Asymmetric Donor–Acceptor 2,7-Disubstituted Fluorenes and Their 9-Diazoderivatives: Synthesis, Optical Spectra and Photolysis
Abstract
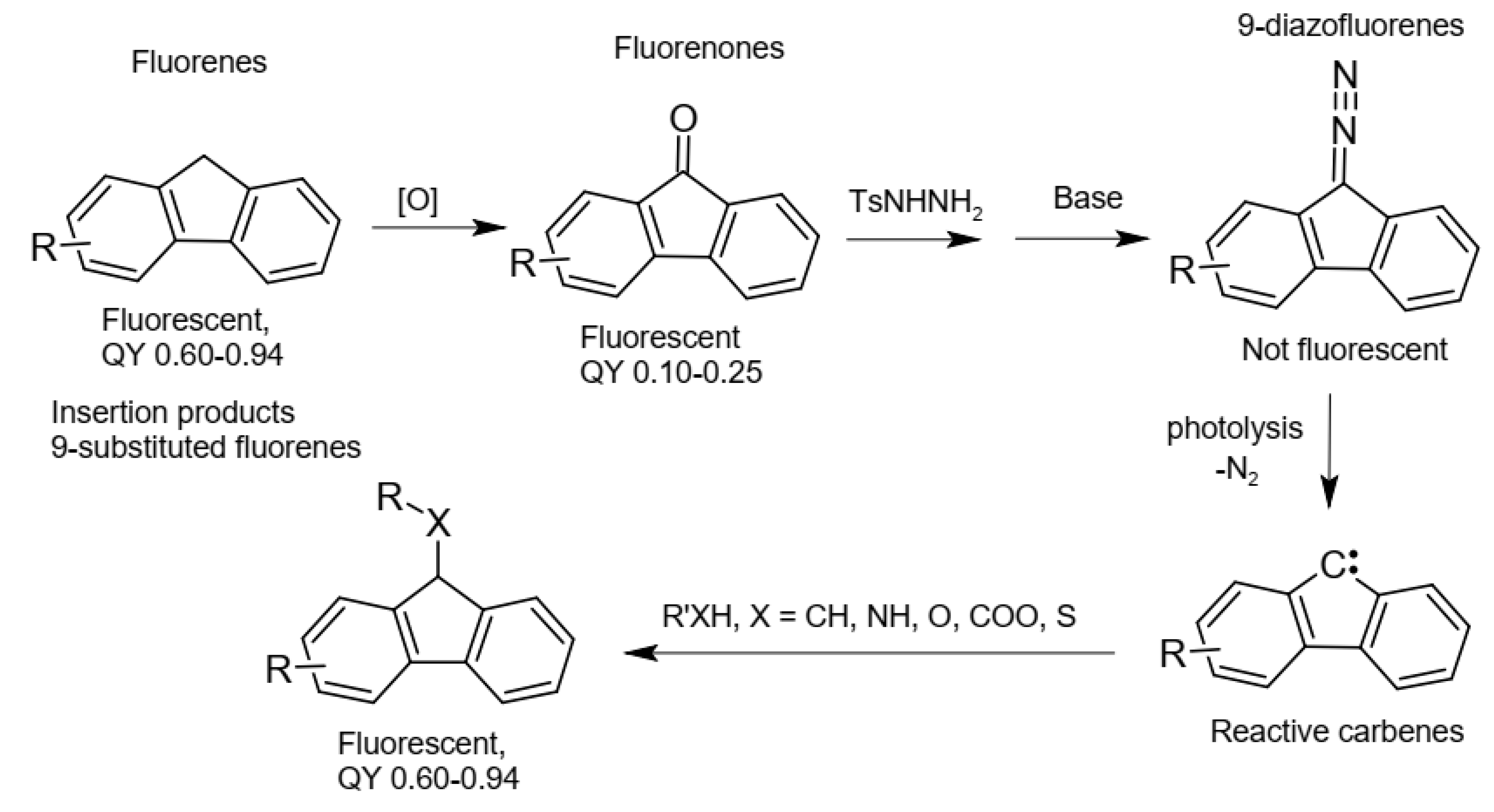
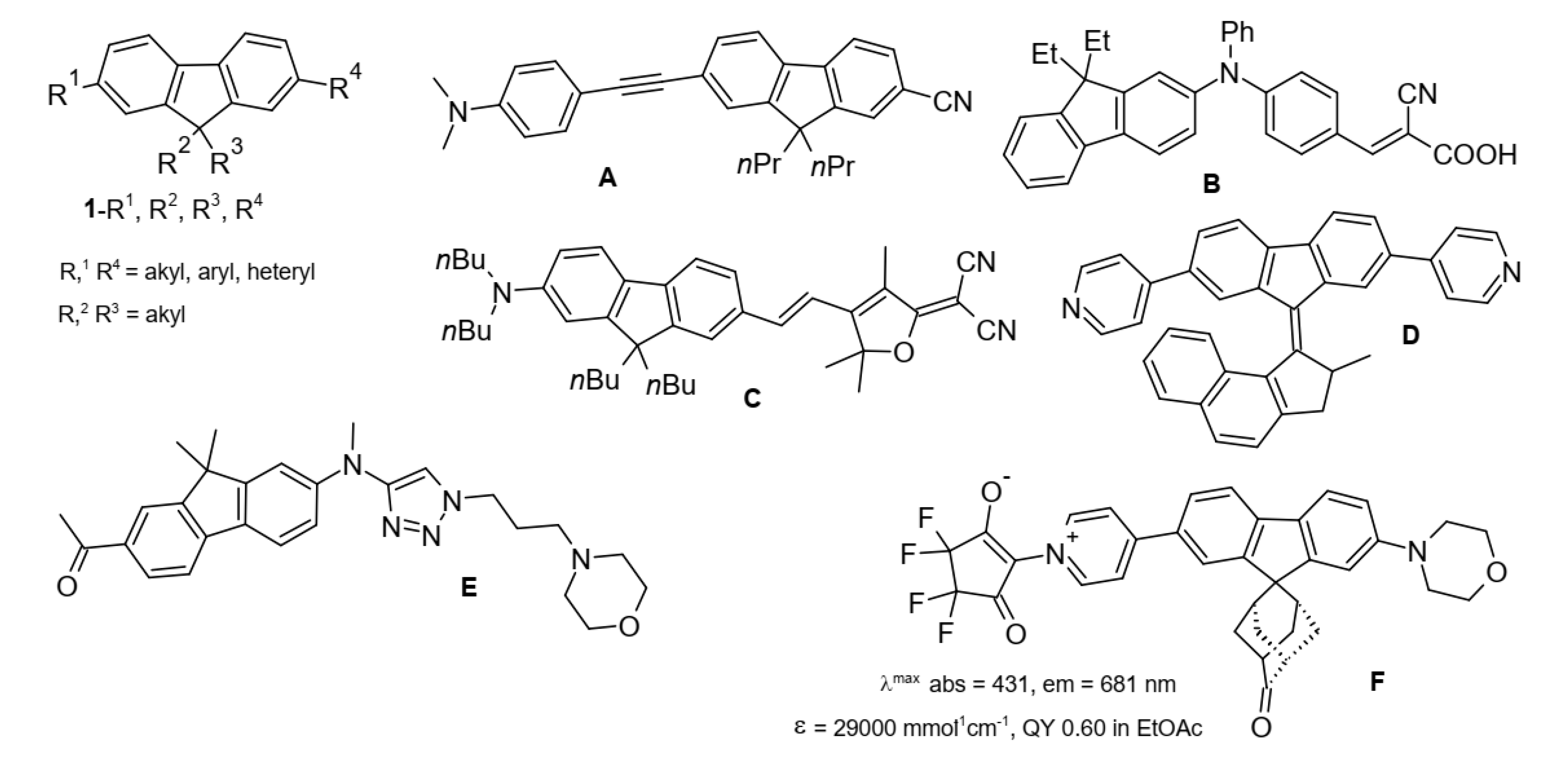
1. Introduction
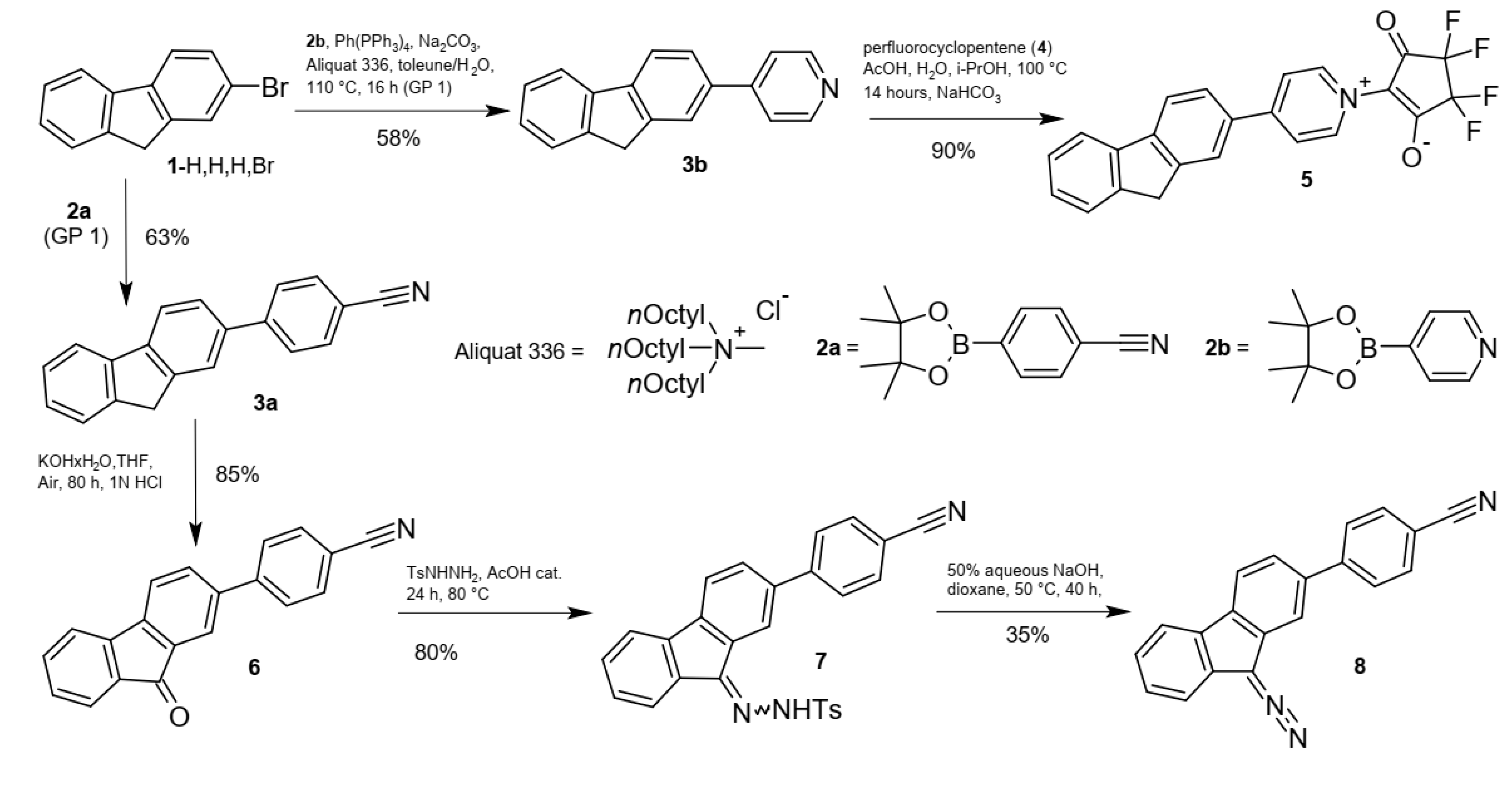
2. Results and Discussion
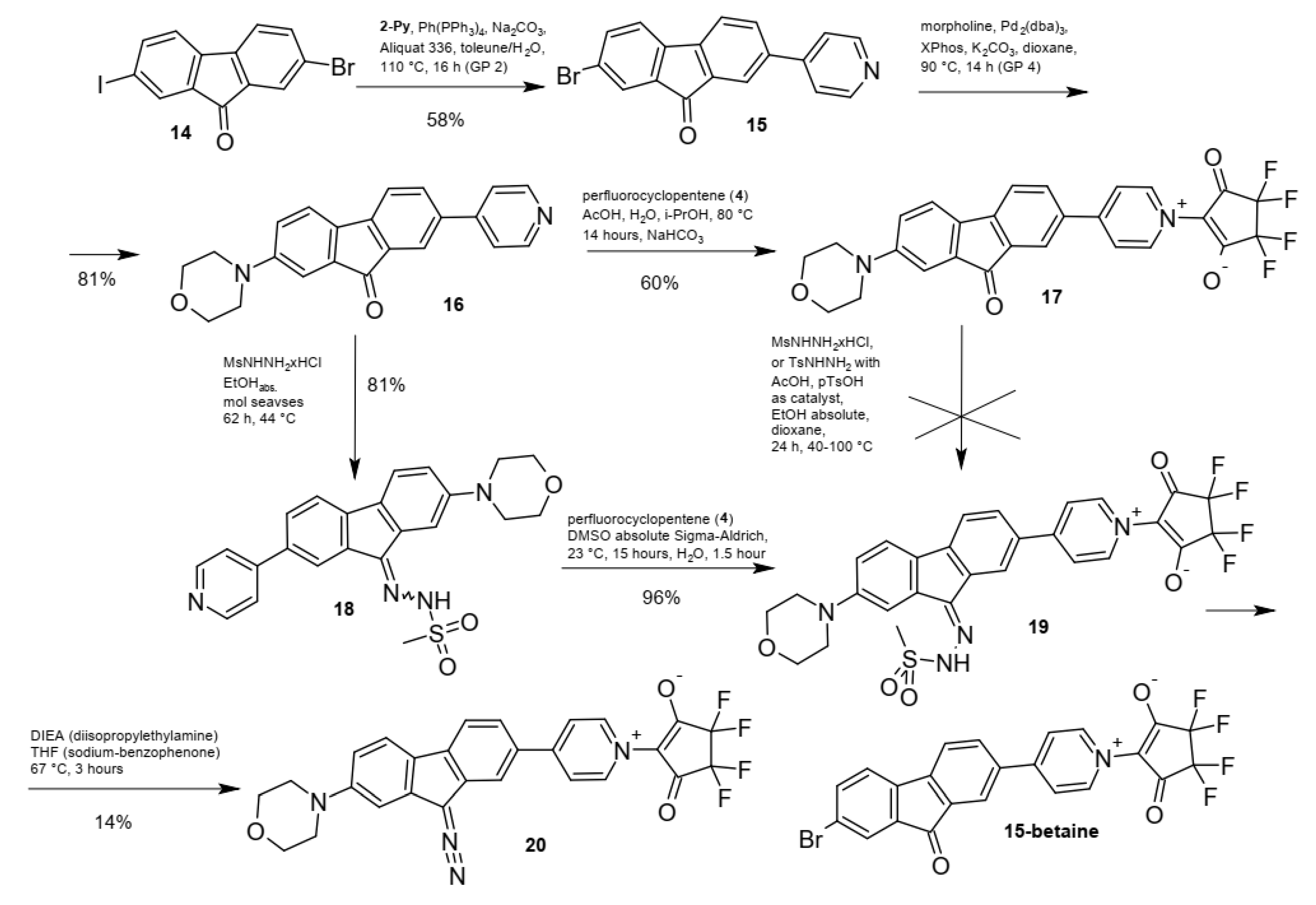
2.1. Synthesis and Spectra
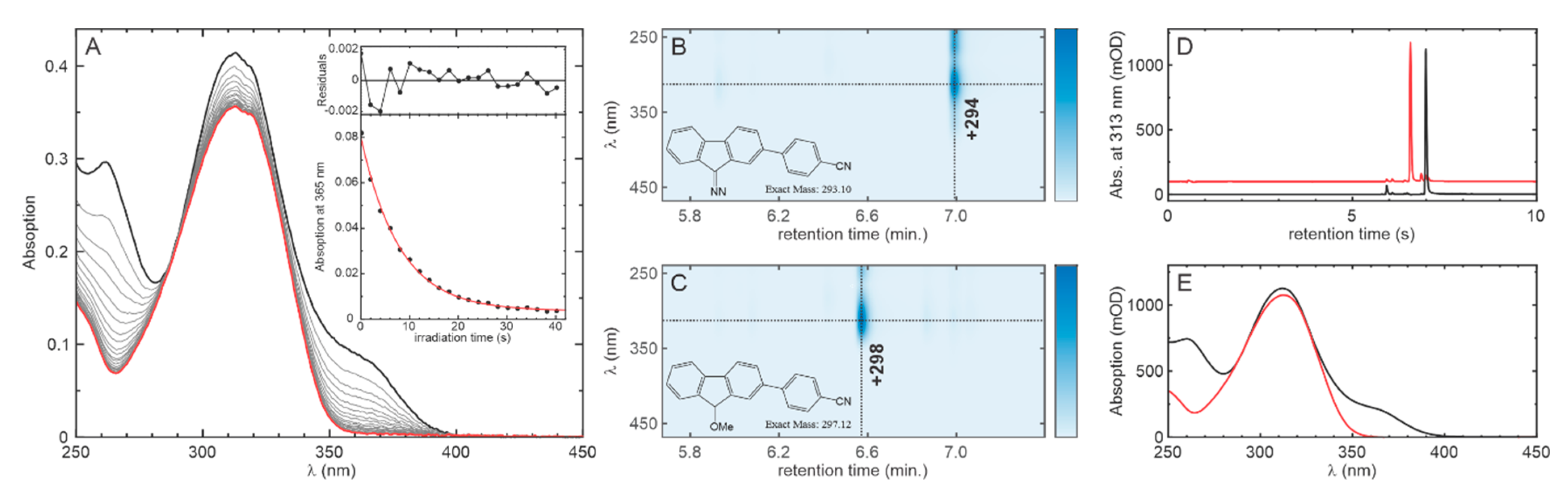
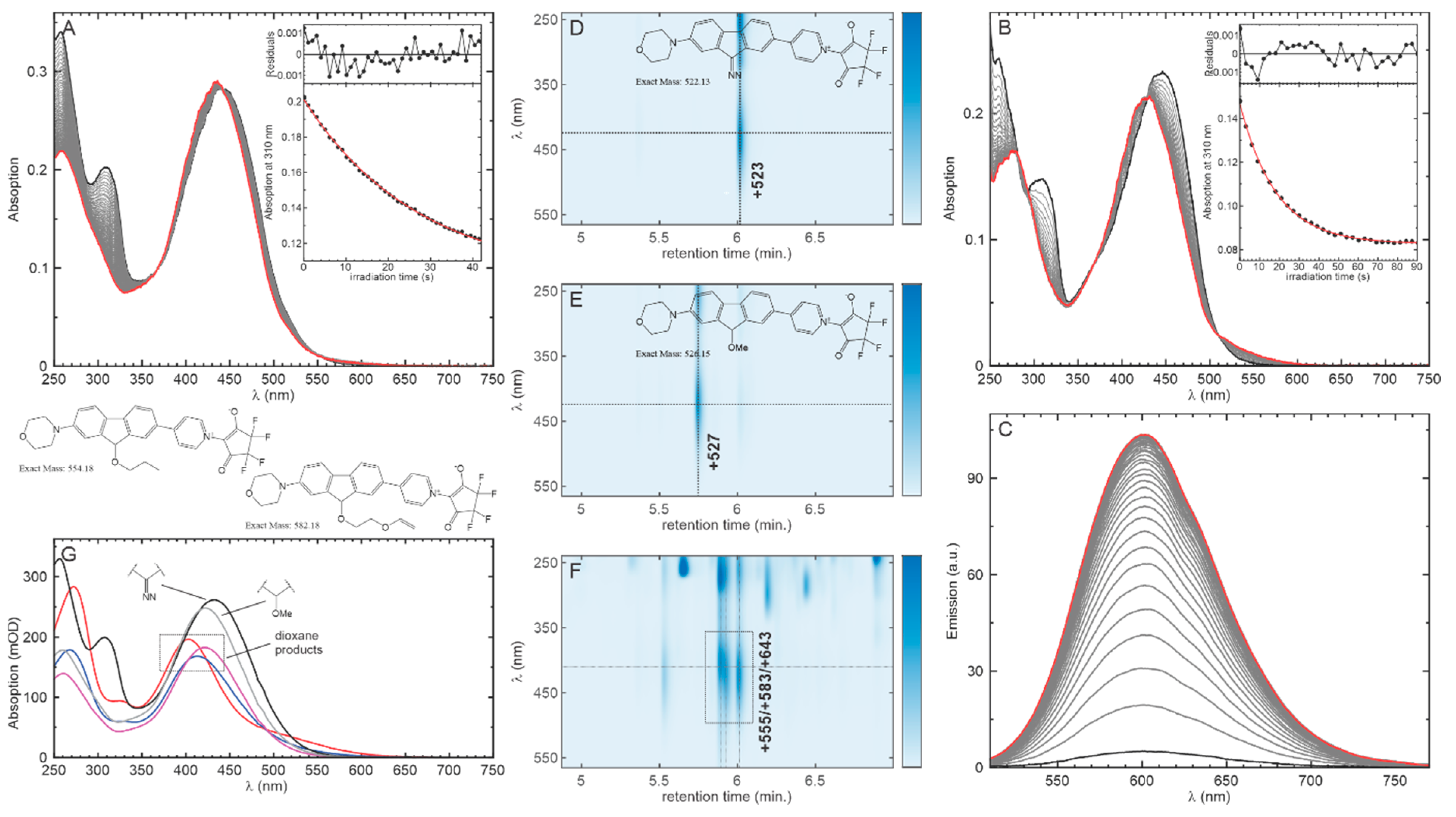
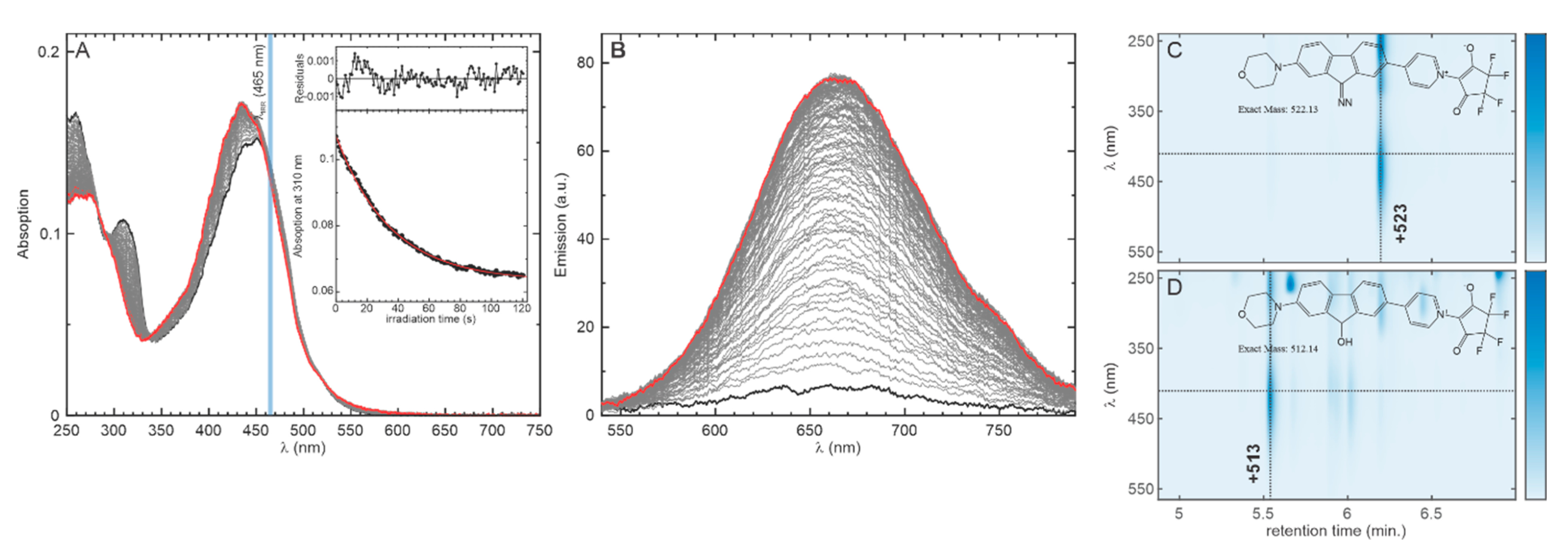
2.2. Photolysis of the Optimized Probe
3. Materials and Methods
3.1. General Procedures
3.1.1. General Protocol 1 (GP1) for Preparation of 2-Substituted Fluorenes
3.1.2. General Protocol 2 (GP2) for Preparation of 7-Substituted 2-Bromofluorenes
3.1.3. General Protocol 3 (GP3) for Preparation of 2,7-Disubstituted Fluorenes
3.1.4. General Protocol 4 (GP4) for Preparation of 2-Amino-7-(4-Pyridyl)-Disubstituted Fluorenes [18]
3.1.5. General Protocol 5 (GP5) for Preparation of Pyridinium Betaines [15]
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurdyukova, I.V.; Ishchenko, A.A. Organic Dyes Based on Fluorene and Its Derivatives. Russ. Chem. Rev. 2012, 81, 258–290. [Google Scholar] [CrossRef]
- Justin Thomas, K.R.; Venkateswararao, A.; Joseph, V.; Kumar, S.; Jou, J.-H. Polarity Tuning of Fluorene Derivatives by Chromophores to Achieve Efficient Blue Electroluminescent Materials. Org. Electron. 2019, 64, 266–273. [Google Scholar] [CrossRef]
- Justin Thomas, K.R.; Baheti, A. Fluorene Based Organic Dyes for Dye Sensitised Solar Cells: Structure–Property Relationships. Mater. Technol. 2013, 28, 71–87. [Google Scholar] [CrossRef]
- Huo, F.Y.; Zhang, H.; Chen, Z.; Qiu, L.; Liu, J.L.; Bo, S.H.; Kityk, I.V. Novel Nonlinear Optical Push-Pull Fluorene Dyes Chromophore as Promising Materials for Telecommunications. J. Mater. Sci.-Mater. Electron. 2019, 30, 12180–12185. [Google Scholar] [CrossRef]
- Danowski, W.; van Leeuwen, T.; Abdolahzadeh, S.; Roke, D.; Browne, W.R.; Wezenberg, S.J.; Feringa, B.L. Unidirectional Rotary Motion in a Metal-Organic Framework. Nat. Nanotechnol. 2019, 14, 488–494. [Google Scholar] [CrossRef]
- Shaya, J.; Corridon, P.R.; Al-Omari, B.; Aoudi, A.; Shunnar, A.; Mohideen, M.I.H.; Qurashi, A.; Michel, B.Y.; Burger, A. Design, Photophysical Properties, and Applications of Fluorene-Based Fluorophores in Two-Photon Fluorescence Bioimaging: A Review. J. Photochem. Photobiol. C-Photochem. Rev. 2022, 52, 100529. [Google Scholar] [CrossRef]
- Shaya, J.; Fontaine-Vive, F.; Michel, B.Y.; Burger, A. Rational Design of Push-Pull Fluorene Dyes: Synthesis and Structure-Photophysics Relationship. Chemistry 2016, 22, 10627–10637. [Google Scholar] [CrossRef]
- Meineke, D.N.H. Fluorescent Dyes and Quenchers with Rigid Linkers. Ph.D. Thesis, Georg-August-University Göttingen, Göttingen, Germany, 2017. [Google Scholar]
- Lala, A.K.; Dixit, R.R.; Koppaka, V. Depth-Dependent Photolabeling of Membrane Hydrophobic Core with 9-Diazofluorene-2-Butyric Acid. Biochim. Biophys. Acta Biomembr. 1989, 978, 333–336. [Google Scholar] [CrossRef]
- Lala, A.K.; Dixit, R.R. Synthesis of Diazofluorene-Based Reagents as Photoactivatable Membrane Probes. J. Chem. Soc. Chem. Commun. 1989, 10, 636–638. [Google Scholar] [CrossRef]
- Wang, J.; Kubicki, J.; Hilinski, E.F.; Mecklenburg, S.L.; Gustafson, T.L.; Platz, M.S. Ultrafast Study of 9-Diazofluorene: Direct Observation of the First Two Singlet States of Fluorenylidene. J. Am. Chem. Soc. 2007, 129, 13683–13690. [Google Scholar] [CrossRef]
- Kobayashi, S.; Tanaka, M.; Ohuchi, K.; Ishikawa, R.; Usui, M.; Sato, T. Compound and Light Emitting Element Using the Same. U.S. Patent Application No. 2022/0123220 A1, 21 April 2022. [Google Scholar]
- Zhou, Z.G.; Yuan, Y.Y.; Xie, Y.R.; Li, M. Green, Efficient and Reusable Bis(Imidazolium) Ionic Liquids Promoted Pd-Catalyzed Aqueous Suzuki Reaction for Organic Functional Materials. Catal. Lett. 2018, 148, 2696–2702. [Google Scholar] [CrossRef]
- He, B.; Huang, J.; Zhang, J.; Liu, X.; Wang, D.; Sung, H.H.Y.; Liu, Y.; Qin, A.; Lam, J.W.Y.; Tang, B.Z. In-Situ Generation of Poly(Quinolizine)s via Catalyst-Free Polyannulations of Activated Diyne and Pyridines. Sci. China Chem. 2022, 65, 789–795. [Google Scholar] [CrossRef]
- Meineke, D.N.; Bossi, M.L.; Ta, H.; Belov, V.N.; Hell, S.W. Bichromophoric Compounds with Orthogonally and Parallelly Arranged Chromophores Separated by Rigid Spacers. Chemistry 2017, 23, 2469–2475. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ji, X.; Jiang, S.; Liu, L.; Weeks, B.L.; Zhang, Z. Highly Efficient Synthesis of 9-Fluorenones from 9H-Fluorenes by Air Oxidation. Green Chem. 2011, 13, 1891–1896. [Google Scholar] [CrossRef]
- Uno, K.; Bossi, M.L.; Belov, V.N.; Irie, M.; Hell, S.W. Multicolour Fluorescent “Sulfide-Sulfone” Diarylethenes with High Photo-Fatigue Resistance. Chem. Commun. Camb. 2020, 56, 2198–2201. [Google Scholar] [CrossRef]
- Butkevich, A.N.; Belov, V.N.; Kolmakov, K.; Sokolov, V.V.; Shojaei, H.; Sidenstein, S.C.; Kamin, D.; Matthias, J.; Vlijm, R.; Engelhardt, J.; et al. Hydroxylated Fluorescent Dyes for Live-Cell Labeling: Synthesis, Spectra and Super-Resolution STED. Chemistry 2017, 23, 12114–12119. [Google Scholar] [CrossRef]
- Newcombe, A.G. Mesyl Derivatives of Hydrazine. Can. J. Chem. 1955, 33, 1250–1255. [Google Scholar] [CrossRef]
- Birman, V.B.; Zhao, Z.; Guo, L. Benzo[b]Fluorenes via Indanone Dianion Annulation: A Short Synthesis of Prekinamycin. Org. Lett. 2007, 9, 1223–1225. [Google Scholar] [CrossRef]
- Maas, G. New Syntheses of Diazo Compounds. Angew. Chem. Int. Ed. 2009, 48, 8186–8195. [Google Scholar] [CrossRef]
- Shen, Y.; Tang, X.; Xu, Y.; Liu, H.; Zhang, S.; Yang, B.; Ma, Y. Enhanced Deep-Red Emission in Donor-Acceptor Molecular Architecture: The Role of Ancillary Acceptor of Cyanophenyl. Chin. Chem. Lett. 2019, 30, 1947–1950. [Google Scholar] [CrossRef]
- Zeng, W.; Ballard, T.E.; Tkachenko, A.G.; Burns, V.A.; Feldheim, D.L.; Melander, C. Mimicking the Biological Activity of Diazobenzo[b]Fluorene Natural Products with Electronically Tuned Diazofluorene Analogs. Bioorg. Med. Chem. Lett. 2006, 16, 5148–5151. [Google Scholar] [CrossRef] [PubMed]
- Nakahama, T.; Kitagawa, D.; Sotome, H.; Ito, S.; Miyasaka, H.; Kobatake, S. Optical Properties and Solvatofluorochromism of Fluorene Derivatives Bearing S,S-Dioxidized Thiophene. Photochem. Photobiol. Sci. 2016, 15, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Aktalay, A.; Jensen, N.; Uno, K.; Bossi, M.L.; Belov, V.N.; Hell, S.W. Supramolecular Complex of Photochromic Diarylethene and Cucurbit[7]Uril: Fluorescent Photoswitching System for Biolabeling and Imaging. J. Am. Chem. Soc. 2022, 144, 14235–14247. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, B.; Jansen, M.; Goerigk, L.; Wong, W.W.H.; Ritchie, C. Highly Fluorescent Pyridinium Betaines for Light Harvesting. Angew. Chem. Int. Ed. Engl. 2017, 56, 13882–13886. [Google Scholar] [CrossRef]

| R2 | Br- | 4-Pyridyl-(Py) | 4-Me2NC6H4-(DmP) | |
|---|---|---|---|---|
| R1 | ||||
| 4-Pyridyl-(Py) | 10-Py,Br (47) | 11-Py,Py (37) ** | 11-Py,DmP (51) | |
| 2-Thienyl-(Th) | 10-Th,Br (41) | 11-Th,Py (62) | 11-Th,DmP (38) | |
| 2-Benzothiophen-2-yl-(BTh) | 10-BTh,Br (79) | 11-BTh,Py (51) | 11-BTh,DmP (92) | |
| 2-Benzothiophen-2-yl-(oxidized) [BTh(O2)] | 10-BTh(O2),Br (54) * | 11-BTh(O2),Py (81) | 11-BTh(O2),DmP (90) | |
| Compound | [nm] | [nm] | εmax [M−1cm−1] | Φfl [%] | LT [ns] |
|---|---|---|---|---|---|
| 3a (Scheme 2) | 317 | 372 | 36,500 | 80 | 1.0, 10.4 * |
| 3b (Scheme 2) | 311 | 352 | 25,700 | --- ** | 0.8 |
| 5 (Scheme 2) | 390 | 474 | 34,400 | 80 | 2.7 |
| 6 (Scheme 2) | 284, 318, 404sh | 504 | 51,200, 13,500, 1000sh | 25 | --- |
| 8 (MeCN) (Scheme 2) | 312 | 384 | 44,800 | --- ** | --- *** |
| 10-Py,Br | 317 | 354 | 33,900 | --- ** | 0.2, 0.9 * |
| 10-BTh(O2),Br | 368 | 450 | 30,900 | 53 | 1.7 |
| 11-Py,Py | 325 | 356, 373 | 50,700 | --- ** | 0.8, 1.6 * |
| 11-Th,Py | 339 | 378, 394 | 49,000 | 84 | 0.9 |
| 10-BTh,Br | 347 | 381, 400 | 57,900 | 84 | 0.8 |
| 11-BTh(O2),Py-betaine | 402 | 466 | 21,600 | 72 | 1.7 |
| 11-Py,DmP | 345 | 449 | 32,300 | 90 | 0.3, 1.4 * |
| 11-BTh,DmP-Me3N+ | 350 | 390, 450 | 11,100 | 73 | 1.6 |
| 11-BTh(O2),DmP | 400 | 555 | 33,300 | 84 | 3.5 |
| 11-BTh(O2),DmP-Me3N+ | 373 | 470 | 17,700 | 72 | 2.6 |
| 12 | 342 | 435 | 24,000 | 94 | 0.2, 1.6 * |
| 13 | 436 | 613 | 31,700 | 84 | 3.1 |
| 15-betaine | 373 | 508 | 31,000 | 9 | 2.7, 5.4 * |
| 17 (MeCN) (Scheme 4) | 407, 505 | 503, 741 | 31,700, 6800 | 0.5 | 2.2, 10.3 * |
| 20 (MeCN) (Scheme 4) | 438 | 500, 738 | 21,500 | 2 | --- *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savchenko, A.I.; Belov, V.N.; Bossi, M.L.; Hell, S.W. Asymmetric Donor–Acceptor 2,7-Disubstituted Fluorenes and Their 9-Diazoderivatives: Synthesis, Optical Spectra and Photolysis. Molecules 2025, 30, 321. https://doi.org/10.3390/molecules30020321
Savchenko AI, Belov VN, Bossi ML, Hell SW. Asymmetric Donor–Acceptor 2,7-Disubstituted Fluorenes and Their 9-Diazoderivatives: Synthesis, Optical Spectra and Photolysis. Molecules. 2025; 30(2):321. https://doi.org/10.3390/molecules30020321
Chicago/Turabian StyleSavchenko, Andrei I., Vladimir N. Belov, Mariano L. Bossi, and Stefan W. Hell. 2025. "Asymmetric Donor–Acceptor 2,7-Disubstituted Fluorenes and Their 9-Diazoderivatives: Synthesis, Optical Spectra and Photolysis" Molecules 30, no. 2: 321. https://doi.org/10.3390/molecules30020321
APA StyleSavchenko, A. I., Belov, V. N., Bossi, M. L., & Hell, S. W. (2025). Asymmetric Donor–Acceptor 2,7-Disubstituted Fluorenes and Their 9-Diazoderivatives: Synthesis, Optical Spectra and Photolysis. Molecules, 30(2), 321. https://doi.org/10.3390/molecules30020321