Structural, Morphological, and Antibacterial Attributes of Graphene Oxide Prepared by Hummers’ and Brodie’s Methods
Abstract
1. Introduction
2. Results
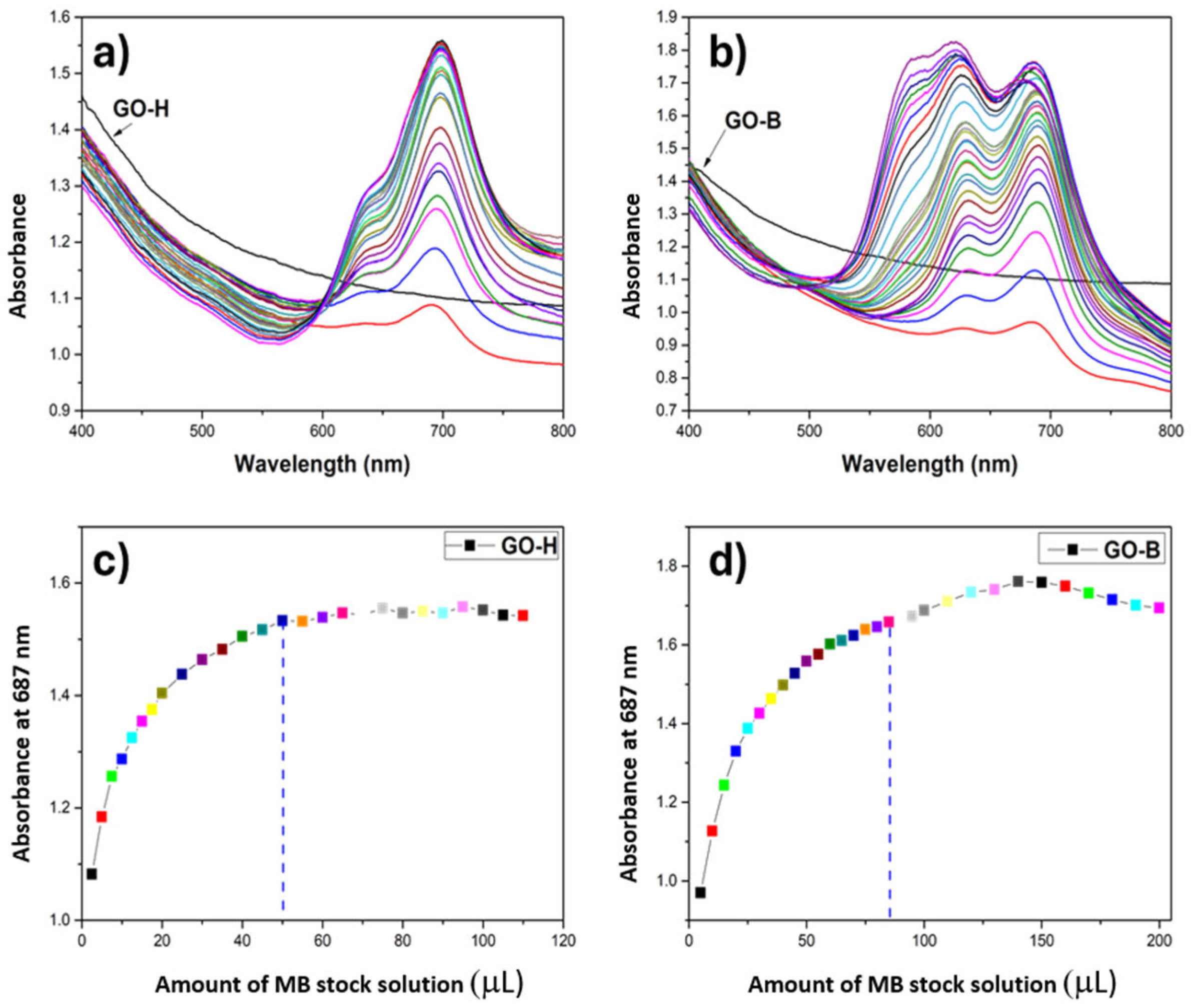
2.1. Structural and Morphological Characterization of GO Samples
2.2. Antibacterial Activity of GO-B and GO-H Samples
2.3. The Impact of GO-H and GO-B on Biofilm Production
2.4. Biofilm Eradication in the Presence of GO-B and GO-H
3. Discussion
4. Conclusions
5. Materials and Methods
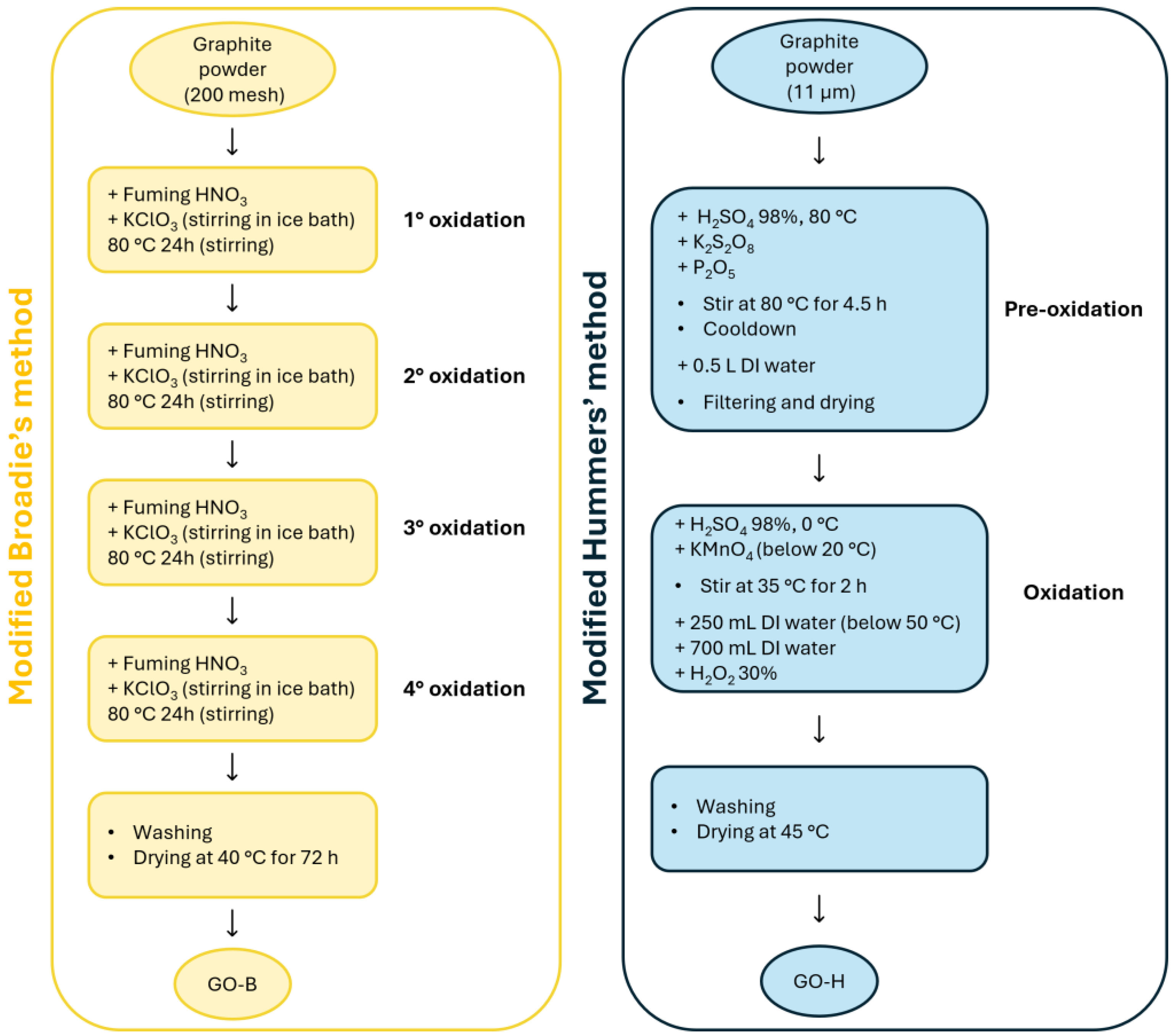
5.1. Materials Used in the Study for Graphite Oxidation
5.2. Graphite Oxidation
5.3. GO Characterization Techniques
5.4. Antibacterial Efficacy of GO-B and GO-H
5.5. GO-B and GO-H Impact on Bacterial Biofilm Formation and Eradication
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Piao, Y.; Chen, B. Self-assembled graphene oxide-gelatin nanocomposite hydrogels: Characterization, formation mechanisms, and pH-sensitive drug release behavior. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 356–367. [Google Scholar] [CrossRef]
- Gao, W. Graphene Oxide. In Reduction Recipes, Spectroscopy, and Applications; Springer: Cham, Switzerland, 2015. [Google Scholar] [CrossRef]
- Shi, L.; Wang, L.; Chen, J.; Chen, J.; Ren, L.; Shi, X.; Wang, Y. Modifying graphene oxide with short peptide via click chemistry for biomedical applications. Appl. Mater. Today 2016, 5, 111–117. [Google Scholar] [CrossRef]
- Alam, S.N.; Sharma, N.; Kumar, L. Synthesis of Graphene Oxide (GO) by Modified Hummers Method and Its Thermal Reduction to Obtain Reduced Graphene Oxide (rGO)*. Graphene 2017, 6, 1–18. [Google Scholar] [CrossRef]
- Brodie, B.C. On the Atomic Weight of Graphite. In Philosophical Transactions of the Royal Society of London; Royal Society: London, UK, 1859; Volume 149, Available online: https://royalsocietypublishing.org/doi/10.1098/rstl.1859.0013 (accessed on 9 December 2024).
- Staudenmaier, L. Verfahren zur Darstellung der Graphitsäure. Eur. J. Inorg. Chem. 1898, 31, 1481–1487. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Botas, C.; Álvarez, P.; Blanco, C.; Blanco, P.; Granda, M.; Santamaría, R.; Romasanta, L.J.; Verdejo, R.; López-Manchado, M.A.; Menéndez, R. Graphene materials with different structures prepared from the same graphite by the Hummers and Brodie methods. Carbon 2013, 65, 156–164. [Google Scholar] [CrossRef]
- Cardoso, C.E.D.; Almeida, J.C.; Lopes, C.B.; Trindade, T.; Vale, C.; Pereira, E. Recovery of rare earth elements by carbon-based nanomaterials—A review. Nanomaterials 2019, 9, 814. [Google Scholar] [CrossRef] [PubMed]
- Muzyka, R.; Drewniak, S.; Pustelny, T.; Sajdak, M.; Drewniak, Ł. Characterization of graphite oxide and reduced graphene oxide obtained from different graphite precursors and oxidized by different methods using raman spectroscopy statistical analysis. Materials 2021, 14, 769. [Google Scholar] [CrossRef]
- Lavin-Lopez, M.D.P.; Romero, A.; Garrido, J.; Sanchez-Silva, L.; Valverde, J.L. Influence of different improved hummers method modifications on the characteristics of graphite oxide to make a more easily scalable method. Ind. Eng. Chem. Res. 2016, 55, 12836–12847. [Google Scholar] [CrossRef]
- Pedrosa, M.; Da Silva, E.S.; Pastrana-Martínez, L.M.; Drazic, G.; Falaras, P.; Faria, J.L.; Figueiredo, J.L.; Silva, A.M. Hummers’ and Brodie’s graphene oxides as photocatalysts for phenol degradation. J. Colloid Interface Sci. 2020, 567, 243–255. [Google Scholar] [CrossRef]
- Talyzin, A.V.; Mercier, G.; Klechikov, A.; Hedenström, M.; Johnels, D.; Wei, D.; Cotton, D.; Opitz, A.; Moons, E. Brodie vs. Hummers graphite oxides for preparation of multi-layered materials. Carbon 2017, 115, 430–440. [Google Scholar] [CrossRef]
- Kumar, P.; Huo, P.; Zhang, R.; Liu, B. Antibacterial properties of graphene-based nanomaterials. Nanomaterials 2019, 9, 737. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E. Toxicity of graphene and graphene oxide nanowalls against bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Lv, M.; Xiu, P.; Huynh, T.; Zhang, M.; Castelli, M.; Liu, Z.; Huang, Q.; Fan, C.; Fang, H.; et al. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat. Nanotechnol. 2013, 8, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Peng, H.; Wang, X.; Shao, F.; Yuan, Z.; Han, H. Graphene oxide exhibits broad-spectrum antimicrobial activity against bacterial phytopathogens and fungal conidia by intertwining and membrane perturbation. Nanoscale 2014, 6, 1879–1889. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Dayem, A.A.; Eppakayala, V.; Kim, J.-H. Oxidative stress-mediated antibacterial activity of graphene oxide and reduced graphene oxide in Pseudomonas aeruginosa. Int. J. Nanomed. 2012, 7, 5901–5914. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Unnikrishnan, B.; Wei, S.-C.; Chou, C.P.; Zhang, L.-Z.; Huang, C.-C. Graphene oxide and carbon dots as broad-spectrum antimicrobial agents—A minireview. Nanoscale Horiz. 2019, 4, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Zou, F.; Zhou, H.; Jeong, D.Y.; Kwon, J.; Eom, S.U.; Park, T.J.; Hong, S.W.; Lee, J. Wrinkled surface-mediated antibacterial activity of graphene oxide nanosheets. ACS Appl. Mater. Interfaces 2017, 9, 1343–1351. [Google Scholar] [CrossRef]
- Perreault, F.; de Faria, A.F.; Nejati, S.; Elimelech, M. Antimicrobial Properties of Graphene Oxide Nanosheets: Why Size Matters. ACS Nano 2015, 9, 7226–7236. [Google Scholar] [CrossRef] [PubMed]
- Mao, A.; Zhang, D.; Jin, X.; Gu, X.; Wei, X.; Yang, G.; Liu, X. Synthesis of graphene oxide sheets decorated by silver nanoparticles in organic phase and their catalytic activity. J. Phys. Chem. Solids 2012, 73, 982–986. [Google Scholar] [CrossRef]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’Homme, R.K.; Aksay, I.A.; Car, R. Raman spectra of graphite oxide and functionalized graphene sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.; Lochab, B. A comparative study of graphene oxide: Hummers, intermediate and improved method. FlatChem 2019, 13, 40–49. [Google Scholar] [CrossRef]
- Emiru, T.F.; Ayele, D.W. Controlled synthesis, characterization and reduction of graphene oxide: A convenient method for large scale production. Egypt. J. Basic Appl. Sci. 2017, 4, 74–79. [Google Scholar] [CrossRef]
- Pulingam, T.; Thong, K.L.; Appaturi, J.N.; Lai, C.W.; Leo, B.F. Mechanistic actions and contributing factors affecting the antibacterial property and cytotoxicity of graphene oxide. Chemosphere 2021, 281, 130739. [Google Scholar] [CrossRef]
- Fallatah, H.; Overton, T.; Ali-Boucetta, H.; Gkatzionis, K. Impact of Environmental Stresses on the Antibacterial Activity of Graphene Oxide (GO) Nanoparticles against P. putida Biofilms. Microorganisms 2023, 11, 609. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.; Kumar, A.; Bekyarova, E.; Al-Hadeethi, Y.; Zhang, X.; Chen, M.; Ansari, M.S.; Cochis, A.; Rimondini, L. Antimicrobial Mechanisms and Effectiveness of Graphene and Graphene-Functionalized Biomaterials. A Scope Review. Front. Bioeng. Biotechnol. 2020, 8, 465–486. [Google Scholar] [CrossRef]
- Farid, M.U.; Guo, J.; An, A.K. Bacterial inactivation and in situ monitoring of biofilm development on graphene oxide membrane using optical coherence tomography. J. Membr. Sci. 2018, 564, 22–34. [Google Scholar] [CrossRef]
- Evseev, Z.I.; Tarasova, L.A.; Vasilieva, F.D.; Egorova, M.N.; Dmitriev, P.S.; Akhremenko, Y.A.; Smagulova, S.A. Comparison of Antimicrobial Properties of Graphene Oxide-Based Materials, Carbon Dots, and Their Combinations Deposited on Cotton Fabrics. Int. J. Mol. Sci. 2024, 25, 5328. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hu, M.; Zeng, T.H.; Wu, R.; Jiang, R.; Wei, J.; Wang, L.; Kong, J.; Chen, Y. Lateral dimension-dependent antibacterial activity of graphene oxide sheets. Langmuir 2012, 28, 12364–12372. [Google Scholar] [CrossRef] [PubMed]
- Nanda, S.S.; Yi, D.K.; Kim, K. Study of antibacterial mechanism of graphene oxide using Raman spectroscopy. Sci. Rep. 2016, 6, 28443. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, I.; Bhattacharya, P.; Talukdar, M.; Neogi, S.; Pal, S.K.; Chakraborty, S. Bactericidal effect of graphene oxide and reduced graphene oxide: Influence of shape of bacteria. Colloids Interface Sci. Commun. 2019, 28, 60–68. [Google Scholar] [CrossRef]
- Olczak, K.; Jakubowski, W.; Szymański, W. Bactericidal Activity of Graphene Oxide Tests for Selected Microorganisms. Materials 2023, 16, 4199. [Google Scholar] [CrossRef] [PubMed]
- Aunkor, T.H.; Raihan, T.; Prodhan, S.H.; Metselaar, H.S.C.; Malik, S.U.F.; Azad, A.K. Antibacterial activity of graphene oxide nanosheet against multidrug-resistant superbugs isolated from infected patients. R. Soc. Open Sci. 2020, 7, 200640. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, R.R.; Mohammad, M.R.; Hussien, A.M.A. Antibacterial Activity and Morphological Characterization of Synthesis Graphene Oxide Nanosheets by Simplified Hummer’s Method. Biosci. Biotechnol. Res. Asia 2018, 15, 627–633. [Google Scholar] [CrossRef]
- Bousiakou, L.G.; Qindeel, R.; Al-Dossary, O.M.; Kalkani, H. Synthesis and characterization of graphene oxide (GO) sheets for pathogen inhibition: Escherichia coli, Staphylococcus aureus and Pseudomonas aeruginosa. J. King Saud Univ. Sci. 2022, 34, 102002. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, M.; Zappacosta, R.; Lodovico, S.; Campli, E.; Siani, G.; Fontana, A.; Cellini, L. Antimicrobial and Antibiofilm Efficacy of Graphene Oxide Against Chronic Wound Microorganisms; NIH: Bethesda, MD, USA, 2018. [Google Scholar] [CrossRef]
- Saeed, S.I.; Vivian, L.; Zalati, C.W.S.C.W.; Sani, N.I.M.; Aklilu, E.; Mohamad, M.; Noor, A.A.M.; Muthoosamy, K.; Kamaruzzaman, N.F. Antimicrobial activities of graphene oxide against biofilm and intracellular Staphylococcus aureus isolated from bovine mastitis. BMC Vet. Res. 2023, 19, 10. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, L.; Wu, H.; Song, X.; Chen, Y.; Liu, D.; Lei, P.; Li, L.; Cui, B. Reactive oxygen species (ROS) dependent antibacterial effects of graphene oxide coatings. Dig. J. Nanomater. Biostructures 2022, 17, 481–489. [Google Scholar] [CrossRef]
- Ezraty, B.; Gennaris, A.; Barras, F.; Collet, J.-F. Oxidative stress, protein damage and repair in bacteria. Nat. Rev. Microbiol. 2017, 15, 385–396. [Google Scholar] [CrossRef]
- Seixas, A.F.; Quendera, A.P.; Sousa, J.P.; Silva, A.F.Q.; Arraiano, C.M.; Andrade, J.M. Bacterial Response to Oxidative Stress and RNA Oxidation. Front. Genet. 2022, 12, 821535. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial activity of graphite, graphite oxide, graphene oxide, and reduced graphene oxide: Membrane and oxidative stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef] [PubMed]
- Masip, L.; Veeravalli, K.; Georgiou, G. The many faces of glutathione in bacteria. Antioxid. Redox Signal. 2006, 8, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, V.; Mijakovic, I.; Pandit, S. Antimicrobial Activity of Graphene Oxide Contributes to Alteration of Key Stress-Related and Membrane Bound Proteins. Int. J. Nanomed. 2022, 17, 6707–6721. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Bai, H.; Lu, G.; Li, C.; Shi, G. Flexible graphene films via the filtration of water-soluble noncovalent functionalized graphene sheets. J. Am. Chem. Soc. 2008, 130, 5856–5857. [Google Scholar] [CrossRef] [PubMed]
- Szabó, T.; Tombácz, E.; Illés, E.; Dékány, I. Enhanced acidity and pH-dependent surface charge characterization of successively oxidized graphite oxides. Carbon 2006, 44, 537–545. [Google Scholar] [CrossRef]
- Gerasymchuk, Y.; Kędziora, A.; Wędzyńska, A.; Tahershamsi, L.; Chernii, V.; Tretyakova, I.; Chernii, S.; Pekhnyo, V.; Korona-Głowniak, I.; Malm, A.; et al. Composite based on graphite oxide, metallic silver and zirconium phthalocyanine coordinated by out-of-plane argininate ligands as photoactive antibacterial additive to endodontic cement. J. Photochem. Photobiol. A Chem. 2021, 418, 113432. [Google Scholar] [CrossRef]
- Gerasymchuk, Y.; Wędzyńska, A.; Stręk, W. Liquid “Syngas” Based on Supercritical Water and Graphite Oxide/TiO2 Composite as Catalyst for CO2 to Organic Conversion. Catal. Lett. 2022, 152, 2840–2851. [Google Scholar] [CrossRef]
- Montes-Navajas, P.; Asenjo, N.G.; Santamaría, R.; Menéndez, R.; Corma, A.; García, H. Surface area measurement of graphene oxide in aqueous solutions. Langmuir 2013, 29, 13443–13448. [Google Scholar] [CrossRef]
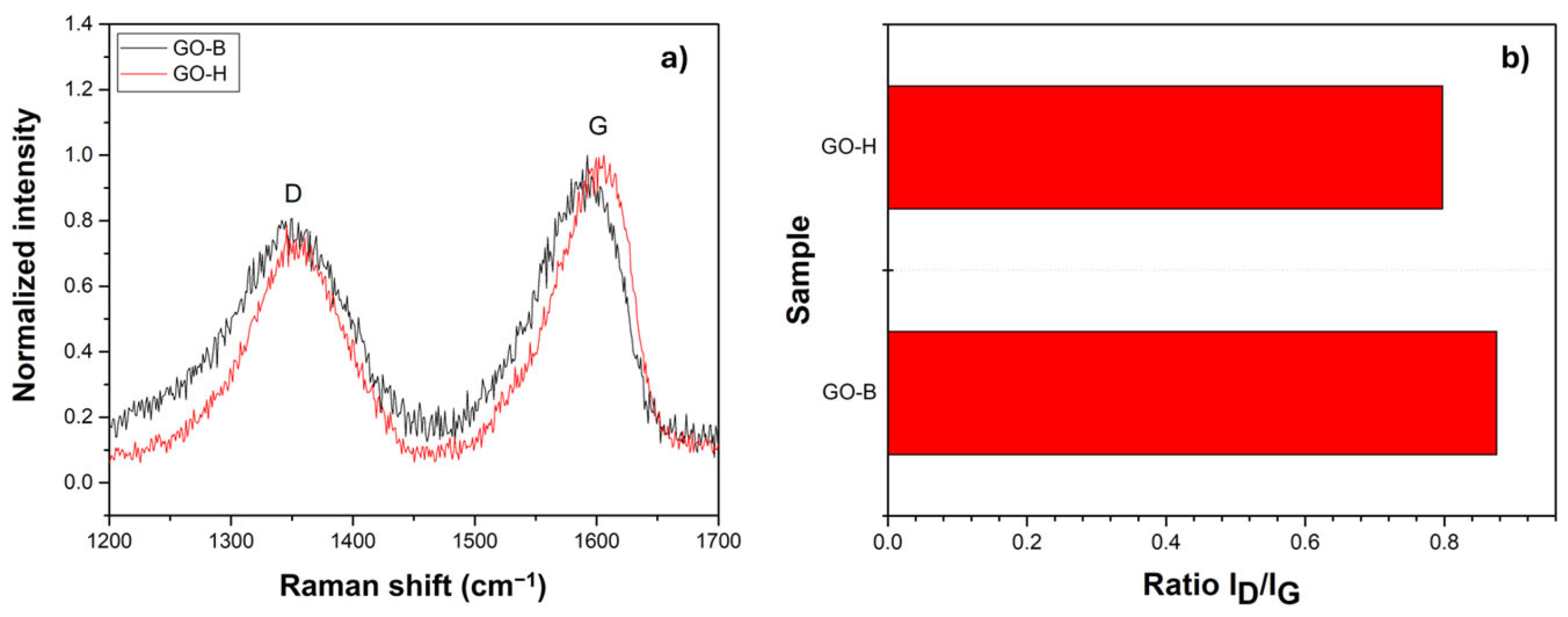
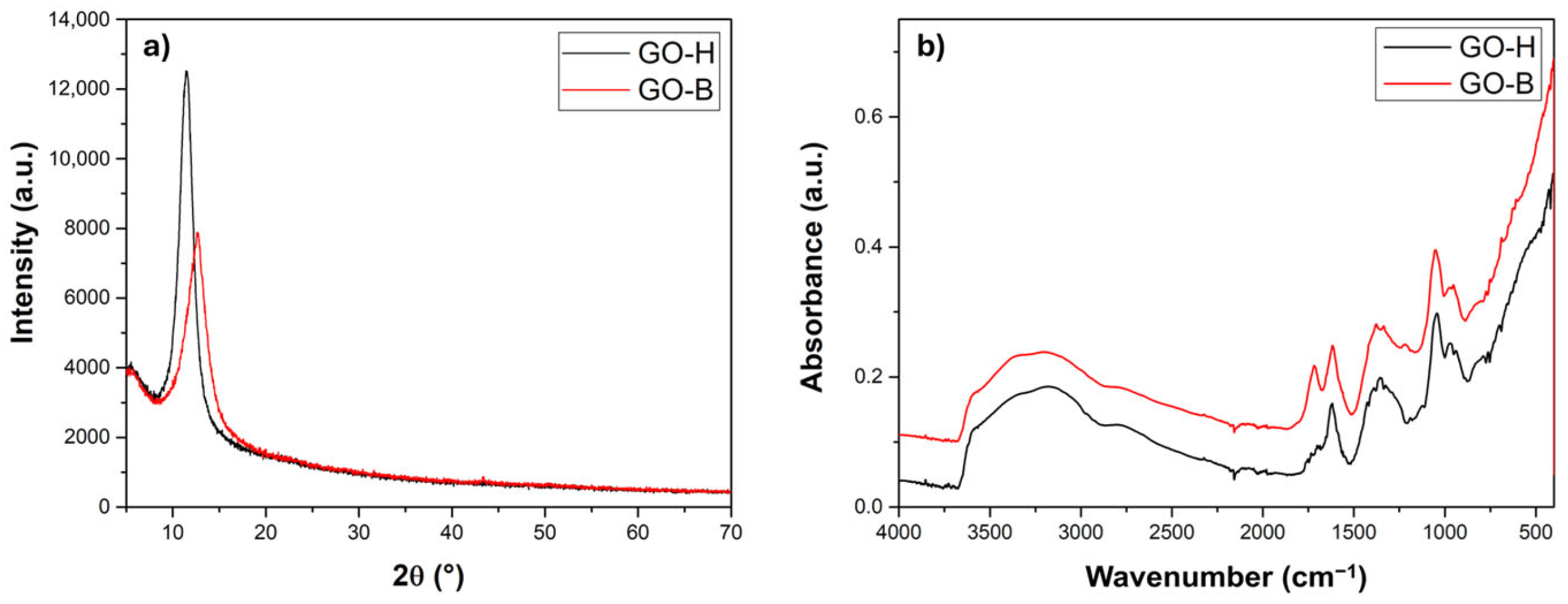
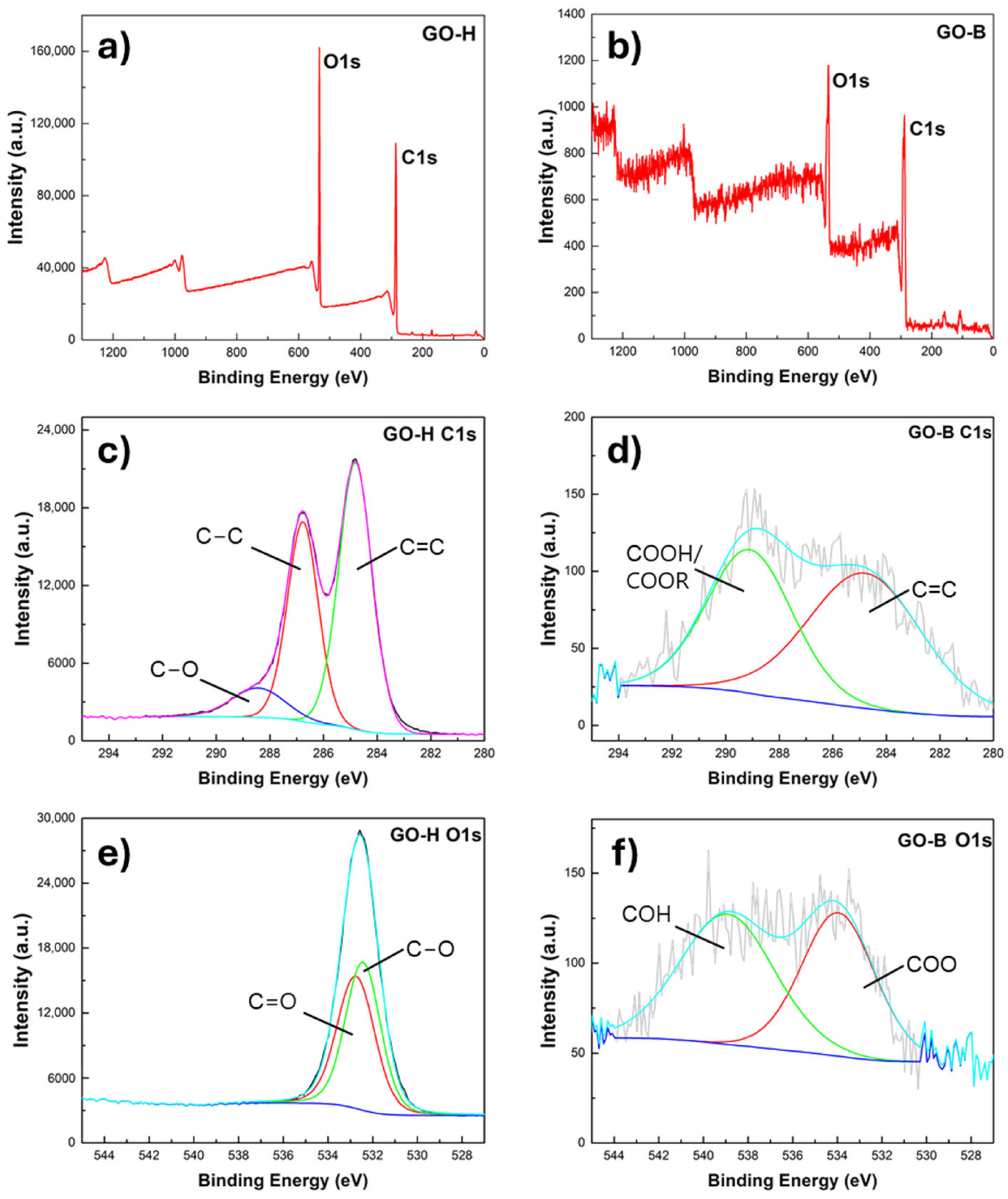
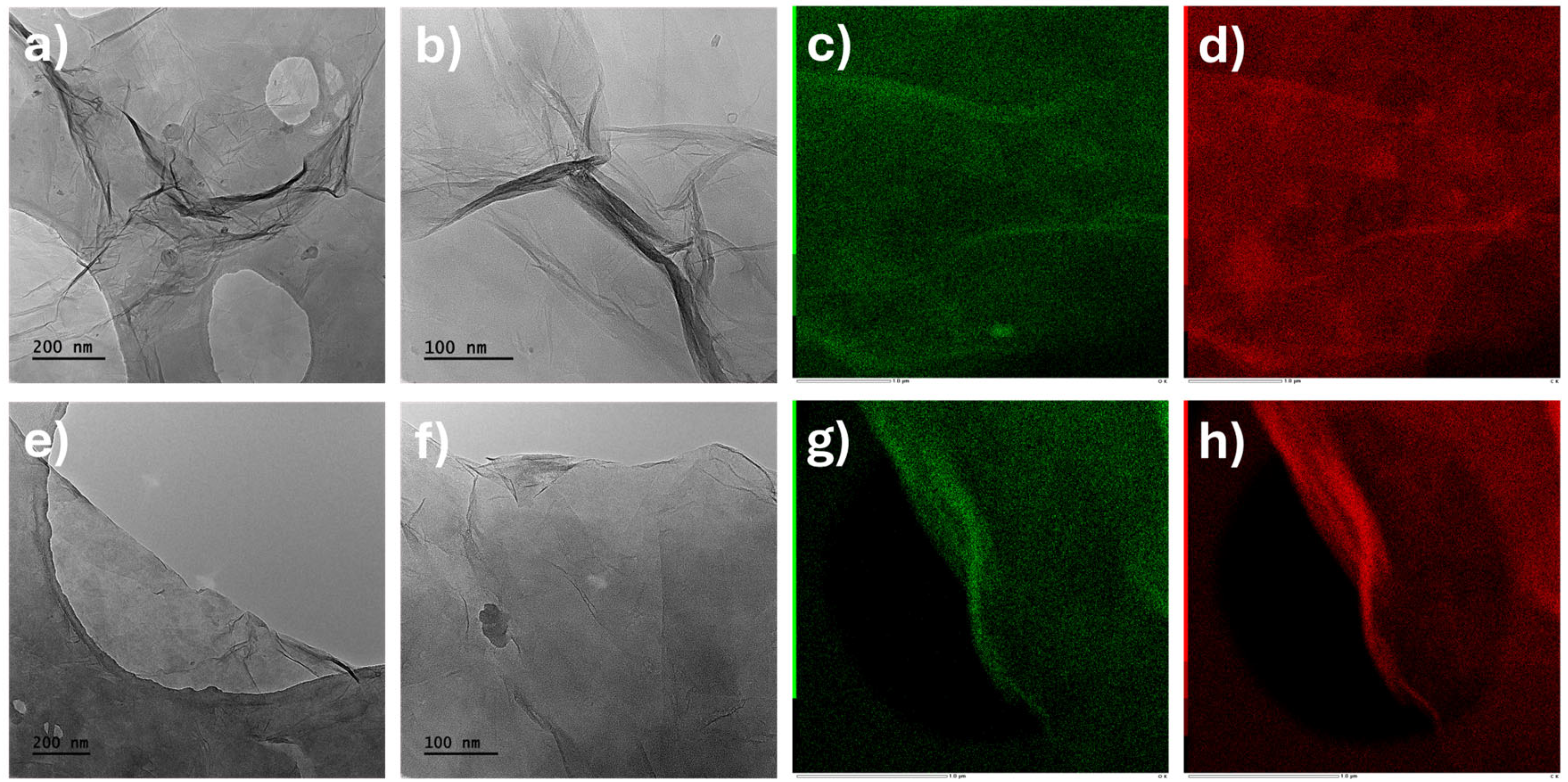


| Signal [cm−1] | Description | GO-H | GO-B |
|---|---|---|---|
| ~700–900 | C-O in -C-O-C- (epoxy group) | 704 | 687 |
| (stretching) | |||
| ~1050–1080 | -C-O-C- (epoxy group), -C-O- (alkoxy groups) | 1043 | 1051 |
| (stretching) | |||
| ~1220 | -C=O, -C-OH (stretching) | – | 1223 |
| ~1400 | -O-H (deformation) | 1378 | 1385 |
| ~1615 | C=C (unoxidized graphitic domain), | 1620 | 1620 |
| -OH, absorbed water (stretching) | |||
| ~1720 | -COOH, -C=O (stretching) | – | 1720 |
| ~2320 | -C-H | 2319 | 2319 |
| ~2800, ~2900 | asymmetric and symmetric CH2 | 2800 sh. | 2800 sh. |
| Stretching | 2900 sh. | 2900 sh. | |
| 2500–3500 | -O-H of carboxyl groups, | 3352 | 3369 |
| 3400 | -O-H, absorbed water | ||
| (stretching) |
| Species | GO-B | GO-H | ||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| S. aureus | 60 | >1000 | 60 | >1000 |
| E. faecalis | 60 | 1000 | 60 | >1000 |
| P. aeruginosa | 30 | 100 | 30 | 100 |
| E. coli | 30 | 100 | 30 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marsala, V.; Gerasymchuk, Y.; Saladino, M.L.; Paluch, E.; Wawrzyńska, M.; Boiko, V.; Li, X.; Giordano, C.; Hreniak, D.; Sobieszczańska, B. Structural, Morphological, and Antibacterial Attributes of Graphene Oxide Prepared by Hummers’ and Brodie’s Methods. Molecules 2025, 30, 240. https://doi.org/10.3390/molecules30020240
Marsala V, Gerasymchuk Y, Saladino ML, Paluch E, Wawrzyńska M, Boiko V, Li X, Giordano C, Hreniak D, Sobieszczańska B. Structural, Morphological, and Antibacterial Attributes of Graphene Oxide Prepared by Hummers’ and Brodie’s Methods. Molecules. 2025; 30(2):240. https://doi.org/10.3390/molecules30020240
Chicago/Turabian StyleMarsala, Vittorio, Yuriy Gerasymchuk, Maria Luisa Saladino, Emil Paluch, Magdalena Wawrzyńska, Vitalii Boiko, Xiang Li, Cristina Giordano, Dariusz Hreniak, and Beata Sobieszczańska. 2025. "Structural, Morphological, and Antibacterial Attributes of Graphene Oxide Prepared by Hummers’ and Brodie’s Methods" Molecules 30, no. 2: 240. https://doi.org/10.3390/molecules30020240
APA StyleMarsala, V., Gerasymchuk, Y., Saladino, M. L., Paluch, E., Wawrzyńska, M., Boiko, V., Li, X., Giordano, C., Hreniak, D., & Sobieszczańska, B. (2025). Structural, Morphological, and Antibacterial Attributes of Graphene Oxide Prepared by Hummers’ and Brodie’s Methods. Molecules, 30(2), 240. https://doi.org/10.3390/molecules30020240







