Formulation Strategies for High-Thermal-Conductivity Organosilicon Potting Adhesive
Abstract
1. Introduction
2. Results and Discussion
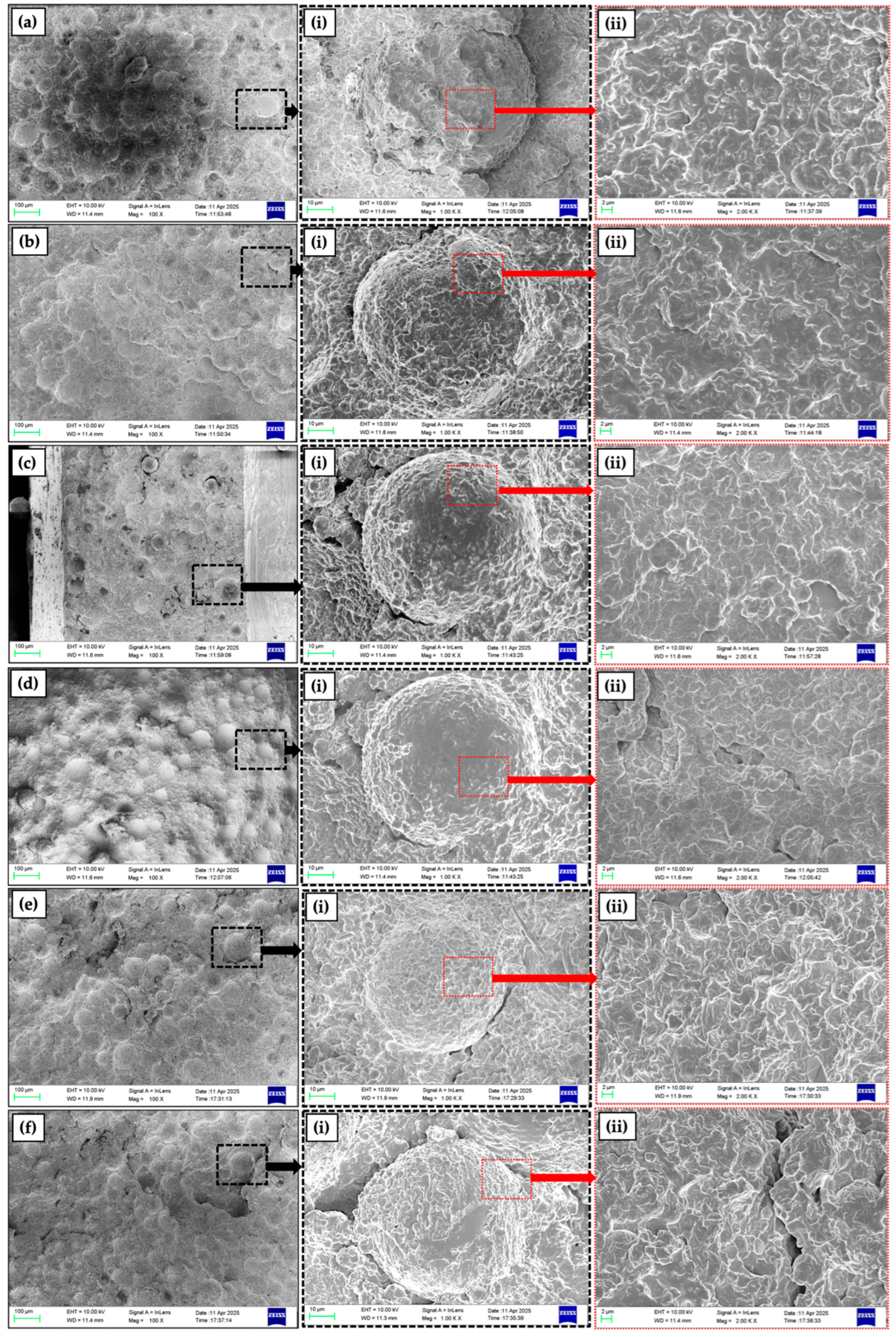
2.1. Structural Analysis
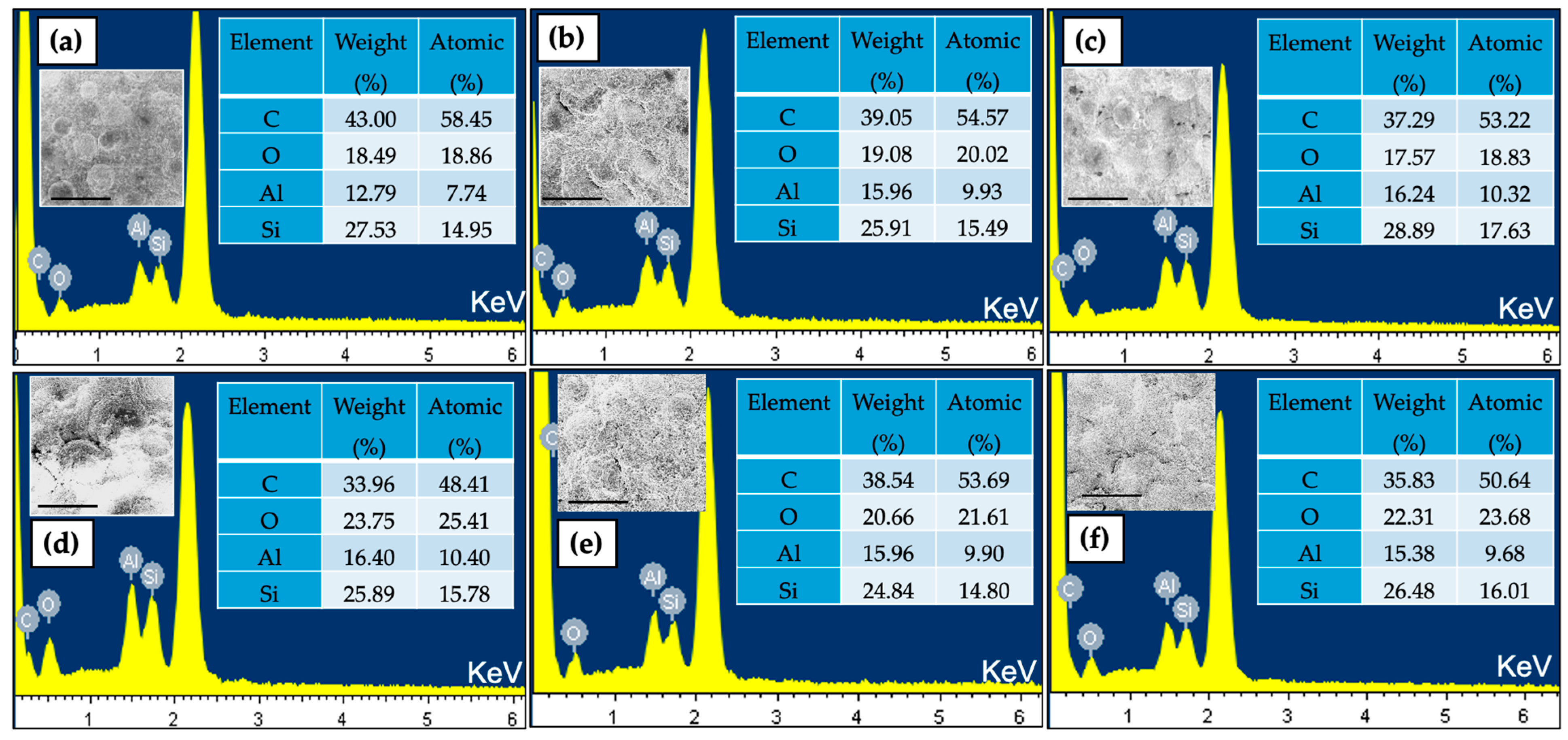
2.2. Elemental Analysis
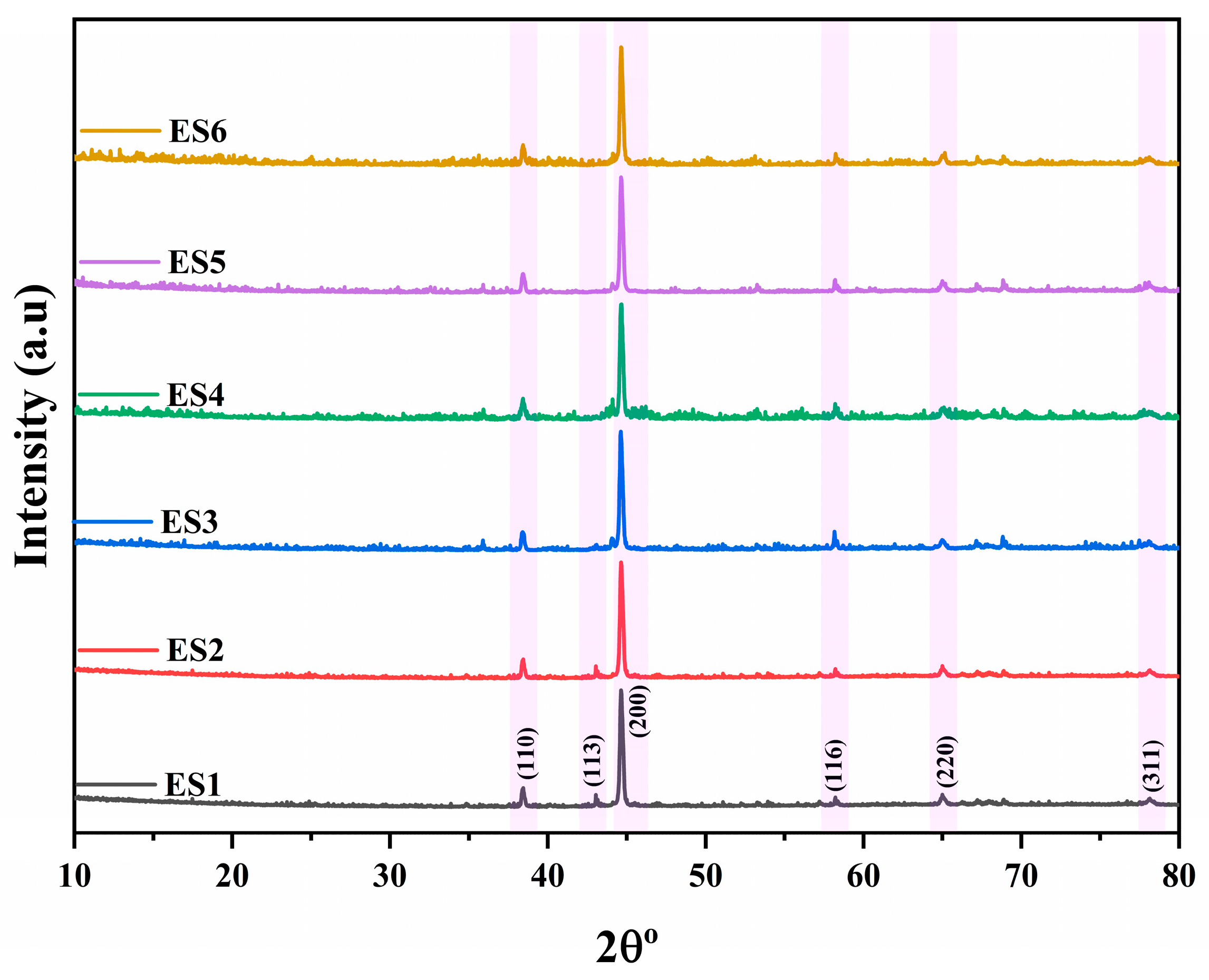
2.3. Phase Analysis
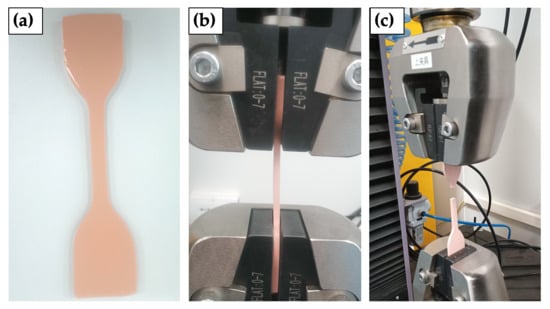
2.4. Mechanical Analysis
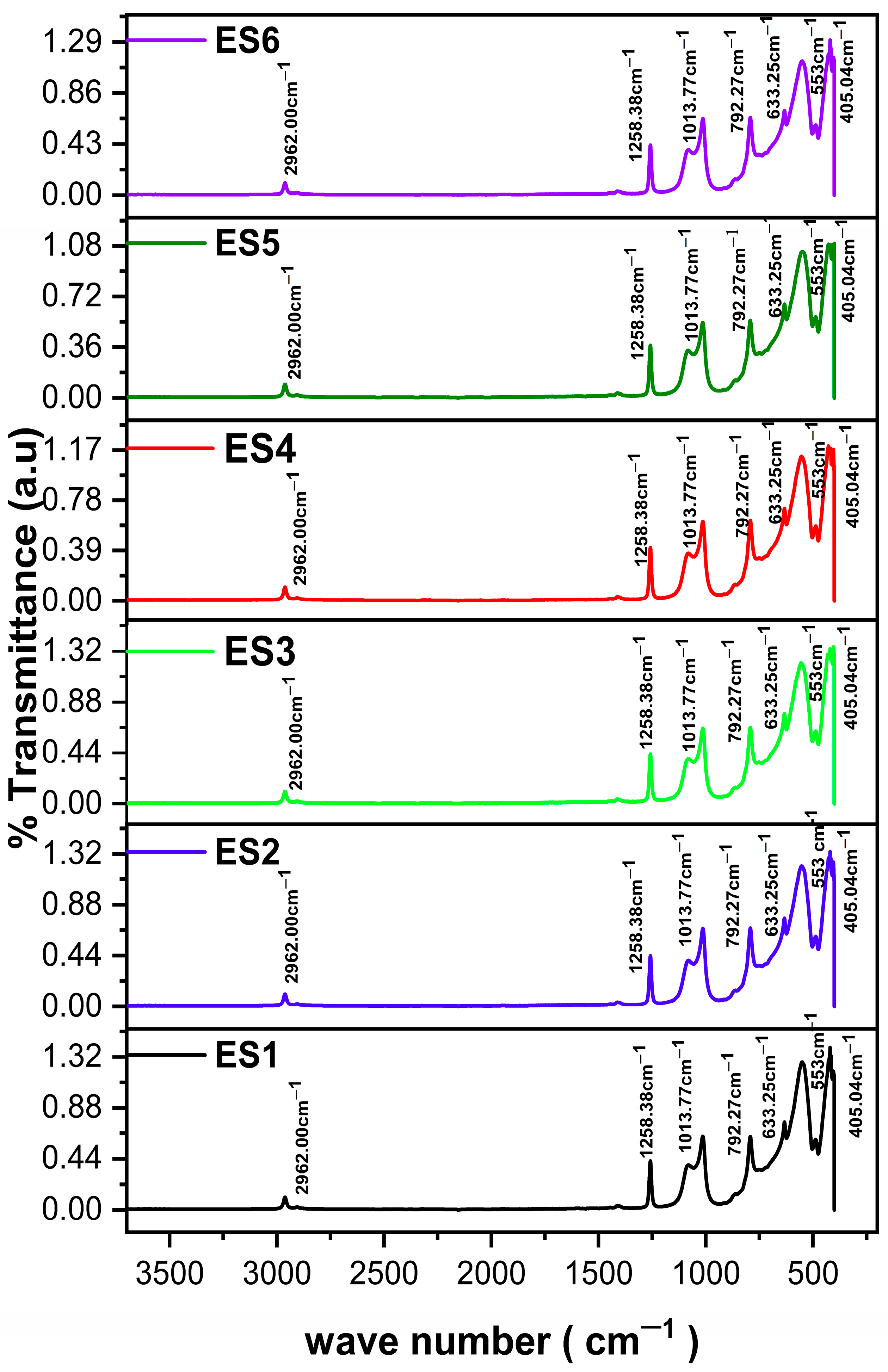
2.5. FT-IR Analysis
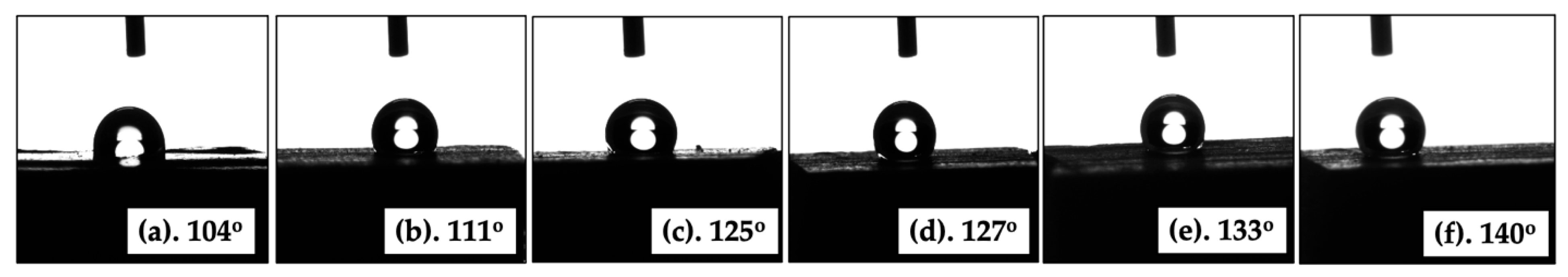
2.6. Water Contact Angle Analysis
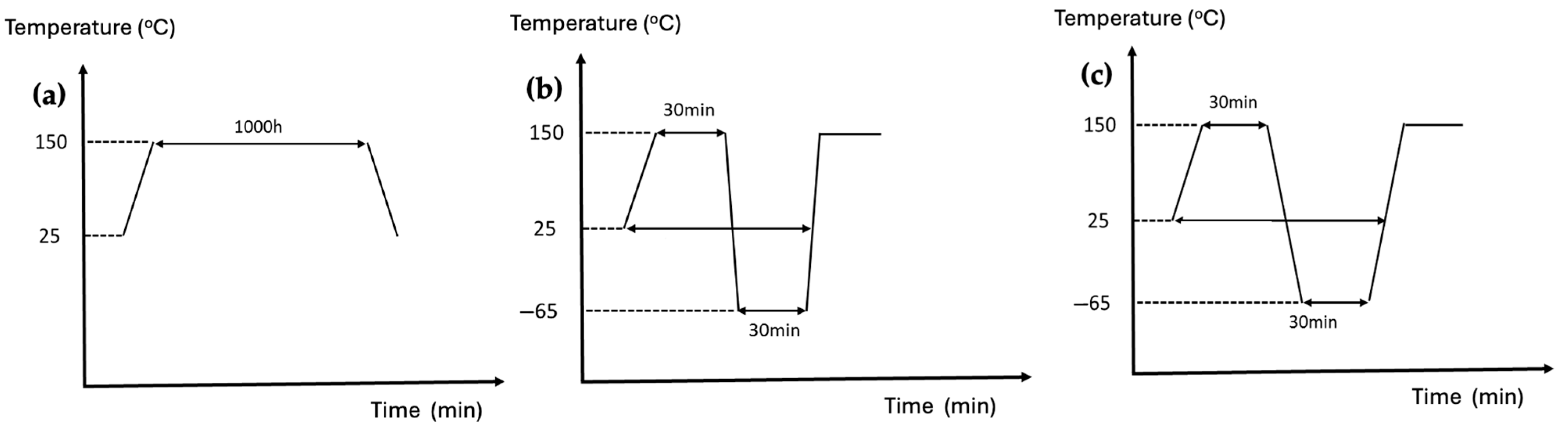
2.7. Environmental Testing
- i.
- Thermal conductivity measurements
- ii.
- High-Temperature Aging Test
- Post-curing of the silicone network, enhancing crosslink density and filler wetting.
- Matrix relaxation and void reduction, leading to improved particle–particle contact.
- Stabilization of interfacial interactions, especially in compositions with balanced LHC-2/DH37 ratios.
- iii.
- Thermal Shock Test
- iv.
- Temperature Cycling Test
- Temperature range: −50 °C to +150 °C
- Dwell time: 30 min at each temperature extreme
- Transition rate: 3 °C/min
- v.
- Discussion of Environmental Test Results
- Post-curing and polymer relaxation at elevated temperature (especially during long high-temp aging) that improve wetting between filler particles and matrix, reducing interfacial thermal resistance.
- Densification/reduction in micro voids by volatilization or stress relaxation, improving particle–particle contact.
- Thermally assisted rearrangement of filler networks (micro-scale displacement or settling) that increases the percolation and connectivity of the conductive filler network.
- Rapid thermal shock can also produce mechanical settling/compaction of fillers and local stress relaxation, giving an intermediate improvement. Temperature cycling (slower, symmetric cycling) produces only modest changes consistent with small, repeated elastic strains that do not strongly reorganize the filler network.
3. Materials and Methods
3.1. Composition of the Encapsulant
3.2. Crosslinking Agents and Chain Extenders
3.3. Properties of the Silicone Resin and Additives
3.4. Preparation Method
3.5. Analysis
4. Conclusions
- The encapsulant enhances thermal conductivity while maintaining good mechanical properties like tensile strength and elongation at break.
- The formulation allows for better shock absorption, easier disassembly, and repairability, making it suitable for use in electronic components that require heat management, like LED and solar power systems.
- The A-component and B-component are mixed at a specific ratio before application, balancing thermal conductivity and mechanical performance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jian, S. IEEE Power Electronics Society southern power electronics conference. IEEE Power Electron. Mag. 2020, 7, 99. [Google Scholar] [CrossRef]
- Cui, P.; Yu, Q.; Chen, J.; Chen, K.; Yin, P. Enhancing adhesive performance of polyvinyl alcohol with sub-nanoscale polyoxotungstate clusters under extreme conditions. Nano Res. 2025, 18, 94907126. [Google Scholar] [CrossRef]
- Iraqui, S.; Kashyap, S.S.; Rashid, M.H. NiFe2O4nanoparticles: An efficient and reusable catalyst for the selective oxidation of benzyl alcohol to benzaldehyde under mild conditions. Nanoscale Adv. 2020, 2, 5790–5802. [Google Scholar] [CrossRef]
- Wei, H.; Gao, Z.; Cao, L.; Li, K.; Yan, X.; Liu, T.; Zhu, M.; Huang, F.; Fang, X.; Lin, J. FePO4supported Rh subnano clusters with dual active sites for efficient hydrogenation of quinoline under mild conditions. Nanoscale 2023, 15, 1422–1430. [Google Scholar] [CrossRef]
- Fu, B.; Qu, X.; Song, Y.; Li, F.; Shi, H.; Lv, Y.; Wang, J.; Jin, H.; Yu, X.; Yang, Y. A polyoxotungstate/W18O49 nanocomposite with a bird-nest-like network supports high-performance electrochromic supercapacitors. Nanoscale 2025, 17, 6103–6114. [Google Scholar] [CrossRef]
- Aezid-Ul-Hassan, N.; Abdul, W.; Rohith, P.; Oliver, S.; Thomas, E.; Bahman, S. Thermal management of fuel cell-battery electric vehicles: Challenges and solutions. Appl. Energy 2025, 387, I125635. [Google Scholar] [CrossRef]
- Boehme, B.; Roellig, M.; Wolter, K.-J. Moisture-induced change of the viscoelastic material properties of adhesives for SHM sensor applications. In Proceedings of the 2010 Proceedings 60th Electronic Components and Technology Conference (ECTC), Las Vegas, NV, USA, 1–4 June 2010; IEEE: New York, NY, USA, 2010; pp. 1885–1892. [Google Scholar] [CrossRef]
- Satpathy, R.; Pamuru, V. Manufacturing of crystalline silicon solar PV modules. In Solar PV Power; Elsevier: Amsterdam, The Netherlands, 2021; pp. 135–241. [Google Scholar] [CrossRef]
- GmbH, S.H. Sealing, Gluing, Potting—Multiple Solutions for the Automotive Industry. Adhes. Adhes. 2019, 16, 14–19. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, W.; Zhao, N.; Xu, J. Electrically insulating PBO/MXene film with superior thermal conductivity, mechanical properties, thermal stability, and flame retardancy. Nat. Commun. 2023, 14, 1–9. [Google Scholar] [CrossRef]
- Broughton, W. Testing the mechanical, thermal and chemical properties of adhesives for marine environments. In Adhesives in Marine Engineering; Elsevier: Amsterdam, The Netherlands, 2012; pp. 99–154. [Google Scholar] [CrossRef]
- Faster electrical potting and sealing with new light curing adhesives. Solder. Surf. Mt. Technol. 2000, 12, 18. [CrossRef]
- Sadaf, B.K.; Nan, l.; Shenggui, C.; Jiahua, L.; Chuang, X.; Xiaohong, S. Influence of nanoparticle size on the mechanical and tribological characteristics of TiO2 reinforced epoxy composites. J. Mater. Res. Technol. 2023, 26, 6001–6015. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Z.; Li, X.; Li, J.; Sun, W.; Zhang, Y. Fully green, strong adhesion and multifunctional adhesive via construction of biomimetic mineralized structure. Chem. Eng. J. 2025, 509, 161539. [Google Scholar] [CrossRef]
- Zhang, C.; Qi, Z.; Ma, Z.; Gong, Y.; Feng, H.; Zang, J.; Du, W.; Shang, L. Sustainably sourced p-coumaric acid-derived bio-based epoxy resins: Functionalized thermosetting epoxy materials with excellent flame retardancy, ultra-high strength, toughness, and transparency. J. Ind. Eng. Chem. 2025, 148, 809–820. [Google Scholar] [CrossRef]
- Xu, Y.; Han, Y.; Li, Y.; Li, J.; Li, J.; Gao, Q. Preparation of a strong, mildew-resistant, and flame-retardant biomimetic multifunctional soy protein adhesive via the construction of an organic-inorganic hybrid multiple-bonding structure. Chem. Eng. J. 2022, 437, 135437. [Google Scholar] [CrossRef]
- Morgan, N.; Zhao, X.; Rouse, J.; Hopkins, D. Characterization of Silicone Gel for High Temperature Encapsulation in High Voltage WBG Power Modules. Int. Symp. Microelectron. 2017, 2017, 000312–000317. [Google Scholar] [CrossRef]
- Wu, Z.; Shangguan, Y.; Zhang, C.; Zheng, Q. Effects of Crosslinking and Silicone Coupling Agent on Properties of EVA Composite Hot Melt Adhesive. Polymers 2021, 13, 4101. [Google Scholar] [CrossRef] [PubMed]
- Krump, H.; Šimor, M.; Hudec, I.; Jaššo, M.; Luyt, A.S. Adhesion strength study between plasma-treated polyester fibres and a rubber matrix. Appl. Surf. Sci. 2005, 240, 268–274. [Google Scholar] [CrossRef]
- Lee, H.K.; Hausmann, L.R. Structural repair and strengthening of damaged RC beams with sprayed FRP. Compos. Struct. 2004, 63, 201–209. [Google Scholar] [CrossRef]
- Slaitas, J.; Valivonis, J. Technological peculiarities of strengthening RC members with prestressed FRP laminates. Compos. Struct. 2022, 290, 115526. [Google Scholar] [CrossRef]
- Custódio, J.; Cabral-Fonseca, S. Advanced fiber-reinforced polymer (FRP) composites for the rehabilitation of timber and concrete structures: Assessing strength and durability. In Advanced Fiber-Reinforced Polymer (FRP) Composites for Structural Applications; Elsevier: Amsterdam, The Netherlands, 2023; pp. 725–809. [Google Scholar] [CrossRef]
- Recent Revisions to Acceptance Criteria for Concrete and Masonry Strengthening Using Externally Bonded FRP Systems (AC125). In SP-275: Fiber-Reinforced Polymer Reinforcement for Concrete Structures 10th International Symposium; American Concrete Institute: Baltimore, MD, USA, 2011. [CrossRef]
- Mitachi, S.; Kimura, K. Novel highly moisture resistant optical adhesives and their high power resistivity. In Proceedings of the 2014 IEEE 64th Electronic Components and Technology Conference (ECTC), Lake Buena Vista, FL, USA, 27–30 May 2014; IEEE: New York, NY, USA, 2014; pp. 230–235. [Google Scholar] [CrossRef]
- Liu, Y.; Li, J. A protocol to further improve the thermal conductivity of silicone-matrix thermal interface material with nano-fillers. Thermochim. Acta 2022, 708, 179136. [Google Scholar] [CrossRef]
- Matt, C.F.; Cruz, M.E. Effective Thermal Conductivity of Composite Materials with 3-D Microstructures and Interfacial Thermal Resistance. Numer. Heat Transf. Part A Appl. 2007, 53, 577–604. [Google Scholar] [CrossRef]
- Mumtaz, N.; Mumtaz, A.; Farooq, Z.; Guo, Q.; Nisa, F.U.; Li, Y.; Artiaga, R. Fillers and Methods to Improve the Effective (Out-Plane) Thermal Conductivity of Polymeric Thermal Interface Materials—A Review. Preprints, 2023; ahead of print. [Google Scholar] [CrossRef]
- Liu, T.; Pei, C.; Zeng, R.; Zhang, J.; Hejna, A.; Kuang, T. Achieving High Thermal Conductivity, Good Electrical Insulation, and Balanced Mechanical Properties in Biodegradable Polymer Composites with Low Filler Content. ACS Appl. Polym. Mater. 2023, 5, 8062–8072. [Google Scholar] [CrossRef]
- Janík, R.; Kohutiar, M.; Dubec, A.; Eckert, M.; Moricová, K.; Pajtášová, M.; Ondrušová, D.; Krbata, M. DMA Analysis of Plasma Modified PVC Films and the Nature of Initiated Surface Changes. Materials 2022, 15, 4658. [Google Scholar] [CrossRef] [PubMed]
- GB/T 528-2009; Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties. National Standard of the People’s Republic of China: Beijing, China, 2009. Available online: https://www.chinesestandard.net/PDF.aspx/GBT528-2009 (accessed on 29 September 2025).

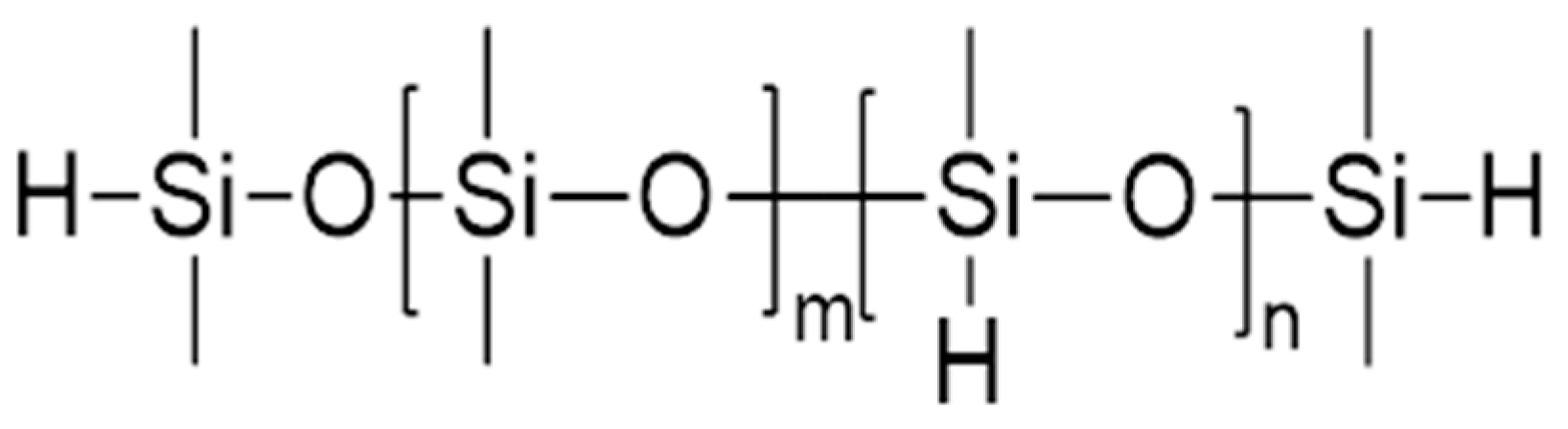

| Sample | Tensile Strength (MPa) | Elongation at Break (%) | Max Force (N) | Cross-Sectional Area (mm2) | Modulus (MPa) | Material Behavior |
|---|---|---|---|---|---|---|
| 1 | 1.86 ± 0.05 | 4644 ± 50 | 38.86 | 20.88 | 0.04 | Highly elastic, low strength |
| 2 | 2.75 ± 0.10 | 3645 ± 100 | 55.40 | 20.16 | 0.08 | Moderate strength, less elastic |
| 3 | 2.56 ± 0.15 | 3744 ± 400 | 49.72 | 19.60 | 0.07 | Balanced properties, high variability |
| 4 | 3.32 ± 0.35 | 2014 ± 380 | 77.57 | 20.88 | 0.16 | High strength, low elongation |
| 5 | 3.10 ± 0.15 | 2510 ± 100 | 70.67 | 21.66 | 0.12 | Optimal balance |
| 6 | 2.79 ± 0.22 | 1920 ± 220 | 63.42 | 21.09 | 0.15 | Reduced elasticity, inconsistent |
| Sample | LHC-2 (B-Side) | DH37 (B-Side) | Ratio LHC-2/DH37 |
|---|---|---|---|
| ES1 | 4.17 | 85.43 | 1:20.5 |
| ES2 | 2.78 | 113.93 | 1:41 |
| ES3 | 1.61 | 134.80 | 1:83.7 |
| ES4 | 1.19 | 146.52 | 1:123.1 |
| ES5 | 0.93 | 151.85 | 1:163.3 |
| ES6 | 0.76 | 155.34 | 1:204.4 |
| Peak Position (cm−1) | Intensity | Functional Group/Vibration | Interpretation |
|---|---|---|---|
| 405.04 | 1.176 | Si–O bending/lattice mode | Indicative of dense siloxane framework or silica domain vibrations |
| 428.23 | 1.202 | Si–O bending | Strong siloxane backbone presence |
| 486.67 | 0.579 | Si–O–Si bending mode | Crosslinking in the siloxane network |
| 553.90 | 1.123 | Si–O rocking/bending | Associated with cage-like or highly ordered Si–O structures |
| 633.25 | 0.717 | Si–C or Si–O–Si framework | Methyl-modified polysiloxane. Possibly related to denser crosslinked siloxane structures. |
| 792.27 | 0.625 | Si–C stretching/rocking | Presence of –CH3 groups on silicon |
| 1013.77 | 0.615 | Si–O–Si-O-Si asymmetric stretching | Main signature of the siloxane backbone (network formation). Indicates more crosslinked or cage-like structures |
| 1258.38 | 0.413 | Si–CH3 bending | Confirms methyl-modified polysiloxane (hydrophobic nature). Methyl group bending in polysiloxane |
| 2962.00 | 0.106 | C–H asymmetric stretching (–CH3, –CH2–) | From organic groups, weak due to low organic content |
| Environmental Test | Unit | ES1 | ES2 | ES3 | ES4 | ES5 | ES6 |
|---|---|---|---|---|---|---|---|
| Thermal Conductivity | (W/(m·K) | 3.111 | 3.151 | 3.212 | 3.203 | 3.184 | 3.167 |
| Temperature cycling | (W/(m·K) | 3.125 | 3.170 | 3.250 | 3.234 | 3.205 | 3.188 |
| Temperature shock | (W/(m·K) | 3.222 | 3.271 | 3.404 | 3.341 | 3.311 | 3.302 |
| High-temperature aging | (W/(m·K) | 3.315 | 3.373 | 3.512 | 3.444 | 3.418 | 3.398 |
| (a) | ||||||||||||
| Composition | Component A | Component B | ||||||||||
| Base polymer | 0.5–10 parts by weight | 0.5–10 parts by weight | ||||||||||
| Silicone resin | 0.15–10 parts by weight | 0.15–10 parts by weight | ||||||||||
| Plasticizer | 0.5–5 parts by weight | 0.5–5 parts by weight | ||||||||||
| Color paste | 0.01–0.2 parts by weight | - | ||||||||||
| Crosslinking agent | - | 0.1–10 parts by weight | ||||||||||
| Thermal conductive filler | 70–120 parts by weight | 70–120 parts by weight | ||||||||||
| Filler treatment agent | 2–8 parts by weight | - | ||||||||||
| Chain extender | - | 0.1–10 parts by weight | ||||||||||
| Catalyst | 0.1–2 parts by weight | - | ||||||||||
| Crosslinking inhibitor | - | 0.01–1 part by weight | ||||||||||
| (b) | ||||||||||||
| Composition | ES1 | ES2 | ES3 | ES4 | ES5 | ES6 | ||||||
| A | B | A | B | A | B | A | B | A | B | A | B | |
| S15 | 42.4 | 42.4 | 42.4 | 42.4 | 42.4 | 42.4 | ||||||
| Vi1305 | 3.6 | 3.6 | 3.6 | 3.6 | 3.6 | 3.6 | ||||||
| RMS-35D | 60 | 60 | 60 | 60 | 60 | 60 | ||||||
| 1657R | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | ||||||
| EL-G302 | 1055 | 895 | 1046 | 1154 | 1085 | 1295 | 1043 | 1457 | 1040 | 1500 | 1044 | 1536 |
| LHC-2 | 4.17 | 2.78 | 1.61 | 1.19 | 0.93 | 0.76 | ||||||
| DH37 | 85.43 | 113.93 | 134.8 | 146.52 | 151.85 | 155.34 | ||||||
| PT-3000 | 7.92 | 7.92 | 7.92 | 7.92 | 7.92 | 7.92 | ||||||
| YZJ-1 | 0.216 | 0.216 | 0.216 | 0.216 | 0.216 | 0.216 | ||||||
| m(EL-G302) = 90.5 wt%; n(SiH)/n(SiVi) = 1.1; n(LHC-2)/n(DH37) = 1/1 | m(EL-G302) = 90.5 wt%; n(SiH)/n(SiVi) = 1.1; n(LHC-2)/n(DH37) = 1/2 | m(EL-G302) = 90.5 wt%; n(SiH)/n(SiVi) = 1.1. n(LHC-2)/n(DH37) = 1/4 | m(EL-G302) = 90.5 wt%; n(SiH)/n(SiVi) = 1.1; n(LHC-2)/n(DH37) = 1/6 | m(EL-G302) = 90.5 wt%; n(SiH)/n(SiVi) = 1.1; n(LHC-2)/n(DH37) = 1/8 | m(EL-G302) = 90.5 wt%; n(SiH)/n(SiVi)= 1.1; n(LHC2)/n(DH37) = 1/10 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Khan, S.B.; Zhang, Z.; Wang, W. Formulation Strategies for High-Thermal-Conductivity Organosilicon Potting Adhesive. Molecules 2025, 30, 4043. https://doi.org/10.3390/molecules30204043
Chen L, Khan SB, Zhang Z, Wang W. Formulation Strategies for High-Thermal-Conductivity Organosilicon Potting Adhesive. Molecules. 2025; 30(20):4043. https://doi.org/10.3390/molecules30204043
Chicago/Turabian StyleChen, Limin, Sadaf Bashir Khan, Zhengjun Zhang, and Weipeng Wang. 2025. "Formulation Strategies for High-Thermal-Conductivity Organosilicon Potting Adhesive" Molecules 30, no. 20: 4043. https://doi.org/10.3390/molecules30204043
APA StyleChen, L., Khan, S. B., Zhang, Z., & Wang, W. (2025). Formulation Strategies for High-Thermal-Conductivity Organosilicon Potting Adhesive. Molecules, 30(20), 4043. https://doi.org/10.3390/molecules30204043