The Effect of β-Glucan on the Release and Antiradical Activity of Phenolic Compounds from Apples in Simulated Digestion
Abstract
1. Introduction
2. Results
2.1. Phenolic Compounds During Simulated Gastrointestinal Digestion
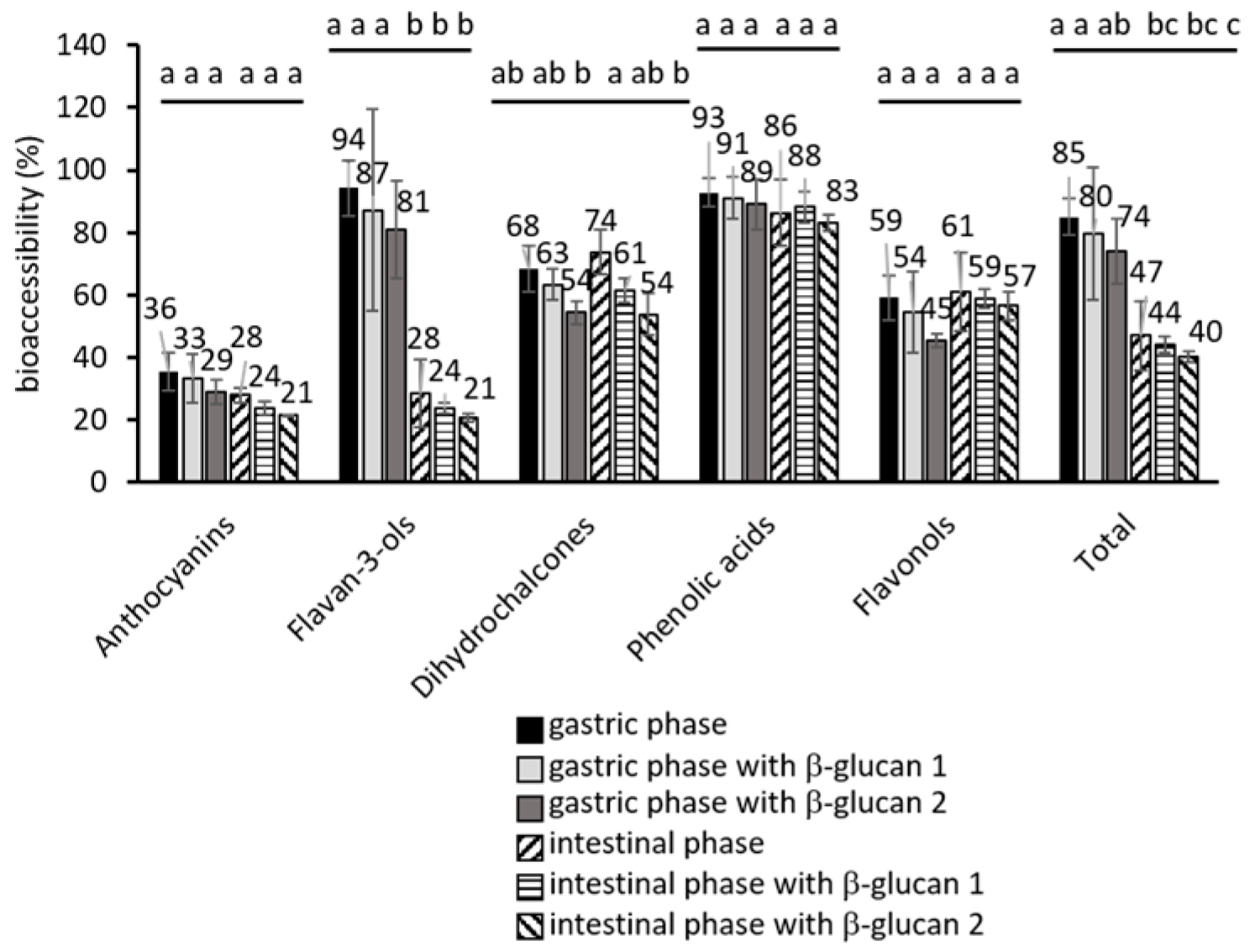
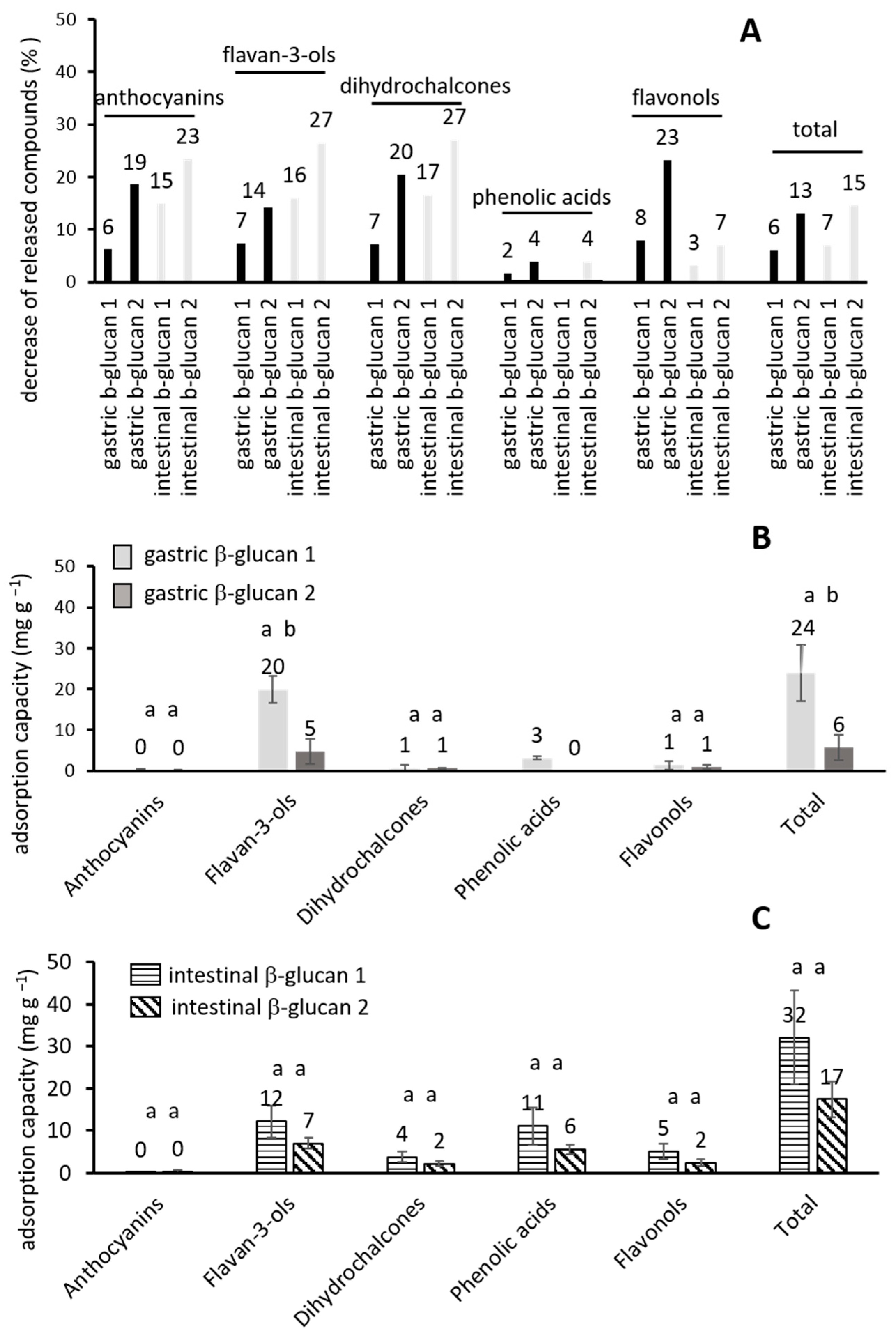
2.2. The Influence of β-Glucan on the Released Phenolic Compounds
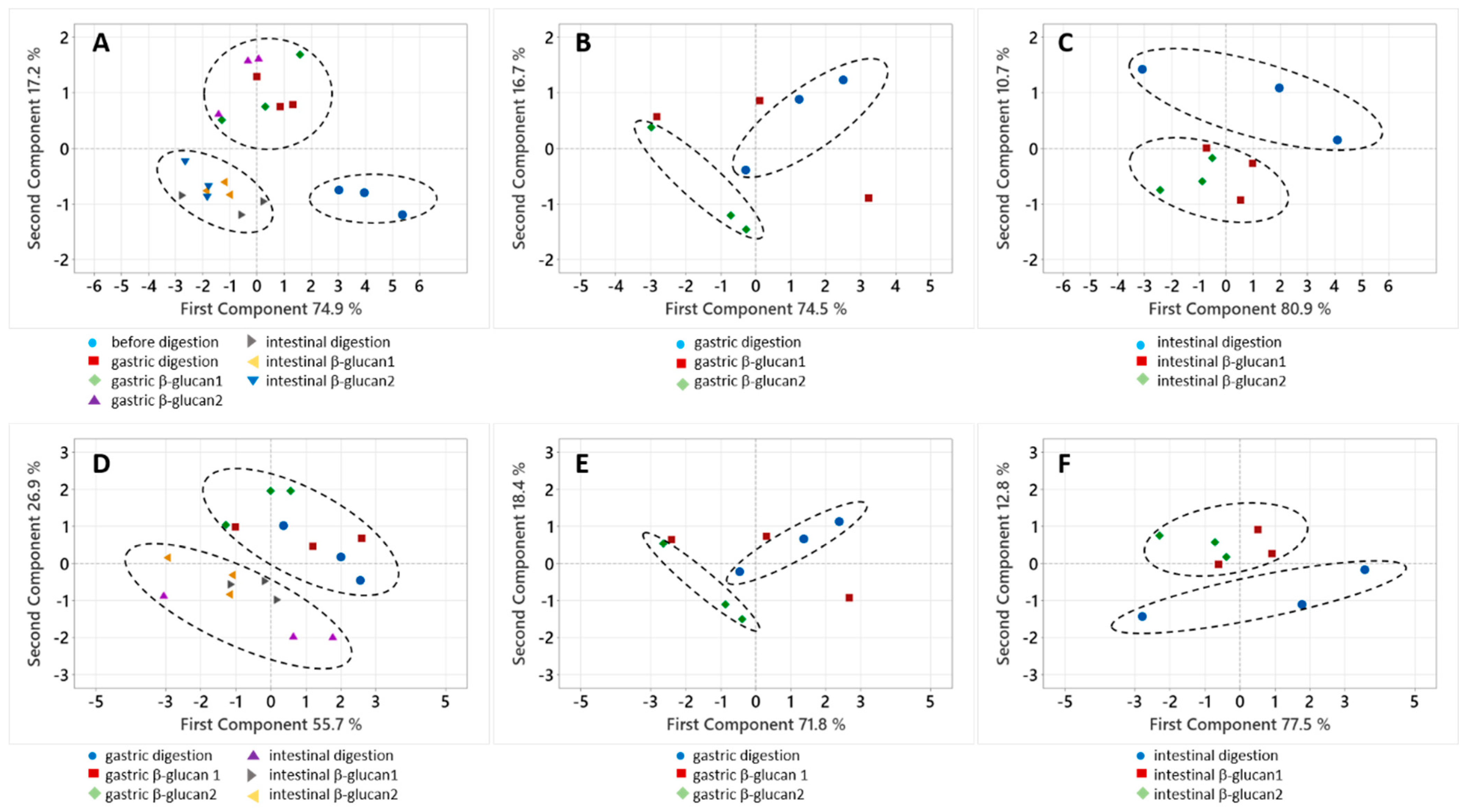
2.3. A Statistical Analysis of the Results
2.4. Antiradical Activity
3. Discussion
3.1. Phenolic Compounds in Native Apples and in Gastric and Intestinal Digestion
3.2. Gastrointestinal Digestion with or Without β-Glucan
3.3. Antiradical Activity of Phenolic Compounds Released in the Simulated Digestion
4. Materials and Methods
4.1. Chemicals and the Preparation of Solutions
4.2. Apple Samples
4.3. Extraction of Phenolic Compounds
4.4. Simulated Digestion
4.5. Bioaccessibility Calculation
4.6. HPLC Method
4.7. DPPH Method
4.8. Statistical Analysis of the Data
5. Conclusions
- One is the potential role of dietary fibers to serve as carriers of phenolic compounds to the lower part of the gastrointestinal tract where they can be released and act beneficially.
- Another is the potential effect of dietary fiber–phenolic compound complexes that might be created inside the stomach and small intestine.
- Furthermore, studies to enlighten whether better health effects might be achieved by consuming phenolic compounds alone, between meals, or with dietary fibers during meals, should be conducted.
- Such specific studies can be focused on phenolic compounds from apples and food sources rich in β-glucans since they are a regular part of the diet.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trautmann, D.; Suazo, F.; Torres, K.; Simón, L. Antitumor effects of resveratrol opposing mechanisms of Helicobacter pylori in gastric cancer. Nutrients 2024, 16, 2141. [Google Scholar] [CrossRef] [PubMed]
- Kurata, R.; Adachi, M.; Yamakawa, O.; Yoshimoto, M. Growth suppression of human cancer cells by polyphenolics from sweetpotato (Ipomoea batatas L.) leaves. J. Agric. Food Chem. 2007, 55, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-L.; Ko, C.-H.; Yan, Y.-Y.; Wang, C.-K. Antiadhesion and anti-inflammation effects of Noni (Morinda citrifolia) fruit extracts on AGS cells during Helicobacter pylori infection. J. Agric. Food Chem. 2014, 62, 2374–2383. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Topi, S.; Bottalico, L.; Charitos, I.A.; Jirillo, E. Current knowledge about gastric microbiota with special emphasis on Helicobacter pylori-related gastric conditions. Curr. Issues Mol. Biol. 2024, 46, 4991–5009. [Google Scholar] [CrossRef]
- Domgnim Mokam, C.E.; Ngenge Tamfu, A.; Mesmine Kuissu, M.; Kucukaydin, S.; Mezui, C.; Enow-Orock, E.G.; Vernyuy Tan, P. Phenolic profile and chronic gastric ulcer healing effects of Cameroonian propolis. Clin. Tradit. Med. Pharmacol. 2024, 5, 200140. [Google Scholar] [CrossRef]
- Beserra, A.M.S.e.S.; Calegari, P.I.; do Carmo Souza, M.; dos Santos, R.A.N.; da Silva Lima, J.C.; Silva, R.M.; Balogun, S.O.; de Oliveira Martins, D.T. Gastroprotective and ulcer-healing mechanisms of ellagic acid in experimental rats. J. Agric. Food Chem. 2011, 59, 6957–6965. [Google Scholar] [CrossRef]
- Zhang, L.; Gui, S.; Wang, J.; Chen, Q.; Zeng, J.; Liu, A.; Chen, Z.; Lu, X. Oral administration of green tea polyphenols (TP) improves ileal injury and intestinal flora disorder in mice with Salmonella typhimurium infection via resisting inflammation, enhancing antioxidant action and preserving tight junction. J. Funct. Food 2020, 64, 103654. [Google Scholar] [CrossRef]
- Che, S.; Yuan, J.; Zhang, L.; Ruan, Z.; Sun, X.; Lu, H. Puerarin prevents epithelial tight junction dysfunction induced by ethanol in Caco-2 cell model. J. Funct. Food 2020, 73, 104079. [Google Scholar] [CrossRef]
- Xu, C.; He, M.; Jiang, Z.; Yang, Q. Inhibition mechanism of buckwheat hulls polyphenols on α-amylase and α-glucosidase using kinetics, spectroscopic and molecular docking approaches. Int. J. Biol. Macromol. 2024, 280, 136046. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Wang, G.; Cao, J.; Yang, X.; Liu, X.; Sun, L. α-amylase inhibition of a certain dietary polyphenol is predominantly affected by the concentration of α-1, 4-glucosidic bonds in starchy and artificial substrates. Food Res. Int. 2022, 157, 111210. [Google Scholar] [CrossRef]
- Jakobek, L.; Matić, P. Phenolic compounds from apples: From natural fruits to the beneficial effects in the in the digestive system. Molecules 2024, 29, 568. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, M. Oxidative stress-induced gastrointestinal diseases: Biology and nanomedicines—A Review. BioChem 2024, 4, 189–217. [Google Scholar] [CrossRef]
- Li, L.; Peng, P.; Ding, N.; Jia, W.; Huang, C.; Ta, Y. Oxidative stress, inflammation, gut dysbiosis: What can polyphenols do in inflammatory bowel disease? Antioxidants 2023, 12, 967. [Google Scholar] [CrossRef]
- Alongi, M.; Lanza, U.; Gorassini, A.; Verardo, G.; Comuzzi, C.; Anese, M.; Manzocco, L.; Nicoli, M.C. The role of processing on phenolic bioaccessibility and antioxidant capacity of apple derivatives. Food Chem. 2025, 463, 141402. [Google Scholar] [CrossRef] [PubMed]
- Corona-Leo, L.S.; Meza-Márquez, O.G.; Hernández-Martínez, D.M. Effect of in vitro digestion on phenolic compounds and antioxidant capacity of different apple (Malus domestica) varieties harvested in Mexico. Food Biosci. 2021, 43, 101311. [Google Scholar] [CrossRef]
- Fernández-Jalao, I.; Balderas, C.; Sánchez-Moreno, C.; De Ancos, B. Impact of an in vitro dynamic gastrointestinal digestion on phenolic compounds and antioxidant capacity of apple treated by high-pressure processing. Innov. Food Sci. Emerg. 2020, 66, 102486. [Google Scholar] [CrossRef]
- Lucas-González, R.; Viuda-Martos, M.; Pérez Álvarez, J.A.; Fernández-López, J. Changes in bioaccessibility, polyphenol profile and antioxidant potential of flours obtained from persimmon fruit (Diospyros kaki) co-products during in vitro gastrointestinal digestion. Food Chem. 2018, 256, 252–258. [Google Scholar] [CrossRef]
- Du, B.; Meenu, M.; Liu, H.; Xu, B. A concise review on the molecular structure and function relationship of β-glucan. Int. J. Mol. Sci. 2019, 20, 4032. [Google Scholar] [CrossRef]
- Feliciano, R.P.; Antunes, C.; Ramos, A.; Serra, A.T.; Figueira, M.E.; Duarte, C.M.M.; de Carvalho, A.; Bronze, M.R. Characterization of traditional and exotic apple varieties from Portugal. Part 1—Nutritional, phytochemical and sensory evaluation. J. Funct. Food 2010, 2, 35–45. [Google Scholar] [CrossRef]
- Koutsos, A.; Lima, M.; Conterno, L.; Gasperotti, M.; Bianchi, M.; Fava, F.; Vrhovsek, U.; Lovegrove, J.A.; Tuohy, K.M. Effects of commercial apple varieties on human gut microbiota composition and metabolic output using an in vitro colonic model. Nutrients 2017, 9, 533. [Google Scholar] [CrossRef]
- Jakobek, L.; Ištuk, J.; Matić, P.; Skendrović Babojelić, M. Interactions of polyphenols from traditional apple varieties ‘Bobovac’, ‘Ljepocvjetka’ and ‘Crvenka’ with β-Glucan during in vitro simulated digestion. Food Chem. 2021, 363, 130283. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L.; Ištuk, J.; Barron, A.; Matić, P. Bioactive phenolic compounds from apples during simulated in vitro gastrointestinal digestion: Kinetics of their release. Appl. Sci. 2023, 13, 8434. [Google Scholar] [CrossRef]
- Quatrin, A.; Rampelotto, C.; Pauletto, R.; Maurer, L.H.; Nichelle, S.M.; Klein, B.; Fritzsche Rodrigues, R.; Maróstica Junior, M.R.; de Souza Fonseca, B.; Ragagnin de Menezes, C.; et al. Bioaccessibility and catabolism of phenolic compounds from jaboticaba (Myrciaria trunciflora) fruit peel during in vitro gastrointestinal digestion and colonic fermentation. J. Funct. Food 2020, 65, 103714. [Google Scholar] [CrossRef]
- Herrero-Martínez, J.M.; Sanmartin, M.; Rosés, M.; Bosch, E.; Ràfols, C. Determination of dissociation constants of flavonoids by capillary electrophoresis. Electrophoresis 2005, 26, 1886–1895. [Google Scholar] [CrossRef]
- Bouayed, J.; Deußer, H.; Hoffmann, L.; Bohn, T. Bioaccessible and dialysable polyphenols in selected apple varieties following in vitro digestion vs. their native patterns. Food Chem. 2012, 131, 1466–1472. [Google Scholar] [CrossRef]
- Narita, Y.; Inouye, K. Degradation kinetics of chlorogenic acid at various pH values and effects of ascorbic acid and epigallocatechin gallate on its stability under alkaline conditions. J. Agric. Food Chem. 2013, 61, 966–972. [Google Scholar] [CrossRef]
- Chebotarev, A.N.; Snigur, D.V. Study of the acid-base properties of quercetin in aqueous solutions by color measurements. J. Anal. Chem. 2015, 70, 55–59. [Google Scholar] [CrossRef]
- Wu, Z.; Li, H.; Ming, J.; Zhao, G. Optimization of adsorption of tea polyphenols into oat β-glucan using response surface methodology. J. Agric. Food Chem. 2011, 59, 378–385. [Google Scholar] [CrossRef]
- Wu, Z.; Ming, J.; Gao, R.; Wang, Y.; Liang, Q.; Yu, H.; Zhao, G. Characterization and antioxidant activity of the complex of tea polyphenols and oat β-glucan. J. Agric. Food Chem. 2011, 59, 10737–10746. [Google Scholar] [CrossRef]
- Sugimoto, K.; Matsuoka, Y.; Sakai, K.; Fujiya, N.; Fujii, H.; Mano, J. Catechins in green tea powder (matcha) are heat-stable scavengers of acrolein, a lipid peroxide-derived reactive carbonyl species. Food Chem. 2021, 355, 129403. [Google Scholar] [CrossRef]
- Valcheva-Kuzmanova, S.; Denev, P.; Eftimov, M.; Georgieva, A.; Kuzmanova, V.; Kuzmanov, A.; Kuzmanov, K.; Tzaneva, M. Protective effects of Aronia melanocarpa juices either alone or combined with extracts from Rosa canina or Alchemilla vulgaris in a rat model of indomethacin-induced gastric ulcers. Food Chem. Toxicol. 2019, 132, 110739. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.T.; Rodrigues, R.F.; Machado, N.M.; Maurer, L.H.; Ferreira, L.F.; Somacal, S.; da Veiga, M.L.; de Ugalde Marques da Rocha, M.I.; Vizzotto, M.; Rodrigues, E.; et al. Natural deep eutectic solvent (NADES)-based blueberry extracts protect against ethanol-induced gastric ulcer in rats. Food Res. Int. 2020, 138, 109718. [Google Scholar] [CrossRef] [PubMed]
- Pastene, E.; Spiesky, H.; García, A.; Moreno, J.; Troncoso, M.; Figueroa, G. In vitro and in vivo effects of apple polyphenols against Helicobacter pylori. J. Agric. Food Chem. 2010, 58, 7172–7179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xu, Y.; Lv, H.; Pang, W.; Wang, J.; Ma, H.; Wang, S. Intestinal pharmacokinetics of resveratrol and regulatory effects of resveratrol metabolites on gut barrier and gut microbiota. Food Chem. 2021, 357, 129532. [Google Scholar] [CrossRef]
- Li, D.; Sun, L.; Yang, Y.; Wang, Z.; Yang, X.; Zhao, T.; Gong, T.; Zou, L.; Guo, Y. Young apple polyphenols postpone starch digestion in vitro and in vivo. J. Funct. Food 2019, 56, 127–135. [Google Scholar] [CrossRef]
- Gong, T.; Yang, X.; Bai, F.; Li, D.; Zhao, T.; Zhang, J.; Sun, L.; Guo, Y. Young apple polyphenols as natural α-glucosidase inhibitors: In vitro and in silico studies. Bioorg. Chem. 2020, 96, 103625. [Google Scholar] [CrossRef]
- Rascón-Chu, A.; Martínez-López, A.L.; Carvajal-Millán, E.; de León-Renova, N.E.P.; Márquez-Escalante, J.A.; Romo-Chacón, A. Pectin from low quality Golden Delicious apples: Composition and gelling capability. Food Chem. 2009, 116, 101–103. [Google Scholar] [CrossRef]
- Patton, D. Cellulose from apple tissue: Isolation, purification and chemical modification. Can. Inst. Food Sci. Technol. J. 1974, 7, 61–64. [Google Scholar] [CrossRef]
- Guzowska, M.; Dziendzikowska, K.; Kopiasz, Ł.; Gajewska, M.; Wilczak, J.; Harasym, J.; Czerwinska, M.; Gromadzka-Ostrowska, J. Oat beta-glucans modulate the gut microbiome, barrier function, and immune responses in an in vivo model of early-stage colorectal cancer. Int. J. Mol. Sci. 2024, 25, 13586. [Google Scholar] [CrossRef]
- Stephen, A.M.; Champ, M.M.J.; Cloran, S.J.; Fleith, M.; van Lieshout, L.; Mejborn, H.; Burley, V.J. Dietary fibre in Europe: Current state of knowledge on definitions, recommendations, intakes and relationship to health. Nutr. Res. Rev. 2017, 30, 149–190. [Google Scholar] [CrossRef]
- Bouayed, J.; Hoffmann, L.; Bohn, T. Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: Bioaccessibility and potential uptake. Food Chem. 2011, 128, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriére, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
| Before Digestion | Gastric Phase | Gastric Phase with β-Glucan 1 | Gastric Phase with β-Glucan 2 | Intestinal Phase | Intestinal Phase with β-Glucan 1 | Intestinal Phase with β-Glucan 2 | |
|---|---|---|---|---|---|---|---|
| Anthocyanins | |||||||
| cyanidin-3-galactoside | 26.0 ± 2.9 a | 7.8 ± 1.4 b | 7.2 ± 1.9 b | 6.4 ± 1.0 b | 9.0 ± 5.2 b | 7.7 ± 0.7 b | 6.9 ± 4.0 b |
| cyanidin-3-glucoside | 6.1 ± 0.7 a | 3.6 ± 0.6 b | 3.5 ± 0.7 b | 2.9 ± 0.3 b | |||
| Total | 32.1 ± 3.6 a | 11.4 ± 2.0 b | 10.7 ± 2.6 b | 9.3 ± 1.3 b | 9.0 ± 5.2 b | 7.7 ± 0.7 b | 6.9 ± 4.0 b |
| Flavan-3-ols | |||||||
| procyanidin B1 | 38.7 ± 2.0 b | 86.4 ± 6.3 a | 76.1 ± 18.5 a | 77.1 ± 14.2 a | |||
| (+)-catechin | 28.0 ± 4.6 a | 26.3 ± 1.0 a | 24.2 ± 5.0 a | 24.0 ± 0.6 a | 23.8 ± 9.8 a | 21.1 ± 1.7 a | 16.6 ± 2.6 a |
| procyanidin B2 | 105.9 ± 7.5 a,b | 152.8 ± 17.5 a | 146.2 ± 77.4 a | 127.6 ± 32.1 a | 24.8 ± 20.9 b,c | 11.9 ± 2.1 c | 11.4 ± 1.7 c |
| (−)-epicatechin | 194.5 ± 18.3 a | 80.4 ± 10.7 b | 73.6 ± 18.4 b | 67.9 ± 11.1 b | 55.8 ± 16.8 b | 54.8 ± 3.5 b | 48.6 ± 2.0 b |
| Total | 367.1 ± 32.4 a | 345.8 ± 35.5 a | 320.1 ± 119.4 a | 296.7 ± 57.9 a | 104.5 ± 47.4 b | 87.7 ± 7.3 b | 76.7 ± 6.3 b |
| Dihydrochalcones | |||||||
| phloretin-2-glucoside | 39.5 ± 4.1 a | 27.0 ± 3.0 b,c | 25.0 ± 2.0 b,c | 21.5 ± 1.5 c | 29.1 ± 2.9 b | 24.3 ± 1.5 b,c | 21.2 ± 2.6 c |
| Total | 39.5 ± 4.1 a | 27.0 ± 3.0 b,c | 25.0 ± 2.0 b,c | 21.5 ± 1.5 c | 29.1 ± 2.9 b | 24.3 ± 1.5 b,c | 21.2 ± 2.6 c |
| Phenolic acids | |||||||
| neochlorogenic acid | 8.7 ± 1.6 a | 9.2 ± 1.5 a | 7.6 ± 0.4 a | ||||
| chlorogenic acid | 110.9 ± 6.5 a | 109.8 ± 3.5 a | 107.6 ± 7.2 a | 106.7 ± 9.3 a | 80.2 ± 8.9 b | 82.3 ± 4.4 b | 79.8 ± 4.4 b |
| cryptochlorogenic acid | 8.8 ± 1.1 a,b | 9.6 ± 1.2 a | 7.6 ± 0.6 b | ||||
| p-coumaroylquinic acid | 28.4 ± 2.8 a | 19.5 ± 2.8 b | 19.3 ± 2.3 b | 17.3 ± 1.9 b | 22.7 ± 3.5 a,b | 21.8 ± 1.4 b | 20.6 ± 0.7 b |
| total | 139.3 ± 9.3 a | 129.2 ± 6.3 a | 126.9 ± 9.5 a | 124.0 ± 11.2 a | 120.3 ± 15.1 a | 122.9 ± 8.5 a | 115.6 ± 6.0 a |
| Flavonols | |||||||
| quercetin-3-galactoside | 9.2 ± 1.7 a | 9.5 ± 2.2 a | 8.0 ± 3.9 a | 6.3 ± 0.3 a | 6.2 ± 1.8 a | 6.2 ± 0.5 a | 6.8 ± 1.7 a |
| quercetin-3-rutinoside | 8.4 ± 1.0 a | 4.5 ± 0.5 b | 4.2 ± 0.8 b | 3.6 ± 0.2 b | 5.0 ± 0.9 b | 4.8 ± 0.3 b | 4.5 ± 0.2 b |
| quercetin-3-glucoside | 28.8 ± 3.6 a | 14.6 ± 1.8 b | 13.7 ± 2.7 b | 11.6 ± 0.8 b | 16.6 ± 3.4 b | 16.0 ± 1.1 b | 15.0 ± 0.5 b |
| quercetin-3-xyloside | 28.6 ± 3.4 a | 13.5 ± 1.9 b | 12.8 ± 2.2 b | 10.8 ± 0.9 b | 16.3 ± 3.3 b | 15.8 ± 1.2 b | 14.7 ± 0.3 b |
| quercetin-3-rhamnoside | 9.2 ± 1.1 a | 7.7 ± 1.3 a,b | 7.0 ± 1.7 a,b | 5.8 ± 0.3 b | 7.2 ± 1.2 a,b | 6.9 ± 0.0 a,b | 6.6 ± 1.3 a,b |
| Total | 84.1 ± 10.9 a | 49.8 ± 7.7 b | 45.7 ± 11.3 b | 38.2 ± 2.5 b | 51.2 ± 10.6 b | 49.6 ± 3.2 b | 47.6 ± 4.0 b |
| TOTAL | 662.1 ± 60.2 a | 563.2 ± 54.5 a | 528.5 ± 144.6 a | 489.7 ± 74.3 a,b | 314.1 ± 81.2 b,c | 292.1 ± 21.2 c | 268.0 ± 22.9 c |
| Sample | % Inhibition | ||
|---|---|---|---|
| 5 Min | 10 Min | 20 Min | |
| before digestion | 14.3 ± 3.9 c | 18.7 ± 3.4 b | 24.1 ± 1.3 b,c,d |
| gastric | 12.0 ± 0.2 c | 14.9 ± 0.2 b | 20.0 ± 0.1 c,d |
| gastric β-glucan 1 | 8.9 ± 1.1 c | 11.6 ± 1.4 b | 16.1 ± 1.8 d |
| gastric β-glucan 2 | 8.2 ± 0.1 c | 10.8 ± 0.1 b | 14.9 ± 0.4 d |
| intestinal | 28.3 ± 7.9 a,b | 32.8 ± 7.2 a | 36.3 ± 6.9 a |
| intestinal β-glucan 1 | 30.4 ± 2.1 a | 33.3 ± 2.1 a | 32.6 ± 1.7 a,b |
| intestinal β-glucan 2 | 15.4 ± 0.0 bc | 19.4 ± 1.4 b | 30.4 ± 1.4 a,b,c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakobek, L.; Kenjerić, D.; Šoher, L.; Matić, P. The Effect of β-Glucan on the Release and Antiradical Activity of Phenolic Compounds from Apples in Simulated Digestion. Molecules 2025, 30, 301. https://doi.org/10.3390/molecules30020301
Jakobek L, Kenjerić D, Šoher L, Matić P. The Effect of β-Glucan on the Release and Antiradical Activity of Phenolic Compounds from Apples in Simulated Digestion. Molecules. 2025; 30(2):301. https://doi.org/10.3390/molecules30020301
Chicago/Turabian StyleJakobek, Lidija, Daniela Kenjerić, Lidija Šoher, and Petra Matić. 2025. "The Effect of β-Glucan on the Release and Antiradical Activity of Phenolic Compounds from Apples in Simulated Digestion" Molecules 30, no. 2: 301. https://doi.org/10.3390/molecules30020301
APA StyleJakobek, L., Kenjerić, D., Šoher, L., & Matić, P. (2025). The Effect of β-Glucan on the Release and Antiradical Activity of Phenolic Compounds from Apples in Simulated Digestion. Molecules, 30(2), 301. https://doi.org/10.3390/molecules30020301







