Hydrogen-Bonded Di(hydroperoxy)alkane Adducts of the Type Cy3P=O·(HOO)2CHR (R = Alkyl)
Abstract
1. Introduction
General Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
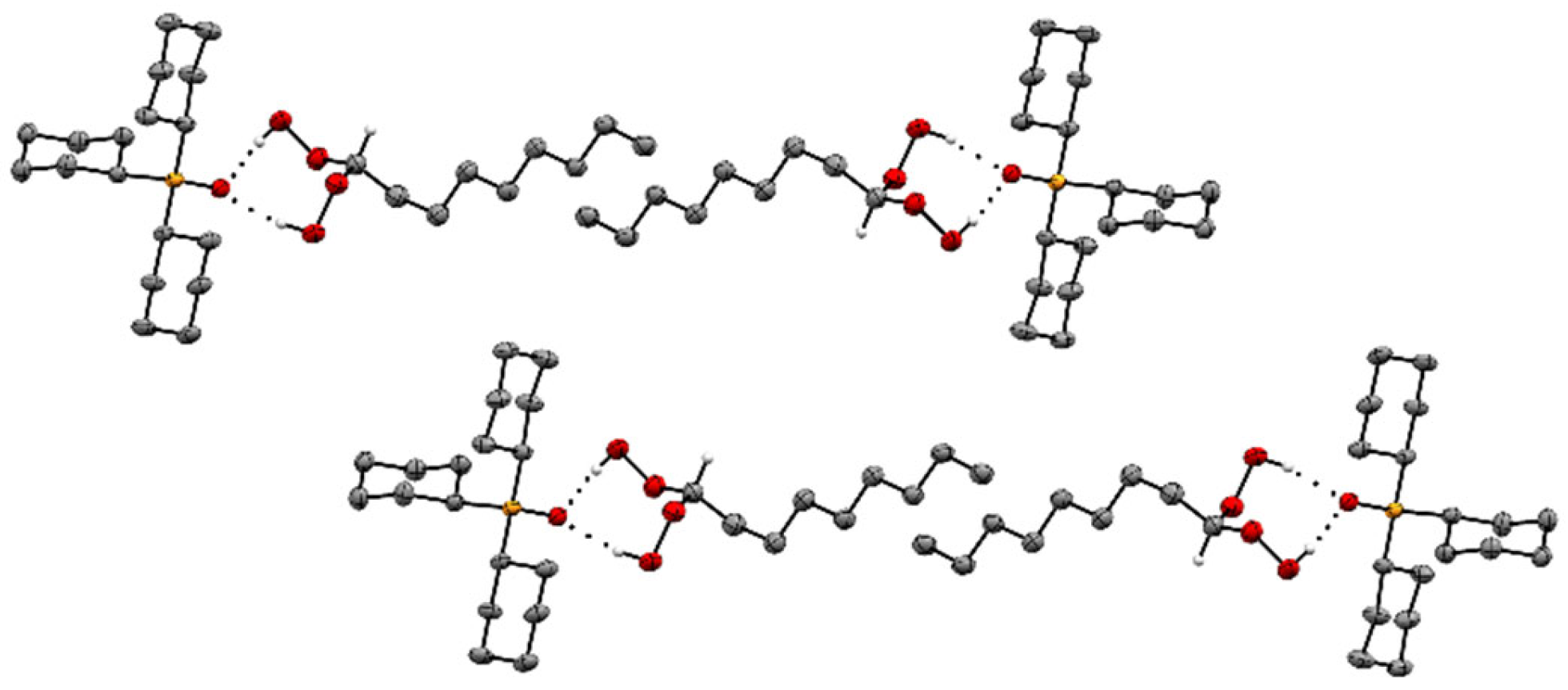
2.2. X-Ray Crystallography of the Adducts Cy3PO·(HOO)2CHR
2.3. NMR Spectroscopy of the Adducts of Cy3PO·(HOO)2CHR
2.4. IR Spectroscopy of the Adducts of Cy3PO·(HOO)2CHR
2.5. Solubilities of the Adducts in Organic Solvents
2.6. Application for the Selective Oxidation of PPh3
3. Experimental Section
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duprey, D.; Cavani, F. Handbook of Advanced Methods and Processes in Oxidation Catalysis; Imperial College Press: London, UK, 2014. [Google Scholar]
- Cavani, F.; Teles, J.H. Sustainability in Catalytic Oxidation: An Alternative Approach or a Structural Evolution? ChemSusChem 2009, 2, 508–534. [Google Scholar] [CrossRef]
- Comyns, A.E. Peroxides and Peroxide Compounds. In Van Nostrand’s Encyclopedia of Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Klassen, N.V.; Marchington, D.; McGowan, H.C.E. H2O2 Determination by the I3− Method and by KMnO4 Titration. Anal. Chem. 1994, 66, 2921–2925. [Google Scholar] [CrossRef]
- Azizi, M.; Maleki, A.; Hakimpoor, F. Solvent, Metal- and Halogen-Free Synthesis of Sulfoxides by Using a Recoverable Heterogeneous Urea-Hydrogen Peroxide Silica-Based Oxidative Catalytic System. Catal. Commun. 2017, 100, 62–65. [Google Scholar] [CrossRef]
- Taliansky, S. Urea-Hydrogen Peroxide Complex. Synlett 2005, 12, 1962–1963. [Google Scholar] [CrossRef][Green Version]
- Ji, L.; Wang, Y.-N.; Qian, C.; Chen, X.-Z. Nitrile-Promoted Alkene Epoxidation with Urea-Hydrogen Peroxide (UHP). Synth. Commun. 2013, 43, 2256–2264. [Google Scholar] [CrossRef]
- Koukabi, N. Sodium Percarbonate: A Versatile Oxidizing Reagent. Synlett 2010, 19, 2969–2970. [Google Scholar] [CrossRef]
- McKillop, A.; Sanderson, W.R. Sodium Perborate and Sodium Percarbonate: Further Applications in Organic Synthesis. J. Chem. Soc. Perkin Trans. 2000, 1, 471–476. [Google Scholar] [CrossRef]
- Habibi, D.; Zolfigol, M.A.; Safaiee, M.; Shamsian, A.; Ghorbani-Choghamarani, A. Catalytic Oxidation of Sulfides to Sulfoxides using Sodium Perborate and/or Sodium Percarbonate and Silica Sulfuric Acid in the Presence of KBr. Catal. Commun. 2009, 10, 1257–1260. [Google Scholar] [CrossRef]
- Arzumanyan, A.V.; Novikov, R.A.; Terent’ev, A.O.; Platonov, M.M.; Lakhtin, V.G.; Arkhipov, D.E.; Korlyukov, A.A.; Chernyshev, V.V.; Fitch, A.N.; Zdvizhkov, A.T.; et al. Nature Chooses Rings: Synthesis of Silicon-Containing Macrocyclic Peroxides. Organometallics 2014, 33, 2230–2246. [Google Scholar] [CrossRef]
- Zha, Q.; Wu, Y. Synthesis of Primary gem-Dihydroperoxides and Their Peroxycarbenium [3 + 2] Cycloaddition Reactions with Alkenes. J. Org. Chem. 2020, 85, 14121–14138. [Google Scholar] [CrossRef] [PubMed]
- Dove, J.E.; Riddick, J. Concerning the preparation of anhydrous hydrogen peroxide. Can. J. Chem. 1968, 46, 330–332. [Google Scholar] [CrossRef]
- Wolanov, Y.; Lev, O.; Churakov, A.V.; Medvedev, A.G.; Novotortsev, V.M.; Prikhodchenko, P.V. Preparation of pure hydrogen peroxide and anhydrous peroxide solutions from crystalline serine perhydrate. Tetrahedron 2010, 66, 5130–5133. [Google Scholar] [CrossRef]
- Cofré, P.; Sawyer, D.T. Redox Chemistry of Hydrogen Peroxide in Anhydrous Acetonitrile. Inorg. Chem. 1986, 25, 2089–2092. [Google Scholar] [CrossRef]
- Kharel, S.; Bhuvanesh, N.; Gladysz, J.A.; Blümel, J. New Hydrogen Bonding Motifs of Phosphine Oxides with a Silanediol, a Phenol, and Chloroform. Inorg. Chim. Acta 2019, 490, 215–219. [Google Scholar] [CrossRef]
- Tupikina, E.Y.; Bodensteiner, M.; Tolstoy, P.M.; Denisov, G.S.; Shenderovich, I.G. P=O Moiety as an Ambidextrous Hydrogen Bond Acceptor. J. Phys. Chem. C 2018, 122, 1711–1720. [Google Scholar] [CrossRef]
- Begimova, G.; Tupikina, E.Y.; Yu, V.K.; Denisov, G.S.; Bodensteiner, M.; Shenderovich, I.G. Effect of Hydrogen Bonding to Water on the 31P Chemical Shift Tensor of Phenyl- and Trialkylphosphine Oxides and α-Amino Phosphonates. J. Phys. Chem. C 2016, 120, 8717–8729. [Google Scholar] [CrossRef]
- Cuypers, R.; Sudhölter, E.J.R.; Zuilhof, H. Hydrogen Bonding in Phosphine Oxide/Phosphate–Phenol Complexes. ChemPhysChem 2010, 11, 2230–2240. [Google Scholar] [CrossRef] [PubMed]
- Bewick, N.A.; Arendt, A.; Li, Y.; Szafert, S.; Lis, T.; Wheeler, K.A.; Young, J.; Dembinski, R. Synthesis and Solid-State Structure of (4-Hydroxy-3,5-diiodophenyl)phosphine Oxides. Dimeric Motifs with the Assistance of O-H···O=P Hydrogen Bonds. Curr. Org. Chem. 2015, 19, 469–474. [Google Scholar] [CrossRef]
- Hoefler, J.C.; Vu, A.; Perez, A.J.; Blümel, J. Immobilized Di(hydroperoxy)propane Adducts of Phosphine Oxides as Traceless and Recyclable Oxidizing Agents. Appl. Surf. Sci. 2023, 629, 157333. [Google Scholar] [CrossRef]
- Hilliard, C.R.; Bhuvanesh, N.; Gladysz, J.A.; Blümel, J. Synthesis, Purification, and Characterization of Phosphine Oxides and Their Hydrogen Peroxide Adducts. Dalton Trans. 2012, 41, 1742–1754. [Google Scholar] [CrossRef]
- Arp, F.F.; Bhuvanesh, N.; Blümel, J. Hydrogen Peroxide Adducts of Triarylphosphine Oxides. Dalton Trans. 2019, 48, 14312–14325. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Cluff, K.J.; Bhuvanesh, N.; Blümel, J. Hydrogen Peroxide and Di(hydroperoxy)propane Adducts of Phosphine Oxides as Stoichiometric and Soluble Oxidizing Agents. Angew. Chem. Int. Ed. 2015, 54, 13341–13345. [Google Scholar] [CrossRef] [PubMed]
- Arp, F.F.; Bhuvanesh, N.; Blümel, J. Di(hydroperoxy)cycloalkane Adducts of Triarylphosphine Oxides: A Comprehensive Study Including Solid-State Structures and Association in Solution. Inorg. Chem. 2020, 59, 13719–13732. [Google Scholar] [CrossRef] [PubMed]
- Arp, F.F.; Ahn, S.H.; Bhuvanesh, N.; Blümel, J. Selective Synthesis and Stabilization of Peroxides via Phosphine Oxides. New J. Chem. 2019, 43, 17174–17181. [Google Scholar] [CrossRef]
- Ahn, S.H.; Bhuvanesh, N.; Blümel, J. Di(hydroperoxy)alkane Adducts of Phosphine Oxides: Safe, Solid, Stoichiometric, and Soluble Oxidizing Agents. Chem. Eur. J. 2017, 23, 16998–17009. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Lindhardt, D.; Bhuvanesh, N.; Blümel, J. Di(hydroperoxy)cycloalkanes Stabilized via Hydrogen Bonding by Phosphine Oxides: Safe and Efficient Baeyer-Villiger Oxidants. ACS Sustain. Chem. Eng. 2018, 6, 6829–6840. [Google Scholar] [CrossRef]
- Arp, F.F.; Ashirov, R.; Bhuvanesh, N.; Blümel, J. Di(hydroperoxy)adamantane Adducts: Synthesis, Characterization and Application as Oxidizers for the Direct Esterification of Aldehydes. Dalton Trans. 2021, 50, 15296–15309. [Google Scholar] [CrossRef] [PubMed]
- Blümel, J. Linkers and Catalysts Immobilized on Oxide Supports: New Insights by Solid-State NMR Spectroscopy. Coord. Chem. Rev. 2008, 252, 2410–2423. [Google Scholar] [CrossRef]
- Guenther, J.; Reibenspies, J.; Blümel, J. Synthesis and Characterization of Tridentate Phosphine Ligands Incorporating Long Methylene Chains and Ethoxysilane Groups for Immobilizing Molecular Rhodium Catalysts. Mol. Catal. 2019, 479, 110629–110642. [Google Scholar] [CrossRef]
- Pope, J.C.; Posset, T.; Bhuvanesh, N.; Blümel, J. The Palladium Component of an Immobilized Sonogashira Catalyst System: New Insights by Multinuclear HRMAS NMR Spectroscopy. Organometallics 2014, 33, 6750–6753. [Google Scholar] [CrossRef]
- Shakeri, E.; Blümel, J. Creating Well-Defined Monolayers of Phosphine Linkers Incorporating Ethoxysilyl Groups on Silica Surfaces for Superior Immobilized Catalysts. Appl. Surf. Sci. 2023, 615, 156380–156387. [Google Scholar] [CrossRef]
- Posset, T.; Guenther, J.; Pope, J.; Oeser, T.; Blümel, J. Immobilized Sonogashira Catalyst Systems: New Insights by Multinuclear HRMAS NMR Studies. Chem. Commun. 2011, 47, 2059–2061. [Google Scholar] [CrossRef][Green Version]
- Hayashi, S.; Jimura, K.; Kojima, N. Adsorption of Trimethylphosphine Oxide on Silicalite Studied by Solid-State NMR. Bull. Chem. Soc. Jpn. 2014, 87, 69–75. [Google Scholar] [CrossRef]
- Machida, S.; Sohmiya, M.; Ide, Y.; Sugahara, Y. Solid-State 31P Nuclear Magnetic Resonance Study of Interlayer Hydroxide Surfaces of Kaolinite Probed with an Interlayer Triethylphosphine Oxide Monolayer. Langmuir 2018, 34, 12694–12701. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, P.J.; Benzie, J.W.; Bakhmutov, V.I.; Blümel, J. Disentangling Different Modes of Mobility of Triphenylphosphine Oxide Adsorbed on Alumina. J. Chem. Phys. 2020, 152, 054718-1–054718-8. [Google Scholar] [CrossRef] [PubMed]
- Kharel, S.; Cluff, K.J.; Bhuvanesh, N.; Gladysz, J.A.; Blümel, J. Structures and Dynamics of Secondary and Tertiary Alkylphosphine Oxides Adsorbed on Silica. Chem. Asian J. 2019, 14, 2704–2711. [Google Scholar] [CrossRef] [PubMed]
- Hilliard, C.R.; Kharel, S.; Cluff, K.J.; Bhuvanesh, N.; Gladysz, J.A.; Blümel, J. Structures and Unexpected Dynamic Properties of Phosphine Oxides Adsorbed on Silica Surfaces. Chem. Eur. J. 2014, 20, 17292–17295. [Google Scholar] [CrossRef] [PubMed]
- Ashirov, R.; Kimball, M.R.; O’Brien, M.; Bhuvanesh, N.; Blümel, J. Aluminum Trichloride Adducts of Phosphine Oxides: A Single Crystal X-Ray and Solid-State NMR Study. Inorg. Chim. Acta 2024, 564, 121952. [Google Scholar] [CrossRef]
- Chrzanowski, J.; Krasowska, D.; Drabowicz, J. Synthesis of Optically Active Tertiary Phosphine Oxides: A Historical Overview and the Latest Advances. Heteroat. Chem. 2018, 29, e21476. [Google Scholar] [CrossRef]
- Kharel, S.; Jia, T.; Bhuvanesh, N.; Reibenspies, J.H.; Blümel, J.; Gladysz, J.A. A Non-templated Route to Macrocyclic Dibridgehead Diphosphorus Compounds: Crystallographic Characterization of a “Crossed-Chain” Variant of in/out Stereoisomers. Chem. Asian J. 2018, 13, 2632–2640. [Google Scholar] [CrossRef]
- Fletcher, M.D. Organophosphorus Reagents, a Practical Approach in Chemistry; Murphy, P.J., Ed.; Oxford University Press: London, UK, 2004; pp. 171–214. ISBN 9780198502623. [Google Scholar]
- Adams, H.; Collins, R.C.; Jones, S.; Warner, C.J.A. Enantioselective Preparation of P-Chiral Phosphine Oxides. Org. Lett. 2011, 13, 6576–6579. [Google Scholar] [CrossRef]
- Swamy, K.C.K.; Kumar, N.N.B.; Balaraman, E.; Kumar, K.V.P.P. Mitsunobu and Related Reactions: Advances and Applications. Chem. Rev. 2009, 109, 2551–2651. [Google Scholar]
- Dembinski, R. Recent Advances in the Mitsunobu Reaction: Modified Reagents and the Quest for Chromatography-Free Separation. Eur. J. Org. Chem. 2004, 2004, 2763–2772. [Google Scholar] [CrossRef]
- Beddoe, R.H.; Andrews, K.G.; Magne, V.; Cuthbertson, J.D.; Saska, J.; Shannon-Little, A.L.; Shanahan, S.E.; Sneddon, H.F.; Denton, R.M. Redox-Neutral Organocatalytic Misunobu Reactions. Science 2020, 365, 910–914. [Google Scholar] [CrossRef] [PubMed]
- The Following CCDC Reference Numbers Contain the Supplementary Crystallographic Data for the Corresponding Compounds 2–6 for This Paper: 2404335 (2), 2404336 (3), 2404337 (4), 2404338 (5), and 2404339 (6). These Data Can Be Obtained Free of Charge from the Cambridge Crystallographic Data Centre. Available online: www.ccdc.cam.ac.uk/data_request/cif (accessed on 19 December 2024).
- Günzler, H.; Gremlich, H.-U. IR Spektroskopie, 4th ed.; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Jeffrey, G.A. An Introduction to Hydrogen Bonding; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Baker, E.N.; Hubbard, R.E. Hydrogen Bonding in Globular Proteins. Prog. Biophys. Mol. Bio. 1984, 44, 97–179. [Google Scholar] [CrossRef]
- Kalinowski, H.-O.; Berger, S.; Braun, S. 13C-NMR-Spektroskopie; Georg Thieme Verlag Stuttgart: New York, NY, USA, 1984. [Google Scholar]
- Hesse, M.; Meier, H.; Zeeh, B. Spektroskopische Methoden in der Organischen Chemie; Georg Thieme Verlag Stuttgart: New York, NY, USA, 1984. [Google Scholar]
- Hoefler, J.C.; Jackson, D.; Blümel, J. Surface-Assisted Selective Air Oxidation of Phosphines Adsorbed on Activated Carbon. Inorg. Chem. 2024, 63, 9275–9287. [Google Scholar] [CrossRef]
- CrysAlisPro Software System, Rigaku Oxford Diffraction 2003. Available online: https://rigaku.com/products/crystallography/x-ray-diffraction/crysalispro (accessed on 19 December 2024).
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure solution with ShelXT. Acta Cryst. 2015, A71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H.J. OLEX2. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Taylor, R.; Macrae, C.F. Mercury. Acta Cryst. 2001, B57, 815–827. [Google Scholar] [CrossRef] [PubMed]
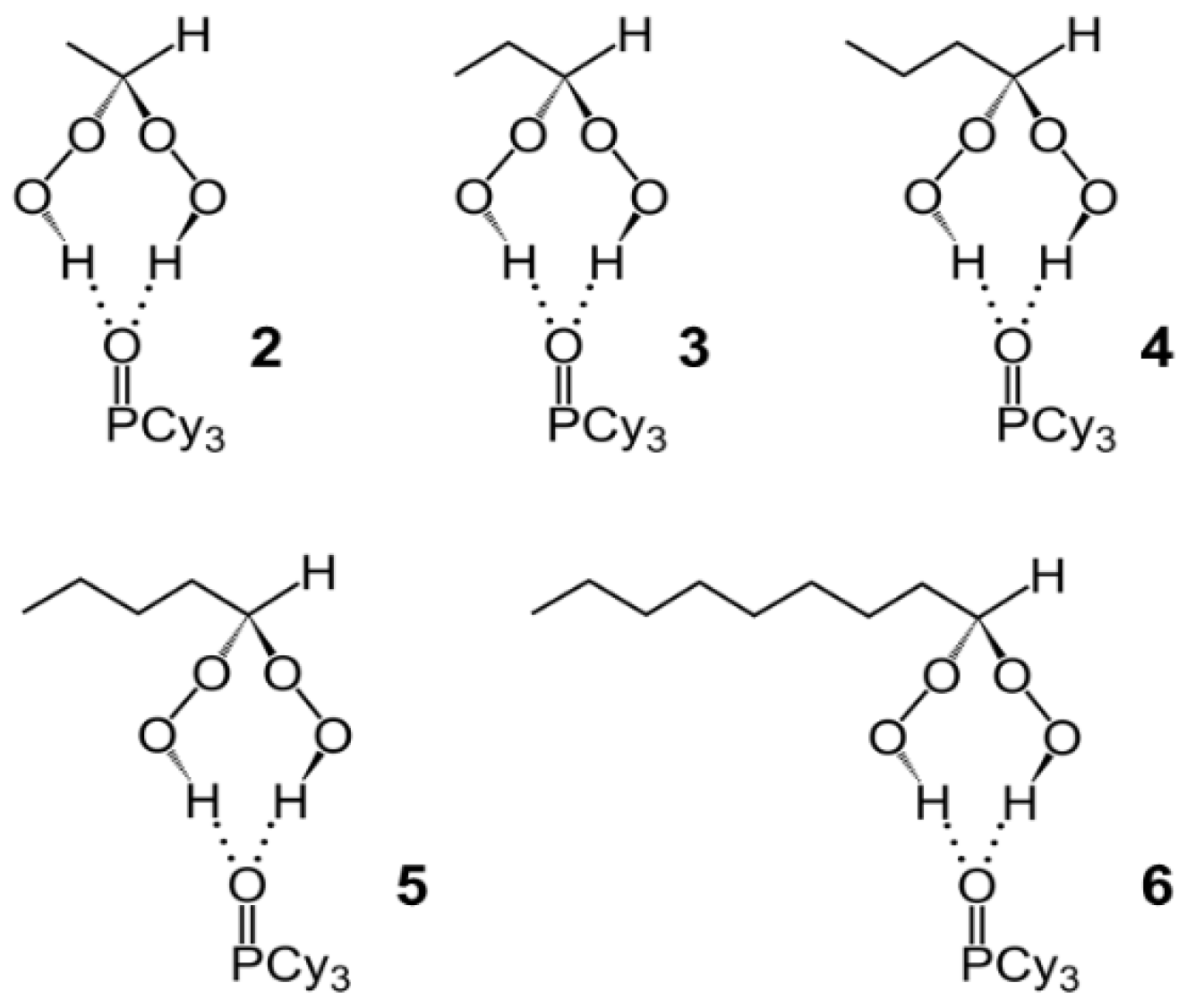
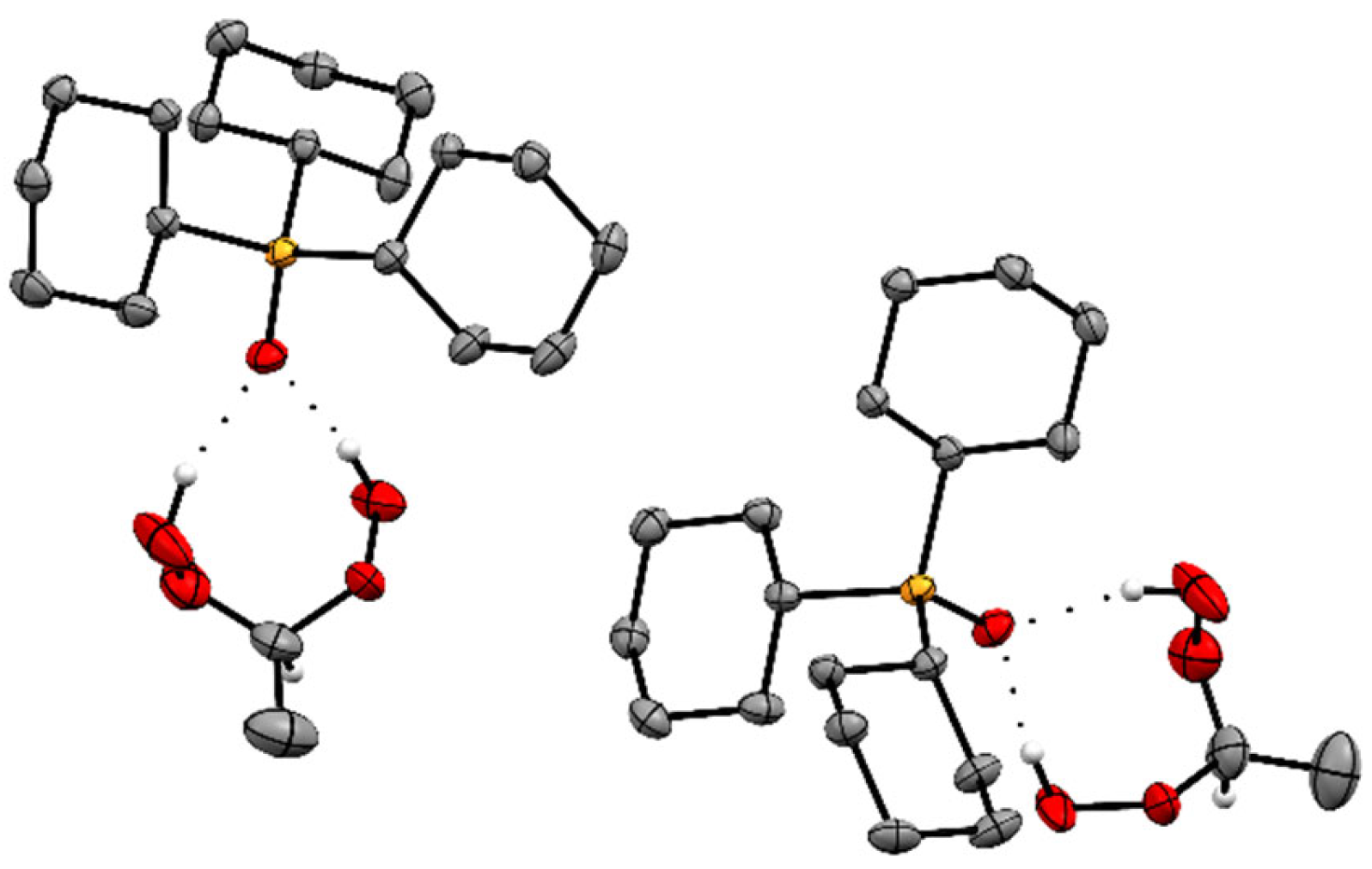


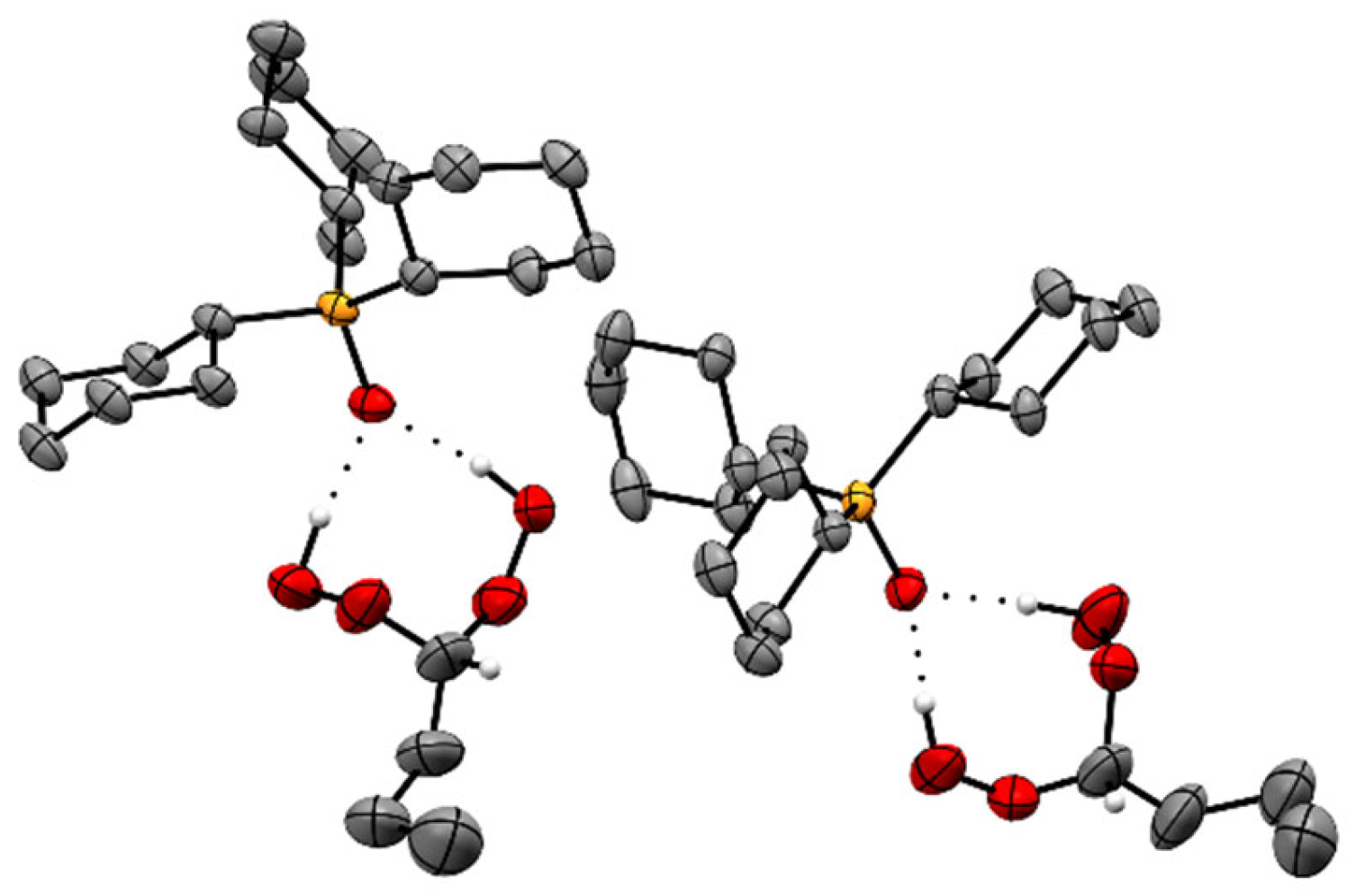

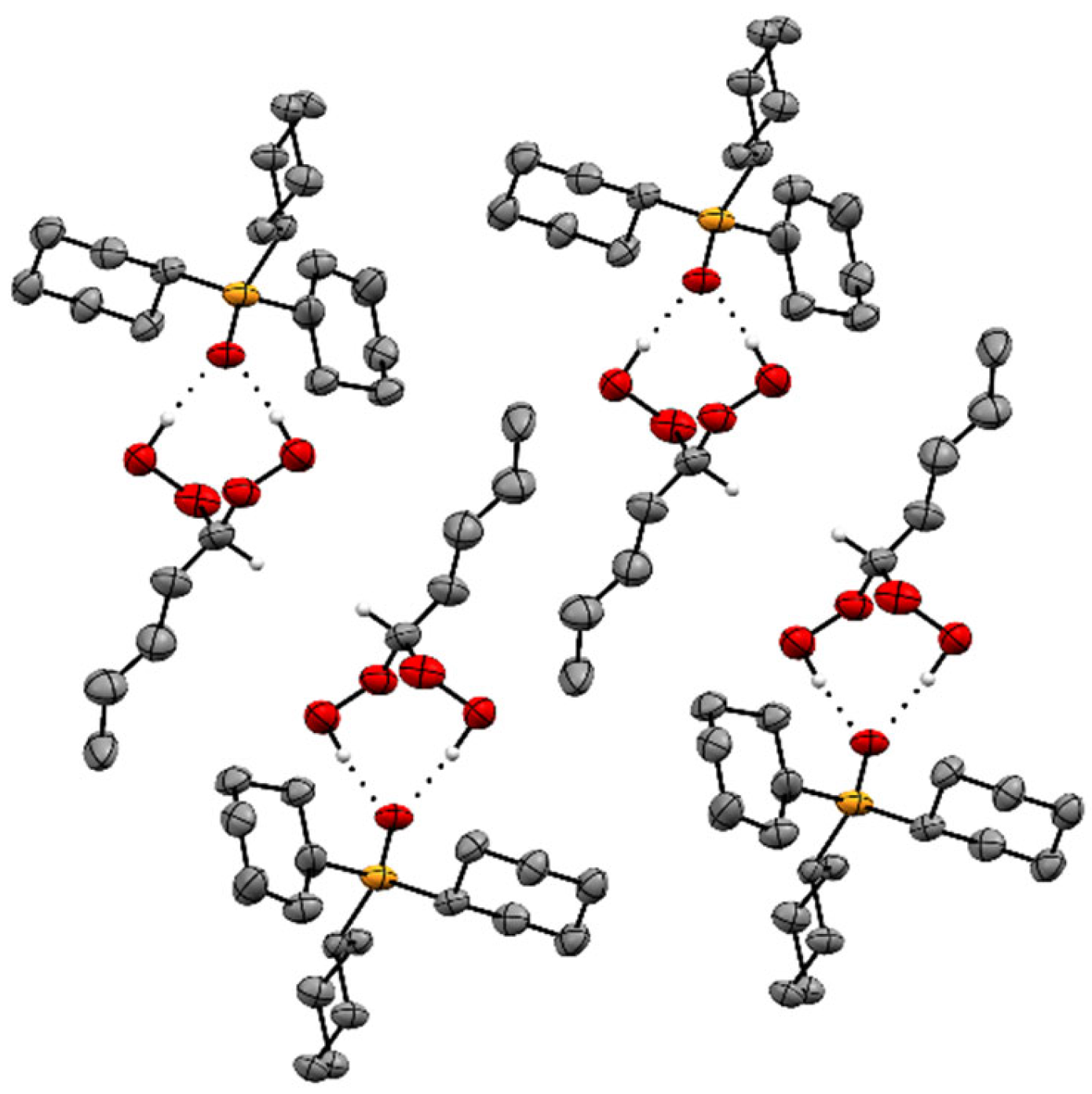

| Adduct | O–H···O (Å) | P=O (Å) | ∆(P=O) (Å) |
|---|---|---|---|
| 2 | 2.689/2.751 | 1.5071 (17) | 0.0169 (17) |
| 3 | 2.719/2.741 | 1.5110 (9) | 0.0208 (9) |
| 4 | 2.711/2.719 | 1.5082 (14) | 0.0180 (14) |
| 5 | 2.701/2.702 | 1.5074 (11) | 0.0172 (11) |
| 6 | 2.712/2.737 | 1.5155 (10) | 0.0253 (10) |
| Cy3PO·(HOO)2CHR | O–C–O (°) | O1–C–C/O2–C–C (°) |
|---|---|---|
| 2 | 114.5 (2) | 103.8 (3)/121.2 (3) |
| 3 | 112.5 (3) | 113.7 (3)/106.5 (3) |
| 4 | 113.6 (3) | 114.3 (5)/119.0 (5) |
| 5 | 113.66 (17) | 114.0 (2)/114.44 (19) |
| 6 | 113.05 (12) | 105.40 (12)/115.43 (12) |
| Cy3PO·(HOO)2CHR | δ(31P) (ppm) | ∆δ(31P) (ppm) |
|---|---|---|
| 2 | 57.35 | 7.44 |
| 3 | 57.05 | 7.14 |
| 4 | 56.96 | 7.05 |
| 5 | 57.20 | 7.29 |
| 6 | 57.43 | 7.52 |
| Cy3PO·(HOO)2CHR | ν(O–H) (cm−1) | ν(P=O) (cm−1) | ∆ν(P=O) (cm−1) |
|---|---|---|---|
| 2 | 3195 | 1129 | 28 |
| 3 | 3193 | 1127 | 30 |
| 4 | 3198 | 1131 | 26 |
| 5 | 3200 | 1131 | 26 |
| 6 | 3196 | 1132 | 25 |
| Cy3PO·(HOO)2CHR | mp (°C) |
|---|---|
| 2 | 87–99 |
| 3 | 84–90 |
| 4 | 100–105 |
| 5 | 84–90 |
| 6 | 89–92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashirov, R.; Todorovic, M.; Bhuvanesh, N.; Blümel, J. Hydrogen-Bonded Di(hydroperoxy)alkane Adducts of the Type Cy3P=O·(HOO)2CHR (R = Alkyl). Molecules 2025, 30, 329. https://doi.org/10.3390/molecules30020329
Ashirov R, Todorovic M, Bhuvanesh N, Blümel J. Hydrogen-Bonded Di(hydroperoxy)alkane Adducts of the Type Cy3P=O·(HOO)2CHR (R = Alkyl). Molecules. 2025; 30(2):329. https://doi.org/10.3390/molecules30020329
Chicago/Turabian StyleAshirov, Rahym, Maya Todorovic, Nattamai Bhuvanesh, and Janet Blümel. 2025. "Hydrogen-Bonded Di(hydroperoxy)alkane Adducts of the Type Cy3P=O·(HOO)2CHR (R = Alkyl)" Molecules 30, no. 2: 329. https://doi.org/10.3390/molecules30020329
APA StyleAshirov, R., Todorovic, M., Bhuvanesh, N., & Blümel, J. (2025). Hydrogen-Bonded Di(hydroperoxy)alkane Adducts of the Type Cy3P=O·(HOO)2CHR (R = Alkyl). Molecules, 30(2), 329. https://doi.org/10.3390/molecules30020329






