Synthesis of Alkyl α-Amino-benzylphosphinates by the Aza-Pudovik Reaction; The Preparation of the Butyl Phenyl-H-phosphinate Starting P-Reagent
Abstract
1. Introduction
2. Results and Discussion
2.1. A Study on the Preparation of Butyl Phenyl-H-phosphinate
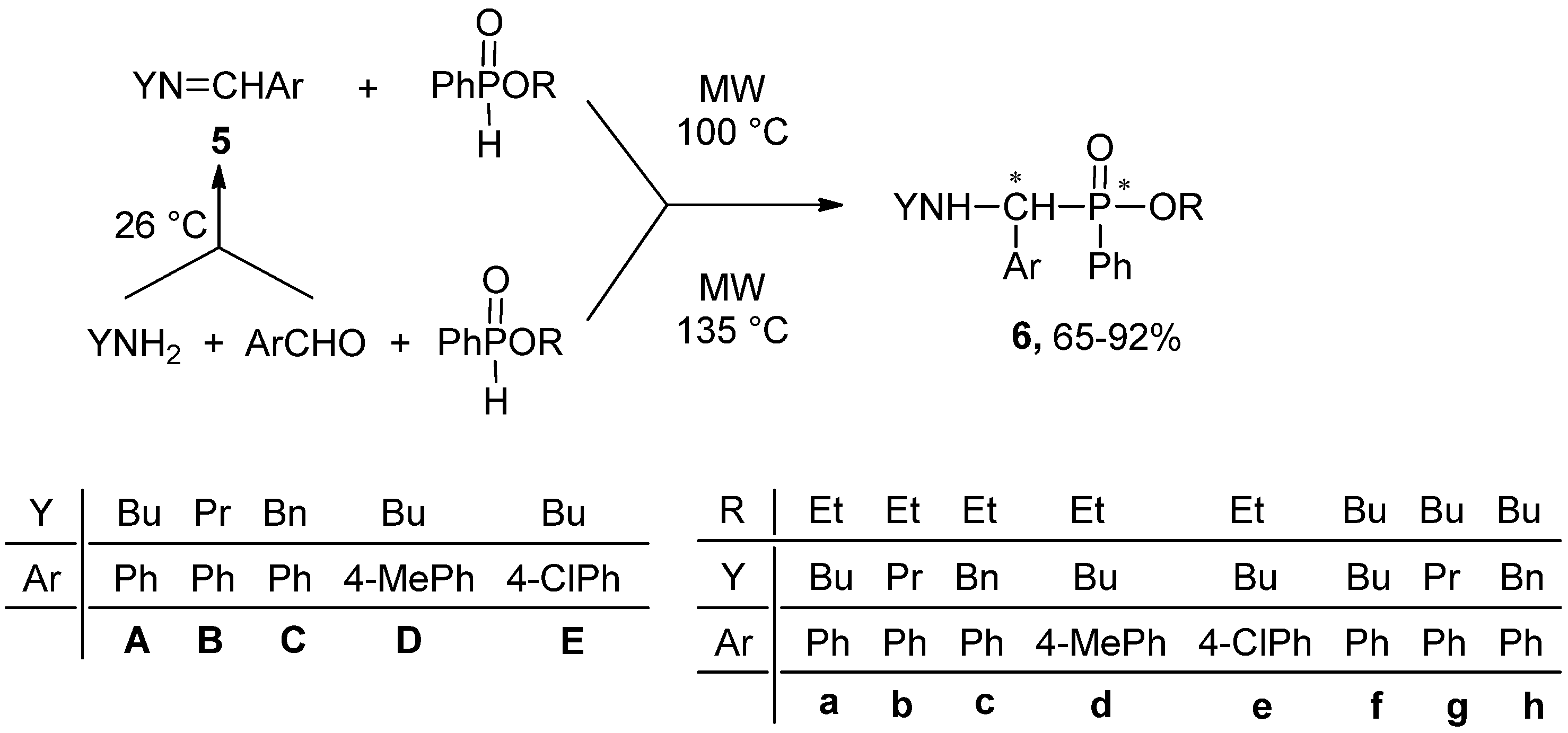
2.2. Synthesis of Alkyl (α-Alkylamino-arylmethyl-)phenylphosphinates
2.3. Cytotoxic Activity of a Few of Alkyl (α-Alkylamino-arylmethyl-)phenylphosphinates
3. Materials and Methods (Experiment)
3.1. General
3.2. The Preparation of Butyl-phenyl-H-phosphinates
3.2.1. Alkylating Esterification
3.2.2. MW-Assisted Esterification
3.2.3. Thermal Esterification
3.3. The Preparation of Alkoxy α-Alkylamino-benzyl-phenylphosphinates
3.3.1. General Procedure for the Synthesis of Alkoxy α-Alkylamino-benzyl-phenylphosphinates (6a–h) by the aza-Pudovik Reaction
3.3.2. General Procedure for the Synthesis of Alkoxy α-Alkylamino-benzyl-phenylphosphinates 6a and 6f by the Kabachnik–Fields Condensation
3.4. Bioactivity Studies
Cell Viability Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kukhar, V.P.; Hudson, H.R. (Eds.) Aminophosphonic and Aminophosphinic Acids: Chemistry and Biological Activity; Wiley: Chichester, UK, 2000. [Google Scholar]
- Grembecka, J.; Mucha, A.; Cierpicki, T.; Kafarski, P. The most potent organophosphorus inhibitors of leucine aminopeptidase. Structure-based design, chemistry, and activity. J. Med. Chem. 2003, 46, 2641–2655. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Royers, C.J.; Fisher, A.J.; Toney, M. Aminophosphonate inhibitors of dialkylglycine decarboxylase: structural basis for slow binding inhibition. Biochemistry 2002, 41, 12320–12328. [Google Scholar] [CrossRef]
- Abdou, M.M. Synopsis of recent synthetic methods and biological applications of phosphinic acid derivatives. Tetrahedron 2020, 76, 131251. [Google Scholar] [CrossRef]
- Rodriguez, J.B.; Gallo-Rodriguez, C. The Role of the Phosphorus Atom in Drug Design. ChemMedChem 2019, 14, 190. [Google Scholar] [CrossRef]
- Sevrain, C.M.; Berchel, M.; Couthon, H.; Jaffres, P.-A. Phosphonic acid: Preparation and applications. Beilstein J. Org. Chem. 2017, 13, 2186–2213. [Google Scholar] [CrossRef]
- Mucha, A.; Kafarski, P.; Berlicki, L. Remarkable Potential of the α-Aminophosphonate/Phosphinate Structural Motif in Medicinal Chemistry. J. Med. Chem. 2011, 54, 5955–5980. [Google Scholar] [CrossRef] [PubMed]
- Kafarski, P.; Lejczak, B. Aminophosphonic Acids of Potential Medical Importance. Curr. Med. Chem. Anti Cancer Agents 2001, 1, 301–312. [Google Scholar] [CrossRef]
- Kang, S.-U.; Shi, Z.-D.; Worthy, K.M.; Bindu, L.K.; Dharmawardana, P.G.; Choyke, S.J.; Bottaro, D.P.; Fisher, R.J.; Burke, T.R., Jr. Examination of phosphoryl-mimicking functionalities within a macrocyclic Grb2 SH2 domain-binding platform. J. Med. Chem. 2005, 48, 3945–3948. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Robbins, B.L.; Srinivas, R.V.; Kim, C.; Bischofberger, N.; Fridland, A. Anti-Human Immunodeficiency Virus Activity and Cellular Metabolism of a Potential Prodrug of the Acyclic Nucleoside Phosphonate 9-R-(2-Phosphonomethoxypropyl)adenine (PMPA), Bis(isopropyloxymethylcarbonyl)PMPA. Antimicrob. Agents Chemother. 1998, 42, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Lassaux, P.; Hamel, M.; Gulea, M.; Delbruck, H.; Mercuri, P.S.; Horsfall, L.; Dehareng, D.; Kupper, M.; Frere, J.-M.; Hoffmann, K.; et al. Mercaptophosphonate Compounds as Broad-Spectrum Inhibitors of the Metallo-β-lactamases. J. Med. Chem. 2010, 53, 4862–4876. [Google Scholar] [CrossRef] [PubMed]
- Haemers, T.; Wiesner, J.; Van Poecke, S.; Goeman, J.; Henschker, D.; Beck, E.; Jomaa, H.; Van Calenbergh, S. Synthesis of α-substituted fosmidomycin analogues as highly potent Plasmodium falciparum growth inhibitors. Bioorg. Med. Chem. Lett. 2006, 16, 1888–1891. [Google Scholar] [CrossRef] [PubMed]
- Maryanoff, B.E. Inhibitors of Serine Proteases as Potential Therapeutic Agents: The Road from Thrombin to Tryptase to Cathepsin G. J. Med. Chem. 2004, 47, 769–787. [Google Scholar] [CrossRef] [PubMed]
- Varga, P.R.; Keglevich, G. Synthesis of α-aminophosphonates and related derivatives; the last decade of the Kabachnik–Fields reaction. Molecules 2021, 26, 2511. [Google Scholar] [CrossRef] [PubMed]
- Naydenova, E.D.; Todorov, P.T.; Troev, K.D. Recent synthesis of aminophosphonic acids as potential biological importance. Amino Acids 2010, 38, 23–30. [Google Scholar] [CrossRef]
- Shastri, R.A. Review on the synthesis of α-aminophosphonate derivatives. Chem. Sci. Trans. 2019, 8, 359–367. [Google Scholar] [CrossRef]
- Sun, P.; Hu, Z.; Huang, Z. Gallium triiodide catalyzed organic reaction: A convenient synthesis of α-amino phosphonates. Synth. Commun. 2004, 34, 4293–4299. [Google Scholar] [CrossRef]
- Xu, F.; Luo, Y.; Deng, M.; Shen, Q. One-pot synthesis of α-amino phosphonates using samarium diiodide as a catalyst precursor. Eur. J. Org. Chem. 2003, 35, 4728–4730. [Google Scholar] [CrossRef]
- Ranu, B.C.; Hajra, A.; Jana, U. General procedure for the synthesis of α-amino phosphonates from aldehydes and ketones using indium(iii) chloride as a catalyst. Org. Lett. 1999, 1, 1141–1143. [Google Scholar] [CrossRef]
- Lee, S.-G.; Park, J.H.; Kang, J.; Lee, J.K. Lanthanide triflate-catalyzed three component synthesis of α-aminophosphonates in ionic liquids. A catalyst reactivity and reusability study. Chem. Commun. 2001, 1698–1699. [Google Scholar] [CrossRef]
- Lee, S.-G.; Lee, J.K.; Song, C.E.; Kim, D.-C. Microwave-assisted Kabachnik-Fields reaction in ionic liquid. Bull. Korean Chem. Soc. 2002, 23, 667–668. [Google Scholar] [CrossRef]
- Matveeva, E.D.; Podrugina, T.A.; Tishkovskaya, E.V.; Tomilova, L.G.; Zefirov, N.S. A Novel Catalytic Three-Component Synthesis (Kabachnick-Fields Reaction) of α-Aminophosphonates from Ketones. Synlett 2003, 2003, 2321–2324. [Google Scholar] [CrossRef]
- Bhagat, S.; Chakraborti, A.K. An Extremely Efficient Three-Component Reaction of Aldehydes/Ketones, Amines, and Phosphites (Kabachnik−Fields Reaction) for the Synthesis of α-Aminophosphonates Catalyzed by Magnesium Perchlorate. J. Org. Chem. 2007, 72, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Sun, W.; Xia, H.-G.; Sun, X. A facile and highly efficient route to α-amino phosphonates via three-component reactions catalyzed by Mg(ClO4)2 or molecular iodine. Org. Biomol. Chem. 2006, 4, 1663–1666. [Google Scholar] [CrossRef] [PubMed]
- Firouzabadi, H.; Iranpoor, N.; Sobhani, S. Metal Triflate-Catalyzed One-Pot Synthesis of α-Aminophosphonates from Carbonyl Compounds in the Absence of Solvent. Synthesis 2004, 2004, 2692–2696. [Google Scholar] [CrossRef]
- Ghosh, R.; Maiti, S.; Chakraborty, A.; Maiti, D.K. In(OTf)3 catalysed simple one-pot synthesis of α-amino phosphonates. J. Mol. Catal. A 2004, 210, 53–57. [Google Scholar] [CrossRef]
- Bhattacharya, A.K.; Kaur, T. An Efficient One-Pot Synthesis of a-Amino Phosphonates Catalyzed by Bismuth Nitrate Pentahydrate. Synlett 2007, 2007, 745–748. [Google Scholar] [CrossRef]
- Zhan, Z.-P.; Li, J.-P. Bismuth(III) Chloride–Catalyzed Three-Component Coupling: Synthesis of α-Amino Phosphonates. Synth. Commun. 2005, 35, 2501–2508. [Google Scholar] [CrossRef]
- Wu, J.; Sun, W.; Wang, W.-Z.; Xiu, H.-G. A Highly Efficient Catalyst FeCl3 in the Synthesis of α-Amino Phosphonates via Three-component Reactions. Chin. J. Chem. 2006, 24, 1054–1057. [Google Scholar] [CrossRef]
- Xu, F.; Luo, Y.; Wu, J.; Shen, Q.; Chen, H. Facile one-pot synthesis of α-amino phosphonates using lanthanide chloride as catalyst. Heteroat. Chem. 2006, 17, 389–392. [Google Scholar] [CrossRef]
- Ravinder, K.; Vijender Reddy, A.; Krishnaiah, P.; Venkataramana, G.; Niranjan Reddy, V.L.; Venkateswarlu, Y. CAN Catalyzed One-Pot Synthesis of α-Amino Phosphonates from Carbonyl Compounds. Synth. Commun. 2004, 34, 1677–1683. [Google Scholar] [CrossRef]
- Kafarski, P.; Gorniak, M.G.V.; Andrasiak, I. Kabachnik-Fields Reaction Under Green Conditions—A Critical Overview. Curr. Green Chem. 2015, 2, 218–222. [Google Scholar] [CrossRef]
- Keglevich, G.; Szekrényi, A. Eco-friendly accomplishment of the extended Kabachnik–Fields reaction; a solvent- and catalyst-free microwave-assisted synthesis of α-aminophosphonates and α-aminophosphine oxides. Lett. Org. Chem. 2008, 5, 616–622. [Google Scholar] [CrossRef]
- Varga, P.R.; Karaghiosoff, K.; Sári, É.V.; Simon, A.; Hegedűs, L.; Drahos, L.; Keglevich, G. New N-acyl-, as well as N-phosphonoylmethyl- and N-phoshinoylmethyl-α-amino-benzylphosphonates by acylation and a tandem Kabachnik–Fields protocol. Org. Biomol. Chem. 2023, 21, 1709–1718. [Google Scholar] [CrossRef]
- Varga, P.R.; Oláhné Szabó, R.; Dormán, G.; Bősze, S.; Keglevich, G. Cytotoxyc activity of α-aminophosphonic derivatives coming from the tandem Kabachnik–Fields reaction and acylation. Pharmaceuticals 2023, 16, 506. [Google Scholar] [CrossRef] [PubMed]
- Bálint, E.; Tóth, R.E.; Keglevich, G. Synthesis of alkyl α-aminomethyl-phenylphosphinates and N,N-bis(alkoxyphenylphosphinylmethyl)amines by the microwave-assisted Kabachnik–Fields reaction. Heteroat. Chem. 2016, 27, 323–335. [Google Scholar] [CrossRef]
- Kiss, N.Z.; Henyecz, R.; Keglevich, G. Continuous flow esterification of a H-phosphinic acid, and transesterification of H-phosphinates and H-phosphonates under microwave conditions. Molecules 2020, 25, 719. [Google Scholar] [CrossRef] [PubMed]
- Kiss, N.Z.; Keglevich, G. Microwave-assisted direct esterification of cyclic phosphinic acids in the presence of ionic liquids. Tetrahedron Lett. 2016, 57, 971–974. [Google Scholar] [CrossRef]
- Ullah, Z.; Khan, A.S.; Muhammad, N.; Ullah, R.; Alqahtani, A.S.; Shah, S.N.; Ghanem, O.B.; Bustam, M.A.; Man, Z. A review on ionic liquids as perspective catalysts in transesterification of different feedstock oil into biodiesel. J. Mol. Liq. 2018, 266, 673–686. [Google Scholar] [CrossRef]



| Entry | 1 (mmol) | T (°C) | t (h) | Conversion (%) | Composition (%) [a] | ||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | |||||
| 1 | 0.70 | 160 | 1.5 | 100 | – | 100 [b] | – |
| 2 | 1.40 | 160 | 2.5 | 86 | 14 | 77 [c] | 9 |
| 3 | 1.40 | 180 | 1.5 | 78 | 22 | 69 | 9 |
| 4 | 2.80 | 185 | 3.5 | 59 | 41 | 54 | 5 |
| 5 | 2.80 | 200 | 4 | 50 | 50 | 45 | 5 |
| Entry | 1 (mmol) | BuOH (Equiv.) | T (°C) | t (h) | Conversion (%) | Composition (%) [a] | ||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 4 | ||||||
| 1 | 1.4 | 15 | Δ | 36 | 98 | 2 | 97 | 1 |
| 2 | 14.1 | 12 | Δ | 48 | 95 [b] | 5 | 86 | 9 |
| 3 | 2.8 | 15 | 110 [c] (oil bath) | 72 | 100 | 100 | – | |
| 4 | 14.1 | 12 | Δ [c] | 60 | 100 [d] | 0 | 100 | – |
| 5 | 3.52 | 12 | Δ [c,e] | 30 | 100 [f] | 0 | 100 | – |
| δP (CDCl3) | δP [Lit.] | [M + H] | HRMS | |
|---|---|---|---|---|
| PhP(O)(OBu)H 2 | 24.8 | 25.1 [36] | 199 | [M + H]found = 190.0888, C10H16O2P requires 199.0888 |
| PhHP(O)OP(O)PhH 3 | −0.90 | – | 267 | |
| PhP(O)(OBu)OH 4 | 20.9 | – | 215 |
| PANC-1 | U266 | |||||
|---|---|---|---|---|---|---|
| Compound | 1 μM | 10 μM | 100 μM | 1 μM | 10 μM | 100 μM |
| 6a | 0.98 ± 0.03 | 1.03 ± 0.12 | 1.20 ± 0.04 x | 1.03 ± 0.08 | 0.95 ± 0.07 | 0.94 ± 0.06 |
| 6b | 0.95 ± 0.13 | 1.17 ± 0.19 | 0.95 ± 0.13 | 1.10 ± 0.05 | 1.15 ± 0.02 x | 0.94 ± 0.07 |
| 6c | 1.04 ± 0.37 | 1.00 ± 0.24 | 1.08 ± 0.14 | 0.73 ± 0.25 z | 1.05 ± 0.12 | 1.06 ± 0.05 x |
| 6d | 1.16 ± 0.11 | 0.97 ± 0.07 | 1.15 ± 0.12 | 1.09 ± 0.08 | 1.18 ± 0.08 x | 0.99 ± 0.02 |
| 6e | 1.05 ± 0.27 | 1.07 ± 0.10 | 1.20 ± 0.17 | 1.13 ± 0.02 x | 1.16 ± 0.03 x | 0.97 ± 0.01 |
| 6f | 0.96 ± 0.02 | 0.93 ± 0.12 | 0.30 ± 0.15 z | 1.06 ± 0.04 | 0.81 ± 0.09 y | 0.91 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajusz, B.; Nagy, D.; Tóth, R.; Szalai, Z.; Gömöry, Á.; Takács, A.; Kőhidai, L.; Keglevich, G. Synthesis of Alkyl α-Amino-benzylphosphinates by the Aza-Pudovik Reaction; The Preparation of the Butyl Phenyl-H-phosphinate Starting P-Reagent. Molecules 2025, 30, 339. https://doi.org/10.3390/molecules30020339
Bajusz B, Nagy D, Tóth R, Szalai Z, Gömöry Á, Takács A, Kőhidai L, Keglevich G. Synthesis of Alkyl α-Amino-benzylphosphinates by the Aza-Pudovik Reaction; The Preparation of the Butyl Phenyl-H-phosphinate Starting P-Reagent. Molecules. 2025; 30(2):339. https://doi.org/10.3390/molecules30020339
Chicago/Turabian StyleBajusz, Bence, Dorka Nagy, Regina Tóth, Zsuzsanna Szalai, Ágnes Gömöry, Angéla Takács, László Kőhidai, and György Keglevich. 2025. "Synthesis of Alkyl α-Amino-benzylphosphinates by the Aza-Pudovik Reaction; The Preparation of the Butyl Phenyl-H-phosphinate Starting P-Reagent" Molecules 30, no. 2: 339. https://doi.org/10.3390/molecules30020339
APA StyleBajusz, B., Nagy, D., Tóth, R., Szalai, Z., Gömöry, Á., Takács, A., Kőhidai, L., & Keglevich, G. (2025). Synthesis of Alkyl α-Amino-benzylphosphinates by the Aza-Pudovik Reaction; The Preparation of the Butyl Phenyl-H-phosphinate Starting P-Reagent. Molecules, 30(2), 339. https://doi.org/10.3390/molecules30020339









