Technology for the Quantitative Identification of Dairy Products Based on Raman Spectroscopy, Chemometrics, and Machine Learning
Abstract
1. Introduction
2. Characterization of Dairy Products Based on Multidimensional Raman Spectroscopy
3. Standardization and Preprocessing of Raman Spectroscopy for Dairy Products
4. Quantitative Recognition of Dairy Products Based on Feature Extraction
5. Quantitative Identification of Dairy Products Based on Similarity Evaluation
6. Quantitative Identification of Dairy Products Based on Machine Learning Algorithms
7. Conclusions and Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smirnova, A.; Konoplev, G.; Mukhin, N.; Stepanova, O.; Steinmann, U. Milk as a complex multiphase polydisperse system: Approaches for the quantitative and qualitative analysis. J. Compos. Sci. 2020, 4, 151. [Google Scholar] [CrossRef]
- Nath, S. Advancements in food quality monitoring: Integrating biosensors for precision detection. Sustain. Food Technol. 2024, 2, 976–992. [Google Scholar] [CrossRef]
- Moore, J.C.; Spink, J.; Lipp, M. Development and application of a database of food ingredient fraud and economically motivated adulteration from 1980 to 2010. J. Food Sci. 2012, 77, R118–R126. [Google Scholar] [CrossRef]
- Yang, Q.; Niu, B.; Gu, S.; Ma, J.; Zhao, C.; Chen, Q.; Guo, D.; Deng, X.; Yu, Y.; Zhang, F. Rapid detection of nonprotein nitrogen adulterants in milk powder using point-scan Raman hyperspectral imaging technology. ACS Omega 2022, 7, 2064–2073. [Google Scholar] [CrossRef]
- Domingo, E.; Tirelli, A.A.; Nunes, C.A.; Guerreiro, M.C.; Pinto, S.M. Melamine detection in milk using vibrational spectroscopy and chemometrics analysis: A review. Food Res. Int. 2014, 60, 131–139. [Google Scholar] [CrossRef]
- Ellis, D.I.; Brewster, V.L.; Dunn, W.B.; Allwood, J.W.; Golovanov, A.P.; Goodacre, R. Fingerprinting food: Current technologies for the detection of food adulteration and contamination. Chem. Soc. Rev. 2012, 41, 5706–5727. [Google Scholar] [CrossRef]
- Liang, W.; Wei, Y.; Gao, M.; Yan, X.; Zhu, X.; Guo, W. Detection of melamine adulteration in milk powder by using optical spectroscopy technologies in the last decade—A review. Food Anal. Methods 2020, 13, 2059–2069. [Google Scholar] [CrossRef]
- Xiu, C.; Klein, K.K. Melamine in milk products in china: Examining the factors that led to deliberate use of the contaminant. Food Policy 2010, 35, 463–470. [Google Scholar] [CrossRef]
- Zhu, H.; Kannan, K. Continuing occurrence of melamine and its derivatives in infant formula and dairy products from the united states: Implications for environmental sources. Environ. Sci. Technol. Lett. 2018, 5, 641–648. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, R.; Chen, H.; Kong, J. Rapid quantification of thiocyanate in milk samples using a universal paper-based sers sensor. Analyst 2022, 147, 5038–5043. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, J.; Wang, H. Microchip-based surface enhanced Raman spectroscopy for the determination of sodium thiocyanate in milk. Anal. Lett. 2015, 48, 1930–1940. [Google Scholar] [CrossRef]
- Nunes, P.P.; Almeida, M.R.; Pacheco, F.G.; Fantini, C.; Furtado, C.A.; Ladeira, L.O.; Jorio, A.; Júnior, A.P.M.; Santos, R.L.; Borges, Á.M. Detection of carbon nanotubes in bovine raw milk through fourier transform Raman spectroscopy. J. Dairy. Sci. 2024, 107, 2681–2689. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Fan, D.; Li, W.; Meng, Y.; Liu, T.C. Rapid evaluation of milk acidity and identification of milk adulteration by Raman spectroscopy combined with chemometrics analysis. Vib. Spectrosc. 2022, 123, 103440. [Google Scholar] [CrossRef]
- Ní Fhuaráin, Á.M.; O Donnell, C.P.; Luo, J.; Gowen, A.A. A review on mir, nir, fluorescence and Raman spectroscopy combined with chemometric modeling to predict the functional properties of raw bovine milk. ACS Food Sci. Technol. 2024, 4, 2258–2271. [Google Scholar] [CrossRef]
- Montgomery, D.C. Introduction to Statistical Quality Control, 7th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Cozzolino, D. The role of vibrational spectroscopy as a tool to assess economically motivated fraud and counterfeit issues in agricultural products and foods. Anal. Methods 2015, 7, 9390–9400. [Google Scholar] [CrossRef]
- Kumar, D.; Verma, A.K.; Chatli, M.K.; Singh, R.; Kumar, P.; Mehta, N.; Malav, O.P. Camel milk: Alternative milk for human consumption and its health benefits. Nutr. Food Sci. 2016, 46, 217–227. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Q.; Gu, S.; Yu, Y.; Deng, X.; Niu, B.; Chen, Q. Rapid identification method of milk powder from different animals based on Raman spectroscopy. J. Dairy Sci. 2025, 108, 136–151. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Yao, Y.; Huo, Z.; Cui, X.; Liu, M.; Zhao, L.; Ge, W. Oligosaccharide profiles as potential biomarkers for detecting adulteration of caprine dairy products with bovine dairy products. Food Chem. 2024, 443, 138551. [Google Scholar] [CrossRef]
- Ostovar Pour, S.; Afshari, R.; Landry, J.; Pillidge, C.; Gill, H.; Blanch, E. Spatially offset Raman spectroscopy: A convenient and rapid tool to distinguish cheese made with milks from different animal species. J. Raman Spectrosc. 2021, 52, 1705–1711. [Google Scholar] [CrossRef]
- Ullah, R.; Khan, S.; Ali, H.; Bilal, M.; Saleem, M.; Mahmood, A.; Ahmed, M. Raman-spectroscopy-based differentiation between cow and buffalo milk. J. Raman Spectrosc. 2017, 48, 692–696. [Google Scholar] [CrossRef]
- Mohammadi, S.; Gowen, A.; Luo, J.; O’Donnell, C. Prediction of milk composition using multivariate chemometric modelling of infrared, Raman, and fluorescence spectroscopic data: A review. Food Control 2024, 165, 110658. [Google Scholar] [CrossRef]
- Silva, M.G.; de Paula, I.L.; Stephani, R.; Edwards, H.G.M.; de Oliveira, L.F.C. Raman spectroscopy in the quality analysis of dairy products: A literature review. J. Raman Spectrosc. 2021, 52, 2444–2478. [Google Scholar] [CrossRef]
- Khan, H.M.H.; Mccarthy, U.; Esmonde-White, K.; Casey, I.; O’Shea, N. Potential of Raman spectroscopy for in-line measurement of raw milk composition. Food Control 2023, 152, 109862. [Google Scholar] [CrossRef]
- Genis, D.O.; Sezer, B.; Durna, S.; Boyaci, I.H. Determination of milk fat authenticity in ultra-filtered white cheese by using Raman spectroscopy with multivariate data analysis. Food Chem. 2021, 336, 127699. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.R.; Oliveira, K.D.S.; Stephani, R.; de Oliveira, L.F.C. Fourier-transform Raman analysis of milk powder: A potential method for rapid quality screening. J. Raman Spectrosc. 2011, 42, 1548–1552. [Google Scholar] [CrossRef]
- Ferreira, J.L.A.; de Almeida, L.F.; Simões, S.D.S.; Diniz, P.H.G.D.; Fernandes, D.D.D.S. Raman spectroscopy-based authentication of powder goat milk adulteration with cow milk. Food Control 2025, 167, 110800. [Google Scholar] [CrossRef]
- Zhang, Z.; Yao, A.; Yue, T.; Niu, M.; Wang, H. Bayesian discriminant analysis of yogurt products based on Raman spectroscopy. J. AOAC Int. 2020, 103, 1435–1439. [Google Scholar] [CrossRef]
- Reiner, J.; Protte, K.; Hinrichs, J. Investigation of the applicability of Raman spectroscopy as online process control during consumer milk production. ChemEngineering 2020, 4, 45. [Google Scholar] [CrossRef]
- Gu, Y.; Zhao, S.; Xiong, L.; Ji, N.; Hong, H.; Zhang, Z. Characterization and discriminant analysis of milk powder based on multidimensional Raman spectroscopy. Food Sci. Technol. Econ. 2022, 47, 97–101. [Google Scholar]
- Khan, U.M.; Sameen, A.; Decker, E.A.; Shabbir, M.A.; Hussain, S.; Latif, A.; Abdi, G.; Aadil, R.M. Implementation of plant extracts for cheddar-type cheese production in conjunction with ftir and Raman spectroscopy comparison. Food Chem. X 2024, 22, 101256. [Google Scholar] [CrossRef]
- Yan, H.; Neves, M.D.G.; Wise, B.M.; Moraes, I.A.; Barbin, D.F.; Siesler, H.W. The application of handheld near-infrared spectroscopy and Raman spectroscopic imaging for the identification and quality control of food products. Molecules 2023, 28, 7891. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Jin, S.; Noda, I.; Jung, Y.M. Continuing progress in the field of two-dimensional correlation spectroscopy (2d-cos): Part iii. Versatile applications. Spectrochim. Acta Part A 2023, 284, 121636. [Google Scholar] [CrossRef] [PubMed]
- Noda, I.; Ozaki, Y. Two-Dimensional Correlation Spectroscopy—Applications in Vibrational and Optical Spectroscopy; John Wiley & Sons Ltd.: Chichester, UK, 2005. [Google Scholar]
- Lafi, A.G.A.; Naser, I.; Abboud, H. Application of two dimensional correlation fourier transform infrared spectroscopy (2d-cos-ftir) to the quantification of carbohydrates in milk powder. J. Food Compos. Anal. 2024, 134, 106541. [Google Scholar] [CrossRef]
- Huang, M.; Yang, R.; Zheng, Z.; Wu, H.; Yang, Y. Discrimination of adulterated milk using temperature-perturbed two-dimensional infrared correlation spectroscopy and multivariate analysis. Spectrochim. Acta Part A 2022, 278, 121342. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, S.; Sha, M.; Liu, J. Characterization of fresh milk products based on multidimensional Raman spectroscopy. J. Appl. Spectrosc. 2021, 87, 1206–1215. [Google Scholar] [CrossRef]
- Grelet, C.; Pierna, J.A.F.; Dardenne, P.; Soyeurt, H.; Vanlierde, A.; Colinet, F.; Bastin, C.; Gengler, N.; Baeten, V.; Dehareng, F. Standardization of milk mid-infrared spectrometers for the transfer and use of multiple models. J. Dairy Sci. 2017, 100, 7910–7921. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, L.; Yue, T.; Liu, J.; Wang, H. Research on the standardization and noise reduction of Raman spectroscopy of milk powder. Food Sci. Technol. Econ. 2018, 43, 57–61. [Google Scholar]
- Bērziņš, K.; Harrison, S.D.L.; Leong, C.; Fraser-Miller, S.J.; Harper, M.J.; Diana, A.; Gibson, R.S.; Houghton, L.A.; Gordon, K.C. Qualitative and quantitative vibrational spectroscopic analysis of macronutrients in breast milk. Spectrochim. Acta Part A 2021, 246, 118982. [Google Scholar] [CrossRef]
- Tian, H.; Chen, S.; Li, D.; Lou, X.; Chen, C.; Yu, H. Simultaneous detection for adulterations of maltodextrin, sodium carbonate, and whey in raw milk using Raman spectroscopy and chemometrics. J. Dairy. Sci. 2022, 105, 7242–7252. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, Z.; Zhang, Y.; Ni, X.; Wang, H. Identification of infant rice cereal based by Raman spectroscopy combined with an extreme learning machine algorithm. J. Anal. Chem. 2024, 79, 447–455. [Google Scholar] [CrossRef]
- Yazgan Karacaglar, N.N.; Bulat, T.; Boyaci, I.H.; Topcu, A. Raman spectroscopy coupled with chemometric methods for the discrimination of foreign fats and oils in cream and yogurt. J. Food Drug Anal. 2019, 27, 101–110. [Google Scholar] [CrossRef]
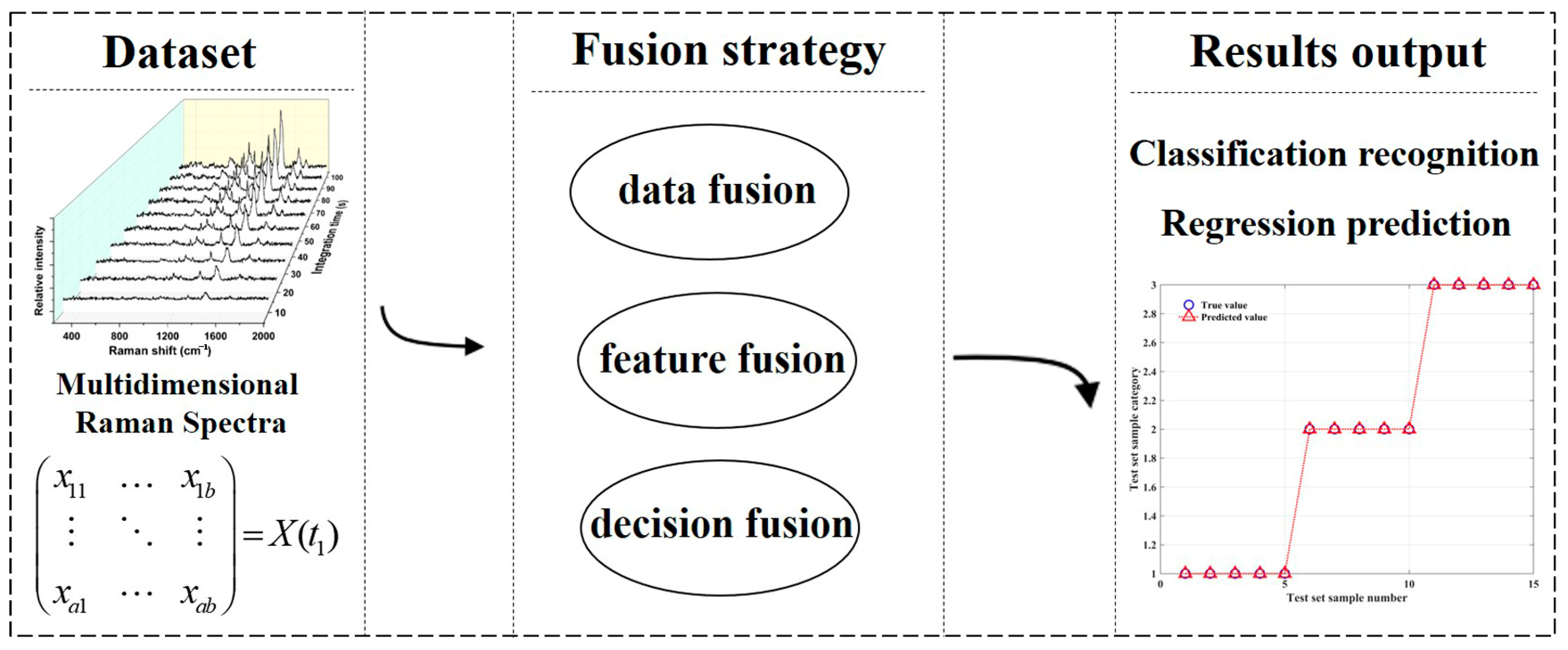
- Zhang, Z. The statistical fusion identification of dairy products based on extracted Raman spectroscopy. RSC Adv. 2020, 10, 29682–29687. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jin, L.; Guo, F.; Ni, X.; Zhao, Y.; Cheng, Y.; Wang, H. Matrix factorization-based dimensionality reduction algorithms—A comparative study on spectroscopic profiling data. Anal. Chem. 2022, 94, 13385–13395. [Google Scholar] [CrossRef]
- Dewantier, G.R.; Torley, P.J.; Blanch, E.W. Identifying chemical differences in cheddar cheese based on maturity level and manufacturer using vibrational spectroscopy and chemometrics. Molecules 2023, 28, 8051. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Jiang, Y.; Zhang, Y.; Zhou, Y.; Zhao, Y.; Guo, F.; Wang, H. Identification of liquid milk adulteration using Raman spectroscopy combined with lactose indexed screening and support vector machine. Int. Dairy J. 2023, 146, 105751. [Google Scholar] [CrossRef]
- Zhang, Z.; Gui, D.; Sha, M.; Liu, J.; Wang, H. Raman chemical feature extraction for quality control of dairy products. J. Dairy Sci. 2019, 102, 68–76. [Google Scholar] [CrossRef]
- Ullah, R.; Khan, S.; Khan, A.; Saleem, M.; Ali, H.; Bilal, M.; Ahmed, M. Infant gender-based differentiation in concentration of milk fats using near infrared Raman spectroscopy. J. Raman Spectrosc. 2017, 48, 363–367. [Google Scholar] [CrossRef]
- de Oliveira Mendes, T.; Manzolli Rodrigues, B.V.; Simas Porto, B.L.; Alves Da Rocha, R.; de Oliveira, M.A.L.; de Castro, F.K.; Dos Anjos, V.D.C.; Bell, M.J.V. Raman spectroscopy as a fast tool for whey quantification in raw milk. Vib. Spectrosc. 2020, 111, 103150. [Google Scholar] [CrossRef]
- Li, J.; Qing, C.; Wang, X.; Zhu, M.; Zhang, B.; Zhang, Z. Discriminative feature analysis of dairy products based on machine learning algorithms and Raman spectroscopy. Curr. Res. Food Sci. 2024, 8, 100782. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, Q.; Noda, I.; Sun, S. Quantitative classification of two-dimensional correlation spectra. Appl. Spectrosc. 2009, 63, 920–925. [Google Scholar] [CrossRef]
- Arroyo-Cerezo, A.; Jiménez-Carvelo, A.M.; González-Casado, A.; Ruisánchez, I.; Cuadros-Rodríguez, L. The potential of the spatially offset Raman spectroscopy (sors) for implementing rapid and non-invasive in-situ authentication methods of plastic-packaged commodity foods—Application to sliced cheeses. Food Control 2023, 146, 109522. [Google Scholar] [CrossRef]
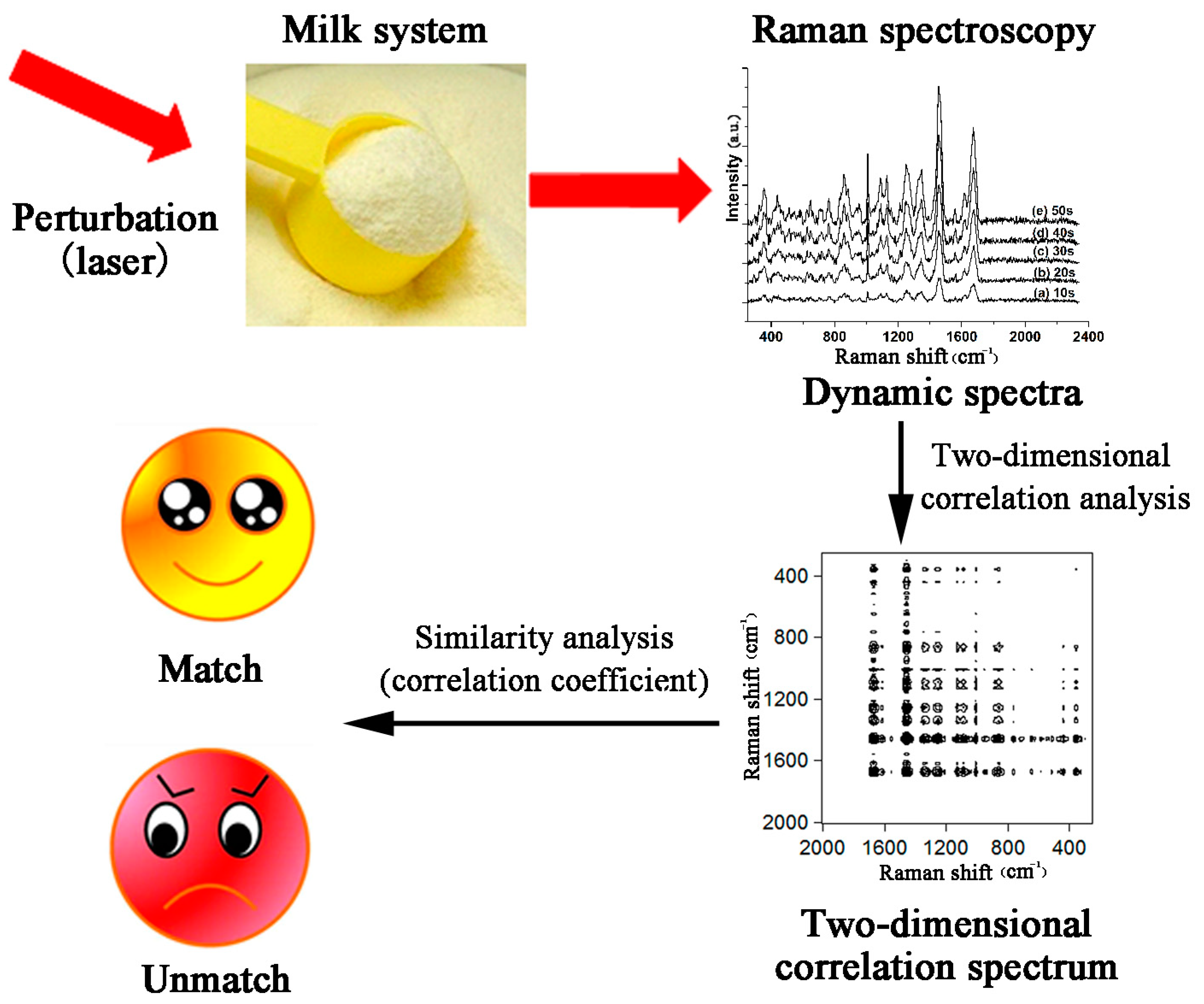
- Zhang, Z.; Sha, M.; Wang, H. Laser perturbation two-dimensional correlation Raman spectroscopy for quality control of bovine colostrum products. J. Raman Spectrosc. 2017, 48, 1111–1115. [Google Scholar] [CrossRef]
- Zhang, Z.; Yue, T.; Ma, J.; Sun, S.; Liu, J.; Wang, H. Identification of yoghurt based on Raman spectroscopy and k nearest neighbor algorithm. Chin. J. Anal. Lab. 2019, 38, 553–557. [Google Scholar]
- Lussier, F.; Thibault, V.; Charron, B.; Wallace, G.Q.; Masson, J. Deep learning and artificial intelligence methods for Raman and surface-enhanced Raman scattering. TrAC Trends Anal. Chem. 2020, 124, 115796. [Google Scholar] [CrossRef]
- Goyal, R.; Singha, P.; Singh, S.K. Spectroscopic food adulteration detection using machine learning: Current challenges and future prospects. Trends Food Sci. Technol. 2024, 146, 104377. [Google Scholar] [CrossRef]
- García-Méndez, S.; De Arriba-Pérez, F.; Somoza-López, M.D.C. Informatics and dairy industry coalition: Artificial intelligence trends and present challenges. IEEE Ind. Electron. Mag. 2024, 18, 30–37. [Google Scholar] [CrossRef]
- Amjad, A.; Ullah, R.; Khan, S.; Bilal, M.; Khan, A. Raman spectroscopy based analysis of milk using random forest classification. Vib. Spectrosc. 2018, 99, 124–129. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi, X.; Zhao, Y.; Zhang, Y.; Wang, H. Brand identification of soybean milk powder based on Raman spectroscopy combined with random forest algorithm. J. Anal. Chem. 2022, 77, 1282–1286. [Google Scholar] [CrossRef]
- Li, W.; Huang, W.; Fan, D.; Gao, X.; Zhang, X.; Meng, Y.; Liu, T.C. Rapid quantification of goat milk adulteration with cow milk using Raman spectroscopy and chemometrics. Anal. Methods 2023, 15, 455–461. [Google Scholar] [CrossRef]
- Yazgan, N.N.; Genis, H.E.; Bulat, T.; Topcu, A.; Durna, S.; Yetisemiyen, A.; Boyaci, I.H. Discrimination of milk species using Raman spectroscopy coupled with partial least squares discriminant analysis in raw and pasteurized milk. J. Sci. Food. Agric. 2020, 100, 4756–4765. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, M.; Xiong, H. Optimized identification of cheese products based on Raman spectroscopy and an extreme learning machine. New J. Chem. 2023, 47, 6889–6894. [Google Scholar] [CrossRef]
- Yaman, H. A rapid method for detection adulteration in goat milk by using vibrational spectroscopy in combination with chemometric methods. J. Food Sci. Technol. 2020, 57, 3091–3098. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Wang, W.; Diao, Y.; Yi, S.; Shang, Y.; Ren, D.; Ge, K.; Gu, Y. Recent advances of artificial intelligence in quantitative analysis of food quality and safety indicators: A review. TrAC Trends Anal. Chem. 2024, 180, 117944. [Google Scholar] [CrossRef]
- Mohammadi, S.; Gowen, A.; O’Donnell, C. Vibrational spectroscopy data fusion for enhanced classification of different milk types. Heliyon 2024, 10, e36385. [Google Scholar] [CrossRef]
- Wang, X.; Esquerre, C.; Downey, G.; Henihan, L.; O’Callaghan, D.; O’Donnell, C. Development of chemometric models using vis-nir and Raman spectral data fusion for assessment of infant formula storage temperature and time. Innov. Food Sci. Emerg. Technol. 2021, 67, 102551. [Google Scholar] [CrossRef]
- Feng, Z.; Liu, D.; Gu, J.; Zheng, L. Raman spectroscopy and fusion machine learning algorithm: A novel approach to identify dairy fraud. J. Food Compos. Anal. 2024, 129, 106090. [Google Scholar] [CrossRef]
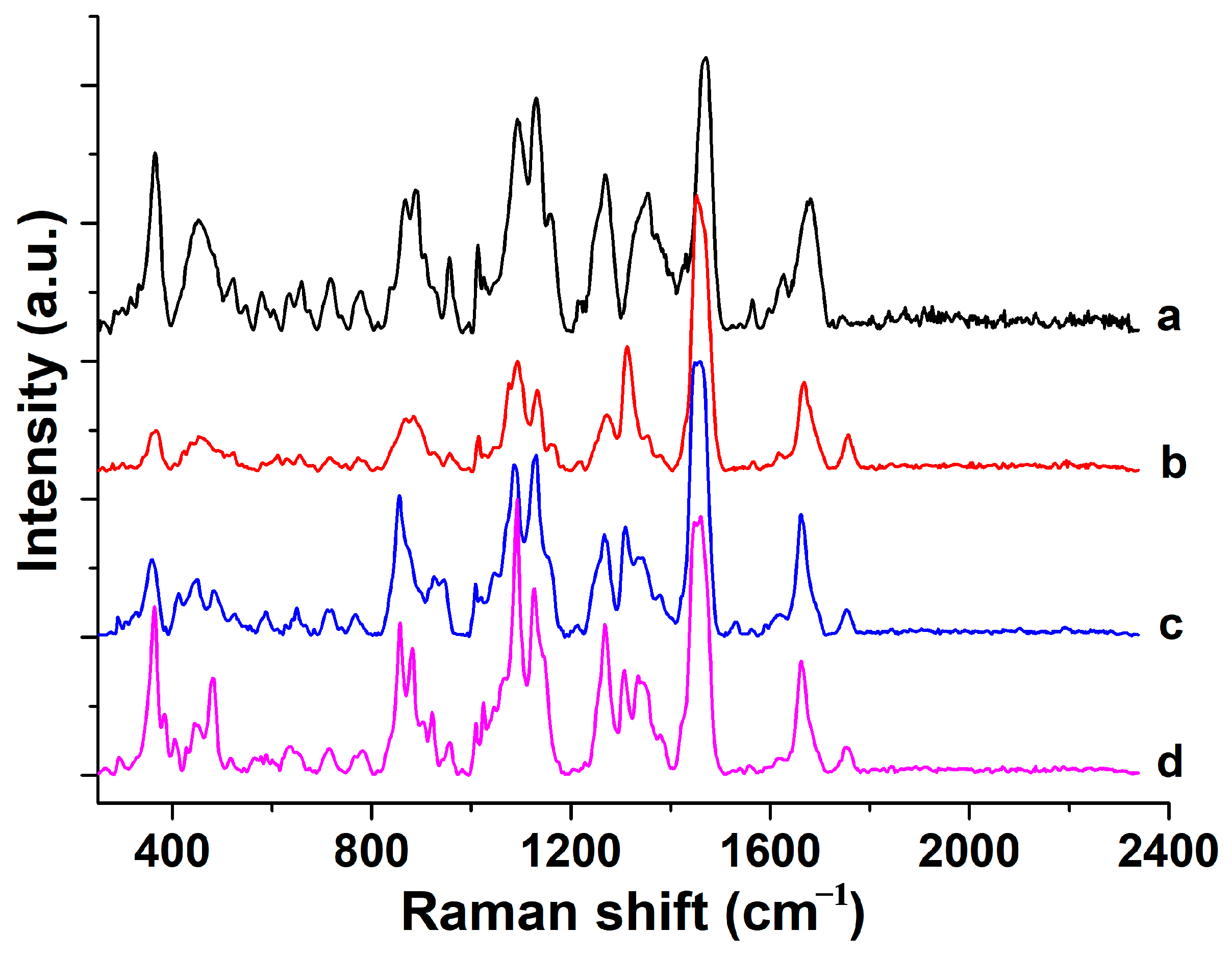
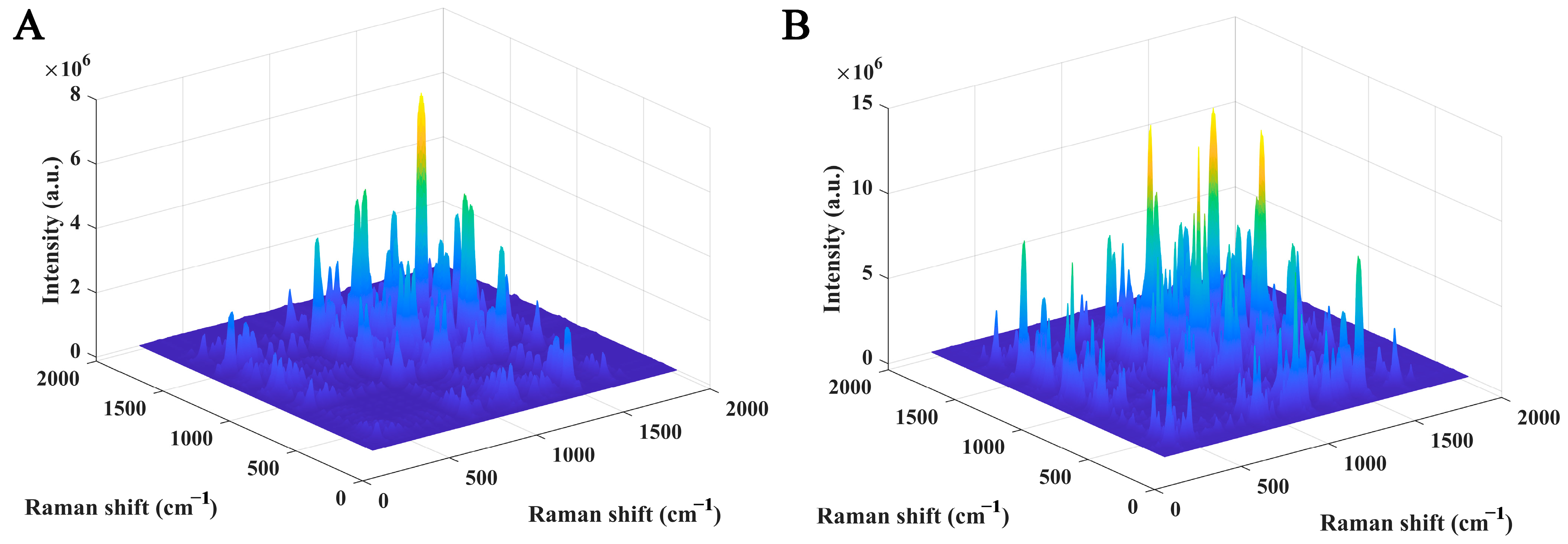
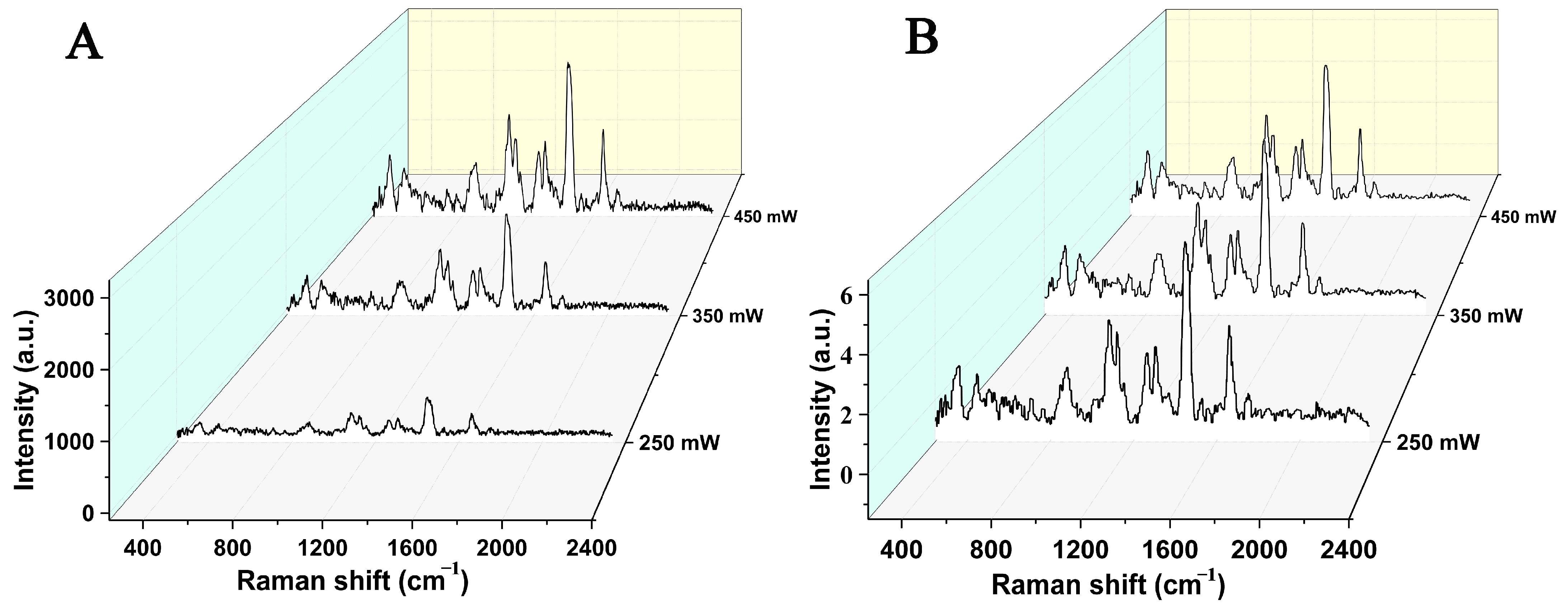
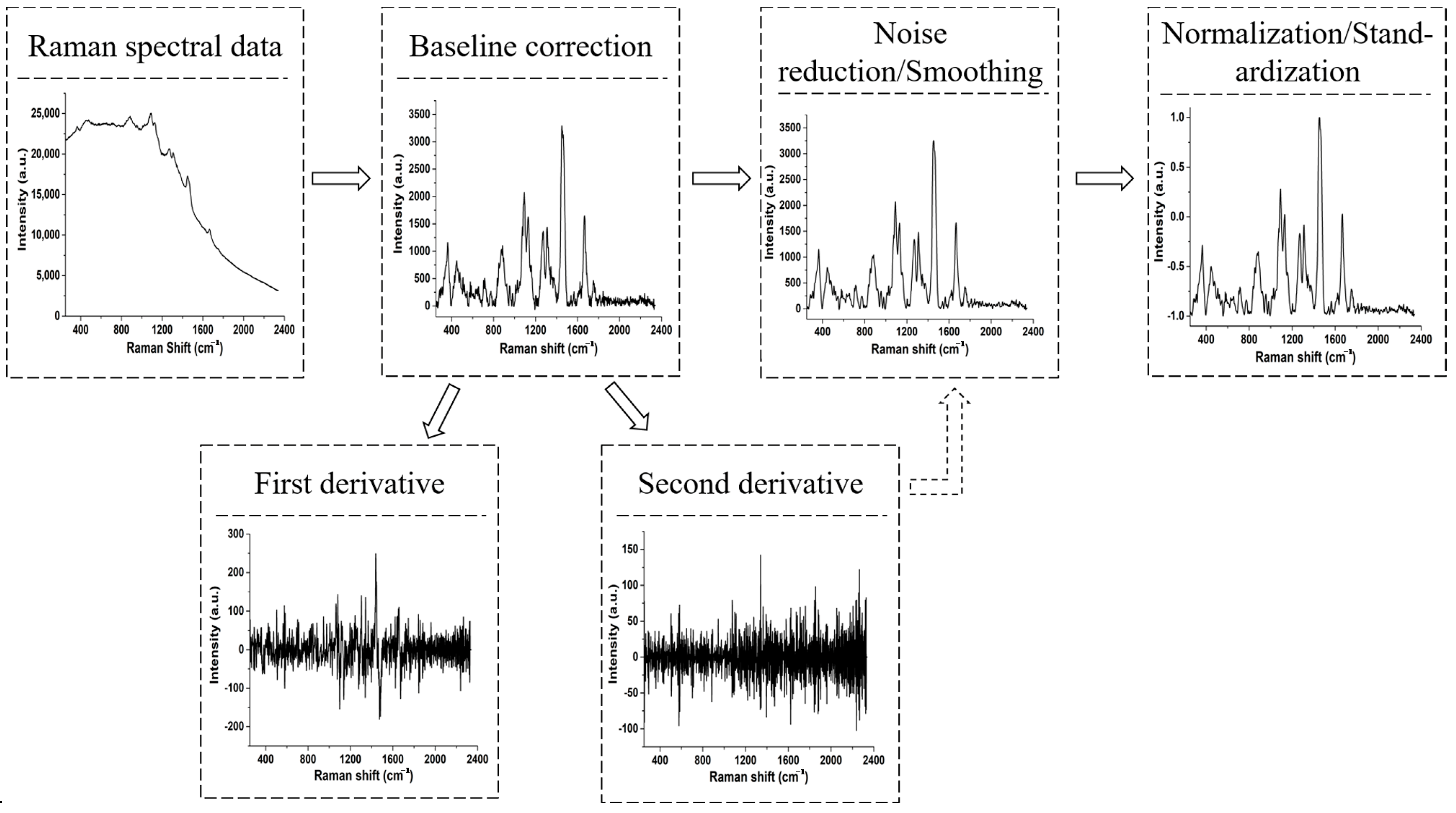
| Raman Shift (cm−1) Brand a | Raman Shift (cm−1) Brand b | Raman Shift (cm−1) Brand c | Raman Shift (cm−1) Brand d | Assignment | Possible Component Attribution |
|---|---|---|---|---|---|
| - | 1757 | 1753 | 1754 | ν (C=O) ester | Fat |
| 1678 | 1667 | 1661 | 1662 | ν (C=O) amide I | Protein |
| 1625 | 1618 | 1616 | 1617 | ν (C=C) ring | Protein |
| 1565 | 1565 | 1562 | 1558 | δ (N–H), ν (C–N) amide II | Protein |
| 1471 | 1453 | 1457 | 1457 | δ (CH2) | Fat, Carbohydrate |
| 1355 | 1353 | 1338 | 1335 | δ (C–H), ν (C–O) | Carbohydrate |
| - | 1311 | 1309 | 1307 | τ (CH2) | Fat |
| 1270 | 1272 | 1267 | 1268 | γ (CH2) | Carbohydrate |
| 1130 | 1131 | 1130 | 1125 | ν (C–O) + ν (C–C) + δ (C–O–H) | Carbohydrate |
| 1091 | 1092 | 1087 | 1093 | ν (C–O) + ν (C–C) + δ (C–O–H) | Carbohydrate |
| 1014 | 1013 | 1008 | 1008 | ν (C=C) ring | Protein |
| 955 | 953 | 946 | 957 | δ (C–O–C) + δ (C–O–H) + ν (C–O) | Carbohydrate |
| 925 | 925 | 925 | 921 | δ (C–O–C) + δ (C–O–H) + ν (C–O) | Carbohydrate |
| 889 | 884 | - | 882 | δ (C–C–H) + δ (C–O–C) | Carbohydrate |
| 866 | 869 | 855 | 857 | δ (C–C–H) + δ (C–O–C) | Carbohydrate |
| 778 | 772 | 767 | 784 | δ (C–C–O) | Carbohydrate |
| 715 | 715 | 713 | 711 | ν (C–S) | Protein |
| 659 | 658 | 650 | - | δ (C–C–O) | Carbohydrate |
| 634 | 631 | 628 | 639 | δ (C–C–O) | Carbohydrate |
| 580 | 580 | 588 | 585 | δ (C–C–O) + τ (C–O) | Carbohydrate |
| 520 | 523 | 525 | 518 | Glucose | Carbohydrate |
| - | - | 482 | 481 | δ (C–C–C) + τ (C–O) | Carbohydrate |
| 452 | 453 | 448 | 445 | δ (C–C–C) + τ (C–O) | Carbohydrate |
| - | - | 409 | 405 | Lactose | Carbohydrate |
| - | - | - | 385 | Lactose | Carbohydrate |
| 364 | 369 | 358 | 365 | Lactose | Carbohydrate |
| - | - | 290 | 294 | Lactose | Carbohydrate |
| Feature Extraction Method | Application Situation | References |
|---|---|---|
| PCA | Classification of male and female buffalo milk samples | [49] |
| PCA | Quantitative determination of whey in raw milk | [50] |
| SPA, UVE, RF, and CARS | Identification of adulteration between pasteurized milk and ultra-high temperature sterilized milk | [47] |
| Raman peak height, peak area, and peak ratio | Quality control of dairy products from different brands | [48] |
| Moving window selection, spectral band selection, and fusion | Identification of different brands of pasteurized milk | [44] |
| Spectral intervals extraction and fusion | Quality discrimination of different brands of dairy products | [51] |
| High-dimensional Raman peak ratio | Identification of fresh milk samples | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.-Y.; Su, J.-S.; Xiong, H.-M. Technology for the Quantitative Identification of Dairy Products Based on Raman Spectroscopy, Chemometrics, and Machine Learning. Molecules 2025, 30, 239. https://doi.org/10.3390/molecules30020239
Zhang Z-Y, Su J-S, Xiong H-M. Technology for the Quantitative Identification of Dairy Products Based on Raman Spectroscopy, Chemometrics, and Machine Learning. Molecules. 2025; 30(2):239. https://doi.org/10.3390/molecules30020239
Chicago/Turabian StyleZhang, Zheng-Yong, Jian-Sheng Su, and Huan-Ming Xiong. 2025. "Technology for the Quantitative Identification of Dairy Products Based on Raman Spectroscopy, Chemometrics, and Machine Learning" Molecules 30, no. 2: 239. https://doi.org/10.3390/molecules30020239
APA StyleZhang, Z.-Y., Su, J.-S., & Xiong, H.-M. (2025). Technology for the Quantitative Identification of Dairy Products Based on Raman Spectroscopy, Chemometrics, and Machine Learning. Molecules, 30(2), 239. https://doi.org/10.3390/molecules30020239





