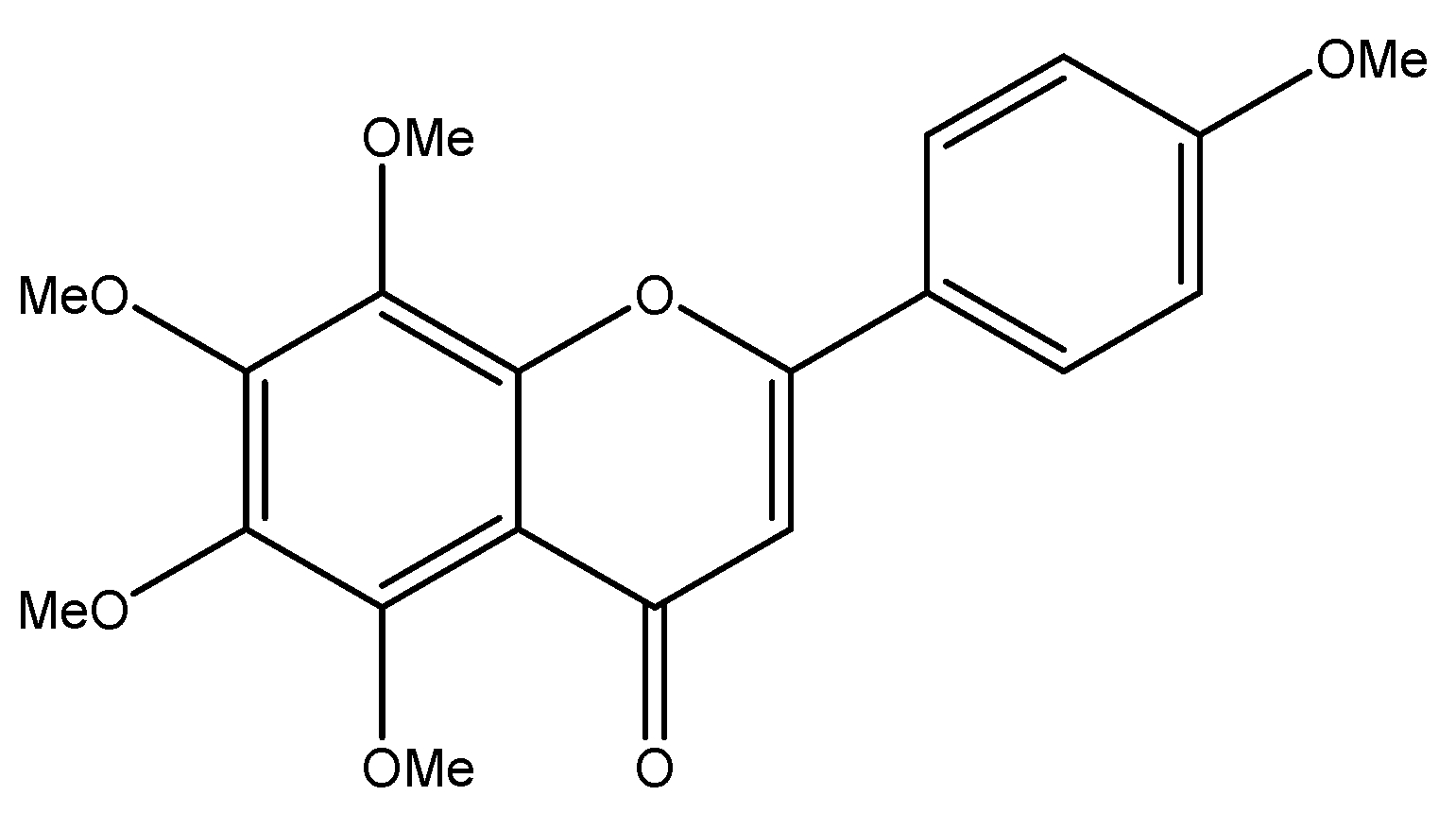
The Anticancer Perspective of Tangeretin: A Small Review
Abstract
1. Introduction
2. The Anticancer Potential of Tangeretin
2.1. Lung Cancer
2.2. Breast Cancer
2.3. Prostate Cancer
2.4. Bladder Cancer
2.5. Leukemia
2.6. Oral Cancer
2.7. Melanoma
2.8. Colorectal Cancer
2.9. Liver Cancer
2.10. Gastric Cancer
2.11. Ovarian Cancer
2.12. Osteosarcoma
3. Other Biological Activities of Tangeretin
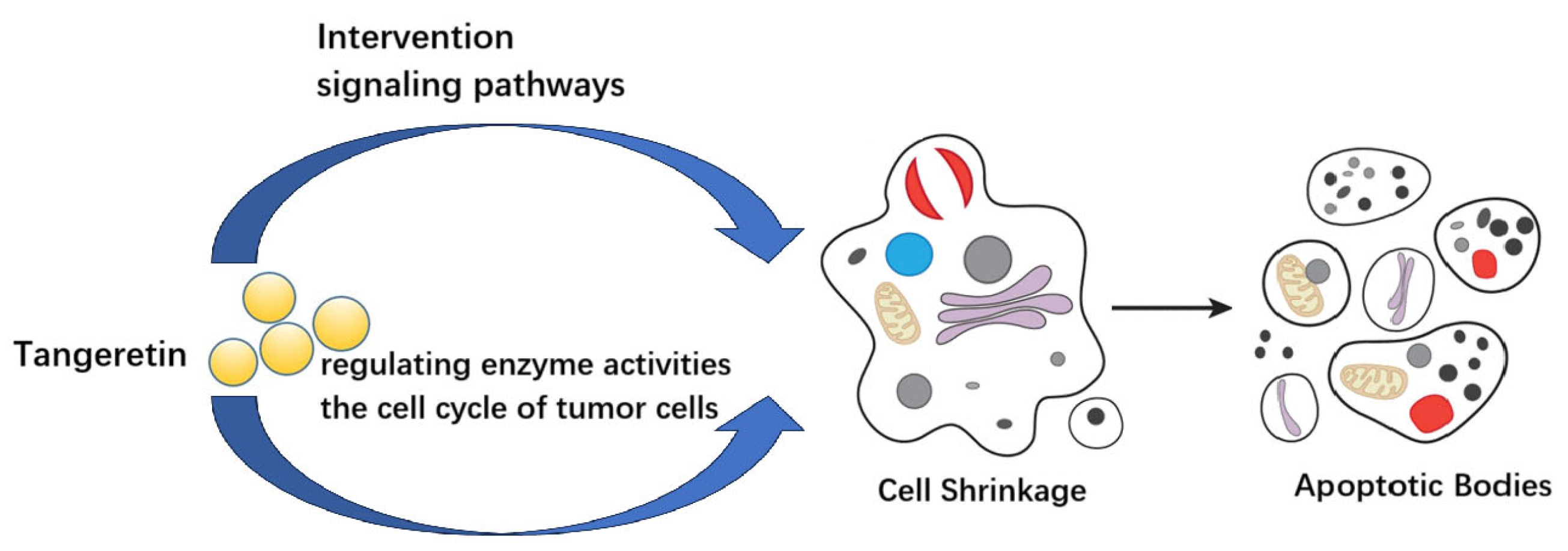
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, S.; Cao, Z.; Prettner, K.; Kuhn, M.; Yang, J.; Jiao, L.; Wang, Z.; Li, W.; Geldsetzer, P.; Baernighausen, T.; et al. Estimates and projections of the global economic cost of 29 cancers in 204 countries and territories from 2020 to 2050. JAMA Oncol. 2023, 9, 465–472. [Google Scholar] [CrossRef]
- Connal, S.; Cameron, J.M.; Sala, A.; Brennan, P.M.; Palmer, D.S.; Palmer, J.D.; Perlow, H.; Baker, M.J. Liquid biopsies: The future of cancer early detection. J. Transl. Med. 2023, 21, 118. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Brianna; Lee, S.H. Chemotherapy: How to reduce its adverse effects while maintaining the potency? Med. Oncol. 2023, 40, 88. [Google Scholar] [CrossRef]
- Bouabdallah, S.; Al-Maktoum, A.; Amin, A. steroidal saponins: Naturally occurring compounds as inhibitors of the hallmarks of cancer. Cancers 2023, 15, 3900. [Google Scholar] [CrossRef]
- Teles, Y.C.F.; Souza, M.S.R.; de Souza, M.D.V. Sulphated flavonoids: Biosynthesis, structures, and biological activities. Molecules 2018, 23, 480. [Google Scholar] [CrossRef] [PubMed]
- Micek, A.; Godos, J.; Del Rio, D.; Galvano, F.; Grosso, G. Dietary flavonoids and cardiovascular disease: A comprehensive dose-response meta-analysis. Mol. Nutr. Food Res. 2021, 65, 2001019. [Google Scholar] [CrossRef] [PubMed]
- Rakha, A.; Umar, N.; Rabail, R.; Butt, M.S.; Kieliszek, M.; Hassoun, A.; Aadil, R.M. Anti-inflammatory and anti-allergic potential of dietary flavonoids: A review. Biomed. Pharmacother. 2022, 156, 113945. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Zhao, Y.; Li, L.; He, Y.; Zhang, X.; Zhu, Y.; Hong, G. Comparative metabolomics of flavonoids in twenty vegetables reveal their nutritional diversity and potential health benefits. Food Res. Int. 2023, 164, 112384. [Google Scholar] [CrossRef] [PubMed]
- Dias, M.C.; Pinto, D.; Silva, A.M.S. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef] [PubMed]
- Naeem, H.; Momal, U.; Imran, M.; Shahbaz, M.; Hussain, M.; Alsagaby, S.A.; Al Abdulmonem, W.; Umar, M.; Mujtaba, A.; El-Ghorab, A.H.; et al. Anticancer perspectives of genistein: A comprehensive review. Int. J. Food Prop. 2023, 26, 3305–3341. [Google Scholar] [CrossRef]
- Azeem, M.; Hanif, M.; Mahmood, K.; Ameer, N.; Chughtai, F.R.S.; Abid, U. An insight into anticancer, antioxidant, antimicrobial, antidiabetic and anti-inflammatory effects of quercetin: A review. Polym. Bull. 2023, 80, 241–262. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-X.; Ma, J.; Li, X.-Y.; Wu, Y.; Shi, H.; Chen, Y.; Lu, G.; Shen, H.-M.; Lu, G.-D.; Zhou, J. Quercetin induces p53-independent cancer cell death through lysosome activation by the transcription factor EB and Reactive Oxygen Species-dependent ferroptosis. Br. J. Pharmacol. 2021, 178, 1133–1148. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Ye, H.; Kamaraj, R.; Zhang, T.; Zhang, J.; Pavek, P. A review on pharmacological activities and synergistic effect of quercetin with small molecule agents. Phytomedicine 2021, 92, 153736. [Google Scholar] [CrossRef]
- Mahapatra, D.M.; Bharti, S.K.; Asati, V. Anti-cancer chalcones: Structural and molecular target perspectives. Eur. J. Med. Chem. 2015, 98, 69–114. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Li, J.; Chen, X.; Fu, X.; Sun, S.; Wu, Q. Chalcone derivatives: Role in anticancer therapy. Biomolecules 2021, 11, 894. [Google Scholar] [CrossRef]
- Rammohan, A.; Reddy, J.S.; Sravya, G.; Rao, C.N.; Zyryanov, G.V. Chalcone synthesis, properties and medicinal applications: A review. Environ. Chem. Lett. 2020, 18, 433–458. [Google Scholar] [CrossRef]
- Chen, J.; Montanari, A.M. Isolation and identification of new polymethoxyflavonoids from dancy tangerine leaves. J. Agric. Food Chem. 1998, 46, 1235–1238. [Google Scholar] [CrossRef]
- Mouly, P.; Gaydou, E.M.; Auffray, A. Simultaneous separation of flavanone glycosides and polymethoxylated flavones in citrus juices using liquid chromatography. J. Chromatogr. A 1998, 800, 171–179. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, Q.; Cong, P.; Zhu, Z. Simultaneous determination of flavonoids in different citrus fruit juices and beverages by high-performance liquid chromatography and analysis of their chromatographic profiles by chemometrics. Anal. Methods 2012, 4, 3748–3753. [Google Scholar] [CrossRef]
- Mitani, R.; Tashiro, H.; Arita, E.; Ono, K.; Haraguchi, M.; Tokunaga, S.; Sharmin, T.; Aida, T.M.; Mishima, K. Extraction of nobiletin and tangeretin with antioxidant activity from peels of Citrus poonensis using liquid carbon dioxide and ethanol entrainer. Sep. Sci. Technol. 2021, 56, 290–300. [Google Scholar] [CrossRef]
- Chien, W.-J.; Saputri, D.S.; Lin, H.-Y. Valorization of Taiwan’s Citrus depressa Hayata peels as a source of nobiletin and tangeretin using simple ultrasonic-assisted extraction. Curr. Res. Food Sci. 2022, 5, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Calomme, M.; Pieters, L.; Vlietinck, A.; Vanden Berghe, D. Inhibition of bacterial mutagenesis by Citrus flavonoids. Planta Medica 1996, 62, 222–226. [Google Scholar] [CrossRef]
- Morley, K.L. Investigating the Cellular and Molecular Effects of Citrus Flavonoids Tangeretin and Nobiletin in Human Cancer Cells. Ph.D. Thesis, University of Western Ontario, London, ON, Canada, 2008. [Google Scholar]
- Abdel-Fattah, M.M.; Mohamed, W.R.; Hassanein, E.H.M.; Arab, H.A.; Arafa, E.A. Role of NF-κB/ICAM-1, JAK/STAT-3, and apoptosis signaling in the anticancer effect of tangeretin against urethane-induced lung cancer in BALB/c mice. Life Sci. 2023, 325, 121749. [Google Scholar] [CrossRef]
- Liu, X.; Chen, L.; Liu, Y.; Zhang, T. Tangeretin sensitises human lung cancer cells to TRAIL-induced apoptosis via ROS-JNK/ERK-CHOP pathwaymediated up-regulation of death receptor 5. Trop. J. Pharm. Res. 2017, 16, 17–29. [Google Scholar] [CrossRef]
- Xie, Y.; Feng, S.-l.; He, F.; Yan, P.-Y.; Yao, X.-J.; Fan, X.-X.; Leung, E.L.-H.; Zhou, H. Down-regulating Nrf2 by tangeretin reverses multiple drug resistance to both chemotherapy and EGFR tyrosine kinase inhibitors in lung cancer. Pharmacol. Res. 2022, 186, 106514. [Google Scholar] [CrossRef]
- Li, Y.R.; Li, S.; Ho, C.-T.; Chang, Y.-H.; Tan, K.-T.; Chung, T.-W.; Wang, B.-Y.; Chen, Y.-K.; Lin, C.-C. Tangeretin derivative, 5-acetyloxy-6,7,8,4-tetramethoxyflavone induces G2/M arrest, apoptosis and autophagy in human non-small cell lung cancer cells in vitro and in vivo. Cancer Biol. Ther. 2016, 17, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Roshini, A.; Jagadeesan, S.; Arivazhagan, L.; Cho, Y.-J.; Lim, J.-H.; Doh, Y.-H.; Kim, S.-J.; Na, J.; Choi, K.H. pH-sensitive tangeretin-ZnO quantum dots exert apoptotic and anti-metastatic effects in metastatic lung cancer cell line. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 477–488. [Google Scholar] [CrossRef]
- Akram, M.; Iqbal, M.; Daniyal, M.; Khan, A.U. Awareness and current knowledge of breast cancer. Biol. Res. 2017, 50, 33. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Kim, D.; Ko, S.; Kim, A.; Mo, K.; Yoon, H. Breast Cancer metastasis: Mechanisms and therapeutic implications. Int. J. Mol. Sci. 2022, 23, 6806. [Google Scholar] [CrossRef]
- Katsura, C.; Ogunmwonyi, I.; Kankam, H.K.N.; Saha, S. Breast cancer: Presentation, investigation and management. Br. J. Hosp. Med. 2022, 83, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rojas, K.; Stuckey, A. Breast Cancer epidemiology and risk factors. Clin. Obstet. Gynecol. 2016, 59, 651–672. [Google Scholar] [CrossRef] [PubMed]
- Periyasamy, K.; Baskaran, K.; Ilakkia, A.; Vanitha, K.; Selvaraj, S.; Sakthisekaran, D. Antitumor efficacy of tangeretin by targeting the oxidative stress mediated on 7,12-dimethylbenz(a) anthracene-induced proliferative breast cancer in Sprague-Dawley rats. Cancer Chemother. Pharmacol. 2015, 75, 263–272. [Google Scholar] [CrossRef]
- Lakshmi, A.; Subramanian, S. Chemotherapeutic effect of tangeretin, a polymethoxylated flavone studied in 7, 12-dimethylbenz(a)anthracene induced mammary carcinoma in experimental. Biochimie 2014, 99, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Arivazhagan, L.; Subramanian, S.P. Tangeretin, a citrus flavonoid attenuates oxidative stress and protects hepatocellular architecture in rats with 7, 12-dimethylbenz(a)anthracene induced experimental mammary carcinoma. J. Funct. Foods 2015, 15, 339–353. [Google Scholar] [CrossRef]
- Lakshmi, A.; Subramanian, S.P. Tangeretin ameliorates oxidative stress in the renal tissues of rats with experimental breast cancer induced by 7,12-dimethylbenz a anthracene. Toxicol. Lett. 2014, 229, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Arivazhagan, L.; Pillai, S.S. Tangeretin, a citrus pentamethoxyflavone, exerts cytostatic effect via p53/p21 up-regulation and suppresses metastasis in 7,12-dimethylbenz(α)anthracene-induced rat mammary carcinoma. J. Nutr. Biochem. 2014, 25, 1140–1153. [Google Scholar] [CrossRef] [PubMed]
- Surichan, S.; Arroo, R.R.; Tsatsakis, A.M.; Androutsopoulos, V.P. Tangeretin inhibits the proliferation of human breast cancer cells via CYP1A1/CYP1B1 enzyme induction and CYP1A1/CYP1B1-mediated metabolism to the product 4′ hydroxy tangeretin. Toxicol. Vitr. 2018, 50, 274–284. [Google Scholar] [CrossRef]
- Periyasamy, K.; Sivabalan, V.; Baskaran, K.; Kasthuri, K.; Sakthisekaran, D. Cellular metabolic energy modulation by tangeretin in 7,12-dimethylbenz(a) anthracene-induced breast cancer. J. Biomed. Res. 2016, 30, 134–141. [Google Scholar] [CrossRef]
- Hermawan, A.; Putri, H.; Hanif, N.; Ikawati, M. Integrative bioinformatics study of tangeretin potential targets for preventing metastatic breast cancer. Evid. Based Complement. Altern. Med. 2021, 2021, 2234554. [Google Scholar] [CrossRef]
- Dai, C.; Heemers, H.; Sharifi, N. Androgen signaling in prostate cancer. Cold Spring Harb. Perspect. Med. 2017, 7, a030452. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.; McManus, J.M.; Sharifi, N. Hormonal therapy for prostate cancer. Endocr. Rev. 2021, 42, 354–373. [Google Scholar] [CrossRef]
- Westaby, D.; Fenor de La Maza, M.d.L.D.; Paschalis, A.; Jimenez-Vacas, J.M.; Welti, J.; de Bono, J.; Sharp, A. A New old target: Androgen receptor signaling and advanced prostate cancer. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 131–153. [Google Scholar] [CrossRef] [PubMed]
- Attard, G.; Parker, C.; Eeles, R.A.; Schroder, F.; Tomlins, S.A.; Tannock, I.; Drake, C.G.; de Bono, J.S. Prostate cancer. Lancet 2016, 387, 70–82. [Google Scholar] [CrossRef]
- Zhu, W.-B.; Xiao, N.; Liu, X.-J. Dietary flavonoid tangeretin induces reprogramming of epithelial to mesenchymal transition in prostate cancer cells by targeting the PI3K/Akt/mTOR signaling pathway. Oncol. Lett. 2018, 15, 433–440. [Google Scholar] [CrossRef]
- Guo, J.J.; Li, Y.J.; Xin, L.L. Tangeretin prevents prostate cancer cell proliferation and induces apoptosis via activation of Notch signalling and regulating the androgen receptor (AR) pathway and the phosphoinositide 3-kinase (PI3k)/Akt/mTOR pathways. Bangladesh J. Pharmacol. 2015, 10, 937–947. [Google Scholar] [CrossRef]
- Wei, G.J.; Chao, Y.H.; Tung, Y.C.; Wu, T.Y.; Su, Z.Y. A Tangeretin derivative inhibits the growth of human prostate cancer LNCaP cells by epigenetically restoring p21 gene expression and inhibiting cancer stem-like cell proliferation. AAPS J. 2019, 21, 86. [Google Scholar] [CrossRef]
- Lopez-Beltran, A.; Cookson, M.S.; Guercio, B.J.; Cheng, L. Advances in diagnosis and treatment of bladder cancer. BMJ Br. Med. J. 2024, 384, e076743. [Google Scholar] [CrossRef] [PubMed]
- Van Hoogstraten, L.M.C.; Vrieling, A.; van der Heijden, A.G.; Kogevinas, M.; Richters, A.; Kiemeney, L.A. Global trends in the epidemiology of bladder cancer: Challenges for public health and clinical practice. Nat. Rev. Clin. Oncol. 2023, 20, 287–304. [Google Scholar] [CrossRef] [PubMed]
- Ascione, C.M.; Napolitano, F.; Esposito, D.; Servetto, A.; Belli, S.; Santaniello, A.; Scagliarini, S.; Crocetto, F.; Bianco, R.; Formisano, L. Role of FGFR3 in bladder cancer: Treatment landscape and future challenges. Cancer Treat. Rev. 2023, 115, 102530. [Google Scholar] [CrossRef]
- Lin, J.-J.; Huang, C.-C.; Su, Y.-L.; Luo, H.-L.; Lee, N.-L.; Sung, M.-T.; Wu, Y.-J. Proteomics analysis of tangeretin-induced apoptosis through mitochondrial dysfunction in bladder cancer cells. Int. J. Mol. Sci. 2019, 20, 1017. [Google Scholar] [CrossRef] [PubMed]
- Ikegawa, T.; Ushigome, F.; Koyabu, N.; Morimoto, S.; Shoyama, Y.; Naito, M.; Tsuruo, T.; Ohtani, H.; Sawada, Y. Inhibition of P-glycoprotein by orange juice components, polymethoxyflavones in adriamycin-resistant human myelogenous leukemia (K562/ADM) cells. Cancer Lett. 2000, 160, 21–28. [Google Scholar] [CrossRef]
- Ishii, K.; Tanaka, S.; Kagami, K.; Henmi, K.; Toyoda, H.; Kaise, T.; Hirano, T. Effects of naturally occurring polymethyoxyflavonoids on cell growth, P-glycoprotein function, cell cycle, and apoptosis of daunorubicin-resistant T lymphoblastoid leukemia Cells. Cancer Investig. 2010, 28, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Abe, K.; Gotoh, M.; Oka, K. Citrus flavone tangeretin inhibits leukaemic HL-60 cell growth partially through induction of apoptosis with less cytotoxicity on normal lymphocytes. Br. J. Cancer 1995, 72, 1380–1388. [Google Scholar] [CrossRef]
- Lust, S.; Vanhoecke, B.; Van Gele, M.; Philippe, J.; Bracke, M.; Offner, F. The flavonoid tangeretin activates the unfolded protein response and synergizes with imatinib in the erythroleukemia cell line K562. Mol. Nutr. Food Res. 2010, 54, 823–832. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Nanavati, R.; Modi, T.G.; Dobariya, C. Oral cancer: Etiology and risk factors: A review. J. Cancer Res. Ther. 2016, 12, 458–463. [Google Scholar] [CrossRef]
- Montero, P.H.; Patel, S.G. Cancer of the oral cavity. Surg. Oncol. Clin. N. Am. 2015, 24, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Warnakulasuriya, S.; Kerr, A.R. Oral Cancer Screening: Past, present, and future. J. Dent. Res. 2021, 100, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Gv, V.; Ranganathan, P., Jr.; Palati, S. Tangeretin’s Anti-apoptotic signaling mechanisms in oral cancer cells: In vitro anti-cancer activity. Cureus 2023, 15, e47452. [Google Scholar] [CrossRef]
- Poniewierska-Baran, A.; Sluczanowska-Glabowska, S.; Malkowska, P.; Sierawska, O.; Zadroga, L.; Pawlik, A.; Niedzwiedzka-Rystwej, P. Role of miRNA in melanoma development and progression. Int. J. Mol. Sci. 2023, 24, 201. [Google Scholar] [CrossRef] [PubMed]
- Manthey, J.A.; Guthrie, N. Antiproliferative activities of citrus flavonoids against six human cancer cell lines. J. Agric. Food Chem. 2002, 50, 5837–5843. [Google Scholar] [CrossRef] [PubMed]
- Martinez Conesa, C.; Vicente Ortega, V.; Yanez Gascon, M.J.; Alcaraz Banos, M.; Canteras Jordana, M.; Benavente-Garcia, O.; Castillo, J. Treatment of metastatic melanoma B16F10 by the flavonoids tangeretin, rutin, and diosmin. J. Agric. Food Chem. 2005, 53, 6791–6797. [Google Scholar] [CrossRef]
- Rodriguez, J.; Yáñez, J.; Vicente, V.; Alcaraz, M.; Benavente-García, O.; Castillo, J.; Lorente, J.; Lozano, J.A. Effects of several flavonoids on the growth of B16F10 and SK-MEL-1 melanoma cell lines: Relationship between structure and activity. Melanoma Res. 2002, 12, 99–107. [Google Scholar] [CrossRef]
- Yoon, H.S.; Ko, H.C.; Kim, S.S.; Park, K.J.; An, H.J.; Choi, Y.H.; Kim, S.J.; Lee, N.H.; Hyun, C.G. Tangeretin triggers melanogenesis through the activation of melanogenic signaling proteins and sustained extracellular signal-regulated kinase in B16/F10 murine melanoma cells. Nat. Prod. Commun. 2015, 10, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Chen, W.J.; Lin-Shiau, S.Y.; Ho, C.T.; Lin, J.K. Tangeretin induces cell-cycle G1 arrest through inhibiting cyclin-dependent kinases 2 and 4 activities as well as elevating Cdk inhibitors p21 and p27 in human colorectal carcinoma cells. Carcinogenesis 2002, 23, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Morley, K.L.; Ferguson, P.J.; Koropatnick, J. Tangeretin and nobiletin induce G1 cell cycle arrest but not apoptosis in human breast and colon cancer cells. Cancer Lett. 2007, 251, 168–178. [Google Scholar] [CrossRef]
- Lee, A.C.-L.; Hsiao, W.-C.; Wright, D.E.; Chong, S.Y.; Leow, S.K.; Ho, C.-T.; Kao, C.-F.; Lo, Y.-C. Induction of GADD45α expression contributes to the anti-proliferative effects of polymethoxyflavones on colorectal cancer cells. J. Funct. Foods 2013, 5, 616–624. [Google Scholar] [CrossRef]
- Bai, Y.; Xiong, Y.; Zhang, Y.-Y.; Cheng, L.; Liu, H.; Xu, K.; Wu, Y.-Y.; Field, J.; Wang, X.-D.; Zhou, L.-M. Tangeretin synergizes with 5-fluorouracil to induce autophagy through MicroRNA-21 in colorectal cancer cells. Am. J. Chin. Med. 2022, 50, 1681–1701. [Google Scholar] [CrossRef]
- Dey, D.K.; Chang, S.N.; Vadlamudi, Y.; Park, J.G.; Kang, S.C. Synergistic therapy with tangeretin and 5-fluorouracil accelerates the ROS/JNK mediated apoptotic pathway in human colorectal cancer cell. Food Chem. Toxicol. 2020, 143, 111529. [Google Scholar] [CrossRef]
- Bao, H.; Zheng, N.; Li, Z.; Zhi, Y. Synergistic effect of tangeretin and atorvastatin for colon cancer combination therapy: Targeted delivery of these dual drugs using RGD peptide decorated nanocarriers. Drug Des. Dev. Ther. 2020, 14, 3057–3068. [Google Scholar] [CrossRef] [PubMed]
- Ting, Y.; Chiou, Y.-S.; Pan, M.-H.; Ho, C.-T.; Huang, Q. In vitro and in vivo anti-cancer activity of tangeretin against colorectal cancer was enhanced by emulsion-based delivery system. J. Funct. Foods 2015, 15, 264–273. [Google Scholar] [CrossRef]
- Yahoo, N.; Dudek, M.; Knolle, P.; Heikenwaelder, M. Role of immune responses in the development of NAFLD- associated liver cancer and prospects for therapeutic modulation. J. Hepatol. 2023, 79, 538–551. [Google Scholar] [CrossRef] [PubMed]
- Anwanwan, D.; Singh, S.K.; Singh, S.; Saikam, V.; Singh, R. Challenges in liver cancer and possible treatment approaches. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188314. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.-H.; Lee, H.-Y.; Chu, Y.-L.; Ho, C.-T.; Sheen, L.-Y. Bitter orange peel extract induces endoplasmic reticulum-mediated autophagy in human hepatoma cells. J. Funct. Foods 2019, 60, 103404. [Google Scholar] [CrossRef]
- Cheng, Z.; Surichan, S.; Ruparelia, K.; Arroo, R.; Boarder, M.R. Tangeretin and its metabolite 4′-hydroxytetramethoxyflavone attenuate EGF-stimulated cell cycle progression in hepatocytes; role of inhibition at the level of mTOR/p70S6K. Br. J. Pharmacol. 2011, 162, 1781–1791. [Google Scholar] [CrossRef]
- Dong, Y.; Cao, A.; Shi, J.; Yin, P.; Wang, L.; Ji, G.; Xie, J.; Wu, D. Tangeretin, a citrus polymethoxyflavonoid, induces apoptosis of human gastric cancer AGS cells through extrinsic and intrinsic signaling pathways. Oncol. Rep. 2014, 31, 1788–1794. [Google Scholar] [CrossRef] [PubMed]
- Yumnam, S.; Raha, S.; Kim, S.M.; Saralamma, V.V.G.; Lee, H.J.; Ha, S.E.; Heo, J.D.; Lee, S.J.; Kim, E.H.; Lee, W.S.; et al. Identification of a novel biomarker in tangeretin-induced cell death in AGS human gastric cancer cells. Oncol. Rep. 2018, 40, 3249–3260. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Zhang, H.; Chen, J.; Cao, J.; Chen, Q.; Li, X.; Sun, C. Polymethoxyflavones from citrus inhibited gastric cancer cell proliferation through inducing apoptosis by upregulating RARβ, both in vitro and in vivo. Food Chem. Toxicol. 2020, 146, 111811. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, L.; Sun, Y.; Wang, T.; Wang, B. Tangeretin enhances radiosensitivity and inhibits the radiation-induced epithelial-mesenchymal transition of gastric cancer cells. Oncol. Rep. 2015, 34, 302–310. [Google Scholar] [CrossRef]
- Moufarrij, S.; Dandapani, M.; Arthofer, E.; Gomez, S.; Srivastava, A.; Lopez-Acevedo, M.; Villagra, A.; Chiappinelli, K.B. Epigenetic therapy for ovarian cancer: Promise and progress. Clin. Epigenetics 2019, 11, 7. [Google Scholar] [CrossRef]
- Cheng, H.; Wang, Z.; Fu, L.; Xu, T. Macrophage Polarization in the development and progression of ovarian cancers: An overview. Front. Oncol. 2019, 9, 421. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Li, B.; Rankin, G.O.; Rojanasakul, Y.; Chen, Y.C. Selecting bioactive phenolic compounds as potential agents to inhibit proliferation and VEGF expression in human ovarian cancer cells. Oncol. Lett. 2015, 9, 1444–1450. [Google Scholar] [CrossRef] [PubMed]
- Arafa, E.-S.A.; Zhu, Q.; Barakat, B.M.; Wani, G.; Zhao, Q.; El-Mahdy, M.A.; Wani, A.A. Tangeretin sensitizes cisplatin-resistant human ovarian cancer cells through down regulation of phosphoinositide 3-kinase/Akt signaling pathway. Cancer Res. 2009, 69, 8910–8917. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.K.; Tellez-Gabriel, M.; Heymann, D. Cancer stem cells in osteosarcoma. Cancer Lett. 2017, 386, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Gianferante, D.M.; Mirabello, L.; Savage, S.A. Germline and somatic genetics of osteosarcoma—Connecting aetiology, biology and therapy. Nat. Rev. Endocrinol. 2017, 13, 480–491. [Google Scholar] [CrossRef]
- Gurunathan, S.; Jeyaraj, M.; Kang, M.-H.; Kim, J.-H. Tangeretin-assisted platinum nanoparticles enhance the apoptotic properties of doxorubicin: Combination therapy for osteosarcoma treatment. Nanomaterials 2019, 9, 1089. [Google Scholar] [CrossRef] [PubMed]
- Satsu, H.; Shibata, R.; Suzuki, H.; Kimura, S.; Shimizu, M. Inhibitory Effect of tangeretin and cardamonin on human intestinal SGLT1 activity in vitro and blood glucose levels in mice in vivo. Nutrients 2021, 13, 3382. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.J.; Chen, J.F.; Ren, W.; Zhu, Y.K.; Zhao, Q.; Zhang, K.N.; Su, D.M.; Qiu, C.; Zhang, W.; Li, K. Citrus flavone tangeretin is a potential insulin sensitizer targeting hepatocytes through suppressing MEK-ERK1/2 pathway. Biochem. Biophys. Res. Commun. 2020, 529, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.Y.; Lee, E.-J.; Park, J.-S.; Jang, S.-E.; Kim, D.-H.; Kim, H.-S. Anti-inflammatory and antioxidant mechanism of tangeretin in activated microglia. J. Neuroimmune Pharmacol. 2016, 11, 294–305. [Google Scholar] [CrossRef]
- Yang, T.S.; Feng, C.W.; Wang, D.Y.; Qu, Y.Y.; Yang, Y.; Wang, Y.L.; Sun, Z.R. Neuroprotective and anti-inflammatory effect of tangeretin against cerebral ischemia-reperfusion onjury in rats. Inflammation 2020, 43, 2332–2343. [Google Scholar] [CrossRef]
| Cancer Type | In Vitro/In Vivo | Experimental Model | Effect | References and Remarks |
|---|---|---|---|---|
| Lung cancer | In vivo | Male BALB/c mice | Decreased the expression of NF-κB/ICAM-1 and JAK/STAT, and promoted caspase-3 signal transduction | [25] |
| In vitro | Human lung cancer cells H1299 and H1975 | Promoted DR4 and DR5 expression | [26] | |
| In vivo and in vitro | Human non-small-cell lung cancer (NSCLC) cell lines NCI-H1819, A549, NCI-H1975, HCC827, and HCT-8 and ABCB1 overexpression-resistant cell line A549/T; female BALB/c nude mouse | Inhibited cellular antioxidant signal Nrf2 | [27] | |
| In vivo and in vitro | NSCLC cell line; BALB/c nude mice without thymus; CL1-5 cells | Induced G2/M stagnation and down-regulated Bcl-2, XIAP, and survivin | [28] (Note: tangeretin derivative 5-AcTMF was used) | |
| In vitro | NCI-H358 metastatic lung cancer cells | Induced DNA damage of H358 metastatic lung cancer cells; decreased expression of MMP2, MMP9, and VEGF proteins | [29] (using tangeretin–ZnO quantum dots) | |
| Breast cancer | In vivo | Female Sprague Dawley rats (Rattus norvegicus) | Decreased the levels of NO, LPO, and CEA and increased the levels of enzymatic antioxidants | [34] |
| In vivo | Female Wistar rats | Resulted in anti-proliferation and a low ER, PR, and HER2/neu expression | [35] | |
| In vivo | Female Wistar rats | Upregulated p53/p21; downregulated PCNA, COX-2, and Ki-67; down-regulated MMPs and VEGF; and inhibited CDK kinase activity | [38] | |
| In vitro | MCF7 and MDA-MB-468 cells | Caused stasis of cancer cell cycle in G1 phase | [39] | |
| In vivo | Female Sprague Dawley rats (Rattus norvegicus) | Inhibited EMP enzyme activity and enhanced TCA enzyme activity | [40] | |
| Prostate cancer | In vitro | Prostate cancer PC-3 and LNCaP cell lines | Targeted the PI3K/Akt/mTOR signaling pathway | [46] |
| Bladder cancer | In vitro | BFTC-905 cells | Induced mitochondrial dysfunction | [52] |
| Leukemia | In vitro | Human promyelocytic leukemia HL-60 cells | Induced apoptosis | [55] |
| In vitro | The human erythroleukemia cell line K562 | Activated UPR in K562 cells; regulated the expression of Bcl-2 family members in K562 cells; blocked G2/M | [56] | |
| Oral cancer | In vitro | KB cells | Regulated pro-apoptotic and anti-apoptotic genes | [60] |
| Melanoma | In vitro | SK-MEL5 human melanoma cells | Resulted in anti-proliferation | [62] |
| In vitro | B16F10 (highly metastatic subline of mouse melanoma B16) | Resulted in anti-proliferation and anti-metastasis | [63] | |
| Colorectal cancer | In vitro | Cell line COLO 205 | Induced G0/G1 cell cycle arrest | [66] |
| In vitro | HT-29 (human colorectal adenocarcinoma) cell line | Induced G1 cell cycle arrest | [67] | |
| In vitro | HCT116 cells | Induced GADD45α expression and anti-proliferation | [68] | |
| In vitro | Human colorectal carcinoma HCT-116 cells (ATCC) and HCT-15 cells (KCLB) | Induced programmed cell death through JNK-mediated signaling pathway; increased DNA damage and inhibited DNA repair; regulated oxidative stress | [70] | |
| Liver cancer | In vitro | Human liver cancer Hep3B | Induced endoplasmic reticulum-mediated autophagy in human hepatoma cells | [75] |
| In vitro | Liver cells isolated from male Wistar-strain rats | Regulated cell cycle progression | [76] | |
| Gastric cancer | In vitro | Human gastric cancer cell line AGS | Caused exogenous and endogenous signaling pathways to induce apoptosis of AGS cells | [77] |
| In vitro | Human AGS gastric cancer cell line | Induced cell death | [78] | |
| In vitro and in vivo | Gastric cancer cell lines AGS, BGC-823, and SGC-7901; BALB/c nude mice (5–6 weeks of age) | Up-regulated RARβ-induced apoptosis | [79] | |
| Ovarian cancer | In vitro | Human ovarian cancer A2780 cells and their homologous cisplatin-resistant A2780/CP70 and cisplatin-sensitive and cisplatin-resistant human ovarian cancer cell lines 2008 and 2008/C13 | Downregulated phosphoinositol 3-kinase/Akt signaling pathway | [84] |
| Osteosarcoma | In vitro | U2OS cells | Resulted in oxidative stress and DNA damage; damaged the integrity of the membrane; increased the levels of NO and carbonyl protein; led to mitochondrial dysfunction; changed the cell morphology | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Yan, X.; Zhuang, J.; Hao, H. The Anticancer Perspective of Tangeretin: A Small Review. Molecules 2025, 30, 300. https://doi.org/10.3390/molecules30020300
Xu Y, Yan X, Zhuang J, Hao H. The Anticancer Perspective of Tangeretin: A Small Review. Molecules. 2025; 30(2):300. https://doi.org/10.3390/molecules30020300
Chicago/Turabian StyleXu, Yuan, Xi Yan, Junpeng Zhuang, and Haijun Hao. 2025. "The Anticancer Perspective of Tangeretin: A Small Review" Molecules 30, no. 2: 300. https://doi.org/10.3390/molecules30020300
APA StyleXu, Y., Yan, X., Zhuang, J., & Hao, H. (2025). The Anticancer Perspective of Tangeretin: A Small Review. Molecules, 30(2), 300. https://doi.org/10.3390/molecules30020300





