Effect of Drying Methods on the Phenolic Profile and Antioxidant Capacity of Pithecellobium dulce (Roxb.) Benth. Aril and Its Inhibitory Properties on Human SW480 Colon Adenocarcinoma Cells
Abstract
1. Introduction
2. Results
2.1. Impact of Drying Methods on the Polyphenolic Composition and Antioxidant Capacity of P. dulce Aril
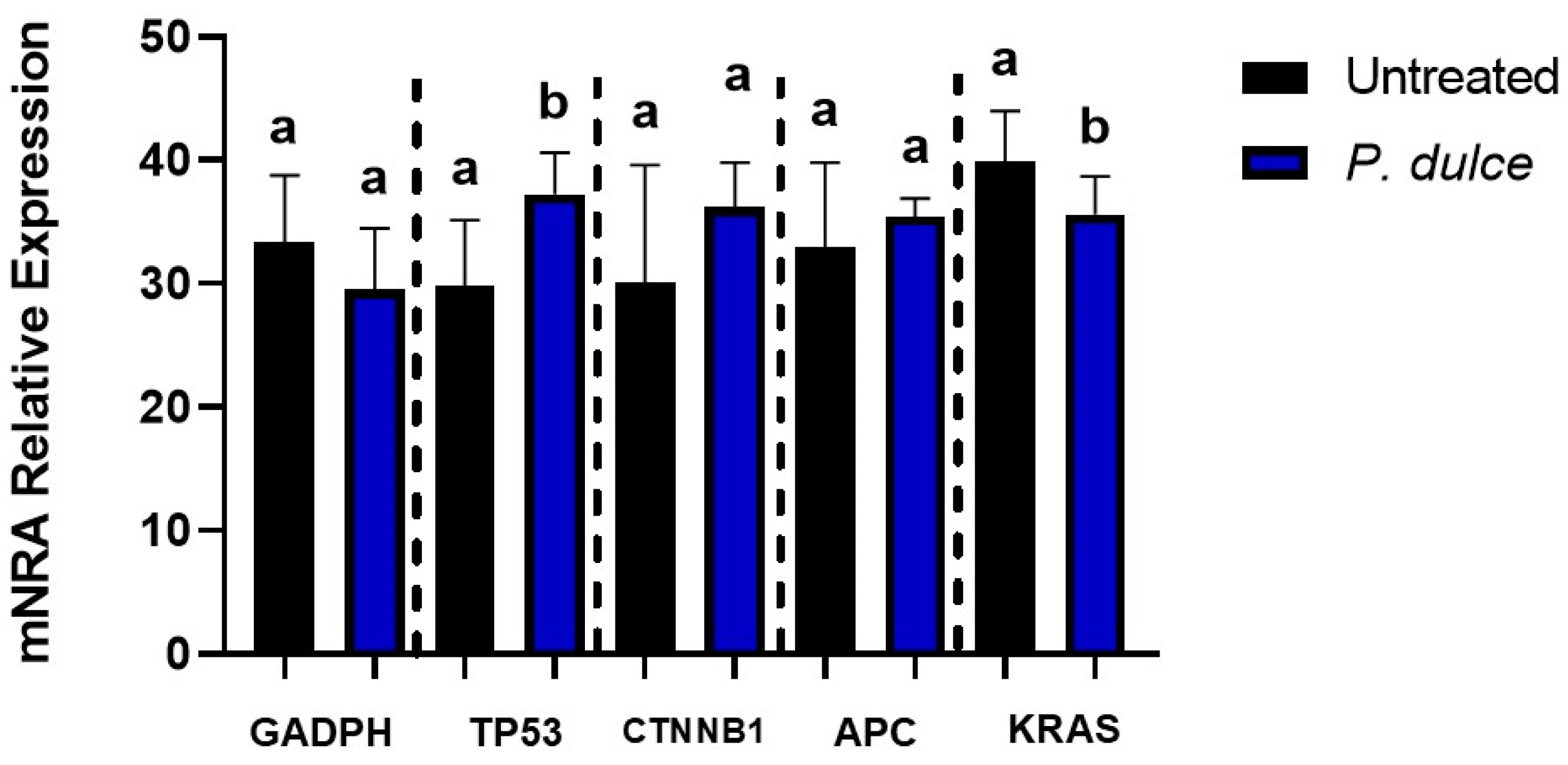
2.2. Effect of P. dulce Extracts on Cellular Metabolic Activity, Apoptosis, Cell Cycle, Necrosis, and the Expression of Pro-Apoptotic and Anti-Apoptotic Genes
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction Process
4.3. Phenolic Compounds Identification and Quantification
4.4. Antioxidant Capacity Determination
4.5. Cell Culture Assays
4.5.1. Quantification of Cellular Metabolic Activity by 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Assay
4.5.2. Cell Necrosis Determination by Lactate Dehydrogenase (LDH) Assay
4.5.3. Apoptosis Quantification by Cell Cytometry
4.5.4. Cell Cycle Analysis by Flow Cytometry
4.5.5. Assessment of p53 and KRAS Gene Expression by qPCR Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An Overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef]
- Briguglio, G.; Costa, C.; Pollicino, M.; Giambò, F.; Catania, S.; Fenga, C. Polyphenols in Cancer Prevention: New Insights (Review). Int. J. Funct. Nutr. 2020, 1, 1–11. [Google Scholar] [CrossRef]
- Abotaleb, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Therapeutic Potential of Plant Phenolic Acids in the Treatment of Cancer. Biomolecules 2020, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- De, S.; Paul, S.; Manna, A.; Majumder, C.; Pal, K.; Casarcia, N.; Mondal, A.; Banerjee, S.; Nelson, V.K.; Ghosh, S.; et al. Phenolic Phytochemicals for Prevention and Treatment of Colorectal Cancer: A Critical Evaluation of In Vivo Studies. Cancers 2023, 15, 993. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Siles, L.; Roman, S.; Fogliano, V.; Siegrist, M. Naturalness and Healthiness in “Ultra-Processed Foods”: A Multidisciplinary Perspective and Case Study. Trends Food Sci. Technol. 2022, 129, 667–673. [Google Scholar] [CrossRef]
- Vargas-Madriz, Á.F.; Kuri-García, A.; Vargas-Madriz, H.; Chávez-Servín, J.L.; Ferriz-Martínez, R.A.; Hernández-Sandoval, L.G.; Guzmán-Maldonado, S.H. Phenolic Profile and Antioxidant Capacity of Pithecellobium Dulce (Roxb) Benth: A Review. J. Food Sci. Technol. 2020, 57, 4316–4336. [Google Scholar] [CrossRef] [PubMed]
- Giada, M.D.L.R. Food Phenolic Compounds: Main Classes, Sources and Their Antioxidant Power; IntechOpen: London, UK, 2013. [Google Scholar]
- Shofian, N.M.; Hamid, A.A.; Osman, A.; Saari, N.; Anwar, F.; Dek, M.S.P.; Hairuddin, M.R. Effect of Freeze-Drying on the Antioxidant Compounds and Antioxidant Activity of Selected Tropical Fruits. Int. J. Mol. Sci. 2011, 12, 4678–4692. [Google Scholar] [CrossRef] [PubMed]
- Shonte, T.T.; Duodu, K.G.; de Kock, H.L. Effect of Drying Methods on Chemical Composition and Antioxidant Activity of Underutilized Stinging Nettle Leaves. Heliyon 2020, 6, e03938. [Google Scholar] [CrossRef]
- Quintero-Castaño, V.D.; Vasco-Leal, J.F.; Cuellar-Nuñez, L.; Luzardo-Ocampo, I.; Castellanos-Galeano, F.; Álvarez-Barreto, C.; Bello-Pérez, L.A.; Cortés-Rodriguez, M. Novel OSA-Modified Starch from Gros Michel Banana for Encapsulation of Andean Blackberry Concentrate: Production and Storage Stability. Starch-Stärke 2021, 73, 2000180. [Google Scholar] [CrossRef]
- De Ancos, B.; Sánchez-Moreno, C.; Zacarías, L.; Rodrigo, M.J.; Sáyago Ayerdí, S.; Blancas Benítez, F.J.; Domínguez Avila, J.A.; González-Aguilar, G.A. Effects of Two Different Drying Methods (Freeze-Drying and Hot Air-Drying) on the Phenolic and Carotenoid Profile of ‘Ataulfo’ Mango by-Products. J. Food Meas. Charact. 2018, 12, 2145–2157. [Google Scholar] [CrossRef]
- Martínez-De La Cruz, I.; Rubí-arriaga, M.; González-huerta, A.; Pérez-lópez, D.D.J.; Franco, O. Frutos y Semillas Comestibles En El Estado de México. Rev. Mex. De Cienc. Agric. 2015, 6, 331–346. [Google Scholar]
- Murugesan, S.; Lakshmanan, D.K.; Arumugam, V.; Alexander, R.A. Nutritional and Therapeutic Benefits of Medicinal Plant Pithecellobium Dulce (Fabaceae): A Review. J. Appl. Pharm. Sci. 2019, 9, 130–139. [Google Scholar] [CrossRef]
- Dhanisha, S.S.; Drishya, S.; Guruvayoorappan, C. Pithecellobium Dulce Inhibits Pulmonary Metastasis Induced by B16F10 Melanoma Cells in C57BL via Regulating EGFR/STAT/NFκB/AKT signaling axis. J. Food Biochem. 2022, 46, e14466. [Google Scholar] [CrossRef] [PubMed]
- Cháirez-Ramírez, M.H.; de la Cruz-López, K.G.; García-Carrancá, A. Polyphenols as Antitumor Agents Targeting Key Players in Cancer-Driving Signaling Pathways. Front. Pharmacol. 2021, 12, 710304. [Google Scholar] [CrossRef] [PubMed]
- Rosa, L.; Silva, N.; Soares, N.; Monteiro, M.; Teodoro, A. Anticancer Properties of Phenolic Acids in Colon Cancer–A Review. J. Nutr. Food Sci. 2016, 6, 1–7. [Google Scholar] [CrossRef]
- Khamchun, S.; Thongboonkerd, V. Cell Cycle Shift from G0/G1 to S and G2/M Phases Is Responsible for Increased Adhesion of Calcium Oxalate Crystals on Repairing Renal Tubular Cells at Injured Site. Cell Death Discov. 2018, 4, 106. [Google Scholar] [CrossRef]
- Oleynikov, I.P.; Azarkina, N.V.; Vygodina, T.V.; Konstantinov, A.A. Mechanism of Inhibition of Cytochrome c Oxidase by Triton X-100. Biochemistry 2021, 86, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Oyinloye, T.M.; Yoon, W.B. Effect of Freeze-Drying on Quality and Grinding Process of Food Produce: A Review. Processes 2020, 8, 354. [Google Scholar] [CrossRef]
- Garcìa, L.M.; Ceccanti, C.; Negro, C.; De Bellis, L.; Incrocci, L.; Pardossi, A.; Guidi, L. Effect of Drying Methods on Phenolic Compounds and Antioxidant Activity of Urtica dioica L. Leaves. Horticulturae 2021, 7, 10. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Murtijaya, J. Antioxidant Properties of Phyllanthus Amarus Extracts as Affected by Different Drying Methods. LWT-Food Sci. Technol. 2007, 40, 1664–1669. [Google Scholar] [CrossRef]
- Liu, J.; Jiao, Z.; Zhang, C.; Yang, W.; Liu, H.; Lv, Z. Efects of Diferent Drying Methods on Phenolic Contents, Antioxidant, and Tyrosinase Inhibitory Activity of Peach Blossoms. J. Food Meas. Charact. 2018, 12, 2339–2348. [Google Scholar] [CrossRef]
- Nunes, J.C.; Lago, M.G.; Castelo-Branco, V.N.; Oliveira, F.R.; Torres, A.G.; Perrone, D.; Monteiro, M. Effect of Drying Method on Volatile Compounds, Phenolic Profile and Antioxidant Capacity of Guava Powders. Food Chem. 2016, 197, 881–890. [Google Scholar] [CrossRef]
- Boeing, J.S.; Barizão, É.O.; e Silva, B.C.; Montanher, P.F.; de Cinque Almeida, V.; Visentainer, J.V. Evaluation of Solvent Effect on the Extraction of Phenolic Compounds and Antioxidant Capacities from the Berries: Application of Principal Component Analysis. Chem. Cent. J. 2014, 8, 48. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of Phenolic Compounds: A Review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Megala, J.; Geetha, A. Free Radical-Scavenging and H+, K+-ATPase Inhibition Activities of Pithecellobium Dulce. Food Chem. 2010, 121, 1120–1128. [Google Scholar] [CrossRef]
- Manna, P.; Bhattacharyya, S.; Das, J.; Ghosh, J.; Sil, P.C. Phytomedicinal Role of Pithecellobium Dulce against Ccl4-Mediated Hepatic Oxidative Impairments and Necrotic Cell Death. Evid.-Based Complement. Altern. Med. 2011, 2011, 1–17. [Google Scholar] [CrossRef]
- Rao, G.N.; Nagender, A.; Satyanarayana, A.; Rao, D.G. Preparation, Chemical Composition and Storage Studies of Quamachil (Pithecellobium Dulce L.) Aril Powder. J. Food Sci. Technol. 2011, 48, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Pío-León, J.F.; Díaz-Camacho, S.; Montes-Avila, J.; López-Angulo, G.; Delgado-Vargas, F. Nutritional and Nutraceutical Characteristics of White and Red Pithecellobium dulce (Roxb.) Benth Fruits. Fruits 2013, 68, 397–408. [Google Scholar] [CrossRef]
- Kubola, J.; Siriamornpun, S.; Meeso, N. Phytochemicals, Vitamin C and Sugar Content of Thai Wild Fruits. Food Chem. 2011, 126, 972–981. [Google Scholar] [CrossRef]
- Suganthi, A.; Josephine, R.M. Evaluating the Chemical Analysis Profile of Some Lesser Known Edible Fruits. Indo Am. J. Pharm. Sci. 2018, 5, 815–820. [Google Scholar] [CrossRef]
- da Silva, G.V.; Machado, B.A.S.; Oliveira, W.P.; de Silva, C.F.G.d.; de Quadros, C.P.; Druzian, J.I.; Ferreira, E.d.S.; Umsza-Guez, M.A. Effect of Drying Methods on Bioactive Compounds and Antioxidant Capacity in Grape Skin Residues from the New Hybrid Variety “BRS Magna”. Molecules 2020, 25, 3701. [Google Scholar] [CrossRef]
- Nowak, D.; Piechucka, P.; Witrowa-Rajchert, D.; Wiktor, A. Impact of Material Structure on the Course of Freezing and Freeze-Drying and on the Properties of Dried Substance, as Exemplified by Celery. J. Food Eng. 2016, 180, 22–28. [Google Scholar] [CrossRef]
- Bhatta, S.; Stevanovic Janezic, T.; Ratti, C. Freeze-Drying of Plant-Based Foods. Foods 2020, 9, 87. [Google Scholar] [CrossRef]
- Li, J.; Li, W.; Deng, Z.; Li, H.; Yu, Y.; Zhang, B. Comparison of Free, Conjugated, and Insoluble-bound Phenolics and Their Antioxidant Activities in Oven-drying and Freeze-drying Bamboo (Phyllostachys edulis) Shoot Tips. J. Food Sci. 2021, 86, 4223–4243. [Google Scholar] [CrossRef] [PubMed]
- Villota, H.; Santa-González, G.A.; Uribe, D.; Henao, I.C.; Arroyave-Ospina, J.C.; Barrera-Causil, C.J.; Pedroza-Díaz, J. Modulatory Effect of Chlorogenic Acid and Coffee Extracts on Wnt/β-Catenin Pathway in Colorectal Cancer Cells. Nutrients 2022, 14, 4880. [Google Scholar] [CrossRef]
- Sithara, T.; Arun, K.B.; Syama, H.P.; Reshmitha, T.R.; Nisha, P. Morin Inhibits Proliferation of SW480 Colorectal Cancer Cells by Inducing Apoptosis Mediated by Reactive Oxygen Species Formation and Uncoupling of Warburg Effect. Front. Pharmacol. 2017, 8, 640. [Google Scholar] [CrossRef] [PubMed]
- Alhamed, A.S.; Alqinyah, M.; Alghaith, A.F.; Algahtani, M.M.; Alqahtani, F.; Nasr, F.A.; Alqahtani, A.S.; Noman, O.M.; Bazaid, A.S.; AlMalki, R.H.; et al. Phytochemical Analysis and Anticancer Activity of the Pithecellobium Dulce Seed Extract in Colorectal Cancer Cells. Open Chem. 2023, 21, 20230362. [Google Scholar] [CrossRef]
- Sharma, M. Selective Cytotoxicity and Modulation of Apoptotic Signature of Breast Cancer Cells by Pithecellobium Dulce Leaf Extracts. Biotechnol. Prog. 2016, 32, 756–766. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Chinkwo, K.; Santhakumar, A.; Johnson, S.; Blanchard, C. Apoptosis Induction Pathway in Human Colorectal Cancer Cell Line SW480 Exposed to Cereal Phenolic Extracts. Molecules 2019, 24, 2465. [Google Scholar] [CrossRef]
- Vargas-Madriz, Á.F.; Luzardo-Ocampo, I.; Moreno-Celis, U.; Roldán-Padrón, O.; Chávez-Servín, J.L.; Vergara-Castañeda, H.A.; Martínez-Pacheco, M.; Mejía, C.; García-Gasca, T.; Kuri-García, A. Comparison of Phytochemical Composition and Untargeted Metabolomic Analysis of an Extract from Cnidoscolus Aconitifolius (Mill.) I. I. Johnst and Porophyllum Ruderale (Jacq.) Cass. and Biological Cytotoxic and Antiproliferative Activity In Vitro. Plants 2023, 12, 1987. [Google Scholar] [CrossRef]
- Dhanisha, S.S.; Drishya, S.; Guruvayoorappan, C. Pithecellobium Dulce Induces Apoptosis and Reduce Tumor Burden in Experimental Animals via Regulating Pro-Inflammatory Cytokines and Anti-Apoptotic Gene Expression. Food Chem. Toxicol. 2022, 161, 112816. [Google Scholar] [CrossRef]
- Mišković Špoljarić, K.; Šelo, G.; Pešut, E.; Martinović, J.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Antioxidant and Antiproliferative Potentials of Phenolic-Rich Extracts from Biotransformed Grape Pomace in Colorectal Cancer. BMC Complement. Med. Ther. 2023, 23, 29. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, S.K. Growth Inhibition by Caffeic Acid, One of the Phenolic Constituents of Honey, in HCT 15 Colon Cancer Cells. Sci. World J. 2012, 2012, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Martin, V.; Plaza-Calonge, M.d.C.; Soriano-Lerma, A.; Ortiz-Gonzalez, M.; Linde-Rodriguez, A.; Perez-Carrasco, V.; Ramirez-Macias, I.; Cuadros, M.; Gutierrez-Fernandez, J.; Murciano-Calles, J.; et al. Gallic Acid: A Natural Phenolic Compound Exerting Antitumoral Activities in Colorectal Cancer via Interaction with G-Quadruplexes. Cancers 2022, 14, 2648. [Google Scholar] [CrossRef]
- Nouri, Z.; Fakhri, S.; Nouri, K.; Wallace, C.E.; Farzaei, M.H.; Bishayee, A. Targeting Multiple Signaling Pathways in Cancer: The Rutin Therapeutic Approach. Cancers 2020, 12, 2276. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lin, S.; Yang, C.; Cai, S.; Li, W. Effect of KRAS Mutations and P53 Expression on the Postoperative Prognosis of Patients with Colorectal Cancer. Mol. Genet. Genom. Med. 2022, 10, e1905. [Google Scholar] [CrossRef]
- Sheikhnia, F.; Rashidi, V.; Maghsoudi, H.; Majidinia, M. Potential Anticancer Properties and Mechanisms of Thymoquinone in Colorectal Cancer. Cancer Cell Int. 2023, 23, 320. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.; Tong, J.H.M.; Chan, A.W.H.; Yu, J.; Kang, W.; To, K.F. Targeting the Oncogenic P53 Mutants in Colorectal Cancer and Other Solid Tumors. Int. J. Mol. Sci. 2019, 20, 5999. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.-Y.; Singh, A.K.; Chan, C.-H.; Deng, Y.-H.; Li, P.-Y.; Su, C.-W.; Wu, C.-Y.; Deng, W.-P. AGA Induces Sub-G1 Cell Cycle Arrest and Apoptosis in Human Colon Cancer Cells through P53-Independent/P53-Dependent Pathway. BMC Cancer 2023, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Anantharaju, P.G.; Gowda, P.C.; Vimalambike, M.G.; Madhunapantula, S.V. An Overview on the Role of Dietary Phenolics for the Treatment of Cancers. Nutr. J. 2016, 15, 99. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.I.; Othman, I.; Abas, F.; Lajis, N.H.; Naidu, R. Mechanism of Apoptosis Induced by Curcumin in Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 2454. [Google Scholar] [CrossRef] [PubMed]
- Meng, M.; Zhong, K.; Jiang, T.; Liu, Z.; Kwan, H.Y.; Su, T. The Current Understanding on the Impact of KRAS on Colorectal Cancer. Biomed. Pharmacother. 2021, 140, 111717. [Google Scholar] [CrossRef] [PubMed]
- Teixeira-Guedes, C.I.; Oppolzer, D.; Barros, A.I.; Pereira-Wilson, C. Phenolic Rich Extracts from Cowpea Sprouts Decrease Cell Proliferation and Enhance 5-Fluorouracil Effect in Human Colorectal Cancer Cell Lines. J. Funct. Foods 2019, 60, 103452. [Google Scholar] [CrossRef]
- Maugeri, A.; Calderaro, A.; Patanè, G.T.; Navarra, M.; Barreca, D.; Cirmi, S.; Felice, M.R. Targets Involved in the Anti-Cancer Activity of Quercetin in Breast, Colorectal and Liver Neoplasms. Int. J. Mol. Sci. 2023, 24, 2952. [Google Scholar] [CrossRef] [PubMed]
- Godínez-Santillán, R.I.; Chávez-Servín, J.L.; García-Gasca, T.; Guzmán-Maldonado, S.H. Caracterización Fenólica y Capacidad Antioxidante de Extractos Alcohólicos de Hojas Crudas y Hervidas de Cnidoscolus Aconitifolius (Euphorbiaceae). Acta Bot. Mex. 2019, 126, 1–15. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Feregrino-Pérez, A.A.; Berumen, L.C.; García-Alcocer, G.; Guevara-Gonzalez, R.G.; Ramos-Gomez, M.; Reynoso-Camacho, R.; Acosta-Gallegos, J.A.; Loarca-Piña, G. Composition and Chemopreventive Effect of Polysaccharides from Common Beans (Phaseolus Vulgaris L.) on Azoxymethane-Induced Colon Cancer. J. Agric. Food Chem. 2008, 56, 8737–8744. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Madriz, Á.F.; Luzardo-Ocampo, I.; Chávez-Servín, J.L.; Moreno-Celis, U.; Roldán-Padrón, O.; Vargas-Madriz, H.; Vergara-Castañeda, H.A.; Kuri-García, A. Comparison of Phenolic Compounds and Evaluation of Antioxidant Properties of Porophyllum Ruderale (Jacq.) Cass (Asteraceae) from Different Geographical Areas of Queretaro (Mexico). Plants 2023, 12, 3569. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, L.R.; Mazza, G. Assessing Antioxidant and Prooxidant Activities of Phenolic Compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Van Den Berg, R.; Haenen, G.R.M.M.; Van Den Berg, H.; Bast, A. Applicability of an Improved Trolox Equivalent Antioxidant Capacity (TEAC) Assay for Evaluation of Antioxidant Capacity Measurements of Mixtures. Food Chem. 1999, 66, 511–517. [Google Scholar] [CrossRef]
- Markossian, S.; Grossman, A.; Arkin, M.; Auld, D.; Austin, C.; Baell, J.; Brimacombe, B.; Chung, T.D.Y.; Coussens, N.P.; Dahlin, J.L.; et al. Assay Guidance Manual [Internet]; National Library Medicine: Bethesda, MD, USA, 2004. [Google Scholar]
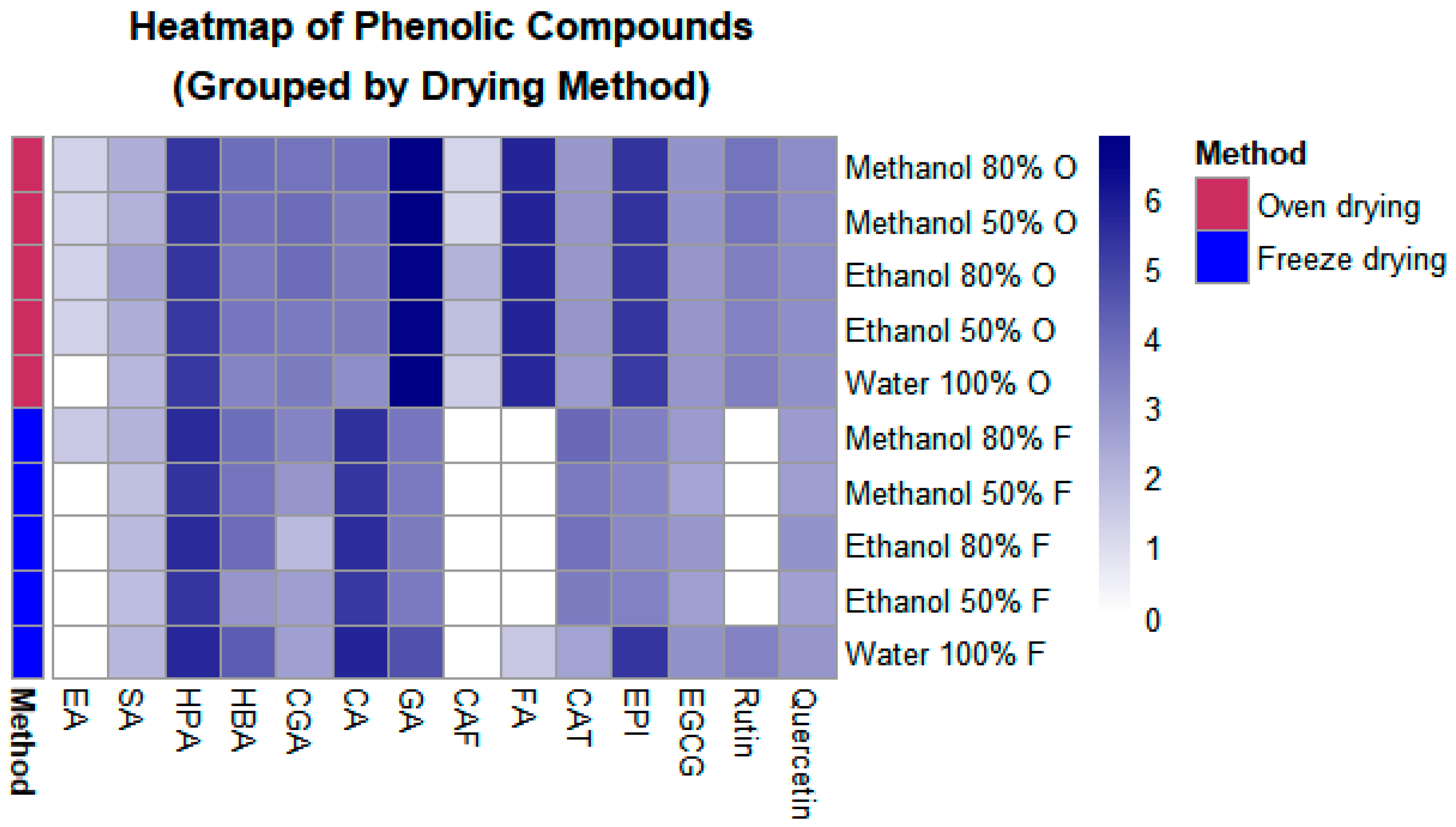
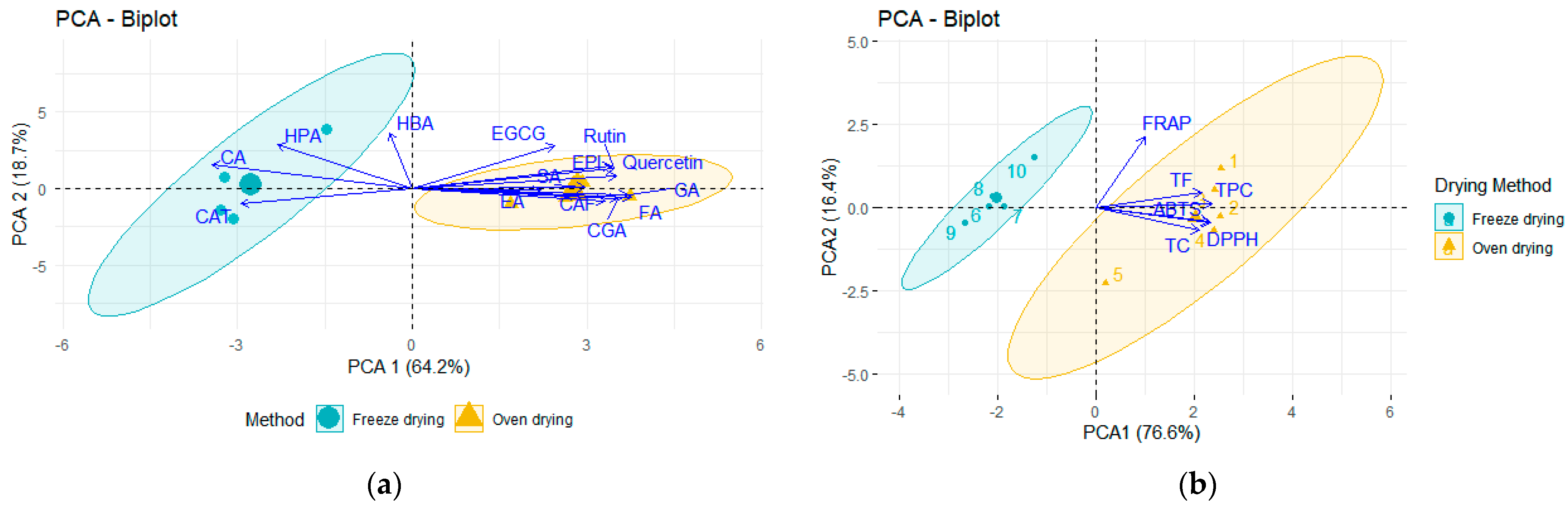
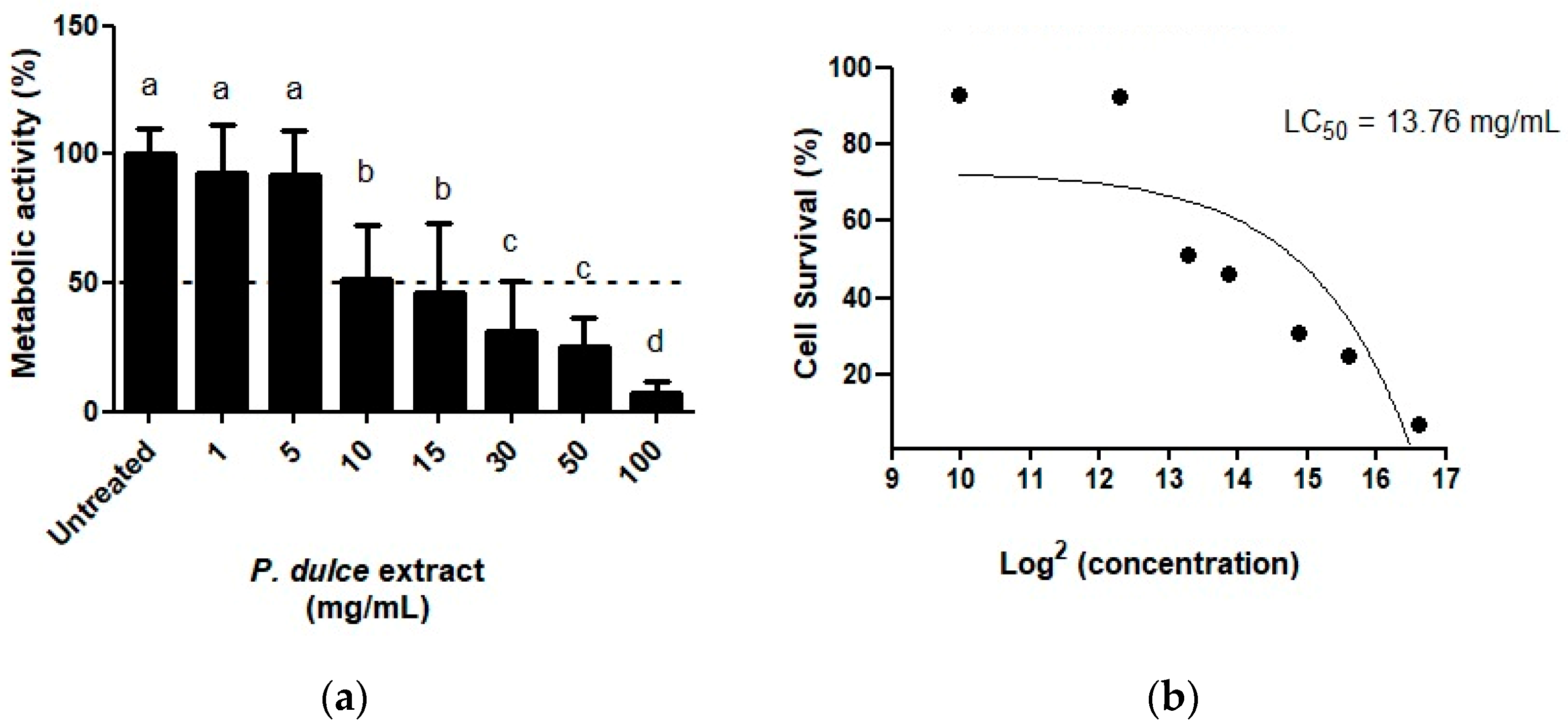
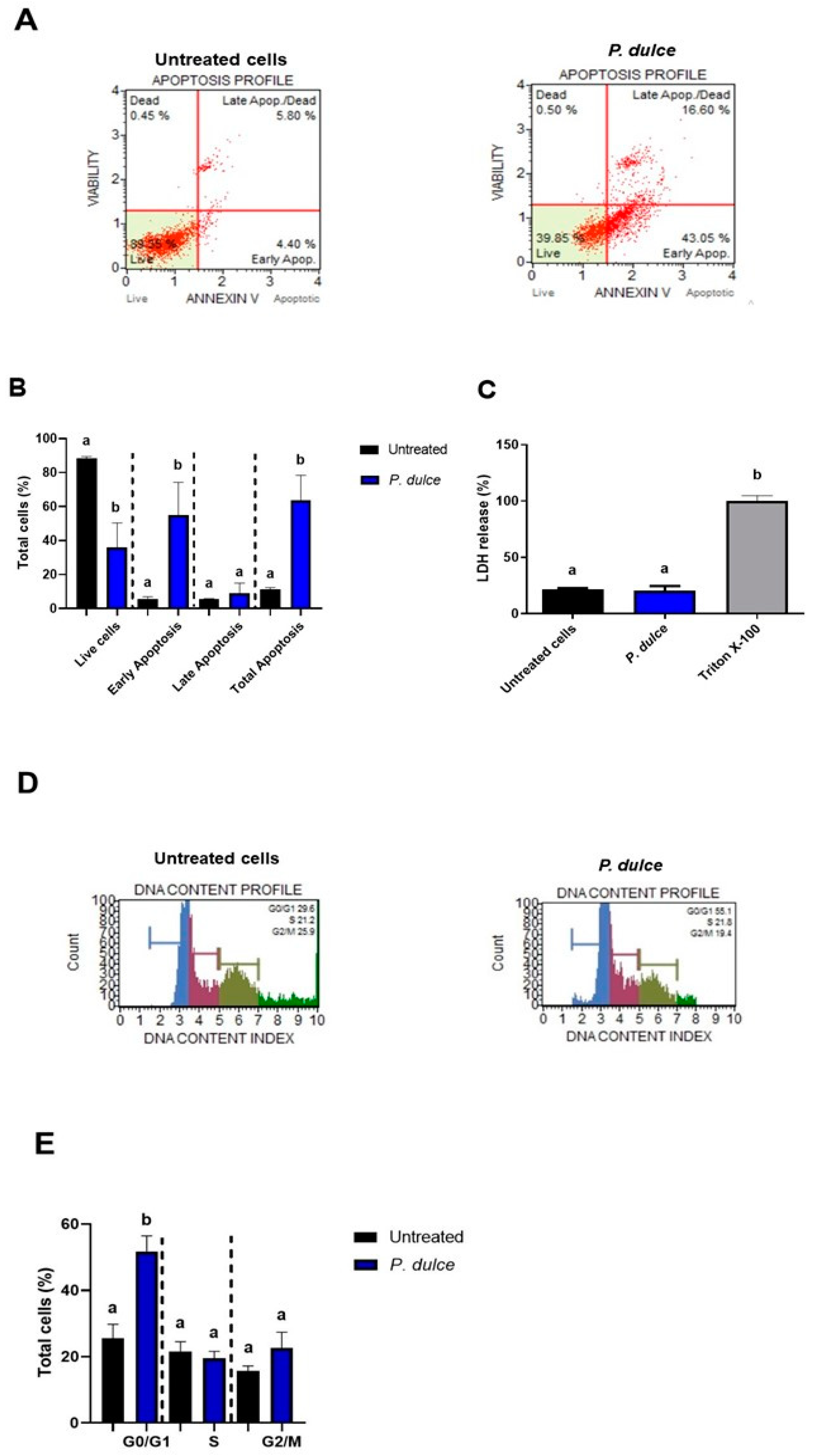
| Extracts | TPC (mg GAE/100 g LE) | TF (mg CE/100 g LE) | CT (mg CE/100 g LE) | |||
|---|---|---|---|---|---|---|
| Oven-Drying | Freeze-Drying | Oven-Drying | Freeze-Drying | Oven-Drying | Freeze-Drying | |
| Aqueous | 892.50 ± 56.67 bA | 799.14 ± 33.94 aA | 5.85 ± 1.94 bA | 5.10 ± 2.40 aA | 0.18 ± 0.07 aA | 0.13 ± 0.03 aA |
| 80% v/v E:W | 1061.01 ± 151.65 abA | 631.72 ± 28.85 bB | 20.93 ± 4.06 aA | 7.10 ± 4.31 aB | 0.16 ± 0.08 aA | 0.07 ± 0.01 bA |
| 50% v/v E:W | 1038.99 ± 45.39 abA | 651.55 ± 36.33 bB | 17.11 ± 3.01 aA | 6.67 ± 1.79 aB | 0.19 ± 0.08 aA | 0.08 ± 0.01 bA |
| 80% M:W | 1149.45 ± 69.27 aA | 637.36 ± 57.32 bB | 18.68 ± 2.52 aA | 7.90 ± 2.73 aB | 0.15 ± 0.07 aA | 0.08 ± 0.01 bA |
| 50% M:W | 1094.50 ± 54.22 abA | 726.53 ± 34.06 abB | 16.84 ± 2.00 aA | 6.05 ± 1.99 aB | 0.17 ± 0.08 aA | 0.09 ± 0.02 abA |
| Extracts | DPPH 1 | FRAP 1 | ABTS 1 | |||
|---|---|---|---|---|---|---|
| Oven-Drying | Freeze-Drying | Oven-Drying | Freeze-Drying | Oven-Drying | Freeze-Drying | |
| Aqueous | 37.82 ± 3.13 aA | 19.64 ± 0.87 bAB | 26.29 ± 1.97 bA | 35.03 ± 3.05 aA | 178.75 ± 9.63 aA | 151.84 ± 7.87 bA |
| 80% v/v E:W | 43.03 ± 3.27 aA | 22.28 ± 0.97 bA | 33.12 ± 6.53 aA | 30.67 ± 1.33 aAB | 190.06 ± 16.27 aA | 159.43 ± 19.13 bA |
| 50% v/v E:W | 42.43 ± 2.32 aA | 19.10 ± 0.83 bB | 30.71 ± 2.69 aA | 27.93 ± 2.06 aB | 200.26 ± 13.66 aA | 143.81 ± 11.87 bA |
| 80% M:W | 43.67 ± 2.62 aA | 21.35 ± 1.30 bAB | 34.97 ± 1.56 aA | 29.66 ± 1.20 bB | 190.16 ± 5.59 aA | 153.15 ± 9.27 bA |
| 50% M:W | 44.63 ± 2.00 aA | 21.44 ± 0.97 bAB | 31.74 ± 0.71 aA | 30.08 ± 0.81 aAB | 202.23 ± 6.32 aA | 158.34 ± 7.69 bA |
| Extract | T | Hydroxybenzoic Acids 1 | Hydroxycinnamic Acids 1 | 4-Hydroxyphenylacetic Acid 1 | Total 1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ellagic Acid | 4-Hydroxybenzoic Acid | Gallic Acid | Sinapic Acid | Chlorogenic Acid | Caffeic Acid | p-Coumaric Acid | Ferulic Acid | ||||
| Aqueous | O | ND | 26.50 ± 1.53 fB | 922.30 ± 218.00 aA | 6.26 ± 0.41 bcA | 33.69 ± 0.71 cA | 2.99 ± 0.60 c | 19.84 ± 2.00 fB | 283.90 ± 0.18 bA | 193.00 ± 0.57 dB | 1488.48 ± 224.00 aA |
| FD | ND | 77.63 ± 2.40 aA | 99.46 ± 4.93 bB | 6.20 ± 0.33 bcA | 11.85 ± 4.98 eB | ND | 300.00 ± 9.13 aA | 3.61 ± 0.03 cB | 289.30 ± 6.82 aA | 788.05 ± 28.63 bB | |
| 80% v/v E:W | O | 2.68 ± 0.02 b | 35.93 ± 4.43 eB | 762.90 ± 8.52 aA | 12.41 ± 3.04 aA | 49.69 ± 4.13 aA | 7.11 ± 0.04 a | 32.93 ± 0.23 efB | 307.90 ± 9.52 a | 202.90 ± 2.09 cdB | 1414.45 ± 32.01 aA |
| FD | ND | 49.35 ± 2.46 bA | 32.29 ± 0.82 bB | 5.56 ± 0.12 bcB | 5.53 ± 0.12 fB | ND | 250.40 ± 3.21 bA | ND | 264.10 ± 3.66 bA | 607.23 ± 10.39 bcB | |
| 50% v/v E:W | O | 2.61 ± 0.05 b | 38.07 ± 0.45 deA | 818.20 ± 45.62 aA | 8.30 ± 0.29 bA | 36.72 ± 0.66 bcA | 4.62 ± 0.12 b | 32.80 ± 1.00 efB | 300.70 ± 4.08 a | 193.9 ± 2.36 dB | 1435.92 ± 54.63 aA |
| FD | ND | 16.34 ± 1.58 gB | 35.67 ± 0.18 bB | 5.11 ± 0.24 cB | 13.03 ± 0.13 eB | ND | 195.20 ± 0.31 dA | ND | 210.00 ± 1.00 cA | 475.35 ± 3.43 cB | |
| 80% M:W | O | 2.59 ± 0.01 bB | 47.12 ± 6.00 bcdA | 867.90 ± 15.15 aA | 8.26 ± 0.53 bA | 42.52 ± 0.10 bA | 2.39 ± 0.03 cd | 45.59 ± 0.07 eB | 308.60 ± 2.91 a | 205.40 ± 0.72 cdB | 1530.37 ± 25.52 aA |
| FD | 3.27 ± 0.19 aA | 47.53 ± 0.85 bcA | 38.21 ± 4.44 bB | 7.21 ± 0.56 bcA | 26.12 ± 0.40 dB | ND | 232.70 ± 8.52 cA | ND | 266.30 ± 8.40 bA | 621.34 ± 23.36 bcB | |
| 50% M:W | O | 2.53 ± 0.01 b | 45.08 ± 0.61 bcdB | 956.50 ± 5.18 aA | 7.75 ± 0.04 bcA | 49.30 ± 1.13 aA | 2.20 ± 0.05 d | 33.44 ± 0.81 dB | 311.60 ± 3.83 a | 211.10 ± 0.65 cA | 1619.50 ± 12.30 aA |
| FD | ND | 40.91 ± 2.48 cdeA | 36.78 ± 2.40 bB | 4.91 ± 0.32 cB | 16.65 ± 0.99 eB | ND | 196.80 ± 10.63 efA | ND | 212.60 ± 10.84 cA | 508.65 ± 27.66 cB | |
| Extract | T | Flavanols 1 | Flavonols 1 | Total | |||
|---|---|---|---|---|---|---|---|
| (+)-Catechin | (−)-Epicatechin | (−)-Epigallocatechin-3-O-gallate | Rutin | Quercetin | |||
| Aqueous | O | 13.87 ± 0.84 efA | 177.10 ± 0.58 dB | 15.74 ± 0.10 cdB | 31.08 ± 0.19 bA | 18.39 ± 0.01 bA | 256.18 ± 1.72 cB |
| FD | 11.00 ± 0.33 fA | 205.80 ± 6.84 bcA | 18.80 ± 0.56 aA | 29.57 ± 1.21 abA | 16.49 ± 0.34 cB | 281.66 ± 9.27 bA | |
| 80% v/v E:W | O | 15.61 ± 0.17 eB | 203.30 ± 1.73 cA | 16.71 ± 0.18 bcA | 30.06 ± 8.76 b | 21.36 ± 0.08 aA | 287.04 ± 10.91 bA |
| FD | 44.39 ± 0.42 bA | 23.32 ± 1.85 fB | 15.34 ± 0.20 dB | ND | 17.59 ± 0.68 bB | 100.64 ± 3.15 deB | |
| 50% v/v E:W | O | 15.77 ± 0.17 e | 199.00 ± 0.81 c | 15.85 ± 0.11 cd | 29.59 ± 0.68 b | 20.66 ± 0.13 a | 280.87 ± 1.91 bA |
| FD | 32.72 ± 0.36 d | 28.99 ± 1.69 ef | 12.09 ± 0.13 f | ND | 12.46 ± 0.02 e | 86.26 ± 2.20 efB | |
| 80% v/v M:W | O | 15.54 ± 0.18 e | 210.90 ± 0.84 ab | 17.63 ± 0.05 b | 40.60 ± 0.43 a | 21.34 ± 0.03 a | 306.01 ± 1.53 aA |
| FD | 55.42 ± 2.02 a | 31.39 ± 1.18 e | 13.90 ± 0.81 e | ND | 13.60 ± 0.49 d | 114.31 ± 4.50 dB | |
| 50% v/v M:W | O | 15.95 ± 0.17 eB | 213.20 ± 0.46 aA | 17.22 ± 0.10 bA | 40.68 ± 0.19 a | 21.47 ± 0.11 aA | 308.52 ± 1.04 aA |
| FD | 36.54 ± 2.17 cA | 25.63 ± 1.67 efB | 10.44 ± 0.70 gB | ND | 12.66 ± 0.58 deB | 85.27 ± 5.12 fB | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Madriz, Á.F.; Kuri-García, A.; Luzardo-Ocampo, I.; Ferriz-Martínez, R.A.; García-Gasca, T.; Saldaña, C.; Vargas-Madriz, H.; Guzmán-Maldonado, S.H.; Chávez-Servín, J.L. Effect of Drying Methods on the Phenolic Profile and Antioxidant Capacity of Pithecellobium dulce (Roxb.) Benth. Aril and Its Inhibitory Properties on Human SW480 Colon Adenocarcinoma Cells. Molecules 2025, 30, 233. https://doi.org/10.3390/molecules30020233
Vargas-Madriz ÁF, Kuri-García A, Luzardo-Ocampo I, Ferriz-Martínez RA, García-Gasca T, Saldaña C, Vargas-Madriz H, Guzmán-Maldonado SH, Chávez-Servín JL. Effect of Drying Methods on the Phenolic Profile and Antioxidant Capacity of Pithecellobium dulce (Roxb.) Benth. Aril and Its Inhibitory Properties on Human SW480 Colon Adenocarcinoma Cells. Molecules. 2025; 30(2):233. https://doi.org/10.3390/molecules30020233
Chicago/Turabian StyleVargas-Madriz, Ángel Félix, Aarón Kuri-García, Ivan Luzardo-Ocampo, Roberto Augusto Ferriz-Martínez, Teresa García-Gasca, Carlos Saldaña, Haidel Vargas-Madriz, Salvador Horacio Guzmán-Maldonado, and Jorge Luis Chávez-Servín. 2025. "Effect of Drying Methods on the Phenolic Profile and Antioxidant Capacity of Pithecellobium dulce (Roxb.) Benth. Aril and Its Inhibitory Properties on Human SW480 Colon Adenocarcinoma Cells" Molecules 30, no. 2: 233. https://doi.org/10.3390/molecules30020233
APA StyleVargas-Madriz, Á. F., Kuri-García, A., Luzardo-Ocampo, I., Ferriz-Martínez, R. A., García-Gasca, T., Saldaña, C., Vargas-Madriz, H., Guzmán-Maldonado, S. H., & Chávez-Servín, J. L. (2025). Effect of Drying Methods on the Phenolic Profile and Antioxidant Capacity of Pithecellobium dulce (Roxb.) Benth. Aril and Its Inhibitory Properties on Human SW480 Colon Adenocarcinoma Cells. Molecules, 30(2), 233. https://doi.org/10.3390/molecules30020233












