 Hz), 3.79 (t, 2H, J = 6.8 Hz), 6.81–6.87 (m, 3H), 7.21–7.26 (m, 2H), 7.68–7.70 (m, 2H), 7.82–7.84 (m, 2H). Anal. calcd for C21H23N3O2: C, 72.18; H, 6.63; N, 12.03. Found: C, 72.32; H, 6.64; N, 12.05.
4.1.18. N-(2-(4-Benzylpiperazin-1-yl)propyl)phtalimide (60)
The title compound was prepared from 41 and 1-benzylpiperazine (50) using the general procedure II to afford 60 as a yellow solid (0.424 g, 94.21%), after trituration with petroleum ether, mp = 132–135 °C, 1H NMR (CDCl3) ppm: 1.86 (qu, 2H, J = 6.8 Hz), 2.34–2.43 (m, 10H), 3.40 (s, 2H), 3.75 (t, 2H, J = 6.8 Hz), 6.81–6.87 (m, 3H), 7.21–7.26 (m, 2H), 7.68–7.70 (m, 2H), 7.82–7.84 (m, 2H). Anal. calcd for C22H25N3O2: C, 72.70; H, 6.93; N, 11.56. Found: C, 72.55; H, 6.92; N, 11.54.
4.1.19. General Procedure III: Synthesis of amino-alkylaryl-piperazines 30–39
To a solution of phtalimide (51–60) (0.724 mmol) in EtOH (3 mL) was added NH2NH2·H2O (5.2 eq), and the resulting solution was reacted under microwave irradiation (abs. low) at 80 °C for 20 min. The resulting suspension was filtered and then washed with EtOH, whose evaporation gave a residue, washed with EtOAc, and filtered. The organic solution was then dried (Na2SO4) and evaporated, providing a crude product that, purified by FC with an appropriate mixture of eluents, allowed us to obtain the desired amine (30–39).
4.1.20. 2-(4-Phenylpiperazin-1-yl)ethyl-1-amine (30)
The title compound was prepared from 51 using the general procedure III to afford 30 as a hygroscopic brown solid (0.058 g, 33.14%), after FC purification (CHCl3/CH3OH 9/1), mp = 90–95 °C, 1H NMR (CDCl3) ppm: 1.71 (br s, 2H, NH2 exch. with D2O), 2.50 (t, 2H, J = 5.8 Hz), 2.59–2.66 (m, 4H), 2.85 (t, 2H, J = 6.6 Hz), 3.17–3.25 (m, 4H), 6.80–6.97 (m, 3H), 7.21–7.33 (m, 2H). Anal. calcd for C12H19N3: C, 70.20; H, 9.33; N, 20.47. Found: C, 70.27; H, 9.34; N, 20.49.
4.1.21. 2-(4-(4-Chlorophenyl)piperazin-1-yl)ethyl-1-amine (31)
The title compound was prepared from 52 using the general procedure III to afford 31 as a hygroscopic pink solid (0.169 g, 84.54%), after FC purification (CHCl3/CH3OH 8/2), mp = 56–58 °C, 1H NMR (CDCl3) ppm: 1.69 (br s, 2H, NH2 exch. with D2O), 2.49 (t, 2H, J = 6.2 Hz), 2.55–2.65 (m, 4H), 2.84 (t, 2H, J = 6.0 Hz), 3.11–3.21 (m, 4H), 6.79–6.89 (m, 3H), 7.17–7.27 (m, 2H). Anal. calcd for C12H18ClN3: C, 60.12; H, 7.57; Cl, 14.79; N, 17.53. Found: C, 60.00; H, 7.55; Cl, 14.76; N, 17.49.
4.1.22. 2-(4-(2-Chlorophenyl)piperazin-1-yl)ethyl-1-amine (32)
The title compound was prepared from 53 using the general procedure III to afford 32 as a hygroscopic pink solid (0.159 g, 79.71%), after FC purification (CHCl3/CH3OH 8/2), mp = 98–100 °C, 1H NMR (CDCl3) ppm: 1.80 (br s, 2H, NH2 exch. with D2O), 2.51 (t, 2H, J = 6.0 Hz), 2.62–2.68 (m, 4H), 2.85 (t, 2H, J = 6.0 Hz), 2.99–3.11 (m, 4H), 6.95–7.07 (m, 2H), 7.23 (dd, 1H, Jo = 8.6 Hz, Jm = 1.6 Hz), 7.36 (dd, 1H, Jo = 7.8 Hz, Jm = 1.4 Hz). Anal. calcd for C12H18ClN3: C, 60.12; H, 7.57; Cl, 14.79; N, 17.53. Found: C, 60.30; H, 7.59; Cl, 14.83; N, 17.58.
4.1.23. 2-(4-(2-Methoxyphenyl)piperazin-1-yl)ethyl-1-amine (33)
The title compound was prepared from 54 using the general procedure III to afford 33 as a hygroscopic pink solid (0.111 g, 56.25%), after FC purification (CHCl3/CH3OH 9/1), mp = 61–64 °C, 1H NMR (CDCl3) ppm: 1.65 (br s, 2H, NH2 exch. with D2O), 2.50 (t, 2H, J = 6.4 Hz), 2.60–2.70 (m, 4H), 2.84 (t, 2H, J = 6.4 Hz), 2.99–3.10 (m, 4H), 3.79 (s, 3H), 6.83–6.89 (m, 1H), 6.90–7.10 (m, 1H). Anal. calcd for C13H21N3O: C, 66.35; H, 8.99; N, 17.86. Found: C, 66.42; H, 9.00; N, 17.88.
4.1.24. 2-(4-(3-Methoxyphenyl)piperazin-1-yl)ethyl-1-amine (34)
The title compound was prepared from 55 using the general procedure III to afford 34 as a hygroscopic pink solid (0.130 g, 66.24%), after FC purification (CHCl3/CH3OH 8/2), mp = 62–65 °C, 1H NMR (CDCl3) ppm: 1.91 (br s, 2H, NH2 exch. with D2O), 2.48 (t, 2H, J = 5.8 Hz), 2.56–2.63 (m, 4H), 2.84 (t, 2H, J = 6.2 Hz), 3.10–3.23 (m, 4H), 3.79 (s, 3H), 6.38–6.50 (m, 2H), 6.54 (d, 1H, J = 8.6 Hz), 7.17 (t, 1H, J = 8.0 Hz). Anal. calcd for C13H21N3O: C, 66.35; H, 8.99; N, 17.86. Found: C, 66.22; H, 8.97; N, 17.82.
4.1.25. 2-(4-(2-Ethoxyphenyl)piperazin-1-yl)ethyl-1-amine (35)
The title compound was prepared from 56 using the general procedure III to afford 35 as a hygroscopic pink solid (0.179 g, 86.57%), after FC purification (CHCl3/CH3OH 9/1), mp = 55–58 °C, 1H NMR (CDCl3) ppm: 1.46 (t, 3H, J = 7.0 Hz), 1.83 (br s, 2H, NH2 exch. with D2O), 2.50 (t, 2H, J = 6.0 Hz), 2.61–2.70 (m, 4H), 2.85 (t, 2H, J = 6.4 Hz), 3.10–3.21 (m, 4H), 4.07 (q, 2H, J = 6.8 Hz), 6.82–6.97 (m, 4H). Anal. calcd for C14H23N3O: C, 67.43; H, 9.30; N, 16.85. Found: C, 67.30; H, 9.28; N, 16.82.
4.1.26. 4-(4-(2-Aminoethyl)piperazin-1-yl)benzonitrile (36)
The title compound was prepared from 57 using the general procedure III to afford 36 as a hygroscopic yellow solid (0.179 g, 86.98%), after FC purification (CHCl3/CH3OH 9/1), mp = 72–75 °C, 1H NMR (CDCl3) ppm: 1.60 (br s, 2H, NH2 exch. with D2O), 2.52 (t, 2H, J = 6.2 Hz), 2.60–2.71 (m, 4H), 2.84 (t, 2H, J = 6.0 Hz), 3.20–3.29 (m, 4H), 6.96–7.04 (m, 2H), 7.44–7.59 (m, 2H). Anal. calcd for C13H18N4: C, 67.80; H, 7.88; N, 24.33. Found: C, 67.94; H, 7.90; N, 24.38.
4.1.27. 2-(4-(3,4-Dichlorophenyl)piperazin-1-yl)ethyl-1-amine (37)
The title compound was prepared from 58 using the general procedure III to afford 37 as a hygroscopic pink solid (0.108 g, 48.25%), after FC purification (CHCl3/CH3OH 8/2), mp = 89–92 °C, 1H NMR (CDCl3) ppm: 1.60 (br s, 2H, NH2 exch. with D2O), 2.48 (t, 2H, J = 6.2 Hz), 2.55–2.65 (m, 4H), 2.84 (t, 2H, J = 5.8 Hz), 3.13–3.21 (m, 4H), 6.74 (dd, 1H, Jo = 8.6 Hz, Jm = 2.4 Hz), 6.95 (d, 1H, J = 2.4 Hz), 7.22–7.30 (m, 1H). Anal. calcd for C12H17Cl2N3: C, 52.57; H, 6.25; Cl, 25.86; N, 15.33. Found: C, 52.62; H, 6.26; Cl, 25.89; N, 15.34.
4.1.28. 2-(4-Phenylpiperazin-1-yl)propyl-1-amine (38)
The title compound was prepared from 59 using the general procedure III to afford 38 as a white solid (0.109 g, 68.55%), after FC purification (CHCl3/CH3OH 8/2), mp = 101–103 °C, 1H NMR (CDCl3) ppm: 1.45 (br s, 2H, NH2 exch. with D2O), 1.65–1.69 (m, 2H), 2.46 (t, 2H, J = 5.6 Hz), 2.59–2.62 (m, 4H), 2.78 (t, 2H, J = 5.6 Hz), 3.19–3.21 (m, 4H), 6.85 (t, 1H, J = 7.2 Hz), 6.93 (d, 2H, J = 7.2 Hz), 7.26 (t, 2H, J = 7.6 Hz). Anal. calcd for C13H21N3: C, 71.19; H, 9.65; N, 19.16. Found: C, 71.33; H, 9.67; N, 19.20.
4.1.29. 2-(4-Benzylpiperazin-1-yl)propyl-1-amine (39)
The title compound was prepared from 60 using the general procedure III to afford 39 as an oil (0.092 g, 54.78%), after trituration with petroleum ether, 1H NMR (CDCl3) ppm 1.59–1.66 (m, 4H), 1.59–1.66 (m, 4H), 2.37 (t, 2H, J = 5.8 Hz), 2.40–2.47 (m, 6H), 2.73 (t, 2H, J = 5.2 Hz), 7.24–7.2316 (m, 5H). Anal. calcd for C14H23N3: C, 72.06; H, 9.93; N, 18.01. Found: C, 72.20; H, 9.95; N, 18.05.
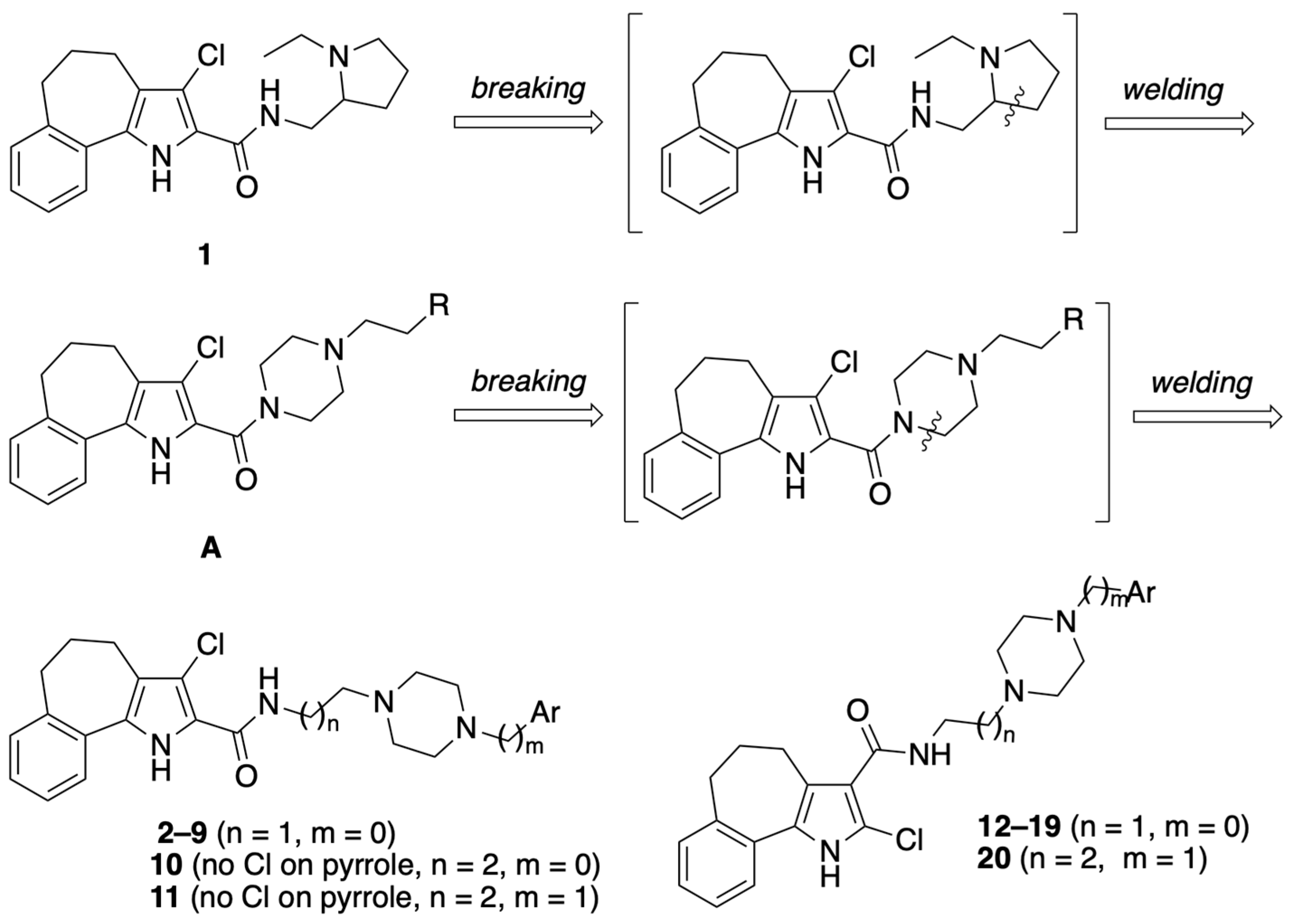
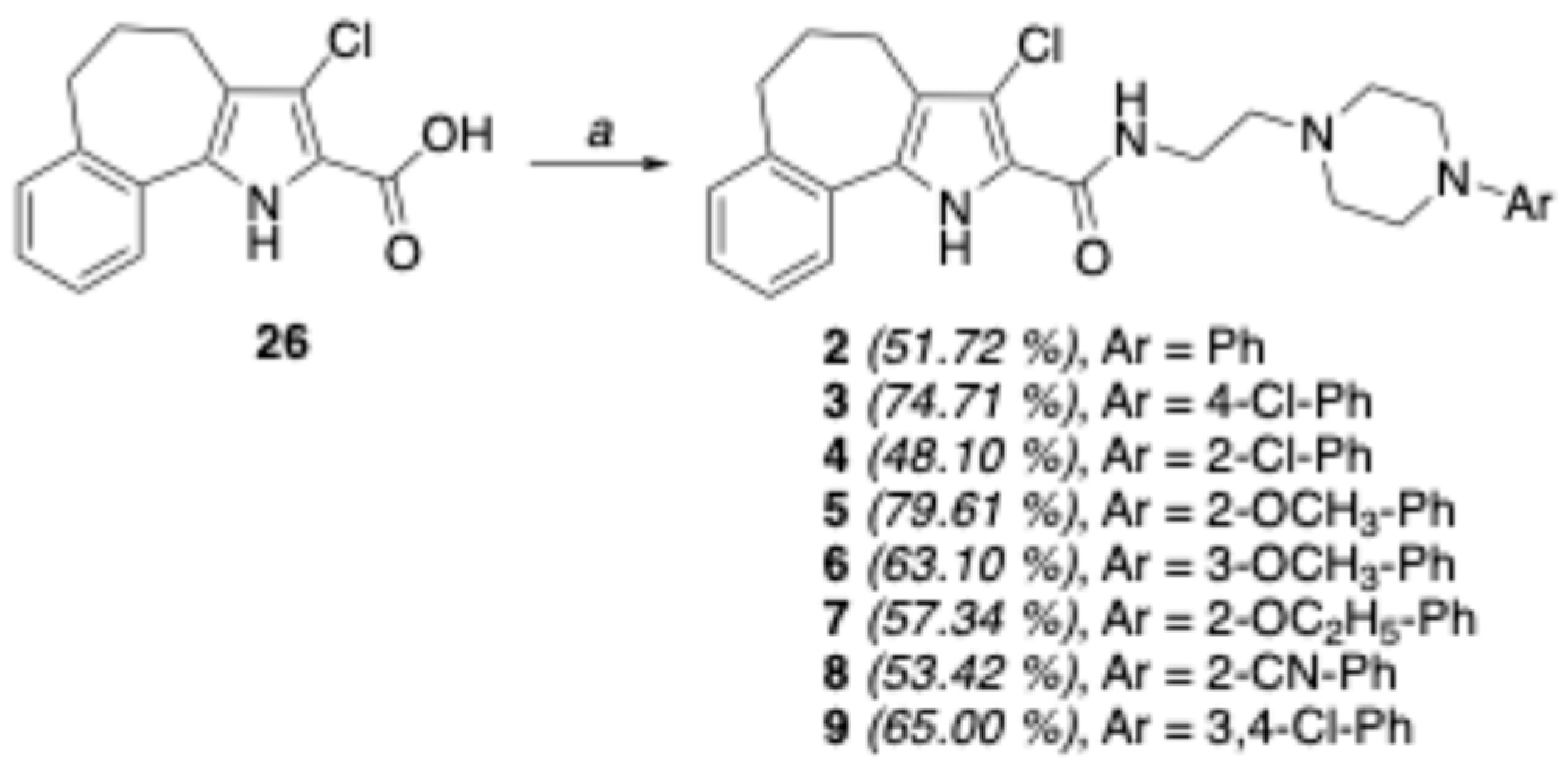
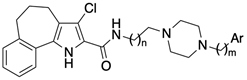
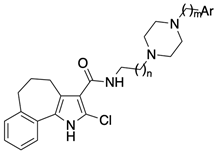
4.1.30. General Procedure IV: Synthesis of carboxamides 2–20
Carboxamides 2–20 were prepared starting from the appropriate acid 26, 28, or 29 (0.38 mmol) and aryl-piperazin-alkyl amines 30–39, as above reported for compound 1. The purification of the crude product by FC yielded the desired final compounds 2–20.
4.1.31. 3-Chloro-N-(2-(4-phenylpiperazin-1-yl)ethyl)-1,4,5,6-tetrahydrobenzo[6,7]cyclohepta[1,2-b]pyrrole-2-carboxamide (2)
The title compound was prepared from acid 26 and 2-(4-phenylpiperazin-1-yl)ethyl-1-amine (30) using the general procedure IV to afford 2 as a beige solid (0.088 g, 51.72%), after FC purification (CHCl3/CH3OH 95/5), mp = 201–203 °C, 1H NMR (CDCl3) ppm: 1.98–2.10 (m, 2H), 2.68–2.80 (m, 10H), 3.20–3.30 (m, 4H), 3.55–3.65 (m, 2H), 6.84–6.90 (m, 1H), 6.95 (d, 1H, J = 7.8 Hz), 7.17–7.19 (m, 2H), 7.23–7.31 (m, 3H), 7.48 (d, 1H, J = 7.6 Hz), 7.58 (br s, 1H, exch. with D2O), 9.26 (br s, 1H, exch. with D2O). 13C NMR (CDCl3) ppm: 25.81 (CH2), 26.57 (CH2), 34.66 (CH2), 36.01 (CH2), 49.29 (CH2 × 2), 52.67 (CH2 × 2), 55.95 (CH2), 115.98 (CH × 2), 117.99 (CH × 2), 119.72 (C), 120.96 (CH), 125.01 (C), 126.46 (C), 127.20 (C), 129.08 (CH), 129.60 (CH), 130.30 (C), 141.34 (CH), 141.48 (C), 151.17 (CH), 159.78 (C). MS (ESI): C26H29ClN4O requires m/z 448.20, found 449.21 [M+H]+. Anal. calcd for C26H29ClN4O: C, 69.55; H, 6.51; Cl, 7.90; N, 12.48. Found: C, 69.51; H, 6.50; Cl, 7.89; N, 12.50.
4.1.32. 3-Chloro-N-(4-(4-chlorophenyl)piperazin-1-yl)ethyl)-1,4,5,6-tetrahydrobenzo[6,7]cyclohepta[1,2-b]pyrrole-2-carboxamide (3)
The title compound was prepared from acid 26 and 2-(4-(4-chlorophenyl)piperazin-1-yl)ethyl-1-amine (31) using the general procedure IV to afford 3 as a beige solid (0.137 g, 74.71%), after FC purification (CHCl3/CH3OH 99/1), mp = 193–195 °C, 1H NMR (CDCl3) ppm: 1.99–2.10 (m, 2H), 2.65–2.79 (m, 10H), 3.14–3.21 (m, 4H), 3.55–3.64 (m, 2H), 6.86 (d, 2H, J = 9.0 Hz), 7.17–7.20 (m, 3H), 7.21–7.26 (m, 3H), 7.29–7.32 (m, 1H), 7.47 (d, 1H, J = 8.6 Hz), 7.50 (br s, 1H, NH exch. with D2O), 9.31 (br s, 1H, exch. with D2O). 13C NMR (CDCl3) ppm: 24.92 (CH2), 25.82 (CH2), 33.92 (CH2), 36.00 (CH2), 49.33 (CH2 × 2), 52.54 (CH2 × 2), 55.95 (CH2), 113.63 (C), 117.18 (CH × 2), 118.00 (CH × 2), 120.99 (C), 124.53 (C), 124.83 (C), 126.54 (C), 127.29 (C), 128.91 (CH × 2), 129.66 (C), 130.23 (C), 141.37 (CH), 149.77 (CH), 159.75 (C). MS (ESI): C26H28Cl2N4O requires m/z 482.16; found 483.17 [M+H]+. Anal. calcd for C26H28Cl2N4O: C, 64.60; H, 5.84; Cl, 14.67; N, 11.59. Found: C, 64.59; H, 5.83; Cl, 14.65; N, 11.48.
4.1.33. 3-Chloro-N-(4-(2-chlorophenyl)piperazin-1-yl)ethyl)-1,4,5,6-tetrahydrobenzo[6,7]cyclohepta[1,2-b]pyrrole-2-carboxamide (4)
The title compound was prepared from acid 26 and 2-(4-(2-chlorophenyl)piperazin-1-yl)ethyl-1-amine (32) using the general procedure IV to afford 4 as a beige solid (0.088 g, 48.10%), after FC purification (CHCl3/CH3OH 99/1), mp = 169–171 °C, 1H NMR (CDCl3) ppm: 1.99–2.13 (m, 2H), 2.64–2.85 (m, 10H), 3.08–3.14 (m, 4H), 3.54–3.62 (m, 2H), 6.95–7.02 (m, 1H), 7.06 (d, 1H, J = 8.0 Hz), 7.08–7.17 (m, 2H), 7.20–7.30 (m, 2H), 7.36 (d, 1H, J = 7.8 Hz), 7.49 (d, 1H, J = 7.6 Hz), 7.59 (br s, 1H, exch. with D2O), 9.33 (br s, 1H, NH exch. with D2O). 13C NMR (CDCl3) ppm: 25.85 (CH2), 26.56 (CH2), 34.66 (CH2), 36.05 (CH2), 51.39 (CH2 × 2), 52.82 (CH2 × 2), 55.96 (CH2), 113.57 (C), 120.28 (CH), 120.93 (CH), 120.95 (C), 123.64 (C), 124.96 (C), 126.50 (CH), 127.22 (CH), 127.52 (CH), 128.67 (CH), 129.56 (CH), 129.57 (CH), 130.29 (C), 130.61 (C), 141.33 (C), 149.14 (C), 159.76 (C). MS (ESI): C26H28Cl2N4O requires m/z 482.16; found 483.17 [M+H]+. Anal. calcd for C26H28Cl2N4O: C, 64.60; H, 5.84; Cl, 14.67; N, 11.59. Found: C, 64.59; H, 5.83; Cl, 14.65; N, 11.48.
4.1.34. 3-Chloro-N-(4-(2-methoxyphenyl)piperazin-1-yl)ethyl)-1,4,5,6-tetrahydrobenzo[6,7]cyclohepta[1,2-b]pyrrole-2-carboxamide (5)
The title compound was prepared from acid 26 and 2-(4-(2-methoxyphenyl)piperazin-1-yl)ethyl-1-amine (33) using the general procedure IV to afford 5 as a beige solid (0.145 g, 79.61%), after FC purification (CHCl3/CH3OH 99/1), mp = 163–165 °C, 1H NMR (CDCl3) ppm: 1.99–2.10 (m, 2H), 2.65–2.80 (m, 10H), 3.10–3.13 (m, 4H), 3.55–3.63 (m, 2H), 3.88 (s, 3H), 6.88 (d, 1H, J = 7.6 Hz), 6.90–7.00 (m, 2H), 7.16–7.20 (m, 2H), 7.21–7.27 (m, 2H), 7.49 (d, 1H, J = 7.2 Hz), 7.60 (br s, 1H, NH exch. with D2O), 9.33 (br s, 1H, NH exch. with D2O). 13C NMR (CDCl3) ppm: 25.79 (CH2), 26.56 (CH2), 34.65 (CH2), 36.02 (CH2), 50.81 (CH2 × 2), 52.87 (CH2 × 2), 55.29 (CH3), 55.96 (CH2), 111.79 (CH), 113.59 (C), 118.11 (CH), 120.90 (CH × 2), 121.03 (C × 2), 122.92 (CH), 125.02 (C), 126.46 (CH), 127.17 (C), 129.57 (CH × 2), 130.32 (C), 141.31 (C), 152.15 (C), 159.78 (C). MS (ESI): C27H31ClN4O2 requires m/z 478.21; found 479.22 [M+H]+. Anal. calcd for C27H31ClN4O2: C, 67.70; H, 6.52; Cl, 7.40; N, 11.70. Found: C, 67.68; H, 6.51; Cl, 7.39; N, 11.68.
4.1.35. 3-Chloro-N-(4-(3-methoxyphenyl)piperazin-1-yl)ethyl)-1,4,5,6-tetrahydrobenzo[6,7]cyclohepta[1,2-b]pyrrole-2cCarboxamide (6)
The title compound was prepared from acid 26 and 2-(4-(3-methoxyphenyl)piperazin-1-yl)ethyl-1-amine (34) using the general procedure IV to afford 6 as a beige solid (0.115 g, 63.10%), after FC purification (CHCl3/CH3OH 98/2), mp = 167–170 °C, 1H NMR (CDCl3) ppm: 1.89–2.05 (m, 2H), 2.64–2.78 (m, 10H), 3.20–3.25 (m, 4H), 3.52–3.59 (m, 2H), 3.80 (s, 3H), 6.46–6.60 (m, 3H), 7.14–7.35 (m, 4H), 7.46 (s, 1H), 7.50 (br s, 1H, NH exch. with D2O), 9.35 (br s, 1H, NH exch. with D2O). 13C NMR (CDCl3) ppm: 25.39 (CH2), 26.31 (CH2), 34.55 (CH2), 35.71 (CH2), 48.88 (CH2 × 2), 52.33 (CH2 × 2), 54.86 (CH3), 55.68 (CH2), 102.02 (CH), 104.15 (CH), 108.45 (CH), 113.35 (C), 120.62 (CH), 124.82 (CH), 126.19 (C), 126.93 (C), 129.32 (CH × 2), 129.80 (CH), 130.00 (C), 141.02 (C), 152.29 (C), 157.03 (C), 159.44 (C), 160.19 (C). MS (ESI): C27H31ClN4O2 requires m/z 478.21; found 479.22 [M+H]+. Anal. calcd for C27H31ClN4O2: C, 67.70; H, 6.52; Cl, 7.40; N, 11.70. Found: C, 67.67; H, 6.51; Cl, 7.38; N, 11.69.
4.1.36. 3-Chloro-N-(4-(2-ethoxyphenyl)piperazin-1-yl)ethyl)-1,4,5,6-tetrahydrobenzo[6,7]cyclohepta[1,2-b]pyrrole-2-carboxamide (7)
The title compound was prepared from acid 26 and 2-(4-(2-ethoxyphenyl)piperazin-1-yl)ethyl-1-amine (35) using the general procedure IV to afford 7 as a white solid (0.107 g, 57.34%), after FC purification (CHCl3/CH3OH 98/2), mp = 110–113 °C, 1H NMR (CDCl3) ppm: 1.47 (t, 3H, J = 7.2 Hz), 1.99–2.10 (m, 2H), 2.65–2.81 (m, 10H), 3.14–3.18 (m, 4H), 3.55–3.65 (m, 2H), 4.08 (q, 2H, J = 6.8 Hz), 6.87–6.98 (m, 4H), 7.17–7.19 (m, 2H), 7.25–7.30 (m, 1H), 7.48 (d, 1H, J = 8.2 Hz), 7.62 (br s, 1H, NH exch. with D2O), 9.30 (br s, 1H, NH exch. with D2O). 13C NMR (CDCl3) ppm: 14.92 (CH3), 25.88 (CH2), 26.54 (CH2), 34.71 (CH2), 36.06 (CH2), 50.75 (CH2 × 2), 52.96 (CH2 × 2), 56.05 (CH2), 63.54 (CH2), 112.50 (CH), 113.63 (C), 118.12 (CH), 120.95 (CH), 121.01 (C), 121.09 (C), 122.73 (CH), 124.85 (CH), 126.55 (CH), 127.25 (CH), 129.46 (C), 129.67 (CH), 130.32 (C), 141.31 (C), 141.37 (C), 151.54 (C), 159.76 (C). MS (ESI): C28H33ClN4O2 requires m/z 492.23, found 493.23 [M+H]+. Anal. calcd for C28H33ClN4O2: C, 68.21; H, 6.75; Cl, 7.19; N, 11.36. Found: C, 68.23; H, 6.76; Cl, 7.21; N, 11.38.
4.1.37. 3-Chloro-N-(2-(4-(2-cyanophenyl)piperazin-1-yl)ethyl)-1,4,5,6-tetrahydrobenzo[6,7]cyclohepta[1,2-b]pyrrole-2-carboxamide (8)
The title compound was prepared from acid 26 and 2-(4-(2-aminoethyl)piperazin-1-yl)benzonitrile (36) using the general procedure IV to afford 8 as a beige solid (0.096 g, 53.42%), after FC purification (CHCl3/CH3OH 99/1), mp = 171–174 °C, 1H NMR (CDCl3) ppm: 1.90–2.05 (m, 2H), 2.66–2.80 (m, 10H), 3.25–3.30 (m, 4H), 3.56–3.63 (m, 2H), 6.96–7.05 (m, 1H), 7.16–7.20 (m, 2H), 7.22–7.27 (m, 2H), 7.45–7.47 (m, 3H), 7.59 (br s, 1H, NH exch. with D2O), 9.40 (br s, 1H, NH exch. with D2O). 13C NMR (CDCl3) ppm: 25.81 (CH2), 26.58 (CH2), 34.64 (CH2), 36.00 (CH2), 51.69 (CH2 × 2), 52.62 (CH2 × 2), 55.86 (CH2), 105.92 (C), 113.63 (C), 118.41 (CH), 118.60 (CH), 120.91 (CH), 120.99 (C), 121.74 (CH), 125.00 (C), 126.49 (C), 127.25 (CH), 129.62 (CH), 130.27 (C), 133.74 (CH), 134.31 (CH), 141.31 (C), 155.54 (C), 159.75 (C). MS (ESI): C27H28ClN5O requires m/z 473.20; found 474.20 [M+H]+. Anal. calcd for C27H28ClN5O: C, 68.42; H, 5.95; Cl, 7.48; N, 14.78. Found: C, 68.43; H, 5.96; Cl, 7.50; N, 14.80.
4.1.38. 3-Chloro-N-(4-(3,4-dichlorophenyl)piperazin-1-yl)ethyl)-1,4,5,6-tetrahydrobenzo[6,7]cyclohepta[1,2-b]pyrrole-2-carboxamide (9)
The title compound was prepared from acid 26 and 2-(4-(3,4-dichlorophenyl)piperazin-1-yl)ethyl-1-amine (37) using the general procedure IV to afford 9 as a light brown solid (0.128 g, 65.00%), after FC purification (CHCl3/CH3OH 99/1), mp = 199–201 °C, 1H NMR (CDCl3) ppm: 1.99–2.10 (m, 2H), 2.65–2.85 (m, 10H), 3.16–3.25 (m, 4H), 3.56–3.62 (m, 2H), 6.76 (dd, 1H, Jo = 8.8 Hz, Jm = 2.8 Hz), 6.94–6.99 (m, 1H), 7.16–7.19 (m, 2H), 7.20–7.31 (m, 2H), 7.46 (s, 1H), 7.50 (br s, 1H, exch. with D2O), 9.30 (br s, 1H, NH exch. with D2O). 13C NMR (CDCl3) ppm: 25.81 (CH2), 26.56 (CH2), 34.65 (CH2), 35.98 (CH2), 48.84 (CH2 × 2), 52.35 (CH2 × 2), 55.94 (CH2), 113.55 (CH), 115.28 (CH), 117.14 (CH), 118.00 (C), 120.93 (CH), 122.11 (C), 124.97 (C), 126.46 (C), 127.24 (C), 129.63 (CH × 2), 130.27 (C), 130.35 (CH), 132.70 (C), 141.35 (C), 150.53 (C), 159.74 (C). MS (ESI): C26H27Cl3N4O requires m/z 516.13; found 517.13 [M+H]+. Anal. calcd for C26H27Cl3N4O: C, 60.30; H, 5.26; Cl, 20.54; N, 10.82. Found: C, 60.29; H, 5.25; Cl, 20.53; N, 10.81.
4.1.39. N-(3-(4-Phenylpiperazin-1-yl)propyl)-1,4,5,6-tetrahydrobenzo[6,7]cyclohepta[1,2-b]pyrrole-2-carboxamide (10)
The title compound was prepared from acid 29 and 2-(4-phenylpiperazin-1-yl)propyl-1-amine (38) using the general procedure IV to afford 10 as a beige solid (0.039 g, 24.07%), without FC purification, mp = 185–187 °C, 1H NMR (CDCl3) ppm: 1.55–1.60 (m, 2H), 1.80–1.83 (m, 2H), 1.95–1.99 (m, 2H), 2.61 (t, 2H, J = 6.0 Hz), 2.67–2.71 (m, 4H), 2.76–2.78 (m, 2H), 3.26–3.29 (m, 4H), 3.55–3.59 (m, 2H), 6.45 (d, 1H, Jm = 2.8 Hz), 6.88 (t, 1H, J = 7.2 Hz), 6.95 (d, 2H, J = 7.6 Hz), 7.14–7.16 (m, 2H), 7.23–7.30 (m, 3H), 7.45 (d, 1H, J = 7.6 Hz), 7.51 (br s, 1H, exch. with D2O), 9.18 (br s, 1H, exch. with D2O). MS (ESI): C27H32N4O requires m/z 428.26; found 429.26 [M+H]+. Anal. calcd for C27H32N4O: C, 75.67; H, 7.53; N, 13.07. Found: C, 75.64; H, 7.51; N, 13.05.
4.1.40. N-(3-(4-Benzylpiperazin-1-yl)propyl)-2-chloro-1,4,5,6-tetrahydrobenzo[6,7]cyclohepta[1,2-b]pyrrole-2-carboxamide (11)
The title compound was prepared from acid 29 and 2-(4-benzylpiperazin-1-yl)propyl-1-amine (39) using the general procedure IV to afford 11 as a white solid (0.052 g, 30.92%) after FC purification (CHCl3/CH3OH 98/2), mp = 103–105 °C, 1H NMR (CDCl3) ppm: 1.65–1.72 (m, 2H), 1.97–2.03 (m, 2H), 2.47–2.50 (m, 10H), 2.75–2.77 (m, 2H), 2.80–2.84 (m, 2H), 3.42–3.47 (m, 4H), 6.50 (d, 1H, Jm = 2.8 Hz), 7.08–7.11 (m, 2H), 7.17–7.19 (m, 3H), 7.24–7.26 (m, 3H), 7.41 (d, 1H, J = 7.6 Hz), 7.67 (br s, 1H, exch. with D2O), 9.18 (br s, 1H, exch. with D2O). 13C NMR (CDCl3) ppm: 24.73 (CH2), 27.57 (CH2), 27.64 (CH2), 34.94 (CH2), 39.97 (CH2), 53.23 (CH2 × 2), 53.28 (CH2 × 2), 58.14 (CH2), 63.34 (CH2), 111.29 (CH), 122.98 (C), 124.87 (CH), 126.07 (C), 126.52 (CH), 126.66 (CH × 2), 127.18 (CH), 128.30 (CH × 2), 129.22 (CH × 2), 129.65 (CH), 130.74 (C), 131.35 (C), 137.93 (C), 141.09 (C), 161.14 (C). MS (ESI): C28H34N4O requires m/z 442.27; found 443.28 [M+H]+. Anal. calcd for C28H34N4O: C, 75.98; H, 7.74; N, 12.66. Found: C, 76.00; H, 7.75; N, 12.68.
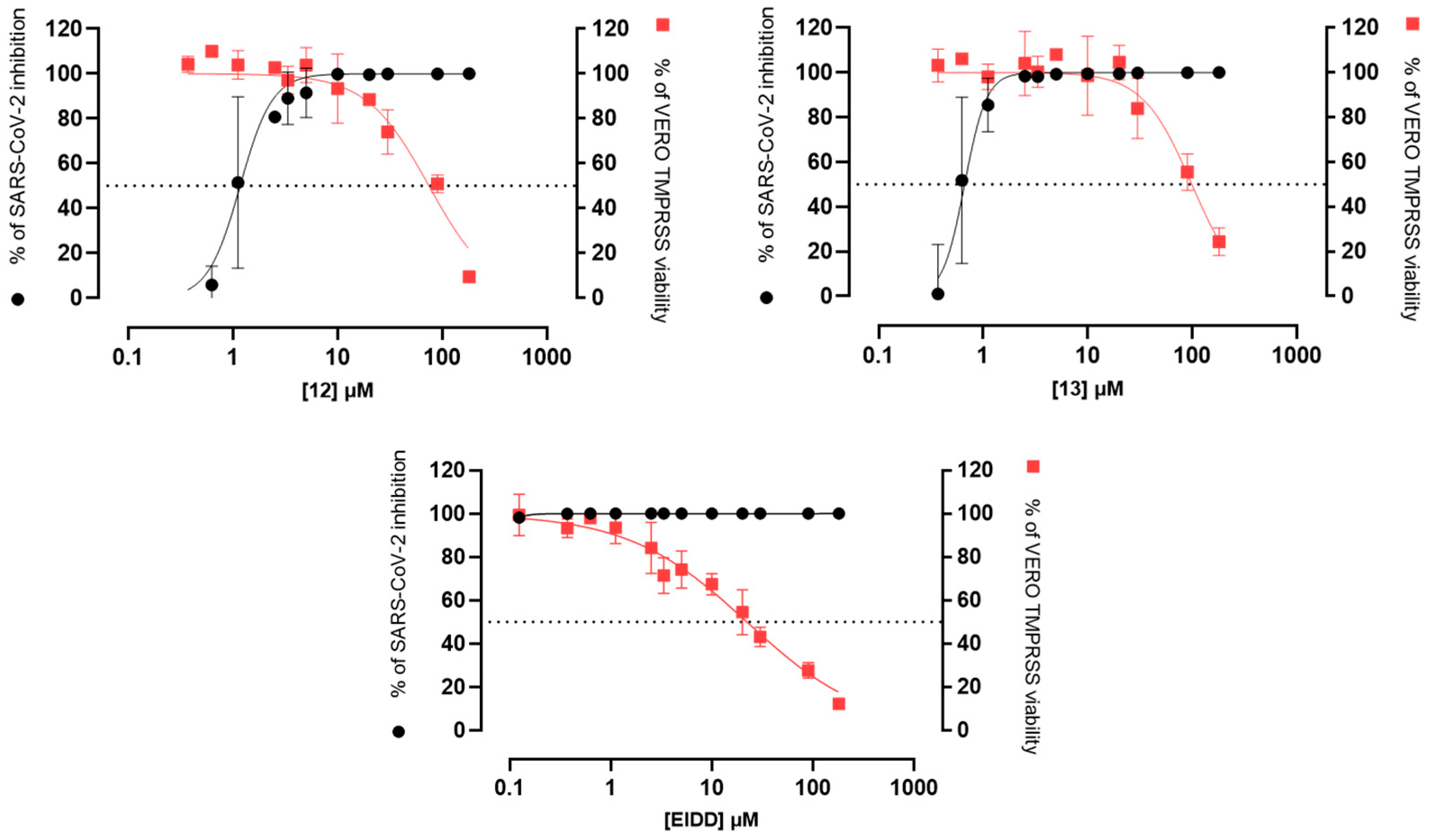
4.1.41. 2-Chloro-N-(2-(4-phenylpiperazin-1-yl)ethyl)-1,4,5,6-tetrahydrobenzo[6,7]cyclohepta[1,2-b]pyrrole-3-carboxamide (12)
The title compound was prepared from acid 28 and 2-(4-phenylpiperazin-1-yl)ethyl-1-amine (30) using the general procedure IV to afford 12 as a brown solid (0.077 g, 45.16%), after FC purification (CHCl3/CH3OH 98/2), mp = 167–170 °C, 1H NMR (CDCl3) ppm: 1.90–2.10 (m, 2H), 2.63–2.80 (m, 8H), 3.10–3.12 (m, 2H), 3.13–3.22 (m, 4H), 3.46–3.58 (m, 2H), 6.90–6.97 (m, 5H, NH exch. with D2O), 7.17–7.36 (m, 4H), 8.29 (br s, 1H, NH exch. with D2O). 13C NMR (CDCl3) ppm: 27.53 (CH2), 27.82 (CH2), 34.71 (CH2), 35.83 (CH2), 49.19 (CH2 × 2), 52.65 (CH2 × 2), 56.17 (CH2), 115.39 (C), 115.95 (CH × 2), 118.01 (CH), 119.74 (CH), 123.65 (C), 124.34 (CH), 126.19 (CH), 126.40 (C), 127.32 (C), 129.09 (CH × 2), 129.38 (CH), 130.60 (C), 141.28 (C), 151.12 (C), 164.10 (C). MS (ESI): C26H29ClN4O requires m/z 448.20, found 449.20 [M+H]+. Anal. calcd for C26H29ClN4O: C, 69.55; H, 6.51; Cl, 7.90; N, 12.48. Found: C, 69.48; H, 6.52; Cl, 7.87; N, 12.46.
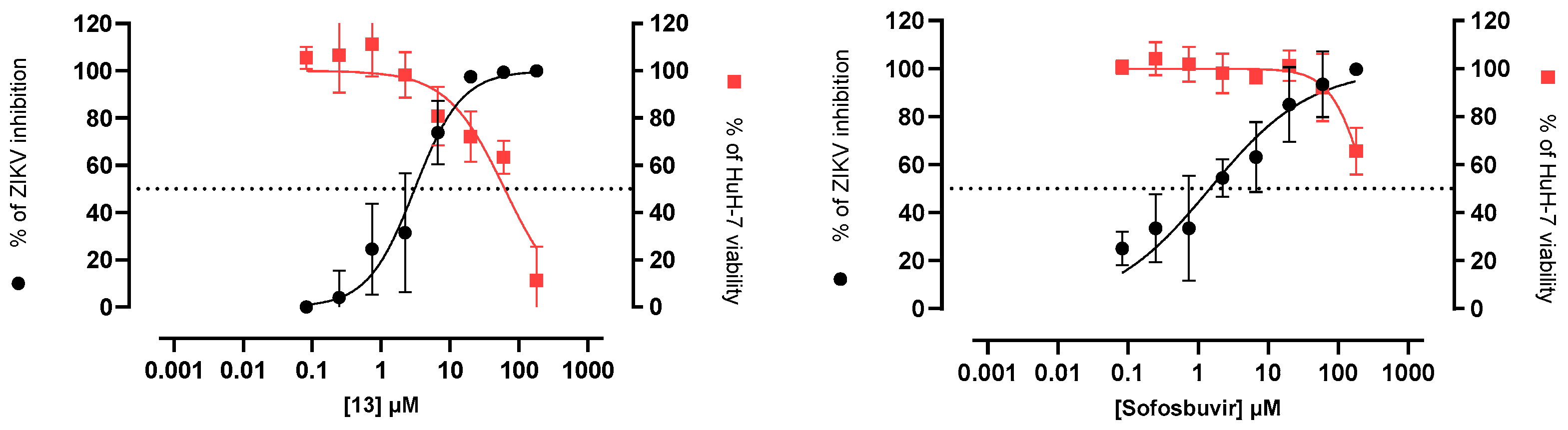
4.1.42. 2-Chloro-N-(4-(4-chlorophenyl)piperazin-1-yl)ethyl)-1,4,5,6-tetrahydrobenzo[6,7]cyclohepta[1,2-b]pyrrole-3-carboxamide (13)
The title compound was prepared from acid 28 and 2-(4-(4-chlorophenyl)piperazin-1-yl)ethyl-1-amine (31) using the general procedure IV to afford 13 as a beige solid (0.083 g, 45.10%), after FC purification (CHCl3/CH3OH 98/2), mp = 200–203 °C, 1H NMR (CDCl3) ppm: 1.99–2.10 (m, 2H), 2.60–2.65 (m, 6H), 2.70–2.78 (m, 2H), 3.01–3.18 (m, 6H), 3.51–3.62 (m, 2H), 6.85 (d, 4H, J = 8.8 Hz), 7.14–7.20 (m, 2H), 7.25–7.31 (m, 3H, NH exch. with D2O), 8.32 (br s, 1H, exch. with D2O). 13C NMR (CDCl3) ppm: 27.55 (CH2), 27.89 (CH2), 34.78 (CH2), 35.89 (CH2), 49.25 (CH2 × 2), 52.60 (CH2 × 2), 56.30 (CH2), 115.22 (C), 115.61 (C), 117.20 (CH × 2), 123.80 (C), 124.26 (CH), 124.61 (C), 126.30 (C), 126.56 (CH), 127.36 (C), 128.97 (CH × 2), 129.21 (CH), 130.62 (C), 141.40 (C), 149.81 (C), 164.04 (C). MS (ESI): C26H28Cl2N4O requires m/z 482.16; found 483.17 [M+H]+. Anal. calcd for C26H28Cl2N4O: C, 64.60; H, 5.84; Cl, 14.67; N, 11.59. Found: C, 64.56; H, 5.83; Cl, 14.66; N, 11.56.
4.1.43. 2-Chloro-N-(4-(2-chlorophenyl)piperazin-1-yl)ethyl)-1,4,5,6-tetrahydrobenzo[6,7]cyclohepta[1,2-b]pyrrole-3-carboxamide (14)
The title compound was prepared from acid 28 and 2-(4-(2-chlorophenyl)piperazin-1-yl)ethyl-1-amine (32) using the general procedure IV to afford 14 as a beige solid (0.045 g, 24.69.10%), after FC purification (CHCl3/CH3OH 98/2), mp = 178–180 °C, 1H NMR (CDCl3) ppm: 1.99–2.10 (m, 2H), 2.60–2.80 (m, 8H), 3.06–3.20 (m, 6H), 3.50–3.60 (m, 2H), 6.94 (br s, 1H, exch. with D2O), 6.98–7.02 (m, 4H), 7.15–7.35 (m, 4H), 8.33 (br s, 1H, NH exch. with D2O). 13C NMR (CDCl3) ppm: 27.50 (CH2), 27.83 (CH2), 34.72 (CH2), 35.86 (CH2), 49.28 (CH2 × 2), 52.69 (CH2 × 2), 56.23 (CH2), 115.57 (CH), 120.28 (C), 120.93 (CH), 120.95 (C), 123.64 (C), 124.96 (CH), 126.50 (C), 127.22 (CH), 127.52 (CH), 128.67 (CH), 129.56 (CH), 129.57 (CH), 130.29 (C), 130.61 (C), 141.33 (C), 149.14 (C), 159.76 (C). MS (ESI): C26H28Cl2N4O requires m/z 482.16; found 483.17 [M+H]+. Anal. calcd for C26H28Cl2N4O: C, 64.60; H, 5.84; Cl, 14.67; N, 11.59. Found: C, 64.61; H, 5.85; Cl, 14.64; N, 11.60.
4.1.44. 2-Chloro-N-(4-(2-methoxyphenyl)piperazin-1-yl)ethyl)-1,4,5,6-tetrahydrobenzo[6,7]cyclohepta[1,2-b]pyrrole-3-carboxamide (15)
The title compound was prepared from acid 28 and 2-(4-(2-methoxyphenyl)piperazin-1-yl)ethyl-1-amine (33) using the general procedure IV to afford 15 as a brown solid (0.098 g, 53.93%), after FC purification (CHCl3/CH3OH 98/2), mp = 88–90 °C, 1H NMR (CDCl3) ppm: 1.98–2.10 (m, 2H), 2.63–2.80 (m, 8H), 3.00–3.20 (m, 6H), 3.52–3.60 (m, 2H), 3.87 (s, 3H), 6.80–6.96 (m, 5H, NH exch. with D2O), 7.12–7.16 (m, 2H), 7.25–7.32 (m, 2H), 8.34 (br s, 1H, NH exch. with D2O). 13C NMR (CDCl3) ppm: 27.53 (CH2), 27.83 (CH2), 34.71 (CH2), 35.84 (CH2), 50.73 (CH2 × 2), 52.85 (CH2 × 2), 55.31 (CH3), 56.22 (CH2), 11.15 (CH), 115.31 (CH), 115.53 (CH), 120.88 (CH), 120.90 (C), 122.95 (CH), 123.63 (CH), 124.26 (C), 126.19 (C), 126.38 (C), 127.26 (CH), 129.39 (C), 130.61 (CH), 141.14 (C), 141.30 (C), 152.18 (C), 164.04 (C). MS (ESI): C27H31ClN4O2 requires m/z 478.21; found 479.22 [M+H]+. Anal. calcd for C27H31ClN4O2: C, 67.70; H, 6.52; Cl, 7.40; N, 11.70. Found: C, 67.69; H, 6.51; Cl, 7.41; N, 11.72.
4.1.45. 2-Chloro-N-(4-(3-methoxyphenyl)piperazin-1-yl)ethyl)-1,4,5,6-tetrahydrobenzo[6,7]cyclohepta[1,2-b]pyrrole-3-carboxamide (16)
The title compound was prepared from acid 28 and 2-(4-(3-methoxyphenyl)piperazin-1-yl)ethyl-1-amine (34) using the general procedure IV to afford 16 as a brown solid (0.104 g, 57.14%), after FC purification (CHCl3/CH3OH 98/2), mp = 89–92 °C, 1H NMR (CDCl3) ppm: 1.99–2.10 (m, 2H), 2.60–2.80 (m, 8H), 3.00–3.20 (m, 6H), 3.50–3.58 (m, 2H), 3.80 (s, 3H), 6.40–6.54 (m, 3H), 6.90 (br s, 1H, NH exch. with D2O), 7.13–7.36 (m, 5H), 8.61 (br s, 1H, NH exch. with D2O). 13C NMR (CDCl3) ppm: 27.53 (CH2), 27.79 (CH2), 34.70 (CH2), 35.81 (CH2), 49.08 (CH2 × 2), 52.57 (CH2 × 2), 55.14 (CH3), 56.12 (CH2), 102.43 (CH), 104.37 (CH), 108.79 (CH), 115.32 (CH × 2), 123.62 (CH), 124.42 (CH), 126.17 (C), 126.37 (C), 127.35 (C), 129.35 (C), 129.76 (CH), 130.63 (C), 141.25 (C), 152.53 (C), 160.47 (C), 164.15 (C). MS (ESI): C27H31ClN4O2 requires m/z 478.21; found 479.22 [M+H]+. Anal. calcd for C27H31ClN4O2: C, 67.70; H, 6.52; Cl, 7.40; N, 11.70. Found: C, 67.68; H, 6.51; Cl, 7.39; N, 11.68.
4.1.46. 2-Chloro-N-(4-(2-ethoxyphenyl)piperazin-1-yl)ethyl)-1,4,5,6-tetrahydrobenzo[6,7]cyclohepta[1,2-b]pyrrole-3-carboxamide (17)
The title compound was prepared from acid 28 and 2-(4-(2-ethoxyphenyl)piperazin-1-yl)ethyl-1-amine (35) using the general procedure IV to afford 17 as a brown solid (0.008 g, 43.09%), after FC purification (CHCl3/CH3OH 98/2), mp = 150–152 °C, 1H NMR (CDCl3) ppm: 1.46 (t, 3H, J = 6.8 Hz), 1.99–2.10 (m, 2H), 2.61–2.80 (m, 8H), 3.00–3.20 (m, 6H), 3.47–3.58 (m, 2H), 4.07 (q, 2H, J = 7.0 Hz), 6.86–6.98 (m, 5H, NH exch. with D2O), 7.14–7.18 (m, 2H), 7.20–7.33 (m, 2H), 8.33 (br s, 1H, NH exch. with D2O). 13C NMR (CDCl3) ppm: 14.91 (CH3), 27.53 (CH2), 27.82 (CH2), 34.73 (CH2), 35.85 (CH2), 50.66 (CH2 × 2), 52.90 (CH2 × 2), 56.23 (CH2), 63.50 (CH2), 112.40 (CH), 115.32 (CH), 115.55 (CH), 120.87 (CH), 122.74 (CH), 123.65 (CH), 124.17 (C), 124.25 (C), 126.19 (CH), 126.39 (C), 127.24 (C), 129.39 (C), 130.62 (CH), 141.19 (C), 141.30 (C), 151.47 (C), 164.02 (C). MS (ESI): C28H33ClN4O2 requires m/z 492.23, found 493.23 [M+H]+. Anal. calcd for C28H33ClN4O2: C, 68.21; H, 6.75; Cl, 7.19; N, 11.36. Found: C, 68.19; H, 6.74; Cl, 7.18; N, 11.34.
4.1.47. 2-Chloro-N-(2-(4-(2-cyanophenyl)piperazin-1-yl)ethyl)-1,4,5,6-tetrahydrobenzo[6,7]cyclohepta[1,2-b]pyrrole-3-carboxamide (18)
The title compound was prepared from acid 28 and 2-(4-(2-aminoethyl)piperazin-1-yl)benzonitrile (36) using the general procedure IV to afford 18 as a beige solid (0.087 g, 48.46%), after FC purification (CHCl3/CH3OH 98/2), mp = 87–90 °C, 1H NMR (CDCl3) ppm: 1.99–2.10 (m, 2H), 2.65–2.81 (m, 8H), 3.11 (t, 2H, J = 7.0 Hz), 3.15–3.27 (m, 4H), 3.54–3.60 (m, 2H), 6.83 (br s, 1H, NH exch. with D2O), 6.96–7.10 (m, 2H), 7.14–7.20 (m, 2H), 7.22–7.31 (m, 2H), 7.42–7.61 (m, 2H), 8.36 (br s, 1H, NH exch. with D2O). 13C NMR (CDCl3) ppm: 27.30 (CH2), 27.84 (CH2), 34.74 (CH2), 35.20 (CH2), 51.52 (CH2 × 2), 52.66 (CH2 × 2), 56.21 (CH2), 105.83 (CH), 115.19 (CH), 15.28 (CH), 118.59 (CH), 121.77 (C), 123.71 (CH), 124.23 (C), 126.22 (C), 126.26 (C), 127.29 (CH), 128.56 (CH), 130.58 (CH), 133.77 (C), 134.34 (C), 141.34 (C), 155.44 (C), 164.02 (C). MS (ESI): C27H28ClN5O requires m/z 473.20, found 471.20 [M+H]+. Anal. calcd for C27H28ClN5O: C, 68.42; H, 5.95; Cl, 7.48; N, 14.78. Found: C, 68.40; H, 5.94; Cl, 7.47; N, 14.76.
4.1.48. 2-Chloro-N-(4-(3,4-dichlorophenyl)piperazin-1-yl)ethyl)-1,4,5,6-tetrahydrobenzo[6,7]cyclohepta[1,2-b]pyrrole-3-carboxamide (19)
The title compound was prepared from acid 28 and 2-(4-(3,4-dichlorophenyl)piperazin-1-yl)ethyl-1-amine (37) using the general procedure IV to afford 19 as a grey solid (0.036 g, 18.46%), after FC purification (CHCl3/CH3OH 99/1), mp = 213–216 °C, 1H NMR (CDCl3) ppm: 1.90–2.10 (m, 2H), 2.30–2.40 (m, 2H), 2.63–2.68 (m, 4H), 2.73–2.80 (m, 2H), 2.85–2.95 (m, 2H), 3.00–3.18 (m, 4H), 3.45–3.58 (m, 2H), 6.06 (br s, 1H, NH exch. with D2O), 6.69–6.79 (m, 3H), 7.93–7.17 (m, 2H), 7.18–7.34 (m, 2H), 8.58 (br s, 1H, exch. with D2O). 13C NMR (CDCl3) ppm: 25.83 (CH2), 26.58 (CH2), 34.67 (CH2), 36.01 (CH2), 48.87 (CH2 × 2), 52.34 (CH2 × 2), 55.92 (CH2), 113.52 (CH), 115.25 (CH), 117.10 (CH), 118.12 (C), 121.93 (CH), 122.21 (C), 124.94 (C), 126.47 (C), 127.32 (C), 129.62 (CH × 2), 130.29 (C), 131.85 (CH), 132.65 (C), 140.96 (C), 151.52 (C), 158.06 (C). MS (ESI): C26H27Cl3N4O requires m/z 516.13; found 517.13 [M+H]+. Anal. calcd for C26H27Cl3N4O: C, 60.30; H, 5.26; Cl, 20.54; N, 10.82. Found: C, 60.33; H, 5.27; Cl, 20.55; N, 10.83.
4.1.49. N-(3-(4-Benzylpiperazin-1-yl)propyl)-2-chloro-1,4,5,6-tetrahydrobenzo[6,7]cyclohepta[1,2-b]pyrrole-3-carboxamide (20)
The title compound was prepared from acid 28 and 2-(4-benzylpiperazin-1-yl)propyl-1-amine (39) using the general procedure IV to afford 20 as a beige solid (0.083 g, 45.81%), after FC purification (CHCl3/CH3OH 95/5), mp = 169–171 °C, 1H NMR (CDCl3) δ: 1H NMR (CDCl3) ppm: 1.65–1.72 (m, 2H), 1.97–2.03 (m, 2H), 2.47–2.50 (m, 10H), 2.75–2.77 (m, 2H), 2.80–2.84 (m, 2H), 3.42–3.47 (m, 4H), 6.50 (d, 1H, Jm = 2.8 Hz), 7.08–7.11 (m, 3H), 7.17–7.19 (m, 2H), 7.24–7.26 (m, 2H), 7.41 (d, 1H, J = 7.6 Hz), 7.67 (br s, 1H, exch. with D2O), 9.18 (br s, 1H, exch. with D2O). 13C NMR (CDCl3) ppm: 25.97 (CH2), 27.35 (CH2), 27.71 (CH2), 34.84 (CH2), 38.52 (CH2), 52.82 (CH2 × 2), 53.14 (CH2 × 2), 56.74 (CH2), 63.02 (CH2), 114.75 (C), 116.42 (C), 123.56 (C), 124.13 (CH), 126.31 (CH), 126.52 (CH), 127.09 (CH), 127.24 (C), 128.22 (CH × 2), 129.29 (CH × 2), 129.40 (CH), 130.66 (C), 137.81 (C), 141.38 (C), 164.11 (C). MS (ESI): C28H33ClN4O requires m/z 476.23, found 477.23 [M+H]+. Anal. calcd for C28H33ClN4O: C, 70.50; H, 6.97; Cl, 7.43; N, 11.74. Found: C, 70.47; H, 6.95; Cl, 7.1; N, 11.73.